11 - Systemic Hypertension
Editors: McPhee, Stephen J.; Papadakis, Maxine A.; Tierney, Lawrence M.
Title: Current Medical Diagnosis & Treatment, 46th Edition
Copyright 2007 McGraw-Hill
> Table of Contents > 14 - Alimentary Tract
function show_scrollbar() {}
14
Alimentary Tract
Kenneth R. McQuaid MD
Symptoms & Signs of Gastrointestinal Disease
Dyspepsia
Dyspepsia refers to acute, chronic, or recurrent pain or discomfort centered in the upper abdomen. The discomfort may be characterized by or associated with upper abdominal fullness, early satiety, burning, bloating, belching, nausea, retching, or vomiting. Heartburn (retrosternal burning) should be distinguished from dyspepsia. Patients with dyspepsia often have heartburn as an additional symptom. When heartburn is the dominant complaint, gastroesophageal reflux is nearly always present. Dyspepsia occurs in 25% of the adult population and accounts for 3% of general medical office visits.
Etiology
A. Food or Drug Intolerance
Acute, self-limited indigestion may be caused by overeating, eating too quickly, eating high-fat foods, eating during stressful situations, or drinking too much alcohol or coffee. Many medications cause dyspepsia, including aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics (metronidazole, macrolides), various diabetes drugs (metformin, alpha-glucosidase inhibitors, amylin analogs, GLP-1 receptor antagonists), cholinesterase inhibitors (donepezil, rivastigmine), corticosteroids, digoxin, iron, and opioids.
B. Luminal Gastrointestinal Tract Dysfunction
Peptic ulcer disease is present in 5 15% of patients with dyspepsia. Gastroesophageal reflux disease is present in up to 20% of patients with dyspepsia, even without significant heartburn. Gastric cancer is identified in 1% but is rare in persons under age 45 years. Other causes include gastroparesis (especially in diabetes mellitus), lactose intolerance or malabsorptive conditions, and parasitic infection (Giardia, Strongyloides).
C. Helicobacter pylori Infection
Chronic gastric infection with H pylori as a cause of dyspepsia remains controversial. The prevalence of H pylori-associated chronic gastritis in patients with dyspepsia without peptic ulcer disease is 20 50%, the same as in the general population.
D. Pancreatic Disease
Pancreatic carcinoma, chronic pancreatitis.
E. Biliary Tract Disease
The abrupt onset of epigastric or right upper quadrant pain due to cholelithiasis or choledocholithiasis should be readily distinguished from dyspepsia.
F. Other Conditions
Diabetes, thyroid disease, renal insufficiency, myocardial ischemia, intra-abdominal malignancy, gastric volvulus or paraesophageal hernia, and pregnancy are sometimes accompanied by dyspepsia.
G. Functional Dyspepsia
This is the most common cause of chronic dyspepsia. Up to two-thirds of patients have no obvious organic cause for their symptoms after evaluation. Symptoms may arise from a complex interaction of increased visceral afferent sensitivity, gastric delayed emptying or impaired accommodation to food, or psychosocial stressors. Although benign, these symptoms may be chronic and difficult to treat.
Clinical Findings
A. Symptoms and Signs
Given the nonspecific nature of dyspeptic symptoms, the history has limited diagnostic utility. It should clarify the chronicity, location, and quality of the discomfort, its relationship to meals, and whether it is relieved by antacids. Concomitant weight loss, persistent vomiting, constant or severe pain, dysphagia, hematemesis, or melena warrants endoscopy or abdominal imaging. Potentially offending medications and excessive alcohol use should be identified and discontinued if possible. The patient's reason for seeking care should be determined. Recent changes in employment, marital discord, physical and sexual abuse, anxiety, depression, and fear of serious disease may all contribute to the development and reporting of symptoms. Patients with functional dyspepsia often are younger, report a variety of abdominal
P.549
and extragastrointestinal complaints, show signs of anxiety or depression, or have a history of use of psychotropic medications.
The symptom profile alone does not differentiate between functional dyspepsia and organic gastrointestinal disorders. Based on the clinical history alone, primary care physicians misdiagnose nearly half of patients with peptic ulcers or gastroesophageal reflux and have < 25% accuracy in diagnosing functional dyspepsia.
The physical examination is rarely helpful. Signs of serious organic disease such as weight loss, organomegaly, abdominal mass, or fecal occult blood are further evaluated. In patients over age 50 years, initial laboratory work should include a blood count, electrolytes, liver enzymes, calcium, and thyroid function tests.
B. Special Examinations
1. Upper endoscopy
Upper endoscopy is the study of choice to diagnose gastroduodenal ulcers, erosive esophagitis, and upper gastrointestinal malignancy. Upper gastrointestinal barium radiography is inferior to endoscopy for the evaluation of dyspepsia. Upper endoscopy is indicated to look for gastric cancer or other serious organic disease in all patients over age 55 years with new-onset dyspepsia and in all patients with alarm features such as weight loss, dysphagia, recurrent vomiting, evidence of bleeding, or anemia. It is also helpful for patients who are concerned about serious underlying disease. For patients born in regions in which there is a higher incidence of gastric cancer, an age threshold of 45 years may be appropriate.
2. Empiric management
In patients younger than 55 years with uncomplicated dyspepsia (in whom gastric cancer is rare), initial noninvasive management strategies should be pursued. In most clinical settings, a noninvasive test for H pylori (IgG serology, fecal antigen test, or urea breath test) should be performed first. Although serologic tests are inexpensive, performance characteristics are poor in low-prevalence populations. If test results are negative in a patient not taking NSAIDs, peptic ulcer disease is virtually excluded. Most of these H pylori-negative patients have functional dyspepsia or atypical gastroesophageal reflux disease and can be treated with an antisecretory agent (proton pump inhibitor) for 4 weeks. For patients who have symptom relapse after discontinuation of the proton pump inhibitor, intermittent or long-term proton pump inhibitor therapy may be considered.
For patients in whom test results are positive for H pylori, antibiotic therapy proves definitive for over 90% of peptic ulcers and may improve symptoms in a small subset (< 10%) of infected patients with functional dyspepsia. Patients with persistent dyspepsia after H pylori eradication can be given a trial of proton pump inhibitor therapy. In clinical settings in which the prevalence of H pylori infection in the population is low (< 10%), it may be more cost-effective to initially treat all young patients with uncomplicated dyspepsia with a 4-week trial of a proton pump inhibitor. Patients who have symptom relapse after discontinuation of the proton pump inhibitor should be tested for H pylori and treated if positive.
Endoscopic evaluation is warranted when symptoms fail to respond to initial empiric management strategies or frequent symptom relapse occurs after discontinuation of antisecretory therapy. Abdominal imaging (ultrasonography or CT scanning) is performed only when pancreatic or biliary tract disease is suspected. Gastric emptying studies are valuable only in patients with recurrent vomiting. Ambulatory esophageal pH testing may be of value when atypical gastroesophageal reflux is suspected.
Treatment of Functional Dyspepsia
Regardless of the initial strategy undertaken for patients with dyspepsia, a significant proportion will have persistent or recurrent symptoms requiring evaluation with endoscopy. Most patients will have no significant findings and will be given a diagnosis of functional dyspepsia.
A. General Measures
Most patients have mild, intermittent symptoms that respond to reassurance and lifestyle changes. Alcohol, caffeine, and fatty foods should be reduced or discontinued. A food diary, in which patients record their food intake, symptoms, and daily events, may reveal dietary or psychosocial precipitants of pain.
B. Pharmacologic Agents
Drugs have demonstrated limited efficacy in the treatment of functional dyspepsia. One-third of patients derive relief from placebo. Antisecretory therapy for 2 4 weeks with either H2-receptor antagonists (ranitidine or nizatidine, 150 mg twice daily; famotidine, 20 mg twice daily; or cimetidine, 400 800 mg twice daily) or proton pump inhibitors (omeprazole, esomeprazole, or rabeprazole 20 mg, lansoprazole 30 mg, or pantoprazole 40 mg) may benefit 10 15% of patients, particularly those with dyspepsia and heartburn ( reflux-like dyspepsia ). Superiority of proton pump inhibitors to H2-antagonists has not been established. Low doses of antidepressants (eg, desipramine or nortriptyline, 10 50 mg at bedtime) are believed to benefit some patients, possibly by moderating visceral afferent sensitivity. However, side effects are common and response is patient specific. Doses should be increased slowly. The prokinetic agent metoclopramide (5 10 mg three times daily) may improve symptoms, but improvement does not correlate with the presence or absence of gastric emptying delay. Long-term metoclopramide use is associated with a high incidence of neuropsychiatric side effects and cannot be recommended. Limited studies to date have not demonstrated efficacy for the prokinetic agent tegaserod.
C. Anti-H pylori Treatment
A meta-analysis has suggested that a small number of patients (< 10%) derive benefit from H pylori eradication therapy.
P.550
D. Alternative Therapies
Psychotherapy and hypnotherapy may be of benefit in selected motivated patients. Herbal therapies (peppermint, caraway) may offer benefit with little risk of adverse effects.
Ford AC et al: Helicobacter pylori test and treat or endoscopy for managing dyspepsia: an individual patient data meta-analysis. Gastroenterology 2005;128:1838.
Gupta S et al: Management of nonsteroidal, anti-inflammatory, drug-associated dyspepsia. Gastroenterology 2005;129:1711.
Talley NJ et al; American Gastroenterological Association: American Gastroenterological Association Medical Position Statement: Evaluation of dyspepsia. Gastroenterology 2005;129: 1753.
Talley NJ et al; Practice Parameters Committee of the American College of Gastroenterology: Guidelines for the management of dyspepsia. Am J Gastroenterol 2005;100:2324.
Timmons S et al: Functional dyspepsia: motor abnormalities, sensory dysfunction, and therapeutic options. Am J Gastroenterol 2004;99:739.
Nausea & Vomiting
Nausea is a vague, intensely disagreeable sensation of sickness or queasiness that may or may not be followed by vomiting and is distinguished from anorexia. Vomiting often follows, as does retching (spasmodic respiratory and abdominal movements). Vomiting should be distinguished from regurgitation, the effortless reflux of liquid or food stomach contents; and from rumination, the chewing and swallowing of food that is regurgitated volitionally after meals.
The medullary vomiting center (which contains histamine H1-receptors and muscarinic cholinergic receptors) may be stimulated by four different sources of afferent input: (1) Afferent vagal and splanchnic fibers from the gastrointestinal viscera are rich in serotonin 5-HT3 receptors; these may be stimulated by biliary or gastrointestinal distention, mucosal or peritoneal irritation, or infections. (2) Fibers of the vestibular system, which have high concentrations of histamine H1 and muscarinic cholinergic receptors. (3) Higher central nervous system centers; here, certain sights, smells, or emotional experiences may induce vomiting. For example, patients receiving chemotherapy may develop vomiting in anticipation of its administration. (4) The chemoreceptor trigger zone, located outside the blood-brain barrier in the area postrema of the medulla, which is rich in opioid, serotonin 5-HT3, neurokinin 1 (NK1) and dopamine D2 receptors. This region may be stimulated by drugs and chemotherapeutic agents, toxins, hypoxia, uremia, acidosis, and radiation therapy. Although the causes of vomiting are many, a simplified list is provided in Table 14-1.
Complications of vomiting include dehydration, hypokalemia, metabolic alkalosis, aspiration, rupture of the esophagus (Boerhaave's syndrome), and bleeding secondary to a mucosal tear at the gastroesophageal junction (Mallory-Weiss syndrome).
Table 14-1. Causes of nausea and vomiting. | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
Clinical Findings
A. Symptoms and Signs
Acute symptoms without abdominal pain are typically caused by food poisoning, infectious gastroenteritis, drugs, or systemic illness. Inquiry should be made into recent changes in medications, diet, other intestinal symptoms, or similar illnesses in family members. The acute onset of severe pain and vomiting suggests peritoneal irritation, acute gastric or intestinal obstruction, or pancreaticobiliary disease. Examination may reveal fever, focal tenderness or rigidity, guarding, or rebound tenderness. Persistent vomiting suggests pregnancy, gastric outlet obstruction, gastroparesis, intestinal dysmotility, psychogenic disorders, and central nervous system or systemic disorders. Vomiting that occurs in the morning before breakfast is common with pregnancy, uremia, alcohol intake, and increased intracranial pressure. Vomiting immediately after meals strongly suggests bulimia or psychogenic causes. Vomiting of undigested food one to several hours after meals is characteristic of gastroparesis or a gastric outlet obstruction; physical examination may reveal a succussion splash. Patients with acute or chronic symptoms should be asked about neurologic symptoms that suggest a central nervous system cause such as headache, stiff neck, vertigo, and focal paresthesias or weakness.
B. Special Examinations
With vomiting that is severe or protracted, serum electrolytes should be obtained to look for hypokalemia, azotemia, or metabolic alkalosis resulting from loss of gastric contents. Flat and upright abdominal radiographs are obtained in patients with severe pain or suspicion of mechanical obstruction to look for free intraperitoneal air or dilated loops of small bowel. If mechanical small intestinal or gastric obstruction is thought likely, a nasogastric tube is placed for relief of symptoms. The cause of gastric outlet obstruction is best demonstrated by upper endoscopy, and the cause of small intestinal obstruction is best demonstrated with barium radiography or abdominal CT imaging. Gastroparesis is confirmed by nuclear scintigraphic studies or 13C-octanoic acid breath tests, which show delayed gastric emptying and either upper endoscopy or barium upper gastrointestinal series showing no evidence of mechanical gastric outlet obstruction. Abnormal liver function tests or elevated amylase or lipase suggest pancreaticobiliary disease, which may be investigated with an abdominal sonogram or CT scan. Central nervous system causes are best evaluated with either head CT or MRI.
Treatment
A. General Measures
Most causes of acute vomiting are mild, self-limited, and require no specific treatment. Patients should ingest
P.551
P.552
clear liquids (broths, tea, soups, carbonated beverages) and small quantities of dry foods (soda crackers). For more severe acute vomiting, hospitalization may be required. Patients unable to eat and losing gastric fluids may become dehydrated, resulting in hypokalemia with metabolic alkalosis. Intravenous 0.45% saline solution with 20 mEq/L of potassium chloride is given in most cases to maintain hydration. A nasogastric suction tube for gastric decompression improves patient comfort and permits monitoring of fluid loss.
B. Antiemetic Medications
Medications may be given either to prevent or to control vomiting (see above). Combinations of drugs from different classes may provide better control of symptoms with less toxicity in some patients. All of these medications should be avoided in pregnancy. (For dosages, see Table 14-2.)
1. Serotonin 5-HT3-receptor antagonists
Ondansetron, granisetron, dolasetron, and palonosetron are effective in preventing chemotherapy- and radiation-induced emesis when initiated prior to treatment. Single-dose administration schedules are as effective as multiple-dose regimens. Although serotonin antagonists are effective for the prevention of postoperative nausea and vomiting, less expensive alternatives (eg, dexamethasone or droperidol) are equally effective.
2. Corticosteroids
Corticosteroids (eg, dexamathesone) have antiemetic properties, but the basis for these effects is unknown. These agents enhance the efficacy of serotonin receptor antagonsists for preventing acute and delayed nausea and vomiting in patients receiving moderately to highly emetogenic chemotherapy regimens. For the prevention of postoperative nausea and vomiting, corticosteroids, serotonin antagonists, and droperidol have efficacy; however, combinations of these agents have additive benefit.
3. Neurokinin receptor antagonists
Aprepitant is a highly selective antagonist for NK1-receptors in the area postrema. It is used in combination with corticosteroids and serotonin antagonists for the prevention of acute and delayed nausea and vomiting with highly emetogenic chemotherapy regimens. Combined therapy with aprepitant prevents acute emesis in 80 90% and delayed emesis in > 70% of patients treated with highly emetogenic regimens.
4. Dopamine antagonists
The phenothiazines, butyrophenones, and substituted benzamides have antiemetic properties that are due to dopaminergic blockade as well as to their sedative effects. High doses of these agents are associated with antidopaminergic side effects, including extrapyramidal reactions and depression. These agents are used in a variety of situations. Cases of QT prolongation leading to ventricular tachycardia (torsade de pointes) have been reported in several patients receiving droperidol, hence electrocardiographic monitoring is recommended before and after administration.
Table 14-2. Common antiemetic dosing regimens. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Antihistamines and anticholinergics
These drugs (eg, meclizine, dimenhydrinate, transdermal scopolamine) may be valuable in the prevention of vomiting arising from stimulation of the labyrinth, ie, motion sickness, vertigo, and migraines. They may induce drowsiness.
6. Sedatives
Benzodiazepines are used in psychogenic and anticipatory vomiting.
7. Cannabinoids
Marijuana has been used widely as an appetite stimulant and antiemetic. Pure 9-tetrahydrocannabinol (THC) is the major active ingredient in marijuana and is available by prescription as
P.553
dronabinol. In doses of 5 15 mg/m2, oral dronabinol is effective in treating nausea associated with chemotherapy, but it is associated with central nervous system side effects in most patients.
Apfel CC et al: A factorial trial of six interventions in the prevention of postoperative nausea and vomiting. N Engl J Med 2004;350:2441.
Hasler WL et al: Nausea and vomiting. Gastroenterology 2003;125:1860.
Sharma R et al: Management of chemotherapy-induced nausea, vomiting, oral mucositis, and diarrhoea. Lancet Oncol 2005;6:93.
