50 - Pacing of the Diaphragm
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XI - The Pleura > Chapter 61 - Fibrothorax and Decortication of the Lung
Chapter 61
Fibrothorax and Decortication of the Lung
Thomas W. Rice
Decortication of the lung is the process of peeling or stripping a constricting membrane from the pleural surfaces. Fowler (1893) and Delorme (1894) were the first physicians to describe freeing an entrapped lung, and in 1896 Delorme first used the term decortication. Decortication departed from the classic approach of aspiration, open drainage, and thoracoplasty for treatment of chronic empyema and fibrothorax. Further refinements of the initial procedure by Lloyd (1908), Lund (1911), Mayo and Beckman (1914), Lilienthal (1915), Ware (1917), and Eggers (1923) included intercostal incision, wide exploration of the pleural cavity, full mobilization of the lung, removal of the fibrous peel (but not the visceral pleura), and suction drainage of the complicated pleural space.
Despite individual surgical successes with decortication for chronic empyema, scant enthusiasm developed for this procedure. Milfeld and colleagues (1978) opined that this was due primarily to inadequacies of anesthesia, antimicrobial agents, blood transfusion, and technical expertise. Lack of therapy for organizing hemothorax complicating penetrating thoracic trauma reactivated interest in decortication during World War II, as documented by Price Thomas and Cleland (1945), Burford (1945) and Samson (1946) and their colleagues, as well as by Samson and Burford (1947) and Tuttle and associates (1946). This experience, coupled with development of effective antituberculous medication, encouraged many surgeons, including Mulvihill and Klopstock (1948), Ackman and Madore (1951), Gurd (1947), and Himmelstein (1948), Gordon (1949), O'Rourke (1949), and Weinberg (1948) and their associates, as well as Weinberg and Davis (1949) and Waterman and Domm (1951), to explore decortication in the treatment of fibrothorax and iatrogenic pneumothorax complicating pulmonary tuberculosis. More than 50 years after its inception, decortication of the lung in the treatment of fibrothorax was finally established. This surgical evolution was the result of one World War and three disease processes.
Today, an established fibrothorax is optimally managed by thoracotomy and decortication of the lung. Timely evacuation of hemothorax, empyema, or large pleural effusions with expansion of the underlying lung is critical in the prevention of fibrothorax. Tube thoracostomy, according to Huang and co-workers (1999), is unlikely to successfully treat parapneumonic effusions if they are loculated or have a leukocyte count of no more than 6,400 per microliter. Failure of thoracentesis or tube thoracostomy should prompt early thoracoscopy or video-assisted thoracic surgery (VATS) drainage of pleural collections. In patients with empyema and hemothorax, videothoracoscopy and VATS, as noted by Angelillo Mackinlay (1996), Davidoff (1996), Hutter (1985), Landreneau (1996), and Sendt (1995) and their associates, as well as by Ridley and Braimbridge (1991) and Silen and Weber (1995), facilitate early irrigation, d bridement, and drainage of the pleural space. Despite earlier diagnosis and minimally invasive abilities to drain the pleural space, entrapment of the lung still occurs, as noted by Steinbrecher and Najmaldin (1999). Once organization has occurred, thoracotomy and decortication remain the treatment of choice, as advocated by Striffeler (1998) and Cassina (1999) and their colleagues.
PATHOPHYSIOLOGY
Samson (1955) pointed out that if pleural fluid is left undrained, regardless of its nature (Table 61-1), an inflammatory response deposits fibrin on the visceral and parietal pleura. As inflammation proceeds, a thin layer of immature blood vessels and loose collagen forms over the pleural surfaces (Fig. 61-1; see Color Fig. 61-1). Organization produces a dense, avascular collagen matrix that walls off the insulting fluid but does not affect the underlying pleura. In this fibrous cavity between the visceral and parietal pleura, the fluid decomposes (Fig. 61-2; see Color Fig. 61-2). The extent of inflammatory reaction is determined by the nature of the fluid and varies from a microscopically thin glistening membrane in transudative pleural effusions to a thick fibrous peel of empyema and hemothorax.
