2 - Lens
Editors: Tasman, William; Jaeger, Edward A.
Title: Wills Eye Hospital Atlas of Clinical Ophthalmology , The, 2nd Edition
Copyright 2001 Lippincott Williams & Wilkins
> Table of Contents > Chapter 2 - Lens
function show_scrollbar() {}
Chapter 2
Lens
S. Gregory Smith
Ralph C. Eagle Jr.
Edward A. Jaeger
William Tasman
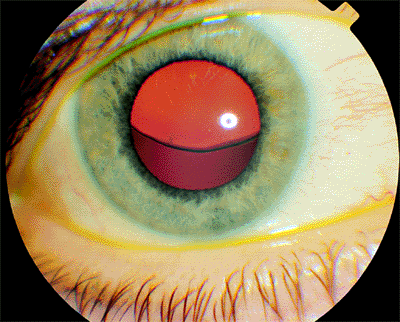 |
| The right eye of a 34-year-old woman with Marfan syndrome shows the typical superior subluxation of the lens. The pupil has been dilated. With a +6.00 7.00 180 glass correction, vision was 20/30. A similar condition affected the left eye. |
P.70
Embryology and Anatomy
The crystalline lens is derived embryologically from the surface ectoderm. Early in embryonic life, at about 2 weeks of gestation, the surface ectoderm overlying the optic vesicle (Fig. 2.1A) thickens, forming the lens plate or placode (Fig. 2.1B).
The placode invaginates, forming a hollow ball of cells called the lens vesicle as the neuroectodermal optic vesicle concurrently invaginates to form the optic cup (Fig. 2.1C, D). The lens vesicle is initially attached to the surface ectoderm, but it separates at about 4 weeks to lie within the anterior portion of the optic cup. The lens vesicle consists of a single layer of cells surrounded by a basal lamina, which becomes the lens capsule.
At about 5 weeks of gestation, the epithelial cells lining the posterior portion of the lens vesicle elongate anteriorly, forming the primary lens fibers. The formation of the primary lens fibers obliterates the cavity of the lens vesicle (Fig. 2.1E, F). In the adult lens, these fibers persist as the central embryonic nucleus. The layer of epithelium anteriorly continues as a monolayer of cuboidal cells throughout life. Normally, no further cellular proliferation occurs in the posterior portion of the lens, and all new lens cells arise from a germinative zone located just anterior to the lens equator, where the anterior lens epithelium terminates.
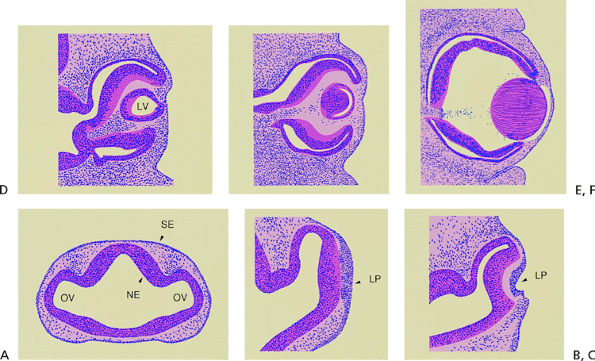 |
Figure 2.1. A: Section through the forebrain of a 4-mm human embryo. The neural ectoderm (NE) is completely internalized, and lateral out-pocketings represent the optic vesicles (OV). The surface ectoderm (SE) is undifferentiated. (Carnegie embryo 6097.) B: The surface ectoderm opposite the optic vesicle has thickened to form the lens placode (LP) at the 4.5-mm embryo stage. (Carnegie embryo 8119.) C: The lens placode and the internalized neural ectoderm begin to invaginate at about the 7-mm embryonic stage. (Carnegie embryo 7394.) D: At the 10-mm embryonic stage, the lens vesicle (LV) has closed completely and is beginning to separate from the surface ectoderm. The optic cup has developed, but a space remains between the two layers of neural ectoderm. (Carnegie embryo 6517; C D adapted from Smelser GK. Embryology and morphology of the lens. Invest Ophthalmol 1965;4:404, with permission.) E: The lens vesicle has separated from the surface ectoderm and lies within the optic cup. The epithelial cells lining the posterior portion of the lens vesicle have elongated anteroposteriorly to form the primary lens fibers at the 15-mm embryonic stage. (Carnegie embryo 6520; adapted from Kleiman NJ, Worgul BV. Lens. In: Tasman W, Jaeger EA. Biomedical foundations of ophthalmology, vol 1. Philadelphia: JB Lippincott; 1994:1; courtesy of Basil V. Worgul, Ph.D., and Victoria Ozamics, New York, NY, with permission.) F: At the 18-mm embryonic stage, the lens vesicle is completely filled by the primary lens fibers. These fibers denucleate and will form the embryonal nucleus. (Carnegie embryo 5537; courtesy of Basil V. Worgul, Ph.D., and Victoria Ozamics, New York, NY.) |
The proliferating equatorial epithelial cells are stimulated to differentiate terminally into mature, highly specialized lens cells, known as secondary lens fibers (Fig. 2.2). The secondary lens fibers begin to form at about 7 weeks of gestation. Seven or 8 mm long and hexagonal in cross section, these elongated, strap-like cells are joined together in a remarkably regular array of complex, interdigitating intercellular connections. Mature lens fibers lack nuclei and other organelles, and their homogeneous cytoplasm is filled with a concentrated solution of ordered crystalline proteins. The absence of vessels, lymphatics, nerves, and connective tissue, and the paucity of extracellular space in the lens also contribute to its transparency.
As new secondary lens fibers form, they elongate anteriorly beneath the lens epithelium and posteriorly beneath the capsule to envelope the embryonic nucleus. In the fetal nucleus, the tips of the fibers meet anteriorly and posteriorly, forming the Y-shaped lens sutures. The anterior Y is upright, and the posterior Y is inverted (Fig. 2.2). The Y sutures lie within the fetal nucleus, just beneath the capsule at birth. As new concentric lamellae of secondary lens fibers
P.71
form after birth, the sutural pattern becomes increasingly complex. A nine-branched stellate pattern is found in the adult nucleus at 20 years of age.
The lens continues to grow throughout life. New concentric lamellae of secondary lens fibers successively form in the periphery of the lens in an onion-like fashion. As new cells form, the older cells are sequestered centrally. The newer cells comprise the lens cortex, and the older cells constitute the nucleus (Fig. 2.3). Lines of discontinuity form within the lens and represent the interface between layers. These lines create the typical slit lamp appearance of the adult lens (Fig. 2.4).
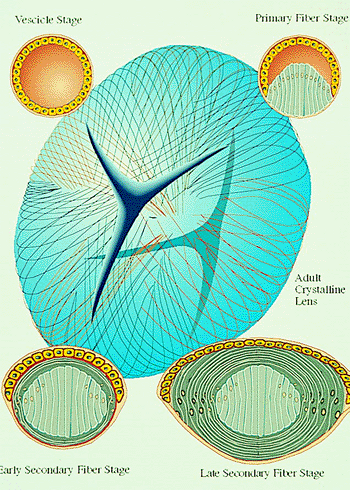 |
Figure 2.2. Lens vesicle development. The upper left portion shows closed vesicle stage. In the upper right portion, the epithelial cells lining the posterior portion of the lens vesicle have elongated anteriorly to form the primary lens fibers. The lower left portion shows the early secondary lens fiber stage. The equatorial cells have elongated into secondary lens fibers and are beginning to engulf the primary fibers. The lower right portion demonstrates the advanced secondary fiber stage. The strap-like secondary fibers have enclosed the future embryonal nucleus. Their ends meet to form the Y sutures (center) and will eventually comprise the fetal nucleus. (Courtesy of Neal H. Atebara, M.D., Honolulu, HI.) |
Inherent in this normal developmental pattern lies the inevitability of senile nuclear cataract. With the passage of time, the older lens fibers in the nucleus gradually degenerate. Cellular membranes dissolve, and soluble crystalline proteins denature and dehydrate. Yellow-brown urochrome
P.72
pigment accumulates, and densification of the nucleus increases its index of refraction. These changes are evident clinically as nuclear sclerosis. The former Y suture lines tend to fade later in life and may not be visible after nuclear sclerosis develops.
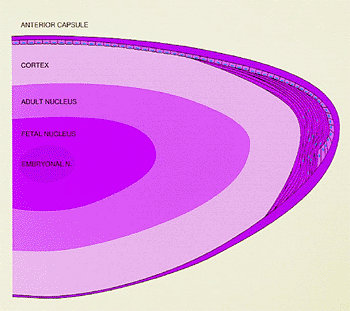 |
Figure 2.3. Schematic drawing of the adult lens showing the nuclear zones, cortex, epithelium, and capsule. New lens fibers are derived from the epithelial cells in the equatorial region. The nuclei remain at the equator, and the cytoplasm extends anteriorly and posteriorly. (From Hogan MJ, Alvarado JA, Weddel JE. Histology of the human eye. Philadelphia: WB Saunders; 1971:642, with permission.) |
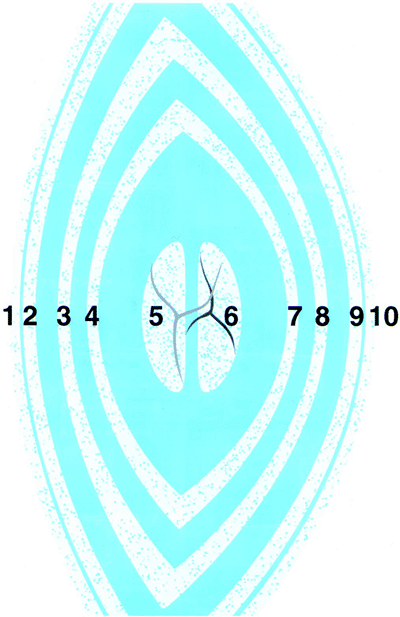 |
Figure 2.4. Schematic optical section of the normal adult lens. (1, anterior capsule; 2, anterior line of disjunction between the lens epithelium and the cortex; 3, anterior surface of the adult nucleus; 4, anterior surface of the fetal nucleus; 5, anterior half of the fetal nucleus, containing the anterior Y suture; 6, posterior half of the fetal nucleus, containing the posterior Y suture; 7, posterior surface of the fetal nucleus; 8, posterior surface of the adult nucleus; 9, posterior line of disjunction; 10, posterior capsule. From Phelps CD. Examination and functional evaluation of the crystalline lens. In: Tasman W, Jaeger EA. Clinical ophthalmology, vol 1. Philadelphia: JB Lippincott; 1994:2, with permission.) |
Adult Cataracts
Classification and Pathophysiology
There has been considerable variation in and some degree of confusion about the terminology used to identify cataracts. The older literature tended to be descriptive in that opacities were labeled according to their appearance, such as cerulean (i.e., blue), stellate, spear, cupuliform (i.e., saucer shaped). Latin-based phraseology was often used, such as cataract pisciformis (i.e., fish-like cataract). Many of these terms are no longer commonly used, and cataracts tend to be described according to their anatomic location: cortical, nuclear, and subcapsular. Frequently, more than one area of the lens is opacified in the same patient.
