145 - Paraesophageal Hiatal Hernia
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXVIII - Mediastinal Infections, Overview of Mass Lesions in the Mediastinum, and Control of Vascular Obstructing Symptomatology > Chapter 170 - Superior Vena Cava Syndrome: Clinical Features, Diagnosis, and Treatment
function show_scrollbar() {}
Chapter 170
Superior Vena Cava Syndrome: Clinical Features, Diagnosis, and Treatment
John F. Greskovich Jr.
Timothy J. Kinsella
The clinical signs and symptoms and radiographic manifestations resulting from compromised blood flow through the superior vena cava (SVC) are easily recognizable and are referred to as the superior vena cava syndrome (SVCS). The SVCS is encountered commonly in association with a variety of thoracic neoplasms, but potential etiologies are numerous and diverse. For malignant etiologies, histologic diagnosis is essential for the institution of appropriate management. Although older medical literature, such as reports from Salsali and Clifton (1965), as well as Davenport (1978) and Hussey (1946) and their associates, have suggested that SVCS is a medical emergency, this is rarely the case. In most instances, a proper diagnostic workup can be completed before therapeutic intervention. The prognosis, in terms of palliation, is usually excellent, but long-term survivorship is achieved only in a minority of patients.
HISTORIC BACKGROUND
The first published description of SVCS is generally credited to William Hunter who, in 1757, described the syndrome as a consequence of a syphilitic aneurysm of the ascending aorta. At autopsy, Hunter noted that the SVC and the innominate vein were both so much compressed by the dilated artery, as hardly to have any thing left of their natural capacity and appearance. One of the first clinical descriptions of SVCS caused by a malignancy was made by William Stokes in 1837. Stokes described a patient whose face was bloated, pale, and slightly edematous with an appearance of the eyes as if the balls were protruded from the sockets, and a marked dilatation of the nostrils during breathing, gave his countenance an expression of distress and suffering. Stokes goes on to describe the physical findings vividly. The right jugular vein was much distended, as were the veins in the right axilla; but this symptom was chiefly remarkable on the surface of the belly, where two vein[s] pursued a remarkably tortuous course turgid and dilated to the size of swan quills.
SVCS was regarded as a relatively rare clinical entity before the mid-20th century. In a review by McIntire and Sykes (1948), the authors provided an extensive review of all literature published on SVCS, and were only able to document 502 cases. McIntire and Sykes felt that superior vena caval obstruction occurs much more frequently than is generally suspected and that many cases are doubtless hidden because of being reported as cases of the primary pathology.
The dominant process causing SVC obstruction before 1948 was infectious. Of the 502 cases reported by McIntire and Sykes (1948), nearly two thirds of cases were from benign etiologies such as syphilitic aortic aneurysms, chronic fibrous mediastinitis from tuberculosis or syphilis, and phlebitis with thrombus formation, whereas only one third of the cases were from primary thoracic tumors. With improved antibiotic management of infectious disease, syphilis and tuberculosis are now extremely rare causes of SVCS. Paralleling the dramatic increase in lung cancer incidence as recorded by Boring and colleagues (1993), thoracic malignancies have become far and away the leading cause of SVCS.
ANATOMY AND PATHOPHYSIOLOGY
A thorough understanding of this entity requires an appreciation of the intricate venous anatomy and physiology of the SVC and the collateral venous pathways. At least nine collateral pathways for drainage of the venous system in the chest are important: (a) azygos-hemiazygos, (b) paravertebral, (c) lateral thoracic, (d) internal, (e) mammary, (f) anterior jugular, (g) thyroidal, (h) thymic, and (i) pericardiophrenic. The five major collateral venous networks of the thorax are discussed on the next page.
P.2546
Venous Drainage of the Thorax
Superior Vena Cava
The fusion of the right and left brachiocephalic (innominate) veins results in the formation of the SVC, which drains the preponderance of venous blood from the upper thorax, upper extremities, and head and neck. The SVC is located in the superior portion of the middle mediastinum to the right of the aorta, and anterior to the trachea and right main-stem bronchus. Its length measures 6 to 8 cm, with the final 2 cm embedded within the pericardial reflection around the right atrium. It originates behind the first right costal cartilage, traverses posterior to the first and second intercostal spaces, and terminates in the right atrium at the level of the third intercostal space. Being a large-caliber (diameter about 2 cm), thin-walled vein lacking valves, it maintains blood at a low pressure and is therefore easily compressible. The SVC is completely encircled by numerous lymph nodes, and disease processes that cause mediastinal nodal enlargement are a leading cause of obstruction of SVC flow. Alternatively, direct tumor extension from mediastinal or lung masses may produce SVC obstruction. The anatomic relationships of the SVC are best understood by reviewing its cross-sectional anatomy.
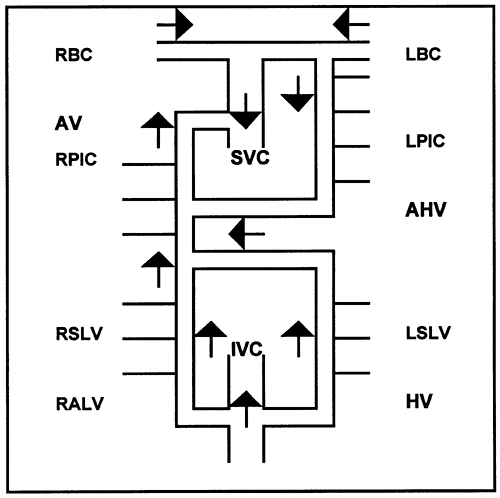 |
Fig. 170-1. Schematic illustration of the azygos venous system. The right and left segmental lumbar veins (RSLV, LSLV) empty into the right ascending lumbar vein (RALV) and the hemiazygos vein (HV). These continue into the azygos vein (AV) and connect with the inferior vena cava (IVC). The right and left posterior intercostals (RPIC, LPIC) empty into the AV and the accessory HV (AHV), the latter draining into the AV inferiorly and left brachiocephalic (LBC) vein superiorly. The LBC and right brachiocephalic (RBC) vein form the superior vena cava (SVC), which receives the AV posteriorly as it enters the right atrium. |
Azygos and Hemiazygos Venous System
The azygos vein is the thoracic continuation of the right ascending lumbar vein running longitudinally anterior to the thoracic vertebral bodies. The azygos vein collects blood from the right posterior intercostal veins and terminates into the posterior aspect of the SVC immediately above the level of the pericardial reflection. In addition, the azygos receives blood from the esophageal, mediastinal, pericardial, and bronchial veins.
On the left, the hemiazygos vein is an intercepting trunk for the lower left posterior intercostal veins and is the thoracic continuation of the left ascending lumbar vein. The hemiazygos vein ascends to the left of the thoracic spine to approximately the eighth thoracic vertebral body, where it crosses over the vertebral column and fuses with the azygos. The accessory hemiazygos vein is an intercepting trunk for the upper left posterior intercostal veins and communicates with the left brachiocephalic vein superiorly. The accessory hemiazygos runs along the left side of the upper thoracic spine and, in addition to the intercostal veins, receives blood from the left bronchial veins. The accessory hemiazygos may join the hemiazygos directly at the level of the eighth vertebral body or may join the azygos directly by crossing over the vertebral column at the level of the seventh thoracic vertebral body. The azygos and hemiazygos veins communicate with the inferior vena cava (IVC) through the right and left ascending lumbar veins, respectively. Under normal physiologic conditions, negative intrathoracic pressure induces cephalad flow into the azygos vein from the lower half of the body.
When venous flow through the SVC is compromised, the azygos venous system provides the principal auxiliary pathway for venous drainage. In supraazygos SVC obstruction, venous return from the upper body is shunted through the accessory hemiazygos, hemiazygos, and interconnecting veins to the azygos, which enters the SVC below the obstruction. In addition, because the azygos system is a low-pressure system with incomplete valves, permitting blood flow reversal, blood may be shunted inferiorly through the ascending lumbar veins and finally into the IVC. The interconnections within this system are schematically presented in Figure 170-1.
Paravertebral Venous System
The paravertebral venous system represents another low-pressure, intercommunicating network capable of altering the direction of blood flow and permitting communication between the SVC and IVC. At any given transverse level, an almost bicircumferential system exists, consisting of an internal plexus within the canal and an external plexus outside the vertebral bodies. Each of the plexuses has anterior and posterior venous arches. An extensive net of interconnecting, valveless, thin-walled tributaries connects the plexuses. These transverse plexuses communicate with a series of valveless, longitudinal veins and can therefore
P.2547
support directional change. Inferiorly, these veins are contiguous with the common iliac and the lumbar veins, which empty into the IVC and the azygos system, respectively. Superiorly, communication is maintained with both the azygos and the SVC through the intercostal veins. Numerous possibilities for variations and directions for flow exist through the paravertebral venous system.
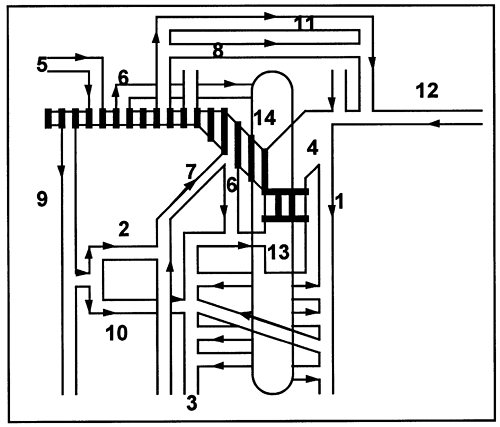 |
Fig. 170-2. Collateral pathways in supraazygos caval occlusion. The hashed areas represent occlusion of the superior vena cava (13) affecting the right side, but not affecting the azygos (3). The pathways include the following: the jugular venous arch (8) to the left subclavian (12), left brachiocephalic (4), and accessory hemiazygos (1); the lateral thoracic (9) to the posterior intercostals (10) into the azygos; the cervical venous network (6) into the vertebral venous plexus (14), which communicates with the azygos as well as the superior vena cava; the lateral thoracic anterior intercostal (2) internal mammary (7) pathway; by way of the cephalic vein (5) into the subclavian (12); and the cerebral sinus route (11). Modified from Muramatsu T, Miyamae T, Dohi Y: Collateral pathways observed by radionuclide superior cavography in 70 patients with superior vena caval obstruction. Clin Nucl Med 16:333, 1991. |
Lateral Thoracic Venous System
The lateral thoracic veins are longitudinally oriented and can provide alternative collateral pathways in the event of SVC obstruction. The lateral thoracic vein typically drains into the axillary vein, which continues as the subclavian vein after passing under the first rib, and as the brachiocephalic vein after junction with the internal jugular vein. With SVC obstruction, the direction of flow is able to reverse within the lateral thoracic vein, shunting blood into the azygos system through the posterior intercostal vein or into the IVC through the anterior intercostal, internal mammary, and superior and inferior epigastric veins. An additional collateral pathway to the IVC involves blood flow from the lateral thoracic vein to the inferior epigastric vein and then to the external iliac vein, which empties into the IVC.
Internal Mammary Venous System
The internal mammary (thoracic) veins provide yet another detour. These veins have connections with both the anterior intercostals and the superior epigastrics, which all run within the thoracic or abdominal wall. The internal mammary vessels course from the subclavian vessels superiorly, descending behind the costal cartilages on the inner aspect of the thoracic wall parallel to the sternum. Collateral flow around an SVC obstruction may occur either through the superior and inferior epigastrics emptying into the external iliac vein and finally the IVC, or through the anterior and posterior intercostal veins into the azygos.
Jugular Venous System
The jugular venous system is an important collateral pathway in supraazygous SVC obstruction. Blood is diverted to the contralateral brachiocephalic vein through the jugular venous arch, a transverse connecting trunk between the anterior jugular veins of either side.
AN ANIMAL MODEL FOR SUPERIOR VENA CAVA SYNDROME
In an elegant series of experiments in dogs, Carlson (1934) documented the effects of ligating the SVC. When the SVC was ligated below the azygos, none of the animals survived. This strongly suggested the importance of the azygos pathway. Other collateral pathways were unable to compensate acutely for sudden, complete SVC occlusion below the azygos. Ligation of the SVC above the azygos resulted in significant acute symptomatology such as cyanosis and stupor. Six of seven dogs recovered rapidly with virtual disappearance of cyanosis within 24 hours. Within 7 to 10 days, collaterals appeared over the thorax and abdomen, permitting survival and recovery. These experiments suggest that the azygos system represents the most important collateral pathway. Patent azygos flow is able to compensate for even acute and complete SVC occlusion. In additional experiments, Carlson was able to document that a two-stage experiment, ligation of the SVC followed 23 to 28 weeks later by ligation of the azygos, was compatible with life, suggesting that other collaterals can support life if allowed to develop over time.
COLLATERAL CIRCULATORY PATHWAYS IN HUMANS
Collateral venous pathways have been described extensively in humans by McIntire and Sykes (1948) and Klassen and associates (1951). Stanford and colleagues (1987) have demonstrated the development of multiple collateral pathways in SVCS using venography. Radionuclide venography (RNV), as noted by Gollub (1980) and Muramatsu (1991)
P.2548
and their associates, has resulted in substantial improvement in the understanding of development of these collateral channels. Muramatsu and colleagues (1991) have identified several major and minor collateral pathways, depending on whether the obstruction was above or at the azygos. These include several of the pathways previously described, but they also demonstrated an important collateral pathway whereby blood is transported to the contralateral brachiocephalic vein through the jugular venous arch (see Fig. 170-2). The most common venous collaterals listed in decreasing order of frequency in 21 patients evaluated using contrast-enhanced, helical computed tomography (CT) scan by Cihangiroglu and co-workers (2001) were: (a) azygos (91%), (b) thoracoepigastric (86%), (c) mediastinal (81%), (d) internal mammary (76%), (e) hemiazygos (71%), (f) lateral thoracic (71%), (g) pericardiophrenic (71%), (h) paravertebral (67%), (i) intercostal (57%), (j) thoracoacromion trunk (57%), (k) capsular/surface liver (52%), (l) bilateral phrenic (52%), (m) thoracodorsal scapular (48%), (n) superficial epigastric (48%), (o) superior epigastric (43%), (p) inferior epigastric (43%), and (q) accessory hemiazygos (38%). The major collateral pathways are summarized in Table 170-1.
