24 - Anesthesia for Pediatric General Thoracic Surgery
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section VII - Pulmonary Resections > Chapter 31 - Segmentectomy and Lesser Pulmonary Resections
Chapter 31
Segmentectomy and Lesser Pulmonary Resections
Joseph LoCicero III
Parenchyma-sparing pulmonary resections are important procedures for many patients. Although lobectomy and pneumonectomy remain standard operations for patients with primary lung cancer who can tolerate them, many authors advocate less than lobectomy as an alternative operation, particularly in patients with limited pulmonary reserve. In cases in which resection of pulmonary metastases may provide prolonged survival, a lesser parenchymal resection is an ideal procedure. These techniques are used for the diagnosis of the indeterminate pulmonary nodule.
SEGMENTECTOMY
Segmentectomy, a form of resection popularized in the mid-20th century, is an anatomic operation because it removes the segmental bronchus back to its primary branch, along with all the lung parenchyma and lymph node groupings drained by the bronchus and its associated segmental pulmonary artery. This technique was used in the treatment of pulmonary tuberculosis, bronchiectasis, and other suppurative pulmonary lesions. After development of effective antituberculous chemotherapy and broad-spectrum antibiotics for suppurative diseases, the operation became less common. Many authors since the 1970s, such as Jensik (1987) and associates (1973, 1979), Shields and Higgins (1974), and Crabbe and colleagues (1989, 1991), suggested its use in early lung cancer. Segmentectomy is an anatomic operation that obeys the principles of cancer surgery by removing the associated lymph nodes. Studies by Read and co-workers (1990) and Warren and Faber (1994) showed that excellent results can be achieved in patients with early (T1N0) cancers. For those patients with primary lung cancer and poor pulmonary function, Kodama (1997) and Cerfolio (1996) and their associates noted that segmentectomy can lead to long-term survival.
The only randomized trial on this subject comes from the Lung Cancer Study Group and was reported by Ginsberg and Rubenstein (1995). However, the report combines both segmentectomy and wedge resection. Together, they have a worse outcome when compared with lobectomy. Several Japanese groups are readdressing this issue. Okada and colleagues (2001) performed segmentectomies in 70 patients with primary lung cancers smaller than 2 cm. They began with a segmentectomy and extended the margins with wedge resections if the intrapulmonary nodes were positive. The 5-year survival rate was 87%. Yoshikawa and associates (2002) reported a multiinstitutional prospective trial of 55 patients with early-stage lung cancer who underwent extended segmentectomy. Results were encouraging. Koike and colleagues (2003) offered segmentectomy to all patients with early-stage lung cancers over a 5-year period and compared results to those in patients who wanted a lobectomy. The 5-year survival rate for 74 patients with segmentectomy was 89.1% and for 159 patients with a lobectomy was 90.1%. Five patients in the segmentectomy group and nine in the lobectomy group had recurrent disease. Thus, controversy over the use of segmentectomy for primary lung cancer remains.
With the resurgence of Mycobacterium tuberculosis in urban areas and the development of drug-resistant atypical strains, this technique remains vitally important to the eradication of suppurative disease while maximally preserving a normally functioning lung. Agasthian (1996) and Ashour (1996) and their associates reported control of bronchiectasis by segmentectomy alone in 13% to 16% of patients. Segmentectomy has been used in patients with severe Aspergillus species and pulmonary mycosis infections. Massard (1993) and Temeck (1994) and their colleagues noted that segmentectomy has significant complications in these patients but that they are equal to complications associated with other types of infections.
Even congenital lesions can be managed with segmental resections. Nuchtern and Harberg (1994) used segmentectomy for intralobar lung cysts. Sapin and associate (1997)
P.497
were able to use segmentectomy in one third of the patients they treated for congenital adenomatoid disease of the lung.
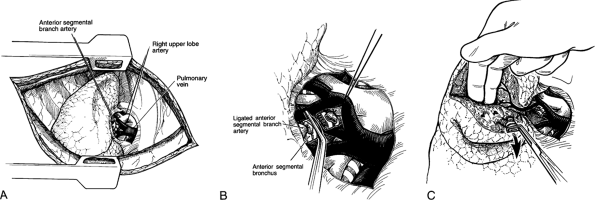 |
Fig. 31-1. A right anterior segmentectomy. A. Isolation of the right upper lobe artery and ligation of the anterior segmental branch. B. Division of the anterior segmental bronchus. C. Traction and finger fracture of the pulmonary parenchyma visualizing the pulmonary vein in the intersegmental plane. |
Technique
Standard lateral thoracotomy position using double-lumen endotracheal tube anesthesia is the most popular approach at present. A posterior approach in the prone position may be used, particularly when one expects copious secretions from a suppurative process. This posterior approach has limited application today and should be undertaken only by those familiar with the special positioning required to avoid brachial plexus injury.
