41 - Chest Wall Deformities
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section X - The Diaphragm > Chapter 51 - Congenital Posterolateral Diaphragmatic Hernias and Other Less Common Hernias of the Diaphragm in Infants and Children
Chapter 51
Congenital Posterolateral Diaphragmatic Hernias and Other Less Common Hernias of the Diaphragm in Infants and Children
Marleta Reynolds
The diagnosis of congenital diaphragmatic hernia can be made in utero with a great deal of accuracy. Early diagnosis allows time for parents to receive genetic and pediatric surgical consultation. Perinatal management can be altered to facilitate optimal care. The combination of early diagnosis and advances in prenatal and postnatal therapies has improved the outcome for neonates born with congenital diaphragmatic hernia. A plethora of research related to congenital diaphragmatic hernia has provided insight into the pathophysiology of this complex anomaly. Infants and older children who are found to have a congenital diaphragmatic hernia are not as severely affected as the neonate and should have 100% predicted survival.
EMBRYOLOGY
The classic congenital diaphragmatic hernia of Bochdalek is a posterolateral defect in the diaphragm thought to be caused by failure of the pleuroperitoneal canal to close at 8 weeks' gestation (Fig. 51-1). Kluth and associates (1996), who studied an animal model of congenital diaphragmatic hernia, believe the hernia results from defective formation of the posthepatic mesenchymal plate portion of the developing diaphragm. Babiuk and Greer (2002), using animal models of congenital diaphragmatic hernia, have shown that the defect results from malformation of the amuscular mesenchymal component of the developing diaphragm rather than a defect in muscularization. They also demonstrated that the defect is a primary process and is not secondary to the development of a hypoplastic lung. The defect occurs on the left side 80% of the time and is occasionally bilateral. The hole can range in size from a 1- to 2-cm round defect to total absence of the hemidiaphragm.
When the intestines return to the abdomen from the yolk sac at 10 weeks' gestation, the intestines and other abdominal viscera may herniate into the chest. The mediastinum is pushed into the contralateral hemithorax. Autopsy studies by the author (1984) and Geggel (1985) and respective colleagues demonstrated pulmonary hypoplasia of both lungs. The ipsilateral lung's weight may be 20% to 50% below normal. Geggel and associates (1985) found the contralateral lung's volume to be 12% to 42% below normal. The pulmonary hypoplasia is characteruzed by a decrease in the number of bronchioles and arterioles, and in the number and size of the alveoli. Excessive muscularization of the pulmonary arterioles also occurs. Kluth and associates (1996) suggested that the pulmonary hypoplasia results from a reduction in spatial proportions of the ipsilateral lung. Miyazaki and colleagues (1998) found strong insulin-like growth factor I in the hypoplastic lungs of affected neonates and proposed that the lung hypoplasia results from a failure to progress in the developing lung.
Bollmann and associates (1995) reported that, in 72% of fetuses diagnosed prenatally with congenital posterolateral diaphragmatic hernia, one or more extradiaphragmatic malformations were present. Eighteen percent of the fetuses studied in their group had chromosomal anomalies. Only 36.3% of the neonates diagnosed postnatally had associated malformations. In another study of fetuses with the diagnosis of congenital diaphragmatic hernia, Witters and associates (2001) identified associated malformations in only 26%; chromosome anomalies were present in 9.5%. Only one infant with associated malformation survived. Congenital heart disease is the most common associated lesion and occurs in 10% to 35% of affected fetuses. When the congenital heart disease is found to coexist with congenital diaphragmatic hernia, Cohen and colleagues (2002) found that there is a 2.9 times higher risk for death. Neural tube
P.762
defects; genitourinary, skeletal, craniofacial anomalies; and abdominal wall defects can also be found. Anomalies of intestinal rotation and fixation are always present. The Congenital Diaphragmatic Hernia Study Group, as noted by Neville and coinvestigators (2002), reported that, in their collected series of 1,833 patients, 23 (1.3%) had associated anomalies consistent with Fryn's syndrome (distal digital hypoplasia, coarse facies, abnormal ears, and congenital diaphragmatic hernia). This particular subset of patients had a 17% suvival rate.
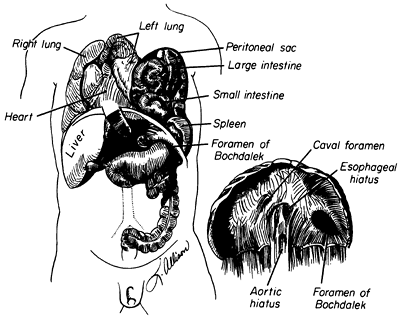 |
Fig. 51-1. Congenital diaphragmatic hernia of Bochdalek. From Shields TW: The diaphragm. In Nora P (ed): Operative Surgery: Principles and Techniques. Philadelphia: Lea & Febiger, 1972. With permission. |
Attempts have been made to identify prognostic factors based on prenatal ultrasound findings. Prenatal diagnosis alone was once considered a bad prognostic factor, but as the numbers of routine ultrasounds increased, the significance of this factor decreased. Albanese and colleagues (1998) reviewed the records of 48 patients with prenatal diagnosis and analyzed the position of the liver on ultrasound and its relation to survival. The group with the liver in the chest (32) required extracorporeal membrane oxygenation (ECMO) support more frequently (53%) than those with the liver in the abdomen (19%). Survival rate was lower in the patients with the liver in the chest (43%) than in patients with the liver in the abdomen (93%). Teixeira and associates (1997) reported that fetal abdominal circumference below the fifth percentile is a poor prognostic sign. Metkus and co-workers (1996) found that the size of the contralateral lung measured as a ratio of lung area to head circumference (LHR) was the only absolute predictor of survival.
A retrospective evaluation of echocardiographic findings in fetuses with congenital diaphragmatic hernia by Sokol and associates (2002) found that pulmonary artery dimensions reflect lung weight. Branch pulmonary artery diameters were not hypoplastic when measured during the early midtrimester. An enlarged contralateral pulmonary artery in midtrimester correlated with postnatal death. Discrepancy in branch pulmonary artery size and a larger main pulmonary artery size correlated with postnatal respiratory morbidity. The hypoplasia of the ipsilateral pulmonary artery was progressive and may play a major role in progressive lung hypoplasia.
