XIX - Physiology
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXIII - Benign Esophageal Disease > Chapter 145 - Paraesophageal Hiatal Hernia
Chapter 145
Paraesophageal Hiatal Hernia
Keith S. Naunheim
Patricia Limpert
The esophageal hiatus is formed by muscle fibers of the right crus of the diaphragm with little or no contribution from the left crus. These fibers overlap inferiorly, where they attach over and along the right side of the median arcuate ligament, which is attached to the lateral aspects of the vertebral bodies. The orifice is therefore teardrop shaped, with the point to the right of the aorta and the rounded portion in the midline close to the connecting portion of the central tendon of the diaphragm. The crural fibers form a tunnel that encloses the esophagus. The phrenicoesophageal ligament is formed by fusion of the endothoracic and endoabdominal fascia at the diaphragmatic hiatus. This ligament holds the distal esophagus in place. The lower esophagus normally resides within the abdomen.
Herniation of abdominal contents through the esophageal hiatus into the thoracic cavity has been recognized for several centuries. Bowditch (1853) reported such a case but credited Ambrose Par with a description of a patient with herniation of the stomach through the esophageal hiatus in 1610. One of the first successful repairs was accomplished by Potempski in 1884 and reported by him in 1889.
CLASSIFICATION
Hiatal hernias are generally classified into four types, the most common of which is the sliding, or type I, hiatal hernia.
Type I Hiatal Hernia
In the sliding type of hiatal hernia, the gastroesophageal junction moves through the esophageal hiatus into the visceral mediastinum so that it occupies an intrathoracic position cephalad to the stomach, which follows it. This process occurs because of circumferential weakening of the phrenicoesophageal ligament. Factors that may contribute to the development of this hernia include increased abdominal pressure (e.g., with pregnancy, obesity, or vomiting) and vigorous esophageal contraction, which may pull the gastroesophageal junction up into the mediastinum. This type of hiatal hernia is frequently accompanied by loss of tone and competence of the lower esophageal sphincter (LES), which may result in gastroesophageal reflux and esophagitis. The LES effects may be related to the loss of mechanical advantage at the gastroesophageal junction when it is displaced into the chest. Many sliding hiatal hernias do not produce symptoms. A peculiar abnormality is incompetence of the gastric cardia or the LES without radiologic evidence of a hiatal hernia, which Hiebert and Belsey (1961) called a patulous cardia. The diagnosis and treatment of reflux esophagitis and type I hiatal hernia are reviewed in Chapter 143.
Type II Hiatal Hernia
The paraesophageal, or type II, hiatal hernia is an uncommon disorder that is distinct from the sliding hiatal hernia. In a paraesophageal hiatal hernia, the phrenicoesophageal membrane is not weakened diffusely but rather is weakened focally, anterior and lateral to the esophagus. The gastric cardia and lower esophagus remain below the diaphragm. The gastric fundus protrudes or rolls through the defect into the mediastinum (Fig. 145-1). Paraesophageal hiatal hernia is by far less common than the sliding hiatal hernia. Hill and Tobias (1968), Ozdemir and colleagues (1973), and Sanderud (1967) reported that this condition accounted for only 3% to 6% of all patients undergoing surgical repair of hiatal hernias. Because most patients with hiatal hernia do not undergo operative repair, probably only 1% to 2% of all hiatal hernias are type II defects. Allen and colleagues (1993) found only 147 patients (0.32%) with paraesophageal hiatal hernias, with 75% or more of the stomach in the chest, among 46,236 patients with hiatal hernia at the Mayo Clinic from 1980 to 1990. In 124 of their patients who underwent operation, 51 patients (41%) had a type II hiatal hernia, 52 patients had a type III (mixed sliding and paraesophageal) hiatal hernia (43%), and 21 patients (17%) had a type IV hiatal hernia (i.e., with herniation of other organs in addition to the stomach).
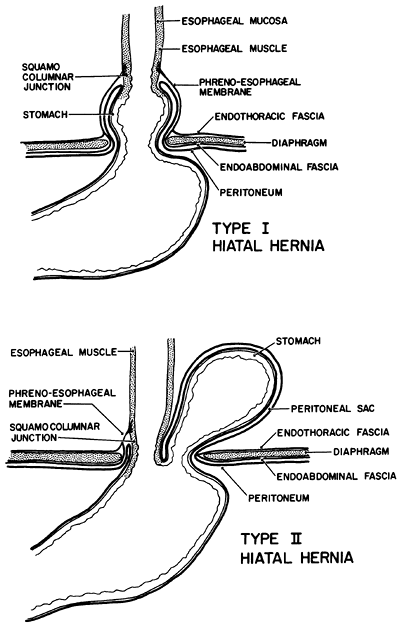 |
Fig. 145-1. Two types of hiatal hernia. Type I hiatal hernia is not a true hernia in that the endoabdominal fascial lining of the abdomen remains intact. In type II hiatal hernia, a defect in the fascia allows a peritoneal sac to pass through the opening in the esophageal hiatus and enter the pleural cavity. |
P.2191
The term parahiatal hernia has been used in the past, but this type of defect may not actually exist. We have never encountered a defect in the diaphragm alongside the esophageal hiatus with protrusion of stomach into the chest and identifiable crural or diaphragmatic fibers between the hernia orifice and the esophageal hiatus.
