117 - Adenoid Cystic Carcinoma and Other Primary Salivary Gland-Type Tumors of the Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXI - Operative Procedures in the Management of Esophageal Disease > Chapter 135 - Free Intestinal Transfer Techniques in Reconstruction of the Esophagus
Chapter 135
Free Intestinal Transfer Techniques in Reconstruction of the Esophagus
Babak J. Mehrara
Joseph J. Disa
The advent of microsurgical techniques has revolutionized pharyngolaryngeal reconstruction. These techniques have enabled reconstructive surgeons to perform reliable single-stage repairs of complex defects with minimal morbidity and mortality. Microvascular free-flap reconstruction with intestinal conduits enables maintenance of oral sections and rapid return of oral feeds and swallowing. Avoidance of dependency on tube feeds has a tremendous impact on quality of life and is particularly important in patients with limited long-term survival, such as those with locally advanced tumors of the pharyngoesophagus.
Microvascular transfer of intestinal tissues for esophageal reconstruction is indicated in the treatment of circumferential or partial defects of the esophagus resulting from surgical resection of pharyngoesophageal or laryngeal cancers, trauma, caustic stenosis, congenital esophageal atresia, congenital tracheoesophageal fistula, and pharyngoesophageal stricture resulting from surgery or radiation therapy. Major reports have been published by Mansour (1990) and Carlson (1993) and their associates, as well as by Coleman (1995), Anthony and colleagues (1997), and Chen and Tang (2000). One of us (JJD) and Cordiero (2001) and colleagues (2003) presented our own experience with these procedures. Although a number of vascularized intestinal conduits have been described and the ideal reconstructive technique is debated, the jejunum remains the most popular choice and is the workhorse in most centers.
HISTORY
Early surgical techniques for reconstruction of the cervical esophagus relied on placement of rigid stents or primary closure and were complicated by high rates of postoperative complications and mortality. Gastric pull-up procedures and pedicled colon or jejunal flaps were also described for reconstruction of the cervical esophagus; however, these procedures carried significant morbidity and mortality risks because of the need for extensive dissection in the abdomen and the chest and ischemic complications in the distal portions of the flap.
In the 1920s, Gilles and Blair popularized the use of random patterned tubed skin flaps that were waltzed to the site of defect by picking up blood supply from the site of insertion. These procedures were multistaged, complicated, and commonly suffered from ischemic complications. Most patients had a controlled pharyngocutaneous fistula for 6 to 12 months, and many died prior to completion of reconstruction. Wookey (1942) improved on these techniques by describing a laterally based tubed random cervical flap that decreased the time necessary for reconstruction. Although this technique was the standard of care until the mid-1960s, its use was limited due to a relatively small amount of available skin (particularly above the hyoid bone), high rates of wound complications and fistula formation, and poor vascular supply after radical neck dissection or radiation therapy.
In 1965, Bakamjian revolutionized cervical esophageal reconstruction when he introduced the use of the deltopectoral flap. The deltopectoral flap, unlike other skin flaps used before, was based on an axial blood supply derived from perforating vessels arising from the internal mammary vessels through the second, third, and fourth intercostal space. This pattern of blood supply enabled reliable flap elevation that could be extended to produce large tube flaps if a delay procedure was utilized. The deltopectoral flap had the additional advantage of a remote blood supply that was not injured by radical neck dissection or previous radiation therapy. Using these flaps, Bakamjian (1965) reported a mortality rate of less than 4% and a fistula rate of approximately 30%. Although these results represented a significant advance in esophageal reconstruction, the use of the deltopectoral flap was still less than ideal because it was a multistage procedure (at least two stages if a delay was not necessary) with a controlled salivary fistula, and because the relatively poor blood supply of the distal portions of the flap did not improve wound healing in the neck.
P.2043
Musculoskeletal flaps, in particular the pectoralis major myocutaneous flap (PMMF), were the next advance in cervical esophageal reconstruction because of their improved blood supply and the potential for single-stage reconstruction immediately after resection, as described by Ariyan (1979a, 1979b). The rich blood supply of these flaps had the potential to improve wound healing in the neck and decreased fistula rates as compared with skin flaps, as noted by Ariyan (1979b) and by Theogaraj and co-workers (1980). The excessive bulk of the PMMF (particularly in women) complicated circumferential reconstruction in some patients and led to wound healing complications at the proximal esophageal anastomosis due to the effect of gravity, making this reconstructive technique less than ideal.