The initial steps in segmentectomy are the same as for any standard resection of the lung. The surgeon must be familiar with intraparenchymal anatomy of the bronchus and pulmonary artery to accomplish a segmentectomy successfully. The hilar structures are identified, and the major fissure is opened. On the right side of the chest, the bronchus is the most posterior hilar structure. As one proceeds into the hilus, the upper lobe branches of the pulmonary artery are the most superior hilar structures (Fig. 31-1). In the major fissure, the pulmonary artery can be exposed, demonstrating the continuation of the main pulmonary artery into the lower lobe. Anterior and posterior branches originate opposite one another and go, respectively, to the middle lobe and the superior segment of the lower lobe, forming a cross. Often, a posterior ascending branch arises from the posterior segmental artery and supplies part of the posterior segment of the upper lobe (Fig. 31-2).
On the left side of the chest, the pulmonary artery crosses superiorly above the left main-stem bronchus to become the most posterior structure in the hilus. The apicoposterior and anterior and segmental branches are located anteriorly and superiorly. A separate posterior segmental branch is often found posteriorly on the main pulmonary artery, just at or above the major fissure (Fig. 31-3). In the major fissure, the lingular branches anteriorly and the superior segment branch posteriorly form a cross on the continuation of the pulmonary artery (Fig. 31-4). The surgeon must be mindful of the high degree of variability of the branches of the pulmonary artery and carefully identify each branch before ligation (see Chapter 5).
The artery and bronchus are ligated and divided. When performing a segmentectomy for suppurative lung disease, it may be best to divide the bronchus first to prevent contamination of the normal lung. Controversy about whether to divide the bronchus or artery first raged through the 1930s and 1940s and has been carefully traced by Grismer and Read (1995). However, in the United States today, the order of division depends on the segment to be removed. For the apical and anterior segment of the upper lobe on the right side, the artery is ligated first and elevated to locate the segmental bronchus. For the posterior right upper lobe segment, it may be easier to divide the bronchus first and then the artery, which may be deep in the parenchyma and not easily isolated anteriorly because of the other segmental branches. On the left side, the arterial branches are more easily isolated first because the bronchus is the middle structure on that side.
Before dividing the bronchus, the segment to be removed should be differentially deflated and inflated to help delineate the intersegmental planes, keeping in mind that filling of the deflated segment may occur from adjacent segments by means of collateral ventilation. With the advent of the double-lumen tube, it is often easier to inflate the entire lung, clamp the bronchus, and allow the rest of the lung to deflate. The segment remains inflated for a longer period,
P.498
even with some loss from collateral ventilation. Once the appropriate segmental bronchus is identified, it is divided. The proximal stump may be closed either with a mechanical stapler or with interrupted fine absorbable sutures. Additional coverage of the stump is usually not necessary.
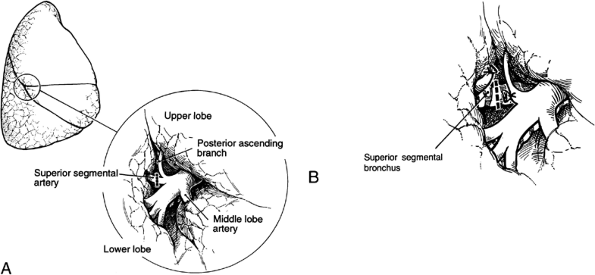 |
Fig. 31-2. A right superior segmentectomy. A. Exposure of the pulmonary artery in the fissure demonstrating the middle lobe artery and the superior segmental artery opposite one other with identification and preservation of the posterior ascending arterial branch. B. Division of the superior segmental bronchus. |
After the bronchus and artery are ligated, traction is placed on the bronchus, and the segment is removed in a retrograde manner. The plane is developed using digital blunt dissection with division of the pleura by scissors or cautery. The pulmonary veins course through the intersegmental planes and provide an excellent anatomic guide. Individual branches of the vein emanating from the segment into the fissure are sequentially divided.
