XIII - The Trachea
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIV - Congenital, Structural, and Inflammatory Diseases of the Lung > Chapter 88 - Surgery for the Management of Mycobacterium Tuberculosis and Nontuberculous Mycobacterial Infections of the Lung
Chapter 88
Surgery for the Management of Mycobacterium Tuberculosis and Nontuberculous Mycobacterial Infections of the Lung
Marvin Pomerantz
Mycobacterial pulmonary disease includes infections with Mycobacterium tuberculosis, a virulent organism that rapidly destroys normal lung tissue and produces an acute illness with significant systematic effects, as well as infections with other mycobacteria that have been called atypical tuberculosis, nontuberculous mycobacterial (NTM) infections, infection from mycobacteria other than tuberculosis (MOTT), and more recently environmental mycobacterial (EM) infections. The latter term is probably the best descriptive name. They have also been described by the color they produce on culture plates or by their speed of growth in culture (rapid growers). In contrast to tuberculosis (TB), they most often infect previously diseased lung. The infections are more indolent and are often ignored or are treated over many years before the disease is eradicated, surgery is required, or lung destruction is so severe that only supportive measures can be used.
In 1997 there were approximately 8 million new cases of TB in the world, as reported by Dye and associates (1999). Fortunately, only 10% to 15% of those exposed to the TB bacillus come down with the clinical disease, as noted by Iseman (2000). The World Health Organization estimates that one-third of the world's population is infected with M. tuberculosis. According to Reichman (2001), TB most likely spread from animals to humans 8,000 to 10,000 years ago This spread from animals to humans is similar to the spread of AIDS in the 20th century. The primary difference is that TB is spread by airborne droplets, not by body fluids or infected blood. The 85% to 90% of people who do not develop symptoms of TB remain a latent population for later activation of the disease, most often at an elderly age, when their immune systems break down.
Robert Koch first identified the TB bacillus in the 1880s. The early treatment of TB gave rise to the sanatoria system in the late 1800s and the early 1900s. Fresh air and rest were presumed to be beneficial for the treatment of TB; however, there was probably more benefit to society from the isolation of the patient than to the individual tuberculous patient from the actual sanatoria treatment.
Surgical therapy for TB began with collapse therapy. This was effective because the M. tuberculosis organism is an obligate aerobe; by collapsing cavitary disease, the organisms, which number in the billions in tuberculous cavities, would be deprived of oxygen and thus die. In 1906 Forlanini claimed credit for introducing collapse therapy; however, it had been performed in a few cases prior to that time by several other surgeons. Various forms of collapse therapy were employed until the introduction of chemotherapy in 1945. The different forms of collapse therapy included thoracoplasty, induced pneumothorax, wax or lucite ball plombage, pneumoperitoneum, and phrenic nerve crush or interruption.
Chemotherapy for TB started with the introduction of streptomycin and p-aminosalicylic acid (PAS) in about 1945. However, it was not until 1952 with the introduction of isoniazid that enduring cures could be obtained. With the advent of the aforementioned drugs, surgical resection replaced collapse therapy in those patients believed to be benefited by the combination of drug therapy and surgery. With the introduction of rifampin into clinical use in 1966, the need for surgery was markedly reduced; thus, the closing of the TB sanatoria began. Today they are essentially nonexistent.
Dye and coauthors (1999) and Farmer (1999) indicate that currently there are still approximately 8 million new cases of TB in the world yearly and an estimated 3 million deaths. The greatest numbers of these cases occur in Southeast Asia, the western Pacific, Africa, the eastern Mediterranean, Latin America, and Eastern Europe. There are currently only about 15,000 new cases of TB in the United States yearly. Forty percent of these cases are foreign-born patients.
P.1252
The current medical treatment of drug-sensitive TB is with isoniazid, rifampin, and a short course of pyrazinamide. Ethambutol is added if resistance is expected until specific sensitivities are obtained. In addition to isoniazid, the first-line drugs used in the treatment of TB are rifampin, pyrazinamide, ethambutol, and streptomycin. Additional drugs used in the treatment of TB as well as other mycobacterial infections include cycloserine, ethionamide, PAS, ofloxacin, clofazimine, clarithromycin, and amikacin. Treatment is usually for about 6 months and must include directly observed therapy (DOT). Without DOT, patients often do not take their medication because of the frequent side effects. Under these circumstances there is a much greater risk of developing drug-resistant TB or multidrug-resistant TB (MDR TB), which implies resistance to isoniazid and rifampin. The two major problems facing those medically treating TB is the development of MDR TB and the synergy between TB and AIDS. Dye and associates (2002) state that in sub-Saharan Africa, TB accounts for over 20% of the deaths in AIDS patients, and TB now accounts for 15% of all AIDS deaths worldwide.
SURGICAL RESECTION OF PARENCHYMAL DISEASE CAUSED BY MULTIDRUG-RESISTANT TUBERCULOSIS
Surgery still plays a role in the treatment of patients with TB. Patients with lungs destroyed by MDR TB (Fig. 88-1) or cavitary disease (Fig. 88-2), with or without positive sputum smears, will require resection; this is currently the most frequent indication for surgery in patients with TB in the United States. Incidentally, treatment of MDR TB may cost up to 100 to 200 times more than treatment of patients with drug-sensitive TB. Decortication alone for management of a trapped lung is sometimes indicated (Fig. 88-3). Other patients who require surgical intervention include patients with bronchopleural fistulae,
P.1253
massive hemoptysis (greater than 600 cc in 24 hours), or bronchostenosis, or those in whom there is a need to rule out cancer.
