146 - Benign Strictures of the Esophagus
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXVIII - Mediastinal Infections, Overview of Mass Lesions in the Mediastinum, and Control of Vascular Obstructing Symptomatology > Chapter 171 - Vein Grafts for the Superior Vena Cava
Chapter 171
Vein Grafts for the Superior Vena Cava
John R. Doty
Donald B. Doty
Obstruction of the superior vena cava (SVC) can present either as a chronic, progressive syndrome or as an acute, life-threatening process. According to Nieto and one of us (DBD) (1986), more than 90% of all cases of SVC syndrome (SVCS) are caused by malignancy, with lung carcinoma and lymphoma as the most common tumors. Lung cancer in particular has a propensity to cause obstruction of the SVC, with reports by Nogiere and associates (1979) and Escalante (1993) that 5% to 15% of patients with bronchial carcinoma develop superior vena cava syndrome (SVCS). Benign causes are less common, with fibrosing mediastinitis and fungal diseases the leading etiologies as noted by Kalweit and colleagues (1996), as well as by Nieto and one of us (DBD) (1986). However, Abner (1993) has reported an increasing number of infectious causes of SVCS in the setting of immunosuppression. As observed by Escalante (1993), thrombosis of the SVC results in the most dramatic presentation and is becoming more common with the increasing use of central venous access catheters and invasive monitoring devices.
The clinical features of SVC obstruction have been discussed in Chapter 170 and are not repeated here. However, the diagnostic tests that should be used to guide medical and surgical therapy may be reiterated for emphasis and include chest radiography, ultrasonography, computed tomography (CT), and contrast venography. According to Stanford and one of us (DBD) (1986), venography is particularly important to properly stratify patients for surgical intervention. Patients with type III (near complete to complete obstruction of the SVC with reversal of azygous blood flow) pattern of obstruction on venography are the best candidates for caval bypass operations.
Most cases of SVCS are treated effectively with nonoperative therapy, as noted in Chapter 170, especially if the etiology is infectious or malignant. Thrombolytic therapy, as noted by Gray and associates (1991), has been shown to be useful in resolving SVC obstruction secondary to clot formation and is most effective in lysis of thrombus associated with a central venous catheter. Percutaneous placement of intravascular stents, as reported by Jackson and Brooks (1995) and Shah and co-workers (1996), also has a role in SVC obstruction secondary to malignancy, but such stents are prone to thrombosis. Wisselink and colleagues (1993) have noted that simple balloon angioplasty yields results that are inferior to those of operative reconstruction and requires multiple attempts to approach the long-term success of surgery.
INDICATIONS FOR SURGERY
The operative indications for surgical treatment of SVC obstruction are not completely defined and depend to some degree on the underlying etiology and the rapidity of the obstructive process. Malignant diseases that result in acute, sudden obstruction and thrombosis of the SVC may not resolve with thrombolytic and radiation therapy. Acute obstruction of the SVC associated with signs of cerebral or laryngeal edema are indicative of death within 6 weeks. Surgical intervention in these patients not only immediately relieves life-threatening symptoms, but also allows definite treatment of the malignancy to proceed with the patient feeling more comfortable. Invasion of the SVC by malignant or benign tumor may be resistant to chemotherapy or radiation therapy because of clot and fibrosis associated with clot resolution, which was stimulated and propagated by the presence of tumor within the bloodstream. Obstruction of the SVC with restriction of blood flow may persist even though there appears to be good response of the tumor to treatment. Some of the benign causes of SVC obstruction are severe and relentless fibrotic processes that result in recurrent and extending obstruction. Clot propagation within the venous system proximal to the primary site of caval obstruction may present the appearance of progression of SVCS in patients with a benign etiology. Some patients with obstruction of the SVC may never develop adequate collateral circulation even though the causative process may be stabilized or arrested. Thus, any patient being considered for surgical reconstruction should have contrast
P.2568
venography documentation of complete obstruction of the SVC with inadequate collateral circulation.
Current recommendations for operative intervention in SVC obstruction are as follows: (a) persistent severe SVCS because of chronic SVC obstruction caused by a benign process, (b) acute SVC obstruction caused by a benign or malignant process with signs of cerebral or laryngeal edema, (c) relief of life-threatening SVCS during palliation of a malignant process, and (d) failure of nonoperative therapy to resolve SVCS caused by SVC obstruction.
