100 - Investigation and Management of Nodules Less Than One Centimeter in Size
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > Section XVII - Other Tumors of the Lung > Chapter 117 - Adenoid Cystic Carcinoma and Other Primary Salivary Gland Type Tumors of the Lung
Chapter 117
Adenoid Cystic Carcinoma and Other Primary Salivary Gland Type Tumors of the Lung
Richard S. D'Agostino
Ronald B. Ponn
The submucosal serous and mucous glands of the tracheobronchial tree are similar to those of the major and minor salivary glands and can give rise to morphologically and biologically analogous tumors. Adenoid cystic carcinoma, mucoepidermoid carcinoma, mixed tumors of salivary gland type, acinic cell carcinoma, and oncocytoma have been included with carcinoid tumors under the misleading rubric bronchial adenomas. However, in contrast with true adenomas, adenoid cystic carcinoma, mucoepidermoid carcinoma, and acinic cell carcinoma are malignant lesions. Although their growth rate is often indolent, local invasion and lymph node metastases ultimately may occur, and, in some cases, biological behavior is aggressive. Mixed tumors and oncocytoma can behave in a benign or malignant fashion. These noncarcinoid, salivary gland type tumors of the lung are uncommon and constitute less than 1% of all primary lung neoplasms.
ADENOID CYSTIC CARCINOMA
Adenoid cystic carcinoma, also called cylindroma, most often arises in the salivary glands. Less frequent primary sites include the lung, breast, skin, uterine cervix, esophagus, and prostate.
Pathology
The majority of pulmonary adenoid cystic carcinomas develop centrally in the trachea and major bronchi. These otherwise uncommon tumors account for 20% to 30% of all primary tracheal malignancies. Dalton and Gatling (1990) and Inoue and colleagues (1991), among others, reported instances of primary adenoid cystic carcinoma arising peripherally and suggested that this may be the case in approximately 10% of patients. Hatton and associates (1993) reported a singular case of peripheral right upper lobe adenoid cystic carcinoma presenting with Pancoast's syndrome. Peripheral lesions, however, are more likely to represent metastases from an extrathoracic primary site. In a review of their own experience and previously reported series, Goldstraw and colleagues (1976) noted local invasion in up to 29% of cases. Hematogenous metastases at the time of presentation are rarely encountered. However, Maziak and co-workers (1996) noted metachronous hematogenous metastases in 44% of their patients.
On macroscopic inspection, adenoid cystic carcinoma appears white, pink, or light tan and is either polypoid or annular. The overlying mucosa is usually intact, although ulceration may occur. Direct transluminal extension is the rule. Malignant submucosal and perineural infiltration is common and often extends a considerable distance proximally and distally from the main tumor mass (Fig. 117-1).
Adenoid cystic carcinomas are histologically similar regardless of their primary site. The following three patterns are recognized: cribriform, tubular, and solid. The cribriform or cylindromatous subtype is the classic finding (Fig. 117-2). Nests and columns of small rounded or polyhedral cells arranged in a sievelike pattern surround glandlike spaces filled with periodic acid Schiff positive material. The tubular pattern is defined by cords of polygonal cells containing central ducts that are separated from surrounding stroma. The solid or basaloid pattern is morphologically the least differentiated and consists of sheets of small cuboidal cells with prominent mitoses and occasional small clusters of larger polygonal cells. Nomori and associates (1988) found that tumors with solid elements are associated with an aggressive clinical course and found the appearance of distant metastases more often than with other patterns. An individual tumor can display more than one pattern (Fig. 117-3).
By electron microscopy, Balazs (1986) showed that most tumors are dimorphous and contain both mature ductal cells
P.1769
and immature myoepithelial cells. A high proportion of immature elements correlated with increased infiltrative potential, whereas more circumscribed tumors had equal populations of ductal and myoepithelial cells. Moran (1995) noted that the immunohistochemical profile of adenoid cystic carcinoma is dominated by the myoepithelial cell proliferation. These tumor cells showed positive staining with both wide-spectrum and low-molecular-weight keratin as well as with vimentin and actin antibodies. Reactivity to S-100 protein has been variable. Staining for glial fibrillary acidic protein was uniformly negative. This latter finding can be used to distinguish adenoid cystic carcinoma from pleomorphic adenoma, a tumor that also has a prominent myoepithelial cell component. Although the histopathologic features of adenoid cystic carcinoma are usually distinctive enough to allow for definitive diagnosis by routine light microscopy, this tumor can be confused with adenocarcinoma, especially when faced with a small biopsy specimen. In such situations, the demonstration of a myoepithelial cell immunophenotype supports the diagnosis of adenoid cystic carcinoma. Lin and co-workers (2002) found eight of nine patients with tracheobronchial adenoid cystic carcinoma negative for the biomarkers p53, HER-2/neu, and COX-2. The one patient positive for HER-2/neu developed distant metastases 4 years postoperatively. DNA flow cytometric analysis demonstrated a higher synthetic phase fraction in their patients with higher grade tumors.
