XI - The Pleura
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XI - The Pleura > Chapter 65 - Diffuse Malignant Mesothelioma
function show_scrollbar() {}
Chapter 65
Diffuse Malignant Mesothelioma
Raja M. Flores
Valerie W. Rusch
Diffuse malignant pleural mesothelioma (MPM) is an uncommon and lethal cancer for which there are limited treatment options. The first report of a primary pleural tumor, presumably an MPM, is attributed to Lieutaud in 1767, but there was no precise pathologic description until Klemperer and Rabin (1937) classified MPMs as either localized or diffuse. Cell culture experiments by Stout and Murray (1942) examined the histologic origin of these tumors. However, MPM was largely regarded as a medical curiosity until 1960, when Wagner and co-workers reported 33 cases of diffuse MPM in asbestos mine workers from the Northwestern Cape Province of South Africa. Subsequent studies, especially work by Selikoff and associates (1965) and Whitwell and Rawcliffe (1971) in the United States, confirmed that asbestos exposure was the major risk factor for MPM. The epidemiology of diffuse MPM is now well understood, but its biological behavior remains an enigma and the treatment of this cancer is still controversial.
The incidence of MPM is increasing because of the large number of individuals who were exposed to asbestos during the 1930s to 1960s in asbestos mines and asbestos-related industries, before the causal relationship between asbestos and MPM was recognized. An estimated 2,000 to 3,000 cases occur annually in the United States. In Western Europe alone, a quarter of a million deaths are projected over the next 30 years, as reported by Peto in 1999. It is important for thoracic surgeons to be knowledgeable about MPM because they are often called on to make the diagnosis and to recommend treatment.
EPIDEMIOLOGY AND INCIDENCE
A relationship between asbestos exposure and interstitial lung disease was first recognized in 1906, when deaths among asbestos textile workers from pneumoconiosis were reported in England and France by Murray (1907) and Auribault (1906). Scattered case reports by Wedler (1943a, 1943b), Cartier and Smith (1952), and Van der Schoot (1958) suggested a link between asbestos exposure and diffuse MPM, but this was not clearly established until the report of Wagner and colleagues in 1960 and Wagner's seminal publication in 1986. Wagner was appointed the Asbestosis Research Fellow at the South Africa Pneumoconiosis Research Unit in 1954 and was charged with determining if all types of asbestos caused the same diseases. An increasing frequency of a fatal pleural tumor in patients hospitalized at the Tuberculosis Hospital in the asbestos mining region of South Africa led Wagner and associates to study the pleural biopsy results of these patients. The surprise finding of MPM in these biopsy samples prompted a careful epidemiologic study. Asbestos exposure was the single factor common to all these cases. Subsequently, Wagner went to Great Britain to study this problem further and he again demonstrated an epidemiologic relationship between asbestos exposure and MPM among asbestos workers and insulators.
The second direct demonstration of a link between asbestos exposure and MPM, described by Layman (1992) and Musk and colleagues (1989), occurred in Western Australia, where approximately 7,000 individuals at the Wittenoom asbestos mines had extensive exposure to asbestos from 1943 to 1966. The first case of MPM was diagnosed in 1960. By the end of 1986, 94 cases of MPM, 141 cases of lung cancer, and 356 successful compensation claims for asbestosis were recorded among former miners and their family members. An additional 692 cases of MPM are expected to occur in this cohort between 1987 and 2020. The Wittenoom asbestos industry is considered the worst industrial disaster in Australian history.
As reported by de Klerk and Armstrong (1992), the type of asbestos fiber plays a critical role in the risk for developing MPM. Asbestos belongs to the family of silicate fibers and includes two mineralogic groups: amphibole and serpentine fibers. Chrysotile asbestos is the only member of the serpentine group, whereas crocidolite, amosite, tremolite, anthophyllite, and actinolite asbestos belong to the amphibole group. Pooley (1987) reported that these minerals differ
P.902
considerably in their structure and composition. Serpentine fibers are large, curly fibers that do not travel beyond the major airways, whereas amphibole fibers are narrow and straight fibers that migrate through the lymphatics of the pulmonary parenchyma and accumulate in the interstitial spaces and the subpleural region. It is the amphibole fibers, especially crocidolite asbestos ( blue asbestos ) that are most clearly associated with MPM. Chrysotile is more closely associated with the development of lung cancer.
Crocidolite asbestos is found only in South Africa and Western Australia but has been exported all over the world for various industrial uses. Chrysotile ( white asbestos ) accounts for 97% of worldwide asbestos production and has been mined principally in the Ural Mountains in Russia, the Quebec Province in Canada, Zimbabwe and Swaziland in South Africa, the Italian Alps, and Cyprus. Churg and DePaoli (1988) and McDonald and co-workers (1989) reported that chrysotile itself is not thought to cause MPM but is often contaminated with amphibole fibers, such as tremolite or amosite ( brown asbestos ). However, controversy over the potential role of chrysotile asbestos in causing mesothelioma persists.
Individuals can be exposed to asbestos in many situations because it has a thousand uses, as reported by Huncharek (1992). However, as discussed by Andersson and Olsen (1985), Malker and associates (1985), and McDonald and McDonald (1980), the areas of the world that have a high incidence of MPM are those with asbestos mines and countries that have shipyards, insulation, construction, and automobile industries that use large amounts of asbestos. In North America, the highest incidence areas include the provinces of Quebec and British Columbia in Canada, which have asbestos mines, and Seattle, Hawaii, San Francisco Oakland, New York New Jersey, New Orleans, and Norfolk, Virginia, all of which have large shipyards or asbestos industries. It is difficult to document a relationship between the duration or intensity of asbestos exposure and the risk for developing MPM, but Levine (1981) has reported that patients with peritoneal MPM usually have a history of heavier exposure than do patients with pleural disease.
Malignant pleural mesothelioma is also caused by other naturally occurring and manufactured silicate fibers that share the physical properties of amphibole asbestos fibers. These properties are a diameter of less than 0.25 m and a length greater than 5.0 m. The most notable example is erionite, a zeolite fiber found in volcanic deposits in central Turkey, where it is the major material for building homes. Baris (1987) found that in Karain, Turkey, a village of 604 persons, MPM was the single most common cause of death, with 62 cases recorded from 1970 to 1981. In this village, MPM frequently occurs in individuals who are in their twenties or thirties because they have been exposed to erionite from birth.
Less common causes of MPM also exist. Lerman and associates (1991) recorded that radiation exposure at periods ranging from 10 to 31 years before the development of MPM is the most clearly documented of these causes (e.g., individuals who received mantle radiation for Hodgkin's disease as young adults). Extravasation of radioactive thorium dioxide (Thorotrast, a contrast agent previously used for radiologic procedures) and exposure to isoniazid in utero are anecdotally reported by Antman (1983, 1984) and Anderson (1985) and their co-workers to cause MPM. A variety of other substances, summarized by Peterson and associates (1984) and Hammar and Bolen (1988) in Table 65-1, are thought to be possible risk factors for MPM, based on epidemiologic or experimental animal studies. However, in contrast to lung cancer, for which asbestos and smoking
P.903
act as synergistic carcinogens, smoking is not a risk factor for MPM.
Table 65-1. Nonasbestos Causes of Mesothelioma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 65-2. Histologic Classification of Mesothelioma | ||
|---|---|---|
|
The peak age for the development of MPM is the sixth decade of life. Because most patients develop MPM as a result of occupational exposure to asbestos, the increased incidence of this disease has occurred in men. In the United States, the incidence in women remains at the baseline level of 3 cases per million population, whereas in men it has risen to 15 cases per million people per year. MPM is predominantly a disease of older adults because of the long latency period (at least 20 years) between exposure to causative agents and the development of cancer, but Fraire and colleagues (1988) reported that it can occur in childhood. In that setting, it is usually idiopathic. MPM sometimes develops in young adults because of exposure to risk factors during childhood, as reported by Kane and colleagues (1990).
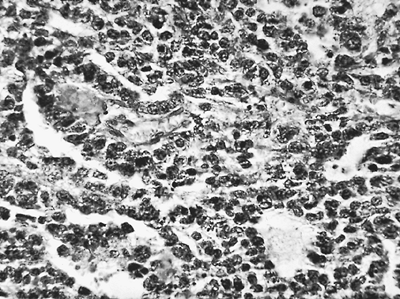 |
Fig. 65-1. Photomicrograph of epithelial type of malignant mesothelioma. |
PATHOLOGY
Malignant pleural mesotheliomas arise from multipotential mesothelial or subserosal cells that can develop into either an epithelioid or a sarcomatoid neoplasm. In contrast to localized fibrous tumors of the pleura described by Scharifker and Kenko (1979), diffuse MPMs always have an epithelioid component. However, they exhibit a wide array of histologic patterns (Table 65-2) and, often, a mixture of epithelioid and sarcomatoid features (Figs. 65-1 and 65-2). In a review of 819 cases, Hillerdal (1983) reported that 50% were of epithelioid type, 34% were of mixed type, and 16% were of the sarcomatoid type. The histologic appearance of MPMs is easily confused with that of other neoplasms and there is often disagreement
P.904
among pathologists when light microscopy is used as the sole method of diagnosis. The rate at which tumors originally diagnosed as MPMs are reclassified ranges from 30% to 84% when these specimens are reviewed by panels of reference pathologists. The usual challenge for the pathologist is to distinguish epithelioid MPMs from metastatic adenocarcinoma. However, as Cantin and associates (1982) reported, very early MPMs can be difficult to distinguish from benign mesothelial hyperplasia, and the rare desmoplastic form of MPM often resembles benign fibrosis because of its predominantly fibroblastic cell type and sparsely cellular appearance.
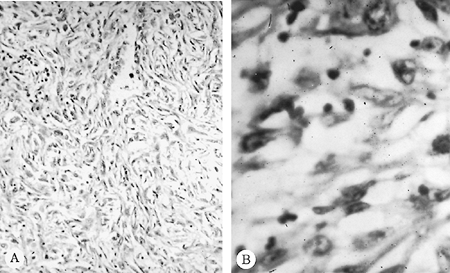 |
Fig. 65-2. Photomicrograph of a malignant mesothelioma of the sarcomatous variety. A. Low-power magnification. B. High-power magnification. |
Table 65-3. Mesothelioma Versus Adenocarcinoma | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Several histochemical stains help to distinguish MPM from other tumors. Pulmonary adenocarcinomas usually stain positively with mucicarmine, whereas MPM does not. Approximately 20% of epithelioid MPMs produce hyaluronic acid, an acidic mucosubstance that can be seen either within or between cells with an Alcian blue or colloidal iron stain. However, immunohistochemistry and electron microscopy are now routinely used to establish a definitive pathologic diagnosis. Battifora and Kopinski (1985), as well as Wirth (1991) and Mezger (1990) and their colleagues, reported that useful immunohistochemical stains include antibodies to high- and low-molecular-weight cytokeratins, to vimentin, to human milk fat globule, to carcinoembryonic antigen, and to Leu-M1. MPMs stain positively for low-molecular-weight cytokeratins, a feature that distinguishes them from sarcomas. Calretinin has recently been added to the standard battery of stains because it is almost always positive in MPM. MPMs rarely stain for carcinoembryonic antigen, a feature that distinguishes them from adenocarcinomas (Table 65-3). If immunohistochemical stains yield equivocal results, electron microscopy usually provides a definitive diagnosis. Burns and associates (1985) have emphasized that the most prominent feature of MPMs is that they have numerous, long, sinuous microvilli, whereas adenocarcinomas have short, straight microvilli that are covered by a fuzzy glycocalyx (Fig. 65-3).