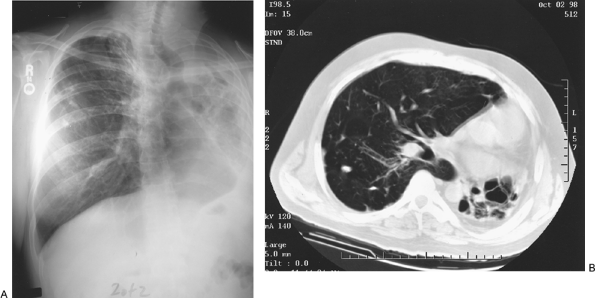 |
Fig. 88-1. A. Totally destroyed left lung caused by tuberculosis. B. Computed tomographic scan demonstrating destroyed left lung. |
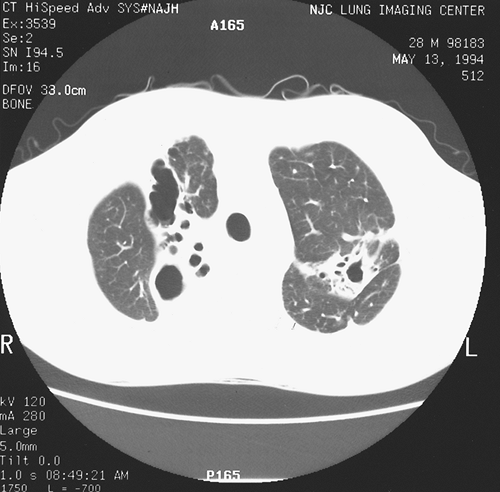 |
Fig. 88-2. Computed tomographic scan shows bilateral upper lobe cavities. |
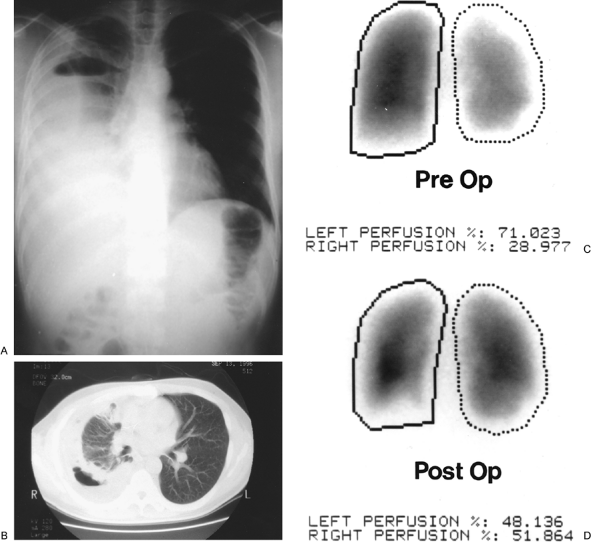 |
Fig. 88-3. A. Posteroanterior view of the chest with trapped right lung. B. Computed tomographic scan of the chest demonstrating thick peel and trapped right lung. C. Perfusion scan prior to decortication showing decreased perfusion to right lung. D. Perfusion scan after decortication revealing normal perfusion of right lung. |
B. J. Pomerantz and colleagues (2001) note that for unknown reasons, when there was total lung destruction, the left lung was involved in three-fourths of the patients in the series studied. Van Leuven and associates (1997) reported an incidence of left-sided pneumonectomies of 80%. Earlier, Ashour and colleagues (1990) reported a like experience (12 of 13 pneumonectomies were performed on the left side); in a later follow-up by Ashour (1997), 16 of 20 pneumonectomies were done on the left. Ashour and associates (1990) suggested that the propensity for left-sided lung destruction in patients with resistant M. tuberculosis infection was possibly related to the smaller caliber (10% to 15%) of the left main-stem bronchus, the tight space surrounding it in its passage through the mediastinum, and the angle of its takeoff from the trachea. Also, the anatomic configuration of the left upper lobe bronchus to the left lower lobe bronchus may
P.1254
play a role. In lobar disease, like most airborne diseases, the right upper lobe is the lobe most frequently involved, followed by the left upper lobe.
Preoperative Preparation
Preparation for surgery is critical, with nutritional support being essential. Most of these patients have lost weight some as many as 30 to 40 pounds. Additional nutrition via gastrostomy or jejunostomy tubes may be needed to ensure that the patient is anabolic prior to surgery. Surgery should not be performed on patients whose albumin level is less than 3.0 g/dL. The patient should be on the best available antimycobacterial therapy based on culture and sensitivity data for approximately 3 months prior to surgery. If bacterial counts from sputum samples are still decreasing, antibiotics can be given for a longer period; or, if a negative sputum result occurs, surgery can be done earlier. In about 50% of surgical patients with MDR TB, negative sputum cultures cannot be obtained and surgery has to be done in spite of positive sputum results.
One of the important principles of surgery is to leave enough viable lung based on pulmonary function tests to ensure that the patient can function reasonably well after surgery. Therefore, pulmonary function tests and ventilation-perfusion scans must be obtained preoperatively to be certain the patient will tolerate the proposed resection. Based on a 70-kg patient, a calculated forced expiratory volume in 1 second (FEV1) of 1 liter should be left after the indicated resections are completed. Regardless of a radiographic appearance of the lung that may suggest that a lobectomy would be adequate, a perfusion scan showing only 15% or less going to what would be the remaining lung on the operative side is an indication for a pneumonectomy.
Improvement in pulmonary hygiene is essential in the preoperative management of mycobacterial patients. The remaining lung, though appearing normal, usually has some degree of infectious involvement. These patients are more apt to develop atelectasis and other pulmonary complications postoperatively. Ideally, patients should not smoke for 1 month prior to surgery. The use of postural drainage, the flutter valve, and incentive spirometers should be taught preoperatively.