In 1907, Alexis Carrel introduced the concept of microvascular tissue transfer by transferring a segment of jejunum to the cervical esophagus in a canine model. Technical difficulties, including the lack of adequate operating microscopes and microsurgical instruments, impeded the clinical use of these techniques until 1959, when Seidenberg and associates reported the first clinical case in which a free jejunum autograft was used to reconstruct a cervical esophageal defect in a patient with recurrent laryngeal cancer. The patient survived for 5 days but died suddenly of a cerebrovascular accident. Autopsy examination at that time, however, revealed that the flap was viable. In 1961, Roberts and Douglas reported the first successful case in which the patient was able to resume oral feeding and swallowing after microvascular jejunal transplantation. That same year, Heibert and Cummings tubularized and transferred a segment of the gastric antrum to reconstruct the cervical esophagus and pharynx. The greater curvature of the stomach together with the greater omentum was first transferred by Baudet in 1979. This flap had the advantages of an extremely long vascular pedicle (>30 cm) and avoided the erosive complications associated with acid secretion by the transplanted gastric antrum. The sigmoid colon flap was reported by Nakayama and associates in 1962; however, concerns about abdominal and neck contamination kept the interest in this flap relatively low.
Since the late 1970s, improvements in microsurgical techniques, instrumentation, and operating microscopes have improved the rates of success with free intestinal transfers, and these techniques have become the mainstay of cervical esophageal reconstruction. Because of the technical considerations, most centers rely primarily on the free jejunal flap for cervical esophagus reconstruction.
MICROVASCULAR TRANSPLANTATION OF THE JEJUNUM
Indications and Contraindications
Microvascular transfer of the jejunum is indicated in the repair of circumferential and near-circumferential ( 50%) defects of the cervical esophagus. Although the jejunum free flap has been used as a patch for smaller defects of the cervical esophagus (<50%), we, as reported by one of us (JJD) and colleagues (2003), prefer to use the free radial forearm flap for these situations. Because of the segmental blood supply of the jejunum, up to 20 cm of jejunum can be harvested based on a single vascular arcade. In general, this length limits the use of the microvascular jejunal transfer to the cervical esophagus. Total and subtotal esophageal reconstructions extending to the thoracic esophagus are usually reconstructed with pedicled colon or gastric pull-up operations. If these options are not available, then reconstruction can be performed using a pedicled jejunal flap with distal revascularization in the neck or a free skin flap as described by Chen and Tang (2000).
Free jejunal transfer is contraindicated in patients with chronic intestinal diseases (e.g., Crohn's disease), grave medical conditions, and chronic liver disease with ascites. The lack of available recipient vessels in the neck also precludes free jejunal transfer. Multiple previous abdominal operations with extensive adhesions are a relative contraindication.
Operative Technique
Before harvesting the jejunum, the reconstructive surgeon must ensure that recipient vessels in the neck are available for microsurgical transfer. These vessels should be in close proximity to each other since it is not feasible to widely separate the mesenteric vein and artery. Arterial supply is reestablished by end-to-side anastomosis to the external carotid artery or end-to-end anastomosis to the lingual, facial, or superior thyroid arteries. The lingual artery should be used only if it is certain that the contralateral lingual artery is intact. Venous drainage is provided by end-to-side anastomosis to the internal jugular vein or end-to-end anastomosis to a suitable side-branch. Vein grafts are avoided at all costs.
Preoperative bowel preparation and preoperative/intraoperative placement of enteral feeding tubes (percutaneous endoscopic gastrostomy, jejunostomy) are not routine, as noted by Flynn and Acland (1979), as well as by Fisher (1985, 1990) and Coleman (1987, 1989) and their colleagues. Broad-spectrum antibiotics are administered preoperatively, and Venodyne boots are placed prior to the induction of general anesthesia.
The jejunum is exposed by a midline upper abdominal incision. Although a vascularized segment of jejunum can be harvested from virtually anywhere between the ligament of Treitz to as far as 150 cm beyond, a portion that is approximately 40 cm distal to the ligament is the usual location chosen, as recommended by McKee and Peters (1978), McCaffrey and Fisher (1987), and Shumrick and Savoury (1988), as well as by Salamoun (1987) and Wang (1986) and their associates. This is based on the fact that the pedicle length of this portion of the jejunum is longer than more
P.2044
proximal or distal portions. In addition, jejunal harvest from this portion of the intestine facilitates intraabdominal bowel reanastomosis and enables the procurement of a second portion of jejunum without proximal ischemic complications if the first segment that was selected is lost due to anastomotic thrombosis.