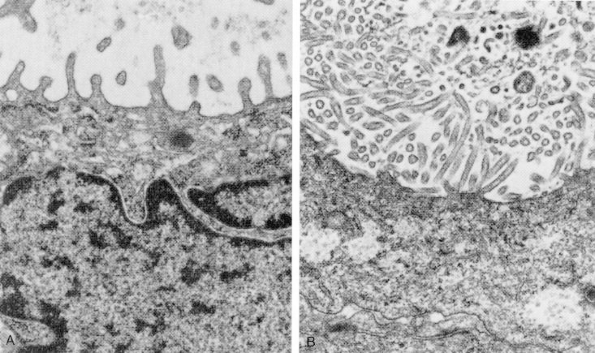 |
Fig. 65-3. Ultrastructural features of pulmonary adenocarcinoma (A), characterized by short, plump microvilli, contrasted with those of mesothelioma (B), in which microvilli are numerous, long, and slender. From Kobzik L. The lung. In Cotran RS, Kumar V, Collins T (eds): Robbins Pathologic Basis of Disease. 6th Ed. Philadelphia: WB Saunders, 1999, p. 753. With permission. |
MOLECULAR BIOLOGY
As summarized by Donington and associates (1995) and by Pass and Mew (1996), relatively little is understood about the biology of diffuse MPM. Similar to other solid tumors,
P.905
MPM is characterized biologically by the presence of multiple cytogenetic abnormalities that include duplications, deletions, and translocations. Asbestos is thought to play a significant role in causing DNA damage, partly through the production of free radicals from the iron contained in asbestos fibers. It also generates free radicals though interaction with monocytes, macrophages, and neutrophils. Nitric oxide, generated by the phagocytosis of asbestos by macrophages, may accelerate changes in DNA by deamination, and may also suppress T-cell activity. Asbestos fibers themselves can directly interfere with spindle apparatus formation during mitosis with resulting aneuploidy.
Taguchi and co-workers (1993) reported multiple clonal alterations in both primary tumors and cell lines, with the most frequent changes being chromosomal losses at 1p21-22, 9p21-22, 3p21, and 6q15-21. Lee and colleagues (1996) and Bell and co-workers (1997) confirmed that 1p21-22 and 6q15-21 were frequent areas of chromosomal loss. Bj rkqvist and associates (1997) reported DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q, and 22q based on comparative genomic hybridization (CGH) analyses on 27 tumor specimens. More losses than gains were observed. All of these studies found that multiple chromosomal changes occurred in combination, suggesting the involvement of a genetic cascade in MPM.
With respect to specific genes, Kumar-Singh and colleagues (1997) identified frequent mutations of the Wilms' tumor 1 gene (WT1) in both primary MPM and cell lines, which is located on 11p13. WT1 encodes a transcription factor that regulates the early growth response 1 (EGR1), the insulin growth factor 1 receptor (IGF-1R), and the epidermal growth factor receptor (EGFR) genes, and is thought to be important in mediating apoptosis. Sekido and associates (1995) and Bianchi and co-workers (1995) reported that the neurofibromatosis gene type 2 (NF2), a tumor suppressor gene located on chromosome 22q12, was mutated in approximately half of MPM tumors examined. Cheng (1994), Xio (1995), and Prins (1998) and their associates found that the p16 and p15 genes located on chromosome 9p21, were deleted in at least 70% of the primary MPM and cell lines examined. These genes play an important role in cell cycle regulation.
Overexpression of the platelet-derived growth factors (PDGF) A and B and of their receptors was noted by Gerwin (1987), and Versnel (1988) and their co-workers. Ohta and coinvestigators (1999a) reported that the vascular endothelial growth factors (VEGF and VEGF-C) and their receptors, which regulate angiogenesis, were overexpressed in at least 50% of MPM tumors and cell lines. The same group of investigators (1999b) found that thrombospondin-1 (TSP-1), which also regulates angiogenesis, was overexpressed in 95% of MPM tumors examined, although this did not appear to correlate with prognosis.
Considerable controversy surrounds the presence of simian virus 40 (SV40) in MPM and its potential role in carcinogenesis. SV40 sequences have been identified in MPM specimens from the United States but not from several other countries, including Finland, as reported by Testa (1998) and Hirvonen (1999) and their associates. It is hypothesized that some individuals may have been exposed to SV40 through contaminated polio vaccines used during the 1950s. SV40 is well known to be tumorigenic in animal models, potentially through inactivation of the p53 and Rb tumor suppressor genes. In the report of an international meeting held to discuss the association of SV40 with human tumors, Klein and co-workers (2002) summarized all of the known information about SV40 in MPM. Clearly, MPM often develops in the absence of SV40, while evidence of SV40 sequences is present in some tumors. Since MPM is a clinically, pathologically, and biologically heterogeneous tumor, it is postulated that SV40 may function as a cocarcinogen, leading to dysregulation of one or more genetic pathways known to be important in MPM tumorigenesis. Carbone and colleagues (2002) proposed a possible synergistic pathogenic mechanism of asbestos and SV40 in the development of MPM (Fig. 65-4).
In summary, the biology of MPM is complex and reflects the interplay of multiple genetic abnormalities, most frequently the deletion or inactivation of various tumor suppressor
P.906
genes. These abnormalities may arise from a multifactorial impact of asbestos fibers on cell cycle regulation and on cellular and immunologic function. SV40 may be present in some MPM and may function as a cocarcinogen, although this is still hypothetical. Clearly, much more investigation is needed to gain a full understanding of the biological mechanisms underlying MPM tumorigenesis.
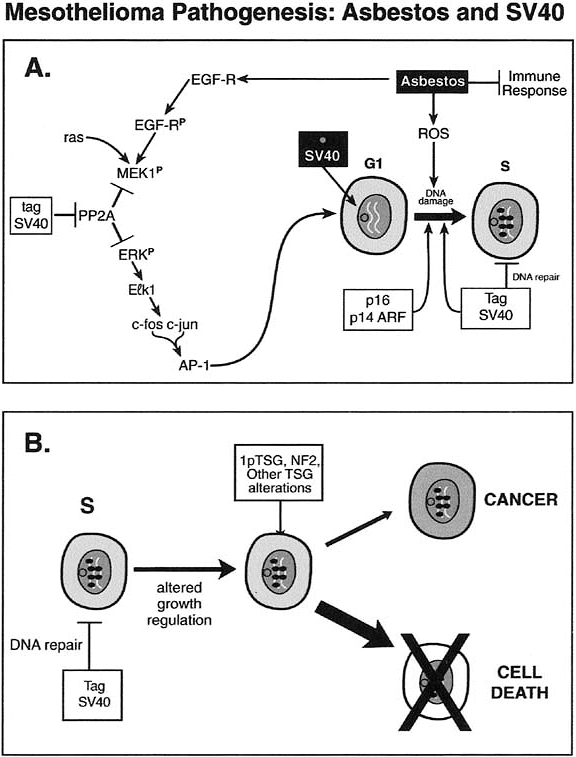 |
Fig. 65-4. Mesothelioma pathogenesis: possible pathogenic mechanisms of asbestos and SV40. From Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol 29:2, 2002. With permission. |
CLINICAL PRESENTATION
The clinical presentation of diffuse MPM is insidious and nonspecific. MPM has traditionally been portrayed as a diffuse, massive tumor that causes excruciating chest pain. In fact, these signs and symptoms are seen only when MPM reaches a locally advanced stage. During the early stages of disease, dyspnea is the predominant symptom and is related to the presence of an effusion. When the effusion is drained, patients are asymptomatic. As the tumor grows, patients develop ill-defined, mild, but continuous chest discomfort. Dyspnea may actually improve during this phase of the disease because, with tumor growth, the pleural surfaces fuse and the effusion resolves. Only when the disease becomes locally advanced does the patient develop severe chest pain, which is related to tumor infiltration of the chest wall and intercostal nerves. This is accompanied by a sense of chest tightness and dyspnea caused by entrapment of the lung by tumor. In the final stages of disease, dyspnea and chest pain become severe and unremitting. These symptoms are related to encasement of the chest wall, lung, and mediastinum, and are occasionally associated with mediastinal shift and compression of the contralateral lung. The tumor may also extend directly though the pericardium, causing a pericardial effusion or myocardial metastases. The symptoms of locally advanced pleural disease may also be compounded by the development of ascites from direct extension of the tumor through the diaphragm or a contralateral pleural effusion from metastatic disease. Elmes and Simpson (1976) and Ruffie and associates (1989) report a variety of other symptoms, such as bone pain, which can occur in terminal patients who develop extrathoracic metastases.
Thus, dyspnea and chest pain are the most common presenting symptoms, occurring in 90% of patients. Weight loss occurs in approximately 30% of patients but is seen only in the advanced stages of the disease. Uncommon symptoms include cough, weakness, anorexia, fever, hemoptysis, hoarseness, dysphagia, and Horner's syndrome. A few cases presenting with a spontaneous pneumothorax have been reported by Sheard and colleagues (1991).
In the early stages of disease, the findings on physical examination are nonspecific. Dullness to percussion and decreased breath sounds may be noted because of the presence of a pleural effusion, but the chest examination is otherwise normal. In the late stages of disease when tumor encases the hemithorax, the excursion of the chest with respiration diminishes and the chest wall is noticeably contracted. Diffuse dullness to percussion and decreased breath sounds are present over the entire hemithorax and there is a subtle fullness of the intercostal spaces. If the tumor has grown through the intercostal spaces or has implanted in the site of a previous thoracentesis or thoracotomy incision, palpable soft tissue masses may be found in the chest wall. Palpable supraclavicular or axillary nodes or an obvious ascites may be present if the tumor has metastasized to these areas, as described by Law and colleagues (1982b).
Paraneoplastic syndromes are uncommon, but autoimmune hemolytic anemia, hypercalcemia, hypoglycemia, the syndrome of inappropriate secretion of antidiuretic hormone, and hypercoagulability not related to thrombocytosis have been reported by Ruffie and associates (1989). Olesen and Thorshauge (1988) found that thrombocytosis, defined as a platelet count of 400,000/mL or greater, occurs in approximately 30% to 40% of patients. This is sometimes associated with a leukemoid reaction, but does not seem to increase the risk for thromboembolic episodes.
Malignant pleural mesothelioma patients often have abnormal electrocardiographic (ECG) and echocardiographic findings. In a review of 64 patients, Wadler and co-workers (1986) found that 55 patients (89%) had an abnormal ECG. Sinus tachycardia was seen in 42% of patients, non-life-threatening ventricular or atrial arrhythmias occurred in 17% of patients, and more than one third of patients had some form of bundle branch block. Although pericardial invasion or myocardial involvement was a common finding at autopsy in these patients, most ECG abnormalities occurred more than 6 months before death, which suggests that they are not solely related to the presence of advanced disease. Echocardiography was somewhat insensitive but highly specific for involvement of the pericardium or myocardium by tumor: three patients who had pericardial effusions by echocardiogram had pericardial and myocardial involvement at autopsy, whereas five patients who had pericardial tumor at autopsy had had a normal echocardiogram.
No tumor markers are routinely used for MPM. Serum hyaluronan may be elevated in some patients, which is not surprising given the positive staining for hyaluronic acid seen in many epithelioid MPMs. In one study of 37 patients, Dahl and colleagues (1989) found that an increase in serum hyaluronan had a sensitivity of 65% and a specificity of 85% as a predictor of progressive disease. Hyaluronan can be measured using a commercial kit, but the kit is not readily available in most hospitals. CA-125 was reported by one of us (VWR) and colleagues (1994) to be elevated in approximately 20% of patients, and its use as a serum marker is therefore limited.
