24 - Nervous System
Editors: McPhee, Stephen J.; Papadakis, Maxine A.; Tierney, Lawrence M.
Title: Current Medical Diagnosis & Treatment, 46th Edition
Copyright 2007 McGraw-Hill
> Table of Contents > 27 - Diabetes Mellitus & Hypoglycemia
function show_scrollbar() {}
27
Diabetes Mellitus & Hypoglycemia
Umesh Masharani MB, BS, MRCP(UK)
Diabetes Mellitus
![]() Essentials of Diagnosis
Essentials of Diagnosis
Type 1 diabetes:
Polyuria, polydipsia, and weight loss associated with random plasma glucose 200 mg/dL.
Plasma glucose of 126 mg/dL or higher after an overnight fast, documented on more than one occasion.
Ketonemia, ketonuria, or both.
Islet autoantibodies are frequently present.
Type 2 diabetes:
Most patients are over 40 years of age and obese.
Polyuria and polydipsia. Ketonuria and weight loss generally are uncommon at time of diagnosis. Candidal vaginitis in women may be an initial manifestation. Many patients have few or no symptoms.
Plasma glucose of 126 mg/dL or higher after an overnight fast on more than one occasion. After 75 g oral glucose, diagnostic values are 200 mg/dL or more 2 hours after the oral glucose.
Hypertension, dyslipidemia, and atherosclerosis are often associated.
Epidemiologic Considerations
In 2002, an estimated 18.2 million people in the United States had diabetes mellitus, of which approximately 1 million have type 1 diabetes and the rest mostly have type 2 diabetes. A third group that was designated as other specific types by the American Diabetes Association (ADA) (Table 27-1) number only in the thousands. Among these are the rare monogenic defects of either B cell function or of insulin action, primary diseases of the exocrine pancreas, endocrinopathies, and drug-induced diabetes. Updated information about the prevalence of diabetes in the United States is available from the Centers for Disease Control and Prevention (http://www.cdc.gov/diabetes/pubs/estimates.htm).
Classification & Pathogenesis
Diabetes mellitus is a syndrome with disordered metabolism and inappropriate hyperglycemia due to either a deficiency of insulin secretion or to a combination of insulin resistance and inadequate insulin secretion to compensate. Type 1 diabetes is due to pancreatic islet B cell destruction predominantly by an autoimmune process, and these patients are prone to ketoacidosis. Type 2 diabetes is the more prevalent form and results from insulin resistance with a defect in compensatory insulin secretion (Table 27-2).
A. Type 1 Diabetes Mellitus
This form of diabetes is immune-mediated in over 90% of cases and idiopathic in less than 10%. The rate of pancreatic B cell destruction is quite variable, being rapid in some individuals and slow in others. Type 1 diabetes is usually associated with ketosis in its untreated state. It occurs at any age but most commonly arises in children and young adults with a peak incidence before school age and again at around puberty. It is a catabolic disorder in which circulating insulin is virtually absent, plasma glucagon is elevated, and the pancreatic B cells fail to respond to all insulinogenic stimuli. Exogenous insulin is therefore required to reverse the catabolic state, prevent ketosis, reduce the hyperglucagonemia, and reduce blood glucose.
The highest incidence of immune-mediated type 1 diabetes is in Scandinavia and northern Europe, where the yearly incidence per 100,000 youngsters 14 years of age or less is as high as 37 in Finland, 27 in Sweden, 22 in Norway, and 19 in the United Kingdom. The incidence of type 1 diabetes generally decreases across the rest of Europe to 10 in Greece and 8 in France. Surprisingly, the island of Sardinia has as high an incidence as Finland (37) even though in the rest of Italy, including the island of Sicily, it is only 10 per 100,000 per year. The United States averages 15 per 100,000, with higher incidences in states more densely populated with persons of Scandinavian descent such as Minnesota. The lowest incidence of type 1 diabetes worldwide was found to be less than 1 per 100,000
P.1220
per year in China and parts of South America. The global incidence of type 1 diabetes is increasing (approximately 3% each year).
Table 27-1. Other specific types of diabetes mellitus. | ||
|---|---|---|
|
Certain HLAs are strongly associated with the development of type 1 diabetes. About 95% of patients with type 1 diabetes possess either HLA-DR3 or HLA-DR4, compared with 45 50% of white controls. HLA-DQ genes are even more specific markers of type 1 susceptibility, since a particular variety (HLA-DQB1*0302) is found in the DR4 patients with type 1, while a protective gene (HLA-DQB1*0602) is often present in the DR4 controls. In addition, most patients with type 1 diabetes at diagnosis have circulating antibodies to islets (islet cell antibodies, ICA), insulin (IAA), glutamic acid decarboxylase (GAD65), and to tyrosine phosphatases (IA-2 and IA2- ). These antibodies facilitate screening of siblings of affected children as well as adults with atypical features of type 2 for an autoimmune cause of their diabetes (Table 27-3). The antibody levels decline with increasing duration of the disease. Also, once patients are treated with insulin, low levels of anti-insulin antibodies develop.
Family members of diabetic probands are at increased lifetime risk for developing type 1 diabetes. The offspring of a mother with type 1 diabetes has a risk of 3%, whereas the risk is 6% if the father is affected. The risk in siblings is related to the number of HLA haplotypes that the sibling shares with the diabetic proband. If one haplotype is shared, the risk is 6% and if two haplotypes are shared, the risk increases to 12 25%. The highest risk is for identical twins, where the concordance rate is 25 50%.
Certain unrecognized patients with a milder expression of type 1 diabetes initially retain enough B cell function to avoid ketosis but as their B cell mass diminishes later in life, dependence on insulin therapy develops. Islet cell antibody surveys among northern Europeans indicate that up to 15% of type 2 patients may actually have this mild form of type 1 diabetes (latent autoimmune diabetes of adulthood; LADA).
1. Immune-mediated type 1 diabetes mellitus
Immune-mediated type 1 diabetes is believed to result from an infectious or toxic insult to persons whose immune system is genetically predisposed to develop a vigorous autoimmune response either against altered pancreatic B cell antigens or against molecules of the B cell resembling the viral protein (molecular mimicry). Extrinsic factors that affect B cell function include damage caused by viruses such as mumps or coxsackie B4 virus, by toxic chemical agents, or by destructive cytotoxins and antibodies released from sensitized immunocytes. Specific HLA immune response genes are believed to predispose patients to a destructive autoimmune response against their own islet cells (autoaggression), which is mediated primarily by cytotoxic T cells. Amelioration of hyperglycemia in patients given an immunosuppressive agent (eg, cyclosporine)
P.1221
shortly after onset of type 1 diabetes lends further support to the pathogenetic role of autoimmunity.
Table 27-2. Clinical classification of common diabetes mellitus syndromes. | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
Table 27-3. Diagnostic sensitivity and specificity of autoimmune markers in patients with newly diagnosed type 1 diabetes mellitus. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
2. Idiopathic type 1 diabetes mellitus
Fewer than 10% of subjects have no evidence of pancreatic B cell autoimmunity to explain their insulinopenia and ketoacidosis. This subgroup has been classified as idiopathic type 1 diabetes and designated as type 1B. Although only a minority of patients with type 1 diabetes fall into this group, most of these are of Asian or African origin. It was recently reported that about 4% of the West Africans with ketosis-prone diabetes are homozygous for a mutation in PAX-4 (Arg133Trp) a gene that is essential for the development of pancreatic islets.
B. Type 2 Diabetes Mellitus
This represents a heterogeneous group of conditions that used to occur predominantly in adults, but it is now more frequently encountered in children and adolescents. More than 90% of all diabetic persons in the United States are included under this classification. Circulating endogenous insulin is sufficient to prevent ketoacidosis but is inadequate to prevent hyperglycemia in the face of increased needs owing to tissue insensitivity. In most cases of this type of diabetes, the cause is unknown.
Tissue insensitivity to insulin has been noted in most type 2 patients irrespective of weight and has been attributed to several interrelated factors. These include a putative (and as yet undefined) genetic factor, which is aggravated in time by additional enhancers of insulin resistance such as aging, a sedentary lifestyle, and abdominal-visceral obesity. In addition, there is an accompanying deficiency in the response of pancreatic B cells to glucose. Both the tissue resistance to insulin and the impaired B cell response to glucose appear to be further aggravated by increased hyperglycemia (glucose toxicity), and both defects are ameliorated by treatment that reduces the hyperglycemia toward normal. Most epidemiologic data indicate strong genetic influences, since in monozygotic twins over 40 years of age, concordance develops in over 70% of cases within a year whenever type 2 diabetes develops in one twin. Attempts to identify genetic markers for type 2 have as yet been unsuccessful, although linkage to a gene on chromosome 2 encoding a cysteine protease, calpain-10, has been reported in a Mexican-American population. However, its association with other ethnic populations and any role it plays in the pathogenesis of type 2 diabetes remain to be clarified.
The degree and prevalence of obesity varies among different racial groups with type 2 diabetes. While obesity is apparent in no more than 30% of Chinese and Japanese patients with type 2, it is found in 60 70% of North Americans, Europeans, or Africans with type 2 and approaches 100% of patients with type 2 among Pima Indians or Pacific Islanders from Nauru or Samoa.
Patients with this most common form of diabetes have an insensitivity to endogenous insulin. When an associated defect of insulin production prevents adequate compensation for this insulin resistance, nonketotic mild diabetes occurs. Hyperplasia of pancreatic B cells is often present and probably accounts for the fasting hyperinsulinism and exaggerated insulin and proinsulin responses to glucose and other stimuli seen early in the disease. After several years' duration of diabetes, chronic deposition of amyloid in the islets may combine with inherited genetic defects to progressively impair B cell function.
The mechanisms underlying the insulin resistance of type 2 diabetes are poorly understood. Obesity is generally associated with abdominal distribution of fat, producing an abnormally high waist-to-hip ratio. This visceral obesity, due to accumulation of fat in the omental and mesenteric regions, correlates with insulin resistance; subcutaneous abdominal fat seems to have less of an association with insulin insensitivity. Exercise may affect the deposition of visceral fat as suggested by CT scans of Japanese wrestlers, whose extreme obesity is predominantly subcutaneous. Their daily vigorous exercise program prevents accumulation of visceral fat, and they have normal serum lipids and euglycemia despite daily intakes of 5000 7000 kcal and development of massive subcutaneous obesity. Several adipokines, secreted by fat cells, can affect insulin action in obesity. Two of these, leptin and adiponectin, seem to increase sensitivity to insulin, presumably by increasing hepatic responsiveness. Two others tumor necrosis factor- , which inactivates insulin receptors, and the newly discovered peptide resistin interfere with insulin action on glucose metabolism and have been reported to be elevated in obese animal models. Mutations or abnormal levels of these adipokines may contribute to the development of insulin resistance in human obesity.
Hyperglycemia per se can impair insulin action by causing accumulation of hexosamines in muscle and fat tissue and inhibiting glucose transport (acquired glucose toxicity). Correction of hyperglycemia reverses this acquired insulin resistance.
C. Other Specific Types of Diabetes Mellitus
1. Maturity-onset diabetes of the young (MODY)
This subgroup is a relatively rare monogenic disorder characterized by non-insulin-dependent diabetes with autosomal dominant inheritance and an age at onset of
P.1222
25 years or younger. Patients are nonobese, and their hyperglycemia is due to impaired glucose-induced secretion of insulin. Six types of MODY have been described. Except for MODY 2, in which a glucokinase gene is defective, all other types involve mutations of a nuclear transcription factor that regulates islet gene expression.
MODY 2 is quite mild, associated with only slight fasting hyperglycemia and few if any microvascular diabetic complications. It generally responds well to hygienic measures or low doses of oral hypoglycemic agents. MODY 3 the most common form accounts for two-thirds of all MODY cases. The clinical course is similar to that of idiopathic type 2 diabetes in terms of microangiopathy and failure to respond to oral agents with time.
2. Diabetes due to mutant insulins
This is a very rare subtype of nonobese type 2 diabetes, with no more than ten families having been described. Since affected individuals were heterozygous and possessed one normal insulin gene, diabetes was mild, did not appear until middle age, and showed autosomal dominant genetic transmission. There is generally no evidence of clinical insulin resistance, and these patients respond well to standard therapy.
3. Diabetes due to mutant insulin receptors
Defects in one of their insulin receptor genes have been found in more than 40 people with diabetes, and most have extreme insulin resistance associated with acanthosis nigricans. In very rare instances when both insulin receptor genes are abnormal, newborns present with a leprechaun-like phenotype and seldom live through infancy.
4. Diabetes mellitus associated with a mutation of mitochondrial DNA
Since sperm do not contain mitochondria, only the mother transmits mitochondrial genes to her offspring. Diabetes due to a mutation of mitochondrial DNA that impairs the transfer of leucine or lysine into mitochondrial proteins has been described. Most patients have a mild form of diabetes that responds to oral hypoglycemic agents; some have a nonimmune form of type 1 diabetes. Two-thirds of patients with this subtype of diabetes have a hearing loss, and a smaller proportion (15%) had a syndrome of myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS).
5. Wolfram's syndrome
Wolfram's syndrome is an autosomal recessive neurodegenerative disorder first evident in childhood. It consists of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, hence the acronym DIDMOAD. It is due to mutations in a gene named WFS1, which encodes a 100.3 KDa transmembrane protein localized in the endoplasmic reticulum. The function of the protein is not known. The diabetes mellitus, which is nonimmune and not linked to specific HLA antigens, usually presents in the first decade together with the optic atrophy. Cranial diabetes insipidus and sensorineural deafness develop during the second decade in 60 75% of patients. Ureterohydronephrosis, neurogenic bladder, cerebellar ataxia, peripheral neuropathy, and psychiatric illness develop later in many patients.
Insulin Resistance Syndrome (Syndrome X; Metabolic Syndrome)
Twenty-five percent of the general nonobese nondiabetic population has insulin resistance of a magnitude similar to that seen in type 2 diabetes. These insulin-resistant nondiabetic individuals are at much higher risk for developing type 2 diabetes than insulin-sensitive persons. In addition to diabetes, these individuals have increased risk for elevated plasma triglycerides, lower high-density lipoproteins (HDLs), and higher blood pressure a cluster of abnormalities termed syndrome X. These associations have now been expanded to include small, dense, low-density lipoprotein (LDL), hyperuricemia, abdominal obesity, prothrombotic state with increased levels of plasminogen activator inhibitor type 1 (PAI-1), and proinflammatory state. These clusters of abnormalities significantly increase the risk of atherosclerotic disease.
It has been postulated that hyperinsulinemia and insulin resistance play a direct role in these metabolic abnormalities, but supportive evidence is inconclusive. Although hyperinsulinism and hypertension often coexist in whites, that is not the case in blacks or Pima Indians. Moreover, patients with hyperinsulinism due to insulinoma are not hypertensive, and there is no fall in blood pressure after surgical removal of the insulinoma restores normal insulin levels. The main value of grouping these disorders as a syndrome, however, is to remind clinicians that the therapeutic goals are not only to correct hyperglycemia but also to manage the elevated blood pressure and dyslipidemia that result in increased cerebrovascular and cardiac morbidity and mortality in these patients. Clinicians aware of this syndrome are more cautious in prescribing therapies that correct hypertension but may raise lipids (diuretics, -blockers) or that correct hyperlipidemia but increase insulin resistance, with aggravation of diabetes (niacin). Finally, the use of long-acting insulins and sulfonylureas that promote sustained hyperinsulinism may have to be moderated, with insulin-sparing drugs such as metformin or a thiazolidinedione being preferable, if the hypothesis behind the insulin resistance syndrome is ever substantiated.
Clinical Findings
The principal clinical features of the two major types of diabetes mellitus are listed for comparison in Table 27-4.
Patients with type 1 diabetes have a characteristic symptom complex. An absolute deficiency of insulin results in accumulation of circulating glucose and fatty acids, with consequent hyperosmolality and hyperketonemia.
Patients with type 2 diabetes may or may not have characteristic features. The presence of obesity or a strongly
P.1223
positive family history for mild diabetes suggests a high risk for the development of type 2 diabetes.
Table 27-4. Clinical features of diabetes at diagnosis. | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
A. Symptoms and Signs
1. Type 1 diabetes
Increased urination is a consequence of osmotic diuresis secondary to sustained hyperglycemia. This results in a loss of glucose as well as free water and electrolytes in the urine. Thirst is a consequence of the hyperosmolar state, as is blurred vision, which often develops as the lenses are exposed to hyperosmolar fluids.
Weight loss despite normal or increased appetite is a common feature of type 1 when it develops subacutely. The weight loss is initially due to depletion of water, glycogen, and triglycerides; thereafter, reduced muscle mass occurs as amino acids are diverted to form glucose and ketone bodies.
Lowered plasma volume produces symptoms of postural hypotension. Total body potassium loss and the general catabolism of muscle protein contribute to the weakness.
Paresthesias may be present at the time of diagnosis, particularly when the onset is subacute. They reflect a temporary dysfunction of peripheral sensory nerves, which clears as insulin replacement restores glycemic levels closer to normal, suggesting neurotoxicity from sustained hyperglycemia.
When absolute insulin deficiency is of acute onset, the above symptoms develop abruptly. Ketoacidosis exacerbates the dehydration and hyperosmolality by producing anorexia and nausea and vomiting, interfering with oral fluid replacement.
The patient's level of consciousness can vary depending on the degree of hyperosmolality. When insulin deficiency develops relatively slowly and sufficient water intake is maintained, patients remain relatively alert and physical findings may be minimal. When vomiting occurs in response to worsening ketoacidosis, dehydration progresses and compensatory mechanisms become inadequate to keep serum osmolality below 320 330 mosm/L. Under these circumstances, stupor or even coma may occur. The fruity breath odor of acetone further suggests the diagnosis of diabetic ketoacidosis.
Hypotension in the recumbent position is a serious prognostic sign. Loss of subcutaneous fat and muscle wasting are features of more slowly developing insulin deficiency. In occasional patients with slow, insidious onset of insulin deficiency, subcutaneous fat may be considerably depleted.
2. Type 2 diabetes
While many patients with type 2 diabetes present with increased urination and thirst, many others have an insidious onset of hyperglycemia and are asymptomatic initially. This is particularly true in obese patients, whose diabetes may be detected only after glycosuria or hyperglycemia is noted during routine laboratory studies. Occasionally, type 2 patients may present with evidence of neuropathic or cardiovascular complications because of occult disease present for some time prior to diagnosis. Chronic skin infections are common. Generalized pruritus and symptoms of vaginitis are frequently the initial complaints of women. Diabetes should be suspected in women with chronic candidal vulvovaginitis as well as in those who have delivered large babies (> 9 lb, or 4.1 kg) or have had polyhydramnios, preeclampsia, or unexplained fetal losses.
Obese diabetics may have any variety of fat distribution; however, diabetes seems to be more often associated in both men and women with localization of fat deposits on the upper segment of the body (particularly the abdomen, chest, neck, and face) and relatively less fat on the appendages, which may be quite muscular. Standardized tables of waist-to-hip ratio indicate that ratios of greater than 0.9 in men and greater than 0.8 in women are associated with an increased risk of diabetes in obese subjects. Mild hypertension is often present in obese diabetics. Eruptive xanthomas on the flexor surface of the limbs and on the buttocks and lipemia retinalis due to hyperchylomicronemia can occur in patients with uncontrolled type 2 diabetes who also have a familial form of hypertriglyceridemia.
B. Laboratory Findings
1. Urinalysis
a. Glucosuria
A specific and convenient method to detect glucosuria is the paper strip impregnated with glucose oxidase and a chromogen system (Clinistix, Diastix), which is sensitive to as little as 0.1% glucose in urine. Diastix can be directly applied to the urinary stream, and differing color responses of the indicator strip reflect glucose concentration.
A normal renal threshold for glucose as well as reliable bladder emptying is essential for interpretation.
b. Ketonuria
Qualitative detection of ketone bodies can be accomplished by nitroprusside tests (Acetest or Ketostix). Although these tests do not detect -hydroxybutyric acid, which lacks a ketone group, the
P.1224
semiquantitative estimation of ketonuria thus obtained is nonetheless usually adequate for clinical purposes.
2. Blood testing procedures
a. Glucose tolerance test
(1) Methodology and normal fasting glucose
Plasma or serum from venous blood samples has the advantage over whole blood of providing values for glucose that are independent of hematocrit and that reflect the glucose concentration to which body tissues are exposed. For these reasons, and because plasma and serum are more readily measured on automated equipment, they are used in most laboratories. If serum is used or if plasma is collected from tubes that lack an agent to block glucose metabolism (such as fluoride), samples should be refrigerated and separated within 1 hour after collection. The glucose concentration is 10 15% higher in plasma or serum than in whole blood because structural components of blood cells are absent.
(2) Criteria for laboratory confirmation of diabetes mellitus
If the fasting plasma glucose level is 126 mg/dL or higher on more than one occasion, further evaluation of the patient with a glucose challenge is unnecessary. However, when fasting plasma glucose is less than 126 mg/dL in suspected cases, a standardized oral glucose tolerance test may be done (Table 27-5).
For proper evaluation of the test, the subjects should be normally active and free from acute illness. Medications that may impair glucose tolerance include diuretics, contraceptive drugs, glucocorticoids, niacin, and phenytoin.
Because of difficulties in interpreting oral glucose tolerance tests and the lack of standards related to aging, these tests are being replaced by documentation of fasting hyperglycemia.
b. Glycated hemoglobin (hemoglobin A1) measurements
Hemoglobin becomes glycated by ketoamine reactions between glucose and other sugars and the free amino groups on the and chains. Only glycation of the N-terminal valine of the beta chain imparts sufficient negative charge to the hemoglobin molecule to allow separation by charge dependent techniques. These charge separated hemoglobins are collectively referred to as hemoglobin A1 (HbA1). The major form of HbA1 is hemoglobin A1c (HbA1c) where glucose is the carbohydrate. HbA1c comprises 4 6% of total hemoglobin A1. The remaining HbA1 species contain fructose-1,6 diphosphate (HbA1a1); glucose-6-phosphate (HbA1a2); and unknown carbohydrate moiety (HbA1b). The hemoglobin A1c fraction is abnormally elevated in diabetic persons with chronic hyperglycemia. Methods for measuring HbA1c include electrophoresis, cation-exchange chromatography, boronate affinity chromatography, and immunoassays. Office-based immunoassays using capillary blood give a result in about 9 minutes and this allows for immediate feedback to the patients regarding their glycemic control.
Table 27-5. The Diabetes Expert Committee criteria for evaluating the standard oral glucose tolerance test.1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Since glycohemoglobins circulate within red blood cells whose life span lasts up to 120 days, they generally reflect the state of glycemia over the preceding 8 12 weeks, thereby providing an improved method of assessing diabetic control. The HbA1c value, however, is weighted to more recent glucose levels (previous month) and this explains why significant changes in HbA1c are observed with short-term (1 month) changes in mean plasma glucose levels. Measurements should be made in patients with either type of diabetes mellitus at 3- to 4-month intervals so that adjustments in therapy can be made if HbA1c is either subnormal or if it is more than 2% above the upper limits of normal for a particular laboratory. In patients monitoring their own blood glucose levels, HbA1c values provide a valuable check on the accuracy of monitoring. In patients who do not monitor their own blood glucose levels, HbA1c values are essential for adjusting therapy. Data from the Diabetes Control and Complications Trial (DCCT) showed that there is a linear relationship between the HbA1c and the mean of seven-point capillary blood glucose profiles (preprandial, postprandial, and bedtime). Thus, mean plasma glucose levels of 170, 205, 240, and 275 mg/dL approximately correlate with HbA1c values of 7%, 8%, 9%, and 10%, respectively. Use of HbA1c for screening is controversial. Sensitivity in detecting known diabetes cases by HbA1c measurements is only 85%, indicating that diabetes cannot be excluded by a normal value. On the other hand, elevated HbA1c assays are fairly specific (91%) in identifying the presence of diabetes.
The accuracy of HbA1c values can be affected by hemoglobin variants or derivatives; the effect depends on the specific hemoglobin variant or derivative and the specific assay used. Immunoassays that use an antibody to the glycated amino terminus of globin do not recognize the terminus of the globin of hemoglobin F. Thus, in patients with high levels of hemoglobin F, immunoassays give falsely low values of HbA1c. Cation-exchange chromatography separates hemoglobin species by charge differences. Hemoglobin variants that co-elute
P.1225
with HbA1c can lead to an overestimation of the HbA1c value. Chemically modified derivatives of hemoglobin such as carbamoylation (in renal failure) or acetylation (high-dose aspirin therapy) can similarly co-elute with HbA1c by some assay methods.
