64 - Localized Fibrous Tumors of the Pleura
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIII - The Trachea > Chapter 76 - Surgical Anatomy of the Trachea and Techniques of Resection and Reconstruction
Chapter 76
Surgical Anatomy of the Trachea and Techniques of Resection and Reconstruction
Hermes C. Grillo
ANATOMY
Functionally, the trachea serves principally as a conduit for ventilation. Viewed in this way, it would seem to be an ideal structure for replacement or reconstruction when involved by surgical disease. Anatomically, however, it presents several unique features that partially account for the difficulty in its surgical management. These features are its unpaired nature, unique structural rigidity, short length, relative lack of longitudinal elasticity, proximity to major cardiovascular structures, and segmental blood supply.
In 1964, I and my colleagues reported that the adult human trachea averages 11.8 cm in length (range, 10 to 13 cm) from the infracricoid level to the top of the carinal spur. Usually, 18 to 22 cartilaginous rings occur within this length, about two rings per centimeter. Occasionally, rings are incomplete or bifid. In an adult man, the internal diameter of the trachea measures about 2.3 cm laterally and 1.8 cm anteroposteriorly. These measurements vary roughly in proportion to the size of the individual and are usually smaller in women. The cross-sectional shape in the adult is nearly elliptic. In infants and children, it is more nearly circular. The configuration may change with disease. Thus, the lower two thirds may be flattened in tracheomalacia or rigidly narrowed from side to side to produce a saber-sheath trachea.
The surgeon usually visualizes the trachea as he or she learned to see it in the thyroidectomy position, with the neck extended, as a structure that is one half cervical and one half thoracic. Mulliken and the author (1968) pointed out that the trachea becomes almost entirely mediastinal when the neck is flexed because the cricoid cartilage drops to the level of the thoracic inlet. This may be the permanent position in aged people because of cervical kyphosis. These simple observations contributed to the development of surgical reconstructive techniques that obviate the requirement for prostheses.
The trachea, when viewed laterally in the upright individual, courses backward and downward at an angle from a nearly subcutaneous position at the infracricoid level to rest against the esophagus and vertebral column at the carina. The larynx and the origin of the esophagus are intimately related anatomically at the cricopharyngeal level. Below this point, the posterior membranous wall of the trachea maintains a close spatial relationship to the esophagus. A distinct, easily separable plane is present below the cricoid level, but a common blood supply is shared. Anteriorly, the thyroid isthmus passes over the trachea in the region of the second ring. The lateral lobes of the thyroid are closely applied to the trachea, and a common blood supply is obtained from the branches of the inferior thyroid artery. Lying in the groove between trachea and esophagus are the recurrent nerves, coursing from beneath the arch of the aorta on the left side and therefore having a longer course in proximity to the trachea there than on the right side, where the nerve has looped around the subclavian artery and then approached the groove. A nonrecurrent nerve rarely is present on the right in conjunction with an anomalous subclavian artery. These nerves enter the larynx between the cricoid and thyroid cartilages just anterior to the inferior cornu of the thyroid cartilage.
The anterior pretracheal plane may be developed easily in the cervical region. Fibrofatty tissue, lymph nodes, and fine branches of the anterior jugular vein are present in front of this plane. The innominate vein lies anteriorly, away from the trachea. The innominate artery, however, crosses over the midtrachea obliquely from its point of origin from the aortic arch to the right side of the neck. In children, the innominate artery is higher and is encountered in the lower part of the neck. In some adults, the artery is unusually high and crosses the trachea at the base of the neck when slight extension is present. Occasionally, a tiny branch of this artery may be encountered in the segment of the artery that crosses the trachea. At the level of the carina,
P.1037
the left main bronchus passes beneath the aortic arch and the right beneath the azygos vein. The pulmonary artery lies just in front of the carina. On either side of the trachea lies fibrofatty tissue containing lymph node chains; a large packet of nodes lies just beneath the carina (see Chapter 6).
The course of the trachea from the anterior cervical position to the posterior mediastinal position with close relationships to major vascular structures makes access to the entire trachea through a single incision difficult. I (1969) emphasized that these anatomic facts demand precise definition of the extent and nature of the tracheal lesions in planning surgical procedures.
The cartilaginous rings give the human trachea its lateral rigidity. The rings extend about two thirds of the circumference. The posterior wall is membranous. The trachea is lined with respiratory mucosa, which is tightly applied to the inner surface of the cartilages grossly. The normal epithelium is columnar and ciliated. The cilia clear particulate matter and secretions. Mucous glands are liberally present. In chronic smokers and in persons with other chronic irritation, squamous metaplasia frequently occurs; in extreme instances, few ciliated cells remain. Such individuals must clear secretions by coughing vigorously. This observation, plus the demonstrated feasibility of cutaneous reconstructions and occasional successes with prosthetic interpositions, makes it clear that ciliated epithelium, although highly desirable, is not essential for tracheal reconstruction. Between the cartilaginous rings and in the membranous wall, the submucosa is fibromuscular.
Considerable contraction of the muscular membranous wall can occur with coughing and with spasm, the tips of the cartilages being drawn inward. Such transient narrowing of the airway may be observed fluoroscopically and during bronchoscopy in normal individuals. Some longitudinal flexibility exists; a degree of elasticity is present that appears to be greater in youth and to decrease with age. Calcification of the rings is seen most often with advancing age, although to a lesser degree than in the cricoid cartilage. Local trauma or operation may lead to calcification. The normal trachea slides easily in its layer of fibrofatty areolar tissue from neck to mediastinum.
