75 - Tracheostomy
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIV - Congenital, Structural, and Inflammatory Diseases of the Lung > Chapter 89 - Thoracic Mycotic and Actinomycotic Infections of the Lung
function show_scrollbar() {}
Chapter 89
Thoracic Mycotic and Actinomycotic Infections of the Lung
John C. Lucke
THORACIC MYCOTIC INFECTIONS
Fungal infections of the lungs are not encountered commonly by most practicing thoracic surgeons. Exceptions are surgeons who practice in the southwestern United States, an area endemic for coccidioidomycosis, or along the Mississippi River Valley, an area endemic for histoplasmosis. But because mycotic lung infections may mimic both lung cancer and tuberculosis, they should be included in the thoracic surgeon's differential diagnoses. Most fungi are opportunistic, causing pulmonary and other systemic infections in humans only when natural host resistance is impaired. Some fungi, however, are true pathogens, causing systemic infections in otherwise healthy patients. These fungi, Histoplasma, Coccidioides, Blastomyces, and Paracoccidioides, are endemic. They usually cause only mild infections, and they are dimorphic. In nature they occur in a mycelial form, but in tissue they are present as yeast, or yeastlike spherules in the case of Coccidioides. These morphologic differences occur in response to changes in temperature. The yeast cells that grow in culture at 37 C survive and reproduce within tissue macrophages. According to Maresca and Kobayashi (1989), the ability of these fungi to adapt to changing environments through temperature-induced metamorphosis helps to explain their roles as pathogens. Sporothrix is another dimorphic fungus that, although primarily a subcutaneous pathogen, can produce pulmonary and other systemic infections.
In addition to surgeons practicing in endemic areas, surgeons managing organ transplant programs are likely to encounter fungal infections in the lungs of patients whose immune systems have been altered.
Two major considerations are worth noting. First, immunologically compromised patients [i.e., patients undergoing chemotherapy for malignancies, patients receiving corticosteroids or aggressive antibiotic therapy for a wide variety of conditions, very young and very old patients, and patients with acquired immunodeficiency syndrome (AIDS)] are more likely to become infected with fungi. Consequently, the incidence of fungal infections has increased significantly (Table 89-1), and the crude mortality for these patients is higher than for other bacterial infections. Second, several new antifungal agents have become available that are effective against many fungi and are less toxic to patients (Table 89-2).
In most instances, the role of the thoracic surgeon in the care of patients with fungal infections of the lungs is adjunctive (i.e., consultative and diagnostic). Both roles are important and require close collaboration with the pathologist and internist. Frequently, however, as noted by Godwin (1983) and Lillington (1982), thoracotomy is necessary because of a strong suspicion of carcinoma, especially in men over 40 years of age.
The laboratory diagnosis of fungal infections is discussed in detail in Chapter 14. The lack of methods for early and accurate diagnosis of many systemic fungal infections is a major concern and frequently the cause of delayed treatment.
Histoplasmosis
Histoplasmosis, probably the most common of all fungal infections of the lungs, is caused by the airborne spore Histoplasma capsulatum. Initially the disease was thought to be rare and almost always fatal. By 1945, however, the relationship between benign pulmonary calcifications and reactivity to skin testing with histoplasmin antigen was established, and widespread, almost universal, subclinical infection with histoplasmosis in certain well-defined geographic areas of the United States was recognized. Some 30 million people were estimated to be infected, mostly along the Mississippi River and its tributaries (Fig. 89-1). Active disease, however, occurs uncommonly, with only 1 in 2,000 patients developing chronic pulmonary disease. Disseminated infection occurs in approximately 1 in 100,000 instances.
Table 89-1. Rank Order of Nosocomial Bloodstream Pathogens and the Associated Crude Mortality Rate Among 49 Hospitals Throughout the United States | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
P.1263
The organisms are found in soil, especially that contaminated by the droppings of fowl or bats. Local outbreaks have been associated with disruption of soil during construction, cleaning attics, bridges or barns, tearing down structures, and cave exploration. In tissue, yeast forms may appear packed in macrophages, or the stained capsules of dead organisms can be identified in the necrotic center of chronic granulomatous lesions (Fig. 89-2).
Wheat and associates (2000) classified histoplasmosis into self-limiting presentations that usually require no treatment and presentations that require treatment with antifungal preparations and possible surgical resection (Table 89-3).
Pathogenesis and Symptomatology
Benign Infection of the Normal Host
In highly endemic areas, essentially the entire population becomes infected, without identifiable symptomatology, and often with conversion from a negative to a positive
P.1264
histoplasmin skin test result as the only evidence of infection. Occasionally in infants and children, rarely in adults, cough, fever, and hilar lymphadenopathy mark the episode. Rarely, the process progresses to the second or third categories. After an unusually heavy exposure to dust containing the spores of H. capsulatum, however, the clinical entity of acute histoplasmosis of either primary or reinfection types is recognized (Fig. 89-3). Radiographically, it is characterized by scattered, sparse to almost confluent, small pneumonic or nodular infiltrates. The process is self-limiting and may leave multiple nodular or calcific buckshot residues. In fewer than 1% of the infections, dissemination or death occurs. When acute histoplasmosis is caused by reinfection, it produces a finer, more miliary granulomatous reaction. The radiographic characteristics occurring in an endemic area after exposure to dust contaminated with fowl or bat droppings, a positive skin test result, and positive serologic findings (complement fixation) aid in confirming the diagnosis. Medical treatment is recommended for patients who remain ill for 2 to 4 weeks or for patients who are dyspneic or hypoxic.
Table 89-2. Antifungal Drugs for Pulmonary and Systemic Infections | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Table 89-3. Indications for Treatment in Patients with Histoplasmosis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
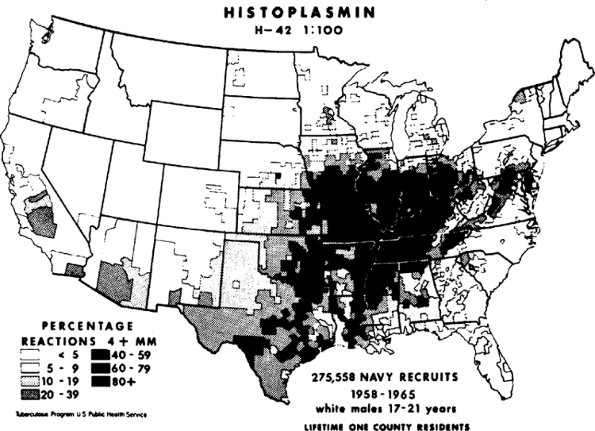 |
Fig. 89-1. Map of the United States showing areas of endemicity for histoplasmosis, based on county of lifetime residence of naval recruits with positive histoplasmin skin test reactivity, shown as a percentage of those treated. From Edwards LB, et al: An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis 99(suppl):1, 1969. With permission. |
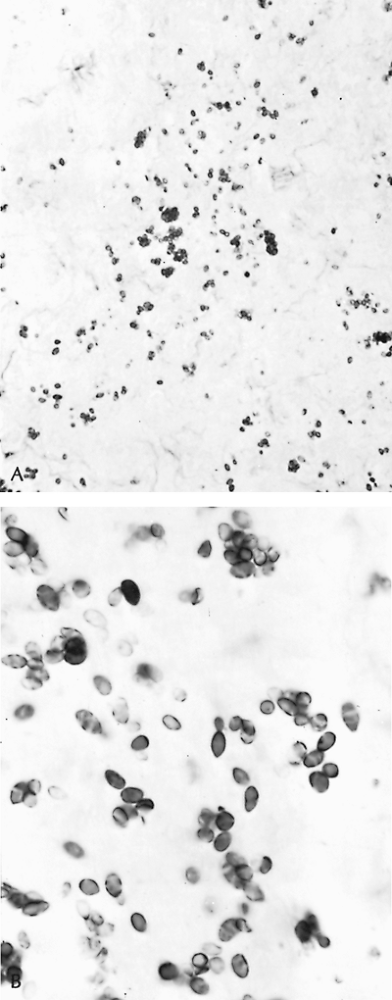 |
Fig. 89-2. Histoplasma capsulatum organisms. A. Nonviable capsules in necrotic area of a granuloma (Gomori's stain, original magnification 780). B. Viable yeast forms in lymph node (original magnification 1,300). |
Opportunistic Infections
Opportunistic infections occur on two bases: immunologic and structural. Because histoplasmosis is often thought
P.1265
of as a disease of the reticuloendothelial system, patients with deficiencies in cellular immunity are particularly susceptible. This deficiency is identifiable in patients receiving immunosuppressive medications, lymphatic or hematopoietic malignancies, and AIDS patients. The pulmonary alveolar macrophage is parasitized by H. capsulatum and provides not only nourishment and protection, but also possibly transportation to all parts of the reticuloendothelial system. Progressive disseminated histoplasmosis is a serious condition, for which full courses of medical therapy are mandatory.
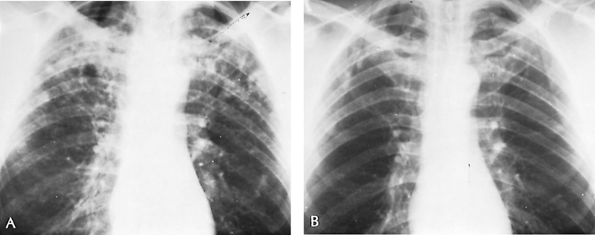 |
Fig. 89-3. Acute histoplasmosis. A. Thoracic radiograph of 48-year-old man who was asymptomatic when this routine radiograph was made. Purified protein derivative and histoplasmin skin test results were both positive. One sputum culture was reported to show Histoplasma capsulatum. Radiograph shows bilateral multiple soft infiltrates. No treatment was given. B. Thoracic radiograph 2 years later shows minimal residual fibrotic nodules. Patient remained well during the subsequent 5 years. |
Chronic pulmonary histoplasmosis is postulated to occur as an opportunistic infection in an area of lung structurally damaged by centrilobular or bullous emphysema. Substantial radiologic evidence shows that chronic obstructive pulmonary disease sets the stage for chronic cavitary histoplasmosis. The organisms are found only in effusions in emphysematous spaces or in the necrotic lining of established cavities (Fig. 89-4).
According to this hypothesis, colonization of an emphysematous space, with spillover (i.e., bronchogenic spread) of
P.1266
antigen-laden effusion into adjacent areas of lung, accounts for the recognized interstitial pneumonitis and necrosis characteristic of this form of the disease. Infection of cavity walls similarly is thought to be secondary to either colonization or to dispersal of the same antigenic material from adjacent colonized spaces that causes, in other areas, pneumonitis and necrosis. Cavitary walls 2 mm or more thick suggest active disease that is likely to persist; thinner-walled cavities usually signify inactivity. Persistent thick-walled (4 mm) cavities appear to promote continuing necrosis and enlargement, the so-called marching cavities.
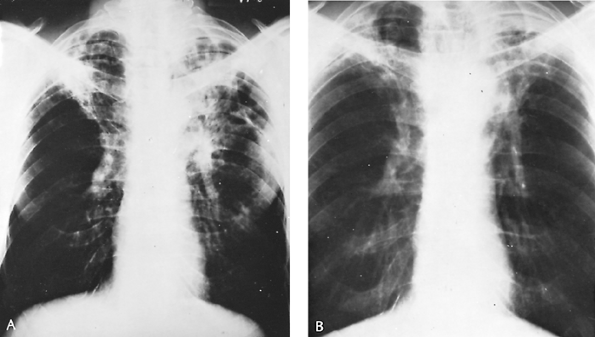 |
Fig. 89-4. Chronic cavitary histoplasmosis in a 53-year-old man. Initially, pulmonary tuberculosis was suspected; ultimately, sputum cultures and animal innoculation studies proved this to be chronic cavitary histoplasmosis. The patient received 2 g of amphotericin B over a 3-month period; ultimately, the lung lesions cleared. A. Thoracic radiograph before treatment. B. Appearance 6 months after completed treatment. |
Symptomatology is nonspecific, resembling pulmonary tuberculosis as well as chronic obstructive lung disease. Cough, sputum, and hemoptysis are common, as are weight loss, low-grade fever, and weakness. Symptoms of pulmonary insufficiency may be prominent.
Because immunologic testing is unreliable, a positive diagnosis requires the demonstration of H. capsulatum in cultures of the sputum, which must be repeatedly and persistently tested for this fungus. The thicker the wall of the cavity, the more readily organisms are identifiable by sputum cultures. In patients with progressive disseminated histoplasmosis, the organism may be seen in circulating phagocytes (buffy coat) or lymph nodes. A bone marrow biopsy and culture often provide the quickest diagnosis. H. capsulatum antigen, when positive in blood, urine, or spinal fluid, is of both prognostic and diagnostic value.
Treatment
Chronic cavitary histoplasmosis should be treated with itraconazole. As noted by Wheat and associates (2000), however, the more severe infection may require amphotericin B. Response rates are 80%; however, the end points are blurred because of the underlying pulmonary disease. Progressive disseminated disease in a normal host should be treated with amphotericin B followed by a course of itraconazole, with a response rate of 85%. Intravenous itraconazole can be used as primary treatment in patients with mild or moderate disease. Immunocompromised patients require life-long treatment with itraconazole following initial treatment with amphotericin B. Johnson and co-workers (2002) found that patients with AIDS were better treated with liposomal amphotericin B. Clinical success was achieved in 88% of patients treated with the liposomal formulation and fewer patients developed nephrotoxicity. Furthermore, Wheat and colleagues (2001) found that fungal clearance was more rapid in the liposomal amphotericin B group.
Progressive disseminated histoplasmosis with prosthetic heart valve involvement has been reported by Kanawaty and associates (1991) as well as Isotalo and co-workers (2001). Surgical replacement of the diseased valve and high-dose amphotericin B is the treatment of choice.
Surgical excision is recommended for localized thick-walled cavities only if no improvement is noted after one or two courses of drug therapy, and if pulmonary function permits. Because chronic obstructive lung disease is almost the sine qua non of opportunistic infection of structurally damaged lung tissue by H. capsulatum; however, the risks and benefits of resection have to be carefully and individually assessed to determine what is in the patient's best interests.