Type III Hiatal Hernia
The type III, or mixed, hiatal hernia is a combination of types I and II: a sliding and rolling hernia. If a type I hiatal hernia enlarges, the attenuated phrenicoesophageal membrane may also weaken focally anteriorly, allowing protrusion of the gastric fundus. Rotation of the stomach may result in the body or fundus obtaining a higher position within the chest than the cardia, a situation usually found only in type II hiatal hernias. Pearson and colleagues (1983) stated that true type II hiatal hernias are rare and suggested that most are, in fact, misdiagnosed type III defects with a supradiaphragmatic LES. Little support exists for this controversial viewpoint, however. How often a type II paraesophageal hiatal hernia becomes a type III hiatal hernia is not known. Frequently, however, when a patient has a large paraesophageal hiatal hernia with rotation of the body and fundus of the stomach into the chest, the gastroesophageal junction is in a location superior to the esophageal hiatus of the diaphragm. In such circumstances, however, the gastroesophageal junction is in the posterior aspect of the hiatus, and the patient does not usually have symptoms of an attenuated intrinsic sphincter with reflux esophagitis.
A type III defect is frequently present when a type II hiatal hernia has been present for many years, presumably due to gradual enlargement of the esophageal hiatus so that the gastroesophageal junction no longer lies within or below the hiatus. Attachments of the gastroesophageal junction remain intact posteriorly.
Evidence increasingly suggests that a type I hiatal hernia is caused by esophageal contraction abnormalities with a pull on the gastroesophageal junction. Patients with severe esophagitis rarely have a paraesophageal herniation, and patients with a large paraesophageal hiatal hernia seldom have significant esophagitis despite a supradiaphragmatic gastroesophageal junction.
Type IV Hiatal Hernia
Progressive enlargement of the diaphragmatic opening eventually can lead to herniation of organs other than the stomach. The transverse colon and omentum are most commonly involved, but the spleen and small bowel also may herniate into the chest.
ANATOMY AND PHYSIOLOGY
In a true paraesophageal hiatal hernia, the lower esophagus and cardia remain fixed below the diaphragm in the posterior aspect of the diaphragmatic hiatus. A focal weakening of the phrenicoesophageal membrane occurs anterior or lateral to the esophagus, and the combination of negative intrathoracic and positive intraabdominal pressure pushes the abdominal viscera through the defect. The protruding organs are covered circumferentially by a layer of peritoneum that forms a true hernia sac, unlike the type I hiatal hernia, in which the stomach forms the posterior wall of the hernia sac.
The intrathoracic migration of the stomach evolves by so-called organoaxial rotation (Fig. 145-2). The lesser curve of the stomach is anchored in the abdomen by the posterior attachments of the lower esophagus, the left gastric artery, and the retroperitoneal fixation of the pylorus and duodenum. These three points define the long axis of the stomach, and they remain relatively fixed in the abdomen in a type II hiatal hernia. The greater curve of the stomach, however, is relatively mobile and rotates about the
P.2192
long axis by moving first anteriorly and then upward, as the hernia evolves. The fundus is the first part of the stomach to protrude upward through the anterior hernia sac. As the hiatal defect enlarges, the body and antrum continue the axial rotation and migrate into the thorax, leaving the cardia and pylorus in the abdomen. The stomach then resides upside down in the chest, with the greater curve pointing cephalad and the cardia remaining below the diaphragm (Fig. 145-3). The stomach initially may occupy a retrocardiac position, but as the hernia enlarges, rotation occurs into the right chest. With huge hernias, most of the stomach lies within the right hemithorax, with the greater curve of the stomach pointing toward the right shoulder. This rotation places an upward tension on the omentum and may facilitate herniation of the transverse colon into the sac. The organoaxial rotation of the stomach is most commonly upward into the chest and to the right. This is the path of least resistance because of the aorta to the left and the heart anterior and to the left. Occasionally, however, the stomach may rotate superiorly but not to the right, so that the greater curve lies transversely behind the heart.
 |
Fig. 145-2. Mechanics of incarceration and strangulation with paraesophageal hiatal hernia. Note that the fundus may prolapse back into the abdomen. From Postlethwait RW (ed): Surgery of the Esophagus. 2nd Ed. East Norwalk, CT: Appleton-Century-Crofts, 1986, p. 256. With permission. |
As with any true anatomic hernia, the potential complications include bleeding, incarceration, volvulus, obstruction, strangulation, and perforation. Gastritis and ulceration have been visualized endoscopically in as many as 30% of the patients who have type II hiatal hernias. Wichterman and colleagues (1979) suggested that these ulcers are the result of poor gastric emptying and torsion of the gastric wall, particularly after repeat incarcerations, which may impair the blood supply and lymphatic drainage. Although brisk bleeding can occur, these ulcers more frequently cause a slow, chronic blood loss and anemia.