This section is not intended to be a compendium of lens opacities, but some of the more common varieties are illustrated. Specific types of lens opacities often have been associated with specific disease entities. Included in this group are the blue dot cortical opacities and posterior subcapsular cataract (PSC) of myotonic dystrophy, the PSC of atopic dermatitis, and the discrete white cortical opacities associated with hypocalcemia. Snowflake opacities located in the anterior and posterior subcapsular cortex have been associated with diabetes mellitus and Down syndrome (Fig. 2.5). Cortical wedges caused by lens fiber swelling may be seen in acute-onset diabetes. More commonly, age-related cataract types occur earlier and more frequently in diabetics (particularly PSC) and Down syndrome patients and progress at a faster rate. Retinal degenerations, such as retinitis pigmentosa and gyrate atrophy, are usually associated with PSCs.
A category of presenile cataracts has been described in which age-related cataracts occur prematurely. Lens opacities may occur earlier in life than normal because of genetic defects, other ocular and systemic diseases, certain medications, trauma, and idiopathic causes.
Much research has been directed toward determining the pathophysiology of cataract development. Extensive studies in animal models have revealed information regarding the fundamental cataractous process, but much still remains a mystery. Datiles and Kinoshita have summarized current thinking on this subject. There is no single process that results in the formation of lens opacities. A wide variety of precipitating factors, including nutritional, environmental, metabolic, and genetic elements, are involved. However, according
P.73
to Datiles and others, there appear to be two fundamental processes that lead to lens opacification. One process occurs in the lens cortex and is characterized by an electrolyte imbalance. The normal lens contains a low-sodium, high-potassium content. The reverse is true in a cortical cataract, leading to increased membrane permeability and hydration of the cortex. The second process occurs in the nucleus, where modification of lens fiber proteins occurs, resulting in higher-molecular-weight protein aggregates. There is no significant change in the electrolyte balance. Oxidation, proteolysis, glycation, and deamidation may be responsible for these changes.
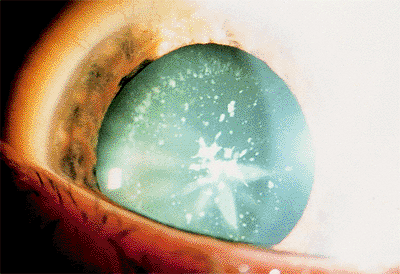 |
Figure 2.5. Discrete flakes in the anterior and posterior cortex in a 26-year-old man with Down syndrome. An incomplete, cortical, star-shaped opacity is also evident. |
This is a gross oversimplification of a complex and poorly understood process, and interested readers should explore the extensive research reports available throughout the literature. Much work remains to be done in this area.
The diabetic cataract presents a better understood pathophysiologic process. Twenty-five years ago, Van Heyninger reported the finding of polyol sorbitol in the lenses of diabetic rats. Sorbitol forms from the reduction of glucose by the enzyme aldose reductase. Because sorbitol does not pass freely through cell membranes, an osmotic pressure gradient forms, resulting in an influx of water into the cell and swelling. Hydration of the cell leads to opacification.
Types of Cataracts
Flecks, Dots, Flakes, and Clubs
When examining the lens with the slit lamp, a wide variety of opacities in the form of multiple white, grayish, or blue dots, flecks, or clubs may be seen (Fig. 2.6).
Clinical Features
These opacities may appear in the anterior or posterior cortex. These may be congenital or develop in the transition to the adult lens. If they occur in a ring configuration near the lens equator, the term coronary cataract has been applied (Fig. 2.7). Their cause remains speculative. They may result from a degeneration of the cytoplasm of lens cells over which normal lens fibers were subsequently formed. Cogan and Kuwabara suggested that these were the result of wart-like excrescences that developed on the back surface of the anterior lens capsule. These eventually degenerate and are displaced inward by newly formed fibers.
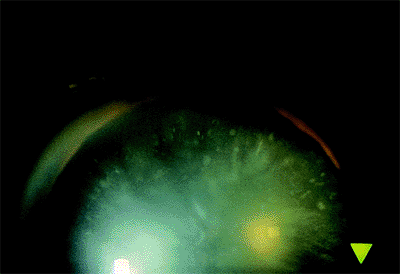 |
Figure 2.6. Discrete dots, clubs, and flecks in the anterior cortex. |
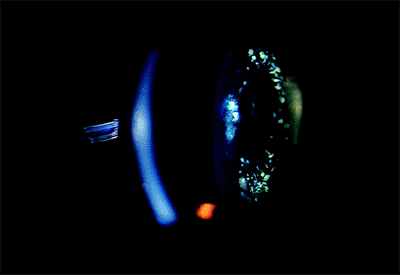 |
Figure 2.7. A coronary cataract is characterized by dot- and club-shaped opacities in a wreath-like configuration in the anterior and posterior cortex. |
These opacities do not affect vision, and their relation to the development of any subsequent age-related cataract remains speculative.
Cortical Cataract
A cortical cataract is characterized by spoke-like opacifications in the periphery of the lens that radiate toward the center (Fig. 2.8).
Clinical Features
These radial opacifications may remain peripheral but often coalesce and encroach on the visual axis. They can occur anterior or posterior to the nucleus. Water clefts and vacuoles, clear areas within the cortex, are frequently observed in the early stages of cataract formation (Fig. 2.9).
Patients with cortical cataracts may become visually symptomatic under bright light conditions. The clinical course is usually one of slow progression, and good visual acuity can be maintained for years.
Management
The decision to recommend cataract removal depends on subjective visual functional impairment, objective measures of visual function, density of the opacity, presence of other ocular disease, and the patient's health.
Nuclear Sclerosis
Nuclear sclerosis is characterized by hardening and yellowing of the lens nucleus.
Clinical Features
Typically a brownish yellow discoloration of the lens is seen centrally (Fig. 2.10). In its more advanced forms, the lens may become reddish, and in the most severe form, it may become black. Patients with nuclear sclerosis may have complaints of monocular diplopia
P.74
P.75
and glare. Typically, patients with nuclear sclerotic cataracts have worsening of their symptoms at night or under low levels of light. Nuclear sclerosis increases the refractive index of the lens, resulting in progressively more myopia or less hyperopia. Although patients may initially have good near vision, as the cataract matures, they may have increasing difficulty reading for extended periods because of multiple images from irregular hardening and refraction of the lens nucleus.
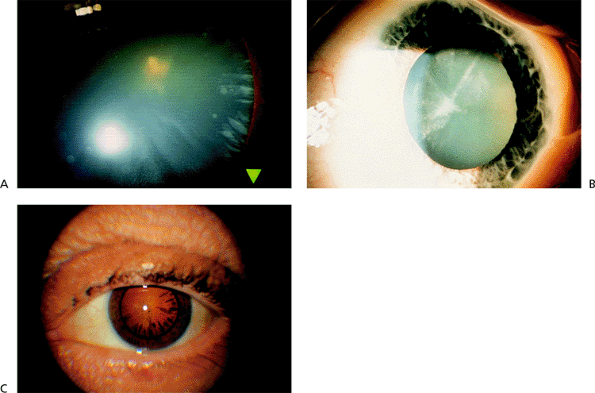 |
Figure 2.8. A: Spoke-like anterior cortical opacities are typical of a beginning cortical cataract in the periphery. B: Further extension of the spokes centrally. C: Spokes seen in retroillumination. |
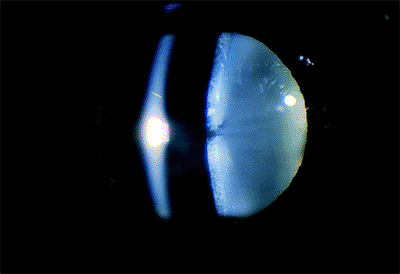 |
Figure 2.9. Water clefts (clear areas) in the anterior cortex. |
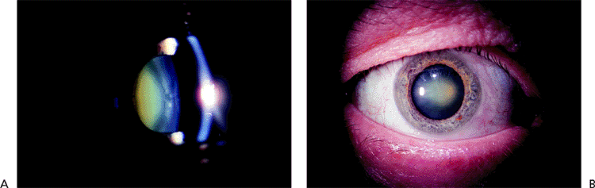 |
Figure 2.10. Yellowish brown discoloration of the nucleus is typical of nuclear sclerosis. Slit lamp cross-sectional view (A) and direct view (B), in which the discoloration is more gray. |
Management
Change in the refractive index of the lens results in a myopic shift and frequently requires a change in the distance prescription. However, the glass change may make the middle range or near vision worse, requiring a detailed explanation by the refractionist. When the best refraction does not provide adequate visual function, surgery is indicated.
Posterior Subcapsular Cataract
Posterior subcapsular cataract is characterized by a proliferation of cells on the posterior capsule of the lens, usually accompanied by opacification of the adjacent posterior cortex.
Clinical Features
The posterior capsular opacification is frequently located in the visual axis but may occur outside of it as well (Fig. 2.11). The affected area appears irregular and looks like the surface of the moon on slit lamp examination. Its growth is often rapid. Fortunately, this is the least common of the three main cataract types. This cataract can be seen in the presence of other types of cataracts and may be associated with uveitis, prolonged steroid use, irradiation, and diabetes. The symptoms of these patients worsen in bright light, and decreased reading vision is an early symptom.
Management
It is important to test the patient's vision under normal and bright light conditions. Initially, the patient has good vision under scotopic testing conditions but later has difficulty under bright light conditions. The indications for cataract surgery are similar to those for other forms of lens opacity.