Any condition that shortens erythrocyte survival or decreases mean erythrocyte age (eg, recovery from acute blood loss, hemolytic anemia) will falsely lower HbA1c irrespective of the assay method used. Alternative methods such as fructosamine (see below) should be considered for these patients. Vitamins C and E are reported to falsely lower test results possibly by inhibiting glycation of hemoglobin.
c. Serum fructosamine
Serum fructosamine is formed by nonenzymatic glycosylation of serum proteins (predominantly albumin). Since serum albumin has a much shorter half-life than hemoglobin, serum fructosamine generally reflects the state of glycemic control for only the preceding 1 2 weeks. Reductions in serum albumin (eg, nephrotic state or hepatic disease) will lower the serum fructosamine value. When abnormal hemoglobins or hemolytic states affect the interpretation of glycohemoglobin or when a narrower time frame is required, such as for ascertaining glycemic control at the time of conception in a diabetic woman who has recently become pregnant, serum fructosamine assays offer some advantage. Normal values vary in relation to the serum albumin concentration and are 1.5 2.4 mmol/L when the serum albumin level is 5 g/dL.
d. Self-monitoring of blood glucose
Capillary blood glucose measurements performed by patients themselves, as outpatients, are extremely useful. In type 1 patients in whom tight metabolic control is attempted, they are indispensable. There are several paper strip (glucose oxidase, glucose dehydrogenase, or hexokinase) methods for measuring glucose on capillary blood samples. A reflectance photometer or an amperometric system is then used to measure the reaction that takes place on the reagent strip. A large number of blood glucose meters are now available. All are accurate, but they vary with regard to speed, convenience, size of blood samples required, and cost. Popular models include those manufactured by LifeScan (One Touch), Bayer Corporation (Glucometer Elite, DEX), Roche Diagnostics (Accu-Chek), Abbott Laboratories (ExacTech, Precision, FreeStyle), and Home Diagnostics (Prestige). A Freestyle Flash meter, for example, requires only 0.3 mL of blood and gives a result in 7 seconds and illustrates how there has been continued progress in this technologic area. Various glucometers appeal to a particular consumer need and are relatively inexpensive, ranging from $50.00 to $100.00 each. The more expensive models compute blood glucose averages and can be attached to printers for data records and graph production. Test strips remain a major expense, costing 50 75 cents apiece. In self-monitoring of blood glucose, patients must prick a finger with a 28 to 30 gauge lancets, which can be facilitated by a small plastic trigger device such as an Autolet (Ames Co.), SoftClix (Boehringer-Mannheim), or Penlet (Lifescan, Inc.). When used for multiple patients, as in a clinic, physician's office, or hospital ward, disposable finger-rest platforms are required to avoid inadvertent transmission of blood-borne viral diseases. Some meters such as the FreeStyle (Abbott Laboratories) have been approved for measuring glucose in blood samples obtained at alternative sites such as the forearm and thigh. There is, however, a 5- to 20-minute lag in the glucose response on the arm with respect to the glucose response on the finger. Forearm blood glucose measurements could therefore result in a delay in detection of rapidly developing hypoglycemia.
The clinician should be aware of the limitations of the self-monitoring glucose systems. First, a few of the older meters (such as the One Touch Profile) are calibrated against whole blood glucose concentrations even though the test strip measures the glucose in the plasma fraction. This means the displayed values are 10% to 15% lower than the laboratory glucose result. Second, increases or decreases in hematocrit can decrease or increase the measured glucose values. The mechanism underlying this effect is not known but presumably it is due to the impact of red cells on the diffusion of plasma into the reagent layer. Third, the meters and the test strips are calibrated over the glucose concentrations ranging from 60 mg/dL to 160 mg/dL, and the accuracy is not as good for higher and lower glucose levels. When the glucose is less than 60 mg/dL, the difference between the meter and the laboratory value may be as much as 20%. Fourth, glucose oxidase-based amperometric systems underestimate glucose levels in the presence of high oxygen tension. This may be important in the critically ill who are receiving supplemental oxygen; under these circumstances, a glucose dehydrogenase-based system may be preferable. The accuracy of data obtained by glucose monitoring requires education of the patient in sampling and measuring procedures as well as in proper calibration of the instruments. Bedside glucose monitoring in a hospital setting requires rigorous quality control programs and certification of personnel to avoid errors.
e. Continuous glucose monitoring systems
Two continuous glucose monitoring systems are currently available for clinical use. The system manufactured by Medtronic Minimed involves inserting a subcutaneous sensor (rather like an insulin pump cannula) that measures glucose concentrations in the interstitial fluid for 72 hours. In the newest version of the system (Guardian RT), the glucose values are available for review by the patient at the time of measurement. There are also options to set alarms for dangerously low or high glucose values. The other system ( Glucowatch ) measures glucose in interstitial fluid extracted through intact skin by applying a low electric current (reverse iontophoresis). This process can cause local skin irritation, and sweating distorts the glucose measurement. Both systems require calibration with finger blood glucose
P.1226
measurements. The main value of these systems appears to be in identifying episodes of asymptomatic hypoglycemia, especially at night.
3. Lipoprotein abnormalities in diabetes
Circulating lipoproteins are just as dependent on insulin as is the plasma glucose. In type 1 diabetes, moderately deficient control of hyperglycemia is associated with only a slight elevation of LDL cholesterol and serum triglycerides and little if any change in HDL cholesterol. Once the hyperglycemia is corrected, lipoprotein levels are generally normal. However, in obese patients with type 2 diabetes, a distinct diabetic dyslipidemia is characteristic of the insulin resistance syndrome. Its features are a high serum triglyceride level (300 400 mg/dL), a low HDL cholesterol (less than 30 mg/dL), and a qualitative change in LDL particles, producing a smaller dense particle whose membrane carries supranormal amounts of free cholesterol. These smaller dense LDL particles are more susceptible to oxidation, which renders them more atherogenic. Since a low HDL cholesterol is a major feature predisposing to macrovascular disease, the term dyslipidemia has preempted the term hyperlipidemia, which mainly denoted the elevated triglycerides. Measures designed to correct the obesity and hyperglycemia, such as exercise, diet, and hypoglycemic therapy, are the treatment of choice for diabetic dyslipidemia, and in occasional patients in whom normal weight was achieved, all features of the lipoprotein abnormalities cleared. Since primary disorders of lipid metabolism may coexist with diabetes, persistence of lipid abnormalities after restoration of normal weight and blood glucose should prompt a diagnostic workup and possible pharmacotherapy of the lipid disorder. Chapter 28 discusses these matters in detail.
Differential Diagnosis
A. Hyperglycemia Secondary to Other Causes
Secondary hyperglycemia has been associated with various disorders of insulin target tissues (liver, muscle, and adipose tissue) (Table 27-6). Other secondary causes of carbohydrate intolerance include endocrine disorders often specific endocrine tumors associated with excess production of growth hormone, glucocorticoids, catecholamines, glucagon, or somatostatin. In the first four situations, peripheral responsiveness to insulin is impaired. With excess of glucocorticoids, catecholamines, or glucagon, increased hepatic output of glucose is a contributory factor; in the case of catecholamines, decreased insulin release is an additional factor in producing carbohydrate intolerance, and with excess somatostatin production it is the major factor.
Table 27-6. Secondary causes of hyperglycemia. | |
|---|---|
|
A rare syndrome of extreme insulin resistance associated with acanthosis nigricans afflicts either young women with androgenic features as well as insulin receptor mutations or older people, mostly women, in whom a circulating immunoglobulin binds to insulin receptors and reduces their affinity to insulin.
Medications such as diuretics, phenytoin, niacin, and high-dose corticosteroids can produce hyperglycemia that is reversible once the drugs are discontinued or when diuretic-induced hypokalemia is corrected. Chronic pancreatitis or subtotal pancreatectomy reduces the number of functioning B cells and can result in a metabolic derangement very similar to that of genetic type 1 diabetes except that a concomitant reduction in pancreatic A cells may reduce glucagon secretion so that relatively lower doses of insulin replacement are needed. Insulin-dependent diabetes is occasionally associated with Addison's disease and autoimmune thyroiditis (Schmidt's syndrome, or polyglandular failure syndrome). This occurs more commonly in women and represents an autoimmune disorder in which there are circulating antibodies to adrenocortical and thyroid tissue, thyroglobulin, and gastric parietal cells.
B. Nondiabetic Glycosuria
Nondiabetic glycosuria (renal glycosuria) is a benign asymptomatic condition wherein glucose appears in the urine despite a normal amount of glucose in the blood, either basally or during a glucose tolerance test. Its cause may vary from an autosomally transmitted genetic disorder to one associated with dysfunction of the proximal renal tubule (Fanconi's syndrome, chronic renal failure), or it may merely be a consequence of the increased load of glucose presented to the tubules by the elevated glomerular filtration rate during pregnancy. As many as 50% of pregnant women normally have demonstrable sugar in the urine, especially during the third and fourth months. This sugar is practically always glucose except during the late weeks of pregnancy, when lactose may be present.
Clinical Trials in Diabetes
A fundamental controversy regarding whether diabetic microangiopathy is related exclusively to the existence
P.1227
and duration of hyperglycemia or whether it reflects a separate genetic disorder have been resolved by the findings of the DCCT and of the United Kingdom Prospective Diabetes Study (UKPDS), which confirmed the beneficial effects of improved glycemic control in both type 1 and type 2 diabetes, respectively (see below). In addition, with increased understanding of the pathophysiology of both type 1 and type 2 diabetes, large prospective studies have been initiated in attempts to prevent onset of these disorders. Investigators with the Diabetes Prevention Trial 1 (DPT-1) and the Diabetes Prevention Program (DPP) have recently reported their findings (see below).
A. Clinical Trials in Type 1 Diabetes
1. Diabetes Prevention Trial-1
This multicenter study sponsored by the National Institutes of Health was designed to determine whether the development of type 1 diabetes mellitus could be prevented or delayed by immune intervention therapy. Daily low-dose insulin injections were administered for up to 8 years in first-degree relatives of type 1 diabetic patients who were selected as being at high risk for development of type 1 diabetes because of detectable islet cell antibodies and reduced early-insulin release. Unfortunately, this immune intervention failed to affect the onset of type 1 diabetes compared with a randomized untreated group. A related study using oral insulin in lower risk first-degree relatives who have islet cell antibodies but whose early insulin release remains intact also failed to show an effect on the onset of type 1 diabetes. After an average of 4.3 years of observation, type 1 diabetes developed in about 35% of persons in both the oral insulin and the placebo groups.
2. The Diabetes Control and Complications Trial
A long-term therapeutic study involving 1441 patients with type 1 diabetes mellitus reported that near normalization of blood glucose resulted in a delay in the onset and a major slowing of the progression of established microvascular and neuropathic complications of diabetes during a follow-up period of up to 10 years. Multiple insulin injections (66%) or insulin pumps (34%) were used in the intensively treated group, who were trained to modify their therapy in response to frequent glucose monitoring. The conventionally treated groups used no more than two insulin injections, and clinical well-being was the goal with no attempt to modify management based on HbA1c determinations or the glucose results.
In half of the patients, a mean hemoglobin A1c of 7.2% (normal: < 6%) and a mean blood glucose of 155 mg/dL were achieved using intensive therapy, while in the conventionally treated group HbA1c averaged 8.9% with an average blood glucose of 225 mg/dL. Over the study period, which averaged 7 years, there was an approximately 60% reduction in risk between the two groups in regard to diabetic retinopathy, nephropathy, and neuropathy. Intensively treated patients had a threefold greater risk of serious hypoglycemia as well as a greater tendency toward weight gain. However, there were no deaths definitely attributable to hypoglycemia in any persons in the DCCT study, and no evidence of posthypoglycemic cognitive damage was detected.
The general consensus of the ADA is that intensive insulin therapy associated with comprehensive self-management training should become standard therapy in patients with type 1 diabetes mellitus after the age of puberty. Exceptions include those with advanced renal disease and the elderly, since in these groups the detrimental risks of hypoglycemia outweigh the benefits of tight glycemic control.
3. Immune Intervention Trials in New-Onset Type 1 Diabetes
At the time of diagnosis of type 1 diabetes, patients still have significant B cell function. This explains why soon after diagnosis patients go into a partial clinical remission ( honeymoon ) requiring little or no insulin. This clinical remission is short-lived, however, and eventually patients lose all B cell function and have more labile glucose control. Attempts have been made to prolong this partial clinical remission using drugs such as cyclosporine, azathioprine, prednisone, and antithymocyte globulin. These drugs have had limited efficacy, and there are concerns about toxicity and the need for continuous treatment.
Newer agents that may induce immune tolerance and appear to have few side effects have been used in new-onset type 1 patients. Three small studies, one with a heat shock protein peptide (DiaPep277) and two with anti-CD3 antibodies, have demonstrated that these agents can preserve endogenous insulin production. Larger phase 2 clinical trials are currently in progress.
B. Clinical Trials in Type 2 Diabetes
While patients with type 2 were not studied in the DCCT, the eye, kidney, and nerve abnormalities are quite similar in both types of diabetes, and it is likely that similar underlying mechanisms apply. Several important differences, however, must be considered. Since patients with type 2 diabetes are generally older with a high incidence of macrovascular disease, an episode of severe hypoglycemia entails much greater risk than it would in a younger patient with type 1 diabetes. Moreover, weight gain may be much greater in obese persons with type 2 diabetes in whom intensive insulin therapy is attempted. These risks take on a greater relevance in older patients with type 2 diabetes because the prevalence of microangiopathy is relatively lower than in those patients with type 1 diabetes; preventing microvascular disease in patients with type 2 diabetes is much less likely to influence morbidity and mortality because of the greater consequences of their macrovascular disease.
To address the issues raised by the DCCT findings as well as a previous concern that sulfonylureas may increase cardiovascular deaths, as reported in 1970 by the University Group Diabetes Program, randomized clinical trials of intensive therapy have been conducted in patients with type 2 diabetes.
P.1228
1. The Diabetes Prevention Program
This study was aimed at discovering whether treatment with either diet and exercise or metformin could prevent the onset of type 2 diabetes in people with impaired glucose tolerance; 3234 overweight men and women aged 25 85 years with impaired glucose tolerance participated in the study. Intervention with a low-fat diet and 150 minutes of moderate exercise (equivalent to a brisk walk) per week reduced the risk of progression to type 2 diabetes by 71% compared with a matched control group. Participants taking 80 mg of metformin twice a day reduced their risk of developing type 2 diabetes by 31%, but this intervention was relatively ineffective in those who were either less obese or in the older age group.
With the demonstration that intervention can be successful in preventing progression to diabetes in these subjects, a recommendation has been made to change the terminology from the less comprehensible impaired glucose tolerance to prediabetes. The latter is a term that the public can better understand and thus respond to by implementing healthier diet and exercise habits.
2. Kumamoto Study
The Kumamoto study involved a relatively small number of patients with type 2 diabetes (n = 110) who were nonobese and only slightly insulin-resistant, requiring less than 30 units of insulin per day for intensive therapy. Over a 6-year period, it was shown that intensive insulin therapy, achieving a mean HbA1c of 7.1%, significantly reduced microvascular end points compared with conventional insulin therapy achieving a mean HbA1c of 9.4%. Cardiovascular events were neither worsened nor improved by intensive therapy, and weight changes were likewise not influenced by either form of treatment.
3. The Veterans Administration Cooperative Study
This investigation involved 153 obese men who were moderately insulin-resistant and who were monitored for only 27 months. Intensive insulin treatment resulted in mean HbA1c differences from conventional insulin treatment (7.2% versus 9.5%) that were comparable to those reported from the Kumamoto Study. However, a difference in cardiovascular outcome in this study has prompted some concern. While conventional insulin therapy resulted in 26 total cardiovascular events, there were 35 total cardiovascular events in the intensively treated group. This difference in the relatively small population was not statistically significant, but when the total events were broken down to major events (myocardial infarction, stroke, cardiovascular death, congestive heart failure, or amputation), the 18 major events in the group treated intensively with insulin were reported to be statistically greater (P = .04) than the ten major events occurring with conventional treatment. While this difference may be a chance consequence of studying too few patients for too short a time, it raises the possibility that insulin-resistant patients with visceral obesity and long-standing type 2 diabetes may develop a greater risk of serious cardiovascular mishap when intensively treated with high doses of insulin. At the end of the study, 64% of the intensively treated group were either receiving (1) an average of 113 units of insulin per day when only two injections per day were used or (2) a mean dosage of 133 units per day when multiple injections were used. Unfortunately, the UKPDS (see below), which did not discern any effect of intensive therapy on cardiovascular outcomes, does not resolve the concern generated by the Veterans Administration Study since their patient population consisted of newly diagnosed diabetic patients in whom the obese subgroup seemed to be less insulin-resistant, requiring a median insulin dose for intensive therapy of only 60 units per day by the twelfth year of the study.
4. The United Kingdom Prospective Diabetes Study
This multicenter study was designed to establish, in type 2 diabetic patients, whether the risk of macrovascular or microvascular complications could be reduced by intensive blood glucose control with oral hypoglycemic agents or insulin and whether any particular therapy was of advantage. A total of 3867 patients aged 25 65 years with newly diagnosed diabetes were recruited between 1977 and 1991, and studied over 10 years. The median age at baseline was 54 years; 44% were overweight (> 120% over ideal weight); and baseline HbA1c was 9.1%. Therapies were randomized to include a control group on diet alone and separate groups intensively treated with either insulin or sulfonylurea (chlorpropamide, glyburide, or glipizide). Metformin was included as a randomization option in a subgroup of 342 overweight patients, and much later in the study an additional subgroup of both normal-weight and overweight patients who were responding unsatisfactorily to sulfonylurea therapy were randomized to either continue on their sulfonylurea therapy alone or to have metformin combined with it.
In 1987, an additional modification was made to evaluate whether tight control of blood pressure with stepwise antihypertensive therapy would prevent macrovascular and microvascular complications in 758 hypertensive patients among this UKPDS population compared with 390 of them whose blood pressure was treated less intensively. The tight control group was randomly assigned to treatment with either an angiotensin-converting enzyme (ACE) inhibitor (captopril) or a -blocker (atenolol). Both drugs were stepped up to maximum dosages of 100 mg/d and then, if blood pressure remained higher than the target level of < 150/85 mm Hg, more drugs were added in the following stepwise sequence: a diuretic, slow-release nifedipine, methyldopa, and prazosin until the target level of tight control was achieved. In the control group, hypertension was conventionally treated to achieve target levels < 180/105 mm Hg, but these patients were not prescribed either ACE inhibitors or -blockers.
a. Results of the UKPDS
Intensive treatment with either sulfonylureas, metformin, combinations of those two, or insulin achieved mean HbA1c levels of
P.1229
7%. This level of glycemic control decreases the risk of microvascular complications (retinopathy and nephropathy) in comparison with conventional therapy (mostly diet alone), which achieved mean levels of HbA1c of 7.9%. Weight gain occurred in intensively treated patients except when metformin was used as monotherapy. No cardiovascular benefit and no adverse cardiovascular outcomes were noted regardless of the therapeutic agent. Hypoglycemic reactions occurred in the intensive treatment groups, but only one death from hypoglycemia was documented during 27,000 patient-years of intensive therapy.
When therapeutic subgroups were analyzed, some unexpected and paradoxical results were noted. Among the obese patients, intensive treatment with insulin or sulfonylureas did not reduce microvascular complications compared with diet therapy alone. This was in contrast to the significant benefit of intensive therapy with these drugs in the total group. Furthermore, intensive therapy with metformin was more beneficial in obese persons than diet alone with regard to fewer myocardial infarctions, strokes, and diabetes-related deaths, but there was no significant reduction by metformin of diabetic microvascular complications as compared with the diet group. Moreover, in the subgroup of obese and nonobese patients in whom metformin was added to sulfonylurea failures, rather than showing a benefit, there was a 96% increase in diabetes-related deaths compared with the matched cohort of patients with unsatisfactory glycemic control on sulfonylureas who remained on their sulfonylurea therapy. Chlorpropamide also came out poorly on subgroup analysis in that those receiving it as intensive therapy did less well regarding progression to retinopathy than those conventionally treated with diet.
Intensive antihypertensive therapy to a mean of 144/82 mm Hg had beneficial effects on microvascular disease as well as on all diabetes-related end points, including virtually all cardiovascular outcomes, in comparison with looser control at a mean of 154/87 mm Hg. In fact, the advantage of reducing hypertension by this amount was substantially more impressive than the benefit achieved by improving the degree of glycemic control from a mean HbA1c of 7.9% to 7%. More than half of the patients needed two or more drugs for adequate therapy of their hypertension, and there was no demonstrable advantage of ACE inhibitor therapy over therapy with -blockers with regard to diabetes end points. Use of a calcium channel blocker added to both treatment groups appeared to be safe over the long term in this diabetic population despite some controversy in the recent literature about its safety in diabetics.
b. Implications of the UKPDS
It appears that glycemic control to levels of HbA1c to 7% shows benefit in reducing total diabetes end points, including a 25% reduction in microvascular disease as compared with HbA1c levels of 7.9%. This reassures those who have questioned whether the value of intensive therapy, so convincingly shown by the DCCT in type 1 diabetes, can safely be extrapolated to older patients with type 2 diabetes. It also argues against the concept of a threshold of glycemic control since in this group there was a benefit from this modest reduction of HbA1c below 7.9% whereas in the DCCT a threshold was suggested in that further benefit was less apparent at HbA1c levels below 8%.
Because of the complexity of the overall design in which many of the original therapy groups received additional medications to achieve glycemic goals but remained assigned to their group, statistical analysis may have been compromised by these multiple crossovers. For instance, in the diet group that was used as a control for all the drug treatment groups, only 58% of their total patient-years were actually drug-free while the remainder consisted of nonintensive therapy with various hypoglycemic drug regimens to avoid unacceptable hyperglycemia. This probably partly explains why the mean HbA1c for this group was only 7.9% on diet alone therapy for over 10 years. In view of these crossovers within treatment groups, caution is suggested regarding several subgroup analyses that are controversial. These include the implication that metformin was superior to insulin or sulfonylureas in reducing diabetes-related end points in obese patients compared with diet therapy even though all three treatment groups achieved the same degree of glycemic control. Conversely, the finding of excess mortality in the subgroup of patients receiving combination therapy with metformin and sulfonylureas need not necessarily preclude this combination in patients doing poorly on sulfonylureas alone, although it certainly indicates a need for clarification of this important question.
Probably the most striking implication of the UKPDS is the benefit to the hypertensive type 2 diabetic patient of intensive control of blood pressure. There was no demonstrable advantage of ACE inhibitor therapy on outcome despite a number of short-term reports in smaller populations, implying that these drugs have special efficacy in reducing glomerular pressure beyond their general antihypertensive effects. Moreover, slow-release nifedipine showed no evidence of cardiac toxicity in this study despite some previous reports claiming that calcium channel blockers may be hazardous in patients with diabetes. Finally, the greater benefit in diabetes end points from antihypertensive than from antihyperglycemic treatments may be that the difference between the mean blood pressures achieved (144/82 mm Hg versus 154/87 mm Hg) is therapeutically more influential than the slight difference in HbA1c (7% versus 7.9%). Greater hyperglycemia in the control group would most likely have rectified this discrepancy in outcomes.
5. The STENO-2 Study
The Steno-2 study was designed in 1990 to validate the efficacy of targeting multiple concomitant risk factors for both microvascular and macrovascular disorders in type 2 diabetes. A prospective,
P.1230
randomized, open, blinded end point design was used where 160 patients with type 2 diabetes and microalbuminuria were assigned to conventional therapy with their general practitioner or to intensive care at the Steno Diabetes Center. The intensively treated group had step-wise introduction of lifestyle and pharmacologic interventions aimed at keeping glycated hemoglobin less than 6.5%, blood pressure less than 130/80 mm Hg; total cholesterol < 175 mg/dL, and triglycerides < 150 mg/dL. All the intensively treated group received ACE inhibitors and if intolerant, an angiotensin II-receptor blocker. The lifestyle component of intensive intervention included reduction in dietary fat intake to less than 30% of total calories; smoking cessation program; light to moderate exercise; daily vitamin-mineral supplement of vitamin C, E, and chromium picolinate. Initially aspirin was only given as secondary prevention to patients with a history of ischemic cardiovascular disease; later all patients received aspirin. After a mean follow-up of 7.8 years, cardiovascular events (eg, myocardial infarction, angioplasties, coronary bypass grafts, strokes, amputations, vascular surgical interventions) developed in 44% of patients in the conventional arm and only in 24% in the intensive multifactorial arm about a 50% reduction. Rates of nephropathy, retinopathy, and autonomic neuropathy were also lower in the multifactorial intervention arm by 62% and 63%, respectively.
The data from the UKPDS and this study provide support for guidelines recommending vigorous treatment of concomitant microvascular and cardiovascular risk factors in patients with type 2 diabetes.
Treatment Regimens
A. Diet
A well-balanced, nutritious diet remains a fundamental element of therapy. However, in more than half of cases, diabetic patients fail to follow their diet. In prescribing a diet, it is important to relate dietary objectives to the type of diabetes. In obese patients with mild hyperglycemia, the major goal of diet therapy is weight reduction by caloric restriction. Thus, there is less need for exchange lists, emphasis on timing of meals, or periodic snacks, all of which are so essential in the treatment of insulin-requiring nonobese diabetics. This type of patient represents the most frequent challenge for the clinician. Weight reduction is an elusive goal that can only be achieved by close supervision and education of the obese patient. See Chapter 29 for dietary management of obesity.
1. American Diabetes Association recommendations
The ADA releases an annual position statement on medical nutrition therapy that replaces the calculated ADA diet formula of the past with suggestions for an individually tailored dietary prescription based on metabolic, nutritional, and lifestyle requirements. They contend that the concept of one diet for diabetes and the prescription of an ADA diet no longer can apply to both major types of diabetes. In their recommendations for persons with type 2 diabetes, the 55 60% carbohydrate content of previous diets has been reduced considerably because of the tendency of high carbohydrate intake to cause hyperglycemia, hypertriglyceridemia, and a lowered HDL cholesterol. In obese type 2 patients, glucose and lipid goals join weight loss as the focus of therapy. These patients are advised to limit their carbohydrate content by substituting noncholesterologenic monounsaturated oils such as olive oil, rapeseed (canola) oil, or the oils in nuts and avocados. This maneuver is also indicated in type 1 patients on intensive insulin regimens in whom near-normoglycemic control is less achievable on higher carbohydrate diets. They should be taught carbohydrate counting so they can administer 1 unit of regular insulin or short-acting insulin analog for each 10 or 15 g of carbohydrate eaten at a meal. In these patients, the ratio of carbohydrate to fat will vary among individuals in relation to their glycemic responses, insulin regimens, and exercise pattern.
