65 - Diffuse Malignant Mesothelioma
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIII - The Trachea > Chapter 77 - Management of Nonneoplastic Diseases of the Trachea
Chapter 77
Management of Nonneoplastic Diseases of the Trachea
Hermes C. Grillo
A wide spectrum of benign conditions that affect the trachea are described under the general headings of lesions that are caused by infection; posttraumatic lesions, including iatrogenic injuries; extrinsic lesions compressing the trachea; and miscellaneous, including a variety of lesions largely of unknown origin.
INFECTION
Tuberculosis
Tuberculosis of the upper airway appears principally to involve the lower trachea, main bronchi, or both. Acute ulcerative tuberculous tracheitis is treated medically. As the acute process heals, stenosis may evolve. Typically, the stenosis shows a pattern of submucosal fibrosis laid down in circumferential manner with marked narrowing or occlusion of the airway. The tracheal cartilages appear to be grossly intact, although peribronchial or peritracheal fibrosis is seen. The lesions may be quite lengthy and thus present a marked or insuperable surgical challenge. Active tuberculosis should be arrested and controlled before surgical resection and reconstruction is performed. In one patient in whom surgery was forced because of acute disease obstructing both the distal trachea and the carina, healing was unsatisfactory, and fatal disruption occurred. In three patients who required carinal resection and reconstruction for excision of mature stenoses, two had excision of the right upper lobe as well with reimplantation of the bronchus intermedius. Complete stenosis of the left main bronchus has been managed by total excision of that bronchus and advancement and reimplantation of the bifurcation of the left upper and lower lobes to the carina, as described by Newton and associates (1991).
Histoplasmosis
Histoplasmosis may affect the airways in several ways. It may produce massive mediastinal fibrosis with involvement of the distal trachea, carina, and main bronchi, or it may involve principally the right bronchial tree in relation to the masses of lymph nodes in the right paratracheal and pretracheal area and in the middle lobe sump. The fibrosing process may extend centrally to involve the right pulmonary artery up to its point of origin even within the pericardium. The lesions may be a composite of airway compression plus intrinsic fibrotic involvement. Massive histoplasmoma at the carina may compress the airway. In such lesions there may be central caseation with a fibrotic capsule that actually involves one or both main bronchial walls intimately. Another presentation is with densely fibrotic and calcified subcarinal and precarinal lymph nodes, which may invade and erode through the wall of the trachea, carina, or bronchi. Broncholiths also occur peripherally in the lobar bronchi. Secondary infection and hemorrhage may follow. More recently, broncholithiasis in general has been associated with histoplasmosis rather than with tuberculosis, as it was in an earlier era. These clinical manifestations have been described by Mathisen and myself (1992).
The organism Histoplasma capsulatum is more often identified by special stains in pathologic material removed at surgery rather than on cultures. Organisms have been identified in fewer than 50% of patients who are presumed to have disease originating from this source. It has been theorized that the continuing fibrotic process is a reaction to products of the infection rather than to viable organisms. Thus, diagnosis is often presumptive, based on pathologic and radiologic findings as well as on a history of exposure and clinical evolution of the disease.
P.1048
Other Inflammatory Disease Processes
A small number of patients have been seen who have suffered from diphtheria in childhood and presented many years later with tracheal stenosis or laryngotracheal stenosis. Because most of these patients had tracheostomies in infancy or early childhood for treatment of the acute disease, it is difficult to differentiate whether the late stenoses were caused by the disease or the treatment. Reconstruction may be possible.
Scleroma is a rare disease from infection with Klebsiella rhinoscleromatis that may involve the airways as well as the nasopharynx. It is found in Mexico and Central America. A rare case of necrosing mucormycosis involving the trachea or carina as well as the lungs may be seen in diabetic patients or in people who are immunosuppressed or undergoing chemotherapy, particularly for lymphomas. Prompt and radical surgical excision with vigorous and prolonged treatment with amphotericin may save some of these patients, as noted by Tedder and associates (1994).
POSTTRAUMATIC LESIONS
Blunt Trauma
Ruptures of the trachea, carina, or main bronchi that are caused by blunt trauma may go unrecognized. Such patients almost always have a history of pneumothorax treated by tube drainage, often bilateral in the case of tracheal rupture. They present with shortness of breath or wheezing. The trachea or bronchus may be reduced to only a tiny opening when the diagnosis is made at last. Treatment consists of prompt excision of stenosis and surgical repair. When the bronchus is injured, every effort is made to salvage the distal lung. This is usually possible unless severe infection has ensued. Deslauriers and associates (1982) have demonstrated adequate function of reimplanted lungs. Functional return appears to be roughly inversely proportional to the length of time that the lung was compromised.
In patients who have suffered tracheal separation caused by blunt injury in the neck and who have been treated by tracheostomy only, total stenosis of the area of separation follows. Both recurrent laryngeal nerves are usually at least temporarily paralyzed and often permanently. Such patients must be evaluated carefully some months after their injury when the local inflammation has subsided. Laryngeal reconstruction, when necessary, with stabilization of the glottic aperture, is generally accomplished first. The larynx is then reconnected to the trachea, as described by Mathisen and the author (1987). An effective although unmodulated voice is obtained. Pharyngoesophageal separation that was not repaired initially is reconstructed at the same time.
Inhalation Burns
Inhalation burns of the larynx, trachea, and bronchi are particularly difficult injuries to manage. The agent may have been chemical, thermal, or a combination of both. These patients often show little damage to the pharynx or supraglottic larynx once the immediate injury has subsided. Persistent damage often commences in the subglottis just below the vocal cords and extends down the airway in a gradually diminishing intensity of injury. The depth of injury and the length of airway injured probably relate to the dose received as well as to the actual injury potential of the agent. Gaissert and I and our colleagues (1993) found that in 18 patients treated for tracheal stenosis caused by inhalation injury, 14 had subglottic strictures as well and 2 had main bronchial stenosis. Although it is sometimes difficult to differentiate later injuries from the intubation with which the patients were treated acutely, three of our patients had laryngotracheal strictures without any history of intubation.
In most cases, the tracheal rings were not destroyed and the injuries were confined to various depths of mucosal and submucosal damage. Attempts at resection of injuries should not be made, especially in the early phase. First, involvement often commences immediately below the cords and involves the entire subglottic larynx, making repair almost impossible. Second, the burned airway responds poorly to early surgery, even where the lesion appears to be limited, much in the way that burned skin elsewhere in the body does (i.e., by the reformation of massive scarring). With appropriately placed splinting, silicone T-tubes, and a great deal of patience, a stable and open airway may usually be obtained in most of these patients in time. Resection, if required, is deferred.
Posttherapeutic Stenosis
Stenosis of the trachea after tracheal reconstruction in most cases is caused by excessive tension on the anastomosis, and this is related to overzealous resection of too great a length of trachea. Dangerous tensions in tracheal resection may be reached at approximately above the 50% level of length of resection in the adult and above the 30% level in the child. Carinal resections are particularly at risk because of their complex nature. Patients chronically on high doses of prednisone are especially at risk if extensive tracheal resection is performed. Unnecessary disturbance of the blood supply to the trachea by extensive circumferential dissection also leads to stenosis or separation. Profuse, hypertrophic granulations at the anastomosis, which were seen when nonabsorbable sutures were used for tracheal repair, have vanished since the introduction of Vicryl sutures.
Stenoses also may result from radiation therapy and laser injury. Brachytherapy has contributed to a number of main bronchial stenoses. The contribution of lasering to tracheal
P.1049
damage is more difficult to assess because the laser is often applied for attempted treatment of preexisting lesions and in conjunction with a tracheostomy performed to safeguard the airway. Whereas laser injury may often be dealt with by subsequent resectional surgery, irradiation injuries may either be surgically uncorrectable when first seen or correctable only with considerable risk.