RADIOLOGIC FEATURES
The radiographic appearance of MPM is variable and is related to the stage of the tumor at diagnosis. In early-stage
P.907
MPM, a large pleural effusion is often the only sign of disease (Fig. 65-5). Subtle pleural thickening or small, discrete, pleural-based masses may be seen on computed tomography (CT) (Fig. 65-6). Subsequently, larger pleura-based masses become evident and are often intermixed with multiloculated effusions. Gotfried and colleagues (1983) note that a dominant pleural-based mass may be the initial presentation in rare cases, but ultimately the involvement of the pleura is always diffuse. Eventually, a thick confluent pleural rind develops, with encasement of the lung and obliteration of the pleural space (Fig. 65-7). Mediastinal adenopathy, direct extension of the tumor into the mediastinum, involvement of the pericardium with pericardial effusion, and extension into the chest wall or through the diaphragm are seen with locally advanced disease (Fig. 65-8). Rabinowitz (1982), Mirvis (1983), Law (1982b), and Alexander (1981) and their colleagues reported that CT permits a far better assessment of the extent of the disease than does standard radiography, which cannot demonstrate many of these abnormalities. CT is currently the most accurate noninvasive way to stage patients, as well as to assess response to treatment and to detect recurrent disease postoperatively. However, as reported by one of us (VWR) and associates (1988), it is often inaccurate in diagnosing chest wall involvement or extension through the diaphragm. It
P.908
was hoped that magnetic resonance (MR) imaging would prove more accurate in this regard, but in a prospective study comparing CT and MR imaging for preoperative staging, Heelan and associates (1999) showed that MR imaging is not significantly better than CT in defining the local extent of tumor. Therefore, CT remains the standard imaging study.
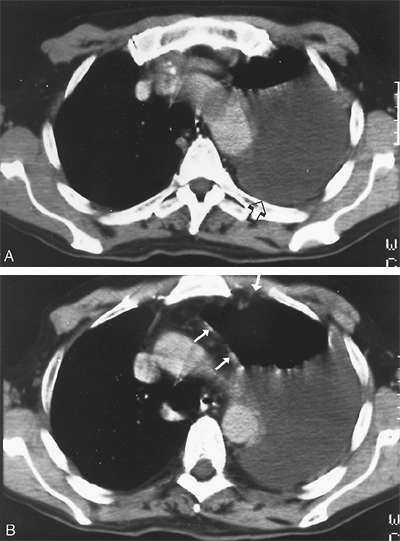 |
Fig. 65-5. CT scans of an early-stage diffuse mesothelioma. A. The CT scan at the level of the aortic arch shows a large left pleural effusion (arrow) with no evidence of pleural disease. B. The second CT scan at the level of the aortopulmonary window shows mild pleural thickening and irregularity (arrows) in addition to the effusion. At thoracotomy, there was diffuse studding of the pleura with tumor nodules that were 1 to 2 mm in diameter. |
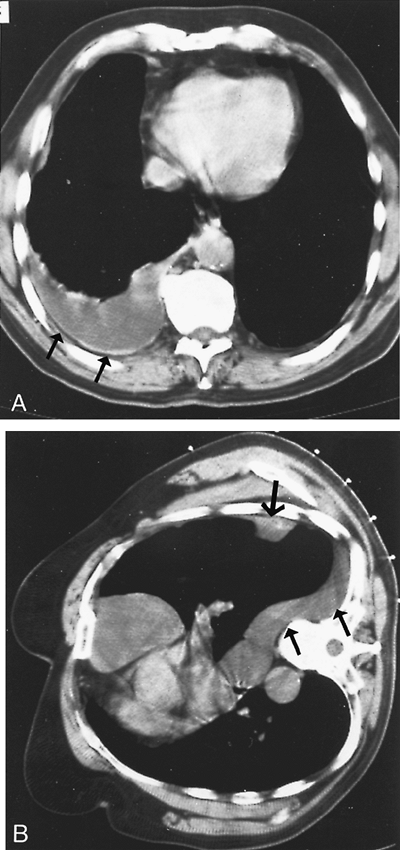 |
Fig. 65-6. CT scans of another early-stage mesothelioma. A. There is a large pleural effusion with diffuse mild pleural thickening and irregularity (arrows). B. A CT scan obtained with the patient in the lateral position shows a dominant chest wall mass (large arrow) and a freely flowing effusion (small arrow). |
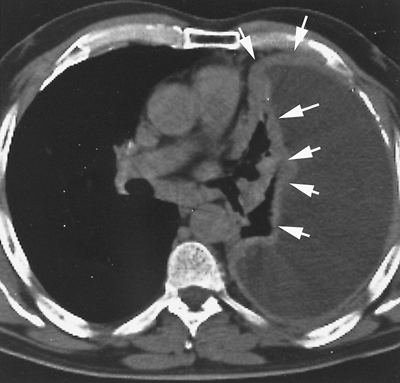 |
Fig. 65-7. CT scan of a more locally advanced mesothelioma. There is a thick confluent pleural peel along the chest wall (large arrows), encasing the collapsed lung and extending into the fissure (small arrows). |
Pass and colleagues (1998) conducted a study to evaluate the impact of preoperative tumor volume by three-dimensional CT reconstructions on outcome in patients undergoing resection for MPM. Forty-eight patients had measurement of preresection solid tumor volume and were staged according to the American Joint Committee on Cancer (AJCC) staging system for MPM, prior to surgical resection. Median survival for preoperative tumor volume less than 100 mL was 22 months versus 11 months for tumor volume greater than 100 mL (p = 0.03). Progressively higher stage was associated with higher median preoperative volume: stage I, 4 mL; stage II, 94 mL; stage III, 143 mL; and stage IV, 505 mL (p = 0.007). Higher tumor volumes were also associated with a greater likelihood of lymph node metastasis. This study showed that preoperative tumor volume assessed by volumetric CT tumor measurement is representative of tumor stage status in MPM and can predict survival.
The utility of positron emission tomographic (PET) scanning in the preoperative staging of MPM has been investigated at Memorial Sloan-Kettering Cancer Center (MSKCC), and at other centers, as reported by B nard and associates (1998). One of us (RMF) and colleagues (2003a) reported on 63 patients who underwent PET scans during their initial evaluation prior to surgical resection. Fluorodeoxyglucose uptake was present in all except one patient with stage IA disease. PET scan was not found to add to the assessment of locoregional disease, because it did not help in identifying patients with T4 unresectable lesions, and did not diagnose lymph node involvement. However, a high standard uptake value (SUV) was associated with a greater likelihood of mediastinal lymph node metastases (N2 disease). More importantly, PET was useful in identifying 10% of the patients as having distant disease that was undetectable by CT scan, thereby preventing inappropriate surgical intervention.
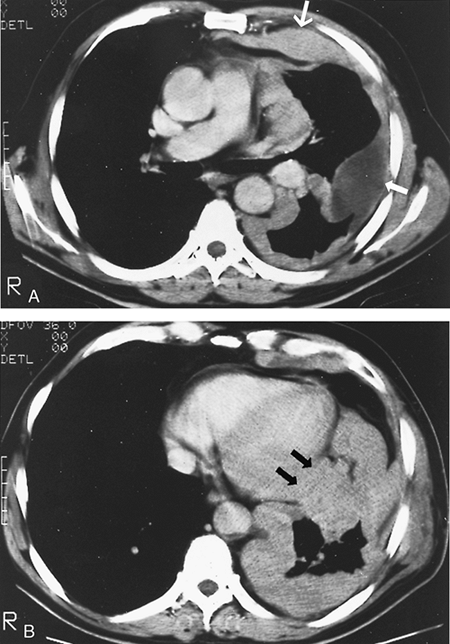 |
Fig. 65-8. CT scan of another locally advanced mesothelioma. A. The CT scan at the level of the pulmonary artery shows a thick irregular confluent pleural peel (large arrow) with a loculated pleural effusion (small arrow). B. The CT scan at the level of the midheart shows massive tumor encasing and collapsing the lung and suggests invasion of the pericardium (arrows). |
In addition to identifying patients with stage IV disease, PET scan findings may have prognostic significance. We recently evaluated 85 MPM patients who underwent PET scanning at diagnosis and found that there was a linear relationship between increasing SUV and decreasing median
P.909
survival. The relative risk for death in patients with an SUV of greater than 4 when compared with an SUV of less than 4 was 3.3 (p = 0.03). In both univariable and multivariate analyses, SUV significantly predicted overall survival. These findings, as reported by one of us (RMF) and coinvestigators (2003b), suggest that PET SUV may be used to stratify patients for treatment.
DIAGNOSIS
Because most patients present with a pleural effusion, a thoracentesis is usually the initial diagnostic procedure. Pleural fluid cytology is positive for malignancy in only 30% to 50% of these patients, and percutaneous pleural biopsy yields a diagnosis of malignancy in up to one third of cases. As emphasized by Battifora and Kopinski (1985), as well as Wirth (1991) and Mezger (1990) and their colleagues, it frequently does not provide a large enough specimen for the immunohistochemical or electron microscopic studies that are critical for a definitive diagnosis. As described by Boutin and associates (1991a) and Bouton and Rey (1993), thoracoscopy is the optimal diagnostic procedure because it yields a diagnosis in at least 80% of patients without committing the patient to a major surgical procedure. The appearance of the pleural space is variable and depends on the extent of disease and the cell type. In the earliest stage of MPM, involvement of the pleura is microscopic, and the only visible finding is a large pleural effusion. As the disease progresses, the thoracoscopic appearance evolves from tumor studding of the parietal pleura with a free pleural space and a large pleural effusion to studding of both parietal and visceral pleurae. The next stage involves larger but still discrete masses with multiloculated pleural effusions and, finally, a confluent irregular sheet of tumor with obliteration of the pleural space. The tumor ranges from soft, friable, and hypervascular to densely fibrotic, depending on the mixture of cell types. No clinical findings are pathognomonic of MPM.
When thoracoscopy is not technically feasible because the pleural space is obliterated by locally advanced tumor, the small incision made for thoracoscopy is extended to a length of 6 cm and is used for open pleural biopsy by resection of a short segment of the overlying rib. Because MPM has a notorious propensity to implant in the chest wall, the incision should be placed in line with a possible subsequent thoracotomy incision so that it can be excised at the time of the definitive operation. Exploratory thoracotomy should be avoided because it exposes patients who have metastatic adenocarcinoma to the unnecessary morbidity of a major operation and complicates definitive surgical resection in patients with MPM. Most important, pleural biopsy specimens should be submitted fresh to the pathologist so that they can be placed in the appropriate fixative for electron microscopy, in case this is required to achieve a definitive diagnosis.
No additional studies or procedures beyond thoracoscopy, CT scan of the chest and abdomen, and PET scan are routinely necessary to diagnose and stage patients with MPM. Bronchoscopy is performed only to exclude the possibility of a primary lung cancer with endobronchial tumor, and it is uniformly normal in patients with MPM, as noted by Lewis and co-workers (1981).
The role of mediastinoscopy in the management of MPM is still unclear. Schouwink and colleagues (2003) examined the usefulness of cervical mediastinoscopy in 43 patients. Of the 43 patients, only 24 went on to thoracotomy for pathologic confirmation; therefore, data were not available on the patients with potentially false-negative mediastinoscopy results. Of the 17 patients with enlarged nodes detected by CT scan, only 6 were confirmed to be positive by cervical mediastinoscopy, emphasizing the fact that lymph nodes that are enlarged on CT are not necessarily malignant. In addition, mediastinoscopy cannot diagnose lymph node metastases that occur frequently in MPM but are in anatomic locations inaccessible to this procedure, including the posterior mediastinal, internal mammary, and peridiaphragmatic regions. Although mediastinoscopy will clearly identify some patients with N2 disease, its role in staging MPM needs further study. Finally, while the presence of N2 disease is generally associated with a worse prognosis, it is not clear that all such patients should be denied surgical resection given current treatment options.