Surgical Technique
Double-lumen endobronchial tubes or bronchial blockers are used to anesthetically isolate the lungs during anesthesia and the surgical procedure. At least one large intravenous line is placed to replace blood if needed. An epidural catheter is placed for use for pain control postoperatively. Alternatively, intrathecal one-dose analgesia can be utilized and then switched to patient-controlled analgesia when the effect wears off.
A posterior lateral thoracotomy incision is routinely employed.1 All grossly involved lung should be removed. Cavitary disease has been found to have 107 to 109 organisms, versus 102 to 104 organisms in nodules; therefore, all cavitary disease should be removed. Additionally, all destroyed lung should be resected. Nodular disease without cavitation does not require resection. Resectional surgery is often done in the extrapleural plane. Dense adhesions are usually found over the apical and posterior segments of the upper lobe and often over the superior segment of the lower lobes. There is often a free plane of dissection over the aortic arch on the left and the azygos vein on the right. When dissecting in the extrapleural plane, care must be taken to avoid the subclavian vessels, the recurrent laryngeal nerve, the esophagus, and the intercostal vessels during dissection posterior to the aorta, as emphasized by Brown and the author (1995). Frequently, once the lung is freed from its adhesions, the hilum can be isolated without difficulty. However, with completion pneumonectomies, vessel ligations may have to be performed within the pericardium.
Bronchial closure can be done either by suture or with staples. In mycobacterial resections, I have found no difference in the incidence of bronchial stump disruption with either closure. Muscle flaps, although thought to be controversial by some, should be used to cover the bronchial closure in patients who still have a positive sputum smear at the time of surgery, in patients who have had a bronchopleural fistula, or if there is polymicrobial contamination. They also may be used if there is a need to fill space after a lobectomy. I and my colleagues (1991) have recommended the latissimus dorsi as the muscle of choice. Using the serratus anterior produces a winged scapula, which may protrude under the posterior portion of the wound in these usually cachetic patients. When only support of the bronchial closure is needed following a pneumonectomy, an intercostal muscle flap may be used. When there is no muscle available due to previous surgery or if there is massive contamination, an omental flap is used. Although there is no controlled series regarding muscle flaps, it is my impression that there are fewer bronchopleural fistulae in patients when a muscle flap is used for the aforementioned reasons. Muscle flaps are not used for middle lobe and lingula resections, segmental resections, or lower lobectomies. In my experience, it is rare to have a bronchial stump break down with these procedures. However, whenever possible in these latter procedures, a pleural flap or pericardial fat pad should be used for bronchial stump coverage.
At the completion of surgery, bronchoscopy is usually done to cleanse the tracheal bronchial tree of secretions that may be present from manipulation of the lung during resection. Intraoperative crystalloid fluid administration should
P.1255
be limited to about 800 to 1,000 cc for pneumonectomies and 1,200 cc for lobectomies.
In very contaminated cases, an Eloesser procedure (1935) is performed after the resection. The Eloesser flap is closed 4 to 6 weeks later after daily packing with Kerlix gauze soaked in half-strength Dakin's solution. At the time of closure, a modified Clagett solution is placed into the pleural cavity.
Postoperative Care
Fluids are restricted for the first 3 to 5 days. Following lobectomy, fluids are restricted to 1,800 cc per 24 hours; after a pneumonectomy, they are restricted to 1,500 cc per 24 hours. Ambulation is encouraged on the first postoperative day. Compression stockings are used, and pulmonary hygiene is continued. Heparin (5,000 units) is given subcutaneously twice daily until the patient is fully ambulating. Ketorolac is used for several days if renal function is normal. Epidural analgesia is continued for 48 to 72 hours, and then patient-controlled analgesia is instituted. Passive range of motion exercises are begun on the upper extremities to prevent a frozen shoulder. Nutrition is emphasized in the postoperative period, as it is preoperatively. When the infected lung is removed, appetite often improves and the patient may become anabolic. However, side effects from the antibiotics may continue to negatively affect caloric intake. Appropriate antimycobacterial drugs are continued for 12 to 24 months as indicated, with some injectable medications being stopped first.
Complications
A dreaded complication following surgery for MDR TB is a bronchopleural fistula. This is more common after right pneumonectomies than left pneumonectomies. Other factors increasing the incidence of bronchopleural fistulae are positive sputum culture at the time of operation, significant polymicrobial contamination, diabetes, and prior chest wall irradiation. The incidence of bronchopleural fistulae if these factors are present is greater than 5%. In contrast, with middle lobe, lingula, lower lobe, and segmental resection, the incidence is much less than 5%. Postpneumonectomy pulmonary edema is a lethal complication and can occur without fluid overload. It is more common after a right pneumonectomy than a left pneumonectomy. Treatment is supportive, with ventilatory assistance, monitoring of pulmonary pressures, diuresis, and other supportive measures, but it often is a lethal complication in patients with MDR TB. If pulmonary hypertension is present, it is treated with nitroglycerine, nitroprusside (Nipride), or nitric oxide.
Atelectasis or pneumonia or both are common after mycobacterial surgery. Unlike cancer patients who undergo surgery, when after resection the remaining lung may be more normal, the mycobacterial patient usually has other diseased lung remaining. Therefore, pulmonary complications are harder to treat in MDR TB patients. Prevention using incentive spirometry, the flutter valve, and physiotherapy is essential.
Wound complications are frequent in these catabolic patients. Seromas often form from the latissimus dissection area when muscle flaps are used. Suction drains should be left in these areas until little drainage (less than 25 cc) is collected in a 24-hour period.
