118 - Benign Tumors of the Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXI - Operative Procedures in the Management of Esophageal Disease > Chapter 136 - Minimally Invasive Esophageal Surgery
function show_scrollbar() {}
Chapter 136
Minimally Invasive Esophageal Surgery
Matthew J. Schuchert
James D. Luketich
During the past decade, dramatic technologic and procedural advances have revolutionized the minimally invasive surgical approach to diseases of the esophagus. Though the basic principles of esophageal surgery remain the same whether performed open or with minimally invasive techniques, successful performance of these advanced procedures requires state-of-the-art instrumentation, specialized training, and experience. Some procedures (e.g., laparoscopic Nissen fundoplication) have become accepted as the standard and have replaced the conventional open repair in most centers.
This chapter summarizes the role of minimally invasive surgery in the treatment of benign and malignant esophageal disorders. Many of these less invasive approaches have achieved operative and short-term results that are comparable to open procedures, but with less morbidity and shorter hospital stays. Long-term results for most of these procedures are either absent or only now becoming available. Very complex procedures such as minimally invasive esophagectomy, redo laparoscopic Nissen fundoplication, and laparoscopic giant paraesophageal hernia repair are routinely being performed in only a limited number of specialized centers. Prospective trials will ultimately be required to identify objectively the benefits and limitations of these minimally invasive approaches.
MINIMALLY INVASIVE RESECTION OF THE ESOPHAGUS
Carcinoma of the Esophagus
Premalignant Lesions (Barrett's Esophagus)
If on endoscopic evaluation, salmon-colored mucosa is found to extend more than 2 cm above the proximal gastric folds, or exists in islands above the squamocolumnar border, the diagnosis of Barrett's esophagus is suspected, and a biopsy is indicated. Barrett's esophagus represents replacement of the normal esophageal squamous epithelium with stratified columnar epithelium. Microscopically, the diagnosis of Barrett's esophagus is established by the presence of intestinal stratified columnar metaplasia containing goblet cells. This condition most commonly arises in the setting of chronic gastroesophageal reflux disease, in which repeated mucosal injury leads to the development of intestinal metaplasia, as summarized by the two of us (MJS, JDL) (2003). Phillips and Wong (1991) have estimated that Barrett's esophagus can be found in 7% to 12% of patients with chronic severe GERD. This abnormal specialized columnar epithelium predisposes patients to the development of mucosal dysplasia and, ultimately, adenocarcinoma. It should therefore be considered a premalignant condition, even if involving only a short segment, with a 50- to 100-fold increased risk for cancer compared with the general population, as discussed by Spechler (2002). Shaheen and associates (2000) estimated the risk for developing adenocarcinoma in patients with Barrett's esophagus to be 0.5% per year. DeMeester and DeMeester (2000) have suggested that the presence of dysplasia indicates the potential for progression from low-grade dysplasia to high-grade dysplasia and ultimately to adenocarcinoma (the metaplasia-dysplasia-carcinoma sequence). The presence of high-grade dysplasia is frequently associated with an unrecognized focus of adenocarcinoma in up to one third of patients. Antireflux surgery, as opposed to medical therapy, has been proposed to induce regression, or halt progression, of intestinal metaplasia, but this concept remains highly controversial and is, at best, speculative at this point.
Staging for Esophageal Cancer
Esophageal cancer affects 12,300 new patients in the United States annually, and more than 11,000 patients will die of this disease each year, as estimated by Robert and co-workers (2000). Most patients present with advanced disease because dysphagia (the most common symptom) does not develop until about two thirds of the esophageal lumen has been obliterated. Therefore, most patients have advanced local, regional, or distant metastases at the time of initial diagnosis.
P.2051
Evidence of extensive local invasion or distant metastases typically precludes curative resection. Therefore, precise preoperative assessment of the extent of disease (staging) will help to identify patients who are unlikely to benefit from radical resection as well as those who may be favorable candidates for aggressive chemotherapy and radiation therapy regimens. In addition, accurate pretreatment staging allows a more rational evaluation and comparison of different treatment modalities. Currently, several noninvasive modalities are employed in the preoperative assessment of patients with esophageal cancer. The most commonly used are computed tomography (CT) and endoscopic ultrasound (EUS). More recent innovations include the application of positron emission tomography (PET) to esophageal cancer staging. These noninvasive methods frequently clinically overstage or understage esophageal cancer because of the lack of pathologic verification. There is difficulty in discriminating normal lymph nodes from metastatic lymph nodes, inflammatory nodules from metastatic nodules, as well as T3 from T4 invasive lesions.
Video-assisted staging for esophageal cancer using minimally invasive techniques dates back to the report of Murray and colleagues (1977), who described mediastinoscopy and minilaparotomy to evaluate for the presence of pathologic lymph node. Dagnini and colleagues (1986) were the first to report the use of laparoscopy for esophageal cancer in 369 patients. They were able to identify intraabdominal metastases in 14% of patients and celiac lymph node metastases in 9.7%, thus preventing unnecessary resection in these patients. Krasna and McLaughlin (1993) were the first to report the utility of thoracoscopic lymph node staging.
Technique of Thoracoscopic Staging.
After induction of general endotracheal anesthesia through a single-lumen tube, flexible bronchoscopy is performed. Endobronchial narrowing or invasion can be ascertained. A double-lumen endotracheal tube is then used to replace the single-lumen tube. The patient is then positioned in the lateral decubitus position. After adequate preparation and drape, a right thoracoscopic approach is preferred because this allows more complete exposure of the esophagus and periesophageal lymph nodes without interference by the aorta. If an indeterminate pulmonary node is present on the left side, then a left-sided video-assisted thoracic surgery (VATS) approach can be chosen. After lung deflation, a 10-mm port is placed at the level of the sixth intercostal space in the midaxillary line for the camera. Two dissecting 5-mm ports are then placed at the fifth and eighth intercostal spaces posteriorly. An additional 5-mm port is positioned anteriorly in the fifth intercostal space to provide retraction of the lung medially. The hemithorax is then systematically inspected to rule out metastases on the parietal pleura, pericardium, and diaphragm. The inferior pulmonary ligament is divided, and the pleura overlying the lower one third of the esophagus is opened. The primary lesion is assessed to determine whether there is T3 or T4 involvement. The dissection is begun by retracting the right upper lobe inferiorly and anteriorly to expose the esophagus and to allow identification of the azygos vein. The inferior pulmonary ligament and the pleura over the lower half of the esophagus are incised. The azygos vein is mobilized carefully with blunt dissection above and below. Rarely, division of the vein is necessary to improve exposure. Lymph node dissection from the right chest provides access to the level 2 and 4 lymph nodes superior to the azygos veins as well as the level 10 hilar lymph nodes located inferior to the azygos vein. In addition, the subcarinal (level 7), paraesophageal (level 8), and inferior pulmonary ligament (level 9) lymph nodes should be sampled for complete staging (Fig. 136-1). The search for lymph nodes is continued until either a positive node is confirmed by frozen section analysis, or multiple lymph nodes are found to be negative. A liberal use of clips is employed to minimize bleeding and lymphatic leakage. All surgical sites are inspected for bleeding. A single 28F straight chest tube can be placed through the inferior port site. Two-lung ventilation is resumed, and the lung is inspected for adequate expansion.
In cases in which preoperative noninvasive staging demonstrates suspicious lymph nodes [e.g. aortopulmonary (level 5)] on the left, left-sided thoracoscopy can be performed. A similar port configuration is employed. The mediastinal pleura overlying the lymph nodes is incised from the phrenic and vagus nerves superiorly to the left main pulmonary artery inferiorly.
Technique of Laparoscopic Staging.
The patient is placed in a comfortable supine position and is prepared as for a standard laparotomy. Abdominal access is obtained as described in Figure 136-2. The abdomen is carefully inspected for signs of diffuse metastasis and peritoneal implants. The surface of the liver is carefully evaluated for hepatic metastases. Intraoperative laparoscopic ultrasound can be performed to enhance the sensitivity of the liver evaluation or to confirm the presence of deep lesions seen on CT. In addition, ultrasound is useful to guide a core-needle biopsy of deep-seated lesions. A liver retractor can be used to elevate the left lateral segment of the liver to assess the gastroesophageal (GE) junction. The gastrohepatic ligament is divided. The lesser sac is entered, and the stomach is retracted to the left. The phrenoesophageal ligament is divided, and the right crus is mobilized to allow exposure of the lesser curvature and parahiatal lymph nodes (levels 15 and 16). The left gastric vessel is identified and can be traced back toward the celiac axis, allowing biopsies of levels 17 to 20. The dissection is carried into the periesophageal and retroesophageal planes until either a diseased (positive) lymph node is detected by frozen section analysis or several benign lymph nodes are histologically verified. As desired, a feeding jejunostomy, Infusaport catheter, or both can be placed at this time.
Results
As experience was gained using the thoracoscopic and laparoscopic staging technique, it became apparent that
P.2052
the success rates were very high. In a multiinstitutional trial by the Cancer and Leukemia Group B, a success rate of more than 90% was achieved, with reported accuracy of laparoscopy and thoracoscopy of 94% and 91%, respectively, as reported by Krasna and associates (1995, 1996).
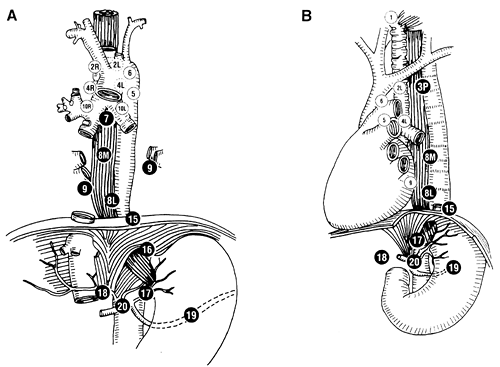 |
Fig. 136-1. Esophageal lymph node staging map. |
In 1999(a), a comparison study was performed by Krasna and co-workers on 88 patients with esophageal cancer who underwent CT, EUS, or both followed by a thoracoscopic-laparoscopic staging procedure to evaluate the role of the various staging modalities. Of these patients, 82 patients received both chest and abdominal CT scans, and 62 patients underwent endoscopic ultrasound. Thoracoscopic staging was completed in 82 patients, and laparoscopic staging was accomplished in 55 patients. Forty-nine patients underwent both thoracoscopic and laparoscopic staging. Thirty-nine (44%) patients did not undergo resection after staging because of an advanced lesion (T4, 13 patients; M1, 3 patients). Three of 42 patients (6.3%) with N0 disease established by thoracoscopy were found at resection to have paraesophageal lymph node involvement (N1). The overall accuracy of thoracoscopic staging was 93.6%, and that of laparoscopic staging, 93.9%. Based on pathologic evaluation of resected specimens, the sensitivity, specificity, and positive predictive value for staging N1 disease in the chest were 62.5%, 100%, and 100% for thoracoscopy; 75%, 75.6%, and 23.1% with CT; and 0%, 51.4%, and 5.5% by EUS respectively. For N1 disease in the abdomen, it was 85%, 100%, and 100% by laparoscopy, 0%, 97.1%, and 0% by CT and 22%, 81.5%, and 28.6% by EUS. Therefore, thoracoscopic-laparoscopic staging has a higher specificity and accuracy than either CT or endoscopic ultrasound, especially for N1 disease in the chest.
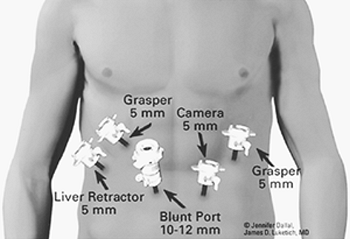 |
Fig. 136-2. Standard abdominal port placement for laparoscopic approach to the lower esophagus (e.g., esophagectomy, Nissen fundoplication, giant paraesophageal hernia repair). |
CT has been shown to be sensitive in detecting lymphadenopathy in the chest but lacks a similar sensitivity in tumor staging. Endoscopic ultrasound has a much higher sensitivity in detecting the depth of tumor invasion but is limited in discerning the extent of advanced lesions, assessing lesions that occlude the esophageal lumen, and evaluating lesions with nodes distant from the esophagus. Minimally invasive staging provides greater accuracy in discerning T3 from T4 tumors and in the assessment of distant metastatic disease such as liver metastases. One of us (JDL) and associates (1997a) also found that minimally invasive staging was superior compared with EUS in detecting lymph node metastases in esophageal cancer. In this study, the sensitivity and
P.2053
specificity for EUS in nodal evaluation were found to be 65% and 66%, respectively. Overall accuracy was 65%. The overall incidence of lymph node metastases as detected by laparoscopy and VATS staging was 81%. In the six cases that laparoscopy and VATS detected N1 disease, in which EUS showed N0 disease, the lymph nodes were less than 1 cm in diameter. Although minimally invasive staging never identified a T4 lesion when a T3 lesion was diagnosed by EUS, the sensitivity was further decreased to 44% for nodal metastases smaller than 1 cm. In 20% to 38% of patients with esophageal cancer, a high-grade malignant stricture precludes passage of an echoendoscope. Dilation of such strictures can be performed, but dilation with subsequent EUS carries a significant risk for perforation, as reported by Van Dam and co-workers (1993). EUS documented no distant metastases, whereas 4 of 26 (15%) patients undergoing minimally invasive staging were found to have liver metastases Lymph nodes are considered positive if they fulfill one of the following criteria: distinct borders, rounded appearance, hypoechogenicity, or size larger than 1 cm. The accuracy of EUS can be enhanced by the use of fine-needle aspiration (FNA) of suspicious celiac lymph nodes, as discussed by Reed and colleagues (1999). If N1 disease can be verified at the time of EUS, then a much less extensive surgical staging procedure is required. However, the number of lymph nodes involved, the location above and below the diaphragm, and the presence of distant metastases are all better assessed by minimally invasive staging and may have more precise bearing on prognostic outcomes. However, laparoscopy and thoracoscopy improve the accuracy of lymph node staging in esophageal cancer and have the additional advantage of evaluating the thoracic and abdominal cavities for metastases. We use EUS as a complementary diagnostic procedure that accurately assesses tumor depth of penetration and that can allow tissue diagnosis by FNA.
The goal of clinical staging is to identify T3 and N1 disease because patients with this stage of disease have poor surgical survival. Esophageal ultrasound is the best clinical instrument for evaluation of the T stage. If T3 or T4 lesions are found and the patient has locally advanced disease, and identification of N1 disease is a luxury, but not essential for management. In patients with a T2 lesion, the prevalence of positive nodes is 50%. A T3 lesion, whether stricturing or not, is associated with positive lymph nodes in 80% of patients. N1 confirmation becomes critical when it affects treatment and survival. Accuracy rates of 65% to 80% have been reported with EUS for nodal status. Although malignant strictures provide a limitation to the use of EUS, the presence of a malignant stricture itself is a reliable predictor of advanced disease, with 90% of such patients exhibiting stage III or IV disease.
PET has also been used in the pretreatment staging of esophageal cancer, as discussed by one of us (JDL) and associates (1997b). In an evaluation of 35 patents with potentially resectable esophageal cancer, PET detected nine sites of distant metastases missed by conventional scanning (CT and bone scan), achieving a sensitivity of 88%, specificity of 93%, and accuracy of 91%. One of us (JDL) and co-workers (1997b) have also demonstrated that PET can identify distant metastases in 20% of cases in which CT scanning and bone scanning were negative, but has limited sensitivity (45%) and accuracy (48%) for lymph node metastases smaller than 1 cm. Compared with other noninvasive staging modalities (such as CT), PET appears to afford superior sensitivity and accuracy. In a study of 91 patients and 100 consecutive PET scans by one of us (JDL) and co-workers (1999), PET scan using 18F-fluorodeoxyglucose was compared with CT scanning in the detection of distant metastases. Minimally invasive staging was used to confirm or refute imaging results, and identified 70 distant metastases in 39 cases. In this study, PET detected 51 metastases in 27 of the 39 cases (69% sensitivity, 93.4% specificity, and 84% accuracy), compared with CT, which detected 26 metastases in 18 of 39 cases (46.1 sensitivity, 73.8% specificity, and 63% accuracy) (p < 0.01). Therefore, PET scanning enhances our ability to detect distant metastases in the preoperative staging of esophageal cancer and is more accurate than CT, but is only 69% sensitive when compared with minimally invasive staging. As CT scanning technology improves, so does the sensitivity of this modality. Wren and co-workers (2002) have demonstrated comparable sensitivity and accuracy when comparing CT to PET in the detection of regional nodal involvement or distant metastatic disease. PET continues to demonstrate superior specificity, however. An analysis of the cost effectiveness of the different preoperative staging modalities (CT, PET, EUS with FNA, and minimally invasive staging) was performed by Wallace and colleagues (2002) to determine which modality (either alone or in combination) is most cost effective in the staging of esophageal cancer. The combination of PET and EUS with FNA was found to be the most cost-effective approach and was associated with a $60,544 per quality-adjusted life-year gained. Interestingly, PET also appears to be predictive of disease-free and overall survival when comparing responses to neoadjuvant therapy before esophagectomy. In a prospective study of 39 patients with esophageal cancer published by Downy and associates (2003), 2-year disease-free and overall survival rates were 38% and 63%, respectively, in patients who had less than a 60% decrease in the standardized uptake value (SUV), and 67% and 89%, respectively, in patients who had a greater than 60% increase in SUV. CT and PET combination modalities may further enhance the utility of these tools in the preoperative staging of esophageal cancer.
Minimally invasive staging of esophageal cancer provides greater sensitivity, specificity, and accuracy than either CT or EUS in the assessment of tumor extent, N1 disease, or the presence of metastases. In addition, histologic examination misses micrometastases in up to 20% of lymph nodes evaluated. In recent years, it has become apparent that combining minimally invasive surgical techniques with new molecular diagnostic techniques might
P.2054
improve the staging of patients with esophageal cancer. Kassis and co-workers (1998) demonstrated that carcinoembryonic antigen (CEA) messenger RNA (mRNA) expression detected by reverse transcriptase polymerase chain reaction (RT-PCR) is more sensitive than histologic examination alone in the detection of nodal metastases in patients with esophageal cancer. Of 73 histologically negative lymph nodes, 36 (49%) contained CEA mRNA, suggestive of the presence of occult micrometastases. When combined with thoracoscopic-laparoscopic staging techniques, RT-PCR detects more lymph node metastases than histopathologic evaluation (77% vs. 48%), and a positive RT-PCR in the setting of negative histologic findings may indicate a poor prognostic outcome, as reported by Krasna and associates (1999b), who have expanded on these findings by determining that the immunohistochemical staining of p53 can also be used for this purpose. Overexpression of p53 protein in the pretreatment esophagogastroduodenoscopy (EGD), as well as in lymph nodes obtained in thoracoscopic-laparoscopic staging, is predictive of poor response to chemoradiation and decreased survival in esophageal cancer patients. Of patients who are p53 negative, up to 75% achieve a complete pathologic response, with median survival of 30 months (double that of patients who are p53 positive).
The staging of esophageal cancer is imprecise. No single noninvasive imaging modality or invasive procedure is an ideal staging method for esophageal cancer. Thoracoscopic-laparoscopic staging techniques help to establish a more accurate assessment of the patient's stage of disease at the time of presentation. This provides optimal information in the assessment of a patient's likelihood of recurrent disease and long-term survival. It provides accurate anatomic information that can be employed in the individualization of radiation fields to the exact tumor area, while selectively sparing noninvolved areas. In addition, pretreatment surgical node staging can also predict the response to induction treatment as well as survival for esophageal cancer patients undergoing trimodality treatment, as suggested by Krasna and Jiao (2000). It provides critical information regarding the presence of lymph node involvement as well as in determining the presence or absence of mediastinal invasion. Perhaps most importantly, it provides a framework that is useful in evaluating and comparing the results of clinical trials evaluating the usefulness of preoperative chemotherapy and radiation therapy. Many surgeons (including our own group) have suggested that the thoracoscopic staging could be omitted in most cases of GE junction adenocarcinoma, inasmuch that only 3 of 26 cases were found for which laparoscopic staging was negative and thoracoscopic staging was positive. Laparoscopic staging alone would permit decreased operative times, length of stay, and complication rates. We have also found that resection is technically more difficult after minimally invasive staging, as well as following multimodality therapy. VATS staging should be employed to evaluate indeterminate thoracic lesions or to maximize accuracy in the face of a negative laparoscopy.
A recent prospective Thoracic Intergroup study (Cancer and Leukemia Group B 9380), published by Krasna and associates (2001), has defined the effectiveness of this technique in managing patients with esophageal cancer. In this study of 134 patients, the feasibility and accuracy of this staging modality was assessed. Thoracoscopic-laparoscopic staging was considered successful if one thoracoscopic lymph node and three laparoscopic lymph nodes were sampled, a confirmed positive node was found, or T4 or M1 disease was documented. If these conditions were met in at least 70% of patients, the method was believed to be feasible. There were no deaths or major complications, the median operative time was 210 minutes (range, 40 to 865 minutes), and the postoperative hospital stay was 3 days (range, 1 to 35 days). Seventy-three percent of patients met the definition of feasibility. Positive lymph node disease was found in 43 patients (32%); 10 patients (13%) were found to have T4, M1 disease. Of note, thoracoscopy was not feasible in this study in 30 of 134 patients. In addition, the number of lymph node stations sampled reliably, as well as the positivity rate of each station, was lower in the chest, when compared with abdominal lymph node stations. The frequency of positive lymph nodes by station was found to be: 2 (10%), 3 (8%), 4 (10%), 7 (10%), 8 (25%), 9 (10%), 10 (10%), 17 (34%), and 20 (27%). Thirty-two patients (24%) were deemed N0 by thoracoscopic-laparoscopic staging. Of these, 13 went directly to surgery without induction therapy. In final pathologic analysis, only 3 of 13 (23%) were found to have N1 disease, achieving a much higher accuracy of staging compared with the conventional noninvasive modalities. In this study, CT, MRI, and EUS incorrectly identified TN staging in 50%, 40%, and 30% of patients, respectively. Thoracoscopic-laparoscopic staging, therefore, is safe and feasible. It doubles the number of positive lymph nodes detected by conventional noninvasive staging. The overall accuracy will be better defined by the analysis of the lymph node negative group on long-term follow-up. Some centers, including our own, favor laparoscopic (without thoracoscopic) staging in combination with the noninvasive modalities of CT and EUS in the evaluation of GE junction tumors. Laparoscopic staging is simpler, is faster, and shortens hospital stays. As noted previously, the greatest yield in surgical staging occurs within the abdominal lymph node stations. EUS provides adequate sensitivity in detecting thoracic lymph node involvement. Thoracoscopy is reserved for those patients with suspicious findings in the chest (e.g., great vessel invasion), or in those with more proximal (predominantly squamous) tumors.