Contraindications to surgical reconstruction include patients in whom collateral circulation has formed adequately to provide upper compartment venous decompression. Patients with large, bulky tumors of the anterior mediastinum are unsuitable candidates for operative intervention, as are patients with limited life expectancy because of advanced malignancy or from associated medical disorders.
VEIN GRAFT OPTIONS
Operative reconstruction of venous drainage patterns in the thorax ideally should be performed with autogenous tissue to provide the optimal long-term outcome. Options for venous conduits include spiral saphenous vein, femoral vein, straight saphenous vein, and composite autogenous vein grafts. In unusual settings and according to individual patient anatomy, alternative venous conduits, such as azygos vein inferior vena cava or jugular vein femoral vein grafts, can be constructed. In the absence of autogenous venous tissue, aortic homograft, venous homograft, and pericardial tube construction can serve as conduit. Prosthetic graft materials are generally inferior to autogenous tissue grafts.
Success with bypass grafting of the SVC depends primarily on two main factors: adequate size of the conduit and proper orientation. The ideal graft should closely match the native SVC in diameter to prevent residual obstructive flow gradients. The graft should be measured carefully for length so that the completed conduit does not have redundancy, which can result in graft kinking and obstruction. Both the inflow and outflow of the graft should be free of intraluminal obstructions, such as atrial trabeculations and venous thrombosis.
Spiral Saphenous Vein Graft
The most extensive experience with venous bypass grafts has been with the spiral saphenous vein graft, a concept developed by Chiu and associates in 1974. The first use of the spiral vein graft in humans in 1976 in a patient with SVC obstruction secondary to granulomatous mediastinitis was reported in 1976 by one of us (DBD) and Baker. Twenty-three years later, this patient remains asymptomatic.
The spiral vein graft is constructed from the patient's own saphenous vein and is used as a bypass graft from the innominate vein to the right atrium (Fig. 171-1). After performing a sternotomy, the distance from the confluence of the left internal jugular vein and the left subclavian vein to the right atrial appendage is measured. The diameter of the innominate vein is measured as the eventual diameter of the spiral vein graft. The saphenous vein is then exposed and its diameter is measured. The length of the saphenous vein to be removed is calculated using the simple formula:

For example, if the innominate vein is 15 mm in diameter, the saphenous vein is 3 mm in diameter, and the length to the right atrial appendage is 8 cm, the proper length of saphenous vein to be harvested is 40 cm (15/3 8).
The saphenous vein is removed and incised in a longitudinal fashion throughout its entire length. A thoracostomy tube is chosen that is the same diameter as the innominate vein as a stent to form the bypass graft. The saphenous vein is flattened and wrapped in a spiral fashion around the stent,
P.2569
and the edges are joined using a continuous suture of 7-0 polypropylene. This forms a large conduit with a diameter that closely matches the innominate vein and a length that approximates the distance from the jugular subclavian confluence to the right atrial appendage.
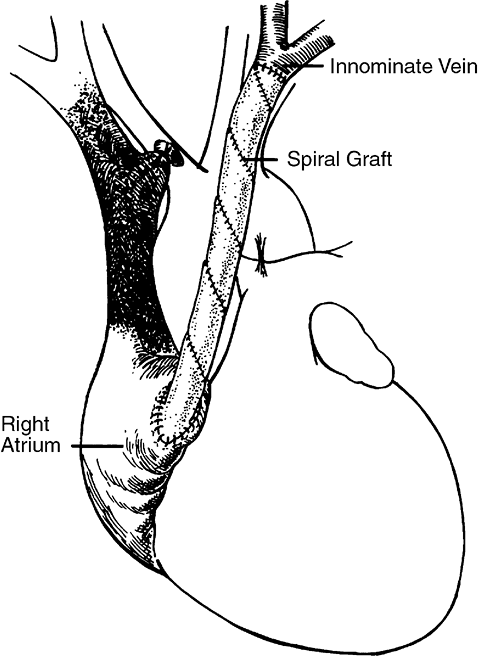 |
Fig. 171-1. Bypass of superior vena cava. A composite spiral saphenous vein graft is anastomosed to the left innominate vein at the jugular subclavian vein confluence. The distal end of the graft is anastomosed to the right atrial appendage to bypass the obstructed superior vena cava. |
Heparin (200 300 U/kg) is administered intravenously, and the innominate vein is ligated at its junction with the SVC. A soft jaw vascular clamp is applied at the jugular subclavian confluence, and the innominate vein is divided. Abnormal intimal tissue and thrombus are removed from the innominate vein. The spiral vein graft is pushed slightly off the stent, and an end-to-end anastomosis is performed to the innominate vein using continuous 7-0 polypropylene suture. The stent is then removed from the graft, and a curved vascular clamp is placed on the right atrial appendage. The tip of the right atrial appendage is excised, and obstructing trabeculae are removed to ensure unrestricted blood flow out of the graft. The graft is anastomosed to the right atrial appendage using continuous 5-0 polypropylene suture.