Excessive Fibrosis: Healed Primary Lesion
The solitary pulmonary nodule, one of the most common lesions seen by thoracic surgeons, in endemic areas is often a histoplasmoma. This benign lesion results from an excessive response to some antigenic stimulus to fibrogenesis in the necrotic center of the small (2 4 mm) primary focus containing H. capsulatum. Over a period of years, concentric layers of collagen are laid down, approximately 1 to 2 mm per year, resulting in a slowly enlarging and thus ominous-appearing nodular mass. Central or target calcification, however, or concentric laminar calcification that gives unequivocal evidence of the benign nature of the granulomatous reaction, can often be seen radiographically. This calcification is by far most commonly caused by histoplasmosis, but also occasionally occurs with tuberculosis and coccidioidomycosis (Fig. 89-5). Although more than 50% of 1,000 solitary pulmonary nodules in adults, mostly men, were benign granulomas, as Steele (1964) reported, 36%
P.1267
were malignant tumors. Therefore, unless the characteristic radiographic findings noted previously are identified by computed tomographic (CT) scan, if not obvious on plain film radiographs of the thorax, exploratory thoracotomy is often indicated, as Godwin (1983) emphasized, especially in men over 40 years of age (Fig. 89-6). Fluorodeoxyglucose positron emission tomographic imaging (FDG PET imaging) and somatostatin analogue scanning have excellent sensitivity but lack sufficient specificity to suggest not performing a biopsy on a noncalcified nodule. Croft and co-workers (2002) evaluated pulmonary nodules with FDG PET in an endemic region and found the negative predictive value to be only 55%. Perioperative drug therapy is not indicated during resection of granulomas. On the other hand, if the diagnosis of a granuloma can be made by the characteristic radiographic findings, or by transbronchial brushing or percutaneous aspiration needle biopsy, or by evidence of an unchanging lesion for a period of several years, neither thoracotomy nor drug therapy may be needed. High-resolution CT imaging, which determines the density of the lesion, also may help. A high Hounsfield unit number (>164) of the lesion, because of microcalcifications, denotes that the lesion is benign and that surgery is unnecessary (see Chapter 93).
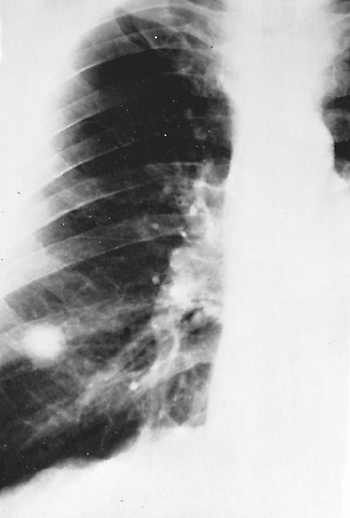 |
Fig. 89-5. Histoplasma granuloma in a male adult. This centrally calcified lesion remained unchanged for 10 years. |
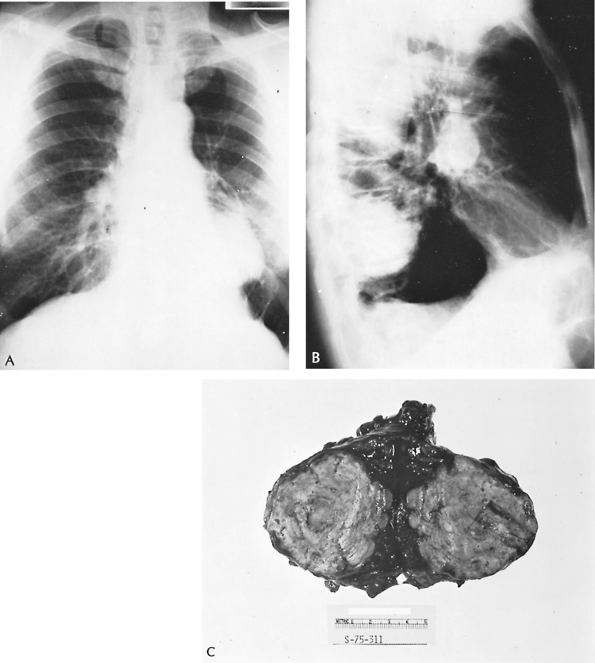 |
Fig. 89-6. Giant histoplasmoma. Posteroanterior (A) and lateral (B) radiographs of an asymptomatic 62-year-old man who was having a routine checkup. Diagnostic studies, including pulmonary angiography and thoracic aortography, did not disclose the nature of the lesion. C. After exploratory thoracotomy on suspicion of bronchial carcinoma, a left lower lobectomy was performed. A large laminated fibrocollagenous granuloma with a necrotic center containing yeast cells morphologically consistent with Histoplasma capsulatum was found. This excessive degree of fibrogenesis is unusual. |
In hilar and mediastinal lymph nodes, this excessive encapsulating fibrogenic response to the antigen of H. capsulatum may have serious consequences, depending on the region involved. In the right paratracheal region, superior
P.1268
vena caval syndrome or right bronchial stenosis may result; subcarinal lymph node involvement may produce stenosis of either or both main bronchi or the pulmonary veins; hilar lymph nodal involvement may cause either pulmonary arterial or bronchial stenosis. Mediastinal granulomas may be produced by the matting together of several large, caseous lymph nodes, presenting as mass lesions, in addition to giving rise to the aforementioned problems. There has been no proven benefit to antifungal or corticosteroid therapy in patients with mediastinal fibrosis as discussed by Mathisen and Grillo (1992). Because of these serious complications and the difficulties in managing them once they appear, excision of resectable, asymptomatic, mediastinal granulomas to forestall such difficult problems has been advocated. Pneumonectomy can be an option when severe mediastinal fibrosis is unilateral. Although pneumonectomy can be lifesaving, it is far more technically difficult and is associated with a high operative mortality rate secondary to dense adhesions and systemic collateral blood supply. Davis and associates (2001) have recently reviewed the subject in detail. Dunn and colleagues (1990) recommend that surgical reconstruction of mediastinal structures should be attempted whenever possible (see Chapters 171 and 172).
Coccidioidomycosis
Coccidioidomycosis is a suppurative and granulomatous infectious disease that primarily attacks the lungs. The disseminated form of the disease is encountered more frequently than in the past because of the large number of immunocompromised patients.
Coccidioidomycosis bears several resemblances to histoplasmosis. This fungus occurs as a soil contaminant in sharply circumscribed geographic areas, resulting in subclinical infection in one third of the population, who are identifiable by a specific skin test. In contrast to histoplasmosis, coccidioidomycosis does not form yeast, but rather undergoes a morphologic change into spherules that are large, thick-walled structures that become filled with endospores. Drutz and Catanzaro (1978) and Ampel and associates (1989) have presented extensive reviews of this disease.
Epidemiology
Fungal organisms occur in a well-defined area of the southwestern United States, including portions of California, Nevada, Arizona, New Mexico, and Texas (Fig. 89-7), as well as in Mexico and Central and South America. Infection results from the inhalation of spore-laden dust. The estimated average annual incidence of symptomatic coccidioidomycosis among susceptible individuals is 0.439. Agricultural and construction workers and others with similar outdoor pursuits are most likely to become infected. Archeologists are at special risk. Crum and associates (2002) reported an outbreak in otherwise healthy U.S. Navy SEALs training in an endemic region, where 45% of the men had serologic evidence of acute coccidioidomycosis. Infectious outbreaks are most common in the late summer and fall, when the soil is dry and the weather is windy. Coccidioidomycosis is being diagnosed with increasing frequency in patients with AIDS, mostly among patients residing in endemic areas, as Galgiani and Ampel (1990) have reported. Patients with hematologic malignancies or who have undergone stem cell transplantation are also at increased risk, as described by Kauffman (2002).
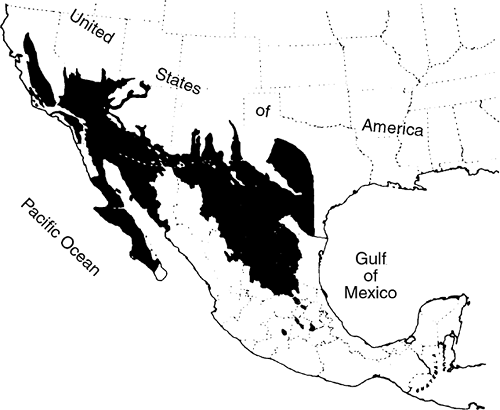 |
Fig. 89-7. Map showing area of endemicity of coccidioidomycosis in the United States and Mexico. From Ochoa GA: Coccidioidomycosis in Mexico. In Ajello CL (ed): Coccidioidomycosis. Tucson: University of Arizona Press, 1967. With permission. |
Unlike most other fungal diseases, infection also may be acquired by inhalation of dust from fomites and from laboratory cultures of Coccidioides immitis. Therefore, such materials must be handled with special precautions.
Pathology
Pathologically, coccidioidomycosis resembles pulmonary tuberculosis. The primary complex is composed of a parenchymal pneumonitis and involvement of a regional lymph node or nodes, which may not be detected clinically. Reactivation of the primary complex may result in reinfection, with the development of caseous nodules, effusions, pneumonic areas, cavities, and calcified, fibrotic, or ossified lung lesions (Fig. 89-8).
Extrapulmonary dissemination to lymph nodes, skin, spleen, liver, kidneys, bones, and meninges is more likely to occur in the primary than in the reinfection phase of the disease and is more likely to afflict blacks, Filipinos, Native Americans, and Mexicans, by factors of 5 to 10 times the incidence seen in whites.
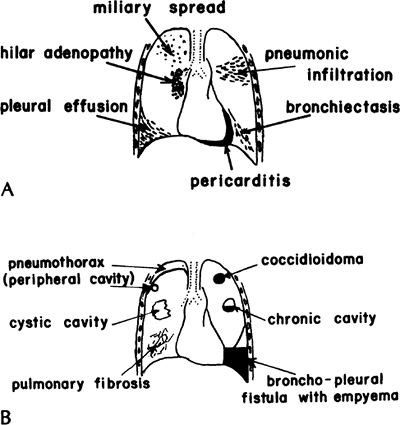 |
Fig. 89-8. Varieties of pulmonary manifestations of coccidioidomycosis. A. Acute stage. B. Chronic stage. From Paulsen GA: Pulmonary surgery in coccidioidal infections. In Ajello L (ed): Coccidioidomycosis. Tucson: University of Arizona Press, 1967. With permission. |
P.1269
The histologic characteristics of coccidioidomycosis lesions are those of granuloma formation with suppuration. With special stains, large (e.g., 15 80 m) spherules, packed with tiny endospores when mature, or the endospores themselves can be seen (Fig. 89-9). Rarely, a mycetoma or fungus ball containing hyphae appears, as Bayer (1976) and Thadepalli (1977) and their associates, as well as Rohatgi and Schmitt (1984), reported, because Coccidioides grows best at body temperature in spherule form.
Symptomatology
Most patients with primary infection have no symptoms. In approximately 25% of patients, either mild or severe symptoms are noted, which are nonspecific, and either referable to the respiratory tract or flulike, with malaise, headaches, and fever. Again, the condition is clustered among blacks and Filipinos. Symptoms may be pleuritic pain and cough productive of mucoid or, rarely, bloody sputum. Erythema multiforme, erythema nodosum, or less specific morbilliform rashes are observed occasionally, especially in women. Mild arthritic manifestations, which are called desert rheumatism, also occur sometimes. The radiographic findings are nonspecific. In the acute stage, miliary lesions, pneumonic infiltrates, hilar adenopathy, or pleural and pericardial effusions may appear. With chronic coccidioidomycosis, solitary nodules representing coccidioidal granulomas; chronic, usually thin-walled cavities, sometimes with an air fluid level; pneumothorax; fibrosis; or empyema may be manifestations of the disease.
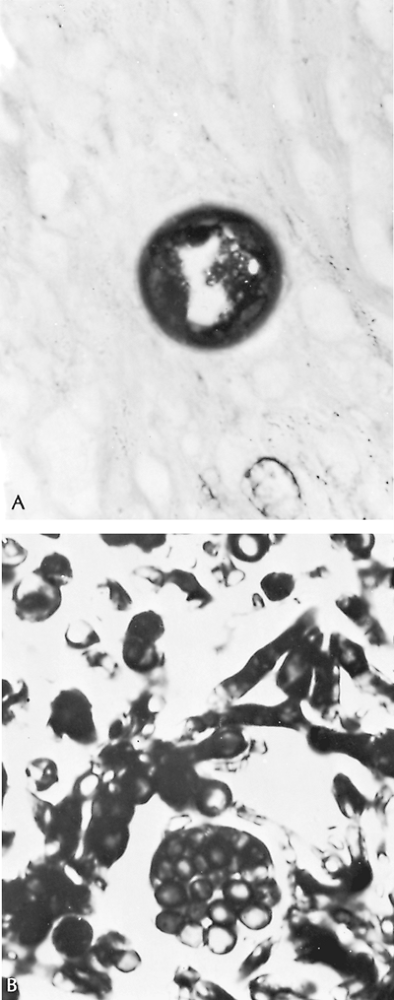 |
Fig. 89-9. A. Old spherule of Coccidioides immitis containing endospores, in an area of pulmonary fibrosis (original magnification 1,300). B. Organisms identified in a coccidioidal fungus ball in a cavitary lesion, showing both hyphae and spore forms (original magnification 1,300). This patient died of extensive cavitary coccidioidomycosis despite amphotericin B therapy 8 years after left pneumonectomy had been performed in an attempt to control his disease. |
P.1270
Diagnosis
A positive culture result of sputum, other body fluid, or tissue is necessary for a definitive diagnosis. Using a rapid diagnostic evaluation of bronchial washings with a Papanicolaou stain with 10% potassium hydroxide digestion, Sarosi and colleagues (2001) were able to make a diagnosis in 9 of 14 patients, when cultures were often negative. In acute coccidioidal pleural effusions, Lonky and associates (1976) noted that cultures of pleural biopsy specimens can be more rewarding than culturing the pleural fluid. Recent conversion of the coccidioidin or spherulin skin test result to positive or positive serology strongly suggests coccidioidomycosis. Although four serologic tests are available, early coccidioidal infection is usually detected only by the tube precipitin or the latex agglutination tests. Pappagianis (2001) has reviewed the value of serologic studies in patients with suspected or confirmed coccidioidomycosis. Quantitation of the level of immunoglobulin G utilizing a coccidioidin from the hyphal phase is useful in prognosis and diagnosis. When coccidioidal disease has disseminated outside of the lungs, the fluid (pleural, cerebrospinal, peritoneal, synovial) can also be tested for antibodies provided the serum is positive.
Treatment
Many patients need no treatment at all. Itraconazole and fluconazole have replaced amphotericin B as the treatment of choice for patients with non life-threatening coccidioidomycosis. Although these triazoles have never been compared directly with amphotericin B, their response rates are comparable. The majority of patients can be treated as outpatients, and the therapy is better tolerated. Fifty-seven percent of patients responded to 400 mg/day of itraconazole treatment as reported by Graybill and associates (1990), whereas 55% of patients with chronic pulmonary coccidioidomycosis responded to 200 to 400 mg/day of fluconazole as reported by Catanzaro and co-workers (1995). Indications for treatment include: (a) prevention of dissemination, especially in black patients, Filipinos, Native Americans, and Mexicans, the threat of which may be indicated by continuing elevation of the titer of the complement fixation test (1:64 or higher); (b) control of cavitary disease with sputum culture results positive for C. immitis; (c) arrest of dissemination of disease; (d) control of progressive chronic pulmonary lesions; (e) coverage during pulmonary resections; and (f) preventive medical coverage in patients with active coccidioidomycosis who require corticosteroids or during pregnancy. For patients with life-threatening illness (e.g., acute respiratory distress, sepsis, or other evidence of overwhelming infection) amphotericin B remains the treatment of choice. After stabilization, therapy can often be switched to itraconazole or fluconazole.