The special problem of obtaining healing after reconstruction in a previously irradiated trachea (when the dosage has exceeded 4,000 cGy approximately 1 year or more earlier) has been largely successfully met by advancement of an omental buttress, as described by Muehrcke and colleagues (1995).
Postintubation Damage
Intubation either with oral or nasal endotracheal tubes or with tracheostomy tubes is most commonly used to deliver mechanical ventilatory support in respiratory failure. Assistance supplied through cuffed tubes has thus far proved to be the only practicable method of management for adults with poor pulmonary or chest wall compliance. High-flow respirators with uncuffed tubes, electrophrenic respirators, and negative-pressure tank respirators have not been satisfactory for managing these severe problems. High-frequency ventilation for long-term use remains developmental. A whole spectrum of tracheal lesions resulting from such treatment (Fig. 77-1) was discerned by Andrews and Pearson (1971) and by the author (1969, 1970). The most common lesions and those most amenable to definitive treatment are those responsible for airway obstruction. Because a single patient may have more than one lesion and because the treatment of these lesions differs, precise definition of the pathologic state is essential in planning treatment.
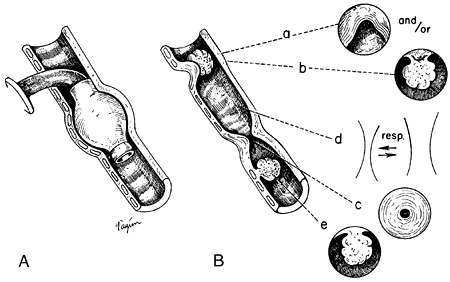 |
Fig. 77-1. Diagram of inflammatory lesions related to cuffed tracheostomy tubes. A. Location of the stoma and the distorting effect of a conventional cuff. B.Lesions developing at corresponding sites of injury. At the stoma, an anterior stricture (a) or a granuloma (b), or a combination, may occur. At the cuff site (c), circumferential stricture occurs. Between the stoma and such a stricture, varying degrees of tracheal malacia may result, with functional occlusion (d). At the site of erosion by the tip of the tube (e), a granuloma may occur. Innominate erosion and a tracheoesophageal fistula are seen at both cuff level and tip level. |
Lindholm (1970) showed that endotracheal tubes may cause injury at the laryngeal level even after only 48 hours of intubation: glottic edema; vocal cord granulomas; erosions, particularly over the arytenoids; formation of granulation tissue; polypoid obstructions; and actual stenosis, particularly at the subglottic intralaryngeal level. Subglottic injury is produced also by cricothyroidotomy and by cricoid erosion caused by high tracheostomy in the presence of kyphosis. Montgomery (1968) noted that subglottic stenosis may be difficult to correct. Sometimes it is impossible.
At the tracheostomy site, granulomas that can obstruct the airway may form during healing. If the tracheostomy stoma has been made too large by turning a large flap or excising a large window in the initial tracheostomy, or if erosion is caused by sepsis and heavy prying equipment, cicatricial healing may produce an anterior A-shaped stenosis that can severely compromise the airway. The posterior wall of the trachea may be relatively intact in these patients. At the level of the inflatable cuff, whether placed on a tracheostomy tube or an endotracheal tube, circumferential erosion of the tracheal wall may occur. If this erosion is deep enough, all the anatomic layers of the trachea may be destroyed, so that cicatricial repair results in a tight circumferential stenosis (Fig. 77-2). Malacia may result also. Below this level, at the point where the tip of the tube may pry against the tracheal wall, additional erosion may occur with formation of granuloma, especially in children, for whom uncuffed tubes are used. In the segment between the stomal and cuff level, varying degrees of chondromalacia with resulting tracheomalacia may occur. Here, the cartilages are not totally destroyed but only thinned. Bacterial infection in this segment of the trachea during the period of ventilatory support probably contributes to this process.
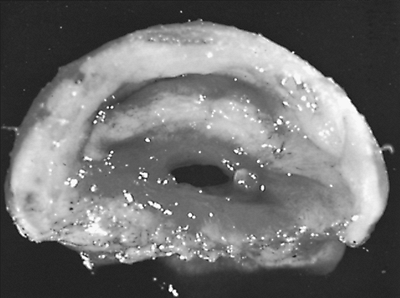 |
Fig. 77-2. Circumferential stenosis at cuff level. This surgical specimen shows the narrow size to which the lumen may be reduced before recognition of symptoms. |
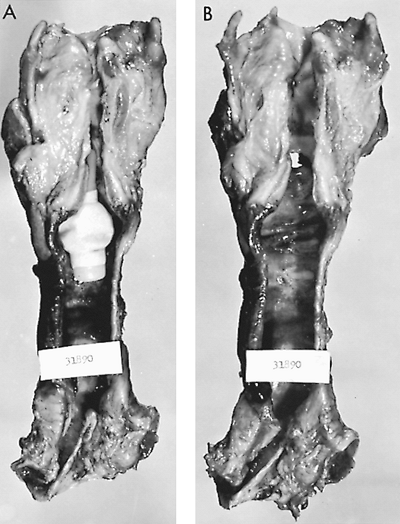 |
Fig. 77-3. Autopsy specimen of larynx and trachea reveals tracheal injury caused by cuffed tracheostomy tube. A.Portex tracheostomy tube had been in place for 19 days. Note the dilatation of the trachea where the cuff had been inflated. B.Inflammatory erosive changes have bared multiple cartilages. Note also a distal erosion caused by the tip of the tube. Similar injuries occur with metal or rubber tubes. From Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970. With permission. |
P.1050
The etiologic basis of the cuff stenosis has been variously attributed to pressure necrosis by the cuff, the irritating quality of materials in rubber and plastic cuffs and tubes, irritant materials produced by gas sterilization, hypotension, and bacterial infection. Studies by Cooper and me (1969a), and earlier by Florange and colleagues (1965), of autopsy specimens of patients who had been on ventilators with inflated cuffs (Fig. 77-3), prospective studies of similar patients by Andrews and Pearson (1971), and analysis of surgically removed lesions caused by cuffs and experimental reproduction of these lesions under controlled conditions by Cooper and me (1969a, 1969b) point to pressure necrosis as the principal etiologic agent. As my associates and I (1971) showed, if standard Rusch cuffs are inflated to just provide a seal at ventilatory pressures of approximately 25 cm H2O, intracuff pressures increase to 180 to 250 mm Hg. Carroll and associates (1969) noted that, although these pressures are not exactly those exerted on the tracheal mucous membrane, high pressures are indeed exerted. The trachea has an elliptic form, so it becomes deformed at the point where a seal is obtained. If perfusion pressures in the patient are lower than normal, necrosis can occur even more easily. The mucosa overlying the cartilage is initially destroyed. The bared cartilages become necrotic and ultimately slough. Attempts at repair after full-thickness damage to the tracheal wall lead only to scar formation. Because the erosion is circumferential, the resultant strictures are also. Even further erosive damage can lead to tracheoesophageal fistula posteriorly or to perforation of the innominate artery anteriorly.
Patients with stenosis and malacia develop symptoms and signs of airway obstruction consisting of dyspnea on exertion, stridor, cough, and obstructive episodes. Hemoptysis does not occur. In a few patients, pneumonia, sometimes bilateral, has been noted. On occasion, a patient, while still intubated, begins to develop obstruction from formation of granulations around the tip of the tube. In most instances, the obstruction appears only after extubation, because the tube splints a cuff stenosis or potential stomal stenosis as long as it remains in place. Any patient developing symptoms of airway obstruction who has been intubated for over 24 hours within the previous 2 years must be considered to have organic obstruction until proved otherwise. Many such patients have been treated for varying lengths of time with the incorrect diagnosis of asthma. Such errors resulted from lack of awareness of these lesions and the fact that in most patients routine radiography of the chest shows normal lung fields.