At MSKCC we conducted a study to determine the utility of laparoscopy in detecting transdiaphragmatic tumor extension when CT findings were equivocal. During a 1-year period, 12 of 36 patients considered for possible thoracotomy and surgical resection had equivocal CT findings of diaphragmatic invasion. All underwent laparoscopy with diaphragmatic and peritoneal biopsies. There were no perioperative complications and the median hospital stay was 1 day. Six patients had biopsy-proven transdiaphragmatic extension, or peritoneal studding of tumor. The other six patients subsequently underwent thoracotomy: three had a complete resection and three had unresectable tumor due to chest wall (n = 2) or mediastinal (n = 1) invasion. In no case was transdiaphragmatic extension of a tumor seen. This experience, as reported by Conlon and colleagues in 1996, demonstrated that laparoscopy is a safe and accurate method for detecting transdiaphragmatic tumor extension when CT fails to do so, and should be considered a standard part of prethoracotomy staging in this subset of patients.
Additional imaging studies, such as MR imaging or bone scans, are indicated only to evaluate specific symptoms (i.e., localized bone pain or laboratory abnormalities, such as an elevated alkaline phosphatase). Our preoperative staging and medical evaluation includes a CT scan of the chest and upper abdomen, a PET scan, a radionuclide stress test, pulmonary function studies (including a diffuse capacity), and a quantitative ventilation-perfusion lung scan. In general, these examinations suffice to determine the extent of disease (Table 65-4).
Table 65-4. Standard Initial Evaluation of Malignant Pleural Mesotheliomas at Memorial Sloan-Kettering Cancer Center | |
|---|---|
|
P.910
NATURAL HISTORY AND STAGING
The successful treatment of any cancer is based on an understanding of its natural history. However, our understanding of the natural history and prognostic factors in MPM is still limited. Until recently, there was not even an accurate, universally accepted staging system. The staging system used most frequently in the past was the one proposed by Butchart and colleagues (1976) (Table 65-5), which had imprecise descriptors for the primary tumor and for the lymph node involvement. A stage I tumor, for instance, could include patients who had minimal pleural studding, a free pleural space, and pleural effusion as well as patients who had a thick, confluent sheet of tumor with obliteration of the pleural space but without invasion of the mediastinum or opposite pleura. Yet clinical experience indicates that the latter is a more locally advanced tumor with a poorer prognosis. In addition, the exact sites, incidence, and prognostic implications of lymph node involvement are still controversial. The inclusion of lymph node involvement in the chest in stage II and lymph node involvement outside the chest in stage III is empiric. Allen and co-workers (1994) did not find any association between lymph node metastases and survival in 96 patients who underwent surgical resection. However, reports from Sugarbaker and associates (1993,1996, 1999) as well as from one of us (VWR) and Venkatraman (1996,1999) indicate that patients who have positive mediastinal nodes have a poorer prognosis.
Table 65-5. Staging Proposed by Butchart and Colleagues | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Dimitrov and McMahon (1987) described several other proposed staging systems, including a tumor-node-metastasis (TNM)-based system by Chahinian (1983). This system was more precise than the Butchart system but did not fully reflect the usual findings at thoracotomy. For instance, the T1 descriptor (involvement of the parietal and visceral pleural surfaces with sparing of the diaphragm) does not reflect what we now know is the pattern of disease progression: tumor studding of all parietal pleural surfaces followed by involvement of the visceral pleura. This pattern of disease progression has been well described by Boutin and colleagues (1993), based on findings at thoracoscopy and careful long-term follow-up of patients with MPM. The greatest tumor burden is almost always in the lower half of the hemithorax and on the diaphragm.
In an effort to improve and unify the staging system for MPM, the Union Internationale Contre le Cancer (UICC) (2002) proposed another TNM-based system. The T-status descriptors were more detailed than in previous systems and the descriptors for nodal involvement in this system were borrowed directly from the international staging system for non small cell lung cancer (NSCLC). However, this system was developed without clinicopathologic correlation.
Because of the lack of a universally accepted staging system, the International Mesothelioma Interest Group (IMIG) (composed of experts from around the world) met to develop an internationally accepted staging system that was based on the available data correlating clinical and pathologic extent of disease with outcome, as reported by one of us (VWR) and colleagues (1995). The IMIG staging system has become universally accepted and was recently adopted by the UICC and the AJCC in the sixth editions of their staging manuals (2002) (Table 65-6).
Another issue confounding the interpretation of the published literature is that most studies have not staged patients initially by CT scan but only by symptoms, physical examination, and chest radiographs. The inaccuracy of such a clinical assessment leads to a heterogeneous patient population that makes it hard to decipher the results of treatment for MPM. Many series also record outcome in small numbers of patients seen over long periods and treated in a highly individualized manner. Because of these factors, reported survival rates vary widely. Law and co-workers (1984) reported a median survival of 18 months for 64 patients treated with supportive care, with no differences in survival according to cell type. Twelve of the 64 patients survived longer than 5 years, but the diagnosis of MPM in this study was based on histology alone, and less than one third of all patients were staged by CT scan. Hulks and colleagues (1989) reported a median survival of 30 weeks for 68 patients treated with supportive care. They based their pathologic diagnosis on immunohistochemistry as well as histology. In addition, CT scanning was not performed and patients were classified principally according to their symptoms. Patients who presented with dyspnea lived significantly longer than did patients who presented with pain, for a median survival of 44 versus 22 weeks, probably reflecting
P.911
the extent of disease at diagnosis. Cell type did not appear to influence survival.
Table 65-6. AJCC/UICC Staging System | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Other researchers, notably Tammilehto (1992) as well as Ruffie (1989), Adams (1986), and Antman (1988) and their colleagues, have tried to identify prognostic factors in MPM. However, this has been done mainly in the setting of retrospective reviews of patients with different stages of disease, treated with widely varying regimens. Contrary to the data reported by Law (1982a) and Hulks (1989) and their associates, epithelioid histology has generally been a favorable prognostic factor. Absence of chest pain and good performance status are also thought to be favorable prognostic factors, but probably only reflect an early stage of the disease. Other factors, including female gender and age younger than 50 years, have incidentally been cited as favorable prognostic factors. In several series, thrombocytosis (defined as a platelet count of >400,000/mL), appears to have a negative impact on survival.
Prognostic factors are critical for patient stratification in clinical trials. For MPM, the prognostic scoring systems of the Cancer and Leukemia Group B (CALGB), as reported by Herndon and colleagues in 1998, and European Organization for Research and Treatment of Cancer (EORTC), as reported by Curran and co-workers (1998), are the most widely used. These systems rate (a) performance status, (b) age, (c) histologic subtype, (d) weight loss, and (e) hematologic parameters as the best prognostic factors for MPM. As biological markers are developed, additional information on MPM may help stratify patients, as suggested by Steele (2002).
Malignant pleural mesothelioma was long thought to be a tumor that remained localized to the chest, as described by Nauta and colleagues (1982). However, several autopsy series disprove this. Ruffie and coinvestigators (1989) found that 45 of 92 patients (49%) had distant metastases at autopsy. The liver was the most common site and the contralateral lung the second most common site of distant disease, but metastases were also found in sites as widely disseminated as the prostate, brain, and thyroid. Elmes and Simpson (1976) found distant metastases in 48 of 148 patients (33%) at autopsy. The metastases were widely disseminated, but the liver and the contralateral lung were once again the most common sites of disease. Similar findings have been reported by Roberts (1976) and by Whitwell and Rawcliffe (1971). The uncommon but definite occurrence of brain and spine metastases has been emphasized in several reports, notably those of Walters and Martinez (1975), as well as those of Kaye (1986) and Ruffie (1989) and their associates. Virtually all patients have advanced local or regional disease at death, and one of us (RMF) and coinvestigators (2003a) indicated that approximately 10% of patients with apparent early-stage disease may have metastasis as demonstrated by PET scan. However, as pointed out by Nauta and colleagues (1982), the symptoms related to locoregional tumor are usually the most difficult to palliate and therefore the most obvious clinically. Patients with MPM face a dual problem: control of the locoregional tumor throughout the course of their disease, as well as prevention of distant metastases as a late manifestation of their cancer.
P.912
TREATMENT
As for any other cancer, the treatment options for MPM include surgery, radiation therapy, chemotherapy, immunotherapy, supportive care, or some combination of these modalities. However, the choice of treatment is influenced by factors that do not apply to some other malignancies: the location and extent of the tumor, and the general medical condition of these patients who are usually older and have significant medical comorbidities. Unfortunately, the assessment of treatment regimens for MPM is hampered by a lack of large prospective clinical trials. Most patients have been treated in a highly individualized manner, and reported series are often small and retrospective.
Radiation Therapy
It is difficult to evaluate the success of radiation therapy as the only treatment because it is usually given in conjunction with surgical resection or chemotherapy. Radiation therapy as the sole treatment is generally used to palliate an area of symptomatic tumor in the chest wall or mediastinum.
According to Brady (1981), Ball and Cruickshank (1990), and Gordon and co-workers (1982), the use of radiation therapy is limited by the volume of the primary tumor that involves the entire hemithorax and by proximity of the tumor to many vital structures that are intolerant of high doses of radiation. For the most part, radiation doses to the affected hemithorax have been kept at 4,500 cGy or less to prevent toxicity to the heart, esophagus, lung, and spinal cord. Maasilta (1991) documented the severe pulmonary toxicity caused by higher-dose hemithoracic irradiation. The radiographic changes and the deterioration of pulmonary function and oxygenation that develop in the year after radiation therapy are compatible with a total loss of lung function on the irradiated side. Sinoff and associates (1982) have shown that the toxicity of irradiation may also be potentiated by the administration of chemotherapy, including drugs such as doxorubicin.
One way to circumvent these problems is to administer radiation therapy as adjuvant treatment after surgical resection of gross tumor. A variety of techniques can be used to minimize the radiation dose to the lung. The largest and most consistent experience with this approach was reported by Hilaris (1984) and Kutcher (1987) and their colleagues at MSKCC. After subtotal resection of gross tumor by pleurectomy-decortication, any residual tumor was implanted intraoperatively with iodine 125 or iridium 192 implants. Patients then received external-beam irradiation to the entire hemithorax using a mixed photon-electron beam technique to a total dose of 4,500 cGy, attempting to spare the underlying lung. In an updated report by Mychalczak and co-workers (1989), 105 patients treated in this manner from 1976 to 1988 at MSKCC had a median survival of 12.6 months, with 1- and 2-year actuarial survival rates of 52% and 23%, respectively. However, the 27 patients who had pure epithelioid histology and minimal gross residual disease requiring only external-beam irradiation without brachytherapy had a median survival of 15 months, and 1- and 2-year survival rates of 68% and 35%, respectively. There were 19 complications, including 12 cases of radiation pneumonitis and 8 patients with pericarditis and tamponade. The most common site of relapse was local and ipsilateral recurrent pleural tumor, seen in 64 of the 105 patients (63%). Both this experience and some experimental work by Soubra and associates (1990) indicate that a low-dose mixed photon-electron beam, although theoretically attractive, does not spare the pulmonary parenchyma, does not provide long-term local control for most patients, and after pleurectomy/decortication is associated with an unacceptable risk for complications.