 |
Fig. 117-1. Perineural infiltration of adenoid cystic carcinoma is characteristic of this tumor and is the route of extension toward the mediastinum. Courtesy of Darryl Carter. |
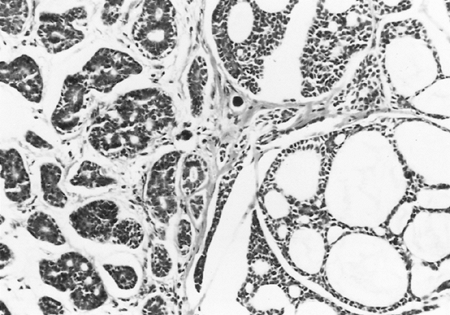 |
Fig. 117-2. Adenoid cystic carcinoma with the typical cribriform or cylindromatous pattern. |
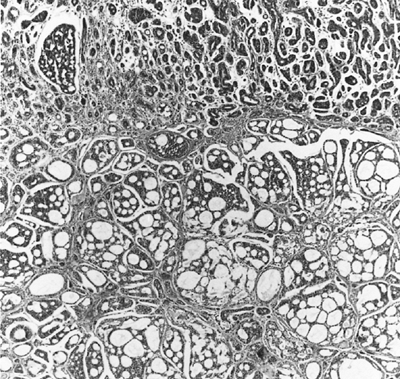 |
Fig. 117-3. Adenoid cystic carcinoma in the bronchial submucosa. The tumor is growing in a basaloid pattern beneath the intact ciliated epithelium. In the deeper zone, it displays the classic cribriform appearance. |
Clinical Features
Adenoid cystic carcinoma typically occurs in patients 40 to 50 years of age, but it has been reported in persons ranging in age from 18 to 82 years. Men and women are affected equally. There is no known association with tobacco use or environmental toxins. Because they are located centrally and are often not apparent on chest radiographs, most tumors are detected only after they produce symptoms. Patients generally describe one or more of the following problems: cough, dyspnea, sputum production, hemoptysis, wheezing, or recurrent pneumonias. In some cases, significant tracheal or main bronchial obstruction occurs early and can be life threatening. However, in many instances the intraluminal component is relatively small despite extensive submucosal and extraluminal spread. This pattern, coupled with typically slow growth, can produce an insidious presentation. An interval of several months to years may elapse
P.1770
between the onset of symptoms and the establishment of a diagnosis. Some patients are treated for prolonged periods for an erroneous diagnosis of asthma. The physical examination may demonstrate abnormal findings generally limited to the chest and may include diminished or absent breath sounds because of complete obstruction of a lobe or an entire lung, a unilateral wheeze from partial blockage of a main bronchus, or stridor produced by tracheal involvement, but the test results also may be negative. Peripheral adenopathy and clubbing are not features of adenoid cystic carcinoma.
Radiographic Features
The standard chest radiograph results may be normal, but often they show atelectasis of a lobe or an entire lung. A hilar or mediastinal mass may be present. Subtle changes in airway contour occasionally are noted, particularly with lesions of the intrathoracic trachea. On the other hand, tumor masses in the cervical trachea are often easily appreciated on plain neck radiographs (Fig. 117-4). Spizarny (1986) and Li (1990) and their colleagues showed that computed tomography (CT) scan accurately defines both intraluminal and transverse extraluminal tumor and is helpful in detecting metastases to the lung or pleura. As with other malignancies, the obliteration of fat planes is not a reliable predictor of local invasion. However, CT scan usually underestimates the longitudinal extent of airway involvement because of partial volume averaging and the tendency for submucosal tumor extension. Although rarely used, conventional tomography offers an excellent assessment of the intraluminal length of gross tumor because it yields images in the coronal and sagittal planes; it is inferior, however, for demonstrating extraluminal tumor mass (Fig. 117-5). Shanley (1991) and Akata (2001) and their colleagues have found magnetic resonance imaging, with its ability to image multiple anatomic planes and to provide variably weighted images, superior to CT for delineating the extent of both submucosal infiltration and mediastinal involvement. In some cases, contrast esophograms may be helpful by showing esophageal compression or invasion.
Diagnosis
Because adenoid cystic carcinomas usually are covered by normal bronchial epithelium, cytologic examination of the sputum is generally unrewarding unless the overlying respiratory epithelium has been eroded. Except for the unusual circumstance of a peripherally situated lesion, these tumors are identified at bronchoscopy, and the diagnosis is
P.1771
established by transmucosal biopsy. The typical bronchoscopic appearance is that of a broad-based polypoidal mass partially or completely obstructing the airway (Fig. 117-6). Alternatively, the tumor may appear as a diffuse, submucosal infiltration causing luminal distortion and obstruction (Fig. 117-7). Although Attar and associates (1985), among others, reported substantial bleeding after biopsy of bronchial tumors in some patients, hemorrhage appears to be more of a problem with carcinoid and mucoepidermoid tumors than with adenoid cystic carcinomas.