Most patients undergoing spiral vein bypass grafting for SVC obstruction can be followed clinically for graft patency. Prompt resolution of symptoms indicates successful decompression, and stenosis or occlusion of the graft is typically heralded by return of the obstructive syndrome. Ultrasonography, CT scanning, and venography, as well as magnetic resonance imaging, as noted by Levitt and associates (1986), are useful adjuncts for evaluating spiral vein bypass grafts when the postoperative clinical presentation is less clear.
Femoral Vein Graft
The first successful bypass operations for SVC obstruction were performed with autologous femoral vein grafts, as reported in the early 1950s by Bricker and McAfee (1952) and Klassen and associates (1951). Since that time, however, the femoral vein has rarely been used as a bypass graft for SVCS. More recently, Gladstone and associates (1985) used autologous femoral vein to construct a bypass graft in two patients, one with mediastinal fibrosis and the other with poorly differentiated carcinoma.
After median sternotomy to confirm feasibility of the bypass operation, the femoral vein is exposed from its junction with the greater saphenous vein as far distally as the adductor hiatus, if necessary. Minor branches are ligated, and the appropriate length of vein is removed distal to the femoral-saphenous junction. After heparinization, the femoral vein graft is interposed between the innominate vein and the right atrial appendage, as described previously for spiral vein graft bypass.
Composite Autologous Vein
Construction of large-caliber venous bypass conduits can be accomplished using the saphenous vein in a composite or paneled manner, first reported by Benvenuto and colleagues in 1961. In this operation, the saphenous vein is divided into several segments, each of which is incised longitudinally. These segments are then flattened, placed on a stent in a paneled or tiled manner, and sewn together to create the conduit.
Saphenous Vein
Intact saphenous vein can be used as a bypass conduit for SVC obstruction. The smaller caliber of the native saphenous vein typically requires construction of more than one graft to provide adequate venous flow. Mitchell and associates (1986) described two patients with mediastinal fibrosis in whom the saphenous vein was used to create a double bypass graft to the proximal SVC. One patient remained asymptomatic at 11 years postoperatively; the other patient thrombosed both grafts 2 weeks after the operation.
Alternative Venous Anastomoses
Occasionally, patients with SVC obstruction can be surgically treated without construction of a venous bypass graft in the thorax. Schramel and Olinde (1961) were the first to describe subcutaneous tunneling of a long saphenous vein bypass conduit to the jugular vein. Taylor and associates (1974) later performed a similar approach, and Vincze and colleagues (1982) described seven patients treated with saphenous-jugular bypass for SVC obstruction caused by lung carcinoma.
Homografts and Pericardial Conduits
On occasion, a venous bypass graft cannot be constructed. Alternative tissue conduits in this setting include aortic homograft, SVC homograft, and pericardial tube graft. Aortic homografts have excellent handling characteristics and perform well when used for replacement in the venous system.