The indications for resective surgery for coccidioidomycosis include localized granulomatous lesions and cavitary disease (Fig. 89-10). Although most undiagnosed solitary pulmonary nodules occurring in patients in the endemic area are coccidioidomas, Read (1972) and Cohen and associates (1972) found 26% to 35% to be malignant. Therefore, in the endemic area, the indications for resection of undiagnosed solitary pulmonary nodules must be individualized, as for histoplasmosis, because a known granuloma does not ordinarily necessitate resection.
Marks and colleagues (1967) reported that localized resections in more than 700 patients were accompanied by a complication rate of approximately 10%, many of them air leaks due to peripheral small bronchopleural fistulae. Spillage of contaminated material into the pleural cavity is associated with a significant increase in complications. For cavitary lesions, in most patients resection is not indicated. When cavities persist for more than 2 to 4 years, however, and are larger than 2 cm in diameter; when they are rapidly enlarging, thick walled, or ruptured, or contain a fungus ball; when they are associated with severe or recurrent hemoptysis; when they occur in diabetic or pregnant patients; and when they coexist with pulmonary tuberculosis, surgical resection, preferably by lobectomy, is indicated, as Nelson (1974) and Baker (1978) and their colleagues, as well as Cunningham and Einstein (1982), suggested. In some patients who are at low surgical risk, resection of persistent cavitary lesions may be recommended in favor of prolonged medical therapy, as discussed in the practice guidelines by Galgiani and Ampel (2000). Some recommend drug coverage with amphotericin B, but it is not clear that the use of amphotericin B has resulted in significantly fewer complications of bronchopleural fistula, empyema, and recurrent cavitation. Intravenous fluconazole may be a reasonable alternative to avoid the potential adverse effects of amphotericin B, as described by Johnson and Sarosi (1995). Resection and biopsy of the undiagnosed coccidioidal granuloma may be accomplished thoracoscopically, as shown in Fig. 89-10.
North American Blastomycosis
North American blastomycosis is a suppurative and granulomatous infectious disease caused by Blastomyces dermatitidis, which is found in tissue as a round, thick-walled, single budding yeast cell, 5 to 20 m in diameter. When originally described by Gilchrist in 1894, it was thought to be a skin disease, but it has since been shown that the lung is the primary site of infection and that skin and other sites are affected because of secondary dissemination.
Epidemiology
North American blastomycosis occurs mostly in the southeastern, South Central, and midwestern states and Canadian provinces that border the Great Lakes. The distribution follows the areas that border the Ohio, Mississippi, and St. Lawrence rivers (Fig. 89-11). This disease is also an
P.1271
airborne infection of exogenous origin, the fungus having been identified in pigeon manure, and is frequently found in soil near bodies of water, which results in exposure to moist soil with decaying vegetation. Thus, the incidence in some studies, as Busey and associates (1964) noted, is highest among persons in close contact with the soil and occupational and recreational activities along streams and rivers. Kitchen and colleagues (1977), however, described an urban epidemic.
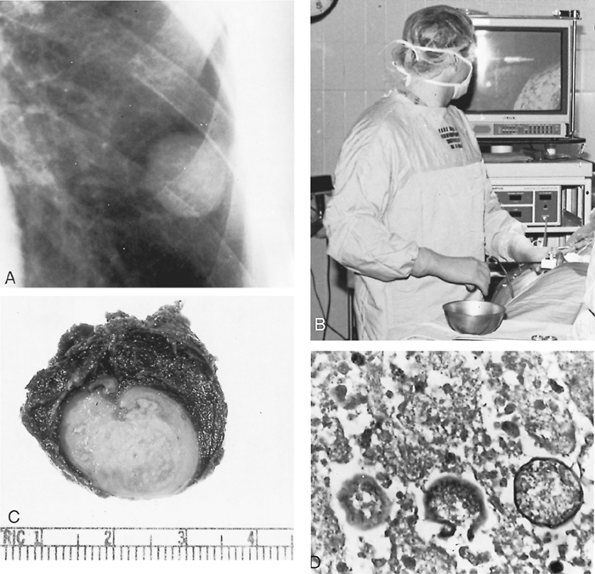 |
Fig. 89-10. Coccidioidal granuloma. A. Thoracic radiograph showing a 2-cm nodule in the left lower lobe of the lung of a 41-year-old man with a 75-pack per year smoking history. He had been stationed in the California desert while in the army. Bronchoscopy was negative. B. The nodule was resected thoracoscopically. The lung and instrument can be visualized on the monitor. C. Resected specimen, which was a coccidioidal granuloma. D. Microscopic sections (original magnification 400) show spherules packed with endospores. |
Pathology and Microbiology
North American blastomycosis characteristically induces a granulomatous and pyogenic reaction with microabscesses and giant cells and, occasionally, caseation, cavitation, and fibrosis. Primarily the lungs, skin, bones, and genitourinary tract are affected. Skin lesions exhibit pseudoepitheliomatous squamous cell proliferation with microabscesses between areas of acanthotic epithelium.
P.1272
Special stains reveal the fungal organisms with the characteristic thick refractile walls (Fig. 89-12). Landis and Varkey (1976) and Laskey and Sarosi (1977) documented endogenous reinfection, as in pulmonary tuberculosis.
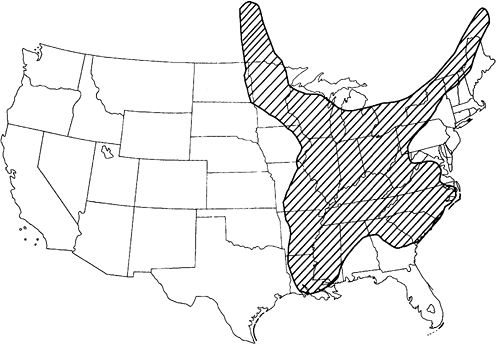 |
Fig. 89-11. Approximate area of endemicity in the United States for North American blastomycosis. Adapted from Menges RW, et al: Clinical and epidemiological studies on 79 canine blastomycosis cases in Arkansas. Am J Epidemiol 81:169, 1965. With permission. |
Symptomatology
The patient with blastomycosis is usually first seen because of symptoms referable to the skin or the lungs, or both. Some patients may present with joint, bone, throat, or prostate involvement as described by Vasquez and co-workers (1998). The cutaneous manifestations begin as single or multiple papules or papulopustules that enlarge slowly, ulcerate, and exhibit elevated, cyanotic edges. Biopsies from such areas are more likely to yield the organisms than are cultures of sputum. The lesions may appear anywhere, on both exposed and unexposed portions of the skin, and are characterized by chronicity (Fig. 89-13). The patient may be unaware of the skin lesions. Healing may occur, leaving a soft noncontracting scar. The weight of clinical and pathologic evidence, as Rabinowitz and associates (1976) pointed out, suggests that a primary pulmonary focus is present in practically all instances of both cutaneous and systemic North American blastomycosis.
After the cutaneous form, the next most common manifestations of blastomycosis are caused by pulmonary involvement. Cough, usually productive only of mucoid sputum, chest pain, and hemoptysis, may occur, or mild fever, malaise, weight loss, weakness, and other nonspecific symptoms may be the only complaints. Physical signs are not characteristic or particularly helpful.
Thoracic Radiographic Findings
Consolidation is characteristic in acute disease but also is common in chronic blastomycosis. Fibronodular lesions, with or without cavitation, reminiscent of pulmonary tuberculosis are also common in chronic disease. Mass lesions, diffuse patterns,
P.1273
pleural involvement, and hilar lymphadenopathy also appear, but less commonly. Kinasewitz and colleagues (1984) recognized that major pleural disease indicates a poor prognosis. Many patients have undergone exploration with the presumptive diagnosis of carcinoma of the lung.
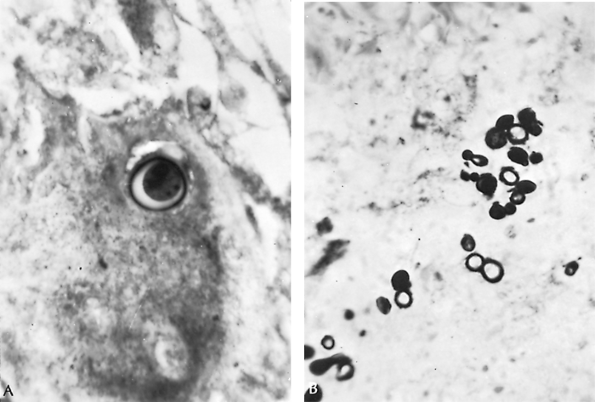 |
Fig. 89-12. Organisms of Blastomyces dermatitidis from resected lung tissue. A. Single thick-walled yeast form with refractile cell wall. B. Multiple yeast forms with single budding characteristic of this fungus (periodic acid Schiff stain, original magnification 1,100). From Takaro T: Thoracic surgery. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
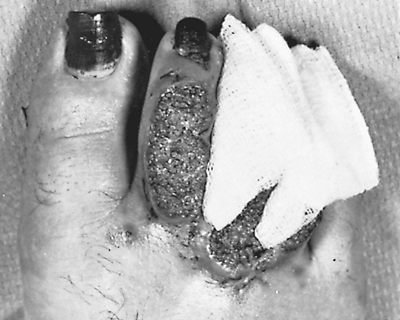 |
Fig. 89-13. Skin lesions of blastomycosis on dorsum of toes. Biopsy of characteristic raised edges showed multiple microabscesses containing Blastomyces dermatitidis. From Takaro T: Thoracic actinomycetic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
Diagnosis
Most commonly, diagnosis is made by cultural demonstration of the organism in the sputum, in material from skin lesions, or from skeletal lesions: abscesses, sinuses, or biopsy material. Sutliff and Cruthirds (1973) advocated the examination of bronchial washings or sputum cytologic techniques for B. dermatitidis; Sanders and associates (1977) confirmed the value of the latter. Lung biopsy may be necessary to make the diagnosis; however, this procedure is occasionally followed by dissemination of the disease. The major differential diagnostic problem in endemic areas of blastomycosis is carcinoma of the lung, which Poe and associates (1972) emphasized. In patients with unrecognized pulmonary blastomycosis, unnecessarily radical operations may be performed with the mistaken diagnosis of tumor. A preoperative diagnosis is therefore important to avoid an unnecessary operation.
Because skin and complement fixation test results may be negative as often as positive, and because of the close antigenic similarity between coccidioidin, histoplasmin, and blastomycin, these serologic intracutaneous diagnostic tests are inadequate and cannot be relied on for a definitive diagnosis.
Treatment
Blastomycosis should be treated whenever the diagnosis can be made unequivocally by identifying the organism. Self-limited disease, however, was described by Sarosi and associates (1986). In any event, a presumptive diagnosis is not an adequate basis for therapy, because the drugs used for treatment are expensive and potentially toxic. Practice guidelines for the management of patients have been published by Chapman and associates (2000). Dismukes and co-workers (1992) and Davies and Sarosi (1997) have shown that most patients with pulmonary blastomycosis can be treated with oral itraconazole. The recommended dose is 400 mg orally for 6 months. Response rates with this therapy are 95%, with more than 90% of patients being cured. Ketoconazole and fluconazole are believed to be less active agents. For cavitary lesions or extensive disease or for systemic dissemination, amphotericin B is preferable (Fig. 89-14).
Resection is indicated when bronchial carcinoma is suspected after efforts have been made, especially in endemic areas, to rule out North American blastomycosis. Drug treatment with amphotericin B or itraconazole should follow surgery when the diagnosis is made only at the time of thoracotomy. Resection of known blastomycotic cavitary lesions is indicated if they persist after adequate drug therapy with amphotericin B or itraconazole, or both, because viable organisms are likely to persist in such lesions, even if they cannot be recovered in the sputum.
Pulmonary blastomycosis is still a serious disease, with a 5-year mortality rate of approximately 20%.
Cryptococcosis
Cryptococcosis is a subacute or chronic infection caused by Cryptococcus neoformans, formerly known as Torula histolytica, which primarily attacks the bronchopulmonary tree but also has a predilection for the central nervous system. This disease, like blastomycosis, histoplasmosis, and coccidioidomycosis, was formerly thought to be a rare and invariably fatal infection, with meningitis the most prominent feature. Bronchopulmonary manifestations are apparently more common than the dreaded meningeal form, however, and since the introduction of amphotericin B and 5-fluorocytosine, even this form can be controlled. According to Minamoto and Rosenberg (1997), cryptococcosis is presently the fourth most common opportunistic infection in patients with AIDS, with approximately 6% to 10% of patients with AIDS infected.
Epidemiology
Cryptococcosis has no known geographic area of endemicity. Two varieties are found throughout the world. Ellis and Pfeiffer (1990) and Levitz (1991) reviewed the infectious characteristics of the two varieties. C. neoformans, serotype A or D, is most common and is most likely to produce disease in immunodeficient patients. C. neoformans vargattii, serotypes B and C, which may have originated in Australia, usually cause disease in normal hosts. Cryptococci may be present in soil and dust contaminated by pigeon
P.1274
droppings; in this regard, it resembles histoplasmosis as well as North American blastomycosis.
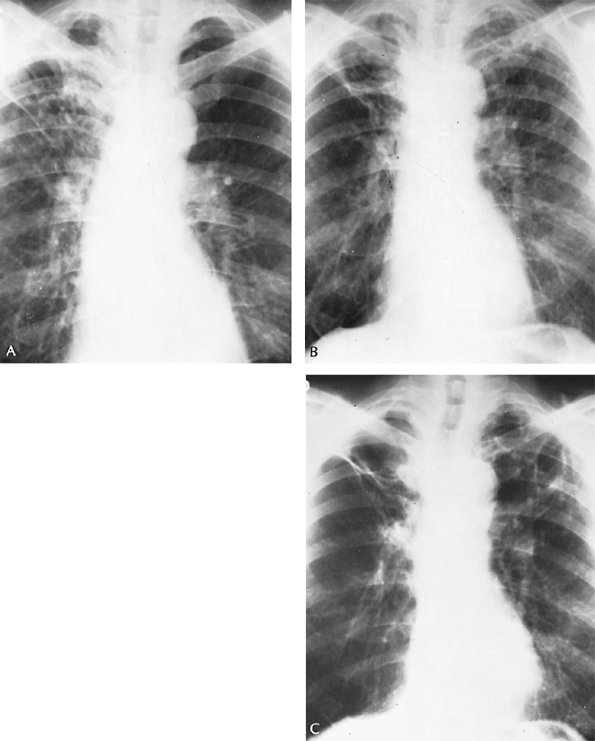 |
Fig. 89-14. Evolution of chronic pulmonary blastomycosis. A. Thoracic radiograph shows extensive right upper lung infiltrative lesion in a man with proven blastomycotic skin lesion of the nostril. Lesion was treated at first with potassium iodide. B. Four years later, bilateral cavitary disease is observed, but no organisms were seen in the sputum. C. Two and a half years later, two thin-walled cavities in the left upper lobe and an emphysematous bulla in the right upper lobe were noted. A number of sputum specimens showed Blastomyces dermatitidis. He was treated with 7 g of 2-hydroxystilbamidine. He has remained well. |
Pathology
The organism C. neoformans is a round, budding yeast form 5 to 20 m in diameter (average 8 10 m) with a sometimes wide gelatinous capsule that surrounds the organism and remains unstained. The usual special stains for fungi are all effective for demonstrating the organisms. The mucicarmine stain is specific. In fresh material, the capsules can be demonstrated by mounting some of the specimen in a drop of diluted india ink (Fig. 89-15).