Minimally Invasive Esophagectomy
Minimally invasive esophagectomy is a complex and technically challenging procedure that is performed in only
P.2055
a few medical centers worldwide. Open esophagectomy approaches (e.g., transhiatal) remain the standard approach in most medical centers, as reviewed by Lee and Miller (1997). A number of open approaches have been devised that have been found historically to be useful in the resection of esophageal cancer. These operations, however, are associated with significant morbidity and mortality rates (in the range of 6% to 7%) even in experienced centers, as reported by Kelsen and co-workers (1998). In a report documenting statistics from a national database, Birkmeyer and colleagues (2002) found that mortality rates from esophagectomy ranged from 8% in high-volume centers to as high as 23 % in low-volume centers. Since the introduction of laparoscopic Nissen fundoplication in 1991, tremendous improvements in instrumentation and optics have fostered the development of minimally invasive approaches to esophageal diseases, including esophagectomy. The earliest descriptions of minimally invasive esophagectomy involved a combination of open surgery with either thoracoscopy or laparoscopy. In 1993, Collard demonstrated that esophageal dissection could be carried out thoracoscopically, when combined with laparotomy for gastric mobilization. There have been multiple subsequent reports of esophagectomy for cancer, performed by thoracoscopy and open laparotomy, including those of Liu (1995), Akaishi (1996), Dexter (1996), and Law (1997) and their associates. These studies demonstrated the feasibility of thoracoscopy-assisted esophagectomy, but the overall benefit was not well established. Depaula and co-workers (1995) were the first to describe a completely laparoscopic transhiatal esophagectomy. Swanstrom and Hansen (1997) published their initial experience in laparoscopic total esophagectomy, reaffirming the feasibility of this approach, but questioning its role as a potential cure for cancer. The first totally laparoscopic esophagectomy at the University of Pittsburgh was performed in 1996, as described by one of us (JDL) and collaborators (1998b). This initial approach has evolved into one combining thoracoscopy and laparoscopy for several reasons. Laparoscopic esophageal mobilization can be tedious and cumbersome through a completely laparoscopic approach. In addition, visualization of paraesophageal structures (such as the inferior pulmonary vein and the main-stem bronchi) and the performance of mediastinal lymph node dissection can be very limited when employing an exclusively transabdominal approach. In the first 77 patients at the University of Pittsburgh undergoing minimally invasive esophagectomy, one of us (JDL) and co-workers (2000a) used a combined thoracoscopic-laparoscopic approach in the majority, achieving a median length of hospital stay of 7 days, and a stage-specific survival similar to or better than open surgery results. The authors have now performed minimally invasive esophagectomy on more than 200 patients with high-grade dysplasia or cancer, as detailed by Fernando (2000, 2002b), Nguyen (1999, 2000a), Litle (2002), and one of us (JDL) (1998b, 2003) and respective co-workers, as well as Pierre and one of us (JDL) (2002). Using a similar approach, Nguyen and co-workers (2000b) recently compared minimally invasive esophagectomy to open approaches as part of a single-institution experience. This report highlights a shorter hospital stay, less morbidity, and similar operative times when compared with open techniques. In some centers, a complete thoracoscopic-laparoscopic approach is not feasible or preferred, and the use of hand-assisted techniques in the approach to esophagectomy has been explored, as detailed by Glasgow and Swanstrom (2001). Although it offers some potential advantage in cases in which organ integrity is important, a hand inserted near the esophageal hiatus or into the mediastinum frequently obscures the view and is generally unnecessary during minimally invasive esophagectomy.
Minimally invasive esophagectomy should be performed by surgeons who have extensive experience in minimally invasive esophageal surgery. Patients must be deemed fit for operation and must have resectable lesions, as characterized by EUS or CT. During the early portion of the learning curve, surgeons should consider performing cases in patients who have high-grade dysplasia, small tumors, a favorable body habitus, and minimal or no prior abdominal or thoracic surgery. As experience increases, the authors have found that previous abdominal or thoracic surgery and preoperative chemoradiation do not represent contraindications to a minimally invasive approach for either staging or resection of esophageal cancer.
Technique of Minimally Invasive Esophagectomy.
The procedure is begun with an on-table EGD to make a final assessment of the tumor's location as well as the suitability of the gastric conduit for reconstruction. If the EGD, EUS, or CT scan findings suggest gastric extension, T4 local invasion, or possible metastases, we perform a staging laparoscopy, thoracoscopy, or both. The patient is intubated with a double-lumen tube to permit single-lung ventilation and is placed in the left lateral decubitus position. With the right lung collapsed, four thoracoscopic ports are introduced (Fig. 136-3). The camera port (30 degrees, 10 mm) is placed at the seventh or eighth intercostal space slightly anterior to the midaxillary line. A 5-mm port is placed at the eighth or ninth intercostal space 2 cm posterior to the posterior axillary line for the ultrasonic coagulating shears (U.S. Surgical Corp., Norwalk, CT). A 10-mm port is then placed in the anterior axillary line at the level of the fourth intercostal space and is used for placement of a fan retractor to assist with anteromedial lung reflection and exposure of the esophageal bed. The last 5-mm port is placed posterior to the tip of the scapula. In most cases, a retracting suture (0-Surgidac, U.S. Surgical Corp., Norwalk, CT) is placed in the central tendon of the diaphragm and brought out of the inferior anterior chest wall through a 1-mm skin nick using the Endo-close device (U.S. Surgical Corp., Norwalk, CT). This traction suture allows downward retraction on the diaphragm without the need for providing manual retraction
P.2056
and provides excellent exposure of the distal esophagus at the level of the diaphragm.
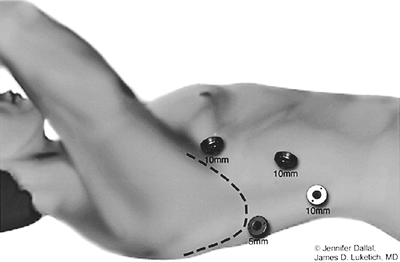 |
Fig. 136-3. Thoracoscopic port placement for minimally invasive esophagectomy. |
Next, the inferior pulmonary ligament is divided. The mediastinal pleura overlying the esophagus is divided, and the entire thoracic esophagus is exposed. The authors generally choose a plane distant to the tumor while dissecting circumferentially around the esophagus. Encircling the esophagus with a Penrose drain facilitates traction and exposure (Fig. 136-4). The azygos vein is then isolated and divided using the Endo-GIA stapler with a vascular load. Care is taken to preserve the pleura above the azygos vein. We believe that this pleural layer helps to maintain the gastric tube in a mediastinal location and may also help to seal the plane around the gastric tube near the thoracic inlet, thereby minimizing the extension of a cervical leak downward into the chest. Circumferential mobilization of the esophagus is then performed (including the surrounding lymph nodes, periesophageal tissue, and fat) from the level of the diaphragm to the thoracic inlet, following the plane along the pericardium and aorta as well as the contralateral mediastinal pleura, up to (but not including) the thoracic duct and azygos vein laterally. The authors do not routinely dissect out the recurrent laryngeal lymph nodes or perform a cervical lymph node dissection. Aortoesophageal vessels are sequentially ligated and divided with the Autoclip and Autosonic shears (U.S. Surgical Corp., Norwalk, CT). Deployment of clips during this dissection (especially laterally) will minimize the risks for bleeding and thoracic duct leak. Using this technique, the thoracoscopic part of this procedure can be performed within 1 to 2 hours in most cases. The intercostal nerves are then blocked with 1 to 2 mL of bupivacaine (0.5%) in dilute epinephrine for control of immediate postoperative pain. A single 28F chest tube is then inserted in the camera port, the lung is reinflated, and the port sites are closed.
The patient is then turned into a comfortable supine position. Access to the abdomen is obtained through a standard five-port technique (see Fig. 136-2). The left lateral segment of the liver is retracted anteriorly to expose the esophageal hiatus using a liver retractor (Diamond-Flex, Snowden-Pencer, Tucker, GA), which is secured into position with a self-retaining system (Mediflex, Velmed, Wexford, PA). The abdominal dissection is initiated with division of the gastrohepatic ligament, allowing exposure of the right crus of the diaphragm. At this stage of the operation, we avoid dividing the phrenoesophageal membrane because early entry into the mediastinum may lead to loss of pneumoperitoneum into the chest cavity and difficulties with exposure. The dissection is then carried over the anterior surface of the esophagus, with care being taken to identify and preserve the anterior vagus trunk. The diaphragmatic attachments of the spleen are taken down at the level of the left crus, which allows the spleen to fall away, greatly facilitating the retroesophageal and left crural portions of the dissection. The short gastric vessels are then divided with the ultrasonic coagulating shears. The dissection continues along the greater curvature of the stomach, preserving the right gastroepiploic vessels. The stomach is then folded over and reflected superiorly, allowing dissection of the undersurface of the stomach and extraction of celiac and gastric vessel lymph nodes. The left gastric artery and vein are then exposed and divided using the Endo-GIA vascular stapler.
 |
Fig. 136-4. Thoracic esophageal mobilization. |
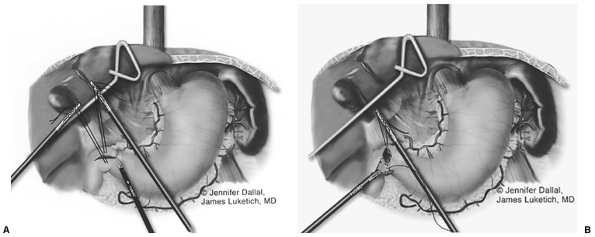 |
Fig. 136-5. Laparoscopic pyloroplasty. |
P.2057
After complete gastric mobilization, a pyloroplasty is then performed using ultrasonic shears, and closed transversely with the Endo-stitch device (2-0, U.S. Surgical Corp., Norwalk, CT) (Fig. 136-5A, B). The lesser curve fat and nodes are dissected en bloc with the stomach. Care is taken to preserve the right gastric vessels. A gastric tube is then constructed using the Endo-GIA II 3.5- or 4.8-mm stapler (U.S. Surgical Corp., Norwalk, CT) (Fig. 136-6). There is some variability in the construction of the gastric tube based on the characteristics of the resected lesion. Variable portions of the stomach may need to be resected as part of the specimen, and a narrow tube may need to be created. If gastric extension is found to be significant, then an adequate margin is ensured, and a chest anastomosis can be performed. For most patients operated on to date, gastric involvement has been minimal. Currently, we prefer a tube measuring 5 to 6 cm in diameter. Extreme caution must be exercised during gastric tube mobilization and stapling to avoid trauma. The gastric tube is attached to the esophagogastric specimen using two 2-0 Endo-stitch sutures. Marking sutures are also placed on the anterior surface of the proximal gastric tube to aid in the prevention of twisting as the tube is brought up into the neck (Fig. 136-7).
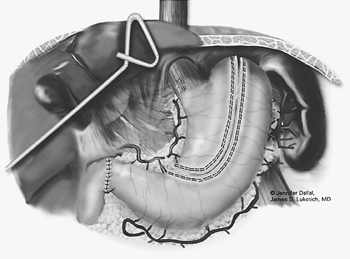 |
Fig. 136-6. Creation of the gastric tube. |
We routinely place a laparoscopic jejunostomy feeding tube during minimally invasive esophagectomy. In most cases, an additional 10-mm port is inserted in the right lower quadrant to facilitate suturing of the jejunum to the anterior abdominal wall. The colon is retracted cephalad, and the ligament of Treitz is identified. The jejunum is then followed distally 25 to 50 cm, and is tacked to the left anterior abdominal wall with an Endo-stitch. A needle jejunostomy feeding catheter kit (Compat Biosystems, Minneapolis, MN) is placed percutaneously into the peritoneal cavity under direct laparoscopic vision and is directed into the isolated loop of jejunum. A guidewire is advanced, and the catheter is threaded over the guidewire. The puncture site is then sealed with three tacking sutures positioned circumferentially. A small amount of air is injected into the lumen to confirm appropriate positioning of the catheter. If there is any concern regarding the intraluminal position of the J tube, an on-table Gastrografin injection can be performed.
The phrenoesophageal membrane is then divided to complete the esophageal mobilization. If necessary, the right and left crura can be divided with the ultrasonic shears to widen the hiatus, allowing passage of the specimen into the chest. This maneuver can help to minimize diaphragmatic compression of the gastric conduit, which is a common cause of delayed gastric emptying postoperatively.
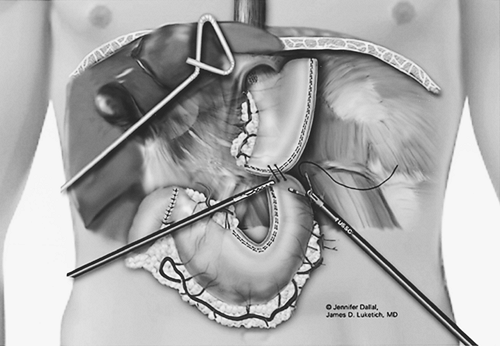 |
Fig. 136-7. The gastric tube is sutured to the specimen to facilitate alignment during pull-up. |
P.2058
A 4- to 6-cm horizontal left neck incision is made 2 fingerbreadths above the sternal notch (Fig. 136-8). The cervical esophagus is mobilized and exposed. A finger or sponge stick is used to retract the thyroid and avoid retraction on the recurrent laryngeal nerve. The dissection is carried distally, until the thoracic dissection plane is encountered. The esophagus is divided 1 to 2 cm below the cricopharyngeus, and the esophagogastric specimen is carefully pulled out of the wound, while the laparoscopic assistant carefully delivers the specimen and gastric tube in proper alignment into the mediastinum (see Fig. 136-8). The specimen is sent to pathology for frozen section analysis of the surgical margins. An anastomosis is then performed between the esophagus and gastric tube using a hand sewn, end-side EEA or side-side technique with an Endo-GIA II stapler (Fig. 136-9). Currently, we prefer the 25-mm EEA stapler. We prefer a very high anastomosis to ensure adequate resection of any tumor or Barrett's involvement, as well as to enable anastomotic leak drainage through the neck incision. A nasogastric tube is passed through the anastomosis distally into the gastric tube for postoperative decompression. Any redundant gastric conduit is then pulled back into the abdomen under direct visualization. The gastric tube is then tacked to the diaphragm to prevent herniation of abdominal contents into the chest, using the Endo-stitch. Care is taken to avoid injury to the gastric vessels. The abdominal instrumentation is withdrawn, and the ports are closed. The skin of the neck is loosely approximated with staples. The completed reconstruction is shown in Figure 136-9.
Results
In the initial 222 cases, there were 186 (84%) men and 36 (26%) women. The median age was 66.5 years
P.2059
(range, 39 to 89 years). Preoperative indications included carcinoma (79%) and high-grade dysplasia (21%). Neoadjuvant chemotherapy was used in 35% of patients and radiation in 16%. Before minimally invasive esophagectomy, esophageal stents were inserted in 6% and feeding tubes (G or J tubes) in 3%, with previous open abdominal surgery having been performed in 25% of the patients. Most cases can be completed using a minimally invasive approach, with open conversion ranging between 0% and 29% in reported series. Reasons for conversion include dense pleural adhesions, bleeding from esophageal and intercostals vessels, and difficulty with an intrathoracic anastomosis. Open thoracotomy is occasionally necessary in the setting of locally advanced tumors. In the initial 222 cases performed at this institution, minithoracotomy was liberally performed in 8 of the first 15 patients, with only four additional thoracotomies used in the next 207 cases. We have subsequently discovered that thoracoscopy permits full esophageal and lymph node dissection. Conversion to open laparotomy was performed in 4 patients because of dense abdominal adhesions. Overall, minimally invasive esophagectomy was successfully completed in 206 patients (93%), with a 30-day operative mortality rate of 1.4% (3 patients).
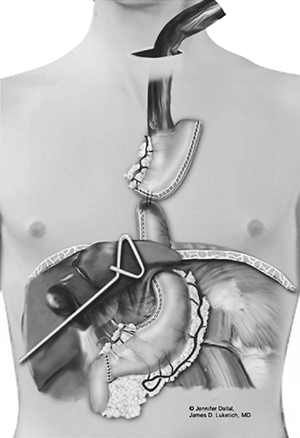 |
Fig. 136-8. Through a low transverse cervical incision, the esophagus is isolated proximally and divided, and the esophagogastric specimen is pulled out of the wound. |
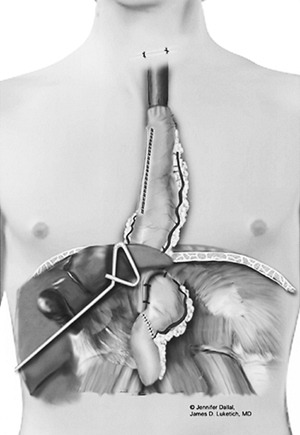 |
Fig. 136-9. Completed cervical anastomosis. |
The most frequent minor complication was atrial fibrillation (12%), followed by pleural effusion (6%), which was treated with bedside thoracentesis or pigtail catheter drainage, as described by Gammie and associates (1999). Major complications occurred in 32% of patients. The most common major complication was anastomotic leak (12%). Most anastomotic leaks were localized to the neck and were managed conservatively. Pneumonia was the second most common major complication, occurring in 8% of patients. Vocal cord palsy (4%), chylothorax (3%), and gastric tip necrosis (3%) were rare, but serious, complications. Early Teflon or Gelfoam injection of the vocal cords in the setting of recurrent laryngeal nerve palsy improves swallowing function and lowers the risk for aspiration. These results compare very favorably with both open and minimally invasive series reported to date. Nguyen and co-workers (2000b) reported a series of 18 combined thoracoscopic and laparoscopic esophagectomies at the University of California, Davis, where the most frequent complications included anastomotic leaks (11%), respiratory failure (11%), pulmonary embolism (6%), delayed gastric emptying (6%), and tracheogastric fistula (6%).
In the only study evaluating the morbidity and mortality of minimally invasive esophagectomy compared with open transhiatal esophagectomy, published by Nguyen and co-workers (2000b), the mean operative time (364 minutes), blood loss (297 mL), and length of intensive care unit stay (6 days) were decreased in the minimally invasive group compared with the open approaches at the same institution. The incidence of respiratory complications (pneumonia, pulmonary embolism, respiratory failure) was similar between the groups. Recently, Nguyen and associates (2003) reported their most recent results of the combined thoracoscopic and laparoscopic esophagectomy that was performed in 41 of 46 patients (38 patients had esophageal carcinoma); variations of the procedure were done in 5 of the 46 patients. There were 2 postoperative deaths (4.3%), and early complications occurred in 11 of the 44 (25%) surviving patients. Late complications [tracheogastric fistula, delayed gastric emptying, esophageal diaphragmatic herniation, and anastomotic stricture (the most common)] occurred in 12 (27.2%) of the surviving patients. In the 38 patients with cancer, the cancer-specific survival rate at 3 years was 57%. In our series, the median intensive care unit stay was 1 day, time to oral intake was 4 days, and hospital stay was 7 days. At a median follow-up period of 19 months, Kaplan-Meier estimates of survival parallel closely that seen in the open literature (Fig. 136-10).
The early results of minimally invasive esophagectomy compare favorably with those of many open series. Orringer and colleagues' series (1999) of 1,085 patients is one of the largest reported and serves as a standard with which to compare. In their series, the overall anastomotic leak rate was 13%, with a perioperative mortality of 4%. Fifty-three percent of the patients were discharged by the
P.2060
tenth postoperative day. More recently, Bailey and co-workers (2003) published their analysis of a prospective Veterans Administration cohort of 1,777 patients, citing a total complication rate of 50% and a mortality rate of 10%. In a recent analysis of esophagectomy outcomes in the national Medicare claims database published by Birkmeyer and co-workers (2002), high-volume hospitals were found to have the lowest mortality rate (8.1%). By comparison, the 30-day operative mortality rate after minimally invasive esophagectomy in our series was 1.3%, with a median hospital stay of 7 days. The low incidence of pneumonia (8%) and acute respiratory distress syndrome (ARDS) (5%) suggests an advantage for the minimally invasive approach. In addition, quality-of-life subjective assessments were found to be similar to preoperative values and population norms.
Minimally invasive esophagectomy is technically demanding with a steep learning curve. Operative times have been shown to decrease from 7 to 8 hours down to 4 to 5 hours after performing 20 operations. Therapeutic outcomes compare quite favorably (and in many instances, superiorly) to most open series. Such encouraging results will serve to broaden the applicability of this technique to higher-risk patient groups such as the elderly population, as reported by Perry and associates (2002). Prospective studies will be required to determine whether postoperative pain, recovery time, and cost are improved. A phase II Intergroup Study (E2202) is currently being developed to evaluate the clinical and oncologic results of minimally invasive esophagectomy for cancer compared with traditional open surgery. Until these results are available, the optimal surgical approach for each patient should be decided based on surgical experience, tumor characteristics, and patient preference.
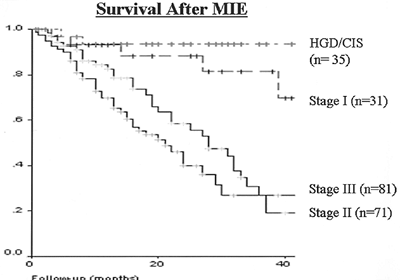 |
Fig. 136-10. Kaplan-Meier survival plot for 222 patients undergoing minimally invasive esophagectomy. |
MINIMALLY INVASIVE TECHNIQUES FOR OTHER ESOPHAGEAL OPERATIONS
Gastric Fundoplication
Antireflux surgery has proved to be an effective means of controlling gastroesophageal reflux disease (GERD), achieving the restoration of the lower esophageal sphincter (LES) function, and abrogating the reflux of gastric contents into the esophagus. It, thus, breaks the cycle of repetitive injury to both the normal and neoplastic esophageal mucosa. The most common procedure performed for the treatment of GERD is gastric fundoplication. Multiple variations have been devised to achieve this end, but the Nissen fundoplication has become the most widely accepted approach with the best long-term results. The goal of surgical therapy is to recreate a functional LES by reestablishing an intraabdominal high-pressure zone at the GE junction, and repairing any associated hiatal hernia. Historically, two problems have prevented the widespread acceptance of antireflux surgery in the treatment of GERD. The first problem was the development of postoperative dysphagia and gas bloating due to an overly competent esophageal high-pressure zone. The second was the perioperative morbidity and mortality associated with the open laparotomy that was previously required to perform antireflux surgery. These problems were overcome by two major developments in the surgical approach to GERD. The first is the recognition that a shorter, looser fundoplication markedly reduces the postoperative sequelae associated with antireflux surgery, as delineated by Donahue and associates (1985). The second was the introduction of laparoscopic Nissen fundoplication, which markedly reduced the morbidity, mortality, postoperative pain, length of stay, and time off from work. The
P.2061
threshold for referring patients to surgery has thus been reduced. Laparoscopic Nissen fundoplication is now one of the most commonly performed laparoscopic surgical procedures and has been shown to be a safe, highly effective alternative to lifelong medical therapy, although most centers have failed to publish their long-term results.
Historically, antireflux surgery has been reserved for patients with severe esophagitis or stricture, or for those refractory to medical therapy. As a result of the development of a minimally invasive surgical approach, with associated reduction in morbidity and mortality, patients with less severe disease are now considered potential surgical candidates. The principal indications for surgical intervention now include symptoms refractory to 8 to 12 weeks of maximal medical therapy, inability or unwillingness to maintain lifelong acid suppression, aspiration or recurrent pneumonia, children with severe esophagitis or failure to thrive, the development of esophageal ulcers, strictures or Barrett's metaplasia.
A thorough preoperative workup is performed to establish that GERD is the underlying cause of the patient's symptoms, to estimate the risk for progressive disease, to elucidate the presence or absence of esophageal shortening, and to evaluate esophageal body function and, occasionally, gastric emptying problems, as outlined by Hagan and Peters (2000). Preoperative evaluation should include a careful history and physical examination, barium esophagram with gastric and duodenal views, EGD, and esophageal manometry. Barium esophagography is an inexpensive test that provides important anatomic information, such as esophageal length, as well as the presence of a hiatal hernia, gastric ulceration, or esophageal stricture. When properly performed, a barium esophagram demonstrating free gastroesophageal reflux is a very specific test for pathologic GERD, although it is not as sensitive as 24-hour pH testing. Esophageal shortening can occur as a consequence of scarring and fibrosis associated with repetitive esophageal injury, as outlined by Gozzetti and co-workers (1987). Upper endoscopy is routinely performed to evaluate for esophagitis as well as the development of Barrett's esophagus or cancer. We also advocate the use of ambulatory 24-hour pH monitoring to document the frequency and duration of reflux episodes, thus providing the most concrete objective evidence of gastroesophageal reflux, as espoused by Johnson and DeMeester (1986). This test may not be necessary in individuals with classic signs of reflux with an initial favorable response to pharmacotherapy, or in those with pathologic signs of reflux, such as esophagitis, stricture, or metaplasia documented by endoscopy. Complications of reflux disease have also been shown to correlate with the presence of abnormal esophageal exposure to bilirubin, which can be directly tested (Bilitec monitoring, Medtronic Functional Diagnostics, Minneapolis, MN), as discussed by Nehra and co-workers (1999). Esophageal function should also be evaluated preoperatively using esophageal motility studies (esophageal manometry). Absent or diminished peristalsis should alert the clinician to the possibility of a primary esophageal motor disorder (e.g., achalasia) that might affect operative decision making. When peristalsis is absent or severely disordered (>50% simultaneous contractions), or if the amplitude of contractions in one or more of the lower esophageal segments is lower than 20 mm Hg, most surgeons would opt for a partial fundoplication, or at least a modification to a very floppy Nissen. The LES pressure is typically low or normal in patients with GERD. Elevated pressures are distinctly unusual and warrant further investigation. Gastric emptying studies may be useful in patients with diabetes, frequent vomiting, or prior esophagogastric surgery.