Symptoms occurred in a few patients within 2 days of extubation; most demonstrated symptoms between 10 and 42 days after extubation, and a few at greater intervals, usually within a few months. If a patient remains sedentary while recovering from the original disease, the airway may shrink to a critical diameter of 4 to 5 mm before symptoms become obvious. At this aperture, fatal obstruction may occur at any time.
Although general improvement has occurred with design of large-volume cuffs, most of these cuffs can still produce tracheal injury if slightly overinflated beyond their resting maximal volume, because of their relatively inextensible materials. Stomal injuries continue to occur for the reasons described. Cricothyroidotomy may lead to severe or irreparable subglottic injury.
Three additional and particularly severe injuries to the airway may occur from intubation. These are tracheoesophageal fistula, tracheoinnominate artery fistula, and subglottic laryngeal or laryngotracheal stenosis. Tracheoesophageal fistula occurs most commonly in patients who have a ventilating cuff in the trachea for a long period of time along with a feeding tube in the esophagus. The two foreign bodies pincer the party wall between trachea and esophagus, leading first to inflammation, which seals one against the other, and then perforation, which may enlarge to include the entire membranous wall of the trachea. Concomitant circumferential injury to the trachea is usually present as well, as pointed out by me and my colleagues (1976), because this is basically a cuff lesion.
Anterior erosion of the trachea may lead to a fistula into the innominate artery. A small number of anterior erosions were seen in the past that were caused by angulation of a
P.1051
tube tip or a high-pressure cuff itself directly eroding through into the artery. More common, although still rare, are erosions of the artery that occur at the inferior margin of a low-placed tracheostomy stoma that is in immediate contiguity with the artery. The inner curve of the tube erodes its way through the arterial wall. It is seen most often in children and young adults in whom tracheostomy is placed too low, because on hyperextension more than one-half of the trachea rises up into the neck. If the stoma is placed with respect to the sternal notch rather than to the cricoid cartilage, the tracheostomy then resides just above the elevated innominate artery. Deslauriers and colleagues (1975) called attention to this complication.
Stenosis of the upper trachea may be associated with a severe subglottic stenosis as well. Stenosis of the subglottic larynx arises from three causes. The principal one is erosion, which is caused by an endotracheal tube that has been left in place for some time. The principal factor at fault may be use of a tube that has a bore too large for the patient. One of the narrowest parts of the upper airway is at the level of the cricoid cartilage. The second most common cause is erosion by a tracheostomy tube upward through the cricoid cartilage to affect the lower anterior larynx. It occurs most commonly in older patients who are kyphotic and in whom the cricoid cartilage is close to the sternal notch. A third cause of subglottic stenosis is the deliberate use of cricothyroidotomy for ventilation. If damage occurs at the stomal level, it is by surgical selection within the larynx. Lesions that involve the subglottic larynx as well as the upper trachea are much more difficult to repair surgically, although single-staged techniques have been devised by myself (1982), as well as by Pearson (1975) and Couraud (1979) and their associates.
Tracheoesophageal fistula becomes manifest by a sudden increase in tracheal secretions and the appearance of any ingested material in the trachea. If the patient is on a respirator, gastric distention may appear. Tracheoinnominate arterial fistulae are rare but may be announced by premonitory hemorrhage or by massive initial hemorrhage. In treating bleeding from a tracheostomy, it is important to differentiate between erosion of tracheal granulations or mucosa and arterial fistula. Sometimes angiography demonstrates a false aneurysm that soon bleeds massively.
EXTRINSIC LESIONS
Goiter
Large goiters, either cervical or mediastinal, may gradually compress the airway sufficiently to cause symptoms. The slow growth of the goiter may deform cartilaginous rings without destroying them. When the goiter is removed, the trachea may remain distorted in shape and narrowed, but clinically significant airway obstruction is rarely present. Quite frequently, removal of the goiter leads to immediate improvement in respiratory symptoms. If, however, sufficient softening of the cartilages has occurred that was caused by the prolonged compression, removing the supporting mass of thyroid tissue actually allows the trachea to collapse with respiratory effort. This is determined by intraoperative bronchoscopy, local examination and palpation in the operative field, and, finally, by observation of the patient in the operating room after extubation. Several methods of managing this problem have evolved, including intubation with an uncuffed tube followed by tracheostomy, preferably with insertion of a silicone T-tube several days later when the wound is sealed, immediate buttressing of the trachea with specially made polypropylene plastic rings, or in the past, by using traction sutures from the tracheal wall tied over either internal or external buttons.
An anterior substernal goiter usually does not exert pressure on the trachea because of its position in front of the great vessels. Katlic and colleagues (1985) reported that the trachea was more likely to be compressed by posterior descending goiters that enter the thoracic strait lateral to the esophagus and trachea.
Vascular Compression
Symptoms of tracheal compression may be produced by congenital vascular rings or by aneurysms of the innominate artery or of an anomalous subclavian artery that passes behind the trachea and esophagus. In children, compression may be produced by the innominate artery itself (see Chapter 79).
Patients with a right aortic arch that rises high, turns down sharply in a hairpin configuration, and descends on the right (accompanied by a left anomalous subclavian artery, often a diverticulum of Kommerell, ligamentum arteriosum, and also with a narrow anteroposterior chest with or without a degree of pectus excavatum) may suffer tracheal compression that becomes severely symptomatic. Excision of the diverticulum and transplantation of the anomalous subclavian may not relieve the obstruction. Aortopexy of the arch or even arch division after aortic bypass may be required.
Mediastinal Masses
Most mediastinal masses that compress the trachea are malignant neoplasms. On rare occasions a large bronchogenic cyst located at the carina actually compresses the airway. Infant tracheal compression may be caused by a large thymic cyst.
Postpneumonectomy Syndrome
After right pneumonectomy, the mediastinum may move completely over to the right axilla and posteriorly, and in so doing, the aortic arch becomes rotated horizontally. This
P.1052
may lead to angulation and compression of the remaining tracheobronchial tree with obstruction either at the carina or in the proximal left main bronchus (Fig. 77-4). The bronchus is actually compressed between the pulmonary artery, which is stretched in front of it, and either the aorta or the vertebral bodies posteriorly. It cannot be predicted which patient will suffer this distortion after pneumonectomy. It was formerly thought that this was principally a syndrome seen in children, but my colleagues and I (1992) described a number of patients in whom the problem appeared after pneumonectomy in adulthood. The reverse situation may be seen after left pneumonectomy in the presence of a right aortic arch. The patient's symptoms may be rapidly progressive and lead to total disability. Rarely, the syndrome may follow left pneumonectomy with a normal aortic arch.