PRESENTATION
A congenital posterolateral diaphragmatic hernia may cause life-threatening respiratory distress in the first hours or days of life. The defect can cause respiratory distress or feeding intolerance in later infancy or childhood or may be identified on a radiograph obtained for unrelated reasons in an asymptomatic patient. The morbidity and mortality associated with a congenital diaphragmatic hernia are directly related to the age of the patient at presentation (Table 51-1).
Some neonates with congenital posterolateral diaphragmatic hernia become symptomatic in the delivery room. The diagnosis is suspected if the abdomen is scaphoid and heart sounds are heard in the right chest. Radiography of the neonate demonstrates gas-filled loops of intestines in the chest (Fig. 51-2). An orogastric tube placed into the stomach to decompress the intestines may appear in the chest, and a paucity of gas exists in the abdomen (Fig. 51-3). Any neonate with respiratory distress at birth who is suspected of having a posterolateral diaphragmatic hernia should be quickly intubated. Mask bagging only increases the distention of the herniated stomach and intestines and further compromises ventilation.
Mechanical ventilation with 100% FIO2 and low airway pressures (less than 25 cm H2O with 5 cm of positive end-expiratory pressure) should be used. Vascular access using an umbilical artery catheter is adequate for arterial blood gas sampling and fluid and drug administration. The neonate should be rapidly transported to a center with a surgeon, a neonatal intensive care unit equipped to care for such an infant, and ECMO capabilities. Profound respiratory acidosis is typically found with the first arterial blood gas. Ventilation is individualized to prevent or treat hypercarbia and hypoxemia. Prolonged hypoxemia, acidosis, and hypercarbia produce pulmonary vasoconstriction and persistent fetal circulation. Myocardial dysfunction may necessitate support with dobutamine and renal perfusion with low-dose dopamine. Because dopamine in higher doses may constrict the pulmonary vasculature, it is used only in low doses. Five percent albumin can be used to treat systemic hypotension in boluses of 10 to 15 mL.
Table 51-1. Infants with Congenital Posterolateral Diaphragmatic Hernia, 1962 1983 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
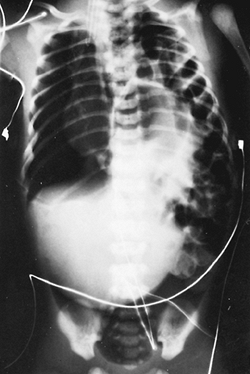 |
Fig. 51-2. This babygram demonstrates multiple loops of intestine on the left side of the chest and a few loops in the abdomen. The mediastinum is shifted to the contralateral side. |
P.763
A congenital poste rolateral diaphragmatic hernia may be found incidentally in an older infant or child. Newman and colleagues (1986) found that the older infant or child with a posterolateral diaphragmatic hernia may present with respiratory or gastrointestinal symptoms. Diagnosis is made with chest radiography or barium studies of the gastrointestinal tract. The hernia should be repaired at the time of diagnosis. The lungs of these children are not hypoplastic, and the operative mortality rate should be 0%.
MANAGEMENT
After the newborn with a congenital posterolateral diaphragmatic hernia is transferred to a center with ECMO capabilities, an effort is made to stabilize the neonate for at least 72 hours before considering operative repair. In the past, hyperventilation and alkalosis were used to control pulmonary hypertension and persistent fetal circulation. Wung and associates (1995) proposed permissive hypercapnia with spontaneous ventilation in an effort to minimize ventilator-induced lung injury (Table 51-2). Boloker and colleagues (2002) reported their experience treating 120 infants using this strategy. The overall survival rate was 76%, and only 13% required ECMO.
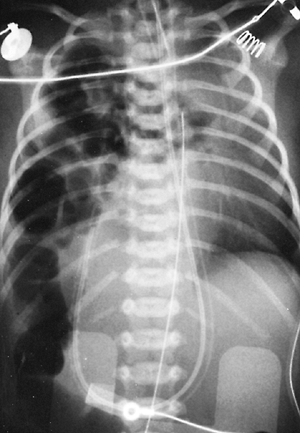 |
Fig. 51-3. Radiograph demonstrates a right-sided diaphragmatic hernia. The orogastric tube is seen in the right side of the chest, identifying the location of the stomach. |
 |
Table 51-2. Management Strategy in Newborns with Congenital Posterolateral Diaphragmatic Hernia |
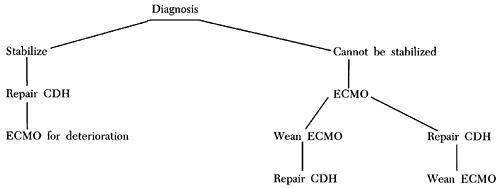 |
Fig. 51-4. Algorithm for a symptomatic baby with congenital diaphragmatic hernia. |
P.764
Inhaled nitric oxide (NO) is a selective pulmonary vasodilator that has been successfully used to treat pulmonary hypertension in newborns. The maximal dose recommended is 20 ppm. If persistent fetal cirulation ensues, NO should be initiated. High-frequency oscillation can also be used. In some infants, these alternative therapies will avoid the need for ECMO.
Hirschl (1996) described the technique of partial liquid ventilation in babies with diaphragmatic hernia. Perfluorocarbon is instilled into the trachea and lungs. Standard mechanical ventilation is performed through the liquid. Because perfluorocarbon can carry more oxygen and carbon dioxide, it can enhance gas exchange.
If these strategies are not successful in reversing persistent fetal circulation, ECMO is considered for newborns who meet institutional criteria (Fig. 51-4).
OPERATIVE CORRECTION
In the past, repair of a congenital posterolateral diaphragmatic hernia was considered a surgical emergency. Newborns were operated upon in the delivery room or were taken directly from the transport ambulance to the operating suite. Retrospective analysis of this treatment plan has shown little advantage or improvement in survival. In fact, Sakai and associates (1987) demonstrated that early operation causes deterioration in pulmonary mechanics. Nakayama and colleagues (1991) showed that preoperative stabilization results in an improvement in pulmonary compliance in a group of neonates with delayed repair of the hernia.