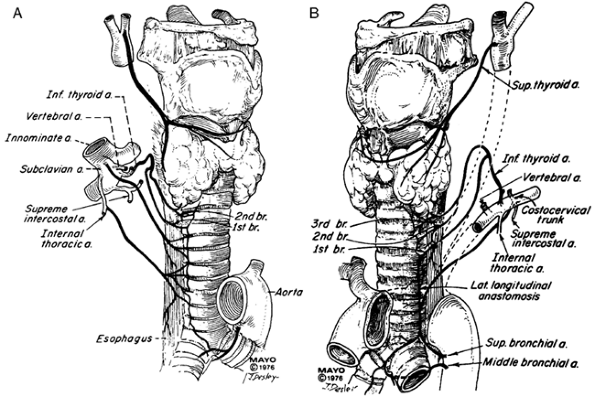 |
Fig. 76-1. Blood supply of the trachea. A. Right anterior view. B. Left anterior view. The right side varies in some respects. From Salassa JR, Pearson BW, Payne WS: Gross and microscopical blood supply of the trachea. Ann Thorac Surg 24:100, 1977. With permission. |
The blood supply of the human trachea is segmental, largely shared with the esophagus and derived principally from multiple branches of the inferior thyroid artery above and the bronchial arteries below. The arteries approach laterally, and fine branches pass anteriorly to the trachea and posteriorly to the esophagus. Miura and the author (1966) noted that the inferior thyroid artery nourishes the upper trachea, usually through a pattern of three principal branches with fine subdivisions and extremely fine collateral vessels, but with many variations, as noted by Salassa and colleagues (1977). The bronchial vessels nourish the lower trachea. Sometimes, the internal mammary artery contributes (Fig. 76-1). Excessive circumferential dissection with division of the lateral pedicles during an operative procedure can easily devascularize the trachea.
METHODS OF RECONSTRUCTION OF THE TRACHEA
The surgical approach to the trachea developed more slowly than other areas of thoracic surgery, as I have reviewed in detail (2003), because of the rarity of tracheal tumors, the anatomic complexities of reconstruction, and the biologic incompatibilities that met efforts at prosthetic reconstruction. Earlier hesitations because of problems of physiologic management during reconstruction proved to be
P.1038
less formidable. The growth in frequency of postintubation benign lesions, as a result of the success of modern respiratory therapy, increased the urgency of developmental work.
The concept of direct end-to-end anastomosis of trachea to trachea was generally accepted as the ideal method of tracheal repair after reconstruction. It was long believed, however, as stated by Belsey (1950), that no more than 2 cm (about four tracheal rings) could be removed and anastomosis consistently made. As a result, lateral resection was done when possible, with attempts made to patch the defect in various ways, using fascia, skin, pericardium, other tissues, and foreign materials. When such a technique was applied to malignant neoplasms, inadequate removal of tumor resulted, with early recurrence. Such patches also failed to heal. Partial cicatrization was an additional factor. Attention was directed early to the development of an artificial trachea.
Prosthetic Replacement
Many materials have been used for prosthetic replacement of the trachea. Most work has been done in animals, but a scattered experience in humans has been reported. Replacements have consisted of tubes made of glass or metal; stainless steel mesh in either tubes or coils and tantalum mesh; Lucite, polyethylene, and other plastic cylinders; and tubes of Ivalon or Marlex mesh and Teflon with combinations of Ivalon or Dacron. More recently, investigators have tried polytetrafluoroethylene and Silastic tubes, often with stainless steel wire or plastic rings to supply rigidity. Early prostheses were usually solid tubes that bridged defects between the two ends of the trachea. Their failure led to use of rigid mesh cylinders that were intended to allow incorporation into the surrounding connective tissue. More recently, flexible meshwork has been supported by splinting plastic rings. These meshwork prostheses were based on the theory that they would be incorporated by connective tissue and that epithelium would then grow down over this bed of new connective tissue; the rigid rings would maintain an open airway. In most experiments, only short prosthetic bridges have been incorporated to any extent. Some of the longer prostheses maintain an open airway, but firm healing with full tissue encasement and epithelialization has not occurred. This basically unhealed state might be acceptable as an airway, but these longer prostheses have been subject, in a high percentage of instances, to occlusion by formation of granulation tissue at the nonhealing ends, strictures at these ends, sepsis causing rejection of the prosthesis, or erosion of major vessels with fatal hemorrhage. An occasional long-term success has occurred, largely as an exception rather than as the rule. The problem is the biologic instability caused by placing a foreign body in a bed of connective tissue adjacent to an epithelium that necessarily is contaminated with bacteria. With a foreign body in place, a chronic abscess is presented to the mediastinum, with the described results. Borrie and Redshaw (1970) attempted to solve these problems by accepting a foreign tube as a permanent airway, but making it with cuffs that could be sutured that they hoped would be incorporated by connective tissue. Neville and associates (1990) reported successes with a similar prosthesis. Vogt-Moykopf and Mickisch (1987) noted the many complications that occur.