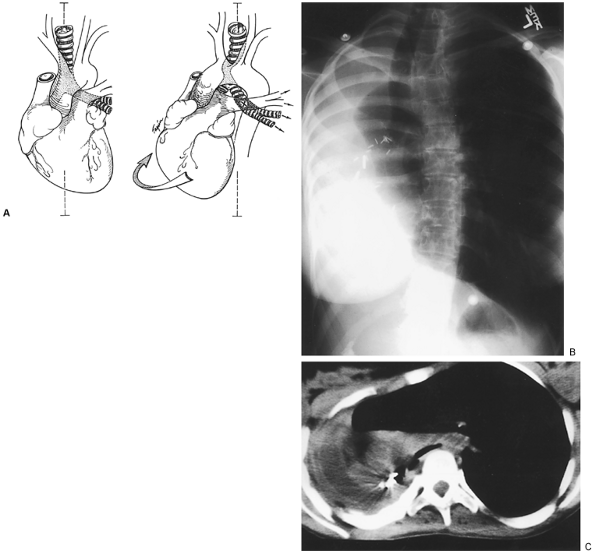 |
Fig. 77-4. Postpneumonectomy syndrome. A.Diagram shows extreme lateral displacement and rotation of heart and aortic arch after right pneumonectomy with left aortic arch. The midline is indicated by a dashed line. The trachea and carina are displaced to the right and posteriorly. The origin of the left main bronchus at the carina is compressed between the pulmonary artery and aorta or vertebral column. The opposite occurs after left pneumonectomy with right aortic arch. From Grillo HC, et al: Postpneumonectomy syndrome: diagnosis, management and results. Ann Thorac Surg 54:638, 1992b. With permission. B.Posteroanterior chest roentgenogram of a 19-year-old woman 11 months following right pneumonectomy for congenital cystic lung with hemorrhage. Note marked mediastinal shift. Lateral radiograph confirmed posterior displacement of heart and arch. The left lung is herniated and overexpanded. C.Computed tomographic scan of the same patient showing the displacement described. The carina is also markedly shifted, and the left main bronchus is compressed over the spine. Postoperative state is pictured in Fig. 77-7. |
MISCELLANEOUS LESIONS
Relapsing Polychondritis
Relapsing polychondritis is a disease of unknown origin and uncertain course. Cartilaginous structures in the body may be affected, most prominently the nasal and ear cartilages and those of the tracheobronchial tree. The airway changes
P.1053
may precede the more diagnostic changes in the nose and ears, sometimes by years. When the lower trachea and bronchi are affected first, the disease manifests itself by progressive airway obstruction with difficulty in clearing secretions and ultimately pulmonary infections. The disease may extend into the segmental bronchi. Relapsing polychondritis also may affect the larynx and uppermost trachea. Here, the cartilages become inflamed and thickened, and constrictive narrowing of the subglottic and subcricoid airways occurs. The disease may then progress distally, but without predictability. Surgical therapy is usually not applicable. Sometimes it is necessary to provide an airway with a tracheostomy tube, and at times stenting with a silicone T-tube or T-Y tube may provide palliation for a time. The disease is unrelenting.
Wegener's Granulomatosis
Wegener's granulomatosis may affect the larynx and trachea with inflammatory lesions that lead to airway obstruction. The rate and extent of involvement are highly unpredictable. With response to medical treatment, an apparently stable stenosis may result.
Sarcoidosis
Sarcoidosis may produce airway obstruction through the mechanism of massive enlargement of mediastinal lymph nodes compressing the airway and distorting it, as well as by intrinsic fibrotic changes in the wall of the trachea and bronchi. A circumferential stenosis results that involves a long segment of trachea and main bronchi, and sometimes, bronchi more distally. These lesions are not amenable to surgical treatment because of their diffuseness and extent, but periodic dilation is effective for some time.
Amyloidosis
Amyloid disease on rare occasions involves the trachea and main bronchi in an extensive process leading to narrowing throughout the tracheobronchial tree. The lesion often is too extensive to permit surgical resection and reconstruction, but localized disease is sometimes resectable.
Tracheopathia Osteoplastica
Tracheopathia osteoplastica manifests itself pathologically by the formation of calcified nodules beneath the mucosa, adjacent to but not actually originating from the cartilages, as described by Young and associates (1980). The involvement may commence in the subglottic larynx and extend throughout the trachea and more distally into the bronchial tree. The trachea often is of saber-sheath configuration. It appears in adults, progressing insidiously. Patients have difficulty in raising their tenacious secretions as the disease progresses. Ultimately, severe obstructive symptoms may ensue. In some patients, however, the disease remains a curiosity and does not seriously impair them. Some reported cases have been discovered incidentally on autopsy. Rarely, patients require surgical relief.
Tracheobronchomegaly
Tracheobronchomegaly (Mounier-Kuhn syndrome) is probably of congenital origin, although it usually becomes clinically manifest in adulthood. The symptoms are progressive dyspnea on exertion and difficulty in raising secretions. The trachea may be hugely widened on radiographic examination, with both unusually elongated cartilages, which are markedly deformed, and a redundant membranous wall. The cartilages tend gradually to assume a reverse curve, which brings the redundant membranous wall up against the cartilages, causing obstruction. The main bronchi are also involved.
Saber-Sheath Trachea
Saber-sheath trachea is a deformity seen usually as an incidental finding in patients with varying degrees of chronic obstructive pulmonary disease later in their life, usually in their fifties and sixties. The radiologic presentation was detailed by Greene and Lechner (1975). The lower two-thirds of the trachea, the intrathoracic trachea, gradually assume a configuration in which the side-to-side diameter diminishes progressively and the anteroposterior diameter increases. The cartilages are not malacic. The configuration of the airway changes. In early stages the deformity causes no difficulty, but as it become more and more marked, the posterior part of the cartilages approximate with attempts to cough and breathe deeply, and the patient finds that he or she cannot clear secretions. The proximal cervical portion of the trachea usually appears quite normal.
Idiopathic Tracheal Stenosis
Idiopathic stenosis presents over a wide spectrum of age, almost exclusively in women, with progressive dyspnea on exertion and wheezing. Patients with idiopathic tracheal stenosis are usually found to have a short stenosis (approximately 2 to 3 cm) involving the uppermost trachea and, in many cases, the subglottic larynx as well. Distally, the trachea appears quite normal. The patients have no history of trauma, infection, inhalation injury, intubation for ventilation, or any other tracheal or airway disease. In a series of 49 patients that my colleagues and I described (1993), only 3 had any systemic symptoms. Two had mild arthralgias, and one had poorly defined arteritis. Many who had been
P.1054
followed for as long as 15 years had never developed any other systemic symptoms.
The stricture itself is roughly circumferential and pathologically shows only chronic inflammation with marked submucosal fibrosis. The cartilages are uninvolved. The pathology is distinct from polychondritis, Wegener's granulomatosis, or any of the aforementioned conditions. The patients do not have mediastinal fibrosis or pathologic processes involving mediastinal lymph nodes. An antinuclear cytoplasmic antibody (ANCA) test is essential to exclude isolated upper airway stenosis due to Wegener's disease. Idiopathic laryngotracheal stenosis characteristically is not progressive, nor does it recur after successful resection, as shown by Ashiku and Mathisen (2003).
In addition to this quite well-defined lesion, some patients present with stenosis involving a large part of the trachea, and there are others in whom the carina or main bronchi, or both, are involved in an undefined inflammatory fibrotic process. In this small group of patients, no other incriminating signs, symptoms, or laboratory findings occur to implicate any known disease or syndrome. The process progresses in some of these patients, sometimes fatally.
Tracheal Malacia
Acquired tracheal and tracheobronchial malacia remain poorly defined for the most part. A segmental area of malacia may result from postintubation injury either at the level of a cuff lesion or in the segment between the stoma and the cuff lesion. With chronic obstructive pulmonary disease, including emphysema and chronic bronchitis, malacia may develop in the lower trachea, main bronchi, and sometimes the more distal bronchi. In this situation the tracheal rings take on the shape of an archer's bow with elongation of the membranous wall. When the patient attempts to expire forcefully or to cough, the membranous wall approximates to the anterior softened and flattened cartilage, causing nearly total obstruction. Patients have great difficulty in raising secretions, which became viscid. Herzog and associates (1987) carefully defined this entity. It is entirely different from the characteristics of saber-sheath trachea.
A smaller number of patients have been seen who have malacia involving a large portion or even the entire trachea wherein the rings are thinned to a point at which they no longer support the airway. The airway almost takes on the appearance of the esophagus. In these patients, the malacia is total, in contrast with the picture just described of anteroposterior collapse. The limited malacia that may result from compression by a goiter or thyroid adenoma has been previously mentioned.