The bowel is transilluminated, and a suitable vascular arcade is selected (Fig. 135-1). Particular attention must be paid to the position of the recipient vessels in the neck in relation to the proximal bowel anastomosis with the cervical esophagus (Fig. 135-2). The distance along a diagonal line from the proximal anastomosis to the position of the recipient vessels is carefully measured and is used to design the vascular pattern that will minimize kinking, stretching, or compression of the anastomosis (Fig. 135-3).
The proximal (i.e., isoperistaltic) portion of the jejunum is marked with a stitch, and dissection begins with meticulous ligation of the mesentery. This should be done in small increments because bunching of the mesentery in large bites decreases the pedicle length. The peritoneum overlying the mesenteric vessels is incised at the point where the vessels begin to branch, and the vessels are carefully dissected to obtain length. The dissection of pedicle, particularly the mesenteric vein, should be performed with great caution because these vessels are extremely fragile and even minor injury can result in a mesenteric hematoma. The pedicle vessels are separated in situ and the bowel is divided using an intestinal stapler (Fig. 135-4). The jejunum is allowed to perfuse for a few minutes prior to ligation of the pedicle vessels.
Once the pedicle is ligated, the flap is quickly transferred to the neck and made ready for the proximal cervical anastomosis. The width of the jejunum may be increased to match the diameter of the cervical esophagus by splitting the jejunum along its antimesenteric border for several centimeters. Alternatively, as suggested by Jones and co-workers (1991), if a larger opening is required (as, for example, during reconstruction of the base of the tongue), the flap may be split along its antimesenteric border and folded and sutured to itself to effectively double its luminal diameter.
 |
Fig. 135-1. A segment of jejunum is transilluminated to demonstrate the vascular pattern. |
 |
Fig. 135-2. A typical cervical esophageal defect. In this case the defect spans between the open arrows. Note clip on lingual artery. Pedicle length (P) necessary in the jejunal flap is estimated by measuring the diagonal distance between the proximal esophageal stump and the location of the recipient vessels. |
The proximal cervical anastomosis is performed with interrupted resorbable sutures such as 2-0 Vicryl. This anastomosis must be done meticulously to avoid postoperative leaks and fistula formation. Some authors prefer to also complete the distal anastomosis prior to revascularization; however, the correct length of jejunum that is necessary may be difficult to judge because the jejunum grows significantly in length after revascularization. In addition, completion of the distal anastomosis may increase the ischemic time, leading to reperfusion injury.
Microvascular anastomosis is performed using standard techniques, and the flap is monitored for reperfusion. Spontaneous and mechanically induced peristalsis should be readily visible. The distal anastomosis is performed with interrupted sutures after the jejunum is trimmed to the appropriate length. Although the distal anastomosis may be performed with automatic stapling devices, there is some evidence, as presented by Schusterman and associates (1990), that these techniques may increase the incidence of stricture formation and stenosis. The patient's neck should be in neutral or slightly flexed position to avoid redundancy.
P.2045
Some authors reinforce the suture line with Lembert-type sutures. The esophageal repair is stented with a nasogastric tube. Closed suction drains are placed in each side of the neck and positioned away from the microvascular and bowel anastomoses.
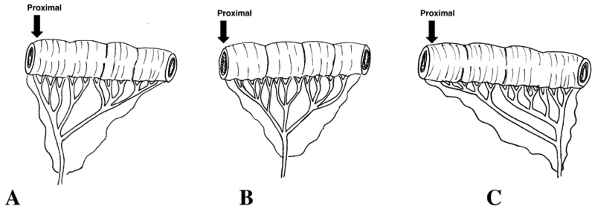 |
Fig. 135-3. Patterns of vascular arcade that may be harvested to minimize kinking, stretching, or compression of the anastomosis. The pattern in (A) would be selected if the recipient vessels were located close to the proximal esophageal stump (e.g., superior thyroid artery and internal jugular vein), whereas the vascular pattern in (C) would be more appropriate if the recipient vessels were located relatively far away from the proximal esophageal stump (e.g., transverse cervical vessels). |
A number of studies have evaluated methods to increase the ischemic tolerance of the transferred jejunum. These studies have relied primarily on pharmacologic manipulations or hypothermia. Fisher and colleagues (1985) theorized that hyperosmolar perfusate infused into the mesenteric artery could stabilize the endothelium. Others, including Nakamura (1975) and Wang (1986) and their co-workers, have attempted perfusion of the flap with heparinized saline. No difference in outcome was noted by McKee and Peters (1978), however, between heparin-perfused and non-heparin-perfused flaps.