Fluid in the pleural space also results in pulmonary compression and, if sufficient in quantity, atelectasis. The compressed atelectatic lung is trapped by inflammatory tissue coating the visceral pleura. The underlying lung is usually unaffected unless the pleural fluid production began as a
P.852
destructive parenchymal process (e.g., tuberculosis, necrotizing pneumonia, or penetrating trauma). The chest wall and diaphragm are similarly coated by the fibrous process that is typically thicker and more exuberant over the parietal pleura than over the visceral pleura. Entrapment of the lung and encasement of the thoracic cage produces a restrictive ventilatory defect and is characterized by reduction of lung volumes [forced vital capacity (FVC), total lung capacity (TLC), vital capacity (VC), forced expiratory volume in 1 second (FEV1), FEV50, FEV25], decreased diffusing capacity of the lung for carbon monoxide (DLCO) and carbon monoxide transfer coefficient (Kco), and an increase of the residual volume (RV)/TLC ratio, as pointed out by Liu and collaborators (1991). The DLCO corrected for these reduced volumes is normal, indicative of extrapulmonary restriction with a normal underlying lung. The alterations of pulmonary function in tuberculous pleural disease generally exceed those of tuberculous parenchymal disease, as documented by Autio (1959).
Table 61-1. Causes of Fibrothorax | |
|---|---|
|
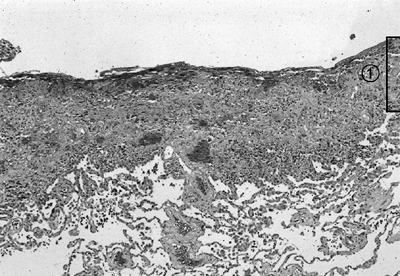 |
Fig. 61-1. Photomicrograph shows that a distinct boundary between early pleuritis (1) and the visceral pleura does not yet exist. Decortication before maturation of the pleural peel will develop a plane between the visceral pleura and pulmonary parenchyma. (Hematoxylin and eosin, original magnification 100.) (See Color Fig. 61-1.) |
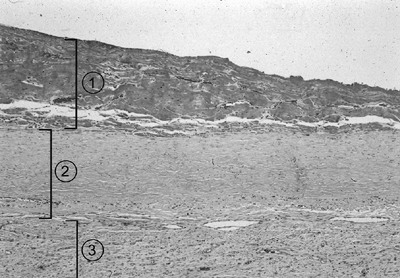 |
Fig. 61-2. Photomicrograph of a mature pleural peel consists of: (1) debris, (2) mature collagen, and (3) a loose vascular matrix overlying the visceral pleura. (Hematoxylin and eosin, original magnification 100.) (See Color Fig. 61-2.) |
Pulmonary vasoconstriction results in a significant reduction of perfusion of the entrapped lung. Perfusion abnormality usually exceeds reduction of ventilation of the involved lung. This mechanism prevents hypoxia if contralateral lung function is preserved. Muller (1959) found that generally there is normal oxygen saturation at rest but moderate reduction with exercise. In severe or bilateral disease, hypoxia, hypercarbia, pulmonary hypertension, and cor pulmonale may result, as noted by Robin and associates (1966).
DIAGNOSIS AND EVALUATION
Although a history of penetrating thoracic trauma, pneumonia, or tuberculosis may be elicited, no inciting cause will be found in 50% of patients according to Deslauriers and Perrault (1995). The most common complaint is progressive dyspnea on exertion. Occasionally, patients may experience chest discomfort ranging from tightness to frank pain, dry nonproductive cough, fatigue, or malaise. Ghoshal and colleagues (1997) have emphasized that cough with expectoration, hemoptysis, or fever should alert the examining physician to underlying pulmonary parenchymal disease. Physical examination will demonstrate unilateral fixation of the chest wall with reduced excursion of the diaphragm. The chest will be dull to percussion with impaired transmission of breath sounds on auscultation.
Findings on chest radiographs, as described by Maffessanti and colleagues (1996), are uniform, dependent radiodensities that involve the diaphragmatic surface and lower lateral chest wall with obliteration of the costophrenic sulcus. This may progress superiorly and in extreme cases can obliterate the pleural space (Fig. 61-3). Chest wall findings include narrowed intercostal spaces and diminished size of the hemithorax with retraction of the mediastinum toward the fibrothorax. Pleural calcifications may occur on the inner aspect of the peel, providing an accurate measurement of the thickness of the rind (Fig. 61-4 and 61-5). The extent of the fibrothorax is
P.853
P.854
best appreciated by computed tomographic (CT) scan, which images the pleural peel as a smooth, symmetric, tissue density extrapulmonary mass (Figs. 61-6 and 61-7). Fibrothorax complicating hemothorax has a heterogeneous character on CT scan (Fig. 61-8). A nodular appearance is suspicious for mesothelioma or pleural metastases; Im (1991) and Maffessanti (1996) and their associates note that a fat component is seen in tuberculous fibrothorax or with chronic steroid administration. More important, CT scans enable surgeons to assess the underlying pulmonary parenchyma for tuberculosis, bronchiectasis, and mass lesions. Rounded atelectasis may masquerade as a carcinoma, but as Cohen and co-workers (1993) demonstrated, it is caused by pleural entrapment of the lung and is a parenchymal finding of fibrothorax.