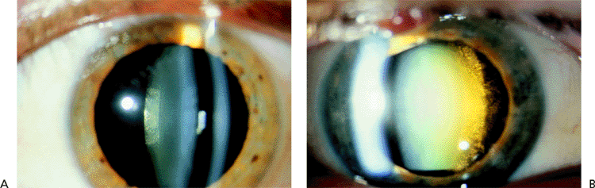 |
Figure 2.11. Posterior subcapsular cataract (PSC) is characterized by opacification of the posterior capsule and adjacent posterior cortex. A: Early PSC and (B) more advanced opacification along with nuclear sclerosis. (Courtesy of Stephen B. Lichtenstein, M.D., Philadelphia, PA.) |
Mature Cataract
In a mature cataract, the lens is white, and no iris shadow is seen on oblique penlight examination.
Clinical Features
Complete opacification of the cortex results in the white appearance of the lens (Fig. 2.12). Sometimes, a sclerotic nucleus can be seen through the cortex on slit lamp examination. The fundus cannot be adequately examined ophthalmoscopically. Typically, this type of cataract is acquired over a long period.
Management
Before cataract surgery, some assessment of retinal function is necessary. The pupils should be checked for an afferent defect. Two-point light discrimination and light projection in each quadrant can be tested. Other tests of macular function include blue field entoptic phenomena and the use of a potential acuity meter and laser interferometry. Unfortunately, these tests are not totally reliable in cases of very dense cataracts. B-scan ultrasonography should be done to rule out other posterior segment pathology. Care is necessary during the anterior capsulotomy, because cortical material may leak out and blur the view. If surgery is deferred for any reason, these patients should be followed regularly. Leakage of lens material into the anterior chamber may result in phacolytic glaucoma, or further swelling of the lens may induce angle closure.
Hypermature or Morgagnian Cataract
A hypermature or morgagnian cataract is a further deterioration of a mature cataract in which the cortex has liquefied.
Clinical Features
The lens is totally opaque and no fundus reflex is visible. The brownish nucleus drops into a dependent position and may be detected inferiorly through the milky cortex on slit lamp examination (Fig. 2.13). The nucleus undergoes partial reabsorption and is smaller than normal.
The lens may spontaneously rupture, allowing liquid cortex to permeate the anterior chamber and resulting in phacolytic glaucoma.
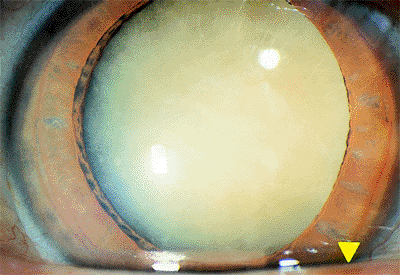 |
Figure 2.12. Mature cataract with complete opacification of the lens. |
P.76
Management
B-scan ultrasound should be done to rule out retinal detachment or intraocular tumor, and the previously described tests can be attempted to assess visual potential. Cataract extraction presents some technical difficulties. The anterior capsule is usually thin, and once incised, it may be obscured by the milky cortex. Because visualization is poor, care must be taken to ensure that the capsulotomy does not extend posteriorly. A manual extracapsular technique is probably the procedure of choice. The nucleus can often be removed with an irrigating vectis through a 7-mm incision. If phacoemulsification is used, a second stab incision should be made to provide a second instrument for manipulation of the nucleus. In the absence of formed cortex, the nucleus is forced directly against the posterior capsule, increasing the risk of a capsular tear.
Plaques and fibrosis are common on the posterior capsule, which may be difficult to remove. Care must be exercised not to rupture the capsule. In most cases, a posterior chamber lens can be placed in the bag.
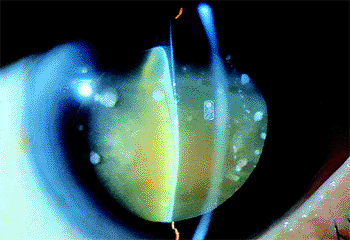 |
Figure 2.13. Hypermature (i.e., morgagnian) cataract. The cortex has liquefied, and the brownish nucleus has dropped into a dependent position. (Courtesy of Jamie E. Nichols, Philadelphia, PA.) |
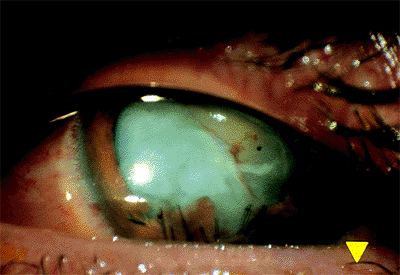 |
Figure 2.14. Traumatic cataract with opacification of the cortex and disruption of the iris due to a penetrating injury. |
Traumatic Cataract
A traumatic cataract occurs when the lens capsule is perforated by a foreign object but may also occur secondary to severe blunt trauma.
Clinical Features
In the initial few days after the trauma, the lens may remain clear, and in some cases, the area of perforation can spontaneously seal. If it does not seal, fluid imbibition occurs, and rapid swelling and opacification of the lens ensues, markedly decreasing vision (Fig. 2.14). Lens opacification associated with blunt trauma can develop months or years after injury.
Management
Surgery is indicated when the lens opacity becomes visually significant, particularly if there is rapid swelling, which may compromise the anterior chamber angle. In doing cataract surgery, care must be taken to identify the original tear in the anterior capsule, and a capsulotomy should be performed in such a way that prevents tearing into the posterior capsule. An initial incision in the anterior capsule with a Vannas scissors often works well, and a can-opener type capsulotomy may be necessary to perform an adequate anterior capsulotomy. In a young patient, the nucleus is usually soft, and phacoemulsification can be readily performed. The lens may need to be placed in the sulcus if the capsular bag is inadequate.
Complicated Cataract
Complicated cataract develops as a result of longstanding intraocular disease, such as chronic uveitis, retinal detachment, prolonged hemorrhage, or tumor.
Clinical Features
Chronic iridocyclitis is the most common cause of complicated cataract. Typically, chronic inflammation and the use of steroid medication result initially in a posterior subcapsular type of opacity. After prolonged inflammation, cortical changes develop that can progress to a mature cataract (Fig. 2.15). Posterior synechiae often are present.
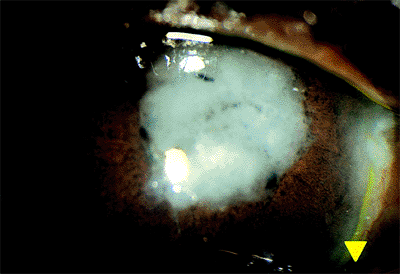 |
Figure 2.15. This complicated cataract was characterized by extensive opacification of the cortex and iris atrophy in a patient with longstanding sarcoid uveitis. |
P.77
Management
Control of the inflammation or other underlying pathology often arrests the cataract development. If vision is significantly decreased and retinal function has not been affected, cataract surgery can be considered. In the case of uveitis, the inflammation may recur after surgery.
Cataract Associated With Medication
Certain drugs, usually administered over a long period, have been associated with cataract formation.
Corticosteroid-Related Cataract
Clinical Features
Corticosteroids given topically or systemically are the most commonly encountered group of drugs causing cataracts. These lesions begin as axially positioned posterior subcapsular opacities (Fig. 2.16). They are dose and duration related, and they rarely begin before 1 year of steroid therapy is completed.
Management
Cessation of the drug may stop or retard lens opacification. In some patients, continued steroid therapy is necessary, resulting in further cataract development. Surgical removal is judged on the basis of the degree of interference with visual function.
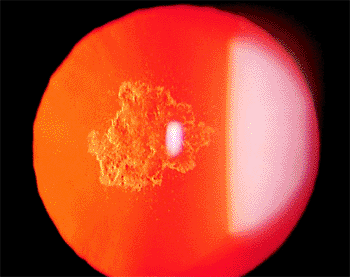 |
Figure 2.16. Posterior subcapsular cataract seen in retroillumination in a 32-year-old patient with severe steroid-dependent asthma. (Courtesy of Stephen B. Lichtenstein, M.D., Philadelphia, PA.) |
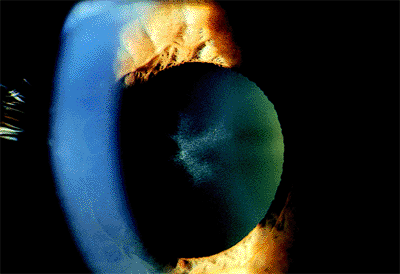 |
Figure 2.17. Stellate anterior subcapsular lens opacity in a patient with a history of high-dose, long-term treatment with a phenothiazine medication for psychiatric dysfunction. (Courtesy of Kit Johnson and M. Lisa McHam, M.D., Boston, MA.) |
Phenothiazine-Related Cataract
Clinical Features
The phenothiazine class of antipsychotic drugs (e.g., chlorpromazine, thioridazine) is frequently used in the treatment of patients with chronic psychiatric conditions. Bilateral stellate anterior subcapsular lens opacities have been associated with phenothiazine therapy (Fig. 2.17). Other ocular findings associated with toxicity include pigment dusting of the corneal endothelium, atrophy of the retinal pigment epithelium, and associated pigmentary retinopathy. The ocular findings seen with phenothiazine toxicity appear to be duration and dose related.
Management
The lens opacities usually do not result in serious visual dysfunction. The pigmentary retinopathy is more of a concern. The ocular findings usually persist even after the cessation of therapy.
Topical Miotic-Related Cataract
Lens opacities have been associated with long-term use of topical miotics for the treatment of esotropia in children. Echothiophate and demecarium bromide have been reported to cause anterior subcapsular vacuoles, as well as posterior cortical and nuclear changes. Cessation of the therapy stops or even reverses the opacities.
Other Diseases of the Lens
Pseudoexfoliation Syndrome
Pseudoexfoliation syndrome (PXS) appears as whitish blue dandruff-like deposits on the anterior lens capsule and pupillary margin. It is found primarily in the elderly, and incidence increases with increasing age.
Clinical Features
Pseudoexfoliation is appreciated best with the pupil dilated. The deposits on the front surface of the lens usually form a target-like configuration. The central bull's-eye corresponds to the diameter of the undilated pupil (Fig. 2.18). Surrounding the central disc is an intermediate
P.78
clear area that is presumably wiped clean by movement of the juxtapupillary iris. Some of the deposit areas may exhibit curled edges or strands. Sheet-like granular areas with curled edges are peripheral to the clear zone. There can be considerable variation in this clinical appearance. Additional deposits occur on the back of the cornea, angle, zonules, and ciliary processes in addition to the pupil margin. Increased iris pigment dispersion occurs in PXS, and iris transillumination defects may be found. The accumulation of pigment anterior to Schwalbe's line is known as Sampaolesi's line and is characteristic of PXS.