In supraazygos obstruction, shunting of blood to the nonoccluded contralateral brachiocephalic vein through the jugular venous arch appears to be the most important pathway. Other major pathways include the lateral thoracic, which shunts blood into the azygos through the posterior intercostals, the vertebral venous plexus, and the internal mammary pathway. In obstructions involving the azygos orifice, the major pathways all shunt blood into the IVC through the lateral thoracic superior epigastric route, the internal mammary inferior epigastric route, the cervical vertebral network azygos route, and the lateral thoracic posterior intercostal azygos route (Fig. 170-3).
Other unusual and collateral pathways include systemic to pulmonary venous shunting, as reported by Gale and colleagues (1990); direct shunting between the right subclavian vein and the left ventricle, demonstrated by Taki and co-workers (1990); collateral channels connecting the upper extremity veins and the portal vein through the paraumbilical veins, resulting in a scintigraphic hot spot in the liver that was recorded by Suneja and Teal (1989); and also through the cerebral sinuses, as shown by the studies of Muramatsu and associates (1991).
P.2549
In addition, left-sided and bilateral SVCs have also been reported by Zellers and colleagues (1989), and these have unusual drainage patterns, including the coronary sinus and pulmonary venous atrium, among other vessels. Obviously, the presence of SVC occlusion in these situations results in rather dramatic and unusual collateralization.
Table 170-1. Collateral Pathways in Superior Vena Cava Syndrome Identified by Radionuclide Superior Cavography | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
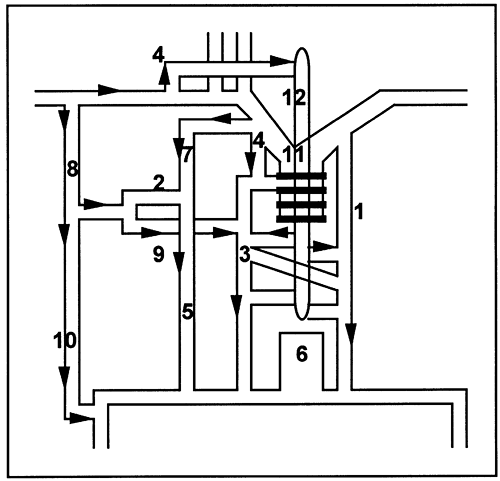 |
Fig. 170-3. Collateral pathways in paraazygos caval occlusion. When the occlusion involves the orifice of the azygos (3), the major collaterals drain into the inferior vena cava (6) or its branches: lateral thoracic (8) superior epigastric (10) inferior vena cava; internal mammary (7) inferior epigastric (5) inferior vena cava; cervical venous network (4) azygos system (3) inferior vena cava; and lateral thoracic posterior intercostal (9) azygos inferior vena cava. Modified from Muramatsu T, Miyamae T, Dohi Y: Collateral pathways observed by radionuclide superior cavography in 70 patients with superior vena caval obstruction. Clin Nucl Med 16:333, 1991. |
ETIOLOGY
Obstruction of blood flow through the SVC can occur from extrinsic compression, thrombus formation, tumor invasion, impedance of venous return from intraluminal pathology, or possibly, a combination of the above. This simple mechanistic model allows for convenient categorization of the numerous potential etiologies of SVCS. Although it is unclear which mechanism predominates, Roswitt and associates (1953) have suggested that even when extrinsic compression is highly suspected, thrombotic events may be present in up to one half of patients. Compromised flow through a low-velocity system will predispose to thrombus formation. Extrinsic compression by tumor, coupled with the use of an intraluminal device, decreases the cross-sectional area for fluid flow and therefore decrease the fluid velocity, as described by Laplace's law. The situation is compounded by the presence of a tumor-associated hypercoagulable state, thrombocytosis, treatment-related dehydration, weight loss, and reduced cardiac output. A rare etiology of thrombotic occlusion is from tumor thrombi, as described by Kumar and Good (1989).
As mentioned earlier, before the mid-20th century, the dominant etiologies of SVCS were from benign sources such as syphilitic aortic aneurysms, chronic fibrous mediastinitis from tuberculosis or syphilis, and phlebitis with thrombus formation. Only one third of the cases in McIntire and Sykes' (1948) series were from primary thoracic tumors, and metastatic disease accounted for only 3%. By 1954, the spectrum had noticeably changed when Schechter (1954) reported that 40% of 274 cases of SVCS were caused by syphilitic aneurysms or tuberculous mediastinitis and that 53% had a malignant etiology. In 1962, Effler and Groves, reporting on a series of 64 patients from the Cleveland Clinic who had developed SVCS since 1950, noted that 16 patients had a benign etiology, including mediastinal granuloma in 7 patients, multinodular goiter in 2 patients, fibrosing mediastinitis in 1 patient, and idiopathic causes in 6 patients. Among the malignant etiologies, lung cancer constituted the most common diagnosis.
Banker and Maddison (1967) reported a literature review of 438 cases from 16 reports published between the years 1951 and 1966. Lung cancer was the most common etiology (65%), followed by mediastinitis (9%), mediastinal tumors (7%), Hodgkin's disease or lymphoma (4%), metastatic lesions (4%), and syphilitic aortic aneurysm (4%). In all, 80% of their cases were from malignant cause. Kamiya and associates (1967) reviewed the Japanese experience in 734 patients from 1949 to 1965 and found that almost three fourths of their patients had an intrathoracic malignancy. By 1979, the proportion of SVCS cases caused by malignancies had escalated to 97%, as reported by Lochridge and colleagues. This changing spectrum is illustrated in Figure 170-4. Although malignant etiologies currently far outnumber the benign causes, Schindler and Vogelzang (1999) predict a resurgence in the percentage of cases secondary to benign etiologies due to the increased use of central venous cannulation.
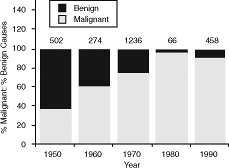 |
Fig. 170-4. Decline in benign causes of superior vena cava syndrome since 1950. The graph is a composite depiction of the declining proportionate contribution of benign diseases as a cause of superior vena cava syndrome. The data are extracted from McIntire and Sykes (1948), Schechter (1954), Effler and Groves (1962), Banker and Maddison (1967), Fincher (1987), and Kamiya (1967), Lochridge (1979), Schraufnagel (1981), Parish (1981), Bell (1986), Chen (1990), and Yellin (1990) and their colleagues and represent the proportions for the preceding decade. The gray bars represent the percentage contribution from malignant causes, and the solid bars represent benign causes. The numbers above the bars represent total patient numbers used to calculate the breakdown. |
Malignant Causes of Superior Vena Cava Syndrome
Thoracic malignancies account for the overwhelming majority of SVCS cases encountered in routine practice. Malignant encroachment on the SVC by a rapidly proliferating tumor is often the primary etiologic factor. In descending order, the most common malignancies responsible for SVCS are small cell lung cancer (SCLC), squamous cell lung cancer, adenocarcinoma, large cell lung cancer, lymphoma, metastases, germ cell tumors, and thymic neoplasms. Lung cancer has been reported by Nieto and Doty (1986) to account for between 67% and 82% of cases of SVCS, lymphomas for 5% to 15%, and metastases for 3% to 20%. In a composite analysis of 267 total cases of SVCS reported by Bell (1986), Chen (1990), Yellin (1990), and their associates, the proportionate distribution was as follows:
P.2550
lung cancer, 69%; miscellaneous, including benign, 16%; lymphoma, 7%; metastases, 4%; germ cell tumors, 2%; and thymic neoplasms, 2% (Fig. 170-5).
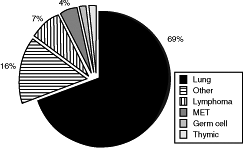 |
Fig. 170-5. Distribution of various malignancies causing superior vena cava syndrome. Data from Bell DR, Woods RL, Levi JA: Superior vena caval obstruction: a 10-year experience. Med J Aust 145:566, 1986; Chen JC, Bongard F, Klein SR: A contemporary perspective on superior vena cava syndrome. Am J Surg 160:207, 1990; and Yellin A, et al: Superior vena cava syndrome. The myth the facts. Am Rev Respir Dis 141:1114, 1990. |
Lung Cancer
As early as 1953, Roswitt and colleagues recognized that the rapidly increasing and significant numbers of patients with inoperable bronchial carcinoma implied that this disease would become the leading cause of SVCS. In a retrospective review of 4,100 lung cancer cases treated at the Mallinckrodt Institute of Radiology between 1965 and 1984, Armstrong and associates (1987) identified 99 patients, or 2.4% of all lung cancer cases, presenting with SVCS. Salsali and Clifton (1969) noted the occurrence of SVCS in 4.2% of 4,960 patients. Overall, 3% to 5% of all lung cancer patients develop SVCS, and 80% of the time the causative mass is located in the right lung.
In a comprehensive literature review, Ahmann (1984) evaluated a total of 1,986 SVCS patients reported in the literature up through 1984. The histologic breakdown from the 1,086 patients who had lung cancer reveals that SCLC was the most frequent histologic diagnosis, accounting for 32% of all cases. Squamous cell carcinoma, undifferentiated carcinoma, adenocarcinoma, and large cell carcinoma accounted for 15%, 8%, 5%, and 4%, respectively. Ahmann further analyzed a subset of 720 cases in which the 1967 World Health Organization (WHO) recommendations for subdividing lung cancer into the four broad categories (epidermoid carcinoma, adenocarcinoma, large cell carcinoma, and small cell carcinoma) were followed. The percentage of cases caused by SCLC in this second analysis increased to 40%. Squamous cell, adenocarcinoma, and large cell accounted for 18%, 9%, and 7%, respectively. Other histologic variants (bronchoalveolar, undifferentiated, anaplastic, squamous, and anaplastic) as well as unproven diagnosis made up the remaining 26%. Considering the fact that 46 unproven cases and 60 anaplastic histologies were in the denominator, small cell carcinoma probably accounted for a number of these cases, and therefore, represents more than 40% of the cases.
Adding to the above analysis, 984 patients with SVCS resulting from lung cancer reported in 21 recent series, the percentage contribution from the various histologies is 39% small cell, 20% squamous, 11% adenocarcinoma, and 8% large cell. SCLC has been responsible historically for 25% of all lung cancer. But more recently, the incidence of small cell carcinoma has decreased and currently is responsible for 20% of the cases as noted by Elias (1997). The inordinate contribution to the development of SVCS can be explained on the basis of the natural history of small cell lung cancer. It is typically a central lesion located in the bronchial submucosa with a high incidence of bulky, hilar, and mediastinal nodal involvement. Because it is located centrally, bronchoscopy is diagnostic in 90% of the cases. Small cell carcinoma is a fast growing histology, having a shorter tumor doubling time than other types of lung cancer, and therefore patients can present more acutely than with non small cell histologies. Whereas SVCS develops approximately 3% of the time with non small cell histologies, Sculier (1986) and Urban (1993) and their associates, as well as Dombernowsky and Hansen (1978), have recorded that SVCS occurs in approximately 10% to 12% of patients with SCLC.
Anterior Mediastinal Masses
The anterior mediastinum houses the thymus gland, great vessels, and lymph nodes. The differential diagnosis for an anterior mediastinal mass in an adult includes thymoma, lymphoma, germ cell tumor, and thyroid carcinoma. Mullen and Richardson (1986) reported the relative frequency of primary anterior mediastinal tumors in 702 adults. In their series, 47% of anterior mediastinal masses were from thymus tumors. Lymphoma, endocrine (thyroid and parathyroid), and germ cell tumors made up 23%, 16%, and 15%, respectively. The most common types of mediastinal masses associated with SVCS are discussed as follows.
Lymphoma
According to Perez-Soler (1984), Bell (1986), Armstrong (1987), Chen (1990), and Yellin (1990) and their co-workers, non Hodgkin's lymphoma (NHL) usually accounts for about 7% to 13% of all causes of SVCS, and as a group constitute the second most common etiologic factor in the development of SVCS. Analogous to the rationale for the inordinate contribution of SCLC to the causation of SVCS, NHL is also rapidly proliferating and frequently
P.2551
produces bulky mediastinal adenopathy leading to a high incidence of SVCS. Conversely, although Hodgkin's disease can produce large mediastinal masses, the relatively slow rate of growth prevents a high rate of SVCS. Perez-Soler and associates (1984) identified 36 cases of SVCS among 915 patients with NHL treated at M. D. Anderson, representing 4% of the NHL cases. The two most common histologies were diffuse large cell lymphoma (64%) and lymphoblastic lymphoma (33%). Lymphoblastic lymphoma, like SCLC, has a high likelihood for the development of SVCS, and overall, 21% of the lymphoblastic lymphoma patients in Perez-Soler's (1984) series had SVCS.