External-beam radiation therapy has also been administered after extrapleural pneumonectomy. At the Dana Farber Cancer Institute and Joint Center for Radiation Therapy, Baldini and associates (1997) gave adjuvant cyclophosphamide, adriamycin (doxorubicin), and cisplatin (CAP) chemotherapy and hemithoracic irradiation to 49 patients who had undergone extrapleural pneumonectomy. After a median radiation dose of 30.6 Gy (range 20 to 41.4 Gy), 16 patients (35%) developed a local recurrence. This high rate of recurrent disease in the ipsilateral hemithorax emphasizes that low- to moderate-dose radiation therapy does not provide adequate local control. The use of high-dose (54-Gy) hemithoracic irradiation as adjuvant therapy after extrapleural pneumonectomy was evaluated in a prospective trial at MSKCC and is discussed in the combined modality treatment section.
The successful use of fast neutron therapy to control local bulky disease has been described in a case report by Blake and co-workers (1985), but this has not yet been confirmed in larger series. Small-series studies have been reported on the intrapleural use of radioactive colloidal compounds, including radioactive gold (198Au) and chromic phosphate (32P), but as described by Brady (1981), these seem ineffective in treating any substantial tumor bulk in the pleural cavity.
The most successful use of irradiation is as an adjuvant therapy to prevent tumor implantation in the chest wall after thoracoscopy. In a small randomized study, Boutin and associates (1995) reported that 8 of 20 patients (40%) who did not receive radiation therapy to the chest wall after thoracoscopy developed tumor implantation compared with none of the 20 patients who did receive radiation. Because the radiation regimen was a short course (700 cGy daily for 3 days), this treatment is worth considering in patients for whom no further surgical intervention is planned. Low and colleagues (1995) confirmed the experience, using this radiation regimen in 20 patients. They found no evidence of local recurrence in irradiated sites.
Overall, it is generally agreed that hemithoracic radiation therapy is not a feasible primary treatment for MPM because the doses of radiation therapy that might be effective in controlling the tumor are not tolerated by the underlying lung or surrounding mediastinal structures. In addition, it is unclear whether radiation therapy even palliates the pain
P.913
caused by locally advanced tumor involving the chest wall. Radiation therapy may be effective in adjuvant treatment, particularly after extrapleural pneumonectomy, when it becomes possible to deliver higher-dose irradiation to the hemithorax. Adjuvant short-course radiation therapy to the chest wall after thoracoscopy seems to prevent the development of chest wall tumor implants after thoracoscopy.
Chemotherapy
Numerous phase II studies of chemotherapeutic agents have been performed in MPM, testing virtually all the currently available drugs. These have been well summarized by Antman and associates (1988) and more recently reviewed in detail by Krarup-Hansen and Hansen (1991), Ong and Vogelzang (1996), and Tomek and colleagues (2003). Response rates as high as 30% to 40% have been reported for either single or multiple agents in small single-institution studies, but in pooled data from multiple studies, response rates are generally in the 20% range. The results of these studies are influenced by the inclusion of patients with varying stages of disease and different MPM cell types, as well as by the lack of use of CT scanning to assess response. Dimitrov (1982), Chahinian (1978), Dabouis (1981), Raghavan (1990), and Umsawasdi (1991) and their associates have shown that active agents include doxorubicin, detorubicin, ifosfamide, cisplatin, carboplatin, mitomycin, methotrexate, edatrexate, 5-azacytidine, and 5-fluorouracil. In the past, combination treatment has not shown clear superiority to a single agent (Table 65-7). However, the response rates for newer chemotherapy drugs in MPM are more encouraging (Table 65-8).
Table 65-7. Single-Agent Chemotherapy Since 1995 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 65-8. Combination Chemotherapy Since 1995 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.914
Byrne and colleagues (1999) conducted a phase II study of combined cisplatin and gemcitabine for patients with advanced measurable MPM. Of the 21 patients treated, 62% had epithelioid tumors and 18 were classified as AJCC stage III or IV. There was a 47.6% [95% confidence interval (CI) 26.2 to 69.0%] response rate with symptom improvement in responding patients. Toxicity was mainly gastroenterologic and hematologic. This study has demonstrated the best response rate to date.
These findings were confirmed in a multicenter study by Nowak and colleagues (2002), where 53 patients with MPM received the cisplatin and gemcitabine regimen. Quality of life and pulmonary function were assessed at each cycle. The best response achieved in 52 assessable patients was partial response, 17 (33%, 95% CI 20 to 46%); stable disease, 31 (60%); and progressive disease, 4 (8%). The median time to disease progression was 6.4 months, median survival from start of treatment was 11.2 months, and median survival from diagnosis was 17.3 months. Vital capacity and global quality of life remained stable in all patients and improved significantly in responding patients.
The results of a phase III international trial that compared cisplatin to cisplatin and pemetrexed (a new multitargeted antifolate) and closed in February 2002 were recently published by Vogelzang and colleagues (2003). During a 2-year period, 448 patients were randomized. The primary end point was overall survival. The study was designed with an 80% power to detect a hazard ratio of 0.67. Overall survival was significantly better in the pemetrexed/cisplatin arm, 12.1 versus 9.3 months, and the overall response rate was 41% versus 17% for the cisplatin-only arm of the trial. An unexpected finding was that folic acid and vitamin B12 supplements improved drug tolerance, thus leading to higher response rates. Pulmonary function tests and quality of life were also better in the cisplatin pemetrexed arm. Additional large randomized phase III chemotherapy trials are in progress, and these are outlined in Table 65-9.
Immunotherapy
Interferons are known to have a direct antiproliferative effect on MPM cell lines. Studies by Sklarin and co-workers (1988) on MPM xenografts in nude mice have shown the efficacy of recombinant human interferon- 2a combined with mitomycin C. These experimental data prompted the development of clinical trials using interferon, either alone or in combination with chemotherapy. However, these trials have not demonstrated response rates that are superior to single-agent cisplatin alone.
The use of interferon- as an intrapleural treatment in patients with early-stage disease has recently been reported by Boutin and associates (1991b). Twenty-two patients were treated with a solution of interferon- (40 106 U) infused into the pleural space twice weekly for 2 months. Response
P.915
was assessed by serial CT scans and repeat thoracoscopy and a 56% overall response rate was observed. These promising initial results will undoubtedly stimulate additional clinical trials. To be effective, however, intrapleural immunotherapy requires a free pleural space and therefore can be administered only to patients with early-stage disease who have a free-flowing effusion and minimal tumor involving the pleural surfaces.
Table 65-9. Randomized Phase II III Studies in Patients with Malignant Pleural Mesothelioma Treated with Chemotherapy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Goey and colleagues (1995) studied intrapleural interleukin 2 (IL-2) in a phase I trial in patients with stage I or II MPM. IL-2 was administered as a continuous infusion using a dose-escalation schedule ranging from 3 104 to 36 106 IU daily. A partial response was seen in 4 of 21 evaluable patients. There were no complete responses, and the median overall survival was 15.6 months.
Surgery
Because of the limitations of radiation therapy and chemotherapy, surgical resection is still a mainstay of treatment for MPM. Three operations have been performed: extrapleural pneumonectomy (also termed pleuropneumonectomy), pleurectomy-decortication, and a palliative limited pleurectomy.
Extrapleural pneumonectomy is an en bloc resection of the pleura, lung, ipsilateral hemidiaphragm, and pericardium. As described by one of us (VWR) and coinvestigators (1994), pleurectomy-decortication is an attempt to remove all gross pleural disease without removing the underlying lung. The hemidiaphragm and pericardium are also removed and reconstructed if necessary. A palliative pleurectomy involves limited resection of the parietal pleura to control a pleural effusion by creating a durable pleurodesis. The details of the surgical technique for these operations are described in other chapters and are not reviewed here (see Chapters 61 and 66).
Another operation performed for strictly palliative purposes is thoracoscopy and talc poudrage. As reported by Ruffie (1989) and Boutin (1991a) and their colleagues, this is highly effective in controlling effusions and provides excellent palliation for patients whose general medical condition precludes more extensive treatment.
Role of Surgical Resection
Complete resection of all gross tumor seems to convey a modest but definite improvement in survival in several large series. However, the value of extrapleural pneumonectomy relative to pleurectomy-decortication remains controversial. Extrapleural pneumonectomy has the aesthetic appeal of removing the tumor en bloc, but either operation, if performed well in properly selected patients, allows the removal of all gross tumor. Conversely, resection of the tumor with wide, microscopically negative margins (as can be achieved with lung, breast, or colon cancer) is simply not feasible in MPM because the margins of resection are vital structures, such as the aorta, cavae, and esophagus.
In an initial report by Butchart and associates (1976), extrapleural pneumonectomy carried an operative mortality rate of 30%. More recent data show a substantial reduction in this mortality rate, probably reflecting better patient selection and improved perioperative care (Table 65-10). Preoperative CT scanning, careful pulmonary function testing, ventilation-perfusion lung scanning, and improved methods of evaluating cardiac function noninvasively now allow selection of patients who have completely resectable tumors and have the cardiopulmonary reserve to tolerate
P.916
the operation safely (see Table 65-4). Intraoperative monitoring and anesthetic management are much better now than they were in the past. In a prospective multiinstitutional study reported by one of us (VWR) and coinvestigators (1991), the operative mortality rate in the early 1990s was 15%. However, as reported by DeLaria (1978), Sugarbaker (1991, 1996,1999), DeValle (1986), and Allen (1994) and their associates, as well as by one of us (VWR) and Venkatraman (1996, 1999), mortality rates as low as 5% have been achieved in single-institution retrospective studies in which patients have been carefully selected and operated on by experienced surgeons.
Table 65-10. Mortality of Extrapleural Pneumonectomy | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
By contrast, McCormack and associates (1982) at MSKCC reported an operative mortality rate of 1.8% for pleurectomy-decortication. These results are similar to what is seen with pulmonary resections for lung cancer, namely, that operative mortality rate is directly linked to the extent of resection and is 5% to 10% for a standard pneumonectomy. Both pleurectomy-decortication and extrapleural pneumonectomy are technically complex and are not frequently performed by most surgeons. Therefore, patients may benefit by referral to centers dedicated to the treatment of MPM.
Controversy exists with regard to surgical treatment centers on the relative value of an extrapleural pneumonectomy versus a pleurectomy-decortication: Is the higher operative mortality of extrapleural pneumonectomy justified by a better overall survival? One problem is that extrapleural pneumonectomy cannot be performed in some patients because they have underlying cardiopulmonary disease that does not permit resection of an entire lung. However, pneumonectomy facilitates some forms of adjuvant treatment, especially postoperative irradiation, which can be administered to a much higher total dose after pneumonectomy than after pleurectomy-decortication.
In addition, some patients do not have a tumor that is technically resectable by pleurectomy-decortication. A confluent sheet of tumor encasing the lung with obliteration of the pleural space is resectable only by extrapleural pneumonectomy. This situation can usually be identified by videothoracoscopy and the preoperative CT scan (see Figs. 65-7 and 65-8). It is not known whether a patient who has early-stage disease that is technically completely resectable by either extrapleural pneumonectomy or by pleurectomy-decortication is better served by one operation versus the other. Ultimately, the long-term outcome of such a patient after complete resection of all gross tumor may be determined not by the operation but by the type and effectiveness of the adjuvant treatment.