Glick (1992) and Suen (1993) and their associates demonstrated that the lungs of fetuses with congenital Bochdalek's diaphragmatic hernias are biochemically premature, and some neonates have been shown to benefit from surfactant replacement therapy during this stabilization period, as reported by Bos and colleagues (1990, 1991). On the other hand, Cogo and coinvestigators (2002) found that surfactant synthesis and kinetics were not altered in the group of infants with diaphragmatic hernia they studied. Mann and associates (2002) reported that the combination of prenatal maternal glucocorticoids and postnatal inhaled NO improved survival as compared with prenatal maternal glucocorticoids used alone in the rat model of diaphragmatic hernia.
Preoperative stabilization has become the standard of care for newborns who present with respiratory distress. This can require up to 7 to 10 days to accomplish, and no benefit has been found in repairing the hernia when the neonate is not stable and still demonstrates persistent fetal circulation.
Once stable, the neonatal ventilator is moved from the neonatal intensive care unit to the operating room for use during the operation, or the surgery is performed in the intensive care unit. Any sudden deterioration of vital signs during transport, in the operating room, or during the postoperative period usually indicates a pneumothorax on the contralateral side. Gibson and Fonkalsrud (1983), as well as Srouji and associates (1981), reported that a contralateral pneumothorax or a pneumomediastinum is associated with an increase in mortality and should be prevented with the use of low airway pressures. Needle aspiration followed by tube thoracostomy with a 10F chest tube should be performed when a pneumothorax is suspected.
The correction of a congenital posterolateral diaphragmatic hernia is performed through a paramedian or subcostal incision. The abdominal viscera are returned to the abdomen from the chest, and the hernia sac, if present, is excised.
P.765
Extralobar pulmonary sequestrations, often an associated malformation in infants with congenital diaphragmatic hernia, are resected at the time of hernia repair. A small diaphragmatic defect is closed with permanent suture and Teflon pledgets. Larger defects can be closed with polytetrafluoroethylene membrane. When the hemidiaphragm is completely absent, a polytetrafluoroethylene membrane can be sutured to the ribs, both anteriorly and posterolaterally. The medial portion of the membrane can be sutured to the contralateral diaphragmatic leaf and the adventitia overlying the aorta and esophagus. A chest tube may be placed in the ipsilateral thorax and attached to a three-way stopcock and closed. The tube can be used to remove fluid or air should this become necessary. Topical thrombin and cryoprecipitate are applied to the surgical field to assist in hemostasis if ECMO is anticipated (Fig. 51-5).
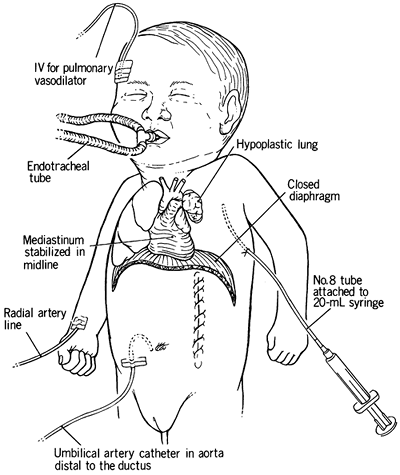 |
Fig. 51-5. Total postoperative management of a poor-risk infant. The mediastinum is stabilized with the pleural catheter, blood gases are monitored above and below the ductus arteriosus, and the intravenous lines are used for the administration of vasodilator drugs. From Ramenofsky MD, Luck SR: Diaphragmatic anomalies. In Raffensperger JC (ed): Swenson's Pediatric Surgery. 4th Ed. East Norwalk, CT: Appleton-Century-Crofts, 1980, p. 675. With permission. |
Controversy continues regarding the best method of thoracic drainage. Suggestions have included no chest tube, bilateral prophylactic chest tubes, underwater seal, and tubes exposed to atmospheric pressure. Tyson and associates (1985) recommended balanced thoracic drainage to maintain normal intrathoracic pressure. The ipsilateral chest tube attached to a three-way stopcock allows removal of air or fluid, depending on the clinical picture and the finding on radiography of the chest. The author prefers this latter method.
Associated intraabdominal anomalies should be corrected at the time of hernia repair if the neonate's condition is stable. If fascial closure causes compromise of respiratory excursion, a ventral hernia can be created by closing the skin only. Occasionally, even the skin cannot be closed, and a silo of Silastic sheeting or polytetrafluoroethylene can be used to contain the abdominal viscera temporarily.
EXTRACORPOREAL MEMBRANE OXYGENATION
ECMO has been successfully used in more than 100 centers around the world to treat reversible respiratory failure in newborn infants. Hardesty (1981), Bartlett (1986), Weber (1987), Redmond (1987), and Langham (1987) and their colleagues reported survival rates among infants with congenital diaphragmatic hernia treated with ECMO ranging from 38% to 77%. This wide range in survival rates probably reflects differences in selection criteria and the experience of the particular center. Standard criteria have not been established, but in general include: (a) alveolar-arterial oxygen gradient (AaDO2) less than 600 for 12 hours, (b) oxygen index greater than 40, (c) acute deterioration (pH less than 7.15 or Pao2 less than 55 mm Hg) for 2 consecutive hours, (d) failure of conventional management, and (e) progressive barotrauma.
Contraindications to the use of ECMO, as described by Bartlett and associates (1986), include preexisting intraventricular hemorrhage, weight less than 2,000 g, and congenital or neurologic abnormalities incompatible with normal life.
Reickert and co-workers (1998) reported the outcome of neonates with congenital diaphragmatic hernia of Bochladek treated at 16 different institutions that used multimodal therapies. The overall survival rate in 411 patients was 69%. The survival rate for neonates placed on ECMO was 55%, and it was 81% for those who did not need ECMO. Currently, ECMO is reserved for those neonates who cannot be stabilized or deteriorate after hernia repair. If ECMO is needed before hernia repair, the hernia can be repaired after ECMO is no longer needed or in a final effort to wean a neonate from ECMO.