Tramer MR: Treatment of postoperative nausea and vomiting. BMJ 2003;327:762.
Hiccups (Singultus)
Though usually a benign and self-limited annoyance, hiccups may be persistent and a sign of serious underlying illness. In patients on mechanical ventilation, hiccups can trigger a full respiratory cycle and result in respiratory alkalosis.
Causes of benign, self-limited hiccups include gastric distention (carbonated beverages, air swallowing, overeating), sudden temperature changes (hot then cold liquids, hot then cold shower), alcohol ingestion, and states of heightened emotion (excitement, stress, laughing). There are over 100 causes of recurrent or persistent hiccups, grouped into the following categories:
Central nervous system: Neoplasms, infections, cerebrovascular accident, trauma.
Metabolic: Uremia, hypocapnia (hyperventilation).
Irritation of the vagus or phrenic nerve: (1) Head, neck: Foreign body in ear, goiter, neoplasms. (2) Thorax: Pneumonia, empyema, neoplasms, myocardial infarction, pericarditis, aneurysm, esophageal obstruction, reflux esophagitis. (3) Abdomen: Subphrenic abscess, hepatomegaly, hepatitis, cholecystitis, gastric distention, gastric neoplasm, pancreatitis, or pancreatic malignancy.
Surgical: General anesthesia, postoperative.
Psychogenic and idiopathic.
Clinical Findings
Evaluation of the patient with persistent hiccups should include a detailed neurologic examination, serum creatinine, liver chemistry tests, and a chest radiograph. When the cause remains unclear, CT of the head, chest, and abdomen, echocardiography, bronchoscopy, and upper endoscopy may help. On occasion, hiccups may be unilateral; chest fluoroscopy will make the diagnosis.
Treatment
A number of simple remedies may be helpful in patients with acute benign hiccups. (1) Irritation of the nasopharynx by tongue traction, lifting the uvula with a spoon, catheter stimulation of the nasopharynx, or eating 1 tsp of dry granulated sugar. (2) Interruption of the respiratory cycle by breath holding, Valsalva's maneuver, sneezing, gasping (fright stimulus), or rebreathing into a bag. (3) Stimulation of the vagus, carotid massage. (4) Irritation of the diaphragm by holding knees to chest or by continuous positive airway pressure during mechanical ventilation. (5) Relief of gastric distention by belching or insertion of a nasogastric tube.
A number of drugs have been promoted as being useful in the treatment of hiccups. Chlorpromazine, 25 50 mg orally or intramuscularly, is most commonly used. Other agents reported to be effective include anticonvulsants (phenytoin, carbamazepine), benzodiazepines (lorazepam, diazepam), metoclopramide, baclofen, gabapentin, and occasionally general anesthesia.
Krakauer EL et al: Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 6 2005. A 58-year-old man with esophageal cancer and nausea, vomiting, and intractable hiccups. N Engl J Med 2005;352:817.
Moretti R et al: Gabapentin as a drug therapy of intractable hiccup because of vascular lesion: a three-year follow up. Neurologist 2004;10:102.
Constipation
The first step in evaluating the patient is to determine what is meant by constipation. Patients may define constipation as infrequent stools (fewer than 3 in a week), hard stools, excessive straining, or a sense of incomplete evacuation. Table 14-3 summarizes the many causes of constipation, which are discussed below.
Common Identifiable Causes of Constipation
A. Poor Dietary and Behavioral Habits
The majority of constipated patients have mild symptoms that cannot be attributed to any structural abnormalities, intestinal motility disorders, or systemic disease. Dietary review will reveal that most of these patients do not consume adequate fiber and fluids. Ingestion of additional 10 12 g of fiber per day either by dietary changes or the addition of commercial fiber supplementation is often all that is needed. At least one or two glasses of fluid should be taken with meals. The elderly are predisposed because of poor eating habits, a variety of medications, decreased colonic motility and, in some cases, inability to sit on a toilet (bed-bound patients).
B. Structural Abnormalities
Constipation may be caused by colonic lesions, such as neoplasms and strictures, that obstruct fecal passage. Diagnostic studies to exclude such lesions are indicated in patients with a family history of colon cancer or inflammatory bowel disease; with alarm symptoms
P.554
or signs, such as hematochezia, weight loss, anemia, or positive fecal occult blood tests (FOBT); and in patients older than 45 50 years with new-onset constipation. Defecatory difficulties also can be due to a variety of anorectal outlet problems that impede or obstruct flow (perineal descent, rectal prolapse, rectocele), some of which may require surgery, and Hirschsprung's disease (usually suggested by lifelong constipation).
Table 14-3. Causes of constipation in adults. | ||
|---|---|---|
|
C. Systemic Diseases
Medical diseases can cause constipation due to neurologic gut dysfunction, myopathies, endocrine disorders, and electrolyte abnormalities such as hypercalcemia or hypokalemia.
D. Medications
Anticholinergic and opioid agents are common causes of constipation.
Causes of Severe or Refractory Constipation
Patients whose constipation cannot be attributed to the above causes and who do not respond to conservative dietary management present difficult management problems. Conceptually, these patients can be divided into three classes.
A. Slow Colonic Transit
Normal colonic transit time is approximately 35 hours; more than 72 hours is significantly abnormal. Slow colonic transit may be part of a more generalized gastrointestinal dysmotility syndrome but most commonly is idiopathic. Colonic inertia is more common in women, some of whom have a history of psychosocial problems or sexual abuse.
B. Pelvic Floor Dysfunction
With normal defecation, the anal sphincter and puborectalis muscle relaxes. Patients with pelvic floor dysfunction women more often than men have a paradoxical contraction of the anal sphincter and pelvic floor during attempted defecation that impedes the bowel movement. They may complain of excessive straining with a sense of incomplete evacuation, the need for digital pressure on the vagina or perineum, or the need for digital disimpaction.
C. Irritable Bowel Syndrome
Patients with primary complaints of abdominal pain, bloating, or a sense of incomplete evacuation may have irritable bowel syndrome. (See below.)
Evaluation
A. Initial Management
All patients should undergo a history and physical examination, including digital rectal examination and stool testing for occult blood. Digital examination should assess for anatomic abnormalities, such as anal stricture, rectocele, rectal prolapse, or perineal descent during straining. In otherwise healthy patients under age 50 without alarm symptoms or signs, it is reasonable to initiate a trial of empiric treatment. Further diagnostic tests should be performed in patients with any of the following: age 50 years or older, severe constipation, signs of an organic disorders, alarm symptoms (hematochezia, weight loss, positive FOBT), a family history of colon cancer or inflammatory bowel disease, and in patients whose symptoms have not responded to empiric management. Laboratory studies should include a complete blood count, serum electrolytes, serum calcium, serum glucose, and serum thyroid-stimulating
P.555
hormone (TSH). A colonoscopy or flexible sigmoidoscopy and barium enema should be obtained to exclude a neoplasm, stricture, or inflammatory bowel disease.
B. Second Level of Investigation
Patients with refractory constipation not responding to conservative measures may require further investigation by means of colonic transit and pelvic floor function studies in order to distinguish slow colonic transit from outlet disorders. Colon transit time is measured by performing an abdominal radiograph 120 hours after ingestion of 24 radio-opaque markers. Retention of > 20% of the markers indicates prolonged transit. Pelvic floor dysfunction and anorectal disorders are assessed with balloon expulsion testing, anal manometry, and defecography.
Standard Treatment of Chronic Constipation
A. Dietary Measures
Proper dietary fluid and fiber intake should be emphasized. Fiber may be given by means of dietary alterations or fiber supplements (Table 14-4). Increased dietary fiber may cause temporary distention or flatulence, which often diminishes over several days. Response to fiber therapy is not immediate, and increases in dosage should be made gradually over 7 10 days. Whereas fiber may benefit most patients, it normally does not benefit patients with severe colonic inertia or outlet disorders.
B. Stool Surfactant Agents
Docusate sodium, 50 200 mg/d, or mineral oil, 14 45 mL/d, may be given orally or rectally to promote softening of stools. Aspiration of mineral oil can cause lipoid pneumonia.
C. Osmotic Laxatives
These agents, used to soften stools, may be given alone or in combination with fiber supplements (Table 14-4). They are commonly used in older nonambulatory patients to prevent constipation and fecal impaction. They are safe and are titrated to a dose that results in soft to semiliquid stools. The less expensive saline laxatives should be tried first before using more expensive osmotic agents, such as nonabsorbable carbohydrates or polyethylene glycol solution.
1. Saline laxatives
Magnesium-containing saline laxatives (milk of magnesia, magnesium sulfate) are the most commonly used agents for the prevention and treatment of chronic constipation. These agents should not be given to patients with renal insufficiency. Sodium phosphate or magnesium citrate may be used for aggressive treatment of acute constipation or as a purgative prior to surgical, endoscopic, or radiographic procedures.
2. Nonabsorbable carbohydrates
Either sorbitol (70%) or lactulose, 15 30 mL once or twice daily, is efficacious for the prevention or treatment of chronic constipation. These malabsorbed sugars are often limited by their propensity to induce bloating, cramps, and flatulence.
3. Polyethylene glycol solution
Polyethylene glycol is a component of solutions traditionally used for colonic lavage prior to colonoscopy (CoLyte, GoLYTELY, NuLytely). Polyethylene glycol 3350 powder (Miralax) is available for the treatment of acute or chronic constipation. Seventeen grams of powder may be mixed in water or juice and taken once or twice daily.
D. Stimulant Agents
These agents stimulate fluid secretion and colonic contraction, resulting in a bowel movement within 6 12 hours after oral ingestion or 15 60 minutes after rectal administration. Common preparations include bisacodyl, senna, cascara, and castor oil (Table 14-4). Colchicine (0.6 mg) or misoprostol (200 400 mcg) two or three times daily may be helpful in some patients with refractory constipation. The prokinetic agent tegaserod (6 mg twice daily) is a 5-HT4-receptor agonist that is approved for the treatment of chronic constipation. In randomized trials of patients with chronic constipation (fewer than three spontaneous bowel movements per week), 40% of patients who received tegaserod experienced an increase in the number of spontaneous bowel movements compared with 25% of those who received placebo. At present, this drug should be reserved for patients who have suboptimal response or side effects with less expensive agents.
Treatment of Fecal Impaction
Severe impaction of stool in the rectal vault may result in obstruction to further fecal flow, leading to partial or complete large bowel obstruction. Predisposing factors include severe psychiatric disease, prolonged bed rest and debility, neurogenic disorders of the colon, and spinal cord disorders. Clinical presentation includes decreased appetite, nausea, and vomiting, and abdominal pain and distention. There may be paradoxical diarrhea as liquid stool leaks around the impacted feces. Firm feces are palpable on digital examination of the rectal vault. Initial treatment is directed at relieving the impaction with enemas (saline, mineral oil, or diatrizoate) or digital disruption of the impacted fecal material. Long-term care is directed at maintaining soft stools and regular bowel movements (as above).
Table 14-4. Pharmacologic management of constipation. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
American College of Gastroenterology Task Force: An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol 2005;100 (Suppl 1):S1.
Cash BD et al: The role of serotonergic agents in the treatment of patients with primary constipation. Aliment Pharmacol Ther 2005;22:1047.
P.556
P.557
Muller-Lissner SA et al: Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005;100:232.
Rao SS et al: Clinical utility of diagnostic tests for constipation in adults: a systematic review. Am J Gastroenterol 2005;100: 1605.
Ramkumar D et al: Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol 2005;100:936.
Wald A: Severe constipation. Clin Gastroenterol Hepatol 2005; 3:432.
Gastrointestinal Gas
Belching
Belching (eructation) is the involuntary or voluntary release of gas from the stomach or esophagus. It occurs most frequently after meals, when gastric distention results in transient lower esophageal sphincter relaxation. Belching is a normal reflex and does not itself denote gastrointestinal dysfunction. Virtually all stomach gas comes from swallowed air. With each swallow, 2 5 mL of air is ingested, and excessive amounts may result in distention, flatulence, and abdominal pain. This may occur with rapid eating, gum chewing, smoking, and the ingestion of carbonated beverages. Chronic excessive belching is almost always caused by aerophagia, common in anxious individuals and institutionalized patients. Evaluation should be restricted to patients with other complaints such as dysphagia, heartburn, early satiety, or vomiting.
Once patients understand the relationship between aerophagia and belching, most can deal with the problem by behavioral modification. Physical defects that hamper normal swallowing (ill-fitting dentures, nasal obstruction) should be corrected. Antacids and simethicone are of no value.
Flatus
The rate and volume of expulsion of flatus is highly variable. Flatus is derived from two sources: swallowed air and bacterial fermentation of undigested carbohydrate. The majority of swallowed air not belched passes through the gut and leaves as flatus. Swallowed air may contribute up to 500 mL of flatus per day (primarily nitrogen). Bacterial fermentation of undigested carbohydrates leads to the additional production of gas, particularly H2, CO2, and methane. The majority of this fermentation takes place in the colon. Under normal circumstances, a small substrate of fermentable substances reaches the colon. These substances include fructose, lactose, sorbitol, trehalose (mushrooms), raffinose, and stachyose (legumes, cruciferous vegetables). Complex starches and fiber may also cause gas. Gas production may be increased with ingestion of these carbohydrates or with malabsorption.
Determining abnormal from normal amounts of flatus is difficult. An initial trial of a lactose-free diet is recommended. Common gas-producing foods should be reviewed and the patient given an elimination trial. These include beans of all kinds, peas, lentils, brussels sprouts, cabbage, parsnips, leeks, onions, beer, and coffee. Fructose intolerance may be more common than previously appreciated. Fructose is present not only in many fruits but is also used commonly as a sweetener or as fructose corn syrup in candy, fruit juices, and soda. Foul odor may be caused by garlic, onion, eggplant, mushrooms, and certain herbs and spices. For patients with persistent complaints, complex starches, and fiber may be eliminated, but such restrictive diets are unacceptable to most patients. Of refined flours, only rice flour is gas-free.
The nonprescription agent Beano ( -d-galactosidase enzyme) reduces gas caused by foods containing raffinose and stachyose, ie, cruciferous vegetables, legumes, nuts, and some cereals. Activated charcoal may afford relief. Simethicone is of no proved benefit.
Complaints of chronic abdominal distention or bloating are common. Some of these patients may produce excess gas. However, many patients have impaired small bowel gas propulsion or enhance visceral sensitivity to gas distention. Many of these patients have an underlying functional gastrointestinal disorder such as irritable bowel syndrome or functional dyspepsia. Reduction of dietary fat, which delays intestinal gas clearance, may be helpful.
Azpiroz F et al: Abdominal bloating. Gastroenterology 2005; 129:1060.
Chitkara DK et al: Aerophagia in adults: a comparison with functional dyspepsia. Aliment Pharmacol Ther 2005;22:855.
Diarrhea
Diarrhea can range in severity from an acute self-limited episode to a severe, life-threatening illness. To properly evaluate the complaint, the physician must determine the patient's normal bowel pattern and the nature of the current symptoms.
Approximately 10 L of fluid enter the duodenum daily, of which all but 1.5 L are absorbed by the small intestine. The colon absorbs most of the remaining fluid, with < 200 mL lost in the stool. Although diarrhea sometimes is defined as a stool weight of more than 200 300 g/24 h, quantification of stool weight is necessary only in some patients with chronic diarrhea. In most cases, the physician's working definition of diarrhea is increased stool frequency (more than three bowel movements per day) or liquidity of feces.
The causes of diarrhea are myriad. In clinical practice, it is helpful to distinguish acute from chronic diarrhea, as the evaluation and treatment are entirely different (Tables 14-5 and 14-7).
Table 14-5. Causes of acute infectious diarrhea. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
1. Acute Diarrhea
Etiology & Clinical Findings
Diarrhea acute in onset and persisting for less than 2 weeks is most commonly caused by infectious agents,
P.558
bacterial toxins (either preformed or produced in the gut), or drugs. Community outbreaks (including nursing homes, schools, cruise ships) suggest a viral etiology or a common food source. Similar recent illnesses in family members suggest an infectious origin. Ingestion of improperly stored or prepared food implicates food poisoning. Day care attendance or exposure to unpurified water (camping, swimming) may result in infection with Giardia or Cryptosporidium. Large Cyclospora outbreaks have been traced to contaminated produce. Recent travel abroad suggests traveler's diarrhea (see Chapter 30). Antibiotic administration within the preceding several weeks increases the likelihood of Clostridium difficile colitis. Finally, risk factors for HIV infection or sexually transmitted diseases should be determined. (AIDS-associated diarrhea is discussed in Chapter 31; infectious proctitis is discussed in this chapter under Anorectal Disorders.) Persons engaging in anal intercourse or oral-anal sexual activities are at risk for a variety of infections that cause proctitis, including gonorrhea, syphilis, lymphogranuloma venereum, and herpes simplex.
The nature of the diarrhea helps distinguish among different infectious causes (Table 14-5).
A. Noninflammatory Diarrhea
Watery, nonbloody diarrhea associated with periumbilical cramps, bloating, nausea, or vomiting suggests a small bowel source caused by either a toxin-producing bacterium (enterotoxigenic Escherichia coli [ETEC], Staphylococcus aureus, Bacillus cereus, Clostridium perfringens) or other agents (viruses, Giardia) that disrupt normal absorption and secretory process in the small intestine. Prominent vomiting suggests viral enteritis or S aureus food poisoning. Although typically mild, the diarrhea (which originates in the small intestine) can be voluminous and result in dehydration with hypokalemia and metabolic acidosis (eg, cholera). Because tissue invasion does not occur, fecal leukocytes are not present.