Combined Modality Treatment
Both extrapleural pneumonectomy and pleurectomy-decortication permit only the removal of gross tumor without wide surgical margins. One of us (VWR) and coinvestigators (1991) have shown that patients treated with surgical resection alone relapse rapidly. Therefore, most treatment regimens have focused on multimodality treatment. It is difficult, however, to evaluate the results of combined modality treatment because most series, including those reported by Achatzy (1989), Chahinian (1982), and Alberts (1988) and their associates, report small numbers of patients treated in a highly individualized manner over long periods. In addition to the MSKCC experience with pleurectomy-decortication and radiation therapy described previously, another large and relatively uniform experience with combined modality treatment has been reported by the Dana Farber Cancer Center. From 1980 to 1997, Sugarbaker and colleagues (1999) performed an extrapleural pneumonectomy in 183 patients, followed by CAP chemotherapy or carboplatin and Taxol and subsequent hemithoracic irradiation (3,000 rad). The overall survival rates were 38% at 2 years and 15% at 5 years. The 2- and 5-year survival rates were 52% and 21%, respectively, for patients with epithelioid cell type and 16% and 0% for patients with sarcomatous or mixed histology tumors. Nodal metastases were a significant adverse prognostic factor. It is difficult to assess how much the irradiation or chemotherapy each contribute to these survival rates because these have not been tested individually as adjuvant treatment. The major problem with this modality, as demonstrated by Baldini and co-workers (1997) was a 35% local recurrence rate.
One of us (VWR) and associates (2001) performed a phase II trial of high-dose hemithoracic radiation after complete resection to determine feasibility and to estimate rates of local recurrence and survival. After complete resection, patients received hemithoracic radiation (54 Gy) and then were followed up with serial CT scanning. Over a 3-year period, 88 patients were entered into the study. The operations performed included 62 extrapleural pneumonectomies (70%) and 5 pleurectomies/decortications; procedures for exploration only were performed in 21 patients. Adjuvant radiation, administered to 57 patients (54 undergoing extrapleural pneumonectomy and 3 undergoing pleurectomy/decortication) at a median dose of 54 Gy, was well tolerated (grade 0 to 2 fatigue, esophagitis), except for one late esophageal fistula. The median survival was 33.8 months for stage I and II tumors but only 10 months for
P.917
stage III and IV tumors (p = 0.04). For the patients undergoing extrapleural pneumonectomy, the sites of recurrence were locoregional in 2, locoregional and distant in 5, and distant only in 30. The aforementioned study in 2001 demonstrated the feasibility at a dose not previously reported and a dramatic reduction in local recurrence rate to approximately 10%. Since our major area of failure was distant, we have now focused our research efforts on the treatment of systemic disease. We are currently conducting a phase II trial of neoadjuvant gemcitabine and cisplatin followed by extrapleural pneumonectomy and adjuvant high-dose radiotherapy (54 Gy) for patients who have bulky stage II or stage III disease at diagnosis.
Several novel treatment strategies have also been investigated. Photodynamic therapy (PDT) seeks to improve local control by eliminating microscopic residual disease immediately after surgical resection. This approach is based on experimental data with MPM cell lines as reported by Keller and co-workers (1990), as well as a previous small clinical trial reported by Ris and colleagues (1991), which suggested the feasibility of this approach. Pass and associates (1994) reported that 42 patients received PDT after maximal surgical tumor debulking using escalating light doses of 15 to 35 J/cm2. There was one death and three serious complications. Overall, this trial demonstrated the feasibility and safety of combined surgical resection and PDT.
Therefore, Pass and associates (1997) conducted a phase III trial comparing maximum debulking surgery and postoperative cisplatin, interferon- 2b, and tamoxifen (CIT) immunochemotherapy with and without intraoperative PDT to determine the impact on local recurrence and survival. Sixty-three patients with localized MPM were randomized to either PDT or no PDT over a 3-year period. The tumors of 15 patients could not be debulked. Patients assigned to PDT (n = 25) and no PDT (n = 23) were similar with respect to age, sex, tumor volume, and histology. The type of resection included 11 pleurectomies and 14 pneumonectomies for the PDT group versus 12 pleurectomies and 11 pneumonectomies for the group without PDT. There was one operative death (hemorrhage), and each group had two bronchopleural fistulae. In addition, postoperative staging was equivalent, and comparable numbers of CIT cycles were delivered. Median survival for the 15 nondebulked patients was 7.2 months, compared with 14 months for the 48 patients on protocol. There were no major differences in median survival (14.4 vs. 14.1 months) or median progression-free time (8.5 vs. 7.7 months), and sites of first recurrence were similar. These results demonstrate that PDT does not prolong survival or increase local control for MPM.
One of us (VWR) and colleagues (1994) tested another approach in a phase II trial at MSKCC. Patients received a single dose of intrapleural cisplatin (75 mg/m2) and mitomycin (8 mg/m2) after complete resection of all gross tumor by pleurectomy-decortication. Additional chemotherapy was administered systemically starting 1 month postoperatively using two cycles of cisplatin 50 mg/m2 per week and mitomycin 8 mg/m2. This approach of surgical resection and short, intensive chemotherapy tried to address the problems of both local control and potential distant metastases. It was based on the established use of intraperitoneal chemotherapy in ovarian cancer and on a smaller but successful experience with intracavitary chemotherapy in both pleural and peritoneal MPM, reported by Lederman (1987), Markman (1986), and Mintzer (1985) and their associates.
A total of 23 patients received the entire treatment of pleurectomy-decortication and adjuvant chemotherapy. Although the treatment was tolerable, it was associated with a high risk for local recurrence (16 of 20 patients). The overall survival was favorable (median survival of 18 months, 40% at 2 years) but the high rate of local relapse after intensive chemotherapy was believed not to warrant further trials of incorporating this approach into treatment.
More recently, Sterman and colleagues (1998) explored the feasibility of treating early-stage MPM with intrapleural gene therapy. Using human MPM tumors growing in the peritoneal cavities of severe combined immunodeficient mice, Hwang and colleagues (1995) reported that the herpes simplex virus thymidine kinase (HSVtk) gene could be successfully transferred to tumor via an adenovirus. Administration of the antiviral drug ganciclovir then led to selective tumor cell death. This treatment approach is currently being evaluated in phase I and II human clinical trials.
SUMMARY
Malignant pleural mesothelioma is an uncommon and generally fatal malignancy. However, during the past decade considerable insight has been gained into the biology and clinical behavior of MPM. Pathologic diagnosis of MPM has become easier, staging methods have improved, and treatment options have increased. As a result, some patients experience longer survival than was the case 10 to 20 years ago. Continuing investigation into the biology and treatment of MPM is vital to improving the prognosis of this difficult neoplasm.
REFERENCES
Achatzy R, et al: The diagnosis, therapy and prognosis of diffuse malignant mesothelioma. Eur J Cardiothorac Surg 3:445, 1989.
Adams VI, et al: Diffuse malignant mesothelioma of pleura. Diagnosis and survival in 92 cases. Cancer 58:1540, 1986.
Alberts AS, et al: Malignant pleural mesothelioma: a disease unaffected by current therapeutic maneuvers. J Clin Oncol 6:527, 1988.
Alexander E, et al: CT of malignant pleural mesothelioma. AJR 137:287, 1981.
Allen KB, Faber LP, Warren WH: Malignant pleural mesothelioma. Extrapleural pneumonectomy and pleurectomy. Chest Surg Clin North Am 4:113, 1994.
American Joint Committee on Cancer: AJCC Cancer Staging Handbook. New York: Springer-Verlag, 2002.
P.918
Andersen MK, et al: Ifosfamide in malignant mesothelioma: a phase II study. Lung Cancer 24:39, 1999.
Anderson KA, et al: Malignant pleural mesothelioma following radiotherapy in a 16-year-old boy. Cancer 56:273, 1985.
Andersson M, Olsen JH: Trend and distribution of mesothelioma in Denmark. Br J Cancer 51:699, 1985.
Antman KH, et al: Malignant mesothelioma following radiation exposure. J Clin Oncol 1:695, 1983.
Antman KH, et al: Mesothelioma following Wilms' tumor in childhood. Cancer 54:367, 1984.
Antman KH, et al: Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965 1985. J Clin Oncol 6:147, 1988.
Auribault M: Bulletin de l'Inspection du Travail 126, 1906.
Aversa SL, et al: Carboplatin and gemcitabine chemotherapy for malignant pleural mesothelioma (MPM): a phase II study of the GSTPV. Ann Oncol 9:117, 1998.
Baas P, et al: Caelyx in malignant mesothelioma: a phase II EORTC study. Ann Oncol 11:697, 2000.
Baldini EH, et al: Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 63:334, 1997.
Ball DL, Cruickshank DG: The treatment of malignant mesothelioma of the pleura: review of a 5-year experience, with special reference to radiotherapy. Am J Clin Oncol 13:4, 1990.
Bamler KJ, Maassen W: Uber die Verteilung der benignen und malignen Pleuraturmoren im Krankengut einer lungenchirurgischen Klinik mit besonderer Berucksichtigung des malignen Pleuramesothelioms und seiner radikalen Behandlung einschliesslich der Ergebnisse des Zwerchfellersatzes mit konservieter Dura mater. Thoraxchir Vask Chir 22:386, 1974.
Baris YI: Asbestos and Erionite Related Chest Diseases. Ankara, Turkey: Semik Ofset Matbaacilik, 1987.
Battifora H, Kopinski MI: Distinction of mesothelioma from adenocarcinoma. An immunohistochemical approach. Cancer 55:1679, 1985.
Belani CP, et al: Docetaxel for malignant mesothelioma: phase II study of the Eastern Oncology Group (ECOG 2595) [Abstract 1829]. Proc Am Soc Clin Oncol 18:474, 1999.
Bell DW, Jhanwar SC, Testa JR: Multiple regions of allelic loss from chromosome arm 6q in malignant mesothelioma. Cancer Res 57:4057, 1997.
B nard F, et al: Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest 114:713, 1998.
Bianchi AB, et al: High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A 92:10854, 1995.
Bischoff HG, et al: Gemcitabine (Gemzar) may reduce tumor load and tumor associated symptoms in malignant pleural mesothelioma: phase II study of the Eastern Cooperative Oncology Group. Proc Ann Meet Am Soc Clin Oncol 17:A1784, 1998.
Bj rkqvist A-M, et al: Recurrent DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q and 22q detected by comparative genomic hybridization in malignant mesothelioma. Br J Cancer 75:523, 1997.
Blake PR, Catterall M, Emerson PA: Pleural mesothelioma treated by fast neutron therapy. Thorax 40:72, 1985.
Boutin C, Rey F: Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1: Diagnosis. Cancer 72:389, 1993.
Boutin C, Rey F, Viallat JR: Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 108:754, 1995.
Boutin C, Viallat JR, Aelony Y: Practical Thoracoscopy. Berlin: Springer-Verlag, 1991a.
Boutin C, et al: Activity of intrapleural recombinant gamma-interferon in malignant mesothelioma. Cancer 67:2033, 1991b.
Boutin C, et al: Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 2: Prognosis and staging. Cancer 72:394, 1993.
Brady LW: Mesothelioma the role for radiation therapy. Semin Oncol 8:329, 1981.
Breau JL, et al: Combination therapy with cisplatinum, adriamycin, bleomycin and mitomycin C plus systemic and intra-pleural hyaluronidase in 25 consecutive cases of stage II, II pleural mesothelioma. Presented at the First International Mesothelioma Conference, Paris, France, 1991.
Burns TR, et al: Ultrastructural diagnosis of epithelial malignant mesothelioma. Cancer 56:2036, 1985.
Butchart EG, et al: Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 31:15, 1976.
Byrne MJ, et al: Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol 17:25, 1999.
Cantin R, Al-Jabi M, McCaughey WT: Desmoplastic diffuse mesothelioma. Am J Surg Pathol 6:215, 1982.
Carbone M, Kratzke RA, Testa JR: The pathogenesis of mesothelioma. Semin Oncol 29:2, 2002.