The current recommendations for both types of diabetes continue to limit cholesterol to 300 mg daily and advise a daily protein intake of 10 20% of total calories. They suggest that saturated fat be no higher than 8 9% of total calories with a similar proportion of polyunsaturated fat and that the remainder of caloric needs be made up of an individualized ratio of monounsaturated fat and of carbohydrate containing 20 35 g of dietary fiber. Poultry, veal, and fish continue to be recommended as a substitute for red meats for keeping saturated fat content low. The present ADA position statement proffers no evidence that reducing protein intake below 10% of intake (about 0.8 g/kg/d) is of any benefit in patients with nephropathy and renal impairment, and doing so may be detrimental.
Exchange lists for meal planning can be obtained from the American Diabetes Association and its affiliate associations or from the American Dietetic Association, 216 W. Jackson Blvd., Chicago, IL 60606 (312 899-0040). Their Internet address is http://www.eatright.org.
2. Dietary fiber
Plant components such as cellulose, gum, and pectin are indigestible by humans and are termed dietary fiber. Insoluble fibers such as cellulose or hemicellulose, as found in bran, tend to increase intestinal transit and may have beneficial effects on colonic function. In contrast, soluble fibers such as gums and pectins, as found in beans, oatmeal, or apple skin, tend to retard nutrient absorption rates so that glucose absorption is slower and hyperglycemia may be slightly diminished. Although its recommendations do not include insoluble fiber supplements such as added bran, the ADA recommends food such as oatmeal, cereals, and beans with relatively high soluble fiber content as staple components of the diet in diabetics. High soluble fiber content in the diet may also have a favorable effect on blood cholesterol levels.
3. Artificial sweeteners
Aspartame (NutraSweet) has proved to be a popular sweetener for diabetic patients. It consists of two amino acids (aspartic acid and
P.1231
phenylalanine) that combine to produce a nutritive sweetener 180 times as sweet as sucrose. A major limitation is that it cannot be used in baking or cooking because of its lability to heat.
The nonnutritive sweetener saccharin continues to be available in certain foods and beverages despite warnings by the Food and Drug Administration (FDA) about its potential long-term carcinogenicity to the bladder. The latest position statement of the ADA concludes that all nonnutritive sweeteners that have been approved by the FDA (such as aspartame and saccharin) are safe for consumption by all people with diabetes. Two other nonnutritive sweeteners have been approved by the FDA as safe for general use: sucralose (Splenda) and acesulfame potassium (Sunett, Sweet One, DiabetiSweet). These are both highly stable and, in contrast to aspartame, can be used in cooking and baking.
Nutritive sweeteners such as sorbitol and fructose have increased in popularity. Except for acute diarrhea induced by ingestion of large amounts of sorbitol-containing foods, their relative risk has yet to be established. Fructose represents a natural sugar substance that is a highly effective sweetener and induces only slight increases in plasma glucose levels. However, because of potential adverse effects of large amounts of fructose (up to 20% of total calories) on raising serum cholesterol and LDL cholesterol, the ADA feels it may have no overall advantage as a sweetening agent in the diabetic diet. This does not preclude, however, ingestion of fructose-containing fruits and vegetables or fructose-sweetened foods in moderation.
B. Drugs for Treating Hyperglycemia
(Table 27-7.) The drugs for treating type 2 diabetes fall into several categories: (1) Drugs that primarily stimulate insulin secretion by binding to the sulfonylurea receptor. Sulfonylureas remain the most widely prescribed drugs for treating hyperglycemia. The meglitinide analog repaglinide and the D-phenylalanine derivative nateglinide also bind the sulfonylurea receptor and stimulate insulin secretion. (2) Drugs that alter insulin action: Metformin works in the liver. The thiazolidinediones appear to have their main effect on skeletal muscle and adipose tissue. (3) Drugs that principally affect absorption of glucose: The -glucosidase inhibitors acarbose and miglitol are such currently available drugs. (4) Drugs that mimic incretin effect or prolong incretin action: Exenatide and DPP 1V inhibitors fall into this category. (5) Other: Pramlintide lowers glucose by suppressing glucagon and slowing gastric emptying.
1. Drugs that primarily stimulate insulin secretion by binding to the sulfonylurea receptor on the beta cell
a. Sulfonylureas
The primary mechanism of action of the sulfonylureas is to stimulate insulin release from pancreatic B cells. Specific receptors on the surface of pancreatic B cells bind sulfonylureas in the rank order of their insulinotropic potency (glyburide with the greatest affinity and tolbutamide with the least affinity). It has been shown that activation of these receptors closes potassium channels, resulting in depolarization of the B cell. This depolarized state permits calcium to enter the cell and actively promote insulin release.
Sulfonylureas are not indicated for use in type 1 diabetes patients since these drugs require functioning pancreatic B cells to produce their effect on blood glucose. These drugs are used in patients with type 2 diabetes, in whom acute administration improves the early phase of insulin release that is refractory to acute glucose stimulation. Sulfonylureas are metabolized by the liver and apart from acetohexamide, whose metabolite is more active than the parent compound, the metabolites of all the other sulfonylureas are weakly active or inactive. The metabolites are excreted by the kidney and, in the case of the second-generation sulfonylureas, partly excreted in the bile. Sulfonylureas are generally contraindicated in patients with severe hepatic or renal impairment. Idiosyncratic reactions are rare, with skin rashes or hematologic toxicity (leukopenia, thrombocytopenia) occurring in less than 0.1% of users.
(1) First-generation sulfonylureas (tolbutamide, tolazamide, acetohexamide, chlorpropamide)
Tolbutamide is supplied as 500-mg tablets. It is rapidly oxidized in the liver to inactive metabolites, and its approximate duration of effect is relatively short (6 10 hours). Tolbutamide is probably best administered in divided doses (eg, 500 mg before each meal and at bedtime); however, some patients require only one or two tablets daily with a maximum dose of 3000 mg/d. Because of its short duration of action, which is independent of renal function, tolbutamide is probably the safest sulfonylurea to use if liver function is normal. Prolonged hypoglycemia has been reported rarely with tolbutamide, mostly in patients receiving certain antibacterial sulfonamides (sulfisoxazole), phenylbutazone for arthralgias, or the oral azole antifungal drugs to treat candidiasis. These drugs apparently compete with tolbutamide for oxidative enzyme systems in the liver, resulting in maintenance of high levels of unmetabolized, active sulfonylurea in the circulation.
Tolazamide is supplied in tablets of 100, 250, and 500 mg. It has a longer duration of action than tolbutamide, lasting up to 20 hours, with maximal hypoglycemic effect occurring between the fourth and fourteenth hours. It is often effective, as are other longer-acting sulfonylureas also, when tolbutamide fails to correct prebreakfast hyperglycemia. Tolazamide is metabolized to several compounds that retain hypoglycemic effects. If more than 500 mg/d is required, the dose should be divided and given twice daily. Doses larger than 1000 mg daily do not improve the degree of glycemic control.
Table 27-7. Drugs for treatment of type 2 diabetes mellitus. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acetohexamide and chlorpropamide are now rarely used. Chlorpropamide has a prolonged biologic effect, and severe hypoglycemia can occur especially in the elderly
P.1232
P.1233
as their renal clearance declines with aging. Its other side effects include alcohol-induced flushing and hyponatremia due to its effect on vasopressin secretion and action.
(2) Second-generation sulfonylureas (glyburide, glipizide, gliclazide, glimepiride)
Glyburide, glipizide, gliclazide, and glimepiride are 100 200 times more potent than tolbutamide. These drugs should be used with caution in patients with cardiovascular disease or in elderly patients, in whom prolonged hypoglycemia would be especially dangerous.
Glyburide is available in 1.25-mg, 2.5-mg, and 5-mg tablets. The usual starting dose is 2.5 mg/d, and the average maintenance dose is 5 10 mg/d given as a single morning dose; maintenance doses higher than 20 mg/d are not recommended. Some reports suggest that 10 mg is a maximum daily therapeutic dose, with 15 20 mg having no additional benefit in poor responders and doses over 20 mg actually worsening hyperglycemia. Glyburide is metabolized in the liver into products with hypoglycemic activity, which probably explains why assays specific for the unmetabolized compound suggest a plasma half-life of only 1 2 hours, yet the biologic effects of glyburide are clearly persistent 24 hours after a single morning dose in diabetic patients. Glyburide is unique among sulfonylureas in that it not only binds to the pancreatic B cell membrane sulfonylurea receptor but also becomes sequestered within the B cell. This may also contribute to its prolonged biologic effect despite its relatively short circulating half-life. A Press Tab formulation of micronized glyburide easy to divide in half with slight pressure if necessary is available in tablet sizes of 1.5 mg, 3 mg, and 6 mg.
Glyburide has few adverse effects other than its potential for causing hypoglycemia, which at times can be prolonged. Flushing has rarely been reported after ethanol ingestion. It does not cause water retention, as chlorpropamide does, but rather slightly enhances free water clearance. Glyburide is absolutely contraindicated in the presence of hepatic impairment and should not be used in patients with renal insufficiency, in elderly patients, or in those who would be put at serious risk from an episode of hypoglycemia.
Glipizide is available in 5-mg and 10-mg tablets. For maximum effect in reducing postprandial hyperglycemia, this agent should be ingested 30 minutes before meals, since rapid absorption is delayed when the drug is taken with food. The recommended starting dose is 5 mg/d, with up to 15 mg/d given as a single daily dose before breakfast. When higher daily doses are required, they should be divided and given before meals. The maximum dose recommended by the manufacturer is 40 mg/d, although doses above 10 15 mg probably provide little additional benefit in poor responders and may even be less effective than smaller doses.
At least 90% of glipizide is metabolized in the liver to inactive products, and 10% is excreted unchanged in the urine. Glipizide therapy is therefore contraindicated in patients with hepatic or renal impairment, who would be at high risk for hypoglycemia; but because of its lower potency and shorter duration of action, it is preferable to glyburide in elderly patients. Glipizide has also been marketed as Glucotrol-XL in 5-mg and 10-mg tablets. It provides extended release during transit through the gastrointestinal tract with greater effectiveness in lowering prebreakfast hyperglycemia than the shorter-duration immediate-release standard glipizide tablets. However, this formulation appears to have sacrificed its lower propensity for severe hypoglycemia compared with longer-acting glyburide without showing any demonstrable therapeutic advantages over glyburide.
Gliclazide (not available in the United States) is another intermediate duration sulfonylurea with a duration of action of about 12 hours. It is available as 80 mg tablets. The recommended starting dose is 40 80 mg/d with a maximum dose of 320 mg. Doses of 160 mg and above are given as divided doses before breakfast and dinner. The drug is metabolized by the liver; the metabolites and conjugates have no hypoglycemic effect. An extended release preparation is available.
Glimepiride is given once daily as monotherapy or in combination with insulin to lower blood glucose in diabetes patients who cannot control their glucose level through diet and exercise. Glimepiride achieves blood glucose lowering with the lowest dose of any sulfonylurea compound, and this tends to increase its cost-effectiveness. A single daily dose of 1 mg/d has been shown to be effective, and the maximal recommended dose is 8 mg. It has a long duration of action with a pharmacodynamic half-life of 5 hours, allowing once-daily administration, which improves compliance. It is completely metabolized by the liver to relatively inactive metabolic products.
b. Meglitinide analogs
Repaglinide is structurally similar to glyburide but lacks the sulfonic acid-urea moiety. It acts by binding to the sulfonylurea receptor and closing the ATP-sensitive potassium channel. It is rapidly absorbed from the intestine and then undergoes complete metabolism in the liver to inactive biliary products, giving it a plasma half-life of less than 1 hour. The drug therefore causes a brief but rapid pulse of insulin. The starting dose is 0.5 mg three times a day 15 minutes before each meal. The dose can be titrated to a maximal daily dose of 16 mg. Like the sulfonylureas, repaglinide can be used in combination with metformin. Hypoglycemia is the main side effect. In clinical trials, when the drug was compared with a long-duration sulfonylurea (glyburide), there was a trend toward less hypoglycemia. Like the sulfonylureas also, repaglinide causes weight gain. Metabolism is by cytochrome P450 3A4 isoenzyme, and other drugs that induce or inhibit this isoenzyme may increase or inhibit (respectively) the metabolism of repaglinide. The drug may be useful in patients with renal impairment or in the elderly. It remains to be shown that this drug has significant advantages over short-acting sulfonylureas.
c. D-Phenylalanine derivative
Nateglinide stimulates insulin secretion by binding to the sulfonylurea
P.1234
receptor and closing the ATP-sensitive potassium channel. This compound is rapidly absorbed from the intestine, reaching peak plasma levels within 1 hour. It is metabolized in the liver and has a plasma half-life of about 1.5 hours. Like repaglinide, it causes a brief rapid pulse of insulin, and when given before a meal it reduces the postprandial rise in blood glucose. The drug is available as 60-mg and 120-mg tablets. The 60-mg dose is used in patients who have mild elevations in HbA1c. For most patients, the recommended starting and maintenance dose is 120 mg three times a day before meals. Like the other insulin secretagogues, its main side effects are hypoglycemia and weight gain. This drug has been approved for use either alone or in combination with metformin.
2. Drugs that alter insulin action
a. Metformin
Metformin (1,1-dimethylbiguanide hydrochloride) is used, either alone or in conjunction with other oral agents or insulin, in the treatment of patients with type 2 diabetes.
Metformin's primary action is on the liver, reducing hepatic gluconeogenesis by activating adenosine monophosphate-activated protein kinase (AMPK), which acts as an intracellular energy sensor, and has a critical role regulating gluconeogenesis. LKB1 is a protein threonine kinase that phosphorylates and activates AMPK; it was recently reported that deletion of LKB1 function in the liver results in hyperglycemia with increased gluconeogenic and lipogenic gene expression. The deletion of LKB1 also eliminated the glucose lowering effect of metformin, providing genetic proof for the hypothesis that metformin lowers glucose levels by AMP kinase activation.
Metformin has a half-life of 1.5 3 hours, is not bound to plasma proteins, and is not metabolized in humans, being excreted unchanged by the kidneys.
Metformin may be used as an adjunct to diet for the control of hyperglycemia and its associated symptoms in patients with type 2 diabetes, particularly those who are obese or are not responding optimally to maximal doses of sulfonylureas. A side benefit of metformin therapy is its tendency to improve both fasting and postprandial hyperglycemia and hypertriglyceridemia in obese diabetics without the weight gain associated with insulin or sulfonylurea therapy. Metformin is not indicated for patients with type 1 diabetes and is contraindicated in diabetics with serum creatinine levels of 1.5 mg/dL or higher, hepatic insufficiency, alcoholism, or a propensity to develop tissue hypoxia.
Metformin is dispensed as 500 mg, 850 mg, and 1000 mg tablets. A 500 mg extended-release preparation is also available. Although the maximal dosage is 2.55 g, little benefit is seen above a total dose of 2000 mg. It is important to begin with a low dose and increase the dosage very gradually in divided doses taken with meals to reduce minor gastrointestinal upsets. A common schedule would be one 500 mg tablet three times a day with meals or one 850 mg or 1000 mg tablet twice daily at breakfast and dinner. One to four tablets of the extended-release preparation can be given once a day.
The most frequent side effects of metformin are gastrointestinal symptoms (anorexia, nausea, vomiting, abdominal discomfort, diarrhea), which occur in up to 20% of patients. These effects are dose-related, tend to occur at onset of therapy, and often are transient. However, in 3 5% of patients, therapy may have to be discontinued because of persistent diarrheal discomfort.
Hypoglycemia does not occur with therapeutic doses of metformin, which permits its description as a euglycemic or antihyperglycemic drug rather than an oral hypoglycemic agent. Dermatologic or hematologic toxicity is rare.
Lactic acidosis has been reported as a side effect but is uncommon with metformin in contrast to phenformin. While therapeutic doses of metformin reduce lactate uptake by the liver, serum lactate levels rise only minimally if at all, since other organs such as the kidney can remove the slight excess. However, if tissue hypoxia occurs, the metformin-treated patient is at higher risk for lactic acidosis due to compromised lactate removal. Similarly, when renal function deteriorates, affecting not only lactate removal by the kidney but also metformin excretion, plasma levels of metformin rise far above the therapeutic range and block hepatic uptake enough to provoke lactic acidosis without associated increases in lactic acid production. Almost all reported cases have involved subjects with associated risk factors that should have contraindicated its use (renal, hepatic, or cardiorespiratory insufficiency, alcoholism, advanced age). Acute renal failure can occur rarely in certain patients receiving radiocontrast agents. Metformin therapy should therefore be temporarily halted on the day of the test and for 2 days following injection of radiocontrast agents to avoid potential lactic acidosis if renal failure occurs.
b. Thiazolidinediones
Drugs of this class of antihyperglycemic agents sensitize peripheral tissues to insulin. They bind a nuclear receptor called peroxisome proliferator-activated receptor gamma (PPAR- ) and affect the expression of a number of genes and regulate the release of the adipokines resistin and adiponectin from adipocytes. Adiponectin secretion is stimulated, which sensitizes tissues to the effects of insulin, and resistin secretion is inhibited, which reduces insulin resistance. Observed effects of thiazolidinediones include increased glucose transporter expression (GLUT 1 and GLUT 4), decreased free fatty acid levels, decreased hepatic glucose output, and increased differentiation of preadipocytes into adipocytes. Like the biguanides, this class of drugs does not cause hypoglycemia. Troglitazone, the first drug in this class to go into widespread clinical use, has been withdrawn from clinical use because of drug-associated fatal liver failure.
Two other drugs in the same class are available for clinical use: rosiglitazone and pioglitazone. Both are effective as monotherapy and in combination with sulfonylureas or metformin or insulin. When used as monotherapy,
P.1235
these drugs lower HbA1c by about 1 or 2 percentage points. When used in combination with insulin, they can result in a 30 50% reduction in insulin dosage, and some patients can come off insulin completely. The combination of a thiazolidinedione and metformin has the advantage of not causing hypoglycemia. Patients inadequately managed on sulfonylureas can do well on a combination of sulfonylurea and rosiglitazone or pioglitazone. About 25% of patients in clinical trials fail to respond to these drugs, presumably because they are significantly insulinopenic.
The thiazolidinediones not only lower glucose but also have effects on lipids and other cardiovascular risk factors. Rosiglitazone therapy is associated with increases in total cholesterol, LDL-cholesterol (15%), and HDL-cholesterol (10%). There is a reduction in free fatty acids of about 8 15%. The changes in triglycerides were generally not different from placebo. The increase in the LDL-cholesterol need not necessarily be detrimental studies with troglitazone showed that there is a shift from the atherogenic small dense LDL particles to larger, less dense LDL particles. Pioglitazone in clinical trials lowered triglycerides (9%) and increased HDL-cholesterol (15%) but did not cause a consistent change in total cholesterol and LDL-cholesterol levels. A prospective randomized comparison of the metabolic effects of pioglitazone and rosiglitazone on patients who had previously taken troglitazone showed similar effects on HbA1c and weight gain. Pioglitazone-treated subjects, however, had lower total cholesterol, LDL-cholesterol, and triglycerides when compared with rosiglitazone. The thiazolidinediones have also been demonstrated to decrease levels of plasminogen activator inhibitor type 1, matrix metalloproteinase 9, C-reactive protein, and interleukin 6. Small prospective studies have also demonstrated that treatment with these drugs lead to improvements in the biochemical and histologic features of nonalcoholic fatty liver disease. These effects make these drugs particularly beneficial for patients with the metabolic syndrome. The thiazolidinediones also may limit vascular smooth muscle proliferation after injury, and there are reports that troglitazone and piogliotazone reduce neointimal proliferation after coronary stent placement. Also, in one double-blind, placebo-controlled study, rosiglitazone was shown to be associated with a decrease in the ratio of urinary albumin to creatinine excretion.
Anemia occurs in 4% of patients treated with these drugs, but this effect may be due to a dilutional effect of increased plasma volume rather than a reduction in red cell mass. Weight gain occurs especially when the drug is combined with a sulfonylurea or insulin. Edema occurs in about 3 4% of patients receiving monotherapy with rosiglitazone or pioglitazone. The edema occurs more frequently (10 15%) in patients receiving concomitant insulin therapy and may result in congestive heart failure. The drugs are contraindicated in diabetic individuals with New York Heart Association class III and IV cardiac status. Rosiglitazone has recently been reported as being associated with new onset or worsening macular edema. Apparently, this is a rare side effect and most of these patients also had peripheral edema. The macular edema resolved or improved once the drug was discontinued. The dosage of rosiglitazone is 4 8 mg daily and of pioglitazone 15 45 mg daily, and the drugs do not have to be taken with food. Rosiglitazone is primarily metabolized by the CYP 2C8 isoenzyme and pioglitazone is metabolized by CYP 2C8 and CYP 3A4.
These two agents have so far not (unlike troglitazone) caused drug-induced hepatotoxicity. The FDA has, however, recommended that patients should not initiate drug therapy if there is clinical evidence of active liver disease or the alanine aminotransferase (ALT) level is 2.5 times greater than the upper limit of normal. Obviously, caution should be used in initiation of therapy in patients with even mild ALT elevations. Liver function tests should be performed prior to initiation of treatment and periodically thereafter.
3. Drugs that affect absorption of glucose
-Glucosidase inhibitors competitively inhibit the -glucosidase enzymes in the gut that digest dietary starch and sucrose. Two of these drugs acarbose and miglitol are available for clinical use. Both are potent inhibitors of glucoamylase, -amylase, and sucrase but have less effect on isomaltase and hardly any on trehalase and lactase. Acarbose binds 1000 times more avidly to the intestinal disaccharidases than do products of carbohydrate digestion or sucrose. A fundamental difference between acarbose and miglitol is in their absorption. Acarbose has the molecular mass and structural features of a tetrasaccharide, and very little (about 2%) crosses the microvillar membrane. Miglitol, however, has a structural similarity with glucose and is absorbable. Both drugs delay the absorption of carbohydrate and lower postprandial glycemic excursion.
a. Acarbose
Acarbose is available as 50-mg and 100-mg tablets. The recommended starting dose of acarbose is 50 mg twice daily, gradually increasing to 100 mg three times daily. For maximal benefit on postprandial hyperglycemia, acarbose should be given with the first mouthful of food ingested. In diabetic patients, it reduces postprandial hyperglycemia by 30 50%, and its overall effect is to lower the HbA1c by 0.5 1%.
The principal adverse effect, seen in 20 30% of patients, is flatulence. This is caused by undigested carbohydrate reaching the lower bowel, where gases are produced by bacterial flora. In 3% of cases, troublesome diarrhea occurs. This gastrointestinal discomfort tends to discourage excessive carbohydrate consumption and promotes improved compliance of type 2 patients with their diet prescriptions. When acarbose is given alone, there is no risk of hypoglycemia. However, if combined with insulin or sulfonylureas, it might increase the risk of hypoglycemia from these agents. A slight rise in hepatic aminotransferases has been noted in clinical trials with acarbose (5% versus 2% in placebo controls, and particularly with doses > 300 mg/d). The levels generally return to normal on stopping the drug.
In the UKPDS, approximately 2000 patients on diet, sulfonylurea, metformin, or insulin therapy were
P.1236
randomized to acarbose or placebo therapy. By 3 years, 60% of the patients had discontinued the drug, mostly because of gastrointestinal symptoms. If one looked only at the 40% who remained on the drug, they had an 0.5% lower HbA1c compared with placebo.
b. Miglitol
Miglitol is similar to acarbose in terms of its clinical effects. It is indicated for use in diet- or sulfonylurea-treated patients with type 2 diabetes. Therapy is initiated at the lowest effective dosage of 25 mg three times a day. The usual maintenance dose is 50 mg three times a day, although some patients may benefit from increasing the dose to 100 mg three times a day. Gastrointestinal side effects occur as with acarbose. The drug is not metabolized and is excreted unchanged by the kidney. Theoretically, absorbable -glucosidase inhibitors could induce a deficiency of one or more of the -glucosidases involved in cellular glycogen metabolism and biosynthesis of glycoproteins. This does not occur in practice because, unlike the intestinal mucosa, which sees a high concentration of the drug, the blood level is 200-fold to 1000-fold lower than the concentration needed to inhibit intracellular -glucosidases. Miglitol should not be used in renal failure, when its clearance would be impaired.
4. Incretins
Oral glucose provokes a threefold to fourfold higher insulin response than an equivalent dose of glucose given intravenously. This is because the oral glucose causes a release of gut hormones, principally glucagon-like peptide 1 (GLP-1) and glucose dependent insulinotropic polypeptide (GIP1), that amplify the glucose-induced insulin release. This incretin effect is reduced in patients with type 2 diabetes. GLP-1 secretion (but not GIP1 secretion) is impaired in patients with type 2 diabetes and when GLP-1 is infused in patients with type 2 diabetes, it stimulates insulin secretion and lowers glucose levels. GLP-1, unlike the sulfonylureas, has only a modest insulin stimulatory effect at normoglycemic concentrations. This means that GLP-1 has a lower risk for hypoglycemia than the sulfonylureas.