B. Inflammatory Diarrhea
The presence of fever and bloody diarrhea (dysentery) indicates colonic tissue damage caused by invasion (shigellosis, salmonellosis, Campylobacter or Yersinia infection, amebiasis) or a toxin (C difficile, E coli O157:H7). Because these organisms involve predominantly the colon, the diarrhea is small in volume (< 1 L/d) and associated with left lower quadrant cramps, urgency, and tenesmus. Fecal leukocytes or lactoferrin usually are present in infections with invasive organisms. E coli O157:H7 is a Shiga toxin-producing noninvasive organism most commonly acquired from contaminated meat that has resulted in several outbreaks of an acute, often severe hemorrhagic colitis. In immunocompromised and HIV-infected patients, cytomegalovirus (CMV) can cause intestinal ulceration with watery or bloody diarrhea.
Infectious dysentery must be distinguished from acute ulcerative colitis, which may also present acutely with fever, abdominal pain, and bloody diarrhea. Diarrhea that persists for more than 14 days is not attributable to bacterial pathogens (except for C difficile) and should be evaluated as chronic diarrhea.
Evaluation
In over 90% of patients with acute noninflammatory diarrhea, the illness is mild and self-limited, responding within 5 days to simple rehydration therapy or antidiarrheal agents; diagnostic investigation is unnecessary. The isolation rate of bacterial pathogens from stool cultures in patients with acute noninflammatory diarrhea is under 3%. Thus, the goal of initial evaluation is to distinguish patients with mild disease from those with more serious illness. If diarrhea worsens or persists for more than 7 days, stool should be sent for fecal leukocyte or lactoferrin determination, ovum and parasite evaluation, and bacterial culture.
Prompt medical evaluation is indicated in the following situations (Figure 14-1): (1) Signs of inflammatory diarrhea manifested by any of the following: fever (> 38.5 C), bloody diarrhea, or abdominal pain. (2) The passage of six or more unformed stools in 24
P.559
hours. (3) Profuse watery diarrhea and dehydration. (4) Frail older patients. (5) Immunocompromised patients (AIDS, posttransplantation). (6) Nosocomial diarrhea (onset more than 3 days after hospitalization).
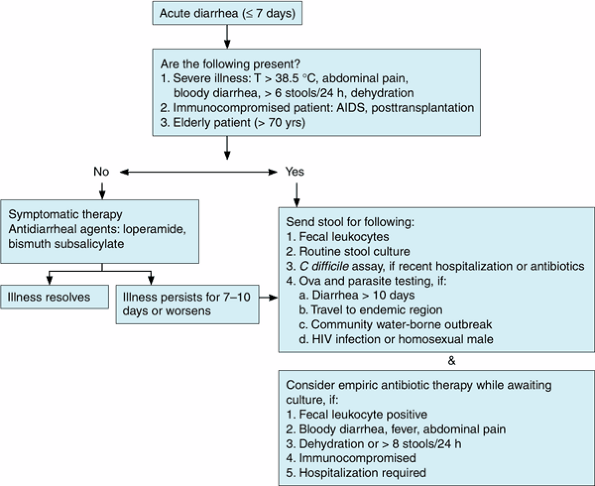 |
Figure 14-1. Evaluation of acute diarrhea. |
Physical examination pays note to the patient's level of hydration, mental status, and the presence of abdominal tenderness or peritonitis. Peritoneal findings may be present in infection with C difficile or enterohemorrhagic E coli. Hospitalization is required in patients with severe dehydration, toxicity, or marked abdominal pain. Stool specimens should be sent for examination for bacterial cultures (Table 14-6).
The rate of positive bacterial cultures in such patients is 60 75%. For bloody stools, the laboratory should be directed to perform serotyping for Shiga-producing E coli O157:H7. Special culture media are required for Yersinia, Vibrio, and Aeromonas. In patients who are hospitalized or who have a history of antibiotic exposure, a stool sample should be tested for C difficile toxin. In patients with diarrhea that persists for more than 10 days, who have a history of travel to areas where amebiasis is endemic, or who engage in oral-anal sexual practices, three stool examinations for ova and parasites should also be performed. The stool antigen detection tests for both Giardia and Entamoeba histolytica are more sensitive than stool microscopy for detection of these organisms. A serum antigen detection test for E histolytica is also available. Cyclospora and Cryptosporidium are detected by fecal acid-fast staining.
Treatment
A. Diet
Most mild diarrhea will not lead to dehydration provided the patient takes adequate oral fluids containing carbohydrates and electrolytes. Patients find it more comfortable to rest the bowel by avoiding high-fiber foods, fats, milk products, caffeine, and alcohol. Frequent feedings of tea, flat carbonated beverages, and soft, easily digested foods (eg, soups, crackers, bananas, applesauce, rice, toast) are encouraged.
Table 14-6. Fecal leukocytes in intestinal disorders. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
B. Rehydration
In more severe diarrhea, dehydration can occur quickly, especially in children, the frail, and the elderly. Oral rehydration with fluids containing glucose, Na+, K+, Cl-, and bicarbonate or citrate is preferred when feasible. A convenient mixture is 1/2 tsp salt (3.5 g),
P.560
1 tsp baking soda (2.5 g NaHCO3), 8 tsp sugar (40 g), and 8 oz orange juice (1.5 g KCl), diluted to 1 L with water. Alternatively, oral electrolyte solutions (eg, Pedialyte, Gatorade) are readily available. Fluids should be given at rates of 50 200 mL/kg/24 h depending on the hydration status. Intravenous fluids (lactated Ringer's injection) are preferred in patients with severe dehydration.
C. Antidiarrheal Agents
Antidiarrheal agents may be used safely in patients with mild to moderate diarrheal illnesses to improve patient comfort. Opioid agents help decrease the stool number and liquidity and control fecal urgency. However, they should not be used in patients with bloody diarrhea, high fever, or systemic toxicity and should be discontinued in patients whose diarrhea is worsening despite therapy. With these provisos, such drugs provide excellent symptomatic relief. Loperamide is preferred, in a dosage of 4 mg initially, followed by 2 mg after each loose stool (maximum: 16 mg/24 h).
Bismuth subsalicylate (Pepto-Bismol), two tablets or 30 mL four times daily, reduces symptoms in patients with traveler's diarrhea by virtue of its anti-inflammatory and antibacterial properties. It also reduces vomiting associated with viral enteritis. Anticholinergic agents (eg, diphenoxylate with atropine) are contraindicated in acute diarrhea because of the rare precipitation of toxic megacolon.
D. Antibiotic Therapy
1. Empiric treatment
Empiric antibiotic treatment of all patients with acute diarrhea is not indicated. Even patients with inflammatory diarrhea caused by invasive pathogens usually have symptoms that will resolve within several days without antimicrobials. Empiric treatment may be considered in patients with non-hospital-acquired diarrhea with moderate to severe fever, tenesmus, or bloody stools or the presence of fecal lactoferrin while the stool bacterial culture is incubating, provided that infection with E coli O157:H7 is not suspected. The drugs of choice for empiric treatment are the fluoroquinolones (eg, ciprofloxacin 500 mg, ofloxacin 400 mg, or norfloxacin 400 mg, twice daily, or levofloxacin 500 mg once daily) for 5 7 days. Alternatives include trimethoprim-sulfamethoxazole, 160/800 mg twice daily; or doxycycline, 100 mg twice daily. Macrolides and penicillins are no longer recommended because of widespread microbial resistance to these agents. Rifaximin, a nonabsorbed oral antibiotic, 200 mg three times daily for 3 days, is approved for empiric treatment of noninflammatory traveler's diarrhea (see Chapter 30).
2. Specific antimicrobial treatment
Antibiotics are not recommended in patients with nontyphoid Salmonella, Campylobacter, E coli O157:H7, Aeromonas, or Yersinia, except in severe disease, because they do not hasten recovery or reduce the period of fecal bacterial excretion. The infectious diarrheas for which treatment is recommended are shigellosis, cholera, extraintestinal salmonellosis, traveler's diarrhea, C difficile infection, giardiasis, and amebiasis. Therapy for traveler's diarrhea, infectious (sexually transmitted) proctitis, and AIDS-related diarrhea is presented in other chapters of this book.
Musher DM et al: Contagious acute gastrointestinal infections. N Engl J Med 2004;350:2417.
Thielman NM et al: Clinical practice. Acute infectious diarrhea. N Engl J Med 2004;350:38.
2. Chronic Diarrhea
Etiology
The causes of chronic diarrhea may be grouped into seven major pathophysiologic categories (Table 14-7).
Table 14-7. Causes of chronic diarrhea. | |
|---|---|
|
P.561
A. Osmotic Diarrheas
As stool leaves the colon, fecal osmolality is equal to the serum osmolality, ie, approximately 290 mosm/kg. Under normal circumstances, the major osmoles are Na+, K+, Cl-, and HCO3-. The stool osmolality may be estimated by multiplying the stool (Na+ + K+) 2. The osmotic gap is the difference between the measured osmolality of the stool (or serum) and the estimated stool osmolality and is normally less than 50 mosm/kg. An increased osmotic gap (> 125 mosm/kg) implies that the diarrhea is caused by ingestion or malabsorption of an osmotically active substance. The most common causes are disaccharidase deficiency (lactase deficiency), laxative abuse, and malabsorption syndromes (see below). Osmotic diarrheas resolve during fasting. Those caused by malabsorbed carbohydrates are characterized by abdominal distention, bloating, and flatulence due to increased colonic gas production.
Disaccharidase deficiencies are common and should be considered in all patients with chronic diarrhea. Lactase deficiency occurs in 75% of nonwhite adults and up to 25% of whites. It may also be acquired after an episode of viral gastroenteritis, medical illness, or gastrointestinal surgery. Sorbitol is commonly used as a sweetener in gums, candies, and some medications that may cause diarrhea in some patients. The diagnosis of sorbitol or lactose malabsorption may be established by an elimination trial for 2 3 weeks.
Ingestion of magnesium- or phosphate-containing compounds (laxatives, antacids) should be considered in enigmatic chronic diarrhea. Surreptitious use should be considered, especially in patients with a long history of undiagnosed medical ailments or employment in the medical field. The fat substitute olestra also causes diarrhea and cramps in occasional patients.
B. Secretory Conditions
Increased intestinal secretion or decreased absorption results in a high-volume watery diarrhea with a normal osmotic gap. There is little change in stool output during the fasting state, and dehydration and electrolyte imbalance may develop. Causes include endocrine tumors (stimulating intestinal or pancreatic secretion), bile salt malabsorption (stimulating colonic secretion), and laxative abuse.
C. Inflammatory Conditions
Diarrhea is present in most patients with inflammatory bowel disease (ulcerative colitis, Crohn's disease, microscopic colitis). A variety of other symptoms may be present, including abdominal pain, fever, weight
P.562
loss, and hematochezia. (See Inflammatory Bowel Disease, below.)
D. Malabsorptive Conditions
The major causes of malabsorption are small mucosal intestinal diseases, intestinal resections, lymphatic obstruction, small intestinal bacterial overgrowth, and pancreatic insufficiency. Its characteristics are weight loss, osmotic diarrhea, steatorrhea, and nutritional deficiencies. Significant diarrhea in the absence of weight loss is not likely to be due to malabsorption. The physical and laboratory abnormalities related to deficiencies of vitamins or minerals are discussed in Chapter 29.
E. Motility Disorders
Abnormal intestinal motility secondary to systemic disorders or surgery may result in diarrhea due to rapid transit or to stasis of intestinal contents with bacterial overgrowth, resulting in malabsorption. Probably the most common cause of chronic diarrhea is irritable bowel syndrome (see Irritable Bowel Syndrome, below).
F. Chronic Infections
Chronic parasitic infections may cause diarrhea through a number of mechanisms. Pathogens most commonly associated with diarrhea include the protozoans Giardia, E histolytica, and Cyclospora as well as the intestinal nematodes. Bacterial infections with Aeromonas and Plesiomonas may uncommonly be a cause of chronic diarrhea.
Immunocompromised patients are susceptible to infectious organisms that can cause acute or chronic diarrhea (see Chapter 31), including Microsporida, Cryptosporidium, CMV, Isospora belli, Cyclospora, and Mycobacterium avium complex.
G. Factitious Diarrhea
Fifteen percent of patients have factitious diarrhea caused by surreptitious laxative abuse or dilution of stool.
Evaluation
The history and physical examination commonly suggest the underlying pathophysiology that guides the subsequent diagnostic workup (Figure 14-2). Important tests are described here. AIDS-associated diarrhea is discussed in Chapter 31.
A. Stool Analysis
1. Twenty-four-hour stool collection for weight and quantitative fecal fat
A stool weight of more than 300 g/24 h confirms diarrhea. A weight > 500 g excludes irritable bowel syndrome, whereas a weight > 1000 1500 g suggests a secretory process. A fecal fat determination in excess of 10 g/24 h indicates a malabsorptive disorder. (See Celiac Sprue and specific tests for malabsorption, below.)
2. Stool osmolality
Stool osmolality less than serum osmolality implies that water or urine has been added to the specimen (factitious diarrhea). A stool pH < 5.6 is consistent with carbohydrate malabsorption.
3. Stool laxative screen
In cases of suspected laxative abuse, stool magnesium, phosphate, and sulfate levels may be measured. Phenolphthalein and bisacodyl can be analyzed in stool water, using chromatographic techniques. Anthraquinones and bisacodyl are sought for in the urine.
4. Fecal leukocytes
The presence of fecal leukocytes or lactoferrin implies inflammatory diarrhea.
5. Stool for ova and parasites
The presence of Giardia and E histolytica may be detected in wet mounts. However, fecal antigen detection tests for Giardia and E histolytica may be a more sensitive and specific method of detection. Cryptosporidium and Cyclospora are found with modified acid-fast staining.
B. Blood Tests
1. Routine laboratory tests
Complete blood count, serum electrolytes, liver function tests, calcium, phosphorus, albumin, TSH, -carotene, and prothrombin time may be of value. Anemia occurs in malabsorption syndromes (folate, iron deficiency [rare], or vitamin B12) as well as inflammatory conditions. Hypoalbuminemia is present in malabsorption, protein-losing enteropathies, and inflammatory diseases. Hyponatremia and nonanion gap metabolic acidosis occur in secretory diarrheas.
2. Other laboratory tests
In patients with suspected malabsorption, serologic testing for celiac sprue includes IgG and IgA antigliadin or tissue transglutaminase antibodies. Secretory diarrheas due to neuroendocrine tumors are rare. When this is suspected, serum vasoactive intestinal peptide (VIP) (VIPoma), calcitonin (medullary thyroid carcinoma), gastrin (Zollinger-Ellison syndrome), and glucagon determinations may be diagnostic. Urine should be sent for 5-hydroxyindoleacetic acid (5-HIAA) (carcinoid), vanillylmandelic acid (VMA), metanephrine, and histamine determinations.
3. Endoscopic examination and mucosal biopsy
Either sigmoidoscopy or colonoscopy with mucosal biopsy is helpful in the detection of inflammatory bowel disease (including microscopic colitis) and melanosis coli (indicative of chronic anthraquinone laxative use). Upper endoscopy with small bowel biopsy is performed when a small intestinal malabsorptive disorder is suspected (celiac sprue, Whipple's disease) and in patients with AIDS to document Cryptosporidium, Microsporida, and M avium-intracellulare infection. If bacterial overgrowth is suspected, the diagnosis is confirmed with noninvasive breath tests (D-[14C]xylose, glucose, or lactulose) or by obtaining an aspirate of small intestinal contents for quantitative aerobic and anaerobic bacterial culture.
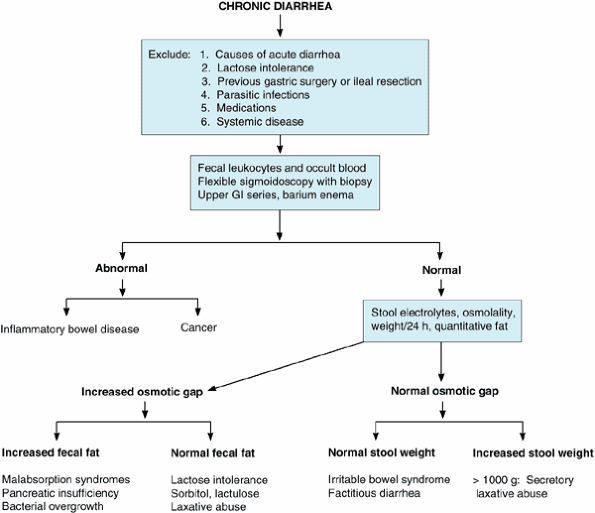 |
Figure 14-2. Decision diagram for diagnosis of causes of chronic diarrhea. |
P.563
4. Other imaging studies
Calcification on a plain abdominal radiograph confirms a diagnosis of chronic pancreatitis, although abdominal CT and endoscopic ultrasonography are more sensitive for the diagnosis of chronic pancreatitis as well as pancreatic cancer. Small intestinal barium radiography is helpful in the diagnosis of Crohn's disease, small bowel lymphoma, carcinoid, and jejunal diverticula. Neuroendocrine tumors may be localized using somatostatin receptor scintigraphy.
Treatment
A number of antidiarrheal agents may be used in certain patients with chronic diarrheal conditions and are listed below. Opioids are safe in most patients with chronic, stable symptoms.