Stevens and colleagues (2002) studied a large group of neonates (2,406) with congenital diaphragmatic hernia treated with ECMO. They found that the survival rate for neonates delivered after 40 weeks' gestation was higher than for those delivered between 38 and 40 weeks' gestation. Their data suggest that fetuses with prenatally diagnosed congenital diaphragmatic hernia should be allowed to come to term before elective delivery.
FACTORS IN SURVIVAL
Several methods have been used to predict survival in infants with congenital posterolateral diaphragmatic hernia. Boix-Ochoa and associates (1977) reported that an arterial blood pH of less than 7.0 with a Pco2 of greater than 100 is an early predictor for a poor outcome. Touloukian and Markowitz (1984) devised a scoring system based on preoperative radiographic findings. The radiographic findings include: (a) the side of the diaphragmatic hernia, (b) location of the stomach, (c) presence of pneumothorax, and (d) relative volume of aerated ipsilateral and contralateral lungs. A total score can be derived for each patient and identifies the high-risk patient.
AaDO2 is used more frequently to predict survival. Harrington (1982) and Manthei (1983) and their associates reported that preoperative and postoperative AaDO2 greater than 500 mm Hg correlated with little chance of survival. Manthei and colleagues (1983) also noted that initial postoperative improvement, as evidenced by a transient decrease in AaDO2, was followed by a sudden increase in AaDO2. Expecting this deterioration allows prompt institution of aggressive measures to prevent and treat the decline in oxygenation.
Bohn and colleagues (1987) used ventilatory parameters to predict survival by plotting Pco2 versus ventilation index (ventilation index = ventilation rate mean airway pressure) in the pre-ECMO era. Wilson and associates (1991) have shown that the best postductal Pco2 and the oxygenative-ventilation index (Pco2/mean airway pressure respiratory rate 100) were predictors of mortality. Infants who did not respond to conventional mechanical ventilation (best postductal Po2 less than 100; best postductal Pco2 greater than 40, with ventilation index greater than 1,000) did not benefit from ECMO.
P.766
FUTURE PROSPECTS
Because the degree of pulmonary hypoplasia determines survival in infants with congenital posterolateral diaphragmatic hernia, investigations of in utero correction began. Harrison (1980a) and Adzick (1985) and their colleagues developed a model for congenital posterolateral diaphragmatic hernia in the fetal lamb and observed that changes identical to those found in humans occurred in the fetal lamb. Harrison and associates (1980b) reported that the in utero correction of the defect in fetal lambs allowed sufficient lung growth for survival. The optimal time predicted for in utero intervention in humans is between 22 and 28 weeks' gestation. As Harrison and co-workers (1985) pointed out, ultrasound diagnosis of other anomalies and chromosomal analysis are prerequisites for maternal intervention. Although technically feasible, the results of clinical trials, such as those by Harrison and colleagues (1993), did not show improved outcome, and in utero repair was abandoned.
Lipsett and associates (1998) have shown that antenatal tracheal occlusion can reverse the lung hypoplasia in a lamb model of congenital diaphragmatic hernia using morphometric analysis. Attempts to apply the technique to the human fetus have been difficult, and since 1995, several investigators have been studying the feasibility of tracheal occlusion as a means of reversing pulmonary hypoplasia in a congenital diaphragmatic hernia experimental model. Problems with open hysterotomy have led to attempts to apply endoscopic techniques to accomplish the same goal. VanderWall and colleagues (1996) described the use of a fetal endoscopic tracheal clip, and Benachi and associates (1997) used endoscopic placement of a tracheal balloon. Benachi and colleagues (1998) reported that although tracheal occlusion of the fetus with congenital diaphragmatic hernia of Bochdalek was feasible and could reverse hypoplasia, a significant reduction in the number of type II pneumocytes and surfactant production was seen in the experimental animals. Wu and associates (2002), studying a rat model, found that delaying the tracheal occlusion until later in gestation successfully accelerated lung growth without type 2 pneumocyte depletion. Harrison and colleagues (1998) reported a series of fetuses treated with open and endoscopic tracheal clip application. Survival was better for those treated with the endoscopic technique (75% vs. 15%). Fauza and associates (2002), in an attempt to decrease further the threat of preterm labor following fetal intervention, introduced a technique using ultrasound-guided tracheal occlusion. Necessary refinements in this technique may make this an attractive alternative to more invasive methods. Whether in utero treatment with tracheal occlusion will prove to be beneficial in a large series of human fetuses with congenital diaphragmatic hernia remains to be seen.
LONG-TERM FOLLOW-UP
Several short- and long-term studies, such as that by Ijsselstijn and colleagues (1997), have identified the long-term morbidity associated with the diagnosis of congenital Bochdalek's diaphragmatic hernia. Jeandot and co-workers (1989) evaluated survivors after 1 to 2 years and found a persistent reduction in perfusion but improved ventilation of the ipsilateral lung based on lung ventilation and perfusion scintigraphy. Chest radiography confirmed continuing changes (Fig. 51-6). Vanamo (1996) and Zaccara (1996) and their associates studied older survivors and found no clinical impairment but identified some reduced exercise tolerance and minor ventilatory impairment. Other sequelae documented by Nobuhara and colleagues (1996) included anterior chest wall deformities in 21%, scoliosis in 10.5%, and gastroesophageal reflux in more than 50%. Kamiyama and associates (2002) found that the incidence of postrepair gastroesophageal reflux was higher in children with direct closure of the hernia when compared with those in whom a patch closure was used. Naik and co-workers (1996) found that morbidity could be correlated with the length of time the infant required ventilatory support or supplemental oxygen. Of those infants requiring ECMO, Stolar and associates (1995) found cognitive outcome to be worse when compared with infants treated with ECMO for other reasons. There is a higher incidence of bronchopulmonary dysplasia (BPD), especially in infants that require ECMO. Guarino and colleagues (2002) demonstrated that the mechanical distention of the hypoplastic lungs results in an increased production of elastin, an important factor in the development of BPD, in the rat model. Sensorineural hearing loss was reported by Nobuhara and colleagues (1996) to develop in 18% of survivors in their study. The author has found a similar incidence in her institution. Recurrent posterolateral diaphragmatic hernia can develop, especially in those infants that require patch repair. Small bowel obstruction can also plague these children at any time.