The principles of fundoplication are centered on the reconstruction of a functional LES zone. The degree of competence of the LES is directly proportional to the length of the intraabdominal esophagus in the absence of intrinsic muscle tone. Formation of an optimal wrap will restore 1.5 to 2 cm of intraabdominal esophagus, which will respond to intraabdominal pressure changes. The resting LES pressure must be approximately three times the resting gastric pressure to overcome gastric distention. The fundus of the stomach should be used to create the wrap, and the vagus nerves must be identified and preserved. These structures are critical in receptive relaxation of the stomach in the setting of deglutition and also play an important role in gastric emptying. Finally, the esophageal peristaltic wave must be able to overcome the resistance of the reconstructed valve.
Complications of laparoscopic fundoplication include bleeding, infection, gastric or esophageal perforation, vagal nerve or splenic injury, pneumomediastinum, dysphagia, bloating, and dumping. The wrap and crural repair may slip or disrupt over time, leading to recurrent hiatal hernia and symptoms as well as the need for reoperation.
Choice of Operation
A variety of antireflux procedures have been developed, all of which have advocates claiming effective results, as described by such innovators as Nissen (1956), Collis (1957), Dor and co-workers (1962), Toupet (1963), Skinner and Belsey (1967), and Hill (1967). The Nissen fundoplication (360-degree fundoplication) has become the most popular repair and has been shown to be effective in comparative studies, achieving more than 90% control of gastric reflux symptoms, as reviewed by DeMeester and co-workers (1974, 1986). First performed by the Swiss surgeon Rudolph Nissen in 1955, it entails the construction of a 360-degree proximal gastric wrap that creates a distal esophageal high-pressure zone to prevent acid reflux. The Toupet (180- to 270-degree posterior) and Dor (anterior) fundoplications employ partial wraps that are useful in the setting of impaired esophageal motility. The Belsey Mark IV (270-degree transthoracic) fundoplication is currently
P.2062
most frequently employed in patients with a history of significant abdominal surgery and a hostile abdomen, as discussed by Orringer and associates (1972); however, short-term results of thoracoscopic Belsey Mark IV fundoplication reported by Nguyen and colleagues (1998) have demonstrated up to a 20% failure rate, which has limited our enthusiasm for this approach. Worldwide, the Nissen fundoplication remains the most popular procedure in the surgical treatment of GERD and shall be the focus of the remaining discussion.
Dallemagne published the first description of a minimally invasive Nissen fundoplication in 1991. During the past decade, this technique has proved safe, effective, and durable, and now is considered by many to be the standard in the surgical approach to GERD. Laparoscopic Nissen fundoplication has been shown to achieve decreased pain, decreased length of hospital stay, and earlier return to work when compared with its open counterpart. From our clinical experience, it is our opinion that good results can only be anticipated if the procedure is performed in a center staffed with surgeons who have a high degree of minimally invasive surgical skills and experience with antireflux surgery.
Technique of Laparoscopic Nissen Fundoplication.
The anesthetized patient is placed in a comfortable supine position. We recommend routinely performing on-table upper endoscopy to gain up-to-the-minute information regarding the endoluminal appearance of the esophagus and stomach, the presence and extent of ulcers, strictures, Barrett's esophagus, or hiatal hernia. Care is taken to minimize insufflation so as not to overdistend the stomach and small bowel. The scope is used to decompress the stomach and is then withdrawn. A urinary catheter is inserted to decompress the bladder. Subcutaneous heparin is administered, and inflatable compression stockings are worn on the lower extremities for perioperative deep venous thrombosis prophylaxis. Many surgeons prefer either the lithotomy or inverted Y position. We favor the standard supine position, with the surgeon standing to the patient's right and the first assistant standing to the patient's left. The patient is placed in a steep reverse-Trendelenburg position. Video monitors are positioned on either side of the head of the table. Access to the abdomen is achieved through a direct cut-down technique or the Varess needle. With a closed (Varess) technique, a 2-mm skin incision is made at the umbilicus or just inferior to the left costal margin. A Varess needle is then inserted into the abdominal cavity. Two pops should be heard as the needle penetrates the fascial layers. Before insufflation, the needle position should be confirmed with the drop test. We prefer to perform a direct, open cut-down approach to minimize the risk for inadvertent injury to the abdominal contents. This approach is particularly useful in patients with prior abdominal surgery, who may have adhesions and distorted anatomy.
Our preferred port placement is demonstrated in Figure 136-2. We begin with placement of a 10-mm blunt port through the right rectus muscle through direct cut-down technique, at a level near the midpoint between the umbilicus and xiphoid. This port may be positioned superiorly in the setting of larger hiatal hernias, where extensive mediastinal dissection is anticipated. Care is taken to preserve the rectus musculature, gently spreading with blunt retractors rather than dividing with electrocautery. This simple step will minimize delayed port-site hernias. After insufflation with carbon dioxide to a pressure of 15 mm Hg, a 5-mm, 30-degree camera is then placed into the abdomen to assess the gastrohepatic terrain, the presence of adhesions or other anatomic considerations. Under direct visualization, a 5-mm port is placed in the midclavicular line just inferior to the left costal margin. A second 5-mm port is placed through the left rectus, 3 to 4 fingerbreadths to the left of the initial 10-mm port. A third 5-mm port is placed along the right costal margin toward the right shoulder. A liver retractor is then introduced through a 5-mm port positioned just inferior to the right costal margin in the midaxillary line. Attachment to a table-mounted mechanical arm facilitates liver retraction (Fig. 136-11). The patient is then placed into steep reverse-Trendelenburg (Fowler) position, allowing gravity to assist in the displacement of the bowel and stomach from the diaphragm. A toothed, noncrushing atraumatic grasper (Snowden-Pencer, Tucker, GA) is used in the surgeon's left hand, and the harmonic scalpel (UltraCision, Inc., U.S. Surgical, or similar) is used in the right hand. The 30-degree camera is positioned in the assistant's left hand to provide enhanced visualization of the retrogastric and retroesophageal structures. The gastrohepatic omentum is then opened, and the caudate lobe of the liver is exposed (Fig. 136-12). An aberrant left hepatic artery branch arising from the left gastric artery may be present in up to 25% of patients and should be avoided if possible, although
P.2063
many are small and can be divided with little consequence. The right crus is exposed, and care is taken to preserve the peritoneal lining over both crura. The phrenoesophageal ligament is divided with the Autosonic shears or harmonic scalpel along its anterior border, with care being taken to identify and preserve the anterior vagus nerve. This tissue is divided from right to left above the epiphrenic fat pad across the crural arch. Dissection is extended further to the left, dividing the gastrophrenic peritoneum separating the gastric fundus from the diaphragm. Dissection of the left crus is carried out posteriorly near the angle of His. Inferior traction on the gastric fundus and epiphrenic fat pad are necessary to expose the left crus adequately. If a hiatal hernia is present, it is reduced fully into the abdominal cavity with gentle traction. All adhesions and attachments of the distal esophagus and proximal stomach are released to facilitate this maneuver. During hiatal mobilization, care must be taken to avoid violation of the pleura, which can often be seen lateral to the esophagus adherent to larger hernia sacs, to prevent pneumothorax.
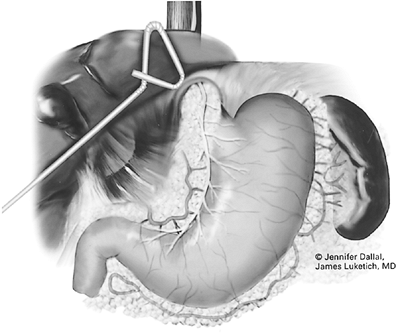 |
Fig. 136-11. Positioning of the liver retractor to expose the esophageal hiatus. |
 |
Fig. 136-12. The gastrohepatic omentum is opened, providing exposure to the caudate lobe and the right crus. |
At this point, division of the short gastric vessels is performed. Whether or not the short gastric vessels need to be divided during a laparoscopic Nissen fundoplication is controversial, and was not performed in the original description by Nissen. A randomized trial by Dalenbak and associates (1998) has demonstrated that failure to divide the short gastric vessels is associated with an increased risk for postoperative dysphagia. We believe the short gastric vessels should be divided to promote full fundic mobilization, to diminish tension on the wrap, and to ensure that the fundus (not the body) is used in the performance of the wrap. Division of the proximal three to four short gastric vessels relieves tension transmitted to the splenic capsule during fundoplication and decreases the risk for postoperative dysphagia by diminishing torque on the completed fundoplication. Dissection is begun by taking down any remaining gastrophrenic adhesions from the angle of His to the tip of the spleen. The assistant grasps the gastrosplenic omentum near the greater curvature and lifts anteriorly, and the surgeon provides countertraction on the stomach. A window is then created into the lesser sac. Once the lesser sac is entered, the assistant places one blade of the grasper in the lesser sac to optimize retraction. The surgeon may then serially take down the greater curvature vessels with a scissors and clips, a bipolar electrosurgical device, or the Autosonic shears or harmonic scalpel (Fig. 136-13). Care must be taken as one advances toward the superior pole of the spleen because the vessel length becomes shorter and the risk for capsular tear greater. Division of the short gastric vessels provides excellent exposure of the inferior portion of the left crus. Further dissection on the left side at this point greatly facilitates the subsequent creation of a retroesophageal window.
Blunt dissection is then used to develop the window between the crura and the esophagus. The posterior vagus nerve is identified and may be left adjacent to the undersurface of the esophagus with subsequent incorporation in the Nissen wrap, or alternatively it may be dissected further and left outside of the wrap. A retroesophagogastric window is created by dissection from right to left under direct vision until the left crus of the diaphragm is identified behind the esophagus. After adequate mobilization of the distal esophagus and gastric fundus, the esophagogastric fat pad is dissected from its close adherence to the anterior aspect of the GE junction. This step allows accurate determination of the true GE junction, identified by the point of splaying of the esophageal muscle fibers. If the esophagus retracts into the mediastinum, it is imperative that mobilization
P.2064
of the esophagus continues by further cephalad dissection. This is especially important in patients with a large hiatal hernia, or in those with a foreshortened esophagus secondary to a peptic stricture. Upon completion of dissection, the fundus should be free to pass behind the esophagus and remain there without retraction into the chest. If the degree of esophageal shortening is deemed to be significant, then consideration of an esophageal lengthening procedure (Collis gastroplasty) should be made (see subsequent section, Laparoscopic Collis Gastroplasty). Tension on the esophagus is released, and a bougie dilator (50F to 54F) is passed. We generally use a 50F bougie for most patients with normal motility. A larger bougie size may be used in the patient with diminished motility. The bougie permits calibration of the wrap, thus preventing excessive narrowing of the esophagus during fundoplication. However, it is clear that it is possible to make a tight wrap around a relatively large bougie, emphasizing that the tension present during creation of the wrap is equally important as bougie size. We generally perform a floppy Nissen, with a generous, but loose, fundic wrap. A noncrushing grasper is then inserted and passed through the retrogastric window. The fundic tip is then grasped and pulled posteriorly through the window (Fig. 136-14A). A shoeshine maneuver allows assessment of wrap orientation and tension (Fig. 136-14B). The line of the divided short gastric vessels should appear on the right side of the esophagus. If adequate mobilization is achieved, the wrap should remain in place after release of the grasper. If the wrap springs back behind the esophagus, then it is too tight, and mobilization is incomplete.
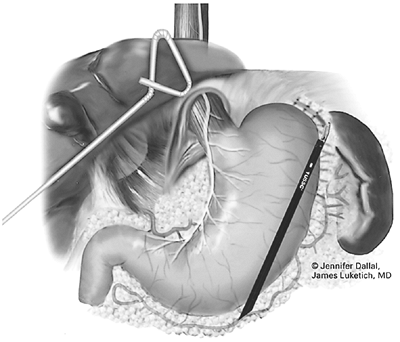 |
Fig. 136-13. Division of the short gastric vessels facilitates fundic mobilization. |
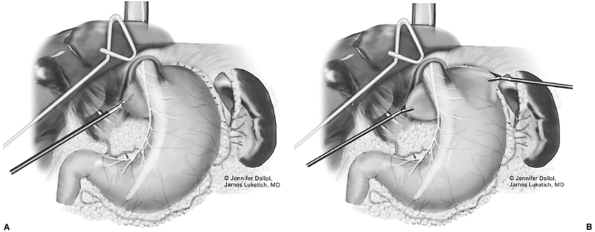 |
Fig. 136-14. A. The gastric fundus is wrapped posteriorly at the level of the gastroesophageal junction. B. Assessment of wrap orientation and tension. |
The importance of a floppy Nissen was highlighted by Donahue and Bombeck (1977), when it was demonstrated that reflux could be prevented without a marked increase in postoperative LES pressures, and with a lower incidence of postoperative dysphagia and bloat. The fundoplication is then secured using three 2-0 nonabsorbable sutures (Surgidac, U.S. Surgical Corp., Norwalk, CT) (Fig. 136-15A). Each stitch should incorporate a full-thickness bite of stomach and a partial-thickness bite of esophagus to prevent slippage of the wrap around the body of the stomach, or into the thoracic cavity. Ideally, the wrap should be 1.5 to 2 cm in length because longer wraps are associated with a significantly higher risk for postoperative dysphagia. These knots are typically tied intracorporeally (e.g., Endo-stitch). Although an extracorporeal technique can be employed, we prefer intracorporeal knot-tying techniques.
The crura are then approximated behind the esophagus using a heavy nonabsorbable suture (0-0 Surgidac) (Fig. 136-15B). The knots are secured using the Endo-stitch device. Pledgets are not routinely used but are helpful in situations of attenuated tissue or when a large defect is present. When the hiatal defect is unable to be closed primarily, a diaphragmatic relaxing incision can be made, or mesh can be deployed, either synthetic (Gore-Tex, W.L. Gore and Associates, Newark, DE) or biological (Surgisys, Cook Biotech, Inc., West Lafayette, IN). The mesh is positioned to close the defect and secured with 2-0 Surgidac sutures or an endoscopic tacking device (Origin Medsystems, Menlo Park, CA). Some surgeons prefer to tack the fundus to the undersurface of the diaphragm in two or three places to prevent migration of the fundoplication into the chest. This is probably not necessary if the esophageal length is adequate, and if the wrap is performed without tension (Fig. 136-15C). In addition, tacking maneuvers will not prevent recurrent hiatal herniation if there is persistent cephalad tension due to esophageal shortening. All blood and irrigation fluid is removed from the left subphrenic area, and all instruments and ports are removed. Before closure, we pass a nasogastric tube
P.2065
under direct vision in complex cases of giant paraesophageal hernia, redo Nissen or Collis-Nissen procedures. We do not routinely pass nasogastric tubes for simple Nissen fundoplications. If a nasogastric tube is inserted, it is generally removed on the first postoperative morning. Small ports of 5-mm diameter are generally not closed, except at the skin level. Larger ports are generally closed under direct laparoscopic visualization with a suture passer to incorporate fascia and peritoneum. Postoperatively, all antacid and antireflux medications can typically be discontinued. We routinely perform a postoperative barium swallow to rule out leak or obstruction and to use as a baseline study for later comparison should clinical problems develop. Failure to obtain this study leaves the clinician without a later comparison film. The patient is started on clear liquids on the morning after surgery. The diet is then gradually advanced to a soft-mechanical (post-Nissen) regimen for about 2 weeks while esophageal edema resolves. Patients are typically discharged to home on postoperative day number 1 or 2.
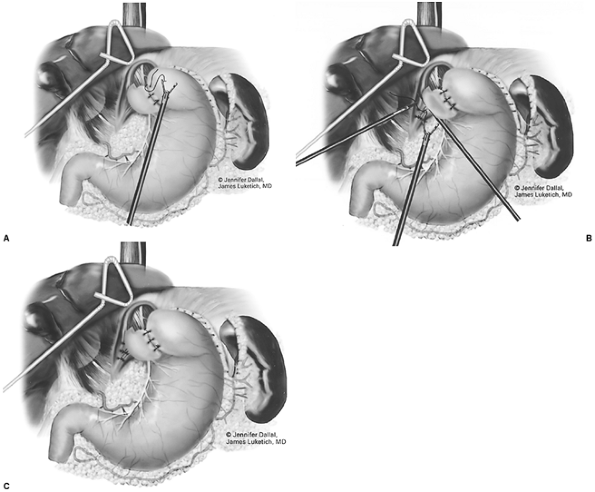 |
Fig. 136-15. A. Suture placement for Nissen fundoplication. B. Closure of the crural defect. C. Completed Nissen fundoplication. |
Results
Antireflux surgery has emerged as a superior option in the long-term treatment of GERD when compared with medical therapy alone. A Veterans Administration randomized trial presented by Spechler (1992) comparing medical (ranitidine, metoclopramide, and sucralfate) and surgical (open Nissen fundoplication) therapy demonstrated that surgical therapy was more effective at controlling symptoms, preventing the development of esophagitis, and restoring a normal esophageal pH. However, with the rapid acceptance of laparoscopic antireflux procedures, as well as the emergence of maintenance proton inhibitor therapy, both treatment arms of this study are now essentially obsolete. Randomized studies will be required to compare the long-term efficacy of more modern medical [proton pump inhibitors (PPIs) and surgical (laparoscopic Nissen) therapeutic modalities].
If surgeons performing laparoscopic Nissen fundoplication maintain the well-established principles of antireflux surgery, patients are likely to enjoy the same long-term control
P.2066
of GERD seen with the open Nissen approach. Many retrospective and nonrandomized studies have documented less postoperative pain, shorter hospitalization, and faster recovery with laparoscopic Nissen fundoplication, as reviewed by Jamieson and colleagues (1994). Most series, including those of Hinder (1994) and Trus (1996) and their associates, report control of reflux symptoms in more than 90% of patients, comparable to the open approach. These concepts have been validated by several prospective, randomized studies. Laine and co-workers (1997) compared 55 patients undergoing open Nissen with 55 patients undergoing laparoscopic Nissen fundoplication. All of the patients in the laparoscopic group, and 86% of the open group, achieved good to excellent control of their reflux symptoms. Mean hospital stay was 3.2 versus 6.4 days, and the mean sick leave was 15.3 days versus 37.2 days, when comparing the laparoscopic to the open groups. Heikkinen and associates (2000) demonstrated significant, but equal, improvement in Gastrointestinal Quality of Life indices in both laparoscopic and open patient cohorts. Although laparoscopic operative times remain slightly higher (98 versus 74 minutes), the laparoscopic approach has been associated with less pain, faster return to normal life, and faster return to work. Heikkinen and co-workers (1999) also found that total costs, including direct and indirect costs arising from lost workdays, are significantly lower ($7,506 vs. $13,118) in the laparoscopic group during prospective, randomized comparison. Earlier hospital discharges, and discontinuation of expensive medication represents substantial savings in health care dollars, and the rapid return to work markedly reduces indirect costs. Postoperative complications occur in 8% to 10% of patients and include ileus (6%), pneumothorax (2%), dysphagia (2%), need for reoperation (0.6%), and perforated viscus (0.2%), comparable or superior to the open technique, as summarized by Pohl and colleagues (2001). The rate of conversion to an open procedure is about 2%. There is also a decrease in the risk for splenic injuries, dropping from about 2% in most open series to 0.1% to 0.2% in most recent laparoscopic series. Mortality is extremely uncommon (<1%) in both laparoscopic and open cohorts. Campos and associates (1999) have identified three clinical parameters that have been independently found to predict a good outcome in laparoscopic Nissen fundoplication: patient presentation with typical symptoms (heartburn, regurgitation), pH test-proven abnormal esophageal acid exposure, and patients who have responded to, but remain dependent on, proton pump inhibitors for symptom relief. Although these data demonstrate that laparoscopic Nissen fundoplication is both safe and effective and bears certain advantages when compared with the open approach, it should be emphasized that most follow-up studies report relatively short-term results.
In addition to controlling typical reflux symptoms, laparoscopic fundoplication has also been shown to improve atypical features of GERD. Interestingly, while the otolaryngologic manifestations of GERD (laryngitis, pharyngitis) usually respond to antisecretory medications, reflux-induced asthma responds convincingly only to antireflux surgery, as discussed by Kahrilas (1996). In a study of 62 patients with both GERD and asthma followed for up to 19.1 years, patients were randomized to antacids (24 control subjects), ranitidine, 150 mg three times daily (22 medical subjects), or Nissen fundoplication (16 surgical subjects). In the surgical group (but not in the medical or control groups), there was an immediate and sustained reduction in acute nocturnal exacerbations of wheezing, coughing, and dyspnea. At 2 years, 75% of the surgical group sustained improvement in their overall asthma status, as compared with 9% in the medical group and 4% in the control group. Despite such subjective symptomatic improvements, Sontag and co-workers (2003) were unable to identify any significant differences in pulmonary function testing, medication requirements, and overall survival.
Antireflux surgery also may favorably affect the progression of Barrett's esophagus, and some reports have even claimed it may lead to its regression, as discussed by Gurski and co-workers (2003). Brand and colleagues (1980) were among the first to describe the complete regression of Barrett's esophagus in patients who have undergone a Nissen fundoplication. In their series, 4 of 10 patients demonstrated complete regression of Barrett's epithelium. In contrast to the relatively unreliable regression of longer-segment Barrett's esophagus (>3 cm), DeMeester and associates (1998) have found that up to 73% of patients with Barrett's metaplasia at the GE junction can achieve complete regression after antireflux surgery. In addition, antireflux surgery is associated with a reduced incidence of dysplasia and adenocarcinoma. McCallum and co-workers (1991) prospectively evaluated 181 patients with Barrett's esophagus treated medically (152 patients) and surgically (29 patients). After a mean follow-up of 62 months in the surgical group, and 49 months in the medical group, there was a significant difference in the progression to dysplasia and adenocarcinoma. Whereas dysplasia was detected in 19.7% of patients in the medical group, it was only seen in 3.4% of patients who had undergone surgery. No patient in the surgical group developed adenocarcinoma, compared with two medically treated patients. In another study by Katz and associates (1998) of 102 patients with Barrett's esophagus with 503 patient-years of endoscopic follow-up, 19 patients developed low-grade dysplasia, 4 developed high-grade dysplasia, and 4 patients progressed to adenocarcinoma with medical treatment alone. None of the patients from this cohort who underwent Nissen fundoplication developed dysplasia or adenocarcinoma. In another comprehensive review of the outcomes in 97 patients with Barrett's esophagus treated with fundoplication during a median follow-up of 5 years, Hofstetter and co-workers (2001) found that regression of low-grade dysplasia to a nondysplastic Barrett's epithelium occurred in 44% of patients. No patient developed high-grade dysplasia or cancer in 410 patient-years of follow-up. In cases in which cancer
P.2067
has been reported after antireflux surgery, there has generally been a failure of surgical therapy, most commonly due to inadequate wrap construction, the added complexity of reoperative gastric surgery, or failure to recognize patients with a shortened esophagus (e.g., in those with paraesophageal hernias).