After removal of the specimen, the raw surfaces of the adjacent segments are inspected, and any significant bleeding is controlled. A sponge is applied to the expanded lung. After 5 to 10 minutes, the sponge is removed, and the lung surface is again inspected. If the dissection of the intersegmental plan has been done carefully, only a few alveolar air leaks will be present. These tend to seal over promptly with reexpansion of the lung during the postoperative period. Any leaking from small bronchi, however, must be recognized and controlled; this type of leak may cause persistent problems and predispose to postoperative space problems and infections. Jensik (1986) advocated covering the raw surfaces with pleural flaps or reconstituting the lung by bringing the adjacent segments together. Such a step generally is unnecessary when anatomic planes are respected and may lead to increased postoperative problems.
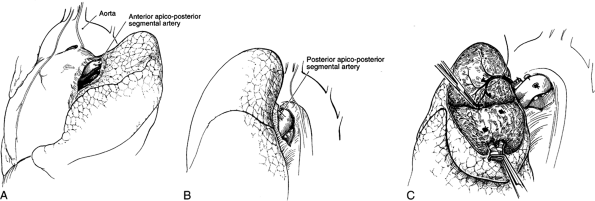 |
Fig. 31-3. A left apicoposterior segmentectomy. A. Identification and ligation of the anterior artery. B. Identification and ligation of the posterior artery. C. Parenchymal dissection of the segment exposing the vein in the intersegmental plane. |
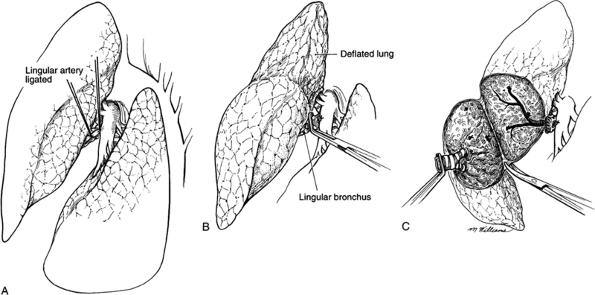 |
Fig. 31-4. A lingulectomy. A. Exposure of the pulmonary artery in the fissure with ligation of the lingular artery exposing the lingular bronchus. B. Identification of the intersegmental plane after deflation of the lung. C. Division of the pleura by sharp dissection. |
P.499
Management of the Pleural Space
Two thoracostomy tubes are placed into the space before the wound is closed, one anteriorly and one posteriorly. These tubes are connected to an underwater drainage system. The amount and timing of the suction to be used is at the discretion of the surgeon. Subsequent management of the pleural tubes is the same as that described for lobectomy.
Physiologic Effects
The physiologic changes after segmentectomy are the same as those noted after a lobectomy. The patient reacts in the same way as after a lobectomy because of the significant dysfunction after handling of the lobe during the operation. Late functional results are related to the number of segments removed. Over the next several months, the patient regains function of the remaining segments.
Morbidity and Mortality
The nonfatal complications are similar to those occurring after a lobectomy. The major ones are prolonged air leak, either peripheral alveolar pleural fistula or a true bronchopleural fistula, empyema, and persistent pleural air space. All are interrelated, and the incidence of any one alone or in combination varies most directly with the disease process and the difficulty experienced in the dissection of the intersegmental planes.
The incidence of persistent pleural air space is as high as 33% in patients with pulmonary tuberculosis, probably owing to the nature of pulmonary tuberculosis. Compared with pleural air spaces occurring after lobectomy, however, only a small percentage of those noted after segmentectomy produce symptoms. When serious complications develop as a result of the space, they are most likely to be septic. The empyema with or without bronchopleural fistula must be treated in the usual manner.
Segmentectomy is essentially a benign procedure, and the mortality rate should approximate 1%. It may, however, be as high as 4% to 6% in patients with poor pulmonary function or in those with previous pulmonary resection, as reported by Jensik (1986).
NONANATOMIC PARENCHYMA-SPARING RESECTIONS
Patients with metastatic lesions of epithelial cell origin and sarcomas are ideal candidates for metastasectomy using nonanatomic resections. These patients may have multiple lesions or may present with additional lesions on subsequent occasions. Anatomic resections may remove a considerable amount of unaffected normal functioning lung, which might render these individuals pulmonary cripples and severely affect their quality of life.