Loperamide: 4 mg initially, then 2 mg after each loose stool (maximum: 16 mg/d).
Diphenoxylate with atropine: One tablet three or four times daily as needed.
Codeine and deodorized tincture of opium: Because of potential habituation, these drugs are avoided except in cases of chronic, intractable diarrhea. Codeine may be given in a dosage of 15 60 mg every 4 hours; tincture of opium, 10 25 drops every 6 hours as needed.
Clonidine: 2-Adrenergic agonists inhibit intestinal electrolyte secretion. Clonidine, 0.1 0.6 mg twice daily, or a clonidine patch, 0.1 0.2 mg/d, may help in some patients with secretory diarrheas, diabetic diarrhea, or cryptosporidiosis.
Octreotide: This somatostatin analog stimulates intestinal fluid and electrolyte absorption and inhibits intestinal fluid secretion and the release of gastrointestinal peptides. It is given for secretory diarrheas due to neuroendocrine tumors (VIPomas, carcinoid) and in some cases of AIDS-related diarrhea. Effective doses range from 50 to 250 mcg subcutaneously three times daily.
Cholestyramine: This bile salt-binding resin may be useful in patients with bile salt-induced diarrhea
P.564
secondary to intestinal resection or ileal disease. A dosage of 4 g once to three times daily is recommended.
Camilleri M: Chronic diarrhea: a review on pathophysiology and management for the clinical gastroenterologist. Clin Gastroenterol Hepatol 2004;2:198.
Headstrom PD et al: Chronic diarrhea. Clin Gastroenterol Hepatol 2005;3:734.
Schiller L: Chronic diarrhea. Gastroenterology 2004;127:287.
Thomas PD et al: Guidelines for the investigation of chronic diarrhea, 2nd edition. Gut 2003;52(Suppl 5):v1.
Gastrointestinal Bleeding
1. Acute Upper Gastrointestinal Bleeding
![]() Essentials of Diagnosis
Essentials of Diagnosis
Hematemesis (bright red blood or coffee grounds ).
Melena in most cases; hematochezia in massive upper gastrointestinal bleeds.
Volume status to determine severity of blood loss; hematocrit is a poor early indicator of blood loss.
Endoscopy diagnostic and may be therapeutic.
General Considerations
There are over 250,000 hospitalizations a year in the United States for acute upper gastrointestinal bleeding, with a mortality rate of 7 10%. Approximately half of patients are over 60 years of age, and in this age group the mortality rate is even higher. Patients seldom die of exsanguination but rather from complications of an underlying disease.
The most common presentation of upper gastrointestinal bleeding is hematemesis or melena. Hematemesis may be either bright red blood or brown coffee grounds material. Melena develops after as little as 50 100 mL of blood loss in the upper gastrointestinal tract, whereas hematochezia requires a loss of more than 1000 mL. Although hematochezia generally suggests a lower bleeding source (eg, colonic), upper gastrointestinal bleeding may present with hematochezia in 10% of cases.
Upper gastrointestinal bleeding is self-limited in 80% of patients; urgent medical therapy and endoscopic evaluation are obligatory in the rest. Patients with bleeding more than 48 hours prior to presentation have a low risk of recurrent bleeding.
Etiology
Acute upper gastrointestinal bleeding may originate from a number of sources. These are listed in order of the frequency and discussed in detail below.
A. Peptic Ulcer Disease
Peptic ulcers account for half of major upper gastrointestinal bleeding with an overall acute mortality rate of 6 10%. However, in North America the incidence of bleeding from ulcers is declining, perhaps due to eradication of H pylori, use of safer NSAIDs, and prophylaxis with proton pump inhibitors in high-risk patients.
B. Portal Hypertension
Portal hypertension causes bleeding from varices (most commonly esophageal; rarely, gastric or duodenal) or portal hypertensive gastropathy. Acute bleeding develops in less than one-third of patients with portal hypertension and varices; however, these lesions account for 10 20% of significant gastrointestinal hemorrhages. If untreated, 50% of varices will rebleed during hospitalization. Due to improved care, the hospital mortality rate of has declined over the past 20 years from 40% to 15%. Nevertheless, a mortality rate of 60 80% is expected at 1 4 years due to recurrent bleeding or other complications of chronic liver disease.
C. Mallory-Weiss Tears
Lacerations of the gastroesophageal junction cause 5 10% of cases of upper gastrointestinal bleeding. Many patients report a history of heavy alcohol use or retching. Less than 10% have continued or recurrent bleeding.
D. Vascular Anomalies
Vascular anomalies are found throughout the gastrointestinal tract and may be the source of chronic or acute gastrointestinal bleeding. They account for 7% of cases of acute upper tract bleeding. Vascular ectasias (angiodysplasias) have a bright red stellate appearance. They may be part of systemic conditions (hereditary hemorrhagic telangiectasia, CREST syndrome) or may occur sporadically. There is an increased incidence in patients with chronic renal failure. Dieulafoy's lesion is an aberrant, large-caliber submucosal artery, most commonly in the proximal stomach that causes recurrent, intermittent bleeding.
E. Gastric Neoplasms
Gastric neoplasms result in 1% of upper gastrointestinal hemorrhages.
F. Erosive Gastritis
Because this process is superficial, it is a relatively unusual cause of severe gastrointestinal bleeding (< 5% of cases) and more commonly results in chronic blood loss. Gastric mucosal erosions are due to NSAIDs, alcohol, or severe medical or surgical illness (stress gastritis).
G. Erosive Esophagitis
Severe erosive esophagitis due to chronic gastroesophageal reflux may rarely cause significant upper gastrointestinal
P.565
bleeding, especially in patients who are bed bound long-term.
H. Others
An aortoenteric fistula complicates 2% of abdominal aortic grafts or can occur as the initial presentation of a previously untreated aneurysm. Usually located between the graft or aneurysm and the third portion of the duodenum, these fistulas characteristically present with a herald nonexsanguinating initial hemorrhage, with melena and hematemesis, or with chronic intermittent bleeding. The diagnosis may be suspected by upper endoscopy or abdominal CT. Surgery is mandatory to prevent exsanguinating hemorrhage. Unusual causes of upper gastrointestinal bleeding include hemobilia (from hepatic tumor, angioma, penetrating trauma), pancreatic malignancy, and pseudoaneurysm (hemosuccus pancreaticus).
Initial Evaluation & Management
A. Stabilization
The initial step is assessment of the hemodynamic status. A systolic blood pressure less than 100 mm Hg identifies a high-risk patient with severe acute bleeding. A heart rate over 100 beats/min with a systolic blood pressure over 100 mm Hg signifies moderate acute blood loss. A normal systolic blood pressure and heart rate suggest relatively minor hemorrhage. Postural hypotension and tachycardia are useful when present but may be due to causes other than blood loss. Because the hematocrit may take 24 72 hours to equilibrate with the extravascular fluid, it is not a reliable indicator of the severity of acute bleeding.
In patients with significant bleeding, two 18-gauge or larger intravenous lines should be started prior to further diagnostic tests. Blood is sent for complete blood count, prothrombin time with international normalized ratio (INR), serum creatinine, liver enzymes, and cross-matching for 2 4 units or more of packed red blood cells. In patients without hemodynamic compromise or overt active bleeding, aggressive fluid repletion can be delayed until the extent of the bleeding is further clarified. Patients with evidence of hemodynamic compromise are given 0.9% saline or lactated Ringer's injection and cross-matched blood. It is rarely necessary to administer type-specific or O-negative blood. Central venous pressure monitoring is desirable in some cases, but line placement should not interfere with rapid volume resuscitation.
A nasogastric tube should be placed in all patients with suspected active upper tract bleeding. The aspiration of red blood or coffee grounds confirms an upper gastrointestinal source of bleeding, though 10% of patients with confirmed upper tract sources of bleeding have nonbloody aspirates especially when bleeding originates in the duodenum. An aspirate of bright red blood indicates active bleeding and is associated with the highest risk of further bleeding, and complications, while a clear aspirate identifies patients at lower initial risk. Efforts to stop or slow bleeding by gastric lavage with large volumes of fluid are of no benefit and expose the patient to an increased risk of aspiration. Periodic reaspiration of the nasogastric tube serves as an indicator of ongoing bleeding or rebleeding.
B. Blood Replacement
The amount of fluid and blood products required is based on assessment of vital signs, evidence of active bleeding
P.566
from nasogastric aspirate, and laboratory tests. Sufficient packed red blood cells should be given to maintain a hematocrit of 25 30%. In the absence of continued bleeding, the hematocrit should rise 4% for each unit of transfused packed red cells. Transfusion of blood should not be withheld from patients with brisk active bleeding regardless of the hematocrit. It is desirable to transfuse blood in anticipation of the nadir hematocrit. In actively bleeding patients, platelets are transfused if the platelet count is under 50,000/mcL and considered if there is impaired platelet function due to aspirin use (regardless of the platelet count). Uremic patients (who also have dysfunctional platelets) with active bleeding are given three doses of desmopressin (DDAVP), 0.3 mcg/kg intravenously, at 12-hour intervals. Fresh frozen plasma is administered for actively bleeding patients with a coagulopathy and an INR > 1.5. In the face of massive bleeding, 1 unit of fresh frozen plasma should be given for each 5 units of packed red blood cells transfused.
C. Initial Triage
A preliminary assessment of risk based on several clinical factors aids in the resuscitation as well as the rational triage of the patient. Clinical predictors of increased risk of rebleeding and death include age over 65 years, comorbid illnesses, shock, and bright red blood in the nasogastric aspirate or on rectal examination.
1. Very low risk
Reliable patients without serious comorbid medical illnesses or advanced liver disease who have normal hemodynamics, no evidence of overt bleeding (hematemesis or melena) within 48 hours, a negative nasogastric lavage, and normal laboratory tests do not require hospital admission and can undergo further evaluation as outpatients as indicated.
2. High risk
Patients with active bleeding manifested by hematemesis or bright red blood on nasogastric aspirate, shock, persistent hemodynamic derangement despite fluid resuscitation, serious comorbid medical illness, or evidence of advanced liver disease require admission to an intensive care unit (ICU). Emergent endoscopy should be performed after adequate resuscitation, usually within 12 hours.
3. Low to moderate risk
All other patients are admitted to a step-down unit or medical ward after appropriate stabilization for further evaluation and treatment. Patients without evidence of active bleeding undergo nonemergent endoscopy usually within 12 24 hours. In some centers, these patients undergo urgent upper endoscopy to help decide appropriate triage. Based on the findings at endoscopy, patients deemed to be at low risk of rebleeding may be discharged and monitored as outpatients.
Subsequent Evaluation & Treatment
Specific treatment of the various causes of upper gastrointestinal bleeding is discussed elsewhere in this chapter. The following general comments apply to most patients with bleeding.
A. History and Physical Examination
The physician's impression of the bleeding source is correct in only 40% of cases. Signs of chronic liver disease implicate bleeding due to portal hypertension, but a different lesion is identified in 25% of patients with cirrhosis. A history of dyspepsia, NSAID use, or peptic ulcer disease suggests peptic ulcer. Acute bleeding preceded by heavy alcohol ingestion or retching suggests a Mallory-Weiss tear, though most of these patients have neither.
B. Upper Endoscopy
Virtually all patients with upper tract bleeding should undergo upper endoscopy. The benefits of endoscopy in this setting are threefold.
1. To identify the source of bleeding
The appropriate acute and long-term medical therapy is determined by the cause of bleeding. Patients with portal hypertension will be treated differently from those with ulcer disease. If surgery is required for uncontrolled bleeding, the source of bleeding as determined at endoscopy will determine the approach.
2. To determine the risk of rebleeding
Patients with a nonbleeding Mallory-Weiss tear, esophagitis, gastritis, and ulcers that have a clean, white base have a very low risk of rebleeding. It may be safe and cost-effective to discharge such patients from the emergency department or from the medical ward with subsequent outpatient follow-up. Patients with ulcers that are actively bleeding or have a visible vessel or who have variceal bleeding require closer observation in an ICU or step down unit.
3. To render endoscopic therapy
Hemostasis can be achieved in actively bleeding lesions with endoscopic modalities such as cautery, injection, or endoclips. About 90% of bleeding or nonbleeding varices can be effectively treated immediately with injection of a sclerosant or application of rubber bands to the varices. Similarly, 90% of bleeding ulcers, angiomas, or Mallory-Weiss tears can be controlled with either injection of epinephrine, direct cauterization of the vessel by a heater probe or multipolar electrocautery probe, or application of an endoclip. Certain nonbleeding lesions such ulcers with visible blood vessels, and angiomas are also treated with these therapies. Specific endoscopic therapy of varices, peptic ulcers, and Mallory-Weiss tears is dealt with elsewhere in this chapter.
C. Acute Pharmacologic Therapies
1. Acid inhibitory therapy
H2-receptor antagonists do not stop acute bleeding or reduce the incidence of rebleeding. Intravenous proton pump inhibitors (omeprazole, lansoprazole, or pantoprazole, 80 mg bolus, followed by 8 mg/h continuous infusion for 72 hours) reduce the risk of rebleeding in patients with peptic ulcers with high-risk features (active bleeding, visible vessel, or adherent clot) after endoscopic treatment. High doses of oral proton pump inhibitors (omeprazole 40 mg or lansoprazole 60 mg, twice daily for 5 days) may also be effective. Pending the results of endoscopic examination, it may be reasonable to initiate therapy with a high-dose proton pump inhibitor (intravenously or orally) in patients with suspected peptic ulcer bleeding.
2. Octreotide
Continuous intravenous infusion of octreotide (100 mcg bolus, followed by 50 100 mcg/h) reduces splanchnic blood flow and portal blood pressures and is effective in the initial control of bleeding related to portal hypertension. It is administered promptly to all patients with active upper gastrointestinal bleeding and evidence of liver disease or portal hypertension until the source of bleeding can be determined by endoscopy.
3. Vasoactive agents
Intravenous vasopressin is no longer used in the treatment of upper gastrointestinal bleeding. In countries where it is available, terlipressin may be preferred to octreotide for the treatment of bleeding related to portal hypertension because of its sustained reduction of portal and variceal pressures and its proven reduction in mortality.
D. Other Treatment
1. Intra-arterial embolization or vasopressin
Angiographic treatment is used rarely in patients with persistent bleeding from ulcers, angiomas, or Mallory-Weiss tears who have failed endoscopic therapy and are poor operative risks.
2. Transvenous intrahepatic portosystemic shunts (TIPS)
Placement of a wire stent from the hepatic vein through the liver to the portal vein provides effective decompression of the portal venous system and control of acute variceal bleeding. It is indicated in patients in whom endoscopic modalities have failed to control acute variceal bleeding.
Adler DG: ASGE Guideline: the role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc 2004; 60;497.
Bardou M et al: Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005;21:677.
Barkun A et al: Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003;139:843.
P.567
Das A et al: Prediction of outcome of acute GI hemorrhage: a review of risk scores and predictive models. Gastrointest Endosc 2004;60:85.
Pavey DA: Endoscopic therapy for upper-GI vascular ectasias. Gastrointest Endosc 2004;59:233.
2. Acute Lower Gastrointestinal Bleeding
![]() Essentials of Diagnosis
Essentials of Diagnosis
Hematochezia usually present.
Ten percent of cases of hematochezia due to upper gastrointestinal source.
Evaluation with colonoscopy in stable patients.
Massive active bleeding calls for evaluation with sigmoidoscopy, upper endoscopy, angiography, or nuclear bleeding scan.
General Considerations
Lower gastrointestinal bleeding is defined as that arising below the ligament of Treitz, ie, the small intestine or colon; however, over 95% of cases arise from the colon. The severity of lower gastrointestinal bleeding ranges from mild anorectal bleeding to massive, large-volume hematochezia. Bright red blood that drips into the bowl after a bowel movement or is mixed with solid brown stool signifies mild bleeding, usually from an anorectosigmoid source, and can be evaluated in the outpatient setting. Serious lower gastrointestinal bleeding is more common in older men. In patients hospitalized with gastrointestinal bleeding, lower tract bleeding is one-fourth as common as upper gastrointestinal hemorrhage and tends to have a more benign course. Patients hospitalized with lower gastrointestinal tract bleeding are less likely to present with shock or orthostasis (< 20%) or to require transfusions (< 40%). Spontaneous cessation of bleeding occurs in over 85% of cases, and hospital mortality is less than 3%.
Etiology
The cause of these lesions depends on both the age of the patient and the severity of the bleeding. In patients under 50 years of age, the most common causes are infectious colitis, anorectal disease, and inflammatory bowel disease. In older patients, significant hematochezia is most often seen with diverticulosis, vascular ectasias, malignancy, or ischemia. In 20% of acute bleeding episodes, no source of bleeding can be identified.
A. Diverticulosis
Hemorrhage occurs in 3 5% of all patients with diverticulosis and is the most common cause of major lower tract bleeding, accounting for 50% of cases. A significant percentage of cases are associated with the use of nonsteroidal anti-inflammatory agents. Although diverticula are more prevalent on the left side of the colon, bleeding more commonly originates on the right side. Diverticular bleeding usually presents as acute, painless, large-volume maroon or bright red hematochezia in patients over age 50 years. More than 95% of cases require less than 4 units of blood transfusion. Bleeding subsides spontaneously in 80% but may recur in up to 25% of patients.
