49 - Diaphragmatic Function, Diaphragmatic Paralysis, and Eventration of the Diaphragm
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XI - The Pleura > Chapter 60 - Tuberculous and Fungal Infections of the Pleura
Chapter 60
Tuberculous and Fungal Infections of the Pleura
Gilbert Massard
Jean-Marie Wihlm
Mycobacterial and fungal infections of the pleural space are uncommon disorders in the Western world. Pleural tuberculosis (TB) is most often a side phenomenon of primary infection and seldom requires operative intervention except for diagnostic purposes. Empyema caused by reactivation of TB rarely occurs in patients who received adequate medical treatment but represents a real challenge to the thoracic surgeon. In a series of 380 empyema patients reported by Weissberg and Refaely (1996), no case of fungal infection was mentioned. Fungal empyema typically occurs in residual pleural spaces after previous resection or radiation therapy; these cases are particularly difficult, marked by chronic illness and consumption.
Although expertise from a large number of cases is obviously lacking, the general guidelines used for treatment of any empyema also apply to tuberculous and fungal empyema. A first step is to clean gross contamination, either with tube thoracostomy or open window thoracostomy. Once the pleural space has been cleaned, a complete and definitive obliteration of the space must be achieved to prevent further relapse of the infection. The procedure of choice is decortication, which requires an expandable underlying lung. Open window thoracostomy may be a life-saving procedure in acutely ill patients but jeopardizes the possibility of subsequent decortication; permanent loss of function must be expected when the lung remains trapped below an armor of fibrotic scar tissue. The common feature of chronic mycobacterial and fungal infections is that often the underlying lung cannot be reexpanded to fill the pleural space, either because of previous partial resection or because of diffuse fibrosis. In the latter patients, the goal of treatment may be achieved either with thoracoplasty or with various pedicled flaps developed from the chest wall muscles or the greater omentum. Extrapleural pneumonectomy for destroyed lung complicated with empyema is a high-risk procedure and cannot be recommended as standard practice.
In this discussion, we describe separately tuberculous empyema, Aspergillus empyema, and finally miscellaneous conditions, because the patients differ considerably. We do not detail the technical aspects of procedures such as decortication, thoracoplasty, and muscle transfers; these procedures are presented in Chapters 61, 62, and 59, respectively.
PLEURAL TUBERCULOSIS
Tuberculous infection of the pleura appears as a disease of the past when one refers to the paucity of recent publications. Indeed, most of the principles defined during the 1950s and 1960s are still valuable today. Langston and colleagues (1967) stated that TB of the pleura may manifest itself clinically and pathologically in a variety of ways. The gamut runs from a thin idiopathic effusion that may yield acid-fast bacilli with difficulty, if at all, to a thick purulent exudate that has positive results on direct smears. All gradations and combinations of extent as well as character of pleural involvement or bacteriologic content are seen, as noted by Langston and associates (1967). Nevertheless, for clarification, we artificially subdivide the subsequent discussion into three groups defined by the natural history of disease:
During primary TB, pleural effusions appear in approximately 8% of patients. The fluid is usually serofibrinous, and this condition should be called tuberculous pleuritis.
During reactivation of TB, pleural infection turns to a true empyema, characterized by an opaque and purulent effusion. Such tuberculous empyemas may be either pure or mixed. In the case of bronchopleural fistula, other microorganisms can infect the pleura together with Mycobacterium tuberculosis.
The particular setting of late complications of collapse therapy for TB, which are infrequently encountered nowadays, presents varying complex problems.
Tuberculous Pleuritis
According to Jereb and colleagues (1991), the pleural space is the second most common site of extrapulmonary TB,
P.841
the first being the lymphatic system. Pleural infection, as noted by Weir and Thornton (1985), is supposed to originate from subpleural pulmonary lesions. The clinical presentation is well known. General signs include low-grade fever, weakness, weight loss, and night sweats. Revealing respiratory symptoms are nonproductive cough, pleuritic chest pain, and dyspnea correlated with the extent of effusion. In the elderly, silent disease is not uncommon. Chest radiography shows a pleural effusion; concomitant parenchymal disease is observed in approximately one-third of cases. At the early stage of disease, the tuberculin skin test result is positive in 75% of patients, but, according to Berger and Mejia (1973), it should be positive in virtually all patients by 2 months, except in patients with acquired immunodeficiency syndrome (AIDS).
Positive diagnosis relies on direct sampling of the pleural fluid and on pleural biopsies. Gross analysis of pleural fluid reveals an exudative fluid with a protein level in excess of 40 g/L and a white cell count of 1 to 6 g/L with predominant lymphocytes. Absence of desquamated mesothelial cells suggests TB, as reported by Weir and Thornton (1985). Glucose level determination is not really useful; cultures take 3 to 6 weeks, and the results are inconsistently positive. Only 30% became positive in Berger and Mejia's (1973) experience. In the study of Caminero and colleagues (1993), determination of IgG antibody levels to mycobacterial antigen 60 with a cut-off value of 150 U/mL had a sensitivity of 50% and a specificity of 100%. The sophisticated determination of adenosine deaminase has been found by Berenguer and associates (1992) to have a poor sensitivity and specificity. Falk (1965) noted that cultures from sputum or gastric content are expected to be negative unless radiologic evidence exists for parenchymal disease. Therefore, the most reliable investigation is pleural biopsy. Bates (1979) and Berger and Mejia (1973) reported that pleural biopsy with the Abrams needle or similar devices yields a positive result in 60% to 80% of patients. According to Yim (1996), video-assisted thoracic surgery achieves a high level of specificity because multiple biopsies increase the diagnostic threshold. Given the remarkably low operative risk, it seems reasonable to proceed with thoracoscopy when direct examination of bacteriologic samples is negative, rather than waiting several weeks for the result of cultures. Histologic evidence of caseating epithelioid granulomas is indicative of TB; Levine and colleagues (1970) recommended that immediate antituberculous therapy should be commenced, although only identification of acid-fast bacilli is completely diagnostic.
In summary, the diagnostic criteria defined by Langston and associates (1967) are still valuable today. Tuberculous pleuritis may be assumed for one or more of the following specific findings: positive tuberculin skin test result; secretions positive for M. tuberculosis, sputum, or gastric content; pleural fluid positive for M. tuberculosis; and pleural disease consistent with tuberculous granuloma on histologic study of biopsy or resected tissue.
