II - Breast
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > III - Musculoskeletal System > 4 - Bone
function show_scrollbar() {}
4
Bone
Andrew E. Rosenberg
Sanford I. Roth
Introduction
Bone tissue along with cartilage, fibrous tissue, fat, blood vessels, nerves, and hematopoietic elements form individual bones. In humans, there are 206 separate bones, which together with their articulations form the skeleton. Anatomically, the skeleton can be divided into the axial skeleton, which includes the skull, vertebral column, ribs, sternum, and hyoid, and the peripheral (or appendicular) skeleton, which consists of the upper and lower limbs and the pelvis (Figure 4.1). The acral skeleton refers to the bones of the hands and feet.
Bone, whether referring to an organ or a type of connective tissue, is composed of a unique biphasic blend of organic and inorganic elements. The quality, quantity, and architectural arrangement of these components determine its ultimate form and function and confer important biological properties. The contributions of bone to mineral homeostasis are vital to life, and its structural characteristics are essential to locomotion and organ protection. Additionally, bones form the framework of our bodies, thereby giving it size and shape and provide a nurturing storehouse for the hematopoietic elements.
Bone the Organ: Gross and Microscopic Anatomy
Bones are rigid (but not brittle), lightweight, usually cylindrical structures, that have a relatively high tensile strength. Tan-white and smooth-surfaced, they are the hardest and strongest structures of the body, being as strong as cast iron but one-third of the weight as a result of their unique structure. Bones are reinforced, asymmetric, hollow structures designed to provide a maximum strength-to-weight ratio (Figure 4.2).
Individual bones are classified according to their size and shape. There are bones that are flat (bilaminar plates), those that are cuboid, and the most common group are bones that are tubular, both long and short (Figure 4.1). Tubular bones are further subdivided anatomically along their long axis into the epiphysis (extends from the base of the articular surface to the region of the growth plate), the metaphysis (extends from the region of the growth plate to where the diameter of the bone becomes significantly narrow), and the diaphysis or shaft (extends from the base of one metaphysis to the base of the opposing metaphysis) (Figure 4.3). In immature or growing bones, the metaphysis is
P.76
separated from the epiphysis by a cartilaginous growth plate, or physis (1). Apophyses, such as the greater and lesser trochanters of the femur, are protuberances that form at large tendoligamentous insertion sites. The medical and forensic determination of skeletal age and growth utilizes the amount and localization of bone ossification, the formulation and size of the secondary ossification centers, and the degree and amount of remodeling (see below).
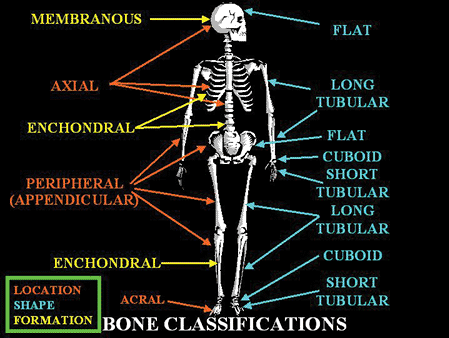 |
Figure 4.1 Diagram of the skeleton illustrating the different shapes and sizes of bones, as well as their method of formation. |
Despite their differences in size and shape, all bones are of similar composition and generally have a periosteum, cortex, and medullary canal that contains variable amounts of cortical (compact) and cancellous bone, fatty and hematopoietic marrow (Figure 4.2), blood vessels, and nerves. For any given bone, the quantity and arrangement of cortical or cancellous bone is directly related to the biomechanical requirements (Wolff's law). For instance, bones exposed to the largest torsional forces are usually long bones, and they are composed roughly of 80% cortical bone and 20% cancellous bone. In contrast, bones that predominately transmit weight-bearing forces, such as the vertebral bodies, consist of 80% cancellous bone and 20% cortical bone.
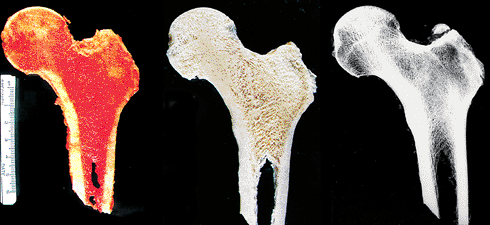 |
Figure 4.2 Gross (left) and macerated (center) longitudinally cut specimen and the accompanying x-ray (right) of a proximal femur, including the head, neck, and upper diaphysis sectioned in the frontal plane. The cortex defines the outer limits of the bone and is thickest along the medial surface (left) of the neck and diaphysis, where load bearing is greatest. The medullary cavity is filled with bony trabeculae and red hematopoietic and yellow fatty marrow. The trabeculae are aligned along the lines of stress; this is especially prominent in the medial portion. The horizontal line at the base of the head on the x-ray represents the accrual of bone that occurred during closure of the epiphyseal growth plate. |
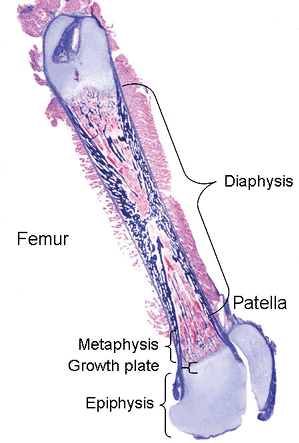 |
Figure 4.3 Whole mount of an immature femur and patella. The long bone is composed of the proximal and distal epiphyses, metaphyses and growth plates, and the intervening diaphysis. |
Woven and Lamellar Bone
Histologically, bone tissue, regardless of whether it is cortical or cancellous, normal or part of a pathologic process, is categorized into woven and lamellar types based on the organization of its type I collagen fibers. In woven bone, the collagen fibers are arranged in an irregular feltwork (Figure 4.4), while in lamellar bone they are deposited in parallel arrays (Figure 4.5).
Woven bone is fabricated during periods of rapid bone growth; it composes the developing bony skeleton during embryogenesis and portions of bones in the growing infant and adolescent. It may also be the predominant type of bone that is formed in a variety of reactive (fracture-callus, infection-involucrum) and neoplastic (Codman's triangle, matrix of bone forming neoplasms) conditions. Woven bone is hypercellular, and the osteocytes and their lacunae
P.77
are large and appear to be distributed in a haphazard fashion as the long axes of the cells parallel the feltlike direction of the neighboring collagen fibers (Figure 4.4). The mineral content of woven bone is higher than that of lamellar bone, and more than 50% of it is deposited outside of the collagen fibers. Overall, this structural organization enables woven bone to resist forces equally in all directions and facilitates rapid formation, mineralization, and resorption. These factors explain why woven bone is weaker, less rigid, and more flexibile than lamellar bone.
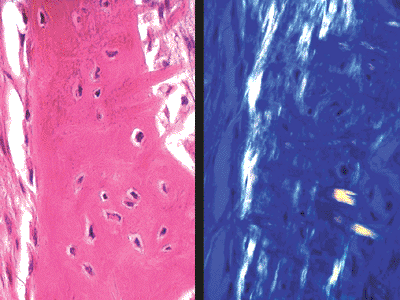 |
Figure 4.4 Woven bone seen on hematoxylin- and eosin-stained slide (left) and with polarized light (right). The collagen fibers are oriented in all planes. There are many osteocytes, and their long axes follow the direction of the neighboring collagen fibers. |
Normally, the entire mature skeleton is composed solely of lamellar bone. Lamellar bone, in contrast to woven bone, is synthesized more slowly, is less cellular, and the osteocytes and their lacunae are smaller and distributed in a more organized fashion along the more regular collagen lamellae (Figure 4.5). Additionally, the process of mineralization of lamellar bone differs from that of woven bone in that it occurs more slowly and continues long after the organic matrix is initially deposited. Furthermore, the mineral deposits are localized almost exclusively within the collagen fibers and are first deposited within the spaces, or hole regions, between the ends of adjacent collagen fibers (2,3). Subsequently, the mineral content increases as a result of enlargement and increase in the number of the apatite crystals. Microradiographs of undemineralized sections reveal varying densities, with the oldest bone being most heavily mineralized (Figure 4.6). Since the mineral and collagen fibers are well-organized and intimately bound to one another, lamellar bone has greater rigidity and tensile strength and less elasticity than woven bone.
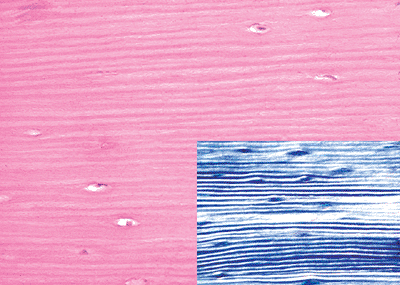 |
Figure 4.5 Lamellar bone as seen on hematoxylin- and eosin-stained slide and with polarization (inset). The collagen fibers are arranged in parallel arrays. There are comparatively fewer osteocytes, and they oriented in the same direction as the collagen fibers. |
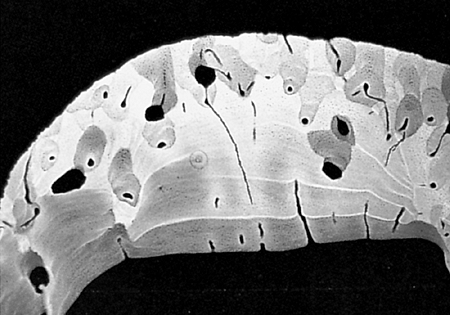 |
Figure 4.6 Microradiograph of the cortex of a 2-month old female. There are various degrees of mineralization, with the radiolucent being the most recently deposited and the radiodense portions representing the oldest. |
Both lamellar and woven bone are made by osteoblasts in discrete quantities or units, which are fastened to one another by cement or reversal lines. Cement lines are thin and intensely basophilic on conventional histologic slides, and comparatively little is known about them. Recent studies, however, have shown that they are collagen poor, have less mineral, an increased calcium-to-phosphorus ratio compared to hydroxyapatite, and are richer in sulfur than is the surrounding bone matrix (4,5). Some investigators have suggested that cement lines represent a residuum of mineralized ground substance that is secreted during the initial reversal phase in the formation of new bone (6).