For metastatectomy, Mountain and colleagues (1984) reported an overall survival rate of 35% in large collected series of patients. McAfee and co-workers (1992) found a 5-year survival rate of 35% for colon carcinoma. Lanza and associates (1992) reported a 35% 5-year survival for breast carcinoma. Pogrebniak and colleagues (1992) were able to extend median survivals of patients with renal cell carcinoma to 43 months.
P.500
Patients who also may benefit from such an approach are those with marginal function who have an early stage I or II lung cancer. In a small series of collected patients with marginal lung function and early cancers, Miller and Hatcher (1987) showed a 5-year survival rate of 35%. Errett and associates (1985), Peters (1982), and Lewis and colleagues (1992) advocated similar approaches for the patient with marginal lung function. Currently, a National Cancer Institute Intergroup study is collecting data on combining video-assisted wedge resection with radiation for primary lung cancer in patients with marginal pulmonary function. Several methods are available for performing nonanatomic resections of the lung, including open or video-assisted stapling, electrocautery, and laser. The techniques for each of these are similar.
Technique
Patients undergoing limited lung resection using any one of the variety of the aforementioned methods may have one of a variety of incisions ranging from a standard posterolateral incision or sternotomy for bilateral disease to the minimally invasive video-assisted thoracic surgical techniques (VATSs). Most patients are placed in the lateral thoracotomy position with double-lumen tube anesthesia. After entry into the chest, thorough inspection is made to ensure that all areas of disease are identified. If the patient has multiple metastatic lesions, each should be addressed separately.
The wedge is best performed by using a U-type rather than a V-type resection. This ensures an adequate margin around the lesion. Two methods can be used. Two parallel staple lines are placed into the lungs, several centimeters separated from the lesion. Several open and endoscopic in-line stapling devices made by a variety of companies are available to perform a nonanatomic wedge resection. The lesion is then elevated, and one or two additional staple lines are placed at the proximal margin. Alternatively, the staple line is begun near the lesion, the edge of the specimen to be removed is elevated, and additional staple lines are fired until the lesion and the surrounding margin are resected. The last firing is usually performed from a different angle (Fig. 31-5). When done thoracoscopically, the latter technique is used. Such a maneuver may require three to five staple applications, but it creates a contoured cut with an adequate margin around the lesion. The use of a video-assisted resection has largely supplanted the subsequent techniques, but they are included for completeness.
Electrocautery may be used to remove a lesion. This device can cut and coagulate simultaneously; the technique for its use was described by Urschel (1986). A linear incision is made over the lesion in the parenchyma, and the lesion is exposed. By using traction and countertraction and applying cautery just beneath the lesion, it can be excised, leaving essentially all normal lung tissue. To accomplish this excision, a setting of at least 70 W must be applied to coagulate the tissue. At this power, resection proceeds with adequate coagulation of small and medium blood vessels. The major disadvantage is that the hot cautery blade may stick to the parenchyma.
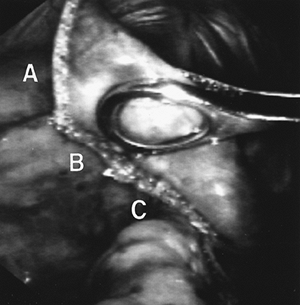 |
Fig. 31-5. A wedge resection. The first staple line begins resection (A). The second staple line provides a generous margin under the lesion (held in forceps) (B). The third staple line completes the margin around the lesion (C). A fourth staple line completes the resection. |
An alternative to this approach was described by Cooper and colleagues (1986). They described the use of a bipolar cautery. When traction and countertraction are applied, small amounts of tissue are grabbed with the bipolar forceps and coagulated. All larger vessels and bronchi are ligated individually. This technique produces good results with minimal air leak or injury to the remaining parenchyma. It is laborious and time consuming, however, and any vessel larger than 1 mm must be ligated individually.