In addition to its insulin stimulatory effect, GLP-1 also has a number of other pancreatic and extrapancreatic effects. It suppresses glucagon secretion and so may ameliorate the hyperglucagonemia that is present in people with diabetes and improve postprandial hyperglycemia. GLP-1 preserves islet integrity and reduces apoptotic cell death of human islet cells in culture. In mice, streptozotocin-induced apoptosis is significantly reduced by coadministration of exendin-4 or exenatide, a GLP-1 receptor agonist. GLP-1 acts on the stomach delaying gastric emptying; the importance of this effect on glucose lowering is illustrated by the observation that antagonizing the deceleration of gastric emptying markedly reduces the glucose lowering effect of GLP-1. GLP-1 receptors are present in the central nervous system, and intracerebroventricular administration of GLP-1 in wild type mice, but not in GLP-1 receptor knockout mice, inhibits feeding. Type 2 diabetic patients undergoing GLP-1 infusion are less hungry; it is unclear whether this is mainly due to a deceleration of gastric emptying or whether there is a central nervous system effect as well.
a. Exenatide
GLP-1 is rapidly proteolysed by dipeptidyl peptidase IV (DPP IV), and therefore for clinical effect would need to be administered as a continuous infusion. Exendin 4 or exenatide is a GLP-1 receptor agonist isolated from the saliva of the Gila Monster (a venomous lizard) that is more resistant to DPP IV action and, when given to type 2 diabetics by subcutaneous injection twice a day, lowers blood glucose and HbA1c levels. Exenatide appears to have the same effects as GLP-1 on glucagon suppression and gastric emptying. In clinical trials, adding exenatide therapy to patients with type 2 diabetes already taking metformin or a sulfonylurea, or both, further lowered the HbA1c value by 0.4% to 0.6% over a 30-week period. These patients also experienced a weight loss of 3 6 pounds. In an open label extension study up to 80 weeks, the HbA1c reduction was sustained and there was further weight loss (to a total loss of about 10 pounds). The main side effect was nausea, affecting over 40% of the patients. The nausea was dose-dependent and declined with time. The risk of hypoglycemia was higher in persons taking sulfonylureas. Exenatide is dispensed as two fixed-dose pens (5 mcg and 10 mcg). It is injected 60 minutes before breakfast and before dinner. Patients should be prescribed the 5 mcg pen for the first month and, if tolerated, the dose can then be increased to 10 mcg twice a day. The drug is less stable than insulin and needs to be refrigerated between injections.
b. Oral DPP IV inhibitors
These agents, which work by prolonging the action of endogenously released GLP-1, are in clinical trials for use in type 2 diabetes.
5. Others
Pramlintide is a synthetic analog of islet amyloid polypeptide (IAPP or amylin). When given subcutaneously, it delays gastric emptying, suppresses glucagon secretion, and decreases appetite. It is approved for use both in type 1 diabetes and in insulin-treated type 2 diabetes. In 6-month clinical studies with type 1 and insulin-treated type 2 patients, those on the drug had an approximately 0.4% reduction in HbA1c and about 1.7 kg weight loss compared with placebo. The HbA1c reduction was sustained for 2 years but some of the weight was regained. The drug is given by injection immediately before the meal. Hypoglycemia can occur, and it is recommended that the short-acting or premixed insulin doses be reduced by 50% when the drug is started. Nausea was the other main side effect, affecting 30 50% of persons but tended to improve with time. In patients with type 1 diabetes, the initial dose of pramlintide is 15 mcg before each meal and titrated up by 15 mcg increments to a maintenance dose of 30 mcg or 60 mcg before each meal. In patients with type 2 diabetes, the starting dose is 60 mcg premeals increased to 120 mcg in 3 to 7 days if no significant nausea occurs.
P.1237
6. Drug combinations
Several drug combinations are available in different dose sizes, including glyburide and metformin (Glucovance); glipizide and metformin (Metaglip); rosiglitazone and metformin (Avandamet); pioglitazone and metformin (ACTOplus Met); and rosiglitazone and glimepiride (Avandaryl). These drug combinations, however, limit the clinician's ability to optimally adjust dosage of the individual drugs and for that reason are not recommended.
7. Safety of the antihyperglycemic agents
The UKPDS has put to rest previous concerns regarding the safety of sulfonylureas. It did not confirm any cardiovascular hazard among over 1500 patients treated intensively with sulfonylureas for over 10 years, compared with a comparable number who received either insulin or diet therapy. Analysis of a subgroup of obese patients receiving metformin also showed no hazard and even a slight reduction in cardiovascular deaths compared with conventional therapy.
The currently available thiazolidinediones have not to date exhibited the idiosyncratic hepatotoxicity seen with troglitazone. However, these drugs can precipitate congestive heart failure and should not be used in patients with New York Heart Association class III and IV cardiac status. Lactic acidosis from metformin (see above) is quite rare and probably not a major problem with its use in the absence of major risk factors such as impaired renal or hepatic disease or conditions predisposing to hypoxia.
D. Insulin
Insulin is indicated for type 1 diabetes as well as for type 2 diabetic patients with insulinopenia whose hyperglycemia does not respond to diet therapy either alone or combined with other hypoglycemic drugs.
With the development of highly purified human insulin preparations, immunogenicity has been markedly reduced, thereby decreasing the incidence of therapeutic complications such as insulin allergy, immune insulin resistance, and localized lipoatrophy at the injection site. However, the problem of achieving optimal insulin delivery remains unsolved with the present state of technology. It has not been possible to reproduce the physiologic patterns of intraportal insulin secretion with subcutaneous injections of short-acting or longer-acting insulin preparations. Even so, with the help of appropriate modifications of diet and exercise and careful monitoring of capillary blood glucose levels at home, it has often been possible to achieve acceptable control of blood glucose by using various mixtures of short- and longer-acting insulins injected at least twice daily or portable insulin infusion pumps.
1. Characteristics of available insulin preparations
Commercial insulin preparations differ with respect to the time of onset and duration of their biologic action (Table 27-8).
a. Species of insulin
Human insulin is produced by recombinant DNA techniques (biosynthetic human insulin) as Humulin (Eli Lilly) and as Novolin (Novo Nordisk). It is dispensed as either regular (R) or NPH (N) formulations. Five analogs of human insulin three rapidly acting (insulin lispro, insulin aspart, insulin glulisine) and two long-acting (insulin glargine and insulin detemir) have been approved by the FDA for clinical use (see below) (Table 27-9). Animal insulins are no longer available in the United States.
b. Purity of insulin
Purified insulin is defined by FDA regulations as the degree of purity wherein proinsulin contamination is less than 10 ppm. All insulins presently available contain less than 10 ppm of proinsulin and are labeled as purified. These purified insulins seem to preserve their potency quite well, so that refrigeration is recommended but not crucial. During travel, reserve supplies of insulin can thus be readily transported for weeks without losing potency if protected from extremes of heat or cold.
c. Concentration of insulin
At present, insulins in the United States are available in a concentration of 100 units/mL (U100), and all are dispensed in 10-mL vials. With the popularity of low-dose (0.5- or 0.3-mL) disposable insulin syringes, U100 can be measured with acceptable accuracy in doses as low as 1 2 units. For use in rare cases of severe insulin resistance in which large quantities of insulin are required, U500 regular human insulin (Humulin R) is available from Eli Lilly.
2. Insulin preparations
Four principal types of insulins are available: (1) rapid-acting insulin analogs with more rapid onset and a shorter duration of action than regular insulin after subcutaneous injection;
P.1238
(2) short-acting regular insulin; (3) intermediate-acting; and (4) long-acting, with slow onset of action (Table 27-9 and Figure 27-1). Rapid-acting insulin analogs and regular insulin are dispensed as clear solutions at neutral pH and contain small amounts of zinc to improve their stability and shelf life. The long-acting insulin analogs are also dispensed as clear solutions; insulin glargine is at acidic pH and insulin detemir is at neutral pH. NPH insulin is dispensed as a turbid suspension at neutral pH with protamine in phosphate buffer. The Lente series of insulin (ultralente and lente) are no longer available in the United States. The rapid-acting insulin analogs, intermediate-acting, and long-acting insulins are designed for subcutaneous administration, while regular insulin can also be given intravenously. Insulin aspart has been approved for intravenous use, but there is no advantage in using this insulin over regular for this purpose.
Table 27-8. Summary of bioavailability characteristics of the insulins. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 27-9. Insulin preparations available in the United States.1 | ||
|---|---|---|
|
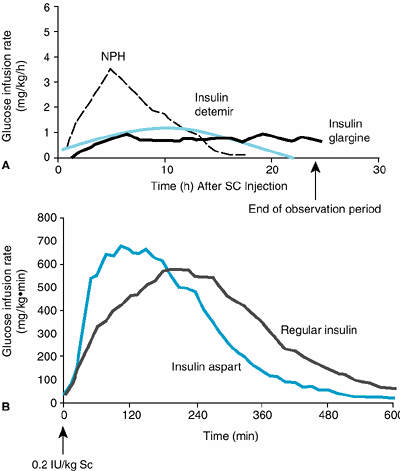 |
Figure 27-1. Extent and duration of action of various types of insulin-euglycemic hyperinsulinemic clamps in normal volunteers. A: Intermediate neutral protamine Hagedorn (NPH) insulin and long-acting insulin analogs. B: Regular insulin and rapid-acting insulin analogs. |
a. Rapid-acting insulin analogs
Insulin lispro (Humalog) is an insulin analog produced by recombinant technology, wherein two amino acids near the carboxyl terminal of the B chain have been reversed in position: Proline at position B28 has been moved to B29 and lysine has been moved from B29 to B28. Insulin aspart (Novolog) is a single substitution of proline by aspartic acid at position B28. Insulin glulisine (Apidra) differs from human insulin in that the amino acid asparagine at position B3 is replaced by lysine and the lysine in position B29 by glutamic acid. These changes result in these three analogs having less tendency to form hexamers, in contrast to human insulin. When injected subcutaneously, the analogs quickly dissociate into monomers and are absorbed very rapidly, reaching peak serum values in as soon as 1 hour in contrast to regular human insulin, whose hexamers require considerably more time to dissociate and become absorbed. The amino acid changes in these analogs do not interfere with their binding to the insulin receptor, with the circulating half-life, or with their immunogenicity, which are all identical with those of human regular insulin.
Clinical trials have demonstrated that the optimal times of preprandial subcutaneous injection of comparable doses of the rapid-acting insulin analogs and of regular human insulin are 20 minutes and 60 minutes, respectively, before the meal. While this more rapid onset of action has been welcomed as a great convenience by diabetic patients who object to waiting as long as 60 minutes after injecting regular human insulin before they can begin their meal, patients must be taught to ingest adequate absorbable carbohydrate early in the meal to avoid hypoglycemia during the meal. Another desirable feature of insulin lispro is that its duration of action remains at about 4 hours irrespective of dosage. This contrasts with regular insulin, whose duration of action is prolonged when larger doses are used.
The rapid-acting analogs are also commonly used in pumps. In a double-blind crossover study comparing insulin lispro with regular insulin in insulin pumps, persons using insulin lispro had lower HbA1c values and improved postprandial glucose control with the same frequency of hypoglycemia. The concern remains that in the event of pump failure, users of the rapid-acting insulin analogs will have more rapid onset of hyperglycemia and ketosis.
b. Short-acting regular insulin
Regular insulin is a short-acting soluble crystalline zinc insulin whose effect appears within 30 minutes after subcutaneous injection and lasts 5 7 hours when usual quantities are administered. Intravenous infusions of regular insulin are particularly useful in the treatment of diabetic ketoacidosis and during the perioperative management of insulin-requiring diabetics. When intravenous insulin is needed for hyperglycemic emergencies, the rapid-acting insulin analogs have no advantage over regular human insulin, which is instantly converted to the monomeric form when given intravenously. Regular insulin is indicated when the subcutaneous insulin requirement is changing rapidly, such as after surgery or during acute infections although the rapid-acting insulin analogs may be preferable in these situations.
For markedly insulin-resistant persons who would otherwise require large volumes of insulin solution, a U500 preparation of human regular insulin is available. Since a U500 syringe is not available, a U100 insulin syringe or tuberculin syringe is used to measure doses. The physician should carefully note dosages in both units and volume to avoid overdosage.
P.1239
c. Intermediate-acting neutral protamine Hagedorn (NPH) insulin
NPH (neutral protamine Hagedorn or isophane) insulin is an intermediate-acting insulin whose onset of action is delayed by combining 2 parts soluble crystalline zinc insulin with 1 part protamine zinc insulin. This produces equivalent amounts of insulin and protamine, so that neither is present in an uncomplexed form ( isophane ).
Its onset of action is delayed to 2 4 hours, and its peak response is generally reached in about 8 10 hours. Because its duration of action is often less than 24 hours (with a range of 10 20 hours), most patients require at least two injections daily to maintain a sustained insulin effect. Occasional vials of NPH insulin have tended to show unusual clumping of their contents or frosting of the container, with considerable loss of bioactivity. This instability is rare and occurs less frequently if NPH human insulin is refrigerated when not in use and if bottles are discarded after 1 month of use.
d. Long-acting insulins
(1) Insulin glargine
This agent is an insulin analog in which the asparagine at position 21 of the A chain of the human insulin molecule is replaced by glycine and two arginines are added to the carboxyl terminal of the B chain. The arginines raise the isoelectric point of the molecule closer to neutral, making it more soluble in an acidic environment. In contrast, human insulin has an isoelectric point of pH 5.4. Insulin glargine is a clear insulin which, when injected into the neutral pH environment of the subcutaneous tissue, forms microprecipitates that slowly release the insulin into the circulation. It lasts for about 24 hours without any pronounced peaks and is given once a day to provide basal coverage. This insulin cannot be mixed with the other human insulins because of its acidic pH. When this insulin was given as a single injection at bedtime to type 1 patients, fasting hyperglycemia was better controlled when compared with bedtime NPH insulin. The clinical trials also suggest that there may be less nocturnal hypoglycemia with this insulin when compared with NPH insulin.
In one clinical trial involving type 2 patients, insulin glargine was associated with a slightly higher progression of retinopathy when compared with NPH insulin. The frequency was 7.5% with the analog and 2.7% with the NPH. This finding, however, was not seen in other clinical trials with this analog. Insulin glargine does have a sixfold greater affinity for IGF-1 receptor compared with the human insulin. There has also been a report that insulin glargine had increased mitogenicity compared with human insulin in a human osteosarcoma
P.1240
cell line. The significance of these observations is not yet clear. Because of lack of safety data, use of insulin glargine during pregnancy is not recommended.
(2) Insulin detemir
This agent is an insulin analog in which the tyrosine at position 30 of the chain has been removed and a 14-C fatty acid chain (tetradecanoic acid) is attached to the lysine at position 29 by acylation. The fatty acid chain makes the molecule more lipophilic than native insulin and the addition of zinc stabilizes the molecule and leads to formation of hexamers. After injection, self-association at the injection site and albumin binding in the circulation via the fatty acid side chain, leads to slower distribution to peripheral target tissues and prolonged duration of action. The affinity of insulin determir is fourfold to fivefold lower than that of human soluble insulin and therefore the U100 formulation of insulin detemir has an insulin concentration of 2400 nmol/mL compared with 600 nmol/mL for NPH. The duration of action for insulin detemir is about 17 hours at therapeutically relevant doses. It is recommended that the insulin be injected once or twice a day to achieve a stable basal coverage. This insulin has been reported to have lower within-subject pharmacodynamic variability compared with NPH insulin and insulin glargine. In vitro studies do not suggest any clinically relevant albumin binding interactions between insulin detemir and fatty acids or protein-bound drugs. Since there is a vast excess (~400,000) of albumin binding sites available in plasma per insulin detemir molecule, it is unlikely that hypoalbuminemic disease states will affect the ratio of bound to free insulin detemir.
e. Mixtures of insulin
Since intermediate insulins require several hours to reach adequate therapeutic levels, their use in patients with type 1 diabetes requires supplements of regular or rapid-acting insulin analogs preprandially. For convenience, regular or rapid-acting insulin analogs and NPH insulin may be mixed together in the same syringe and injected subcutaneously in split dosage before breakfast and supper. It is recommended that the regular insulin or rapid-acting insulin analog be withdrawn first, then the NPH insulin and that the injection be given immediately after loading the syringe. Stable premixed insulins (70% NPH and 30% regular or 50% of each) are available as a convenience to patients who have difficulty mixing insulin because of visual problems or impairment of manual dexterity. Premixed preparations of insulin lispro and NPH insulins are unstable because of exchange of insulin lispro with the human insulin in the protamine complex. Consequently, the soluble component becomes over time a mixture of regular and insulin lispro at varying ratios. In an attempt to remedy this, an intermediate insulin composed of isophane complexes of protamine with insulin lispro was developed called NPL (neutral protamine lispro). This insulin has the same duration of action as NPH insulin. Premixed combinations of NPL and insulin lispro (eg, 75:25, 50:50, and 25:75 of NPL:insulin lispro) have been tested. Both 75% NPL/25% insulin lispro mixture (Humalog Mix 75/25) and 50% NPL/50% insulin lispro mixture (Humalog Mix 50/50) are available for clinical use. Similarly, a 70% insulin aspart protamine/30% insulin aspart (NovoLogMix 70/30) is available. The main advantages of these mixtures is that they can be given within 15 minutes of starting a meal and they are superior in controlling the postprandial glucose rise after a carbohydrate rich meal. These benefits have not translated into improvements in HbA1c levels when compared with the usual 70% NPH/30% regular mixture.
The longer-acting insulin analogs cannot be mixed with either regular insulin or the rapid-acting insulin analogs.
3. Methods of insulin administration
a. Insulin syringes and needles
Plastic disposable syringes are available in 1-mL, 0.5-mL, and 0.3-mL sizes. The low-dose 0.3-mL syringes have become increasingly popular, because many diabetics do not take more than 30 units of insulin in a single injection except in rare instances of extreme insulin resistance. Two lengths of needles are available: short (8 mm) and long (12.7 mm). Long needles are preferable in obese patients to reduce variability of insulin absorption. Ultrafine needles as small as 31 gauge reduce the pain of injections. Disposable syringes may be reused until blunting of the needle occurs (usually after three to five injections). Sterility adequate to avoid infection with reuse appears to be maintained by recapping syringes between uses. Cleansing the needle with alcohol may not be desirable since it can dissolve the silicone coating and can increase the pain of skin puncturing.
Any part of the body covered by loose skin can be used, such as the abdomen, thighs, upper arms, flanks, and upper buttocks. Preparation with alcohol is no longer required prior to injection as long as the skin is clean. Rotation of sites continues to be recommended to avoid delayed absorption when fibrosis or lipohypertrophy occurs from repeated use of a single site. However, considerable variability of absorption rates from different sites, particularly with exercise, may contribute to the instability of glycemic control in certain type 1 patients if injection sites are rotated too frequently in different areas of the body. Consequently, it is best to limit injection sites to a single region of the body and rotate sites within that region. The abdomen is recommended for subcutaneous injections, since regular insulin has been shown to absorb more rapidly from there than from other subcutaneous sites. The effect of anatomic regions appears to be much less pronounced with the analog insulins.
b. Insulin pen injector devices
Insulin pens eliminate the need for carrying insulin vials and syringes. Cartridges of insulin lispro, insulin aspart, insulin glargine, regular insulin, NPH insulin, and 70% NPH/30% regular insulin are available for reusable pens (Novo Nordisk,
P.1241
Becton Dickinson, and Sanofi Aventis pens). Disposable prefilled pens are also available for insulin lispro, NPH, 70% NPH/30% regular, 75% NPL/25% insulin lispro, 50% NPL/50% insulin lispro, and 70% insulin aspart protamine/30% insulin aspart. Thirty-one gauge needles (5, 6, and 8 mm long) for these pens make injections almost painless.
c. Insulin pumps
In the United States, Medtronic Mini-Med, Animas, and Deltec Cozmo insulin infusion pumps are available for subcutaneous delivery of insulin. These pumps are small (about the size of a pager) and very easy to program. They offer many features, including the ability to set a number of different basal rates throughout the 24 hours and to adjust the time over which bolus doses are given. They also are able to detect pressure build-up if the catheter is kinked. Improvements have also been made in the infusion sets. The catheter connecting the insulin reservoir to the subcutaneous cannula can be disconnected, allowing the patient to remove the pump temporarily (eg, for bathing). The great advantage of continuous subcutaneous insulin infusion (CSII) is that it allows for establishment of a basal profile tailored to the patient. The patient therefore is able to eat with less regard to timing because the basal insulin infusion should maintain constant blood glucose between meals. Also the ability to adjust the basal insulin infusion makes it easier for the patient to manage glycemic excursions that occur with exercise.
CSII therapy is appropriate for patients who are motivated, mechanically inclined, educated about diabetes (diet, insulin action, treatment of hypoglycemia and hyperglycemia), and willing to monitor their blood glucose four to six times a day. Known complications of CSII include ketoacidosis, which can occur when insulin delivery is interrupted, and skin infections. Another disadvantage is its cost and the time demanded of physicians and staff in initiating therapy.
d. Inhaled insulin
A novel method for delivering a preprandial powdered form of insulin by inhalation (Exubera) has been approved by the FDA. In the clinical trials that led to the approval of this preparation, approximately 2500 adult patients with type 1 and type 2 diabetes were studied. The inhaled insulin was as effective as subcutaneous regular insulin in controlling postprandial glucose excursions, but the studies did not compare inhaled insulin with the short-acting insulin analogs.
Pharmacokinetic studies of the inhaled preparation show that it is rapidly absorbed and its onset of action is 32 minutes, compared with 48 and 41 minutes for regular insulin and insulin lispro, respectively. However, the metabolic effect is slower, and the duration of action is longer than insulin lispro and comparable to regular insulin. The bioavailability of inhaled insulin is about 10%, and so patients would need to inhale about 300 400 units of insulin a day. Inhaled insulin is administered 10 minutes prior to meals using a combination of 1- and 3-mg unit doses; 1 mg of inhaled insulin is equivalent to 3 units subcutaneous insulin injection.
Because consecutive inhalation of three 1-mg blisters is associated with a 30 40% greater insulin exposure than one 3-mg dose blister, the two regimens are not interchangeable. A small decrease in pulmonary function (forced expiratory volume in 1 second [FEV1] and single-breath diffusing capacity for carbon monoxide [DLCO]) was seen in the first few months of use, but in the phase 3 studies lasting 2 years, patients did not experience clinically significant effects on pulmonary function. The clinical trials excluded patients with pulmonary disorders. Inhaled insulin does result in higher insulin antibody titers than subcutaneous insulin, but the studies so far have not shown any clinical impact on dosage or control. Other side effects associated with Exubera therapy include cough, shortness of breath, sore throat, and dry mouth. Smokers have higher insulin levels than nonsmokers, so this insulin preparation is contraindicated in patients who smoke or who have discontinued smoking for less than 6 months. The FDA has recommended pulmonary function tests at baseline, after 6 months of treatment and every year thereafter, even if there are no pulmonary symptoms.
E. Transplantation
Pancreas transplantation at the time of renal transplantation is becoming more widely accepted. Patients undergoing simultaneous pancreas and kidney transplantation have an 85% chance of pancreatic graft survival and a 92% chance of renal graft survival after 1 year. Solitary pancreatic transplantation in the absence of a need for renal transplantation should be considered only in those rare patients who fail all other insulin therapeutic approaches and who have frequent severe hypoglycemia or who have life-threatening complications related to their lack of metabolic control.
Islet cell transplantation is a minimally invasive procedure, and investigators in Edmonton, Canada, have reported initial insulin independence in a small number of patients with type 1 diabetes who underwent this procedure. Using islets from multiple donors and corticosteroid-free immunosuppression, percutaneous transhepatic portal vein transplantation of islets was achieved in over 20 subjects. Although all of the initial cohort was able to achieve insulin independence posttransplantation (some for more than 2 years of follow-up), a decline in insulin secretion has occurred over time and the subjects have again required supplemental insulin. All patients had complete correction of severe hypoglycemic reactions, leading to a marked improvement in overall quality of life. Even if long-term insulin independence is demonstrated, wide application of this procedure for the treatment of type 1 diabetes is limited by the dependence on multiple donors and the requirement for potent long-term immunotherapy.
General Considerations in Treatment of Diabetes
Insulin-treated patients with diabetes can have a full and satisfying life. However, free diets and unrestricted
P.1242
activity are still not advised. Until new methods of insulin replacement are developed that provide more normal patterns of insulin delivery in response to metabolic demands, multiple feedings with carbohydrate counting will continue to be recommended, and certain occupations potentially hazardous to the patient or others will continue to be prohibited because of risks due to hypoglycemia. The American Diabetic Association can act as a patient advocate in case of employment questions.
Exercise increases the effectiveness of insulin, and moderate exercise is an excellent means of improving utilization of fat and carbohydrate in diabetic patients. A judicious balance of the size and frequency of meals with moderate regular exercise can often stabilize the insulin dosage in diabetics who tend to slip out of control easily. Strenuous exercise can precipitate hypoglycemia in an unprepared patient, and diabetics must therefore be taught to reduce their insulin dosage in anticipation of strenuous activity or to take supplemental carbohydrate. Injection of insulin into a site farthest away from the muscles most involved in exercise may help ameliorate exercise-induced hypoglycemia, since insulin injected in the proximity of exercising muscle may be more rapidly mobilized.