MISCELLANEOUS CONGENITAL DIAPHRAGMATIC HERNIAS
Hernias through the Central Tendon of the Diaphragm
Hernias can occur through the central portion of the diaphragm. Partial or localized eventration of the diaphragm with marked thinning of the tissues to form a ring and a hernial sac can also occur. When such a hernia occurs on the right side, a mushroomlike projection of liver that has grown through the opening in the right diaphragmatic leaf may be found. On radiography of the chest, this projection is occasionally misinterpreted as a diaphragmatic tumor. Differentiation may be made by instituting a pneumoperitoneum, after which air appears to surround the liver protrusion.
P.767
Repair of this type of hernia is unnecessary if clear identification can be made by the pneumoperitoneum. If not, exploration is required to rule out the possibility of a primary tumor of the diaphragm.
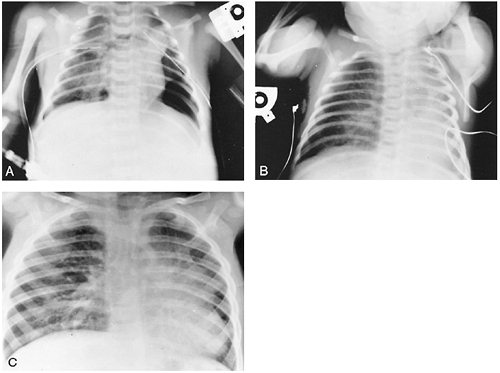 |
Fig. 51-6. A. Early postoperative radiograph shows a small left lung. B. One month later, hyperinflation of the contralateral lung is seen, and the mediastinum has shifted into the ipsilateral chest. C. Even 2 years after repair of the diaphragmatic hernia, the contralateral lung and mediastinum are still in the ipsilateral chest. The child was asymptomatic at the time. |
If the hernia is on the left side, the stomach is occasionally herniated through the central portion of the diaphragm; it usually is identified as an air-containing cyst on the top of the diaphragm. The hernia may be associated with a partial absence of the pericardium, and the stomach and small intestine may herniate into the pericardial sac and cause cardiac symptoms. In general, when such a defect occurs through the central portion of the left hemidiaphragm or into the pericardial sac, the hernia should be repaired as soon as it is discovered. In infants and children, the repair usually is accomplished through an abdominal approach, similar to that described for the repair of the foramen of Bochdalek hernia. In contrast, in the older child and adult, repair is accomplished through a thoracic approach.
Hernia in Infants and Children
A paraesopParaesophagealhageal hernia diagnosed in infancy or childhood may be congenital or acquired. The acquired variety usually occurs as a complication of the surgical treatment of gastroesophageal reflux. A congenital paraesophageal hernia first may be suggested by the findings on chest radiography (Fig. 51-7A). An upper gastrointestinal series demonstrates the herniated stomach and other abdominal contents in the chest (Fig. 51-7B). In some children, anemia may develop from gastritis, or esophagitis and vomiting from obstruction or incarceration. The symptoms depend on the size of the defect, the position of the stomach, the amount of obstruction at the gastroesophageal junction or pylorus, and the presence of other viscera within the hernia sac.
Surgery is indicated in all cases and should include reduction of the intestinal contents, excision of the sac, reapproximation of the diaphragmatic crura, and, in some cases,
P.768
as suggested by Jawad and associates (1998), an antireflux procedure or gastropexy should be carried out (see Chapter 144). Minimally invasive techniques have similar results when compared with open techniques.
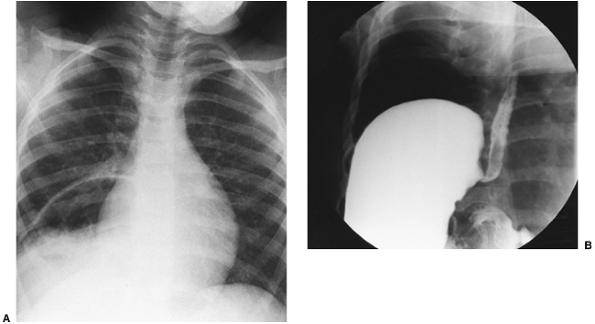 |
Fig. 51-7. A. Chest radiograph illustrating the presence of a large cystic air space located behind the right side of the cardiac shadow. B. The barium swallow revealed the stomach to be present in a large paraesophageal hiatus hernia. |
Foramen of Morgagni Hernia
Anatomy
On each side of the sternum is a potential space, known as the foramen of Morgagni, or the space of Larrey, through which passes the internal mammary artery to become the superior epigastric artery. This triangular space is between the muscular fibers originating from the xiphisternum and the costal margin that insert on the central tendon of the diaphragm. The left space is less likely to develop a hernia because it is protected by the pericardial sac. The ligamentum teres defines the medial border of the hernia through either space.
Most often in infants and children, a foramen of Morgagni hernia contains only a piece of omentum that is caught up in the defect that may enlarge over a period of time. Rarely, a portion of one of the abdominal viscera, often the stomach, is contained in the hernia (Fig. 51-8). A portion of the liver, as reported by Newman and Davis (1989), may be present in the hernia sac.
Incidence
Hernias through the foramen of Morgagni are uncommon at any age but are even rarer in the child than in the adult. Berman and associates (1989) reported only 15 infants and children with foramen of Morgagni hernias collected over a 20-year period at the Hospital for Sick Children in Toronto. Pokorny and associates (1984) reported only five hernias of this type in infants in a 6-year period at the Texas Children's Hospital. The unusual occurrence of foramen of Morgagni hernias in identical twins was recorded by Harris and associates (1993).