Cortical (Compact) Bone
Cortical bone, also known as dense compact bone, is hard and tan-white (Figures 4.2, 4.7). Its thickness depends on its location and mechanical requirements, being thickest in
P.78
areas exposed to large torsional and weight-bearing forces, such as the middiaphysis of the femur or tibia, and thinnest where the transmission of weight-bearing forces is paramount, as seen adjacent to articular surfaces and in vertebral bodies (Figure 4.2).
 |
Figure 4.7 Longitudinal section through cortical bone. The cortex is tan-white and solid. The round hole within it represents the pathway of the nutrient artery. |
During the early stages of growth and development, cortical bone is constructed entirely of woven bone. Over time, it is gradually remodeled until it is composed of pure lamellar bone in the mature skeleton. Adult cortical bone is composed of three different architectural patterns of lamellar bone: circumferential, concentric, and interstitial (Figure 4.8). The circumferential lamellae form outer and inner envelopes to the cortex and consist of several subperiosteal and endosteal layers that are oriented parallel to the long axis of the bone. They are the first cortical lamellae to be deposited, and in young individuals comprise almost the entire cortex. As mechanical stresses on the bone increase, many of the circumferential lamellae (except for several lamellae just beneath the periostum and along the endosteum) are replaced by the concentric lamellae of the haversian systems (Figures 4.8,4.9,4.10,4.11,4.12).
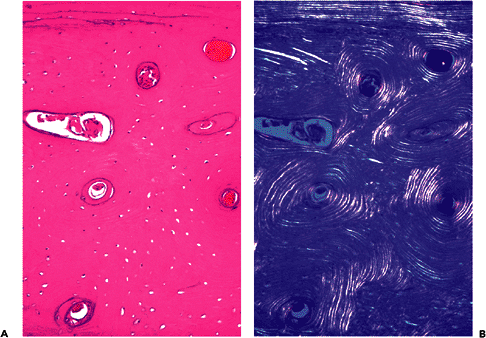 |
Figure 4.8 Cross sections of cortex with circumferential, concentric, and interstitial lamellae. The circumferential lamellae are beneath the periosteum, the concentric lamellae surround the haversian canals containing blood vessels, and the interstitial lamellae fills the intervening spaces (A, H&E; B, polarized light). |
Haversian systems, or osteons, are created by osteoclastic resorption of the circumferential lamellae that usually begins on the endosteal surface of the cortex, and less
P.79
frequently on the periosteal surface. As the bone resorption proceeds, it penetrates into the cortex and forms a canal (Volkmann's canal) perpendicular or at an angle to the long axis of the bone (Figures 4.10, 4.11). The numerous osteoclasts located in the head of the canal form the cutting cone, and the canal they generate is filled with vessels, nerves, and mesenchymal cells (including stem cells) enmeshed in a loose connective tissue stroma. Within a short distance, the osteoclastic activity becomes concentrated on one side of the canal; and, as a consequence, the direction of the cutting cone becomes aligned with the long axis of the bone. The burrowing osteoclasts elongate the canal, and in their wake newly formed osteoblasts deposit lamellae of bone in a targetlike, or concentric, fashion. The collagen fibers in any one lamella are oriented parallel to each other; however, their pitch is slightly different from those in adjacent lamellae, and this enhances the
P.80
biomechanical strength of the cortex. In due course, the accrual of concentric lamellae reduces the diameter of the haversian canal so that in the end it is small and contains nutritional blood vessels and nerve twigs (Figure 4.8).
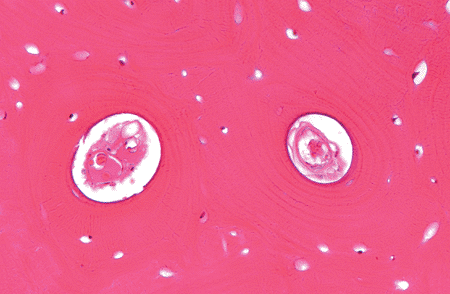 |
Figure 4.9 Two adjacent mature haversian systems containing the central canal, blood vessels, and surrounding concentric lamellae. Empty lacunae are seen in areas of the interstitial lamellae. |
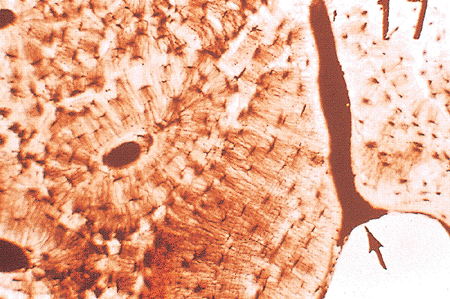 |
Figure 4.10 Ground, unstained section of mineralized, compact, cortical bone. The arrow points to a Volkmann's canal arising from the endosteum. Canaliculi connect cells in adjacent circumferential lamellae. (This slide was prepared by Glimcher MJ, Roth SI, Schiller AL, as part of a course in Pathophysiology of Bone for the Harvard Medical School, Boston, MA.) |
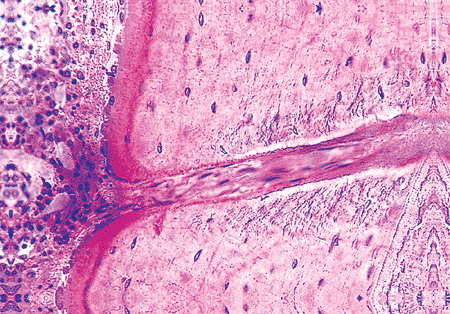 |
Figure 4.11 A forming Volkmann's canal coursing through the cortex of the bone. The canal is angled with respect to the bone lamellae and is filled with connective tissue. Osteoid and osteoblasts, indicating new bone formation, are present around the endosteal opening of the canal, but none are seen in the canal. The cortex shows circumferential lamellar bone with regularly placed osteocytes. (Undemineralized bone section.) |
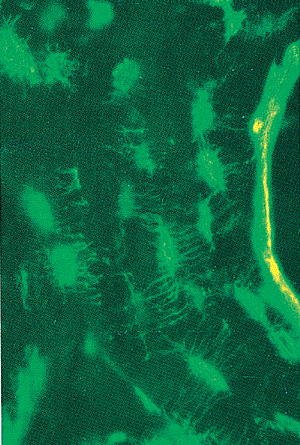 |
Figure 4.12 An undemineralized section of cortical bone, stained in vivo, with tetracycline. A layer of tetracycline appears at the mineralization front of the haversian system where new bone is being formed. The osteocyte lacunae and the canaliculi of their connecting dendritic processes are visible as bright green areas. (Unstained, fluorescent light.) |
Individual haversian systems are relatively self-contained metabolic units because nutritional support of their cells, especially the bone cells, depends upon the process of diffusion from their central vessels. Consequently, osteocyte viability is not sustainable beyond a certain distance from the vessels, which imposes a biologic limit on the maximal number of lamellae contained within any haversian system. Also, the integrated network of osteocytes is generally limited to the osteon within which it develops, as osteocytic cytoplasmic processes generally contact only those that dwell in the same system, with cement lines defining the physical boundaries of every haversian system (Figures 4.10, 4.12).
Mature haversian canals are long and cylindrical, range from 25 to 125 m in diameter (average 50 m), and are widest nearest the medullary cavity. They form an intricate, branching, spiraling, and interconnecting network that courses thoughout the cortex. The number of haversian systems in a particular bone is variable and is determined by age, the amount of mechanical stress and weight that the bone is subjected to over time, and other biological and genetic factors (6,7).
Between the haversian systems are the interstitial lamellae, which are somewhat irregular, geometric-shaped units of lamellar bone (Figure 4.9). They fill the spaces between active haversian systems and help glue, or anneal, them to one another, which is important in maintaining cortical integrity. The osteocytes confined to the interstitial lamellae may lose their access to nutritional sources and consequently undergo necrosis, leaving behind empty lacunae.
The endosteum is the loose areolar connective tissue that immediately abuts the osteoblasts along the inner surface of the cortex and along the medullary surfaces of the trabeculae.
Cancellous (Trabecular, or Spongy) Bone
Cancellous bone, also known as trabecular or spongy bone, is tan-white and fenestrated. It is composed of interconnecting plates and struts of trabecular bone and is located within the medullary cavity (Figure 4.2). In the adult, it is the fourth type of lamellar bone, and the lamellae are oriented parallel to the long axis of the bone (Figure 4.13). In developing bone, it is composed of significant amounts of woven bone; and, when initially formed, it contains a central core of calcified cartilage (primary spongiosa). Cancellous bone is deposited in relation to lines of mechanical stress (Wolff's law) to provide added support and distribute large weight-bearing forces along a variety of different pathways (Figure 4.2). Accordingly, cancellous bone is most abundant in the weight-bearing ends of bones, such as the epiphyses and vertebral bodies, and is present in only small amounts in the middiaphysis of tubular bones. Small trabeculae are avascular, while larger ones may contain haversian systems, including concentric lamellae. The surfaces of mature trabeculae are typically lined by endosteum composed of quiescent osteoblasts or surface-lining cells (Figure 4.14). In three dimensions, the trabeculae are usually interconnecting plates (Figure 4.15). The global surface area of cancellous bone is very large, which facilitates remodeling and the ability of the skeleton to rapidly respond to the metabolic demands of the body. The mature trabeculae are heavily mineralized with a thin (1 3 m) layer of osteoid beneath the inactive flattened osteoblasts.
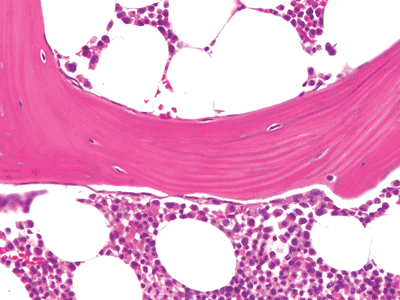 |
Figure 4.13 A mature trabeculum composed of lamellar bone. The lamellae are oriented in the same direction as the trabeculum. |
Periosteum
The periosteum consists of a thin layer of tan-white connective tissue that covers the outer surface of all cortices. In children it is relatively loosely attached, whereas in adults it is firmly anchored to the bone. The periosteum is composed of an outer fibrous layer and an inner cellular (cambium)
P.81
layer (Figure 4.16). The fibrous layer contains fibroblasts and broad collagen fibers that are continuous with those of the joint capsule, tendons, and muscle fascia. At tendoligamentous insertion sites, the collagen fibers of the tendoligamentous structure pierce the periosteum and become anchored in the bone (Sharpey's fibers). Spindle-shaped fibroblasts and osteoprogenitor cells occupy the cambium layer, which is generally the most cellular during growth and development. The number of osteoprogenitor cells present depends on the age of the individual and the amount of bone cell activity in any particular region; they are especially numerous during periods of active bone formation.