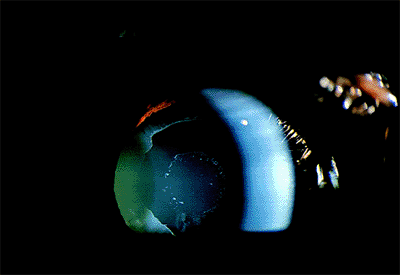 |
Figure 2.18. Typical appearance of the anterior surface of the lens capsule in a patient with pseudoexfoliation syndrome. (Courtesy of Robert Bailey, M.D., Philadelphia, PA.) |
The zonules are weakened in PXS. Rarely, subluxation of the lens occurs in the case of a mature cataract and longstanding PXS (Fig. 2.19). Exfoliation material is produced by the lens epithelium and the nonpigmented epithelium of the ciliary processes. Evidence suggests the PXS may be the ocular manifestation of an otherwise benign disorder of elastic tissue.
An increased prevalence of open-angle glaucoma is associated with PXS. Presumably, this is the result of deposition of the material and pigment within the drainage system. Although figures vary widely, one study reported that 15% of patients with PXS also had elevated intraocular pressure. The longer the duration of PXS, the more likely is the development of increased intraocular pressure.
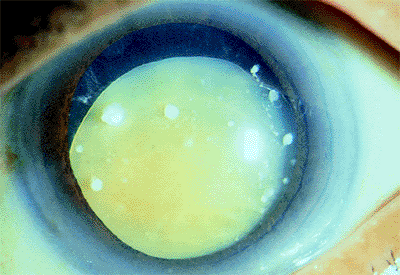 |
Figure 2.19. Inferior subluxation of the lens in an 82-year-old patient with longstanding pseudoexfoliation and a mature cataract. (Courtesy of Jamie E. Nichols, Philadelphia, PA.) |
Management
Patients with PXS should be followed periodically for the development of glaucoma. Cataract surgery must be approached with caution in patients with PXS. The iris is more rigid, and the pupil does not dilate well, making surgery more difficult. The zonules are weakened and are more prone to rupture during cataract extraction, resulting in vitreous loss. If phacoemulsification is preformed, it should be under low flow and with rotary movements of the nucleus. The lens can be safely removed without disinserting of the capsular bag in most cases.
Subluxation of the Lens
Weakening or rupture of a segment of the zonules results in displacement of the lens (i.e., ectopia lentis) in a direction opposite to the disinsertion. Many diseases and trauma are associated with lens subluxation.
Clinical Features
All degrees of subluxation may be seen. Classically, iridodonesis is seen on slit lamp examination with eye movement. Phakodonesis may also be observed. The body of the lens is displaced, and stretched or absent zonules may be noticed in the affected segment when the pupil is dilated. In severe cases, the lens may be displaced into the vitreous cavity or anterior chamber.
The most common causes of subluxation of the lens are Marfan syndrome, homocystinuria, and trauma. Subluxation occurs in 80% of those with Marfan syndrome, and typically, the lens is displaced superiorly (Fig. 2.20). In homocystinuria, the zonular weakness is generalized rather than segmental, resulting in a gravity-induced downward displacement of the lens. Other systemic abnormalities that have been associated with ectopia lentis include Weill-Marchesani
P.79
syndrome, sulfite oxidase deficiency, and syphilis.
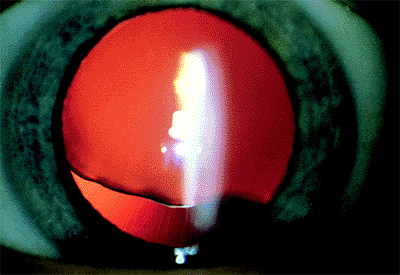 |
Figure 2.20. Superior subluxation of the lens in a 32-year-old woman with Marfan syndrome. Notice the irregular inferior border of the lens created by variable zonular traction. (Courtesy of Robert Bailey, M.D., Philadelphia, PA.) |
Management
A conservative approach is indicated in the management of patients with subluxation of the lens. In particular, patients with Marfan syndrome may be followed for many years, even though the observer is uncomfortable with the degree of subluxation. Periodic refraction and observation are necessary. If the lens is subluxated into the vitreous, observation alone still may be indicated, and the patient fitted with a soft contact lens. Subluxation into the anterior chamber may result in an acute glaucoma. Sometimes, if the pupil is dilated and the patient placed in a supine position, the lens returns to the posterior chamber, in which case pilocarpine should be instilled. In other cases, it may be wise to remove the lens at this time. Any eye sustaining blunt trauma should be examined for zonular rupture and lens subluxation.
If cataract surgery must be undertaken, the surgeon must pay close attention to the zonules, because they may further disinsert during the extraction. An anterior vitrectomy is often necessary. Because the capsular support is often lacking, the surgeon should be prepared to suture a posterior chamber lens to the sulcus or the iris, use an anterior chamber lens, or consider contact lens aphakic correction.
Microspherophakia
Microspherophakia is characterized by a small, round lens.
Clinical Features
The dilated examination reveals the entire lens and supporting zonules to be within the pupillary space (Fig. 2.21). Patients with microspherophakia frequently develop a pupillary block-type glaucoma caused by anterior prolapse of the lens. This commonly occurs at nighttime, causing pain and discomfort. The situation is usually relieved by placing the patient in a supine position, permitting the lens to fall back into the posterior chamber. However, progressive peripheral anterior synechiae may form.
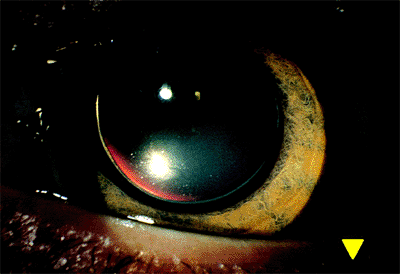 |
Figure 2.21. In microspherophakia, the entire lens appears within the dilated pupil and has been displaced into the anterior chamber. |
Microspherophakia is commonly associated with Weill-Marchesani syndrome, in which the lens is displaced superior temporally. Other features of this syndrome include small stature, brachydactyly, deafness, and a high degree of myopia.
Management
Patients with microspherophakia should undergo gonioscopy to determine whether peripheral anterior synechiae are present. A peripheral laser iridotomy should be considered to prevent repeated attacks of pupillary block glaucoma and progressive closure of the filtration angle by peripheral anterior synechiae. The zonules are loose, and the lens may eventually disinsert, resulting in dislocation. If cataract surgery is undertaken, the surgeon should be prepared to suture the intraocular lens to the iris or sclera. An anterior chamber lens is usually contraindicated in these patients because of peripheral anterior synechiae.
Anterior Lenticonus
The term lenticonus refers to a developmental anomaly in which the surface of the axial portion of the lens protrudes like a cone. If this localized protrusion is spherical, the term lentiglobus is used.
Clinical Features
Anterior lenticonus is a rare condition in which the front surface of the lens is conically shaped (Fig. 2.22). The lens capsule thins or even disappears at the apex of the cone. This abnormal lens shape may result in a high degree of lenticular myopia. Focal opacification of the lens may develop within the area of the cone. The condition may be discovered because of poor vision on a screening examination, strabismus, or a distorted fundus reflex.
Anterior lenticonus has been associated with Alport's syndrome, a hereditary disorder of basement membrane collagen that is transmitted as an autosomal dominant condition with variable penetrance. Other features of this syndrome include hemorrhagic nephritis and nerve deafness. Anterior polar cataracts and an albipunctatus-like fundus appearance have been associated with this syndrome.
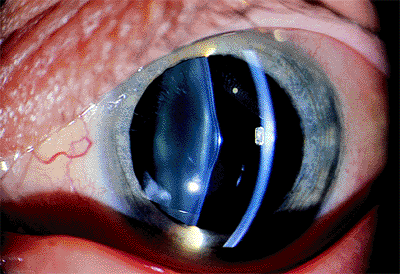 |
Figure 2.22. Localized cone-like protrusions of the lens surface occur in anterior lenticonus. (Courtesy of Stephen B. Lichtenstein, M.D., Philadelphia, PA.) |
P.80
Management
Visual symptoms depend on the degree of distortion and anisometropia created by the cone. If minimal and discovered early, significant amblyopia may be avoided. Frequent refraction is necessary. If the lens opacity becomes significant, it is managed as any other developmental cataract.
Posterior Lenticonus
Posterior lenticonus is an uncommon abnormality in which the back surface of the lens undergoes progressive posterior bowing. The condition begins in early infancy and is usually unilateral and sporadic, although bilateral and familial cases have been reported. The cause is unknown, but trauma, traction from the hyaloid system, and inflammation have been suggested.
Clinical Features
Posterior lenticonus is seen initially as a transparent, usually axial, conical, posterior projection of the posterior lens in a normal-sized eye. If the projection is more generalized, the term lentiglobus has been used. The initial clinical findings are an oil droplet appearance in the central red reflex on ophthalmoscopy and a pathognomonic scissors movement on retinoscopy (Fig. 2.23A). The axial refraction is markedly myopic, and the peripheral error may be hyperopic.
Anisometropia or optical distortion may result in amblyopia. Strabismus affects a significant percentage of patients. Cataracts develop in the most severe cases, beginning with opacification of the posterior cortex, which may obscure the underlying defect (Fig. 2.23B).
Management
Amblyopia detected in the oil droplet, clear lens stage or found postoperatively should be managed in the usual fashion. Significant lens opacification presents all of the therapeutic challenges of unilateral congenital cataract. Management techniques have included lens extraction with refractive correction by intraocular lens implantation, a contact lens, aphakic glass correction, or epikeratoplasty. Visual results may be reasonably good (i.e., 49% achieved 20/20 to 20/40 in one series) if the condition is detected early.