B. Vascular Ectasias
Vascular ectasias (or angiodysplasias) occur throughout the upper and lower intestinal tracts and cause painless bleeding ranging from melena or hematochezia to occult blood loss. They are responsible for 5 10% of cases of lower gastrointestinal bleeding, where they are most often seen in the cecum and ascending colon. They are flat, red lesions (2 10 mm) with ectatic peripheral vessels radiating from a central vessel, and are most common in patients over 70 years and in those with chronic renal failure. Bleeding in younger patients more commonly arises from the small intestine.
Most colonic ectasias are degenerative lesions that are felt to arise from chronic colonic mucosal contraction obstructing venous mucosal drainage. Over time, the mucosal capillaries dilate and become incompetent. The cause of gastric and small intestinal ectasias is unknown. Some are congenital, part of an inherited syndrome such as hereditary hemorrhagic telangiectasia, or related to autoimmune disorders, typically scleroderma. Ectasias can be identified in up to 6% of subjects over age 60 years, so their mere presence does not prove that the lesion is the source of bleeding, as active bleeding is seldom seen.
C. Neoplasms
Benign polyps and carcinoma are associated with chronic occult blood loss or intermittent anorectal hematochezia. However, colonic neoplasms may cause up to 10% of acute lower gastrointestinal hemorrhage. After endoscopic removal of colonic polyps, important bleeding may occur up to 2 weeks later in 0.3% of patients. Although many patients can be treated conservatively (ie, without colonoscopy), prompt colonoscopy generally is recommended to treat postpolypectomy hemorrhage and minimize the need for transfusions.
D. Inflammatory Bowel Disease
Patients with inflammatory bowel disease (especially ulcerative colitis) often have diarrhea with variable amounts of hematochezia. Bleeding varies from occult blood loss to recurrent hematochezia usually mixed with stool. Symptoms of abdominal pain, tenesmus, and urgency are often present.
E. Anorectal Disease
Anorectal disease commonly results in small amounts of bright red blood noted on the toilet paper, streaking
P.568
of the stool, or dripping into the toilet bowl. The bleeding is slight and seldom results in significant blood loss. Painless bleeding is commonly caused by internal hemorrhoids. Bleeding associated with pain during bowel movements suggests an anal fissure.
F. Ischemic Colitis
This condition is seen commonly in older patients, most of whom have atherosclerotic disease. Most cases occur spontaneously due to transient episodes of nonocclusive ischemia. Ischemic colitis may also occur in 5% of patients after surgery for ileoaortic or abdominal aortic aneurysm. In young patients, colonic ischemia may develop due to vasculitis, coagulation disorders, estrogen therapy, and long distance running. Ischemic colitis results in hematochezia or bloody diarrhea associated with mild cramps. In most patients, the bleeding is mild and self-limited.
G. Others
Radiation-induced proctitis causes anorectal bleeding that may develop months to years after pelvic radiation. Endoscopy reveals multiple rectal telangiectasias. Acute infectious colitis (see Acute Diarrhea, above) commonly causes bloody diarrhea. Rare causes of lower tract bleeding include vasculitic ischemia, solitary rectal ulcer, NSAID-induced ulcers in the small bowel or right colon, small bowel diverticula, and colonic varices.
Evaluation & Management
The color of the stool helps distinguish upper from lower gastrointestinal bleeding, especially when observed by the physician. Brown stools mixed or streaked with blood predict a source in the rectosigmoid or anus. Large volumes of bright red blood suggest a colonic source; maroon stools imply a lesion in the right colon or small intestine; and black stools (melena) predict a source proximal to the ligament of Treitz. Although 10% of patients admitted with self-reported hematochezia have an upper gastrointestinal source of bleeding (eg, peptic ulcer), this almost always occurs in the setting of massive hemorrhage with hemodynamic instability. Painless large-volume bleeding usually suggests diverticular bleeding or vascular ectasias. Bloody diarrhea associated with cramping abdominal pain, urgency, or tenesmus is characteristic of inflammatory bowel disease, infectious colitis, or ischemic colitis.
Important considerations in management include exclusion of an upper tract source, anoscopy and sigmoidoscopy, colonoscopy, nuclear bleeding scans and angiography, and small intestine push enteroscopy or capsule imaging.
A. Exclusion of an Upper Tract Source
A nasogastric tube with aspiration should be considered, especially in patients with hemodynamic compromise. Aspiration of red blood or dark brown ( coffee grounds ) guaiac-positive material strongly implicates an upper gastrointestinal source of bleeding. If blood is not seen and bile is aspirated, an upper source is found in only 1% of patients.
B. Anoscopy and Sigmoidoscopy
In otherwise healthy patients without anemia under age 45 years with small-volume bleeding, anoscopy and sigmoidoscopy are performed to look for evidence of anorectal disease, inflammatory bowel disease, or infectious colitis. If a lesion is found, no further evaluation is needed immediately unless the bleeding persists or is recurrent. In patients over age 45 years with small-volume hematochezia, the entire colon must be evaluated with colonoscopy to exclude tumor.
C. Colonoscopy
In those patients with stable vital signs whose lower gastrointestinal bleeding appears to have stopped (ie, no rectal bleeding within 4 hours of evaluation), elective colonoscopy should be performed to determine the probable site of bleeding within 24 hours of admission after adequate resuscitation and routine colonic lavage. For patients with signs of severe or active lower gastrointestinal bleeding (defined as pulse 100 beats/min or higher, systolic blood pressure < 100 mm Hg), urgent colonoscopy is performed within 6 12 hours of admission after administration of a rapid, high-volume colonic lavage solution, given until the effluent is clear of blood and clots (GoLYTELY, CoLYTE, NuLYTE, 4 8 L given orally or by nasogastric tube over 3 5 hours). At urgent colonoscopy, the probable site of bleeding can be identified in 70 85% of patients, and a high-risk lesion can be identified and treated in up to 20%. Alternatively, many physicians choose first to obtain a nuclear bleeding scan to determine whether there is active bleeding. If no bleeding is detected on the scan, a colonoscopy should be done. If bleeding is detected, angiography should be performed.
D. Nuclear Bleeding Scans and Angiography
Significant continued bleeding occurs in only 15% of patients but may limit the diagnostic effectiveness of colonoscopy. In such patients, either angiographic embolization or surgery may become necessary to control the bleeding. Technetium-labeled red blood cell scanning can detect significant active bleeding and localize it to the small intestine, right colon, or left colon. Because bleeding may be slow or intermittent, less than half of studies are diagnostic, and the accuracy of a positive study is only 78%. Nuclear bleeding studies are more apt to be positive in patients who are passing bright red or maroon stools at the time of the scan. Selective mesenteric angiography requires more brisk bleeding (0.5 1 mL/min) for a positive result than technetium scans and leads to major complications in up to 3% of patients. Accordingly, angiograms are
P.569
performed only in patients with positive technetium scans or with hemodynamically significant, ongoing bleeding. Localization of an actively bleeding vessel is possible in up to 80%.
E. Small Intestine Push Enteroscopy or Capsule Imaging
Less than 5% of acute episodes of lower gastrointestinal bleeding arise from the small intestine, eluding diagnostic evaluation with upper endoscopy and colonoscopy. Because of the difficulty of examining the small intestine and its relative rarity as a source of acute bleeding, evaluation of the small bowel is not usually pursued in patients during the initial episode of acute lower gastrointestinal bleeding. However, the small intestine is investigated in patients with unexplained recurrent hemorrhage of obscure origin. (See Occult & Obscure Gastrointestinal Bleeding below.)
Treatment
A. Discontinue Aspirin and NSAIDs
Up to 80% of patients with lower gastrointestinal tract bleeding have recently ingested aspirin or NSAIDs, which may potentiate bleeding through inhibition of platelet function. These agents should be discontinued. Platelet transfusion (3 6 units) should be administered for persistent bleeding.
B. Therapeutic Colonoscopy
Until recently, colonoscopy served a largely diagnostic role in the patient with lower gastrointestinal bleeding. High-risk lesions (eg, diverticulum with active bleeding or a visible vessel, or a vascular ectasia) may now be treated endoscopically with epinephrine injection, cautery (bipolar or heater probe), or application of metallic endoclips. In severe diverticular hemorrhage with high-risk lesions identified at colonoscopy, rebleeding occurs in half of untreated patients compared with virtually no rebleeding in patients treated endoscopically. Radiation proctitis is effectively treated with applications of cautery therapy to the rectal telangiectasias, preferably with an argon plasma coagulator.
C. Intra-arterial Vasopressin or Embolization
Selective mesenteric arterial infusion of vasoconstrictors (eg, vasopressin) may arrest bleeding in up to 80% of patients with active bleeding from a diverticulum or vascular ectasia, but bleeding recurs in up to 50%. Selective arterial embolization with microcoils is now preferred by most angiographers because it provides definitive control of bleeding in up to 90% with reduced risk of bowel ischemia (< 10%) compared with other embolic agents. Embolization may be preferred to surgery in patients with continued bleeding who are poor surgical candidates.
D. Surgical Treatment
Surgery is indicated in patients with ongoing bleeding that requires more than 4 6 units of blood within 24 hours or more than 10 total units. Most such hemorrhages are caused by a bleeding diverticulum or vascular ectasia. With increasing experience with urgent colonoscopy and angiographic embolization, the need for surgical treatment appears to be decreasing. Preoperative localization of the bleeding site by nuclear scan or angiography allows limited resection of the bleeding segment of small intestine or colon. When accurate localization is not possible or when emergency surgery is required for massive hemorrhage, total abdominal colectomy with ileorectal anastomosis is required with significantly higher morbidity and mortality than limited resections.
Surgery may also be indicated in patients with two or more hospitalizations for diverticular hemorrhage depending on the severity of bleeding and the patient's other comorbid conditions.
Davila RE et al; Standards of Practice Committee: ASGE Guideline: the role of endoscopy in the patient with lower-GI bleeding. Gastrointest Endosc 2005;62:656.
Elta GH: Urgent colonoscopy for acute lower-GI bleeding. Gastrointest Endosc 2004;59:402.
Jensen D: Management of patients with severe hematochezia with all current evidence available. Am J Gastroenterol 2005;100:2403.
Khanna A et al: Embolization as first-line therapy for diverticulosis-related massive lower gastrointestinal bleeding: evidence from a meta-analysis. J Gastrointest Surg 2005;9:343.
Simpson PW et al: Use of endoclips in the treatment of massive colonic diverticular bleeding. Gastrointest Endosc 2004;59: 433.
Strate LL et al: Validation of a clinical prediction rule for severe acute lower intestinal bleeding. Am J Gastroenterol 2005; 100:1821.
Villavicencio R et al: Efficacy and complications of argon plasma coagulation for hematochezia related to radiation therapy. Gastrointest Endosc 2002;55:70.
3. Occult & Obscure Gastrointestinal Bleeding
Occult gastrointestinal bleeding refers to bleeding that is not apparent to the patient. Chronic gastrointestinal blood loss of less than 100 mL/d may cause no appreciable change in stool appearance. Thus, occult bleeding in an adult is identified by a positive FOBT or iron deficiency anemia in the absence of visible blood loss. FOBT may be performed in patients with gastrointestinal symptoms or as a screening test for colorectal neoplasia (see Colorectal Cancer Screening, below). From 1% to 2.5% of patients in screening programs have a positive FOBT.
In the United States, 2% of men and 5% of women have iron deficiency anemia (serum ferritin < 30 45 mcg/L). In premenopausal women, iron deficiency anemia is most commonly attributable to menstrual and pregnancy-associated iron loss; however, a gastrointestinal source of chronic blood loss is present in 10%. Among men and postmenopausal women, a
P.570
potential gastrointestinal cause of blood loss can be identified in the colon in 15 30% and in the upper gastrointestinal tract in 35 55%; a malignancy is present in 10%. Iron deficiency on rare occasions is caused by malabsorption (especially celiac disease) or malnutrition.
Obscure gastrointestinal bleeding refers to occult or overt bleeding of unknown origin that persists or recurs after initial endoscopic evaluation with upper endoscopy and colonoscopy. Obscure-occult bleeding is manifested by recurrent positive FOBTs or recurrent iron deficiency anemia, or both. Obscure-overt bleeding is manifested by persistent or recurrent visible evidence of gastrointestinal bleeding (hematemesis, hematochezia, or melena). Up to 5% of patients admitted to hospitals with clinically overt gastrointestinal bleeding do not have a cause identified on upper endoscopy or colonoscopy.
Causes of Occult or Obscure Gastrointestinal Blood Loss
Occult blood loss may arise from anywhere in the gastrointestinal tract. The most common causes are (1) neoplasms; (2) vascular abnormalities (vascular ectasias, portal hypertensive gastropathy); (3) acid-peptic lesions (esophagitis, peptic ulcer disease, erosions in hiatal hernia); (4) infections (nematodes, especially hookworm; tuberculosis); (5) medications (especially NSAIDs or aspirin); and (6) other causes such as inflammatory bowel disease.
Obscure bleeding (either occult or overt) most commonly arises from lesions in the small intestine. In up to one-third of cases, however, a source of bleeding has been overlooked in the upper or lower tract on prior endoscopic studies. Hematemesis or melena suggests a source proximal to the ligament of Treitz: vascular ectasias, Dieulafoy's vascular malformation, portal hypertensive gastropathy, gastroduodenal varices, or hepatic and pancreatic lesions. In the small intestine, the most common causes of occult or overt obscure bleeding are vascular ectasias and NSAID-induced erosions and ulcerations. Other causes include small bowel neoplasms (stromal tumors, carcinoid, lymphoma, adenocarcinoma) and Meckel's diverticula-associated ulceration.
Evaluation
All adults over age 40 45 years with positive FOBTs or iron deficiency anemia should undergo colonoscopy or upper endoscopy. Endoscopic evaluation is also recommended in premenopausal women and younger men with gastrointestinal symptoms (abdominal pain, dyspepsia or heartburn, change in bowel habits, weight loss), a positive family history of gastrointestinal cancer, or anemia that is disproportionate to the estimated menstrual blood loss. The presence and nature of gastrointestinal symptoms help guide the choice of initial study. After evaluation of the upper and lower gastrointestinal tract with upper endoscopy and colonoscopy, the origin of positive FOBT or iron deficiency anemia remains unexplained in 30 50% of patients. Patients with occult bleeding who have a negative initial endoscopic evaluation may be given a trial of iron supplementation and closely observed. Most require no further evaluation. For recurrent or persistent chronic gastrointestinal blood loss or anemia that responds poorly to iron supplementation, further evaluation is pursued for a source of obscure bleeding (as described below). Where possible, NSAIDs and aspirin should be discontinued.
A. Colonoscopy
Unless patients have symptoms referable to the upper gastrointestinal tract, the colon should be evaluated first. Colonoscopy detects over 95% of colorectal polyps and cancer and permits polypectomy, tumor biopsy, or endoscopic cautery of vascular ectasias. The finding on colonoscopy of a significant lesion clearly consistent with chronic bleeding (mass lesion, large ulceration, bleeding ectasias) obviates the need for upper endoscopy. Barium enema examinations are less accurate than colonoscopy, missing up to 50% of significant adenomas. Furthermore, up to 30 40% of patients with a positive FOBT will have a polyp or mass lesion detected on barium enema, which then necessitates colonoscopic evaluation. For these reasons, barium enema can no longer be recommended except in patients in whom colonoscopy is contraindicated or where expertise in colonoscopy is not available.
B. Upper Endoscopy
Upper endoscopy should be performed first in patients with symptoms referable to the upper gastrointestinal tract (heartburn, dyspepsia, dysphagia, vomiting, weight loss). Upper endoscopy should also be performed in patients with iron deficiency anemia after colonoscopy. In asymptomatic patients with a positive FOBT without iron deficiency anemia whose colonoscopic examination is negative, significant abnormalities are found in almost 50% of patients on upper endoscopy. Although the cost-effectiveness of evaluation of the upper gastrointestinal tract in this setting is uncertain, upper endoscopy is recommended by many experts. Owing to its lower diagnostic accuracy, upper gastrointestinal radiography should be performed only in patients in whom upper endoscopy is contraindicated or where a gastroenterologist is unavailable.
C. Evaluation of Occult or Overt Obscure Bleeding
Upper endoscopy and colonoscopy should be repeated to ascertain that a lesion in these regions has not been overlooked. Abdominal CT may be considered to exclude an hepatic or pancreatic source of bleeding. Small bowel push enteroscopy permits visualization of the upper one-third of the small intestine and permits thermocoagulative treatment of vascular ectasias or bleeding ulcers, when identified. If enteroscopy is unrevealing, video capsule
P.571
endoscopy of the small intestine should be performed. This device captures up to 8 hours of video images of the small intestine, which are transmitted to a portable recorder for subsequent viewing. Capsule endoscopy is superior to radiographic studies (standard small bowel follow through, enteroclysis, or CT enterography) for the detection of small bowel abnormalities, demonstrating possible sources of occult bleeding, most commonly vascular abnormalities and ulcers, in over 50% of patients in whom these studies were unrevealing. Capsule imaging is unable to provide precise localization and is obscured in the setting of significant active bleeding. Laparotomy with intraoperative enteroscopy of the entire small bowel is reserved for patients with obscure gastrointestinal bleeding that is believed to arise from a source in the small intestine, especially for those patients in whom a possible source has been identified on capsule imaging, and who have required multiple transfusions or repeated hospitalizations. A new double-balloon enteroscope is available that allows visualization of most or all of the small intestine via the oral or anal routes with the ability to obtain biopsies or apply thermocoagulation. As this technique becomes more widely available, it may obviate the need for intraoperative endoscopy in many patients.