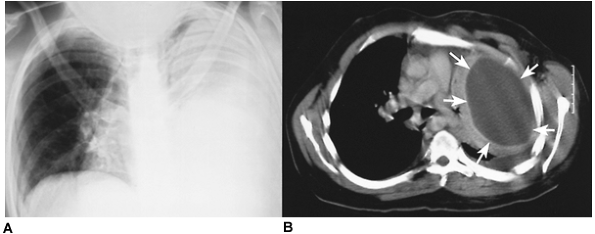 |
Fig. 61-3. Empyema. A. Nearly complete opacification of the left hemithorax in a patient with cerebral palsy who is recovering from Staphylococcus pneumonia. B. A midthoracic computed tomographic section demonstrates a homogeneous pleural fluid collection with enhancing margins [ split pleura sign (arrows)]. |
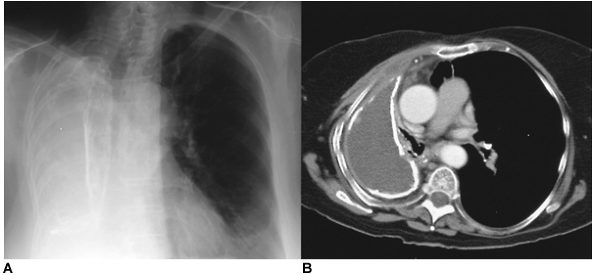 |
Fig. 61-4. Tuberculous empyema. A. A chronic tuberculous empyema filling the right hemithorax. B. A computed tomographic section at the pulmonary hilum demonstrates a calcified tuberculous empyema cavity. The right main bronchus is visualized, but there is destruction of the right lung. |
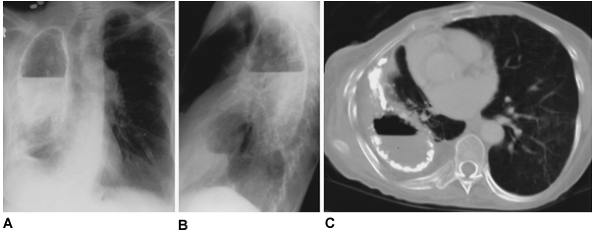 |
Fig. 61-5. Tuberculous empyema with bronchopleural fistula. A, B. Posteroanterior and lateral chest roentgenograms of a tuberculous empyema with calcified margins and an air fluid level. Aerated pulmonary parenchyma is visualized inferior to the empyema cavity. C. A midthoracic computed tomographic section demonstrates the calcified empyema cavity with an air fluid level. Although significant volume loss has occurred, a compressed aerated right lung without parenchymal destruction is seen. |
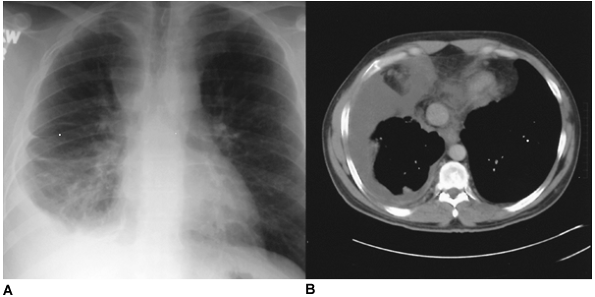 |
Fig. 61-6. Neglected pleural effusion. A. An idiopathic chronic right pleural effusion. B. A computed tomographic section of the lower chest demonstrates a homogeneous low-density fluid collection. At thoracoscopy, a constricting pleural peel was found that required decortication. |
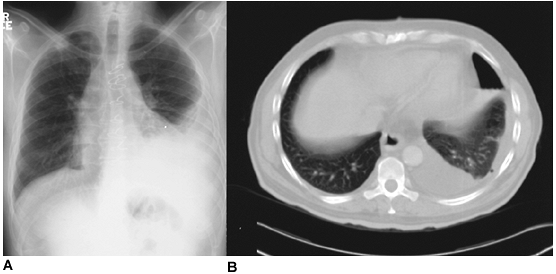 |
Fig. 61-7. Neglected pleural effusion. A. A chronic left pleural effusion complicating coronary artery bypass surgery. B. A lower thoracic computed tomographic section demonstrates a hydropneumothorax following thoracentesis. A constricting pleural peel is suggested due to the inability of thoracentesis to evacuate the pleural collection and expand the lung. The evacuation of fluid by thoracentesis from this rigid cavity required replacement with an equal volume of air. |
Preoperative physiologic evaluation includes conventional pulmonary function testing with spirometry, diffusion studies (DLCO), arterial blood gas analysis, and exercise testing. These evaluations are useful to quantify the degree of respiratory impairment and may also be used for comparisons in postoperative follow-up. A quantitative perfusion scan will confirm the unilateral reduction of pulmonary perfusion.