Chemically fixed tracheal tissue grafts are essentially bioprostheses, subject sooner or later to resorption and cicatricial replacement. Only short experimental allografts have survived when indirectly revascularized, most often by omentum. Longer grafts have been accomplished with direct revascularization, both arterial and venous. This requires concurrent transplantation of thyroid gland and esophagus to preserve what is common blood supply. The usual immunologic problems are present, and a major concern is that the principal need for tracheal replacement today is for extensive malignant tumors, usually a contraindication to transplantation. Despite all efforts, there is no viable tracheal prosthesis.
Anatomic Mobilization
Perhaps most crucial to the evolution of mobilization techniques for tracheal reconstruction was recognition that the cervical trachea, as seen in the hyperextended surgical thyroid position, may be delivered into the mediastinum by cervical flexion. A few reports of clinical resections greater than 2 cm appeared, but few systematic studies of the anatomic potential are recorded. Michelson and associates (1961) noted that careful mobilization of the entire trachea in eight cadavers allowed for anastomosis with 1 lb of tension, after resection of 4 to 6 cm, with an additional 2.5 to 5.0 cm obtained by division of the left main bronchus.
Detailed anatomic studies in cadavers attempted to answer the surgical questions of how much trachea could be resected and primary anastomosis made when the trachea was approached in progressive fashion from either a cervical or a transthoracic approach, depending on the location of the lesion. In one study, Mulliken and the author (1968) mobilized the trachea through a cervicomediastinal approach, carefully preserving the lateral tissue that bears the blood supply. Using a standard tension of 1000 to 1200 g for approximation, it was possible, with the neck in 15 to 35 degrees of flexion, to resect an average length of 4.5 cm (about seven rings) and to increase this by 1.4 cm by entering the pleural space and mobilizing the right hilus (Fig. 76-2A). With greater degrees of cervical flexion, even longer resections are possible. Suprahyoid laryngeal release, described by Montgomery (1974), adds 1.0 to 1.5 cm while minimizing the difficulties in swallowing that attended earlier techniques for release. Alternating lateral division of the intercartilaginous ligaments of the trachea to obtain extension has been proposed experimentally but not applied clinically to any extent. This technique has the disadvantages of probable interference with tracheal blood supply
P.1039
and the need for extensive tracheal exposure to obtain a rather limited extension of length.
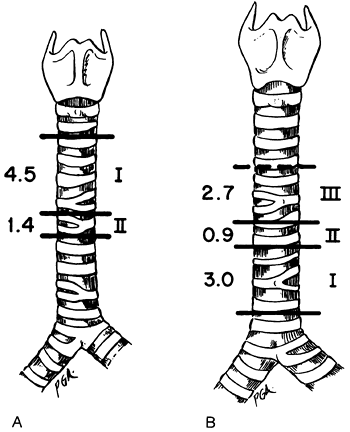 |
Fig. 76-2. The amounts of trachea that can be removed and yet permit primary anastomosis. A. Cervicomediastinal mobilization permitted removal of 4.5 cm under 1,000-g tension, with cervical flexion. Intrathoracic dissection permitted removal of an additional 1.4 cm. B. Transthoracic hilar dissection and division of the pulmonary ligament, with the cervical spine in the neutral position, permitted removal of 3 cm, intrapericardial dissection an additional 0.9 cm, and division of the left main bronchus with reimplantation in the bronchus intermedius an additional 2.7 cm. The use of cervical flexion has demonstrated that the area designated I may be significantly greater than 3 cm. From Grillo HC: Surgical approaches to the trachea. Surg Gynecol Obstet 129:347, 1969. With permission. |
In approaching the lower half of the trachea, I and my colleagues (1964) accomplished mobilization progressively by first freeing the hilus of the right lung and dividing the pulmonary ligament; second, freeing the pulmonary vessels from their pericardial attachments; and third, transplanting the left main bronchus, which is held in place by the arch of the aorta, to the bronchus intermedius. In these earlier studies, the neck was held in the neutral position. At tensions under 1000 g, the first maneuver allowed for resection of 3 cm and the second for 0.9 cm additionally; the radical measure of bronchial implantation permitted an additional 2.7 cm (Fig. 76-2B). It has since become clear that cervical flexion, combined with hilar and pericardial mobilization plus division of the pulmonary ligament, allows lengths of 5 to 6 cm to be removed by the transthoracic approach. These figures represent only guidelines. The length of trachea that may be resected safely in an individual varies widely with age, posture, bodily habitus, extent of disease, and prior tracheal surgery. Bronchial implantation has been reserved for carinal excision or similar complex maneuvers to avoid adding another unnecessary risk to operation. Reimplantation of the left main bronchus into the bronchus intermedius was first used clinically by Barclay and associates (1957).
The limits of safety with varying anastomotic tensions have not been established in humans. Cantrell and Folse (1961) found in dogs that tensions below 1700 g permitted safety from disruption after anastomosis. In anatomic studies in the cadaver, we found that an average tension of 675 g only was required for approximation (maximum, 1000 g) after a 7-cm resection. Such clinical measurements as we have made show tensions of about 600 g in resections of 4 to 5 cm in length. Although about one half of the adult trachea is safely resectable in most adults, with the caution expressed, Wright and associates (2002) observed clinically that the more fragile juvenile trachea is at risk if resection exceeds about one third.
Anatomic and clinical observations show that great attention must be paid to the lateral blood supply in tracheal mobilization. This fine segmental supply cannot be disrupted safely, particularly for anastomosis of a long distal segment to a short proximal segment; the distal segment must not be freed circumferentially.