Cartier P, Smith WE: Survey of some current British and European studies of occupational tumor problems. Arch Indust Hygiene Occup Med 5:242, 1952.
Chahinian AP: Therapeutic modalities in malignant pleural mesothelioma. In Chretien J, Hirsch A (eds): Diseases of the Pleura. New York: Masson, 1983, p. 224.
Chahinian AP, et al: Diffuse pulmonary malignant mesothelioma. Response to doxorubicin and 5-azacytidine. Cancer 42:1687, 1978.
Chahinian AP, et al: Diffuse malignant mesothelioma. Prospective evaluation of 69 patients. Ann Intern Med 96:746, 1982.
Chahinian AP, et al: Randomised phase II trial of cisplatin with mitomycin or doxorubicin for malignant mesothelioma by the Cancer and Leukemia Group. J Clin Oncol 11:1559, 1993.
Cheng JQ, et al: p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res 54:5547, 1994.
Churg A, DePaoli L: Environmental pleural plaques in residents of a Quebec chrysotile mining town. Chest 94:58, 1988.
Conlon KC, Rusch VW, Gillern S: Laparoscopy: an important tool in the staging of malignant pleural mesothelioma. Ann Surg Oncol 3:489, 1996.
Cowan JD, et al: Phase II trial of five day intravenous infusion vinblastine sulfate in patients with diffuse malignant mesothelioma: a Southwest Oncology Group Study. Invest New Drugs 6:247, 1988.
Curran D, et al: Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer Experience. J Clin Oncol 16:145, 1998.
Dabouis G, Le Mevel B, Corroller J: Treatment of diffuse pleural malignant mesothelioma by cis dichloro diammine platinum (C.D.D.P.) in nine patients. Cancer Chemother Pharmacol 5:209, 1981.
Dahl IMS, et al: A longitudinal study of the hyaluronan level in the serum of patients with malignant mesothelioma under treatment. Hyaluronan as an indicator of progressive disease. Cancer 64:68, 1989.
de Klerk NH, Armstrong BK: The epidemiology of asbestos and mesothelioma. In Henderson DW et al (eds): Malignant Mesothelioma. New York: Hemisphere, 1992, p. 223.
DeLaria GA, et al: Surgical management of malignant mesothelioma. Ann Thorac Surg 26:375, 1978.
DeValle MJ, et al: Extrapleural pneumonectomy for diffuse, malignant mesothelioma. Ann Thorac Surg 42:612, 1986.
Dimitrov NV, McMahon S: Presentation, diagnostic methods, staging, and natural history of malignant mesothelioma. In Antman K, Aisner J (eds): Asbestos-Related Malignancy. Orlando: Grune & Stratton, 1987, p. 225.
Dimitrov NV, et al: High-dose methotrexate with citrovorum factor and vincristine in the treatment of malignant mesothelioma. Cancer 50: 1245, 1982.
Donington JS, Mew DJ, Pass HI: Malignant pleural mesothelioma: newer aspects of carcinogenesis, molecular genetics, and prospects for future therapies. Surg Oncol 4:175, 1995.
Eisenhauer EA, et al: A phase II study of VP-16 and cisplatin in patients with unresectable malignant mesothelioma. An NCI Canada Clinical Trials Group Study. Invest New Drugs 6:327, 1988.
Elmes PC, Simpson MJC: The clinical aspects of mesothelioma. Q J Med 45:427, 1976.
Faber LP: Extrapleural pneumonectomy for diffuse, malignant mesothelioma. Ann Thorac Surg 58:1782, 1994.
Fizazi K, et al: Combination raltitrexed (Tomudex)-oxaliplatin: a step forward in the struggle against mesothelioma? The Institut Gustave Roussy experience with chemotherapy and chemoimmunotherapy in mesothelioma. Eur J Cancer 36:1514, 2000.
Flores RM, et al: FDG-PET predicts survival in patients with malignant pleural mesothelioma [Abstract]. Proc ASCO 22:620, 2003a.
Flores RM, et al: Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 126:11, 2003b.
P.919
Fraire AE, et al: Mesothelioma of childhood. Cancer 62:838, 1988.
Gerwin BI, et al: Comparison of production of transforming growth factor- and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res 47:6180, 1987.
Giaccone G, et al: Phase II trial of ZD473 in patients with mesothelioma relapsing after one prior chemotherapy regimen [Abstract A2781]. Proc Ann Mtg Am Soc Clin Oncol 20:257b, 2001.
Goey SH, et al: Intrapleural administration of interleukin 2 in pleural mesothelioma: a phase I-II study. Br J Cancer 72:1283, 1995.
Gordon W Jr, et al: Radiation therapy in the management of patients with mesothelioma. Int J Radiat Oncol Biol Phys 8:19, 1982.
Gotfried MH, Quan SF, Sobonya RE: Diffuse epithelial pleural mesothelioma presenting as a solitary lung mass. Chest 84:99, 1983.
Hammar SP, Bolen JW: Pleural neoplasms. In Dail DH, Hammar SP (eds): Pulmonary Pathology. New York: Springer-Verlag, 1988, p. 973.
Heelan RT, et al: Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. Am J Radiol 172:1039, 1999.
Herndon JE II, et al: Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 113:723, 1998.
Hilaris BS, et al: Pleurectomy and intraoperative brachytherapy and postoperative radiation in the treatment of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 10:325, 1984.
Hillerdal G: Malignant mesothelioma 1982: review of 4710 published cases. Br J Dis Chest 77:321, 1983.
Hirvonen A, et al: Simian virus 40 (SV40)-like DNA sequences not detectable in Finnish mesothelioma patients not exposed to SV40-contaminated polio vaccines. Mol Carcinog 26:93, 1999.
Hughes A, et al: Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J Clin Oncol 20:3533, 2002.
Hulks G, Thomas JS, Waclawski E: Malignant pleural mesothelioma in western Glasgow 1980 6. Thorax 44:496, 1989.
Huncharek M: Changing risk groups for malignant mesothelioma. Cancer 69:2704, 1992.
Hwang HC, et al: Gene therapy using adenovirus carrying the herpes simplex-thymidine kinase gene to treat in vivo models of human malignant mesothelioma and lung cancer. Am J Respir Cell Mol Biol 13:7, 1995.
Kane MJ, Chahinian AP, Holland JF: Malignant mesothelioma in young adults. Cancer 65:1449, 1990.
Kaye JA, et al: Malignant mesothelioma with brain metastases. Am J Med 80:95, 1986.
Keller SM, Taylor DD, Weese JL: In vitro killing of human malignant mesothelioma by photodynamic therapy. J Surg Res 48:337, 1990.
Kindler HL, et al: Edatrexate (10-ethyl-deaza-aminopterin) (NSC #626715) with or without leucovorin rescue for malignant mesothelioma. Sequential phase II trials by the cancer and leukemia group B. Cancer 86:1985, 1999.
Kindler HL, et al: CPT-11 in malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B (CALGB 9733). Proc Ann Meet Am Soc Clin Oncol 27:A1978, 2000.
Kindler HL, et al: Gemcitabine for malignant mesothelioma. A phase II trial by the Cancer and Leukemia Group B. Lung Cancer 31:311, 2001.
Klein G, Powers A, Croce C: Association of SV40 with human tumors. Oncogene 21:1141, 2002.
Klemperer P, Rabin CB: Primary neoplasms of the pleura. A report of five cases. Arch Pathol 11:385, 1937.
Knuuttila A, et al: Docetaxel and irinotecan (CPT-11) in the treatment of malignant pleural mesothelioma a feasibility study. Anticancer Drugs 11:257, 2000.
Kobzik L: The lung. In Cotran RS, Kumar V, Collins T (eds): Robbins Pathologic Basis of Disease. 6th Ed. Philadelphia: WB Saunders, 1999, p. 753.
Krarup-Hansen A, Hansen HH: Chemotherapy in malignant mesothelioma: a review. Cancer Chemother Pharmacol 28:319, 1991.
Kumar-Singh S, et al: WT1 mutation in malignant mesothelioma and WT1 immunoreactivity in relation to p53 and growth factor receptor expression, cell type transition, and prognosis. J Pathol 181:67, 1997.
Kutcher GJ, et al: Technique for external beam treatment for mesothelioma. Int J Radiat Oncol Biol Phys 13:1747, 1987.
Law MR, Hodson ME, Heard BE: Malignant mesothelioma of the pleura: relation between histological type and clinical behaviour. Thorax 37: 810, 1982a.
Law MR, et al: Computed tomography in the assessment of malignant mesothelioma of the pleura. Clin Radiol 33:67, 1982b.
Law MR, et al: Malignant mesothelioma of the pleura: a study of 52 treated and 64 untreated patients. Thorax 39:255, 1984.
Layman L: The blue asbestos industry at Wittenoom in Western Australia: a short history. In Henderson DW, et al (eds): Malignant Mesothelioma. New York: Hemisphere, 1992, p. 305.
Lederman GS, et al: Long-term survival in peritoneal mesothelioma. The role of radiotherapy and combined modality treatment. Cancer 59:1882, 1987.
Lee W-C, et al: Loss of heterozygosity analysis defines a critical region in chromosome 1p22 commonly deleted in human malignant mesothelioma. Cancer Res 56:4297, 1996.
Lerman Y, et al: Radiation associated malignant pleural mesothelioma. Thorax 46:463, 1991.
Levine RL: Asbestos: An Information Resource (NIH Publication No. 81 1681). Bethesda, MD: National Institutes of Health, 1981.
Lewis RJ, Sisler lGE, Mackenzie JW: Diffuse, mixed malignant pleural mesothelioma. Ann Thorac Surg 31:53, 1981.
Low EM, et al: Prevention of tumour seeding following thoracoscopy in mesothelioma by prophylactic radiotherapy. Clin Oncol 7:317, 1995.
Maasilta P: Deterioration in lung function following hemithorax irradiation for pleural mesothelioma. Int J Radiat Oncol Biol Phys 20:433, 1991.
Maksymiuk AW, et al: Phase II trial of topotecan for the treatment of mesothelioma. Am J Clin Oncol 21:610, 1998.
Malker HSR, et al: Occupational risks for pleural mesothelioma in Sweden, 1961 79. J Natl Cancer Inst 74:61, 1985.
Markman M, et al: Cisplatin administered by the intracavitary route as treatment for malignant mesothelioma. Cancer 58:18, 1986.
Martensson G, Sorenson S: A phase II study of vincristine in malignant mesothelioma a negative report. Cancer Chemother Pharmacol 24: 133, 1989.
McCormack PM, et al: Surgical treatment of pleural mesothelioma. J Thorac Cardiovasc Surg 84:834, 1982.
McDonald AD, McDonald JC: Malignant mesothelioma in North America. Cancer 46:1650, 1980.
McDonald JC, et al: Mesothelioma and asbestos fiber type. Evidence from lung tissue analyses. Cancer 63:1544, 1989.
Mezger J, Lamerz R, Permanetter W: Diagnostic significance of carcinoembryonic antigen in the differential diagnosis of malignant mesothelioma. J Thorac Cardiovasc Surg 100:860, 1990.
Middleton GW, et al: Good symptom relief with palliative MVP (mitomycin-C, vinblastine and cisplatin) chemotherapy in malignant mesothelioma. Ann Oncol 9:269, 1998.
Mintzer DM, et al: Phase II trial of high-dose cisplatin in patients with malignant mesothelioma. Cancer Treat Rep 69:711, 1985.
Mirvis S, et al: CT of malignant pleural mesothelioma. AJR 140:655, 1983.