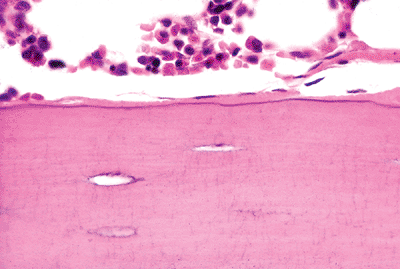 |
Figure 4.14 Quiescent osteoblasts lining a trabecular surface. |
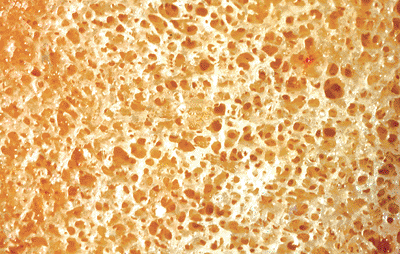 |
Figure 4.15 Gross photograph of a macerated portion of cancellous bone. The trabeculae are interconnecting plates. |
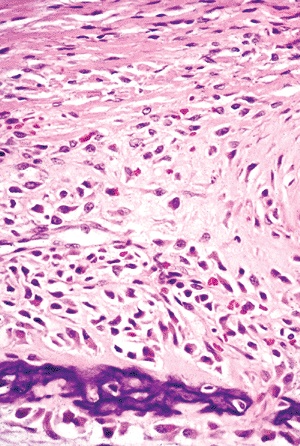 |
Figure 4.16 The outer fibrous layer of a fetal periosteum consists of thick bundles of horizontally oriented collagen fibers. The inner cellular cambium layer contains spindle-shaped, osteoprogenitor cells. The maturing osteoblasts steadily become more polyhedral and acquire increasing amounts of amphophilic cytoplasm. A portion of intramembraneous woven bone is seen forming the new cortex. |
Vascular Supply and Innervation
Bones are vascular organs and require a vascular supply for viability. Bones receive their blood supply from three main sources: (a) large nutrient arteries (one to two per bone), (b) metaphyseal and epiphyseal vessels, and (c) periosteal vessels. Nutrient arteries enter long bones in the diaphysis, traverse the cortex through foramina, and divide into ascending and descending branches within the medullary cavity. Smaller branch arteries, arterioles, capillaries, venules, and veins (Figure 4.17) course throughout the medullary cavity, nourish the fatty and hematopoietic marrow, and extend into haversian canals, where they supply the inner two-thirds of the cortex. At the ends of growing bones, they terminate as small arteries that give rise to capillary loops at the bases of epiphyseal growth plates. The epiphyseal and metaphyseal vessels access bone through small apertures and provide blood flow to regions of the epiphysis and metaphysis in the mature skeleton and to the secondary centers of ossification during active enchondral ossification. The periosteal vessels are small and are believed to nourish the outer third of the cortex. The venous drainage system bone is composed of medullary sinusoids that empty into a central venous sinus, which merges with nutrient veins.
Bones are innervated largely by nonmylineated nerves that are derived from the autonomic nervous system, and their function is to control blood flow. Larger nerve branches are usually associated with arterial vessels (Figure 4.17),
P.82
whereas small groups of fibers can be found adjacent to vessels in haversian systems. Nerves supplying the periosteum contain sensory elements and are the source of the sensation of bone pain.
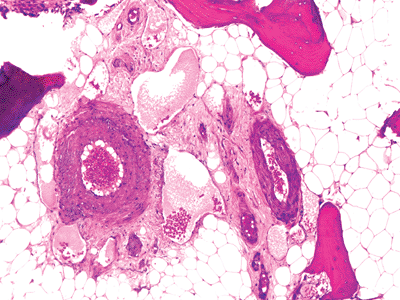 |
Figure 4.17 Small arteries, surrounded by dilated capillaries and nerves in an area of fatty marrow. |
Bone the Tissue: Organic and Inorganic Components
The special biphasic amalgamation of organic and inorganic materials found in bone distinguishes it from all other tissues in the body. The organic component consists of proteins and bone cells, and the inorganic element is a specialized, calcium-poor form of apatite, resembling hydroxyapatite [Ca10(PO4)6(OH)2] in which the hydroxyl residues are replaced by phosphate and carbonate ions. The integration of the mineral phase with the organic matrix (primarily collagen) provides bone with hardness, strength, and limited elasticity (2).
Organic Components
Proteins
The organic component accounts for approximately 35% of the wet weight of bone; and, of this, collagen is responsible for 90%. Collagen is the primary structural protein of bone, and the overwhelming majority (90%) is type I (8); type V collagen is present in much smaller amounts, and there are only trace quantities of collagens III, XI, and XIII (9). Type III collagen may be increased in pathologic conditions (10). The numerous large type I collagen molecules are produced by osteoblasts; aside from their contribution to structural support, they also anchor many of the other constituents (11).
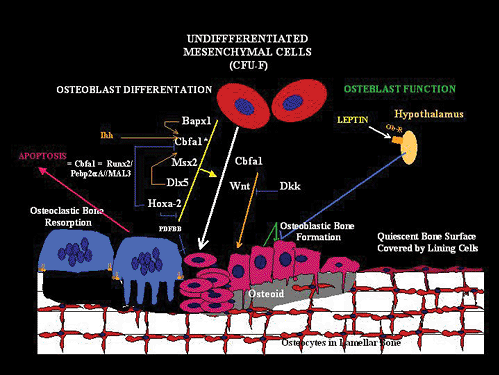 |
Figure 4.18 Diagram of osteoblast formation and metabolism. (Bagpipe, the vertebrate homologue of the Drosophila gene; Bapx1, a member of the NK2 class of homeobox genes; Cbfa1, core binding factor 1; F-CFU, colony-forming unit fibroblasts; Dlx5, homeodomain transcription factor; Dkk, Wnt antagonist Dickkopf2; HOX2a, homeobox gene; Ihh, Indian hedgehog gene; MSx2, homeodomain protein; Ob-R, leptin receptor; PDFBB, prostate-derived factor BB; BMP, a member of the bone morphogenic protein; RUNX2/Pebp2aA//MAL3, runt homology domain protein; Wnt, secreted glycoproteins that function as ligands for members of the Frizzled family of seven transmembrane-domain receptors.) |
The noncollagenous proteins are grouped according to their function as adhesion proteins, calcium-binding proteins, mineralization proteins, enzymes, cytokines, growth factors, and receptors. These proteins mediate all aspects of bone cell activity and are extremely important to the biological success of bone as a tissue. Many of these substances are synthesized and secreted by osteoblasts, and others are derived and concentrated from the serum. The most abundant of the osteoblast-produced noncollagenous proteins is osteocalcin, which functions as a regulator of mineralization. Osteocalcin is made solely by osteoblasts, and its quantification in serum has made it an important clinical marker of bone formation (11).
Osteoprogenitor Cells
Osteoprogenitor cells are derived from tissue-bound mesenchymal stem cells that have developed into fibroblastic colony-forming units (F-CFU) (12,13,14,15,16). They are located in the periosteum, the haversian system, and the Volkmann's and medullary canal. Osteoprogenitor cells are primitive determined mesenchymal cells that have the capacity to produce only osteoblasts. The process of osteoblast differentiation and maturation is complex and involves a variety of different factors (Figure 4.18). By light microscopy, osteoprogenitor cells appear as generic spindle cells and do
P.83
not have any distinguishing morphologic features; therefore, they cannot be identified with certainty in ordinary histologic sections (Figure 4.16). Because bone can be formed in skin, soft tissue, muscle, and viscera, in both experimental and pathologic conditions, osteoprogenitor cells or induceable stem cells are likely present in these sites as well.
Osteoblasts
Osteoblasts are vital to bone tissue and are the cells responsible for the production, transport, and arrangement of most of the components of the organic matrix (osteoid). Additionally, they initiate and regulate matrix mineralization and use autocrine and paracrine mechanisms to control the activity of neighboring osteoblasts, osteocytes, and osteoclasts (3,12,14,17). Immunohistochemical and biochemical studies reveal the presence of alkaline phosphatase, osteopontin, and osteocalcin within their cytoplasm, constitutively expressed RANKL (see section entitled Osteoclasts), and receptors for parathyroid hormone (PTH), prostoglandins, vitamin D3, estrogens, and cytokines, including colony-stimulating factor 1 (CFS-1), on their cell membranes (8,18,19,20,21).
Osteoblasts cover all bone surfaces, where their lifespan may range from months to many years. Their metabolic state is closely related to their morpholgy; they are spindle-shaped when quiescent and large and polyhedral when rapidly producing bone. Metabolically active osteoblasts vary in size from 10 to 80 m (average 20 30 m) and have abundant amphophilic to basophilic cytoplasm that is in intimate contact with the bone (Figures 4.19, 4.20). Multiple cytoplasmic processes extend from the cells into and through the bone, contacting adjacent osteoblasts and osteocytes via nexus (gap) junctions. The nucleus is polarized away from the matrix surface and often has a conspicuous nucleolus and a prominent perinuclear halo that represents a well-developed Golgi apparatus. The cells flatten and elongate as their synthetic activity diminishes and remain lining the resting bone surfaces (Figures 4.14, 4.20).