All diabetic patients must receive adequate instruction on personal hygiene, especially with regard to care of the feet, skin, and teeth. All infections (especially pyogenic ones) provoke the release of high levels of insulin antagonists such as catecholamines or glucagon and thus bring about a marked increase in insulin requirements. Supplemental regular insulin is often required to correct hyperglycemia during infection.
Steps in the Management of the Diabetic Patient
A. Diagnostic Examination
Any features of the clinical picture that suggest end-organ insensitivity to insulin, such as visceral obesity, must be identified. The family history should document not only the incidence of diabetes in other members of the family but also the age at onset, whether it was associated with obesity, and whether insulin was required. An attempt should be made to characterize the diabetes as type 1 or type 2, based on the clinical features present and on whether or not ketonuria accompanies the glycosuria. For the occasional patient, measurement of islet cell, glutamic acid decarboxylase (GAD65), insulin antibodies, and ICA 512 antibodies can help distinguish between type 1 and type 2 diabetes. Many patients in whom type 1 diabetes is newly diagnosed still have significant endogenous insulin production, and C peptide levels may not reliably distinguish between type 1 and type 2 diabetes. Other factors that increase cardiac risk, such as smoking history, presence of hypertension or hyperlipidemia, or oral contraceptive pill use, should be recorded.
Laboratory diagnosis should document fasting plasma glucose levels above 126 mg/dL or postprandial values consistently above 200 mg/dL and whether ketonuria accompanies the glycosuria. A glycohemoglobin measurement is useful for assessing the effectiveness of future therapy. Some flexibility of clinical judgment is appropriate when diagnosing diabetes mellitus in the elderly patient with borderline hyperglycemia.
Baseline values include fasting plasma triglycerides, total cholesterol and HDL-cholesterol, electrocardiography, renal function studies, peripheral pulses, and neurologic, podiatric, and ophthalmologic examinations to help guide future assessments.
B. Patient Education (Self-Management Training)
Since diabetes is a lifelong disorder, education of the patient and the family is probably the most important obligation of the clinician who provides initial care. The best persons to manage a disease that is affected so markedly by daily fluctuations in environmental stress, exercise, diet, and infections are the patients themselves and their families. The teaching curriculum should include explanations by the physician or nurse of the nature of diabetes and its potential acute and chronic hazards and how they can be recognized early and prevented or treated. Self-monitoring of blood glucose should be emphasized, especially in insulin-requiring diabetic patients, and instructions must be given on proper testing and recording of data. Patients should be provided with algorithms they can use to adjust the timing and quantity of their insulin dose, food, and exercise in response to measured blood glucose values. The targets for blood glucose control should be elevated appropriately in elderly patients since they have the greatest risk if subjected to hypoglycemia and the least long-term benefit from more rigid glycemic control. Advice on personal hygiene, including detailed instructions on foot care as well as individual instruction on diet and specific hypoglycemic therapy, should be provided. Patients should be told about community agencies, such as Diabetes Association chapters, that can serve as a continuing source of instruction. Finally, vigorous efforts should be made to persuade new diabetics who smoke to give up the habit, since large vessel peripheral vascular disease and debilitating retinopathy are less common in nonsmoking diabetic patients.
C. Therapy
Treatment must be individualized on the basis of the type of diabetes and specific needs of each patient. However, certain general principles of management can be outlined for hyperglycemic states of different types.
1. Type 2 diabetes
a. The obese patient with type 2 diabetes
The most common type of diabetic patient is obese, is non-insulin-dependent, and has hyperglycemia because of insensitivity to normal or elevated circulating levels of insulin.
P.1243
(1) Weight reduction
Treatment is directed toward achieving weight reduction, and prescribing a diet is only one means to this end. Behavior modification to achieve adherence to the diet as well as increased physical activity to expend energy is also required. Cure can be achieved by reducing adipose stores, with consequent restoration of tissue sensitivity to insulin, but weight reduction is hard to achieve and even more difficult to maintain with our current therapies. The presence of diabetes with its added risk factors may motivate the obese diabetic to greater efforts to lose weight. (See also Chapter 29.)
(2) Hypoglycemic agents
If the patient is not able to achieve target glycemic control with weight management and exercise, then pharmacologic therapy is indicated. The choice of initial agent depends on a number of factors, including comorbid conditions, adverse reactions to the medications, ability of the patient to monitor for hypoglycemia, drug cost, and patient and physician preferences. Metformin is advantageous because apart from lowering glucose without the risk of hypoglycemia, it also lowers triglycerides and promotes some modest weight loss. The drug, however, cannot be used in patients with renal failure, and gastrointestinal side effects develop in some patients at even the lowest doses. Thiazolidinediones improve peripheral insulin resistance and lower glucose without causing hypoglycemia. They also have been reported to improve nonalcoholic fatty liver disease, have beneficial effects on the lipid profile and some other cardiovascular risk factors, decrease microalbuminuria, and reduce neointimal tissue hyperplasia after coronary artery stent placement. These drugs, however, can cause fluid retention and are contraindicated in patients with heart failure. They also very commonly increase weight, which patients find distressing, affecting adherence. The drugs are also contraindicated in patients with active liver disease and in patients with liver enzymes 2.5 times the upper limit of normal. Sulfonylureas have been available for many years and their use in combination with metformin is well established. They do, however, have the propensity of causing hypoglycemia and weight gain. The -glucosidase inhibitors have modest glucose lowering effects and have gastrointestinal side effects. Exenatide has a lower risk of hypoglycemia than the sulfonylureas and promotes weight loss. However, it needs to be given by injection, causes nausea, and is contraindicated in patients with gastroparesis. Exenatide is also expensive and lacks long-term safety data.
For most obese patients with mild type 2 diabetes, metformin is the first-line agent. If it proves to be inadequate, then a second agent should be added. In those patients where the problem is hyperglycemia after a carbohydrate rich meal (such as dinner), then a short-acting secretagogue before meals may suffice to get the glucose levels into the target range. Patients with nonalcoholic fatty liver disease or microalbuminuria may be candidates for one of the thiazolidinediones. Subjects who are very concerned about weight gain may benefit from a trial of exenatide. If two agents are inadequate, then a third agent is added, although data regarding efficacy of such combined therapy are limited. Experienced clinicians have found that instead of maximizing the dose of each agent before adding another agent, some patients are more tolerant of submaximal combinations of drugs. Insulin therapy should be instituted if combination of oral agents (and exenatide) fail to restore euglycemia. Weight-reducing interventions should continue and may allow for simplification of this regimen in the future.
When the combination of oral agents (and exenatide) fail to achieve euglycemia in patients with type 2 diabetes, various insulin regimens may be effective. There is no consensus about how insulin therapy should be instituted. One proposed regimen is to continue the oral combination therapy and then simply add a bedtime dose of NPH or long-acting insulin analog (insulin glargine or insulin detemir) to reduce excessive nocturnal hepatic glucose output and improve fasting glucose levels. If the patient does not achieve target glucose levels during the day, then daytime insulin treatment can be initiated. A convenient insulin regimen under these circumstances is a split dose of 70/30 NPH/regular mixture (or Humalog Mix 75/25 or NovoLogMix 70/30) before breakfast and before dinner. If this regimen fails to achieve satisfactory glycemic goals or is associated with unacceptable frequency of hypoglycemic episodes, then a more intensive regimen of multiple insulin injections can be instituted as in patients with type 1 diabetes. Metformin principally reduces hepatic glucose output and the thiazolidinediones improve peripheral insulin resistance, so it is a reasonable option to continue these drugs when insulin therapy is instituted. The sulfonylureas also have been shown to be of continued benefit. Thus, the continued use of the oral drugs may permit the use of lower doses of insulin and simpler regimens. There is no data on the continued administration of exenatide under these circumstances.
b. The nonobese patient with type 2 diabetes
Nonobese patients with type 2 diabetes frequently have increased visceral adiposity the so-called metabolically obese normal weight patient and the treatment algorithm is much the same as in the obese patient except there is not as much emphasis on weight loss. However, exercise remains an important aspect of treatment. Persons who do not have central obesity or insulin resistance should be evaluated for other types of diabetes such as latent autoimmune diabetes of adulthood (LADA) or maturity onset diabetes of the young (MODY). Patients with LADA can initially be treated with oral agents but require insulin within a few years, so experienced clinicians often prescribe insulin for these patients when the diagnosis is made.
2. Type 1 diabetes
Traditional once- or twice-daily insulin regimens are usually ineffective in type 1 patients without residual endogenous insulin. In these patients, information and counseling based on the findings of the DCCT (see above) should be provided
P.1244
about the advantages of taking multiple injections of insulin in conjunction with self-blood glucose monitoring. If near-normalization of blood glucose is attempted, at least three or four measurements of capillary blood glucose and three or four insulin injections are necessary.
Table 27-10. Examples of intensive insulin regimens using rapid-acting insulin analogs (insulin lispro, aspart, or glulisine) and NPH, or insulin glargine in a 70-kg man with type 1 diabetes.1,2,3 | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
A combination of rapid-acting insulin analogs and long-acting insulin analogs allows for more physiologic insulin replacement. The rapid-acting insulin analogs have been advocated as a safer and much more convenient alternative to regular human insulin for preprandial use. In a study comparing regular insulin with insulin lispro, daily insulin doses and hemoglobin A1c levels were similar, but insulin lispro improved postprandial control, reduced hypoglycemic episodes, and improved patient convenience compared with regular insulin. However, because of their relatively short duration (no more than 3 4 hours), the rapid-acting insulin analogs need to be combined with longer-acting insulins to provide basal coverage and avoid hyperglycemia prior to the next meal. In addition to carbohydrate content of the meal, the effect of simultaneous fat ingestion must also be considered a factor in determining the rapid-acting insulin analog dosage required to control the glycemic increment during and just after the meal. With low-carbohydrate content and high-fat intake, there is an increased risk of hypoglycemia from insulin lispro within 2 hours after the meal. Table 27-10 illustrates some regimens that might be appropriate for a 70-kg person with type 1 diabetes eating meals providing standard carbohydrate intake and moderate to low fat content.
Multiple injections of NPH insulin can be mixed in the same syringe as the insulin lispro, insulin aspart, and insulin glulisine. Insulin glargine is usually given once in the evening to provide 24-hour coverage. This insulin cannot be mixed with any of the other insulins and must be given as a separate injection. There are occasional patients in whom insulin glargine does not seem to last for 24 hours, and in such cases it needs to be given twice a day. Insulin detemir may also need to be given twice a day to get adequate 24-hour basal coverage.
Continuous subcutaneous insulin infusion (CSII) by portable battery-operated open loop devices currently provides the most flexible approach, allowing the setting of different basal rates throughout the 24 hours and permitting patients to delay or skip meals and vary meal size and composition. The dosage is usually based on providing 50% of the estimated insulin dose as basal and the remainder as intermittent boluses prior to meals. For example, a 70-kg man requiring 35 units of insulin per day may require a basal rate of 0.7 units per hour throughout the 24 hours with the exception of 3 AM to 8 AM, when 0.8 units per hour might be appropriate (for the dawn phenomenon). The meal bolus would depend on the carbohydrate content of the meal and the premeal blood glucose value. One unit per 15 g of carbohydrate plus 1 unit for 50 mg/dL of blood glucose above a target value (eg, 120 mg/dL) is a common starting point. Further adjustments to basal and bolus dosages would depend on the results of blood glucose monitoring. The majority of patients use the rapid-acting insulin analogs in the pumps. One of the more difficult therapeutic problems in managing patients with type 1 diabetes is determining the proper adjustment of insulin dose when the prebreakfast blood glucose level is high. Occasionally, the prebreakfast hyperglycemia is due to the Somogyi effect, in which nocturnal hypoglycemia leads to a surge of counterregulatory hormones to produce high blood glucose levels by 7 AM. However, a more common cause for prebreakfast hyperglycemia is the waning of circulating insulin levels by the morning. Also, the dawn phenomenon reduced tissue sensitivity to insulin between 5 AM and 8 AM is present in as many as 75% of type 1 patients and can aggravate the hyperglycemia.
Table 27-11. Prebreakfast hyperglycemia: Classification by blood glucose and insulin levels. | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
P.1245
Table 27-11 shows that diagnosis of the cause of prebreakfast hyperglycemia can be facilitated by self-monitoring of blood glucose at 3 AM in addition to the usual bedtime and 7 AM measurements. This is required for only a few nights, and when a particular pattern emerges from monitoring blood glucose levels overnight, appropriate therapeutic measures can be taken. The Somogyi effect can be treated by eliminating the dose of intermediate insulin at dinnertime and giving it at a lower dosage at bedtime or by supplying more food at bedtime. When a waning insulin level is the cause, then either increasing the evening dose or shifting it from dinnertime to bedtime (or both) can be effective. A bedtime dose either of insulin glargine or insulin detemir provides more sustained overnight insulin levels than human NPH and may be effective in managing refractory prebreakfast hyperglycemia. If this fails, insulin pump therapy may be required. When the dawn phenomenon alone is present, the dosage of intermediate insulin can be divided between dinnertime and bedtime; when insulin pumps are used, the basal infusion rate can be increased (eg, from 0.8 unit/h to 0.9 unit/h from 6 AM until breakfast).
Acceptable Levels of Glycemic Control
See above for a discussion of the DCCT and the UKPDS and their implications for diabetes therapy. A reasonable aim of therapy is to approach normal glycemic excursions without provoking severe or frequent hypoglycemia. What has been considered acceptable control includes blood glucose levels of 90 130 mg/dL before meals and after an overnight fast, and levels no higher than 180 mg/dL 1 hour after meals and 150 mg/dL 2 hours after meals. Glycohemoglobin levels should be no higher than 1% above the upper limit of the normal range for any particular laboratory. It should be emphasized that the value of blood pressure control was as great as or greater than glycemic control in type 2 patients as regards microvascular as well as macrovascular complications.
Complications of Insulin Therapy
A. Hypoglycemia
Hypoglycemic reactions, the most common complication of insulin therapy, may result from delay in taking a meal or unusual physical exertion. With more type 1 patients attempting tight control, this complication has become even more frequent. In older diabetics, in those taking only longer-acting insulins, and often in those attempting to maintain euglycemia on infusion pumps, autonomic counterregulatory responses are less readily elicited during hypoglycemia, and central nervous system dysfunction may occur, ie, mental confusion, bizarre behavior, and ultimately coma. Even focal neurologic deficits mimicking stroke may be observed. More rapid development of hypoglycemia from the effects of regular insulin causes signs of autonomic hyperactivity, both sympathetic (tachycardia, palpitations, sweating, tremulousness) and parasympathetic (nausea, hunger), that may progress to coma and convulsions. Except for sweating, most of the sympathetic symptoms of hypoglycemia are blunted in patients receiving -blocking agents for angina or hypertension. Though not absolutely contraindicated, these drugs must be used with caution in insulin-requiring diabetics, and 1-selective blocking agents are preferred.
1. Altered awareness of hypoglycemia
Since autonomic responses correlate strongly with awareness of hypoglycemia, many poorly controlled diabetics whose nervous systems have adapted to chronic hyperglycemia may trigger adrenergic alarms at levels of blood glucose above the usual hypoglycemic range. Conversely, type 1 patients overtreated with insulin may be unaware of critically low levels of blood glucose because of an adaptive blunting of their alarm systems owing to repeated episodes of hypoglycemia. This has been shown to be reversible if higher average blood glucose levels are maintained in these patients to avoid recurrent hypoglycemia over a period of several weeks.
As evidenced by results of the DCCT, the risk of frequent severe hypoglycemic episodes is greatly increased when normalization of the blood glucose is attempted with presently available methods of insulin delivery, and this is independent of the species of insulin used. Near normalization is therefore a safer target for therapy to avoid hypoglycemic unawareness.
2. Lack of glucagon response in type 1
For unexplained reasons, patients with type 1 lose their glucagon responses to hypoglycemia (but not to amino
P.1246
acids in protein-containing meals) within a year or so after developing diabetes. These patients then rely predominantly on the sympathetic nervous system to counterregulate hypoglycemia and are at special risk in later years when aging, autonomic neuropathy, or frequent hypoglycemic episodes blunt their sympathetic responses.
3. Prevention and treatment of hypoglycemia
Because of the potential danger of insulin-induced reactions, the diabetic patient should carry packets of table sugar or a candy roll at all times for use at the onset of hypoglycemic symptoms. Tablets containing 3 g of glucose are available (Dextrosol). The educated patient soon learns to take the amount of glucose needed and avoids the excess that may occur with eating candy or drinking orange juice, causing very high hyperglycemia. A glucagon emergency kit (1 mg) should be provided to every diabetic receiving insulin therapy, and family or friends should be instructed how to inject it intramuscularly in the event that the patient is unconscious or refuses food. An identification MedicAlert bracelet, necklace, or card in the wallet or purse should be carried by every diabetic receiving hypoglycemic drug therapy. The telephone number for the MedicAlert Foundation International in Turlock, California, is 800-ID-ALERT and the Internet address is http://www.medicalert.org.
All of the manifestations of hypoglycemia are rapidly relieved by glucose administration. If more severe hypoglycemia has produced unconsciousness or stupor, the treatment is 50 mL of 50% glucose solution by rapid intravenous infusion. If intravenous therapy is not available, 1 mg of glucagon injected intramuscularly will usually restore the patient to consciousness within 15 minutes to permit ingestion of sugar. If the patient is stuporous and glucagon is not available, small amounts of honey or syrup or glucose gel (15 g) can be inserted within the buccal pouch, but, in general, oral feeding is contraindicated in unconscious patients. Rectal administration of syrup or honey (30 mL per 500 mL of warm water) has been effective.
B. Immunopathology of Insulin Therapy
At least five molecular classes of insulin antibodies are produced during the course of insulin therapy in diabetes, including IgA, IgD, IgE, IgG, and IgM. With the increased therapeutic use of purified pork and especially human insulin, the various immunopathologic syndromes such as insulin allergy, immune insulin resistance, and lipoatrophy have become quite rare since the titers and avidity of these induced antibodies are generally quite low. However, in parts of the world where less purified forms of beef insulin are still used, these disorders remain a clinical concern among some insulin-treated patients.
1. Insulin allergy
Insulin allergy, or immediate-type hypersensitivity, is a rare condition in which local or systemic urticaria is due to histamine release from tissue mast cells sensitized by adherence of anti-insulin IgE antibodies. In severe cases, anaphylaxis results. When only human insulin has been used from the onset of insulin therapy, insulin allergy is exceedingly rare. Antihistamines, corticosteroids, and even desensitization may be required, especially for systemic hypersensitivity. There have been case reports of successful use of insulin lispro in those rare patients who have a generalized allergy to human insulin or insulin resistance due to a high titer of insulin antibodies.
2. Immune insulin resistance
Most insulin-treated patients develop a low titer of circulating IgG anti-nsulin antibodies that neutralize to a small extent the action of insulin. With the old animal insulins, a high titer of circulating antibodies sometimes developed, resulting in extremely high insulin requirements often more than 200 units daily. This is now rarely seen with the switch to highly purified pork or human insulins and has not been reported with the analogs.
C. Lipodystrophy at Injection Sites
Atrophy of subcutaneous fatty tissue leading to disfiguring excavations and depressed areas may rarely occur at the site of injection. This complication results from an immune reaction, and it has become rarer with the development of pure insulin preparations. Injection of these preparations directly into the atrophic area often results in restoration of normal contours. Lipohypertrophy, on the other hand, is a consequence of the pharmacologic effects of insulin being deposited in the same location repeatedly. It can occur with purified insulins and as well. Rotation of injection sites will prevent lipohypertrophy. There is a case report of a patient who had intractable lipohypertrophy with human insulin but no longer had the problem when he switched to insulin lispro.
Chronic Complications of Diabetes
Late clinical manifestations of diabetes mellitus include a number of pathologic changes that involve small and large blood vessels, cranial and peripheral nerves, the skin, and the lens of the eye. These lesions lead to hypertension, renal failure, blindness, autonomic and peripheral neuropathy, amputations of the lower extremities, myocardial infarction, and cerebrovascular accidents. These late manifestations correlate with the duration of the diabetic state subsequent to the onset of puberty. In type 1 diabetes, end-stage renal disease develops in up to 40% of patients, compared with less than 20% of patients with type 2 diabetes. As regards proliferative retinopathy, it ultimately develops in both types of diabetes but has a slightly higher prevalence in type 1 patients (25% after 15 years' duration). In patients with type 1 diabetes, complications from end-stage renal disease are a major cause of death, whereas patients with type 2 diabetes are more likely to have macrovascular diseases leading to myocardial infarction and stroke as the main causes of death. Cigarette use adds significantly to the risk of
P.1247
both microvascular and macrovascular complications in diabetic patients.
A. Ocular Complications
1. Diabetic cataracts
Premature cataracts occur in diabetic patients and seem to correlate with both the duration of diabetes and the severity of chronic hyperglycemia. Nonenzymatic glycosylation of lens protein is twice as high in diabetic patients as in age-matched nondiabetic persons and may contribute to the premature occurrence of cataracts.
2. Diabetic retinopathy
Three main categories exist: background, or simple, retinopathy, consisting of microaneurysms, hemorrhages, exudates, and retinal edema; preproliferative retinopathy with arteriolar ischemia manifested as cotton-wool spots (small infarcted areas of retina); and proliferative, or malignant, retinopathy, consisting of newly formed vessels. Proliferative retinopathy is a leading cause of blindness in the United States, particularly since it increases the risk of retinal detachment. Vision-threatening retinopathy virtually never appears in type 1 patients in the first 3 5 years of diabetes or before puberty. Up to 20% of patients with type 2 diabetes have retinopathy at the time of diagnosis. Annual consultation with an ophthalmologist should be arranged for patients who have had type 1 diabetes for more than 3 5 years and for all patients with type 2 diabetes, because many were probably diabetic for an extensive period of time before diagnosis. Patients with any macular edema, severe nonproliferative retinopathy, or any proliferative retinopathy require the care of an ophthalmologist. Extensive scatter xenon or argon photocoagulation and focal treatment of new vessels reduce severe visual loss in those cases in which proliferative retinopathy is associated with recent vitreous hemorrhages or in which extensive new vessels are located on or near the optic disk. Macular edema, which is more common than proliferative retinopathy in patients with type 2 diabetes (up to 20% prevalence), has a guarded prognosis, but it has also responded to scatter therapy with improvement in visual acuity if detected early. Avoiding tobacco use and correction of associated hypertension are important therapeutic measures in the management of diabetic retinopathy. There is no contraindication to using aspirin in patients with proliferative retinopathy.
3. Glaucoma
Glaucoma occurs in approximately 6% of persons with diabetes. It is responsive to the usual therapy for open-angle disease. Neovascularization of the iris in diabetics can predispose to closed-angle glaucoma, but this is relatively uncommon except after cataract extraction, when growth of new vessels has been known to progress rapidly, involving the angle of the iris and obstructing outflow.
B. Diabetic Nephropathy
As many as 4000 cases of end-stage renal disease occur each year among diabetic people in the United States. This is about one-third of all patients being treated for end-stage renal disease and represents a considerable national health expense.
The cumulative incidence of nephropathy differs between the two major types of diabetes. Patients with type 1 diabetes have a 30 40% chance of having nephropathy after 20 years in contrast to the much lower frequency in type 2 diabetes patients, in whom only about 15 20% develop clinical renal disease. However, since there are many more individuals affected with type 2 diabetes, end-stage renal disease is much more prevalent in type 2 than in type 1 diabetes in the United States and especially throughout the rest of the world. Improved glycemic control and more effective therapeutic measures to correct hypertension and with the beneficial effects of ACE inhibitors can reduce the development of end-stage renal disease among diabetics.
Diabetic nephropathy is initially manifested by proteinuria; subsequently, as kidney function declines, urea and creatinine accumulate in the blood.
1. Microalbuminuria
Sensitive radioimmunoassay methods of detecting small amounts of urinary albumin have permitted detection of microgram concentrations in contrast to the less sensitive dipstick strips, whose minimal detection limit is 0.3 0.5%. Conventional 24-hour urine collections, in addition to being inconvenient for patients, also show wide variability of albumin excretion, since several factors such as sustained erect posture, dietary protein, and exercise tend to increase albumin excretion rates. For these reasons, a timed overnight urine collection or albumin-creatinine ratio in an early morning spot urine collected upon awakening is preferable. Normal subjects excrete less than 15 mcg/min during overnight urine collections; values of 20 mcg/min or higher are considered to represent abnormal microalbuminuria. In the early morning spot urine, a ratio of albumin (mcg/L) to creatinine (mg/L) of < 30 mcg/mg creatinine is normal, and a ratio of 30 300 mcg/mg creatinine suggests abnormal microalbuminuria. At least two of three timed overnight or early morning spot urine collections over a 3- to 6-month period should be abnormal before a diagnosis of microalbuminuria is justified.
Subsequent renal failure can be predicted by persistent urinary albumin excretion rates exceeding 30 mcg/min. Increased microalbuminuria correlates with increased levels of blood pressure and increased LDL cholesterol, and this may explain why increased proteinuria in diabetic patients is associated with an increase in cardiovascular deaths even in the absence of renal failure. Glycemic control as well as a low-protein diet (0.8 g/kg/d) may reduce both the hyperfiltration and the elevated microalbuminuria in patients in the early stages of diabetes and those with incipient diabetic nephropathy. Antihypertensive therapy also decreases microalbuminuria. Evidence from some studies but not the UKPDS supports a specific role for ACE inhibitors in reducing intraglomerular pressure in addition to their lowering of
P.1248
systemic hypertension. An ACE inhibitor (captopril, 50 mg twice daily) in normotensive diabetics impedes progression to proteinuria and prevents the increase in albumin excretion rate. Since microalbuminuria has been shown to correlate with elevated nocturnal systolic blood pressure, it is possible that normotensive diabetic patients with microalbuminuria have slightly elevated systolic blood pressure during sleep, which is lowered during antihypertensive therapy. This action may contribute to the reported efficacy of ACE inhibitor drugs in reducing microalbuminuria in normotensive patients.