 |
Fig. 117-4. Anteroposterior (A) and lateral (B) neck radiographs show an endotracheal mass (arrows). Biopsy confirmed adenoid cystic carcinoma. |
 |
Fig. 117-5. Anteroposterior tomogram shows an adenoid cystic carcinoma involving the lateral wall of the distal trachea and right main-stem bronchus. |
 |
Fig. 117-6. Bronchoscopic view of an extensive polypoid adenoid cystic carcinoma of the distal trachea, the larger component of which obstructs the left bronchial orifice. Courtesy of John Beamis. |
 |
Fig. 117-7. Bronchoscopic appearance of an infiltrating adenoid cystic carcinoma causing distortion and partial obstruction of the distal trachea. Courtesy of John Beamis and Sergio Cavaliere. |
Treatment
The treatment of choice is resection when feasible. It cannot be overemphasized that submucosal and perineural infiltration commonly extends beyond macroscopic tumor boundaries. Intraoperative frozen section examination of resection margins should be performed routinely as a method of ensuring adequate tumor clearance. Conlan and associates (1978) and Grillo and Mathisen (1990) found that tumor spread to hilar and mediastinal lymph nodes does not preclude long-term survival. In the case of adenoid cystic carcinoma of the trachea, Grillo and Mathisen stressed that lymph nodes adjacent to the tumor should be resected and distant nodes sampled, but that extensive dissection should be avoided because it may jeopardize anastomotic healing. They also pointed out that, in some cases, one must accept a microscopically positive margin rather than extend the resection and produce tension on the suture line.
Tumors involving a lobar bronchus can often be managed with lobectomy, using bronchoplastic techniques where applicable. However, submucosal spread in the main-stem bronchus may mandate a pneumonectomy. Extensive tracheal or carinal involvement presents more complex situations.
P.1772
Maziak and colleagues (1996) noted carinal involvement in 13 of 38 patients. In such situations, the risks of resection must be considered carefully because nonresectional treatment methods can provide effective palliation for long periods. Perelman and Koroleva (1987) recommended preoperative transmucosal bronchoscopic biopsies at different levels above and below an obvious tumor as a guide to therapeutic planning. Pearson and associates (1984), Grillo and Mathisen (1990), and Perelman and Koroleva (1987) described their extensive experience with these situations. The techniques of bronchial sleeve lobectomy and tracheal and carinal resections are discussed in Chapters 28, 30, and 75.
Many patients with adenoid cystic carcinoma are not candidates for resection, and others are at high risk for postoperative local recurrence because of incomplete resection. Most adenoid cystic carcinomas are radiosensitive. Therefore, radiation therapy is the primary treatment for extensive inoperable cancers and for patients in whom resection carries an unacceptable risk. Fields and colleagues (1989) noted that in patients treated with radiation therapy alone, the rate of complete response was significantly related to a tumor dose of 6,000 cGy or more. Conventional low linear energy-transfer radiation therapy (photons or electrons) has also been applied as an adjunct to resection. Pearson and colleagues (1984) administered 3,000 to 3,500 cGy preoperatively. Patients with incomplete resection received additional treatment within 1 to 2 months of operation. Similarly, Perelman and Koroleva (1987) delivered preoperative and postresection irradiation in doses ranging from 3,000 to 6,500 cGy. Grillo and Mathisen (1990) referred most patients in their large series for postoperative irradiation (4,500 6,500 cGy) and noted a low incidence of local recurrence. However, it is worth noting that the results of treatment for incompletely resected or inoperable salivary gland adenoid cystic carcinoma with conventional radiation therapy have been suboptimal. Douglas and associates (1996), among others, have used high linear energy-transfer forms of radiation, such as fast neutrons, for advanced or inoperable head and neck adenoid cystic carcinoma with an apparent two- to threefold increase in locoregional control over conventional radiation therapy. Although Battermann and Mijnheer (1986), in an investigational trial of neutron radiation therapy for lung metastases of varying primary histologies, found one of the highest relative biological effectiveness ratios for adenoid cystic carcinoma, there has been no subsequent reported experience with this modality for primary pulmonary adenoid cystic carcinoma.
For many patients with unresectable or recurrent disease, tumor debulking by neodymium:yttrium-aluminum-garnet (Nd:YAG) laser ablation may provide satisfactory palliation. Diaz-Jimenez and colleagues (1990) reported laser treatment in five patients with unresectable adenoid cystic carcinoma causing tracheal and main-stem bronchial obstructions of 75% to 90% of luminal diameter. One to two laser applications provided palliation for up to 33 months in four cases. The remaining patient was treated on multiple occasions over the course of 49 months before dying of hemoptysis. Brutinel and co-workers (1987) found that four of their eight patients treated with Nd:YAG laser were asymptomatic at 6 months, and Huber and co-workers (1986) reported a single patient treated with laser and radiation therapy who was symptom free 1 year later. Treatment may be affected with either the fiberoptic or rigid bronchoscope, although the latter is favored by most clinicians because it allows easier tumor debulking by morcellation, rapid control of hemorrhage, and protection of the contralateral airway if so needed.