Another peculiarity of tracheal reconstruction depends on the relative rigidity of the anterolateral walls. Transverse wedging of the anterior wall of the trachea may buckle the posterior wall into a partially obstructing valve. Circumferential resection, which may, however, be beveled, is most often preferable.
SURGERY OF THE TRACHEA
Anesthesia
The airway must be under full control at all times during reconstructive surgery of the trachea, so that hasty maneuvers are unnecessary and hypoxia does not occur. The patient should preferably breathe spontaneously during the operation and always at its conclusion so that ventilatory support is not necessary postoperatively. Cardiopulmonary bypass has been used for tracheal surgery, but it is not necessary for relatively simple resection and, as noted by Geffin and colleagues (1969), presents real hazards for more complex procedures requiring extensive manipulation of the lung. Procedures are explained carefully to the patients before the operation. Induction is carried out slowly and gently, especially in a patient with a highly obstructed trachea. If a benign stenosis presents an airway diameter of less than 5 mm, dilatation is performed, and an endotracheal tube is passed beyond the lesion, to prevent arrhythmia caused by CO2 buildup during the early stages of operation. Occasionally, a nearly obstructing tumor has required prompt bronchoscopy with a ventilating bronchoscope shortly after induction, with subsequent intubation. Obstructing tumor may be cored out with the rigid bronchoscope aided by biopsy forceps. Frequent monitoring of blood gases and electrocardiography
P.1040
are essential. Bronchoscopic examination should be done by the surgeon and observed by the anesthetist, who must deal with this airway until surgical access distal to the lesion has been obtained. If tracheostomy is already present, induction is simplified. Initial dissection is always done carefully to avoid increasing the degree of obstruction by roughness or pressure. The area below the obstruction is isolated first, so that a transection of the trachea can be performed at any point and an airway can be introduced across the operative field, should the degree of obstruction increase. Sterile anesthesia tubing, connectors, and endotracheal tubes are available in the operative field. I have not found it necessary to make distal incisions in the tracheobronchial tree for insertion of ventilatory catheters but, rather, have proceeded as described. If transthoracic resection is performed close to the carina, the endotracheal tube is passed into the left main bronchus and that lung alone is ventilated; if the Po2 decreases toward unsatisfactory levels, a previously isolated right pulmonary artery is temporarily clamped to eliminate the shunt through the right lung. This is rarely required. Slow increase in shunting may occur during prolonged operation because of low tidal ventilation, increasing atelectasis, and aspiration of secretions and must be guarded against, as noted by Wilson (1987). High-frequency ventilation is a useful adjunct, especially in complex carinal reconstruction, as reported by El-Baz and associates (1982).
Surgical Approaches
Lesions in the upper half of the trachea that are known to be benign are best approached cervically (Fig. 76-3A). If a malignant lesion is present, be prepared for the cervicomediastinal and, possibly, thoracic approach. Placement of the cervical incision depends on the pathologic state, the presence of existing stomas, and the possible need for sternotomy. If a postoperative temporary tracheostomy stoma may be required after a difficult laryngotracheal anastomosis, then the incision must be planned so that a stoma can be made away from the incision. If the initial dissection through the neck indicates need for further exposure, the upper sternum is split to a point just beyond the angle of Louis; horizontal division of the sternum into an intercostal space is not necessary. Because the great vessels present anteriorly, division of more than the upper sternum is not helpful; division simply allows room to maneuver in managing the more distal trachea (Fig. 76-4B). Innominate vein division also adds nothing.
Rarely, this incision must be extended through the fourth intercostal space on the right to permit additional mobilization of the intrathoracic trachea by freeing the hilus of the right lung. Such an incision permits wide exposure of the entire trachea from cricoid to carina. This is almost never necessary in benign stenosis. If extirpative surgery and terminal tracheostomy are expected, the incision, as I have stated (1965), should avoid a vertical limb even if sternal division is needed. A long-segment cutaneous tracheal replacement also may be so fashioned. Such circumstances are unusual but should be kept in mind in planning extensive procedures.
Neoplastic lesions of the lower half of the trachea are most easily approached directly through a high right thoracotomy incision (Fig. 76-3B). It is possible to excise even low benign lesions from the anterior approach described. Cervical flexion devolves sufficient trachea into the mediastinum so that lower tracheal tumors are usually approachable completely through the right side of the chest without a sternal component. The fourth intercostal space or fourth rib resection is used. Median sternotomy with dissection between the superior vena cava and aorta, and anterior and posterior pericardial division, provides access to the lower trachea and carina, but the exposure is poor for extensive dissection or complex reconstruction.