The most serious complication of the type II hiatal hernia is gastric volvulus associated with incarceration and strangulation. Hill and Tobias (1968) and Ozdemir (1973) and Wichterman (1979) and their colleagues reported that approximately 30% of patients with paraesophageal hernias present with gastric volvulus, but this complication may not be so common today. Perdikis and colleagues (1997) reported this complication in only 6% of patients with type II hiatal hernias, and some series have reported no patients with this complication. After a meal, the fundus may prolapse down from the hernia sac and back into the abdomen (see Fig. 145-2). This twists and angulates the stomach in its midportion just proximal to the antrum, resulting in partial or complete obstruction. Distention of the intrathoracic stomach and further rotation of the fundus can result in obstruction at the gastroesophageal junction. Still further twisting may lead to pyloric obstruction, which results in an incarcerated gastric segment and closed-loop obstruction. If unchecked, this process ultimately leads to strangulation, necrosis, and perforation. Unless this process is recognized and corrected, the resulting mediastinitis and shock are fatal.
Allen and colleagues (1993) reported five patients who required emergency operations for suspected strangulation. Three of these patients had gastric necrosis, and one died. Borchardt's triad (1904) of chest pain, retching with an inability to vomit, and inability to pass a nasogastric tube indicates volvulus of the stomach and was present in three of Allen and colleagues' patients (1993). Twenty-three of
P.2193
their patients were followed without surgery. Four of these patients developed progressive symptoms, and one died of aspiration.
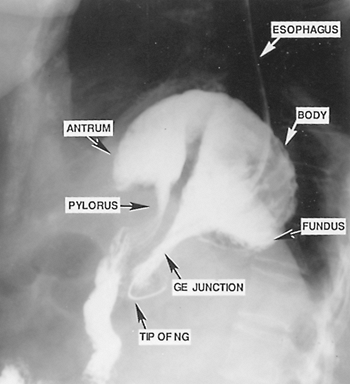 |
Fig. 145-3. Barium study of the stomach that demonstrates the upside-down appearance of the stomach in the thoracic cavity. Note the nasogastric tube (NG) extending through the length of the esophagus with the tip at the gastroesophageal junction (GE) below the esophageal hiatus. The fundus, body, and antrum of the stomach are above the diaphragm. |
Type III defects frequently present when a type II hiatal hernia has been present for many years. This may be due to gradual enlargement of the hiatus so that the gastroesophageal junction no longer lies within or below the hiatus. The attachment of the gastroesophageal junction remains intact posteriorly. Most of these hernias are very large when the diagnosis is made. It may be that the symptoms are so mild or nonspecific when the hernia is small that the patients do not seek medical attention. It may also be that once this type of hernia begins to develop, it progresses rapidly to a large size because of negative intrathoracic and positive intraabdominal pressures.
SYMPTOMS
Many type II hiatal hernias cause few or no symptoms and remain undiagnosed for years until recognized on a routine chest radiograph. Chronic bleeding from gastritis or ulceration of the intrathoracic gastric segment may lead to iron-deficiency anemia, resulting in fatigue and exertional dyspnea. Most patients, however, present with complaints of postprandial discomfort, caused by an intrathoracic gastric segment that becomes dilated by food and swallowed air. Frequently, these complaints have been present for many years. Patients usually describe sensations of substernal fullness or pressure, which is often mistaken for angina. This discomfort is frequently accompanied by nausea and is somewhat relieved by belching or regurgitation.
Although Pearson and colleagues (1983) reported that most of their patients had severe symptoms, it is probably because of the high percentage of mixed (type III) hiatal hernias in their patient population. Ellis and colleagues (1986) noted that symptoms of gastroesophageal reflux were very uncommon in their patients with type II hiatal hernias. Fuller and colleagues (1996) reported that reflux-related symptoms (e.g., heartburn, regurgitation) were predominant in only 27% of patients, and that hernia-related symptoms (e.g., dysphagia, distention, anemia, or bleeding) were predominant in 73% of patients. True dysphagia is uncommon. Finally, a large type III or IV hiatal hernia may occupy a portion of the thoracic cavity and result in postprandial respiratory symptoms of breathlessness with a sense of suffocation. Symptoms may be mild despite a huge hernia. Many patients become accustomed to and tolerate these gas-bloat symptoms well.
When gastric volvulus and obstruction occur, patients present in extreme distress. Most such patients give a long history of complaints but have never sought medical advice. The chief complaints at the time of presentation are severe pain and pressure in the chest or the epigastric region. The discomfort is usually accompanied by nausea and may be misdiagnosed as an acute myocardial infarction. Vomiting may occur, but more frequently the patient complains of retching and an inability to regurgitate. The patient may also complain of the inability to swallow saliva. If the volvulus is allowed to progress, strangulation of the intrathoracic portion of stomach occurs, resulting in a toxic clinical picture including fever, third-spacing of fluid, and hypovolemic shock. The mortality rate in this situation approaches 50%. Kafka (1994) and Oliver (1990) and their colleagues reported acute hemorrhagic pancreatitis in association with paraesophageal hernia, either because of herniation of the pancreatic head or distortion of the pancreatic duct with impaired drainage.
DIAGNOSIS
The diagnosis of paraesophageal hiatal hernia is usually first suspected because of an abnormal chest radiograph. The most frequent finding is a retrocardiac air bubble with or without an air fluid level (Fig. 145-4). In a giant paraesophageal hernia, the hernia sac and its contents occasionally protrude into the right thoracic cavity. The differential diagnosis includes mediastinal cyst or abscess and dilated obstructed esophagus, as in end-stage achalasia. A barium study of the upper gastrointestinal tract is the diagnostic study of choice. The pathognomonic finding is an upside-down stomach in the chest (see Fig. 145-3). The radiologist must pay careful attention to the position of the cardia. This not only confirms the diagnosis of a type II defect but may be important in deciding whether an antireflux procedure should be performed at the same time as the anatomic hernia
P.2194
repair. A barium enema may help determine if any portion of colon is involved.