Symptoms
A small foramen of Morgagni hernia usually is unrecognized and asymptomatic in infants and young children. Larger ones, often because of the presence of an abdominal viscus in the hernia, may produce respiratory symptoms. Neonates with large hernias can present with severe respiratory distress similar to the dramatic clinical picture associated with the more common posterolateral Bochdalek's hernia. In addition, neonates and infants with foramen of Morgagni hernia may have associated congenital anomalies, such as Down's, Turner's, or Noonan's syndrome, pentalogy of Cantrell, pectus deformities, bowel malrotation, and genitourinary malformations.
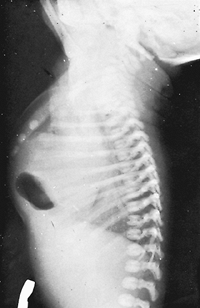 |
Fig. 51-8. Lateral chest radiograph in an infant with a congenital foramen of Morgagni hernia that was found to contain most of the stomach at the time of repair. |
P.769
A foramen of Morgagni hernia, however, is more often symptomatic in older children and adolescents. Exercise and other athletic activity, as noted by Valases and Sills (1988), may precipitate the occurrence of symptoms. The older child or adolescent with a foramen of Morgagni hernia may complain of dull pain in the right subcostal area. Rarely, intermittent, partial intestinal obstruction may occur, including gastric volvulus, but complete obstruction is uncommon. Dyspnea is observed only occasionally in patients past early childhood.
Diagnosis
Radiographic studies of the chest reveal a density, either solid or containing air, adjacent to the right or left side of the heart. A computed tomographic (CT) examination of an anterior cardiophrenic mass may reveal the presence of bowel, thus confirming the diagnosis. Sonographic and magnetic resonance (MR) imaging may at times be valuable, as noted in the report of Newman and Davis (1989). Contrast studies of the large intestine and often of the upper gastrointestinal tract may be indicated in the evaluation of some patients.
Surgical Repair
Surgical repair is recommended for all hernias because of the lifetime risk of intestinal obstruction. In infants and young children, an open repair is indicated. The abdominal approach for surgical repair of this hernia is chosen if the diagnosis is known preoperatively. A subcostal incision or a right epigastric paramedian incision may be used. With the latter incision, the rectus muscle is retracted laterally to expose the posterior rectus and transversalis fascia. After the abdomen has been opened, the contents of the hernia are reduced into the peritoneal cavity, and the margins of the hernial sac are identified. As noted, the ligamentum teres defines the medial border of the hernia. The hernia sac is removed when possible. The repair of the muscular defect is made with interrupted mattress sutures, and usually it is necessary to pull the diaphragm up to the posterior part of the sternum and to the posterior rectus sheath. After repair, the abdomen is closed. In most patients, it is not necessary to enter into the chest or to drain the pleural space. Ponsky and colleagues (2002) have described the use of minimally invasive techniques to repair foramen of Morgagni hernias in children.
Rarely, in children, a foramen of Morgagni hernia is encountered while the anterior mediastinum is being explored for an undiagnosed mediastinal mass in either of the anterior cardiophrenic angles. As soon as the mass has been identified as a foramen of Morgagni hernia, the sac is opened and explored, and the contents are reduced into the peritoneal cavity. The repair is then accomplished in a manner similar to that just described. In this instance, it is also best to suture the diaphragm to the posterior part of the sternum and the rectus sheath. On occasion, because of the size of the defect and a deficiency of available tissue, it is necessary to sew in a small piece of plastic mesh or a soft tissue Gore-Tex patch. Recurrence of a foramen of Morgagni hernia is rare.
Peritoneal Pericardial Hernia
A rare diaphragmatic defect between the peritoneal cavity and pericardial sac also has been reported. Ake and colleagues (1991) reported the prenatal diagnosis of a fetus with such a hernia. The infant was asymptomatic at birth, and the hernia was repaired through the abdomen on the fifth day of life. Milne and associates (1990) reported two patients with an identical defect associated with a giant omphalocele. The author has treated an infant with this defect who presented at birth with severe respiratory distress, hypotension, and persistent fetal circulation (Fig. 51-9). Urgent repair was done; at operation, the stomach was found inside the pericardium. Reduction of the stomach immediately improved cardiac function. The defect was closed with interrupted Ti-Cron pledget sutures. The patient had no further difficulty postoperatively. Milne and associates (1990) suggested that this defect is secondary to failure of fusion of the pars sternalis portion of the septum transversum in the development of the diaphragm. Repair may be performed through the abdomen or chest. The abdominal approach is preferred.
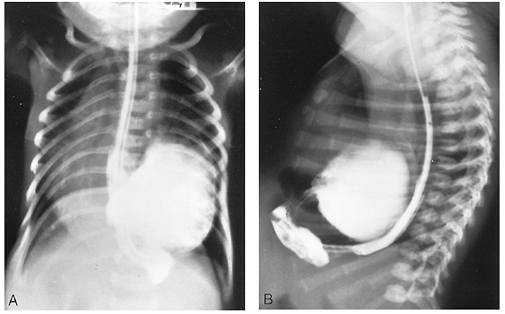 |
Fig. 51-9. Anteroposterior (A) and lateral radiographic (B) views of a barium swallow in an infant with a peritoneal pericardial hernia. |
P.770
REFERENCES
Adzick NS, et al: Correction of congenital diaphragmatic hernia in utero. IV. An early gestation fetal lamb model for pulmonary vascular morphometric analysis. J Pediatr Surg 20:673, 1985.
Ake E, et al: In utero sonographic diagnosis of diaphragmatic hernia with hepatic protrusion into the pericardium mimicking an intrapericardial tumour. Prenat Diagn 11:719, 1991.
Albanese CT, et al: Fetal liver position and perinatal outcome for congenital diaphragmatic hernia. Prenat Diagn 18:1138,1998.
Babiuk RP, Greer JJ: Diaphragm defects occur in a CDH hernia model independently of myogenesis and lung formation. Am J Physiol Lung Cell Mol Physiol 283:L1310, 2002.