TREATMENT
Decortication of the lung is indicated for patients with symptomatic extraparenchymal restrictive disease secondary to fibrothorax. An open surgical procedure is considered if thoracentesis, tube drainage, or thoracoscopy have failed to drain the pleural space and expand the lung and if malignant pleural disease has been excluded. Precise timing of the operation depends on the underlying disease process. In patients with hemothorax, decortication is performed more than 6 weeks after the injury. This allows maturation of the pleural peel and establishes a plane of dissection. If, after conventional therapy, the lung is not completely expanded (especially if there is >50% compression or the apex is collapsed), Samson and co-workers (1946) strongly believe that decortication is indicated. When pleural drainage fails to resolve an empyema, decortication will reexpand the lung, control the pleural space, and eliminate infection. Decortication is indicated in patients with tuberculosis if pleural disease persists after chronic antituberculous therapy. If thoracentesis fails to yield fluid or if no changes are seen on chest radiography, decortication should be considered. There should be considerable opacification on the lateral projection and opacification of at least one fourth to one third of the posteroanterior roentgenogram. Decortication is also indicated for complications following collapse therapy and plombage.
The operation is contraindicated in patients with main bronchial obstruction, pulmonary destruction, uncontrolled sepsis, contralateral disease, chronic debilitation, or prohibitive concomitant organ system dysfunction. Fibrothorax must be differentiated from mesothelioma and malignancies
P.855
metastatic to the pleural space. Thoracentesis, percutaneous biopsy, or thoracoscopy should precede thoracotomy if the diagnosis of fibrothorax is in question.
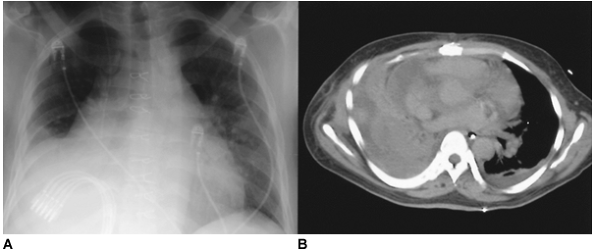 |
Fig. 61-8. Traumatic hemothorax. A. A right hemothorax complicates a gunshot wound to the upper abdomen that caused liver injury. Tube thoracostomy was used ineffectively to treat this traumatic hemothorax. B. Computed tomography demonstrates a heterogeneous pleural collection typical of hemothorax. |
Bronchoscopy is performed initially to exclude endobronchial processes that prohibit expansion of the entrapped lung. A double-lumen endotracheal tube (vs. a single-lumen tube with or without a bronchial blocker) allows variations in ventilation that may facilitate decortication, that is, positive pressure ventilation, pressurization of the lung without ventilation (clamped endotracheal lumen at the end of positive pressure expansion of the lung), or no ventilation. The preferred operative approach is in the lateral decubitus position with a posterolateral thoracotomy. The early reports of Rudstr m and Thoren (1955) and Samson (1955) advocated resection of one or two ribs for improved exposure. This may be necessary when intercostal spaces are so severely contracted that the ribs touch or overlap. However, rib resection is generally not required if the parietal pleura is bluntly dissected from the chest wall in the area of the incision, before the rib retractor is inserted, as described by Waterman and Domm (1951) (Fig. 61-9). Entry into the thorax at the sixth or seventh interspace, according to Williams (1950), provides better exposure of the lower lobe and diaphragm where the fibrothorax may be more dense. Savage and Fleming (1955) suggest that a second, superior intercostal incision (in the fourth or fifth interspace) may improve exposure of the upper thorax with this low intercostal approach.