As reported by Personne and associates (1986), laser photocoagulation and radiation therapy are often used in combination for the palliation of symptoms related to adenoid cystic carcinoma. Laser ablation should be considered before radiation therapy in patients with critical proximal airway obstruction because irradiation in this setting may produce enough edema to cause asphyxia. Munsch and colleagues (1987) reported the dramatic case of a young woman with extensive unresectable tracheal involvement who developed life-threatening respiratory distress after the start of radiation therapy. Oxygenation was maintained by partial cardiopulmonary bypass during which time the tracheal tumor was sufficiently debulked through the rigid bronchoscope to allow tracheal stenting with a silicone prosthesis. Endoluminal brachytherapy is another method that appears to have a palliative role. Ryan and associates (1986) reported prolonged survival with combinations of Nd:YAG laser, external beam irradiation, and endobronchial brachytherapy. Chin and associates (1991) also reported a favorable result in a patient treated with laser and brachytherapy.
We are aware of no published data regarding the efficacy of systemic chemotherapy for pulmonary adenoid cystic carcinoma. Chemotherapy is used for the palliative treatment of salivary gland adenoid cystic carcinoma. However, because this tumor is generally slow growing, the response rates are frequently disappointing. Methotrexate, cyclophosphamide, bleomycin, vincristine, cisplatin, 5-fluorouracil, adriamycin, and epirubicin have all shown some degree of activity when used as single agents. Dimery (1990) and Licitra (1996) and their associates, among others, have reported the use of combination chemotherapy in salivary gland adenoid cystic carcinomas. The combination regimens have generally consisted of cyclophosphamide, doxorubicin, and cisplatin, with or without 5-fluorouracil. Objective response rates range from 18% to 40%, and the median duration of response is generally less than 12 months.
Prognosis
Because adenoid cystic carcinoma tends to grow slowly and recur locally, prolonged survival with persistent disease is not unusual. As noted, purely palliative therapy may offer
P.1773
long-term, symptom-free survival. Carter and Eggleston (1979) compiled the results of several studies including patients who were treated both definitively and palliatively and noted that the 5-, 10-, and 20-year survival rates were 85%, 55%, and 20%, respectively. Studies of patients who have undergone resection for central adenoid cystic carcinomas, often with adjuvant radiation therapy, document favorable results. Perelman and Koroleva (1987) reported a 66% survival rate at 5 years and a 56% survival rate at 15 years. Pearson and associates (1974) reported that 8 of 14 patients (57%) were free of recurrent tumor from 2 to 18 years after therapy. In an update of that experience, Maziak and associates (1996) reported a 79% 5-year and a 51% 10-year survival rate for 32 patients treated with primary resection. Twenty-six of these patients received adjuvant radiation therapy. These investigators found a statistically nonsignificant trend for an increased 10-year survival rate in those patients who underwent complete resection (69% vs. 39% for incomplete resection). Kanematsu and colleagues (2002) noted a 91% 5-year and 76% 10-year local recurrence-free survival rate in 11 patients who underwent resection. Included in that group were 6 patients with residual microscopic tumor, 5 of whom received postoperative radiation therapy. Grillo and Mathisen (1990) reported that only 7 of 60 patients who underwent surgery (12%) had died of their tumors during the 26-year study period and that 75% of those who survived surgery were alive and tumor free at varying postoperative intervals. However, local recurrence can occur many years after complete resection.
MUCOEPIDERMOID CARCINOMA
Mucoepidermoid carcinoma, first reported by Smetana and associates (1952), is an uncommon lesion that accounts for approximately 1% of primary malignant bronchial gland tumors and less than 0.2% of all lung neoplasms. It is morphologically similar to the mucoepidermoid tumor of the salivary glands.
Pathology
Like their salivary gland counterparts, bronchial mucoepidermoid carcinomas are classified as high grade or low grade on the basis of their histologic appearance. Most mucoepidermoid carcinomas arise beyond the carina, usually main-stem bronchi, but occasionally in lobar or segmental airways. Unlike adenoid cystic carcinoma, tracheal involvement is uncommon. Most tumors appear grossly as intrabronchial polypoid masses without a significant extraluminal component. Penetration of the bronchial wall and a more infiltrative growth pattern are usually associated with the high-grade malignant tumors.
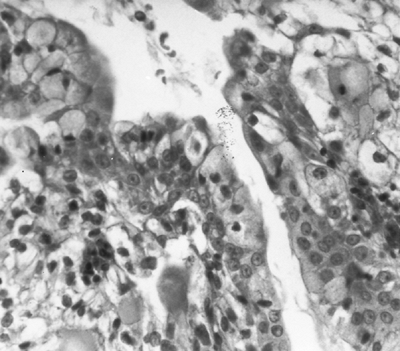 |
Fig. 117-8. High-power photomicrograph shows clear cells and mucin-secreting cells in a low-grade mucoepidermoid carcinoma. Courtesy of Irwin Nash. |
Histologically, mucoepidermoid carcinomas comprise varying mixtures of clear non-mucus-secreting columnar cells and mucus-rich goblet cells (Fig. 117-8), along with basaloid, transitional, and epidermoid cells. Although low-grade malignant tumors can have mixed cystic and solid histologic characteristics, the cystic component usually predominates. Microscopic invasion of the bronchial submucosa is common, but extension into pulmonary parenchyma is unusual. Although mild cytologic atypia can be seen, mitoses are rare. Metastasis to hilar or mediastinal lymph nodes is unusual.