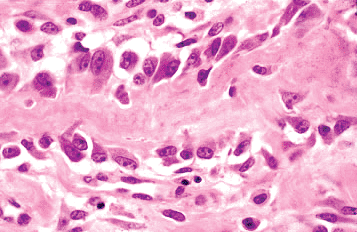 |
Figure 4.19 Metabolically active osteoblasts lining a trabeculum of woven bone. Some osteoblasts are in various stages of being surrounded by matrix and becoming osteocytes. |
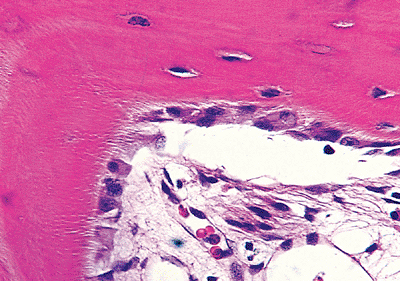 |
Figure 4.20 Metabolically active osteoblasts forming lamellar bone. The thin layer of osteoid cannot be identified in this demineralized sections. At the right of the micrograph, the osteoblasts are becoming inactive, flattening, and being incorporated into the bone as osteocytes. The osteocytes of the lamellar bone are spindle-shaped. The dendrites are identifiable extending from the osteoblast bodies into the bone where they are seen as clear streaks perpendicular to the bone surface represent the canaliculi containing the dendrites of the osteocytes and osteoblasts. |
Ultrastructurally, the cytoplasm of productive osteoblasts contains extensive, granular endoplasmic reticulum, a large prominent Golgi apparatus, and numerous mitochondria and lysosomes (8,9,14,22). In contrast, the cytoplasm of inactive osteoblasts resembles that of quiescent fibroblasts (14).
Osteocytes
Osteoblasts enveloped by matrix become osteocytes, and their half-life is estimated to be as long as 25 years (23). The cell body, nucleus, and surrounding scant cytoplasm reside within a lacunar space. The nuclei are comparatively small and are not always visible in every plane of section; therefore, in most slides of bone tissue, random lacunae appear empty. Osteocytes have numerous long and delicate cytoplasmic processes (dentrites), similar to the neuritic processes (axons) of neurons (Figures 4.12, 4.21). These cell processes traverse the matrix through small tunnels termed canaliculi and provide a very large surface area of contact between the osteocyte and the matrix and extracellular fluid that bathes each cell (22). Osteocyte cell processes connect to those of neighboring osteocytes and to surface osteoblasts via gap junctions. Gap junctions facilitate the transfer of small molecules and biologically generated electrical potentials from cell to cell. In this manner, osteocytes communicate with one another and form a complex and integrated network throughout bone tissue.
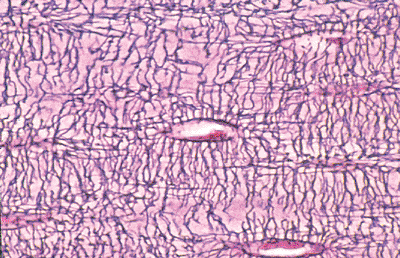 |
Figure 4.21 Osteocytes within lacunar space. Numerous cell processes course through the matrix and contact those of neighboring osteocytes. |
P.84
The number, size, shape, and position of osteocytes varies according to the type of bone they inhabit. In woven bone, they are numerous, large, and plump (Figure 4.4). Their arrangement appears disorganized because their long axes parallel the direction of the neighboring collagen fibers, which in sections of woven bone appears random. In lamellar bone, osteocytes are comparatively fewer in number, smaller, more spindle-shaped, and appear in sections to be more regularly organized because the cells are oriented in the same direction as the surrounding lamellae (Figure 4.5).
The repertoire of biological activity possessed by osteocytes helps them maintain bone tissue and allows bone to be responsive to the mechanical and metabolic demands of the body. For instance, osteocytes are mechanosensory cells that translate mechanical forces into biological activity (23,24). The detection of physical forces stimulates osteocytes to produce and release intercellular messengers that target precursor cells, osteoblasts, and osteoclasts (23). These cells, in turn, respond by remodeling the bone regionally and allowing it to change its mass and structure according to demands of the external physical environment (Wolff's law) (25,26). The widespread distribution of osteocytes and their cell processes is fundamental to another important role of theirs, namely, mineral homeostasis (27). Osteocytes generate and respond to microfluxes in ion concentrations and mediate the exchange of calcium and other ions between the bone matrix and extracellular fluid. In certain conditions, they may even be able to rapidly release calcium and phosphorus from the mineralized matrix by a process termed osteocytic osteolysis, which manifests histologically as enlarged lacunar spaces (28). All in all, their aggregrate activity likely influences the systemic metabolism of calcium and phosphous (27).
Osteoclasts
Osteoclasts are terminally differentiated, multinucleated cells responsible for bone resorption. They are mobile effector cells that have a lifespan of only several weeks. By the time they are recognizable by light microscopy, they are mature and biologically active and can be found residing within resorption pits (Howship's lacunae) produced by their digestion of mineralized bone matrix (Figure 4.22).
Osteoclasts are 40 to 100 m in diameter and are polarized with one portion of the cell membrane intimately attached to the bone and the remainder exposed to the extracellular fluid in its microenvironment. The segment of cell membrane that actually adheres or seals to bone is laden with V 3 integrins. The integrins bind to specific extracellular bone matrix proteins (vitronectin, osteopontin, and bone sialoprotein) previously deposited by osteoblasts, and in this manner the osteoclast can anchor to the bone surface. A network of interconnecting actin filaments that produces a clear area in the cytoplasm links the sealing zone of the osteoclast cell membrane to the nuclei (14,29). On average, osteoclasts have 4 to 20 nuclei (8), though the number may range from 2 to as many as 100. In normal circumstances, however, the amount is usually not greater than 12. The nuclei and adjacent prominent Golgi apppartus tend to congregate away from the bone-resorbing surface, and are surrounded by abundant amphophilic cytoplasm.
The cytoplasm in the vicinity of the resorbing surface is rich in tartrate-resistant acid phosphatase, carbonic anhydrase, and membrane-bound lysosomes. The adjacent cell membrane, which also directly apposes the bone-resorbing surface, has numerous fingerlike extensions that effectively increase its surface area and form the so-called brush border.
The lysosomes fuse with the brush border and release their contents into the resorption pit, which begins the actual process of bone digestion. Metabolic activation of osteoclasts is initiated by anchorage, and this process generates
P.85
a stimulatory signal that is transmitted to the nuclei by the actin network. The nuclei, now activated, orchestrate the complex and transitory cytoplasmic and cell membrane modifications required for bone digestion. Importantly, mineralized bone or cartilage is more efficiently resorbed by osteoclasts than is nonmineralized bone or cartilage. Focal, or partial, demineralization of collagen fibers appears to be one of the first steps in matrix resorption and is followed by catabolism of noncollagenous proteins and, lastly, the degradation of collagen fibers themselves. Once osteoclast activity ceases and the cell moves to another targeted site, macrophages meander into the base of the resorption pit and phagocytize the organic remnants.
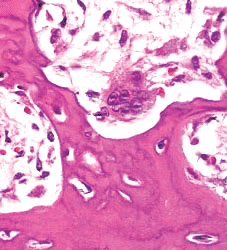 |
Figure 4.22 Osteoclast located within a resorption pit on a trabeculum (Howship's lacunae). |
Osteoclasts are derived from mononuclear, hematopoietic progenitor cells of the granulocytic-macrophage colony-forming (GM-CFU) and macrophage colony-forming units (M-CFU) (16,29,30,31,32,33,34,35,36,37,38,39,40,41,42) (Figure 4.23). The mononuclear preosteoclasts undergo primary fusion to form multinucleated osteoclasts, which are capable of acquiring and shedding nuclei throughout their short lifespans (40). A variety of cytokines and growth factors are critical to their development, maturation, and activity and include interleukin (IL)-1, IL-3, IL-6, IL-11, tumor necrosis factor (TNF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage colony-stimulating factor (M-CSF) (43). These factors work by either stimulating osteoclast progenitor cells or participating in a paracrine system in which osteoblasts and marrow stromal cells play a central role.
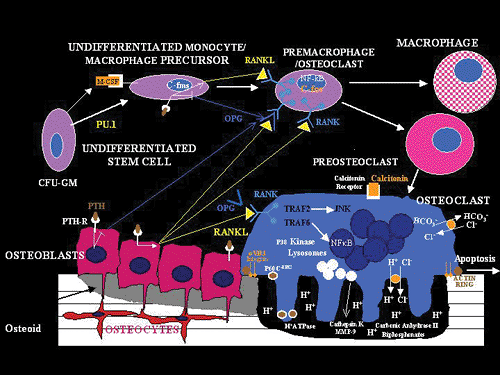 |
Figure 4.23 Diagram of the formation of osteoclasts and their relationship to osteoblasts and undifferentiated mesenchymal cells. ( V 3, V 3 integrin; C-fms, gene for M-CSF receptor; C-fos (murine osteosarcoma viral oncogene homolog); CFU-GM, colony-forming unit for the granulocyte-macrophage series; Cl-, chloride ion; CSF-1, colony-stimulating factor-1; H+, hydrogen ion; H+ATPase, H+-adenosine triphosphatase; HCO3-, carbonate ion; IL-1, interleukin-1; JNK, c-Jun N-terminal kinase; M-CSF, monocyte-macrophage colony-stimulating factor; MMP-9, matrix metaloproteinase 9; NF , nuclear factor kappa beta; OPG, osteoprotegerin; ORA, XX; P6OCsrc, protein tyrosine kinase; PTH, parathyroid hormone; PTH-R, Parathyroid Hormone Receptor; Pu.1, a member of the ets family that is exclusively expressed by hematopoietic cells; RANK, receptor activator factor of nuclear factor kappa beta; RANKL, RANK ligand/osteoclast differentiation factor; TNFR, tumor necrosis factor receptor; TRAF2, tumor necrosis factor receptor associated factor 2; TRAF6, tumor necrosis factor receptor associated factor 6. |
This system is essential to bone metabolism, and its mediators include the molecules RANK (receptor activator for nuclear factor ), RANK ligand (RANKL), and osteoprotegerin (OPG) (43). RANK is a member of the TNF family of receptors expressed mainly on cells of macrophage/monocytic lineage, such as preosteoclasts. When this receptor binds its specific ligand (RANKL) through cell-to-cell contact, a series of signal cascades are activated and osteoclastogenesis is initiated. RANKL is produced by and expressed on the cell membranes of osteoblasts and marrow stromal cells. It's expression may be influenced by other osteotropic factors, and its major role in bone metabolism is stimulation of
P.86
osteoclast formation, differentiation, activation, and survival. The actions of RANKL can be blocked by another member of the TNF family of receptors, osteoprotegerin, which is a soluble protein produced by a number of tissues, including bone, hematopoietic marrow cells, and immune cells. Osteoprotegerin inhibits osteoclastogenesis by acting as a decoy receptor that binds to RANKL, thus preventing the interaction of RANK with RANKL. The interplay between bone cells and these molecules permits osteoblasts and stromal cells to control osteoclast development. This ensures the tight coupling of bone formation and resorption vital to the success of the skeletal system and provides a mechanism for a wide variety of biologic mediators (hormones, cytokines, growth factors) to influence the homeostasis of bone tissue.