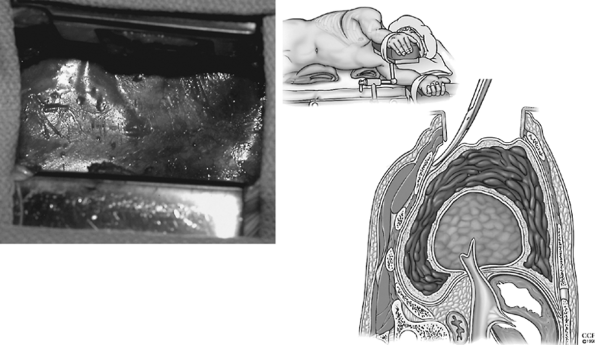 |
Fig. 61-9. The parietal pleura is bluntly dissected from the chest wall in the area of the incision, before the rib retractor is inserted. |
According to Wright and associates (1949), the need for parietal pleurectomy and freeing of the chest wall, diaphragm, and mediastinum spurred great debate. Parietal pleurectomy was discouraged by Samson and Burford (1947) and Tuttle and colleagues (1947) because of increased operative time and the potential for excessive blood loss. However, many physicians, including Ackman and Madore (1951) and Waterman and Domm (1957), recognized that freeing these structures allowed the movement necessary for ventilation. Today, most surgeons would add at least a partial or, if possible, complete decortication of the parietal pleura. The surgeon proceeds with blunt dissection of the parietal peel (Fig. 61-10). The plane of dissection is between the endothoracic fascia and the parietal pleura. The dissection may be carried beyond the chest wall to include the diaphragm and mediastinal pleura. Decortication of the diaphragm and mediastinum may be facilitated by delaying this portion of the parietal dissection of the lung until decortication of the lung has been completed. These two surfaces may then be freed retrograde from the pulmonary hilum and antegrade from the chest wall. Caution must be taken to prevent injury to the diaphragm or phrenic nerve, as noted by Waterman and Domm (1951) and Mayo and associates (1982). In areas of dense fibrosis with obliteration of the plane of dissection, patches of fibrous tissue may be left on these structures. No advantage is gained if aggressive freeing of the diaphragm or mediastinum near the phrenic nerve results in diaphragmatic dysfunction.
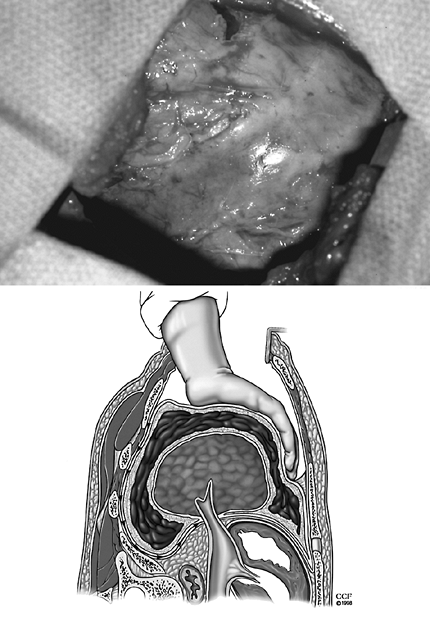 |
Fig. 61-10. Blunt dissection of the parietal peel occurs in the plane between the endothoracic fascia and the parietal pleura. |
P.856
The thickened parietal peel is incised and the pleural cavity is entered (Fig. 61-11). An empyectomy with preservation of the integrity of the cavity was recommended by Samson (1955) for tuberculous empyema. In nontuberculous empyema, entrance into the cavity causes temporary contamination of the operative field and is generally unavoidable. Perioperative antibiotics and complete expansion of the lung with obliteration of any potential pleural space minimizes postoperative empyema. The pleural cavity is entered and evacuated of fluid and debris. This material is cultured. Pulmonary decortication begins with incision of the fibrous peel overlying the visceral pleura. Many layers in the fibrous peel may be divided before the glistening gray visceral pleura is encountered. Unlike parietal decortication, the plane of visceral decortication lies between the visceral pleura and the fibrous peel. The plane of dissection is initiated with a blunt spatula-type instrument. The peel is firmly grasped and gentle traction applied. Blunt dissection is performed with Kittner dissectors or a gauze-covered finger (Fig. 61-12). Gentle reexpansion of the lung may facilitate this process, but pulmonary collapse may expedite the freeing of the entrapped lung without visceral pleural damage. Decortication may not be possible in areas of underlying parenchymal disease or where pulmonary injury resulted from penetrating trauma. In these cases, the pleural peel may be left on the pulmonary surface or the underlying lung may be resected if it is destroyed by the underlying disease process. If the correct plane is not identified, decortication includes the visceral pleura and pulmonary parenchyma. Excessive bleeding and large air leaks from deep parenchymal injury must be controlled. Fortunately, the injury is usually limited to the subpleural parenchyma and is controlled with reexpansion of the lung.