High-grade tumors more commonly show areas of solid growth, but mixed solid and cystic or predominantly glandular growth can be seen (Fig. 117-9). Cellular atypia, mitotic activity, areas of necrosis, and abrupt transition from one cell type to another are characteristic. The high-grade variant of mucoepidermoid carcinoma occasionally can be difficult to distinguish from adenosquamous carcinoma of the lung. Klacsmann and colleagues (1979) proposed several criteria by which this differentiation can be made. The diagnostic confusion is compounded by infrequent reports, such as that of Barsky and associates (1983), of mucoepidermoid tumors with low-grade histologic features that clinically behave in an aggressive fashion.
Clinical Features
Although some investigators have noted an equal dispersion among sexes, Turnbull and colleagues (1971) and Vadasz and Egervary (2000) have noted that men are affected two to three times as frequently as women. The average age of patients in published series varies widely from
P.1774
35 to 53 years, with patients as young as 3 years and as old as 75 years having been reported. Yousem and Hochholzer (1987) found that more than 50% of low-grade lesions occurred in patients younger than 30 years of age, whereas the majority of high-grade tumors were found in people over 30 years of age. As with adenoid cystic carcinoma, there is no apparent relationship to tobacco use or exposure to environmental toxins.
 |
Fig. 117-9. Mucoepidermoid carcinoma consisting predominantly of solid nests of squamous cells with foci of glandular elements and mucin production (upper right). |
Most patients present with cough, dyspnea, wheezing, hemoptysis, or obstructive pneumonia. The average duration of symptoms is between 8 and 18 months, but some individuals report symptoms for many years. An occasional patient is asymptomatic. The occurrence of pain, weight loss, or other constitutional symptoms suggests a more aggressive or disseminated tumor. The results of physical examination may be normal, or signs of partial or total airway obstruction may be noted.
 |
Fig. 117-10. Computed tomographic scan shows a well-circumscribed mass arising from the lateral tracheal wall with no apparent extraluminal component. Biopsy documented a low-grade mucoepidermoid carcinoma. |
Radiographic Features
The radiographic appearance of mucoepidermoid carcinoma is similar to that described for adenoid cystic tumors. The chest radiograph results may be normal or may show signs of whole lung, lobar, or segmental obstruction with or without an associated proximal mass. A distinct intraluminal tumor, however, is generally identified by CT scan (Fig. 117-10), which may also demonstrate significant destruction of chronically obstructed parenchymal areas (Fig. 117-11). CT scan is also valuable for assessing local invasion and nodal metastases in cases of high-grade tumors. Mucoepidermoid carcinoma rarely presents as an isolated peripheral pulmonary nodule or mass.
Diagnosis
As with adenoid cystic tumors, the diagnosis of mucoepidermoid carcinoma is made most often by bronchoscopic biopsy. The tumors appear as soft, well-demarcated polypoid masses and generally have a small base (Fig. 117-12). Conlan and associates (1978) reported hemorrhage following biopsy in 2 of 12 patients in their series, but neither required special measures for control. Because the overlying
P.1775
mucosa is usually intact, sputum cytology results are negative in most cases.
 |
Fig. 117-11. Computed tomographic scan shows a polypoid mass arising from the left lower lobe bronchus and extending into but not invading the distal main-stem bronchus. The lower lobe is destroyed and shrunken. Biopsy showed a mucoepidermoid carcinoma. |
 |
Fig. 117-12. Endoscopic view of a well-circumscribed polypoid mucoepidermoid carcinoma incompletely obstructing the left main-stem bronchus. |
Treatment
Most patients present with low-grade tumors that are either localized or minimally invasive. These lesions are best treated by complete resection, often using bronchoplastic techniques to conserve as much normal lung as possible. Hilar and mediastinal node sampling is also performed. Patients with the high-grade variant should be managed similarly, but they frequently have unresectable disease attributable to extensive local invasion. Occasionally, patients with low-grade tumors are found to have microscopically positive margins after resection. The management and ultimate fate of such patients is uncertain at this time. Heitmiller and associates (1989) have used reoperation and resection successfully in such instances.
The role of radiation therapy, either as the primary therapy or as preoperative or postoperative adjuvant treatment, is undefined. Turnbull and colleagues (1971), as well as Heitmiller and associates (1989), noted radiation therapy to be ineffective in altering the course of high-grade tumors. However, Leonardi and associates (1978) reported one patient who had sufficient regression of an endobronchial tumor after treatment with 3,000 cGy to allow resection by pneumonectomy. As with adenoid cystic carcinoma, no data are available concerning the efficacy of chemotherapy. Endobronchial laser and brachytherapy should be considered for palliation of obstructive symptoms in some patients, but clinical experience with these methods in mucoepidermoid carcinoma is minimal.