The natural history of tuberculous pleuritis is usually benign, with spontaneous resorption even if untreated. Usual management includes antituberculous treatment, repeated thoracentesis as required, and close observation. Most often, patients respond favorably. However, depending on the individual patient's immunologic status, excessive production of exudative material may start a diffuse thickening of the visceral pleura, leading to an entrapment of the lung, regardless of whether adequate antituberculous treatment is used (Fig. 60-1). Such residual pleural disease is a threat for reactivation of TB or further development of a bronchopleural fistula, and therefore decortication should be considered.
Indications for decortication rely on careful examination of medical imaging. Previously, the indication for decortication was confirmed with lateral chest radiography. It was assumed that pleural disease seen on a posteroanterior projection but not visible on lateral films was diffuse around the pleural sac, and that a minor thickness of the pleural peel occurred; such cases were expected to clear progressively, with return of normal motion of the chest wall. On the other hand, an effusion visible on both anteroposterior and lateral views corresponded to a large posterior pocket with a relatively thick pleural peel, by virtue of dependent accumulation. Such encapsulated pleural processes are not likely to resolve completely unless small at the onset. In cases with a thick pleural peel, decortication was recommended by Langston and colleagues (1967). Computed tomographic (CT) scan has confirmed this approach and has replaced bronchography for assessment of the underlying lung (Fig. 60-2).
Determination of the appropriate timing of surgical intervention was clearly defined in 1967, and no obvious reason exists to change anything in Langston and associates' (1967) criteria: (a) Decortication is indicated when thoracentesis fails to yield fluid or fails to alter radiographic appearance; (b) the extent of pleural involvement should be
P.842
equivalent to one-third or one-fourth of the hemithorax and cast a clearly discernible shadow in the posterior basal gutter; and (c) decortication should be made as early as is consistent with good judgment (i.e., after 2 to 4 months of drug therapy).
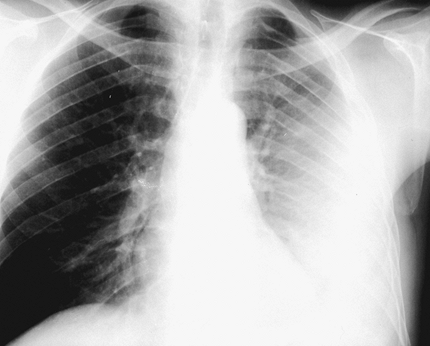 |
Fig. 60-1. Posteroanterior chest radiograph showing a large left pleural effusion. Needle biopsy disclosed tuberculous granuloma initially. The effusion failed to resolve after 4 months of adequate treatment. |
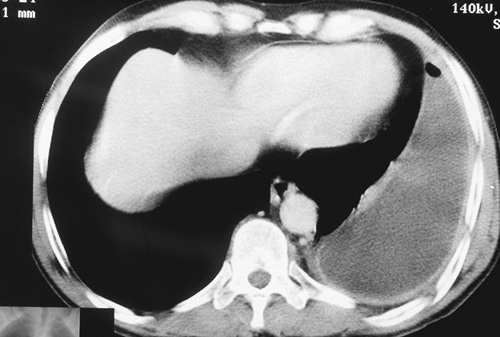 |
Fig. 60-2. Same patient as seen in Fig. 60-1. Computed tomographic scan demonstrated a thickened visceral pleural peel entrapping the lung and requiring decortication for reexpansion. |
Technical aspects of decortication are discussed in Chapter 61. In our opinion, a generous thoracotomy is still required. It remains preferable to proceed with the lung inflated for precise peeling of the visceral pleura to achieve optimal reexpansion. At present, this seems not to be easily reproducible with minimally invasive alternatives to a standard thoracotomy, although Yim (1996) and others have reported that isolated cases have been managed successfully by video-assisted thoracic surgery techniques.
Tuberculous Empyema
Pleural reactivation of TB has been a threat in patients who did not receive major antituberculous drug therapy. Moreover, chronic empyema is frequently complicated by bronchopleural fistula, leading to a so-called mixed empyema characterized by a contamination with both M. tuberculosis and common pyogens. Chronic empyema following TB adequately treated with antituberculous antibiotics is seldom associated with reactivation of TB in the pleural space. For example, none of the 22 patients treated by Garcia-Yuste and co-workers (1998) had any evidence of ongoing mycobacterial infection.
Diagnosis is easy in patients with known sequelae of TB. Low-grade fever, constitutional complaints, and increasing dyspnea with or without chest pain are the major symptoms. Abundant sputum suggests bronchopleural fistula. On chest radiography, the obvious finding is an increase in the extent of the pleural involvement; the appearance of an air fluid level heralds a bronchopleural fistula. Thoracentesis yields purulent fluid, which should be sent routinely for both bacterial and fungal cultures. Thoracentesis may be difficult in patients with a calcified pleura and requires an experienced operator.
As soon as empyema is confirmed, adequate drainage should be instituted. Our preference is the tube thoracostomy, whereas others prefer immediate open thoracostomy. We limit open thoracostomy to the severely ill patient with a poor operative risk, because it may jeopardize subsequent decortication. In patients with documented reactivation of TB, the classic principle to convert sputum cultures with medical treatment before resection should still be applied as recommended by Treasure and Seaworth (1995).
The next step is to plan for definitive treatment, which implies major thoracic surgery with often complicated outcomes (Table 60-1). Unfortunately, video-assisted thoracic surgery has no place, although it should be useful for those patients who are debilitated, thus making them poor candidates for conventional open surgery.
The first question is to determine whether the underlying lung is reexpandable. Ideally, one should prefer the most conservative approach, which consists of reexpanding the lung with decortication. The CT scan is most helpful in the evaluation of the entrapped lung; bronchography is no longer required. Areas with cavitations or large cystic bronchiectases obviously will not reexpand. Conversely, Mouroux and associates (1996), as well as Treasure and Seaworth (1995),
P.843
have found that a patent bronchopleural fistula does not preclude decortication.