Miller and colleagues (1981) have reported a significant association between the extent of sclerosis in a mediastinal lymphoma and the development of SVCS. All of Miller's diffuse histiocytic lymphoma patients with sclerosis who had disease confined to the mediastinum developed SVCS and were relatively resistant to radiation therapy and chemotherapy. Lazzarino and associates (1993) from Italy confirmed the association of primary mediastinal B-cell lymphoma (MBL) with sclerosis with the high incidence of SVCS. In this series of 30 patients, MBL was noted to be a rapidly enlarging, bulky, mediastinal mass associated with SVCS in 57% of the cases. Further, an additional 23% of patients showed subclinical vena cava compression on CT scan. Lazzarino and co-workers confirmed a high incidence of SVCS (47%) in an update in 1997 of 106 patients with primary MBL.
Germ Cell Neoplasms
Primary mediastinal malignant germ cell tumors (GCTs) are an extremely rare entity, and yet these neoplasms account for almost 2% of malignant causes of SVCS as noted in the aforementioned series. The explanation resides in the rapid growth rate of these tumors and their predilection for the anterior mediastinum. All primary mediastinal GCTs have a short tumor doubling time except for the mature teratoma. Symptoms associated with mediastinal GCT are chest pain, dyspnea, cough, and SVCS. Holbert and Libshitz (1986) have observed that almost 20% of patients with primary mediastinal malignant GCTs develop SVCS.
Thymic Neoplasms
Thymoma is the most common tumor of the anterior mediastinum. A malignant thymoma is one that is invasive beyond the capsule of the normal thymus, and the malignant descriptor is not related to its cytologic appearance. Malignant behavior occurs in about one third of thymomas, but invasion of the great vessels is rare. A thymoma's slow growth rate and lack of invasiveness are responsible for its lower rate of inducing SVCS. When it does occur, SVCS is more from extrinsic compression than from vascular invasion. Rarely, direct invasion into the right atrium, as reported by Fujio and co-workers (1985), leads to the development of SVCS. Airan and colleagues (1990) have reported a case of intracardiac infiltration by a malignant thymoma, leading to SVCS. Pollack and associates (1992) reported 36 patients treated at M. D. Anderson for thymoma between 1962 and 1987. Only 3 of 36 (8%) patients developed SVCS from thymoma.
Metastasis
Metastatic disease to the lungs or to the mediastinal lymph nodes accounts for 4% of SVCS caused by malignancies. Metastatic breast cancer is the most frequent metastatic etiology, possibly related to metastases to the internal mammary chain of nodes. With increasing survival of patients with metastatic disease, this cause is likely to also increase in frequency and must always be borne in mind when addressing the differential diagnoses for SVCS.
Uncommon Malignant Causes of Superior Vena Cava Syndrome
A number of case reports (see Reading References) have documented the large variety of tumors that have been known to cause SVCS, most reporting tumors of hematogenous origin such as leukemic infiltrates, granulocytic sarcomas, and plasmacytomas. These various uncommon malignancies are listed in Table 170-2.
Cases of Superior Vena Cava Syndrome in Children
In the pediatric population, SVCS is extremely rare. In a review of the world literature, published by Issa and colleagues (1983), a total of 175 pediatric cases of SVCS were
P.2552
identified, most associated with benign iatrogenic causes such as caval catheters, ventriculoatrial shunts, and cardiovascular surgery, but lymphoproliferative diseases also contributed to the development of the syndrome. Small infants are especially susceptible to catheter-associated vascular thrombosis. Moore and associates (1985) reported that in infants weighing less than 10 kg, thrombotic complications are noted 15% to 20% of the time, compared with only 4% in those weighing more than 10 kg. Grisoni and associates (1986) noted that premature and low-birth-weight infants were at highest risk, with an overall 21% thrombosis rate. Catheter-induced sepsis and SVCS were seen in 37% and 5%, respectively, of low-birth-weight infants (<1,500 g) as reported by Pandit and colleagues (1999). Ladefoged and co-workers (1981) noted more catheter-induced thrombotic events with polyvinyl chloride catheters (1.6 events per catheter-year) than with Silastic ones (0.5 events per catheter-year). Effman and co-workers (1978) reported a higher incidence of catheter-induced thrombosis if the central venous catheter was placed outside of the SVC (15%) versus within the SVC (0%). Williams and colleagues (1998) have reported that central lines may result in late vascular occlusion in children with tumors. In their study, they evaluated children with cancer who had a central line placed and removed previously. Spiral thoracic CT scans with three-dimensional angiographic reconstructions in 25 children revealed an occlusion rate of 12%. In this study, the median time from removal of the central line was 7.4 months, suggesting that post central line thrombotic events are a possible cause of SVC occlusion in children with cancer.
Table 170-2. Uncommon Malignancies Producing Superior Vena Cava Syndrome | ||
|---|---|---|
|
In adolescence, mediastinal tumors are the main cause of SVCS. In a study of 607 children by D'Angio and colleagues (1965), only nine (1.5%) cases of SVCS were reported, all in boys. Anterior mediastinal tumor types in 179 children were reported by Mullen and Richardson (1986). Lymphomas were by far the most common, representing 45% of the histologies, followed by germ cell tumors (24%), thymus tumors (17%), and mesenchymal tumors (15%). Yellin and co-workers (1992) recorded that lymphomas are the most common malignant cause of SVCS in the pediatric population and, as in adults, non-Hodgkin's lymphoma, particularly the diffuse large cell and the lymphoblastic subtypes, predominate.
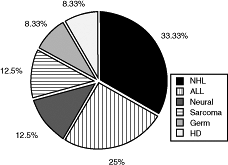 |
Fig. 170-6. Malignancies causing superior vena cava syndrome in children. ALL, acute lymphoblastic leukemia; HD, Hodgkin's disease; NHL, non Hodgkin's lymphoma. Data from Ingram L, Rivera GK, Shapiro DN: Superior vena cava syndrome associated with childhood malignancy: analysis of 24 cases. Med Pediatr Oncol 18:476, 1990. |
Neurogenic tumors, such as neuroblastoma and ganglioneuroma, thymoma, sarcoma, and teratoma, also have been reported to cause SVCS. Ingram and associates (1990) have analyzed the proportion of various mediastinal masses that lead to SVCS in a cohort reported from St. Jude's Childrens' Hospital (Fig. 170-6). Malignant GCTs, with a 20% rate, have the highest proportionate incidence of SVCS. Of a total of 114 children with germ cell neoplasms, 10 presented with mediastinal masses of whom two (20%) developed SVCS.
Nonmalignant Causes of Superior Vena Cava Syndrome
Nonmalignant etiologies of SVCS, although significantly less common than in the first half of the decade, continue to remain an important causative factor for SVCS. An analysis of the series published by Bell (1986), Chen (1990), and Yellin (1990) and their associates suggests that benign causes may be responsible for about 12% of SVCS
P.2553
cases. Although an extremely large number of conditions fall in this category, the most common include mediastinal fibrosis and central venous devices like dialysis catheters, pacer wires, and central venous lines (see Reading References). The frequency of pacemaker-induced SVCS varies but is quite small, being between 1 in 1,000 and 1 in 40,000, as reviewed by Musumeci and co-workers (2000). The latter group is becoming increasingly common because of the widespread use of these devices and is a particularly common cause in the pediatric population, as noted by Moore and colleagues (1985). Mahajan and associates (1975) reviewed the benign causes of SVCS in a series of 16 patients and found that mediastinal fibrosis from histoplasmosis was the most frequent cause, accounting for 12 of 16 cases. Other causes included retrosternal goiter, aortic aneurysm, and congestive heart failure. A tabulation of several nonmalignant causes of SVCS is presented in Table 170-3.
Table 170-3. Nonmalignant Causes of Superior Vena Cava Syndrome | ||
|---|---|---|
|
CLINICAL FEATURES
Is This a Medical Emergency?
As mentioned in this chapter's introduction, SVCS had long been considered a potentially life-threatening medical emergency. This belief was based on the assumption that sudden, dramatic occlusion of the SVC and the azygos system would result in the so-called wet brain syndrome of Effler and Groves (1962), the development of cerebral edema secondary to venous obstruction. Roswitt and associates (1953) have eloquently described this as follows:
The superior vena cava obstruction syndrome in bronchogenic cancer is a grave symptom-complex which grows in severity as the venous pressure mounts in the great vein and its tributaries. The patient experiences progressive dyspnea, cough and orthopnea, aggravated greatly in the prone position. He is soon able to breath only in the erect posture and dares not lie down. There is progressive edema of the head, neck and upper extremities and a peculiar reddish cyanosis of the skin which grows more intensely on recumbency. As the cerebral venopressure rises, the patient suffers headaches, vertigo, drowsiness, stupor and unconsciousness. Unless decompression therapy with X-ray irradiation and/or nitrogen mustard is effective, death comes finally as a result of cerebral anoxemia, failure of the respiratory center or strangulation/edema of the glottis and respiratory passages.
Although this description is colorful and graphic, it is probably far from the truth in most cases. In publications by Schraufnagel and associates (1981) and Ahmann (1984), this picture of SVCS has been challenged. The latter author, in a review of almost 2,000 cases, was able to find only one patient in whom death was directly attributable to SVC obstruction. Many examples of long-term survival with unrelieved SVCS were reported by him. The widely described central nervous system symptoms are, in most instances, secondary to brain metastases, according to Yellin and colleagues (1990). Thomas and co-workers (1991) have reviewed the possibility of sudden death in SVCS resulting from massive angioinvasion by tumor. To date, only eight cases have been well documented, all as a consequence of anaplastic or follicular carcinoma of the thyroid. Other than these exceptional situations, no reasonable grounds exist to believe that unrelieved SVCS is fatal in and of itself.
Further, diagnostic procedures to establish a histologic diagnosis are paramount to successful therapy. Temporizing, emergency mediastinal radiation therapy is not only unwarranted in most cases as just described but could also preclude proper histologic interpretation. Loeffler and associates (1986) reported that 8 of 19 (42%) patients were not able to have a histologic diagnosis established at the time of biopsy. Seven of eight patients had to receive empiric therapy for what was thought to be the most likely diagnosis on clinical grounds.
Clinical Presentation
The clinical identification of a patient with SVCS is routinely simple because the symptoms and signs are typical and unmistakable. A review of 426 patients in publications by Schraufnagel (1981), Parish (1981), Chen (1990), Yellin (1990), and Armstrong (1987) and their associates shows that the most common symptoms in descending order are dyspnea (54%), suffusion (54%), cough (29%), and arm or facial swelling (23%). Less common symptoms include chest pain, dysphagia, syncope, obtundation, hemoptysis, and headache. Particularly notable is the extremely low rate of obtundation (1.6%), once again belying the noxious reputation of SVCS. Assuming a recumbent position, bending over, or coughing exacerbate symptoms from a transient elevation in venous pressure.
In a similar analysis of presenting signs in a series of 256 patients from the studies of Parish (1981), Chen (1990), and Armstrong (1987) and their colleagues, the most common signs in descending order were facial and extremity edema (66%), engorged neck (60%) and chest (58%) veins, cyanosis (21%), and plethora (17%). Hirschmann and Raugi (1992) noted that among the earliest and most prominent signs of SVCS are numerous, dilatated, vertically oriented, tortuous cutaneous veins above the inferior rib cage margin. By occluding a venule with two fingers, stripping the blood along the vessel, and then releasing one of the fingers, one can determine the direction of blood flow. For veins above the umbilicus, instead of the normal cephalad course, the drainage is commonly caudad. Less common signs include Horner's syndrome, vocal cord paralysis, and abnormal cardiac murmurs. Rare presentations described in the literature range from an unusual case, published by Warren and co-workers (1990), of bilateral chylothoraces associated with SVCS to the development of acute macular neuroretinopathy as a consequence of hypoperfusion from SVCS recorded by Leys and associates (1991). In an analysis of 724 biopsy-proven cases of SCLC,
P.2554
Urban and associates (1993) found 87 cases of SVCS. Patients presenting with SVCS from SCLC had a considerably higher risk for synchronous brain metastases (22% vs. 11%) but the same median survival (42 vs. 40 weeks) as those without brain metastases at diagnosis. Such findings may, in part, explain the neurologic symptoms that in the past have been ascribed to the cerebral edema from SVCS.
The aforementioned studies reveal a clear preponderance of SVCS in men, with a male-to-female ratio of 1.4:1. The mean age was 53.6 years. In most patients, the syndrome is insidious, with slow development of symptoms. A short interval to presentation is highly correlated with either an underlying malignancy or catheter-induced thrombotic occlusion, whereas nonmalignant etiologies other than catheters are associated with long-standing symptoms. In this group of patients, the median time from onset of first symptom to actual presentation ranged from 3.2 to 6.5 weeks for patients with malignant disease, and from 60 to 168 weeks for patients with nonmalignant conditions. This clearly underscores the fact that occlusion of the SVC is a relatively slow process, allowing sufficient time for development of collateral circulation.