Inorganic Component
Mineral
The primary mature inorganic mineral of bone is a calcium deficient varient of hydroxyapatite [Ca10(PO4)6(OH)2], in which the hydroxyl groups have been largely replaced by phosphate and carbonate groups (44). There is minimal water in the mature crystals (44). It is the body's major reservoir for calcium and phosphate and contains more than 99% of the body's calcium and 85% of the body's phosphorus (1,9,44). Also harbored within the bone crystals are 95% of the body's sodium, 50% of the body's magnesium, and trace amounts of other essential minerals (1,9,44).
The process of mineralization varies according to the type of tissue. In cartilage and possibly woven bone, it begins with the production of numerous small vesicles (matrix vesicles) derived from chondroblasts and osteoblasts (1,2,45). The matrix vesicles are 2 to 4 m in diameter and are the sites where mineral is first observed. Although there is some uncertainty regarding the initial structure and composition of the mineral, crystals of hydroxyapatite, as well as amorphous calcium phosphate and brushite have been identified (2,3,12,18,45,46,47,48). The primary crystals serve as a nidus for the deposition of larger aggregates, which are then deposited in and around the collagen fibers.
In lamellar bone, matrix vesicles are not present, and the process of mineralization is controlled by osteoblasts. The regulatory steps are complex and incompletely understood, though numerous proteins, growth factors, and cytokines are involved (2,3,12). Mineralization begins with the deposition of mineral in the spaces ( holes ) between the ends of adjacent collagen molecules. It is still unclear whether the first deposits are hydroxyapatite, amorphous calcium phosphate, or a nonapatite crystalline calcium phosphate (octocalcium phosphate, [Ca8(HPO4)2PO4)4. 5H2O]) (44). Regardless of their initial form, the aggregates grow and form crystalline bone apatite. Initially, the crystals are situated within the collagen fibrils, but eventually they also develop outside the fibrils and fibers (44). The shapes of the mature crystals are are not known with certainty. High resolution transmission electron microscopy shows that they have the features of thin plates, whereas, studies with small-angle x-ray scattering suggest that they are needlelike (44). Once the crystals are deposited in bone, they remain there for days to years, only to be dissolved at a future time during bone resorption, when the calcium and phosphorous are released into the extracellular fluid and become available for other biological activities.
Mineralization of osseous organic matrix takes approximately two weeks, therefore, the surfaces of bone are covered by a layer of unmineralized bone, called osteoid (Figures 4.11, 4.19, 4.20). The width of this layer is dependent on the relative rate of bone formation. In inactive regions, the bone is nearly fully mineralized and is covered by a thin osteoid seam (1 5 m in thickness), whereas, in foci of rapid bone deposition, the osteoid layer may be more than several times thicker. The actual zone of mineralization can be detected by the systemic administration of the antibiotic tetracycline, which binds to the bone at the mineralization front and can be visualized with flourescent microscopy (Figure 4.12).
Bone Formation, Growth, and Remodeling
From the time that skeleton formation begins in the embryo until the stage that adult stature is attained, the bones of the body undergo a marked increase in size, refinement of their shape, and enhancement of their contour. Bone is a rigid structure that cannot grow interstitially and only enlarges by the apposition of new bone on its surface. Appositional growth alone is adequate for portions of the skeleton that enlarge slowly during maturation, such as the skull, and the diameter of long bones; however, it is insufficient for bones that must increase in size at a more rapid rate, such as the length of long and short tubular bones of the extremities, the vertebrae, and the ribs. Cartilage, in contrast, exhibits both appositional and interstitial growth; that is, it increases its volume and enlarges in all dimensions by adding new cells and elaborating freshly synthesized extracellular matrix. Consequently, the growth in length of tubular bones in embryos and prepubertal children occurs as growing cartilage is replaced by bone, with the majority of the increase in bone length derived from the cartilage primordium represented in the anlage and growth plate (physis). In the case of callus, bone formation occurs both intramembranously in the fibrous callus and by enchondral cartilage replacement.
The genetic code for skeletal morphogenesis is encrypted in the homeobox genes. Homeobox genes contain the DNA library of a repository of transcriptional regulators essential for growth and differentiation. The expression of homeobox genes occurs in a specific order and temporal sequence; and, regarding the skeletal system, their activation results in the
P.87
generation of localized cellular condensations of primitive mesenchyme at the sites of future bones. The mesenchymal condensations are the earliest precursors of individual bones and are critical to the formation of the skeleton. They begin to develop just prior to day 40 of gestation and, depending upon their anatomic location, are derived from cells that migrate from the cranial neural crest (craniofacial skeleton), paraxial mesoderm (axial skeleton), or the lateral plate mesoderm (appendicular skeleton) (49). Shortly after being formed, usually by the seventh week of gestation, the mesenchymal cells in the condensations begin to alter their genetic expression and assume the morphology of matrix-forming cells. Those cells that mature into chondrocytes form a cartilage model or anlage of the future bone, which is fundamental to the process of enchondral ossification, whereas those that develop directly into osteoblasts produce bone via the mechanism of intramembranous ossification. The mature bone tissue formed from either enchondral or intramembranous ossification are grossly and histologically indistinguishable.
Enchondral Ossification
Initially, the newly formed cartilage anlage is avascular and has the crude shape of the adult bone (Figure 4.24). The mesenchyme surrounding the anlage forms the perichondrium (Figure 4.25), which is the precursor to the periosteum that develops once ossification begins (see below). This process is initiated in each bone at a specific time, and this temporal sequence is the same in all humans.
Growth of the anlage occurs both interstitially and appositionally as a result of the proliferation of chondrocytes and the accumulation of secreted extracellular matrix (Figures 4.25,4.26,4.27). The matrix is composed of proteoglycans and type II collagen with smaller amounts of collagen types IX, X, XI, and XIII (2). As this process continues, three events occur at very nearly the same time in every bone (50):
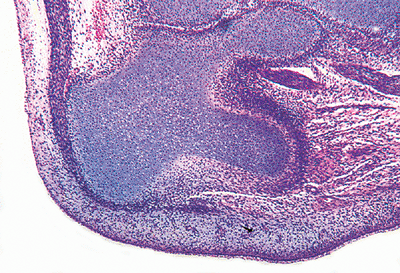 |
Figure 4.24 Cartilage anlage of the os calcis (calcaneus). The cartilage model is the approximate shape of the adult bone. The attachment site of the Achilles tendon and the tibial-calcaneal joint are present. |
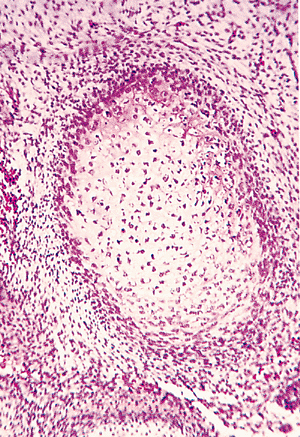 |
Figure 4.25 Cross section of the cartilage anlage of the embryonic femur. The chondrocytes are regular with little hypertrophy. The perichondrium is composed of undifferentiated spindle cells with little intervening stroma, surrounded by a loose mesenchymal tissue. |
The mesenchymal stems cells of the perichondrium, located around the midportion of the cartilaginous shaft, produce a layer of osteoblasts that deposit a collar of woven mineralized bone on the surface of the anlage. This heralds the transformation of the perichondrium into periosteum. The periosteum, osteoblasts, and the thin
P.88
surface layer of bone form the primary center of ossification and delineate the middle region of the diaphysis (Figures 4.28,4.29,4.30,4.31).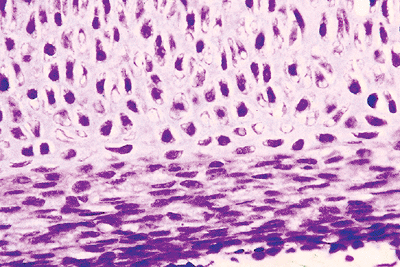
Figure 4.26 Cartilage anlage of femur in an embryo. The perichondrium is in intimate contact with the cartilage.
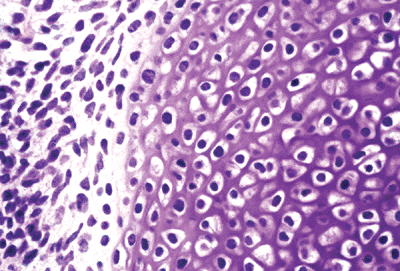
Figure 4.27 Cells of mesechymal condensation surrounding an area in which they have differentiated into hyalin cartilage anlage. The chondrocytes show early hypertrophy.
The chondrocytes in the center of the anlage shaft become encased by the periosteal shell of bone and begin to hypertrophy and swell (Figures 4.27,4.28,4.29,4.30,4.31). The cell enlargement is accompanied by an increase in intracellular glycogen and in the perichondrocyte depostion of type X collagen, and soon thereafter the chondrocytes undergo apoptotic necrosis (51,52,53,54). Concurrently, the surrounding matrix mineralizes, largely via matrix vesicles, although some crystallization may occur within collagen fibers.
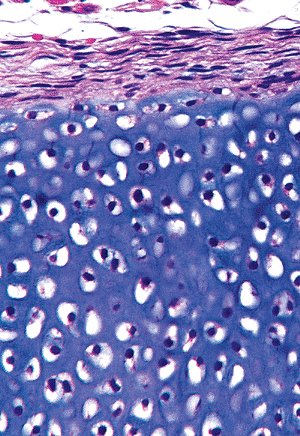
Figure 4.28 The early periosteum about the diaphysis of a cartilage anlage, showing the perichondrium surrounding the hypertrophied chondrocytes.
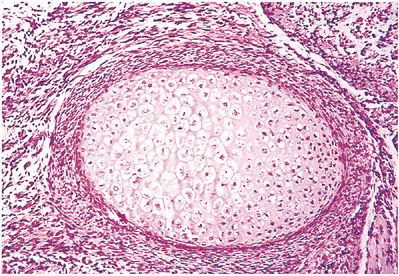
Figure 4.29 Cross section of the diaphysis of an embryonic femur. The thin, collarlike primary center of ossification is between the hypertrophying chondrocytes and the periosteal cells.