For patients with hemodynamically significant acute bleeding, angiography may be helpful for localization and embolization of a bleeding vascular abnormality.
Gralnek I: Obscure-overt gastrointestinal bleeding. Gastroenterology 2005;128:1424.
Hartmann D et al: A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc 2005;61:826.
May A et al: Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc 2005;62:62.
Pannazio M et al: Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive patients. Gastroenterology 2004;126:643.
Triester SL et al: A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005;100:2407.
Diseases of the Peritoneum
Approach To The Patient With Ascites
Etiology of Ascites
The term ascites denotes the pathologic accumulation of fluid in the peritoneal cavity. Healthy men have little or no intraperitoneal fluid, but women normally may have up to 20 mL depending on the phase of the menstrual cycle. The causes of ascites may be classified into two broad pathophysiologic categories: that which is associated with a normal peritoneum and that which occurs due to a diseased peritoneum (Table 14-8). The most common cause of ascites is portal hypertension
P.572
secondary to chronic liver disease, which accounts for over 80% of patients with ascites. The management of portal hypertensive ascites is discussed in Chapter 15. The most common causes of nonportal hypertensive ascites include infections (tuberculous peritonitis), intra-abdominal malignancy, inflammatory disorders of the peritoneum, and ductal disruptions (chylous, pancreatic, biliary).
Table 14-8. Causes of ascites. | |||||
|---|---|---|---|---|---|
|
Clinical Features
A. Symptoms and Signs
The history usually is one of increasing abdominal girth, with the presence of abdominal pain depending on the cause. Because most ascites is secondary to chronic liver disease with portal hypertension, patients should be asked about risk factors for liver disease, especially alcohol consumption, transfusions, tattoos, injection drug use, a history of viral hepatitis or jaundice, and birth in an area endemic for hepatitis. A history of cancer or marked weight loss arouses suspicion of malignant ascites. Fevers may suggest infected peritoneal fluid, including bacterial peritonitis (spontaneous or secondary). Patients with chronic liver disease and ascites are at greatest risk for developing spontaneous bacterial peritonitis. In immigrants, immunocompromised hosts, or severely malnourished alcoholics, tuberculous peritonitis should be considered.
Physical examination should emphasize signs of portal hypertension and chronic liver disease. Elevated jugular venous pressure may suggest right-sided congestive heart failure or constrictive pericarditis. A large tender liver is characteristic of acute alcoholic hepatitis or Budd-Chiari syndrome. The presence of large abdominal wall veins with cephalad flow also suggests portal hypertension; inferiorly directed flow implies hepatic vein obstruction. Signs of chronic liver disease include palmar erythema, cutaneous spider angiomas, gynecomastia, and Dupuytren's contracture. Asterixis secondary to hepatic encephalopathy may be present. Anasarca results from cardiac failure or nephrotic syndrome with hypoalbuminemia. Finally, firm lymph nodes in the left supraclavicular region or umbilicus may suggest intra-abdominal malignancy.
The physical examination is relatively insensitive for detecting ascitic fluid. In general, patients must have at least 1500 mL of fluid to be detected reliably by this method. Even the experienced clinician may find it difficult to distinguish between obesity and small-volume ascites. Abdominal ultrasound establishes the presence of fluid.
B. Laboratory Testing
1. Abdominal paracentesis
Abdominal paracentesis is performed as part of the diagnostic evaluation in all patients with new onset of ascites to help determine the cause. It should also be performed to diagnose bacterial peritonitis in all patients admitted to the hospital with cirrhosis and ascites (in whom the prevalence of bacterial peritonitis is 10 30%) and when patients with known ascites develop clinical deterioration (fever, abdominal pain, rapid worsening of renal function, or worsened hepatic encephalopathy).
a. Inspection
Cloudy fluid suggests infection. Milky fluid is seen with chylous ascites due to high triglyceride levels. Bloody fluid is most commonly attributable to a traumatic paracentesis, but up to 20% of cases of malignant ascites are bloody.
b. Routine studies
(1) Cell count
A white blood cell count is the most important test. Normal ascitic fluid contains < 500 leukocytes/mcL and < 250 polymorphonuclear neutrophils (PMNs)/mcL. Any inflammatory condition can cause an elevated ascitic white count. A PMN count of > 250/mcL (neutrocytic ascites) with a percentage of > 75% of all white cells is highly suggestive of bacterial peritonitis, either spontaneous primary peritonitis or secondary peritonitis (ie, caused by an intra-abdominal source of infection, such as a perforated viscus or appendicitis). An elevated white count with a predominance of lymphocytes arouses suspicion of tuberculosis or peritoneal carcinomatosis.
(2) Albumin and total protein
The serum-ascites albumin gradient (SAAG) is the best single test for the classification of ascites into portal hypertensive and nonportal hypertensive causes (Table 14-8). Calculated by subtracting the ascitic fluid albumin from the serum albumin, the gradient correlates directly with the portal pressure. An SAAG 1.1 g/dL suggests underlying portal hypertension, while gradients < 1.1 g/dL implicate nonportal hypertensive causes.
The accuracy of the SAAG exceeds 95% in classifying ascites. It should be recognized, however, that approximately 4% of patients have mixed ascites, ie, underlying cirrhosis with portal hypertension complicated by a second cause for ascites formation (such as malignancy or tuberculosis). Thus, a high SAAG is indicative of portal hypertension but does not exclude concomitant malignancy.
The ascitic fluid total protein provides some additional clues to the cause. An elevated SAAG and a high protein level (> 2.5 g/dL) are seen in most cases of hepatic congestion secondary to cardiac disease or Budd-Chiari syndrome. However, an increased ascitic fluid protein is also found in up to 20% of cases of uncomplicated cirrhosis. Two-thirds of patients with malignant ascites have a total protein level > 2.5 g/dL.
(3) Culture and Gram stain
The best technique consists of the inoculation of aerobic and anaerobic blood culture bottles with 5 10 mL of ascitic fluid at the patient's bedside, which increases the sensitivity for detecting bacterial peritonitis to over 85% in patients with neutrocytic ascites (> 250 PMNs/mcL), compared with approximately 50% sensitivity by conventional agar plate or broth cultures.
c. Optional studies
Other laboratory tests are of utility in some specific clinical situations. Glucose and
P.573
lactate dehydrogenase (LDH) may be helpful in distinguishing spontaneous from secondary bacterial peritonitis (see below). Glucose levels are reduced in patients with tuberculous peritonitis. An elevated amylase may suggest pancreatic ascites or a perforation of the gastrointestinal tract with leakage of pancreatic secretions into the ascitic fluid. Perforation of the biliary tree is suspected with an ascitic bilirubin concentration that is greater than the serum bilirubin. An elevated ascitic creatinine suggests leakage of urine from the bladder or ureters. Ascitic fluid cytologic examination is ordered if peritoneal carcinomatosis is suspected.
C. Imaging
Abdominal ultrasound is useful in confirming the presence of ascites and in the guidance of paracentesis. Both ultrasound and CT imaging are useful in distinguishing between causes of portal and nonportal hypertensive ascites. Doppler ultrasound and CT can detect thrombosis of the hepatic veins (Budd-Chiari syndrome) or portal veins. In patients with nonportal hypertensive ascites, these studies are useful in detecting lymphadenopathy and masses of the mesentery and of solid organs such as the liver, ovaries, and pancreas. Furthermore, they permit directed percutaneous needle biopsies of these lesions. Ultrasound and CT are poor procedures for the detection of peritoneal carcinomatosis.
D. Laparoscopy
Laparoscopy is an important test in the evaluation of some patients with nonportal hypertensive ascites (low SAAG) or mixed ascites. It permits direct visualization and biopsy of the peritoneum, liver, and some intra-abdominal lymph nodes. Cases of suspected peritoneal tuberculosis or suspected malignancy with nondiagnostic CT imaging and ascitic fluid cytology are best evaluated by this method.
Krige JE et al: ABC of diseases of the liver, pancreas, and biliary system: portal hypertension 2. Ascites, encephalopathy, and other conditions. BMJ 2001;322:416.
Runyon BA: AASLD Practice Guideline. Management of adult patients with ascites due to cirrhosis. Hepatology 2004; 39:841.
Spontaneous Bacterial Peritonitis
![]() Essentials of Diagnosis
Essentials of Diagnosis
A history of chronic liver disease and ascites.
Fever and abdominal pain.
Peritoneal finding on examination uncommonly encountered.
Neutrocytic ascites (> 250 white blood cells/mcL) with neutrophilic predominance.
General Considerations
Spontaneous bacterial infection of ascitic fluid occurs in the absence of an apparent intra-abdominal source of infection. It is seen with few exceptions in patients with ascites caused by chronic liver disease. Translocation of enteric bacteria across the gut wall or mesenteric lymphatics leads to seeding of the ascitic fluid, as may bacteremia from other sites. Approximately 20 30% of cirrhotic patients with ascites develop spontaneous peritonitis; however, the incidence is greater than 40% in patients with ascitic fluid total protein < 1 g/dL, probably due to decreased ascitic fluid opsonic activity.
Virtually all cases of spontaneous bacterial peritonitis are caused by a monomicrobial infection. The most common pathogens are enteric gram-negative bacteria (E coli, Klebsiella pneumoniae, Enterococcus species) or gram-positive bacteria (Streptococcus pneumoniae, viridans streptococci). Anaerobic bacteria are not associated with spontaneous bacterial peritonitis.
Clinical Findings
A. Symptoms and Signs
Eighty to 90 percent of patients with spontaneous bacterial peritonitis are symptomatic; in many cases the presentation is subtle. Spontaneous bacterial peritonitis may be present in 20% of patients hospitalized with chronic liver disease in the absence of any suggestive symptoms or signs.
The most common symptoms are fever and abdominal pain, present in two-thirds of patients. Spontaneous bacterial peritonitis may also present with a change in mental status due to exacerbation or precipitation of hepatic encephalopathy, or sudden worsening of renal function. Physical examination typically demonstrates signs of chronic liver disease with ascites. Abdominal tenderness is present in less than 50% of patients, and its presence suggests other processes.
B. Laboratory Findings
The most important diagnostic test is abdominal paracentesis. Ascitic fluid should be sent for cell count, and blood culture bottles should be inoculated at the bedside; Gram stain is insensitive. An ascitic fluid total protein of more than 1 g/dL is evidence against spontaneous bacterial peritonitis.
In the proper clinical setting, an ascitic fluid PMN count of > 250 cells/mcL (neutrocytic ascites) is presumptive evidence of bacterial peritonitis. The percentage of PMNs is greater than 50 70% of the ascitic fluid white blood cells and commonly approximates 100%. Patients with neutrocytic ascites are presumed to be infected and should be started regardless of symptoms on antibiotics. Although 10 30% of patients with neutrocytic ascites have negative ascitic bacterial cultures ( culture-negative neutrocytic ascites ), it is presumed that these patients have bacterial
P.574
peritonitis and should be treated empirically. Occasionally, a positive blood culture identifies the organism when ascitic fluid is sterile.
Differential Diagnosis
Spontaneous bacterial peritonitis must be distinguished from secondary bacterial peritonitis, in which ascitic fluid has become secondarily infected by an intra-abdominal infection. Even in the presence of perforation, clinical symptoms and signs of peritonitis may be lacking in up to 30% of patients owing to the separation of the visceral and parietal peritoneum by the ascitic fluid. Causes of secondary bacterial peritonitis include appendicitis, diverticulitis, perforated peptic ulcer, and perforated gallbladder. Secondary bacterial infection accounts for 3% of cases of infected ascitic fluid.
Ascitic fluid total protein, LDH, and glucose are useful in distinguishing spontaneous bacterial peritonitis from secondary infection. Up to two-thirds of patients with secondary bacterial peritonitis have at least two of the following: decreased glucose level (< 50 mg/dL), an elevated LDH level (greater than serum), and total protein > 1 g/dL. Ascitic neutrophil counts > 10,000/mcL also are suspicious; however, most patients with secondary peritonitis have neutrophil counts within the range of spontaneous peritonitis. The presence of multiple organisms on ascitic fluid Gram stain or culture is diagnostic of secondary peritonitis.
If secondary bacterial peritonitis is suspected, plain films, abdominal CT imaging, and water-soluble contrast studies of the upper and lower gastrointestinal tracts should be obtained to look for evidence of an intra-abdominal source of infection. If these studies are negative and secondary peritonitis still is suspected, repeat paracentesis should be performed after 48 hours of antibiotic therapy to confirm that the PMN count is decreasing. Secondary bacterial peritonitis should be suspected in patients in whom the PMN count is not below the pretreatment value at 48 hours.
Neutrocytic ascites may also be seen in some patients with peritoneal carcinomatosis, pancreatic ascites, or tuberculous ascites. In these circumstances, however, PMNs account for less than 50% of the ascitic white blood cells.
Prevention
Up to 70% of patients who survive an episode of spontaneous bacterial peritonitis will have another episode within 1 year. Prophylactic therapy with norfloxacin, 400 mg/d; ciprofloxacin, 750 mg weekly; or trimethoprim-sulfamethoxazole, one double-strength tablet daily has been shown to reduce the rate of recurrent infections to less than 20% and is recommended. Prophylaxis should be considered also in patients who have not had prior bacterial peritonitis but are at increased risk of infection due to low-protein ascites (total ascitic protein < 1 g/dL). Although improvement in survival in cirrhotic patients with ascites treated with prophylactic antibiotics has not been shown, decision analytic modeling suggests that in patients with prior bacterial peritonitis or low ascitic fluid protein, the use of prophylactic antibiotics is a cost-effective strategy.
Treatment
Empiric therapy for spontaneous bacterial peritonitis should be initiated with a third-generation cephalosporin such as cefotaxime (dosage: 2 g intravenously every 8 12 hours depending on renal function), which covers 98% of causative agents of this disorder. If enterococcus infection is suspected, ampicillin may be added. Because of a high risk of nephrotoxicity in patients with chronic liver disease, aminoglycosides should not be used. Although the optimal duration of therapy is unknown, a course of 5 10 days is sufficient in most patients, or until the ascites fluid PMN count decreases to < 250 cells/mcL. Renal failure develops in up to 40% of patients and is a major cause of death. In patients given intravenous albumin, 1.5 g/kg on day 1 and 1 g/kg on day 3, the incidence of renal failure and mortality are reduced both during hospitalization and at follow-up. Patients with suspected secondary bacterial peritonitis should be given broad-spectrum coverage for enteric aerobic and anaerobic flora with a third-generation cephalosporin and metronidazole pending identification and definitive (usually surgical) treatment of the cause. In fact, the most effective treatment for spontaneous bacterial peritonitis is liver transplant.
Prognosis
The mortality rate of spontaneous bacterial peritonitis exceeds 30%. However, if the disease is recognized and treated early, the rate is less than 10%. As the majority of patients have underlying severe liver disease, many may die of liver failure, hepatorenal syndrome, or bleeding complications from portal hypertension.
Gines P et al: Management of cirrhosis and ascites. N Engl J Med 2004;350:1646.
Runyon BA: AASLD Practice Guideline. Management of adult patients with ascites due to cirrhosis. Hepatology 2004; 39:841.
Runyon BA: The evolution of ascitic fluid analysis in the diagnosis of spontaneous bacterial peritonitis. Am J Gastroenterol 2003;98:1675.
Sheer TA et al: Spontaneous bacterial peritonitis. Dig Dis 2005;23:39.
Soares-Weiser K et al: Evidence based case report. Antibiotic treatment for spontaneous bacterial peritonitis. BMJ 2002; 324:100.
Tuberculous Peritonitis
Tuberculosis occurs in extrapulmonary sites in 20% of non-HIV-infected people and up to 70% of those infected
P.575
with HIV. Although tuberculous involvement of the peritoneum accounts for less than 2% of all causes of ascites in the United States, it remains a significant problem in the developing world. In Western countries, its incidence is higher among those with HIV disease, immigrants from underdeveloped countries, the urban poor, patients with cirrhosis, and nursing home residents.
The presenting symptoms include low-grade fever, abdominal pain, anorexia, and weight loss. Most patients have symptoms for months before the diagnosis is established. On physical examination, patients may have generalized abdominal tenderness and distention. There may be clinically evident ascites or suggestion of an abdominal mass. Ultrasonography or CT imaging of the abdomen reveals free or loculated ascites in > 80% of patients and also may demonstrate lymphadenopathy or peritoneal, mesenteric, or omental thickening.
The diagnosis thus can be difficult to establish, particularly in patients with underlying cirrhosis with ascites and in patients without ascites (in whom the diagnosis may not be suspected). Chest radiographs are abnormal in over 70%, but active tuberculous pulmonary disease is evident in less than 20% of patients. Skin tests are positive in 50%. Smears of ascitic fluid for acid-fast bacilli are usually negative, and cultures are positive in only 35%. Other findings are an ascitic fluid total protein > 3.0 g/dL, LDH > 90 units/L, or mononuclear cell-predominant leukocytosis > 500/mcL each has a sensitivity of 70 80% but limited specificity. Several studies report that ascites adenosine deaminase activity 30 IU/L has sensitivity and specificity levels of > 90% for the diagnosis of tuberculous peritonitis.