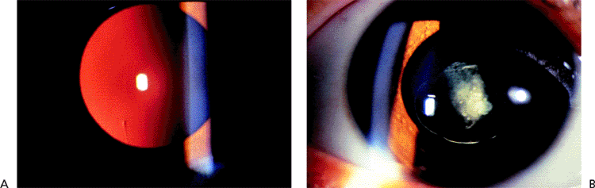 |
Figure 2.23. A: Oil droplet appearance of the posterior lens surface is seen against the red fundus reflex on slit lamp examination in a 4-year-old boy with posterior lenticonus. B: Axial opacification in the posterior cortex is seen in the other eye of the same patient. (Courtesy of Jonathan S. Myers, M.D., Philadelphia, PA.) |
Common Complications of Cataract Surgery
Wound Gape and Dehiscence
Clinical Features
On slit lamp examination, a space is seen between the anterior and posterior edges of the wound; the space results from inadequate suturing techniques, excessive cautery, or overheating during phacoemulsification (Fig. 2.24A). Iris tissue can become incarcerated in the wound (Fig. 2.24B). Wound gape may be noticed immediately after surgery or may occur after minor trauma during the wound healing process. The patient often notices a decrease in vision as a result of the development of plus cylinder astigmatism at 180 degrees. The wound leak can also lead to the development of a flat or shallow anterior chamber and choroidal detachment. The patient is also at greater risk for the development of endophthalmitis and epithelial downgrowth. A wound leak is thought to be an inciting factor in the development of pupillary block. These complications were much more common after intracapsular cataract extraction. Although they may occur in extracapsular-type procedures, particularly in complicated cases with vitreous loss, the advent of small-incision surgery has greatly reduced their incidence.
Management
Seidel's test should be done to rule out the presence of a wound leak. With the patient looking downward, a fluorescein strip wetted with a topical anesthetic should be used to paint the incision area. Under a cobalt light, the leak produces a bright ribbon of the dye flowing downward. Keratometric and refractive readings should be obtained. If the Seidel's test result is positive, the iris is exposed, or the astigmatism marked, resuturing of the wound should be considered.
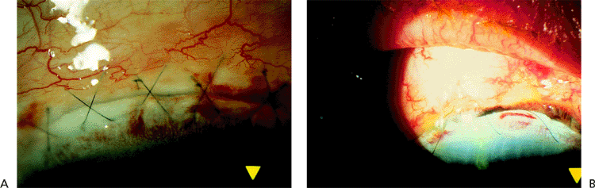 |
Figure 2.24. A: A gap can be seen between the wound edges. B: Brownish tissue indicates the inclusion of iris in the wound. |
P.81
Iris Prolapse
Clinical Features
An iris prolapse appears as a dark brown bulge of tissue through the cataract wound (Fig. 2.25). This may be the result of faulty wound closure but more frequently results from increased intraocular pressure during the immediate postoperative period. Direct trauma may also cause iris prolapse.
Management
Continued exposure of the iris increases the risk of intraocular infection or persistent low-grade inflammation, which can result in cystoid macular edema. The intraocular pressure should be controlled. In most cases, surgical management consists of excising the prolapsed iris, constricting the remaining iris with Miochol, and resuturing the wound.
Wound Bleb
Clinical Features
A bleb may form with a limbal or fornix-based flap if the conjunctival wound has sealed and a scleral wound leak remains. It is rare in modern small-incision surgery. Initially, the conjunctiva may appear edematous over the area of wound dehiscence. With continued leakage, the bleb may enlarge, producing conjunctival thinning (Fig. 2.26). Blebs may develop later in the wound-healing process as well. If large, the bleb itself may result in a scratchy, foreign body sensation, and it may induce refractive changes. More importantly, the patient needs to be observed for all of the complications associated with wound leak previously mentioned.
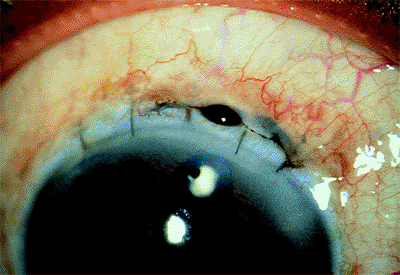 |
Figure 2.25. Iris prolapse. |
Management
Seidel's test should be done. Sometimes, a wound leak detected in the immediate postoperative period can be treated by a pressure patch and Diamox to reduce aqueous secretion. If the bleb remains small, observation may be indicated. However, continued evidence of leakage or hypotony dictates surgical repair of the wound.
Blebs that develop late or are of long duration present more difficult problems. If asymptomatic in a quiet eye, no action is necessary. If the bleb is large and thinning and if leakage or chronic inflammation is present, more aggressive action is indicated. Cryotherapy applied to the bleb may thicken the conjunctiva and shrink the bleb. The application of trichloroacetic acid to the bleb has been used in the past. Autologous blood has been injected into the bleb to try to initiate scar formation. Surgical repair is difficult because the wound leak has formed a fistula with an endothelial cell lining. Creation of a scleral flap to cover the wound has been
P.82
tried. The most definitive treatment involves the use of a lamellar patch of donor corneal tissue. A 5-mm trephine is used to create a one-third depth bed over the fistula. A similar button is obtained from the donor cornea and sutured in place.
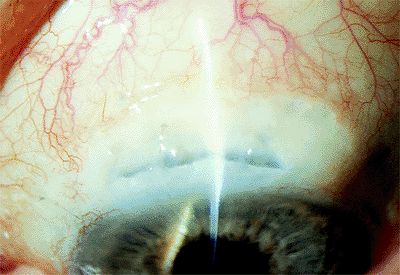 |
Figure 2.26. Conjunctival bleb resulting from a wound leak. (Courtesy of Stephen B. Lichtenstein, M.D., Philadelphia, PA.) |
Corneal Edema and Bullous Keratopathy
A decrease in the integrity of the corneal endothelium may result in persistent corneal edema or bullous keratopathy after cataract surgery.
Clinical Features
The endothelium is a single cell layer that does not have the ability to reproduce, although the existing cells can expand to maintain the integrity of the layer. Cataract surgery results in a decrease in the corneal endothelial cell count. Endothelial cell loss rates of 8% or less during surgery have been reported. A critical cell count may be reached (400 to 600 cells/mm2), at which point the cellular pump mechanism is insufficient to preserve the proper electrolyte and fluid balance, and the cornea becomes edematous. This situation may occur in the immediate postoperative period or take many years to develop.
The edema can begin in a focal area in the peripheral or central cornea with thickening of the stroma and bedewing of the epithelium. Symptoms include decreased vision, intermittent pain, a scratchy sensation, tearing, and sensitivity to lights. As the edema becomes widespread, symptoms increase, and vision becomes blurred. The cornea loses its luster, thickens, and appears cloudy (Fig. 2.27). The epithelium becomes edematous, with areas of breakdown and subepithelial fluid accumulation. This condition is called bullous keratopathy.
Cataract surgery is the most common precipitating factor leading to the development of corneal edema. If an implant has been inserted, the term pseudophakic bullous keratopathy is used. Conditions that predispose to bullous keratopathy include preoperative corneal disease (such as cornea guttata), poor surgical technique, excessive irrigation and manipulation, vitreous touch, prolonged postoperative inflammation, and anterior chamber lenses.
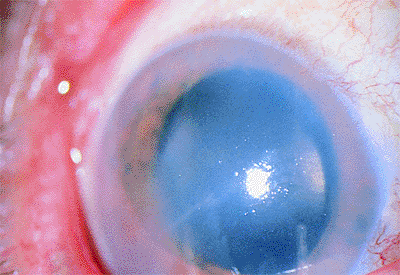 |
Figure 2.27. Pseudophakic bullous keratopathy associated with a closed loop anterior chamber lens developed 4 years after cataract surgery. |
Management
In the early stages of bullous keratopathy, the patient can be maintained on hyperosmotic drops and ointment. Any elevation of intraocular pressure should be controlled. If the edema becomes generalized and the vision compromised, corneal transplant and lens exchange should be considered. The decision to proceed should be based on the patient's desires, general health, life expectancy, absence of other ocular pathology, and status of the other eye.
An anterior chamber lens should be removed if peripheral anterior synechiae are present and corneal decompensation has occurred. The endothelial cells can migrate off the peripheral anterior synechiae and touch the lens material. In these eyes, a sutured posterior chamber lens should be considered.
Residual Cortical and Nuclear Fragments
Occasionally, remnants of the lens cortex or nuclear fragments remain in the eye after cataract surgery (Fig. 2.28A, B).
Clinical Features
Cortical remnants may appear small or
P.83
may be hidden by the iris at the time of surgery but swell into larger, fluffy, white opacities in the postoperative period. They often migrate into the anterior chamber. However, cortex in the anterior chamber is relatively benign and usually dissolves in several weeks. Cortex that is located between the intraocular lens and the posterior capsule or between the peripheral anterior and posterior capsule takes longer to be reabsorbed and may result in early opacification of the capsule or a Soemmering's ring. Cortex in the vitreous also takes longer to resolve. Increased inflammatory reactions sometimes develop in these eyes.
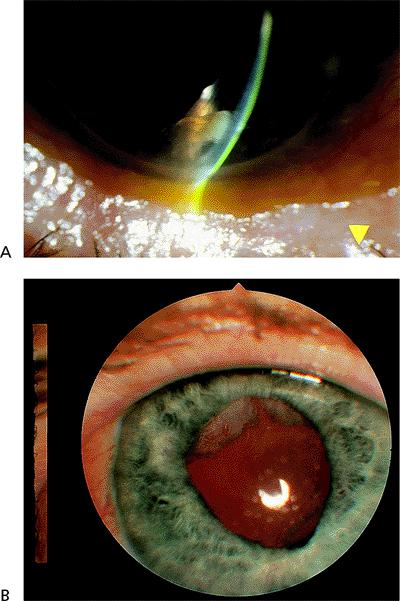 |
Figure 2.28. A: A residual fragment of nucleus seen in the anterior chamber after a complicated cataract extraction. B: A patient demonstrating residual cortical material that is starting to proliferate across the surface of the intraocular lens. The patient complained of glare at nighttime. |
Nuclear fragments in the anterior chamber or vitreous may remain for an extended period and cause marked inflammation. One of the most dreaded complications is the rupture of the posterior capsule, with loss of the entire nucleus or a large fragment into the vitreous (Fig. 2.29).
Management
Most cortex in the anterior chamber reabsorbs. The inflammatory reaction and any elevation of intraocular pressure can usually be controlled medically. Excessive cortex between the intraocular lens and the capsule may need to be removed surgically.
The management of nuclear fragments depends on their size and associated reaction. Fragments in the anterior chamber frequently damage the corneal endothelium and are very slow to dissolve. The patient should be followed with periodic cell counts. If decreasing, the fragment should be removed. Small nuclear fragments in the vitreous can be observed, but larger ones should be removed by a retinal surgeon. Many surgeons think that a lens can be inserted at the time of surgery even if the nucleus cannot be completely removed.
Peaked Pupil
The peaked pupil is not round but appears peaked or shaped like a teardrop, with the apex pointed toward the cataract wound.