2. Progressive diabetic nephropathy
Progressive diabetic nephropathy consists of proteinuria of varying severity occasionally leading to nephrotic syndrome with hypoalbuminemia, edema, and an increase in circulating LDL cholesterol as well as progressive azotemia. In contrast to all other renal disorders, the proteinuria associated with diabetic nephropathy does not diminish with progressive renal failure (patients continue to excrete 10 11 g daily as creatinine clearance diminishes). As renal failure progresses, there is an elevation in the renal threshold at which glycosuria appears.
Hypertension develops with progressive renal involvement, and coronary and cerebral atherosclerosis seems to be accelerated. Approximately two-thirds of adult patients with diabetes have hypertension. Once diabetic nephropathy has progressed to the stage of hypertension, proteinuria, or early renal failure, glycemic control is not beneficial in influencing its course. In this circumstance, antihypertensive medications, including ACE inhibitors, and restriction of dietary protein to 0.8 g/kg body weight per day are recommended. ACE inhibitors have been shown to protect against deterioration in renal function in type 1 diabetic patients with clinical nephropathy. This beneficial effect appears to be due to improved glomerular hemodynamics that cannot be explained only by the antihypertensive action of these drugs. Captopril (25 mg three times daily) has shown a 50% reduction in the risk of the combined end points of death, dialysis, and transplantation in type 1 subjects with diabetic nephropathy and clinical proteinuria. During initiation of ACE inhibitor therapy, an increment in serum creatinine greater than 2 mg/dL due to a rapid fall in intraglomerular pressure or the occurrence of persistent hyperkalemia (above 6 mEq/L) due to hyporeninemic hypoaldosteronism is an indication to stop this medication.
Dialysis has been of limited value in the long-term treatment of renal failure due to diabetic nephropathy. At present, experience in renal transplantation especially from related donors is more promising and is the treatment of choice in cases where there are no contraindications such as severe cardiovascular disease.
C. Diabetic Neuropathy
Diabetic neuropathies are the most common complications of diabetes affecting up to 50% of older patients with type 2 diabetes.
1. Peripheral neuropathy
a. Distal symmetric polyneuropathy
This is the most common form of diabetic peripheral neuropathy where loss of function appears in a stocking-glove pattern and is due to an axonal neuropathic process. Longer nerves are especially vulnerable, hence the impact on the foot. Both motor and sensory nerve conduction is delayed in the peripheral nerves, and ankle jerks may be absent.
Sensory involvement usually occurs first and is generally bilateral, symmetric, and associated with dulled perception of vibration, pain, and temperature. The pain can range from mild discomfort to severe incapacitating symptoms (see below). The sensory deficit may eventually be of sufficient degree to prevent patients from feeling pain. Patients who have a sensory neuropathy should therefore be examined with a 5.07 Semmes Weinstein filament and those who cannot feel the filament must be considered at risk for unperceived neuropathic injury.
The denervation of the small muscles of the foot result in clawing of the toes and displacement of the submetatarsal fat pads anteriorly. These changes, together with the joint and connective tissue changes, alter the biomechanics of the foot and increase plantar pressures. This combination of decreased pain threshold, abnormally high foot pressures, and repetitive stress (such as from walking) can lead to calluses and ulcerations in the high-pressure areas such as over the metatarsal heads. Peripheral neuropathy, autonomic neuropathy, and trauma also predisposes to the development of Charcot's arthropathy. An acute case of Charcot's foot arthropathy presents with pain and swelling, and if left untreated, leads to a rocker bottom deformity and ulceration. The early radiologic changes show joint subluxation and periarticular fractures. As the process progresses, there is frank osteoclastic destruction leading to deranged and unstable joints particularly in the midfoot. Not surprisingly, the key issue for the healing of neuropathic ulcers in a foot with good vascular supply is mechanical unloading. In addition, any infection should be treated with debridement and appropriate antibiotics; healing duration of 8 10 weeks is typical. Occasionally, when healing appears refractory, platelet-derived growth factor (Regranex) should be considered for local application. Once ulcers are healed, therapeutic footwear is key to preventing recurrences. Custom molded shoes are reserved for patients with significant foot deformities. Other patients with neuropathy may require accommodative insoles that distribute the load over as wide an area as possible. Patients with foot deformities and loss of their protective threshold should get regular care from a podiatrist. Patients should be educated on appropriate footwear and those with loss of their protective threshold should be instructed to inspect their feet daily for reddened areas, blisters, abrasions, or lacerations.
b. Isolated peripheral neuropathy
Involvement of the distribution of only one nerve ( mononeuropathy )
P.1249
or of several nerves ( mononeuropathy multiplex ) is characterized by sudden onset with subsequent recovery of all or most of the function. This neuropathology has been attributed to vascular ischemia or traumatic damage. Cranial and femoral nerves are commonly involved, and motor abnormalities predominate. The patient with cranial nerve involvement usually has diplopia and single third, fourth, or sixth nerve weakness on examination but the pupil is spared. A full recovery of function occurs in 6 12 weeks. Diabetic amyotrophy presents with onset of severe pain in the front of the thigh. Within a few days or weeks of the onset of pain, weakness and wasting of the quadriceps develops. As the weakness appears, the pain tends to improve. Management includes analgesia and improved diabetes controls. The symptoms improve over 6 18 months.
c. Painful diabetic neuropathy
Hypersensitivity to light touch and occasionally severe burning pain, particularly at night, can become physically and emotionally disabling. Amitriptyline, 25 75 mg at bedtime, has been recommended for pain associated with diabetic neuropathy. Dramatic relief has often resulted within 48 72 hours. This rapid response is in contrast to the 2 or 3 weeks required for an antidepressive effect. Patients often attribute the benefit to having a full night's sleep. Mild to moderate morning drowsiness is a side effect that generally improves with time or can be lessened by giving the medication several hours before bedtime. This drug should not be continued if improvement has not occurred after 5 days of therapy. If amitriptyline's anticholinergic effects are too troublesome, then nortriptyline can be used. Desipramine in doses of 25 150 mg/d seems to have the same efficacy as amitriptyline. Tricyclic antidepressants in combination with the phenothiazine, fluphenazine have been shown in two studies to be efficacious in painful neuropathy, with benefits unrelated to relief of depression. Gabapentin (900 1800 mg/d in three divided doses) has also been shown to be effective in the treatment of painful neuropathy and should be tried if the tricyclic drugs prove ineffective. Pregabalin, a congener of gabapentin, has been shown in an 8-week study to be more effective than placebo in treating painful diabetic peripheral neuropathy. However, this drug was not compared with an active control. Also because of its abuse potential, it has been categorized as a schedule V controlled substance. Duloxetine a serotonin and norepinephrine reuptake inhibitor, has been approved for the treatment of painful diabetic neuropathy. In clinical trials, this drug reduced the pain sensitivity score by 40 50%. Capsaicin, a topical irritant, has been found to be effective in reducing local nerve pain; it is dispensed as a cream (Zostrix 0.025%, Zostrix-HP 0.075%) to be rubbed into the skin over the painful region two to four times daily. Gloves should be used for application since hand contamination could result in discomfort if the cream comes in contact with eyes or sensitive areas such as the genitalia.
Diabetic neuropathic cachexia is a syndrome characterized by a symmetric peripheral neuropathy associated with profound weight loss (up to 60% of total body weight) and painful dysesthesias affecting the proximal lower limbs, the hands, or the lower trunk. Treatment is usually with insulin and analgesics. The prognosis is generally good, and patients typically recover their baseline weight with resolution of the painful sensory symptoms within 1 year.
2. Autonomic neuropathy
With autonomic neuropathy, there is evidence of postural hypotension, decreased cardiovascular response to Valsalva's maneuver, gastroparesis, alternating bouts of diarrhea (particularly nocturnal) and constipation, inability to empty the bladder, and impotence. Gastroparesis should be considered in type 1 diabetic patients in whom unexpected fluctuations and variability in their blood glucose levels develops after meals. Impotence due to neuropathy differs from psychogenic impotence in that the latter may be intermittent (erections occur under special circumstances), whereas diabetic impotence is usually persistent; aortoiliac occlusive disease may contribute to this problem.
a. Management of autonomic neuropathy
There is no consistently effective treatment for diabetic autonomic neuropathy. Metoclopramide has been of some help in treating diabetic gastroparesis over the short term, but its effectiveness seems to diminish over time. It is a dopamine antagonist that has central antiemetic effects as well as a cholinergic action to facilitate gastric emptying. It can be given intravenously (10 mg three or four times a day, 30 minutes before meals and at bedtime) or orally (20 mg of liquid metoclopramide) before breakfast and dinner. Drowsiness, restlessness, fatigue, and lassitude are common adverse effects. Tardive dyskinesia and extrapyramidal effects also occur. Because cisapride has caused life-threatening cardiac arrhythmias, including 80 deaths, it has been withdrawn from the market in the United States. Erythromycin appears to bind to motilin receptors in the stomach and has been found to improve gastric emptying in doses of 250 mg three times daily. Gastric electrical stimulation has been reported to improve symptoms and quality of life indices in patients with gastroparesis refractory to pharmacologic therapy. Diarrhea associated with autonomic neuropathy has occasionally responded to broad-spectrum antibiotic therapy, although it often undergoes spontaneous remission. Refractory diabetic diarrhea is often associated with impaired sphincter control and fecal incontinence. Therapy with loperamide, 4 8 mg daily, or diphenoxylate with atropine, two tablets up to four times a day, may provide relief. In more severe cases, tincture of paregoric or codeine (60-mg tablets) may be required to reduce the frequency of diarrhea and improve the consistency of the stools. Clonidine has been reported to lessen diabetic diarrhea; however, its usefulness is limited by its tendency to lower blood pressure in these patients who already have autonomic neuropathy, resulting in orthostatic hypotension. Constipation usually
P.1250
responds to stimulant laxatives such as senna. Bethanechol in doses of 10 50 mg three times a day has occasionally improved emptying of the atonic urinary bladder. Catheter decompression of the distended bladder has been reported to improve its function, and considerable benefit has been reported after surgical severing of the internal vesicle sphincter. Mineralocorticoid therapy with fludrocortisone, 0.2 0.3 mg/d, and elastic stockings or pressure suits have reportedly been of some help in patients with orthostatic hypotension occurring as a result of loss of postural reflexes.
b. Management of erectile dysfunction
There are medical, mechanical, and surgical treatments available for treatment of erectile dysfunction. Penile erection depends on relaxation of the smooth muscle in the arteries of the corpus cavernosum, and this is mediated by nitric oxide-induced cyclic 3 ,5 -guanosine monophosphate (cGMP) formation. cGMP-specific phosphodiesterase type 5 (PDE5) inhibitors impair the breakdown of cGMP and improve the ability to attain and maintain an erection. Sildenafil (Viagra), vardenafil (Levitra), and tadalafil (Cialis) have been shown in placebo-controlled clinical trials to improve erections in response to sexual stimulation. The recommended dose of sildenafil for most patients is one 50-mg tablet taken approximately 1 hour before sexual activity. The peak effect is at 1.5 2 hours, with some effect persisting for 4 hours. Patients with diabetes mellitus using sildenafil reported 50 60% improvement in erectile function. The maximum recommended dose is 100 mg. The recommended doses of both vardenafil and tadalafil is 10 mg. The doses may be increased to 20 mg or decreased to 5 mg based on efficacy and side effects. Tadalafil has been shown to improve erectile function for up to 36 hours after dosing. In clinical trials, only a few adverse effects have been reported transient mild headache, flushing, dyspepsia, and some altered color vision. Priapism can occur with these drugs and patients should be advised to seek immediate medical attention if an erection persists for longer than 4 hours. The PDE5 inhibitors potentiate the hypotensive effects of nitrates and their use is contraindicated in patients who are concurrently using organic nitrates in any form. Caution is advised for men who have suffered a heart attack, stroke, or life-threatening arrhythmia within the previous 6 months; men who have resting hypotension or hypertension; and men who have a history of cardiac failure or have unstable angina. Rarely, a decrease in vision or permanent visual loss has been reported after PDE5 inhibitor use.
Intracorporeal injection of vasoactive drugs causes penile engorgement and erection. Drugs most commonly used include papaverine alone, papaverine with phentolamine, and alprostadil (prostaglandin E1). Alprostadil injections are relatively painless, but careful instruction is essential to prevent local trauma, priapism, and fibrosis. Intraurethral pellets of alprostadil avoid the problem of injection of the drug.
External vacuum therapy (Erec-Aid System) is a nonsurgical treatment consisting of a suction chamber operated by a hand pump that creates a vacuum around the penis. This draws blood into the penis to produce an erection which is maintained by a specially designed tension ring inserted around the base of the penis and which can be kept in place for up to 20 30 minutes. While this method is generally effective, its cumbersome nature limits its appeal.
In view of the recent development of nonsurgical approaches to therapy of erectile dysfunction, resort to surgical implants of penile prostheses is becoming less common.
D. Cardiovascular Complications
1. Heart disease
Microangiopathy occurs in the heart and may explain the etiology of congestive cardiomyopathies in diabetic patients who do not have demonstrable coronary artery disease. More commonly, however, heart disease in patients with diabetes is due to coronary atherosclerosis. Myocardial infarction is three to five times more common in diabetic patients and is the leading cause of death in patients with type 2 diabetes. Cardiovascular disease risk is increased in patients with type 1 diabetes as well, although the absolute risk is lower than in patients with type 2 diabetes. Premenopausal women who normally have lower rates of coronary artery disease lose this protection once diabetes develops. The increased risk in patients with type 2 diabetes reflects the combination of hyperglycemia, hyperlipidemia, abnormalities of platelet adhesiveness, coagulation factors, hypertension, oxidative stress, and inflammation. Large intervention studies of risk factor reduction in diabetes are lacking, but it is reasonable to assume that reducing these risk factors would have a beneficial effect. Lowering LDL cholesterol reduces first events in patients without known coronary disease and secondary events in patients with known coronary disease. These intervention studies included some patients with diabetes, and the benefits of LDL cholesterol lowering was apparent in this group. The National Cholesterol Education Program clinical practice guidelines have designated diabetes as a coronary risk equivalent and have recommended that patients with diabetes should have an LDL cholesterol goal of < 100 mg/dL.
The ADA also recommends lowering blood pressure to 130/80 mm Hg or less. The Heart Outcomes Prevention Evaluation (HOPE) study randomized 9297 high-risk patients who had evidence of vascular disease or diabetes plus one other cardiovascular risk factor to receive ramipril or placebo for a mean of 5 years. Treatment with ramipril resulted in a 25% reduction of the risk of myocardial infarction, stroke, or death from cardiovascular disease. The mean difference between the placebo and ramipril group was 2.2 mm Hg systolic and 1.4 mm Hg diastolic blood pressure. The reduction in cardiovascular event rate remained significant after adjustment for this small difference in blood pressure. The mechanism underlying
P.1251
this protective effect of ramipril is unknown. Patients with type 2 diabetes who already have cardiovascular disease or microalbuminuria should therefore be considered for treatment with an ACE inhibitor. More clinical studies are needed to address the question of whether patients with type 2 diabetes who do not have cardiovascular disease or microalbuminuria would specifically benefit from ACE inhibitor treatment.
Aspirin at a dose of 81 325 mg daily has been shown to effectively inhibit thromboxane synthesis by platelets and reduce the risk of diabetic atherothrombosis without increasing risks of gastrointestinal hemorrhage. Use of low-dose enteric-coated aspirin is recommended in diabetic adults with evident macrovascular disease or in those with increased cardiovascular risk factors or those older than 30 years. Contraindications for aspirin therapy are patients with aspirin allergy, bleeding tendency, recent gastrointestinal bleeding, or active hepatic disease. The Early Treatment Diabetic Retinopathy Study (ETDRS) showed that aspirin does not influence the course of proliferative retinopathy. There was no statistically significant difference in the severity of vitreous/preretinal hemorrhages or their rate of resolution between the aspirin and placebo groups. Thus, it appears that there is no contraindication to aspirin use to achieve cardiovascular benefit in diabetic patients who have proliferative retinopathy.
2. Peripheral vascular disease
Atherosclerosis is markedly accelerated in the larger arteries. It is often diffuse, with localized enhancement in certain areas of turbulent blood flow, such as at the bifurcation of the aorta or other large vessels. Clinical manifestations of peripheral vascular disease include ischemia of the lower extremities, impotence, and intestinal angina.
The incidence of gangrene of the feet in diabetics is 30 times that in age-matched controls. The factors responsible for its development, in addition to peripheral vascular disease, are small vessel disease, peripheral neuropathy with loss of both pain sensation and neurogenic inflammatory responses, and secondary infection. In two-thirds of patients with ischemic gangrene, pedal pulses are not palpable. In the remaining one-third who have palpable pulses, reduced blood flow through these vessels can be demonstrated by plethysmographic or Doppler ultrasound examination. Prevention of foot injury is imperative. Agents that reduce peripheral blood flow such as tobacco and propranolol should be avoided. Control of other risk factors such as hypertension is essential. Cholesterol-lowering agents are useful as adjunctive therapy when early ischemic signs are detected and when dyslipidemia is present. Patients should be advised to seek immediate medical care if a diabetic foot ulcer develops. Improvement in peripheral blood flow with endarterectomy and bypass operations is possible in certain patients.
E. Skin and Mucous Membrane Complications
Chronic pyogenic infections of the skin may occur, especially in poorly controlled diabetic patients. Eruptive xanthomas can result from hypertriglyceridemia, associated with poor glycemic control. An unusual lesion termed necrobiosis lipoidica diabeticorum is usually located over the anterior surfaces of the legs or the dorsal surfaces of the ankles. They are oval or irregularly shaped plaques with demarcated borders and a glistening yellow surface and occur in women two to four times more frequently than in men.
Shin spots are not uncommon in adult diabetics. They are brownish, rounded, painless atrophic lesions of the skin in the pretibial area. Candidal infection can produce erythema and edema of intertriginous areas below the breasts, in the axillas, and between the fingers. It causes vulvovaginitis in most chronically uncontrolled diabetic women with persistent glucosuria and is a frequent cause of pruritus.
While antifungal creams containing miconazole or clotrimazole offer immediate relief of vulvovaginitis, recurrence is frequent unless glucosuria is reduced.
F. Special Situations
1. Insulin replacement during surgery
A prospective trial in surgical ICU patients reported that aggressive treatment of hyperglycemia (blood glucose > 110 mg/dL) reduced mortality and morbidity. Only a small number of persons in this study (204 of 1548) had a diagnosis of diabetes preoperatively, and so it seems that hyperglycemia per se (and not complications of diabetes) was an important cause of postoperative complications. Keeping blood glucose values as close to normal as possible is therefore the goal in the postsurgical patient in the ICU. When surgical patients leave the ICU, target glucose values between 100 mg/dL and 200 mg/dL may be appropriate, although this view is based on clinical observations rather than conclusive evidence. The same investigators performed a similar prospective trial in 1200 medical ICU patients and reported that aggressive treatment of hyperglycemia reduced morbidity (decreased acquired renal injury and increased early weaning from mechanical ventilation) but not mortality. Again, as in the surgical ICU study, only a small number of persons (16.9%) had a diagnosis of diabetes at admission. One of the inclusion criteria for the study was that patients would only be enrolled if they were likely to stay in the ICU for 3 days or longer. Some patients stayed in the ICU less than 3 days, and in that subgroup, intensive insulin therapy was associated with increased mortality. On the other hand, patients who stayed in the ICU for 3 days or longer had reduced mortality (and morbidity). Clearly, further studies are needed to address this observation.
During major surgery and in the immediate recovery period in patients with type 1 diabetes and in most patients with type 2 diabetes 5% dextrose in physiologic saline containing 20 mEq of potassium chloride should be infused intravenously at a rate of 100 200 mL/h with regular human insulin (25 units/250 mL 0.9% saline) into the intravenous tubing at a rate of 1 3 units/h. The patient's blood glucose should be monitored every hour
P.1252
initially and the rates of insulin or dextrose adjusted to maintain blood glucose values between 80 mg/dL and 110 mg/dL in the ICU and 100 200 mg/dL on the wards.
Type 2 patients facing minor surgical procedures not requiring general anesthesia who have previously been controlled on oral agents or diet alone do not generally require insulin infusions. Glucose-containing solutions should be avoided during surgery in these patients, and blood glucose levels should be monitored every 4 hours. Regular human insulin or one of the rapid-acting insulin analogs should be administered subcutaneously if needed to maintain blood glucose below 200 mg/dL (see Chapter 3).
2. Pregnancy and the diabetic patient
Several features distinguish the management of diabetics during pregnancy from the general therapy of diabetes. These include the following: (1) Oral hypoglycemic agents are contraindicated. (2) Weight reduction is not advised, since fetal nutrition can be adversely affected. (3) Intensive insulin therapy with frequent self-monitoring of blood glucose is generally recommended to improve the likelihood of having healthy normal babies. Every effort should be made, utilizing multiple injections of insulin or a continuous infusion of insulin by pump, to maintain near-normalization of fasting and preprandial blood glucose values while avoiding hypoglycemia. Glycohemoglobin should be maintained in the normal range.
Since many diabetic pregnancies persist beyond the expected term or because the infants are usually large and hydramnios may be present it has been suggested that the baby should be delivered early (at 37 38 weeks), especially if glycemic control during pregnancy has been inadequate (eg, glycohemoglobin > 10%). There is a present trend away from elective cesarean section and toward induction of labor. See Chapter 18 for further details.
Prognosis
The DCCT showed that the previously poor prognosis for as many as 40% of patients with type 1 diabetes is markedly improved by optimal care. DCCT participants were generally young and highly motivated and were cared for in academic centers by skilled diabetes educators and endocrinologists who were able to provide more attention and services than are usually available. Improved training of primary care providers may be beneficial.
For type 2 diabetes, the UKPDS documented a reduction in microvascular disease with glycemic control, although this was not apparent in the obese subgroup. Cardiovascular outcomes were not improved by glycemic control, although antihypertensive therapy showed benefit in reducing the number of adverse cardiovascular complications as well as in reducing the occurrence of microvascular disease among hypertensive patients. In patients with visceral obesity, successful management of type 2 diabetes remains a major challenge in the attempt to achieve appropriate control of hyperglycemia, hypertension, and dyslipidemia. Once safe and effective methods are devised to prevent or manage obesity, the prognosis of type 2 diabetes with its high cardiovascular risks should improve considerably.
In addition to poorly understood genetic factors relating to differences in individual susceptibility to development of long-term complications of hyperglycemia, it is clear that in both types of diabetes, the diabetic patient's intelligence, motivation, and awareness of the potential complications of the disease contribute significantly to the ultimate outcome.
American Association of Diabetes Educators http://www.aadenet.org/
American Diabetes Association http://www.diabetes.org/home
American Dietetic Association http://www.eatright.org
Abraira C et al: Intensive insulin therapy in patients with type 2 diabetes: implications of the Veterans Affairs (VA CSDM) feasibility trial. Am Heart J 1999;138:S360.
American Diabetes Association Position Statement: Diabetes nephropathy. Diabetes Care 2004;27(Suppl 1):S79.
American Diabetes Association Position Statement: Preventive foot care in people with diabetes. Diabetes Care 2004;27 (Suppl 1):S63.
American Diabetes Association: Standards of Medical Care in Diabetes. Diabetes Care 2006;29:476.
Atkinson MA et al: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221.
DeFronzo RA et al: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092.
Diabetes Prevention Trial - Type 1 Diabetes Study Group: Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854.
Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ 1998;317: 713.
Frank RN: Diabetic retinopathy. N Engl J Med 2004;350:48.
Gaede P et al: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003; 348:383.
Genuth S et al: Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160.
Harris R et al: Screening adults for type 2 diabetes: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2003;138:215.
Hebel G et al: Hypoglycemia: pathophysiology and treatment. Endocrinol Metab Clin North Am 2000;29:725.
Holst JJ et al: Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199.
P.1253
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352: 837.
Juvenile Diabetes Foundation http://www.jdf.org/index.html
Knowler WC et al: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393.
Lipshultz LI et al: Treatment of erectile dysfunction in men with diabetes. JAMA 1999;281:465.
Ohkubo Y et al: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103.
Owens DR et al: Insulins today and beyond. Lancet 2001;358: 739.
Poncelet AN: Diabetic polyneuropathy. Risk factors, patterns of presentation, diagnosis, and treatment. Geriatrics 2003;58: 16.
Rosenstock J et al: Inhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2005;143:549. Summary for patients in: Ann Intern Med 2005;143:I28
Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med 2003;138:212
Setter SM et al: Metformin hydrochloride in the treatment of type 2 diabetes mellitus: a clinical review with a focus on dual therapy. Clin Ther 2003;25:2991.
Shichiri M et al: Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000;23(Suppl 2):B21
Snow V et al: The evidence base for tight blood pressure control in the management of type 2 diabetes mellitus. Ann Intern Med 2003;138:587
Stumvoll M et al: Clinical features of insulin resistance and beta cell dysfunction and the relationship to type 2 diabetes. Clin Lab Med 2001;21:31
Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998;317: 703.