 |
Fig. 61-11. The thickened parietal peel is incised and the pleural cavity evacuated of fluid and debris. |
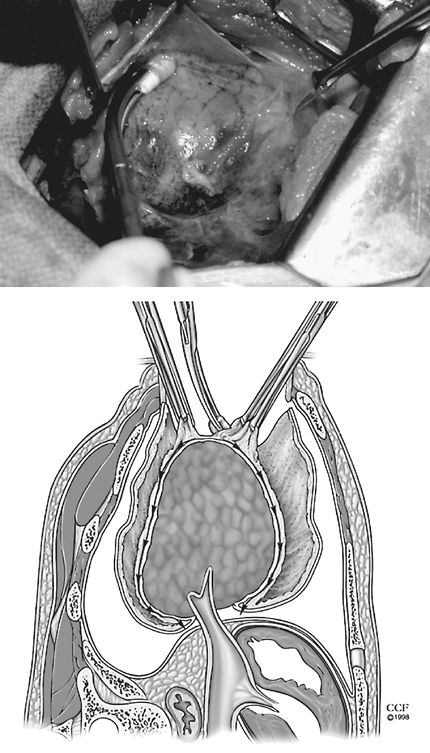 |
Fig. 61-12. Unlike parietal decortication, the plane of visceral decortication lies between the visceral pleura and the fibrous peel. The plane of dissection is initiated with a blunt spatula-type instrument. The peel is firmly grasped and gentle traction applied. Blunt dissection is executed with Kittner dissectors or gauze-covered fingers. |
P.857
The pleural space is drained with two or three chest tubes. They are positioned low in the thoracic cavity and pass (a) posteriorly into the costovertebral sulcus to the apex of the thorax, (b) anteriorly into the hilum to the apex of the thorax, and (c) inferiorly along the diaphragm into the posterior costophrenic sulcus. On completion of the operation, the patient should be reintubated with a large, single-lumen endotracheal tube and bronchoscopy performed to free the airway of secretions. A short period of positive pressure ventilation may facilitate pulmonary expansion. Chest tube suction should be sufficient to evacuate the pleural space of blood and air. Excessive suction fostering air leaks is not a major concern in the early postoperative period.
RESULTS
Mayo (1985) reported that the operative mortality rate ranges from 0% to 8% and is dependent on severity of illness and general state of health. Morbidity is the result of underlying disease processes or operative complications. Sepsis, wound infection, empyema, prolonged air leak, and bronchopleural fistula are the most common complications. These postoperative problems are minimized by meticulous surgical techniques that control air leaks and bleeding and assure complete reexpansion of the lung with obliteration of the pleural space.
Patton (1952), Siebens (1956), and Barker (1965) and their associates have found that absence of underlying parenchymal disease is the best predictor of improved pulmonary function after decortication. Operative complications such as phrenic nerve injury or empyema may diminish benefits of decortication as noted by Siebens and colleagues (1956). Improvement following decortication may be less in patients with tuberculosis than in patients with pyogenic infection or trauma as noted by Morton and colleagues (1970). Both Carroll (1951) and Patton (1952) and their collaborators reported that a shorter duration of pleural disease is associated with better results; however, restoration of pulmonary function has been reported more than 50 years after onset of tuberculosis by Petty (1961), Hughes (1975), and Massard (1995) and their co-workers. Ultimate gain in pulmonary function may be limited by the presence of pleural infection as observed by Patton and associates (1952). Continued postoperative improvement in pulmonary function has been reported for up to 3 years by these latter researchers. A recent report by Ilic (1996) of early decortication for traumatic hemothorax showed stable postoperative pulmonary function immediately after chest tube removal and at 6 months postoperatively. Barker and co-workers (1965) noted that normalization of the chest radiograph correlates with functional improvement. Rzyman and colleagues (2002) recorded that lung function as assessed by spirometry and scintigraphy improves significantly after decortication but remains impaired, never returning to predicted normal values. Early decortication, as reported by Carey (1998) and Powell (2001) and their coinvestigators, results in shorter duration of therapy, lower incidence of recurrence, and fewer complications.
Failure to improve pulmonary function after decortication may be the result of inappropriate patient selection, operative difficulties, or postoperative complications. Expansion of a severely damaged lung or inability to expand a fibrotic lung will not improve pulmonary function. Persistent restriction may result from inability to free the chest wall or diaphragm. Damage of the phrenic nerve or diaphragm will impair ventilation and may worsen pulmonary function. Decortication in the parenchymal plane deep to the visceral pleura causes increased bleeding and air leak and may result in postoperative bronchopleural fistula and recurrence of hemothorax and empyema.
P.858
REFERENCES
Ackman FD, Madore P: Decortication preceding thoracoplasty for the elimination of long-standing tuberculous empyema. J Thorac Surg 22:358, 1951.
Angelillo Mackinlay TA, et al: VATS d bridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg 61:1626, 1996.
Autio V: The reduction of respiratory function by parenchymal and pleural lesions. A bronchospirometric study of patients with unilateral involvement. Acta Tuberc Scand 37:112, 1959.
Barker WL, Neuhaus H, Langston HT: Ventilatory improvement following decortication in pulmonary tuberculosis. Ann Thorac Surg 1:532, 1965.
Burford TH, Parker EF, Samson PC: Early pulmonary decortication in the treatment of posttraumatic empyema. Ann Surg 122:163, 1945.