Other complications include postoperative bleeding [often a frequent complication in many of the reported series, such as those of Treasure and Seaworth (1995) and van Leuven and associates (1997), among others], injury to the recurrent laryngeal nerve, and empyema. All of these complications occur more commonly in MDR TB surgery because of the adhesive infected nature of the disease process as well as the debilitated catabolic condition of the patient. Despite the possibility of a high complication rate following these surgical procedures, B. J. Pomerantz and colleagues of my group (2001) reported only 20 adverse events in 178 resections in 170 patients (11.2%) with MDR TB. The mortality rate was 3.3%. Moreover, in patients with MDR TB who are good surgical candidates, cure can be obtained in over 90% of cases if antimycobacterial agents are continued for the appropriate time postoperatively.
ENVIRONMENTAL MYCOBACTERIAL INFECTIONS
Other mycobacterial lung infections, as noted earlier, have gone through a series of name changes. The term environmental mycobacteria (EM) now seems the most appropriate since, unlike M. tuberculosis, these organisms are found free in water and soil. Environmental mycobacterial infections seem to be increasing in absolute numbers as well as in recognition as a major cause of pulmonary disease. Most frequently they infect patients with previously diseased lungs, and the infection has a more indolent course than in patients infected with M. tuberculosis. Lung damage due to previous TB, bronchiectasis, and chest irradiation for breast carcinoma or other disease is found in many patients with EM infections. Additionally, Iseman and DeGroote (2004) report that genetic testing has found that an increasing number of these patients are afflicted with heterozygous cystic fibrosis or an 1-antitrypsin deficiency. Cilia dysfunction has also been recognized in a number of patients with EM infections. Another common finding in EM patients is acid reflux or esophageal dysmotility, either symptomatic or unrecognized. At least 50% of EM patients will have reflux or esophageal dysmotility, and the latter may even occur in as many as 75% of the patients.
Other conditions or findings associated with EM infections include slender body habitus, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and rheumatoid arthritis. Environmental mycobacterial infections, unlike TB, are not transmitted from person to person; therefore, good epidemiologic data are not available. In the United States, a high proportion of patients with EM infection
P.1256
are white. The reason for this is not clear. Another finding is that there is an overwhelming majority of women relative to the number of men with these infections. Again, the reasons for this are not clear.
The most common EM infection is caused by the Mycobacterium avian complex (MAC). This includes Mycobacterium avian and Mycobacterium intracellulare, which are almost indistinguishable on culture and therefore are grouped together as MAC. Mycobacterium avian complex is widespread, and infection usually advances slowly. The indolent nature of these infections often leads to delay in therapy as the disease progresses from lobe to lobe.
Other slow-growing EM infections are caused by M. kansasii, M. xenopi, M. malmoense, and M. simiae. Lang-Lazdunski and colleagues (2001) in Paris, France, reported the therapeutic reaction in nine patients with M. xenopi infection who were not responding to medical therapy. Satisfactory results were seen in eight patients, and a poor result due to persistently positive sputum culture in one patient. Nine additional patients were operated upon with a diagnostic intent. In one patient, the sputum culture became positive and remained so despite medical therapy. However, in neither of the patients with persistently positive sputum culture was there progression of the pulmonary disease. The role of resection in this group of patients remains somewhat obscure unless there is progressive pulmonary disease that does not respond to medical therapy.
Rapid-growing EM producing significant lung pathology include M. abscessus and M. chelonae. These EM infections seem to be increasing in number in the United States.
The medical treatment of EM infections, as with TB, is a multidrug regimen based on specific culture data. Resistance and intolerance to antimycobacterial drugs are high. Some of the complications of the various drugs used in the treatment of EM infections as well as TB are listed in Table 88-1. Patients infected with rapid growers are more difficult to treat because of poor bacteriocidal antibiotic effectiveness against these organisms. Iseman and DeGroote (2004) observed that although patients with AIDS may become infected with M. avian, AIDS patients do not show increased infection with rapid growers.
Iseman and associates (1991), as well as the author and colleagues (1996), noted that there appears to be a propensity of EM infections to involve the lingula or middle lobe, or both, of slender, older women (Fig. 88-4). This distribution of EM disease has been termed the Lady Windermere syndrome by Reich and Johnson (1992). Chalermskulrat and colleagues (2002) have recently reviewed the salient features and the medical treatment of this condition. Often these women will have skeletal abnormalities such as scoliosis or pectus excavatum. Also, patients with middle lobe or lingula disease frequently have mitral prolapse. Surgical resection is frequently required due to the irreversible lung destruction in the middle lobe or lingula in these older women.
P.1257
I have not found this syndrome in men, though if it occurs it must be rare.
Table 88-1. Side Effects of Antimycobacterial Drugs | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||
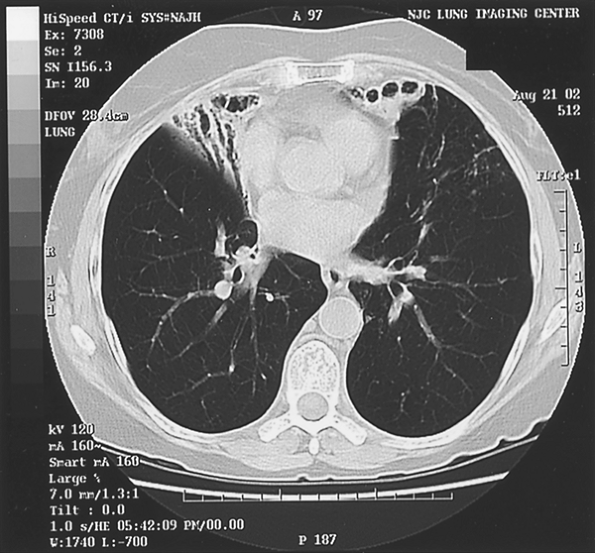 |
Fig. 88-4. Patient infected with Mycobacterium avian complex with middle lobe and lingula disease. |
Surgical Resection in Environmental Mycobacterial Disease
Surgical intervention for patients with EM infections is similar to that for patients with MDR TB. After appropriate antibiotic therapy, resection should be performed if there is a destroyed lung, extensive cavitary disease, or severe involvement of the right middle lobe or lingula. Muscle flaps are used for the same indications as in patients with MDR TB; however, they are not used following resection of the middle lobe or lingula, or in most cases following the resection of a lower lobe.