Bartlett RH, et al: Extracorporeal membrane oxygenation (ECMO) in neonatal respiratory failure. 100 cases. Ann Surg 204:236, 1986.
Benachi A, et al: Tracheal obstruction in experimental diaphragmatic hernia: an endoscopic approach in the fetal lamb. Prenat Diagn 17:629, 1997.
Benachi A, et al: Lung growth and maturation after tracheal occlusion in diaphragmatic hernia. Am J Respir Crit Care Med 157:921, 1998.
Berman L, et al: The late-presenting pediatric Morgagni hernia: a benign condition. J Pediatr Surg 24:970, 1989.
Boix-Ochoa J, et al: The important influence of arterial blood gases on the prognosis of congenital diaphragmatic hernia. World J Surg 1:783, 1977.
Bohn DJ, et al: Ventilatory predictors of pulmonary hypoplasia in congenital diaphragmatic hernia confirmed by morphologic assessment. J Pediatr 111:423, 1987.
Bollmann R, et al: Associated malformations and chromosomal defects in congenital diaphragmatic hernia. Fetal Diagn Ther 10:52, 1995
Boloker J, et al. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/ elective repair. J Pediatr Surg 37:357, 2002.
Bos AP, et al: Congenital diaphragmatic hernia: impact of prostanoids in the perioperative period. Arch Dis Child 65:994, 1990.
Bos AP, et al: Surfactant replacement therapy in high-risk congenital diaphragmatic hernia. Lancet 338:1279, 1991.
Cogo PE, et al: Surfactant synthesis and kinetics in infants with congenital diaphragmatic hernia. Am J Respir Crit Care Med 166:154, 2002.
Cohen MS, et al: Influence of congenital heart disease on survival in children with congenital diaphragmatic hernia. J Pediatr 141:25, 2002.
Fauza DO, et al: Ultrasound-guided fetal tracheal occlusion. J Pediatr Surg 37:300, 2002.
Geggel RL et al: Congenital diaphragmatic hernia: arterial structural changes and persistent pulmonary hypertension after surgical repair. J Pediatr 107:457, 1985.
Gibson C, Fonkalsrud EW: Iatrogenic pneumothorax and mortality in congenital diaphragmatic hernia. J Pediatr Surg 18:555, 1983.
Glick PL, et al: Pathophysiology of congenital diaphragmatic hernia. II: The fetal lamb CDH model is surfactant deficient. J Pediatr Surg 27: 382, 1992.
Guarino N, et al: Effect of mechanical ventilation on the pulmonary expression and production of elastin in nitrofen-infuced diaphragmatic hernia in rats. J Pediatr Surg 37:1253, 2002.
Hardesty RL, et al: Extracorporeal membrane oxygenation. Successful treatment of persistent fetal circulation following repair of congenital diaphragmatic hernia. J Thorac Cardiovasc Surg 81:556, 1981.
Harrington J, Raphaely RC, Downes JJ: Relationship of alveolar-arterial oxygen tension difference in diaphragmatic hernia of the newborn. Anesthesiology 56:473, 1982.
Harris GJ, Soper RT, Kimura KK: Foramen of Morgagni hernia in identical twins: is this an inheritable defect? J Pediatr Surg 28:177, 1993.
Harrison MR, Jester JA, Ross NA: Correction of congenital diaphragmatic hernia in utero. I. The model: intrathoracic balloon produces fatal pulmonary hypoplasia. Surgery 88:174, 1980a.
Harrison MR, et al: Correction of congenital diaphragmatic hernia in utero. II. Simulated correction permits fetal lung growth with survival at birth. Surgery 88:260, 1980b.
Harrison MR, et al: Fetal diaphragmatic hernia: fetal but fixable. Semin Perinatol 9:103, 1985.
P.771
Harrison MR, et al: Correction of congenital diaphragmatic hernia in utero: VI. Hard-earned lessons. J Pediatr Surg 28:1411, 1993.
Harrison MR, et al: Correction of congenital diaphragmatic hernia in utero: IX: fetuses with poor prognosis (liver herniation and low lung-to-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J Pediatr Surg 33:1017, 1998.
Hirschl RB: Innovative therapies in the management of newborns with congenital diaphragmatic hernia. Semin Pediatr Surg 5:256, 1996.
Ijsselstijn H, et al: Long-term pulmonary sequelae in children with congenital diaphragmatic hernia. Am J Respir Crit Care Med 155:174, 1997.
Jawad AJ, et al: Congenital para-oesophageal hiatal hernia in infancy. Pediatr Surg Int 13:91, 1998.
Jeandot R, et al: Lung ventilation and perfusion scintigraphy in the follow-up of repaired congenital diaphragmatic hernia. Eur J Nucl Med 15:591, 1989.
Kamiyama M, et al: Gastroesophageal reflux after repair of congenital diaphragmatic hernia. J Pediatr Surg 37:1681, 2002.
Kluth D, et al: Embryology of congenital diaphragmatic hernia. Semin Pediatr Surg 5:224, 1996.
Langham MR Jr, et al: Extracorporeal membrane oxygenation following repair of congenital diaphragmatic hernias. Ann Thorac Surg 44:247, 1987.
Lipsett J, et al: Effect of antenatal tracheal occlusion on lung development in the sheep model of congenital diaphragmatic hernia: a morphometric analysis of pulmonary structure and maturity. Pediatr Pulmonol 25:257, 1998.
Mann O, et al: Effect of prenatal glucocorticoids and postnatal nitric oxide inhalation on survival of newborn rats with nitrofen-induced congenital diaphragmatic hernia. J Pediatr Surg 37:730, 2002.
Manthei U, Vaucher Y, Crowe CP Jr: Congenital diaphragmatic hernia: immediate preoperative and postoperative oxygen gradients identify patients requiring prolonged respiratory support. Surgery 93:83, 1983.
Metkus AP, et al: Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg 31:148, 1996.
Milne LW, et al: Pars sternalis diaphragmatic hernia with omphalocele: a report of two cases. J Pediatr Surg 25:726, 1990.