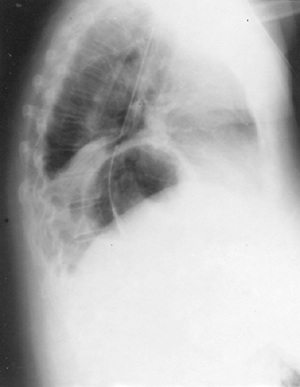 |
Fig. 145-4. Retrocardiac air bubble and type II hiatal hernia. Note the wedge of atelectatic lung compressed by a large hernia sac. |
After the presence of the paraesophageal hiatal hernia has been established radiographically, one must determine whether it has a functional effect on the competence of the LES. This is best accomplished by endoscopy and esophageal function testing.
Although symptoms of gastroesophageal reflux may be uncommon in patients with a pure type II hiatal hernia, they are occasionally present and may indicate pathologic peptic esophagitis. Preoperative esophageal testing may help confirm or refute this suspicion. Esophageal manometry is useful for determining the location of the LES, which marks the gastroesophageal junction, an area that can be difficult to locate on barium study. An LES at a supradiaphragmatic level suggests a huge paraesophageal hiatal hernia or a type III (mixed paraesophageal and sliding hiatal) hernia, which is more likely to have a component of reflux and esophagitis. Ambulatory 24-hour esophageal pH testing can help to identify gastroesophageal reflux, which is best treated by a fundoplication procedure at the time of surgical correction (see Chapter 143). Walther and colleagues (1984) found pH evidence for pathologic reflux in 9 (60%) of 15 patients with type II hiatal hernias. Fuller and colleagues (1996) found similar evidence in 69% of their patients.
The role of esophagoscopy in the evaluation of patients with type II hiatal hernias remains unclear. Pearson and colleagues (1983) performed endoscopy on all 51 patients with primary incarcerated giant hiatal hernias. They found that 30% had grade 1 esophagitis, and another 30% had grade 2 to 4 esophagitis, but virtually all these patients had type III hiatal hernias. Ellis and colleagues (1986) reported a series that included 39 patients with primary type II defects and found only 5 patients (13%) with endoscopic evidence of mild to moderate esophagitis, an incidence similar to that reported by Walther and colleagues (1984). More recently, Fuller and colleagues (1996) found esophagitis in 5 of 15 patients (33%) with type II hiatal hernias, including 3 with grade 2 esophagitis, 1 with grade 3 esophagitis, and 1 with stricture. Significantly, these investigators also reported that endoscopy did not identify pathologic reflux in 58% of their patients who had gastroesophageal reflux demonstrated by pH monitoring.
Apparently, pure type II hiatal hernias are not frequently associated with an incompetent LES or significant gastroesophageal reflux, which probably occurs more frequently in patients with type III defects. Preoperative endoscopy and esophageal motility studies can help to establish the location of the gastroesophageal junction and LES with relation to the diaphragm. The combination of esophageal pH testing and endoscopy may determine whether significant gastroesophageal reflux or pathologic esophagitis is present. These tests should be used before elective operation for any patient with a type II hiatal hernia and symptoms of gastroesophageal reflux. They should also be used routinely for any patient with known or suspected type III hernia with a supradiaphragmatic LES.
TREATMENT
There is no acceptable medical treatment regimen for patients with paraesophageal hiatal hernia. Patients followed expectantly are at great risk, as noted by Skinner and Belsey (1967), who found a 29% mortality rate in 21 patients treated without operation. Because of the serious and life-threatening nature of the complications of this disorder, the presence of the hernia is, in itself, a surgical indication.
When a patient with a type II hiatal hernia presents with gastric volvulus and obstruction, decompression with a nasogastric tube must be performed promptly. In the absence of signs of toxicity, an operation can then be scheduled at the earliest convenience. The inability to decompress a gastric volvulus in this situation constitutes a surgical emergency and mandates immediate operative intervention, whether or not signs of toxicity are present.
Operative Approaches
Although the necessity for operation is universally recognized, there is still controversy about which operation should be performed and by which operative approach. The repair can be performed easily through either an abdominal or thoracic approach and can be performed laparoscopically as well. Each of these approaches has strong proponents. Regardless of the approach, however, the operative principles for hernia repair apply: reduction of the hernia, resection of the sac, and closure of the defect.
Advocates of the thoracic approach emphasize the ease of intrathoracic dissection of the hernia contents and sac. In patients with type III defects, the thoracic approach allows a thorough dissection of the esophagus in cases of moderate to severe esophageal shortening. This approach may allow reduction of a fundoplication beneath the esophageal hiatus without the need for an esophageal lengthening procedure. The proponents of a transthoracic repair, however, usually neglect to note the increased morbidity and discomfort associated with the thoracotomy approach. In addition, a transthoracic repair may allow the stomach to rotate organoaxially after it is pushed back into the peritoneal cavity. This then produces a volvulus of the body of the stomach in which the greater curve of the stomach adheres to the liver. Wichterman and colleagues (1979) reported two patients in whom this occurred; these patients required a laparotomy postoperatively to correct the volvulus.