Murray M: Report of the Departmental Committee on Compensation for Industrial Diseases. London: HMSO, 1907.
Musk AW, et al: The incidence of malignant mesothelioma in Australia, 1947 1980. Med J Aust 150:242, 1989.
Mychalczak BR, et al: Results of treatment of malignant pleural mesothelioma with surgery, brachytherapy, and external beam irradiation [Abstract]. Endocurie Hypertherm Oncol 5:245, 1989.
Nakano T, et al: Cisplatin in combination with irinotecan in the treatment of patients with malignant pleural mesothelioma: a pilot phase II clinical trial and pharmacokinetic profile. Cancer 85:2375, 1999.
Nauta RJ, et al: Clinical staging and the tendency of malignant pleural mesotheliomas to remain localized. Ann Thorac Surg 34:66, 1982.
Nowak AK, et al: A mutlicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer 87:491, 2002.
Oh Y, et al: Phase II study of intravenous Doxil in malignant pleural mesothelioma. Invest New Drugs 18:243, 2000.
Ohta Y, et al: VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer 81:54, 1999a.
Ohta Y, et al: Thrombospondin-1 expression and clinical implications in malignant pleural mesothelioma. Cancer 85:2570, 1999b.
Olesen LL, Thorshauge H: Thrombocytosis in patients with malignant pleural mesothelioma. Cancer 62:1194, 1988.
Ong ST, Vogelzang NJ: Chemotherapy in malignant pleural mesothelioma: a review. J Clin Oncol 14:1007, 1996.
P.920
Pass HI, Mew DJY: In vitro and in vivo studies of mesothelioma. J Cell Biochem Suppl 24:142, 1996.
Pass HI, et al: Intrapleural photodynamic therapy: results of a phase I trial. Ann Surg Oncol 1:28, 1994.
Pass HI, et al: Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann Surg Oncol 4:628, 1997.
Pass HI, et al: Preoperative tumor volume is associated with outcome in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 115:310, 1998.
Pennucci MC, et al: Combined cisplatin, doxorubicin, and mitomycin for the treatment of advanced pleural mesothelioma: a phase II FONICAP trial. Italian Lung Cancer Task Force. Cancer 79:1897, 1997.
Peterson JT Jr, Greenberg SD, Buffler PA. Non-asbestos-related malignant mesothelioma. A review. Cancer 54:951, 1984.
Peto J, et al: The European mesothelioma epidemic. Br J Cancer 79:666, 1999.
Pinto C, et al: Combination chemotherapy with mitoxantrone, methotrexate, and mitomycin (MMM regimen) in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol 24:143, 2001.
Pooley FD: Asbestos mineralogy. In Antman K, Aisner J (eds): Asbestos-Related Malignancy. Orlando, FL: Grune & Stratton, 1987, p. 3.
Prins J-B, et al: The gene for the cyclin-dependent-kinase-4 inhibitor, CDKN2A, is preferentially deleted in malignant mesothelioma. Int J Cancer 75:649, 1998.
Rabinowitz JG, et al: A comparative study of mesothelioma and asbestosis using computed tomography and conventional chest radiography. Radiology 144:453, 1982.
Raghavan D, et al: Phase II trial of carboplatin in the management of malignant mesothelioma. J Clin Oncol 8:151, 1990.
Ris H-B, et al: Photodynamic therapy with chlorins for diffuse malignant mesothelioma: initial clinical results. Br J Cancer 64:1116, 1991.
Roberts GH: Distant visceral metastases in pleural mesothelioma. Br J Dis Chest 70:246, 1976.
Ruffie P, et al: Diffuse malignant mesothelioma of the pleura in Ontario and Quebec: a retrospective study of 332 patients. J Clin Oncol 7:1157, 1989.
Rusch VW: The International Mesothelioma Interest Group: a proposed new international TNM staging system for malignant pleural mesothelioma. Chest 108:1122, 1995.
Rusch VW, Godwin JD, Shuman WP: The role of computed tomography scanning in the initial assessment and the follow-up of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 96:171, 1988.
Rusch VW, Piantadosi S, Holmes EC: The role of extrapleural pneumonectomy in malignant pleural mesothelioma. A Lung Cancer Study Group trial. J Thorac Cardiovasc Surg 102:1, 1991.
Rusch VW, Venkatraman E: The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 111:815, 1996.
Rusch VW, Venkatraman ES: Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 68:1799, 1999.
Rusch VW, et al: A phase II trial of pleurectomy/decortication followed by intrapleural and systemic chemotherapy for malignant pleural mesothelioma. J Clin Oncol 12:1156, 1994.
Rusch VW, et al: A phase II trial of surgical resection and adjuvant high dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 122:788, 2001.
Sahmoud T, et al: Etoposide in malignant pleural mesothelioma: two phase II trials of the EORTC Lung Cancer Treatment Group. Eur J Cancer 33:2211, 1997.
Samson MK, et al: Randomized comparison of cyclophosphamide, imidazole carboxamide, and adriamycin versus cyclophosphamide and adriamycin in patients with advanced stage malignant mesothelioma: a Sarcoma Intergroup Study. J Clin Oncol 5:86, 1987.
Samuels BL, et al: Dihydro-5-azacytidine and cisplatin in the treatment of malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Cancer 82:1578, 1998.
Scagliotti G, et al: Phase II study of ALIMTA (pemetrexed disodium, MTA) single agent in patients with malignant pleural mesothelioma. Eur J Cancer 37:20, 2001.
Scharifker D, Kenko M: Localized fibrous mesothelioma of pleura (submesothelial fibroma). A clinicopathologic study of 18 cases. Cancer 43:627, 1979.
Schouwink JH, et al: The value of chest computer tomography and cervical mediastinoscopy in the preoperative assessment of patients with malignant pleural mesothelioma. Ann Thorac Surg 75:1715, 2003.
Sekido Y, et al: Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res 55:1227, 1995.
Selikoff IJ, et al: Relation between exposure to asbestos and mesothelioma. N Engl J Med 272:560, 1965.
Sheard JDH, et al: Pneumothorax and malignant mesothelioma in patients over the age of 40. Thorax 46:584, 1991.
Sinoff C, et al: Combined doxorubicin and radiation therapy in malignant pleural mesothelioma. Cancer Treat Rep 66:1605, 1982.
Sklarin NT, et al: Augmentation of activity of cis-diamminedichloroplatinum (II) and mitomycin C by interferon in human malignant mesothelioma xenografts in nude mice. Cancer Res 48:64, 1988.
Sorensen PG, et al: Randomized trial of doxorubicin versus cyclophosphamide in diffuse malignant pleural mesothelioma. Cancer Treat Rep 69I:1431, 1985.
Soubra M, et al: Physical aspects of external beam radiotherapy for the treatment of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 18:1521, 1990.
Steele JP: Prognostic factors in mesothelioma. Semin Oncol 29:36, 2002.
Steele JP, et al: Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 18:3912, 2000.
Steele JP, et al: Phase II trial of liposomal daunorubicin in malignant pleural mesothelioma. Ann Oncol 12:497, 2001.
Sterman DH, Kaiser LR, Albelda SM: Gene therapy for malignant pleural mesothelioma. Hematol Oncol Clin North Am 12:553, 1998.
Stout AP, Murray MR: Localized pleural mesothelioma. Arch Pathol 34:951, 1942.
Sugarbaker DJ, et al: Extrapleural pneumonectomy, chemotherapy, and radiotherapy in the treatment of diffuse malignant pleural mesothelioma. J Thorac Cardiovasc Surg 102:10, 1991.
Sugarbaker DJ, et al: Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol 11:1172, 1993.
Sugarbaker DJ, et al: Extrapleural pneumonectomy in the multimodality therapy of malignant pleural mesothelioma. Results in 120 consecutive patients. Ann Surg 224:288, 1996.
Sugarbaker DJ, et al: Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results of 183 patients. J Thorac Cardiovasc Surg 117:54, 1999.
Taguchi T, et al: Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res 53:4349, 1993.
Tammilehto L: Malignant mesothelioma: prognostic factors in a prospective study of 98 patients. Lung Cancer 8:175, 1992.
Testa JR, et al: A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res 58:4505, 1998.
Thodtmann R, et al: Preliminary results of a phase I study with MTA (LY231514) in combination with cisplatin in patients with solid tumors. Semin Oncol 26:89, 1999.
Tomek S, et al: Chemotherapy for malignant pleural mesothelioma: past results and recent developments. Br J Cancer 88:167, 2003.
Umsawasdi T, et al: A case report of malignant pleural mesothelioma with long-term disease control after chemotherapy. Cancer 67:48, 1991.
Union Internationale Contre le Cancer: TNM Classification of Malignant Tumours. New York: Wiley-Liss, 2002.
Van der Schoot HC: Asbestosis en pleuragezwellen. Ned Tijdschr Geneeskd 102:1125, 1958.
Van Haarst JW, et al: Multicenter phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma (MPM) [Abstract 56]. Program and abstracts of the 9th World Conference on Lung Cancer, Tokyo, Japan, September 11, 2000.
van Meerbeck J, et al: Paclitaxel for malignant pleural mesothelioma: a phase II study of the EORTC Lung Cancer Cooperative Group. Br J Cancer 74:961, 1996.
van Meerbeck JP, et al: A phase II study of gemcitabine in patients with malignant pleural mesothelioma. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. Cancer 85:2577, 1999.
P.921
Versnel MA, et al: Expression of c-sis (PDGF B-chain) and PDGF A-chain genes in ten human malignant mesothelioma cell lines derived from primary metastatic tumor. Oncogene 2:601, 1988.
Vogelzang NJ, et al: High-dose paclitaxel plus G-CSF for malignant mesothelioma: CALGB phase II study 9234. Ann Oncol 10:597, 1999.
Vogelzang N, et al: Phase III randomized trial of onconase vs doxorubicin in patients with unresectable malignant mesothelioma [Abstract]. Proc ASCO 19:577, 2000.
Vogelzang NJ, et al: Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21:2636, 2003.
Vorobiof DA, et al: Taxotere in malignant pleural mesothelioma. A Phase II clinical trial [Abstract 58]. Program and Abstracts of the 9th World Conference on Lung Cancer, September 11, 2000.
Wadler S, et al: Cardiac abnormalities in patients with diffuse malignant pleural mesothelioma. Cancer 58:2744, 1986.
Wagner JC: Mesothelioma and mineral fibers. Cancer 57:1905, 1986.
Wagner JC, et al: Diffuse pleural mesotheliomas and asbestos exposure in Northwestern Cape Province. Br J Ind Med 17:260, 1960.
Walters JL Jr, Martinez AJ: Malignant fibrous mesothelioma. Metastatic to brain and liver. Acta Neuropathol (Berl) 33:173, 1975.
Wedler HW: Asbestose und Lungenkrebs. Dtsch Med Wochenschr 69:575, 1943a.
Wedler HW: Uber den Lungenkrebs bei Asbestose. Dtsch Arch Klin Med 191:189, 1943b.
White SC, et al: Randomised phase II study of cisplatin-etoposide versus infusional carboplatin in advanced non-small-cell lung cancer and mesothelioma. Ann Oncol 11:201, 2000.
Whitwell F, Rawcliffe RM: Diffuse malignant pleural mesothelioma and asbestos exposure. Thorax 26:6, 1971.
Wirth PR, Legier J, Wright GL Jr: Immunohistochemical evaluation of seven monoclonal antibodies for differentiation of pleural mesothelioma from lung adenocarcinoma. Cancer 67:655, 1991.
Xio S, et al: Codeletion of p15 and p16 in primary malignant mesothelioma. Oncogene 11:511, 1995.
EAN: 2147483647
Pages: 203