Hypothermia can significantly increase the ischemic tolerance of the jejunum. Lillehei and associates (1959) demonstrated that cooling the flap to 5 C increases the ischemic time to as much as 5 hours. This finding has led some authors, such as Shumrick and Savoury (1988), to use elaborate cooling devices such as glass rods in the lumen of the
P.2046
transferred jejunum through which cool saline is continuously instilled. Most authors do not routinely use cooling devices, however, since the jejunum can reliably survive up to 2 hours during euthermic ischemia, as shown by Lillehei and associates (1959) as well as by Mullens and Pezacki (1971). In fact, it has been proposed by Reuther and colleagues (1984) that 2 hours of euthermic ischemia is beneficial because it may decrease mucus production.
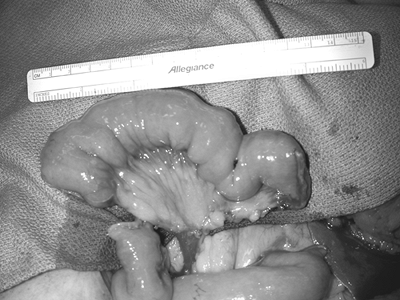 |
Fig. 135-4. The segment of jejunum is ready for harvest. The jejunum has been stapled and divided both proximally and distally with a GIA stapler, and the recipient vessels have been dissected and separated. |
Postoperative Monitoring and Care
Postoperative monitoring of the free jejunum flap may be difficult because the flap is buried. In general, we use either conventional monitoring techniques or implantable Doppler devices. Some authors have recommended the use of a monitor segment brought out along the neck incision; however, one of us (JJD) and co-workers (1999) have found that this technique can be unreliable. It has been suggested by Hidalgo (1998) and one of us (JJD) (1999) and respective associates that monitoring of jejunal microvascular flaps may be irrelevant since these flaps tolerate ischemia poorly and are rarely salvageable at the time of reexploration.
Circumferential dressing or tape (e.g., tracheostomy ties) should be avoided. The patient is kept warm and well hydrated, and urine output is carefully monitored. Some authors advocate the use of aspirin for 5 days postoperatively to prevent microvascular thrombosis.
The esophageal repair is stented and the stomach is decompressed for 4 to 5 days with a nasogastric tube, following which tube feeds are initiated. Although Gastrografin or barium swallows are routinely used by many authors to assess postoperative leaks, a recent review of our experience by Cordeiro and colleagues (1999) demonstrated that the barium swallow was no more predictive of a postoperative leak than clinical exam alone. Thus, false-negative and false-positive findings with the swallow test made the interpretation of these results difficult. We therefore use clinical judgment to determine the initiation of oral feeds and reserve radiologic studies as an adjunctive study (Fig. 135-5). Oral feeds are initiated with clear liquids and advanced as tolerated.
Results of Esophageal Repair with Jejunal Microvascular Transfer
Shangold and associates (1991) reviewed 633 reported cases of microvascular jejunal transfers in the literature. This study demonstrated a 4.4% overall perioperative mortality and a 91.1% overall success rate. Fistulae were noted in 18% of patients and occurred most commonly along the proximal anastomosis. Two-thirds of all fistulae resolved with conservative management. Nearly 82% of patients were able to maintain their nutrition completely with oral feeds.
A recent review of 90 consecutive free jejunum transfers by one of us (JJD) and colleagues (2003) demonstrated a 98% flap success rate. The mean hospital stay was 19 days. Nearly 65% of patients resumed an unrestricted diet, and 88% maintained adequate oral nutrition. Ten percent of patients developed postoperative fistulae, most of which healed spontaneously. Fistula formation was significantly more common in patients who were treated with preoperative radiation therapy. One patient died of respiratory failure, and one patient developed abdominal wall dehiscence. The overall early complication rate was 11%. The most common late complication was stricture formation (8%).