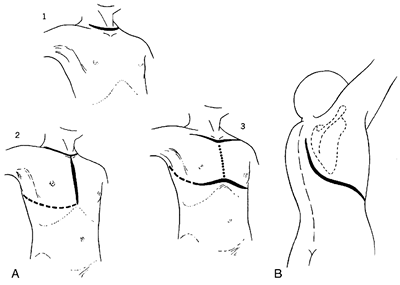 |
Fig. 76-3. A. Incisions for approach to the upper portion of the trachea. (1) Cervical incision allows access to upper trachea and to the mediastinum with somewhat limited exposure. (2) Median sternotomy, usually carried only through the upper one third of the sternum, allows more extended dissection into the mediastinum. Extension of the incision to the right fourth intercostal space (dotted line) allows exposure of the entire trachea from cricoid to carina and permits mobilization of the hilus. (3) Cervicomediastinal approach is here carried out beneath a bipedicled anterior skin flap. The flap is kept intact in case it is necessary to fashion a mediastinal tracheostomy. Such an incision is rarely needed. B. Incision for approach to the lower trachea and carina. The thorax is entered through the fourth intercostal space or the bed of the resected fourth rib. The high incision shown permits the scapula to be drawn out of the way. From Grillo HC: Surgical approaches to the trachea. Surg Gynecol Obstet 129:347, 1969. With permission. |
Reconstruction of the Upper Trachea
The upper flap is raised with or without circumcising an existing tracheostomy incision or including it in the original
P.1041
incision; individualization is required in each patient. Many existing tracheostomy stomas, even if they are to be allowed to close spontaneously later, usually have to be remade in another opening in the skin because of changed postoperative relationships between trachea and overlying skin. If the lesion is high, benign, and short, only a limited field is required. Dissection is confined chiefly to the midline, the upper flap being raised to the level of the cricoid and the lower to the sternal notch to allow dissection in the pretracheal plane as needed. Dense scar is often present in association with benign stenosis, and dissection is done close to the trachea to avoid damage to the recurrent nerves, especially near the cricoid. Isolation of the nerves is avoided because this would increase the danger of injury. Freeing the trachea below the lesion early allows easy establishment of airway control and expedites dissection of a cicatrized segment from the esophagus. Mobilization is made as required before and behind the trachea both proximally and distally. Tentative approximation with traction sutures, while the neck is flexed by the anesthetist, demonstrates whether approximation may be accomplished or whether further dissection is needed. A single layer of anastomotic sutures is placed in interrupted fashion so that the knots are tied on the outside. Fine No. 4-0 absorbable polymeric sutures are preferred. In many instances, the sutures become inaccessible to direct vision during tying and must not break (Fig. 76-4). The anterior approach also may be used for tumor, but in this situation, sternotomy is often required for adequate removal of paratracheal tissue. In this instance, the recurrent nerves are usually identified and preserved, if they are not involved by tumor.
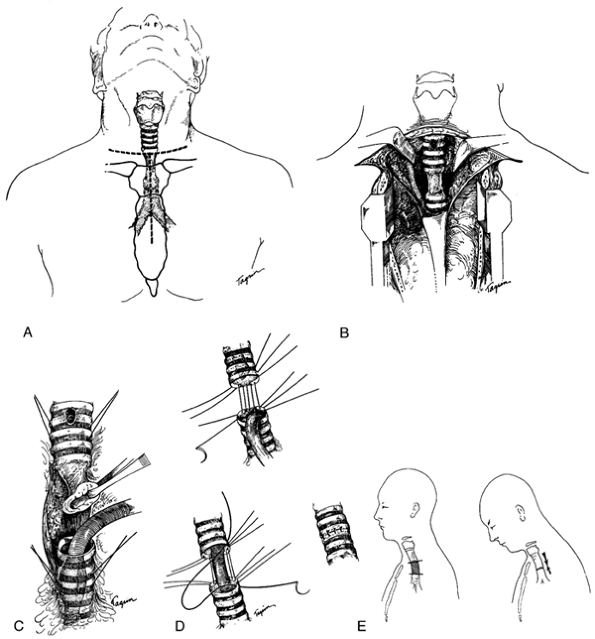 |
Fig. 76-4. Reconstruction of the upper trachea. A. The collar incision is often adequate for benign lesions in the upper and middle trachea. Partial division of the sternum allows access to the mediastinum over the great vessels. B. The innominate vein is retracted but not divided because greater exposure is not so obtained owing to the posterior position of the lower trachea. The pleura is intact. C. Direct intubation has been performed after division of the trachea below an adherent stenotic lesion. Dissection is now simplified. Traction sutures are shown and also the scar of the previous tracheostomy. D. Details of placement of sutures. Interrupted sutures passing through the cartilage and membranous wall are used. Knots are tied on the outside. E. Diagram to indicate that the majority of mobilization in the approach to the upper trachea is obtained by cervical flexion with downward devolution of the trachea and a lesser amount by upward movement of the distal trachea. A D, From Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970. With permission. |
When benign stenosis of the upper trachea also involves the subglottic larynx, one-stage reconstruction is possible. As I (1982a) reported, the technique is complex. The anterior subglottic larynx is resected and, in cases in which the stenosis is circumferential, the posterior cricoid lamina is bared but preserved in order to protect the recurrent laryngeal nerves (Fig. 76-5). The distal tailored trachea is advanced to replace the anterior subglottic laryngeal wall with cartilages and to resurface the posterior cricoid plate with membranous tracheal wall. As I and my colleagues have reported (1992), results are generally favorable. Stenting of the anastomosis is not necessary if the repair is precise.