A capillary network originating from periosteal vessels forms and, with the aid of osteoclastic (chondroclastic) resorption, penetrates the woven bone of the primary center of ossification (Figure 4.32) into the mineralized cartilage. The capillaries are the precursor to the future nutrient vessels and are accompanied by pericytes and other primitive mesenchymal cells, including osteoprogenitor and osteoclast progenitor cells.
As the cartilaginous core of the bone undergoes continued resorption, osteoblasts derived from perivascular stem cells deposit layers of osteoid on the residual longitudinally oriented
P.89
struts of mineralized cartilage. These trabeculae, composed of a central cartilaginous core covered by a rim of bone, are the first (or primary) trabeculae formed, and together they form the primary spongiosa. The spaces that develop as a consequence of the cartilage resorption then coalesce and form the medullary cavity, which is initially filled with loose connective tissue. Eventually, it becomes occupied by varying amounts of adipose tissue and hematopoietic elements. This complex process begins within the center of the shaft and progresses toward both ends of the bone. When complete with primary trabeculae and an adjacent secondary center of ossification (see below), this is recognized as the fully developed growth plate (the physis) (Figures 4.33,4.34,4.35,4.36).
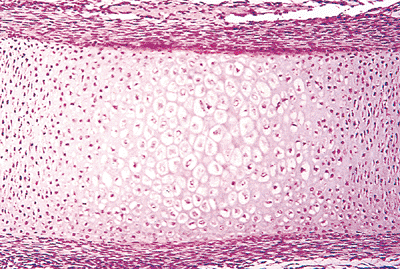 |
Figure 4.30 Longitudinal section of the primary center of ossification in an embryonic femur. The cellular layer of the periosteum is producing osteoblasts, which have formed a layer of pink osteoid. The outer spindle cells of the periosteum are oriented longitudinally along the femoral shaft. The underlying chondrocytes show hypertrophy. |
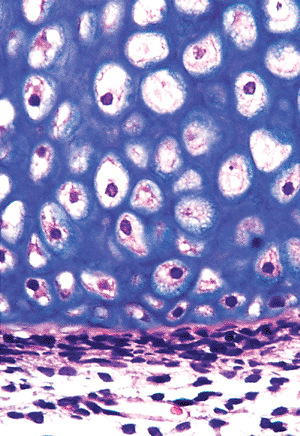 |
Figure 4.31 Primary center of ossification. A thin pink layer of osteoid, containing flattened osteocytes, separates the hypertrophied chondrocytes from the periosteal osteoblasts. |
 |
Figure 4.32 Primary center of ossification with capillary proliferation indicating the early formation of the nutrient artery. Between these capillaries, new trabecular membranous woven bone is being formed: in the cambium layer of the periosteum. The outer fibrous layer of the periosteum is more cellular than in the adult. |
The fully developed growth plate is structured and has been divided into five merging regions that correspond to different stages of chondrocyte maturation (Figures 4.34, 4.36). As the chondrocytes pass through the different stages, they do not literally move within the matrix but mature in the position they occupy when first formed. Important regulators of this sequence of chondrocyte growth and maturation are the Indian hedgehog gene and parathyroid hormone related protein (PTHrP) (54,55,56). The zones include: (a) a region of resting or reserve chondrocytes located nearest the ends of the bone; (b) a region of proliferating chondrocytes that become arranged in spiral columns; (c) a region of chondrocyte hypertrophy; (d) a region of chondrocyte apoptotic necrosis and matrix mineralization; and (e) a region of cartilage resorption by osteoclasts (chondroclasts) that tunnel into the mineralized matrix and leave behind residual longitudinal struts of cartilage that parallel the long axis of the bone. The orientation of the struts is determined by the preexisting columnar arrangement of the chondrocytes in the proliferative and hypertrophied zones (Figures 4.34, 4.36). These cartilaginous struts act as scaffolding for newly deposited woven bone. These struts of mineralized cartilage covered by newly formed woven bone are the primary trabeculae (Figures 4.36,4.37,4.38). The rate of growth differs for each physeal plate and is greatest in the growth plate of the distal femur,
P.90
P.91
followed by that of the proximal tibia. In diseases in which mineralization of the cartilage is impaired (rickets), removal of the cartilage is handicapped, and the zone of hypertrophy becomes massively and irregularly thickened. While most tubular long bones have two epiphyseal growth plates, other bones (such as the ribs and some of the phalanges, carpals, tarsals, metacarpals, and metatarsals) have only a single physis. The growth plates, located at the diaphyseal-epiphyseal junctions of the bones, are delineated peripherally by a circumferential, thin collar of membrane bone that is a continuation of the primary center of ossification and is called the ring of Ranvier (Figures 4.34, 4.35) (57).
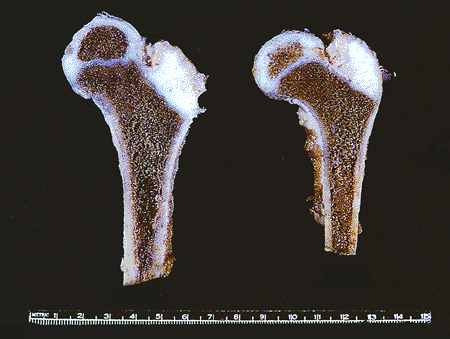 |
Figure 4.33 Gross photograph of the femoral heads of 3.5-year-old male. The secondary centers of ossification in the femoral heads are separated from the primary centers by the epiphyseal growth plates (the physes). The secondary center of ossification of the apophysis of the greater trochanter has not as yet formed. The metaphyses and diaphyses resemble their adult shapes. |
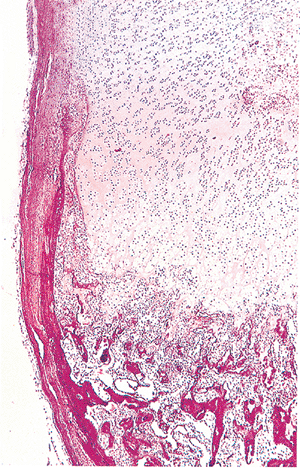 |
Figure 4.34 Photomicrograph of the epiphyseal growth plate of the costochondral junction from a 2-month-old male. The growth plate is surrounded by the ring of Ranvier. No secondary center is present. Primary trabeculae with central cartilaginous cores are seen in the metaphysis and upper diaphysis. |
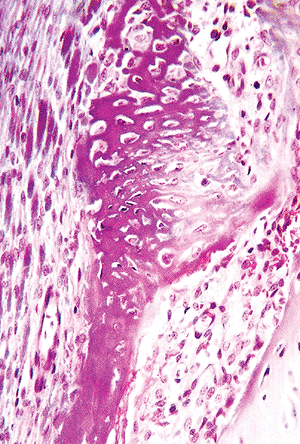 |
Figure 4.35 Ring of Ranvier, composed of bone that forms by the process of intramembranous ossification beneath the periosteum on the left and delineates the peripheral portion of the epiphyseal growth plate near the metaphysis. |
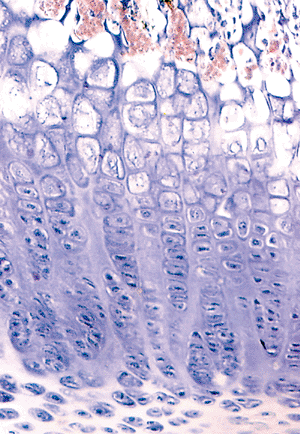 |
Figure 4.36 Photomicrograph of a maturing epiphyseal growth plate, showing the reserve zone (top), the proliferating zone, the hypertrophied zone, and zone of mineralization of the cartilage. The primary trabeculae are oriented vertically and are supporting the base of the growth plate. |
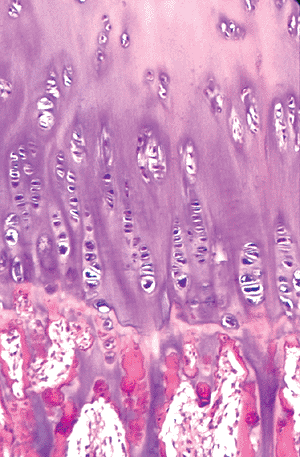 |
Figure 4.37 Junction between the mineralized cartilage columns and the primary trabeculae with the mineralized cartilage cores covered by a layer of woven bone. |
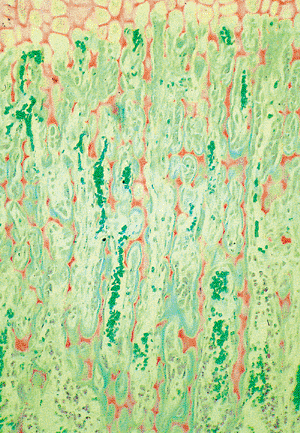 |
Figure 4.38 Longitudinal section of the epiphyseal growth plate, showing the zone of mineralization of the cartilage (orange) and the cartilage struts are being covered by a thin layer of osteoid (blue-green). Capillaries with numerous erythrocytes (green) are present between the primary bone trabeculae. (Goldner's stain.) (This slide was prepared by Glimcher MJ, Roth SI, Schiller AL, as part of a course in the Pathophysiology of Bone for the Harvard Medical School, Boston, MA.) |
Concurrent with continued appositional and interstitial growth of epiphyseal and growth plate cartilage are dramatic changes in the cortex. As the bone increases in diameter, subperiosteal bone is deposited while the bone along the endosteum is resorbed so that the cortical thickness remains proportionally uniform and the medullary cavity enlarges. The bone that first forms the cortex is woven in nature; but, within the first several years of life, the fabricated bone is lamellar. Variation in the rate of formation and resorption changes the shape of the bone. This is most pronounced in a region just distal to the base of the growth plate, known as the cut back zone. The cut back zone is rich in subperiosteal osteoclasts, which reduce the diameter of the bone to that of the diaphysis, and this results in funnelization of the bone. At the same time, the cortical thickness is maintained or increases by appositional new bone formation on the endosteal surface of the cortex. During growth and development, the diameter of the diaphysis continues to enlarge and in specific sites becomes asymmetric. This process is dynamic and not only determines the eventual diameter of the bone but controls the thickness and contour of the cortex. The increase in diaphyseal diameter is the result of periosteal osteoblastic bone formation. The thickness of the cortex is maintained by endosteal osteoclastic bone resorption. Conditions altering the balance of bone formation and resorption may cause abnormally thickened or significantly thinned (osteoporotic) cortices.