Theile and co-workers (1995) evaluated 201 consecutive cases of free jejunal transfer performed for reconstruction of pharyngolaryngectomy defects over a 16-year period (1977 to 1993). The flap success rate was 97%. All microvascular failures were successfully salvaged with a second free jejunum flap. The perioperative mortality rate was 4.5%. No deaths were attributable to leaks, flap failure, or abdominal complications. Anastomotic leaks were observed in 6.5%, and most (67%) closed spontaneously. Leaks that did not close with conservative management were treated successfully with a pectoralis major myocutaneous flap. Nearly 90% of patients in whom follow-up was available were able to swallow well and maintain their nutritional status. Stricture formation was noted in 7.5% of patients.
The incidence of abdominal complications reported by Thiele and associates (1995) was low (2.5%) and consisted of minor wound-healing complications or adhesive bowel obstruction. One patient had intraperitoneal bleeding necessitating reoperation. The authors reported six late failures (ranging from 3 months to 31 months). These patients presented with progressive dysphagia and were noted to have necrotic or fibrotic segments of jejunum on endoscopy. Five of these patients required replacement of the initial flap.
Comparisons of the complication rates of free jejunal reconstruction of cervical esophageal defects with gastric pull-up procedures have been published by Schusterman and associates (1990) and demonstrated significant advantages in favor of microvascular jejunal transfer. These advantages include decreased flap failure, decreased fistula formation, decreased hospital stay, and earlier resumption of oral alimentation.
Management of Complications
Thrombosis and flap loss is most common in the first and second postoperative days. Due to the poor ischemic tolerance of the jejunum, thrombosed flaps are rarely salvageable. In these instances, early d bridement and repeat free jejunum transfer is usually the best approach.
Postoperative salivary leaks and fistulae after free jejunal transfer can usually be treated conservatively with maintenance of NPO status, dressing changes, and wound care. This complication is more common in patients previously treated with radiation therapy. Larger, more persistent leaks may respond to advancement of wound edges, local flap
P.2047
closure with T-tube decompression of the bowel segment as suggested by Vogel and Strong (1978), or pectoralis myocutaneous flap reinforcement of the closure.
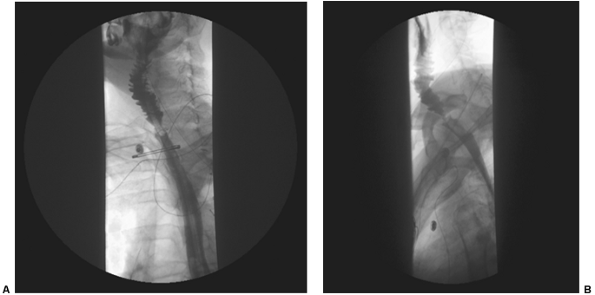 |
Fig. 135-5. Posteroanterior and lateral views of a typical esophagogram after cervical esophageal reconstruction with a free jejunal flap. Note widely open anastomoses and no extravasation of contrast material. Also note plica circulares of the jejunal segment. |
Strictures usually occur at the distal esophageal anastomosis and can be identified by endoscopy or radiologic procedures. Strictures may be due to fibrosis of the distal anastomosis or may result from kinking of elongated bowel segments. Endoscopic dilation may provide some relief in cases of fibrosis. Excision of the redundant segment and reclosure is occasionally necessary.
Postoperative dysphagia may be due to residual dyskinetic peristalsis. Uncoordinated or persistent peristalsis may result in delayed emptying of the jejunal segment, particularly with solids. There are currently no good solutions for this problem. Nakamura and colleagues (1975) have shown that motility decreases with time, a mechanism that may be responsible for the clinically improved swallowing that is noted in most patients over time. Harashina and co-workers (1985) have reported the use of a jejunal free flap split along its antimesenteric border and doubled on itself to increase the diameter of the jejunal conduit and to disrupt the circular muscles of the bowel segment.
MICROVASCULAR TRANSPLANTATION OF THE COLON
Indications and Contraindications
In general, microvascular transfer of the colon (left or right) is considered secondary to that of the jejunum and is used in most instances when the jejunum is not available. This bias is likely due primarily to the potential problem of intraabdominal or cervical contamination with bowel contents. In addition, the harvest of the colon requires a longer abdominal incision, is technically more difficult, and lacks a reliable backup in case of microvascular thrombosis (i.e., a second portion of colon cannot be harvested). Advocates of
P.2048
microvascular colon flaps, such as Smith and co-workers (1987a, 1987b), argue that these flaps are superior to jejunal flaps because they have larger pedicle vessels, have less mucus secretion and thinner mucosa, demonstrate less persistent peristaltic activity, and are more resistant to warm ischemia. These potential advantages cannot be evaluated in a meaningful manner, however, because large series utilizing vascularized colonic transfers have yet to be reported.