Resectional surgery by experienced surgeons can be done with an operative mortality of less than 5%. Many pneumonectomies and upper lobe resections are done in an extrapleural plane. It is important to not enter the mycobacterial cavities. When cavities are entered and the contents spilled, contamination of the pleural cavity occurs.
Complications After Surgery for Environmental Mycobacterial Disease
Mycobacterial surgery for EM disease is fraught with a high complication rate. All patients operated upon are medical failures. Factors that have been found to increase the complication rate are diabetes, low albumin level, chest wall irradiation, positive sputum smear, polymicrobial contamination, and redo thoracotomies. There is a high incidence of bronchopleural fistulae, approximately 20%, following right pneumonectomy for EM disease. This is lowered by muscle and omental flaps. Wound complications are frequent in patients infected with M. chelonae and M. abscessus. Pain out of proportion to visible findings in these patients often indicates an underlying infection. Postpneumonectomy pulmonary edema occurs in similar numbers as in pneumonectomies for other diseases and is more common following right pneumonectomy. If it occurs, it tends to be a lethal complication in environmental mycobacterial surgery.
Nutrition is emphasized postoperatively, as it is preoperatively. The continuation of antimycobacterial drugs and narcotics often makes the patient anorexic, which must be addressed with supplemental calories. Pulmonary rehabilitation is continued for a long time after mycobacterial surgery. The debilitated patient takes longer to recover than the healthier patient being operated upon for other diseases.
Surgical Results
Results following surgery for EM disease are less satisfactory than for MDR TB. The EM patient has a more chronic disease, which often involves other areas of the lung that are not resected. Reactivation of EM infections should be treated aggressively. Patients with EM infection should probably be treated and operated upon earlier to produce better results.
Summary
Tuberculosis will probably not disappear. With 3 million deaths from TB a year and 30% to 40% of the world's population representing a latent source, epidemics can be expected. The number of patients with MDR TB is increasing, unfortunately mostly in countries with limited funds for therapy. The spread of MDR TB is thus becoming a worldwide problem. The rising number of EM infections will likewise remain a source of concern for both medical and surgical physicians. The increasing number of patients with EM infections appears to be due both to increasing recognition as well as to an absolute increase in numbers. Earlier surgical intervention in these patients is recommended.
OTHER SURGICAL INDICATIONS IN PATIENTS WITH MYCOBACTERIA TUBERCULOSIS INFECTION
Mycotic Infection and Life-Threatening Hemoptysis in Patients with Tuberculosis
Superimposed fungal disease is most commonly caused by Aspergillus fumigatus, but infections with Monosporium apiospermum organisms were reported by Jung and colleagues (1977).
P.1258
Intracavitary mycetoma are seen with either of these infections. Hemoptysis is a common complaint, and massive life-threatening hemorrhage may occur, particularly in those patients in whom the underlying disease process is pulmonary TB. In a review by Stoller (1992) of 12 series of massive hemoptysis, active TB was present in 22.5% to 49% of the cases and inactive TB was present in 17% to 51%, with a combined total of 18% to 76% of cases. Although a more conservative approach, as Faulkner (1978) and Jewkes (1983) and their associates suggested, may be appropriate in patients with minor episodes of hemoptysis and mycetoma not associated with underlying TB, those patients with TB usually require an eventual resection (elective, if possible) to control the problem.
Massive life-threatening hemoptysis in patients with TB may occur also in the absence of a fungal infection because of erosion into a bronchial artery or, more rarely, because of pulmonary arterial bleeding caused by rupture of a Rasmussen's aneurysm in cavitary TB.
The management of massive hemoptysis and the timing of surgical intervention pose difficult problems. Initially, the patient should be positioned to minimize aspiration of the blood as much as possible. Bronchoscopy is necessary to identify the site of bleeding, and its role in the management of massive hemoptysis has been stressed by Dweik and Stoller (1999). Gourin and Garzon (1974) recommended prompt surgical resection for any individual who has bled more than 600 mL in 24 hours or less. With such a course of action, the mortality rate was 18%, as compared with a 75% rate in those treated conservatively after bleeding this amount in 16 hours. When active bleeding is present at the time of operation, these authors recommended single-lung anesthesia with balloon occlusion of the bronchus of the bleeding lung over the use of a double-lumen endotracheal tube for the conduct of anesthesia. Garzon and associates (1982) repeated this recommendation. McCollum and associates (1975), however, stated that the double-lumen tube is satisfactory. Gottlieb and Hillberg (1975) advocated endobronchial tamponade with a Fogarty balloon catheter to control the bleeding, either before surgical intervention or if such intervention is contraindicated.