Whether to perform routinely a complete (Nissen) or partial (Toupet) wrap remains a matter of debate. Proponents of partial fundoplication argue that a total fundoplication is associated with a higher rate of postoperative bloating and dysphagia. However, several recent reports by Horvath (1999) and Fernando (2002a) and their associates have clearly shown that there is an unacceptably high prevalence of recurrent reflux (up to 46% of patients) within 2 to 3 years after partial fundoplications. However, there are equally strong clinical studies that favor partial wraps, achieving results similar to those seen with complete wraps. Several prospective, controlled series have documented similar short- and long-term efficacy of partial wraps in the open and laparoscopic settings, as discussed by Lundell (1996) and Laws (1997) and their co-workers, respectively. In a study of 110 patients completing a median follow-up of 11.5 years by Hegedorn and co-workers (2002), 54 patients underwent a total wrap, and 56 underwent a partial posterior fundoplication. Control of heartburn (88% vs. 92%) and acid regurgitation (90% vs. 94%) was comparable between the two groups. No difference in dysphagia was observed; however, a significant increase in the prevalence of flatus and postprandial bloating was noted in the Nissen group. In a compelling randomized study of 200 patients by Zornig and colleagues (2002), cases were stratified by the presence (in 50 patients) or absence (in 50 patients) of esophageal dysmotility in both the Nissen (100 patients) and Toupet (100 patients) fundoplication groups. Symptomatic relief was achieved in 88% of the Nissen group and in 90% of the Toupet group, with a notably high incidence of dysphagia in the Nissen group (30 vs. 11 patients) that did not correlate with preoperative motility status. Surprisingly, Fibbe and associates (2001) have found that performance of a partial fundoplication in the presence of dysmotility has not achieved statistically superior results when compared with Nissen, leading authors such as Rydberg and colleagues (1999) to suggest that tailoring the wrap based on preoperative motility testing may not be necessary. These studies have incited a waxing popularity of partial fundoplication in the treatment of GERD, achieving similar control of reflux symptoms, while avoiding the aforementioned adverse postoperative sequelae. Other reports have shown, however, that Toupet fundoplication may be associated with a high-degree of late failure (by both clinical and objective measures) when employed as a primary treatment for GERD. Jobe and co-workers (1997) reported a prospective series of laparoscopic Toupet fundoplications performed on all patients with documented reflux. Although the procedure was safe and well tolerated, a symptomatic failure rate was obtained in 20% of patients, with abnormal DeMeester scores documented in 59% at a mean of 22 months. Toupet fundoplication was found to fail more often in patients with severe GERD than in patients with uncomplicated or mild disease. In another study by Horvath and co-workers (1999), a preoperative DeMeester score higher than 50 yielded 86% sensitivity for predicting failure of the Toupet wrap. In our opinion, the partial fundoplication appears to result in a higher rate of recurrence, and we do not use it routinely. In the setting of poor motility, the use of partial fundoplication still has a role and should be considered as an option in patients with motility abnormalities.
Recurrent herniation of an intact or partially disrupted wrap is the most common cause of failure in the laparoscopic experience. Why this occurs so commonly is a multifactorial phenomenon and includes the limitation of esophageal mobilization through a laparoscopic approach, breakdown of the crural closure, or even reduced adhesion formation after laparoscopic surgery. Horgan and colleagues (1999a) have proposed that the key technical factors preventing recurrence are effective crural closure, a thorough transhiatal esophageal mobilization, attention to the geometry of the fundoplication, and anchoring of the wrap to the surrounding tissues. Obesity is also being identified as a key factor in recurrence of symptoms. In a study of 224 patients undergoing antireflux surgery by Perez and co-workers (1999), patients with a body mass index (BMI) of more than 30 had a significantly higher recurrence rate (27%) than those with a BMI from 25 to 30 (5% to 8%). Other surgeons have shown that the addition of a Collis gastroplasty yields superior results in patients with complex GERD, especially those with large hiatal hernias. In an impressive series, Maziak and associates (1998) showed a 2% recurrence rate following antireflux surgery employing a Collis gastroplasty at long-term follow-up. In our opinion, the Collis gastroplasty decreases postoperative recurrences in patients with GERD associated with giant hiatal hernias, long-segment Barrett's esophagus, strictures, or in the redo setting.
Reoperative surgery for recurrent reflux is complex and is traditionally performed by open methods. As surgeons gain laparoscopic experience, these procedures are increasingly being performed in a minimally invasive fashion. In one of the largest series of patients undergoing laparoscopic reoperative antireflux surgery, One of us (JDL) and associates (2002) reported the experience of 80 patients followed over a 5-year period. The most common symptoms at the time of presentation were recurrent heartburn (53%), dysphagia (25%), and regurgitation (22%). Key factors in the success of reoperative antireflux surgery include careful patient selection and extensive antireflux surgical experience. Careful examination of the patient's symptoms and a careful evaluation of the preoperative assessment before the original procedure are helpful in determining whether a fundoplication was originally warranted. Review of the operative note may help to identify difficulties encountered at the time of the original operation as well as the adequacy of the mobilization of the distal esophagus, gastric vessels,
P.2068
and esophageal fat pad. Failure in mobilizing the fat pad can lead to difficulty in identifying the GE junction, and the creation of a malpositioned wrap over a tubularized portion of the stomach, rather than the distal esophagus. Barium studies will identify anatomic abnormalities and will aid in evaluating the position and integrity of the wrap. Manometry will elucidate underlying motility disorders, and 24-hour pH testing will provide an objective measure of recurrent reflux. In the series published by one of us (JDL) and co-workers (2002), reoperative antireflux surgery was able to be completed laparoscopically in 97.5% of cases, with only two conversions. The most common problems identified at surgery were mediastinal migration of the wrap (60%) and abnormal wrap geometry (14%). A Nissen-Collis procedure was performed in most (42) patients. Toupet fundoplications were performed in 6 patients secondary to abnormal esophageal motility. Complications occurred in 16 patients, the most common of which was minor gastric perforation (all of which were repaired intraoperatively). Two reoperations were required in patients with a bile leak and a leak from a pyloroplasty. The median length of stay was 2.5 days. Health-related quality-of-life scores were rated as excellent in 65% of patients and satisfactory in 17%. Laparoscopic reoperative antireflux surgery can thus be performed safely and effectively in centers experienced in minimally invasive surgery of the esophagus.
The cost effectiveness of laparoscopic antireflux surgery has been well established. Several analyses have been performed comparing laparoscopic fundoplication with long-term medical therapy. Heudebert and co-workers (1997) found that laparoscopic surgery was the most cost-effective form of treatment for patients requiring life-long medical therapy with proton pump inhibitors. This cost effectiveness will be further enhanced by the developing trend of ambulatory laparoscopic fundoplication. In a study of 61 patients undergoing laparoscopic fundoplication, Milford and Paluch (1997) found that 92% of patients could be successfully treated and discharged on the same day, with all of the patients requiring a hospitalization being discharged on the first postoperative day. Fernando and co-workers (2002c) have furthered these observations by demonstrating a substantial improvement in the quality of life of patients undergoing laparoscopic antireflux surgery. Laparoscopic Nissen fundoplication has thus emerged as the procedure of choice for most surgical candidates with GERD. It is cost-effective, can be performed with little morbidity, and improves quality of life.
Laparoscopic Collis Gastroplasty
Acquired Shortening of the Esophagus
Acquired shortening of the esophagus is a known sequela of complicated gastroesophageal reflux disease. It is most commonly encountered in the setting of ulcerative esophagitis, peptic strictures, paraesophageal herniation, and after failed antireflux operations. These clinical conditions can result in the development of transmural inflammation leading to repeated cycles of injury and repair over time, which ultimately results in scarring and contraction of the esophagus and cephalad displacement of the GE junction. Such shortening can reduce or eliminate the ability to achieve adequate esophageal mobilization and length during fundoplication, ultimately resulting in wrap slippage and failure.
The overall incidence of acquired shortening of the esophagus remains unknown, and some authors question its very existence. In a review of the open and laparoscopic literature, the frequency of esophageal shortening ranges widely from the 60% reported by Pearson and Todd (1987) to the 0% reported by Hill and co-workers (1970) and some laparoscopic series. Horvath and colleagues (2000) have estimated that esophageal shortening can be demonstrated in 10% of patients undergoing antireflux surgery. Its prevalence is perhaps greatest in the setting of giant paraesophageal herniation, whereby the GE junction is chronically displaced within the mediastinum in 77% to 100% of patients, as reported by Altorki and associates (1998). When present, the rate of recurrence is markedly increased (up to 37%) compared with an overall reflux recurrence rate of 3% to 5% in patients undergoing routine antireflux surgery, as initially demonstrated by Skinner and Belsey (1967).
As discussed previously, an adequate fundoplication mandates the maintenance of 2 cm of tension-free intraabdominal esophagus. Unrecognized esophageal shortening is a major cause of recurrent herniation, which is the most common cause of fundoplication failure. It is also the primary explanation for a slipped Nissen fundoplication. In many such instances the initial repair is constructed around proximal tubularized stomach rather than the terminal esophagus. Although there is no ideal method of firmly establishing the presence of a shortened esophagus, the combination of radiographic studies (barium swallow) and endoscopic findings will alert the surgeon to situations in which esophageal shortening is likely (Fig. 136-16). Large hiatal hernias (>5 cm) that fail to reduce during barium roentgenography, and the presence of esophageal strictures, are most likely to be associated with esophageal shortening. Adequate intraoperative assessment requires complete dissection of the esophagogastric fat pad overlying the GE junction. It should be kept in mind that the presence of pneumoperitoneum elevates the diaphragm, which could provide the false impression that an adequate length of intraabdominal esophagus is present. Similarly, downward retraction through a Penrose drain looped around the GE junction, as well as the downward pressure produced by the presence of a bougie, could provide the illusion of an additional 2 to 3 cm of intraabdominal length. When a shortened esophagus is encountered, maximal mobilization of the distal esophagus should be performed. Altorki and co-workers (1998)
P.2069
contend that most cases can be successfully managed in this way. If a tension-free 2- to 3-cm segment of intraabdominal esophagus cannot be obtained with these maneuvers, however, then an esophageal lengthening procedure should be performed.
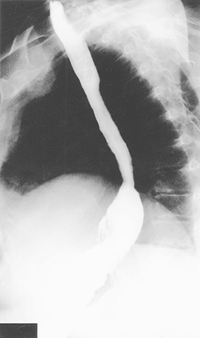 |
Fig. 136-16. Barium esophagram demonstrating shortening of the esophagus. |
Many treatment options have been delineated in the management of esophageal shortening, including esophagopexy, esophagectomy, transthoracic fundoplication, and lengthening procedures. Hill (1967) has long recommended an esophagogastropexy (Hill procedure) as a treatment for all patients with GERD, including those with esophageal shortening. Although the reported results are excellent, this approach has failed to obtain widespread acceptance because of its perceived complexity. On occasion, total esophagectomy with gastric pull-up may be the best option for a severely damaged, dysfunctional esophagus. However, this approach is typically reserved for extremely severe or recalcitrant cases. Intrathoracic fundoplication can provide good control of reflux, as documented by Moghissi (1983). However, there are often significant complications associated with this iatrogenically created herniated wrap, including pain, dysphagia, bleeding, ulcerations, and perforation, as outlined by Mansour and associates (1981). The complexity and associated complication rates of the aforementioned procedures have promoted the development of esophageal lengthening techniques that have become the standard of treatment of the short esophagus. Allen and Matthews (1993) described a circular esophagomyotomy to promote esophageal length in the setting of fundoplication. This method failed to achieve clinical acceptance because of its technical difficulty, perceived patient risk, and compromise of distal esophageal motility.
Originally described by Collis in 1957, the Collis gastroplasty creates a tube of neoesophagus using the gastric cardia and fundus, allowing reduction of the GE junction below the diaphragm and recreation of the angle of His. This neoesophagus remains in an intraabdominal location without tension, effectively reconstructing a physiologic antireflux barrier. As discussed by Adler (1990), however, a Collis gastroplasty alone does not consistently achieve control of reflux without an associated wrap. It soon became apparent that the fundoplication methods of Nissen (1961), as well as Skinner and Belsey (1967), although effective in controlling reflux in most circumstances, were associated with an unacceptably high recurrence rate in patients with a short esophagus. Pearson and colleagues (1971) were the first to describe a transthoracic Collis-Belsey combination for patients with a shortened esophagus, highlighting the importance or restoring the GE junction to an intraabdominal position without undue tension. The transthoracic approach allows complete mobilization of the esophagus and efficient repair of the diaphragmatic hiatal defect, while providing adequate exposure of the proximal stomach for fundoplication. Using the combined Collis-Belsey transthoracic approach, Pearson and Henderson (1976) reported excellent results in 76% of patients followed from 5 to 12 years. Orringer and Sloan (1978), however, found problems with the long-term control of reflux and advocated a transthoracic Collis-Nissen procedure as an alternative. The excellent results reported by Stirling and Orringer (1989) (up to 88% symptomatic reflux control at 10 years of follow-up) have held up favorably over time, making the Collis-Nissen procedure the current standard for patients with esophageal shortening. The Collis-Belsey and Collis-Nissen procedures have traditionally been performed through the chest because of the difficulty in achieving complete esophageal mobilization as well assessing proximal esophageal tension through an abdominal incision. In 1986, Steichen described an effective open Collis-Nissen procedure using newly developed gastrointestinal stapling devices; this has gradually become the preferred approach for esophageal lengthening, while avoiding the attendant complications of thoracotomy. With the advent of laparoscopy, the traditional transabdominal and transthoracic approaches have been supplanted by minimally invasive techniques. The diagnosis of a shortened esophagus can be confirmed, esophageal mobilization can be safely performed high into the mediastinum, intraabdominal esophageal length and tension can be reliably assessed, and a Collis gastroplasty can be performed as needed in conjunction with a Nissen fundoplication. Although the laparoscopic approach has largely replaced transthoracic methods, indications for a transthoracic approach include long proximal strictures, failed previous laparoscopic repairs, and a hostile upper abdomen.
P.2070
A Nissen fundoplication (as described) is then typically performed encompassing the gastroplasty staple line without excessive tension. This approach has been validated clinically and is considered the standard of treatment for the short esophagus. The possibility of performing a minimally invasive Collis gastroplasty has recently emerged as an alternative to open procedures in patients with esophageal shortening, as described by Johnson (1998) and one of us (JDL) (2000b) and respective associates. The laparoscopic approach creates the gastroplasty segment by first making a circular gastrotomy about 5 cm distal to the angle of His, with subsequent firing of a linear stapler toward the left lateral esophageal margin to create the neoesophageal segment. Although some authors contend that thorough esophageal mobilization can afford the additional 2 to 3 cm of esophageal length needed to avoid an esophageal lengthening procedure, Gastal and colleagues (1999) found that up to 50% of patients suspected of having a short esophagus still will ultimately require a gastroplasty.
A careful preoperative assessment is performed to identify those patients at risk for esophageal shortening. Patients with a long history of GERD and those who have developed recurrent symptoms after a failed antireflux operation are at high risk for esophageal shortening. Barium esophagography will identify patients with large hiatal or giant paraesophageal hernias as well as those with strictures (all suggesting the likelihood of a shortened esophagus). Esophagogastroduodenal endoscopy typically reveals esophagitis (possibly Barrett's), strictures, and displacement of the GE junction more than 5 cm above the diaphragmatic hiatus. Manometry frequently documents a hypotensive LES with variable degrees of esophageal dysfunction. Several preoperative clinical features have been identified that have been found independently to predict the need for a Collis gastroplasty. In a retrospective analysis by Urbach and associates (2001), logistical regression modeling identified that the presence of a stricture, paraesophageal hernia, or Barrett's esophagus and the need for redo antireflux surgery were independently associated with the need for gastroplasty.
Technique of Laparoscopic Collis Gastroplasty.
After induction of anesthesia, upper endoscopy is routinely performed to assess for the presence of esophagitis, strictures, and hiatal herniation as well as to document any apparent shortening of the esophagus. Standard laparoscopic port placement is employed as shown in Figure 136-2. After the abdomen is insufflated, the patient is placed in a steep reverse-Trendelenburg position. The GE junction and distal esophagus are circumferentially mobilized as previously described for Nissen fundoplication. The esophageal fat pad is then carefully reflected to the right of the esophagus, with care being taken to identify and preserve the anterior vagus nerve. The esophagus is then circumferentially mobilized from the surrounding tissues of the distal mediastinum and the diaphragmatic hiatus, frequently up to the level of the carina. If the GE junction does not remain below the diaphragmatic hiatus with an adequate segment of tension-free intraabdominal esophagus (ideally, 2 to 3 cm), a Collis gastroplasty is then performed before fundoplication. An esophageal bougie is placed into the stomach and is aligned along the lesser curvature (Fig. 136-17). The diameter of the bougie is determined by the patient's history, size, and esophageal manometry results. Assuming adequate motility, a 50F bougie is typically employed. A large tapered needle attached to a No. 2 paracostal Vicryl suture is straightened and secured to the point of a No. 25 EEA stapler anvil. At a level corresponding to the desired point of esophageal lengthening, the needle is then passed anteriorly through the stomach immediately adjacent to the bougie (Fig. 136-18). The needle serves as a guide for the passage of the anvil, which is carefully pulled through the posterior and anterior stomach walls, respectively. It is important to maintain outward and downward traction on the gastric fundus during this maneuver to optimize positioning of the anvil. Electrocautery can be employed sparingly to assist in passage of the anvil tip. The EEA stapler is then inserted through the right paramedian port and is attached to the anvil. The EEA stapler is fired, creating a circular defect in the stomach wall (Fig. 136-19). The Endo-GIA II stapler is then deployed in a cephalad direction, positioned snugly against the bougie, allowing the creation of the desired length of tension-free, intraabdominal neoesophagus (Fig. 136-20). The staple line is carefully inspected for leaks by direct visualization and endoscopic insufflation. The neoesophagus is then wrapped by the mobilized gastric fundus, achieving a 2- to 3-cm floppy Collis-Nissen fundoplication (Fig. 136-21). The
P.2071
bougie is then removed, and a nasogastric tube is inserted under direct visualization. The crura are then reapproximated posteriorly, as described in the Nissen fundoplication above (see Fig. 136-15B).
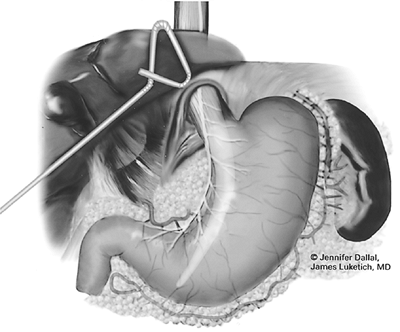 |
Fig. 136-17. Alignment of bougie along lesser curve prior to Collis gastroplasty. |
 |
Fig. 136-18. A. Position of EEA anvil during Collis gastroplasty. B. Delivery of EEA anvil during Collis gastroplasty. |
The nasogastric tube is typically removed on postoperative day 1, and a barium swallow is performed to rule out leak and obstruction. If the barium study demonstrates good passage of contrast without leakage, the patients are started on clear liquids. Patients are usually discharged to home on postoperative day 1 or 2 after receiving nutritional counseling.
Results
Decades of experience with open fundoplications have identified several key principles essential for successful surgical outcomes in the treatment of chronic GERD. Among the most important include thorough preoperative testing, routine division of the short gastric vessels, closure of the crura, and the creation of a tension-free fundoplication while maintaining 2.5 to 3 cm of intraabdominal esophagus. The defining feature of a tension-free hiatal hernia repair is the recognition and proper treatment of an intrinsically shortened esophagus. Failure to recognize or treat a shortened esophagus can lead to hiatal disruption
P.2072
and wrap slippage, accounting for up to one third of surgical failures after open or laparoscopic fundoplication, as discussed by Jobe and co-workers (1998). Excellent long-term results have been achieved in patients undergoing open surgery for complicated gastroesophageal reflux disease when the Collis gastroplasty is performed with a partial or complete fundoplication. More recently, minimally invasive techniques have been developed that duplicate the open results, with greater than 90% control of reflux symptoms. Swanstrom and associates (1996) were the first to report a series of three patients who underwent a minimally invasive Collis-Nissen procedure, employing a combined laparoscopic-thoracoscopic approach. A potential advantage of the thoracoscopic approach is that the stapler can be angled through the chest and placed flush against the esophageal bougie before firing, closely approximating the orientation of the open procedure. The disadvantage of this approach is the requirement for thoracic incisions, which produce increased pain; decreased postoperative respiratory function; and the requirement for chest tube drainage.
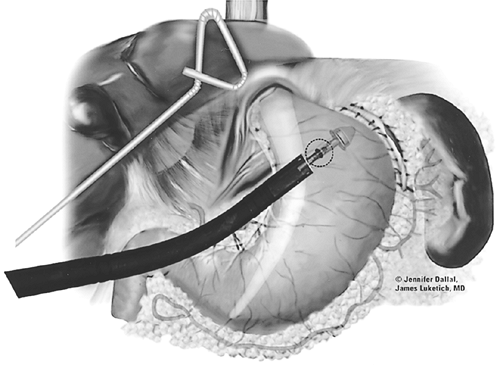 |
Fig. 136-19. Creation of circular gastric staple line. |
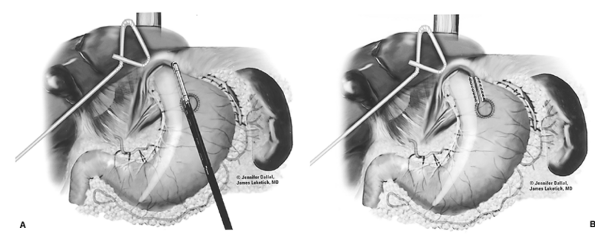 |
Fig. 136-20. Completion of the neoesophagus using a linear stapler. |
 |
Fig. 136-21. Creation of a floppy Collis-Nissen wrap. |
In 1998, Johnson and co-workers were the first to report a series of nine patients in whom a Collis gastroplasty was performed using an exclusively laparoscopic approach. The EEA and GIA technique (as outlined) was performed successfully without the need for thoracoscopy. The mean surgical time was 294 minutes, and the average length of stay was 3 days. There were no deaths and no significant complications in this initial series.
In the largest series of laparoscopic Collis-Nissen fundoplication reported to date by one of us (JDL) and co-workers (2000b), 50 consecutive patients underwent Collis- Nissen antireflux surgery at the University of Pittsburgh. Subjective follow-up results were good to excellent in 96% of patients. There were no deaths, and the median hospital stay was 3 days. Complications of the laparoscopic Collis procedure included dysphagia, staple line leaks, and pneumothorax. In this series, five (10%) intraoperative complications occurred: one vagus nerve injury and four pneumothoraces. There were four (8%) major postoperative complications (two pulmonary embolisms and two staple line leaks). The control of reflux symptoms and the low complication rate compare favorably with published open series.
Theoretic concerns regarding the complications of Collis gastroplasty have been raised. Staple line leaks have been reported, although their incidence is less than 2% in both the open and endoscopic series. A second concern arises from the potential for impaired esophageal motility of the neoesophageal segment. This immotile segment may be at
P.2073
risk for dilation and may play a role in the development of postoperative dysphagia, although this theoretic relationship has not been clearly established in the open or laparoscopic literature. An additional concern is the disposition of a segment of gastric mucosa proximal to the newly created high-pressure zone. Jobe and co-workers (1998) documented that in 7 of 15 patients undergoing a Collis-Nissen procedure, parietal cells could be identified that continued to secrete acid, as indicated by an abnormal postoperative DeMeester scores. Interestingly, the presence of such acid secretion did not correlate with patient symptoms.
Several authors advocate an extended mediastinal dissection as an alternative to gastroplasty in the treatment of patients with esophageal shortening, claiming that in most instances, meticulous esophageal mobilization provides adequate intraabdominal length for a tension-free fundoplication. In a retrospective analysis of 205 patients undergoing laparoscopic Nissen fundoplication, O'Rourke and colleagues (2003) identified 133 patients who underwent a type I (<5 cm) mediastinal esophageal mobilization, and compared the outcomes with the remaining 72 patients who underwent a type II (>5 cm) esophageal mobilization. Failure rates were found to be equivalent between the groups (10%). The authors concluded that aggressive transmediastinal mobilization of the esophagus could provide a success rate similar to that achieved in patients without esophageal shortening, with the Collis gastroplasty being reserved for patients with an irreducible esophagus.
Laparoscopic antireflux procedures have become increasing popular and prevalent and are being performed by an ever-increasing number of practitioners. This will undoubtedly increase the number of patients with a short esophagus who are referred for surgical treatment. The Collis-Nissen procedure has an established excellent long-term success rate for this complex problem. With the development and refinement of laparoscopic Collis techniques, conversion to a laparotomy or a thoracostomy when a short esophagus is encountered is no longer necessary. The addition of a laparoscopic Collis gastroplasty during Nissen fundoplication provides an important tool in the management of patients with acquired shortening of the esophagus, allowing the creation a tension-free intraabdominal segment of neoesophagus that enhances the results of fundoplication.
Giant Paraesophageal Hernia Repair
Paraesophageal hernias represent a subtype of hiatal hernia. The most common form of hiatal hernia is the simple or sliding (type I) hiatal hernia (95%), in which the GE junction migrates above the diaphragmatic hiatus, frequently associated with incompetence of the LES. The remaining forms of hiatal hernia can be classified as paraesophageal hernias (5%). Type II paraesophageal hernias are characterized by the position of the GE junction below the diaphragm, with a portion of the fundus and greater curvature migrating through a hiatal defect alongside the esophagus. This type of hernia is rare, with Maziak and associates (1998) estimating a prevalence of only 3% among all paraesophageal hernias. In type III paraesophageal hernias, both the GE junction and fundus protrude through a hiatal defect. Type IV hernias are defined by herniation of the entire stomach, omentum, or transverse colon into the mediastinum. Giant paraesophageal hernias (GPEHs) are defined by the presence of greater than one third of the stomach within the chest.