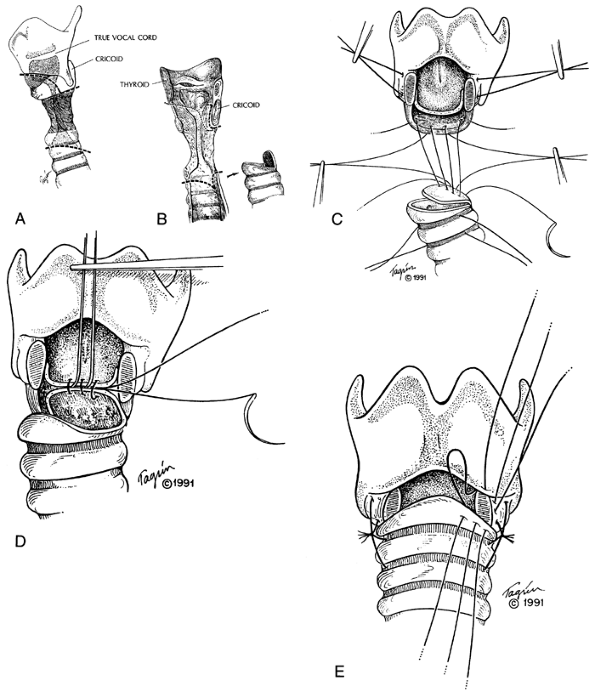 |
Fig. 76-5. Technique of laryngotracheal resection and reconstruction. A. External lines of division of the larynx and trachea are indicated by dashed lines. The anterior cricoid arch is removed. B. When the subglottic intralaryngeal stenosis is circumferential, scar is removed from the front of the posterior cricoid lamina, baring the cartilage. The residual posterior cricoid lamina protects the recurrent laryngeal nerves. Distally, the trachea is beveled over the length of one cartilage to fit the anterolateral subglottic defect that has been created. A broad-based flap of membranous tracheal wall is fashioned to resurface the bared cricoid plate. C. The posterior flap is fixed to the lower margin of the cricoid plate with four extraluminal sutures (4-0 Tevdek). The lateral traction sutures (2-0 Vicryl) are shown also in the larynx proximally and in the trachea distally. D. Posterior mucosal anastomotic sutures (4-0 Vicryl) are placed with knots to lie behind the mucosa. Traction sutures are omitted in this diagram for simplicity. E. After placement of all the posterior and posterolateral anastomotic sutures as far anteriorly as the lateral stay sutures, the patient's neck is flexed, the stay sutures are tied, the sternal fixing Tevdek sutures are tied, and then the posterior mucosal sutures are tied. The anterior and anterolateral anastomotic sutures are then placed and finally tied serially. A, B, From Grillo HC: Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 33:3, 1982a. With permission. C E, From Grillo HC, Mathisen DJ, Wain JC: Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 53:54, 1992. With permission. |
P.1042
P.1043
Reconstruction of the Lower Trachea
After confirmation of the extent of a tumor, anatomic mobilization is usually accomplished before severing the trachea. If obstruction appears to be imminent during mobilization, the trachea is transected and distally intubated. If the line of transection is supracarinal, the left main bronchus is intubated. Access to the subcarinal lymph nodes and lower paratracheal nodes is excellent. The recurrent nerves reach a point adjacent to the trachea promptly and should be sacrificed deliberately only if required. Cervical flexion by the anesthetist devolves a fair segment of trachea into the chest even in the lateral position, and this, in combination with the mobilization maneuvers earlier noted, permits end-to-end anastomosis (Fig. 76-6). Complex maneuvers may be necessary for excision and reconstruction of carinal lesions or lesions involving the right main-stem bronchus or upper lobe bronchus (Fig. 76-7). In general, my principle is to excise the tumor with a satisfactory margin and then use a suitable reconstruction for the specific situation. As I have described (1965, 1970, 1982b), a second-layer flap is always placed around intrathoracic anastomoses, usually a carefully pedicled pericardial fat pad. I described specific techniques of carinal reconstruction in detail (1982b). Laryngeal release is not helpful in carinal resection. Mitchell and others (1999) summarized our more recent experiences with carinal reconstruction.
Tracheostomy is avoided after tracheal reconstruction to avoid drying of secretions or injury to the anastomosis. On rare occasions, it may be necessary, temporarily, after laryngotracheal anastomosis.