With the development of the technique of bronchial artery embolization, the necessity of surgical intervention before controlling the bleeding site has been reduced. At present, the appropriate therapeutic plan is to identify the side and lobar origin of the bleeding by bronchoscopy; endobronchial tamponade is then established as necessary to prevent flooding of the remaining noninvolved lung. With the bleeding temporarily controlled, bronchial arteriography is performed to identify the bleeding area, and the appropriate vessel is then embolized with Gelfoam.
Eckstein and co-workers (1986) and Shetty and Magillijan (1986) reviewed the technique, results, and potential complications of this procedure, such as mediastinal hematoma and neurologic damage. Mal and colleagues (1999) reported immediate control of the bleeding episode in 77% of 56 patients; long-term control was achieved in 45% by embolization alone. In the earlier series reported by Uflacker and associates (1983), bleeding was controlled by embolization alone in 26 (78%) of 33 patients; in the remaining 7, surgical resection was required following the embolization. In the presence of a mycetoma and residual pulmonary disease, however, the rebleed rate was high (43%). This was accompanied by a high mortality rate if interval resection was not or could not be carried out. Thus, even though the bleeding is controlled by the initial embolization, elective resection of the involved area should be carried out when the patient's pulmonary function and the underlying disease are suitable for such a course of action. With this therapeutic approach, Uflacker and associates (1983) reported only an overall 7% to 9% mortality rate for the management of massive hemoptysis. A modified plombage thoracoplasty with the use of a plastic tissue expander, such as reported by Talamonti and associates (1989), has been suggested as a method of controlling hemoptysis associated with a mycetoma in a patient who is unable to withstand a surgical resection (W. Fry, personal communication, 1991).
Tuberculous Bronchial Stricture
Bronchial stricture associated with previous endobronchial TB is uncommon. In North America, tuberculous endobronchitis per se is rare, as noted by Matthews and associates (1984). The incidence of endobronchitis appears somewhat higher in Japan. Ozawa (1981) and Tanaka (1981) and their colleagues reported an incidence of 4.7% to 7.5% of patients with parenchymal TB. In Hong Kong, So and colleagues (1982) reported an incidence of 18%. With subsequent healing of the area of endobronchitis, stenosis of the involved bronchus can develop, as previously noted. Stricture can also be caused by enlarged granulomatous lymph nodes. Clinical symptoms or radiographic findings resulting from the bronchial obstruction can occur. At times, carcinoma may be considered as the cause of obstruction when evidence of TB cannot be identified by bronchial biopsy or sputum cultures.
When carcinoma cannot be excluded or when distal parenchymal destruction has occurred, resection of the stenotic bronchus and the distal involved lung parenchyma is necessary. When the stenosis is symptomatic, lung destruction is absent, and the diagnosis of tuberculous stenosis is known, resection of the area of bronchial stenosis with bronchoplastic reconstruction is the preferred method of treatment, as noted by Ozawa and associates (1981). Watanabe and colleagues (1988) reported nine operative interventions (three lobectomies, one pneumonectomy, and five upper lobe sleeve lobectomies; Fig. 88-5) for these complications, with excellent results. Recently, Watanabe and co-workers (1997) updated their experience with 19 patients with bronchial stenosis due to TB. Twelve patients
P.1259
underwent surgery that included five resections and nine bronchoplastic procedures with either a right or left upper sleeve lobectomy. No deaths or complications were recorded. Han and associates (1992) reported the successful use of a Gianturco self-expanding metallic stent to correct a short but tight stenosis of the left main-stem bronchus that was the result of TB endobronchitis and that had caused a total atelectasis of the left lung. Watanabe and colleagues (1997) had poor results in two patients in whom internal splinting was attempted.
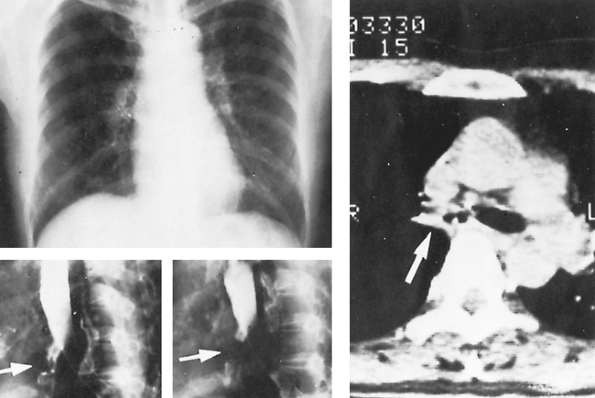 |
Fig. 88-5. A 50-year-old woman had cough and exertional dyspnea. The chest radiograph was normal, but bronchography and computed tomographic scanning revealed stenosis of the right main-stem bronchus (arrows) from healed tuberculous endobronchitis. Right upper sleeve lobectomy with bronchoplastic repair resulted in complete relief of symptoms. From Watanabe Y, Murakami S, Iwa T: Bronchial stricture due to endobronchial tuberculosis. Thorac Cardiovasc Surg 36: 27, 1988. With permission. |
Other Surgical Indications
In patients with known or presumed TB, the question of the presence of malignant disease poses a problem, especially among patients with peripheral nodules, those who have a radiographic change in previously stable disease but whose sputum culture remains negative, and those with an undiagnosed pulmonary lesion but a positive tuberculin test. The management of these individuals requires appropriate diagnostic investigation, which may include video-assisted thoracoscopic exploration or even a thoracotomy. In the experience of Whyte and associates (1989), suspected malignancy was the most common reason (77%) for resections in 31 patients with subsequently proved or known (16%) pulmonary TB.