DIAGNOSTIC STUDIES
Tracheal lesions often are recognized late despite a prolonged period of symptoms. As physicians become aware of the possibility of tracheal lesions, they are increasingly suspicious of a diagnosis of adult-onset asthma. Appropriate radiographic examinations [fluoroscopy, tomography, computed tomographic (CT) scans] are used to rule out the possibility of a tracheal lesion in any patient who has obstructive airway symptoms but radiographic demonstration of normal lung fields. Rarely, even specialized techniques fail to reveal an unusual lesion, and bronchoscopic examination is indicated.
Radiographic Examination of the Trachea
Radiographic studies of the trachea are used not only to rule in or out the presence of a tracheal lesion but also to define the location, extent, and sometimes the character of the lesion (Fig. 77-5). Furthermore, these studies demonstrate the involvement of paratracheal structures by neoplastic lesions. I (1970) have found the following radiographs to be helpful.
Lateral films of the neck with the chin raised demonstrate most lesions of the upper half of the trachea. Careful technique shows the cartilaginous structures of the larynx as well as the trachea and the relationship of the trachea to the vertebral column posteriorly. If the patient has an existing tracheostomy stoma or has a tracheostomy scar, a radiopaque marker placed on the skin at this level helps to identify its relationship to inflammatory posttracheostomy lesions.
Anteroposterior views of the airway from larynx to carina, using a copper filter, provide useful overall assessment. Oblique views throw the tracheal air column into relief. Fluoroscopy demonstrates malacia and clarifies vocal cord function.
Tracheal laminography helps give precise measurement of the linear extent of lesions and their relative distances from landmarks such as the vocal cords and the carina. A magnifying effect occurs in the radiographs, but at the same time the trachea is somewhat foreshortened because of its oblique passage through the chest. When viewing all radiographs, as well as during bronchoscopy, it should be noted that the vocal cords are not the lower border of the larynx. Approximately 1.5 to 2.0 cm of larynx lies between the vocal cords and the inferior border of the cricoid cartilage. Planning for operative procedures must take this into account.
Contrast studies of the trachea add little information, except with a tracheoesophageal fistula, and this is better shown by barium esophagography.
CT is valuable only in showing the mediastinal extent of a tumor. It is of little use in assessing benign stenosis except in special cases such as goiter, vascular lesions, or histoplasmosis. Inspiratory and expiratory computed tomography scans help to clarify dynamic states such as tracheobronchomalacia and postpneumonectomy syndrome.
If a patient with tracheal stenosis still has a tracheostomy tube in place, it should be removed during radiographic examination to obtain useful information. Even if a tube has
P.1055
been in place for many months, it should be removed cautiously, with provision made for immediate reinsertion. Emergency equipment including suctioning devices and a range of replacement tubes should be available. A competent physician must be present to perform such intubation under slight difficulty. The airway can become nearly totally obstructed within 20 minutes after removal of a tube. Occasionally, considerable force is required for reinsertion of an airway. Weber and I (1978) described the radiographic findings in tracheal tumors and stenosis.
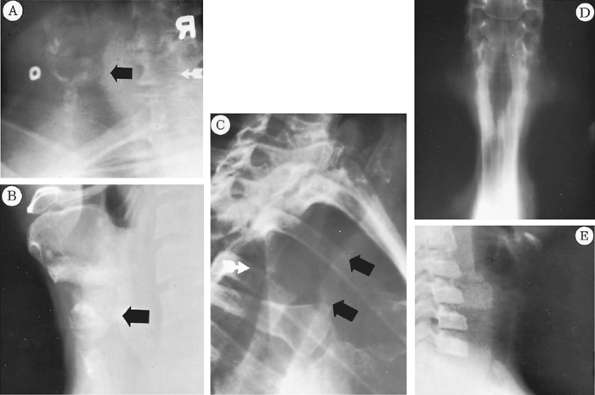 |
Fig. 77-5. Radiographs showing various injuries from tracheostomy tubes. A.Lateral view of the neck. The circular opaque marker is on the skin at the tracheostomy site. The black arrow points to a large inflammatory granuloma occluding the tracheal lumen. Some narrowing of the trachea is seen at this level. Endoscopic removal alone was required. B.Similar view showing an anterior stomal stricture. A deep indentation of the anterior trachea is seen at the level marked by the arrow. Resection and reconstruction were necessary. From Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970. With permission. C.Detail of left anterior oblique view of the chest showing a lengthy midtracheal stenosis marked by the two black arrows. From Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970. With permission. D.Laminogram showing the stenosis in C. The upper narrowing is at the laryngeal level and is normal. E.Lateral neck view with hyperextension to demonstrate granuloma in a child's trachea at the level of the tube's tip. Ventilatory support without a cuff had been given after a cardiac operation in this child. |
Bronchoscopy
Bronchoscopic examination is required, sooner or later, in all of these patients. When a lesion is known to be present, whether it is neoplastic or inflammatory, and when all else points to its surgical correctability, bronchoscopy is best deferred until preparations have been made for definitive treatment of the lesion. The trauma of bronchoscopy in a patient who is subtotally obstructed may precipitate complete obstruction. Little is lost by delaying the bronchoscopy until the time of definitive operation. Frozen sections may be obtained for histologic diagnosis. In the presence of most obstructive lesions, the requirements for resection are clear at the outset. The bronchoscopy is done with the patient under general anesthesia, permitting unhurried, atraumatic examination and manipulation.
Bronchoscopic examination and removal are all that is required in patients with polypoid granulomas at the stomal site or at the site of the tube tip. Esophagoscopy is performed also when neoplasms are examined. Rigid bronchoscopy, under general inhalation anesthesia, using pediatric bronchoscopes serially, is used to dilate severe stenosis for
P.1056
emergency relief. Urgent operation is almost never required. Obstructing tumors may similarly be relieved in an emergency situation or if time is needed to assess a patient, by coring out tumor tissue with the tip of the bronchoscope assisted with biopsy forceps. In 40 years, I never encountered dangerous bleeding or obstruction with this technique. The use of laser has been unnecessary for either benign or malignant lesions.
Other Diagnostic Studies
Pulmonary function studies in patients with obstructing lesions of the trachea confirm a high degree of airway obstruction. Measurements are sometimes useful in clarifying the presence of parenchymal disease and may alter the extent of the operative approach. Obstructing lesions generally require surgical relief. Function studies provide a useful basis for measurement of results, especially forced expiratory volume in 1 second, peak expiratory flow rate, and flow-volume loops.
Bacteriologic cultures are made from tracheal secretions and tracheostomy wounds. Antibiotic sensitivities guide the prophylactic program for perioperative protection.
OPERATIVE VERSUS NONOPERATIVE TREATMENT
The preferred treatment of benign obstruction of the trachea is resection and reconstruction when the patient can tolerate it. With careful evaluation, planning, and execution, most patients with lesions such as postintubation tracheal stenosis can be successfully treated operatively when they have recovered from the primary disease that led to the stenosis. A properly conducted anesthesia and operative repair from the anterior approach do not have great physiologic impact on the patient. Nonoperative methods for temporizing are, however, available. When the disease is not malignant, undue risks must not be taken. Rarely, the medical condition may preclude even the relatively benign procedure required for correction. If the patient has serious neurologic or psychiatric deficits that prevent cooperation in the postoperative phase, reconstruction is best deferred. The patient and anesthesia should be selected to avoid the need for ventilatory support postoperatively. If ventilatory support is needed postoperatively in a shortened trachea, the cuff might rest against the anastomosis and lead to dehiscence.