Symptoms of paraesophageal hernia include those found in GERD (e.g., heartburn, regurgitation). Chest pain (especially postprandial) is a common finding and is frequently mistaken for anginal symptoms. Postprandial distress, nausea, bloating, and anemia are also commonly encountered. Symptoms can be progressive and can result in catastrophic complications with nonsurgical management. In a classic report by Skinner and Belsey (1967) of a group of minimally symptomatic patients with a GPEH undergoing nonsurgical observation, 26% ultimately died of catastrophic complications, including torsion, gangrene, perforation, and massive hemorrhage. Haas and co-workers (1990) reviewed a series of 21 patients with intrathoracic volvulus, of whom 8 were asymptomatic at the time of initial diagnosis. Ten patients from this group ultimately required emergency surgery, 5 for strangulation and 1 for perforation, with an associated 40% operative mortality rate. This has led many to argue that symptomatology is not a reliable predictor of who might progress to acute complications, and that elective surgery should be performed on most patients with GPEH, in particular those with organoaxial rotation diagnosed on barium esophagram. Prompt elective repair is thus recommended after diagnosis to avoid the development of such complications. When the repair is performed electively, excellent control of symptoms (>90%) and a death rate of less than 1% to 2% can be achieved, as reported by Maziak and colleagues (1998).
The surgical technique involves reduction of the herniated contents back into the abdomen, excision of the hernia sac, and closure of the hiatal defect. In the absence of reflux symptoms, the need to perform an antireflux procedure is debatable. However, up to 60% of patients with type III hernias have been shown to have hypotensive LES pressures and abnormal 24-hour pH monitoring studies, as discussed by Walther and associates (1984). Maziak and co-workers (1998) found that 83% of patients with GPEH have a current or remote history of significant GERD. In addition, Williamson and co-workers (1993) have found that about 20% of patients will have reflux symptoms postoperatively when an antireflux procedure is not performed. Such findings support the routine performance of an antireflux procedure with paraesophageal hernia repair.
Traditionally, repair of giant paraesophageal hernia has been performed through an open laparotomy or thoracotomy. Increasingly, repair of GPEH is being performed using minimally invasive techniques, as reviewed by
P.2074
Buenaventura and co-workers (2000). Preoperative assessment includes a barium esophagram that ordinarily establishes the diagnosis (Fig. 136-22). Upper endoscopy allows assessment of the gastric anatomy as well as the extent of mucosal injury. Esophageal manometry and 24-hour pH studies are not routinely performed because the degree of anatomic distortion frequently interferes with the reliable performance of these studies.
 |
Fig. 136-22. Giant paraesophageal hernia. |
Technique of Giant Paraesophageal Hernia Repair
Positioning and port placement are the same as for Nissen fundoplication. The abdominal port incisions can be shifted slightly cephalad in the case of a GPEH to facilitate ease of mediastinal dissection. The left lateral segment of the liver is retracted anteriorly with a 5-mm flexible retractor (Snowden Pencer, Genzyme, Tucker, GA) and is secured to a stationary holding device (Mediflex, Islanda, NY). After exposure, the herniated stomach is reduced back into the abdomen using atraumatic graspers (Snowden Pencer) in a hand-over-hand fashion (Fig. 136-23). Dissection is begun by dividing the gastrohepatic ligament just medial to the lesser curvature of the stomach using the harmonic scalpel (Ethicon, Cincinnati, OH) or the ultrasonic shears (U.S. Surgical Corp, Norwalk, CT). The right crus of the diaphragm is thereby exposed. The dissection is carried anteriorly
P.2075
over the surface of the esophagus, with care being taken to identify and preserve the anterior vagus nerve. Inferior traction on the gastric fundus and epiphrenic fat pad are then performed to allow exposure of the anterior portion of the left crus. A retrogastric-retroesophageal window is then created to expose the posterior portion of the left crus. At this point, division of the short gastric vessels is performed. Once the greater curvature is completely mobilized, the hernia sac is excised, beginning sharply at the diaphragmatic hiatus. Using a combination of sharp and blunt dissection, the hernia sac is then separated from the intrathoracic cavity (Fig. 136-24). Careful attention must be paid to the avoidance of pleural tears. The surgeon and the anesthesiologist must communicate closely during this portion of the procedure because changes in blood pressure or inspiratory pressures may indicate the development of a tension pneumothorax. After reduction of the sac, it is excised and removed. In some patients with extensive adhesions of the hernia sac, a lighted bougie may facilitate identification of the esophageal wall and vagal nerves.
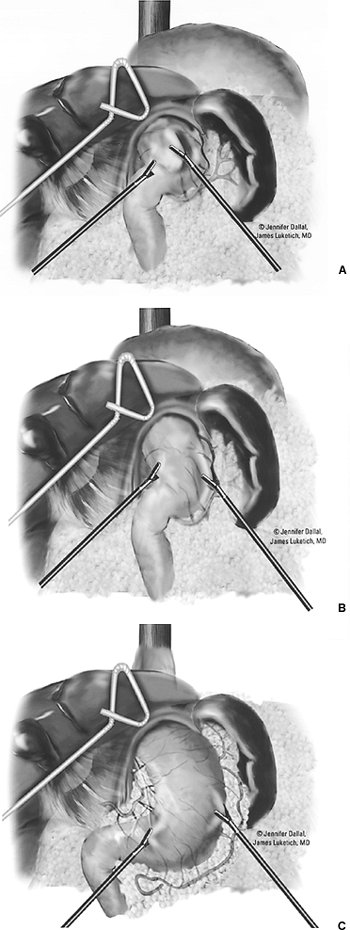 |
Fig. 136-23. Reduction of paraesophageal hernia. |
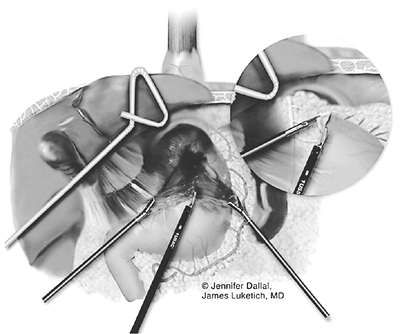 |
Fig. 136-24. Excision of paraesophageal hernia sac and mobilization of the esophagogastric fat pad. |
Careful identification of the gastroesophageal junction after fat pad excision frequently reveals a shortened esophagus in the setting of a GPEH (see Fig. 136-24). The esophageal fat pad is carefully and completely mobilized medially, sweeping the anterior vagus to the right of the esophagus. The distal esophagus is then mobilized at the level of the diaphragmatic hiatus circumferentially to determine whether esophageal shortening is present. The surgeon then assesses for tension by pulling on the stomach caudally once the gastroesophageal junction has been mobilized. If the esophagogastric junction does not remain below the diaphragmatic hiatus with an adequate segment of intraabdominal esophagus, a Collis gastroplasty is added before fundoplication. The neoesophagus is wrapped with the mobilized gastric fundus, and a 2- to 3-cm floppy Nissen fundoplication is fashioned. Care is taken to cover the point of overlap of the EEA circular staple line and the Endo-GIA II line with the gastric wrap because this represents the weak point of the neoesophageal staple line. The crura are reapproximated posteriorly to complete the surgical procedure as outlined previously. The hiatal hernia defect is closed primarily by approximating the crura below the esophagus with interrupted 0-0 braided polyester suture (Surgidac) using the Endo-stitch. In most cases, the crura are approximated primarily without excess tension. In unusual cases of an excessively large defect, a patch of Gore-Tex or Surgisys is used to reinforce the closure. A gastrostomy or a gastropexy is not routinely performed, although this technique can be useful after hernia reduction in patients unfit for the definitive conventional repair. Before closing, endoscopy is routinely performed with intraluminal insufflation to rule out esophageal or gastric leaks. A nasogastric tube is placed under laparoscopic guidance. After surgery, the nasogastric tube is typically removed on postoperative day 1, and a barium swallow is obtained to evaluate the repair and verify the absence of a leak. If no leak is present, clear liquids are started. If a clear liquid diet is tolerated, patients are usually discharged to home on postoperative day 2. Advancement to a soft diet occurs over the subsequent 2 to 3 weeks.
Results
Although laparoscopic Nissen fundoplication has become a well-established procedure in the treatment of GERD, laparoscopic management of GPEH is somewhat controversial. Early studies of laparoscopic repair of GPEH were promising, but concerns have arisen about the safety and efficacy of a minimally invasive approach in handling this complex problem. Dahlberg and co-workers (2001) reported the Mayo clinic experience in 37 patients undergoing laparoscopic repair of GPEH. Successful repair was accomplished laparoscopically in 35 of the 37 patients. Intraoperative injuries included two splenic injuries and one crural tear. Postoperatively, there were two cases of esophageal leak and one small bowel obstruction at a port site. The mortality rate was 5.4%. In follow-up, 4 of 37 patients (12.9%) developed recurrent paraesophageal herniation. The authors concluded that laparoscopic repair of GPEH is a technically challenging operation associated with significant morbidity and mortality. Similar results were obtained in a series of 60 patients by Weichman and colleagues (2001). In this series, reoperation for recurrent herniation was performed in 5.5% of patients, with an associated 1.9% mortality rate. They also concluded that laparoscopic repair of GPEH is a technically challenging procedure, but that with increasing experience and technical refinements, results could approximate the open approach.
As experience with advanced laparoscopic techniques has developed, several series have emerged demonstrating good results after laparoscopic repair of GPEH. Horgan and co-workers (1999b) reported that laparoscopic paraesophageal hernia repair was a technically difficult but effective operation that resulted in a mean hospital stay of 4 days and
P.2076
a single perioperative mortality out of 41 patients. Edye and co-workers (1998) reported on 55 patients with no perioperative mortality and acceptable symptomatic outcomes, although the details of hospital stay were not reported. Swanstrom and colleagues (1999) reported a mean operative time of 4 hours and a hospital stay of 3 days in 52 patients with good outcomes.
One of us (JDL) and associates (2000c) published the outcomes of 100 consecutive laparoscopic repairs of GPEH from July 1995 to February 2000. The median surgical time was 3.67 hours (range, 2.0 to 11.5 hours). The median length of stay was 2 days. Complete sac removal and crural repair were performed in all patients. The crural repair was primary in 96 patients; 4 had a mesh repair. All but one of the patients underwent an antireflux procedure. There were 72 Nissen procedures and 27 Collis-Nissen fundoplications. There were three conversions to open procedures (two cases of severe adhesions, inability to reduce the stomach safely laparoscopically in one patient). Intraoperative complications included pneumothorax requiring a chest tube (4 patients), esophageal perforation (5 patients), and gastric perforation (3 patients). The perforations were small and easily repaired laparoscopically. The 30-day mortality rate was 0%. In a separate report from the University of Pittsburgh, Schauer and colleagues (1998) demonstrated that laparoscopic repair of moderately sized and giant paraesophageal hernias resulted in less blood loss, shorter intensive care unit stays, faster recovery from ileus, and shorter length of hospital stay when compared with patients undergoing open repair.
In the largest series reported to date, Pierre and co-workers (2002) updated the University of Pittsburgh experience in the laparoscopic repair of 203 consecutive GPEHs. Median follow-up was 18 months. The most common symptoms included heartburn (47%), dysphagia (35%), epigastric pain (26%), and vomiting (23%). Laparoscopic procedures included 69 Nissens, 112 Collis-Nissens, and 19 other procedures. Only three patients were converted to open procedures nonurgently, secondary to adhesions. Median length of stay was 3 days. Complications (minor or major) occurred in 57 of 203 patients (28%). There were six postoperative esophageal leaks (3%), and only one death. Five patients (2.5%) required reoperation for recurrent hiatal hernia. Good to excellent results were obtained in 92% of patients based on postoperative questionnaire. The mean postoperative GERD health-related quality-of-life score was 2.4 (scale: 0 to 45; 0 = no symptoms, 45 = worst).
As the experience with antireflux surgery in the setting of GPEH has increased, we have come to recognize the important role of esophageal shortening in this condition. As described previously, 75% to 90% of patients with true GPEH have a GE junction located well above the diaphragmatic hiatus. Altorki and co-workers (1998) have reported excellent short-term results in greater than 90% of patients with complete esophageal mobilization to the aortic arch and the addition of a Belsey antireflux procedure. In contrast, Maziak and colleagues (1998) found that an esophageal lengthening procedure was required in 80% of their patients after careful assessment of the GE junction and its relationship to the diaphragm. In this series of 94 patients, only 2 patients required reoperation and subsequent gastroplasty at a mean follow-up of 8 years. Both of the recurrences had unrecognized esophageal shortening at the time of the first operation. On the basis of such results, an esophageal lengthening procedure as part of the repair of GPEH should be considered the standard in the management of GPEH. As such, the rate of Collis gastroplasties has tripled from 27 in 100, as reported in our initial series, to 86 in 103. In total, 113 patients (56%) received a Collis gastroplasty in the University of Pittsburgh series.
In addition to esophageal lengthening, proper reconstruction of the hiatal defect is critical in the long-term success of the repair. In most patients, the crura can be approximated primarily without undue tension. In most open series, however, a recurrence rate of up to 10% can be seen, as reported by Altorki and co-workers (1998). The radiographic recurrence rate has been reported to be higher using a laparoscopic approach, ranging from 23% to 42%, as estimated by Hashemi and colleagues (2000). Crural breakdown and wrap migration have been implicated in up to two thirds of these failures, as discussed by Horgan and associates (1999a). Failure rates are particularly prominent when the hiatal defect exceeds 5 cm. Several authors advocate mesh cruroplasty in this situation. In a series of 52 consecutive patients undergoing mesh cruroplasty in the setting of GPEH repair, Champion and Rock (2003) used a prosthetic mesh onlay positioned over the hiatal closure. During an average follow-up of 25 months, only one (1.9%) recurrence was detected, and no prosthetic erosion. Longer-term analysis will be necessary to determine the overall efficacy of this approach.
In summary, laparoscopic repair of GPEH is feasible, safe, and effective in centers with extensive experience in minimally invasive esophageal surgery. The laparoscopic approach should adhere to the principles established in open series: complete stomach reduction, complete hernia sac excision, close assessment for esophageal shortening, and crural repair. Liberal use of the Collis gastroplasty may reduce the incidence of recurrent herniation and improve long-term functional results.
MINIMALLY INVASIVE MANAGEMENT OF MOTILITY DISORDERS
Laparoscopic Heller Myotomy
Since the introduction of laparoscopic cardiomyotomy for achalasia in 1991, as described by Shimi and colleagues, minimally invasive approaches have been developed in the treatment of most, if not all, esophageal motility disorders, including esophageal spasm, nutcracker esophagus, hypertensive LES, and both Zenker's and epiphrenic diverticula.
P.2077
Achalasia
Achalasia is the most common primary motility disorder of the esophagus. It is characterized by loss of esophageal peristalsis and failure of the LES to relax completely upon deglutition, leading to ineffective esophageal propulsion and increased outflow resistance. Therapeutic approaches are devised to relieve the distal esophageal outflow obstruction in order to improve transit across the LES. Improvement in esophageal peristalsis, however, rarely occurs. The goal of therapy is to reduce the resting and swallow-induced residual pressures of the LES. Pharmacologic therapies such as calcium channel blockers and nitrates have been employed, but rarely relieve symptoms in a sustained fashion. Local injection of botulinum toxin has been demonstrated to alleviate symptoms, but its effect is largely temporary (Table 136-1). This has led authors to conclude that this therapy should be reserved for those who are prohibitive candidates for pneumatic dilation or myotomy. Sphincter disruption through pneumatic balloon dilation can also be effective, although there is a high prevalence of recurrent dysphagia within the first several months to years, necessitating repeated redilation. The first successful operation performed for achalasia was reported in 1914 by the German surgeon Ernst Heller, describing an anterior and a posterior longitudinal incision of the esophageal smooth muscle using a transthoracic approach. Heller's' myotomy was subsequently modified by the Dutch surgeon Zaaijer in 1923 using a single anterior myotomy incision. Surgical myotomy has risen to be the most effective modality in the treatment of achalasia, producing maximal relief of symptoms with minimal morbidity and mortality. The only available prospective randomized trial comparing open surgical myotomy with pneumatic dilation in 81 patients by Csendes and co-workers (1989) demonstrates that surgical treatment yields superior long-term outcomes (95% excellent results at a median follow-up of 62 months compared with a 65% success rate in the pneumatic dilation group at a mean of 58 months).
Such shortcomings of the nonsurgical modalities, in combination with the established long-term efficacy of laparoscopic myotomy, have promoted the emergence of surgery as the primary therapeutic option in the treatment of achalasia. Several recent reviews have compared the efficacy of these treatment options and have found surgical myotomy to be the most globally efficient form of therapy. Of the surgical modalities, thoracoscopic and laparoscopic myotomy has been associated with the excellent symptomatic improvement on par with open approaches, although long-term data are not yet available, as discussed by Vaezi and Richter (1998). Morbidity and mortality rates among all of these modalities are now nearly identical.
Laparoscopic distal esophageal (Heller) myotomy is thus emerging as the preferred primary approach in the treatment of achalasia. There are no prospective, randomized studies comparing the efficacy of laparoscopic Heller myotomy with pneumatic dilation or botulinum toxin injections. In the only available prospective study comparing surgery (open) with pneumatic dilation by Csendes and co-workers (1989), 91% of the patients in the surgical group and 51% of the patients in the dilation group had complete or near-complete symptom resolution at 5 years of follow-up. In a review of the literature examining surgical and nonsurgical modalities in the treatment of achalasia by Spiess and Kahrilas (1998), laparoscopic myotomy was found to achieve better weighted response rates (92%) than open approaches (84% to 85%) or nonsurgical therapy (32% to 72%) over a 1.1- to 7.6-year mean follow-up. The principal limitation of dilation is the 1% to 3% risk for perforation, whereas the morbidity of thoracotomy-laparotomy is the major limitation of open myotomy. Surgical morbidity is significantly reduced by laparoscopic techniques.
Patients with suspected esophageal dysmotility should undergo a careful preoperative evaluation to identify the underlying physiologic and anatomic abnormalities, which will be useful in helping to guide decision making. Barium esophagography is useful in imaging the contours of the esophageal mucosa, the extent of dilation, and the effectiveness of bolus transport. It provides an anatomic roadmap in evaluating esophageal motility disorders. Distal esophageal dilation with a rapid tapering results in a bird's beak deformity in the setting of achalasia (Fig. 136-25).
P.2078
Upper endoscopy allows examination of the esophageal mucosa and is useful in identifying strictures or tumor at the GE junction that might mimic achalasia (pseudoachalasia). Esophageal manometry is critical in the diagnosis of achalasia. The typical manometric features include an aperistaltic esophageal body, hypertensive LES, and incomplete relaxation of the LES.
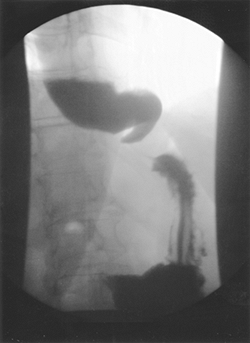 |
Fig. 136-25. Bird's beak deformity typical of achalasia. |
Table 136-1. Comparison of Outcomes in the Treatment of Achalasia | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||
Additional factors that should be investigated include a history of prior nonsurgical treatment as well as the degree of esophageal dilation or tortuosity (e.g., sigmoidal megaesophagus). Prior pneumatic dilation or botulinum toxin (Botox) injection can complicate subsequent myotomy. Submucosal scarring is frequently present (especially after botulinum toxin injections), making smooth separation of the muscles more challenging and increasing the likelihood of mucosal breach, as highlighted by Patti and colleagues (1999a). Patients with a markedly dilated or sigmoid esophagus do not appear to benefit from myotomy with fundoplication, and esophagectomy is frequently recommended, as advocated by Peters and associates (1995). Patti and co-workers (1999b) have suggested, however, that esophageal dilation and tortuosity are not necessarily absolute contraindications to myotomy for achalasia. Of 66 patients with achalasia, 19 were noted to have a dilated esophagus (>6 cm), of which 12 also had a sigmoidal pattern. The conclusions of their analysis suggest that myotomy was no more difficult to perform, complications and length of stay were similar to the other surgically treated patients, and the early relief of dysphagia was similar. None of these patients went on to require esophagectomy. The authors contend that myotomy with fundoplication should still be considered the primary treatment modality regardless of the shape or diameter of the esophagus, with esophagectomy being reserved for those with persistent symptoms not amenable to a second myotomy.
Both thoracoscopic and laparoscopic approaches have been devised. Over time, it has become increasingly evident, however, that the thoracoscopic approach is associated with a higher rate of persistent dysphagia as well as reflux. In a review of five prospective studies on the surgical treatment of achalasia, Vaezi and Richter (1998) found persistent dysphagia in up to 18% of patients and secondary reflux in 50% in patients treated with thoracoscopic distal esophageal myotomy. In a comparison of thoracoscopic Heller myotomy with laparoscopic Heller myotomy and Dor fundoplication (30 subjects per group) by Patti and co-workers (1998), dysphagia was completely relieved in 77% of patients in the laparoscopic group, compared with 70% in the thoracoscopic group. Patients left the hospital sooner (42 vs. 84 hours) and had a lower incidence of postoperative reflux (3% vs. 20%) in the laparoscopic Heller-Dor group. Stewart and colleagues (1999) studied the outcomes in 24 patients treated with thoracoscopic myotomy and 63 patients treated by laparoscopic myotomy with or without fundoplication. Relief of dysphagia was achieved in 90% of those treated laparoscopically, compared with only 31% of those treated thoracoscopically.
Technique of Heller Myotomy and Fundoplication.
The patient is placed in a comfortable supine position. The table is positioned in steep reverse-Trendelenburg, allowing the abdominal viscera to fall inferiorly toward the pelvis and out of the visual and operative field. The patient's arms may be padded and tucked, or abducted to 90 degrees on arm boards. The surgeon stands to the patient's right (or between the legs), with the assistant working at the patient's left. The port sites, hiatal dissection, fundic mobilization and crural closure are performed as described in the Nissen fundoplication.
The procedure begins with the division of the gastrohepatic ligament, allowing exposure of the right crus. The crus is separated from the esophagus with division of the phrenoesophageal ligament, and the dissection is carried anteriorly over the surface of the esophagus, with care taken to identify and preserve the anterior vagus nerve. The anterior portion of
P.2079
the left crus is similarly mobilized with division of splenic attachments, allowing the spleen to fall away from the stomach and hiatus. The dissection is then directed posteriorly, with identification of the crural decussation. The esophagus is mobilized from the posterior portion of the left crus, thus opening a retrogastric window. The short gastric vessels are then divided with the Ultrasonic shears about one third the way around the greater curvature of the stomach. Next, identification and mobilization of the gastroesophageal fat pad are performed, promoting exposure of the GE junction. Dissection is generally initiated along the lesser curvature, extending obliquely toward the angle of His. The anterior vagus nerve is identified and swept medially with the fat pad.
The distal esophageal myotomy is performed next. An epinephrine solution (diluted 1:30,000) is then injected into the muscular layer, as described by Kuster (1998), providing a plane of separation between the mucosa and submucosal layers. The myotomy is typically initiated 1 to 2 cm above the GE junction (ideally in an area proximal to any prior botulinum toxin injections or pneumatic dilation). Either scissors of hook electrocautery can be used to initiate the incision into the longitudinal and circular muscle fibers. The longitudinal fibers are encountered first and are separated along their length. The scissor (or hook) is then insinuated beneath the underlying circular muscle, which is then divided with exposure of the underlying submucosal plane. The circular muscle is divided along this plane proximally for a distance of 4 to 6 cm, separating the musculature from the underlying mucosa. The myotomy is then carried distally across the GE junction onto the proximal stomach for about 1 to 2 cm. After completion of the myotomy, the muscle is then separated bluntly from the esophageal mucosa for up to 50% of the esophageal circumference. Disruption of the mucosa can occur and is easily detected by endoscopic insufflation. Breaches of the mucosa can be repaired laparoscopically with interrupted 2-0 sutures.