Clinical Features in the Pediatric Population
Issa and associates (1983) emphasized that unlike the adult with SVCS, the child with SVCS truly constitutes a medical emergency because in the child, the SVC is tightly locked in a tiny thoracic compartment in close proximity to the tracheobronchial tree, and airway compromise is a frequent accompaniment when an underlying malignancy is suspected. D'Angio and co-workers (1965) have described the superior mediastinal syndrome as opposed to SVCS wherein complete venous blockade or total obstruction or the airway can develop with dramatic suddenness. In addition, Neuman and colleagues (1984) point out that distinct, potentially life-threatening risks exist from anesthesia in these children because tracheal compression is observed in more than one half of the cases. Therefore, whereas judicious minimally invasive procedures are justifiable, more aggressive and invasive procedures should be undertaken only in a major tertiary care center with expertise in this special situation. In a report by Arya and co-workers (2002), all 21 children between 5 and 12 years old with SVCS had a tissue diagnosis obtained. Respiratory distress was a presenting sign in 19 of 21 children with a median duration of 14 days. Dysphagia was present in 7 of 21 patients. Almost all children were found to have lymphoproliferative disorders (lymphoblastic lymphoma in 7, lymphoblastic leukemia in 12, Hodgkin's lymphoma in 1, and Langerhans' cell histiocytosis in 1). Bone marrow biopsy, CT-guided mediastinal biopsy, and lymph node biopsy established the diagnosis in all children. There were no complications during diagnostic procedures performed under local anesthesia; however, 2 of 3 children consciously sedated with ketamine for bone marrow examination had respiratory arrest, resulting in one death. In both cases, CT documented tracheal compression and pericardial effusion. Ingram and co-workers (1990) suggest that in the clinically serious case, the overzealous desire to establish a histologic diagnosis should be superseded by an empiric pretherapy debulking option using radiation therapy.
DIAGNOSTIC WORKUP
Historically, the following statement by Goodman (1975) describes the sentiment with respect to the diagnostic workup of SVCS patients: The pitfalls in the management of SVCS relate to overzealous efforts to establish the site of obstruction and to determine a specific histopathologic diagnosis. The authors go on to state, These efforts may lead to life-threatening complications, such as respiratory obstruction, aspiration, and hemorrhage. This judgment reflects three assumptions: (a) diagnostic studies, particularly invasive ones, are associated with undue risk of life-threatening complications; (b) SVCS is a medical emergency, and an extensive diagnostic workup delays emergent therapy, leading to a poorer outcome; and (c) the most common etiologies of SVCS are malignant neoplasms, predominantly those expected to respond rapidly and dramatically to a course of radiation therapy, and therefore the exact histopathologic definition is moot in most cases.
Wudel and Nesbitt (2001), Brigden (2001), Kumar and Good (1989), Ahmann (1984), Schraufnagel (1981), Yellin (1990), and their associates have challenged this traditional view on several grounds. Histologic diagnosis is essential for the institution of appropriate management, and Ahmann (1984) has emphasized the need for a thorough but expeditious diagnostic workup based on his following conclusions:
Diagnostic studies, even invasive ones, are not associated with undue morbidity. In an analysis of 843 diagnostic procedures in patients with SVCS, including 217 bronchoscopies, 197 contrast venographies, 120 lymph node biopsies, 119 thoracotomies, 96 nuclear venographies, 53 mediastinoscopies, and 41 other miscellaneous studies, he found only 10 reported complications (10 of 843; 1.2%).
SVCS may be an acute condition in some patients, but there is little reported clinical or experimental data to support the concept that an unrelieved obstruction of the SVC is life threatening except in the rare circumstance when the superior mediastinal mass also results in tracheal obstruction.
The spectrum of etiologic factors that can lead to SVCS is so varied that a policy of instituting emergent radiation therapy without a diagnosis may result in some patients with benign diseases receiving unnecessary irradiation and some with malignant tumors, such as small cell lung cancer or lymphoma, receiving radiation therapy alone who would be better treated by modern chemotherapy, or possibly combined-modality chemoradiation.
P.2555
A variety of diagnostic tests are available for the assessment of SVCS, and the actual workup has to be individualized to the particular clinical situation. Chen and co-workers (1990) have proposed an algorithm for evaluation of SVCS, starting with a chest CT and working up toward more invasive procedures. Minimally invasive studies such as CT, magnetic resonance (MR) imaging, ultrasonography, radionuclide scintigraphy, and contrast venography are helpful in determining the location and extent of obstruction. Tests for establishing a histologic diagnosis include sputum cytology, bronchoscopy, thoracentesis, supraclavicular lymph node biopsy, and bone marrow biopsy. CT-guided needle biopsy and mediastinoscopy, a procedure that should be considered in many cases of SVCS, or a cervical substernal extended mediastinoscopy (see Chapter 161) may be a definitive diagnostic procedure. Video assisted thoracoscopy surgery (VATS) or thoracotomy can be performed if all other procedures fail to establishing a histologic diagnosis.
Chest Radiography
Chest radiography is often one of the earliest studies obtained, and although not diagnostic of SVCS, it often establishes the presence of a mediastinal mass as noted by a widened superior mediastinum. Parish and colleagues (1981) noted normal chest radiographs in only 16% of SVCS patients reviewed from the Mayo Clinic. Right-sided lesions, as reported by Roswitt and co-workers (1953), typically outnumber those on the left by a ratio of more than 4:1. The presence of an aortic nipple (i.e., a dilatated left superior intercostal vein that produces a shadow overlying the aorta) has been reported by Carter and associates (1985) to be predictive of SVCS. Other findings include pleural effusion, hilar mass, pulmonary infiltrates, cardiomegaly, and nodes that enlarged, calcified, or both.
Computed Tomography
Chest CT is probably the most frequently used imaging technique for SVCS; it provides a detailed radiographic analysis of the SVC, its tributaries, and other critical anatomic structures. Chen and associates (1990) have reported a diagnostic accuracy of 100% for chest CT. CT is particularly useful in evaluating extrinsic compression and also provides detail regarding collateral circulation. Schwartz and colleagues (1986) used CT scanning with contrast in 18 patients with SVCS and found only one false-negative result. Engel and associates (1983) have stated that a CT diagnosis of SVCS may be made based on the presence of both the so-called direct sign (nonopacification of central venous structures such as the SVC or brachiocephalic vein) and the indirect sign (opacification of collateral venous channels). In an analysis of 36 patients with SVCS, Trigaux and Van Beers (1990) reported that opacification of the subcutaneous anterior channel was highly diagnostic and specific (96%) for SVCS.
Bechtold and colleagues (1985) have suggested that CT also may be able to predict the subsequent evolution of the syndrome in asymptomatic patients. Raptopoulos (1986) has classified SVC obstruction into five distinct grades that roughly correspond to the severity of clinical findings: grade 0 SVC narrowing without clinical evidence of SVCS; grade I moderate SVC narrowing without collaterals; grade II severe SVC narrowing with the azygos vein serving as partial collateral; grade III SVC obstruction above the azygos arch; and grade IV SVC obstruction at or below the level of the azygos arch.
Moncada and associates (1984) also have suggested that the addition of CT phlebography to standard CT provides accurate delineation of the location and extent of SVC obstruction. In addition, this procedure may allow for better definition of radiation therapy portals, permit accurate localization for biopsy, and document collateral circulation. The main limitation of CT is the necessity for intravenous contrast with the possibility of attendant contrast reactions, which can theoretically be of major consequence in a patient with compromised cardiorespiratory status and the possibility of adverse effects on renal function.
Magnetic Resonance Imaging
Magnetic resonance (MR) imaging provides multiplanar anatomic detail that allows for easy visualization of the extrinsic mass in transverse, sagittal, and coronal planes. Medera and co-workers (1988) have stated that coronal MR imaging offers the earliest indication of an impending obstruction because it is exquisitely sensitive in detecting the aortic nipple. Flowing blood generates a signal void and therefore appears black on T1-weighted images, providing a natural contrast to examine vascular structures such as the SVC. However, Loehr and associates (1993) and others have pointed out that MR imaging suffers from several disadvantages, including cost, increased scanning time, lack of availability in some locations, lack of adequate pulmonary visualization, inability to resolve calcific patterns, significantly greater artifacts from clips, degradation by respiratory and cardiac motion, and the lack of general availability of MR-compatible patient equipment. In a comparison of three cross-sectional imaging modalities, CT, MR, and ultrasound, Khimji and Zeiss (1992) have suggested that MR imaging has potential and apparent advantages in the evaluation of a patient with SVCS.
Sonography
Sonographic techniques for the evaluation of SVCS, although not frequently used, have been described and, in the pregnant patient, may represent a safe alternative to potentially
P.2556
teratogenic imaging. Ultrasonography is useful in evaluating the patency of the jugular, subclavian, and axillary veins. The normal subclavian vein shows rhythmic motion with respiration, collapsing with inspiration. In SVCS, Gooding and associates (1986) have shown that this transient collapse is not evident. Unfortunately, evaluation of intrathoracic veins is not accomplished with standard external transthoracic ultrasound. Imaging of the SVC and its surrounding organs, particularly in the acutely ill patient, can be accomplished by transesophageal echocardiography (TEE). Transesophageal echocardiography allows visualization of the SVC at several levels up to the right atrium, thereby permitting evaluation of the location of the obstruction, and is therefore far more accurate than transthoracic echocardiography. In all three patients described by Ayala and colleagues (1992), transthoracic echocardiography failed to visualize the SVC adequately, but transesophageal echocardiography was able to provide accurate diagnosis.
Contrast Venography
Contrast venacavography (phlebography, angiocardiography) is an essential procedure when surgical intervention is contemplated and is a fundamental aspect of evaluation before endovascular stent placement. It found application in the evaluation of SVCS as early as the 1940s by Neuhoff and associates (1949) and allowed for the first true assessment of the site and nature of the obstruction as well as the pattern of collateral development. When combined with pressure measurements, it permits additional physiologic assessment and has been described by Roswitt and colleagues (1953) as affording unequaled precision for localization of venous obstruction in bronchial carcinoma and for determining the extent and efficiency of the collateral circulation. Dyet and Moghisi (1980) as well as Stanford and colleagues (1987) have demonstrated that venography is capable of providing accurate information regarding vessel patency, the degree of thrombus, extent of collateralization, and the level of obstruction, information particularly useful if a surgical procedure is being contemplated. These studies have clearly documented that obstruction of the SVC at the junction of the azygos is the most common presentation, occurring 72% of the time.
Goodman (1975) has suggested that venography should be avoided because interruption of the integrity of the vessel wall with the considerably elevated vascular pressure could result in excessive hemorrhage. In the Mayo study reported by Parish and co-workers (1981), superior venacavography was not found to be a useful procedure in making the diagnosis, and in their experience, the elevated venous pressure was believed to make the procedure hazardous. In his literature review, however, Ahmann (1984) was able to document only one case of relatively minor and transient toxicity resulting from contrast venography in 197 cases (0.5%), thus challenging the notion that venography is an excessively morbid procedure in the setting of SVCS. From a practical standpoint, Dyet and Moghisi (1980) suggest that venography may find application in only the small number of patients who are being considered for surgery.
Radionuclide Venography
Radionuclide venography (RNV), also referred to as scintigraphy, is a minimally invasive alternative to contrast venography and is performed using technetium 99m (99mTc) DPTA. Although this technique, as shown by Gollub (1980) and Muramatsu (1991) and their associates, provides information analogous to contrast venography (site of obstruction, vessel patency, routes of collateral flow), it does not allow adequate assessment of vessel caliber and potential graft sites, information that is crucial if surgery is contemplated. In view of this, scintigraphy cannot be considered a routine diagnostic study for the evaluation of SVCS. In an analysis of 220 RNV studies of the upper extremity in patients with indwelling central venous catheters, Podoloff and Kim (1992) found obstruction in 123 patients, 26 of whom also underwent contrast venography. Significant agreement was noted in 19 cases (73%), but there were seven false-positive cases (27%) with RNV. Further analysis of these cases revealed that, if the RNV diagnosis is based solely on the slow-flow pattern, a high likelihood exists of false-positive results. In a report on 20 patients with SVCS secondary to lung cancer, Maxfield and Meckstroth (1969) reported that RNV provided prognostic information for radiation therapy. The safety of RNV has been documented by the complete lack of morbid events in 96 studies documented by Ahmann (1984).
Establishment of a Histologic Diagnosis
Once considered almost dangerous by Goodman (1975), modern studies by Ahmann (1984), Kumar and Good (1989), and Fincher (1987), as well as Schraufnagel (1981), Parish (1981), Bell (1986), Chen (1990), Yellin (1990) and their associates all call for a thorough effort at establishing a tissue diagnosis. Commonly used procedures include sputum cytology, bronchoscopy, supraclavicular lymph node biopsy, thoracentesis, mediastinoscopy, bone marrow biopsy, and thoracotomy. Sputum cytology can establish the diagnosis in two thirds of patients, as seen in the study by Armstrong and colleagues (1987). Cytologic diagnosis of small cell carcinoma is as accurate as tissue diagnosis. Bronchoscopy was the most frequently used procedure in Armstrong and colleagues' (1987) study, resulting in a histologic diagnosis 60% of the time. In the presence of a pleural effusion, thoracentesis yields a diagnosis of malignancy in 71% of cases. Biopsy of a palpable supraclavicular lymph node also has a high yield, with positive findings of malignancy in 87% of the cases. SCLC and NHL have a relatively
P.2557
high incidence of bone marrow involvement, and bone marrow biopsy should be considered in select cases. The routine use of bone marrow biopsy in small cell lung cancer patients who have normal bone scans and lactate dehydrogenase (LDH) values has fallen out of favor. Adjei and associates (1999) reported a less than 5% incidence of positive bone marrow biopsies in small cell lung cancer patients with normal bone scans and LDH. Bone marrow biopsy should be considered if one is suspicious of NHL or if LDH or bone scan is abnormal.