The management of postresectional complications of persistent empyema or bronchopleural fistulae is discussed in Chapters 37 and 59. At times, when the initial operation has been less than a pneumonectomy, a completion pneumonectomy may become the procedure of choice. Although a completion pneumonectomy entails low morbidity and mortality rates when carried out for recurrent or a new pulmonary malignancy, such procedures have high rates of postoperative complications and deaths when done because of extension or recurrent inflammatory disease in the remaining ipsilateral lung tissue. McGovern and colleagues (1988) noted a mortality rate of 28% when completion pneumonectomy was performed for inflammatory disease, versus only 9.2% for lung cancer. Al-Kattan and Goldstraw (1995) also noted a higher mortality rate after a completion pneumonectomy for benign inflammatory disease, although Gregoire and associates (1993) did not find this difference in mortality in benign versus malignant disease. However, the technical aspects of the procedure are frequently difficult in patients with benign disease, and, at times, intrapericardial ligation of the vascular supply to the remaining lung becomes necessary. Support of the closure of the bronchial stump by a transposed muscle flap or an omental flap is also recommended in those cases with persistent or recurrent MDR TB or EM disease or their complications that require a completion pneumonectomy.
Surgery for Pulmonary Tuberculosis in Children
In children, the indications for surgical intervention are few. A caseous pneumonic process may resolve more slowly than in the adult; the decision concerning the advisability of operation frequently should be postponed for many months or years. In children, in addition to the indications noted in adults, surgical treatment is required in the management of certain complications of progressive primary pulmonary TB. These complications are an uncontrolled, progressively enlarging primary parenchymal lesion with or without cavitation, persistent distal lobar or segmental atelectasis as the result of postprimary bronchostenosis,
P.1260
and clinically significant bronchiectasis (Fig. 85-6). Infrequently, bronchial obstruction may persist because of enlarged, involved peribronchial lymph nodes. With failure of response to medical therapy and persistence of symptoms, particularly respiratory distress, Nakvi and Nohl-Oser (1979) suggested excising or evacuating the caseous material to relieve the obstruction. Worthington and associates (1993) reported this approach in 13 children.
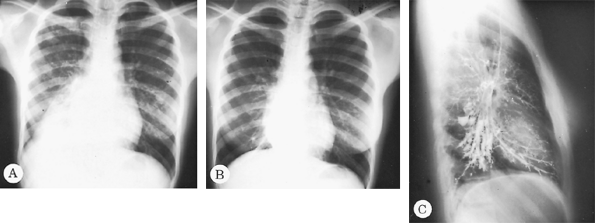 |
Fig. 88-6. A. Posteroanterior radiograph of a 15-year-old girl with bilateral pulmonary tuberculosis and atelectasis of the right lower lobe. B. Same view, after 11 months of treatment, shows clearing of bilateral infiltrate but a completely atelectatic right lower lobe behind the border of the right side of the heart. C. Lateral view of the bronchogram showing extensive bronchiectatic changes throughout the entire right lower lobe. |
Although in the past the incidence of surgical intervention was approximately 5% in children with pulmonary TB under the age of 16 years, as Lees and associates (1967) reported, at present it is rare. Lowe and associates (1980) reported that in a series of 140 children with pulmonary TB, surgical intervention was necessary in only 2 of these individuals, for an incidence of only 1.4%. However, Hewiston and Von Oppell (1997) reported on 161 children under the age of 13 in South Africa who underwent 168 procedures for pulmonary TB or its complications. Surgical decompression of obstructing enlarged lymph nodes (25 acute, 11 chronic) was required and was successful in all but 3 of the chronic cases. Pulmonary resection was required in 72 patients with postprimary tuberculous parenchymal drainage, with a mortality of 2.7% and a morbidity of 16.7%.
REFERENCES
al-Kattan K, Goldstraw P: Completion pneumonectomy: indications and outcome. J Thorac Cardiovasc Surg 110:1125, 1995.
Ashour M: Pneumonectomy for tuberculosis. Eur J Cardiothorac Surg 12: 209, 1997.
Ashour M, et al: Unilateral post-tuberculous lung destruction: the left bronchus syndrome. Thorax 45:210, 1990.
Brown J, Pomerantz M: Extrapleural pneumonectomy for tuberculosis. Chest Surg Clin N Am 5:289, 1995.
Chalermskulrat W, Gilbey JG, Donohue JF: Nontuberculous mycobacteria in women, young and old. Clin Chest Med 23:675, 2002.
Connery CP, et al: Median sternotomy for pneumonectomy in patients with pulmonary complications of tuberculosis. Ann Thorac Surg 75: 1613, 2003.
Dweik RA, Stoller JK: Role of bronchoscopy in massive hemoptysis. Clin Chest Med 20:89, 1999.
Dye C, Watt CJ, Bleed D: Low access to a highly effective therapy: a challenge for international tuberculosis control. Bull World Health Organ 80:437 2002.
Dye C, et al: Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677, 1999.
Eckstein MR, Waltman AC, Athanasoulis CA: The management of massive hemoptysis: control by angiographic methods. In Kittle CF (ed): Current Controversies in Thoracic Surgery. Philadelphia: WB Saunders, 1986, p. 255.
Eloesser L: An operation for tuberculous empyema. Surg Gynecol Obstet 60:1096, 1935.
Farmer P: Infections and Inequalities: The Modern Plagues. Berkeley: University of California Press, 1999.
Faulkner SL, et al: Hemoptysis and pulmonary aspergilloma: operative versus nonoperative treatment. Ann Thorac Surg 25:389, 1978.
Forlanini C: Zur behandlung der lungenschwindsucht durch kunstlich erzengten pneumothorax. Deutsche Med Wochenschr 32:1401, 1906.