This mode of infection is airborne, and the respiratory tract is the portal of entry. McDonnell and Hutchins (1985) described four basic morphologic patterns in the lungs: pulmonary granulomas; granulomatous pneumonias; diffuse alveolar and interstitial cryptococci accompanied by greater
P.1275
or lesser degrees of inflammatory response; and many alveolar and intravascular organisms in the lungs, in which the primary route of infection was uncertain. The lesions are often solid, and the cut surface of the granuloma may be glaring or shiny, as in a mucoid carcinoma. Central necrosis and cavitation may occur but are uncommon, and calcification is rare.
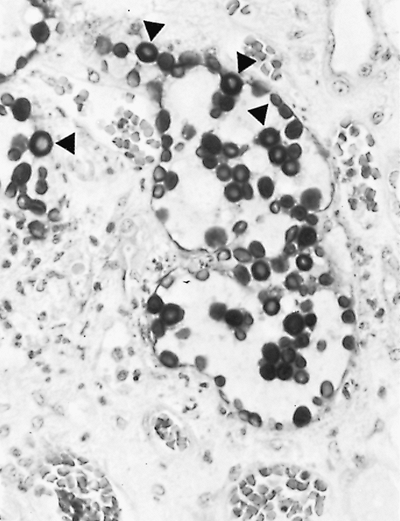 |
Fig. 89-15. Organisms of Cryptococcus neoformans show thick capsules (arrows) (periodic acid Schiff stain, original magnification 780). |
Symptomatology
Pulmonary symptoms are nonspecific, insidious, or absent. Cough, bloody sputum, low-grade fever, weakness, and lethargy sometimes occur. Spontaneous remission is believed to occur in some cases. Because cryptococcosis is often an opportunistic infection, the manifestations of the primary disease may overshadow those of cryptococcosis, and the diagnosis may become apparent only after abnormalities on thoracic radiography call attention to the lungs, or symptoms referable to the central nervous system suggest meningitis.
Diagnosis
Cryptococcus may be isolated from sputum, bronchial washings, bronchial brushings, or percutaneous needle aspiration of the lungs, as well as from cerebrospinal fluid, where it should specifically be sought. Often, the diagnosis is made from a resected lung specimen or at autopsy. No specific skin test for cryptococcosis is available, and serologic tests are of limited value.
The radiographic features of pulmonary cryptococcosis are not sufficiently characteristic to be of diagnostic help. Infiltrative, mass, nodular, and diffuse miliary lesions have all been described (Fig. 89-16). Pleural effusion is unusual, but is recognized more frequently than it once was, as noted by Epstein and associates (1972) as well as Littman and Walter (1968).
Kerkering and colleagues (1981) pointed out that the diagnosis of cryptococcosis is often missed because it is not considered often enough in the differential diagnosis of abnormalities found on thoracic radiography.
Treatment
Medical
The specific antifungal agents effective against C. neoformans are amphotericin B, 5-fluorocytosine (Ancobon, ICN Pharmaceuticals, Costa Mesa, CA, U.S.A.), ketoconazole, and fluconazole. Practice guidelines have been provided by Saag and associates (2000). Treatment of pulmonary disease depends on the immune status of the patient. Patients with normal immunity and uncomplicated cryptococcal pneumonia usually require no treatment. Before the proven efficacy of the oral azoles, patients were treated with amphotericin B and 5-fluorocytosine as described by Minamoto and Rosenberg (1997). Although the Mycoses Study Group and the AIDS clinical trials group found no statistical difference between this therapy and fluconazole (200 400 mg/day), patients receiving amphotericin B had a lower mortality rate and had negative culture results earlier. Fluconazole may be more effective and still well tolerated in these patients when higher doses (800 mg/day) are used as suggested by Duswald and co-workers (1997).
Even the dreaded meningeal form is no longer uniformly fatal. Recovery with drug treatment, sometimes requiring multiple courses, is now the rule rather than the exception.
Surgical
Cryptococcal involvement of the lung in the normal host is often nodular and may resemble pulmonary neoplasm. If the diagnosis is confirmed, a course of medical therapy is warranted, as it will usually be curative. Often the diagnosis is not established until the time of surgical resection. Once the diagnosis is established, resection is not recommended unless the lesion can be easily removed by wedge resection, as discussed by Johnson and Sarosi (1995). Most groups recommend treatment after pulmonary resection unless the lesion was completely resected.
Aspergillosis
In aspergillosis, the usually saprophytic Aspergillus fumigatus, or some other member of the species, has given
P.1276
P.1277
rise to one of three clinical syndromes: Aspergillus hypersensitivity lung disease, aspergilloma (fungus ball), or invasive pulmonary aspergillosis. Considerable clinical overlap occurs between these syndromes, and the clinical manifestations are closely related to the immune status of the patient. In the past, only the aspergilloma was of special interest to surgeons. Because of the extremely poor results of medical therapy, however, several investigators are advocating the benefits of surgical therapy in immunosuppressed patients with invasive pulmonary infections.
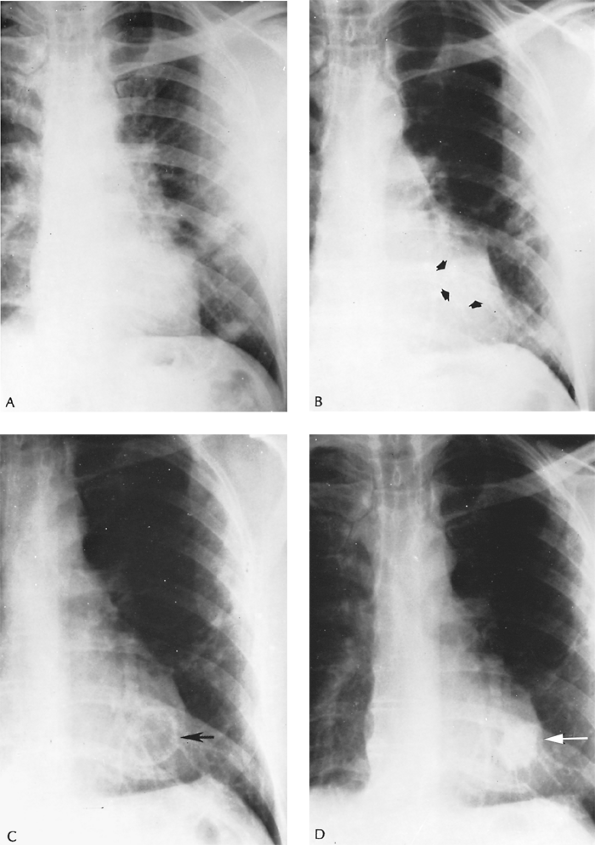 |
Fig. 89-16. Evolution of a cryptococcoma. A. Routine thoracic radiograph of a 54-year-old man with no symptoms referable to the chest shows scattered infiltrative lesions in the left lung. An open lung biopsy was performed after an unrewarding diagnostic workup; biopsies showed multiple small granulomas containing numerous encapsulated yeast forms characteristic of cryptococci. Mouse virulence tests were confirmatory. Examination of spinal fluid showed no organisms. A course of treatment with amphotericin B was discontinued after 1.5 g had been administered, because of thrombosed veins. B. Four months later, a solitary nodule behind the cardiac shadow is seen (arrows); the infiltrative lesions have regressed. C. Eighteen months later, a thin-walled cavity is seen at the site of the solitary nodule (arrow). D. Forty months after the process was first noted, a dense, shrunken, irregular nodular lesion is noted (arrow). The patient remained without pulmonary symptoms. |
Pathogenesis
In the three clinical forms of aspergillosis, A. fumigatus is the fungus most commonly isolated, but Aspergillus flavus, Aspergillus niger, Aspergillus nidulans, and Aspergillus clavatus are other identified species. These filamentous fungi are found in soil and on decaying vegetation and produce airborne spores that are especially abundant at certain times of the year. Arnow and associates (1978) pointed out that hospital air can become contaminated with Aspergillus spores both from within (e.g., false ceilings) and without (e.g., ventilation systems), a serious consideration for immunosuppressed patients. In pathologic materials, usually only the coarse, fragmented, septate, branching hyphae appear either as short strands or as ball-like clusters and can be identified only by isolation and culture (Fig. 89-17). Because aspergilli are saprophytic and ubiquitous, unless the material has been obtained under aseptic conditions, a diagnosis of aspergillosis cannot safely be made simply by culturing the fungi.
Most of the surgically resected lesions of aspergillosis are aspergillomas. A fungus ball is actually a matted sphere of hyphae, fibrin, and inflammatory cells, which appears grossly as a round or oval, friable, gray, red, brown, or yellow necrotic-looking mass. It is ordinarily found lying in an upper lobe cavity, the wall of which is often smooth and may be thick or thin with relatively little evidence of inflammatory reaction. The cavitary disease results from previous chronic lung disease such as tuberculosis, sarcoidosis, histoplasmosis, bronchiectasis, bronchogenic cyst, chronic lung abscess, or cavitating carcinoma. Rarely, no obvious evidence of preexisting pulmonary damage exists (Fig. 89-18). Robinson and associates (1995) found that resected pulmonary tissue from patients with invasive aspergillosis grossly consisted of hemorrhagic necrosis and varying degrees of cavitation filled with mycotic lung sequestrum and surrounding lung consolidation. This widespread necrosis of the tissue creates multiple wedge-shaped areas of infarction. Microscopically, the specimens showed hemorrhagic infarction with necrotic lung invaded by hyphae. Methenamine silver
P.1278
staining revealed abundant septate hyphae, often with blood vessel invasion and thrombosis.
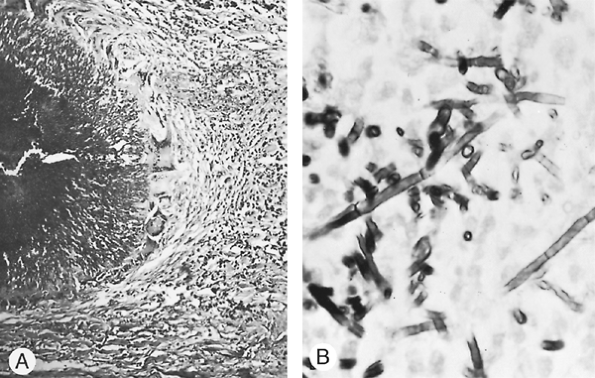 |
Fig. 89-17. Organisms of Aspergillus fumigatus in tissues. A. Small colony of aspergilli found in a resected carcinomatous lesion of the lung. Note mycelia radiating outward from the darker center of the colony (original magnification 250). From Takaro T: Lung infections and interstitial pneumonopathies. In Sabiston DC, Spencer FC (eds): Gibbon's Surgery of the Chest. Philadelphia: WB Saunders, 1976. With permission. B. Close-up of coarse, septate, fragmented mycelia of A. fumigatus. Round bodies are mycelia seen from the end (Gomori's stain, original magnification 950). From Takaro T: Thoracic actinomycotic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
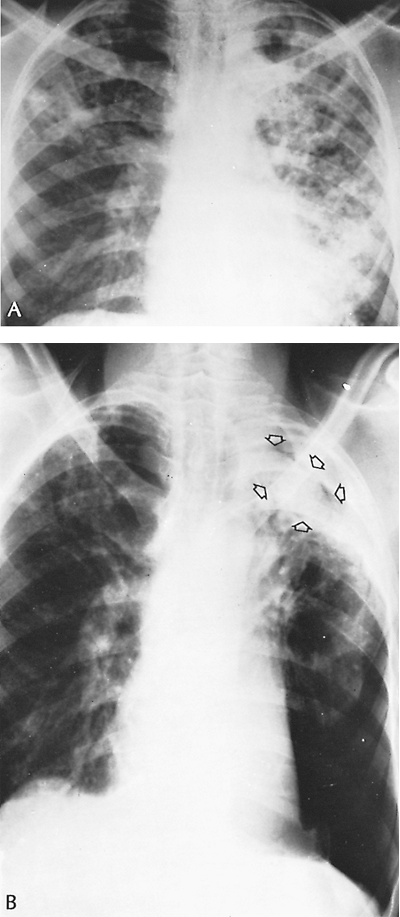 |
Fig. 89-18. Evolution of an aspergilloma. A. Thoracic radiograph in 1959 from a 30-year-old man with advanced cavitary pulmonary tuberculosis. He received multiple drug therapy for many months, and sputum was last positive in 1962. B. Radiograph obtained in 1973 (14 years later) because of occasional mild hemoptysis shows aspergilloma, or fungus ball. Note radiolucent space between fungus ball and cavity wall (arrows). Severe chronic obstructive lung disease precluded resection, although the disease appeared to be localized. Chronic hemoptysis continued. Little change occurred in the patient's condition or the radiographs over a 2-year period. The patient ultimately died 16 years after the radiograph shown in A was obtained and 2 years after the discovery of the fungus ball of extensive pulmonary infection. Patients with this type of complex aspergilloma often do not do well after surgical therapy, but medical treatment also has little to offer. |
Symptomatology
In certain cases, symptoms may be attributable to the aspergilloma. Cough with bloody or blood-streaked sputum, however, frequently occurs, although histopathologic examination of the resected lesion may reveal no bleeding point. Sometimes, hemoptysis is severe to exsanguinating. The first symptoms of the invasive form of aspergillosis usually are those of acute pulmonary infection with fever, cough, dyspnea, and often pleuritic chest pain. Symptoms can initially resemble a pulmonary embolus with a combination of dyspnea, chest pain, and hemoptysis. If not recognized and treated early in the immunosuppressed patient, manifestations of worsening pulmonary function and distant spread may rapidly progress.
Diagnosis
Finding Aspergillus in the sputum alone does not confirm the diagnosis of aspergillosis. If the clinical picture of aspergilloma is present, however, and sputum culture results are positive for Aspergillus, the diagnosis is probable. Griepp (1975) reported that transtracheal aspirates or direct lung aspirates by thin-walled 18-gauge needles may provide a definitive diagnosis and are especially useful in immunosuppressed patients.