After completion of the myotomy, a fundoplication is performed. Either an anterior hemifundoplication augmenting the angle of His (Dor) or a posterior partial fundoplication (Toupet) can be performed (Fig. 136-26). The Dor fundoplication is generally viewed as easier to perform and does not require circumferential esophagogastric mobilization.
Dor Fundoplication.
The anterior (Dor) fundoplication is fashioned by accentuating the angle of His and suturing the medial anterior fundus to the left cut edge of the esophageal musculature with three or four interrupted 2-0 Surgidac sutures. Care is taken to include the left crus in the superior-most suture to achieve tacking of the wrap to the diaphragm. A second column of sutures is then placed between the fundus of the stomach and the right edge of the myotomy, completing the fundic wrap over the myotomy.
Toupet Fundoplication.
In contradistinction to the Dor, the Toupet fundoplication involves a 270-degree posterior wrap. Once the hiatus has been dissected, the crura have been closed, and the fundus has been completely mobilized, the posterior fundus is passed behind the esophagus. The fundus is first secured to the right crural margin using interrupted 2-0 Surgidac sutures. Additional sutures are placed between the posterior lip of the fundus and the right lateral wall of the esophageal myotomy. The anterior fundic lip is sutured to the left crural margin, with additional sutures between the fundus and the left lateral wall of the esophageal myotomy.
Choice of Antireflux Procedure.
Few data are available to guide the choice of anterior or posterior hemifundoplication. Both approaches provide an adequate antireflux mechanism with minimal outflow resistance. Comparable short-term results have been published for both techniques. Some surgeons believe that the anterior wrap adds the additional benefit of covering the exposed mucosa. Other surgeons believe that the Toupet fundoplication is a better antireflux operation. Raiser and associates (1996) performed a retrospective evaluation of 39 patients treated with laparoscopic Heller myotomy and either anterior (10 patients) or posterior (29 patients) fundoplication. In that study, patients undergoing a posterior (Toupet) fundoplication experienced less postoperative heartburn at long-term follow-up (27% vs. 57% in the Dor group; p < 0.005). There was a similar trend toward decreased dysphagia at long-term follow-up (Severity of Dysphagia Score, 0.55 versus 1.43 in the Dor group), although this trend did not attain statistical significance. Most surgeons, in the end, base their decision on personal preference. Further studies are needed to clarify the objective benefits of one over the other.
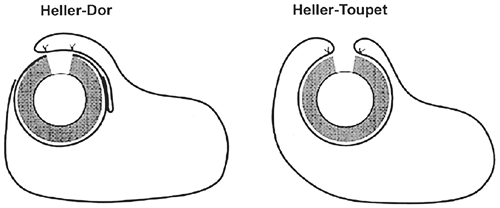 |
Fig. 136-26. Comparison of the anterior (Dor) and posterior (Toupet) fundoplication. From Vaezi MF, Richter JE: Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol 27:21, 1998. With permission. |
P.2080
Results
One of us (JDL) and associates (1999) reported a series of 28 patients with achalasia treated with either laparoscopic or thoracoscopic Heller myotomy, with good to excellent results achieved in 89% of this group. Balaji and Peters (2002) calculated from a variety of published series that laparoscopic cardiomyotomy produces good to excellent clinical response rates in 94% of 254 treated patients (83% to 100%), and reduces LES pressure by 59% (42% to 72%) on average. These results are durable, and are far superior to the endoscopic and medical alternatives. Long-term outcomes for open transabdominal myotomy have been well documented by Mattioli and colleagues (1996), with a durable success rate greater than 85%. Similar results have been achieved with laparoscopic Heller myotomy and either Dor or Toupet fundoplication, as published by Patti (1999b) and Yamamura (2000) and their co-workers. In a study of more than 100 patients, Patti and co-workers (1999b) documented relief of dysphagia in 93% of patients at a mean follow-up of 23 months. Vaezi and Richter (1998) reviewed a series of published reports compiled to date identifying 254 patients with an average success rate of 93% at 2.5 years. Conversion to an open procedure ranges from 0% to 5% of cases. Intraoperative complications are rare, with mucosal perforation being the most common (<5%), especially in patients with a history of botulinum toxin injection. The incidence of objective reflux postoperatively, as evidenced by abnormal esophageal acid exposure, ranges from 6% to 10%. Postoperative manometry consistently demonstrates a reduction in the LES resting pressure to an average of 10 to 11 mm Hg, as documented by Swanstrom and Pennings (1995), and reductions in the degree of fluid retention and esophageal diameter are achieved, as reported by Finley (2001) and Rosati (1998) and their colleagues, respectively.
Heller myotomy and fundoplication can be performed either thoracoscopically or laparoscopically. Drawbacks of the thoracoscopic approach in the treatment of achalasia include the technical difficulty of performing the myotomy while operating in a plane perpendicular to the esophagus, as well as judging the appropriate extension of the myotomy onto the anterior surface of the stomach. There is greater postoperative pain and longer hospital stay when compared with the laparoscopic approach, as discussed by Nguyen and associates (2000c). The laparoscopic approach provides better visualization of the GE junction and simplifies the anesthetic management of these patients, avoiding the need for a double-lumen endotracheal tube and single-lung ventilation. Stewart and co-workers (1999) performed a retrospective analysis of 24 patients undergoing a thoracoscopic myotomy with 63 patients undergoing laparoscopic myotomy. Laparoscopic myotomy was associated with a reduction in operative time, a decrease in the conversion to an open procedure, better relief of dysphagia (90% vs. 31%), and a shortened hospital course when compared with the thoracoscopic group. Patti and co-workers (1998) reported similar results. These outcomes make laparoscopic Heller myotomy and fundoplication an attractive surgical option in the treatment of patients with achalasia.
The choice of best antireflux procedure to perform after laparoscopic cardiomyotomy remains controversial. The most commonly used procedures include the floppy Nissen fundoplication as well as the Dor and Toupet fundoplications. A recent study by Raiser and co-workers (1996) found a higher rate of GERD after the Dor fundoplication (57%) when compared with the Toupet procedure (27%). Hunter and colleagues (1997) also support the use of the Toupet fundoplication on the basis that it holds the myotomy open and provides a better antireflux barrier. Vaezi and Richter (1998) contend that in patients with a markedly dilated esophagus, the Dor procedure is preferred because the Toupet wrap creates a posterior bar, impeding esophageal emptying in these patients. Therefore, no clearcut advantage has been established of one technique over another, and the choice of fundoplication remains largely the surgeon's preference.
Patients with end-stage achalasia who have a markedly dilated, sigmoidal esophagus have a higher failure rate after myotomy, leading some authors to advocate esophagectomy as the ideal approach in this setting. Patti and colleagues (1999b) have reported good to excellent results, however, in a series of 7 patients with end-stage achalasia and a dilated esophagus. Patients that fail this approach, or in those with a megaesophagus (sigmoid, >8 cm in diameter), may benefit from esophagectomy.
Spastic Dysmotility
A long esophageal myotomy can be performed in patients experiencing dysphagia secondary to spastic motor disorders characterized by segmental or generalized simultaneous waveforms, especially in patients whose symptoms are refractory to medical therapy. This group of disorders includes diffuse esophageal spasm, vigorous achalasia, and nonspecific motor disorders associated with a midesophageal or epiphrenic diverticulum. The decision to operate is weighed against such factors as the severity of the patient's symptoms, diet, lifestyle adjustments, and nutritional status.
Diffuse esophageal spasm is rare (occurring five times less frequently than achalasia). It is characterized by dysphagia, frequently associated with chest pain. It differs fundamentally from achalasia in that it represents a primary disease of the esophageal body, produces a lesser degree of dysphagia, causes more chest pain, and usually has a lower impact on the patient's overall condition. Manometry identifies residual peristaltic waveform activity in excess of that seen in achalasia. The LES is usually normal in tone and relaxation. A hypertensive sphincter with poor relaxation may also be present.
Nutcracker esophagus is manifested by intermittent chest pain and dysphagia, with manometric findings characterized
P.2081
by peak amplitudes greater than 2 standard deviations above the normal range, frequently exceeding 400 mm Hg. Esophageal myotomy has been shown to be beneficial in patients with diffuse esophageal spasm, as discussed by Ellis (1992) as well as Patti and Pellegrini (1996), but is of questionable benefit in patients with nutcracker esophagus, as determined by Shimi and co-workers (1992). Long esophageal myotomy is best performed thoracoscopically through the left chest. Three or four thoracoscopic ports may be used, and with suitable lung retraction, the muscular layers can be divided along the entire length of the affected esophagus down to the GE junction. Patti and co-workers (2001) compared the effectiveness of thoracoscopic long esophageal myotomy in 10 patients to medical therapy alone in 30 patients with spastic motor disorders. Eighty percent of the patients in the surgical group and 26% of patients in the medical group obtained good or excellent results. Optimal results are obtained when the length of myotomy is guided by manometric findings and when the myotomy is carried through the LES. Hemifundoplication (either Dor or Toupet) is routinely performed as part of the procedure.
Esophageal Diverticulectomy
Diverticula of the esophagus are divided into three principal types: Zenker's, traction, and epiphrenic. Zenker's diverticulum (pharyngeal pouch) represents 60% to 65% of all esophageal diverticula and occurs two to three times more frequently in men than in women. It is found most frequently in patients in their 70s and probably is a product of the aging process. Zenker's diverticula are located in the dorsal wall of the hypopharynx between the inferior pharyngeal constrictor muscles of the pharynx and the transverse fibers of the cricopharyngeus muscle (i.e., the upper esophageal sphincter), the narrowest part of the alimentary tract. This area, known as Killian's triangle, is an area of natural weakness containing only scant muscle fibers. The first pharyngeal pouch was described by Ludlow in 1769, who reported an abnormal dilation of the posterior pharyngeal wall in a postmortem examination of a patient who had complained of dysphagia during life. Zenker and von Ziemssen (1877) provided a concise pathologic description of 34 patients with a protrusion of pharyngeal mucosa from the dorsal wall, immediately proximal to the transition from the hypopharynx into the esophagus. Developing in the posterior midline, this diverticulum displaces to one side, usually to the left, as it enlarges. It is thought that displacement occurs because the normal cervical esophagus bulges to the left and because the common carotid artery is further removed (i.e., lateral) in the left neck than in the right. The development of a Zenker's diverticulum is thought to result from a defect in the upper esophageal sphincter. It is believed that there is motor dyscoordination between the closing of the glottis and nasopharynx and the failure of the cricopharyngeal muscle of the upper esophageal sphincter to relax during swallowing. This causes an increase in the intrabolus pressure and mimics the concept of pulsion diverticula developing in a high-pressure hypopharynx. The cause of the incomplete upper esophageal sphincter opening still is unknown. Other hypotheses about Zenker's pathophysiology include spasm of the cricopharyngeus muscle and cricopharyngeal achalasia. Initially, no symptoms may be present; however, eventually patients with Zenker's diverticula present with dysphagia, which is the most common symptom. Other symptoms include regurgitation of undigested food (often eaten hours earlier), halitosis, an unpleasant taste, odynophagia, globus sensation, cough, neck pain, and weight loss. Some patients experience a gurgling sound as air passes through the diverticulum, known as Boyce's sign. Regurgitation, often occurring at night when the patient is lying down, can lead to choking, aspiration pneumonia, and lung abscesses in some patients. Although rare, there is a potential for squamous cell carcinoma to develop in patients with Zenker's diverticula. The cause is believed to be related to chronic irritation of the diverticular wall as a result of prolonged food retention. This is found in 0.5% of patients presenting with Zenker's diverticula.
No medical treatment exists for Zenker's diverticula. Surgery is the only way to relieve patients' symptoms and improve their quality of life. The presence of a Zenker's diverticulum is confirmed by barium swallow (Fig. 136-27). Differing opinions exist concerning the utility of upper endoscopy for diagnosis. Some researchers suggest that diagnosis
P.2082
should be based on patient history and confirmed by barium swallow, and that an upper endoscopy is not indicated. Other researchers suggest using both barium swallow and upper endoscopy for diagnosis. Esophagoscopy is avoided because it carries a significant risk for diverticular perforation.
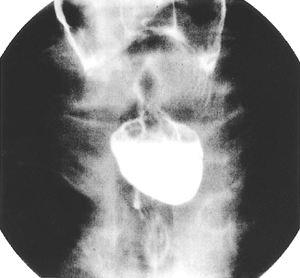 |
Fig. 136-27. Zenker's diverticulum. The posterior hypopharyngeal mucosa herniates through attenuated muscle fibers at a level above the cricopharyngeus (Killian's dehiscence), creating a characteristic pharyngeal pouch. From Nguyen HC, Urquhart AC: Zenker's diverticulum. Laryngoscope 107:1436, 1997. With permission. |
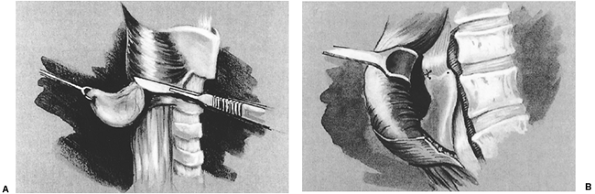 |
Fig. 136-28. Diverticulectomy (A) and diverticulopexy (B) are performed in conjunction with cricopharyngeal myotomy in the treatment of Zenker's diverticulum. From Nguyen HC, Urquhart AC. Zenker's diverticulum. Laryngoscope 107:1436, 1997. With permission. |
The current principles of surgical management include cricopharyngeal myotomy accompanied by diverticulectomy or diverticulopexy (Fig. 136-28).
Cricopharyngeal myotomy is used for small diverticula. The most current literature suggests that diverticula treated by myotomy should be smaller than 2 cm because, in this instance, the diverticular pouch frequently disappears after cricopharyngeal myotomy. The best results are achieved with a 6-cm myotomy extending for at least 2 cm along the hypopharynx. After myotomy, there is a decrease in resistance to food passage and a broader force distribution in the area of the myotomy, so that intrabolus pressure is decreased. Advantages to this procedure include removal of the pathologic constrictive effect of the cricopharyngeus, avoidance of a suture line, rapid restoration of postoperative feeding, and a shorter hospital stay.
For larger diverticula, the cricopharyngeal myotomy is combined with other surgical procedures:
Diverticulopexy with cricopharyngeal myotomy
The first successful outcome of this procedure occurred in 1966. This procedure involves inverting the diverticular sac and suturing it to the prevertebral fascia so that it cannot fill. The literature suggests that this technique can be used successfully on diverticula ranging in size from 1 to 4 cm. With this procedure, oral intake is possible on the first postoperative day, and the patient has a short hospital stay. Possible disadvantages of diverticulopexy include diverticular sac prolapse and the very rare risk of missing a diverticular carcinoma.
Diverticulectomy and cricopharyngeal myotomy
Diverticulectomy for Zenker's diverticula has been performed for almost a century. This procedure involves complete excision of the diverticular sac and has been perfected recently by the use of stapling devices. This procedure is recommended when the diverticular sac is greater than 4 cm. One theoretic advantage to this procedure is that it produces a specimen to rule out the rare occurrence of cancer. Until 1969, a diverticulectomy was performed without myotomy. Since that time, it has been recommended that myotomy be performed with diverticulectomy to decrease the rate of recurrence.
Endoscopic Diverticulotomy
An endoscopic approach to this problem was first developed in 1917, when Mosher published the results of four patients in which he divided the common septum between the esophagus and the pouch. The initial enthusiasm for this approach was tempered when his seventh patient developed mediastinitis and died secondary to esophageal leakage. Dohlman reintroduced endoscopic treatment in 1960 in his historic paper on 100 patients with no deaths or serious complications, using cautery to divide the cricopharyngeus. Thus, the endoscopic diverticulotomy is often called the Dohlman procedure. This procedure consists of dividing the septum between the cervical esophagus and the diverticular pouch. By dividing the septum, food freely drains from the pouch to the esophagus. This division can be accomplished by electrosurgery, laser, and, more recently, a stapling device. The advantages to this method are a shorter surgical
P.2083
time and hospital stay and a faster return to oral intake. One study showed that surgical time averaged 11 minutes, with a hospital stay of 2.2 days. Other researchers have published similar results, with patients being discharged within 2 days. This procedure reduces surgical risk in older adults or debilitated patients with medical problems who might not survive a longer, open procedure. Disadvantages include a historically higher recurrence rate. The procedure requires specialized expertise and equipment because of the risk for esophageal perforation. The procedure provides no specimen for pathologic evaluation. The literature suggests that endoscopic diverticulectomy is a promising technique for treating Zenker's diverticula; however, no long-term clinical results are available for evaluation.
In 1993, Collard from Belgium and Martin-Hirsch form the United Kingdom and their associates were the first to report the use of endoscopic diverticulostomy for the treatment of Zenker's diverticula. In a large series of 102 patients, Narne and co-workers (1999) reported symptomatic improvement in all patients at a mean follow-up of 23 months. Postoperative morbidity and mortality were minimal, and conversion to an open procedure was required in only three patients. Oral feeding was generally begun on the first postoperative day, and the median hospital stay was 3 days. In a series of 74 patients, Cook and co-workers (2000) reported symptomatic improvement in 96% and complete symptom relief in 74% of patients. In this series, the average hospital stay was 1 day, with most patients being released on the day of surgery. The average operative time was 35 minutes. In the largest published series in the world's literature of 159 patients, Chang and colleagues (2003) reported the cumulative results of endoscopic staple diverticulostomy. Symptoms were eliminated or improved in 98% of patients. The average length of hospital stay was 0.76 days, with initiation of the diet in the immediate postoperative setting. There was no mortality. The most common complication was chipped teeth (7.3%), with only one perforation, one case of aspiration pneumonia, and one case of transient vocal cord palsy. The recurrence rate, however, was 11.8% at a mean follow-up of 32.2 months, considerably higher than the mean recurrence rate of 5% reported in open series. Overall, patients have shorter hospital stays, shorter return to diet, and lower complication and mortality rates when compared with open techniques.
Technique of Endoscopic Stapled Diverticulostomy.
After induction of general anesthesia, the diverticulum is visualized by rigid laryngoscopy. The Weerda double-lipped rigid laryngoscope (diverticuloscope, Karl Storz, Germany) can be positioned so that one lip lies in the esophageal lumen and the other lip within the diverticulum. An endoscopic linear stapler is then introduced, and the common septum between the posterior wall of the esophagus and the diverticulum is divided over a length of 30 mm (Fig. 136-29). More than one firing of the stapler may be required depending on the size of the diverticulum. Patients are usually started on clear liquids and discharged to home within 24 hours of surgery. Complications are very rare (<3%) and include perforation of the diverticulum. Endoscopic stapled diverticulectomy is safe and effective and results in less operative time, earlier feeding, shorter hospital stays, and possible fewer complications when compared with open techniques. Patients with cervical spine problems, diverticula smaller than 2 cm, or limited extension of the neck are not suitable candidates for this approach and are probably better served with an open approach.
 |
Fig. 136-29. Endoscopic stapled diverticulectomy. The common septum between the posterior wall of the esophagus and the diverticulum is divided with a linear stapler. |
Epiphrenic Diverticulum
Epiphrenic diverticula occur just proximal to the LES and occur more often in middle-aged men. These diverticula are considered false diverticula because the mucosa herniates through the esophageal muscular wall. They are associated with motor abnormalities (e.g., achalasia), are classified as pulsion diverticula, and may occur in association with a hiatal hernia. Symptoms include dysphagia and esophageal reflux. Symptomatic patients are treated surgically with a combined diverticulectomy and myotomy positioned opposite the diverticulum.
Epiphrenic diverticula typically result as a complication of an underlying esophageal motility disorder, as reviewed by Ott and colleagues (1994). Dysphagia with regurgitation is the predominant symptom complex in most patients. Dysphagia alone occurs in 25% of patients. Of interest, about 25% of patients present with predominantly pulmonary complications (recurring pneumonia, aspiration, lung abscess). Other, less frequent clinical symptoms include chest pain and hiccups. The median duration of symptoms is on the order of 10 years. The diagnosis of epiphrenic diverticulum is secured by videoesophagography (Fig. 136-30) or EGD. Epiphrenic diverticula protrude predominantly to the right (about 70%) and can achieve a wide rang of
P.2084
sizes (2 to 10 cm). Multiple diverticula can also be encountered. In a study of 21 consecutive patients with epiphrenic diverticula by Nehra and associates (2002), motor abnormalities were detected in all 21 patients through stationary or ambulatory esophageal motility studies. The most common abnormality was achalasia (43%), followed by diffuse esophageal spasm (24%), hypertensive LES (21%), and nutcracker esophagus (10%). Opinions vary regarding the need and indications for surgical treatment. Given the high rate of pulmonary complications (25% to 45%), authors such as Nehra (2002) and Altorki (1993) and their colleagues have concluded that all patients with this condition undergo surgical intervention, regardless of the degree of their symptoms. Epiphrenic diverticula can be resected through a thoracoscopic or laparoscopic approach.
 |
Fig. 136-30. Epiphrenic diverticulum. From Feo CV, et al: Laparoscopic approach for esophageal achalasia with epiphrenic diverticulum. Surg Laparosc Endosc Percutan Tech 11:112, 2001. With permission. |
Technique of Transthoracic Epiphrenic Diverticulectomy.
After double-lumen intubation and deflation of the left lung, access to the left chest is gained by placement of a port in the seventh interspace in the midaxillary line. Two additional 5-mm ports are placed for camera access and instrumentation. A fan retractor is used to retract the lung anteromedially. The inferior pulmonary ligament is divided, and the lung is retracted superiorly. The pleura overlying the esophagus is incised for a distance of 10 cm, and the underlying diverticulum is dissected free from the surrounding structures. Unless necessary, the esophagus is not circumferentially mobilized. An esophageal bougie is placed (54F to 60F), and the neck of the diverticulum is stapled using an Endo-GIA stapling device. The muscular layer is closed over the mucosal staple line with interrupted sutures. Suspension and pexy of the diverticulum can be performed if the diverticulum neck is wide or if the diverticulum is in close approximation to the vertebral column. The suspended diverticulum is dissected free from surrounding structures and is sutured to the vertebral fascia with interrupted nonabsorbable sutures in a manner that allows dependent drainage.
Esophageal myotomy is performed as described previously and is oriented on the opposite side of the esophagus in reference to the diverticulum. The proximal limit of the myotomy is determined by the extent of the underlying motility abnormality. The phrenoesophageal ligament is divided. After mobilizing the esophagogastric fat pad, the myotomy is carried distally onto the surface of the stomach for at least 2 cm.
After completing the myotomy, a fundoplication is performed (such as a Dor or Nissen fundoplication). If extensive dissection of the hiatus is required to mobilize the diverticulum, or if the stomach cannot be reduced into the abdomen without deforming the GE junction, then a modified Belsey Mark IV partial fundoplication can be performed.
Technique of Laparoscopic Epiphrenic Diverticulectomy.
The patient is placed in a comfortable supine position. The abdomen is entered through a direct cut-down technique as described before. Pneumoperitoneum is established, and four additional 5-mm ports are placed under direct vision for instrumentation (see Fig. 136-2). A 30-degree scope is recommended. After division of the gastrohepatic ligament, dissection is begun along the right crus and is carried anteriorly over the esophagus to expose the anterior portion of the left crus. The retroesophageal plane is developed. Care is taken to identify and preserve the anterior and posterior vagus trunks. The short gastric arteries are divided with the Ultrasonic shears, and mobilization along the left crus is completed. The dissection is then carried into the mediastinum until the diverticular pouch is reached. A flexible endoscope is advanced into the esophageal lumen and positioned at the level of the diverticular neck. The pouch is then carefully separated from the mediastinal structures until the neck is completely cleared from all adherent tissue (Fig. 136-31A). The pleural folds are identified and swept away from the field of dissection to avoid entry into the pleural space. An Endo-GIA II stapler is used to divide the base of the sac (Fig. 136-31B). This can be performed after placement of an esophageal bougie or with endoscopic visualization. The muscular layer is closed over the staple line with interrupted sutures. A myotomy is performed on the contralateral aspect of the esophagus (Fig. 136-31C). The longitudinal and circular muscle fibers are divided, and the submucosal plane is carefully dissected, as was previously described for achalasia. The myotomy is extended proximally, above the upper limit of the diverticular neck and the underlying motor abnormality, with the assistance of the Ultrasonic shears or hook cautery. The myotomy is extended distally from 1 to 2 cm onto the anterior surface of the stomach. The hiatus is closed with interrupted 0-0 Surgidac sutures.