The safety of performing a mediastinoscopy in a SVCS patient has historically been questioned, and many clinicians considered SVCS as an absolute contraindication to the procedure. Painter and Karpf (1983) have reported five complications in nine (56%) patients when performing mediastinoscopy. Callejas and colleagues (1991) attempted diagnostic mediastinoscopy in eight patients with SVCS and described two (25%) complications, one wound infection and one carotid artery injury requiring median sternotomy. There was no mediastinoscopy-related mortality. These complication rates are higher than expected for routine mediastinoscopy. In contrast, in the series of 53 mediastinoscopies compiled by Ahmann (1984), the morbidity was much lower, at 3 in 53 (6%). Mineo and co-workers (1999) reported an excellent outcome with mediastinoscopy in 80 SVCS patients who were either undiagnosed by lesser techniques (51 patients), required biopsy after empiric radiation or chemotherapy failed (17 patients), or were seen on an urgent basis (12 patients). No perioperative mortality occurred, and a definitive diagnosis was made in all 80 patients. Five cases of significant bleeding were reported, but only one required sternotomy. Four cases of bleeding were documented to be from tear lesions of the minor venous vessels. Bleeding was controlled in these four patients by local compression, metallic clips, or oxidized regenerated cellulose. No airway obstruction or cardiovascular collapse after general anesthesia occurred. A thoracotomy is diagnostic if all other procedures have failed. According to Painter and Karpf (1983), thoracotomy as a procedure has the highest diagnostic yield at 98%, followed by mediastinoscopy with 77%, and thoracentesis with 73%. Other procedures have lower yields, as illustrated in Figure 170-7.
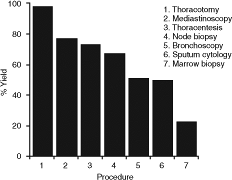 |
Fig. 170-7. The diagnostic yield of various procedures in superior vena cava syndrome. Data from Schraufnagel DE, et al: Superior vena caval obstruction. Is it a medical emergency? Am J Med 70:1169, 1981; Armstrong BA, et al: Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys 13:531, 1987; and Painter TD, Karpf M: Superior vena cava syndrome: diagnostic procedures. Am J Med Sci 285:2, 1983. |
CT-guided needle biopsy or ultrasound-guided needle aspiration biopsy suggested by Chen and colleagues (1992), percutaneous atherectomy reported by Dake and associates (1990), and thoracoscopic biopsy also have been used. To a lesser extent, tumor markers can lead to a diagnosis of mediastinal germ cell tumor. Significantly elevated serum -human chorionic gonadotropin, -fetoprotein, or both may constitute sufficient evidence for germ cell histology if a definitive tissue diagnosis is not feasible.
THERAPEUTIC CONSIDERATIONS
Goals of Therapy
The major therapeutic goals in the management of SVCS are palliation of symptoms in the incurable situation and aggressive management in the potentially curable situation. When considering the most common causes of SVCS, SCLC and non small cell lung cancer (NSCLC), and NHL, histologic diagnosis and staging are vital to determine whether palliation or cure is the goal. When the etiology is nonmalignant, relief of the SVC obstruction is the goal. In all patients, minimizing treatment-related morbidity is an important secondary goal.
Taking into consideration that about 80% of SVCS is caused by malignancy, the most commonly used therapeutic options include radiation therapy and chemotherapy, either alone or in combination. However, other measures such as surgical reconstruction, vascular stents, angioplasty, thrombolytic therapy, anticoagulation, corticosteroids, diuretics, and other medical and supportive measures are used in the management of SVCS. In all instances in which an underlying neoplasm is suspected, histology-directed therapy initiated as soon as possible is most likely to achieve reversal of symptomatology.
Radiation Therapy
Radiation therapy is perhaps the most frequently used definitive therapeutic modality in the management of SVCS. In a composite analysis of 381 patients with SVCS reported in five different studies (Fig. 170-8), radiation
P.2558
therapy was found to be the most frequently used therapeutic modality. As stand-alone therapy, it was used in 53% of cases, and overall, it was used in 68% of cases. The reason for the frequent application of radiation therapy is its efficacy in managing bronchial carcinoma, small and non small cell lymphoma, NHL, GCTs, thymic malignancies, and metastatic tumors. It is also relatively easy to apply even in very frail patients. The three major radiation therapy factors that have to be addressed in the therapeutic management of SVCS are the total dose, fractionation schema, and field size. Because of the paucity of well-controlled prospective randomized trials, these issues are often a matter of institutional practice. Ultimately, the stage and histology determine the dose, fractionation, field size, and use of concurrent chemotherapy.
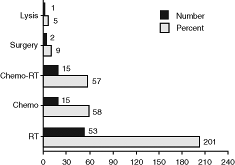 |
Fig. 170-8. Frequency of therapeutic options in superior vena cava syndrome. Chemo, chemotherapy; Chemo-RT, combined chemo-radiotherapy; Lysis, thrombolytic therapy; RT, radiotherapy. From Schraufnagel DE, et al: Superior vena caval obstruction. Is it a medical emergency? Am J Med 70:1169, 1981; Parish JM, et al: Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 36:407, 1981; Yellin A, et al: Superior vena cava syndrome. The myth the facts. Am Rev Respir Dis 141:1114, 1990; and Armstrong BA, et al: Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys 13:531, 1987. |
Fractionation
Whether the initial fractions of radiation should be larger than conventional fraction size is a matter of considerable debate. Critics of large fractions have cited the following as evidence: (a) lack of any prospective randomized trials supporting the use of large fractions; (b) potential for enhanced late tissue toxicity; (c) theoretic possibility of radiation-induced edema that could lead to clinical deterioration; (d) the fact that most tumors in this location are relatively radiosensitive and that conventional fraction sizes of 1.8 to 2 Gy are effective. Proponents, such as Davenport (1978) and Armstrong (1987) and their co-workers, as well as Goodman (1975), of large fraction radiation during the early phase have recommended using about three fractions of 3 to 4 Gy each followed by conventional fractions. In a series of animal experiments and clinical trials, Rubin (1963) and Scarantino (1979) and their colleagues have suggested that the use of initial large-fraction radiation is more efficacious in SVCS. In a few small studies in which large versus small fractions have been evaluated, large-fraction radiation appears to produce faster relief. For example, Armstrong and associates (1987) reported that improvement occurred in 70% of patients in less than 2 weeks in those who received initial high-dose fractions (300 to 400 cGy/d for 3 days), compared with 56% of those receiving conventional fraction radiation, although this difference was not statistically significant (p = 0.09). Fisherman and Bradfield (1973) and Scarantino and associates (1979) have reported on nonrandomized groups of patients treated with conventional versus high-dose fractions and have suggested faster symptomatic resolution in the latter group.
Dose and Use of Concurrent Chemotherapy
The total dose required is a function of the tumor histology and stage. The overall goal of therapy based on the above two factors may be definitive or palliative. Once again, a prospective randomized dose-seeking trial has not been performed, but Armstrong and associates (1987) have reported on their institutional experience at varying dose levels. A dose below 20 Gy resulted in a 50% response rate, whereas higher doses produced response rates of 86%, 93%, and 100% at dose levels of 20 to 40 Gy, 40 to 60Gy, and more than 60 Gy, respectively. When these data are plotted on a semilog graph and a polynomial curve is fitted, a strong suggestion exists for a dose-response relationship mimicking the classic sigmoid radiation-response curves. It would appear that there is indeed a steep dose-response curve from 20 to 40 Gy and a plateau effect thereafter. Based on such retrospective data, a minimum dose of 40 Gy would be considered necessary for SVCS. The criticism of this analysis is that all histologies are included.
Management of Specific Malignancies
Non Small Cell Lung Cancer.
For unresectable NSCLC, Perez and colleagues (1982) have reported that 60 Gy in conventional fractions of 2 Gy per day result in improved 3-year survival and intrathoracic control than 40 or 50 Gy. Radiation doses of 60 to 63 Gy in 1.8- to 2-Gy fractions, combined with chemotherapy, is more effective than radiation alone. Dillman and co-workers (1996) reported the results of a randomized trial in which induction chemotherapy (vinblastine and cisplatin) followed by radiation therapy (60 Gy at 2 Gy per fraction) improved median survival (9.6 vs. 13.7 months) and 5- to 7-year survival (6% vs.
P.2559
17%) over radiation alone (60 Gy at 2 Gy per fraction). Concurrent chemoradiation therapy is more efficacious than sequential treatment at the cost of increased acute radiation effects. Komaki and associates (2000) presented the initial results from a randomized trial comparing sequential chemoradiation (Dillman's regimen) and the same chemoradiation given concurrently. The concurrent regimen showed a statistically significant improved median survival (14.6 vs. 17 months) and 3-year survival rate (16% vs. 26%) favoring the concurrent regimen. Therefore, the standard of care for good performance status, unresectable, NSCLC patients is concurrent chemoradiation.
Small Cell Lung Cancer.
Similar to unresectable NSCLC, treatment of limited-stage SCLC has been the best results with combined chemotherapy and radiation therapy. Two meta-analyses, one by Pignon and associates (1992) and one by Warde and Payne (1992), have shown that limited-stage SCLC clearly benefits when thoracic irradiation is added to chemotherapy. Studies by Murray and colleagues (1993) and by Turrisi and Glover (1990) suggest that upfront irradiation, as opposed to delayed irradiation, is more efficacious. Further, accelerated, hyperfractionated radiation therapy has been proved in a phase III, randomized trial to improve survival and locoregional control in limited-stage, SCLC compared with conventional fractionation. In this latter instance, the total dose was 45 Gy, given as 1.5 Gy twice a day over 3 weeks concurrent with chemotherapy (cisplatin and etoposide) versus the same total dose given at 1.8 Gy once a day over 5 weeks. Turrisi and colleagues reported the results of this study in 1999 with the accelerated, hyperfractionated regimen improving 5-year survival significantly from 16% to 26%. One criticism of this study is that the conventional, once-a-day fractionation arm of the study only used the same total dose as the accelerated arm. But it is still only conjecture that higher doses of conventional fractionation can obtain the same results.
Non Hodgkin's Lymphoma.
For NHL patients presenting with SVCS, Perez-Soler and associates (1984) have shown excellent symptom relief with the use of chemotherapy, radiation therapy, or a combination of the two. All patients achieved complete relief of symptoms from SVCS within 2 weeks. The addition of thoracic irradiation decreased local relapse in the large cell lymphoma group compared with chemotherapy alone. In 22 patients with large cell lymphoma, 18 (81%) had a complete response to therapy, but relapse occurred in 68% of patients. In 8 patients with lymphoblastic lymphoma, the complete response rate was 100%. Relapse occurred distantly in 6 (75%) patients with lymphoblastic lymphoma. Median survival for large cell and lymphoblastic patients was 21 and 19 months, respectively.
A more modern review by Petersdorf and Wood (2000) does not recommend mediastinal radiation therapy for lymphoblastic lymphoma. They emphasize the use of modern anthracycline-based chemotherapy regimens for induction, followed by consolidation, central nervous system prophylaxis with intrathecal chemotherapy with or without cranial radiotherapy, and maintenance therapy. Reports of a 95% complete response rate and a 58% 3-year freedom-from-relapse rate with such aggressive regimens have been made. Petersdorf and Wood (2000) also made recommendations for the other main group of lymphomas causing SVCS: primary mediastinal large cell B-cell lymphoma. The optimal chemotherapy regimen has not been determined but none have been shown to be better than standard CHOP (cyclophosphamide, hydroxydaunomycin, Oncovin [vincristine], prednisone). Survival of these patients has improved over the past few decades (5-year survival rate, 38% to 59%), and involved-field radiation therapy has shown the ability to decrease mediastinal relapses in some reviews. A recent report on this entity by Van Besien and associates (2001) confirms improving survival with modern chemotherapy with or without involved-field irradiation. Progression-free survival rates range from 38% to 88%. It has been customary in many centers to consolidate patients with involved-field radiation therapy, 30 to 50 Gy, with the total dose dependent on amount of previous chemotherapy given and response to chemotherapy.
Field Size
The third crucial issue in radiation therapy for SVCS is field shaping. Obviously, every effort should be made to encompass the full extent of the tumor in every case. If palliation is the goal for an incurable cancer, it may be reasonable to target only the mass that is responsible for the SVC obstruction, especially when treatment of massive thoracic tumor may lead to unacceptable lung toxicity. Attention also should be directed to regional lymph nodes in specific situations. More important, cases of failure of radiation therapy have been described by Kumar and Good (1989) in which the reason for failure is a thrombus extending beyond the radiation portal, and when this is included in the field, prompt response is achieved.
With NHL, involved-field radiation typically means treatment of the regions involved by the NHL and possibly the adjoining lymph node station. For definitive treatment of limited-stage SCLC, radiation fields should encompass all gross tumor as well as the ipsilateral hilar, mediastinal, and supraclavicular lymph nodes with a 1- to 1.5-cm margin of normal tissue. For definitive treatment of NSCLC, the fields are similar to those for small cell, but typically the margins of normal tissue included are slightly larger (1.5 to 2 cm). Supraclavicular radiation is typically recommended for upper lobe lesions or bulky mediastinal lymphadenopathy, one of which is typically the cause of SVCS. Armstrong and co-workers (1987) showed a 33% (two of six) failure rate in the supraclavicular fossa if not
P.2560
treated, compared with a 9% (8 of 91) failure rate if it was treated in a population of NSCLC patients.
Chemotherapy
Roswitt and colleagues (1953) reported some of the earliest clinical experience with chemotherapy in the form of combined-modality therapy using radiation therapy and nitrogen mustard in patients with NSCLC with SVCS. However, Armstrong and associates (1987) have reported that in NSCLC with SVCS, the addition of chemotherapy does not produce a survival advantage. Since that time, the role of chemotherapy for NSCLC/SCLC and lymphoma has expanded with more active agents and combinations.