Miyazaki E, et al: Altered insulin-like growth factor I mRNA expression in human hypoplastic lung in congenital diaphragmatic hernia. J Pediatr Surg 33:1476, 1998.
Naik S, et al: Prediction of morbidity during infancy after repair of congenital diaphragmatic hernia. J Pediatr Surg 31:1651, 1996.
Nakayama DK, Motoyama EK, Tagge EM: Effect of preoperative stabilization on respiratory system compliance and outcome in newborn infants with congenital diaphragmatic hernia. J Pediatr 118:793, 1991.
Neville HL, et al: Fryns syndrome in children with congenital diaphragmatic hernia. The Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg 37:1685, 2002.
Newman B, Davis PL: Sonographic and magnetic resonance imaging of an anterior diaphragmatic hernia. Pediatr Radiol 20:110, 1989.
Newman BM, et al: Presentation of congenital diaphragmatic hernia past the neonatal period. Arch Surg 121:813, 1986.
Nobuhara KK, et al: Long-term outlook for survivors of congenital diaphragmatic hernia. Clin Perinatol 23:873, 1996.
Pokorny WJ, McGill CW, Harberg FJ: Morgagni hernias during infancy. Presentation and associated anomalies. J Pediatr Surg 19:394, 1984.
Ponsky TA, et.al.: Laparoscopy is useful in the diagnosis and management of foramen of Morgagni hernia in children. Surg Laparosc Endosc Percutan Tech 12:375, 2002.
Ramenofsky MD, Luck SR: Diaphragmatic anomalies. In Raffensperger JC (ed): Swenson's Pediatric Surgery. 4th Ed. East Norwalk, CT: Appleton-Century-Crofts, 1980, p. 675.
Redmond CR, et al: Extracorporeal membrane oxygenation for respiratory and cardiac failure in infants and children. J Thorac Cardiovasc Surg 93:199, 1987.
Reickert CA, et al: Congenital diaphragmatic hernia survival and use of extracorporeal life support at selected level III nurseries with multimodality support. Surgery 123:305, 1998.
Reynolds M, Luck SR, Lappen R: The critical'' neonate with diaphragmatic hernia: a 21-year perspective. J Pediatr Surg 19:364, 1984.
Sakai H, et al: Effect of surgical repair on respiratory mechanics in congenital diaphragmatic hernia. J Pediatr 111:432, 1987.
Shields TW: The diaphragm. In Nora P (ed): Operative Surgery: Principles and Techniques. Philadelphia: Lea & Febiger, 1972.
Sokol J, et.al: Fetal pulmonary artery diameters and their association with lung hypoplasia and postnatal outcome in congenital diaphragmatic hernia. Am J Obstet Gynecol 186:1085, 2002.
Srouji MN, Buck B, Downes JJ: Congenital diaphragmatic hernia: deleterious effects of pulmonary interstitial emphysema and tension extrapulmonary air. J Pediatr Surg 16:45, 1981.
Stevens TP, et.al.: Survival in early and late term infants with congenital diaphragmatic hernia treated with extracorporeal membrane oxygenation. Pediatrics 110:590, 2002.
Stolar CJJ, Crisafi MA, Driscoll YT: Neurocognitive outcome for neonates treated with extracorporeal membrane oxygenation: are infants with congenital diaphragmatic hernia different? J Pediatr Surg 30:366, 1995.
Suen HC, et al: Biochemical immaturity of lungs in congenital diaphragmatic hernia. J Pediatr Surg 28:471, 1993.
Sydorak RM, et al: Congenital diaphragmatic hernia and hydrops: A lethal association. J Pediatr Surg 37:1678, 2002.
Teixeira J et al: Abdominal circumference in fetuses with congenital diaphragmatic hernia: correlation with hernia content and pregnancy outcome. J Ultrasound Med 16:407, 1997.
Touloukian RJ, Markowitz RI: A preoperative x-ray scoring system for risk assessment of newborns with congenital diaphragmatic hernia. J Pediatr Surg 19:252, 1984.
Tyson KR, Schwartz MZ, Marr CC: Balanced'' thoracic drainage is the method of choice to control intrathoracic pressure following repair of diaphragmatic hernia. J Pediatr Surg 20:415, 1985.
Valases C, Sills C: Anterior diaphragmatic hernia (hernia of Morgagni). Case report. NJ Med 85:603, 1988.
Vanamo K, et al: Long-term pulmonary sequelae in survivors of congenital diaphragmatic defects. J Pediatr Surg 31:1096, 1996.
VanderWall KJ, et al: Fetal endoscopic ( Fetendo ) tracheal clip. J Pediatr Surg 31:1101, 1996.
Weber TR, et al: Neonatal diaphragmatic hernia. An improving outlook with extracorporeal membrane oxygenation. Arch Surg 122:615, 1987.
Wilson JM, et al: Congenital diaphragmatic hernia: predictors of severity in the ECMO era. J Pediatr Surg 26:1028, 1991.
Witters I, et.al: Associated malformations and chromosome anomalies in 42 cases of prenatally diagnosed diaphragmatic hernia. Am J Med Genet 103:278, 2001.
Wu J, et al: Pulmonary effects of in utero tracheal occlusion are dependent on gestational age in a rabbit model of diaphragmatic hernia. J Pediatr Surg 37:11, 2002.
Wung JT, et al: Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg 30:406, 1995.
Zaccara A, et al: Maximal oxygen consumption and stress performance in children operated on for congenital diaphragmatic hernia. J Pediatr Surg 31:1092, 1996.
Reading References
Baran EM, et al: Foramen of Morgagni hernias in children. Surgery 62:1076, 1967.
Bently G, Lister J: Retrosternal hernia. Surgery 57:567, 1965.
Breaux CW Jr, et al: Improvement in survival of patients with congenital diaphragmatic hernia utilizing a strategy of delayed repair after medical and/or extracorporeal membrane oxygenation stabilization. J Pediatr Surg 26:333, 1991.
Weber TR, et al: Congenital diaphragmatic hernia beyond infancy. Am J Surg 162:643, 1991.
EAN: 2147483647
Pages: 203