Other Methods of Decompression of Superior Vena Cava Obstruction
The surgeon also should be familiar with alterations in venous flow patterns that may exist in the setting of SVC obstruction. Contrast venography is important not only for demonstrating the degree of obstruction of the SVC and its tributaries, but also for evaluation of patency and flow in the azygos system. As Stanford and one of us (DBD) (1986) noted, patients with the type III pattern have reversal of azygous blood flow and are most amenable to venous reconstruction. Cooley and Hallman (1964) reported a case of SVC obstruction in which contrast venography demonstrated
P.2570
retrograde flow through the azygos system in 1963. This patient was treated with side-to-side anastomosis of the azygos vein to the inferior vena cava, which resolved the SVC obstruction. Shimokawa and associates (1996) have described extracorporeal axillofemoral venous bypass as a useful temporizing measure to reduce venous pressure in the upper thorax prior to general anesthesia.
RESULTS OF THE USE OF THE VARIOUS BYPASS METHODS
After Spiral Saphenous Vein Grafts
Long-term follow-up of patients receiving spiral saphenous vein grafts for SVC obstruction has demonstrated excellent results. Our series reported by one of us (JRD) and colleagues (1999) of patients treated with spiral vein bypass grafting for benign disease showed patency in 14 of 16 patients. Three of these individuals have shown long-term patency of spiral vein grafts at 20, 21, and 23 years. Alimi and associates (1998) reported similar excellent patency rates at 5 years, although a few patients have required subsequent percutaneous interventions to maintain graft patency.
Spiral vein bypass grafting is useful also in the setting of SVC obstruction from malignancy. One of us (DBD) (1982) reported six patients with bronchial carcinoma in whom spiral vein grafting was done, and the symptoms of SVC obstruction were relieved in all. Long-term survival in these patients is understandably reduced because of the underlying malignant process, but acute mortality from SVCS was abolished in this series. All but one patient died from metastatic carcinoma, and all patients had patent grafts at the time of death. Smith and Brantigan (1983) also have reported successful use of a spiral vein bypass graft in the setting of SVC obstruction from bronchial carcinoma.
The spiral vein graft has been shown by Brandt and associates (1985) to be useful in children as well as adults, as evidenced by their case report of successful treatment of SVC obstruction secondary to intraatrial baffle for transposition of the great arteries. The saphenous vein was of adequate caliber in this child to form a spiral vein bypass graft from the innominate vein to the left atrial appendage. Anderson and Li (1983) have used a spiral vein conduit as an interposition graft to reconstruct the SVC after resection of a leiomyosarcoma; the graft was patent on CT scan 10 months postoperatively.
After Femoral Vein Grafts
Femoral vein grafts used for bypass of SVC obstruction have been shown to be patent up to 18 months after operation, as reported by Marshall and Kouchoukos (1988). Addition of a ringed polytetrafluoroethylene tube around the graft in their case provided external support to prevent recurrent fibrosis in a patient who had previously been treated with a spiral vein bypass. There has been some concern about leg edema after the removal of the femoral vein, but this has not been problematic in the published reports.
After Composite Autologous Vein Grafts
Both the initial report by Benvenuto and colleagues (1961) and a subsequent study by Miller and Sullivan (1973) demonstrated graft patency at 1 year. Scherck and associates (1974) reviewed 11 case reports in which autogenous vein grafts were used in various methods to bypass or reconstruct the SVC. Follow-up ranged from 8 days to 5 years, and overall graft patency was 70%.
After Saphenous Vein Graft
Larsson and Lepore (1992) reported three patients with thymoma in whom the saphenous vein was used for bypass grafting. Two patients had double-bypass graft construction to the right atrium, and the third patient had single-bypass graft construction to the proximal SVC. All patients had patent grafts at follow-up from 8 to 10 months.
After Alternative Venous Anastomoses
In Vincze and co-workers' (1982) study, all patients were free of obstructive symptoms at the time of death, ranging from 2 to 14 months. Graham and associates (1995) have reported a series of three patients undergoing subcutaneous jugulofemoral bypass; all patients had relief of SVC obstruction at follow-up ranging from 6 weeks to 13 months.
After Homografts and Pericardial Conduits
Scherck and associates (1974) demonstrated an overall graft patency of nearly 90% on review of 17 patients using aortic homograft to reconstruct the SVC. Ohri and colleagues (1997) reported a single case of SVC obstruction caused by multidrug-resistant tuberculosis treated with aortic homograft reconstruction; the graft was patent at 6 months. Oyarzun and McCormick (1998) used a pulmonary homograft for SVC reconstruction with good short-term results. Moore and co-workers (1961) examined the potential application of SVC homograft as a possible conduit for the treatment of SVC obstruction; graft patency was approximately 70% in their experimental model. Use of SVC homograft in a human has been reported only once, and this was by Tice and Zerbino (1972); the graft was patent on follow-up at 1 year.