Those who suggest an abdominal approach point out that the procedure is easily performed through the abdomen and that additional abdominal procedures can be undertaken simultaneously. In addition, this approach allows placement of a gastrostomy tube, which obviates the need for a postoperative nasogastric tube and may also decrease the risk of recurrent volvulus. The only type of patient in whom this approach might prove difficult is one with a proven type III hiatal hernia with known gastroesophageal reflux and a
P.2195
foreshortened esophagus. In this case, the thoracic approach may be a better alternative. Familiarity with the dissection of the esophagus (as for transhiatal esophagectomy), however, allows mobilization of most of the esophagus through an enlarged esophageal hiatus.
Advocates of the laparoscopic procedure emphasize the less invasive nature of this approach. Particularly for elderly patients who may have comorbid conditions, the laparoscopic hernia repair may offer a safe, less traumatic alternative to a conventional operation and may result in a quicker recovery. Although many primary care physicians may be reluctant to refer asymptomatic or mildly symptomatic patients with type II hiatal hernias for conventional surgery, they might be more willing to refer patients for laparoscopic procedures. Even the strongest proponents of this approach, however, recognize that the laparoscopic operation is technically difficult, particularly regarding excision of the hernia sac. Critics of the laparoscopic approach point out that even in experienced hands, the laparoscopic approach may be associated with greater morbidity (e.g., operative complications or conversion to an open procedure) and lead to more postoperative difficulties that require treatment (e.g., second operation, esophageal dilation for dysphagia, gastroesophageal reflux).
Should an Antireflux Procedure Be Included?
The indications for an antireflux procedure at the time of correction of the anatomic hernia remain controversial. Many authors, including Pearson (1983) and Ozdemir (1973) and their colleagues, have written that they routinely perform an antireflux procedure in all patients, regardless of the presence or absence of gastroesophageal reflux symptoms. Allen and associates (1993) reported that nearly 95% of their patients with intrathoracic stomach underwent a transthoracic repair with the addition of an uncut Collis-Nissen fundoplication, Belsey Mark IV fundoplication, or Nissen fundoplication. The remainder of their patients underwent an abdominal repair with an antireflux procedure. Thus, they routinely performed an antireflux procedure despite the fact that only 15% of their patients had esophagitis. They reported excellent results for all types of repair. At the other extreme, Hill and Tobias (1968) espoused simple anatomic repair alone and reported excellent results with no recurrences and no postoperative gastroesophageal reflux in 19 patients.
Other authors have reported a selective approach for the inclusion of an antireflux procedure. Ellis and colleagues (1986) suggested that patients with type II hiatal hernia should undergo preoperative endoscopy, esophageal manometry, and pH testing. This group also suggested that only patients with symptoms or objective evidence of gastroesophageal reflux should be considered for an antireflux repair, usually a Belsey Mark IV fundoplication or a loose Nissen fundoplication.
Williamson and colleagues (1993) reviewed 117 patients with paraesophageal hiatal hernias. The most common presenting symptom was epigastric or substernal pain in 76% of patients. Only 17 patients underwent antireflux procedures in addition to anatomic repair of the hernia. The antireflux procedures were performed for esophagitis determined by symptoms and by esophagoscopy, with a hypotensive LES (>10 mm Hg) or positive 24-hour pH monitoring. Postoperatively, however, 2 of their patients (1.7%) developed severe gastroesophageal reflux symptoms and findings, and 17 others (14.5%) developed mild and controllable symptoms. They reported the development of a recurrent hernia in 10 of 117 patients, with good to excellent results in 86% of patients.
Fuller and colleagues (1996) considered the role of fundoplication in 15 patients with type II hiatal hernia. These authors concluded that with careful preoperative assessment via endoscopy and pH monitoring, objective evidence of gastroesophageal reflux was present in the majority of patients and that an antireflux procedure was indicated for most, if not all, patients.
Operative Technique: Conventional Abdominal Approach
We prefer and recommend the abdominal approach through an upper midline incision. The left lobe of the liver is mobilized and retracted to the right. The contents of the hernia sac are reduced back into the peritoneal cavity by gentle traction. If resistance is encountered while the hernia contents are being reduced, a small rubber catheter inserted in the hernia sac allows entry of air as downward tension is placed on the contents of the sac. This decreases the suction effect that holds the viscera in the chest. Occasionally, in cases of a tight incarceration, the hiatal ring itself may have to be incised to allow return of the organs to the abdominal cavity. This can be performed safely on the left side of the crus posteriorly along the side of the aorta.
The hernia sac is dissected free from the thoracic cavity and resected. Once this has been accomplished, the dead space in the mediastinum disappears as the lungs expand. No drainage of this space is necessary. The large diaphragmatic defect is located anterior to the lower esophagus. In type II hiatal hernias, the gastroesophageal junction usually remains within the abdomen, bound posteriorly by fibrous attachments. Care is taken during the ensuing dissection not to take down this posterior attachment, which maintains the LES in an intraabdominal position. A circumferential dissection is performed on the lower 4 to 8 cm of esophagus through the enlarged esophageal hiatus. With anterior traction on the lower esophagus, the crural repair can begin at the level of the aorta and continue anteriorly until the esophagus is returned to its normal anatomic position at the anterior rim of the esophageal hiatus. This repair is usually performed with simple, interrupted nonabsorbable 0 sutures
P.2196
placed at 1-cm intervals. These sutures must be placed well back from the apparent crural edge due to the attenuated nature of the crura.