Levitz (1989) has stated that precipitating antibodies against A. fumigatus in the serum, skin sensitivity to Aspergillus antigen, or characteristic radiographic opacities are confirmatory evidence. An aspergilloma can sometimes be identified radiographically as a mass shifting within a cavity or cyst on changes in position of the patient. Thus, in a radiograph of the thorax exposed in the upright position, a crescentic radiolucency above a rounded radiopaque lesion suggests aspergilloma (Monad's sign) (Fig. 89-19).
Treatment
Medical
Successful medical therapy depends more on the patient's underlying pulmonary disease and immune status than on the type of treatment instituted. Allergic bronchopulmonary aspergillosis is usually treated with steroids. Adding itraconazole has been found to help reduce the steroid dose required.
Aspergillomas are usually treated expectantly and directed at the prevention of life-threatening hemoptysis. Some groups have had good results using transthoracic or transbronchial installation of therapeutic agents, as described by Giron and associates (1998). Remy and co-workers (1977) reported that embolization of the bronchial arteries in a few patients with massive or repeated hemoptysis
P.1279
caused by aspergilloma resulted in initial remission in four of six cases, but recurrence of bleeding occurred in three of the four initially controlled, probably secondary to multiple collateral channels. Treatment of aspergilloma with amphotericin B and itraconazole has shown some efficacy, but is rarely currative. Denning and Stevens (1990) noted that medical treatment has been generally unsatisfactory for invasive aspergillosis and that results may be improved in neutropenic patients when a higher dose of amphotericin B is used (1.0 1.5 mg/kg/day). Herbrecht and co-workers (2002) reported encouraging results in a randomized nonblinded trial comparing intravenous voriconazole and amphotericin B for invasive aspergillosis. The treatment success rate and 12-week survival rate were statistically better in the voriconazole treatment group (52.8% vs. 31.8% and 70.8% vs. 57.9%, respectively). Furthermore, the severe adverse event rate was statistically higher in the amphotericin group.
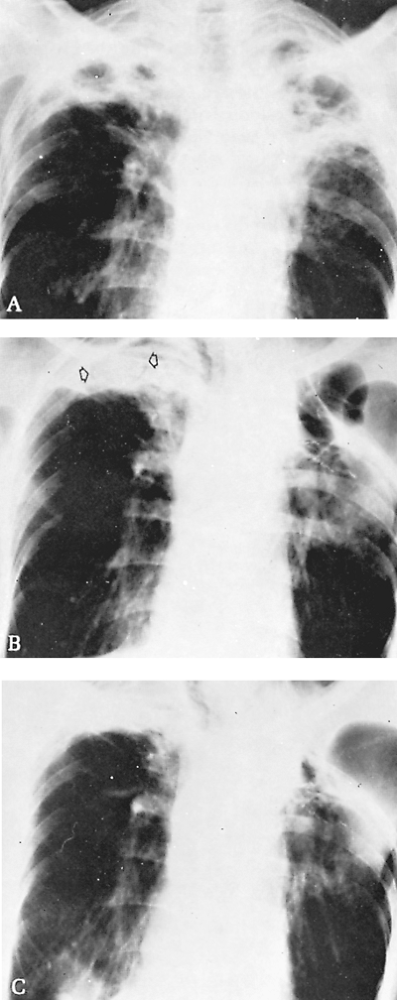 |
Fig. 89-19. Evolution of bilateral cavitary disease with colonization by aspergilli; surgical treatment. A. Thoracic radiograph of a 55-year-old man with symptoms of gradually increasing weakness and weight loss. No positive findings were found on workup other than the destructive lung lesions. Pulmonary ventilatory function was only mildly impaired; segmental resection of the left upper lobe and four-rib thoracoplasty were performed. Pathologic findings were nonspecific inflammatory disease with a cavity containing soft, brown masses, which proved to be colonies of Aspergillus. A chronic bronchocutaneous fistula developed and gradually closed after 3 months. B. Two years later, a distinct new fungus ball can be identified in the right upper cavity (arrows), with the characteristic radiolucent area around the ball. There were few symptoms. C. Four years later, radiograph reveals regression of the disease process in the right lung without specific therapy. |
Surgical
Surgical treatment is controversial. According to Daly and colleagues (1986), management philosophy is more readily directed when aspergillomas are classified as either simple (i.e., thin-walled localized cysts with little surrounding parenchymal disease) or complex (i.e., thick-walled cavities associated with gross evidence of parenchymal disease). Because the complication rate after surgery in the latter group is high, symptoms of hemoptysis or cough, or both, should be significant enough to warrant the risks of surgery. On the other hand, patients with simple aspergillomas with even minimal symptoms may be offered surgery because the risk is lower and the likelihood of long-term cure is much improved.
Individualizing surgical management is clearly critical, as Battaglini (1985) and Butz (1985) and their colleagues emphasized. Daly and colleagues (1986) suggested, in patients with peripheral and complex aspergillomas, the obliteration of the cavity by transposing muscle from the chest wall into the cavity may be the procedure of choice. Babatasi (2000) and Regnard (2000) and their associates have each reviewed large series of patients. Both groups have had excellent survival with acceptable complication rates in patients with aspergillomas. In patients unfit for pulmonary resection, cavernostomy is performed despite the high operative risk. Both series describe bleeding and incomplete reexpansion following lobectomy as the most common complications. Al-Kattan and co-workers (2001) have suggested that posttuberculous aspergillomas are associated with a higher surgical risk than those seen in immunocompromised patients. Niwa and colleagues (1995) advocate arteriography before resection of aspergillomas and, if indicated, embolization of bronchial arteries or subclavian artery branch ligation to reduce intraoperative blood loss.
Aspergillus empyema, or pleural aspergillosis, was reported by Herring and Pecora (1976) and others and has been treated by intrapleural amphotericin B, or nystatin,
P.1280
and by pleural drainage, pleurectomy, thoracoplasty, and repair of bronchopleural fistulae.
Surgery has been suggested by Lupinetti and associates (1992) to have a role in salvaging patients with invasive Aspergillus infection after bone marrow transplantation and for other immunodeficient patients. Successful resection of the involved lung will allow further treatment with cytotoxic drugs without recurrence of aspergillosis as described by Pidhorecky and colleagues (2000). Empyema and bronchopleural fistula are the main complications. Endo and co-workers (2001) suggest intrathoracic transposition of chest wall muscles in patients with large residual pleural spaces. Patients being evaluated for bone marrow transplantation, or any procedure that may reduce the patient's immune system, should be screened for possible cavitary lung disease that could be harboring indolent Aspergillus. Robinson and colleagues (1995) have had good results operating on immunocompromised patients with hematologic disease or after liver transplantation. Eleven of 16 patients survived after resection of invasive Aspergillus. Temeck and associates (1994) found that angioinvasion in the resected specimen correlated with operative mortality in immunosuppressed patients without human immunodeficiency virus.
Candidiasis (Moniliasis)
Candidiasis is an acute, subacute, or chronic superficial fungus infection caused by species of Candida, usually Candida albicans, which commonly affects the skin or the oral, bronchial, or vaginal mucosa. Much less commonly, it can also be a deep or systemic infection, involving the lungs, blood, endocardium, meninges, or almost any other organ. Other fungi of this genus that are occasionally pathogenic to humans are Candida guiliermondii, Candida stellatoidea, Candida parakrusei, Candida tropicalis, and Candida glabrata.
Epidemiology
Candida occurs in the oropharynx of many normal individuals. It is a common hospital and laboratory contaminant, probably of universal distribution.
Pathogenesis
Candida organisms appear in fresh or potassium hydroxide preparations or on Gram's stain as small (2.5 4.0 m) oval, thin-walled, budding cells with or without mycelial elements (Fig. 89-20). An acute or chronic granulomatous reaction may result. In systemic infections, both mycelial and yeast forms may appear in clusters surrounded by polymorphonuclear leukocytes, forming microabscesses. The fungi also may invade the tissues and blood vessel walls, with little evidence of inflammatory reaction in some instances. Thomas (1977) and Wray (1973) and their associates reported costal chondritis as well as osteomyelitis of the sternum caused by Candida.
Clinical Picture and Diagnosis
The importance of this opportunistic fungus infection lies in the slowly but steadily increasing incidence of disseminated disease noted since the 1970s. This phenomenon is essentially iatrogenic; before the advent of broad-spectrum antibiotics, disseminated disease with invasion of internal organs, septicemia, and endocarditis were almost unheard of.
In the presence of intensive or prolonged antibiotic therapy, especially with multiple drugs, or of immunosuppressive therapy after organ transplantation, the normal bacterial flora of patients may be suppressed, allowing an overgrowth of the often present saprophytic species of Candida. This type of superinfection also occurs in patients with AIDS. Invasion then takes place through any portal of entry: the skin, blood (by way of needles or catheters used for intravenous therapy), lungs, or gastrointestinal tract. In the presence of altered host immunity or inhibited inflammatory response for any of various reasons, Candida pneumonia, abscess, which Rubin and Alroy (1977) reported, or septicemia and generalized infection may result, often with a fatal outcome. Orringer and Sloan (1978)
P.1281
observed monilial esophagitis with stricture formation, and Spear and colleagues (1976) reported tracheal obstruction associated with Candida fungus ball. Thus, although the presence of a species of Candida in the sputum of many healthy persons ordinarily is of no diagnostic or prognostic importance, the same cannot be said for the finding of Candida in bronchial or lung biopsies, in blood, or in deep tissue spaces. One cannot lightly dismiss such reports as reflecting laboratory contamination, especially not in immunosuppressed or otherwise compromised patients who have symptoms and signs of pneumonia, or of septicemia. Because of the high mortality rate associated with Candida septicemia, Anaissie and Solomkin (1994) and Blumberg and associates (2001) recommend early presumptive antifungal therapy for patients with known risk factors who remain febrile despite receiving broad-spectrum antibiotic therapy who are known to have at least one culture showing Candida species.
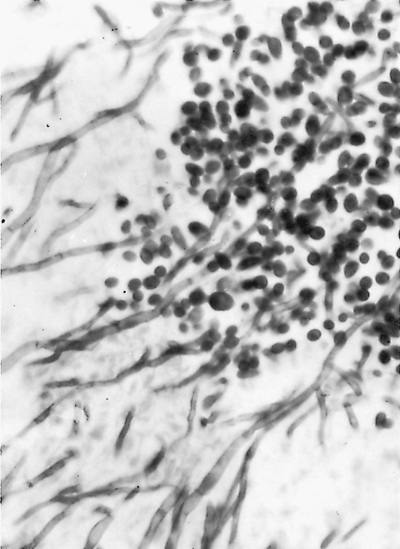 |
Fig. 89-20. Organisms of Candida albicans show both yeast and mycelial forms (Gomori's stain, original magnification 1,000). From Takaro T: Thoracic actinomycetic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
As in the case of the other opportunistic fungi such as A. fumigatus, C. neoformans, and Mucor, Blumberg found that the most significant risk factors for Candida bloodstream infections in patients in the surgical intensive care unit were prior surgery, acute renal failure, utilization of parenteral nutrition, and the presence of a triple-lumen catheter. Trick and co-workers (2002) found that there has been a significant increase in the incidence of Candida glabrata bloodstream infections in intensive care unit patients.
Treatment
Early diagnosis and vigorous treatment are critical for favorable outcome. Strinden and colleagues (1985) reported long-term survival in six of eight patients with septic thrombosis of the central veins caused by Candida who had intensive therapy with amphotericin B. Rex and co-workers (1994) from the Mycoses Study Group described a randomized trial comparing amphotericin B and fluconazole in the treatment of candidemia in patients who were not neutropenic. Fluconazole was found to be an equally effective treatment with less toxicity. Similarly, in a prospective randomized trial that evaluated invasive candidal infections, Anaissie and co-workers (1996) found fluconazole to be equally effective and better tolerated than amphotericin B. Overall response rates were 66% and 64% for fluconazole and amphotericin B, respectively. However, significant toxicity occurred in 35% versus 5% in favor of fluconazole. Susceptibility testing should be performed if non-albicans species are found.
Sporotrichosis
Sporotrichosis is a mycotic infection caused by Sporotrichum schenckii, a cigar-shaped, dimorphic organism that stains bright red with periodic acid Schiff stain. The disease is usually characterized by cutaneous and lymphatic involvement and less often by pulmonary disease. The causative organism is a saprophyte under normal conditions and is widely distributed in plants and soil. Thus, florists are especially susceptible. An epidemic involving miners exposed to infested mine timbers in South Africa was reported in 1947. Dixon and colleagues (1991) reported that in 1988, 84 forestry workers exposed to a single source of sphagnum moss were identified in a major epidemic in the United States. Heller and Fuhrer (1991) reported disseminated sporotrichosis in five patients with AIDS.
Pulmonary infection produces nonspecific symptoms resembling those of pulmonary tuberculosis: fever, hemoptysis, malaise, and weight loss. Similarly, radiographic findings mimic tuberculosis in the wide variety of presenting patterns, including hilar lymphadenopathy, pleural effusion, lobular consolidation, fibrosis, and multiple nodules. Michelson (1977) as well as Jay (1977) and Jung (1979) and their associates reported localized cavitary disease in more than 50 patients. It is necessary not only to culture the organism from sputum, bronchial washings, or lung tissue, but also to establish its pathogenicity for animals, because the organism is a saprophyte. The diagnostic value of serum agglutination tests for Sporotrichum is not established, but direct fluorescent antibody reliably identifies the organisms in specimens and tissue biopsy samples, according to Rohatgi (1980) (Fig. 89-21).
The response to antimicrobials of patients with pulmonary sporotrichosis is generally poor. Response rates for both amphotericin B and itraconazole are a disappointing 20% to 50%, as reported by Kauffman (1996). Initial therapy with itraconazole can be instituted for non life-threatening infections. Ramirez and associates (1998) feel itraconazole is as effective as amphotericin B for pulmonary sporotrichosis, but there are too few patients to evaluate with a randomized study. Patients who are acutely ill should receive amphotericin B, which can be switched to itraconazole if a good response is obtained. Surgery can occasionally play a useful role for localized disease, but many patients have underlying severe obstructive pulmonary disease that precludes operation, as noted by Kauffman (1995).
Zygomycosis (Mucormycosis)
This rare but potentially lethal fungal infection is caused by genera belonging to the class Zygomycetes. These fungi are characterized structurally by broad (6 50 m) nonseptate but branching hyphae (Fig. 89-22) that are difficult to culture. Among disease-causing organisms in this group are species of Absidia, Rhizopus, Mucor, Mortierella, and Basidiobolus. These organisms are generally saprophytes, occurring as molds on manure and foods, and producing spores that can be inhaled. They are not ordinarily pathogenic to humans, but can be pathogenic in immunocompromised patients. In the lungs, the disease is characterized
P.1282
by blood vessel invasion, thrombosis, and infarction, with marked tissue destruction, cavitation, and abscess formation.