Clinical Features
A peaked pupil may result from a variety of intraoperative circumstances. Adhesion to or entrapment of the iris in the wound can result in an updrawn, peaked pupil. The inclusion of vitreous strands, cortical fragments, and capsular fragments in the wound can produce a similar appearance (Fig. 2.30). Adhesions of the posterior iris surface to the underlying capsule, intraocular lens positioning hole, or haptic loop with subsequent contracture can tent the pupil. Sphincter tears during manual delivery of the nucleus and inadvertent inclusion of the iris in the phacoemulsification tip can result in iris distortion.
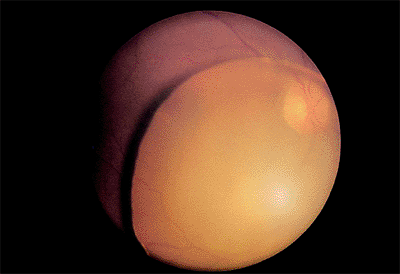 |
Figure 2.29. The entire nucleus has fallen into the vitreous cavity during cataract surgery. (Courtesy of Terry Tomer, Philadelphia, PA.) |
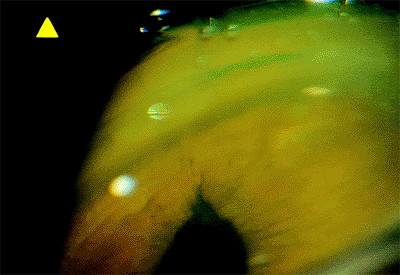 |
Figure 2.30. Gonioscopic view of a peaked pupil caused by a strand of vitreous extending to the wound. |
Tenting was a relatively common phenomenon associated with closed-loop anterior chamber lenses. The haptic loop compressed the last roll of the iris against the ciliary sulcus (Fig. 2.31). The resulting vertical distortion of the pupil often became progressive over many years as peripheral anterior synechiae continued to form.
Management
Meticulous attention to detail, especially wound hygiene, during cataract surgery is the key to preventing a peaked pupil. This is especially true if capsule disruption has occurred. Small strands of vitreous, cortex, and capsule are difficult to see during surgery. Weck sponge cleaning can detect unseen strands. Irregularities in the pupil before and after pharmacologic miosis should arouse a high degree of suspicion. The wound should be swept internally
P.84
from a side port incision at the conclusion of a complicated case.
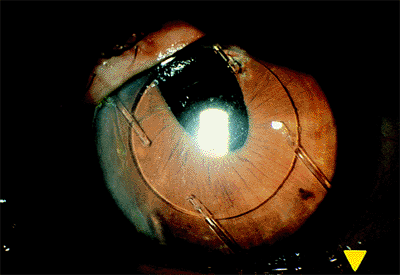 |
Figure 2.31. The closed loop anterior chamber lens has compressed the superior iris against the angle. |
The peaked pupil is usually a benign condition, but associated low-grade inflammation and cystoid macular edema may occur. Isolated strands can be severed with the yttrium-argon-garnet (YAG) laser if the pupil is cosmetically a problem or cystoid macular edema is persistent. Vitrectomy may be necessary in cases of severe and persistent cystoid macular edema.
Atonic Pupil
Postoperatively, the pupil may remain semidilated and weakly reactive to light.
Clinical Features
The postoperative atonic pupil most frequently occurs in cases in which the pupil does not dilate well preoperatively because of iris sphincter fibrosis or chronic miotic therapy. At surgery, the sphincter is stretched during manual nuclear expression or during phacoemulsification, resulting in muscle tears. Sometimes, multiple marginal sphincterectomies are performed to enlarge the pupil and facilitate removal of the nucleus. The resulting pupil is enlarged and has an irregular margin (Fig. 2.32). Rarely, the pupil may remain enlarged and poorly reactive postoperatively even after uneventful surgery. In these cases, interruption of the vascular or nerve supply to the iris may be causative factors.
Management
An atonic pupil seldom requires definitive action. If the enlargement exposes the optic edge or a positioning hole, the patient may notice glare or related visual symptoms. If necessary, pilocarpine may partially constrict the pupil.
Pupillary Capture
In pupillary capture, part of the optic edge of a posterior chamber lens lies anterior to the iris and has been captured by the iris (Fig. 2.33).
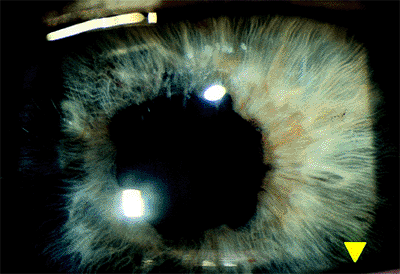 |
Figure 2.32. The atonic pupil is characterized by an enlarged, irregular, and poorly reactive pupil after cataract extraction. This frequently occurs in patients on long-term miotic therapy. |
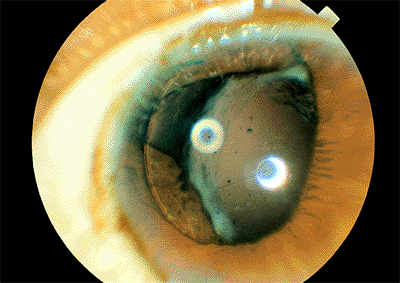 |
Figure 2.33. Pupil capture. A portion of the optic edge of a posterior chamber lens lies anterior to the iris. |
Clinical Features
This condition can occur at the time of surgery if the intraocular lens is not positioned properly and the iris remains dilated. Pupil capture is more likely to occur when one haptic loop is in the bag and the other is in the sulcus. Pupil capture also can develop if the integrity of the posterior capsule has been compromised and vitreous forces a portion of the optic anteriorly. Trauma in the immediate postoperative period can result in pupil capture.
Management
The condition usually is benign, although the pupil distortion may be a cosmetic concern. There is an increased risk of cystoid macular edema. Occasionally, the condition can be treated by dilating the pupil, placing the patient in a supine position that permits the lens to fall back into the posterior chamber, and constricting the pupil with pilocarpine. If surgical repositioning is to be done, it should be done early, before adhesions between the iris and posterior capsule develop.
Posterior Capsule Disruption
The integrity of the posterior capsule may be disrupted during surgery. This can result from a tear in the capsule or a rupture of the zonules attaching the capsule to the ciliary body (i.e., zonular dehiscence).
Clinical Features
Small tears in the capsule may be difficult to recognize at the time of surgery, but the edges of the tear usually can be seen against the red retinal reflex. Absence of the normal circular light reflex created by slight posterior pressure with the irrigation and aspiration tip on an intact capsule is another indication of capsule disruption. If the tear extends, linear folds develop at the edge. Tears may result from inclusion of the capsule in the phacoemulsification, the irrigation and aspiration instruments, or excessive capsule polishing.
Zonular dehiscence may result from excessive pressure during manual nucleus expression, excessive nuclear manipulation during phacoemulsification, or unrecognized inclusion of the peripheral capsule in the aspiration instrument during cortical cleanup. Typically, the folds are seen at the edge of the remaining capsule (Fig. 2.34).
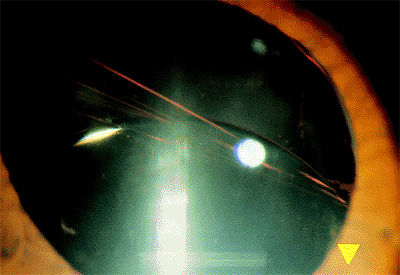 |
Figure 2.34. In zonular dehiscence, rupture of the inferior zonules has resulted in the typical folds seen at the edge of the remaining attached capsule. The intraocular lens is decentered inferiorly, resulting in the appropriately named sunset syndrome. |
P.85
Management
It is essential to recognize the presence and extent of capsule disruption at the time of surgery to avoid inserting a posterior chamber lens without adequate support. In some cases, a posterior lens may still be inserted into the remaining capsular bag, but in others, the lens should be placed in the sulcus. If capsule support is totally inadequate, other options include a posterior chamber lens sutured to the sclera or iris, an anterior chamber lens, or no intraocular lens and subsequent aphakic contact lens correction.
Optic Decentration
The optic edge of a posterior chamber lens may become decentered with respect to the visual axis. This may be caused by one haptic loop being placed in the bag and one in the sulcus. As the capsular bag contracts, the implant is pulled to one side. More commonly, a tear in the posterior capsule or a segmental dehiscence of the zonules is involved. The haptic may be placed inadvertently through the capsular tear. Contracture of the remaining capsule and unopposed pull of the zonules result in displacement of the optic edge.
Clinical Features
If the decentration is minor, no symptoms may be experienced. Glare from the lens edge or images from a positioning hole in the optic edge may be noticed if these elements fall within the pupillary space. As the lens further decenters, monocular diplopia is experienced, and marked blurred vision occurs when the optic disappears from the visual axis. Frequently, as the lens progressively decenters, increased ocular inflammation becomes evident.
Sunrise Syndrome
When the optic edge decenters superiorly, the condition is referred to as a sunrise syndrome.
Clinical Features
This condition develops when the lens is placed in the bag and a zonular dehiscence is present inferiorly, or the superior haptic is through a superior capsular tear. As the capsule contracts, the lens is drawn superiorly (Fig. 2.35). The same decentration may occur, but to a lesser extent, if the vertically oriented inferior loop is in the bag and the superior loop is in the sulcus.
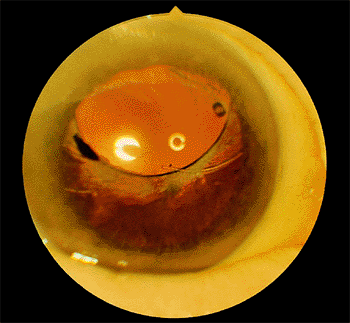 |
Figure 2.35. In this case of sunrise syndrome, the posterior chamber lens was placed in the capsular bag, but the inferior zonules had ruptured; with subsequent contracture of the capsule, the optic edge was decentered superiorly. |
Sunset Syndrome
In the sunset syndrome, the intraocular lens is displaced inferiorly.
Clinical Features
In addition to zonular dehiscence and capsule contraction, gravity also is a factor (Fig. 2.36). Any inferior capsular tear or a zonular rupture inferiorly in which the loop is vertically oriented and not in the bag also results in a sunset syndrome.
East-West Decentration
Nasal or temporal displacement of the intraocular lens can occur.