If the patient has objective evidence of significant reflux esophagitis preoperatively, an antireflux procedure is now performed. If the posterior attachments of the lower esophagus are taken down during dissection, it is likely that the LES has been disturbed and will be incompetent postoperatively. In these patients, we also perform an antireflux procedure at this time; our procedure of choice is a loose Nissen fundoplication. If there is doubt about whether the gastroesophageal junction is below the esophageal hiatus, it is best to mobilize the junction and the lower esophagus. Sufficient mobilization allows the junction to be brought 4 to 5 cm below the esophageal hiatus. The esophageal hiatus can then be narrowed or repaired by approximating the crura, beginning posteriorly over the aorta and behind the esophagus. This displaces the esophagus anteriorly into its normal position as it passes through the esophageal hiatus.
If no fundoplication is to be performed, the stomach is fixed within the peritoneal cavity by two methods. The first is a modified Hill suture plication, in which three interrupted nonabsorbable sutures are placed between the lesser curve of the stomach and the preaortic fascia (Fig. 145-5). These sutures hold the gastroesophageal junction within the abdominal cavity and prevent the development of a type I (sliding) hiatal hernia postoperatively. If the esophagus has been mobilized during the repair, these sutures can be attached to the crural repair posteriorly. The second technique is a Stamm gastrostomy, which serves two functions. First, it eliminates the need for a nasogastric tube. Many patients with incarcerated type II hiatal hernias have a prolonged period of postoperative gastric stasis, and a gastrostomy allows continued drainage without the discomfort or complications of an indwelling nasogastric tube. Second, the gastrostomy fixes the stomach to the anterior abdominal wall, thereby maintaining its position within the abdominal cavity and preventing an intraabdominal gastric volvulus, a reported complication of transthoracic repairs. The gastrostomy tube can be removed 8 to 12 days after the operation.
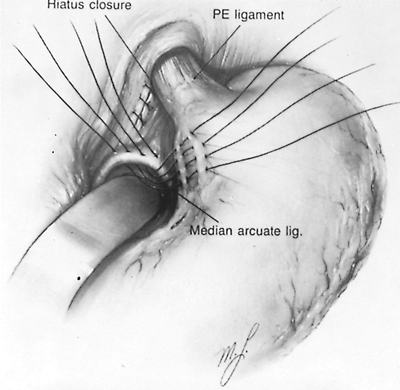 |
Fig. 145-5. Hill suture plication after reduction of the paraesophageal hernia (PE) and repair of the hiatal defect to maintain the position of the gastroesophageal junction within the abdominal cavity. From Postlethwait RW: Surgery of the Esophagus. 2nd Ed. East Norwalk, CT: Appleton-Century-Crofts, 1986, p. 245. With permission. |
If gangrene or perforation is found at the time of operation, all devascularized tissue must be resected and all infected tissue must be d brided. Broad-spectrum antibiotics that include anaerobic coverage are strongly advised in this setting because of the possibility of perforation and mediastinal contamination.
Operative Technique: Laparoscopic Approach
Laparoscopic repair of paraesophageal hiatal hernia was first described by Congreve in 1992. Larger series of patients have recently been reported by Perdikis (1997), Willekes (1997), and Luketich (2000) and their colleagues. The operation is usually performed supine, in steep Trendelenburg's position (Fig. 145-6). Pneumatic stockings are
P.2197
applied before the procedure to minimize the risk of deep vein thrombosis. Five sites of trocar access are routinely employed. These same trocar sites are utilized for the laparoscopic approach to fundoplication in the management of pathologic gastroesophageal disease.
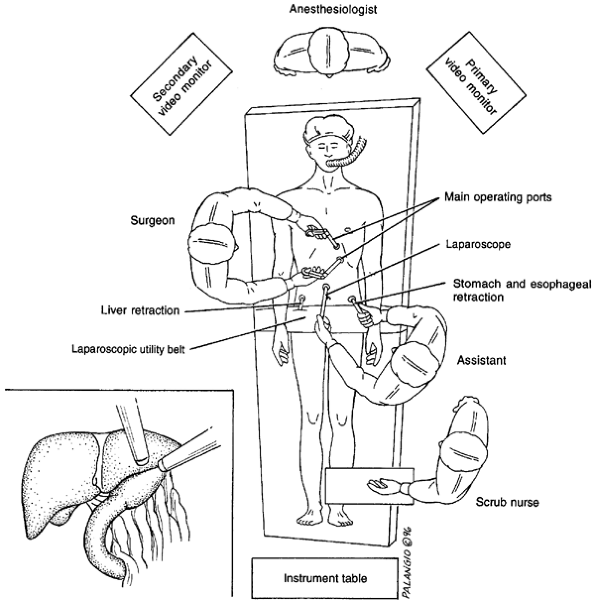 |
Fig. 145-6. Operative setup for laparoscopic repair of paraesophageal hernia. From Willekes CL, et al: Laparoscopic repair of paraesophageal hernia. Ann Surg 225:31, 1997. With permission. |
After abdominal insufflation is achieved, the initial trocar access used for the laparoscopic camera is established in a midline location 5 to 8 cm above the umbilicus. The second trocar access is placed in the right upper quadrant 3 cm below the costal margin in a far lateral position; this is used to introduce a liver retractor. A left upper quadrant site is established for the right-handed endoscopic instrument access to accomplish hiatal dissection. A fourth trocar access is placed at the level of the umbilicus in the left anterior axillary line, used for gastric fundus retraction. A final trocar is inserted in the midline or just to the right of midline slightly inferior to the xiphoid. This site is utilized to introduce the left-handed endoscopic dissecting instrumentation.