Temporizing methods available are repetitive bronchoscopic dilation of a stenosis or reinstitution of a tracheostomy, dilation of the stricture, and passage of a tracheostomy tube or a silicone tube through the lesion to splint the airway. Lesions in the immediate supracarinal position are not easily managed in this way. A tube long enough to remain seated often causes episodes of obstruction when it is near the carina, and a T-Y tube or a silicone dynamic stent may lead to bronchial granulations. Generally, however, it is wiser to use a tube for a permanent airway than to undertake a hazardous reconstruction that has a high risk for failure. Cooper and colleagues (1989) and Gaissert and associates (1993) have detailed the uses and results of T-tube management of complex airway problems.
Repeated dilation and splinting have been proposed as definitive methods for treating tracheal stenosis. In most severe lesions in which the whole thickness of the tracheal wall has been converted to scar tissue, even prolonged stenting for many years does not lead to permanent recovery. Numerous patients have been treated this way. Despite repeated trials, it has been impossible, with only rare exceptions, to remove the splinting tube. When lesser degrees of damage have occurred, either in the completeness of a stricture of the circumference of the trachea or in the depth of the tracheal wall, a period of prolonged splinting may, on occasion, result in an adequate airway after removal of the splint. Such a result has been reported in children.
Toty and colleagues (1987) pointed out that laser treatment can lead to cure only in granuloma, also easily removed by bronchoscopy, and thin, weblike stenosis. Such stenoses are rare. The principal effect of the laser in these lesions has been to delay definitive treatment and, sometimes, to worsen the lesion. Particularly to be deplored is reestablishment of tracheostomy to permit laser treatment, which is usually ineffective for permanent cure.
Expandable stents, even when coated, may cause severe complications over time, such as granulations and stenosis. Although acceptable for palliation in fatal neoplasms that are not otherwise manageable, stents in benign disease may convert a surgically curable lesion to an incurable one, as our group has determined in our experience.
Few, if any, patients with postintubation tracheal stenosis cannot be repaired successfully when first identified. Successive failed or inappropriate therapies make such patients' stenoses unreconstructable.
Prevention of Postintubation Tracheal Stenosis
The incidence of stenosis at the stomal level can be reduced by careful placement of the stoma, avoidance of large apertures, elimination of heavy and prying ventilatory connecting equipment, and meticulous care of the tracheostomy.
Many proposals have been made to reduce the formerly inevitable occurrence of some stenoses at the cuff level. These methods included use of double-cuff tubes, changes in materials and sterilization techniques, attempts to avoid cuffs altogether, use of disk and sponge seals instead of cuffs, use of spacers to relocate the cuff level periodically, and prestretching of plastic cuffs. The only promising methods, accepting the present need for cuffs in the management of adult patients in severe respiratory failure, have been intermittent inflation of cuffs cycled to the respirator, described by Arens and colleagues (1969), and, more simply, the development of large-volume,
P.1057
low-pressure cuffs that conform to the shape of the trachea rather than deforming it, described by Cooper and me (1969b) and by me and my co-workers (1971) (Fig. 77-6). Such a cuff provides a seal at intracuff pressures of 33 mm Hg compared with 270 mm Hg in a comparative Rusch standard cuff. Thus, in a series of 45 patients in whom such a cuff was compared, on a randomized basis, with standard cuffs, 25 patients with the soft cuff showed one-half as much damage, scaled on the basis of endoscopic observations at the time of deflation of the cuff, as 20 patients with standard cuffs. All severe damage was in the standard group. The incidence of cuff stenosis has decreased markedly as equipment has improved, but low-pressure cuffs must be inflated carefully to avoid converting them to high-pressure cuffs. Failure to do so has continued to produce a steady flow of stenoses requiring reconstruction.
 |
Fig. 77-6. A.Diagram of the mechanism of pressure necrosis by a tracheostomy cuff and its avoidance. (a) Normal elliptic shape of the trachea. When a conventional cuff is inflated, it may expand in circular fashion in its widest diameter but at this point fails to occlude the basically irregularly elliptic shape of the trachea. (b) Further distention has been required to effect a seal. At this point the trachea is deformed by the cuff, and much of the considerable intracuff tension is transmitted to the tracheal wall. (c) A large-volume, low-pressure cuff has been inflated with a minimal amount of air. The cuff conforms to the irregular shape of the lumen and provides a seal at low intracuff pressures. Correspondingly low pressures are transmitted to the tracheal wall. B.Comparison of a standard cuff and a large-volume, low-pressure cuff. On the left, the large-volume cuff is shown spontaneously filled with air. No stretch has been placed on the rubber of the cuff wall at this point. The volume is sufficient to occlude most adult tracheas. On the right, a Rusch cuff has been distended with 8 mL of air. It is tense and eccentric. The stretching of the rubber has created a hard structure that exerts considerable pressure on the trachea, which it must deform to provide a seal. From Grillo HC, et al: A low pressure cuff for tracheostomy tubes to minimize tracheal injury: a comparative clinical trial. J Thorac Cardiovasc Surg 62:898, 1971. With permission. |
Cricothyroidotomy should be avoided. Although laryngeal injury is rare, it may not be correctable when it occurs. Tracheal injuries are usually reparable when they first occur. Inappropriate treatment serves to make some incorrectable.
RESULTS OF TREATMENT
I and my colleagues (1995) have had a large experience with postintubation injury. Between 1965 and early 1992, 104 of the referred patients had undergone prior reconstructive attempts or other major tracheal procedures. Two hundred fifty-one had postintubation stenoses at the site of an inflatable cuff. There were 178 stomal stenoses and 38 stomal and cuff stenoses, and in 36 the origin was uncertain. Sixty-two had involvement of the subglottic larynx. One presented with a tracheoinnominate arterial cuff fistula. Corrective reconstructive surgery was effected through the cervical route alone in 350 of these patients, with the addition of an upper sternotomy in 145, and through the transthoracic route in 6, and a skin tube replacement was constructed in 1 patient, for a total of 521 operations in 503 patients.
In 440 patients, the results were good or excellent. An excellent result denotes an anatomically and functionally normal airway. The patient suffers no limitation whatsoever because of the airway, and essentially no narrowing is demonstrated at the anastomotic site on either radiographic or bronchoscopic examination. Patients classified as having a good result have no functional difficulty whatsoever but may have a minimal anatomic narrowing that is definable on either radiographic study or bronchoscopic examination.
The results in 31 patients were classified as satisfactory. These patients are able to carry out all of their normal daily activities but have enough narrowing of the airway to limit major physical effort.
Twenty patients had treatments listed as failures. Causes of failure included inadequate appreciation of existing neurologic dysphagia, cardiac decompensation requiring postoperative
P.1058
ventilation, unappreciated severe laryngeal dysfunction, and restenosis. Five deaths occurred. In four of the patients who died, the patients were sent to us on respirators, and hence reconstruction was contraindicated but undertaken because no therapeutic alternative existed. The other developed bilateral pneumonia and could not be weaned from postoperative respiratory support.
My colleagues and I (1986) described the complications of tracheal surgery for both benign stenosis and neoplasms in detail. Donahue and colleagues (1997) noted surprisingly that the outcome was good or satisfactory in 92% of 75 patients operated upon after unsuccessful initial repairs.
Pearson and Andrews (1971) reported 60 patients with tracheal stenosis. In 34 the stenosis was at the stomal level, and in 26 at the cuff level. Thirty-seven segmental resections were performed. In 33 of the patients, the results were good, and in 1 the result was fair. There was one failure and two operative deaths. Six of the patients developed significant restenosis, and re-resection was performed with good results in all but one of the group. Laryngeal release was used as an adjunctive procedure in 5 of these patients.