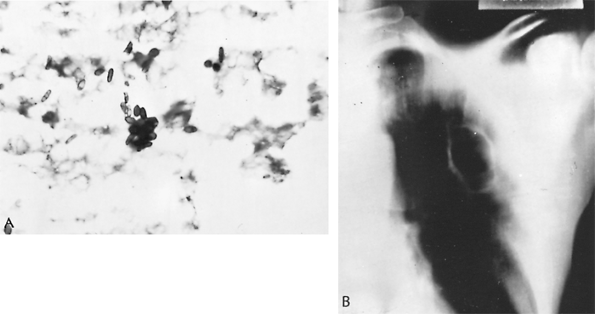 |
Fig. 89-21. Sporotrichosis. A. Cigar-shaped organisms of Sporothrix schenckii seen in resected lung specimen (periodic acid Schiff stain, original magnification 1,100). B. Thoracic radiograph showing cavitary lesion of pulmonary sporotrichosis. From Scott SM, et al: Pulmonary sporotrichosis. N Engl J Med 265:453, 1961. With permission. |
Zygomycosis is usually a rapidly fatal disease, occurring especially in acidotic diabetics, in patients with lymphomas or leukemias, or in persons receiving intensive or prolonged antimetabolite, antibiotic, or corticosteroid therapy. Extensive necrosis of areas around the face (e.g., paranasal sinuses, orbit, mucous membranes) and of the lung and brain may occur in addition to cutaneous and subcutaneous infection. Murray (1975) reported massive fatal pulmonary hemorrhage. Gartenberg and associates (1978) reported two patients with necrotizing chest wall infections after aortocoronary bypass operation in which Elastoplast (Beiersdorf, AG, Hamburg, Germany) dressings were used; both patients died. Although amphotericin B is the only agent with some evidence of efficacy, of 18 survivors with pulmonary mucormycosis 11 were managed by surgery alone in the series of Bigby and colleagues (1986).
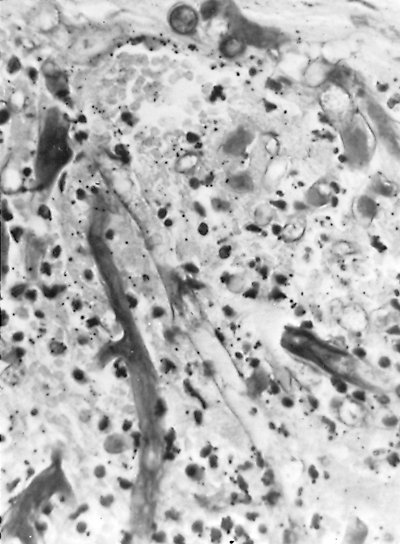 |
Fig. 89-22. Phycomycosis (mucormycosis). Broad, nonseptate hyphae of a phycomycete, probably Mucor, invading thrombosed pulmonary arterial wall (hematoxylin and eosin stain, original magnification 330). From Takaro T: Thoracic actinomycetic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
Early recognition, control of diabetes, and termination of antimetabolite, antibiotic, or corticosteroid therapy are all important. Parfrey (1986) noted that, with increasing premortem diagnosis, allowing more vigorous management both surgically and medically, the prognosis of this grave infection is improving. Zygomycosis has occurred after renal, hepatic, and cardiac transplantation. Pulmonary zygomycosis after renal transplantation is a lethal complication, usually associated with corticosteroid-induced diabetes. Donado-Una and colleagues (2002) favor surgery when there are persistent cavitations following treatment with amphotericin, since this finding portends a high risk for late relapse. Tedder and associates (1994) reported the mortality rate in patients who underwent surgical resection as 11%, whereas those treated medically had a 68% mortality rate. Resection is generally indicated if the patient can tolerate the required operation.
Pulmonary Monosporosis (Pseudallescheriasis)
This rare mycotic infection is caused by Pseudallescheria boydii (Monosporium apiospermum). This inhabitant of soil appears to act as a secondary invader of
P.1283
previously damaged lung tissue, such as a tuberculous cavity, cyst, or bronchial saccule. Sometimes, but not characteristically, a fungus ball is formed. Amphotericin B has not been effective. Terrell and Hughes (1992) reported miconazole as the drug of choice. Goldberg and colleagues (1993) demonstrated the effectiveness of itraconazole in the treatment of simultaneous pulmonary aspergillosis and monosporosis. Jung and associates (1977) summarized the localized resections that were performed in 10 patients, with two deaths. Conservative management is recommended for asymptomatic patients without cavitary disease or bronchiectasis. Resection is advocated for good-risk patients with localized cavitary disease, or to help make a definitive diagnosis when bronchial carcinoma is suspected.
Paracoccidioidomycosis (South American Blastomycosis)
Paracoccidioidomycosis is a chronic granulomatous infection involving the skin, mucous membranes, lymph nodes, and visceral organs, including the lungs. It is caused by Paracoccidioides brasiliensis, a soil saprophyte.
Primary infection usually occurs during the first two decades of life and lays dormant in the liver, spleen, and adrenal glands. It is sometimes confused with Hodgkin's disease. In the chronic form, the lungs are involved in 80% of the cases. Paracoccidioidomycosis is endemic in South and Central America, where it is the most commonly occurring deep systemic fungal infection, and was not recognized outside these areas before the 1970s, as noted by Bouza and colleagues (1977). The organisms resemble B. dermatitidis in tissue (Fig. 89-23). Infection results from inhalation of conidia. In one third of patients, the disease is limited to the lungs and may resolve spontaneously. Cavitary pulmonary disease occurs and is fatal unless treated. In developing countries, sulfonamides are most commonly used because of cost. As discussed by Bethlem and associates (1999), the current treatment of choice is itraconazole, which is usually given at 200 mg/day for 6 months. Ketoconazole and amphotericin B have similar activities, but have more toxicity. Surgical resection apparently has no place in this disease, other than lung biopsy, because bilateral disseminated and polymorphic lung lesions seem to be the rule.
Opportunistic Fungal Infections
Opportunistic infections occur in the lungs of patients receiving cancer chemotherapy, recipients of organ transplants, AIDS patients, and other immunocompromised patients. Factors predisposing to fungal infection include immunosuppressive drugs that compromise cell-mediated immunity, corticosteroids and antineoplastic drugs that contribute to myelosuppression and neutropenia, antibiotics, intravenous catheters, parenteral nutrition with lipids, and diabetes.
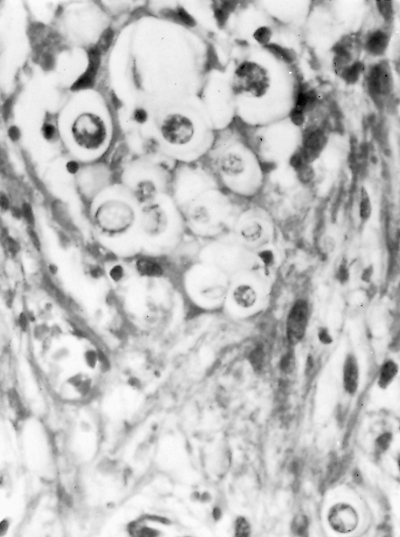 |
Fig. 89-23. Paracoccidioidomycosis (South American blastomycosis). Organisms of Paracoccidioides brasiliensis, in tissue. Note superficial resemblance to Blastomyces dermatitidis. From Takaro T: Thoracic actinomycetic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
Neutropenia (>1,000 cells per microliter) associated with corticosteroids and cancer chemotherapy is the major factor responsible for fungal infection in cancer patients. The most common fungal infections in cancer patients are candidiasis and aspergillosis. Anaissie and Bodey (1990) pointed out that other fungi may be present, such as Fusarium species, Trichosporon beigelii, Torulopsis glabrata, and Zygomycetes. The current approach to patients with antibiotic-resistant fever is intravenous amphotericin B. Ellis and co-workers (1995) showed that fluconazole may be a safer alternative; however, it may have inferior results in this patient population.
Pulmonary infections are a common problem in transplant patients. When the infections are caused by fungi, the mortality is high. The fungi usually associated with organ and bone marrow transplantation are Aspergillus species, H. capsulatum, C. immitis, C. neoformans, Candida species, P. boydii, Mucoraceae, and P. brasiliensis.
The human immunodeficiency retrovirus, by causing selective depression of T4 lymphocytes, impairs cell-mediated immunity, but does not in patients with AIDS; cryptococcosis is the most common systemic fungal infection and does not affect neutrophil function or blood monocyte phagocytosis. Because these functions are not impaired, disseminated candidiasis and invasive aspergillosis are uncommon in patients with AIDS. In a review of fungal infections in patients with AIDS, Diamond (1991) noted that mucocutaneous candidiasis is present as an initial manifestation of AIDS in
P.1284
most cases, that histoplasmosis and coccidioidomycosis are commonly encountered in endemic areas, and that blastomycosis is uncommon.
Emerging Fungal Pathogens
Some fungi previously considered harmless colonizers are now recognized as pathogens that can cause opportunistic infections. Within the Phaeohyphomycoses, the dermatiacious fungi, are the genera Curvularia, Bipolaris, Exserohilum, and Alternaria, all of which have caused human infections. The Hyalohyphomycoses are the nondermatiacious molds of which there are three pathogenic genera: Fusarium, Scopulariopsis, and Pseudallescheria. Disseminated Fusarium infection resembles that of Aspergillus.
Anaissie and Bodey (1989) recorded that among the less common yeasts responsible for opportunistic infection are T. beigelii, which causes systemic infections in neutropenic patients; Malassezia furfur, which causes infection in debilitated patients receiving intravenous lipids; and Hansenula species, which is associated with intravenous catheter contamination.
Antimycotic Drugs
As stated previously, not all patients with proven infections require treatment with antimycotic agents. Several antifungal agents are available, and others are under study.
Amphotericin B
As shown in Table 89-2, amphotericin B, which was one of the first drugs developed for systemic and deep fungal infections, still has the widest spectrum of effectiveness. Amphotericin A and B are fermentation products of the actinomycete Streptomyces nodosa. They are complex lipophilic organic compounds (i.e., polyenes). Only amphotericin B has been developed for clinical use. Polyenes bind to ergosterol, which is present in the cell membranes of fungi, causing disruption and ion leakage.
Amphotericin B is poorly absorbed from the gastrointestinal tract and is effective only by the intravenous route. Because of its toxicity, it should be used only if the diagnosis is reasonably certain and spontaneous cure is unlikely. Suggested modes of administration include giving the patient a test dose of 1 mg of the drug in 250 mL of 5% glucose in water over a 20- to 30-minute period intravenously. On subsequent days, the dose is increased, advancing rapidly to a daily dose of approximately 0.5 to 0.6 mg/kg body weight in 500 mL 5% dextrose in water over a 3- to 4-hour period. Fresh material should be made daily. Drug administration is begun only after obtaining baseline laboratory data (i.e., complete blood cell count, urinalysis, blood urea nitrogen, creatinine, serum potassium, and liver function study results). Hermans (1977) suggested that a flow sheet recording data to be obtained on a continuing basis is helpful. Certain toxic side effects (e.g., headache, nausea, vomiting, fever, hypotension, delirium) may be ameliorated or prevented by adding 25 to 50 mg of hydrocortisone to the infusion bottle, unless the patient is already receiving corticosteroids. Blood pressure and pulse are monitored every half hour for the duration of the infusion; renal function is monitored twice weekly at first, until azotemia stabilizes, preferably at a blood urea nitrogen level below 50 mg/dL and serum creatinine below 2 mg/dL. Then, it can be checked once a week. Pentoxifylline may help to alleviate nephrotoxicity. A total dose of 3 g of amphotericin B is a common goal, but this regimen may be individualized in accordance with the severity of the patient's illness and the known prognosis without therapy of each fungal infection. Hermans (1977) recommended 2.5 g for histoplasmosis, 1.5 g for blastomycosis, and 2 g for nonmeningitic pulmonary coccidioidomycosis. According to Bennett (1974), as well as others, significant permanent reduction of renal function is unlikely in adults at total doses of up to 4 g. The use of liposomal amphotericin B allows larger doses of drug to be given with less toxicity. Sorkine and co-workers (1996) found that patients in the intensive care unit had a lower frequency of drug-associated fever, rigors, hypotension, and nephrotoxicity with this preparation.
5-Fluorocytosine
Some fungi contain cytosine deaminase, which converts 5-fluorocytosine into 5-fluorouracil, which inhibits DNA and RNA synthesis. The range of effectiveness of flucytosine is narrower than that of amphotericin B, but it provides more effective treatment for pulmonary and meningeal cryptococcosis when used with amphotericin B, and it also allows use of lower doses of the latter. This oral preparation is used in a dose of 150 mg/kg body weight, in divided doses, for several weeks to months. Besides mild side effects similar to those of amphotericin B, 5-fluorocytosine can cause leukopenia, anemia, thrombocytopenia, and, occasionally, pancytopenia. Rarely are these side effects serious enough to require discontinuation of treatment. Harder and Hermans (1975), however, noted that when used in combination with amphotericin B, the renal damage caused by 5-fluorocytosine may lead to excessive blood levels of flucytosine.
Azoles
The azole compounds include miconazole, ketoconazole, fluconazole, itraconazole, and voriconazole. The azoles, by inhibiting the enzyme cytochrome P-450, which converts lanosterol to ergosterol, interfere with fungal cell membrane synthesis. The azoles should never be used to treat pregnant women because of their embryotoxic and teratogenic potential.
Miconazole has limited use but is the primary drug for invasive P. boydii infections.
Ketoconazole is an oral antifungal agent with relatively low toxicity. Ketoconazole is effective against histoplasmosis
P.1285
and blastomycosis in immunocompetent patients with non life-threatening disease and against paracoccidioidomycosis. Butman and colleagues (1991) have successfully used ketoconazole prophylactically in heart transplant patients with documented exposure. Because ketoconazole increased cyclosporine levels, the amount of cyclosporine required was reduced significantly, which helped reduce costs.
Fluconazole is a wide-spectrum antifungal agent that can be given orally or intravenously. It is the drug of choice for most Candida, cryptococcal, and coccidioidomycosis infections. Indications for use may expand as higher doses are used in clinical trials.
Itraconazole is the drug of choice for paracoccidioidomycosis, sporotrichosis, and blastomycosis. It is useful for treating candidiasis, histoplasmosis, and meningeal cryptococcosis in patients with AIDS. It is also effective against aspergillosis. There is some evidence that itraconazole may actually be antagonistic to amphotericin B as described by Schaffner and co-workers (1993).
THORACIC ACTINOMYCETIC INFECTIONS
Actinomycosis
Actinomycetic infections, including actinomycosis and nocardiosis, have traditionally, albeit mistakenly, been classified and treated along with true fungal infections of the lungs in many textbooks. Actinomyces literally means ray fungus, for the radiating filaments in microcolonies, the formation of branching hyphae, and the production of spores. The actinomycetes, however, are considered closer to bacteria than to fungi and clearly respond to antibacterial rather than to antifungal agents. In the interests of common usage and expectation, however, they are considered in this chapter.