Prognosis
Patients with low-grade tumors who undergo complete resection can be expected to be cured of their disease. In contrast, Turnbull (1971), Leonardi (1978), and Heitmiller (1989) and their associates all reported 100% mortality rates at 11 to 28 months in cases of high-grade mucoepidermoid carcinoma. Similarly, Vadasz and Egervary (2000) noted a 31% 5-year survival rate in 29 patients with high-grade tumors, and all 5 of their patients with mediastinal lymph node involvement died within 5 years. The prognosis is intermediate for patients with low-grade tumors who have undergone operation but have microscopically involved bronchial resection margins. The role of adjuvant treatment in this group is unknown. Serial radiographic and bronchoscopic surveillance is indicated to detect local recurrence.
MIXED TUMOR OF SALIVARY GLAND TYPE
Mixed tumor of salivary gland type, also known as pleomorphic adenoma, is rarely encountered in the lung and tracheobronchial tree. Payne and associates (1965) were the first to report two cases of mixed tumors arising in bronchial glands. In a literature review, Moran (1995) could identify only 16 reported instances of this tumor. Patients range in age from 35 to 74 years, and there is no apparent gender predilection. Symptoms of bronchial obstruction such as cough, dyspnea, and wheezing are most common, but some patients have had fever, weight loss, and pleural effusion. Other patients are asymptomatic, and the lesion is discovered incidentally. Chest radiographs can show a pulmonary mass, postobstructive atelectasis or pneumonitis, or an associated pleural effusion. Reported tumors have ranged in size from 2 to 16 cm. Macroscopically, these tumors are soft to rubbery in texture and have a gray-white myxoid appearance. Most parenchymal tumors are well circumscribed. Endobronchial tumors present as a polypoid mass with some degree of bronchial obstruction.
Histologically, these are biphasic neoplasms showing admixtures in varying proportions of epithelial and stromal elements. Moran and colleagues (1994) have identified three distinct histologic patterns: (a) the classical mixed tumor with prominent glandular component and chondromyxoid stroma; (b) the solid variant with few glandular structures and predominant solid sheets of myoepithelial cells set against a myxoid background; and (c) obvious cytologic features of malignancy with trabeculae of myoepithelial cells set in a prominent myxoid background. Immunohistochemically,
P.1776
these tumors react strongly with low-molecular-weight keratin antibodies and with antibodies against muscle-specific actin.
Because mixed tumors of the lung can be either benign or malignant, careful histopathologic evaluation is mandatory. The therapeutic goal is complete surgical resection. Most of the reported patients were treated with lobectomy, bilobectomy, or pneumonectomy. Patients with small circumscribed and completely resected lesions have done well. However, two reported patients with large poorly circumscribed masses and histologic features of malignancy have developed recurrent disease despite resection. For such patients the role of adjuvant therapy needs to be defined.
ACINIC CELL CARCINOMA
Fechner and co-workers (1972) were the first to report a primary pulmonary neoplasm that was histologically and ultrastructurally identical to acinic cell carcinomas arising in the salivary glands. Since then, only 14 other instances of this entity have been reported. These tumors usually occur in adults, but reported ages of patients range from 12 to 75 years. There is no apparent sex predilection. These lesions can present as an endobronchial or endotracheal mass or as a peripheral nodule and range in size between 1 and 4.5 cm. Presenting symptoms correspond to the size and location of the tumor. Endobronchial lesions produce symptoms of bronchial obstruction such as cough or wheezing. Peripheral lesions may be totally asymptomatic and discovered as incidental radiographic findings. Macroscopically, these tumors are usually well circumscribed, although not encapsulated, and tan-white or yellow in color.
Histologically, four different growth patterns are recognized: solid, acinar, microcystic, and papillary-cystic. Additionally, oncocytic, clear, and vacuolated cell populations are observed. In a detailed pathologic analysis of five pulmonary acinic cell carcinomas, Moran and colleagues (1992) found these lesions to stain positively with low-molecular-weight and broad-spectrum keratin antibodies as well as epithelial membrane antigen antibodies. Stains for vimentin, S-100 protein, chromogranin, and lysozyme were uniformly negative. Electron microscopy shows abundant zymogen-type cytoplasmic granules characteristic of acinar-type secretory cells. These ultrastructural and immunohistochemical characteristics can be of use in differentiating pulmonary acinic cell carcinomas from other morphologically similar primary and metastatic lung tumors.
Most reported cases have been managed by primary surgical resection including segmentectomy, lobectomy, bilobectomy, and tracheal resection. The results of treatment appear to be excellent because there have been no reported deaths from this lesion. Ansari and associates (1996) reported endoscopic laser tumor ablation to improve airway patency before tracheal resection. Horowitz and Kronenberg (1994) reported a case of tracheal acinic cell carcinoma removed through the bronchoscope with electrocauterization of the tumor base. This patient has been followed for 8 years without evidence of recurrence. Ukoha and colleagues (1999) have reported the only documented case of primary pulmonary acinic cell carcinoma metastatic to interlobar lymph nodes. Their patient was treated with lobectomy, mediastinal lymphadenectomy, and adjuvant radiation therapy and remained without recurrence 20 months postoperatively.