The free colon flap is contraindicated in patients with known colonic diseases (e.g., diverticulitis, cancer, polyposis, ulcerative colitis), grave medical conditions, or a history of extensive intraabdominal surgery. In addition, the use of this flap in elderly patients with atherosclerosis should be avoided. The use of preoperative angiograms to assess the patency of the mesenteric vessels in high-risk patients (e.g., elderly, smokers, diabetics) has been suggested by Chen and Tang (2000).
Operative Technique and Results
Patients are given routine bowel preparations and preoperative antibiotics. A midline laparotomy from xiphoid to pubis is used to expose the abdominal contents. The mesocolon of the sigmoid or transverse colon is transilluminated, and a suitable vascular arcade is selected. Approximately 10 to 14 cm of bowel can be harvested based on a single vascular arcade. The mesentery usually measures 10 to 15 cm.
Mesenteric dissection continues carefully to its root, and the pedicle vessels are exposed. The bowel is transected with an automatic stapling device, and the flap is transferred to the head and neck for inset and microvascular anastomosis. Proximal esophageal anastomosis is in general easier than with the free jejunum because of the larger diameter of the colon. Distal esophageal segment anastomosis is more difficult, however, because of a larger size mismatch between the colon and the cervical esophagus. The esophageal repair is stented with a nasogastric tube, and the wound is drained with closed suction drains as described for the jejunum.
Postoperative monitoring and care of colonic free flaps is similar to that of the jejunum. Enteral feeds are usually delayed until postoperative ileus has completely resolved.
Unlike the jejunum, no large series of microsurgical colon transfers have been reported in the literature; however, small series have reported good results, with success rates similar to the jejunum. The findings of barium swallow studies have led some advocates of colonic flaps, including Smith and co-workers (1987a, 1987b), to claim that these flaps are a more physiologic method of cervical esophageal reconstruction. These studies demonstrate that after swallowing, the initial action of the transplanted colon is to dilate and then return to baseline diameter. In contrast, following swallowing, the initial action of the transplanted jejunum is to contract. These contractions are thought to be a source of postoperative dysphagia. According to Chen and Tang (2000), the beneficial effects of colonic dilation on swallowing have not been consistently demonstrated.
MICROVASCULAR TRANSPLANTATION OF THE STOMACH
Indications and Contraindications
The greater curvature of the stomach together with the greater omentum can be transferred based on the right gastroepiploic vessels, as advocated by Baudet (1979) and by Panje (1987), Moran (1989), and Guedon (1994) and their colleagues. Up to one-half of the circumference of the stomach can be harvested without significant limitations in stomach capacity or ischemic complications in the remaining segments. The use of this flap has been suggested by Harii and Omori (1973), Baudet (1979), and Guedon and associates (1994) for large defects of the floor of the mouth, for defects of the base of the tongue, and as a tubed flap for cervical esophageal reconstruction. The primary advantage of this flap is the long vascular pedicle (up to 30 cm) that can be harvested and used for anastomosis to distant vessels (e.g., axillary vessels) without the need for vein grafting, as noted by Guedon and co-workers (1994). In addition, the transplanted greater omentum can be used to provide bulk and coverage of exposed great vessels. The transplanted segment of greater curvature of the stomach does not appear to produce large amounts of acidic secretions, and most patients have oral secretions that have normal physiologic pH.
Gastric flaps are contraindicated in patients with a previous history of gastric ulcer disease, grave medical conditions, and chronic liver disease with ascites. Multiple previous abdominal operations with extensive adhesions are a relative contraindication.
Operative Technique and Results
The stomach is approached via a midline vertical or transverse upper abdominal incision. The attachments of the greater omentum to the transverse colon are divided, and the omentum is mobilized. The portion of the greater curvature of the stomach to be harvested is marked in a tapered fashion to enable primary closure. The pylorus is preserved. The gastric segment can be harvested using automatic stapling devices to minimize spillage of gastric contents. The right gastroepiploic vessels are then dissected to their origin or as far as necessary. The patient is kept NPO for 5 to 7 days with nasogastric tube decompression. Monitoring of the transplanted segment and evaluation of cervical esophageal healing are performed as described for free jejunal transplantation.