Carey JA, et al: Empyema thoracis: a role for open thoracotomy and decortication. Arch Dis Child 79:510, 1998.
Carroll D, et al: Pulmonary function following decortication of the lung. Am Rev Tuberc 63:231, 1951.
Cassina PC, et al: Video-assisted thoracoscopy in the treatment of pleural empyema: staged-based management and outcome. J Thorac Cardiovasc Surg 117:234, 1999.
Cohen AM, et al: Rounded atelectasis and fibrotic pleural disease: the pathologic continuum. J Thorac Imaging 8:309, 1993.
Davidoff AM, et al: Thoracoscopic management of empyema in children. J Laparoendosc Surg 6(suppl):51, 1996.
Delorme E: Nouveau traitment des empy mes chroniques. Gaz Hop 67:94, 1894.
Delorme E: Du traitment des empyemes chronique par la decortication du poumon. Presented at the Dixieme Congres Francis de Chirugie, Vol. 379, 1896.
Deslauriers J, Perrault LP: Fibrothorax and decortication. In Pearson FG, et al (eds): Thoracic Surgery. New York: Churchill Livingston, 1995, p. 1107.
Dietrick RB, Sade RM, Pak JS: Results of decortication in chronic empyema with special reference to paragonimiasis. J Thorac Cardiovasc Surg 82:58, 1981.
Eggers C: Radical operation for chronic empyema. Ann Surg 77:327, 1923.
Fairfax AJ, McNabb WR, Spiro SG: Chylothorax: a review of 18 cases. Thorax 41:880, 1986.
Fowler GR: A case of thoracoplasty for removal of a large fibrous growth from the interior of the chest, the result of an old empyema. Med Record 44:838, 1893.
Ghoshal AG, et al: Fibrothorax problem, profile and prevention. J Indian Med Assoc 95:610, 1997.
Gordon J, Brook R, Welles ES: Decortication in pulmonary tuberculosis including studies of respiratory physiology. J Thorac Surg 18:337, 1949.
Gurd FB: Decortication in chronic empyema of tuberculous origin. J Thorac Surg 16:587, 1947.
Himmelstein A, Miscall L, Kirschner PA: Decortication in tuberculosis. Surg Clin North Am 28:1601, 1948.
Huang HC, et al: Predicting factors for outcome of tube thoracostomy in complicated parapneumonic effusion for empyema. Chest 115:751, 1999.
Hughes RL, et al: Evaluation of unilateral decortication. A patient successfully treated 44 years after onset of tuberculosis. Ann Thorac Surg 19:704, 1975.
Hutter JA, Harari D, Braimbridge MV: The management of empyema thoracis by thoracoscopy and irrigation. Ann Thorac Surg 39:517, 1985.
Ilic N: Functional effects of decortication after penetrating war injuries to the chest. J Thorac Cardiovasc Surg 111:967, 1996.
Im JG, et al: Apical opacity associated with pulmonary tuberculosis: high-resolution CT findings. Radiology 178:727, 1991.
Landreneau RJ, et al: Thoracoscopy for empyema and hemothorax. Chest 109:18, 1996.
Lilienthal H: Empyema. Exploration of the thorax with primary mobilization of the lung. Ann Surg 62:309, 1915.
Liu CT, et al: Pulmonary function in patients with pleural effusions of varying magnitude and fibrothorax. Panminerva Med 33:86, 1991.
Lloyd S: The surgical treatment of unresolved pneumonia. NY Postgrad Med School Hosp 177, 1908.
Lund FB: The advantage of the so-called decortication of the lung in empyema. JAMA 57:693, 1911.
Maffessanti M, Bortolotto P, Grotto M: Imaging of pleural diseases. Monaldi Arch Chest Dis 51:138, 1996.
Massard G, et al: Decortication is a valuable option for late empyema after collapse therapy. Ann Thorac Surg 60:888, 1995.
Mayo P: Early thoracotomy and decortication for nontuberculous empyema in adults with and without underlying disease. A twenty-five year review. Am Surg 51:230, 1985.
Mayo CH, Beckman EH: Visceral pleurectomy for chronic empyema. Am Surg 59:884, 1914.
Mayo P, Saha SP, McElvein RB: Diaphragmatic avulsion following decortication. A case review of acute nontuberculous empyema. Ala J Med Sci 19:81, 1982.
Milfeld DJ, Mattox KL, Beall AC Jr: Early evacuation of clotted hemothorax. Am J Surg 136:686, 1978.
Morton JR, Boushy SF, Guinn GA: Physiological evaluation of results of pulmonary decortication. Ann Thorac Surg 9:321, 1970.