Another tool for resection is the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, an excellent cutting and coagulating tool for the surgeon. One major advantage of the Nd:YAG laser is that it does not have to touch the tissue, and thus no sticking occurs. At a power setting of 80 W, it can coagulate vessels up to 2 mm in diameter and seal air leaks from bronchi up to 1 mm in diameter. It produces a considerable smoke plume, which must be evacuated through filtered suction. The technique for removing a lesion was described by me and my colleagues (1989) (Fig. 31-6). Traction and countertraction are applied, and the laser is used to excise the entire lesion. Because the laser produces considerable shrinkage of tissue, one must aim about 1 to 2 cm from the lesion. Larger blood vessels not coagulated by the laser should be individually ligated. One
P.501
theoretical advantage of the laser is that because of its depth of penetration, it may be destroying additional small micrometastasis up to 4 mm away from the lesion. One disadvantage is that the dessicated lung tissue at the resection margin produces a scar that is radiologically visible for many months. For that reason, follow-up chest computed tomographic (CT) scans are necessary for an indefinite period of time to observe for recurrence.
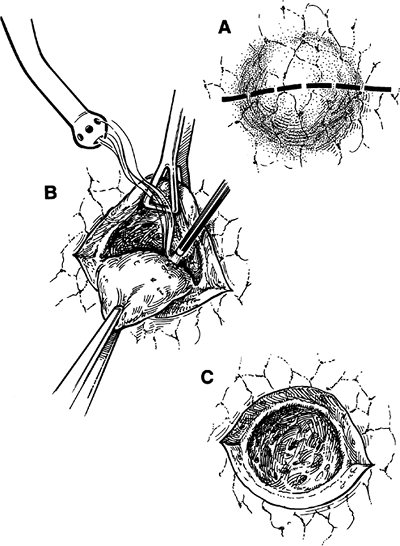 |
Fig. 31-6. A laser resection of a pulmonary nodule. A. Parenchymal incision down to the lesion. B. Lasering of the parenchyma using traction and countertraction. C. Open crater after laser excision. |
Management of the Pleural Space
Thoracostomy tubes are placed into the space before the wound is closed. These tubes are connected to an underwater drainage system. Air leak with staple lines is usually minimal. But for coagulation or laser resection, when negative suction is applied to the tubes, significant air leak may result, particularly on positive-pressure ventilation. It may be best to withhold suction until the patient is in the postanesthesia care unit. When a patient is breathing spontaneously, suction may be applied cautiously. Once the lung is reexpanded, suction may be necessary to maintain this expansion. Subsequent management of the pleural tubes is the same as that described for lobectomy.
Postoperative care is similar to that after lobectomy and segmentectomy. During the early postoperative period, chest radiography frequently shows a halo around the site where the laser or cautery is used. This area eventually collapses and disappears, a process that occurs over several months. Such a scar may be difficult to evaluate radiographically, and the follow-up of malignant lesions, even by computed tomographic scans, is difficult. It is necessary to obtain a scan in the early postoperative period so that this area may be evaluated in comparison with later postoperative studies.
Physiologic Effects
The physiologic changes subsequent to a wedge resection in the absence of postoperative pleural complications are minimal. Those seen are related more directly to the thoracotomy incision than to the removal of a small portion of lung. Early in the postoperative period, the lung volume is restricted. Inspiratory capacity and expiratory reserve volume are decreased. Alveolar hypoventilation, with carbon dioxide retention and some degree of respiratory acidosis, sometimes occurs. Again, because of pain, compliance may be reduced, and the chest wall may be stiff, resulting in an increased work of breathing. This situation is usually noted within the first few hours of operation but returns gradually to normal within 1 to 2 weeks after operation.
Morbidity and Mortality
The morbidity after a nonanatomic parenchyma-sparing resection is minimal. When present, complications are most often the result of either retention of secretions or pleural problems. Persistent air spaces occur in up to 10% of cases. Most of these spaces produce no symptoms and require no treatment.
The mortality rate after nonanatomic parenchyma-sparing resection is near zero in patients with benign inflammatory disease and no more than 0.5% in those with malignant disease or pulmonary tuberculosis. Reports by Saltman and LoCicero (1997) and Tovar and associates (1998) on the video-assisted methods show that such procedures can be performed even as outpatient procedures.
REFERENCES
Agasthian T, et al: Surgical management of bronchiectasis. Ann Thorac Surg 62:976, 1996.
Ashour M, et al: Surgery for unilateral bronchiectasis: results and prognostic factors. Tuber Lung Dis 77:168, 1996.
Cerfolio RJ, et al: Lung resection in patients with compromised pulmonary function. Ann Thorac Surg 62:348, 1996.
Cooper JD, et al: Precision cautery excision of pulmonary lesions. Ann Thorac Surg 41:51, 1986.