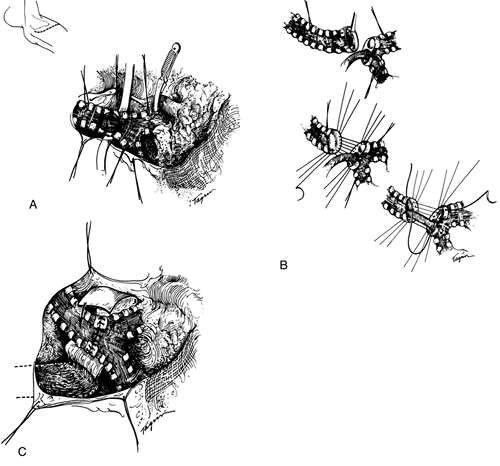 |
Fig. 76-6. Reconstruction of the lower trachea. A. Exposure through a thoracotomy. Hilar mobilization has been accomplished and also circumferential dissection of the trachea. When a tumor is present, paratracheal nodal tissue is excised with the specimen. Traction sutures have been placed proximally and distally. The lines of resection are shown. A clamp may be placed on the pulmonary artery later if the patient fails to maintain adequate oxygenation on intubation of the left main bronchus alone. This step is rarely necessary B. Details of management of resection and suturing. Intubation has been carried out across the operative field, and the specimen is then removed. After placement of sutures on the anterior and lateral walls of the trachea, an elongated endotracheal tube is passed from above into the left main bronchus, and the balance of the posterior sutures are placed before they are tied. C. After completion of the anastomosis, which is facilitated by flexion of the patient's neck, a second layer of vascularized tissue is placed around the anastomosis. From Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970. With permission. |
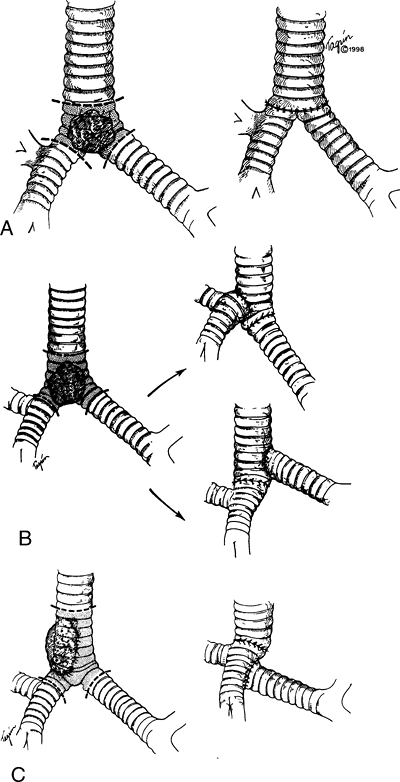 |
Fig. 76-7. Tracheal reconstruction after carinal resection. A. When the lesion is of limited extent, a neocarina may be fashioned. B. Following carinal resection with removal of a segment of trachea less than 4 cm, reconstruction is most frequently performed by implanting the left main bronchus into the stump of the devolved lower trachea. The right main bronchus is then implanted preferentially in a side opening fashioned in the lower portion of the trachea. C. When a longer segment of trachea has been resected, it frequently is usually to mobilize the right lung and elevate the right main bronchus to reach the trachea, which also has been devolved as far distally as it will go. The left main bronchus is anastomosed to the bronchus intermedius. Preservation of blood supply for all of these segments of airway is essential for the repair to heal successfully. From Grillo HC: Tracheal tumors: surgical management. Ann Thorac Surg 26:112, 1978. With permission. |
P.1044
Complex Methods
One sees few benign lesions or potentially curable malignant lesions that require resection of lengths of trachea and still leave a functional larynx, in which end-to-end reconstruction may not be done by present methods. In a rare instance, one can fashion a replacement of cervical trachea by fashioning an invaginated horizontally bipedicled tube of full-thickness skin supported by fully buried polypropylene rings, as I suggested in 1965. Results have been no more dependable than the use of prostheses. The best alternatives are T tubes for benign lesions and irradiation for malignant lesions nonresectable because of length.
CONGENITAL TRACHEAL STENOSIS
Short-segment congenital tracheal stenosis is effectively managed by resection and reconstruction, bearing in mind
P.1045
that length of resection must be limited in the juvenile trachea, as pointed out by Wright and colleagues (2002) of my group. 5-0 Vicryl sutures are employed for anastomosis. A short stenotic bridge bronchus is also treated by resection with side-to-side anastomosis (Fig. 76-8).
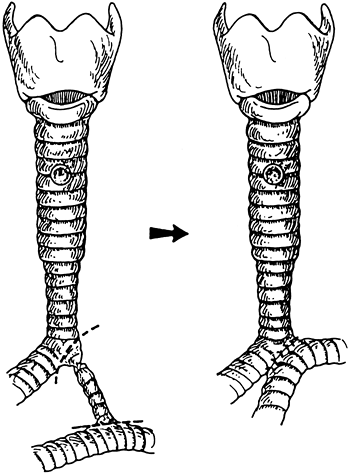 |
Fig. 76-8. Short stenotic anomalous bridge bronchus treated by resection and side-to-side anastomosis. In this patient, the more proximal tracheal stenosis was not sufficiently narrow to require repair. In another patient, a longer stenotic bridge bronchus was widened by slide technique. Resection of this anomaly was described by Cantrell and Guild (1964). From Grillo HC, et al: Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg 123:145, 2002. With permission. |
Long congenital stenosis may be managed by slide tracheoplasty, as I (1994) have suggested. The long stenotic segment is transected at its midpoint; the upper half of stenosis is divided vertically posteriorly and the lower half anteriorly. Corners are trimmed, the two segments slid together, and anastomosis performed (Fig. 76-9). The tracheal circumference is thus doubled, the cross-sectional area is quadrupled, tension is acceptable, and blood supply remains adequate. Advantages of this direct method over prior use of patches of cartilage or pericardium are: (a) trachea reconstructed with trachea, minimizing granulation tissue or necrosis; (b) immediate epithelization of lumen; (c) generally prompt extubation postoperatively; and (d)
P.1046
elimination of the need for cardiopulmonary bypass except when pulmonary artery sling or other cardiac anomalies are present. Wright and associates (2002) of my group have demonstrated long-term growth of the trachea repaired in this way.