P.2085
A partial fundoplication is then performed as we have described. Diverticulopexy is preferred in patients in whom the diverticulum is juxtaposed to the spinal column, where they tend to be more proximal and wide mouthed.
Results.
Although it is considered that symptomatic patients should undergo surgery, several reports highlight significant morbidity and mortality. In a series of 33 patients from the Mayo Clinic, Benacci and co-workers (1993) reported a complication rate of 33%, and a mortality rate of 9.1%. Although most diverticula are right sided, a left thoracoscopy or laparoscopic approach is preferred. Mobilization of the diverticulum is easily achieved, and the left chest or abdominal approach provides superior access to the cardia for the extension of the myotomy and construction of the fundoplication. For wide-mouthed diverticula, a thoracoscopic diverticulopexy
P.2086
is recommended. It is important to perform a myotomy in addition to resection or diverticulopexy. Failure to do so encourages a higher incidence of recurrence or suture line leak ranging from 10% to 20%. The extent of myotomy remains somewhat controversial. Streitz and co-workers (1992) advocated performing a myotomy only in the region of the motor abnormality, sparing the LES unless it was hypertensive, thereby avoiding the need for an antireflux procedure. They further argued that this approach reduces the overall complication rate, and patients experience fewer postoperative reflux symptoms. Our belief is that the myotomy should include the entire sphincter zone and the proximal length of motor abnormality, as determined by the preoperative manometric findings. The myotomy is extended to the neck of the diverticulum. Failure to extend the myotomy over the length of motor abnormality can lead to persistence of symptoms. If the sphincter is divided, it becomes necessary to perform an antireflux procedure. In patients with impaired motility, especially those with achalasia, a partial fundoplication may be preferred because it creates less outflow obstruction. Both thoracoscopic and laparoscopic approaches have been used successfully to treat this process. Either approach addresses the primary principles of treatment in this condition: the removal of the pouch, the treatment of the underlying motor disorder, and prevention of postsurgical reflux. The laparoscopic approach facilitates the hiatal dissection and wrap formation, and it usually provides adequate visualization of the diverticulum, as discussed by Myers and Dempsey (1998). Also, the laparoscopic approach allows optimal alignment of the stapler cartridge along the longitudinal axis of the esophagus, minimizing redundant remnants after stapling, as well as optimal visualization of the GE junction, which allows greater ease in performing the myotomy. A thoracoscopic approach is preferred in patients with diverticula located more than a few centimeters from the GE junction, or in those who require a long myotomy, as summarized by Stuart (1996) and Saw (1998) and their colleagues. Dilation typically fails to alleviate symptoms in patients with epiphrenic diverticulum and incurs the potential risk for perforation. Some authors believe that the presence of a diverticulum should represent a contraindication to dilation and that surgical intervention is warranted. Patients with multiple diverticula may require esophagectomy.
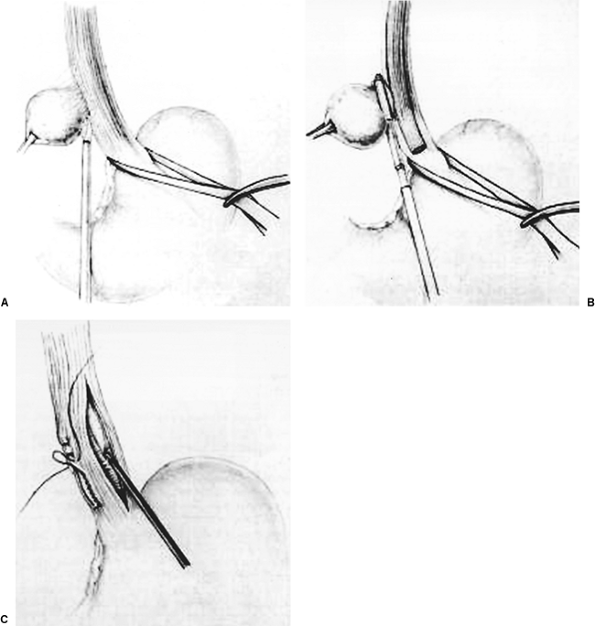 |
Fig. 136-31. Laparoscopic epiphrenic diverticulectomy. A. The diverticular pouch is completely dissected until the neck is free. B. Resection of the diverticulum with a linear stapler. C. The staple line is buttressed, and a myotomy is performed on the contralateral wall. From Rosati R, et al: Diverticulectomy, myotomy, and fundoplication through laparoscopy: a new option to treat epiphrenic esophageal diverticula? Ann Surg 227:174, 1998. With permission. |
Traction Diverticulum
Traction diverticula, which usually are true diverticula, result from forces arising outside the gastrointestinal wall. These diverticula are uncommon and usually a response to periesophageal inflammation. Occurring in old age with equal frequency in men and women, traction diverticula are believed to develop as a result of traction on the esophagus from adhesions originating from an inflammatory disease in the chest. Etiologies of this diverticulum include mediastinal inflammatory (sarcoid, tuberculosis) and malignant (lymphoma) processes. Traction diverticula are benign and frequently may be symptomatic. Indications for repair would include refractory symptomatology. The optimal surgical approach is diverticulectomy by means of thoracoscopy, as described for epiphrenic diverticulum.
MYOMECTOMY
Benign Tumors
Benign tumors of the esophagus are a rare entity. Autopsy studies suggest a prevalence of 0.45% to 0.59%, and they account for 5% of all esophageal neoplasms, as estimated by Plachta (1962). Benign tumors can be classified as mucosal-intraluminal and extraluminal-intramural, as described by Avezzano and associates (1990). Intraluminal tumors can be resected using endoscopic techniques. VATS is the ideal approach for intramural tumors, as summarized by Bardini and Asolati (1997). EUS represents a superior modality in the detection and staging of smooth muscle tumors of the esophagus, benign and malignant, as highlighted by Tio and co-workers (1990). CT is also widely used to characterize size and location of the tumor.
Sauerbruch (1932) is credited with the first resection of a benign esophageal neoplasm. Everitt and co-workers were the first to describe this procedure using a minimally invasive technique in 1992. Both VATS and laparoscopic approaches have been shown to be safe and effective with reduced surgical trauma and decreased length of hospital stay, as summarized by Bonavina (1995) and Roviaro (1998) and their associates.
The principal indications for this procedure are the presence of symptoms and the desire to exclude malignancy. Some surgeons advocate removing all leiomyomas regardless of size. Others rely on size as the principal determinant for resection, with cutoffs for resection ranging from 3 to 5 cm, as discussed by Yamada and co-workers (1992). Malignant transformation to leiomyosarcoma is rare. The correlation between tumor size and malignancy in esophageal neoplasms has not been well established, unlike other gastrointestinal stromal tumors. Yamada and colleagues (1992) identified five endoscopic and ultrasonographic characteristics that are predictive of malignant myogenic tumors: (a) tumor diameter of at least 3 cm, (b) nodular shape, (c) depth of ulceration more than 5 mm, (d) a heterogeneous internal echo, and (e) the presence of an anechoic area. If an asymptomatic tumor fails to conform to at least three of these criteria, then the mass likely represents a benign neoplasm and does not require resection. Other surgical indications include intraluminal pedunculated neoplasms that are not amenable to endoscopic removal. We favor minimally invasive surgical resection of all symptomatic leiomyomas in appropriate-risk patients. Asymptomatic tumors larger than 2 cm and those that display growth should be resected. Small, incidental, asymptomatic leiomyomas smaller than 2 cm can ordinarily be observed.
Intrathoracic leiomyomas can routinely be accessed through the right chest. Leiomyomas of the distal third of
P.2087
the esophagus can be resected from the left chest if this approach is desirable. Neoplasms located in the intraabdominal esophagus or at the GE junction can be removed through laparoscopy.
Technique of VATS Myomectomy.
After intubation with a double-lumen endotracheal tube, the patient is positioned in the left lateral decubitus position, and the right lung is deflated. The surgeon stands on the right side of the table, and the assistant stands on the left. On-table endoscopy is routinely performed to confirm the precise location and appearance of the tumor. Four thoracoscopic ports are introduced into the right hemithorax in a fashion similar to the thoracoscopic mobilization of minimally invasive esophagectomy (see Fig. 136-3). The camera port (10 mm) is placed in the seventh intercostal space just anterior to the midaxillary line. A second 10-mm port is placed anteriorly at the level of the fourth intercostal space and is used for lung retraction. Two 5-mm working ports are placed in the eighth interspace at the level of the posterior axillary line, and just inferior to the scapular tip. For distal intrathoracic tumors, a retraction stitch can be placed (0-0 Surgidac) using the Endo-stitch suturing device. The suture is pulled out through the skin with the assistance of an Endo-close device at a level just above the diaphragm through a 1-mm skin incision. This suture displaces the diaphragm inferiorly, providing excellent exposure of the distal esophagus. A fan retractor is deployed and pulls the lung anteriorly and superiorly. The inferior pulmonary ligament is then taken down with the Ultrasonic coagulating shears. The mediastinal pleura is then incised. Care is taken to identify and preserve the vagal trunks and branches. The azygos vein may be divided with an Endo-GIA stapler if additional exposure is required. The esophagus is mobilized circumferentially. Penrose drains may be placed around the esophagus to facilitate dissection. The esophageal muscularis propria is divided with electrocautery or with Ultrasonic shears to expose the underlying esophageal leiomyoma (Fig. 136-32A). A 0-0 silk suture may be place into the mass to assist with retraction. The mass is then enucleated using a combination of blunt and sharp dissection with the Ultrasonic shears or Endo-peanut (Fig. 136-32B). Care is taken to preserve
P.2088
the integrity of the underlying esophageal mucosa. After enucleation, the tumor is placed into a bag and removed. The right pleural cavity is irrigated, and endoscopic insufflation is performed to assess for any breech in the mucosa. If a perforation is identified, it can be repaired over a bougie with interrupted stitches. After removal of the tumor, the myotomy is reapproximated with interrupted 2-0 Surgidac (Fig. 136-32C). A 28F chest tube is inserted, and the lung is reexpanded. Patients routinely undergo a barium swallow on postoperative day 1. A clear liquid diet is initiated if no esophageal leak is identified. Patients are usually discharged on the second or third postoperative day.
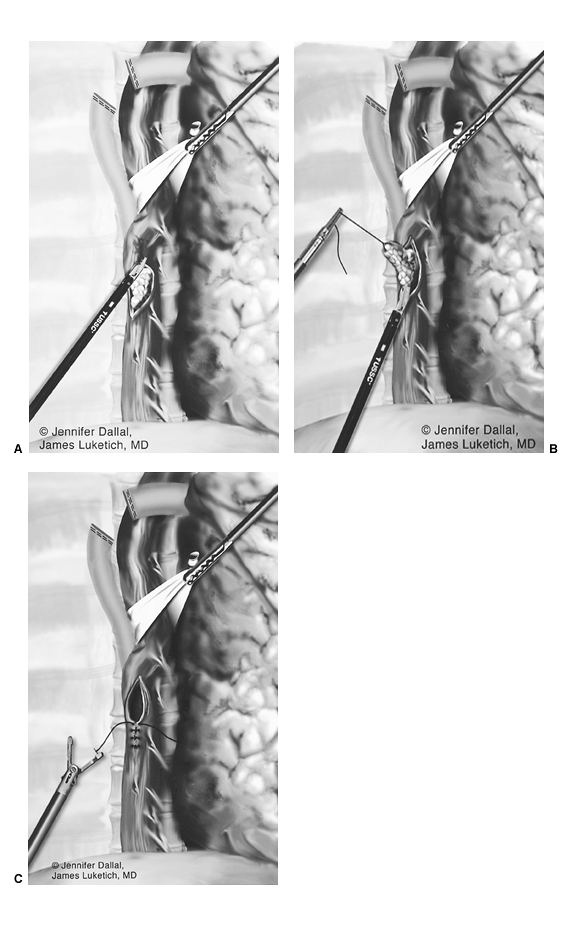 |
Fig. 136-32. Thoracoscopic leiomyomectomy. A. A superficial myotomy permits exposure of the esophageal leiomyoma. B. The leiomyoma is excised while preserving the integrity of the underlying mucosa.C. The myotomy is closed with interrupted sutures. |
Technique of Laparoscopic Myomectomy.
For intraabdominal or GE junction leiomyomas, our standard laparoscopic approach to the esophageal hiatus is used. Five abdominal ports are placed as illustrated previously (see Fig. 136-2). Pneumoperitoneum is established to 15 mm Hg. The left lateral segment of the liver is retracted anteriorly using a diamond flex retractor (Genzyme, Tucker, GA) and is secured into position with the Mediflex self-retaining system (Welmed Inc., Wexford, PA). The distal esophagus is mobilized by dissection of the gastrohepatic and phrenoesophageal ligaments. The right and left crura are exposed. Short gastrics are divided as necessary. The gastroesophageal fat pad is reflected to the patient's right, preserving both the anterior and posterior vagal trunks. The enucleation of the leiomyoma is then performed as previously described. If the patient is documented to have reflux disease, a floppy Nissen wrap is performed over a 50F bougie. For gastric leiomyomas, a wedge resection of the stomach using the Endo-GIA stapler may be performed.
Results.
Esophagectomy can be performed in patients with a very large or annular leiomyoma that is not amenable to enucleation, when the esophageal mucosa is badly ulcerated or damaged during enucleation and cannot be repaired in a satisfactory manner, when multiple or diffuse leiomyomas are found, and when the presence of a leiomyosarcoma is confirmed on biopsy. Minimally invasive esophagectomy as described is an ideal approach for these lesions.
Samphire and associates (2003) reviewed the minimally invasive surgery experience in resecting gastrointestinal stromal tumors of the esophagus and GE junction at the University of Pittsburgh Medical Center. Nine patients were resected between December 1995 and August 2001. The tumors were equally distributed in the middle esophagus (3 patients), the distal esophagus (3 patients), and the GE junction (3 patients). The most common presenting symptoms were dysphagia and heartburn (33%), followed by abdominal pain (11%) and bleeding (11%). The six tumors in the middle and distal thirds of the esophagus were treated with a right VATS and enucleation. The GE junction tumors were approached laparoscopically with enucleation. There were no major morbidities and no mortality. One patient required repair of a small mucosal perforation sustained during the myomectomy after being recognized intraoperatively. There were no postoperative leaks detected. The mean hospital stay was 2.3 days. All tumors were leiomyomas, with an average size of 2.73 cm (0.9 to 8 cm). There has been no evidence of tumor recurrence in any patient at a mean follow-up of 10 months (range, 1 to 34 months).
Minimally invasive resection of esophageal leiomyomas is safe and effective, with low morbidity and low mortality. To date, there have been no reported deaths in patients undergoing minimally invasive enucleation. Among the potential complications encountered with this procedure is esophageal leak, typically resulting from mucosal perforation during the enucleation process. In all of the thoracoscopic literature, only one case has been reported of a recognized and repaired intraoperative perforation, without a postoperative leak. There has been no reported case of a leiomyoma recurrence in either the open or VATS literature.
P.2089
GERD can also occur after enucleation, resulting from disturbance of esophageal motility or the LES mechanism, as noted by Ala-Kulju and Salo (1987). The most common reported complication in the VATS literature has been the development of an esophageal pseudodiverticulum, arising as an outpouching of the esophageal mucosa through the unapproximated outer muscular layers, as reported by Bardini and Asolati (1997) and Roviaro and associates (1998).
In addition to esophageal leiomyoma, minimally invasive techniques can be employed in the treatment of granular cell tumors of the esophagus as well as hemangiomas. Granular cell tumors are rare tumors that arise from neural cells of the esophageal wall. Pathologically, they exhibit staining and electron microscopic characteristics similar to Schwann cells, as documented by Coutinho (1985) and Giacobbe (1988) and their colleagues. These tumors typically reside in the submucosal layer and are most commonly found in the distal esophagus. Multifocal lesions have been demonstrated in about 20% of cases. Management of the tumors remains somewhat controversial. Some authors contend that they should be excised at the time of diagnosis because of the difficulty distinguishing benign from malignant forms, whereas others recommend biopsy and endoscopic follow-up of benign, asymptomatic tumors. Symptomatic tumors and those displaying rapid growth should be removed. Historically, a transthoracic approach has been employed. No minimally invasive resections have yet been reported, although the minimally invasive approach can certainly be used. Hemangiomas are rare vascular tumors that originate in the esophageal submucosa. Hemangiomas are encountered predominantly in the midesophagus. Hemangiomas have been treated with endoscopic resection, as described by Yoshikane (1995), sclerotherapy as reported by Aoki (1997), radiation therapy, and open thoracotomy. There is also a reported case of VATS resection of hemangioma by Ramo and co-workers (1997).
In conclusion, minimally invasive surgery in the treatment of benign tumors of the esophagus has many advantages, including shorter hospital stay, decreased need for postoperative analgesia, and improved cosmesis as compared with open approaches. A VATS or laparoscopic approach should be considered the treatment of choice in centers experienced in minimally invasive esophageal surgery.
REFERENCES
Adler RH: Collis gastroplasty: origin and evolution. Ann Thorac Surg 50: 839, 1990.
Akaishi T, et al: Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg 112:1533, 1996.
Ala-Kulju K, Salo A: Smooth muscle tumours of oesophagus. Scand J Thorac Cardiovasc Surg 21:65, 1987.
Allen SM, Matthews HR: Circular myotomy and Belsey repair for acquired shortening of the oesophagus. Eur J Cardiothorac Surg 7:645, 1993.
Altorki NK, Sunagawa M, Skinner DB: Thoracic esophageal diverticula. Why is operation necessary? J Thorac Cardiovasc Surg 105:260, 1993.
Altorki NK, Yankelvitz D, Skinner DB: Massive hiatal hernias: the anatomic basis of repair. J Thorac Cardiovasc Surg 115:828, 1998.
Aoki T, et al: Successful treatment of an esophageal hemangioma by endoscopic injection sclerotherapy: report of a case. Surg Today 27:450, 1997.
Avezzano EA, et al: Giant fibropolyps of the esophagus. Am J Gastroenterol 85:299, 1990.
Bailey SH, et al: Outcomes after esophagectomy: a ten year prospective cohort. Ann Thorac Surg 75:217, 2003.
Balaji NS, Peters JH: Minimally invasive surgery for esophageal motility disorders. Surg Clin North Am 82:763, 2002.
Bardini R, Asolati M: Thoracoscopic resection of benign tumors of the esophagus. Int Surg 82:5, 1997.
Benacci JC, et al: Epiphrenic diverticulum: results of surgical treatment. Ann Thorac Surg 55:1109, 1993.
Birkmeyer JD, et al: Hospital volume and surgical mortality in the United States. N Engl J Med 346:1128, 2002.
Bonavina L, et al: Surgical therapy of esophageal leiomyoma. J Am Coll Surg 181:257, 1995.
Brand DL, et al: Regression of columnar esophageal (Barrett's) epithelium after anti-reflux surgery. N Engl J Med 302:844, 1980.
Buenaventura PO, et al: Laparoscopic repair of giant paraesophageal hernia. Semin Thorac Cardiovasc Surg 12:179, 2000.
Campos GM, Peters JH, DeMeester TR: Multivariate analysis of the factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 3:292, 1999.
Champion JK, Rock D: Laparoscopic mesh cruroplasty for large paraesophageal hernias. Surg Endosc 17:551, 2003.
Chang CY, Payyapilli RJ, Scher RL: Endoscopic staple diverticulostomy for Zenker's diverticulum: review of literature and experience in 159 consecutive cases. Laryngoscope 113:957, 2003.
Collard JM, et al: En bloc and standard esophagectomies by thoracoscopy. Ann Thorac Surg 56:675, 1993.
Collis JL: An operation for hiatus hernia with short esophagus. J Thorac Surg 34:768, 1957.
Cook RD, et al: Endoscopic staple-assisted esophagodiverticulostomy: an excellent treatment of choice for Zenker's diverticulum. Laryngoscope 110:2020, 2000.
Coutinho DS, et al: Granular cell tumors of the esophagus: a report of two cases and review of the literature. Am J Gastroenterol 80(10):758, 1985.
Csendes A, et al: Late results of a prospective randomized study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut 30:299, 1989.
Dagnini G, et al: Laparoscopy in abdominal staging of esophageal carcinoma. Gastrointest Endosc 32:400, 1986.
Dahlberg PS, et al: Laparoscopic repair of large paraesophageal hernia. Ann Thorac Surg 72:1125, 2001.
Dalenbak J, et al: Improved functional outcome after laparoscopic fundoplication by complete gastric fundus mobilization. Gastroenterology 114:A1384, 1998.
Dallemagne B, et al: Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc 1:138, 1991.
DeMeester SR, DeMeester TR: Columnar mucosa and intestinal metaplasia of the esophagus. Fifty years of controversy. Ann Surg 231:303, 2000.
DeMeester SR, et al: The impact of an anti-reflux procedure on intestinal metaplasia of the cardia. Ann Surg 228:547, 1998.
DeMeester TR, Bonavina L, Albertucci M: Nissen fundoplication for gastroesophageal reflux disease. Evaluation of primary repair in 100 consecutive patients. Ann Surg 204:9, 1986.
DeMeester TR, Johnson LS, Kent AH: Evaluation of current operations for the prevention of gastroesophageal reflux. Ann Surg 180:511, 1974.
Depaula AL, et al: Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc 5:1, 1995.
Dexter SP, Martin IG, McMahon MJ: Radical thoracoscopic esophagectomy for cancer. Surg Endosc 10:147, 1996.
Dohlman GO: The endoscopic operation for esophageal diverticula. Arch Otolaryngol 71:744, 1960.
Donahue PE, Bombeck CT: The modified Nissen fundoplication: reflux prevention without gas bloat. Chir Gastroenterol 11:15, 1977.
Donahue PE, et al: The floppy Nissen fundoplication. Effective long-term control of pathologic reflux. Arch Surg 120:663, 1985.
P.2090
Dor J, et al:. L'interet de la technique de Nissen modifiee dans la prevention du reflux apres cardiomyotomie extramuqueuse de Heller. Mem Acad Chir (Paris) 88:877, 1962.
Downy RJ, et al: Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 21:428, 2003.
Edye M, et al: Sac excision is essential to adequate laparoscopic repair of paraesophageal hernia. Surg Endosc 12:1259, 1998.
Ellis FH Jr: Esophagomyotomy for noncardiac chest pain resulting from diffuse esophageal spasm and related disorders. Am J Med 92:129S, 1992.
Everitt NJ, Glinatsis M, McMahon MJ: Thoracoscopic enucleation of leiomyoma of the esophagus. Br J Surg 79:643, 1992.
Feo CV, et al: Laparoscopic approach for esophageal achalasia with epiphrenic diverticulum. Surg Laparosc Endosc Percutan Tech 11:112, 2001.
Fernando HC, Christie NA, Luketich JD: Thoracoscopic and laparoscopic esophagectomy. Semin Thorac Cardiovasc Surg 12:195, 2000.
Fernando HC, et al: Outcomes of laparoscopic Toupet compared to laparoscopic Nissen fundoplication. Surg Endosc 16:905, 2002a.
Fernando HC, et al: Outcomes of minimally invasive esophagectomy (MIE) for high-grade dysplasia of the esophagus. Eur J Cardiothorac Surg 22:1, 2002b.
Fernando HC, et al: Quality of life after antireflux surgery compared with nonoperative management for severe gastroesophageal reflux disease. J Am Coll Surg 194:23, 2002c.
Fibbe C, et al: Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized clinical and manometric study. Gastroenterology 121:5, 2001.
Finley RJ, et al: Laparoscopic Heller myotomy improves esophageal emptying and the symptoms of achalasia. Arch Surg 136:892, 2001.
Gammie JS, et al: The pigtail catheter for pleural drainage: a less invasive alternative to tube thoracostomy. JSLS 3:57, 1999.
Gastal OL, et al: Short esophagus: analysis of predictors and clinical implications. Arch Surg 134:633, 1999.
Giacobbe A, et al: Granular cell tumor of the esophagus. Am J Gastroenterol 83:1398, 1988.
Glasgow RE, Swanstrom LL: Hand-assisted gastroesophageal surgery. Semin Laparosc Surg 8:135, 2001.