Table 60-1. Treatment Plan for Chronic Mycobacterial or Fungal Empyema | |
|---|---|
|
The second question is to determine whether parenchymal resection is required. Metatuberculous lungs are relatively stiff, and loss of volume leads to residual pleural space, which represents a factor for persistent empyema postoperatively. Therefore, combined parenchymal resections should strictly adhere to the classic indications for resection as reported by Pomerantz (1991) and Mouroux (1996) and their colleagues as well as by Treasure and Seaworth (1995): multiple drug-resistant disease, threat of hemoptysis, and infectious complications such as bronchiectasis or aspergilloma. The current criteria for drug resistance are clinical or radiologic evidence of progressive disease, and persistent mycobacteria on sputum examination after 3 months of a four-drug treatment; at an earlier stage, bacillar casts may be visible on direct examination of smears, but fail to grow in culture. When the remaining lung is extensively destroyed, extrapleural pneumonectomy is to be considered. We would, however, raise some admonitions against a difficult and potentially dangerous procedure. Publications by Halezeroglu (1997) and Conlan (1995) and their associates, as well as by Odell and Henderson (1985), conclude that both previous TB and coexistent empyema are significant risk factors for procedure-related morbidity and mortality.
As we and our colleagues (1995b) have pointed out, the postoperative course after decortication with or without additional parenchymal resection may be complicated mainly by pleural space disease. Prolonged air leaks ultimately seal with prolonged drainage, provided that the lung is completely expanded. Persisting pleural spaces after decortication may be managed either with muscle plombage or with thoracoplasty. Thoracoplasty has an unwarranted bad name and should be rehabilitated in patients with marked malnutrition; a four-rib thoracoplasty with a stable and retracted mediastinum may be expeditiously carried out, as noted by Hopkins and associates (1985), compared with the preparation and transfer of two or three muscle flaps. Rather than performing an immediate thoracoplasty as in former years, we prefer to test the expansion potential of the remaining lung and to proceed with second-stage thoracoplasty only when it proves to be necessary.
Creating an open window thoracostomy initially is an important decision, because decortication cannot be performed in a second stage. However, Garcia-Yuste and colleagues (1998) suggest that two-stage management with thoracostomy and subsequent muscle plombage of the cleaned residual space is a reasonable alternative in cases unsuitable for decortication. Poor general health status and extensive calcifications precluding decortication are indications for this type of management. In the case of a largely destroyed but asymptomatic lung, two-stage myoplasty may help avoid the temptation to proceed with extrapleural pneumonectomy.
Regardless of the type of surgical management decided on, adjuvant antituberculous treatment is mandatory in cases of documented reactivation of TB.
Late Pleural and Extrapleural Complications of Collapse Therapy
Until the early 1960s, when major antituberculous drugs became available and began the end of a long-standing plague, the only active treatment for TB was the so-called collapse procedures. The common objective of these procedures was to collapse cavitated lung tissue and to obtain scarring of the tuberculous area progressively. Most physicians used to these treatments have retired, and few patients still survive. Thoracic surgeons of the younger generation have had virtually no exposure to such patients.
The first stage of collapse therapy was creation of an artificial intrapleural pneumothorax; because of the spontaneous resorption of the intrapleural air, reinjections of air were required at 2-week intervals. The first indication for thoracoscopic surgery was apical adhesiolysis to promote apical collapse, as reported by Dumarest and co-workers in 1945. When extensive apical adhesions precluded adequate collapse with intrapleural pneumothorax, extrapleural pneumolysis was the preferred procedure. Similarly, as carried out by Roberts (1948), the collapse space was maintained by periodic injections of air. After 2 to 3 years of treatment, the injections of air were discontinued, and the space progressively filled with serous fluid and retracted to a small and permanent residual space.
The considerable burden of pneumothorax, and the infectious risk from repeated thoracenteses, led to the conception of extramusculoperiosteal plombage, also called the birdcage operation, which was most popular between 1948 and 1955, as reported by Wilson and co-workers (1956) and Shepherd (1985). In this operation, the periosteum and intercostal muscles were stripped off the ribs, similar to thoracoplasty, and pushed inside the chest cavity to collapse the underlying cavity. The collapse was maintained with methyl methacrylate balls packed between the denuded ribs and the surface of pneumolysis. Initially, extraperiosteal pneumolysis was believed to avoid thoracoplasty. However, the many infectious complications, as well as the frequently reported migrations of material, necessitated that the plombage be removed several months later and thoracoplasty be performed to obliterate the extraperiosteal space. In Chicago and some areas of the Pacific Northwest of the United States, the use of paraffin as the plombage material became common during the same time period. Lees (1951) and Fox (1962) and their associates reviewed their extensive experience with this material. Infectious complications were rare, but migration of the wax plomb occurred in more than 25% of the patients from as early as 5 to 6 months to as late as 10 years or more. A thoracoplasty was rarely necessary after the removal of the migrated plomb, in contrast to the Lucite plombage. All these procedures rapidly vanished with the advent of antituberculous chemotherapy.
Late infectious complications at the site of previous intrapleural or extrapleural pneumothorax present as either progressively acquired swelling of the residual pocket (Fig. 60-3)
P.844
or appearance of an air fluid level because of a bronchopleural fistula. One-half of the patients are symptomatic and complain of fever, increasing dyspnea, pain, or hemoptysis; productive cough usually indicates bronchopleural fistula. In asymptomatic patients, the diagnosis is made when comparing consecutive surveillance chest films, as pointed out by us and our associates (1995a). Surprisingly, the pleural fluid is sterile on culture in more than one-half the patients. Furthermore, the incidence of proven tuberculous empyema is relatively low. In our experience (1995a), 13 of 28 patients had positive microbiology results, and only 4 had proved pleural TB. Similarly, Schmid and De Haller (1986) observed a single case of reactivated TB in a series of 15 patients. These observations are consistent with earlier data reported by Neff and Buchanan (1975), which showed that tuberculous empyema was a rather usual early complication of collapse therapy but did not contribute significantly to the late morbidity.
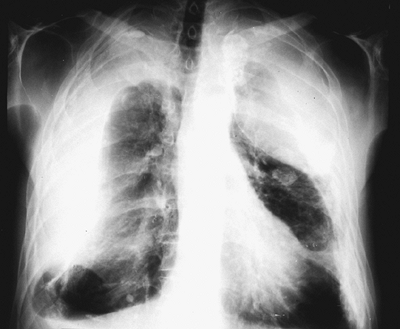 |
Fig. 60-3. Bilateral tension effusion at the site of a right-sided intrapleural and a left-sided extrapleural pneumothorax. |
The preferred mode of treatment of late empyema is decortication. The underlying lung is expected to reexpand because no previous parenchymal resection has been performed. Simple open window drainage is not satisfactory per se but is a fair treatment for debilitated and otherwise inoperable patients. On the other extreme, the risk of extrapleural pneumonectomy is certainly prohibitive in this particular setting. Both sequelae of TB and coexisting empyema are significant risk factors, as noted by Halezeroglu (1997) and the authors (1996) and associates. Furthermore, these patients are in their seventh decade of life and frequently present with significant respiratory or cardiovascular comorbidity. Thoracoplasty should be restricted to either space problems following decortication or diffuse and heavy calcifications obliterating the extrapleural dissection plane.