In patients with suspected tuberculous peritonitis, laparoscopy establishes the diagnosis. In over 90% of patients, characteristic peritoneal nodules are visible, and granulomas are seen on peritoneal biopsy. Peritoneal cultures require at least 4 6 weeks and are positive in less than two-thirds of patients.
Treatment of tuberculosis is discussed in Chapter 9.
Sanai FM et al: Systematic review: tuberculous peritonitis presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther 2005;22:685.
Malignant Ascites
Two-thirds of cases of malignant ascites are caused by peritoneal carcinomatosis. The most common tumors causing carcinomatosis are primary adenocarcinomas of the ovary, uterus, pancreas, stomach, colon, lung, or breast. The remaining one-third are due to lymphatic obstruction or portal hypertension due to hepatocellular carcinoma or diffuse hepatic metastases. Patients present with nonspecific abdominal discomfort and weight loss associated with increased abdominal girth. Nausea or vomiting may be caused by partial or complete intestinal obstruction. Abdominal CT may be useful to demonstrate the primary malignancy or hepatic metastases but seldom confirms the diagnosis of peritoneal carcinomatosis. In patients with carcinomatosis, paracentesis demonstrates a low serum ascites-albumin gradient (< 1.1 mg/dL), an increased total protein (> 2.5 g/dL), and an elevated white cell count (often both neutrophils and mononuclear cells) but with a lymphocyte predominance. Cytology is positive in over 95%, but laparoscopy may be required in patients with negative cytology to confirm the diagnosis and to exclude tuberculous peritonitis, with which it may be confused. Malignant ascites attributable to portal hypertension usually is associated with an increased serum ascites-albumin gradient (> 1.1 g/dL), a variable total protein, and negative ascitic cytology. Ascites caused by peritoneal carcinomatosis does not respond to diuretics.
Patients may be treated with periodic large-volume paracentesis for symptomatic relief. Intraperitoneal chemotherapy is sometimes used to shrink the tumor, but the overall prognosis is extremely poor, with only 10% survival at 6 months. Ovarian cancers represent an exception to this rule. With newer treatments consisting of surgical debulking and intraperitoneal chemotherapy, long-term survival from ovarian cancer is possible.
Adam RA et al: Malignant ascites: past, present, and future. J Am Coll Surg 2004;198:999.
Familial Mediterranean Fever
This is a rare autosomal recessive disorder of unknown pathogenesis that almost exclusively affects people of Mediterranean ancestry, especially Sephardic Jews, Armenians, Turks, and Arabs. Patients lack a protease in serosal fluids that normally inactivates interleukin-8 and the chemotactic complement factor 5A. Symptoms present in most patients before the age of 20 years. It is characterized by episodic bouts of acute peritonitis that may be associated with serositis involving the joints and pleura. Peritoneal attacks are marked by the sudden onset of fever, severe abdominal pain, and abdominal tenderness with guarding or rebound tenderness. If left untreated, attacks resolve within 24 48 hours. Because symptoms resemble those of surgical peritonitis, patients may undergo unnecessary exploratory laparotomy. Colchicine, 0.6 mg two or three times daily, has been shown to decrease the frequency and severity of attacks. Secondary amyloidosis (AA protein) with renal or hepatic involvement may occur in 25% of cases and is the main cause of death. Colchicine prevents or arrests further progression of amyloidosis development. In the absence of amyloidosis, the prognosis is excellent. The diagnosis of familial Mediterranean fever still is based on clinical criteria. Although the gene responsible for familial Mediterranean fever (MEFV) has been identified, commercial genetic tests fail to identify one of the known gene mutations in up to one-third of patients. Genetic testing is most useful to confirm the diagnosis in patients with atypical symptoms.
P.576
Mor A et al: Abdominal and digestive system associations of familial Mediterranean fever. Am J Gastroenterol 2003;98: 2594.
Simon A et al: Mediterranean fever a not so unusual cause of abdominal pain. Best Pract Res Clin Gastroenterol 2005; 19:199.
Mesothelioma
Primary malignant mesothelioma is a rare tumor. Over 70% of cases have a history of asbestos exposure. Presenting symptoms and signs include abdominal pain or bowel obstruction, increased abdominal girth, and small to moderate ascites. The chest radiograph reveals pulmonary asbestosis in over 50%. The ascitic fluid is hemorrhagic, with a low serum-ascites albumin gradient. Cytology is often negative. Abdominal CT may reveal sheet-like masses involving the mesentery and omentum. Diagnosis is made at laparotomy or laparoscopy. The prognosis is extremely poor, but long-term survivors have been described with a combination of surgical debulking of tumor followed by heated intraoperative intraperitoneal chemotherapy and early postoperative intraperitoneal chemotherapy. Multicystic and well-differentiated papillary mesotheliomas are associated with a long survival with surgical treatment alone.
Hassan R et al: Nonpleural mesotheliomas: mesothelioma of the peritoneum, tunica vaginalis, and pericardium. Hematol Oncol Clin North Am 2005;19:1067.
Miscellaneous Peritoneal Diseases
Chylous ascites is the accumulation of lipid-rich lymph in the peritoneal cavity. The ascitic fluid is characterized by a milky appearance with a triglyceride level > 1000 mg/dL. The usual cause in adults is lymphatic obstruction or leakage caused by malignancy, especially lymphoma. Nonmalignant causes include postoperative trauma, cirrhosis, tuberculosis, pancreatitis, and filariasis.
Pancreatic ascites is the intraperitoneal accumulation of massive amounts of pancreatic secretions due either to disruption of the pancreatic duct or to a pancreatic pseudocyst. It is most commonly seen in patients with chronic pancreatitis and complicates up to 3% of cases of acute pancreatitis. Because the pancreatic enzymes are not activated, pain often is absent. The ascitic fluid is characterized by a high protein level (> 2.5 g/dL) but a low SAAG. Ascitic fluid amylase levels are in excess of 1000 units/L. In nonsurgical cases, initial treatment consists of bowel rest, total parenteral nutrition (TPN), and octreotide to decrease pancreatic secretion. Persistent leakage requires treatment with either endoscopic placement of stents into the pancreatic duct or surgical drainage.
Bile ascites is caused most commonly by complications of biliary tract surgery, percutaneous liver biopsy, or abdominal trauma. Unless the bile is infected, bile ascites usually does not cause abdominal pain, fever, or leukocytosis. Paracentesis reveals yellow fluid with a ratio of ascites bilirubin to serum bilirubin greater than 1.0. Treatment depends on the location and rate of bile leakage. Postcholecystectomy cystic duct leaks may be treated with endoscopic sphincterotomy or biliary stent placement to facilitate bile flow across the sphincter of Oddi. Other leaks may be treated with percutaneous drainage by interventional radiologists or with surgical closure.
Le Moine O et al: Endoscopic management of pancreatic fistula after pancreatic and other abdominal surgeries. Best Pract Res Clin Gastroenterol 2004;18:957.
Leong RW et al: Chylous ascites caused by portal vein thrombosis treated with octreotide. J Gastroenterol Hepatol 2003;18: 1211.
Diseases of the Esophagus
Evaluation of Esophageal Disorders
Symptoms
Heartburn, dysphagia, and odynophagia almost always indicate a primary esophageal disorder.
A. Heartburn
Heartburn (pyrosis) is the feeling of substernal burning, often radiating to the neck. Caused by the reflux of acidic (or, rarely, alkaline) material into the esophagus, it is highly specific for gastroesophageal reflux disease.
B. Dysphagia
Difficulties in swallowing may arise from problems in transferring the food bolus from the oropharynx to the upper esophagus (oropharyngeal dysphagia) or from impaired transport of the bolus through the body of the esophagus (esophageal dysphagia). The history usually leads to the correct diagnosis.
1. Oropharyngeal dysphagia
The oropharyngeal phase of swallowing is a complex process requiring elevation of the tongue, closure of the nasopharynx, relaxation of the upper esophageal sphincter, closure of the airway, and pharyngeal peristalsis. A variety of mechanical and neuromuscular conditions can disrupt this process (Table 14-9). Problems with the oral phase of swallowing cause drooling or spillage of food from the mouth, inability to chew or initiate swallowing, or dry mouth. Pharyngeal dysphagia is characterized by an immediate sense of the bolus catching in the neck, the need to swallow repeatedly to clear food from the pharynx, or coughing or choking during
P.577
meals. There may be associated dysphonia, dysarthria, or other neurologic symptoms.
Table 14-9. Causes of oropharyngeal dysphagia. | |
|---|---|
|
2. Esophageal dysphagia
Esophageal dysphagia may be caused by mechanical lesions obstructing the esophagus or by motility disorders (Table 14-10). Patients with mechanical obstruction experience dysphagia, primarily for solids. This is recurrent, predictable, and, if the lesion progresses, will worsen as the lumen narrows. Patients with motility disorders have dysphagia for both solids and liquids. It is episodic, unpredictable, and nonprogressive.
C. Odynophagia
Odynophagia is sharp substernal pain on swallowing that may limit oral intake. It usually reflects severe erosive disease. It is most commonly associated with infectious esophagitis due to Candida, herpesviruses, or CMV, especially in immunocompromised patients. It may also be caused by corrosive injury due to caustic ingestions and by pill-induced ulcers.
Diagnostic Studies
A. Upper Endoscopy
Endoscopy is the study of choice for evaluating persistent heartburn, odynophagia, and structural abnormalities detected on barium esophagography. In addition to direct visualization, it allows biopsy of mucosal abnormalities and dilation of strictures.
B. Videoesophagography
Oropharyngeal dysphagia is best evaluated with rapid-sequence videoesophagography.
C. Barium Esophagography
Patients with esophageal dysphagia often are evaluated first with a radiographic barium study to differentiate between mechanical lesions and motility disorders, providing important information about the latter in particular. In patients with esophageal dysphagia and a suspected motility disorder, barium esophagoscopy should be obtained first. In patients in whom there is a high suspicion of a mechanical lesion, many clinicians will proceed first to endoscopic evaluation because it better identifies mucosa lesions (eg, erosions) and permits mucosal biopsy and dilation. However, barium study is more sensitive for detecting subtle esophageal narrowing due to rings, achalasia, and proximal esophageal lesions.
D. Esophageal Manometry
Esophageal motility may be assessed using manometric techniques. They are indicated (1) to determine the location of the lower esophageal sphincter to allow precise placement of a conventional electrode pH probe; (2) to establish the etiology of dysphagia in patients in whom a mechanical obstruction cannot be found, especially if a diagnosis of achalasia is suspected by endoscopy or barium study; (3) for the preoperative assessment of patients being considered for antireflux surgery to exclude an alternative diagnosis (eg, achalasia) or possibly to assess peristaltic function in the esophageal body.
E. Esophageal pH Recording
Esophageal pH may be monitored continuously by means of a small pH electrode that is passed transnasally
P.578
and placed 5 cm above the lower esophageal sphincter. The probe is attached to a portable pH device capable of recording pH for up to 24 hours. The recording provides information about the amount of esophageal acid reflux and on the temporal correlations between symptoms and reflux. A wireless system now is available in which a radiotelemetry pH capsule that is attached to the mucosa of the esophagus transmits data to a pager-sized receiver worn by the patient.
Table 14-10. Causes of esophageal dysphagia. | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Traditional pH monitoring devices provide information about the amount of esophageal acid reflux but not nonacid reflux. New techniques using multichannel intraluminal impedance allow assessment of gas and nonacid liquid reflux. Although not yet widely available, they may be useful in evaluation of patients with atypical reflux symptoms or persistent symptoms despite therapy.
Achem SR et al: Dysphagia in aging. J Clin Gastroenterol 2005; 39:357.
Arora AS: Management strategies for dysphagia with a normal-appearing esophagus. Clin Gastroenterol Hepatol 2005;3:299.
Pandolfino JE et al; American Gastroenterological Association: American Gastroenterological Association medical position statement: Clinical use of esophageal manometry. Gastroenterology 2005;128:207.
Pandolfino JE et al; American Gastroenterological Association: AGA Technical review on the clinical use of esophageal manometry. Gastroenterology 2005;128:209.
P.579
Prakash C et al: Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2005;3:329.
Streets CG et al: Ambulatory 24-hour pH esophageal monitoring. Why, when, and what to do. J Clin Gastroenterol 2003;37: 14.
Tutuian R et al: Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J Clin Gastroenterol 2003;37:206.
Gastroesophageal Reflux Disease
![]() Essentials of Diagnosis
Essentials of Diagnosis
Heartburn; may be exacerbated by meals, bending, or recumbency.
Typical uncomplicated cases do not require diagnostic studies.
Endoscopy demonstrates abnormalities in < 50% of patients.
Barium esophagography seldom helpful.
General Considerations
Gastroesophageal reflux disease affects 20% of adults, who report at least weekly episodes of heartburn, and up to 10% complain of daily symptoms. Although most patients have mild disease, esophageal mucosal damage (reflux esophagitis) develops in up to 50% and more serious complications develop in a few others. Several factors may contribute to gastroesophageal reflux disease.
A. Incompetent Lower Esophageal Sphincter
The antireflux barrier at the gastroesophageal junction depends on intrinsic lower esophageal sphincter pressure, the intra-abdominal location of the sphincter, and the extrinsic compression of the sphincter by the crural diaphragm. In most patients, baseline lower esophageal sphincter pressures are normal (10 30 mm Hg). In patients without hiatal hernias, about 70% of reflux episodes occur during relaxations of the lower esophageal sphincter that occur spontaneously ( transient relaxations ) or as prolonged relaxation after swallowing. The remaining events occur during periods of low sphincter pressure ( hypotensive sphincter). A small number of patients with more severe involvement (especially those with strictures) have chronically incompetent sphincters (< 10 mm Hg), resulting in free reflux or stress reflux during lifting, bending, or abdominal straining.
B. Hiatal Hernia
Hiatal hernias are common and usually cause no symptoms. In patients with gastroesophageal reflux, however, they are associated with higher amounts of acid reflux and delayed esophageal acid clearance leading to more severe esophagitis, especially Barrett's esophagus. Increased reflux episodes occur during normal swallowing-induced relaxation, periods of sphincter hypotension, and straining due to reflux of acid from the hiatal hernia sac into the esophagus.
C. Irritant Effects of Refluxate
Esophageal mucosal damage is related to the potency of the refluxate and the amount of time it is in contact with the mucosa. Acidic gastric fluid (pH < 4.0) is extremely caustic to the esophageal mucosa and is the major injurious agent in the majority of cases. In some patients, reflux of bile or alkaline pancreatic secretions may be contributory.
D. Abnormal Esophageal Clearance
Acid refluxate normally is cleared and neutralized by esophageal peristalsis and salivary bicarbonate. During sleep, swallowing-induced peristalsis is infrequent, prolonging acid exposure to the esophagus. One-third of patients with severe gastroesophageal reflux disease also have diminished peristaltic clearance. Certain medical conditions such as scleroderma are associated with diminished peristalsis. Sj gren's syndrome, anticholinergic medications, and oral radiation therapy may exacerbate gastroesophageal reflux disease due to impaired salivation.
E. Delayed Gastric Emptying
Impaired gastric emptying due to gastroparesis or partial gastric outlet obstruction potentiates gastroesophageal reflux disease.
Clinical Findings
A. Symptoms and Signs
The typical symptom is heartburn. This most often occurs 30 60 minutes after meals and upon reclining. Patients often report relief from taking antacids or baking soda. When this symptom is dominant, the diagnosis is established with a high degree of reliability. Many patients, however, have less specific dyspeptic symptoms with or without heartburn. Overall, a clinical diagnosis of gastroesophageal reflux has a sensitivity of 80% but a specificity of only 70%. Severity is not correlated with the degree of tissue damage. In fact, some patients with severe esophagitis are only mildly symptomatic. Patients may complain of regurgitation the spontaneous reflux of sour or bitter gastric contents into the mouth. Dysphagia occurs in one-third of patients and may be due to erosive esophagitis, abnormal esophageal peristalsis, or the development of an esophageal stricture.
Atypical manifestations of gastroesophageal disease are being recognized with increasing frequency. These include asthma, chronic cough, chronic laryngitis, sore throat, and noncardiac chest pain. Gastroesophageal reflux may be either a causative or an exacerbating factor in up to 50% of these patients, especially those with refractory symptoms. Because many of these patients do not have heartburn or regurgitation, the diagnosis often is overlooked.
Physical examination and laboratory data are normal in uncomplicated disease.
B. Special Examinations
Patients with typical symptoms of heartburn and regurgitation and with uncomplicated disease may be treated empirically for 4 weeks for gastroesophageal reflux disease without the need for diagnostic studies. Further investigation is required in patients with complicated disease and those unresponsive to empiric therapy.
1. Upper endoscopy
Upper endoscopy with biopsy is the standard procedure for documenting the type and extent of tissue damage in gastroesophageal reflux. Fifty percent of patients with proved acid reflux will have visible mucosal damage (known as reflux esophagitis), characterized by single or multiple erosions or ulcers in the distal esophagus at the squamocolumnar junction. However, endoscopy is normal in up to 50% of symptomatic patients with gastroesophageal reflux, a condition sometimes referred to as nonerosive reflux disease (NERD). The Los Angeles classification grades esophageal abnormalities on a scale of A (one or more isolated mucosal breaks 5 mm that do not extend between the tops of two mucosal folds) to D (one or more mucosal breaks that involve at least 75% of the esophageal circumference). Initial medical therapy for gastroesophageal reflux disease is guided by the presence of symptoms, not the endoscopic findings. Hence, endoscopy is not warranted for most patients with typical symptoms suggesting uncomplicated reflux disease. Endoscopy should be performed in patients whose symptoms have not responded after initial empiric therapy and patients with symptoms suggesting complicated disease (dysphagia, odynophagia, occult or overt bleeding, or iron deficiency anemia).