Similar to the situation with microvascular transplantation of the colon, few large series using the greater curvature of the stomach exist. Guedon and associates (1994)
P.2049
reported on a series of 18 patients. Vascular anastomosis was performed to the axillary vessels, and all flaps survived. Three patients developed postoperative fistulae, all of which healed spontaneously. All patients were able to swallow and maintain oral nutrition. Panje and colleagues (1987) reported a series of seven patients, of which five had successful reconstruction of the oral cavity and pharynx with microvascular gastric flaps. In addition, they found that in contrast to the plica circularis of the jejunum, the rugae of the transplanted stomach flatten over time and may therefore have less potential for food trapping.
CONCLUSIONS
Microvascular transplantation of the intestines is a highly effective method for reconstruction of cervical esophageal defects. These procedures enable one-stage reconstruction with minimal morbidity and mortality. The vast majority of patients resume swallowing and can maintain adequate nutrition without the need for tube feeds. Although the jejunum is the workhorse for reconstruction in most centers, alternative flaps are available in appropriate circumstances.
REFERENCES
Anthony JP, et al: Reconstruction of partial laryngopharyngectomy defects. Head Neck 19:541, 1997.
Ariyan S: The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstr Surg 63:73, 1979a.
Ariyan S: Further experiences with the pectoralis major myocutaneous flap for the immediate repair of defects from excisions of head and neck cancers. Plast Reconstr Surg 64:605, 1979b.
Bakamjian V: A two stage method for pharyngoesophageal reconstruction with a primary pectoral skin flap. Plast Reconstr Surgery 36:173, 1965.
Baudet J: Reconstruction of the pharyngeal wall by free transfer of the greater omentum and stomach. Int J Microsurg 1:53, 1979.
Carlson GW, et al: Reconstruction of the hypopharynx and cervical esophagus. Curr Probl Surg 30:427, 1993.
Carrel A: The surgery of blood vessels, etc. Johns Hopkins Hosp Bull 190:18, 1907.
Chen HC, Tang YB: Microsurgical reconstruction of the esophagus. Semin Surg Oncol 19:235, 2000.
Coleman JJ 3rd: Reconstruction of the pharynx and cervical esophagus. Semin Surg Oncol 11:208, 1995.
Coleman JJ 3rd, et al: Ten years experience with the free jejunal autograft. Am J Surg 154:394, 1987.
Coleman JJ 3rd, et al: Jejunal free autograft: analysis of complications and their resolution. Plast Reconstr Surg 84:589, 1989.
Cordeiro PG, et al: Barium swallows after free jejunal transfer: should they be performed routinely? Plast Reconstr Surg 103:1167, 1999.
Disa JJ, Cordeiro PG: Reconstruction of the hypopharynx and cervical esophagus. Clin Plast Surg 28:349, 2001.
Disa JJ, Cordeiro PG, Hidalgo DA: Efficacy of conventional monitoring techniques in free tissue transfer: an 11-year experience in 750 consecutive cases. Plast Reconstr Surg 104:97, 1999.
Disa J, et al: Microvascular reconstruction of the hypopharynx: defect classification, treatment algorithm, and functional outcome based on 165 consecutive cases. Plast Reconstr Surgery 111:652, 2003.
Fisher SR, et al: Pharyngoesophageal reconstruction using free jejunal interposition grafts. Arch Otolaryngol 111:747, 1985.
Fisher SR, et al: Free jejunal interposition graft for reconstruction of the esophagus. Head Neck 12:126, 1990.
Flynn MB, Acland RD: Free intestinal autografts for reconstruction following pharyngolaryngoesophagectomy. Surg Gynecol Obstet 149:858, 1979.
Guedon CE, et al: Use of gastro-omental free flaps in major neck defects. Am J Surg 168:491, 1994.
Harashina T, et al: Reconstruction of cervical oesophagus with free double-folded intestinal graft. Br J Plast Surg 38:483, 1985.
Harii K, Omori S: Use of the gastroepiploic vessels as recipient or donor vessels in the free transfer of composite flaps by microvascular anastomoses. Plast Reconstr Surg 52:541, 1973.
Hidalgo DA, et al: A review of 716 consecutive free flaps for oncologic surgical defects: refinement in donor-site selection and technique. Plast Reconstr Surg 102:722, 1998.