Actinomycosis is a chronic infectious disease usually caused by Actinomyces israelii, rarely by Actinomyces bovis, and characterized by chronic suppuration, chronic sinus formation, and the discharge of purulent material containing yellow-brown sulfur granules. These granules are actually microcolonies of the tangled hyphae of actinomycetes (Fig. 89-24). Because the organisms are anaerobic or microaerophilic, they require culturing under anaerobic conditions. They also require culturing of material from closed tissue spaces or from draining sinuses or abscesses; isolation from sputum or secretions from the mouth is not proof of infection, because the organism may reside normally in the oral cavity of humans.
Clinical Features
The clinical syndromes are cervicofacial (55%), thoracic (15%), abdominal (20%), and mixed organs (10%). Thoracic actinomycosis usually results from bronchopulmonary infection after entry of organisms into the lungs through the oropharynx. Characteristically, the pleura and chest wall are involved, but this is usually preceded by some type of pulmonary infiltrate or by dense hilar lymphadenopathy. The varieties of lung involvement are nonspecific, although Hsieh and colleagues (1993) emphasized that some of the presentations of actinomycosis may suggest bronchial carcinoma. The disease process may show extensions from one of the anatomic areas to the adjacent area, usually from the cervical or abdominal areas to the thoracic area (Fig. 89-25).
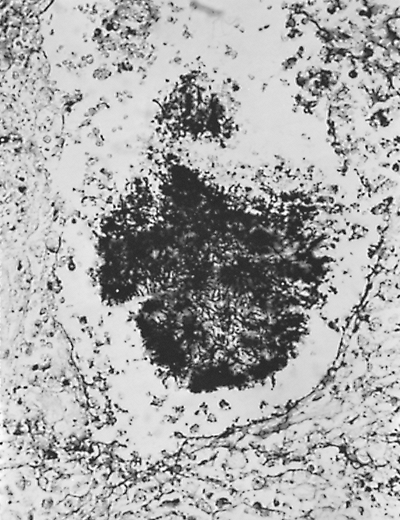 |
Fig. 89-24. Actinomycotic granule in a microabscess from a patient with actinomycosis. Note branching filaments in center of granule (methenamine silver, original magnification 200). |
Conant and Wechsler (1992) emphasized the usefulness of the thoracic CT evaluation. The lesions usually demonstrate an area of low attenuation with associated air bronchograms. The CT scan is more sensitive in detecting bone involvement and pleural involvement, as well as mediastinal and hilar lymphadenopathy. Rarely, as Shinagawa and associates (2002) reported, the pericardium may be involved.
The diagnosis of actinomycosis is difficult: first, because the disease is uncommon enough that it is often not suspected; second, because, being unsuspected, the anaerobic causative organism is often not given the appropriate cultural conditions; and third, because the radiographic resemblance to bronchial carcinoma often compels exploratory thoracotomy for diagnosis. In a review by Endo and colleagues (2002), 77% of patients had poor oral hygiene, 92% of patients were symptomatic, and 77% had hemoptysis. Sometimes a course of penicillin can be diagnostic, as the lesion resolves.
Treatment
The drug of choice is penicillin. The dense fibrous tissue surrounding the colonies of organisms requires the use of high doses of antibiotics for long periods. McQuarrie and Hall (1968) recommended 20 million U of penicillin daily for 1 to 3 months. The tetracyclines, as Feingold and co-workers (1985) reported, are generally an adequate substitute when the patient is allergic to penicillin. The prognosis
P.1286
after effective treatment is good, as noted in a case report by Duhra and associates (1992).
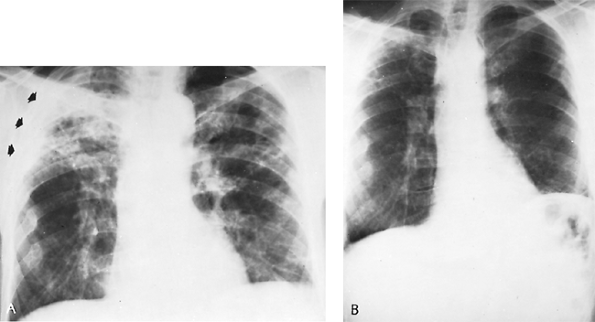 |
Fig. 89-25. Chronic thoracoabdominal actinomycosis. A. Fifty-year-old man with bilateral apical infiltrative disease was treated for presumptive diagnosis of pulmonary tuberculosis until left-sided pain, pleural fluid, and elevated diaphragm led to thoracotomy. A splenic actinomycetic abscess pointing through the diaphragm was removed; lung biopsy of extensive fibronodular disease also showed actinomycosis. Note pleural reaction, right upper lung (arrows), and an elevated diaphragm, left. The patient was treated with 20 million U of penicillin intravenously for 1 month, followed by 4 weeks of the drug, 2.4 million U daily intramuscularly. B. Two years later, the disease process has cleared; except for elevated left diaphragm, chest radiograph is nearly normal. The disease did not recur over a 4-year follow-up period. |
Exploratory thoracotomy on suspicion of carcinoma is the most common indication for surgical intervention in patients with actinomycosis. Adequate and prolonged drug therapy is important postoperatively to prevent reactivation of disease or the development of empyema. If a preoperative diagnosis has been made, and excisional surgery is possible, for removal of a destroyed lobe, for example, it should be performed under adequate drug coverage. Foley and associates (1971) reported that sometimes only drainage of an abscess or empyema may be possible, or necessary, but radical excision of sinus tracts may also sometimes be feasible. Primary actinomycetic empyema is rare, as Harrison and Thomas (1979) and Merdler and associates (1983) noted, but George and colleagues (1985) reported that the empyema may require decortication or pleural drainage. On rare occasions, surgery may be required to control massive hemorrhage. Hamer and co-workers (1992), however, reported success with selective bronchial artery embolization.
Nocardiosis
Nocardiosis is a chronic infectious disease usually caused by Nocardia asteroides, but occasionally also by Nocardia brasiliensis and Nocardia madurae. Some clinical features are similar to actinomycosis (e.g., chronic draining sinuses, sulfur granules in the exudate), but some differ, such as hematogenous dissemination from a pulmonary focus or central nervous system involvement, as Frazier (1975) and Krick (1975) and their colleagues pointed out.
The organisms are aerobic and occur not only in microcolonies, but also in coccobacillary or filamentous forms that are acid fast and gram positive (Fig. 89-26A). The former feature, as well as radiographic similarities, led in the past to confusion with the acid-fast bacilli of Mycobacterium tuberculosis.
Nocardia is widely distributed in soil, grains, and grasses, without a specific area of endemicity. It does not often occur as a saprophyte in humans; thus, its presence in sputum, together with evidence of lung involvement, is presumptive evidence of disease.
P.1287
The disease process varies widely, ranging from benign, self-limited suppurative infections of the skin and subcutaneous tissues to pulmonary or generalized systemic infections. Balikian and associates (1978) reported the radiographic features to be nonspecific pulmonary infiltrates resembling those of pulmonary tuberculosis, including solitary nodules and cavitation (see Fig. 89-26). Empyema caused by Nocardia is also not rare. Central nervous system involvement (i.e., brain abscess or meningitis), which is ominous, may be the presenting picture, with minimal pulmonary disease.
The disease occurs primarily in immunosuppressed patients (85% of instances), including patients receiving organ transplants, being treated for malignancies, or those with AIDS. In the compromised host, cavitation or hematogenous dissemination, or both, may be accelerated. In the absence of a predisposing condition leading to the immunosuppressed state, nocardiosis may mimic bronchial carcinoma.
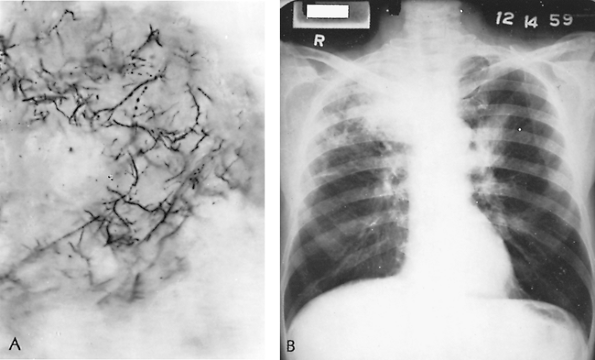 |
Fig. 89-26. Nocardiosis. A. Organisms of Nocardia asteroides (Gram's stain, original magnification 1,000). B. Radiograph of thorax showing bilateral pulmonary pneumonic infiltration. Nocardia asteroides was recovered from the sputum. The patient recovered after a course of sulfadiazine. From Takaro T: Thoracic actinomycetic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978. With permission. |
The diagnosis may be difficult to establish short of percutaneous lung aspiration, thoracoscopic or open lung biopsy, or exploratory thoracotomy. Gomori's stain is useful, and aerobic culture media are necessary.
Many agents have antimicrobial activity in the medical treatment of Nocardia infection. The most common drugs utilized are sulfonamides, minocyline, amikacin, and carbapenems. Tripodi and associates (2001) used ciprofloxacin followed by trimethoprim-sulfamethoxazole to successfully treat heart transplant patients. Prolonged treatment, at least 2 to 3 months, but sometimes longer, may be required. Surgical treatment may be required either to help make the diagnosis or, less frequently, to effect a cure. Drainage of empyemas and abscesses is indicated; with the use of specific drug coverage, this procedure has become safer.
REFERENCES
Al-Kattan K, et al: Surgery for pulmonary aspergilloma in post-tuberculous vs. immuno-compromised patients. Eur J Cardiothorac Surg 20:728, 2001.
Ampel NM, Wieden MA, Galgiani JN: Coccidioidomycosis: clinical update. Rev Infect Dis 2:897, 1989.
Anaissie E, Bodey GP: Nosocomial fungal infections. Old problems and new challenges. Infect Dis Clin North Am 3:867, 1989.
Anaissie EJ, Bodey GP: Fungal infections in patients with cancer. Pharmacotherapy 10(suppl):164, 1990.
Anaissie EJ, Solomkin JS: Algornion and explanation: approach to the surgical patient at risk for candidiasis infection IX. In: Fungal Infection. Scientific American, New York, 1994.
Anaissie EJ, et al: Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clin Infect Dis 23:964, 1996.
Arnow PM, et al: Pulmonary aspergillosis during hospital renovation. Am Rev Respir Dis 118:49, 1978.
Babatasi G, et al: Surgical treatment of pulmonary aspergilloma: current outcome. J Thorac Cardiovasc Surg 119:906, 2000.
Baker EJ, Hawkins JA, Waskow EA: Surgery for coccidioidomycosis in 52 diabetic patients, with special reference to related immunologic factors. J Thorac Cardiovasc Surg 75:680, 1978.
Balikian JP, Herman PG, Kopit S: Pulmonary nocardiosis. Radiology 126:569, 1978.
Battaglini JW, et al: Surgical management of symptomatic pulmonary aspergilloma. Ann Thorac Surg 39:512, 1985.
Bayer AS, et al: Unusual syndromes of coccidioidomycosis. Diagnostic and therapeutic considerations: a report of 10 cases and review of the English literature. Medicine 55:131, 1976.
Bennett JE: Chemotherapy of systemic mycoses. Parts I and II. N Engl J Med 290:30, 320, 1974.
Bethlem EP, et al: Paracoccidioidomycosis. Curr Opin Pulm Med 5:319, 1999.
Bigby TD, et al: Clinical spectrum of pulmonary mucormycosis. Chest 89:435, 1986.
Blumberg HM, et al: Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the nemis prospective multicenter study. Clin Infect Dis 33:177, 2001.
Bouza E, et al: Paracoccidioidomycosis (South American blastomycosis) in the United States. Chest 72:100, 1977.
Busey JF, et al: Blastomycosis. I. A review of 198 collected cases in Veterans Administration Hospitals. Am Rev Respir Dis 89:659, 1964.
Butman SM, et al: Prospective study of the safety and financial benefit of ketoconazole as adjunctive therapy to cyclosporine after heart transplantation. J Heart Lung Transplant 10:351, 1991.
Butz RO, Zvetina JR, Leininger BJ: Ten-year experience with mycetomas in patients with pulmonary tuberculosis. Chest 87:356, 1985.
Catanzaro A, et al: Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. Am J Med 98:249, 1995.
Chapman SW, et al: Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis 30:679, 2000.
Cohen SL, Gale AM, Liston HE: Report of a pilot study on noncalcified discrete pulmonary coin lesions in a coccidioidomycosis endemic area. Ariz Med 29:40, 1972.
Conant EF, Wechsler RJ: Actinomycosis and nocardiosis of the lung. J Thorac Imaging 7:75, 1992.
Croft DR, et al: FDG-PET imaging and the diagnosis of non-small cell lung cancer in a region of high histoplasmosis prevalence. Lung Cancer 36:297, 2002.
P.1288
Crum N, et al: Coccidioidomycosis outbreak among United States Navy SEALS training in a Coccidioides immitis endemic area Coalinga, California. J Infect Dis 186:865, 2002.
Cunningham RT, Einstein H: Coccidioidal pulmonary cavities with rupture. J Thorac Cardiovasc Surg 84:172, 1982.
Daly RC, et al: Pulmonary aspergilloma. Results of surgical treatment. J Thorac Cardiovasc Surg 92:981, 1986.
Davies SF, Sarosi GA: Epidemiological and clinical features of pulmonary blastomycosis. Semin Respir Infect 12:206, 1997.
Davis AM, Pierson RN, Loyd JE: Mediastinal fibrosis. Semin Respir Infect 16:119, 2001.
Denning DW, Stevens DA: Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis 12:1147, 1990.
Diamond RD: The growing problem of mycoses in patients infected with the human immunodeficiency virus. Rev Infect Dis 13:480, 1991.
Dismukes WE, et al: Itraconazole therapy for blastomycosis and histoplasmosis. Am J Med 93:489, 1992.
Dixon DM, et al: Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. J Clin Microbiol 29:1106, 1991.
Donado-una JR, et al: Persistent cavitations in pulmonary mucormycosis after apparently successful amphotericin B. Eur J Cardiothorac Surg 21:940, 2002.
Drutz DJ, Catanzaro A: Coccidioidomycosis. Parts I and II. Am Rev Respir Dis 117:559, 727, 1978.
Duhra P, Ilchyshyn A, Bell R: Thoracic actinomycosis. J R Soc Med 85:44, 1992.
Dunn EJ, et al: Surgical implications of sclerosing mediastinitis: a report of six cases and review of the literature. Chest 97:338, 1990.
Duswald KH, Penk A, Pittrow L: High-dose therapy with fluconazole 800 mg day-1. Mycoses 40:267, 1997.
Edmond MB, Wallace SE, McClish DK, et al: Nosocomial bloodstream infections in the United States hospitals: a three year analysis. Clin Infect Dis 29:239, 1999.
Edwards LB, et al: An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis 99(suppl):1, 1969.
Ellis DH, Pfeiffer TJ: Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 336:923, 1990.