Clinical Features
The same circumstances that result in a vertical malposition of the lens also may cause horizontal
P.86
displacement (Fig. 2.37). A temporal zonular dehiscence with in-the-bag, horizontally oriented loops results in a nasally displaced optic. The lens also may be pushed nasally if there is a nasal capsular tear and the haptic loop is forced through the opening.
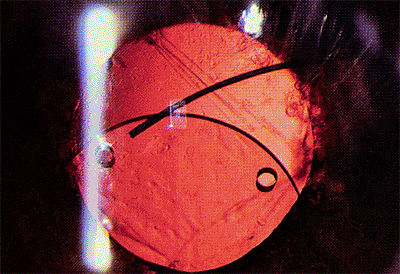 |
Figure 2.36. In this case of sunset syndrome, the inferior portion of the posterior capsule has been disrupted, and the optic edge has decentered inferiorly. |
Management
Definitive action depends on the severity of the associated symptoms, degree of displacement, and persistent inflammation. If the symptoms are minor, the patient may be treated medically with pilocarpine. Frequently, this is not sufficient, and surgical repositioning or replacement of the lens is necessary.
If sufficient capsular support is possible, the lens may be repositioned. However, it may be more advantageous to remove the original lens and replace it with a sulcus-fixated, large (7-mm) optic lens. Usually, fibrosis has fused the peripheral anterior and posterior capsular flaps.
If the capsular support is inadequate, consideration should be given to anchoring the lens with an iris fixation (i.e., McCannel's) suture or a scleral suture, or to replacing the lens with an anterior chamber variety. Removal of the lens with visual correction by contact lens is also an option.
Posteriorly Dislocated Intraocular Lens
Posterior chamber intraocular lenses may dislocate into the vitreous at the time of surgery or sometime later because of the loss of capsular support (Figs. 2.38, 2.39).
Clinical Features
Dislocation into the vitreous may occur suddenly or gradually. Often, one haptic loop is hung up on residual peripheral capsule, creating a hinge-like situation. Vision is blurred as the intraocular lens disappears from the visual axis. Although the lens may remain inert in many cases, some exhibit chronic inflammation.
Management
Dislocated intraocular lenses may be retrieved from the surface of the retina and removed by vitreoretinal surgical techniques. Perfluorocarbon liquids are sometimes helpful in elevating the intraocular lens from the retinal surface. A posterior chamber lens can be sutured into the sulcus, or an anterior chamber lens can be inserted.
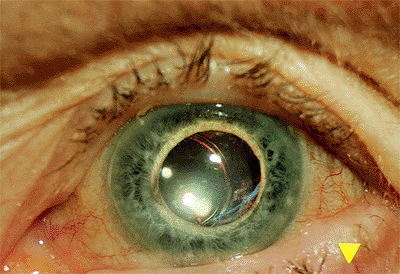 |
Figure 2.37. East-west decentration. The posterior chamber lens was inadvertently placed through a tear in the capsule nasally, resulting in medial decentration of the optic. |
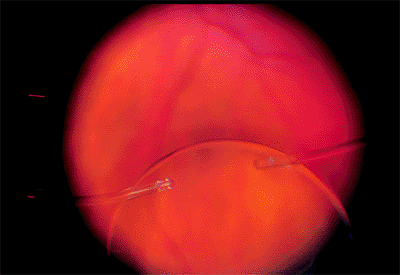 |
Figure 2.38. The intraocular lens has been lost in the vitreous and can be seen outlined on the retinal surface. (Courtesy of Terry Tomer, Philadelphia, PA.) |
Clinical Features
Subluxated posterior chamber lenses still do occur even with incision surgery. We have recently seen a case in which an acrylic lens was used in a patient with 4+ nuclear sclerotic cataract, small pupil, and PXS. The posterior capsule was torn during the procedure and because of the tear and the extent of the tear, it was elected to put the foldable acrylic lens in the ciliary sulcus. The patient recovered 20/20 vision and did well for 3 months but then presented with sudden loss of vision in that eye. At this point, he was found to have the lens subluxated into the vitreous. No posterior capsule was now visible within the pupil. The intraocular lens had one haptic still attached to the capsular bag remnant and was just barely visible inferiorly with the indirect ophthalmoscope.
Management
With repair of this type of problem, it is important that the patient undergo specular microscopy to determine the health of the corneal endothelium prior to the repair. It is also important that the retina be thoroughly evaluated. These surgeries should be performed with two surgeons available: one skilled in posterior vitrectomy, and the other skilled in anterior segment surgical techniques such as suturing in a posterior chamber lens. Both sets of skills are
P.87
necessary as the intraocular lens may appear to be retrievable when the patient is vertical at the slit lamp; however, when the patient is in the supine position, the intraocular lens will drop down into the vitreous and may prove impossible for the anterior segment surgeon to easily retrieve. Once the intraocular lens has been retrieved, our recommendation is to suture a new implant into position. The haptics will sometimes deform after being in the eye and this will contribute to another subluxation if the same lens is used. A variety of suturing techniques can be used for suturing in the intraocular lens, and a list of references is shown below.
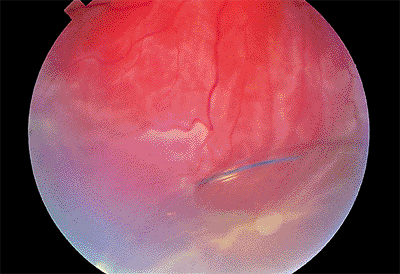 |
Figure 2.39. A posterior chamber lens has dislocated into the vitreous, resulting in associated retinal detachment. (Courtesy of Brian P. Connolly, Philadelphia, PA.) |
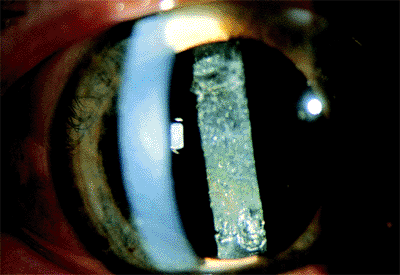 |
Figure 2.40. Opacification of the posterior capsule. (Courtesy of Stephen B. Lichtenstein, M.D., Philadelphia, PA.) |
Posterior Capsule Opacification
After extracapsular cataract extraction, proliferation of lens epithelial cells can lead to posterior capsule opacification (Fig. 2.40).
Clinical Features
Posterior capsule opacification occurs in 50% of patients within 5 years of surgery with some IOL types. The posterior capsule can become opacified as a result of continued proliferation of lens cells from the residual anterior lens epithelium or from residual fibrosis that could not be removed at the time of surgery. Blurred vision and increasing glare are the primary symptoms. Sometimes, tension striae develop in the capsule and become symptomatic. Other causes of blurred vision, particularly macular pathology, must be ruled out. The clarity of the examiner's direct ophthalmoscopic view of the fundus is a clinical indicator of the effect of the capsular opacification.
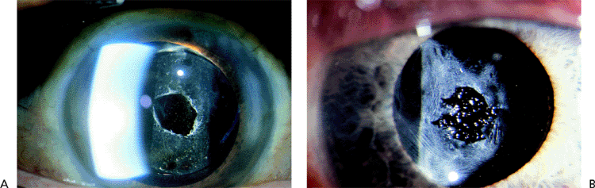 |
Figure 2.41. A: YAG laser capsulotomy. (B) Pitting of the lens can occur during YAG laser capsulotomy. (Courtesy of Stephen B. Lichtenstein, M.D., Philadelphia, PA.) |
Management
A YAG laser capsulotomy should be done when the patient is symptomatic (Fig. 2.41A). The improvement in visual acuity is often dramatic. The patient must be observed for an increase in intraocular pressure, which is usually transient. There is an increased risk of retinal detachment, particularly in myopic patients, after YAG capsulotomy. Retinal detachment has occurred in 1% to 3% of patients. Pitting of the intraocular lens may occur because of difficulty in focusing the laser beam. Although this may be bothersome to the surgeon, it seldom interferes with vision (Fig. 2.41B).
Cortical Pearl Formation
Clinical Features
Cortical pearl formation used to be very frequent in the time of extracapsular cataract surgery with no intraocular lens. With the use of smaller anterior capsulotomies, this problem has started to resurface. The small anterior capsulotomy results in cortical proliferation that can extend across the posterior surface of the intraocular lens even after a YAG capsulotomy previously has been performed. As a cortical proliferation at the edges progresses, the patients will often complain of glare, particularly at night.
Management
The treatment for cortical pearl formation is to use the YAG laser and disrupt the cortical proliferative material. This usually resolves the problem; however, in time, it may recur. In our experience, the rate of recurrence is approximately 3 in 1,000.
Capsule Contraction Syndrome
Capsule contraction syndrome is characterized by a progressive reduction in the diameter of the anterior capsulotomy after cataract surgery (Fig. 2.42).
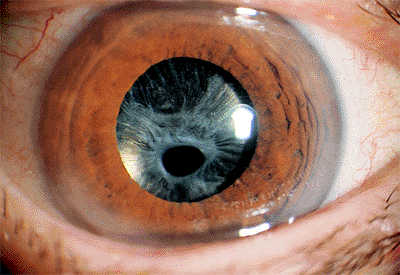 |
Figure 2.42. Capsule contraction syndrome. The anterior capsule has contracted over the lens and displaced the remaining opening inferior to the visual axis. (Courtesy of James A. Davison, M.D., Marshalltown, IA.) |
P.88
Clinical Features
Most cases are associated with a capsulorrhexis-type anterior capsulotomy. Davison surmised the reduction of the anterior capsule opening to be the result of the contraction of residual lens epithelial cells that have undergone fibrous dysplasia. The contraction may occur in the weeks immediately after surgery or at a later time. In Davison's experience, contraction occurred more frequently in eyes with pseudoexfoliation, poorly dilated pupils, small capsulorrhexis, and chronic uveitis.
Capsule contraction may greatly reduce the size of the opening, displace the opening from the visual axis, and result in a malposition of the intraocular lens. Cases of zonular rupture as a result of bag contracture have been reported.
Management
If the capsular contracture is recognized early, YAG laser radial anterior capsulotomies may reduce the sphincter effect. A larger capsulorrhexis or can-opener type capsulotomy with a deliberate superior radial tear may be considered at the time of surgery for predisposed patients. The use of an intraocular lens with a large optic and more rigid haptic may help oppose capsule contraction forces.