Initial experience with tube grafts constructed from autogenous pericardium was disappointing in the experimental setting. Scherck and associates (1974) reviewed three series of experimental pericardial tube grafts; no grafts were patent.
P.2571
The use of autogenous pericardial tubes in humans, however, has been more encouraging. Zembala and colleagues (1986) created a pericardial tube graft between the innominate vein and the right atrial appendage in a patient with malignant teratoma; the graft remained patent for 11 months. Piccione and associates (1990) successfully used autologous pericardium in six patients to reconstruct portions of the SVC after resection for carcinoma, and all patients were relieved of obstructive symptoms. Lemmer and co-workers (1989) created conduits from pedicles of right atrium and pericardium to reconstruct the SVC in two pediatric patients; the grafts remained patent for 24 and 43 months, respectively.
REFERENCES
Abner A: Approach to the patient who presents with superior vena cava obstruction. Chest 103(suppl):394, 1993.
Alimi YS, et al: Reconstruction of the superior vena cava: benefits of postoperative surveillance and secondary endovascular interventions. J Vasc Surg 27:287, 1998.
Anderson RP, Li W: Segmental replacement of superior vena cava with spiral vein graft. Ann Thorac Surg 36:85, 1983.
Benvenuto R, et al: Composite venous graft for replacement of the superior vena cava. Arch Surg 84:570, 1961.
Brandt B III, Hiratzka LF, Marvin WJ Jr: Spiral vein graft: an alternative method for relief of superior vena caval obstruction following the Mustard repair. J Thorac Cardiovasc Surg 89:943, 1985.
Bricker EM, McAfee CA: Femoral vein graft following bilateral internal jugular vein resection. Surgery 32:114, 1952.
Chiu CJ, Terzis J, MacRae ML: Replacement of superior vena cava with the spiral composite vein graft. Ann Thorac Surg 17:555, 1974.
Cooley DA, Hallman GL: Superior vena caval syndrome treated by azygos vein inferior vena cava anastomosis. J Thorac Cardiovasc Surg 47:325, 1964.
Doty DB: Bypass of superior vena cava. Six years experience with spiral vein graft for obstruction of superior vena cava due to benign and malignant disease. J Thorac Cardiovasc Surg 83:326, 1982.
Doty DB, Baker WH: Bypass of superior vena cava with spiral vein graft. Ann Thorac Surg 22:490, 1976.
Doty JR, Flores JH, Doty DB: Superior vena cava obstruction: bypass using spiral vein graft. Ann Thorac Surg 67:1111, 1999.
Escalante CP: Causes and management of superior vena cava syndrome. Oncology 7:61, 1993.
Gladstone DJ, et al: Relief of superior vena caval syndrome with autologous femoral vein used as a bypass graft. J Thorac Cardiovasc Surg 89: 750, 1985.
Graham A, et al: Subcutaneous jugulofemoral bypass: a simple surgical option for palliation of superior vena cava obstruction. J Cardiovasc Surg (Torino) 36:615, 1995.
Gray BH, et al: Safety and efficacy of thrombolytic therapy for superior vena cava syndrome. Chest 99:54, 1991.
Jackson JE, Brooks DM: Stenting of superior vena caval obstruction. Thorax 50(suppl 1):31, 1995.
Kalweit G, et al: Mediastinal compression syndromes due to idiopathic fibrosing mediastinitis report of three cases and review of the literature. Thorac Cardiovasc Surg 44:105, 1996.
Klassen KP, Andrews NC, Curtis GH: Diagnosis and treatment of superior vena cava obstruction. Arch Surg 63:311, 1951.
Larsson S, Lepore V: Technical options in reconstruction of large mediastinal veins. Surgery 111:311, 1992.
Lemmer JH Jr, et al: Pedicled right atrial-pericardial tissue conduit for bypass of the obstructed superior vena cava in children. J Thorac Cardiovasc Surg 98:417, 1989.