SCLC and NHL are generally systemic in nature and chemosensitive. With the use of chemotherapy only for SVCS caused by SCLC, Urban and colleagues (1993) have described a response rate of 81% in 87 patients. In a smaller series of 22 patients with SCLC causing SVCS, Dombernowsky and Hansen (1978) reported symptomatic resolution in all patients with a median response time of 7 days. In a nonrandomized series of 56 patients with SVCS resulting from SCLC, Maddox and co-workers (1983) noted reversal of SVCS in 64% with radiation, 83% with combined therapy, and 100% with chemotherapy.
Perez-Soler and associates (1984) have advocated the use of chemotherapy in SVCS caused by NHL. In the study from M. D. Anderson Hospital, 28 patients were treated with radiation therapy, chemotherapy, or a combination. All patients achieved an excellent response within 2 weeks of therapy, but no survival advantage was apparent from any modality. In the study from Washington University Mallinckrodt Institute reported by Armstrong and colleagues (1987), 11 lymphoma patients were treated with radiation therapy only and 6 with chemotherapy and radiation therapy. Actuarial survival at 5 years was 41%, and no difference by therapy was apparent.
Anticoagulation
Because more than one half of the patients with SVCS actually have a thrombotic occlusion as part of their syndrome, it is appealing to consider the use of anticoagulants in these patients. Anticoagulants have often been used in conjunction with other measures for the treatment of SVCS, but proper documentation of their effectiveness in a controlled fashion is not forthcoming. It is, in fact, quite possible that anticoagulation may actually interfere with invasive diagnostic testing and therefore cannot be recommended routinely. Adelstein and colleagues (1988) have documented an increased risk for thromboembolic events after initiation of chemotherapy and therefore recommend beginning anticoagulation together with cytostatic agents. Urban and associates (1993) have proposed that a possible explanation of a lack of survival disadvantage in his group of 87 SCLC patients with SVCS, who also had a higher incidence of brain metastases, compared with the larger group without SVCS is the use of anticoagulation in the former group. This explanation is based on data, including a randomized trial conducted by Chahinian and co-workers (1988), suggesting that both heparin, as noted by Lebeau and associates (1991), and warfarin improve the outcome in SCLC.
Pacemaker-induced SVCS is a rare condition that was reported by Goudevenos and co-workers (1989) and managed by anticoagulation. Thrombosis, as well as fibrotic occlusion and also infectious vegetations, can cause SVC occlusion from any indwelling system. Of the approximately 30 cases reported, only one was not anticoagulated, and this patient died of pulmonary emboli. Of the 19 patients who were treated initially with anticoagulation, 14 (74%) improved clinically, but did not develop recanalization. Whether this represents successful therapy from anticoagulation or simply the development of compensatory collaterals is unclear. Parenthetically, if thrombolytic therapy is not contemplated, indwelling systems that cause SVCS should, in general, be removed, and particularly if any infection is suspected. Catheter removal should be accompanied by anticoagulation to avoid embolization.
Thrombolytic Therapy
Gray and colleagues (1991) have summarized the literature pertaining to thrombolytic therapy of SVCS. In their own series of 16 patients, they were able to achieve complete clot lysis in nine (56%). Eleven of these patients had a central venous line that was present with the thrombotic SVCS, and in this group, eight (73%) recanalized. They concluded that urokinase is probably superior to streptokinase, and a short duration of symptoms (less than 5 days) is predictive of a good response. In all patients in whom the lysis was successful, catheter function was preserved, and there was no need to remove these devices. Recombinant tissue-type plasminogen activator has also been reported as being useful in catheter-associated thrombotic SVCS by Greenberg and associates (1991).
Angioplasty and Endovascular Stenting
Goudevenos (1989), Meinertz (1989), and Grace (1991) and their colleagues reported that percutaneous transluminal angioplasty has been used in a small number of patients with pacemaker-related thrombotic or fibrotic occlusion. The technique appears to be promising and may either delay or avoid the need for reconstructive surgery in some patients. Advances in percutaneous interventional vascular
P.2561
techniques have produced endovascular stents that are being used in the management of benign and malignant SVCS. In a series of six patients with malignant SVCS, Solomon and colleagues (1991) were able to achieve complete resolution in five. Rosch and associates (1992) described complete symptom relief in all of their patients using the Gianturco-Rosch self-expandable Z stents. Twenty of 22 patients had a malignant etiology for their SVCS, with advanced symptomatology, and 13 of these patients had received radiation therapy. Hochrein and co-workers (1998) have reviewed and summarized the literature on this subject. They conclude that response rates range from 68% to 100%; complications are usually minor and uncommon; symptomatic relief is prompt and dramatic; and although reocclusion occurs in 4% to 45% of patients, further therapeutic options can be used for these patients. In addition, these endovascular techniques also can be combined with endovascular thrombectomy, as reported by Yedlicka and co-workers (1991), and thrombolysis, used by Kee and colleagues (1998). In more recent reports, Lanciego and associates (2001) have reported complete or partial resolution of symptoms from SVCS in all 52 patients within 72 hours of stenting. At follow-up, six obstructions from thrombus or tumor were resolved by a second intervention. One partial migration into the right atrium, two incorrect placements, and four stent shortenings were relieved with coaxial prostheses. Smayra and co-workers (2001) reported on 49 stents placed in 30 patients for malignant (53%), benign (17%), or hemodialysis-associated SVCS. Reocclusion was seen in 13 patients of whom 8 patients were from the hemodialysis group. Primary and secondary patency rates at 1 year for malignant, benign, and hemodialysis patients were 74%, 50%, 22%, and 74%, 75%, 56%, respectively.
Surgical Reconstruction
Because of the relatively short life expectancy of many patients with malignant SVCS, surgical reconstruction and bypass techniques have been reserved primarily for patients with nonmalignant SVCS. With improvements in the management of malignant SVCS, however, more patients are becoming longer-term survivors, and a cohort of patients with recurrent SVCS is emerging. A number of surgical interventions have been described for this group of patients. Such methods can be categorized as either autografts (most commonly the saphenous vein), venous homografts, or artificial grafts such as polytetrafluoroethylene (Gore-Tex) or Dacron, or vascular bypass techniques.
Venous autografts produce the best results but are not always feasible because of the lack of availability of adequate lengths of spare vein. Commonly used veins include the saphenous, left innominate, internal jugular, femoral, azygos, and umbilical veins (see Chapter 171). Other autologous alternatives include the use of a pedicle created from the right atrium or the pericardium, and even aortic homografts. Venous homografts, although more readily available, have a late patency rate of only 10% to 37%. Their inherent antigenicity elicits an inflammatory reaction characterized by intimal thickening and perivascular fibrosis. Synthetic grafts do not have an antigenicity problem but are susceptible to intraluminal thrombosis because of their surface properties. In humans, Chiou and associates (1990) reported the patency rate of polytetrafluoroethylene grafts to be 62%, but better patency rates have been achieved by other groups (see Chapter 172).
Chiou and colleagues (1990) have described the successful use of such grafts in the management of patients with malignant thymoma with venous invasion. Dartevelle (1987, 1991, 1995), Nakahara (1988), Masuda (1988), and Shimizu (1992) and their associates report success with the use of a polytetrafluoroethylene prosthesis, particularly when no thrombosis of the SVC is present. Such tumors previously would have been considered inoperable. The technique also has been used successfully in children by Lemmer and associates (1989). Using spiral vein grafts constructed from the saphenous vein, Doty and colleagues (1990) were able to demonstrate long-term patency in seven of nine grafts for up to 15 years when used in the nonmalignant situation. Piccione and associates (1990) were able to achieve patency in six of six patients with malignant SVCS using autologous pericardial graft. Larsson and Lepore (1992) and Gloviczki and co-workers (1990), in two independent reports, have described the use of various graft techniques in a total of 28 patients, 12 with underlying malignancy. Graham and associates (1995) have described a technique in which palliation of SVCS is achieved by performing a jugulofemoral bypass, using the long saphenous vein tunneled subcutaneously from the groin to the neck. This procedure effectively uses the ICV circulation to bypass SVC occlusion. Therefore, surgical reconstruction remains a viable option in patients with an underlying benign etiology and may even prove beneficial in selected patients with malignancies.
Medical Management
Nonspecific medical measures that are likely to provide comfort and stabilize the patient include bed rest, head elevation, supplemental oxygen, and judicious administration of fluids, all which reduce venous pressure and cardiac output. Diuretics with salt restriction have been empirically used to reduce edema but could potentially lead to dehydration and thrombosis. Corticosteroids have been used routinely by some to block the inflammatory reaction resulting from radiotherapy, but Green and associates (1963), in an experimental model, have debunked this fallacy, and the use of corticosteroids must be considered of unproven benefit except in those patients with concomitant
P.2562
brain metastases or laryngeal edema. Thrombolytic therapy is effective in SVCS induced by indwelling catheters.
OUTCOME
Symptomatic Relief
Whereas it is expected that almost all patients with SVCS resulting from nonmalignant causes achieve symptomatic relief with appropriate therapy, only 50% to 70% of patients with underlying malignancy achieve such relief. In an analysis of 367 such patients in five series (Table 170-4), clinical response was noted in 248 patients (68%). Of 166 patients treated with radiation therapy only, the response rate was 69%, whereas in the 45 patients treated with chemotherapy only, the response rate was 84%. In general, response is achieved within 2 weeks but may be a function of the initial radiation fraction size, as reported by Armstrong and colleagues (1987). When larger fraction size is used, 70% respond within 2 weeks, as compared with 56% when the fraction size is smaller.
Venographic Response
In an effort to document the objective venographic response to therapy, Ahmann (1984) evaluated the literature for serial venographic and autopsy evidence of recanalization. Of 35 cases with serial venograms, clinical response was noted in 30 of 35 (86%). Although 19 patients of 35 (54%) were noted to have improved flow on follow-up venograms, 16 (46%) demonstrated no response. Similarly, in 99 cases with autopsy results, persistent obstruction was noted in 75 (76%). The discordance between verifiable restoration of flow dynamics and clinical response is probably explained by the development of an adequate collateral circulation.
SURVIVAL
The survival of a patient with SVCS is in large measure dependent on the underlying condition. The presence of SVCS itself does not specifically affect survival in most instances. In a review of the five series noted in Table 170-4, the mean survival for patients with malignancy ranged from 12.0 to 40.2 weeks. In the Armstrong and associates series (1987), the median survival of all patients was 22 weeks. A significant effect of histology was noted, with the 5-year survival rate decreasing from 41% for lymphoma to 5% for small cell lung cancer to 2% for NSCLC. Figure 170-9 is a composite plot of survival information for various causes of SVCS compiled from the literature.
Table 170-4. Response Rates for Malignant Superior Vena Cava Syndrome | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
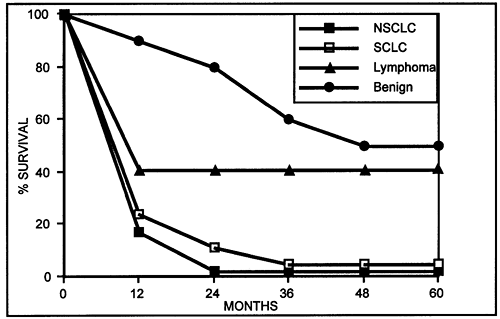 |
Fig. 170-9. Survival of patients with superior vena cava syndrome. Data from Armstrong BA, et al: Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys 13:531, 1987. |
REFERENCES
Adelstein DJ, et al: Thromboembolic events in patients with malignant superior vena cava thrombosis and the role of anticoagulation. Cancer 62:2258, 1988.
Adjei A, Marks RS, Bonner JA: Current guidelines for the management of small cell lung cancer. Mayo Clin Proc 74:809, 1999.
Ahmann FR: A reassessment of the clinical implications of the superior vena caval syndrome. J Clin Oncol 2:961, 1984.
Airan B, et al: Malignant thymoma presenting as intracardiac tumor and superior vena caval obstruction. Ann Thorac Surg 50:989, 1990.
Armstrong BA, et al: Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys 13:531, 1987.
Arya L, et al: Superior vena cava syndrome. Indian J Pediatr 69:293, 2002.
Ayala K, et al: Diagnosis of superior vena caval obstruction by transesophageal echocardiography. Chest 101:874, 1992.
Banker VP, Maddison FE: Superior vena cava syndrome secondary to aortic disease: report of two cases and review of the literature. Dis Chest 51:656, 1967.
Bechtold RE, et al: Superior vena caval obstruction: detection using CT. Radiology 157:485, 1985.
Bell DR, Woods RL, Levi JA: Superior vena caval obstruction: a 10-year experience. Med J Aust 145:566, 1986.
Boring CC, Squires TS, Tong T: Cancer statistics, 1993. CA Cancer J Clin 43:7, 1993.
Brigden M: Hematologic and oncologic emergencies. Doing the most good in the least time. Postgrad Med 109:143, 2001.
Callejas MA, et al: Mediastinoscopy as an emergency diagnostic procedure in superior vena cava syndrome. Scand J Thorac Cardiovasc Surg 25:137, 1991.
P.2563
Carlson HA: Obstruction of the superior vena: an experimental study. Arch Surg 29:669, 1934.
Carter MM, et al: The aortic nipple as a sign of impending superior vena caval obstruction. Chest 87:775, 1985.
Chahinian AP, et al: A randomized trial of anticoagulation with warfarin and of alternating chemotherapy in extensive small cell lung cancer by the Cancer and Leukemia Group. J Clin Oncol 7:993,1988.
Chen JC, Bongard F, Klein SR: A contemporary perspective on superior vena cava syndrome. Am J Surg 160:207, 1990.