Ellis ME, et al: Systemic amphotericin B versus fluconazole in the management of antibiotic resistant neutropenic fever preliminary observations from a pilot, exploratory study. J Infect 30:141, 1995.
Endo S, et al: Surgical outcome of pulmonary resection in chronic necrotizing pulmonary aspergillosis. Ann Thorac Surg 72:889, 2001.
Endo S, et al: Surgical considerations for pulmonary actinomycosis. Ann Thorac Surg 74:185, 2002.
Epstein R, Cole R, Hung KK Jr: Pleural effusion secondary to pulmonary cryptococcosis. Chest 61:296, 1972.
Feingold SM, George WL, Mulligan ME: Anaerobic Infections. Part II. In Cotsonas NJ Jr (ed): Disease-a-Month. Chicago: Year Book, 1985.
Foley TF, Dines DE, Dolan CT: Pulmonary actinomycosis: report of 18 cases. Minn Med 54:593, 1971.
Frazier AR, Rosenow EC III, Roberts GD: Nocardiosis: a review of 25 cases occurring during 24 months. Mayo Clin Proc 50:657, 1975.
Galgiani JN, Ampel NM: Coccidioides immitis in patients with human immunodeficiency virus infections. Semin Respir Infect 5:151, 1990.
Gartenberg G, et al: Hospital-acquired mucormycosis (Rhizopus rhizopodiformis) of skin and subcutaneous tissue. N Engl J Med 299:1115, 1978.
George RB, Penn RL, Kinasewitz GT: Mycobacterial, fungal, actinomycotic and nocardial infections of the pleura. Clin Chest Med 6:63, 1985.
Giron J, et al. CT-guided percutaneous treatment of inoperable pulmonary aspergilloma. Apropos of 42 cases. J Radiol 79:139, 1998. [Article in French] Godwin JD: The solitary pulmonary nodule. Radiol Clin North Am 21:709, 1983.
Goldberg SL, et al: Successful treatment of simultaneous pulmonary Pseudallescheria boydii and Aspergillus terreus infection with oral itraconazole. Clin Infect Dis 16:803, 1993.
Graybill JR, et al: Itraconazole treatment of coccidioidomycosis. Am J Med 89:282, 1990.
Griepp RB: In discussion of Henderson RD, et al: Surgery in pulmonary aspergillosis. J Thorac Cardiovasc Surg 70:1088, 1975.
Hamer DH, Schwab LE, Gray R: Massive hemoptysis from thoracic actinomycosis successfully treated by embolization. Chest 101:1442, 1992.
Harder EJ, Hermans PE: Treatment of fungal infections with flucytosine. Arch Intern Med 135:231, 1975.
Harrison RN, Thomas DJB: Acute actinomycetic empyema. Thorax 12:406, 1979.
Heller HM, Fuhrer J: Disseminated sporotrichosis in patients with AIDS: case report and review of the literature. AIDS 5:1243, 1991.
Herbrecht R, et al: Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408, 2002.
Hermans PE: Antifungal agents used for deep-seated mycotic infections. Mayo Clin Proc 52:687, 1977.
Herring M, Pecora D: Pleural aspergillosis: a case report. Am Surg 42:300, 1976.
Hsieh MJ, et al: Thoracic actinomycosis. Chest 104:366, 1993.
Isotalo PA, et al: Prosthetic valve fungal endocarditis due to histoplasmosis. Can J Cardiol 17:297, 2001.
Jay SJ, Platt MR, Reynolds RC: Primary pulmonary sporotrichosis. Am Rev Respir Dis 115:1051, 1977.
Johnson P, Sarosi G: The endemic mycoses: surgical considerations. Semin Thorac Cardiovasc Surg 7:95, 1995.
Johnson PC, et al: Saftey and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis. Ann Intern Med 137:105, 2002.
Jung JY, et al: The role of surgery in the management of pulmonary monosporosis: a collective review. J Thorac Cardiovasc Surg 73:139, 1977.
Jung JY, et al: Role of surgery in the management of pulmonary sporotrichosis. J Thorac Cardiovasc Surg 77:234, 1979.
Kanawaty DS, Stalker JB, Munt PW: Nonsurgical treatment of histoplasma endocarditis involving a bioprosthetic valve. Chest 99:253, 1991.
Kauffman CA: Old and new therapies for sporotrichosis. Clin Infect Dis 21:981, 1995.
Kauffman CA: Role of azoles in antifungal therapy. Clin Infect Dis 22:5148, 1996.
Kauffman CA: Endemic mycoses in patients with hematologic malignancies. Semin Respir Infect 16:106, 2002.
Kerkering TM, Duma RJ, Shadomy S: The evolution of pulmonary cryptococcosis. Ann Intern Med 94:611, 1981.
Kinasewitz GT, Penn RL, George RB: The spectrum and significance of pleural disease in blastomycosis. Chest 86:580, 1984.
Kitchen MS, Reiber CD, Eastin GB: An urban epidemic of North American blastomycosis. Am Rev Respir Dis 115:1063, 1977.
Krick JA, Stinson EB, Remington JS: Nocardia infection in heart transplant patients. Ann Intern Med 82:18, 1975.
Landis FB, Varkey B: Late relapse of pulmonary blastomycosis after adequate treatment with amphotericin B: case report. Am Rev Respir Dis 113:77, 1976.
Laskey W, Sarosi GA: Endogenous reinfection in blastomycosis. Am Rev Respir Dis 115:266, 1977.
Levitz SM: Aspergillosis. Infect Dis Clin North Am 3:1, 1989.
Levitz SM: The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Rev Infect Dis 13:1163, 1991.
Lillington GA: Pulmonary nodules: solitary and multiple. Clin Chest Med 3:361, 1982.
Littman ML, Walter JE: Cryptococcosis: current status. Am J Med 45:922, 1968.
Lonky SA, et al: Acute coccidioidal pleural effusion. Am Rev Respir Dis 114:681, 1976.
Lupinetti FM, et al: Pulmonary resection for fungal infection in children undergoing bone marrow transplantation. J Thorac Cardiovasc Surg 104:684, 1992.
Maresca B, Kobayashi GS: Dimorphism in Histoplasma capsulatum: a model for the study of cell differentiation in pathogenic fungi. Microbiol Rev 53:186, 1989.
Marks TS, Spence WF, Baisch BF: Limited resection for pulmonary coccidioidomycosis. In Ajello L (ed): Coccidioidomycosis. Tucson: University of Arizona Press, 1967, p. 73.
Mathisen DJ, Grillo HC: Clinical manifestation of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg 54:1053, 1992.
McDonnell JM, Hutchins GM: Pulmonary cryptococcosis. Hum Pathol 16:121, 1985.
McQuarrie DG, Hall WH: Actinomycosis of the lung and chest wall. Surgery 64:905, 1968.
P.1289
Menges RW, et al: Clinical and epidemiological studies on 79 canine blastomycosis cases in Arkansas. Am J Epidemiol 81:169, 1965.
Merdler C, et al: Primary actinomycetic empyema. South Med J 76:411, 1983.
Michelson E: Primary pulmonary sporotrichosis. Ann Thorac Surg 24:83, 1977.
Minamoto GY, Rosenberg AS: Fungal infections in patients with acquired immunodeficiency syndrome. Med Clin North Am 81:381, 1997.
Murray HW: Pulmonary mucormycosis with massive fatal hemoptysis. Chest 68:65, 1975.
Nelson AR: The surgical treatment of pulmonary coccidioidomycosis. Curr Probl Surg 11:1, 1974.
Niwa H, et al: Subclavian artery branch ligation reduces hemorrhage during resection of pulmonary aspergilloma. Ann Thorac Surg 59:1234, 1995.
Ochoa GA: Coccidioidomycosis in Mexico. In Ajello CL (ed): Coccidioidomycosis. Tucson: University of Arizona Press, 1967.
Orringer MB, Sloan H: Monilial esophagitis. Ann Thorac Surg 26:364, 1978.
Pappagianis D: Serologic studies in coccidioidomycosis. Semin Respir Infect 16:242, 2001.
Parfrey NA: Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine 65:113, 1986.
Paulsen GA: Pulmonary surgery in coccidioidal infections. In Ajello L (ed): Coccidioidomycosis. Tucson: University of Arizona Press, 1967.
Pidhorecky I, Urschel J, Anderson T: Resection of invasive pulmonary aspergillosis in immunocompromised patients. Ann Surg Oncol 7:312, 2000.
Poe RH, et al: Pulmonary blastomycosis versus carcinoma challenging differential. Am J Med Sci 263:145, 1972.
Rabinowitz JG, Busch J, Buttram WR: Pulmonary manifestations of blastomycosis: radiological support of a new concept. Radiology 120:25, 1976.
Ramirez J, et al: Chronic cavitary pulmonary sporotrichosis: efficacy of oral itraconazole. J Ky Med Assoc 96:103, 1998.
Read CT: Coin lesion, pulmonary, in the Southwest (solitary pulmonary nodules). Ariz Med 29:775, 1972.
Regnard J, et al: Aspergilloma: a series of 89 surgical cases. Ann Thorac Surg 69:898, 2000.
Remy J, et al: Treatment of hemoptysis by embolization of bronchial arteries. Radiology 122:33, 1977.
Rex JH, et al: A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med 331:1325, 1994.
Robinson LA, et al: Pulmonary resection for invasive Aspergillus infections in immunocompromised patients. J Thorac Cardiovasc Surg 109:1182, 1995.
Rohatgi PK: Pulmonary sporotrichosis. South Med J 73:1611, 1980.
Rohatgi PK, Schmitt RG: Pulmonary coccidioidal mycetoma. Am J Med Sci 287:27, 1984.
Rubin AHE, Alroy GG: Candida albicans abscess of lung. Thorax 32:373, 1977.
Saag MS, et al: Practice guidelines for the management of cryptococcal disease. Clin Infect Dis 30:710, 2000.
Sanders JS, et al: Exfoliative cytology in the rapid diagnosis of pulmonary blastomycosis. Chest 72:193, 1977.
Sarosi GA, Davies SF, Phillips JR: Self-limited blastomycosis: a report of 39 cases. Semin Respir Infect 1:40, 1986.
Sarosi GA, et al: Rapid diagnostic evaluation of bronchial washings in patients with suspected coccidioidomycosis. Semin Respir Infect 16:238, 2001.
Schaffner A, et al: Amphotericin B refractory aspergillosis after itraconazole: evidence for significant antagonism. Mycoses 36:421, 1993.
Scott SM, et al: Pulmonary sporotrichosis. N Engl J Med 265:453, 1961.
Shinagawa N, et al: Pulmonary actinomycosis followed by pericarditis and intractable pleuritis. Intern Med 41:319, 2002.
Sorkine P, et al: Administration of amphotericin B in lipid emulsion decreases nephrotoxicity: results of a prospective, randomized, controlled study in critically ill patients. Crit Care Med 24:1311, 1996.
Spear RK, Walker PD, Lampton LM: Tracheal obstruction associated with a fungus ball. Chest 70:662, 1976.
Steele JD (ed): Treatment of Mycotic and Parasitic Diseases of the Chest. Springfield, IL: Charles C Thomas, 1964.
Strinden WD, Helgerson RB, Maki DG: Candida septic thrombosis of the great central veins associated with central catheters. Ann Surg 202:653, 1985.
Sutliff WD, Cruthirds TP: Blastomyces dermatitidis in cytologic preparations. Am Rev Respir Dis 108:149, 1973.
Takaro T: Lung infections and interstitial pneumonopathies. In Sabiston DC, Spencer FC (eds): Gibbon's Surgery of the Chest. Philadelphia: WB Saunders, 1976.
Takaro T: Thoracic actinomycetic and mycotic infections. In Goldsmith HS (ed): Practice of Surgery. Hagerstown, MD: Harper & Row, 1978.
Tedder BK, et al: Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg 57:1044, 1994.
Temeck BK, et al: Thoracotomy for pulmonary mycoses in non HIV-immunosuppressed patients. Ann Thorac Surg 58:333, 1994.
Terrell CL, Hughes CE: Antifungal agents used for deep-seated mycotic infections. Mayo Clin Proc 67:69, 1992.
Thadepalli H, et al: Pulmonary mycetoma due to Coccidioides immitis. Chest 71:429, 1977.
Thomas FE Jr, et al: Candida albicans infection of sternum and costal cartilages: combined operative treatment and drug therapy with 5-fluorocytosine. Ann Thorac Surg 23:163, 1977.
Tripodi MF, et al: Treatment of pulmonary nocardiosis in heart-transplant patients: importance of susceptibility studies. Clin Transplant 15:415, 2001.
Trick WE, et al: Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989 1999. Clin Infect Dis 35:627, 2002.
Vasquez JE, et al: Blastomycosis in Northeast Tennessee. Chest 114:436, 1998.
Wheat LJ, et al: Practice guidelines for the management of patients with histoplasmosis. Clin Infect Dis 30:688, 2000.
Wheat LJ, et al: Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus intraconazole. Antimicrob Agents Chemother 45:2345, 2001.
Wheat JL, et al: State-of the-art review of pulmonary infections. Semin Respir Infect 17:158, 2002.
Wray TM, Bryant RE, Killen DA: Sternal osteomyelitis and costochondritis after median sternotomy. J Thorac Cardiovasc Surg 65:227, 1973.
Reading References
Baracco GJ, Dickinson GM: Pulmonary nocardiosis. Curr Infect Dis Rep 3:286, 2001.
Bronnimann DA, et al: Coccidioidomycosis in the acquired immunodeficiency syndrome. Ann Intern Med 106:372, 1987.
Davies SF, Sarosi GA: Blastomycosis. Eur J Clin Microbiol Infect Dis 8:474, 1989.
Espinel-Ingroff A, Shadomy S: In vitro and in vivo evaluation of antifungal agents. Eur J Clin Microbiol Infect Dis 8:352, 1989.
Gal AA, et al: The pathology of pulmonary cryptococcal infections in the acquired immunodeficiency syndrome. Arch Pathol Lab Med 110:502, 1986.
Idigbe EO, Onubogu C, John EKO: Human pulmonary nocardiosis. Microbios 69:163, 1992.
Johnston PG, et al: Late recurrent Candida endocarditis. Chest 99:1531, 1991.
Kauffman CA, et al: Guidelines for the management of patients with sporotrichosis. Clin Infect Dis 30:684, 2000.
Murray HW, Littman ML, Roberts RB: Disseminated paracoccidioidomycosis (South American blastomycosis) in the United States. Am J Med 56:209, 1974.
Polak A, Hartman PG: Antifungal chemotherapy are we winning? In Jucker E (ed): Progress in Drug Research. Basel, Switzerland: Hoffmann-La Roche, 1991.
Rippon JW (ed): Medical Mycology: the Pathogenic Fungi and the Pathogenic Actinomycetes. Philadelphia: WB Saunders, 1988.
Saubolle MA: Fungal pneumonias. Semin Respir Infect 15:162, 2000.
EAN: 2147483647
Pages: 203