The management of acquired benign tracheoesophageal fistula depends on whether the adjacent segment of trachea is circumferentially damaged, as it almost always is in postintubation lesions, or whether the posterior wall fistula is the sole tracheal pathology, as it usually is in fistulae that are caused by foreign bodies. In the first case, concomitant tracheal resection is done with lateral excision of the fistula in the esophageal wall. In the latter, tracheal resection is unnecessary, and both membranous tracheal wall and esophageal wall are precisely repaired after division of the fistulae. Healthy tissue, such as strap muscles from the anterior approach or intercostal transthoracically, is always interposed between the two suture lines to prevent recurrence. Mathisen and associates (1991) noted excellent results after repair of 27 postintubation fistulae, with one death after anastomotic separation consequent to an extensive tracheal resection. Three deaths also occurred after transthoracic repair of distal posttraumatic fistulae in the presence of established mediastinal sepsis. All three required postoperative ventilation. Prompt recognition and repair following the initial injury would likely have been successful.
I reported the results, with my colleagues (1992a), on single-stage laryngotracheal resection and repair of postintubation subglottic stenosis involving the larynx and upper trachea in 50 patients. An additional 30 patients had stenoses in the same location from other causes: trauma, 7; idiopathic, 19; and miscellaneous, 4. Long-term results were excellent in 18 patients, good in 51, satisfactory in 8, and failed in 2. One died of acute myocardial infarction. Maddaus (1992) and Couraud (1979) and their colleagues have produced similar encouraging results.
Mathisen and I (1987) found that 16 of 17 patients treated for laryngotracheal stenosis resulting from trauma had good airways and voices, despite the initial presence of vocal cord paralysis in 14. Four also had esophageal injury requiring repair. Eight needed intralaryngeal procedures before laryngotracheal repair.
Gaissert and co-workers (1993) found that complex laryngotracheal strictures caused by burns responded well in many cases to prolonged stenting (mean, 28 months), with recovery of a functional airway and voice in most patients. In a few, resection of subglottic stenosis was necessary. Early tracheal resection was best avoided. Of 16 patients treated, 9 required no airway support, 4 have permanent tracheal tubes, 2 died (1 from respiratory failure and 1 from an unrelated cause), and 1 was lost to follow-up.
After a failed attempt at tracheal reconstruction with postoperative stenosis, it is best to wait for a prolonged period to permit resolution of the scar and inflammation in the operative field. A minimum of 4 months and preferably 6 months should be allowed. In the meanwhile, it may be necessary to insert a tracheal T-tube or tracheostomy tube to maintain the airway. Reoperation is often extremely difficult. It is surprising, however, to find in some situations in which there appeared to be tension at the original anastomosis that re-resection of a limited stricture may be done with the finding that no apparent tension exists at the time of the second repair. This, however, is not universally true. The greatest enemy of secondary resection is the possibility of anastomotic tension, which may have led to the first failure. Much, therefore, depends on the individual history of the patient.
Histoplasmosis can present nearly insuperable problems in airway management. In a description of the manifestations of mediastinal fibrosis and histoplasmosis, Mathisen and the author (1992) listed nine patients who had undergone tracheobronchoplastic procedures: right carinal pneumonectomy in four, carinal reconstruction in one, sleeve lobectomy in three, and main bronchial sleeve resection in one. Three died postoperatively, one from anastomotic separation after extended resection, and two from postpneumonectomy respiratory distress syndrome.
Mitchell and associates (1999) summarized our experiences with 143 carinal resections with reconstruction. Sixteen were for benign or inflammatory strictures of a variety of etiologies.
With my colleagues, I (1992b) presented the results of surgical attempts to treat severe postpneumonectomy syndrome in 11 adults. Ten underwent mediastinal repositioning (Fig. 77-7). Five who did not also develop tracheobronchomalacia did well. Another died from presumed pulmonary embolism. Four suffered malacic obstruction unrelieved by repositioning. Aortic division with bypass to relieve compression and resection of malacic airway in these desperately ill patients produced only one success. Clearly, correction must be done early, before malacia develops. Since then an additional 11 patients have undergone mediastinal repositioning with insertion of saline-filled prostheses to prevent recurrence. All have survived with improvement; none displayed malacic airways.
Extrinsic compression caused by substernal or intrathoracic goiter is generally relieved by thyroidectomy, without
P.1059
the need for tracheal procedures, as shown by Katlic and colleagues (1985) in a series of 80 patients. Dyspnea was present preoperatively in 28% and stridor in 16%; 79% had tracheal deviation. Flow-volume loops showed tracheal obstruction. No deaths occurred. The procedure is well tolerated even by frail and aged patients: only a few required tracheal splinting. No effective medical treatment exists.
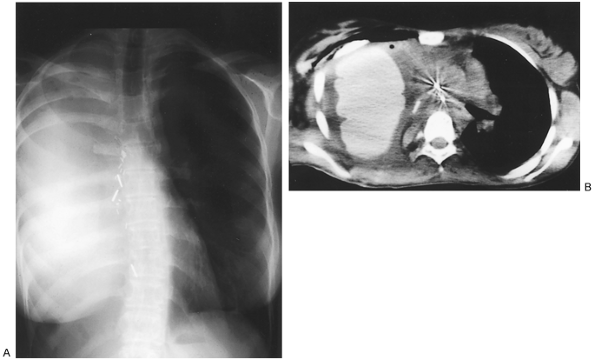 |
Fig. 77-7. Correction of postpneumonectomy syndrome. Same patient as seen in Fig. 77-4, postoperatively. A. Posteroanterior chest roentgenogram. The trachea and mediastinum are restored to midline, and the left lung volume is reduced to normal. B.Computed tomographic scan shows normalized mediastinal anatomy with widely open left main bronchus. The prosthesis necessary to maintain correction is clearly visible in the right hemithorax. |
Ashiku and Mathisen (2003) treated 73 patients with idiopathic laryngotracheal stenosis by one-stage resection and reconstruction. Nineteen (26%) of 72 achieved normal airway and voice; 47 (64%) had some expected diminution of voice projection or quality of singing voice because of laryngeal reshaping. Median follow-up of 8 years revealed no progression of disease or recurrence, the latter casting doubt on suspicion of pharyngolaryngeal reflux as a possible cause.
For severe obstructive tracheopathia osteoplastica, a tracheal fissure from the cricoid to the carina may be performed with insertion of a T-tube or T-Y tube for splinting. Once the trachea has been divided anteriorly, because the membranous wall is not involved by the disease process, it is possible to hinge the two anterolateral walls on either side outward so that a wide lumen is created by the T-tube. The tracheal wall can then be sutured together again. The T-tube is allowed to remain in place for 4 to 6 months to allow firm healing of the trachea in an open position. The tube is removed, having established an adequate airway. Prior attempts have been made to use the laser but failed.
An attempt at splinting and shortening the posterior membranous wall combined with an attempt to reshape the reverse curve of the cartilages failed in one patient with Mounier-Kuhn disease in whom it was attempted. It was necessary to insert an in-lying permanent tracheal T-tube. Two other patients were treated in similar fashion since that time, and all achieved satisfactory palliative results.
In two patients with such extreme saber-sheath trachea that they were unable to clear secretions, the trachea was splinted with special external polypropylene ring splints. The tracheal wall was sutured to the splints, pulling it outward. The sternohyoid muscles were turned down to embed the rings against the tracheal wall to maintain correction after nonabsorbable sutures ultimately pull through. The procedure permitted the patients to clear secretions with cough, which they had not been able to do before.
In patients with tracheobronchial malacia affecting the lower two-thirds of the trachea and main bronchi, who have softened, splayed out cartilages in an archer's bow configuration and redundant membranous tracheal wall, reshaping the trachea was proposed and described by Herzog and colleagues (1987). A strip of splinting material is placed along the membranous wall of the trachea in a width corresponding to estimated normal. The corners of the cartilages on either side are sutured to the splint, and the membranous wall is quilted to the splint as well. Pulling the two ends of the cartilages together posteriorly causes the cartilages to arch forward, recreating a more nearly normal cross-sectional
P.1060
configuration. The redundant membranous wall is fixed to the splint posteriorly so that it cannot pout forward to obstruct the lumen. Herzog originally used fascia lata and eventually moved to use of Gore-Tex (W.L. Gore & Associates, Flagstaff, AZ, U.S.A.). Other materials that have been used include lyophilized bone and perforated plastic splints.