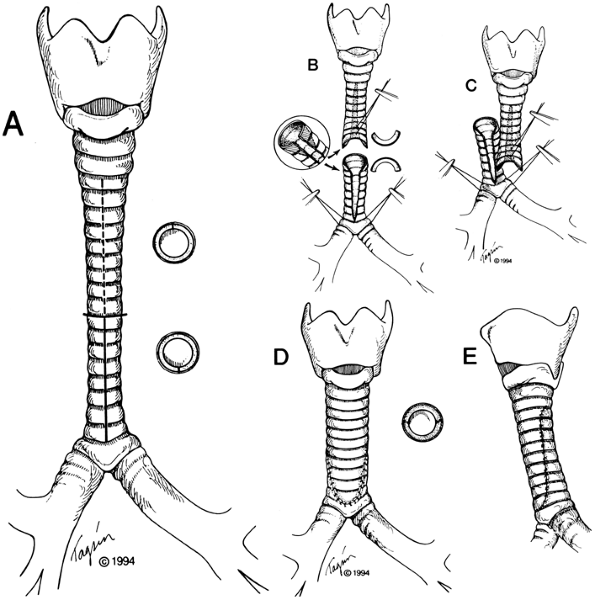 |
Fig. 76-9. Technique of slide tracheoplasty. A. The extent of stenosis is identified precisely. The stenotic segment is divided transversely in its midpoint after circumferential dissection at that locus only. The upper stenotic segment is incised vertically posteriorly, and the lower segment is incised anteriorly for the full length of stenosis. B. The right-angled corners produced by these divisions are trimmed above and below. A stay suture near the tip of the superior flap is helpful, as are traction sutures at the tracheobronchial angles or main bronchi below. Minimal dissection of lateral blood supply is performed. C. The two ends are slid together after placement of individual anastomotic sutures around the entire oblique circumference of the tracheoplasty. D, E. The circumference is doubled, resulting in quadrupled cross-sectional area. From Grillo HC: Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann Thorac Surg 58:613, 1994. With permission. |
REFERENCES
Barclay RS, McSwan N, Welsh TM: Tracheal reconstruction without the use of grafts. Thorax 12:177, 1957.
Belsey R: Resection and reconstruction of the intrathoracic trachea. Br J Surg 38:200, 1950.
Borrie J, Redshaw NR: Cervical tracheal reconstruction in sheep, using Silastic prostheses with subterminal suture cuffs. Proc Univ Otago Med School 48:32, 1970.
Cantrell JR, Folse JR: The repair of circumferential defects of the trachea by direct anastomosis: experimental evaluation. J Thorac Cardiovasc Surg 42:589, 1961.
Cantrell JR, Guild HC: Congenital stenosis of the trachea. Am J Surg 108: 297,1964.
El-Baz N, et al: One-lung high-frequency ventilation for tracheoplasty and bronchoplasty. Ann Thorac Surg 34:564, 1982.
Geffin B, Bland J, Grillo HC: Anesthetic management of tracheal resection and reconstruction. Anesth Analg 48:884, 1969.
Grillo HC: Circumferential resection and reconstruction of mediastinal and cervical trachea. Ann Surg 162:374, 1965.
Grillo HC: Surgical approaches to the trachea. Surg Gynecol Obstet 129: 347, 1969.
Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970.
Grillo HC: Tracheal tumors: surgical management. Ann Thorac Surg 26:112, 1978.
Grillo HC: Primary reconstruction of airway resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 33:3, 1982a.
Grillo HC: Carinal reconstruction. Ann Thorac Surg 34:356, 1982b.
Grillo HC: Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann Thorac Surg 58:613, 1994.
Grillo HC: Tracheal replacement: a critical review. Ann Thorac Surg 73: 1995, 2002.
Grillo HC: Development of tracheal surgery: a historical review. I. Techniques of tracheal surgery. Ann Thorac Surg 75:610, 2003.
Grillo HC, Dignan EF, Miura T: Extensive resection and reconstruction of mediastinal trachea without prosthesis or graft: an anatomical study in man. J Thorac Cardiovasc Surg 48:741, 1964.
Grillo HC, Mathisen DJ, Wain JC: Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 53:54, 1992.
Grillo HC, et al: Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg 123:145, 2002.
Michelson E, et al: Experiments in tracheal reconstruction. J Thorac Cardiovasc Surg 41:784, 1961.
Mitchell JD, et al: Clinical experience with carinal resection. J Thorac Cardiovasc Surg 117:39, 1999.
Miura T, Grillo HC: The contribution of the inferior thyroid artery to the blood supply of the human trachea. Surg Gynecol Obstet 123:99, 1966.
Montgomery WW: Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 99:255, 1974.
Mulliken J, Grillo HC: The limits of tracheal resection with primary anastomosis. Further anatomical studies in man. J Thorac Cardiovasc Surg 55:418, 1968.
Neville WE, Bolandowski PJ, Kotia GG: Clinical experience with the silicone tracheal prosthesis. J Thorac Cardiovasc Surg 99:604, 1990.
Salassa JR, Pearson B, Payne WS: Growth and microscopical blood supply of the trachea. Ann Thorac Surg 23:100, 1977.
Vogt-Moykopf I, Mickisch GH: Prosthetic replacement of the trachea: Discussion. In Grillo HC, Eschapasse H (eds): International Trends in General Thoracic Surgery. Vol. 2. Philadelphia: WB Saunders, 1987, p. 147.
Wilson RS: Anesthetic management for tracheal reconstruction. In Grillo HC, Eschapasse H (eds): International Trends in General Thoracic Surgery. Vol. 2. Philadelphia: WB Saunders, 1987, p. 3.
Wright CD, et al: Pediatric tracheal surgery. Ann Thorac Surg 74:308, 2002.
EAN: 2147483647
Pages: 203