Decortication is well tolerated, although it is complicated by prolonged air leaks in most patients. We and our colleagues (1995b) found that the average drainage time was 16 days; however, drainage time was increased to a mean of 20 days in symptomatic patients and in patients with positive microbiology results. The reexpansion potential of such chronically entrapped lungs is surprising but may be anticipated from careful inspection of CT of the underlying lung (Fig. 60-4). Technical details do not diverge from those of any decortication. Briefly, double-lumen tube intubation is mandatory to prevent flooding of the opposite lung. A generous posterolateral thoracotomy is necessary for adequate exposure. Resection of the fifth or sixth rib is usually necessary because of the considerable retraction of the rib cage; the extrapleural plane is entered through the posterior periosteum. The parietal pleura is freed up to the mediastinal reflections, which requires considerable strength in case of extensive calcifications. Overenthusiastic dissection along the paraspinal gutter may lead to avulsion of the azygos vein on the right side or of intercostal vessels on the left side. The lung is identified ideally at the anterior mediastinal recess. However, when this adequate cleavage plane is not easily found, the pleural pocket should be entered and the visceral pleura incised
P.845
directly over the lung; the thickened pleura is progressively freed and excised from the center to the periphery. One should refrain from resecting any lung, because the previous tuberculous areas have scarred over the years. We recommend triple drainage connected to strong suction (100 to 150 mm Hg), because air leaks stop only when the apposition of pleural surfaces is restored. Because compliance of such lungs is decreased, strong suction is required to shift the mediastinum and elevate the diaphragm. Antituberculous therapy is mandatory and should be guided by sensitivity studies. The functional recovery after such procedures is debated. We do not anticipate any restoration of lung function. In our opinion, the aim of the operation is to use the lung as a natural prothesis to fill the pleural space and to resolve the empyema and, of course, avoid extrapleural pneumonectomy.
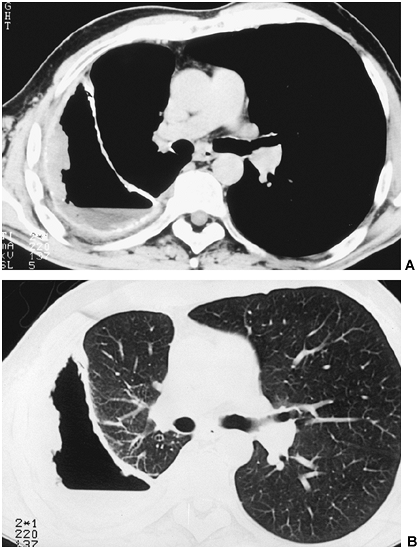 |
Fig. 60-4. Patient with tuberculous empyema complicating a previous intrapleural pneumothorax. A. Mediastinal window. A broad pleural peel is clearly outlined. B. Parenchymal window obviates relatively normal underlying lung tissue prone to reexpansion after decortication. |
Late complications occurring in extraperiosteal plombage cavities were more frequently frank infections; in 1997 we reported eight proved infections in a total of 10 exudates; four of them were tuberculous empyemas. When infection occurs, the usual finding is recent onset of swelling along the thoracoplasty incision or in the subclavicular area; infection facilitates erosion of the devitalized ribs and migration of the plombage material, which can be palpated beneath the skin. The procedure of choice is removal of the plombage and immediate thoracoplasty (Figs. 60-5 and 60-6). The approach is made through the previous thoracoplasty incision. Ablation of the devitalized, sometimes partly eroded ribs, is usually simple. We emphasize that the first rib should be resected to prevent any residual space, which could be the bed for relapse of infection; this technical step is in agreement with Hopkins and associates (1985). Intraoperative fluoroscopy is mandatory to check for complete removal of any foreign material; Lucite balls may be embedded into calcified tissues at the floor of the collapse space and be hidden from the surgeon's eye or fingers. Calcifications of the floor of the collapse space usually determine a peripheral rim, which must be trimmed away to provide adequate collapse. Double drainage for irrigation is placed before standard closure in layers. Naturally, adjuvant antituberculous therapy is mandatory.
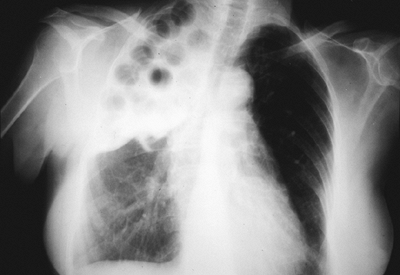 |
Fig. 60-5. Extraperiosteal plombage with methyl methacrylate balls. Late infectious complication with exudative distention of the collapse space. |
 |
Fig. 60-6. Same patient as seen in Fig. 60-5. Freshly removed plombage material. |
ASPERGILLUS EMPYEMA
Pleural infection with Aspergillus fumigatus is a rather infrequent disease; Kearon and colleagues (1987) listed only 30 cases in an exhaustive review of the literature. Even in the immunodeficient setting of lung transplantation, Aspergillus empyema remains rare. In a series of 31 graft recipients with positive Aspergillus culture results reported by Westney and colleagues (1996), only 1 patient developed Aspergillus empyema. Contamination of the pleural space with Aspergillus spores or hyphae results either from direct intrapleural seeding during surgery or by aspiration of aerosolized particles through a bronchopleural fistula. Fungal growth requires a persistent pleural space, which provides excellent atmospheric conditions, with an ambient temperature of 37 C, a moisture of 100%, and abundant proteins readily available for digestion by fungal catalase or chymotrypsin.
Pleural aspergillosis presents in two clinical pictures that differ considerably. Acute Aspergillus empyema develops usually during the immediate postoperative course after an intrapleural thoracic procedure and presents with findings similar to any postoperative pleural infection. Late Aspergillus empyema presents similarly to pulmonary aspergilloma as a chronic process of the saprophytic type. The following section refers mainly to our personal data, originally published in 1992(a).
Acute Aspergillus Empyema
As noted by Herring and Pecora (1976), acute Aspergillus empyema most often occurs after a surgical procedure undertaken
P.846
to treat an aspergilloma. We have reported seven cases of acute pleural infection (1992a). Five of them resulted from gross intraoperative contamination, with four patients being operated on for a known aspergilloma. A fifth patient was operated on for spontaneous hemopneumothorax and underwent resection of an apical bulla; retrospectively, a small mycetoma was identified by review of the preoperative CT scan.