Gore-Tex gives an excellent initial result. After some months, fluid may accumulate between the Gore-Tex and the membranous wall where sutures had pulled through, because Gore-Tex does not become enmeshed in scar tissue. Strips of pericardium tend to attenuate with time. Marlex mesh has pores large enough to permit ingrowth of connective tissue that fuses it to the membranous wall, providing permanent correction. The procedure does not correct underlying obstructive pulmonary disease, from which most of these patients suffer, but improves the patients' ability to raise secretions with more effective cough. The lower two-thirds of the trachea, right main bronchus, bronchus intermedius, and left main bronchus are splinted. Improvement in sputum clearance was observed in 15 patients.
My colleagues and I (2002) reported eight consecutive patients with long-segment congenital tracheal stenosis, between 10 days and 23 years of age, corrected by slide tracheoplasty. Only four with concomitant vascular or cardiac problems needed bypass. All had good results and minimal granulations, and long-term growth was excellent in four infants and small children followed from 1.5 to 7.25 years. Most were extubated after surgery and required no ventilation postoperatively.
REFERENCES
Andrews MJ, Pearson FG: The incidence and pathogenesis of tracheal injury following cuffed tube tracheostomy with assisted ventilation: an analysis of a two-year prospective study. Ann Surg 173:249, 1971.
Arens JF, Oschner JL, Gee C: Volume-limited intermittent cuff inflation for long-term respiratory assistance. J Thorac Cardiovasc Surg 58:837, 1969.
Ashiku SK, Mathisen DJ: Idiopathic laryngotracheal stenosis. Chest Surg Clin N Am 13:257, 2003.
Carroll R, Hedden M, Safar P: Intratracheal cuffs: performance characteristics. Anesthesia 31:275, 1969.
Cooper JD, Grillo HC: The evolution of tracheal injury due to ventilatory assistance through cuffed tubes: a pathologic study. Ann Surg 169:334, 1969a.
Cooper JD, Grillo HC: Experimental production and prevention of injury due to cuffed tracheal tubes. Surg Gynecol Obstet 129:1235, 1969b.
Cooper JD, et al: Use of silicone stents in the management of airway problems. Ann Thorac Surg 47:371, 1989.
Couraud L, et al: Int r t de la r section cricoidienne dans le traitement des st noses cricotrach ales apr s intubation. Ann Chir Thorac Cardiovasc 33:242, 1979.
Couraud L, et al: Post traumatic disruption of the laryngo-tracheal junction. Eur J Cardiothorac Surg 3:441, 1989.
Deslauriers J, et al: Innominate artery rupture. A major complication of tracheal surgery. Ann Thorac Surg 20:671, 1975.
Deslauriers J, et al: Diagnosis and long-term follow-up of major bronchial disruptions due to nonpenetrating trauma. Ann Thorac Surg 33:32, 1982.
Donahue DM, et al: Reoperative tracheal resection and reconstruction for unsuccessful repair of postintubation stenosis. J Thorac Cardiovasc Surg 114:934, 1997.
Florange W, Muller J, Forster E: Morphologie de la n crose trach ale apr s trach otomie et l'utilisation d'une prosth se respiratoire. Anesth Analg 22:693, 1965.
Gaissert HA, Lofgren RH, Grillo HC: Upper airway compromise after inhalation injury. Complex strictures of larynx and trachea and their management. Ann Surg 218:672, 1993.
Greene RE, Lechner GL: Saber-sheath trachea: a clinical and functional study of marked coronal narrowing of the intrathoracic trachea. Radiology 115:265, 1975.
Grillo HC: The management of tracheal stenosis following assisted respiration. J Thorac Cardiovasc Surg 57:52, 1969.
Grillo HC: Surgery of the trachea. Curr Probl Surg 7:3, 1970.
Grillo HC: Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 33:3, 1982.
Grillo HC, Mathisen DJ, Wain JC: Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 53:54, 1992a.
Grillo HC, Moncure AC, McEnany MT: Repair of inflammatory tracheoesophageal fistula. Ann Thorac Surg 22:112, 1976.
Grillo HC, Zannini P, Michelassi F: Complications of tracheal reconstruction. J Thorac Cardiovasc Surg 91:322, 1986.
Grillo HC, et al: A low pressure cuff for tracheostomy tubes to minimize tracheal injury: a comparative clinical trial. J Thorac Cardiovasc Surg 62:898, 1971.
Grillo HC, et al: Postpneumonectomy syndrome: diagnosis, management and results. Ann Thorac Surg 54:638, 1992b.
Grillo HC, et al: Idiopathic laryngotracheal stenosis and its management. Ann Thorac Surg 56:80, 1993.
Grillo HC, et al: Postintubation tracheal stenosis: treatment and results. J Thorac Cardiovasc Surg 109:486, 1995.
Grillo HC, et al: Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction, with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg 123:145, 2002.
Herzog H, et al: Surgical therapy for expiratory collapse of the trachea and large bronchi. In Grillo HC, Eschapasse H (eds): International Trends in General Thoracic Surgery: Major Challenges. Philadelphia: WB Saunders, 1987, p. 74.
Katlic MR, Grillo HC, Wang CA: Substernal goiter. Analysis of 80 Massachusetts General Hospital cases. Am J Surg 149:283, 1985.
Lindholm CE: Prolonged endotracheal intubation. Acta Anaesth Scand Suppl 33:1, 1970.
Maddaus MA, et al: Subglottic tracheal resection and synchronous laryngeal reconstruction. J Thorac Cardiovasc Surg 104:1443, 1992.
Mathisen DJ, Grillo HC: Laryngotracheal trauma. Ann Thorac Surg 43: 254, 1987.
Mathisen DJ, Grillo HC: Clinical manifestation of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg 54:1053, 1992.
Mathisen DJ, et al: Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 52:759, 1991.
Mitchell JD, et al: Clinical experience with carinal resection. J Thorac Cardiovasc Surg 117:39,1999.
Montgomery WW: The surgical management of supraglottic and subglottic stenosis. Ann Otol Rhinol Laryngol 77:534, 1968.
Muehrcke DD, Grillo HC, Mathisen DJ: Reconstructive airway operation after irradiation. Ann Thorac Surg 59:14, 1995.
Newton JR Jr, Grillo HC, Mathisen DJ: Main bronchial sleeve resection with pulmonary conservation. Ann Thorac Surg 52:1272, 1991.
Pearson FG, Andrews MJ: Detection and management of tracheal stenosis following cuffed tube tracheostomy. Ann Thorac Surg 12:359, 1971.
Pearson FG, et al: Primary tracheal anastomosis after resection of the cricoid cartilage with preservation of recurrent laryngeal nerves. J Thorac Cardiovasc Surg 70:806, 1975.
Tedder M, et al: Pulmonary mucormycosis: results of medical and surgical therapy. J Thorac Cardiovasc Surg 57:1044, 1994.
Toty L, et al: Laser treatment of postintubation lesions. In Grillo HC, Eschapasse H (eds): International Trends in General Thoracic Surgery. Vol. 2. Philadelphia: WB Saunders, 1987, p. 31.
Weber AL, Grillo HC: Tracheal stenosis: an analysis of 151 cases. Radiol Clin North Am 16:291, 1978.
Young RH, Sandstrom RE, Mark GJ: Tracheopathia osteoplastica: clinical, radiologic, and pathological correlations. J Thorac Cardiovasc Surg 79:537, 1980.
EAN: 2147483647
Pages: 203