Chen CH, et al: Ultrasonically guided needle aspiration biopsy in the diagnosis of advanced superior vena cava syndrome. Zhonghua Yi Xue Za Zhi (Taipei) 50:119, 1992.
Chiou GT, et al: Reconstruction of superior vena cava in invasive thymoma. Chest 97:502, 1990.
Cihangiroglu M, Lin BH, Dachman AH: Collateral pathways in superior vena caval obstructin as seen on CT. J Comput Assist Tomogr 25:1, 2001.
Dake MD, et al: The cause of superior vena cava syndrome; diagnosis with percutaneous atherectomy. Radiology 174:957, 1990.
D'Angio GJ, Mitus A, Evans AE: The superior mediastinal syndrome in children with cancer. AJR Am J Roentgenol 93:537, 1965.
Dartevelle PG, et al: Replacement of the superior vena cava with polytetrafluoroethylene grafts combined with resection of mediastinal-pulmonary malignant tumors. Report of thirteen cases. J Thorac Cardiovasc Surg 94:361, 1987.
Dartevelle PG, et al: Long-term follow-up after prosthetic replacement of the superior vena cava combined with resection of mediastinal-pulmonary malignant tumors. J Thorac Cardiovasc Surg 102:259, 1991.
Dartevelle PG, Macchiarini P, Chapelier A: Technique of superior vena cava resection and reconstruction. Chest Surg Clin N Am 5:345, 1995.
Davenport D, et al: Radiation therapy in the treatment of superior vena caval obstruction. Cancer 42:2600, 1978.
Dillman R, et al: Improved survival in stage III non-small cell lung cancer: seven year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 88:1210, 1996.
Dombernowsky P, Hansen HH: Combination chemotherapy in the management of superior vena caval obstruction in small cell anaplastic carcinoma of the lung. Acta Med Scand 204:513, 1978.
Doty DB, Doty JR, Jones KW: Bypass of superior vena cava. Fifteen years' experience with spiral vein graft for obstruction of superior vena cava caused by benign disease. J Thorac Cardiovasc Surg 99:889, 1990.
Dyet JF, Moghisi K: Role of venography in assessing patients with superior vena caval obstruction caused by bronchial carcinoma for bypass operations. Thorax 35:628, 1980.
Effler DB, Groves LK: Superior vena caval obstruction. J Thorac Cardiovasc Surg 43:574,1962.
Effman EL, et al: Radiographic aspects of total parenteral nutrition during infancy. Radiology 127:195, 1978.
Elias A: Small cell lung cancer: State-of-the-art therapy in 1996. Chest 112:251S, 1997.
Engel IA, et al: CT diagnosis of mediastinal and thoracic inlet venous obstruction. AJR Am J Roentgenol 141:521, 1983.
Fincher RE: Superior vena cava syndrome: experience in a teaching hospital. South Med J 80:1243, 1987.
Fisherman WH, Bradfield JS: Superior vena caval syndrome: response with initially high daily dose irradiation. South Med J 66:677, 1973.
Fujio A, et al: Intracaval and intraatrial growth of thymoma. Report of a long surviving case. Nippon Kyobu Shikkan Gakkai Zasshi 23:934, 1985.
Gale B, et al: Systemic to pulmonary venous shunting in superior vena cava obstruction. Unusual myocardial and thyroid visualization. Clin Nucl Med 15:246, 1990.
Gloviczki P, et al: Reconstruction of the vena cava and of its primary tributaries: a preliminary report. J Vasc Surg 11:373, 1990.
Gollub S, Hirose T, Klauber J: Scintigraphic sequelae of superior vena caval obstruction. Clin Nucl Med 5:89, 1980.
Gooding GA, et al: Obstruction of the superior vena cava or subclavian veins: sonographic diagnosis. Radiology 159:663, 1986.
Goodman R: Superior vena cava syndrome, clinical management. JAMA 231:58, 1975.
Goudevenos JA, et al: Pacemaker-induced superior vena cava syndrome: report of four cases and review of the literature. Pacing Clin Electrophysiol 12:1890, 1989.
Grace AA, Sutters M, Schofield PM: Balloon dilatation of pacemaker induced stenosis of the superior vena cava. Br Heart J 65:225, 1991.
Graham A, et al: Subcutaneous jugulofemoral bypass: a simple surgical option for palliation of superior vena cava obstruction. J Cardiovasc Surg 36:615, 1995.
Gray BH, et al: Safety and efficacy of thrombolytic therapy for superior vena cava syndrome. Chest 99:54, 1991
Green J, Rubin P, Holzwasser G: The experimental production of superior vena caval obstruction. Radiology 81:406, 1963.
Greenberg S, Kosinski R, Daniels J: Treatment of superior vena cava thrombosis with recombinant tissue type plasminogen activator. Chest 99:1298, 1991.
Grisoni ER, Mehta SK, Connors AF: Thrombosis and infection complicating central venous catheterization in neonates. J Pediatr Surg 21:772, 1986.
Hirschmann JV, Raugi GJ: Dermatologic features of the superior vena cava syndrome. Arch Dermatol 128:953, 1992.
Hochrein J, et al: Percutaneous stenting of superior vena cava syndrome: a case report and review of the literature. Am J Med 104:78, 1998.
Holbert BL, Libshitz HI: Superior vena caval syndrome in primary germ cell tumors. Can Assoc Radiol J 37:182, 1986.
Hunter W: The history of an aneurysm of the aorta with some remarks on aneurysms in general. Med Observ Inq 1:323, 1757.
Hussey HH, Katz S, Yater WM: The superior vena caval syndrome: report of thirty-five cases. Am Heart J 31:1, 1946.
Ingram L, Rivera GK, Shapiro DN: Superior vena cava syndrome associated with childhood malignancy: analysis of 24 cases. Med Pediatr Oncol 18:476, 1990.
Issa PY, et al: Superior vena cava syndrome in childhood: report of ten cases and review of the literature. Pediatrics 71:337, 1983.
Kamiya K, et al: Superior vena cava syndrome. Review of the literature and a case report. J Vasc Dis 4:59, 1967.
Kee ST, et al: Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology 206: 187, 1998.
Khimji T, Zeiss J: MRI versus CT and US in the evaluation of a patient presenting with superior vena cava syndrome. Case report. Clin Imaging 16:269, 1992.
Klassen KP, Andrews NC, Curtis GM: Diagnosis and treatment of superior vena caval obstruction. AMA Arch Surg 63:311, 1951.
Komaki R, et al: Sequential vs. concurrent chemotherapy and radiation therapy for inoperable non-small cell lung cancer: analysis of failures in a phase III study (RTOG 9410). Int J Radiat Oncol Biol Phys 48:113, 2000[abst].
Kumar PP, Good RR: Need for invasive diagnostic procedures in the management of superior vena cava syndrome. J Natl Med Assoc 81:41, 1989.
Ladefoged K, et al: Long-term parenteral nutrition. 2. Catheter-related complications. Scand J Gastroenterol 16:913, 1981.
Lanciego C, et al: Stenting as first option for endovascular treatment of malignant superior vena cava syndrome. AJR Am J Roentgenol 177: 585, 2001.
Large SR, et al: Surgical pathology of thymomas: 20 year experience. Thorax 41:51, 1986.
Larsson S, Lepore V: Technical options in reconstruction of large mediastinal veins. Surgery 111:311, 1992.
Lazzarino M, et al: Primary mediastinal B-cell lymphoma with sclerosis: an aggressive tumor with distinctive clinical and pathologic features. J Clin Oncol 11:2306, 1993.
Lazzarino M, et al: Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol 15:1646, 1997.
Lebeau B, Chastang CI, Brechot JM: Subcutaneous heparin treatment increases complete response rate and overall survival in small cell lung cancer. Lung Cancer 7[Suppl]:129, 1991.
Lemmer JH Jr, et al: Pedicled right atrial-pericardial tissue conduit for bypass of the obstructed superior vena cava in children. J Thorac Cardiovasc Surg 98:417, 1989.
Leys M, et al: Acute macular neuroretinopathy after shock. Bull Soc Belge Ophtalmol 241:95, 1991.
Lochridge SK, Knibble WP, Doty DB: Obstruction of the superior vena cava. Surgery 85:14, 1979.
Loeffler J, et al: Emergency prebiopsy radiation for mediastinal masses: impact on subsequent pathologic diagnosis and outcome. J Clin Oncol 4:716, 1986.
P.2564
Loehr SP, et al: MRI evaluation of the mediastinum. 1. Anterior mediastinum. MRI Decisions 24 32, May/June 1993.
Maddox AM, et al: Superior vena cava obstruction in small cell bronchogenic carcinoma. Cancer 52:2165, 1983.
Mahajan V, et al: Benign superior vena cava syndrome. Chest 68:32, 1975.
Masuda H, et al: Total replacement of superior vena cava because of invasive thymoma: seven years' survival. J Thorac Cardiovasc Surg 95: 1083, 1988.
Maxfield WS, Meckstroth GR: Technetium-99m superior vena cavography. Radiology 92:913,1969.
McIntire TT, Sykes EM: Obstruction of the superior vena cava: a review of the literature and report of two personal cases. Ann Intern Med 30:925, 1948.
Medera M, Meydam K, Schmitt WG: MRI visualization of the aortic nipple. Cardiovasc Intervent Radiol 11:29, 1988.
Meinertz T, et al: Percutaneous recanalization and dilation of a thrombotically occluded superior vena cava in a patient with a peritoneovenous shunt. J Hepatol 9:91, 1989.
Miller JB, et al: Diffuse histiocytic lymphoma with sclerosis: a clinicopathologic entity frequently causing superior vena caval obstruction. Cancer 47:748, 1981.
Mineo TC, et al: Mediastinoscopy in superior vena cava obstruction: analysis of 80 consecutive patients. Ann Thorac Surg 68:223, 1999.
Moncada R, et al: Evaluation of the superior vena cava syndrome by axial CT and CT phlebography. AJR Am J Roentgenol 143:731, 1984.
Moore RA, et al: Clinically silent venous thrombosis following internal and external jugular central venous cannulation in pediatric cardiac patients. Anesthesiology 62:640, 1985.
Mullen B, Richardson J: Primary anterior mediastinal tumors in children and adults. Ann Thorac Surg 42:338, 1986.
Muramatsu T, Miyamae T, Dohi Y: Collateral pathways observed by radionuclide superior cavography in 70 patients with superior vena caval obstruction. Clin Nucl Med 16:332, 1991.
Murray N, et al: Importance of timing for thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 11:336,1993.
Musumeci M, et al: Non malignant superior vena cava syndrome. Int J Dermatol 39:934, 2000.
Nakahara K, et al: Thymoma: results with complete resection and adjuvant postoperative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 95:1041, 1988.
Neuhoff H, Sussman ML, Nabatoff RA: Angiocardiography in the differential diagnosis of pulmonary neoplasms. Surgery 25:178, 1949.
Neuman GC, et al: The anesthetic management of the patient with an anterior mediastinal mass. Anesthesiology 60:144, 1984.
Nieto AF, Doty DB: Superior vena cava obstruction, clinical syndrome, etiology, and treatment. Curr Probl Cancer 10:441, 1986.
Painter TD, Karpf M: Superior vena cava syndrome: diagnostic procedures. Am J Med Sci 285:2, 1983.
Pandit PB, et al: Complications associated with surgically placed central venous catheters in low birth weight neonates. J Perinatol 19:106, 1999.
Parish JM, et al: Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc 36:407, 1981.
Perez CA, et al: Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer 50:1091, 1982.
Perez-Soler R, et al: Clinical features and results of management of superior vena cava syndrome secondary to lymphoma. J Clin Oncol 2:260, 1984.
Petersdorf SH, Wood DE: Lymphoproliferative disorders presenting as mediastinal neoplasms. Semin Thorac Cardiovasc Surg 12:290, 2000.
Piccione W Jr, Faber LP, Warren WH: Superior vena caval reconstruction using autologous pericardium. Ann Thorac Surg 50:417, 1990.
Pignon JP, et al: A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618, 1992.
Podoloff DA, Kim EE: Evaluation of sensitivity and specificity of upper extremity radionuclide venography in cancer patients with indwelling central venous catheters. Clin Nucl Med 17:457, 1992.
Pollack A, et al: Thymoma: treatment and prognosis. Int J Radiat Oncol Biol Phys 23:1037, 1992.
Raptopoulos V: Computed tomography of the superior vena cava. Crit Rev Diagn Imaging 25:373, 1986.
Rosch J, et al: Gianturco-Rosch expandable Z-stents in the treatment of superior vena cava syndrome. Cardiovasc Intervent Radiol 15:319, 1992.
Roswitt B, Kaplan G, Jacobson HG: The superior vena cava obstruction syndrome in bronchogenic carcinoma. Radiology 61:722, 1953.
Rubin P, et al: Superior vena caval syndrome. Slow low dose versus rapid high dose schedules. Radiology 81:388, 1963.
Salsali M, Clifton EE: Superior vena caval obstruction with carcinoma of the lung. Surg Gynecol Obstet 121:783, 1965.
Salsali M, Clifton EE: Superior vena caval obstruction in carcinoma of the lung. N Y State J Med 69:2875, 1969.
Saunders MI, Dische S: Continuous, hyperfractionated, accelerated radiotherapy (CHART) in non-small cell carcinoma of the bronchus. Int J Radiat Oncol Biol Phys 19:1211, 1990.
Scarantino C, et al: The optimum radiation schedule in treatment of superior vena caval obstruction: importance of 99mTc scintiangiograms. Int J Radiat Oncol Biol Phys 5:1987, 1979.