The most common operation preceding acute Aspergillus empyema is partial lung resection such as lobectomy or segmentectomy. One of our personal cases (1992a) occurred after diagnostic thoracoscopy for a metastatic pleural effusion; this procedure was complicated with multiple parenchymal fistulae. Another case reported by Purcell and Corris (1995) was a patient with bronchopulmonary aspergillosis who developed Aspergillus fumigatus empyema subsequent to a spontaneous pneumothorax. Nakanishi and colleagues (1996) noted that percutaneous instillation of antifungal agents into a parenchymal cavity hosting a mycetoma is also known to foster pleural seeding.
A threat of pneumonectomy exists in patients with aspergilloma. These operations are characterized by major intraoperative difficulties because of the usually dense and highly vascular adhesions. Virtually all patients operated on by our group (1996) had excessive intraoperative bleeding, and six of eight developed postpneumonectomy empyema. However, only a single case had the pleural space contaminated with Aspergillus. After this experience, we recommend removal of the fungus ball and a thoracoplasty to obliterate the parenchymal cavity when feasible, as described in 1992(a), rather than proceeding with an extrapleural pneumonectomy.
The most common clinical finding after partial lung resection is prolonged air leak and persistent drainage of fluid; in addition, fever and weight loss are usual. The residual pleural space hosting the infection is not always apparent on standard chest radiography. More particularly, anterior spaces most often require CT studies to be identified. After pneumonectomy, the signs of empyema are well known to all thoracic surgeons: General malaise, fever, and pallor identify the patient at risk. Infection is confirmed by elevated white blood cell count and persisting high C-reactive protein levels, as reported by Icard and associates (1994). Chest radiography shows a rapid increase of the pleural air fluid level because of increased exudation of pleural fluid; the mediastinum is occasionally shifted toward the contralateral lung.
Diagnosis of Aspergillus empyema is easily confirmed by appropriate analysis of pleural fluid samples. Serodiagnosis is less reliable, because it may be negative at the early stages of the disease.
Cure of acute Aspergillus empyema requires sterilization and complete and definitive obliteration of the pleural space. Various strategies such as pleural lavage through a chest tube, surgical d bridement, and open window thoracostomy achieve a satisfactory gross cleaning of the pleural space. But the final decision as to how to obliterate the space depends on the reexpansion potential of the remaining lung. After resections less than lobectomy, expansion is clearly possible as soon as air leaks have sealed. In such patients, conservative management with antifungal treatment has a fair chance for success. According to Chatzimichalis and co-workers (1998), because of the hyperemia of the acutely infected pleura, the tissue penetration of itraconazole is satisfactory and sufficiently high local concentrations are easily obtained, similar to invasive pulmonary aspergillosis. A lone case of success of treatment with aerosolized liposomal amphotericin B has been reported by Purcell and Corris (1995); high local tissue concentrations are expected in the presence of a bronchopleural fistula.
The problem is different in patients who have undergone a lobectomy. Clearly, intraoperative seeding results from parenchymal tears because of a difficult dissection. This means that such patients had complex aspergilloma, as pointed out by Daly and colleagues (1986), and that the remaining lung is expectedly sclerotic with reduced reexpansion potential. In favorable cases, institution of a pneumoperitoneum sufficiently increases the diaphragm to obtain contact of the pleural surfaces. In the event of larger pleural spaces, aggressive management is mandatory to shorten the spontaneous evolution. In our opinion, apical spaces are best dealt with by thoracoplasty. Muscle flaps are often disappointingly thin in emaciated patients marked by chronic illness; further, the largest flap (i.e., the latissimus dorsi) usually has been sacrificed during the initial thoracotomy. None of the six patients we treated with thoracoplasty developed recurrent disease (1992a).
Management of Aspergillus empyema after pneumonectomy does not differ from management of postpneumonectomy empyema in general. Once the diagnosis is established, the pleural space must be drained. Aggressive management is mandatory to obtain quick cleaning of gross purulent material. Most authors proceed with an open window thoracostomy as described by Clagett and Geraci (1963). In our current practice, we prefer surgical d bridement followed by irrigations through a chest tube. It is questionable whether a tedious cleaning by video-assisted thoracic surgery techniques offers any advantage over a short thoracotomy in this situation. To obliterate the cleaned pleural space, the debate is between the proponents of muscle plombage procedures, such as Shirakusa (1989, 1990) and Pairolero (1990) and their colleagues, as well as Ali and Unruh (1990), and the partisans of thoracoplasty, including Horrigan and Snow (1990) as well as Gr goire (1987) and Hopkins (1985) and their associates.
Chronic Aspergillus Empyema
Chronic Aspergillus empyema is fostered by a residual pleural space communicating with the bronchial tree. The cavity is penetrated by aerosolized fungal material similar
P.847
to parenchymal cavities. Further development of the fungus is favored by progressive erosion of the surrounding structures by its proteolytic enzymes. As we have shown (1992a), the most frequent setting is previous partial lung resection for TB or lung cancer. Adjuvant or neoadjuvant radiation therapy has been retrospectively identified in four of our six patients operated for lung cancer (1992a) and, as suggested by Utley (1993), might be a contributing factor. In addition, occasional Aspergillus empyemas are encountered in patients with sequelae of previous collapse therapy, as previously noted.
The common problem in these patients is that medical therapy with itraconazole is likely to fail. The first reason is that tissue penetration in chronic lesions surrounded by fibrotic scar tissue is expected to be low, as pointed out by Chatzimichalis and associates (1998), similar in this aspect to an aspergilloma. The second reason is that the possibility of infection persists as long as a residual pleural space exists. The obligate operative management is one of the most challenging situations in general thoracic surgery. The precarious health status of patients with Aspergillus empyema is best described by Krakowka and colleagues (1970), who reported five deaths during treatment in a series of 10 patients.
Two clinical presentations are discussed, corresponding to postresectional empyema and empyema in previous collapse spaces. Chronic postresectional empyema may be detected on routine chest radiography but is usually symptomatic. The symptoms are similar to those of pulmonary aspergilloma: hemoptysis, bronchorrhea, dyspnea, and chest pain. Chest radiography shows either a partial hydropneumothorax indicating a bronchopleural fistula or a progressive thickening of known pleural sequelae that was noted by Libshitz and associates (1974). True intrapleural megamycetomas are uncommon. Empyema in previous intrapleural or extrapleural collapse spaces or residual pockets after tuberculous pleurisy may be silent and appear as an enlargement of the pleural thickening on sequential surveillance chest radiography. Otherwise, fever and chest pain are the usual symptoms. Cough and increased sputum indicate bronchopleural fistula, which is characterized by an air fluid level on chest films (Figs. 60-7 and 60-8).