ONCOCYTOMA
Oncocytoma is the most infrequently occurring of all salivary gland type pulmonary neoplasms, and only a handful of cases have been documented. This tumor is composed of oncocytes, a descriptive term for epithelial cells with abundant granular eosinophilic cytoplasm and relatively small nuclei. A variety of organelles can impart an eosinophilic granular appearance to the cytoplasm by light microscopy, and a true oncocytoma is defined by the sole ultrastructural criterion of marked mitochondrial hyperplasia. The cause of the mitochondrial hyperplasia is unknown. Oncocytes are normally found in mature organs forming part of the lining epithelium of glandular ducts and acini. They have been found in the salivary glands, thyroid, buccal mucosa, breast, trachea, kidney, and gastrointestinal tract, as well as in other locations. According to Hamperl (1962), oncocytes represent a special metaplastic transformation of epithelial cells, and their numbers increase in frequency with the advancing age of the individual. Tashiro and associates (1995) demonstrated immunoreactivity to cytokeratin and vimentin but not to alpha-actin. Oncocytomas are different from oncocytic carcinoids, which demonstrate biphasic populations of oncocytic and carcinoid cells or the presence of neurosecretory granules by electron microscopy.
Fechner and Bentinck (1973) reported the first case of a true pulmonary oncocytoma confirmed by electron microscopy. Subsequent isolated instances of pulmonary oncocytoma have been reported by Santos-Briz and colleagues (1977), Fernandez and Nyssen (1982), Cwierzyk and associates (1985), and Tesluk and Dajee (1985). Patients range in age from 22 to 68 years, and most are men. The presenting symptoms have been cough, pneumonitis, or chest discomfort. One patient's tumor was discovered as an incidental radiographic finding. Chest radiographs can show a well-circumscribed parenchymal mass or an area of infiltrate distal to an obstructed bronchus. These tumors are yellowish tan or reddish brown in color. Patients were treated with local excision or with lobectomy, and there have been no reported instances of recurrence. Nielsen (1985) reported the only case of bronchial oncocytoma with an infiltrative growth pattern and a microscopic metastasis to a parabronchial lymph node. The patient underwent lobectomy and remained without recurrence 2 years later.
P.1777
REFERENCES
Akata S, et al: Multiplanar reconstruction MR image of primary adenoid cystic carcinoma of the central airway (MPR of central airway adenoid cystic carcinoma). Clin Imaging 25:332, 2001.
Ansari MA, et al: Upper airway obstruction secondary to acinic cell carcinoma of the trachea: use of Nd:YAG laser. Chest 110:1120, 1996.
Attar S, et al: Bronchial adenoma: a review of 51 patients. Ann Thorac Surg 40:126, 1985.
Balazs M: Adenoid cystic (cylindromatous) carcinoma of the trachea: an ultrastructural study. Histopathology 10:425, 1986.
Barsky SH, et al: Low-grade mucoepidermoid carcinoma of the bronchus with high-grade biological behavior. Cancer 51:1505, 1983.
Battermann JJ, Mijnheer BJ: The Amsterdam fast neutron radiotherapy project: a final report. Int J Radiat Oncol Biol Phys 12:2093, 1986.
Brutinel WM, et al: A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest 91:159, 1987.
Carter D, Eggleston J: Fascicle 17. In: Tumors of the Lower Respiratory Tract. Washington, DC: Armed Forces Institute of Pathology, 1979.
Chin HW, et al: Endobronchial adenoid cystic carcinoma. Chest 100:1464, 1991.
Conlan AA, et al: Adenoid cystic carcinoma (cylindroma) and mucoepidermoid carcinoma of the bronchus. Factors affecting survival. J Thorac Cardiovasc Surg 76:369, 1978.
Cwierzyk TA, et al: Pulmonary oncocytoma. Report of a case with cytologic, histologic and electron microscopic study. Acta Cytol 29:620, 1985.
Dalton L, Gatling RR: Peripheral adenoid cystic carcinoma of the lung. South Med J 83:577, 1990.
Diaz-Jimenez JP, Canela-Cardona M, Maestre-Alcacer J: Nd:YAG laser photoresection of low-grade malignant tumors of the tracheobronchial tree. Chest 97:920, 1990.
Dimery I, et al: Fluorouracil, doxorubicin, cyclophosphamide and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J Clin Oncol 8:1056, 1990.
Douglas JG, et al: Neutron radiotherapy for adenoid cystic carcinoma of minor salivary glands. Int J Radiat Oncol Biol Phys 36:87, 1996.
Fechner RE, Bentinck BA: Ultrastructure of bronchial oncocytoma. Cancer 31:1451, 1973.
Fechner RE, Bentinck BA, Askew JB: Acinic cell tumor of the lung: a histologic and ultrastructural study. Cancer 29:501, 1972.
Fernandez MA, Nyssen J: Oncocytoma of the lung. Can J Surg 25:332, 1982.
Fields JN, Rigaud G, Emani BN: Primary tumors of the trachea. Results of radiation therapy. Cancer 63:2429, 1989.
Goldstraw P, et al: The malignancy of bronchial adenoma. J Thorac Cardiovasc Surg 72:309, 1976.
Grillo HC, Mathisen DJ: Primary tracheal tumors: treatment and results. Ann Thorac Surg 49:69, 1990.
Hamperl H: Benign and malignant oncocytoma. Cancer 15:1019, 1962.