P.502
Crabbe MM, Patrissi GA, Fontenelle LJ: Minimal resection for bronchogenic carcinoma: should this be standard therapy? Chest 95:968, 1989.
Crabbe MM, Patrissi GA, Fontenelle LJ: Minimal resection for bronchogenic carcinoma: an update. Chest 99:1421, 1991.
Errett LE, et al: Wedge resection as an alternative procedure for peripheral bronchogenic carcinoma in poor-risk patients. J Thorac Cardiothorac Surg 90:656, 1985.
Ginsberg RJ, Rubinstein LV: Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60:615, 1995.
Grismer JT, Read RC: Evolution of pulmonary resection techniques and review of bronchus first method. Ann Thorac Surg 60:1133, 1995.
Jensik RJ: The extent of resection for localized lung cancer: segmental resection. In Kittle CF, (ed): Current Controversies in Thoracic Surgery. Philadelphia: WB Saunders, 1986.
Jensik RJ: Miniresection of small peripheral carcinomas of the lung. Surg Clin North Am 67:951, 1987.
Jensik RJ, Faber LP, Kittle CF: Segmental resection for bronchogenic carcinoma. Ann Thorac Surg 28:475, 1979.
Jensik RJ, et al: Segmental resection for a lung cancer. A fifteen year experience. J Thorac Cardiothorac Surg 66:563, 1973.
Kodama K, et al: Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 114:347, 1997.
Koike T, et al: Intentional limited pulmonary resection for peripheral T1 N0 M0 small-sized lung cancer. J Thorac Cardiovasc Surg 125:924, 2003.
Lanza LA, et al: Long-term survival after resection of pulmonary metastases of the breast. Ann Thorac Surg 54:244, 1992.
Lewis RJ, et al: Video-assisted thoracic surgical resection of malignant lung tumors. J Thorac Cardiothorac Surg 104:1679, 1992.
LoCicero J III, et al: Laser-assisted parenchyma-sparing resection. J Thorac Cardiothorac Surg 97:732, 1989.
Massard G, et al: Surgical treatment of pulmonary and bronchial aspergilloma. Ann Chir 47:147, 1993.
McAfee MK, et al: Colorectal lung metastases: results of surgical excision. Ann Thorac Surg 53:780, 1992.
Miller JI, Hatcher CR Jr: Limited resection of bronchogenic carcinoma in the patient with marked impairment of pulmonary function. Ann Thorac Surg 44:340, 1987.
Mountain CF, McMutery MJ, Hermes KE: Surgery for pulmonary metastasis: a 20-year experience. Ann Thorac Surg 38:323, 1984.
Nuchtern JG, Harberg FJ: Congenital lung cysts. Semin Pediatr Surg 3: 233, 1994.
Okada M, et al: Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg 71:956, 2001.
Peters RM: The role of limited resection in carcinoma of the lung. Am J Surg 143:706, 1982.
Pogrebniak HW, et al: Renal cell carcinoma: resection of solitary and multiple metastasis. Ann Thorac Surg 54:33, 1992.
Read RC, Yoder G, Schaeffer RC: Survival after conservative resection for T1 N0 M0 non-small cell lung cancer. Ann Thorac Surg 499:391, 1990.
Saltman AE, LoCicero J: Short stay thoracic surgery. J Resp Crit Care Med 155:A482, 1997.
Sapin E, et al: Congenital adenomatoid disease of the lung: prenatal and perinatal management. Pediatr Surg Int 12:126, 1997.
Shields TW, Higgins GA Jr: Minimal pulmonary resection in treatment of carcinoma of the lung. Arch Surg 108:420, 1974.
Temeck BK, et al: Thoracotomy for pulmonary mycoses in non-HIV-immunosuppressed patients. Ann Thorac Surg 58:333, 1994.
Tovar EA, et al: One day admission for lobectomy: an incidental result of a clinical pathway. Ann Thorac Surg 65:803, 1998.
Urschel HC, in discussion of Cooper, et al: Precision cautery excision of pulmonary lesions. Ann Thorac Surg 41:53, 1986.
Warren WH, Faber LP: Segmentectomy vs. lobectomy in patients with stage I pulmonary carcinoma: five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 107:1087, 1994.
Yoshikawa K, et al: Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg 73:1055, 2002.
EAN: 2147483647
Pages: 203