Just like the conventional open procedure, the operation is performed in four major stages: reduction of the hernia, excision of the hernia sac, crural repair, and fundoplication, if necessary. Reduction of the herniated abdominal organs is performed using a hand-over-hand technique using atraumatic graspers. Dissection of the hernia sac can be difficult, and complete removal of the sac is controversial. Although removing the entire sac may mean a more difficult operation with the possibility of increasing the risk of complications, Edye and associates (1998) found decreased recurrence with complete resection of the hernia sac. After the excision of the hernia sac, the crura are repaired with several interrupted nonabsorbable sutures. Although the use of prosthetic materials has also been described, it should be avoided if at all possible due to the risk of erosion and endoluminal migration. A fundoplication, if warranted, can then be performed. Laparoscopic gastrostomy can also be used to fix the stomach to the anterior abdominal wall if fundoplication is not performed.
Results
Elective surgical repair of paraesophageal hernias is safe. In a collective review of 502 patients having the open procedure (Table 145-1), the operative mortality rate was less than 0.5%, a figure similar to that quoted for repair of sliding hiatal hernias. Emergency procedures in cases of gastric volvulus, however, carry a much higher mortality rate of approximately 14%. This marked increase in operative risk underscores the need for elective repair at the time of the initial diagnosis.
The postoperative complications are the same as those for antireflux procedures, with two additions. First, in patients with gastric volvulus and obstruction, pulmonary complications apparently increase, probably because of episodes of regurgitation and aspiration. Second, prolonged gastric stasis may persist for a period of 7 to 10 days after operative repair because of lingering inflammation and edema in the released gastric segment.
Table 145-1. Operative Mortality of Open Paraesophageal Hiatal Hernia Repair | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
The long-term results, as noted by Martin and colleagues (1997), are generally excellent, regardless of whether an antireflux procedure is performed in addition to simple repair. Hill and Tobias (1968) performed simple repair and had no recurrence or gastroesophageal reflux in 22 patients over a 15-year follow-up period. Wichterman and colleagues (1979), who routinely performed concomitant antireflux procedures, noted identical results. Recurrent type I hernias with gastroesophageal reflux, however, have been reported in 10% of cases by Ozdemir and colleagues (1973), in 8% by Pearson and colleagues (1983), and in 8% by Sanderud and colleagues (1967) despite fundoplication at the time of initial repair. Simultaneous fundoplication is therefore apparently ineffective prophylaxis against recurrent herniation with resultant gastroesophageal reflux. More appropriately, fundoplication might be used selectively in patients with documented gastroesophageal reflux.
Allen and colleagues (1993) reported excellent results in 60% of their patients, good results in 33%, fair results in 5.2%, and poor results in 1.7%. In Williamson and colleagues' (1993) report, the results were considered excellent in 53.9%, good in 29.5%, fair in 4.3%, and poor in 12.1%. The poor results in this series resulted mainly from symptomatic recurrence of the paraesophageal hiatal hernia in 10% and the infrequent development of severe gastroesophageal reflux in 2% of the patients who did not have a concomitant antireflux procedure at the time of hernia repair.
For patients undergoing laparoscopic repair, a collective review of 631 patients (Table 145-2) reveals that the operative
P.2198
mortality rate is approximately 1%. Conversion to an open procedure occurs in approximately 5% of patients. The range of complications is similar to that reported for open repair and includes gastric perforation, esophageal perforation, pneumothoraces, small-bowel obstruction, pulmonary embolus, splenic injury, and adult respiratory distress syndrome.
Table 145-2. Mortality and Morbidity of Laparoscopic Paraesophageal Hiatal Hernia Repair | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Schauer and colleagues (1998) retrospectively compared the results of patients undergoing open and laparoscopic repair of paraesophageal hernias. They noted that laparoscopic patients had a significantly lower requirement for pain medication, decreased time to oral intake, fewer intensive care unit stays, a lower rate of complications, and an overall shorter length of stay when compared with the open patients. Although Hashemi and associates (2000) found no difference in the complication rate, they also found a faster return to a regular diet and shorter hospital stay with laparoscopy.
The results of laparoscopic repair of paraesophageal hiatal hernias appear to be quite acceptable in the early postoperative period. Edye (1998) and Luketich (2000) and their colleagues have raised questions regarding the possibility of increased risk of recurrent herniation when utilizing this technique. This has led Luketich and colleagues (2000) to suggest frequent utilization of a Collis gastroplasty at the time of paraesophageal hiatal hernia repair. Because of the relatively recent introduction of laparoscopic repair, long-term results are somewhat limited. Horgan and associates (1999) have published the longest follow-up, averaging 36 months (range 1 to 57), which reveals good outcomes at this intermediate interval. Subjective testing reveals good symptomatic relief, and radiologic or endoscopic tests have shown less than 7% recurrence. Nevertheless, long-term follow-up from other institutions is required before this procedure can be labeled as a gold standard for paraesophageal hiatal hernia repair. Until that time, both thoracotomy and laparotomy remain acceptable techniques that have stood the test of time.