Van den Berghe G et al: Intensive insulin therapy in the critically ill patient. N Engl J Med 2001;345:1359.
Van den Berghe G et al: Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449.
Vijan S et al: Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med 2003;138:593
Yki-Jarvinen H: Thiazolidinediones. N Engl J Med 2004;351: 1106.
Yusuf S et al: Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145. Erratum in: 2000;342:1376. N Engl J Med 2000;342:748
Wajchenberg BL: Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21: 697.
Weissberg-Benchell J et al: Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079
Zinman B et al: American Diabetes Association: Physical activity/exercise and diabetes. Diabetes Care 2004;27(Suppl 1):S58.
Diabetic Coma
Coma may be due to a variety of causes not directly related to diabetes. Certain causes directly related to diabetes require differentiation: (1) Hypoglycemic coma resulting from excessive doses of insulin or oral hypoglycemic agents. (2) Hyperglycemic coma associated with either severe insulin deficiency (diabetic ketoacidosis) or mild to moderate insulin deficiency (hyperglycemic hyperosmolar state). (3) Lactic acidosis associated with diabetes, particularly in diabetics stricken with severe infections or with cardiovascular collapse.
Diabetic Ketoacidosis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Hyperglycemia > 250 mg/dL.
Acidosis with blood pH < 7.3.
Serum bicarbonate < 15 mEq/L.
Serum positive for ketones.
General Considerations
Diabetic ketoacidosis may be the initial manifestation of type 1 diabetes or may result from increased insulin requirements in type 1 diabetes patients during the course of infection, trauma, myocardial infarction, or surgery. It is a life-threatening medical emergency with a mortality rate just under 5% in individuals under 40 years of age, but with a more serious prognosis in the elderly, who have mortality rates over 20%. The National Data Group report an annual incidence of five to eight episodes of diabetic ketoacidosis per 1000 diabetic persons. Ketoacidosis may develop in patients with type 2 diabetes when severe stress such as sepsis or trauma is present. Diabetic ketoacidosis has been found to be one of the more common serious complications of insulin pump therapy, occurring in approximately 1 per 80 patient-months of treatment. Many patients who monitor capillary blood glucose regularly ignore urine ketone measurements, which would signal the possibility of insulin leakage or pump failure before serious illness develops. Poor compliance is one of the most common causes of diabetic ketoacidosis, particularly when episodes are recurrent.
Clinical Findings
A. Symptoms and Signs
The appearance of diabetic ketoacidotic coma is usually preceded by a day or more of polyuria and polydipsia associated with marked fatigue, nausea and
P.1254
vomiting, and, finally, mental stupor that can progress to coma. On physical examination, evidence of dehydration in a stuporous patient with rapid deep breathing and a fruity breath odor of acetone would strongly suggest the diagnosis. Hypotension with tachycardia indicates profound fluid and electrolyte depletion, and mild hypothermia is usually present. Abdominal pain and even tenderness may be present in the absence of abdominal disease. Conversely, cholecystitis or pancreatitis may occur with minimal symptoms and signs.
B. Laboratory Findings
(Table 27-12.) Glycosuria of 4+ and strong ketonuria with hyperglycemia, ketonemia, low arterial blood pH, and low plasma bicarbonate are typical of diabetic ketoacidosis. Serum potassium is often elevated despite total body potassium depletion resulting from protracted polyuria or vomiting. Elevation of serum amylase is common but often represents salivary as well as pancreatic amylase. Thus, in this setting, an elevated serum amylase is not specific for acute pancreatitis. Serum lipase may be useful if the diagnosis of acute pancreatitis is being seriously considered. Azotemia may be a better indicator of renal status than serum creatinine, since multichannel chemical analysis of serum creatinine (SMA-6) is falsely elevated by nonspecific chromogenicity of keto acids and glucose. Most laboratories, however, now routinely eliminate this interference. Leukocytosis as high as 25,000/mcL with a left shift may occur with or without associated infection. The presence of an elevated or even a normal temperature would suggest the presence of an infection, since patients with diabetic ketoacidosis are generally hypothermic if uninfected.
Complications
Hyperglycemia and ketoacidemia are due to insulin lack, hyperglucagonemia, and elevated levels of the stress hormones catecholamines, cortisol, and growth hormone.
A. Hyperglycemia
Hyperglycemia results from increased hepatic production of glucose as well as diminished glucose uptake by peripheral tissues. Hepatic glucose output is a consequence of increased gluconeogenesis resulting from insulinopenia as well as from an associated hyperglucagonemia.
B. Ketoacidemia
Ketoacidemia represents the effect of insulin lack at multiple enzyme loci. Insulin lack associated with elevated levels of growth hormone, catecholamines, and glucagon contributes to an increase in lipolysis from adipose tissue and in hepatic ketogenesis. In addition, there is evidence that reduced ketolysis by insulin-deficient peripheral tissues contributes to the ketoacidemia. The only true keto acid present is acetoacetic acid, which, along with its by-product acetone, is measured by nitroprusside reagents (Acetest and Ketostix). The sensitivity for acetone, however, is poor, requiring over 10 mmol, which is seldom reached in the plasma of ketoacidotic subjects although this detectable concentration is readily achieved in urine. Thus, in the plasma of ketotic patients, only acetoacetate is measured by these reagents. The more prevalent -hydroxybutyric acid has no ketone group and is therefore not detected by conventional nitroprusside tests. This takes on special importance in the presence of circulatory collapse during diabetic ketoacidosis, wherein an increase in lactic acid can shift the redox state to increase -hydroxybutyric acid at the expense of the readily detectable acetoacetic acid. Bedside diagnostic reagents would then be unreliable, suggesting no ketonemia in cases where -hydroxybutyric acid is a major factor in producing the acidosis. A combined glucose and ketone meter (Precision, Medisense) that is able to measure blood -hydroxyubtyrate concentration on capillary blood is available.
C. Fluid and Electrolyte Depletion
Hyperglycemia results in an osmotic diuresis and dehydration and secondary loss of electrolytes. Ketonuria similarly causes loss of water and electrolytes. Balance studies during withdrawal of insulin and treatment in patients with type 1 diabetes show that on average the water depletion is about 5 L; sodium, 300 500 mmol; potassium, 270 400 mmol; chloride, 100 400 mmol.
Drowsiness is fairly common but frank coma only occurs in about 10% of patients. There is a correlation between the degree of depression of the sensorium and extracellular osmolarity. When serum hyperosmolality exceeds 320 330 mosm/L, central nervous system depression or coma may ensue. Coma in a diabetic patient with a lower osmolality should prompt a search for cause of coma other than hyperosmolality.
Treatment
A. Prevention
Education of diabetic patients to recognize the early symptoms and signs of ketoacidosis has done a great deal to prevent severe acidosis. Urine ketones should be measured in patients with signs of infection or in insulin pump-treated patients when capillary blood glucose is unexpectedly and persistently high. When heavy ketonuria and glycosuria persist on several successive examinations, supplemental regular insulin should be administered and liquid foods such as lightly salted tomato juice and broth should be ingested to replenish fluids and electrolytes. The patient should be instructed to contact the physician if ketonuria persists, and especially if vomiting develops or if appropriate adjustment of the infusion rate on an insulin pump does not correct the hyperglycemia and ketonuria. In juvenile-onset diabetics, particularly in the teen years, recurrent episodes of severe ketoacidosis often indicate poor compliance with the insulin regimen, and these patients will require intensive family counseling.
P.1255
B. Emergency Measures
If ketosis is severe, the patient should be placed in the hospital for correction of the hyperosmolality as well as the ketoacidemia. An ICU or, at the least, a step-down unit is preferable for more severe cases.
1. Therapeutic flow sheet
One of the most important steps in initiating therapy is to start a flow sheet listing vital signs and the time sequence of diagnostic laboratory values in relation to therapeutic maneuvers. Indices of the metabolic defects include urine glucose and ketones as well as arterial pH, plasma glucose, acetone, bicarbonate, serum urea nitrogen, and electrolytes. Serum osmolality should be measured or estimated and tabulated during the course of therapy.
A convenient method of estimating effective serum osmolality is as follows (normal values in humans are 280 300 mosm/kg):
![]()
These calculated estimates are usually 10 20 mosm/kg lower than values measured by standard cryoscopic techniques in patients with diabetic coma. Urea is freely permeable across cell membranes and therefore not included in calculations of effective serum osmolality. One physician should be responsible for maintaining this therapeutic flow sheet and prescribing therapy. An indwelling urinary catheter is required in all comatose patients but should be avoided if possible in a fully cooperative diabetic because of the risk of introducing bladder infection. Fluid intake and output should be recorded. Gastric intubation is recommended in the comatose patient to correct the commonly associated gastric dilatation that may lead to vomiting and aspiration. The patient should not receive sedatives or narcotics.
2. Insulin replacement
Only regular insulin should be used initially in all cases of severe ketoacidosis, and it should be given immediately after the diagnosis is established. Regular insulin can be given in a loading dose of 0.15 unit/kg as an intravenous bolus to prime the tissue insulin receptors. Following the initial bolus, doses of insulin as low as 0.1 unit/kg/h are continuously infused or given hourly as an intramuscular injection; this is sufficient to replace the insulin deficit in most patients. Replacement of insulin deficiency helps correct the acidosis by reducing the flux of fatty acids to the liver, reducing ketone production by the liver, and also improving removal of ketones from the blood. Insulin treatment reduces the hyperosmolality by reducing the hyperglycemia. It accomplishes this by increasing removal of glucose through peripheral utilization as well as by decreasing production of glucose by the liver. This latter effect is accomplished by direct inhibition of gluconeogenesis and glycogenolysis, as well as by lowered amino acid flux from muscle to liver and reduced hyperglucagonemia.
The insulin dose should be piggy-backed into the fluid line so the rate of fluid replacement can be changed without altering the insulin delivery rate. If the plasma glucose level fails to fall at least 10% in the first hour, a repeat loading dose is recommended. The availability of bedside glucometers and of laboratory instruments for rapid and accurate glucose analysis (Beckman or Yellow Springs glucose analyzer) has contributed much to achieving optimal insulin replacement. Rarely, a patient with immune insulin resistance is encountered, and this requires doubling the insulin dose every 2 4 hours if hyperglycemia does not improve after the first two doses of insulin.
3. Fluid replacement
In most patients, the fluid deficit is 4 5 L. Initially, 0.9% saline solution is the solution of choice to help reexpand the contracted vascular volume and should be started in the emergency department as soon as the diagnosis is established. The saline should be infused rapidly to provide 1 L/h over the first 1 2 hours. After the first 2 L of fluid have been given, the intravenous infusion should be at the rate of 300 400 mL/h. Use 0.9 % ( normal ) saline unless the serum sodium is greater than 150 mEq/L, when 0.45% ( half normal ) saline solution should be used. The volume status should be very carefully monitored. Failure to give enough volume replacement (at least 3 4 L in 8 hours) to restore normal perfusion is one of the most serious therapeutic shortcomings adversely influencing satisfactory recovery. Excessive fluid replacement (more than 5 L in 8 hours) may contribute to acute respiratory distress syndrome or cerebral edema. When blood glucose falls to approximately 250 mg/dL, the fluids should be changed to a 5% glucose solution to maintain serum glucose in the range of 250 300 mg/dL. This will prevent the development of hypoglycemia and will also reduce the likelihood of cerebral edema, which could result from too rapid decline of blood glucose. Intensive insulin therapy should be continued until the ketoacidosis is corrected.
4. Sodium bicarbonate
The use of sodium bicarbonate in management of diabetic ketoacidosis has been questioned since clinical benefit was not demonstrated in one prospective randomized trial and because of the following potentially harmful consequences: (1) development of hypokalemia from rapid shift of potassium into cells if the acidosis is overcorrected, (2) tissue anoxia from reduced dissociation of oxygen from hemoglobin when acidosis is rapidly reversed (leftward shift of the oxygen dissociation curve), and (3) cerebral acidosis resulting from lowering of cerebrospinal fluid pH. It must be emphasized, however, that these considerations are less important when severe acidosis exists. It is therefore recommended that bicarbonate be administered to diabetic patients in ketoacidosis if the arterial blood pH is 7.0 or less, with careful monitoring to prevent overcorrection. One or two ampules of sodium bicarbonate (one ampule contains 44 mEq/50 mL) should be added to 1 L of 0.45% saline. (Note: Addition of sodium bicarbonate
P.1256
to 0.9% saline would produce a markedly hypertonic solution that could aggravate the hyperosmolar state already present.) This should be administered rapidly (over the first hour). It can be repeated until the arterial pH reaches 7.1, but it should not be given if the pH is 7.1 or greater since additional bicarbonate would increase the risk of rebound metabolic alkalosis as ketones are metabolized. Alkalosis shifts potassium from serum into cells, which could precipitate a fatal cardiac arrhythmia.
5. Potassium
Total body potassium loss from polyuria and vomiting may be as high as 200 mEq. However, because of shifts of potassium from cells into the extracellular space as a consequence of acidosis, serum potassium is usually normal to slightly elevated prior to institution of treatment. As the acidosis is corrected, potassium flows back into the cells, and hypokalemia can develop if potassium replacement is not instituted. If the patient is not uremic and has an adequate urinary output, potassium chloride in doses of 10 30 mEq/h should be infused during the second and third hours after beginning therapy as soon as the acidosis starts to resolve. Replacement should be started sooner if the initial serum potassium is inappropriately normal or low and should be delayed if serum potassium fails to respond to initial therapy and remains above 5 mEq/L, as in cases of renal insufficiency. An ECG can be of help in monitoring the patient's potassium status: High peaked T waves are a sign of hyperkalemia, and flattened T waves with U waves are a sign of hypokalemia. Foods high in potassium content should be prescribed when the patient has recovered sufficiently to take food orally. Tomato juice has 14 mEq of potassium per 240 mL, and a medium-sized banana provides about 10 mEq.
6. Phosphate
Phosphate replacement is seldom required in treating diabetic ketoacidosis. However, if severe hypophosphatemia of less than 1 mg/dL (< 0.32 mmol/L) develops during insulin therapy, a small amount of phosphate can be replaced per hour as the potassium salt. Correction of hypophosphatemia helps restore the buffering capacity of the plasma, thereby facilitating renal excretion of hydrogen. It also corrects the impaired oxygen dissociation from hemoglobin by regenerating 2,3-diphosphoglycerate. However, three randomized studies in which phosphate was replaced in only half of a group of patients with diabetic ketoacidosis did not show any apparent clinical benefit from phosphate administration. Moreover, attempts to use the phosphate salt of potassium as the sole means of replacing potassium have led to a number of reported cases of severe hypocalcemia with tetany. To minimize the risk of inducing tetany from too-rapid replacement of phosphate, the average deficit of 40 50 mmol of phosphate should be replaced intravenously at a rate no greater than 3 4 mmol/h in a 60 70-kg person. A stock solution (Abbott) provides a mixture of 1.12 g KH2PO4 and 1.18 g K2HPO4 in a 5-mL single-dose vial (this equals 22 mmol of potassium and 15 mmol of phosphate). One-half of this vial (2.5 mL) should be added to 1 L of either 0.45% saline or 5% dextrose in water. Two liters of this solution, infused at a rate of 400 mL/h, will correct the phosphate deficit at the optimal rate of 3 mmol/h while providing 4.4 mEq of potassium per hour. (Additional potassium should be administered as potassium chloride to provide a total of 10 30 mEq of potassium per hour, as noted above.) If the serum phosphate remains below 2.5 mg/dL after this infusion, a repeat 5-hour infusion can be given.
7. Hyperchloremic acidosis during therapy
Because of the considerable loss of keto acids in the urine during the initial phase of therapy, substrate for subsequent regeneration of bicarbonate is lost and correction of the total bicarbonate deficit is hampered. A portion of the bicarbonate deficit is replaced with chloride ions infused in large amounts as saline to correct the dehydration. In most patients, as the ketoacidosis clears during insulin replacement, a hyperchloremic, low-bicarbonate pattern emerges with a normal anion gap. This is a relatively benign condition that reverses itself over the subsequent 12 24 hours once intravenous saline is no longer being administered.
8. Treatment of associated infection
Antibiotics are prescribed as indicated. Cholecystitis and pyelonephritis may be particularly severe in these patients.
Prognosis
The frequency of deaths due to diabetic ketoacidosis has been dramatically reduced by improved therapy of young diabetics, but this complication remains a significant risk in the aged and in patients in profound coma in whom treatment has been delayed. Acute myocardial infarction and infarction of the bowel following prolonged hypotension worsen the outlook. A serious prognostic sign is renal failure, and prior kidney dysfunction worsens the prognosis considerably because the kidney plays a key role in compensating for massive pH and electrolyte abnormalities. Cerebral edema has been reported to occur rarely as metabolic deficits return to normal. This is best prevented by avoiding sudden reversal of marked hyperglycemia. Maintaining glycemic levels of 200 300 mg/dL for the initial 24 hours after correction of severe hyperglycemia reduces this risk.
Kitabchi AE et al: Management of hyperglycemic crises in patients with diabetes. Diabetes Care 2001;24:131.
Hyperglycemic Hyperosmolar State
![]() Essentials of Diagnosis
Essentials of Diagnosis
Hyperglycemia > 600 mg/dL.
Serum osmolality > 310 mosm/kg.
No acidosis; blood pH above 7.3.
Serum bicarbonate > 15 mEq/L.
Normal anion gap (< 14 mEq/L).
P.1257
General Considerations
This second most common form of hyperglycemic coma is characterized by severe hyperglycemia in the absence of significant ketosis, with hyperosmolality and dehydration. It occurs in patients with mild or occult diabetes, and most patients are at least middle-aged to elderly. Accurate figures are not available as to its true incidence, but from data on hospital discharges it is rarer than diabetic ketoacidosis even in older age groups. Lethargy and confusion develop as serum osmolality exceeds 310 mosm/kg, and coma can occur if osmolality exceeds 320 330 mosm/kg. A committee of the ADA has recommended replacing the previous name of this disorder (hyperglycemic, hyperosmolar, nonketotic coma) with the consensus term hyperglycemic hyperosmolar state. Underlying renal insufficiency or congestive heart failure is common, and the presence of either worsens the prognosis. A precipitating event such as infection, myocardial infarction, stroke, or recent operation is often present. Certain drugs such as phenytoin, diazoxide, corticosteroids, and diuretics have been implicated in its pathogenesis, as have procedures associated with glucose loading such as peritoneal dialysis.
Pathogenesis
A partial or relative insulin deficiency may initiate the syndrome by reducing glucose utilization of muscle, fat, and liver while inducing hyperglucagonemia and increasing hepatic glucose output. With massive glycosuria, obligatory water loss ensues. If a patient is unable to maintain adequate fluid intake because of an associated acute or chronic illness or has suffered excessive fluid loss, marked dehydration results. As plasma volume contracts, renal insufficiency develops, and the resultant limitation of renal glucose loss leads to increasingly higher blood glucose concentrations. Severe hyperosmolality develops that causes mental confusion and finally coma. It is not clear why ketosis is virtually absent under these conditions of insulin insufficiency, although reduced levels of growth hormone may be a factor, along with portal vein insulin concentrations sufficient to restrain ketogenesis.
Clinical Findings
A. Symptoms and Signs
Onset may be insidious over a period of days or weeks, with weakness, polyuria, and polydipsia. The lack of features of ketoacidosis may retard recognition of the syndrome and delay therapy until dehydration becomes more profound than in ketoacidosis. Reduced intake of fluid is not an uncommon historical feature, due to either inappropriate lack of thirst, nausea, or inaccessibility of fluids to elderly, bedridden patients. Lethargy and confusion develop, progressing to convulsions and deep coma. Physical examination confirms the presence of profound dehydration in a lethargic or comatose patient without Kussmaul respirations.
B. Laboratory Findings
(Table 27-12.) Severe hyperglycemia is present, with blood glucose values ranging from 600 mg/dL to 2400 mg/dL. In mild cases, where dehydration is less severe, dilutional hyponatremia as well as urinary sodium losses may reduce serum sodium to 120 125 mEq/L, which
P.1258
protects to some extent against extreme hyperosmolality. However, as dehydration progresses, serum sodium can exceed 140 mEq/L, producing serum osmolality readings of 330 440 mosm/kg. Ketosis and acidosis are usually absent or mild. Prerenal azotemia is the rule, with serum urea nitrogen elevations over 100 mg/dL being typical.
Table 27-12. Laboratory diagnosis of coma in diabetic patients. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Treatment
A. Saline
Fluid replacement is of paramount importance in treating nonketotic hyperglycemic coma. The onset of hyperosmolarity is more insidious in elderly people without ketosis than in younger individuals with high serum ketone levels, which provide earlier indicators of severe illness (vomiting, rapid deep breathing, acetone odor, etc). Consequently, diagnosis and treatment are often delayed until fluid deficit has reached levels of 6 10 L.
If hypovolemia is present as evidenced by hypotension and oliguria, fluid therapy should be initiated with 0.9% saline. In all other cases, 0.45% saline appears to be preferable as the initial replacement solution because the body fluids of these patients are markedly hyperosmolar. As much as 4 6 L of fluid may be required in the first 8 10 hours. Careful monitoring of the patient is required for proper sodium and water replacement. Once blood glucose reaches 250 mg/dL, fluid replacement should include 5% dextrose in either water, 0.45% saline solution, or 0.9% saline solution. The rate of dextrose infusion should be adjusted to maintain glycemic levels of 250 300 mg/dL in order to reduce the risk of cerebral edema. An important end point of fluid therapy is to restore urinary output to 50 mL/h or more.
B. Insulin
Less insulin may be required to reduce the hyperglycemia in nonketotic patients as compared to those with diabetic ketoacidotic coma. In fact, fluid replacement alone can reduce hyperglycemia considerably by correcting the hypovolemia, which then increases both glomerular filtration and renal excretion of glucose. An initial dose of 0.15 unit/kg is followed by an insulin infusion of 1 2 units/h, which is titrated to lower blood glucose levels by 50 70 mg/dL per hour.
C. Potassium
With the absence of acidosis, there may be no initial hyperkalemia unless associated renal failure is present. This results in less severe total potassium depletion than in diabetic ketoacidosis, and less potassium replacement is therefore needed. However, because initial serum potassium is usually not elevated and because it declines rapidly as a result of insulin's effect on driving potassium intracellularly, it has been recommended that potassium replacement be initiated earlier than in ketotic patients, assuming that no renal insufficiency or oliguria is present. Potassium chloride (10 mEq/L) can be added to the initial bottle of fluids administered if the patient's serum potassium is not elevated.
D. Phosphate
If severe hypophosphatemia (serum phosphate < 1 mg/dL [< 0.32 mmol/L]) develops during insulin therapy, phosphate replacement can be given as described for ketoacidotic patients (at 3 mmol/h).
Prognosis
The overall mortality rate of hyperglycemic, hyperosmolar, nonketotic coma is more than ten times that of diabetic ketoacidosis, chiefly because of its higher incidence in older patients, who may have compromised cardiovascular systems or associated major illnesses and whose dehydration is often excessive because of delays in recognition and treatment. (When patients are matched for age, the prognoses of these two hyperglycemic emergencies are reasonably comparable.) When prompt therapy is instituted, the mortality rate can be reduced from nearly 50% to that related to the severity of coexistent disorders.
Hyperglycemic crises in patients with diabetes mellitus. Diabetes Care 2001;24:154.
Trence DL et al: Hyperglycemic crisis in diabetes mellitus type 2. Endocrinol Metab Clin North Am 2001;30:817.
Lactic Acidosis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Severe acidosis with hyperventilation.
Blood pH below 7.30.
Serum bicarbonate < 15 mEq/L.
Anion gap > 15 mEq/L.
Absent serum ketones.
Serum lactate > 5 mmol/L.
General Considerations
Lactic acidosis is characterized by accumulation of excess lactic acid in the blood. Normally, the principal sources of this acid are the erythrocytes (which lack enzymes for aerobic oxidation), skeletal muscle, skin, and brain. Conversion of lactic acid to glucose and its oxidation principally by the liver but also by the kidneys represent the chief pathways for its removal. Overproduction of lactic acid (tissue hypoxia), deficient removal (hepatic failure), or both (circulatory collapse) can cause accumulation. Lactic acidosis is not uncommon in any severely ill patient suffering from cardiac decompensation, respiratory or hepatic failure, septicemia,
P.1259
or infarction of bowel or extremities. With the discontinuance of phenformin therapy in the United States, lactic acidosis in patients with diabetes mellitus has become uncommon but occasionally occurs in metformin-treated patients (see above) and it still must be considered in the acidotic diabetic, especially if the patient is seriously ill.
Clinical Findings
A. Symptoms and Signs
The main clinical feature of lactic acidosis is marked hyperventilation. When lactic acidosis is secondary to tissue hypoxia or vascular collapse, the clinical presentation is variable, being that of the prevailing catastrophic illness. However, in the idiopathic, or spontaneous, variety, the onset is rapid (usually over a few hours), blood pressure is normal, peripheral circulation is good, and there is no cyanosis.