Gozzetti G, et al: Pathophysiology and natural history of acquired short esophagus. Surgery 102:507, 1987.
Gurski RR, et al: Barrett's esophagus can and does regress after antireflux surgery: a study of prevalence and predictive features. J Am Coll Surg 196:706, 2003.
Haas O, et al: Surgical results of intrathoracic gastric volvulus complicating hiatal hernia. Br J Surg 77:1739, 1990.
Hagan JA, Peters JH: Minimally invasive approaches to antireflux surgery. Semin Thorac Cardiovasc Surg 12:157, 2000.
Hashemi M, et al: Laparoscopic repair of large Type III hiatal hernia: objective follow-up reveals high recurrence rate. J Am Coll Surg 190:553, 2000.
Hegedorn C, et al: Long-term efficacy of total (Nissen-Rossetti) and posterior partial (Toupet) fundoplication: Results of a randomized clinical trial. J Gastrointest Surg 6:540, 2002.
Heikkinen TJ, et al: Comparison of costs between laparoscopic and open Nissen fundoplication: a prospective randomized study with a 3 month follow-up. J Am Coll Surg 188:368, 1999.
Heikkinen TJ, et al: Comparison of laparoscopic and open Nissen fundoplication 2 years after operation: a prospective, randomized trial. Surg Endosc 14:1019, 2000.
Heller E: Extramukise kerkioplastic beim chronischen kardiospasmus mit dilatation des oesophagus. Mitt Grenzgeb Med Chir 27:141, 1914. [article in German]
Heudebert GR, et al: Choice of long-term strategy for the management of patients with severe esophagitis: A cost utility analysis. Gastroenterology 112:1078, 1997.
Hill LD: An effective operation for hiatal hernia: an eight-year appraisal. Ann Surg 166:681, 1967.
Hill LD, Gelfand M, Bauermeister D: Simplified management of reflux esophagitis with stricture. Ann Surg 172:638, 1970.
Hinder RJ, et al: Laparoscopic Nissen fundoplication is an effective treatment for gastrointestinal reflux disease. Ann Surg 220:472, 1994.
Hofstetter WL, et al: Long-term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg 234:532, 2001.
Horgan S, et al: Failed antireflux surgery: what have we relearned from re-operations? Arch Surg 1134:809, 1999a.
Horgan S, et al: Repair of paraesophageal hernias. Am J Surg 177:354, 1999b.
Horvath KD, Swanstrom LL, Jobe BA: The short esophagus: pathophysiology, incidence, presentation and treatment in the era of laparoscopic antireflux surgery. Ann Surg 232:630, 2000.
Horvath KD, et al: Laparoscopic Toupet fundoplication is an inadequate procedure for patients with severe reflux disease. J Gastrointest Surg 3: 583, 1999.
Hunter JG, et al: Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg 225:655, 1997.
Jamieson GG, et al: Laparoscopic Nissen fundoplication. Ann Surg 220: 137, 1994.
Jobe BA, Horvath KD, Swanstrom LL: Postoperative function following laparoscopic Collis gastroplasty for shortened esophagus. Arch Surg 133(8):867, 1998.
Jobe BA, et al: Evaluation of laparoscopic Toupet fundoplication as a primary repair for all patients with medically resistant gastroesophageal reflux. Surg Endosc 11:1080, 1997.
Johnson AB, Oddsdottir M, Hunter JG: Laparoscopic Collis gastroplasty and Nissen fundoplication. A new technique for the management of esophageal foreshortening. Surg Endosc 12:1055, 1998.
Johnson LF, DeMeester TR: Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol 8[Suppl 1]:52, 1986.
Kahrilas PJ: Gastroesophageal reflux disease. JAMA 276:983, 1996.
Kassis ES, et al: Detection of occult lymph nodal metastases in esophageal cancer by minimally invasive staging combined with molecular diagnostic techniques. J Surg Laparosc 2:331, 1998.
Katz D, et al: The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett's esophagus. Am J Gastroenterol 93: 536, 1998.
Kelsen DP, et al: Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339:1979, 1998.
Krasna MJ, Jiao X: Thoracoscopic and laparoscopic staging for esophageal cancer. Semin Thorac Cardiovasc Surg 12:186, 2000.
Krasna MJ, McLaughlin JS: Thoracoscopic lymph node staging for esophageal cancer. Ann Thorac Surg 56:671, 1993.
Krasna MJ, et al: Thoracoscopic staging of esophageal cancer: a prospective multi-institutional trial. Ann Thorac Surg 60:1337, 1995.
Krasna MJ, et al: Combined thoracoscopic/laparoscopic staging of esophageal cancer. J Thorac Cardiovasc Surg 111:800, 1996.
Krasna MJ, et al: The role of thoracoscopic staging of esophageal cancer patients. Eur J Cardiothorac Surg 16[Suppl 1]:S31, 1999a.
Krasna MJ, et al: p53 Gene protein expression predicts results of trimodality therapy in esophageal cancer patients. Ann Thorac Surg 68:2021, 1999b.
Krasna MJ, et al: CALGB 9380: a prospective trial of the feasibility of thoracoscopy/laparoscopy in staging esophageal cancer. Ann Thorac Surg 71:1073, 2001.
Kuster GG: Local epinephrine facilitates laparoscopic Heller myotomy. Surg Endosc 12:79, 1998.
Laine S, et al: Laparoscopic vs. conventional Nissen fundoplication. Surg Endosc 11:441, 1997.
Law S, et al: Thoracoscopic esophagectomy for esophageal cancer. Surgery 122:8, 1997.
Laws HL, et al: A randomized prospective comparison of the Nissen fundoplication versus the Toupet fundoplication for gastroesophageal reflux disease. Ann Surg 225:647, 1997.
Lee RB, Miller JI: Esophagectomy for cancer. Surg Clin North Am 77: 1169, 1997.
Litle VR, Buenaventura PO, Luketich JD: Minimally invasive resection for esophageal cancer. Surg Clin North Am 82:711, 2002.
Liu HP, et al: Video-assisted endoscopic esophagectomy with stapled intrathoracic esophagogastric anastomosis. World J Surg 19:745, 1995.
Ludlow A: A case of obstructed deglutition from a preternatural dilatation of and bag formed in the pharynx. Medical observations and enquiries by a society of physicians in London. Vol. 3. 2nd Ed. 1769:85 101.
Luketich JD: Surgery for motility disorders of the esophagus. In Carrau RL, Murry T (eds.): Comprehensive Management of Swallowing Disorders. San Diego: Singular Publishers Group, 1999:337.
Luketich JD, Grondin SC, Pearson FG: Minimally invasive approaches to acquired shortening of the esophagus: laparoscopic Collis-Nissen gastroplasty. Semin Thorac Cardiovasc Surg 12:173, 2000b.
P.2091
Luketich JD, Nguyen NT, Schauer PR: Laparoscopic transhiatal esophagectomy for Barrett's esophagus with high grade dysplasia. JSLS 2:75, 1998a.
Luketich JD, et al: Bronchoesophagopleural fistula after photodynamic therapy for malignant mesothelioma. Ann Thorac Surg 62:283, 1996.
Luketich JD, et al: Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 114;817, 1997a.
Luketich JD, et al: Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg 64:765, 1997b.
Luketich JD, et al: Minimally invasive approach to esophagectomy. J Soc Laparoendosc Surg 2:243, 1998b.
Luketich JD, et al: Evaluation of distant metastases in esophageal cancer. Ann Thorac Surg 68:1133, 1999.
Luketich JD, et al: Minimally invasive esophagectomy. Ann Thorac Surg 70:906, 2000a.
Luketich JD, et al: Laparoscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg 232:608, 2000c.
Luketich JD, et al: Outcomes after minimally invasive reoperation for gastroesophageal reflux disease. Ann Thorac Surg 74:328, 2002.
Luketich JD, et al: Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg 238:486, 2003.
Lundell L, et al: Long-term results of a prospective randomized comparison of total fundic wrap (Nissen-Rossetti) or semifundoplication (Toupet) for gastro-oesophageal reflux. Br J Surg 83:830, 1996.
Mansour K, et al: Complications of intrathoracic Nissen fundoplication. Ann Thorac Surg 32:173, 1981.
Martin-Hirsch DP, Newbegin CJ: Autosuture GIA gun: a new application in the treatment of hypopharyngeal diverticula. Laryngol Otol 107:723, 1993.
Mattioli S, et al: Surgery for esophageal achalasia: long-term results with three different techniques. Hepatogastroenterology 43:492, 1996.
Maziak DE, Todd TR, Pearson FG: Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg 115:53, 1998.
McCallum R, et al: Role of anti-reflux surgery against dysplasia in Barrett's esophagus. Gastroenterology 100:A121, 1991
Milford MA, Paluch TA: Ambulatory laparoscopic fundoplication. Surg Endosc 11:1150, 1997.
Moghissi I: Intrathoracic fundoplication for reflux stricture associated with short esophagus. Thorax 28:36, 1983.
Mosher HP: Webs and pouches of the esophagus: their diagnosis and treatment. Surg Gynecol Obstet 25:175, 1917.
Murray GF, Wilcox BR, Stared PIK: The assessment of operability of esophageal carcinoma. Ann Thorac Surg 23:393, 1977.
Myers MS, Dempsey DT: Laparoscopic resection of esophageal epiphrenic diverticulum. J Laparoendosc Adv Surg Tech A 8:201, 1998.
Narne S, et al: Endoscopic diverticulectomy for the treatment of Zenker's diverticulum: results in 102 patients with staple-assisted endoscopy. Ann Otol Rhinol Laryngol 108:810, 1999.
Nehra D, et al: Toxic bile acids in gastroesophageal reflux disease: influence of gastric acidity. Gut 44:98, 1999.
Nehra D, et al: Physiologic basis for the treatment of epiphrenic diverticulum. Ann Surg 235:346, 2002.
Nguyen HC, Urquhart AC: Zenker's diverticulum. Laryngoscope 107:1436, 1997.
Nguyen NT, Schauer PR, Luketich JD: Combined laparoscopic and thoracoscopic approach to esophagectomy. J Am Coll Surg 188:328, 1999.
Nguyen NT, Schauer P, Luketich JD: Minimally-invasive esophagectomy for Barrett's esophagus with high-grade dysplasia. Surgery 12:195, 2000a.
Nguyen NT, Wang P, Follette D: Laparoscopy or thoracoscopy for achalasia. Semin Thorac Cardiovasc Surg 12:201, 2000c.
Nguyen NT, et al: Preliminary results of thoracoscopic Belsey Mark IV anti-reflux procedure. Surg Laparosc Endosc 8:185, 1998.
Nguyen NT, et al: Comparison of minimally-invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 135:920, 2000b.
Nguyen NT, et al: Thoracoscopic management of postoperative esophageal leak. J Thorac Cardiovasc Surg 121:391, 2001.
Nguyen NT, et al: Thoracoscopic and laparoscopic esophagectomy for benign and malignant disease: lessons learned from 46 consecutive procedures. J Am Coll Surg 197:902, 2003.
Nissen R: Eine einfache operation zur beeinflussung der refluxoesophagitis. Schweix Med Wochenschr 86:590, 1956. [article in German]
Nissen R: Gastropexy and fundoplication in surgical treatment of hiatal hernia. Am J Dig Dis 6:954, 1961.
O'Rourke RW, et al: Extended transmediastinal dissection: an alternative to gastroplasty for short esophagus. Arch Surg 138:735, 2003.
Orringer MB, Marshall B, Iannettoni MD: Transhiatal esophagectomy: clinical experience and refinements. Ann Surg 230:392, 1999.
Orringer MB, Skinner DB, Belsey RHR: Long term results of the Mark IV operation for hiatal hernia and analyses of recurrences and their treatment. J Thorac Cardiovasc Surg 63:25, 1972.
Orringer MB, Sloan H: Combined Collis-Nissen reconstruction of the esophagogastric junction. Ann Thorac Surg 25:16, 1978.
Ott DJ, et al: Achalasia associated with esophageal diverticula: prevalence and potential implications. J Clin Gastroenterol 18:343, 1994.
Patti MG, Pellegrini CA: Endoscopic surgical treatment of primary oesophageal motility disorders. J R Coll Surg Edinb 41:137, 1996.
Patti MG, Pellegrini CA, Arcrito M: Comparison of medical and minimally invasive surgical therapy for primary esophageal motility disorders. Arch Surg 130:609, 2001.
Patti MG, Pellegrini CA, Horgan S: Minimally invasive surgery for achalasia: an 8-year experience with 168 patients. Ann Surg 230:587, 1990.
Patti MG, et al: Comparison of laparoscopic and thoracoscopic Heller myotomy for achalasia. J Gastrointest Surg 2:561, 1998.
Patti MG, et al: Effects of previous treatment on results of laparoscopic Heller myotomy for achalasia Dig Dis Sci 44:2270, 1999a.
Patti MG, et al: Laparoscopic Heller myotomy relieves dysphagia in achalasia when the esophagus is dilated. Surg Endosc 13:843, 1999b.
Pearson FG, Henderson RD: Long-term follow-up of peptic strictures managed by dilatation, modified Collis gastroplasty and Belsey hiatus hernia repair. Surgery 80:396, 1976.
Pearson FG, Langer B, Henderson RD: Gastroplasty and Belsey hiatus hernia repair: an operation for the management of peptic stricture with acquired short esophagus. J Thorac Cardiovasc Surg 61:50, 1971.
Pearson FG, Todd TR: Gastroplasty and fundoplication for complex reflux problems: long-term results. Ann Surg 206:473, 1987.
Perez AR, Moncure AC, Rattner DW: Obesity is a major cause of failure for both transabdominal and transthoracic antireflux operations. 100th Annual Meeting of the American Gastroenterological Association. Gastroenterology 116:A1343, 1999.
Perry Y, et al: Minimally invasive esophagectomy in the elderly. JSLS 6:299, 2002.
Peters JH, et al: Esophageal resection with colon interposition for end-stage achalasia. Arch Surg 130:632, 1995.
Phillips RW, Wong RK: Barrett's esophagus. Natural history, incidence, etiology and complications. Gastroenterol Clin North Am 20:791, 1991
Pierre AF, Luketich JD: Technique and role of minimally invasive esophagectomy for premalignant and malignant diseases of the esophagus. Surg Oncol Clin North Am 11:337, 2002.
Pierre AF, Luketich JD, Fernando HC: Results of laparoscopic repair of giant paraesophageal hernias: 200 consecutive patients. Ann Thorac Surg 74:1909, 2002.
Plachta A: Benign tumors of the esophagus. Am J Gastroenterol 28:639, 1962.
Pohl D, et al: Management and outcome of complications after laparoscopic antireflux operations. Arch Surg 136:399, 2001.
Raiser F, et al: Heller myotomy via minimal access surgery: an evaluation of anti-reflux procedures. Arch Surg 131:593, 1996.
Ramo OJ, et al: Treatment of a submucosal hemangioma of the esophagus using simultaneous video-assisted thoracoscopy and esophagoscopy: description of a new minimally invasive technique. Endoscopy 29:S27, 1997.
Reed CE, et al: Esophageal cancer staging: Improved accuracy by endoscopic ultrasound of celiac lymph nodes. Ann Thorac Surg 67:319, 1999.
Robert T, et al: Cancer statistics, 2000. CA Cancer J Clin 50:7, 2000.
Rosati R, et al: Evaluating results of laparoscopic surgery for esophageal achalasia. Surg Endosc 12:270, 1998.
Roviaro GC, et al: Videothoracoscopic treatment of esophageal leiomyoma. Thorax 53:190, 1998.
Rydberg L, et al: Tailoring antireflux surgery: A randomized clinical trial. Word J Surg 23:612, 1999.
Samphire J, Nafteaux P, Luketich JD: Minimally-invasive techniques for resection of benign esophageal tumors. Semin Thorac Cardiovasc Surg 15:35, 2003
Sauerbruch F: Presentations in the field of thoracic surgery. Arch F Klin Chir 173:457, 1932.
Saw EC, McDonald TP, Kam NT: Video-assisted thoracoscopic resection of an epiphrenic diverticulum with esophagomyotomy and partial fundoplication. Surg Laparosc Endosc 8:145, 1998.
P.2092
Schauer PR, et al: Comparison of laparoscopic versus open repair of paraesophageal hernia. Am J Surg 176:659, 1998.
Schuchert MJ, Luketich JD: Barrett's esophagus: current status of treatment. Adv Surg 37:179, 2003.
Shaheen NJ, et al: Is there publication bias in reporting cancer risk in Barrett's esophagus? Gastroenterology 119:333, 2000.
Shimi S, Mathanson LK, Cuschieri A: Laparoscopic cardiomyotomy for achalasia. J R Coll Surg Edinb 36:152, 1991.
Shimi SM, Nathanson LK, Cushieri A: Thoracoscopic long esophageal myotomy for nutcracker esophagus: initial experience of a new surgical approach. Br J Surg 79:533, 1992.
Skinner DB, Belsey RH: Surgical management of esophageal reflux and hiatus hernia: long-term results with 1,030 patients. J Thorac Cardiovasc Surg 53:33, 1967.
Sontag SJ, et al: Asthmatics with gastroesophageal reflux: long-term results of a randomized trial of medical and surgical antireflux therapies. Am J Gastroenterol 98:987, 2003.
Spechler SJ: Comparison of medical and surgical therapy for complicated gastroesophageal reflux disease in veterans. N Engl J Med 326:786, 1992.
Spechler SJ: Barrett's esophagus. N Engl J Med 346:836, 2002.
Spiess AE, Kahrilas PJ: Treating achalasia: from whalebone to laparoscope. JAMA 280:638, 1998.
Steichen FM: Abdominal approach to the Collis gastroplasty and Nissen fundoplication. Surg Gynecol Obstet 162:372, 1986.
Stewart KC, et al: Thoracoscopic versus laparoscopic modified Heller myotomy for achalasia: efficacy and safety in 87 patients. J Am Coll Surg 189:164, 1999.
Stirling MC, Orringer MB: Continued assessment of the combined Collis-Nissen operation. Ann Thorac Surg 47:224, 1989.
Streitz JM Jr, Glick ME, Ellis FH Jr: Selective use of myotomy for treatment of epiphrenic diverticula. Manometric and clinical analysis. Arch Surg 127:585, 1992.
Stuart RC, et al: Thoracoscopic resection of esophageal diverticulum: a case report. J Royal Coll Surg Edinb 41:118, 1996.
Swanstrom LL, Hansen P: Laparoscopic total esophagectomy. Arch Surg 132:943, 1997.
Swanstrom LL, Marcus DR, Galloway GQ: Laparoscopic Collis gastroplasty is the treatment of choice for the shortened esophagus. Am J Surg 171:477, 1996.
Swanstrom LL, Pennings J: Laparoscopic esophagomyotomy for achalasia. Surg Endosc 9:286, 1995.
Swanstrom LL, et al: Esophageal motility and outcomes following laparoscopic paraesophageal hernia repair and fundoplication. Am J Surg 177: 359, 1999.
Tio TL, Tyt GNJ, den Hartog Jager FCA: Endoscopic ultrasonography for the evaluation of smooth muscle tumors in the upper gastrointestinal tract: an experience with 42 cases. Gastrointest Endosc 36:342, 1990.
Toupet A: Technique d'oesophago-gastroplastie avec phreno-gastropexie appliquee dans la cure radicale des hernies hiatales et comme complement de l'operation d Heller dans les cardiospasmes. Mem Acad Chir 89:394, 1963.
Trus TL, et al: Intermediate follow-up of laparoscopic antireflux surgery. Am J Surg 171:32, 1996.
Urbach DR. et al: Preoperative determinants of an esophageal lengthening procedure in laparoscopic antireflux surgery. Surg Endosc 15:1408, 2001.
Vaezi MF, Richter JE: Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol 27:21, 1998.
Van Dam J, et al: High-grade malignant stricture is predictive of esophageal tumor stage. Cancer 71:2910, 1993.
Wallace WB, et al: An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography and thoracoscopy/laparoscopy. Ann Thorac Surg 74:1026, 2002.
Walther B, et al: Effect of paraesophageal hernia on sphincter function and the implication on surgical therapy. Am J Surg 147:111, 1984.
Wiechmann RJ, et al: Laparoscopic management of giant paraesophageal herniation. Ann Thorac Surg 71:1080, 2001.
Williamson WA, Ellis FH, Streitz JM: Paraesophageal hiatal hernia: is an antireflux procedure necessary? Ann Thorac Surg 56:447, 1993.
Wren SM, Stijns P, Srinivas S: Positron emission tomography in the initial staging of esophageal cancer. Arch Surg 137:1001, 2002.
Yamada Y, Kida M, Sakaguchi T: A study on myogenic tumors of the upper gastrointestinal tract by endoscopic ultrasonography with special reference to the differential diagnosis of benign and malignant lesions. Dig Endosc 4:396, 1992.
Yamamura MS, et al: Laparoscopic Heller myotomy and anterior fundoplication for achalasia results in a high degree of patient satisfaction. Arch Surg 135:902, 2000.
Yoshikane H, et al: Hemangioma of the esophagus: endoscopic imaging and endoscopic resection. Endoscopy 27:267, 1995.
Zaaijer JH: Cardiospasm in the aged. Ann Surg 77:615, 1923.
Zenker FA, von Ziemssen H: Krankheiten des Oesophagus. In von Ziemssen H (eds): Handbuch der Speciellen Pathologie and Therapie. Vol. 7 (Suppl). Leipzig: FCW Vogel, 1877, pp. 1 87.
Zornig C, et al: Nissen vs. Toupet laparoscopic fundoplication. Surg Endosc 16:758, 2002.
Reading References
DeMeester TR, Stein HJ: Surgical treatment of gastroesophageal reflux disease. In Castell DO (ed): The Esophagus. Boston: Little, Brown, 1992, p. 579.
DeMeester TR, et al: Esophageal function in patients with angina-type chest pain and normal coronary angiograms. Ann Surg 196:488, 1982.
Dodds WJ, et al: Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 307:1547, 1982.
Eastwood GL, Castell DO, Higgs RH: Experimental esophagitis in cats impairs lower esophageal sphincter pressure. Gastroenterology 69:146, 1975.
Ferraro P, Duranceau A: Medical management of gastroesophageal reflux disease. Chest Surg Clin N Am 11:517, 2001.
Gillison EW, et al: The significance of bile in esophagitis. Surg Gynecol Obstet 134:419, 1972.
Howard JM, et al: Macroscopic healing of esophagitis does not improve esophageal motility. Dig Dis Sci 39:648, 1994.
Howard PJ, Heading RC: Epidemiology of gastroesophageal reflux disease. World J Surg 16:288, 1992.
Kadakia SC, et al: Effect of cigarette smoking on gastroesophageal reflux measured by 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol 90:1785, 1995.
Liebermann-Meffert D, et al: Muscular equivalent of the lower esophageal sphincter. Gastroenterology 76:31, 1979.
Luketich JD, et al: Minimally invasive surgical biopsy confirms PET findings in esophageal cancer. Surg Endosc 11:1213, 1997.
Luketich JD, et al: Detection of micrometastases in histologically negative lymph nodes in esophageal cancer. Ann Thorac Surg 66:1715, 1998.
Mayer EM, Grabowski CJ, Fisher RS: Effect of graded doses of alcohol upon esophageal motor function. Gastroenterology 75:1133, 1978.
Mittal RK: Hiatal hernia: myth or reality? Am J Med 103:33S, 1997.
Mittal RK, Balaban DH: The esophagogastric junction. N Engl J Med 336: 924, 1997.
Orlando RC: Esophageal epithelial defenses against acid injury. Am J Gastroenterol 89:S48, 1994.
Schoeman MN, et al: Mechanisms of gastroesophageal reflux in ambulant healthy human subjects. Gastroenterology 108:83, 1995.
Skinner DB: Pathophysiology of gastroesophageal reflux. Ann Surg 202: 546, 1985.
Spechler SJ: Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion 51[Suppl 1]:24, 1992.
Stein HJ, et al: Complications of gastroesophageal reflux disease: role of the lower esophageal sphincter, esophageal acid and acid/alkaline exposure, and duodenogastric reflux. Ann Surg 216:35, 1992.
Vitale GC, et al: The effect of alcohol on nocturnal gastroesophageal reflux. JAMA 258:2077, 1987.
EAN: 2147483647
Pages: 203