Levitt RG, et al: Magnetic resonance imaging of spiral vein graft bypass of superior vena cava in fibrosing mediastinitis. Chest 90:676, 1986.
Marshall WG Jr, Kouchoukos NT: Management of recurrent superior vena caval syndrome with an externally supported femoral vein bypass graft. Ann Thorac Surg 46:239, 1988.
Miller RE, Sullivan FJ: Superior vena caval obstruction secondary to fibrosing mediastinitis. Ann Thorac Surg 15:483, 1973.
Mitchell IM, et al: Surgical treatment of idiopathic mediastinal fibrosis: report of five cases. Thorax 41:210, 1986.
Moore TC, et al: Superior vena caval replacement. 5. Use of fresh homografts of superior vena cava. J Thorac Cardiovasc Surg 42:379, 1961.
Nieto AF, Doty DB: Superior vena cava obstruction: clinical syndrome, etiology, and treatment. Curr Probl Cancer 10:441, 1986.
Nogiere C, Mincer F, Botstein C: Long survival in patients with bronchogenic carcinoma complicated by superior vena cava obstruction. Chest 75:325, 1979.
Ohri SK, Lawrence DR, Townsend ER: Homograft as a conduit for superior vena cava syndrome. Ann Thorac Surg 64:531, 1997.
Oyarzun JR, McCormick JR: Homograft and SVC syndrome. Ann Thorac Surg 65:1836, 1998.
Piccione W Jr, Faber LP, Warren WH: Superior vena caval reconstruction using autologous pericardium. Ann Thorac Surg 50:417, 1990.
Scherck JP, Kerstein MD, Stansel HC Jr: The current status of vena caval replacement. Surgery 76:209, 1974.
Schramel R, Olinde HDH: A new method of bypassing the obstructed vena cava. J Thorac Cardiovasc Surg 41:375, 1961.
Shah R, et al: Stenting in malignant obstruction of superior vena cava. J Thorac Cardiovasc Surg 112:335, 1996.
Shimokawa S, et al: Extracorporeal venous bypass: a beneficial device in operation for superior vena caval syndrome. Ann Thorac Surg 62:1863, 1996.
Smith ER, Brantigan CO: Bypass of superior vena cava obstruction using spiral vein graft. J Cardiovasc Surg (Torino) 24:259, 1983.
Stanford W, Doty DB: The role of venography and surgery in the management of patients with superior vena cava obstruction. Ann Thorac Surg 41:158, 1986.
Taylor GA, et al: Bypassing the obstructed superior vena cava with a subcutaneous long saphenous vein graft. J Thorac Cardiovasc Surg 68:237, 1974.
Tice DA, Zerbino V: Clinical experience with preserved human allografts for vascular reconstruction. Surgery 72:260, 1972.
Vincze K, Kulka F, Csorba L: Saphenous-jugular bypass as palliative therapy of superior vena cava syndrome caused by bronchial carcinoma. J Thorac Cardiovasc Surg 83:272, 1982.
Wisselink W, et al: Comparison of operative reconstruction and percutaneous balloon dilatation for central venous obstruction. Am J Surg 166:2004, 1993.
Zembala M, et al: Pericardial tube for obstruction of superior vena cava by malignant teratoma. J Thorac Cardiovasc Surg 91:469, 1986.
Reading References
Doty DB, Doty JR, Jones KW: Bypass of superior vena cava. Fifteen years' experience with spiral vein graft for obstruction of superior vena cava caused by benign disease. J Thorac Cardiovasc Surg 99:889, 1990.
Inoue T, et al: Surgical treatment of pacemaker induced left innominate vein occlusion using a spiral vein graft. Pacing Clin Electrophysiol 24: 1566, 2001.
Narayan D, Brown L, Thayer JO: Surgical management of superior vena caval syndrome in sarcoidosis. Ann Thorac Surg 66:946, 1998.
Singh S, Sherif H, Reul GJ: Reconstruction of the superior vena cava with the aid of an extraluminal venovenous jugulo-atrial shunt. Tex Heart Inst J 27:38, 2000.
Uwabe K, et al: Thrombectomy and SVC reconstruction due to infective thrombus. J Cardiovasc Surg (Torino) 43:91, 2002.
EAN: 2147483647
Pages: 203