Hatton MQF, Allen MB, Cooke NJ: Pancoast syndrome: an unusual presentation of adenoid cystic carcinoma. Eur Respir J 6:271, 1993.
Heitmiller RF, et al: Mucoepidermoid lung tumors. Ann Thorac Surg 47:394, 1989.
Horowitz Z, Kronenberg J: Acinic cell carcinoma of the trachea. Auris Nasus Larynx 21:193, 1994.
Huber RM, et al: Adenoid cystic carcinoma masquerading as asthma: resection by laser. J Respir Dis 69:195, 1986.
Inoue H, et al: Peripheral pulmonary adenoid cystic carcinoma with substantial submucosal extension to the proximal bronchus. Thorax 46:147, 1991.
Kanematsu T, et al: Treatment outcome of resected and nonresected primary adenoid cystic carcinoma of the lung. Ann Thorac Cardiovasc Surg 8:74, 2002.
Klacsmann PG, Olson JL, Eggleston JC: Mucoepidermoid carcinoma of the bronchus. Cancer 43:1720, 1979.
Leonardi HK, et al: Tracheobronchial mucoepidermoid carcinoma. Clinicopathological features and results of treatment. J Thorac Cardiovasc Surg 76:431, 1978.
Li W, Ellerbroek NA, Libshitz HI: Primary malignant tumors of the trachea. A radiologic and clinical study. Cancer 66:894, 1990.
Licitra L, et al: Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol 7:640, 1996.
Lin CM, et al: Adenoid cystic carcinoma of the trachea and bronchus a clinicopathologic study with DNA flow cytometric analysis and oncogene expression. Eur J Cardiothorac Surg 22:621, 2002.
Maziak DE, et al: Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 112:1522, 1996.
Moran CA: Primary salivary gland-type tumors of the lung. Semin Diagn Pathol 12:106, 1995.
Moran CA, Suster S, Koss MN: Acinic cell carcinoma of the lung ( Fechner tumor ). A clinicopathologic, immunohistochemical and ultrastructural study of five cases. Am J Surg Pathol 16:1039, 1992.
Moran CA, et al: Benign and malignant salivary gland-type mixed tumors of the lung. Clinicopathologic and immunohistochemical study of eight cases. Cancer 73:2481, 1994.
Munsch C, Westaby S, Sturridge M: Urgent treatment for a nonresectable, asphyxiating tracheal cylindroma. Ann Thorac Surg 43:663, 1987.
Nielsen AL: Malignant bronchial oncocytoma: case report and review of the literature. Hum Pathol 16:852, 1985.
Nomori H, et al: Adenoid cystic carcinoma of the trachea and main-stem bronchus. A clinical, histopathologic, and immunohistochemical study. J Thorac Cardiovasc Surg 96:271, 1988.
Payne WS, Schier J, Woolner LB: Mixed tumors of the bronchus (salivary gland type). J Thorac Cardiovasc Surg 49:663, 1965.
Pearson FG, Todd TRJ, Cooper JD: Experience with primary neoplasms of the trachea and carina. J Thorac Cardiovasc Surg 88:511, 1984.
Pearson FG, et al: Adenoid cystic carcinoma of the trachea. Ann Thorac Surg 18:16, 1974.
Perelman MI, Koroleva NS: Primary tumors of the trachea. In Grillo H, Eschapasse H (eds): International Trends in General Thoracic Surgery. Vol. 2. Philadelphia: WB Saunders, 1987.
Personne C, et al: Indications and technique for endoscopic laser resections in bronchology: a critical analysis based upon 2,284 resections. J Thorac Cardiovasc Surg 91:710, 1986.
Ryan KL, Lowy J, Harrell JH: Management of adenoid cystic carcinoma. Chest 89(suppl):503, 1986.
Santos-Briz A, et al: Oncocytoma of the lung. Cancer 40:1330, 1977.
Shanley DJ, Daum-Kowalski R, Embry RL: Adenoid cystic carcinoma of the airway: MR findings. AJR 156:1321, 1991.
Smetana HF, Iverson L, Swann LL: Bronchogenic carcinoma, an analysis of 100 autopsy cases. Milit Surg 111:335, 1952.
Spizarny DL, et al: CT of adenoid cystic carcinoma of the trachea. AJR 146:1129, 1986.
Tashiro Y, et al: Pulmonary oncocytoma: report of a case in conjunction with an immunohistochemical and ultrastructural study. Pathol Int 45:448, 1995.
Tesluk H, Dajee A: Pulmonary oncocytoma. J Surg Oncol 29:173, 1985.
Turnbull AD, et al: Mucoepidermoid tumors of bronchial glands. Cancer 28:539, 1971.
Ukoha OO, et al: Acinic cell carcinoma of the lung with metastasis to lymph nodes. Chest 115:591, 1999.
Vadasz P, Egervary M: Mucoepidermoid bronchial tumors: a review of 34 operated cases. Eur J Cardiothorac Surg 17:566, 2000.
Yousem SA, Hochholzer L: Mucoepidermoid tumors of the lung. Cancer 60:1346, 1987.
EAN: 2147483647
Pages: 203