Hiebert C, Cummings GJ: Successful replacement of the cervical esophagus by transplantation and revascularization of a free graft of the gastric antrum. Ann Surg 154:103, 1961.
Jones NF, Eadie PA, Myers EN: Double lumen free jejunal transfer for reconstruction of the entire floor of mouth, pharynx and cervical oesophagus. Br J Plast Surg 44:44, 1991.
Lillehei R, et al: The physiologic response of the small bowel of the dog to ischemia including prolonged in-vitro preservation of the bowel with successful replacement and survival. Ann Surg 150:543, 1959.
Mansour KA, Picone AL, Coleman JJ 3rd: Surgery for high cervical esophageal carcinoma: experience with 11 patients. Ann Thorac Surg 49:597, 1990.
McCaffrey TV, Fisher J: Effect of radiotherapy on the outcome of pharyngeal reconstruction using free jejunal transfer. Ann Otol Rhinol Laryngol 96:22, 1987.
McKee DM, Peters CR: Reconstruction of the hypopharynx and cervical esophagus with microvascular jejunal transplant. Clin Plast Surg 5:305, 1978.
Moran WJ, et al: Free gastroomental flap for head and neck reconstruction: assessment in an animal model. Am J Otolaryngol 10:55, 1989.
Mullens J, Pezacki E: Reconstruction of the cervical esophagus by revascularized autografts of intestine. An experimental study using the Inokuchi stapler and reporting the use of revascularized intestine to construct an artificial human larynx. Int Surg 55:157, 1971.
Nakamura T, Inokuchi K, Sugimachi K: Use of revascularized jejunum as a free graft for cervical esophagus. Jpn J Surg 5:92, 1975.
Nakayama K, et al: A simple new apparatus for small vessel anastomosis (free autograft of the sigmoid included). Surgery 52:918, 1962.
Panje WR, et al: Immediate free gastro-omental flap reconstruction of the mouth and throat. Ann Otol Rhinol Laryngol 96:15, 1987.
Reuther JF, Steinau HU, Wagner R: Reconstruction of large defects in the oropharynx with a revascularized intestinal graft: an experimental and clinical report. Plast Reconstr Surg 73:345, 1984.
Roberts R, Douglas F: Replacement of the cervical esophagus and hypopharynx by a revascularized free jejunal autograft: report of a case successfully treated. N Engl J Med 264:342, 1961.
Salamoun W, et al: Free jejunal transfer for reconstruction of the laryngopharynx. Otolaryngol Head Neck Surg 96:149, 1987.
Schusterman MA, et al: Reconstruction of the cervical esophagus: free jejunal transfer versus gastric pull-up. Plast Reconstr Surg 85:16, 1990.
Seidenberg B, et al: Immediate reconstruction of the cervical esophagus by a revascularized isolated jejunal segment. Ann Surg 149:162, 1959.
Shangold LM, Urken ML, Lawson W: Jejunal transplantation for pharyngoesophageal reconstruction. Otolaryngol Clin North Am 24:1321, 1991.
Shumrick DA, Savoury LW: Recent advances in laryngo, pharyngo, esophageal reconstruction. Acta Otolaryngol Suppl 458:190, 1988.
Smith RW, et al: Experimental assessment of free jejunal and colonic grafts of the esophagus. Arch Otolaryngol Head Neck Surg 113:187, 1987a.
Smith RW, et al: Jejunum versus colon for free oesophageal reconstruction: an experimental radiological assessment. Br J Plast Surg 40:181, 1987b.
Theile DR, et al: Free jejunal interposition reconstruction after pharyngolaryngectomy: 201 consecutive cases. Head Neck 17:83, 1995.
Theogaraj SD, et al: The pectoralis major musculocutaneous island flap in single-stage reconstruction of the pharyngoesophageal region. Plast Reconstr Surg 65:267, 1980.
Vogel DH, Strong MS: Use of a T-tube in management of a pharyngeal fistula after laryngectomy. Plast Reconstr Surg 62:563, 1978.
Wang TD, Sun YE, Chen Y: Free jejunal graft for reconstructing the pharynx and cervical esophagus. Chin Med J (Engl) 99:941, 1986.
Wookey H: Surgical treatment of carcinoma of the pharynx and upper esophagus. Surg Gynecol Obstet 75:499, 1942.
EAN: 2147483647
Pages: 203