A positive diagnosis relies on direct identification of Aspergillus species or serodiagnosis. Sampling of pleural fluid is easy in patients with hydropneumothorax only. Fortunately, serodiagnosis is most reliable because it is nearly always positive in patients with chronic infection. Our current criteria for Aspergillus infection are the following: either at least two precipitations on immunoelectrophoresis or a single precipitation with positive catalase activity. In our previous series (1992a), the average number of precipitations was 7.2 (range, 2 to 15); all but one patient had a positive result for catalase activity.
Treatment options differ between postresectional and postcollapse empyema. In any event, the underlying lung should be left in place when feasible, because a significantly increased risk for mortality and major morbidity exists after pneumonectomy in patients with preoperative empyema; this has been estimated to be close to 40% by Halezeroglu (1997) and Conlan (1995) and their associates, as well as by Odell and Henderson (1985). Furthermore, according to McGovern and colleagues (1988), the risk of completion pneumonectomy in this situation is prohibitive. The operative mortality of 9.4% after completion pneumonectomy for cancer was increased to 27.6% after completion pneumonectomy for benign disease in the Mayo Clinic experience. Similarly, the prevalence of empyema was 20.7% and 9.3%, respectively, for benign and malignant disease, and the prevalence of bronchopleural fistula was 17.2% and 3.1%. Therefore, further resection is only legitimized when persisting infectious lesions such as lung abscess or bronchiectases would otherwise jeopardize the outcome. In extreme situations, completion pneumonectomy and immediate thoracoplasty have been advocated by Utley (1993).
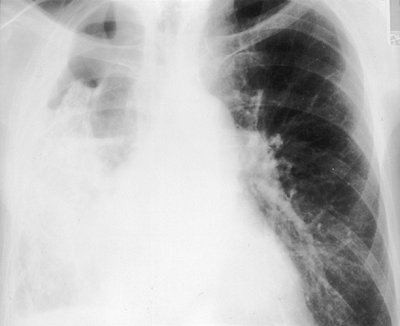 |
Fig. 60-7. Aspergillus empyema caused by bronchopleural fistula complicating an intrapleural pneumothorax. Note the apical pneumothorax and basal effusion, determining a midthoracic air fluid level. Extensive pleural calcifications are visible; translucent trapped lung is packed against the hilum. |
In postresectional empyema, the remaining lobe is usually fibrosed because of previous TB or radiation therapy. Therefore, decortication is likely to fail. Muscle transfers are less than optimal in these malnourished patients with marked chronic illness; besides, as previously noted, the latissimus dorsi muscle has usually been sacrificed during the initial thoracotomy. Although some successes have been obtained with omental transfer, as reported by Shirakusa and associates (1990), we would add some caveats to this technique. In malnourished patients, the tissue volume is spare. Harvest of the omentum exposes the patient to the additional risk of laparotomy. When the omentum is used, one should avoid a direct passage through the diaphragm to prevent herniation
P.848
of abdominal viscera, as we noted (1992b). It is mandatory to use indirect tunneling, either through Morgagni's hiatus or through the phrenohepatic ligaments as suggested by Jurkiewicz and Arnold (1977).
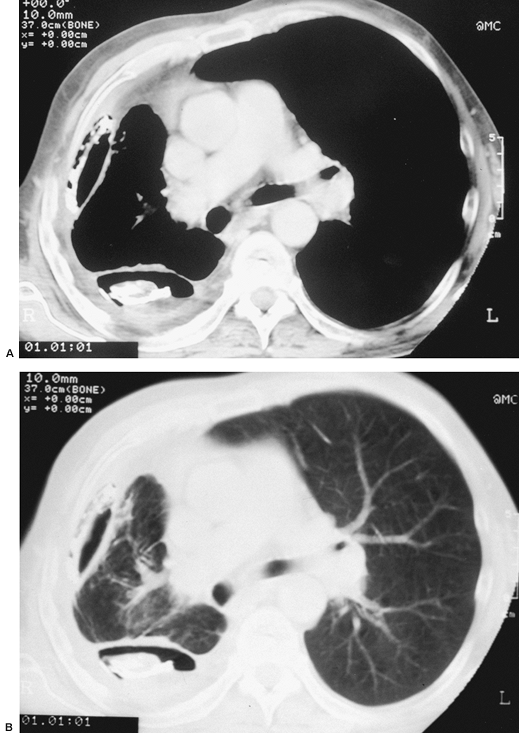 |
Fig. 60-8. Aspergillus empyema caused by bronchopleural fistula complicating an intrapleural pneumothorax. Same patient as seen in Fig. 60-7. A. Mediastinal window: thickened parietal and visceral pleura with extensive calcifications. B. Parenchymal window: The trapped lung tissue is nearly normal and did reexpand after decortication. |
The most appropriate management should include careful curettage of all fungal material, followed by a retailoring of the chest wall with thoracoplasty. Many authors, such as Gr goire and colleagues (1987), recommend leaving the first rib in place to avoid scoliosis. However, others, including Loynes (1972), believe that scoliosis is determined by extensive rib resections and resection of the transverse processes of the vertebra. We stress the need to routinely resect the first rib, as do Hopkins and colleagues (1985) and Horrigan and Snow (1990). Incomplete apical collapse is a concern when the first rib is left in place. Of course, such operations are risky because of the disabled status of these patients. In our experience, mortality of thoracoplasty has been 7%, caused by respiratory infection. More than one-half the patients in our series (1992a) experienced major perioperative bleeding (>1,500 mL) because of the usual hypervascularization of aspergillized cavities; preoperative embolization as suggested by Hughes and associates (1986) usually fails to reduce intraoperative bleeding because the perilesional vascular network is supplied by multiple pedicles, as pointed out by Chen and colleagues (1997). We suggest generous rib resection, because more than 40% of our patients had persistent space problems after thoracoplasty.
The postoperative course is usually prolonged, with an average hospital stay of 49 days in our experience; this is the result of the disease rather than of the procedure used. However, long-term results show no evidence for recurrent infection at a mean follow-up of 7 years. Thoracoplasty offers the possibility for a one-stage cure. Management with open window thoracostomy followed by muscle flap transfer or omentoplasty to fill the residual space seems less aggressive than thoracoplasty, but duration of the complete treatment plan takes at best 6 months, as reported by Shirakusa and colleagues (1989).