In most long bones, a similar process subsequently develops in the middle of the epiphysis, and this region is the secondary center of ossification (Figures 4.33, 4.39). A few long bones have a similar growth center in the apophyses. The maturation and replacement of the cartilage anlage in a secondary center is identical to that which occurs in the diaphysis except that the maturation proceeds from the center centrifugally, toward the periphery. This means that the growing area of the secondary center is, at first, a sphere. Eventually, the enlarging primary and secondary centers of ossification approach one another, entrapping a cylindrical segment of residual cartlage anlage and delineating the final form of the physis.
Continued growth of the primary and secondary centers of ossification results in the mergence of their reserve zones. At this time, a plate of bone demarcating the
P.92
secondary center from the forming growth plate is deposited. From then on, the centrifugal growth of the epiphysis is hemispheric. The cartilage located at the base of the true articular cartilage is responsible for progressive epiphyseal enlargement, and it has the architectural organization of a physis. Variation in the subarticular growth results in concordant shapes of the ends of the adjacent bones about the joint spaces. The epiphysis receives its nutrition primarily from blood vessels within the bone and its adjacent periosteum, whereas, the true articular cartilage is nourished by synovial fluid.
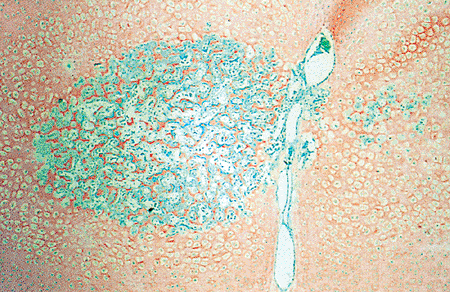 |
Figure 4.39 Photomicrograph of the secondary center of ossification of the femoral head. The nutrient vessels from the ligamentum teres are seen. Osteoid (green) is seen on the cartilage trabeculae (orange) in the spherical center. (Goldner's stain.) |
In the apophyseal cartilage, located on the surface of the bone, a secondary-like center of ossification appears and is responsible for the development of apophyseal bone of the iliac crests, the greater and lesser trochanters of the femur (Figure 4.34), and the tibial tuberosities, to name a few.
Once enchondral ossification is well underway at the growth plate, modeling of the newly formed bone begins. The primary spongiosa undergoes complete osteoclastic resorption, and secondary trabeculae composed solely of lamellar bone are deposited. The expanding medullary cavity becomes largely free of spicules of cancellous bone in much of the diaphysis and fills with adipose tissue and the hematopoietic marrow. Subperiosteal bone deposition and endosteal resorption of the cortex maintains a proper, tubular shape, and mechanical forces exerted by weight bearing and muscle attachments alter the rate of these processes in specific regions, which help sculpt the contour of the bone.
Several hormones, including parathyroid hormone, growth hormone, somatomedins, thyroid hormone, androgens, estrogens, and adrenal cortical hormones, are essential regulators of bone growth. At puberty, low doses of androgens and estrogens cause an increase in cell division in the proliferative zone of the growth plate and the secondary center of ossification. This is accompanied by an increase in the rate of cartilage maturation, mineralization, osteoclastic removal, and formation of primary trabeculae. In toto, these effects produce the so-called growth spurt seen at puberty. As estrogen and androgen levels increase and growth hormone and somatomedin levels fall off, chondrocyte proliferation decreases while maturation and bone formation proceed. This leads to a diminution or thinning of the growth plate, and eventually all of the cartilage of the growth plate undergoes complete enchondral ossification, leaving little or no evidence of its previous existence. At this time, the growth plate is considered closed, and all additional bone growth is appositional (Figure 4.40) (1,14). Cessation of growth of the secondary centers of ossification occurs in a similar fashion. However, a remnant of mineralized growth cartilage, which is the tide mark cartilage, persists at the base of the articular surface. It is demarcated from the true articular cartilage by a thin undulating layer of more densely mineralized matrix, known as the tidemark (Figure 4.41). The biologic potential of the tidemark cartilage persists as increases in hormones, such as growth hormone in the setting of acromegaly, can reactivate the process of enchondral ossification and produce additional growth in the adult. In normal circumstances, however, the vestige of the growth cartilage remains dormant and functions as an anchor of the true articular cartilage to the subchondral bone plate.
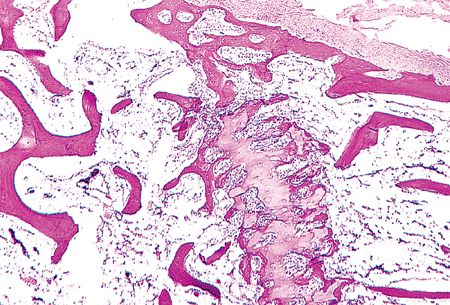 |
Figure 4.40 Closing epiphyseal growth plate in a 16-year-old boy. The periphery of the plate has been bridged by bone connecting the diaphysis and the secondary center of ossification. Cellular proliferation in the physis has ceased while the maturation process continues. The secondary center of ossification is at the top of the micrograph above the remnant of the physis. |
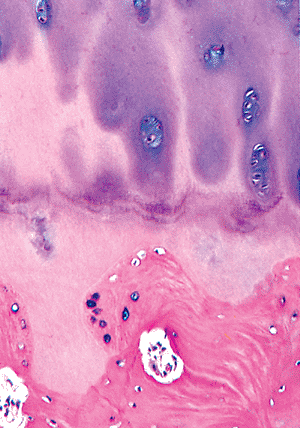 |
Figure 4.41 Section of base of articular cartilage in the region of the tidemark. The tidemark separates the articular nonmineralized cartilage (above) from the mineralized cartilage remnant of the physis and the lamellar bone of the subchondral plate (below). |
 |
Figure 4.42 Intramembranous bone from a fetal skull. The osteoblasts are large along the randomly formed trabeculae. The osteocytes and their lacunae are large, round, and irregularly spaced in the trabeculae. (Trichrome stain.) |
P.93
Intramembranous Ossification
Intramembranous ossification, or bone growth, refers to the process of bone formation in which the tissue occupying the site of the future bone or bone tissue is a fibrouslike membrane. The membrane is rich in osteoprogenitor cells and in normal situations forms the mesenchymal condensations in the developing embryo, the periosteum in the fetus, child, and adult, and the thin layer of fibrous tissue adjacent to all active bone-forming sites. The osteoprogenitor cells within the membrane produce offspring that differentiate into mature osteoblasts that directly deposit bone matrix (Figures 4.19, 4.42). Large portions of the flat bones of the skull, including the frontal, parietal, occipital, and temporal bones, form by this process (1,14). Also, since the cortices of all bones are largely created by osteoblasts derived from the cambium layer of the periosteum, all bones, in at least some part, are formed by intramembranous ossification. Growth of membranous bone occurs only by the apposition of new bone, and the medullary cavites of membranous bones are created and maintained by endosteal osteoclastic activity. Initially, the marrow spaces of these bones are composed of highly vascularized loose connective tissue, which is eventually replaced by adipose and hematopoietic tissues.
Modeling and Remodeling
The processes of bone formation and resorption are tightly coupled, and their balance determines skeletal mass at any point in time (58). As the skeleton grows and enlarges (undergoes modeling) during childhood and young adulthood, bone formation predominates, whereas after the third or fourth decades bone resorption prevails. The breakdown and renewal of bone fundamental to the formation and maintenance of the skeleton is called remodeling. Remodeling is a dynamic process involving the removal and replenishment of both cortical and trabecular bone; it continues throughout life to maintain bone mass, skeletal integrity, and skeletal function. (39,59). This process is complex and at least partially controlled by the central nervous system through hormones (such as leptins) and by mechanically induced microdamage. It depends on the integrated actions of osteoblasts, osteocytes, and osteoclasts (60). Together these cells form the functional or basic multicellular unit of bone (BMU, or bone remodeling unit of Frost) and, in adults, are responsible for remodeling approximately 10% of the skeleton on an annual basis (Figures 4.43, 4.44) (61). This feat is accomplished by approximately 1 million BMUs that are active at any one time, and which likely first target sites that are experiencing fatigue and microdamage (33). The process may begin on any bony surface and incorporates three phases of cell activity: activation, bone resorption, and bone formation (62).
Many pathologic conditions of bone result from abnormalities in bone remodeling (1,9). These disorders may be generalized, in the form of a metabolic bone disease, or localized to small regions of the skeleton or individual bones. For instance, the diminished bone mass in postmenopausal osteoporosis, hyperparathyroidism, and hyperthyroidism results from increased osteoclastic bone resorption, which is not adequately compensated for by an appropriate amount of new bone formation. The lytic lesions in early Paget's disease or those caused by metastases and myeloma result from localized increased osteoclastic bone resorption, which is significantly greater in amount than any new bone that is deposited. The goal of therapy for this broad spectrum of diseases is to restore bone mass, balance bone formation and resorption, and protect and maintain strutural integrity.