REFERENCES
Allen MS, et al: Intrathoracic stomach: presentation and results of operation. J Thorac Cardiovasc Surg 105:253, 1993.
Beardsley JM, Thompson WR: Acutely obstructed hiatal hernia. Ann Surg 159:49, 1964.
Borchardt M: Zur Pathogie und Therapie des Magen Volvulus. Arch Klin Chir 74:243, 1904.
Bowditch HI: Peculiar case of diaphragmatic hernia. Buffalo Med J Month Rev 9:1, 1853.
Carter R, Brewer LA 3rd, Hinshaw DB: Acute gastric volvulus: a study of 25 cases. Am J Surg 140:99, 1980.
Congreve DP: Laparoscopic paraesophageal hernia repair. J Laparoendosc Surg 2:45, 1992.
Dahlberg PS, et al: Laparoscopic repair of large paraesophageal hiatal hernia. Ann Thorac Surg 72:1125, 2001.
Edye MB, et al: Durability of laparoscopic repair of paraesophageal hernia. Ann Surg 228:528, 1998.
Ellis FH Jr, Crozier RE, Shea JA: Paraesophageal hiatus hernia. Arch Surg 121:416, 1986.
Fuller CB, et al: The role of fundoplication in the treatment of type II paraesophageal hernia. J Thorac Cardiovasc Surg 111:655, 1996.
Gantert WA, et al: Laparoscopic repair of paraesophageal hiatal hernias. J Am Coll Surg 186:428, 1998.
Hashemi M. et al: Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg 190:553, 2000.
Hiebert CA, Belsey R: Incompetency of the gastric cardia without radiologic evidence of hiatal hernia. J Thorac Cardiovasc Surg 42:352, 1961.
Hill LD, Tobias JA: Paraesophageal hernia. Arch Surg 96:735, 1968.
Horgan S, et al: Repair of paraesophageal hernias. Am J Surg 177:354, 1999.
Kafka NJ, Leitman IM, Tromba J: Acute pancreatitis secondary to incarcerated paraesophageal hernia. Surgery 115:653, 1994.
Kercher KW, et al: Minimally invasive management of paraesophageal herniation in the high-risk surgical patient. Am J Surg 182:510, 2001.
Khaitan L, et al: Laparoscopic paraesophageal hernia repair has an acceptable recurrence rate. Am Surg 68:546, 2002.
Landreneau RJ, et al: Clinical spectrum of paraesophageal herniation. Dig Dis Sci 37:537, 1992.
Luketich JD, et al: Laparoscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg 232:608, 2000.
P.2199
Martin TR, Ferguson MK, Naunheim KS: Management of giant paraesophageal hernia. Dis Esophagus 10:47, 1997.
Oliver MJ, Wilson AR, Kapila L: Acute pancreatitis and gastric volvulus occurring in a congenital diaphragmatic hernia. J Pediatr Surg 25:1240, 1990.
Ozdemir IA, Burke WA, Ikins PM: Paraesophageal hernia: a life-threatening disease. Ann Thorac Surg 16:547, 1973.
Pearson FG, et al: Massive hiatal hernia with incarceration: a report of 53 cases. Ann Thorac Surg 35:45, 1983.
Perdikis G, et al: Laparoscopic paraesophageal hernia repair. Arch Surg 132:586, 1997.
Postlethwait RW (ed): Surgery of the Esophagus. 2nd Ed. East Norwalk, CT: Appleton-Century-Crofts, 1986, p. 256.
Postlethwait RW: Surgery of the Esophagus. 2nd Ed. East Norwalk, CT: Appleton-Century-Crofts, 1986, p. 245.
Potempski P: Nuovo processo operativo per lar riduzione cruenta della ernie diaframmatiche de trauma e per la sutura delle ferite del diaframma. Bul Reale Accad Med Roma 15:191, 1889.
Sanderud A: Surgical treatment for the complications of hiatal hernia. Acta Chir Scand 133:223, 1967.
Schauer PR, et al: Comparison of laparoscopic versus open repair of paraesophageal hernia. Am J Surg 176:659, 1998.
Skinner DB, Belsey RHR: Surgical management of esophageal reflux and hiatus hernia: long-term results with 1,030 patients. J Thorac Cardiovasc Surg 53:33, 1967.
Swanstrom LL, et al: Esophageal motility and outcomes following laparoscopic paraesophageal hernia repair and fundoplication. Am J Surg 177:359, 1999.
Walther B, et al: Effect of paraesophageal hernia on sphincter function and its implication on surgical therapy. Am J Surg 147:111, 1984.
Wichterman K, et al: Giant paraesophageal hiatal hernia with intra-thoracic stomach and colon: the case for early repair. Surgery 86:497, 1979.
Wiechmann RJ, et al: Laparoscopic management of giant paraesophageal herniation. Ann Thorac Surg 71:1080, 2001.
Willekes CL, et al: Laparoscopic repair of paraesophageal hernia. Ann Surg 225:31, 1997.
Williamson WA, et al: Paraesophageal hiatal hernia: is an antireflux procedure necessary? Ann Thorac Surg 56:447, 1993.
EAN: 2147483647
Pages: 203