Treatment of long-term complications of residual collapse spaces is initiated with tube thoracostomy and irrigations. CT scan reveals some expandable underlying parenchyma in most cases, although perfusion scan shows a dramatic decrease of perfusion (see Fig. 60-8). Knowing the risk of pneumonectomy through an empyema (1995a), we advocate decortication in the latter cases. Inexperienced surgeons might be frightened by the sight of the residual lung with multiple parenchymal leaks, as it presents at the conclusion of the operation. Triple drainage with strong suction, and a good deal of patience, lead finally to sealing of the leaks and cure of the patient. One should remember that postoperative drainage is significantly prolonged in symptomatic patients and patients with positive microbiology findings, as we have noted (1995b).
MISCELLANEOUS CONDITIONS
Several other fungal diseases, such as blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis, may occasionally involve the pleura during acute pulmonary infection with pleural perforation. Patients with AIDS are particularly prone to such infections. The main problem in these patients is appropriate microbiological diagnosis. With adequate antifungal treatment, the infection should resolve without any need for surgical management except for drainage of large effusions.
Two agents, Coccidioides immitis and Candida albicans, are of particular interest. Coccidioidomycosis may cause diffuse lung destruction similar to TB and may result in similar surgical problems. The presence of yeast cells within a pleural effusion suggests the presence of an esophagopleural fistula.
P.849
Coccidioidomycosis
Pleural infection with C. immitis seldom has been referred to in the literature. A review published by Drutz and Catanzaro (1978a, 1978b) describes two situations depending on the natural history of disease. An acute infection appears in 40% of infected patients and mimics an influenza-like acute respiratory infection. The pleural effusion that may appear simultaneously to the pulmonary infiltrate is usually sterile. Approximately 5% of patients develop irreversible pulmonary lesions such as cavitations or bronchiectases. Rupture of a cavitation into the pleural space leads to a coccidioidal or mixed empyema. Amphotericin B is the basis of treatment; surgical indications are carried out along identical guidelines as for pleural TB.
The difficulties of chronic pulmonary coccidioidomycosis with empyema are perfectly illustrated by a case reported by Utley (1993). This patient presented with bronchopleural fistula and simultaneous granulomatous infection of the lung and pleural space 10 years after left lower lobectomy for coccidioidomycosis. Operative intervention was prompted by major hemoptysis. Completion pneumonectomy was required because of cavitations in the remaining lobe. A mass closure of hilar vessels and bronchus with transfixing mattress sutures was used because of inability to individually close the vessels and bronchus because of fibrous scarring of the hilum, and the pleural space was obliterated by an immediate thoracoplasty.
Candida albicans
Though usually seen in immunocompromised individuals, such as patients with AIDS or transplant recipients, as noted by Emery and associates (1991), empyema with yeast cells should suggest an esophageal fistula. Typically, microbiology reveals combined infection together with saprophytic oropharyngeal bacteria or Gram-negative bacilli. Jones and Ginsberg (1992) noted that diagnosis of an esophagopleural fistula is usually simple in case of spontaneous or iatrogenic disruption.
Sehti and Takaro (1978) reported that an esophagopleural fistula complicates approximately 0.5% of pneumonectomies. This complication is relatively unknown, although Takaro and colleagues reported its occurrence in their collective review in 1960. Intraoperative injury causing a direct tear, or devascularization with subsequent necrosis, leads to an early fistula that becomes apparent during the postoperative recovery period. The empyema is managed by insertion of a chest tube, and the diagnosis is often established by simple observation of draining saliva or food particles. Recurrent cancer or inflammatory changes are the cause of late esophagopleural fistulae and are revealed by the occurrence of an empyema. We and our associates (1994) observed the presence of tiny, sinuous tracts that are sometimes tedious to close and require careful barium swallow studies in various positions, especially lateral decubitus with the pneumonectomy space in the dependent position. Curative treatment may be offered to any patient without recurrent cancer and requires two steps: closure of the esophageal defect by direct repair reinforced with a myoplasty or omentoplasty, and eradication of the empyema by definitive obliteration of the pneumonectomy space. This is achieved with either thoracoplasty or muscle flap transfers, as we (1994) and Engelman (1970) and colleagues have suggested.
REFERENCES
Ali I, Unruh H: Management of empyema thoracis. Ann Thorac Surg 50:355, 1990.
Bates JH: Diagnosis of tuberculosis. Chest 76:757, 1979.
Berenguer J, et al: Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med 326:668, 1992.
Berger HW, Mejia E: Tuberculous pleurisy. Chest 63:88, 1973.
Caminero JA, et al: Diagnosis of pleural tuberculosis by detection of specific IgG anti-antigen 60 in serum and pleural fluid. Respiration 60:58, 1993.
Chatzimichalis A, et al: Surgery for aspergilloma: a reappraisal. Ann Thorac Surg 65:927, 1998.
Chen JC, et al: Surgical management for pulmonary aspergilloma: a 28 year experience. Thorax 52:810, 1997.
Clagett OT, Geraci J: A procedure for the management of post-pneumonectomy empyema. J Thorac Cardiovasc Surg 45:141, 1963.
Conlan AA, et al: Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg 110:1118, 1995.
Daly RC, et al: Pulmonary aspergilloma. Results of surgical treatment. J Thorac Cardiovasc Surg 92:981, 1986.
Drutz DJ, Catanzaro A: Coccidioidomycosis. Part I. Am Rev Respir Dis 117:559, 1978a.
Drutz DJ, Catanzaro A: Coccidioidomycosis. Part II. Am Rev Respir Dis 117:727, 1978b.
Dumarest J, et al: La Pratique du Pneumothorax Th rapeutique. Paris: Masson, 1945.
Emery RW, et al: Treatment of end-stage chronic obstructive pulmonary disease with double lung transplantation. Chest 99:533, 1991.
Engelman RM, Spencer FC, Berg P: Postpneumonectomy esophageal fistula. Successful one-stage repair. J Thorac Cardiovasc Surg 59:871, 1970.
Falk A: Tuberculosis pleurisy with effusion. Diagnosis and results of chemotherapy. Postgrad Med 38:631, 1965.
Fox RT, et al: Extraperiosteal plomb thoracoplasty. J Thorac Cardiovasc Surg 44:371, 1962.