B. Laboratory Findings
Plasma bicarbonate and blood pH are quite low, indicating the presence of severe metabolic acidosis. Ketones are usually absent from plasma and urine or at least not prominent. The first clue may be a high anion gap (serum sodium minus the sum of chloride and bicarbonate anions [in mEq/L] should be no greater than 15). A higher value indicates the existence of an abnormal compartment of anions. If this cannot be clinically explained by an excess of keto acids (diabetes), inorganic acids (uremia), or anions from drug overdosage (salicylates, methyl alcohol, ethylene glycol), then lactic acidosis is probably the correct diagnosis. (See Chapter 21 also.) In the absence of azotemia, hyperphosphatemia may be a clue to the presence of lactic acidosis for reasons that are not clear. The diagnosis is confirmed by demonstrating, in a sample of blood that is promptly chilled and separated, a plasma lactic acid concentration of 5 mmol/L or higher (values as high as 30 mmol/L have been reported). Normal plasma values average 1 mmol/L, with a normal lactate/pyruvate ratio of 10:1. This ratio is greatly exceeded in lactic acidosis.1
Treatment
Aggressive treatment of the precipitating cause of lactic acidosis is the main component of therapy, such as ensuring adequate oxygenation and vascular perfusion of tissues. Empiric antibiotic coverage for sepsis should be given after culture samples are obtained in any patient in whom the cause of the lactic acidosis is not apparent.
Alkalinization with intravenous sodium bicarbonate to keep the pH above 7.2 has been recommended by some in the emergency treatment of lactic acidosis; as much as 2000 mEq in 24 hours has been used. However, there is no evidence that the mortality rate is favorably affected by administering bicarbonate, and its use remains controversial. Hemodialysis may be useful in cases where large sodium loads are poorly tolerated.
Prognosis
The mortality rate of spontaneous lactic acidosis is high. The prognosis in most cases is that of the primary disorder that produced the lactic acidosis.
Footnote
1In collecting samples, it is essential to rapidly chill and separate the blood in order to remove red cells, whose continued glycolysis at room temperature is a common source of error in reports of high plasma lactate. Frozen plasma remains stable for subsequent assay.
Forsythe SM et al: Sodium bicarbonate for the treatment of lactic acidosis. Chest 2000;117:260.
Salpeter S et al: Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 2003:CD002967.
The Hypoglycemic States
Spontaneous hypoglycemia in adults is of two principal types: fasting and postprandial. Symptoms begin at plasma glucose levels in the range of 60 mg/dL and impairment of brain function at approximately 50 mg/dL. Fasting hypoglycemia is often subacute or chronic and usually presents with neuroglycopenia as its principal manifestation; postprandial hypoglycemia is relatively acute and is often heralded by symptoms of neurogenic autonomic discharge (sweating, palpitations, anxiety, tremulousness).
Differential Diagnosis (Table 27-13)
Fasting hypoglycemia may occur in certain endocrine disorders, such as hypopituitarism, Addison's disease, or myxedema; in disorders related to liver malfunction, such as acute alcoholism or liver failure; and in instances of renal failure, particularly in patients requiring dialysis. These conditions are usually obvious, with hypoglycemia being only a secondary feature. When fasting hypoglycemia is a primary manifestation developing in adults without apparent endocrine disorders or inborn metabolic diseases from childhood, the principal diagnostic possibilities include (1) hyperinsulinism, due to either pancreatic B cell tumors or islet hyperplasia or surreptitious administration of insulin (or sulfonylureas), and (2) hypoglycemia due to non-insulin-producing extrapancreatic tumors.
Table 27-13. Common causes of hypoglycemia in adults.1 | ||
|---|---|---|
|
Postprandial (reactive) hypoglycemia may be classified as early (within 2 3 hours after a meal) or late (3 5 hours after eating). Early, or alimentary, hypoglycemia occurs when there is a rapid discharge of ingested carbohydrate into the small bowel followed
P.1260
by rapid glucose absorption and hyperinsulinism. It may be seen after gastrointestinal surgery and is particularly associated with the dumping syndrome after gastrectomy. In some cases, it is functional and may represent overactivity of the parasympathetic nervous system mediated via the vagus nerve. Rarely, it results from defective counterregulatory responses such as deficiencies of growth hormone, glucagon, cortisol, or autonomic responses.
Alcohol-related hypoglycemia is due to hepatic glycogen depletion combined with alcohol-mediated inhibition of gluconeogenesis. It is most common in malnourished alcohol abusers but can occur in anyone who is unable to ingest food after an acute alcoholic episode followed by gastritis and vomiting.
Immunopathologic hypoglycemia is an extremely rare condition in which anti-insulin antibodies or antibodies to insulin receptors develop spontaneously. In the former case, the mechanism appears to relate to increasing dissociation of insulin from circulating pools of bound insulin. When antibodies to insulin receptors are found, most patients do not have hypoglycemia but rather severe insulin-resistant diabetes and acanthosis nigricans. However, during the course of the disease in these patients, certain anti-insulin receptor antibodies with agonist activity mimicking insulin action may develop, producing severe hypoglycemia.
Factitious hypoglycemia is self-induced hypoglycemia due to surreptitious administration of insulin or sulfonylureas.
Hypoglycemia due to Pancreatic B Cell Tumors
![]() Essentials of Diagnosis
Essentials of Diagnosis
Hypoglycemic symptoms frequently neuroglycopenic (confusion, blurred vision, diplopia, anxiety, convulsions).
Immediate recovery upon administration of glucose.
Blood glucose < 40 mg/dL with a serum insulin level of 6 microunit/mL or more.
General Considerations
Fasting hypoglycemia in an otherwise healthy, well-nourished adult is rare and is most commonly due to an adenoma of the islets of Langerhans. Ninety percent of such tumors are single and benign, but multiple adenomas can occur as well as malignant tumors with functional metastases. Adenomas may be familial, and multiple adenomas have been found in conjunction with tumors of the parathyroids and pituitary (multiple endocrine neoplasia type 1 [MEN 1]).
Clinical Findings
A. Symptoms and Signs
The most important prerequisite to diagnosing an insulinoma is simply to consider it, particularly in relatively healthy-appearing persons who have fasting hypoglycemia associated with some degree of central nervous system dysfunction such as confusion or abnormal behavior. A delay in diagnosis can result in unnecessary treatment for psychomotor epilepsy or psychiatric disorders and may cause irreversible brain damage. In longstanding cases, obesity can result as a consequence of overeating to relieve symptoms.
Whipple's triad is characteristic of hypoglycemia regardless of the cause. It consists of (1) a history of hypoglycemic symptoms, (2) an associated fasting blood glucose of 40 mg/dL or less, and (3) immediate recovery upon administration of glucose. The hypoglycemic symptoms in insulinoma often develop in the early morning or after missing a meal. Occasionally, they occur after exercise. They typically begin with evidence of central nervous system glucose lack and can include blurred vision or diplopia, headache, feelings of detachment, slurred speech, and weakness. Personality and mental changes vary from anxiety to psychotic behavior, and neurologic deterioration can result in convulsions or coma. Sweating and palpitations may not occur.
Hypoglycemic unawareness is very common in patients with insulinoma. They adapt to chronic hypoglycemia by increasing their efficiency in transporting glucose
P.1261
across the blood-brain barrier, which masks awareness that their blood glucose is approaching critically low levels. Counterregulatory hormonal responses as well as neurogenic symptoms such as tremor, sweating, and palpitations are therefore blunted during hypoglycemia. If lack of these warning symptoms prevents recognition of the need to eat to correct the problem, patients can lapse into severe hypoglycemic coma. However, symptoms and normal hormone responses during experimental insulin-induced hypoglycemia have been shown to be restored after successful surgical removal of the insulinoma. Presumably with return of euglycemia, adaptive effects on glucose transport into the brain are corrected, and thresholds of counterregulatory responses and neurogenic autonomic symptoms are therefore restored to normal.
B. Laboratory Findings
B cell adenomas do not reduce secretion in the presence of hypoglycemia, and the critical diagnostic test is to demonstrate inappropriately elevated serum insulin levels at a time when hypoglycemia is present. A reliable serum insulin level (radioimmunoassay) of 6 microunit/mL or more in the presence of blood glucose values below 40 mg/dL is diagnostic of inappropriate hyperinsulinism. Immunochemiluminometric assays (ICMA) have sensitivities of less than 1 microunit/mL, and with these assays, the cutoffs for insulinomas is insulin level 3 microunit/mL or higher. Other causes of hyperinsulinemic hypoglycemia must be considered, including factitious administration of insulin or sulfonylureas. Factitious use of insulin will result in suppression of endogenous insulin secretion and a low C-peptide levels. An elevated circulating proinsulin level in the presence of fasting hypoglycemia is characteristic of most B cell adenomas and does not occur in factitious hyperinsulinism. Thus, C-peptide and proinsulin levels (by ICMA) of > 200 pmol/L and > 5 pmol/L, respectively, are characteristic of insulinomas.
In patients with epigastric distress, a history of renal stones, or menstrual or erectile dysfunction, a serum calcium, gastrin, or prolactin level may be useful in screening for MEN 1 associated with insulinoma.
C. Diagnostic Tests
1. Prolonged fasting
Prolonged fasting under hospital supervision until hypoglycemia is documented is probably the most dependable means of establishing the diagnosis, especially in men. In 30% of patients with insulinoma, the blood glucose levels often drop below 40 mg/dL after an overnight fast, but some patients require up to 72 hours to develop symptomatic hypoglycemia. However, the term 72-hour fast is actually a misnomer in most cases since the fast should be immediately terminated as soon as symptoms appear and laboratory confirmation of hypoglycemia is available. In normal male subjects, the blood glucose does not fall below 55 60 mg/dL during a 3-day fast. In contrast, in normal premenopausal women who have fasted for only 24 hours, the plasma glucose may fall normally to such an extent that it can reach values as low as 35 mg/dL. In these cases, however, the women are not symptomatic, presumably owing to the development of sufficient ketonemia to supply energy needs to the brain. Insulinoma patients, on the other hand, become symptomatic when plasma glucose drops to subnormal levels, since inappropriate insulin secretion restricts ketone formation. Moreover, the demonstration of a nonsuppressed insulin level 6 microunit/mL using a RIA assay (> 3 microunit/mL using an ICMA assay) in the presence of hypoglycemia suggests the diagnosis of insulinoma. If hypoglycemia does not develop in a male patient after fasting for up to 72 hours and particularly when this prolonged fast is terminated with a period of moderate exercise insulinoma must be considered an unlikely diagnosis. A suggested protocol for the supervised fast is shown in Table 27-14.
2. Stimulation tests
Stimulation with pancreatic B cell secretagogues such as tolbutamide, glucagon, or leucine is generally not needed in most cases if basal insulin is found to be nonsuppressible and therefore inappropriately elevated during fasting hypoglycemia.
Intravenous glucagon (1 mg over 1 minute) can be useful in patients with borderline fasting inappropriate hyperinsulinism. A serum insulin rise above baseline of 200 microunit/mL or more at 5 and 10 minutes strongly suggests insulinoma, although poorly differentiated tumors may not respond. Glucagon has the advantage over tolbutamide of correcting rather than provoking hypoglycemia
P.1262
during stimulation testing and is diagnostic in 60 70% of patients with insulinoma. False-negative results can occur if the tumor is poorly differentiated and agranular.
Table 27-14. Suggested hospital protocol for supervised fast in diagnosis of insulinoma. | |
|---|---|
|
D. Preoperative Localization of B Cell Tumors
After the diagnosis of insulinoma has been unequivocally made by clinical and laboratory findings, studies to localize the tumor should be initiated. The focus of attention should be directed to the pancreas since that is where virtually all insulinomas originate.
A spiral CT angiography of the pancreas should be performed to rule out a large tumor of the pancreas or hepatic metastases from a malignant islet cell tumor. Endoscopic ultrasonographic examination of the pancreas can also be helpful in identifying the pancreatic lesions. Because of the small size of these tumors (averaging 1.5 cm in diameter in one large series), imaging studies do not necessarily identify all the tumors. Patients who do not have an obvious tumor on imaging (but have a definite biochemical diagnosis) should undergo selective calcium-stimulated angiography. In this test, angiography is combined with injections of calcium gluconate into the gastroduodenal, splenic, and superior mesenteric arteries and insulin levels are measured in the hepatic vein effluent. Calcium stimulates insulin release from insulinomas but not normal islets, and so a step-up of insulin levels regionalizes the hyperinsulinism to the head of the pancreas for the gastroduodenal artery, to the uncinate process for the superior mesenteric artery, and to the body and tail of the pancreas for the splenic artery. This technique may also provide data that are particularly helpful when multiple insulinomas are suspected (MEN 1) and it has become a major tool in confirming the diagnosis of diffuse islet hyperplasia in the recently described noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) (see below). Since diazoxide might interfere with this test, it should be discontinued for at least 48 72 hours before sampling. An infusion of dextrose may be required, therefore, and patients should be closely monitored during the procedure to avoid hypoglycemia (as well as hyperglycemia, which could affect insulin gradients). These studies combined with careful intraoperative ultrasonography and palpation by a surgeon experienced in insulinoma surgery identifies up to 98% of tumors.
Treatment
A. Surgical Measures
It is imperative that the surgeon be convinced that the diagnosis of insulinoma has been unequivocally made by clinical and laboratory findings. Only then should surgery be considered, as there is no justification for exploratory operation just as there is none for the use of current localization techniques as a preoperative diagnostic tool. Resection by a surgeon with previous experience in removing pancreatic B cell tumors is the treatment of choice. In patients with a single benign adenoma, 90 95% have a successful cure at the first surgical attempt when intraoperative ultrasound is used by a skilled surgeon. Blood glucose should be monitored throughout surgery, and 10% dextrose in water should be infused at a rate of 100 mL/h or faster. In cases where the diagnosis has been established but no adenoma is located after careful palpation and use of intraoperative ultrasound, it is no longer advisable to blindly resect the body and tail of the pancreas, since a nonpalpable tumor missed by ultrasound is most likely embedded within the fleshy head of the pancreas that is left behind with subtotal resections. Most surgeons prefer to close the incision and schedule a selective arterial calcium stimulation with hepatic venous sampling to locate the tumor site prior to a repeat operation. Laparoscopy using ultrasound and enucleation has been successful with a single tumor of the body or tail of the pancreas, but open surgery remains necessary for tumors in the head of the pancreas.
B. Diet and Medical Therapy
In patients with inoperable functioning islet cell carcinoma and in approximately 5 10% of MEN 1 cases when subtotal removal of the pancreas has failed to produce cure, reliance on frequent feedings is necessary. Since most tumors are not responsive to glucose, carbohydrate feedings every 2 3 hours are usually effective in preventing hypoglycemia, although obesity may become a problem. Diazoxide, 300 600 mg (or 3 mg to 8 mg/kg; 50 mg/mL oral suspension) daily orally in two or three divided doses, is the treatment of choice. Hydrochlorothiazide, 25 50 mg daily, should also be prescribed to counteract the sodium retention and edema secondary to diazoxide therapy as well as to potentiate its hyperglycemic effect. If patients are unable to tolerate diazoxide because of gastrointestinal upset, hirsutism, or edema, the calcium channel blocker verapamil may be beneficial in view of its inhibitory effect on insulin release from insulinoma cells. Octreotide, a potent long-acting synthetic octapeptide analog of somatostatin, has been used to inhibit release of hormones from a number of endocrine tumors. A dose of 50 mcg of octreotide injected subcutaneously twice daily has been tried in cases where surgery failed to remove the source of hyperinsulinism. However, its effectiveness is limited since its affinity for somatostatin receptors of the pancreatic B cell is very much less than for those of the anterior pituitary somatotrophs for which it was originally designed as treatment for acromegaly. When hypoglycemia persists after attempted surgical removal of the insulinoma and if diazoxide or verapamil is poorly tolerated or ineffective, multiple small feedings may be the only recourse until more selective somatostatin receptor agonists are available. Streptozocin can decrease insulin secretion in islet cell carcinomas, and effective doses have been delivered via selective arterial catheter so that the undue renal toxicity that characterized early experience is less of a problem.
Prognosis
When insulinoma is diagnosed early and cured surgically, complete recovery is likely, although brain damage
P.1263
following prolonged severe hypoglycemia is not reversible. A significant increase in survival rate has been shown in streptozocin-treated patients with islet cell carcinoma, with reduction in tumor mass as well as decreased hyperinsulinism.
Grant CS: Surgical aspects of hyperinsulinemic hypoglycemia. Endocrinol Metab Clin North Am 1999;28:533.
Hirshberg B et al: Forty-eight-hour fast: the diagnostic test for insulinoma. J Clin Endocrinol Metab 2000;85:3222.
Service FJ: Diagnostic approach to adults with hypoglycemic disorders. Endocrinol Metab Clin North Am 1999;28: 519.
Tucker ON et al: The management of insulinoma. Br J Surg 2006;93:264.
Persistent Islet Hyperplasia (Noninsulinoma Pancreatogenous Hypoglycemia Syndrome)
In a very small number of patients with organic hyperinsulinism, islet hyperplasia rather than an adenoma is present. These patients typically have documented hyperinsulinemic hypoglycemia after meals but not with fasting up to 72 hours. The patients have a positive response to calcium-stimulated angiography. A gradient-guided partial pancreatectomy leads to clinical remission, and the pathology of the pancreas shows evidence of islet hyperplasia and nesidioblastosis. These patients do not have mutations in the Kir 6.2 and SUR1 genes, which has been reported in children with familial hyperinsulinemic hypoglycemia.
Hypoglycemia due to Extrapancreatic Tumors
These rare causes of hypoglycemia include mesenchymal tumors such as retroperitoneal sarcomas, hepatocellular carcinomas, adrenocortical carcinomas, and miscellaneous epithelial-type tumors. The tumors are frequently large and readily palpated or visualized on CT scans or MRI.
The expression and release of an incompletely processed insulin-like growth factor-2 (IGF-2) has provided the best explanation for the clinical manifestations of hypoglycemia in these cases. A larger immature form of the IGF-2 molecule is released which binds to a carrier protein but not to an acid-labile component of serum which inactivates normal IGF-2. This immature IGF-2 complex therefore remains active and binds to insulin receptors in muscle to promote glucose transport and to insulin receptors in liver and kidney to reduce glucose output. It also binds to receptors for IGF-1 in the pancreatic B cell to inhibit insulin secretion. Serum levels of IGF-2 may be increased but often are normal in quantity, despite the presence of the immature, higher-molecular-weight form of IGF-2, which can only be detected by special laboratory techniques. Laboratory diagnosis depends on documenting fasting hypoglycemia associated with undetectable serum insulin levels.
The prognosis for these tumors is generally poor, and surgical removal should be attempted when feasible. Dietary management of the hypoglycemia is the mainstay of medical treatment, since diazoxide is usually ineffective.
Le Roith D: Tumor-induced hypoglycemia. N Engl J Med 1999; 341:757.
Postprandial Hypoglycemia (Reactive Hypoglycemia)
Postgastrectomy Alimentary Hypoglycemia
Reactive hypoglycemia following gastrectomy is a consequence of hyperinsulinism resulting from rapid gastric emptying of ingested food. Symptoms result from adrenergic hyperactivity in response to the hypoglycemia. Treatment consists of more frequent feedings with smaller portions of less rapidly assimilated carbohydrate combined with more slowly absorbed fat and protein. Cases of hypoglycemia have been reported in patients who have undergone Roux-en-Y gastric bypass surgery for the treatment of obesity. The hypoglycemia occurs after meals and can be severe. Some of these patients are found to have islet cell hyperplasia.
Functional Alimentary Hypoglycemia
This syndrome is classified as functional when no postsurgical explanation exists for the presence of early alimentary type reactive hypoglycemia. It is most often associated with chronic fatigue, anxiety, irritability, weakness, poor concentration, decreased libido, headaches, hunger after meals, and tremulousness. However, most patients with these symptoms do not have hypoglycemia after a mixed meal. (See Chronic Fatigue Syndrome in Chapter 2.)
Indiscriminate use and overinterpretation of glucose tolerance tests have led to an unfortunate tendency to overdiagnose functional hypoglycemia. As many as one-third or more of normal subjects have blood glucose levels as low as 40 50 mg/dL with or without symptoms during a 4-hour glucose tolerance test. Accordingly, to increase diagnostic reliability, hypoglycemia should preferably be documented during a spontaneous symptomatic episode accompanying routine daily activity, with clinical improvement following feeding. Oral glucose tolerance tests are overly sensitive and mixed meals are relatively insensitive in detecting postprandial reactive hypoglycemia. It has been shown that a high-carbohydrate breakfast has proved useful in differentiating persons with postprandial reactive hypoglycemia from normal controls. The test resulted in reactive hypoglycemia to levels below 59 mg/dL in 47% of 38 subjects, in contrast to only 2.2% of the 43 controls. This test was
P.1264
found to be much more sensitive than a standard mixed meal, which was also given to these two groups.
In patients with documented postprandial hypoglycemia on a functional basis, there is no harm and occasional benefit in reducing the proportion of carbohydrate in the diet while increasing the frequency and reducing the size of meals. Support and mild sedation should be the mainstays of therapy, with dietary manipulation only an adjunct.
Late Hypoglycemia (Occult Diabetes)
This condition is characterized by a delay in early insulin release from pancreatic B cells, resulting in initial exaggeration of hyperglycemia during a glucose tolerance test. In response to this hyperglycemia, an exaggerated insulin release produces a late hypoglycemia 4 5 hours after ingestion of glucose. These patients are usually quite different from those with early hypoglycemia occurring 2 3 hours after glucose ingestion, often being obese and frequently having a family history of diabetes mellitus.
In obese patients, treatment is directed at weight reduction to achieve ideal weight. Like all patients with postprandial hypoglycemia, regardless of cause, these patients often respond to reduced carbohydrate intake with multiple, spaced, small feedings. They should be considered potential diabetics and advised to have periodic medical evaluations.
Service GJ et al: Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353:249.
Alcohol-Related Hypoglycemia
Fasting Hypoglycemia after Ethanol
During the postabsorptive state, normal plasma glucose is maintained by hepatic glucose output derived from both glycogenolysis and gluconeogenesis. With prolonged starvation, glycogen reserves become depleted within 18 24 hours and hepatic glucose output becomes totally dependent on gluconeogenesis. Under these circumstances, a blood concentration of ethanol as low as 45 mg/dL can induce profound hypoglycemia by blocking gluconeogenesis. Neuroglycopenia in a patient whose breath smells of alcohol may be mistaken for alcoholic stupor. Prevention consists of adequate food intake during ethanol ingestion. Therapy consists of glucose administration to replenish glycogen stores until gluconeogenesis resumes.
Postethanol Reactive Hypoglycemia
When sugar-containing soft drinks are used as mixers to dilute alcohol in beverages (gin and tonic, rum and cola), there seems to be a greater insulin release than when the soft drink alone is ingested and a tendency for more of a late hypoglycemic overswing to occur 3 4 hours later. Prevention would consist of avoiding sugar mixers while ingesting alcohol and ensuring supplementary food intake to provide sustained absorption.
Factitious Hypoglycemia
Factitious hypoglycemia may be difficult to document. A suspicion of self-induced hypoglycemia is supported when the patient is associated with the health professions or has access to insulin or sulfonylurea drugs taken by a diabetic member of the family. The triad of hypoglycemia, high immunoreactive insulin, and suppressed plasma C peptide immunoreactivity is pathognomonic of exogenous insulin administration. Demonstration of circulating insulin antibodies supports this diagnosis in suspected cases. When sulfonylureas are suspected as a cause of factitious hypoglycemia, a plasma level of these drugs to detect their presence may be required to distinguish laboratory findings from those of insulinoma. Unfortunately, the newer sulfonylurea, glimepiride, and other insulinotropic hypoglycemic drugs like repaglinide and nateglinide are not detected in the standard assays for sulfonylureas.
Immunopathologic Hypoglycemia
This rare cause of hypoglycemia, documented in isolated case reports, may occur as two distinct disorders: one associated with spontaneous development of circulating anti-insulin antibodies and another associated with antibodies to insulin receptors, in which the antibodies apparently have agonist capabilities. This latter disorder is extremely rare, having been documented in no more than five cases. However, development of anti-insulin antibodies has been reported in over 200 patients most of whom were being treated with methimazole for thyrotoxicosis. In Western countries, 23 cases have been reported and include patients with a lupus-like syndrome or with various paraproteinemias. The hypoglycemia occurs 3 4 hours after meals following an initial postprandial hyperglycemic phase that is due to the antibodies interfering with the exit of insulin from the plasma to reach its target tissues. Later, after most of the meal is absorbed, inappropriate high levels of insulin dissociate from this antibody-bound compartment, resulting in hypoglycemia. Insulin levels in excess of 1000 pmol/L are observed at time of hypoglycemia, and these persons have high titers of insulin autoantibodies.
Redmon JB et al: Autoimmune hypoglycemia. Endocrinol Metab Clin North Am 1999;28:603.
Drug-Induced Hypoglycemia
A number of drugs apart from the sulfonylureas can occasionally cause hypoglycemia, especially when ingested in large amounts. These include quinine, quinidine, disopyramide, and salicylates. ACE inhibitors,
P.1265
when taken with antidiabetic drugs, can cause hypoglycemia possibly by improving insulin sensitivity. Recently, it has been reported that the use of gatifloxacin in diabetic patients is associated with serious hypoglycemic and hyperglycemic reactions.
Ten to 20 percent of patients receiving pentamidine for Pneumocystis jiroveci pneumonia develop symptomatic hypoglycemia, particularly when the drug is administered intravenously. This apparently is due to lytic destruction of pancreatic B cells, causing acute hyperinsulinemia and hypoglycemia. It is later followed by insulinopenia and hyperglycemia, which occasionally is persistent. Since other drugs have been found to be as effective in treating Pneumocystis pneumonia, pentamidine administration and its hypoglycemic sequelae are less frequently encountered.
EAN: 2147483647
Pages: 49