Muller C: Cardiopulmonary hemodynamics in chronic lung disease with special reference to pulmonary tuberculosis, cardiac catheterization studies at rest and on exercise. Scand J Clin Lab Invest 11(Suppl 44):1, 1959.
Mulvihill DA, Klopstock R: Decortication of the nonexpandable postpneumothorax tuberculous lung. J Thorac Surg 17:723, 1948.
O'Rourke P, O'Brien EJ, Tuttle WL: Decortication of the lung in patients with pulmonary tuberculosis. Am Rev Rep Dis 59:30, 1949.
Patton WE, Watson TR, Gaensler EA: Pulmonary function before and at intervals after surgical decortication of the lung. Surg Gynecol Obstet 95:477, 1952.
Petty TL, Filley GF, Mitchell RS: Objective functional improvement by decortication after twenty years of artificial pneumothorax for pulmonary tuberculosis. Am Rev Respir Dis 84:572, 1961.
Powell LL, et al: Improved patient outcome after surgical treatment for loculated empyema. Am J Surg 179:1, 2000.
Price Thomas C, Cleland WP: Decortication in clotted and infected haemothoraces. Lancet 1:327, 1945.
Rice DC, et al: Silicone thorax: a complication of tube thoracostomy in the presence of mammary implants. Ann Thorac Surg 60:1417, 1995.
Ridley PD, Braimbridge MV: Thoracoscopic d bridement and pleural irrigation in the management of empyema thoracis. Ann Thorac Surg 51:461, 1991.
Robin ED, et al: Pulmonary hypertension and unilateral pleural constriction with speculation on pulmonary vasoconstrictive substance. Arch Intern Med 118:391, 1966.
Rudstr m P, Thoren L. Decortication of the lung. Acta Chir Scand 110:437, 1955.
Rzyman W, et al: Decortication in chronic pleural empyema effect on lung function. Eur J Cardiothorac Surg 21:502, 2002.
Samson PC: Some surgical considerations in pulmonary decortication. Am J Surg 89:364, 1955.
Samson PC, Burford TH: Total pulmonary decortication. Its evolution and present concepts of indications and operative technique. J Thorac Surg 16:127, 1947.
Samson PC, et al: The management of war wounds of the chest in a base center. The role of early pulmonary decortication. J Thorac Surg 15:1, 1946.
Savage T, Fleming HA: Decortication of the lung in tuberculous disease. A study of 43 cases. Thorax 10:293, 1955.
Sendt W, F rster E, Hau T: Early thoracoscopic d bridement and drainage as definite treatment for pleural empyema. Eur J Surg 161:73, 1995.
Shapiro DH, Anagnostopoulos CE, Dineen JP: Decortication and pleurectomy for the pleuropulmonary complications of pancreatitis. Ann Thorac Surg 9:76, 1970.
Siebens AA, et al: The physiologic effects of fibrothorax and the functional results of surgical treatment. J Thorac Surg 32:53, 1956.
Silen ML, Weber TR: Thoracoscopic d bridement of loculated empyema thoracis in children. Ann Thorac Surg 59:1166, 1995.
Steinbrecher HA, Najmaldin AS: Thoracoscopy for empyema in children. J Pediatr Surg 33:708, 1998.
Sterling GM, Herbert A: Lung en cuirasse: restrictive pleurisy associated with asbestos exposure. Thorax 35:715, 1990.
Striffeler H, et al: Video-assisted thoracoscopic surgery for fibropurulent pleural empyema in 67 patients Ann Thorac Surg 65:319,1998.
Tuttle WM, Langston HT, Crowley RT: The treatment of organizing hemothorax by pulmonary decortication. J Thorac Surg 16:117, 1947.
Ware MW: The trend of surgery in empyema of the thorax. Ann Surg 65:320, 1917.
Waterman DH, Domm SE: Decortication of the unexpandable pneumothorax lung. Dis Chest 19:1, 1951.
Weinberg J, Davis JD: Pleural decortication in pulmonary tuberculosis. Am Rev Tuberc 60:288, 1949.
P.859
Weinberg J, Horner JC, Davis JD: Decortication of the unexpanded tuberculous lung following induced pneumothorax. Surg Clin North Am 28:1591, 1948.
Willekes CL, Widman WD: Empyema from lost gallstones: a thoracic complication of laparoscopic cholecystectomy. J Laparoendosc Surg 6:123, 1996.
Williams MK: The technique of pulmonary decortication and pleurolysis. J Thorac Surg 20:652, 1950.
Wright GW, et al: Physiologic observations concerning decortication of the lung. J Thorac Surg 18:372, 1949.
Yellin A, et al: Fibrothorax associated with a ventriculopleural shunt in a hydrocephalic child. J Pediatr Surg 27:1525, 1992.
EAN: 2147483647
Pages: 203