Nonencapsulated Anterior Capsular Flaps
Clinical Features
For the vast majority of patients, having an anterior capsular flap present within the pupillary space does not cause any difficulty. However, we have recently seen a patient who underwent topical clear corneal cataract extraction in the left eye. An anterior capsular tear was encountered, which extended to the edge of the posterior capsule. An intraocular lens was still implanted in the capsular bag, and postoperatively this patient had pain and mild cystoid macular edema. There was no vitreous to the wound, but a portion of the anterior capsule was visible within the pupillary space. This anterior capsule was freely moveable. With pupil constriction, the capsule seemed to offer very little resistance. The patient's visual acuity, however, did drop to the 20/60 level because of cystoid macular edema.
Management
The patient's eye was dilated and the YAG laser was used to cut the anterior capsule flap completely from the remainder of the anterior capsule. This flap then fell into the anterior chamber and appeared to have vitreous attached to the capsular flap itself. The patient's symptoms resolved completely with no discomfort, and visual acuity returned to the 20/20 level.
Cystoid Macular Edema
Cystoid macular edema is a condition in which fluid accumulates within the sensory retina in the macular area. It may occur after intraocular surgery, such as cataract and filtration procedures, and after retinal detachment surgery. Many other conditions, including diabetes, peripheral uveitis, retinitis pigmentosa, and angiomatous retinae, are associated with cystoid macular edema.
Clinical Features
The patient complains of decreased or hazy vision. Refraction may show a hyperopic shift, and fine macular detail is hazy ophthalmoscopically. However, the classic characteristic of cystoid macular edema is a petaloid appearance caused by leakage of the fluorescein dye in the outer plexiform layer (Fig. 2.43). The cause is still obscure, but there is evidence suggesting that inflammation plays a role. This hypothesis is reinforced by the more frequent occurrence of cystoid macular edema when complications of intraocular surgery, such as a vitreous wick, have occurred, and by cases associated with peripheral uveitis.
Management
Where there have been no surgical complications, cystoid macular edema after cataract surgery may frequently improve, even without treatment. The use of topical antiinflammatory drops may prove helpful, but after treatment is discontinued, cystoid macular edema may return. When there has been vitreous incarceration in the wound, a vitrectomy may be necessary.
Pseudophakic Retinal Detachment
Patients are at higher risk for retinal detachment after cataract surgery.
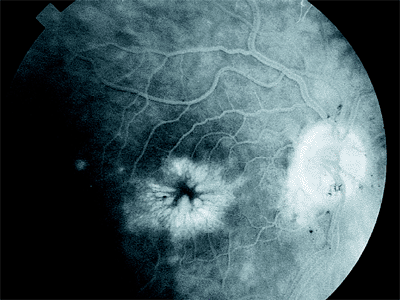 |
Figure 2.43. Typical petaloid appearance of cystoid macular edema caused by leakage of the fluorescein dye during angiography. |
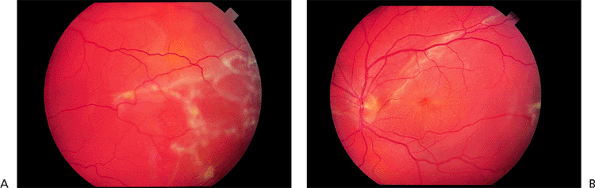 |
Figure 2.44. Pseudophakic retinal detachment at (A) the temporal periphery and (B) the posterior pole. |
P.89
Clinical Features
Symptoms and findings of pseudophakic retinal detachment are similar to those in phakic patients (Fig. 2.44). Retinal detachment is more common when complications of surgery such as vitreous loss have occurred, or after YAG laser capsulotomy. Patients at greatest risk for retinal detachment after YAG laser capsulotomy are those with high degrees of myopia, lattice degeneration, or a history of retinal detachment in the fellow eye.
Management
Most pseudophakic retinal detachments are caused by small breaks above the horizontal meridian near the ora serrata. Multiple breaks are common. Occasionally larger equatorial breaks occur (Fig. 2.45), and rarely a giant retinal break may be noted.
Endophthalmitis
Clinical Features
Patients with endophthalmitis occurring within the first week after intraocular surgery may present with or without pain. Early cases are characterized by inflammation and minimal hypopyon. The fundus may still be visualized. In these instances, it is difficult to differentiate between a purely inflammatory process and an infectious one. The level of discomfort, degree of injection and chemosis, and presence or absence of vitreous cells are factors that may help make this distinction. In mild cases, hourly steroids may be tried to determine if the condition improves. Close observation is essential. If the patient has more severe pain and there is fibrin on the anterior surface of the implant with a hypopyon, the condition is probably infectious (Fig. 2.46). A view of the retina may be difficult because of vitreous involvement.
One of the most frequent pathogens encountered in postoperative endophthalmitis is Staphylococcus epidermidis. This organism is less virulent than others, and many of these patients usually do well. When the organism is more virulent, such as Staphylococcus aureus or a gram-negative rod, the prognosis is considerably more guarded.
Management
When the first edition of Atlas of Clinical Ophthalmology was published, endophthalmitis after intraocular surgery was usually treated with vitrectomy accompanied by culture of the vitreous and anterior chamber aspirates. Since that time, the results of the Endophthalmitis Vitrectomy Study Group (EVS) were published. The study showed that for patients with hand motion or better vision,
P.90
there was no difference in outcomes between immediate three ports pars plana vitrectomy versus anterior chamber and vitreous taps followed by injection of intravitreal antibiotics. However, when visual acuity presentation was light perception only, better visual results were obtained when a vitrectomy was done. The EVS treatment outcomes demonstrated no difference in final visual acuity or media clarity outcomes whether or not systemic antibiotics were used.
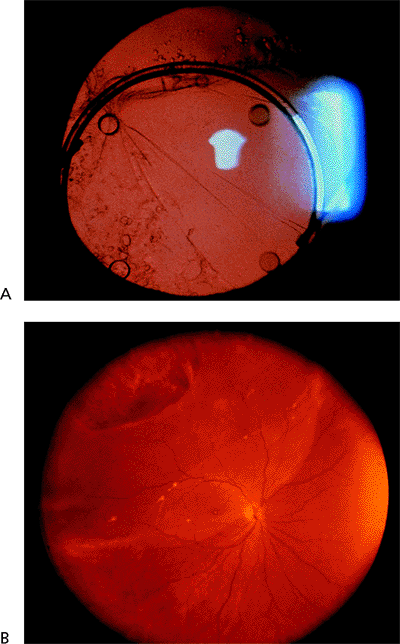 |
Figure 2.45. A: Pseudophakia and (B) large equatorial break with retinal detachment. |
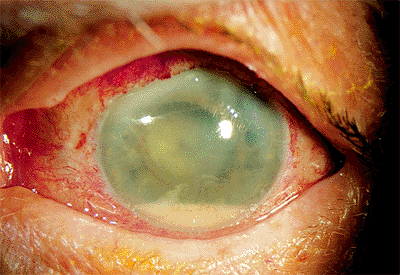 |
Figure 2.46. Acute endophthalmitis is characterized by hazy cornea, hypopyon, and exudative reaction over the implant surface in a patient 6 days after cataract surgery. |
Bibliography
Berliner ML. Biomicroscopy of the eye, vol II. New York: Paul B. Hoeber; 1949:1091.
Boyd-Monk H, Steinmetz CG. Nursing care of the eye. Norwalk: Appleton & Lange; 1991:13.
Charlton JR, Weinstein GW. Cataract surgery. In: Tasman W, Jaeger EA. Clinical ophthalmology, vol 6. Philadelphia: JB Lippincott; 1994:1.
Cheng KP, Hiles DA, Biglan AW, et al. Management of posterior lenticonus. J Pediatr Ophthalmol Strabismus 1991;28:143.
Cogan DG, Kuwabara T. Pathology of cataracts in Mongoloid idiocy. A new concept of pathogenesis of cataracts of the coronary-cerulean type. Doc Ophthalmol 1962;16:73.
Cordes FC. Types of congenital and juvenile cataracts. In: Haik GM. Symposium on diseases and surgery of the lens. St. Louis: CV Mosby; 1957:43.
Datiles MB, Kinoshita JH. Pathogenesis of cataracts. In: Tasman W, Jaeger EA. Duane's clinical ophthalmology, vol 1. Philadelphia: JB Lippincott; 1994:1.
Davison JA. Capsule contraction syndrome. J Cataract Refract Surg 1993;19:582.
Delamere NA, Paterson CA. The crystalline lens. In: Tasman W, Jaeger EA. Foundations of clinical ophthalmology, vol II. Philadelphia: JB Lippincott; 1994:1.
Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. Arch Ophthalmol 1995;113:1479.
Gibbs ML, Jacobs M, Wilkie DM, et al. Posterior lenticonus: clinical patterns and genetics. J Pediatr Ophthalmol Strabismus 1993;30:171.
Jaffe NS, Jaffe MS, Jaffe GF. Cataract surgery and its complications. 5th ed. St. Louis: CV Mosby; 1990:359.
Kleiman NJ, Worgul BV. Lens. In: Tasman W, Jaeger EA. Foundations of clinical ophthalmology, vol 1. Philadelphia: JB Lippincott; 1994:1.
Kozart DM, Yanoff M. Intraocular pressure statistics in 100 conservative patients with exfoliation syndrome. Ophthalmology 1982;89:214.
La Piana FG. Renal disease. In: Tasman W, Jaeger EA. Duane's clinical ophthalmology, vol 5. Philadelphia: JB Lippincott; 1994:4.
Parks MM, Johnson DA, Reed GW. Long-term visual results and complications in children with aphakia. Ophthalmology 1993;100:826.
Schotzer-Schrehardt U, Neuman GO. A histopathologic study of zonular instability in pseudoexfoliative syndrome. Am J Ophthalmol 1994;118:730.
Skuta GL. Pseudoexfoliation syndrome, pigment dispersion syndrome and the associated glaucomas. In: Tasman W, Jaeger EA. Duane's clinical ophthalmology, vol 3. Philadelphia: JB Lippincott; 1994:1.
Smith SG, Holland E, Peterson J. Peripheral anterior synechiae formation with anterior chamber intraocular lenses. Ophthalmic Surg 1992;23:315.
Smith SG, Lindstrom RL. Intraocular lens complications and their management. Slack 1988:167.
Spencer WH. Lens. In: Spencer WH. Ophthalmic pathology, an atlas and textbook, vol 1. 3rd ed. Philadelphia: WB Saunders; 1985:423.
Van Heyninger R. Formation of polyols by the lens of the rat with sugar cataract. Nature 1959;184:194.
EAN: 2147483647
Pages: 17