Garzon AA, Cerruti MM, Golding MR: Exsanguinating hemoptysis. J Thorac Cardiovasc Surg 84:829, 1982.
Gottlieb LS, Hillberg R: Endobronchial tamponade therapy for intractable hemoptysis. Chest 67:482, 1975.
Gourin A, Garzon AA: Operative treatment of massive hemoptysis. Ann Thorac Surg 18:52, 1974.
Gregoire J, et al: Indications, risks and results of completion pneumonectomy. J Thorac Cardiovasc Surg 105:918, 1993.
Han JK, et al: Bronchial stenosis due to endobronchial tuberculosis: successful treatment with self-expanding metallic stent. AJR Am J Roentgenol 159:971, 1992.
Hewiston JP, Von Oppell UO: Role of thoracic surgery for childhood tuberculosis. World J Surg 21:468, 1997.
Iseman MD: A Clinician's Guide to Tuberculosis. Philadelphia: Lippincott Williams & Wilkins, 2000.
Iseman MD, DeGroote M: Environmental mycobacterium (EM) infections.In Gorbach SL, Bartlett JG, Blacklow NR (eds): Infectious Diseases. 3rd Ed. Philadelphia: Lippincott Williams & Wilkins, 2004.
Iseman MD, et al: Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease due to Mycobacterium avian complex. Am Rev Respir Dis 144:914, 1991.
P.1261
Jewkes J, et al: Pulmonary aspergilloma: analysis of prognosis in relationship to hemoptysis and survey of treatment. Thorax 38:572, 1983.
Jung JY, et al: The role of surgery in the management of pulmonary monosporosis. A collective review. J Thorac Cardiovasc Surg 73:139, 1977.
Lang-Lazdunski L, et al: Pulmonary resection for Mycobacterium xenopi pulmonary infection. Ann Thorac Surg 72:1877, 2001.
Lees WM, Fox RT, Shields TW: Pulmonary surgery for tuberculosis in children. Ann Thorac Surg 4:327, 1967.
Lowe JE, et al: Pulmonary tuberculosis in children. J Thorac Cardiovasc Surg 80:221, 1980.
Mal H, et al: Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest 115:996, 1999.
Matthews JI, Matarese SL, Carpenter JL: Endobronchial tuberculosis simulating lung cancer. Chest 86:642, 1984.
McCollum WB, et al: Immediate operative treatment for massive hemoptysis. Chest 67:152, 1975.
McGovern EM, et al: Completion pneumonectomy: indications, complications and results. Ann Thorac Surg 46:141, 1988.
Nakvi AJ, Nohl-Oser HC: Surgical treatment of bronchial obstruction in primary tuberculosis in children: report of seven cases. Thorax 34:464, 1979.
Ozawa K, et al: Bronchial tuberculosis a clinical study on 26 cases. Jpn J Chest Dis 40:42, 1981.
Pomerantz BJ, et al: Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg 121:448, 2001.
Pomerantz M, et al: Surgical management of resistant Mycobacterium tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg 52:1108, 1991.
Pomerantz M, et al: Resection of the right middle lobe and lingula for mycobacterial infections. Ann Thorac Surg 62:990, 1996.
Reich JM, Johnson RE: Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 101:1605, 1992.
Reichman LB: Timebomb: The Global Epidemic of Multi-Drug Resistant Tuberculosis. New York: McGraw-Hill, 2001.
Shetty PC, Magillijan DJ: The management of massive hemoptysis: treatment by bronchial artery embolization. In Kittle CF (ed): Current Controversies in Thoracic Surgery. Philadelphia: WB Saunders, 1986, p. 261.
So SY, Lam WK, Yu DYC: Rapid diagnosis of suspected pulmonary tuberculosis fiberoptic bronchoscopy. Tubercule 63:195, 1982.
Stoller JK: Diagnosis and management of massive hemoptysis: a review. Respir Care 37:564, 1992.
Talamonti MS, et al: A new method of extraperiosteal plombage for atypical pulmonary tuberculosis. Chest 96:237, 1989.
Tanaka K, et al: Tracheo-bronchial tuberculosis. Jpn J Chest Dis 40:1015, 1981.
Treasure RI, Seaworth BJ: Current role of surgery in Mycobacterium tuberculosis. Ann Thorac Surg 59:1405, 1995.
Uflacker R, et al: Management of massive hemoptysis by bronchial artery embolization. Radiology 146:627, 1983.
van Leuven M, et al: Pulmonary resection as an adjunct in the treatment of multiple drug-resistant tuberculosis. Ann Thorac Surg 63:1368, 1997.
Watanabe Y, Murakami S, Iwa T: Bronchial stricture due to endobronchial tuberculosis. Thorac Cardiovasc Surg 36:27, 1988.
Watanabe Y, et al: Treatment of bronchial stricture due to endobronchial tuberculosis. World J Surg 21:480, 1997.
Whyte RI, et al: Recent surgical experience for pulmonary tuberculosis. Respir Med 83:357, 1989.
Worthington MG, et al: Surgical relief of acute airway obstruction due to pulmonary tuberculosis. Ann Thorac Surg 56:1054, 1993.
Connery and colleagues (2003) have recently suggested the use of a median sternotomy incision for access to the hilar structures in patients with tuberculosis who require a pneumonectomy. Unfortunately, this is not the problem in most patients with MDR TB because the mediastinal area is only infrequently involved by the inflammatory process. The problem is the freeing up of the dense adhesions between the infected or destroyed lung and the chest wall. The extrapleural approach via a posterior lateral thoracotomy advocated by Pomerantz remains, in the opinion of many, the most satisfactory approach in these cases. The Senior Editor
EAN: 2147483647
Pages: 203