Schechter MM: The superior vena cava syndrome. Am J Med Sci 227:46, 1954.
Schindler N, Vogelzang R: Superior vena cava syndrome. Experience with endovascular stents and surgical therapy. Surg Clin North Am 79:683, 1999.
Schraufnagel DE, et al: Superior vena caval obstruction. Is it a medical emergency? Am J Med 70:1169, 1981.
Schwartz EE, Goodman LR, Haskin ME: Role of CT scanning in superior vena cava syndrome. Am J Clin Oncol 9:71, 1986.
Sculier JP, et al: Superior vena caval obstruction syndrome in small cell lung cancer. Cancer 57:847, 1986.
Shimizu N, et al: The surgical treatment of invasive thymoma. Resection with vascular reconstruction. J Thorac Cardiovasc Surg 103:414, 1992.
Smayra T, et al: Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol 24:388, 2001.
Solomon N, Wholey MH, Jarmolowski CR: Intravascular stents in the management of superior vena cava syndrome. Cathet Cardiovasc Diagn 23:245, 1991.
Stanford W, et al: Superior vena cava obstruction: a venographic classification. AJR Am J Roentgenol 148:259, 1987.
Stokes W: A Treatise on the Diagnosis and Treatment of Diseases of the Chest. I. Diseases of the Lung and Windpipe. Dublin: Hodges Smith 1837, p. 370.
Suneja SK, Teal JS: Discrepant sulfur colloid and radioparticle liver uptake in superior vena cava obstruction: case report. J Nucl Med 30:113, 1989.
Taki J, et al: Shunt between right subclavian vein and the left heart in superior vena cava obstruction due to lung cancer. Clin Nucl Med 15:251, 1990.
Thomas S, Sawhney S, Kapur BM: Case report: bilateral massive internal jugular vein thrombosis in carcinoma of the thyroid: CT evaluation. Clin Radiol 43:433, 1991.
Trigaux JP, Van Beers B: Thoracic collateral venous channels: normal and pathologic CT findings. J Comput Assist Tomogr 14:769, 1990.
Turrisi AT III, Glover DJ: Thoracic radiotherapy variables: influence on local control in small cell lung cancer limited disease. Int J Radiat Oncol Biol Phys 19:1473, 1990.
Urban T, et al: Superior vena cava syndrome in small-cell lung cancer. Arch Intern Med 153:384, 1993.
Van Besien K, Kelta M, Bahaguna P: Primary mediastinal B-cell lymphoma: a review of pathology and management. J Clin Oncol 19:1855, 2001.
Warde P, Payne D: Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10:890, 1992.
Warren WH, Altman JS, Gregory SA: Chylothorax secondary to obstruction of the superior vena cava: a complication of the LeVeen shunt. Thorax 45:978, 1990.
Williams JA, et al: Late vascular occlusion of central lines in pediatric malignancies. Pediatrics 101:E71, 1998.
Wudel LC Jr, Nesbitt JC: Superior vena cava syndrome. Curr Treat Options Oncol 2:77, 2001.
Yedlicka JW Jr, et al: Thrombectomy with the transluminal endarterectomy catheter (TEC) system: experimental study and case report. J Vasc Interv Radiol 2:343, 1991.
Yellin A, et al: Superior vena cava syndrome. The myth the facts. Am Rev Respir Dis 141:1114, 1990.
P.2565
Yellin A, et al: Superior vena cava syndrome associated with lymphoma. Am J Dis Child 146:1060, 1992.
Zellers TM, Hagler DJ, Julsrud PR: Accuracy of two-dimensional echocardiography in diagnosing left superior vena cava. J Am Soc Echocardiogr 2:132, 1989.
READING REFERENCES
Of General Interest
Armstrong P, Hayes DF, Richardson PH: Transvenous biopsy of carcinoma of bronchus causing superior vena caval syndrome. Br Med J 1: 662, 1975.
Avasthi RB, Moghissi K: Malignant obstruction of the superior vena cava and its palliation: a report of four cases. J Thorac Cardiovasc Surg 74: 244, 1977.
Clemente C: Anatomy, A Regional Atlas of the Human Body. 3rd Ed. Baltimore: Urban & Schwarzenberg, Inc., 1987.
Cohen DJ, et al: Management of patients with malignant thymoma. J Thorac Cardiovasc Surg 87:301, 1984.
Geller W: The mandate for chemotherapeutic decompression in superior vena caval obstruction. Radiology 81:385, 1963.
Ghosh BV, Cliffton EE: Malignant tumors with superior vena cava obstruction. N Y State J Med 73:283, 1973.
Kane RC, et al: Superior vena caval obstruction due to small cell anaplastic lung carcinoma. JAMA 235:1717, 1976.
Raszka WV Jr, Smith FR, Pratt SR: Superior vena cava syndrome in infants. Clin Pediatr 28:195, 1989.
Rosenbloom SE: Superior vena obstruction in primary cancer of the lung. Ann Intern Med 31:470, 1949.
Salsali M, Cliffton EE: Superior vena caval obstruction with lung cancer. Ann Thorac Surg 6:437, 1968.
Shimm DS, Lugue GL, Tigsby LC: Evaluating the superior vena cava syndrome. JAMA 245:951, 1981.
Skinner DB, Salzman EV, Scannell JG: The challenge of superior vena caval obstruction. J Thorac Cardiovasc Surg 49:824, 1965.
Swayne LC, Kaplan IL: Gallium SPECT detection of neoplastic intravascular obstruction of the superior vena cava. Clin Nucl Med 14:823, 1989.
Szur L, Bromley LL II: Obstruction of the superior vena cava in carcinoma of bronchus. BMJ 2:1273, 1956.
Urschel HC, Paulson DL: Superior vena caval obstruction. Dis Chest 49: 155, 1966.
Warwick R, Williams PL: Angiology. In Warwick R, Williams PL (eds): Gray's Anatomy. 35th Ed. Edinburgh: Longman, 1973, p. 700.
Uncommon Neoplasms as Causes of Superior Vena Cava Syndrome
Aggarwal P, et al: Mediastinal carcinoid tumor with unusual manifestations. Postgrad Med J 65:327, 1989.
Bishop WT, et al: Malignant primary cardiac tumour presenting as superior vena cava obstruction syndrome. Can J Cardiol 6:259, 1990.
Davis SR, et al: Superior vena cava syndrome caused by an intrathoracic plasmacytoma. Cancer 68:1376, 1991.
de Mayolo JA, et al: Superior vena cava obstruction in a patient with chronic lymphocytic leukemia and lung cancer. Am J Clin Oncol 15: 352, 1992.
Hwang MH, et al: Cardiac lymphoma associates with superior vena caval syndrome and cardiac tamponade: case history. Angiology 41:328, 1990.
Korobkin M, Gasano VA: Intracaval and intracardiac extension of malignant thymoma: CT diagnosis. J Comput Assist Tomogr 13:348, 1989.
Kuten A, Jose B, O'Shea PA: Superior vena cava syndrome secondary to histiocytosis-X in a child: case report. Med Pediatr Oncol 7:225, 1979.
Martin AA, et al: Superior vena cava syndrome associated with malignant mesothelioma. J La State Med Soc 149:33, 1991.
Nishida H, et al: Metastatic right atrial tumor in colon cancer with superior vena cava syndrome and tricuspid obstruction. Heart Vessels 6:125, 1991.
Rutegard J, Granstrand M, Aberg T: Intracaval paraganglioma causing superior vena cava syndrome. Eur J Cardiothorac Surg 6:337, 1992.
Sunderrajan EV, et al: Leiomyosarcoma in the mediastinum presenting as superior vena cava syndrome. Cancer 53:2553, 1984.
Thomas S, Sawhney S, Kapur BM: Case report: bilateral massive internal jugular vein thrombosis in carcinoma of the thyroid: CT evaluation. Clin Radiol 43:433, 1991.
Varma S, et al: Cytodiagnosis of granulocytic sarcoma presenting as superior vena cava syndrome in acute myeloblastic leukemia. A case report. Acta Cytol 36:371, 1992.
Nonneoplastic Causes of Superior Vena Cava Syndrome
Antonelli D, Rosenfeld T, Kaveh Z: Intermittent superior caval venous syndrome due to permanent transvenous electrode. Int J Cardiol 23:125, 1989.
Aujla N, McCauley J, Sorkin M: Superior vena cava syndrome due to subclavian hemodialysis catheters. Mil Med 155:274, 1990.
Beers TR, Burnes J, Fleming CR: Superior vena caval obstruction in patients with gut failure receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr 14:474, 1990.
Belcastro S, et al: Thrombosis of the superior vena cava due to a central catheter for total parenteral nutrition. JPEN J Parenter Enteral Nutr 14: 31, 1990.
Caglar MK, Tolboom J: Successful treatment of superior vena cava syndrome with urokinase in an infant with a central venous catheter. Helv Paediatr Acta 43:483, 1989.
Cengiz K, et al: Intrathoracic goiter with hyperthyroidism, tracheal compression, superior vena cava syndrome, and Horner's syndrome. Chest 97:1005, 1990.
Coelho Filho JC, Pereira J, Rabello A Jr: Mediastinal and pulmonary entomophthoromycosis with superior vena cava syndrome: case report. Rev Inst Med Trop Sao Paulo 31:430, 1989.
Fincher RM, Sherman EB: Superior vena caval obstruction due to sarcoidosis. South Med J 79:1306, 1986.
Gomes MN, Hufnagel CA: Superior vena cava obstruction: a review of the literature and report of two cases due to benign intrathoracic tumors. Ann Thorac Surg 20:344, 1975.
Goudevenos JA, et al: Pacemaker-induced superior vena cava syndrome: report of four cases and review of the literature. Pacing Clin Electrophysiol 12:1890, 1989.
Kulpati DD, et al: Fibrosing mediastinitis a rare cause of superior vena caval obstruction. Indian J Chest Dis Allied Sci 31:291, 1989.
Lagerstorm CF, et al: Chronic fibrosing mediastinitis and superior vena caval obstruction from blastomycosis. Ann Thorac Surg 54:764, 1992.
Maggiano HJ, et al: Superior vena cava syndrome after open heart surgery. Cleve Clin J Med 59:93, 1992.
Mazzetti H, et al: Superior vena cava occlusion and/or syndrome related to pacemaker leads. Am Heart J 125:831, 1993.
McFalls EO, et al: Pseudoaneurysm formation with superior vena caval syndrome 7 years after aortic composite graft placement. Ann Thorac Surg 48:704, 1989.
Meinertz T, et al: Percutaneous recanalization and dilation of a thrombotically occluded superior vena cava in a patient with a peritoneovenous shunt. J Hepatol 9:91, 1989.
Murakami Y, et al: Superior vena cava syndrome as a complication of DDD pacemaker implantation. Clin Cardiol 13:298, 1990.
Park JY, Chung-Park M, Snow M: Intravascular papillary endothelial hyperplasia of superior vena cava: a rare cause of the superior vena cava syndrome. Thorax 46:272, 1991.
Pitchenik AE, Zaunbrecher F: Superior vena cava syndrome caused by Nocardia asteroides. Am Rev Respir Dis 117:795, 1978.
Preston CI, Poynton CH, Williams LB: Intermittent superior vena cava syndrome caused by a Hickman catheter. Clin Oncol 4:60, 1992.
P.2566
Raszka WV Jr, Smith FR, Pratt SR: Superior vena cava syndrome in infants. Clin Pediatr 28:195, 1989.
Rosin MD, Ridley PD, Maxwell PH: Rupture of a pseudoaneurysm of a saphenous vein coronary arterial bypass graft presenting with superior caval venous obstruction. Int J Cardiol 25:121, 1989.
Sanghvi S, et al: Angioimmunoblastic lymphadenopathy presenting as superior vena caval obstruction. Chest 100:1721, 1991.
Seetharaman M, et al: Filarial mediastinal lymphadenitis. Another cause of superior vena caval syndrome. Chest 94:871, 1988.
Shimizu N, et al: The surgical treatment of invasive thymoma. Resection with vascular reconstruction. J Thorac Cardiovasc Surg 103:414, 1992.
Sola JE, et al: Atypical thrombotic and septic complications of totally implantable venous access devices in patients with cystic fibrosis. Pediatr Pulmonol 14:239, 1992.
Thomas I, Helmold ME, Nychay S: Beh et's disease presenting as superior vena cava syndrome. J Am Acad Dermatol 26:863, 1992.
Urschel HC Jr, et al: Sclerosing mediastinitis: improved management with histoplasmosis titer and ketoconazole. Ann Thorac Surg 50:215, 1990.
van der Brink H, et al: Superior vena cava syndrome caused by systemic lupus erythematosus in a patient with long-standing rheumatoid arthritis. J Rheumatol 17:240, 1990.
Walsh GC, Norton GI, Baird MM, et al: Idiopathic thrombosis of the superior vena cava. Can Med Assoc J 76:292, 1957.
Weber HS, et al: Pulmonary venous collaterals secondary to superior vena cava stenosis: a rare cause of right to left shunting following repair of a sinus venosus atrial septal defect. Pediatr Cardiol 10:49, 1989.
Yavuzer S, et al: Aneurysms of an aberrant right subclavian artery: a rare cause of the superior vena cava syndrome. Vasa 18:69, 1989.
Zufferey P, Chapuis L, Monti M: Superior vena cava syndrome due to chronic granulomatous mediastinitis. Vasa 19:341, 1990. [Anatomic features were derived from Warwick R, Williams PL. Gray's Anatomy, 35th Ed. New York: Churchill Livingstone, 1973.]
EAN: 2147483647
Pages: 203