Garcia-Yuste M, et al: Open-window thoracostomy and thoracomyoplasty to manage chronic pleural empyema. Ann Thorac Surg 65:818, 1998.
Gr goire J, et al: Thoracoplasty: its forgotten use in the management of non-tuberculous post-pneumonectomy empyema. Can J Surg 30:343, 1987.
Halezeroglu S, et al: Factors affecting postoperative morbidity and mortality in destroyed lung. Ann Thorac Surg 64:1635, 1997.
Herring M, Pecora D: Pleural aspergillosis: a case report. Am Surg 42:300, 1976.
Hopkins RA, et al: The modern use of thoracoplasty. Ann Thorac Surg 40:181, 1985.
Horrigan TP, Snow NJ: Thoracoplasty: current application to the infected pleural space. Ann Thorac Surg 50:695, 1990.
Hughes CF, Waugh R, Lindsay D: Surgery for pulmonary aspergilloma: preoperative embolization of the bronchial circulation. Thorax 41:324, 1986.
Icard P, et al: Utility of C-reactive protein measurements for empyema diagnosis after pneumonectomy. Ann Thorac Surg 57:933, 1994.
Jereb JA, et al: Tuberculosis morbidity in the United States: final data, 1990, in CDC surveillance summaries. MMWR Morb Mortal Wkly Rep 40:23, 1991.
Jones WG, Ginsberg RJ: Esophageal perforation: a continuing challenge. Ann Thorac Surg 53:534, 1992.
Jurkiewicz MJ, Arnold PG: The omentum: an account of its use in the reconstruction of the chest wall. Ann Surg 185:548, 1977.
P.850
Kearon MC, et al: Pleural aspergillosis in a 14-year-old boy. Thorax 42: 477, 1987.
Krakowka P, Rowinska E, Halweg H: Infection of the pleura by Aspergillus fumigatus. Thorax 25:245, 1970.
Langston HT, Barker WL, Graham AA: Pleural tuberculosis. J Thorac Cardiovasc Surg 54:511, 1967.
Lees WM, et al: Results in 278 patients who had the modern type of thoracoplasty for tuberculosis. J Thorac Surg 22:329, 1951.
Levine H, et al: Diagnosis of tuberculous pleurisy by culture of pleural biopsy specimen. Arch Intern Med 126:269, 1970.
Libshitz HI, Atkinson GW, Israel HL: Pleural thickening as a manifestation of Aspergillus superinfection. AJR Am J Roentgenol 120:883, 1974.
Loynes RD: Scoliosis after thoracoplasty. J Bone Joint Surg 54:484, 1972.
Massard G, et al: Pleuropulmonary aspergilloma: clinical spectrum and results of surgical treatment. Ann Thorac Surg 54:1149, 1992a.
Massard G, et al: Eventration diaphragmatique compliquant l' piplooplastie apr s r section pari tale thoracique. Ann Chir Plast Esth t 37:329, 1992b.
Massard G, et al: Esophagopleural fistula: an early and long-term complication after pneumonectomy. Ann Thorac Surg 58:1437, 1994.
Massard G, et al: Early and long-term results after completion pneumonectomy. Ann Thorac Surg 59:196, 1995a.
Massard G, et al: Decortication is a valuable option for late empyema after collapse therapy. Ann Thorac Surg 60:888, 1995b.
Massard G, et al: Pneumonectomy for chronic infection is a high-risk procedure. Ann Thorac Surg 62:1033, 1996.
Massard G, et al: Long-term complications of extraperiosteal plombage. Ann Thorac Surg 64:220, 1997.
McGovern EM, et al: Completion pneumonectomy: indications, complications and results. Ann Thorac Surg 46:141, 1988.
Mouroux J, et al: Surgical management of pleuropulmonary tuberculosis. J Thorac Cardiovasc Surg 111:662, 1996.
Nakanishi Y, et al: Empyema following the percutaneous instillation of antifungal agents in patients with aspergillosis. Intern Med 35:657, 1996.
Neff TA, Buchanan BD: Tension pleural effusion: a delayed complication of pneumothorax therapy in tuberculosis. Am Rev Respir Dis 111:543, 1975.
Odell JA, Henderson BJ: Pneumonectomy through empyema. J Thorac Cardiovasc Surg 89:423, 1985.
Pairolero PC, et al: Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg 99:958, 1990.
Pomerantz M, et al: Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg 52:1108, 1991.
Purcell IF, Corris PA: Use of nebulized liposomal amphotericin B in the treatment of Aspergillus fumigatus empyema. Thorax 50:1321, 1995.
Roberts ATM: Extrapleural pneumolysis: a review of 128 cases. Thorax 3:166, 1948.
Schmid FG, De Haller R: Late exudative complications of collapse therapy for pulmonary tuberculosis. Chest 89:822, 1986.
Sehti GK, Takaro T: Esophagopleural fistula following pulmonary resection. Ann Thorac Surg 25:74, 1978.
Shepherd MP: Plombage in the 1980s. Thorax 40:328, 1985.
Shirakusa T, et al: Surgical treatment of aspergilloma and Aspergillus empyema. Ann Thorac Surg 48:779, 1989.
Shirakusa T, et al: Use of pedicled omental flap in treatment of empyema. Ann Thorac Surg 50:420, 1990.
Takaro T, Walkup HE, Okano T: Esophagopleural fistula as a complication of thoracic surgery. J Thorac Cardiovasc Surg 40:179, 1960.
Treasure RL, Seaworth BJ: Current role of surgery in mycobacterium tuberculosis. Ann Thorac Surg 59:1405, 1995.
Utley JR: Completion pneumonectomy and thoracoplasty for bronchopleural fistula and fungal empyema. Ann Thorac Surg 55:672, 1993.
Weir MR, Thornton GF: Extrapulmonary tuberculosis: experience of a community hospital and review of the literature. Am J Med 79:467, 1985.
Weissberg D, Refaely Y: Pleural empyema: 24-year experience. Ann Thorac Surg 62:1026, 1996.
Westney GE, et al: Aspergillus infection in single and double lung transplant recipients. Transplantation 61:915, 1996.
Wilson NJ, et al: Extraperiosteal plombage thoracoplasty: operative technique and results with 161 cases with unilateral surgical problems. J Thorac Surg 32:797, 1956.
Yim AP: The role of video-assisted thoracoscopic surgery in the management of pulmonary tuberculosis. Chest 110:829, 1996.
EAN: 2147483647
Pages: 203