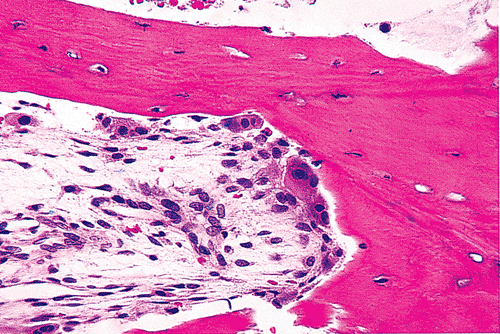 |
Figure 4.43 Basic multicellular unit (bone remodeling unit of Frost) of bone. Osteoclasts form the leading edge of the bone resorption ( the cutting cone ), and just behind them are mononuclear macrophages and osteoblasts. The newly created space is filled with a vascular loose connective tissue. |
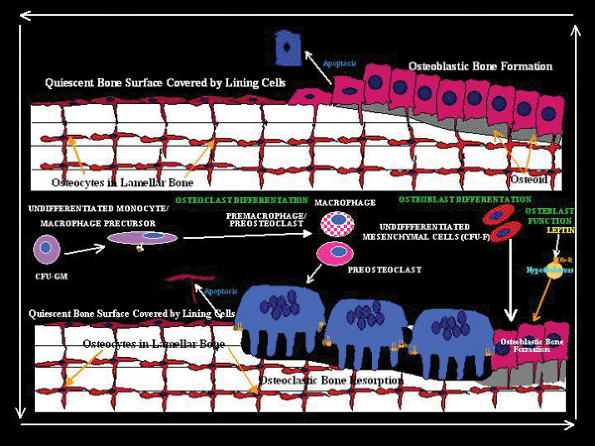 |
Figure 4.44 Drawing of the basic multicellular unit (bone remodeling unit of Frost) of bone. The resting osteoblasts are replaced by osteoclasts. New osteoblastic bone fills the resorbed lacunae of the haversian canal. |
P.94
References
1. Howell D, Dean D. The biology, chemistry, and biochemistry of the mammalian growth plate. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York: Raven Press; 1992:313 353.
2. Glimcher MJ. Mechanism of calcification: role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec 1989;224:139 153.
3. Glimcher MJ. The nature of the mineral component of bone and the mechanism of calcification. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York: Raven Press; 1992:265 286.
4. Huffer WE. Morphology and biochemistry of bone remodeling: possible control by vitamin D, parathyroid hormone, and other substances. Lab Invest 1988;59:418 442.
5. Burr DB, Martin RB. Errors in bone remodeling: toward a unified theory of metabolic bone disease. Am J Anat 1989;186:186 216.
6. Schaffler MB, Burr DB, Frederickson RG. Morphology of the osteonal cement line in human bone. Anat Rec 1987;217:223 228.
7. Ortner DJ. Aging effects on osteon remodeling. Calcif Tissue Res 1975;18:27 36.
8. Baron R. General principles of bone biology. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. Washington, DC: American Society for Bone and Mineral Research; 2003:1 8.
9. Robey P, Bianco P, Termine JD. The cellular biology and molecular biochemistry of bone formation. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York: Raven Press; 1992:241 263.
10. Veis A, Sabsay B. The collagen of mineralized matrices. In: Peck W, ed. Bone and Mineral Research. Vol. 5. Amsterdam: Elsevier; 1988:1 63.
11. Young MF. Bone matrix proteins: their function, regulation, and relationship to osteoporosis. Osteoporos Int 2003;14(suppl 3):S35 S42.
12. Lian, J, Stein GS, Canalis E, Robey PG, Boskey A. Bone formation: Osteoblast lineage cells, growth factors, matrix proteins, and the mineralization process. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 4th ed. Philadephia: Lippincott Williams & Wilkins; 1999:14 29.
13. Manolagas SC, Jilka, RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 1995;332:305 311.
14. Marks SC Jr, Popoff, SN. Bone cell biology: the regulation of development, structure, and function in the skeleton. Am J Anat 1988;183:1 44.
15. Friedenstein A. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. In: Thienfelder S, ed. Immunology of Bone Marrow Transplantation. Berlin: Springer-Verlag; 1980:19 29.
16. Jotereau FV, Le Douarin NM. The development relationship between osteocytes and osteoclasts: a study using the quail-chick nuclear marker in endochondral ossification. Dev Biol 1978;63:253 265.
17. Leblond CP. Synthesis and secretion of collagen by cells of connective tissue, bone, and dentin. Anat Rec 1989;224:123 138.
18. Lian J, Stein GS, Aubin JE. Bone formation: maturation and functional activities of osteoblast lineage. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. Washington, DC: American Society for Bone and Mineral Research. 2003:13 27.
19. Jilka RL. Are osteoblastic cells required for the control of osteoclast activity by parathyroid hormone? Bone Miner 1986;1:261 266.
20. Marks SC Jr. Osteoclast biology: lessons from mammalian mutations. Am J Med Genet 1989;34:43 54.
21. Rouleau MF, Mitchell J, Goltzman D. In vivo distribution of parathyroid hormone receptors in bone: evidence that a predominant osseous target cell is not the mature osteoblast. Endocrinology 1988;123:187 191.
22. Baron R. Anatomy and ultrastructure of bone. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 1999:3 10.
23. Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol 2004;36:1 8.
P.95
24. Iqbal J, Zaidi M. Molecular regulation of mechanotransduction. Biochem Biophys Res Commun 2005;328:751 755.
25. Wolff J. Die Lehre von der functionellen Knochengestalt. Arch f. path. Anat. 1899;155:256 315.
26. Wolff J. Gesetz der Transformation der Knochen. Berlin: Hirschwald; 1892.
27. Cullinane DM. The role of osteocytes in bone regulation: mineral homeostasis versus mechanoreception. J Musculoskelet Neuronal Interact 2002;2:242 244.
28. Belanger LF. Osteocytic osteolysis. Calcif Tissue Res 1969;4:1 12.
29. Raisz L. Mechanisms and regulation of bone resorption by osteoclastic cells. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York: Raven Press;1992:287 311.
30. Friedenstein, A. Determined and inducible osteogenic precursor cells. In: Elliott K, Fitzsimons D, eds. Hard Tissue Growth, Repair and Remineralization. Ciba Foundation Symposium. 11th ed. Amsterdam: Elsevier-Excerpta Medica; 1973:169 185.
31. Fallon MD, Teitelbaum SL. The interpretation of fluorescent tetracycline markers in the diagnosis of metabolic bone diseases. Hum Pathol 1982;13:416 417.
32. Chambers T. The origin of the osteoclast. In: Peck W, ed. Bone and Mineral Research. Vol. 6. Amsterdam: Elsevier; 1989:1 25.
33. Mundy G, Roodman D. Osteoclast ontogeny and function. In: Peck W, ed. Bone and Mineral Research. Vol. 5. Amsterdam: Elsevier; 1987:209 279.
34. Walker DG. Congenital osteopetrosis in mice cured by parabiotic union with normal siblings. Endocrinology 1972;91:916 920.
35. Walker DG. Osteopetrosis cured by temporary parabiosis. Science 1973;180:875.
36. Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 1975;190:784 785.
37. Walker DG. Spleen cells transmit osteopetrosis in mice. Science 1975;190:785 787.
38. Walker DG. Control of bone resorption by hematopoietic tissue. The induction and reversal of congenital osteopetrosis in mice through use of bone marrow and splenic transplants. J Exp Med 1975;142:651 663.
39. Dempster D. Bone remodeling. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York: Raven Press; 1992:355 380.
40. Stout SD, Teitelbaum SL. Histomorphometric determination of formation rates of archaeological bone. Calcif Tissue Res 1976;21:163 169.
41. Reddy SV. Regulatory mechanisms operative in osteoclasts. Crit Rev Eukaryot Gene Expr 2004;14:255 270.
42. Quinn JM, Gillespie MT. Modulation of osteoclast formation. Biochem Biophys Res Commun 2005;328:739 745.
43. Blair HC, Robinson LJ, Zaidi M. Osteoclast signalling pathways. Biochem Biophys Res Commun 2005;328:728 738.
44. Glimcher MJ. The nature of the mineral phase of bone: biological and clinical implications. In: Avioloi L, Krane S, eds. Metabolic Bone Disease and Clinically Related Disorders. 3rd ed. San Diego: Academic Press; 1998:23 50.
45. Robey P, Boskey A. Extracellular matrix and biomineralization of bone. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. Washington, DC: American Society for Bone and Mineral Research; 2003:38 45.
46. Canalis E. Osteogenic growth factors. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. Washington, DC: American Society for Bone and Mineral Research; 2003:28 31.
47. Posner AS. The mineral of bone. Clin Orthop Relat Res 1985:200:87 89.
48. Posner A. Bone mineral and the mineralization process. In: Peck W, ed. Bone and Mineral Research. Vol. 6. Amsterdam: Elsevier; 1988:65 116.
49. Olsen BR. Bone morphogenesis and embryological development. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases of Mineral Metabolism. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2003:9 13.
50. Mundy G, Chen D, Oyajobi BO. Bone remodeling. In: Favus MJ, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. Washington, DC: American Society for Bone and Mineral Research; 2003:46 58.
51. Linsenmayer TF, Eavey RD, Schmid TM. Type X collagen: a hypertrophic cartilage-specific molecule. Pathol Immunopathol Res 1988;7:14 19.
52. Poole AR, Matsui Y, Hinek A, Lee ER. Cartilage macromolecules and the calcification of cartilage matrix. Anat Rec 1989;224:167 179.
53. Poole AR, Pidoux I. Immunoelectron microscopic studies of type X collagen in endochondral ossification. J Cell Biol 1989;109:2547 2554.
54. Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun 2005;328:658 665.
55. van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev 2003;24:782 801.
56. Kronenberg HM. Developmental regulation of the growth plate. Nature 2003;423:332 336.
57. Shapiro F, Holtrop ME, Glimcher MJ. Organization and cellular biology of the perichondrial ossification groove of ranvier: a morphological study in rabbits. J Bone Joint Surg Am 1977;59:703 723.
58. Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol 2000;16:191 220.
59. Canalis E, McCarthy T, Centrella M. The regulation of bone formation by local growth factors. In: Peck W, ed. Bone and Mineral Research. Vol. 6. Amsterdam: Elsevier; 1989:27 56.
60. Takeda S. Central control of bone remodeling. Biochem Biophys Res Commun 2005;328:697 699.
61. Pogoda P, Priemel M, Rueger JM, Amling M. Bone remodeling: new aspects of a key process that controls skeletal maintenance and repair. Osteoporos Int 2005;16(suppl 2):S18 S24.
62. Eriksen EF. Normal and pathological remodeling of human trabecular bone: three dimensional reconstruction of the remodeling sequence in normals and in metabolic bone disease. Endocr Rev 1986;7:379 408.
- ERP Systems Impact on Organizations
- ERP System Acquisition: A Process Model and Results From an Austrian Survey
- Distributed Data Warehouse for Geo-spatial Services
- Intrinsic and Contextual Data Quality: The Effect of Media and Personal Involvement
- Healthcare Information: From Administrative to Practice Databases