2. Barium esophagography
This study plays a limited role. In patients with severe dysphagia, it is sometimes obtained prior to endoscopy to identify a stricture.
3. Ambulatory esophageal pH monitoring
Ambulatory pH monitoring is the best study for documenting acid reflux, but it is unnecessary in most patients. Dual-channel transnasal probes are sometimes used to measure reflux in the proximal esophagus or hypopharynx in patients with atypical reflux symptoms, especially laryngitis, cough, and asthma. Esophageal pH monitoring is indicated in the following situations: (1) to document abnormal esophageal acid exposure in a patient being considered for antireflux surgery who has a normal endoscopy (ie, no evidence of reflux esophagitis); (2) to evaluate patients with a normal endoscopy who have reflux symptoms unresponsive to therapy with a proton pump inhibitor; (3) to detect either abnormal amounts of reflux or an association between reflux episodes and atypical symptoms such as noncardiac chest pain, asthma, chronic cough, laryngitis, and sore throat. It is recommended that most patients with atypical symptoms first be given an empiric trial of antireflux therapy with a double-dose proton pump inhibitor for 2 3 months. If symptoms fail to improve, a pH study is then performed.
Differential Diagnosis
Symptoms of gastroesophageal reflux disease may be similar to those of other diseases such as esophageal motility disorders, peptic ulcer, functional dyspepsia, and angina pectoris. Reflux erosive esophagitis may be confused with pill-induced damage, radiation esophagitis, or infections (CMV, herpes, Candida).
Complications
A. Barrett's Esophagus
This is a condition in which the squamous epithelium of the esophagus is replaced by metaplastic columnar epithelium containing goblet and columnar cells (specialized intestinal metaplasia). Present in up to 10% of patients with chronic reflux, it arises from chronic reflux-induced injury to the esophageal squamous epithelium. Barrett's esophagus is suspected at endoscopy from the presence of orange, gastric type epithelium that extends upward from the stomach into the distal tubular esophagus in a tongue-like or circumferential fashion. Biopsies obtained at endoscopy confirm the diagnosis. Three types of columnar epithelium may be identified: gastric cardiac, gastric fundic, and specialized
P.580
intestinal metaplasia. Only the latter is believed to carry an increased risk of neoplasia.
Barrett's esophagus does not provoke specific symptoms but gastroesophageal reflux does. Most patients have a long history of reflux symptoms, such as heartburn and regurgitation. Dysphagia due to impaired motility is common. Paradoxically, one-third of patients report minimal or no symptoms of gastroesophageal reflux disease, suggesting decreased acid sensitivity of Barrett's epithelium. Indeed, over 90% of individuals with Barrett's esophagus in the general population do not seek medical attention. Barrett's esophagus may be complicated by stricture formation or acid-peptic ulceration, which can bleed.
Barrett's esophagus should be treated with long-term proton pump inhibitors. Surgical fundoplication may be desirable in some situations. Medical or surgical therapy may prevent progression, but there is no convincing evidence that regression occurs in most patients. Endoscopic ablation of Barrett's epithelium with cautery probes or photodynamic therapy (using photosensitizers and laser energy) results in partial or complete regression of columnar epithelium but is not recommended outside of clinical trials for uncomplicated disease.
The most serious complication of Barrett's esophagus is esophageal adenocarcinoma. It is believed that most adenocarcinomas of the esophagus and many such tumors of the gastric cardia arise from dysplastic epithelium in Barrett's esophagus. Current clinical guidelines recommend screening endoscopy in patients with longstanding reflux symptoms (over 5 years) to look for Barrett's esophagus and surveillance endoscopy every 3 5 years in patients with Barrett's esophagus to look for dysplasia or adenocarcinoma. Although there is a 40-fold increased risk compared with patients without Barrett's esophagus, adenocarcinoma of the esophagus remains a relatively uncommon malignancy in the United States (7000 cases/year). Given the large number of adults with chronic GERD relative to the small number in whom adenocarcinoma develops, it is unclear whether screening or surveillance for Barrett's esophagus is cost-effective. Among patients with Barrett's esophagus who do not have dysplasia at initial diagnosis, the estimated annual incidence of adenocarcinoma is < 0.5% per year. It is controversial whether these low-risk patients merit chronic surveillance.
In contrast, patients with low-grade dysplasia require repeat endoscopic surveillance in 3 6 months to screen for coexisting high-grade dysplasia or cancer. If low-grade dysplasia persists (which occurs in < 25% of patients), endoscopic surveillance should be repeated yearly. The treatment of patients with high-grade dysplasia or superficial mucosal cancers is controversial and evolving rapidly. Because 10 40% of patients may progress to (or already have) invasive adenocarcinoma, esophagectomy generally has been recommended for patients deemed to have a low operative risk. For patients with high-grade dysplasia who are deemed to be at high risk for esophagectomy, photodynamic therapy has been demonstrated to reduce the incidence of adenocarcinoma by 50% (28% vs 13%). Alternatively, patients with high-grade dysplasia may undergo close endoscopic surveillance with biopsy every 3 6 months, reserving surgery or photodynamic therapies for treatment of invasive adenocarcinoma.
The high morbidity and mortality rates (40% and 2 9%, respectively) associated with esophagectomy has led many centers to offer endoscopic mucosal resections rather than esophagectomy for selected patients with focal areas of high-grade dysplasia or even small (< 2 cm) mucosal adenocarcinomas that have undergone staging with endoscopic ultrasonography. Provided there is no evidence of invasion of the muscularis mucosa or submucosa, endoscopic mucosal snare resection of focal lesions can be safely performed. If pathologic assessment of the tissue specimen demonstrates submucosal invasion, esophagectomy should be recommended. Residual Barrett's epithelium may be treated with photodynamic therapy to reduce the risk of tumor recurrence.
B. Peptic Stricture
Stricture formation occurs in about 10% of patients with esophagitis. It is manifested by the gradual development of solid food dysphagia progressive over months to years. Often there is a reduction in heartburn because the stricture acts as a barrier to reflux. Most strictures are located at the gastroesophageal junction. Strictures located above this level usually occur with Barrett's metaplasia. Endoscopy with biopsy is mandatory in all cases to differentiate peptic stricture from malignant causes of esophageal stricture (esophageal carcinoma). Active erosive esophagitis is often present. Up to 90% of symptomatic patients are effectively treated with dilation with flexible weighted bougies (passed under fluoroscopic guidance), graduated polyvinyl catheters passed over a wire placed at the time of endoscopy or fluoroscopically, or balloons passed fluoroscopically or through an endoscope. Dilation is continued over one to several sessions. A luminal diameter of 13 17 mm is usually sufficient to relieve dysphagia. Long-term therapy with a proton pump inhibitor is required to decrease the likelihood of stricture recurrence. Some patients require intermittent dilation to maintain luminal patency, but operative management for strictures that do not respond to dilation is seldom required. Refractory strictures may benefit from endoscopic injection of triamcinolone into the stricture.
Treatment
A. Medical Treatment
The goal of treatment is to provide symptomatic relief, to heal esophagitis (if present), and to prevent complications. In the majority of patients with uncomplicated
P.581
disease, empiric treatment is initiated based on a compatible history without the need for further confirmatory studies. Patients not responding and those with suspected complications undergo further evaluation with upper endoscopy or esophageal pH recording.
Patients with known erosive esophagitis, complications (such as a peptic stricture or Barrett's esophagus), or suspected atypical manifestations (such as asthma or laryngitis) are treated initially with a proton pump inhibitor (see below). In most other patients, treatment may proceed in the following stepwise fashion.
1. Mild, intermittent symptoms
Gastroesophageal reflux is a lifelong disease that requires lifestyle modifications as well as medical intervention. The best advice is to avoid lying down within 3 hours after meals, the period of greatest reflux. Elevating the head of the bed on 6-inch blocks or a foam wedge to reduce reflux and enhance esophageal clearance is recommended, especially for patients with nocturnal and atypical symptoms. Patients should be advised to avoid acidic foods (tomato products, citrus fruits, spicy foods, coffee) and agents that relax the lower esophageal sphincter or delay gastric emptying (fatty foods, peppermint, chocolate, alcohol, and smoking). Weight loss, avoidance of bending after meals, and reduction of meal size may also be helpful.
Antacids are the mainstay for rapid relief of occasional heartburn; however, their duration of action is less than 2 hours. Many are available over the counter. Those containing magnesium should not be used in renal failure, and patients with this condition should be cautioned appropriately. Gaviscon is an alginate-antacid combination that decreases reflux in the upright position.
All H2-receptor antagonists are available in over-the-counter formulations: cimetidine 200 mg, ranitidine and nizatidine 75 mg, famotidine 10 mg all of which are half of the typical prescription strength. When taken for active heartburn, these agents have a delay in onset of at least 30 minutes; antacids provide more immediate relief. However, once these agents take effect, they provide heartburn relief for up to 8 hours. When taken before meals known to provoke heartburn, these agents reduce the symptom. A combination agent containing famotidine 10 mg and antacid (calcium carbonate and magnesium hydroxide) is available.
2. Moderate symptoms
Patients with typical reflux symptoms that occur several times per week or daily and with uncomplicated disease should be treated empirically with an H2-receptor antagonist or a proton pump inhibitor. Standard twice-daily prescription doses of H2-receptor antagonists are ranitidine or nizatidine 150 mg, famotidine 20 mg, or cimetidine 400 800 mg. These agents reduce 24-hour acidity by over 60%. Ranitidine and cimetidine are available in less expensive generic formulations. Treatment twice daily affords improvement in up to two-thirds of patients. Given their superior efficacy and once-daily dosing, proton pump inhibitors increasingly are prescribed as first-line therapy for mild to moderate symptoms in preference to beginning with an H2-receptor antagonist (see below for dosing). However, generic H2-receptor antagonists are significantly less expensive than proton pump inhibitors and provide effective symptomatic relief in most patients with mild to moderate reflux symptoms.
Patients whose symptoms persist despite 6 weeks of standard doses of H2-receptor antagonist therapy should be treated with a proton pump inhibitor (once daily omeprazole or rabeprazole 20 mg, lansoprazole 30 mg, esomeprazole or pantoprazole 40 mg). The decision to prescribe proton pump inhibitors is based on the presence of persistent symptoms, not endoscopic findings. Omeprazole 20 mg is now available both as an over-the-counter formulation and as a generic formulation available by prescription.
In those who achieve good symptomatic relief with either an H2-receptor antagonist or a proton pump inhibitor, therapy should be discontinued after 8 12 weeks. Patients whose symptoms relapse may be treated with either continuous therapy, intermittent 2 4 week courses, or on demand therapy (ie, drug taken until symptoms abate) depending on symptom frequency and patient preference. Many patients have their symptoms controlled adequately with intermittent or on demand courses of therapy rather than continuous maintenance treatment.
3. Severe symptoms and erosive disease
For patients with severe symptoms and for patients who undergo endoscopy and have documented erosive esophagitis, Barrett's esophagus, or peptic stricture, the optimal initial therapy is a proton pump inhibitor (omeprazole or rabeprazole 20 mg, lansoprazole 30 mg, pantoprazole or esomeprazole 40 mg) once daily. Proton pump inhibitors given once daily provide symptom relief and healing of esophagitis in over 80% and given twice daily provide relief in over 95% of patients compared with under 50% with standard doses of H2-receptor antagonists and are therefore the drugs of choice for severe or erosive disease. Because there appears to be little difference between these agents in efficacy or side effect profiles, the choice of agent is determined by cost. Esomeprazole, the S-isomer of omeprazole, provides slightly greater inhibition of 24-hour gastric acidity than the other agents, resulting in a small (5%) improvement in esophagitis healing rates compared with other proton pump inhibitors. Approximately 10 20% of patients fail to achieve symptom relief with a once-daily dose within 2 4 weeks and require a higher dosage (twice-daily) proton pump inhibitor. Therefore, some recommend initiating therapy with a twice-daily dose of proton pump inhibitor, reducing therapy after 2 4 weeks to a once-daily dose. The initial course of therapy is usually 8 12 weeks. As tissue healing correlates well with symptom resolution, repeat endoscopy is warranted in patients only if they fail to respond to once-daily or twice-daily proton pump inhibitor therapy.
P.582
After discontinuation of proton pump inhibitor therapy, relapse of symptoms occurs in 80% of patients within 1 year the majority of relapses occurring within the first 3 months. Therefore, chronic therapy to maintain symptom remission is required in most but not all patients. Patients with severe erosive esophagitis, Barrett's esophagus, or peptic stricture should be maintained on chronic therapy with a proton pump inhibitor at a dose sufficient to provide complete symptom relief. In other patients, a trial off proton pump inhibitors should be considered. Patients with prolonged symptomatic remissions (over 3 months) may be treated effectively with intermittent 4- to 8-week courses of acute proton pump inhibitor therapy. Patients with prompt recurrence of symptoms (within 3 months) require chronic maintenance therapy with either a proton pump inhibitor or an H2-receptor antagonist. The therapy should be stepped down to the lowest dose that is effective in controlling reflux symptoms.
The maintenance doses of proton pump inhibitors may escalate over time, with over 20% of patients eventually requiring double or triple doses of proton pump inhibitors to control symptoms.
4. Extraesophageal reflux manifestations
Establishing a causal relationship between gastroesophageal reflux and extraesophageal symptoms (eg, asthma, hoarseness, cough) can be difficult. Although ambulatory esophageal pH testing can document the presence of increased acid esophageal reflux, it does not prove a causative connection. A trial of a twice-daily proton pump inhibitor for 2 3 months helps determine whether these symptoms improve after acid suppression.
5. Unresponsive disease
Approximately 10 20% of patients with gastroesophageal reflux symptoms do not respond to once-daily doses of proton pump inhibitors, and 5% do not respond to twice-daily doses. These patients undergo endoscopy prior to escalation of therapy. The presence of active erosive esophagitis usually is indicative of inadequate acid suppression and can almost always be treated successfully with higher proton pump inhibitor doses (eg, omeprazole 40 mg twice daily). Truly refractory esophagitis may be caused by gastrinoma with gastric acid hypersecretion (Zollinger-Ellison syndrome), pill-induced esophagitis, resistance to proton pump inhibitors, and medical noncompliance. Patients without endoscopically visible esophagitis should undergo esophageal pH monitoring to determine the amount of esophageal acid reflux and to assess whether the symptoms are acid related. If the pH study shows a normal amount of acid reflux, treatment with a low-dose tricyclic antidepressant (eg, imipramine or nortriptyline 25 mg at bedtime) may be beneficial. Where clinically available, impedance monitoring may be useful to document nonacid reflux.
B. Surgical Treatment
Surgical fundoplication affords good to excellent relief of symptoms and healing of esophagitis in over 85% of properly selected patients and may now be performed laparoscopically with low complication rates in most instances. Cost-effectiveness studies suggest that aggregate medical costs exceed surgical costs after 10 years. Although patient satisfaction is high, over 50% of patients require intermittent or continuous acid-suppression medication after fundoplication. Furthermore, over 30% of patients develop new symptoms of dysphagia, bloating, increased flatulence, or dyspepsia. Within 5 10 years after surgery, typical reflux symptoms occur in 10 30% of patients. Surgical treatment is not recommended for patients who are well controlled with medical therapies but should be considered (1) for otherwise healthy patients with extraesophageal manifestations of reflux, as these symptoms often require high doses of proton pump inhibitors and may be more effectively controlled with antireflux surgery; (2) for those with severe reflux disease who are unwilling to accept lifelong medical therapy due to its expense, inconvenience, or theoretical risks; and (3) for patients with erosive disease who are intolerant of or resistant to proton pump inhibitors.
C. Endoscopic Therapy
There are three types of endoscopic modalities that are approved for the treatment of mild to moderate gastroesophageal reflux disease, one of which was recently removed from the market by the manufacturer. It is believed that these techniques may decrease the frequency of reflux events or the volume of refluxate by changing the anatomic or mechanical properties of the gastroesophageal junction. The first uses an endoscopic sewing machine to place sutures below the gastroesophageal junction in the region of the gastric cardia, creating a mucosal plication that may mimic a surgical fundoplication. The second uses an intraesophageal balloon catheter from which needle electrodes are inserted through the mucosa and into the muscularis at multiple levels of the distal esophagus and cardia, permitting application of radiofrequency wave current. It is believed that this current may disrupt neural reflex pathways from the stomach to the esophagus, resulting in a decrease in transient relaxations of the lower esophageal sphincter. The third involves endoscopic injection of a biocompatible, sponge-like nonresorbable polymer to bolster the lower esophageal sphincter. This compound was recently removed from the marketplace by the manufacturer because of complications. To date, two prospective controlled studies have been performed, which compared radiofrequency wave energy and polymer injection to sham therapy. After 3 6 months, more patients in the active treatment groups had improvement in heartburn and quality of life, but there were no differences in esophageal acid exposure times. The polymer injection also resulted in a reduced usage of proton pump inhibitors. Uncontrolled studies demonstrate modest improvement in symptoms and reduction in use of antisecretory medications, but less impact on esophageal acid reflux as assessed by pH
P.583
testing. With all techniques, minor, temporary postprocedure adverse events (chest pain) are common, and rare but serious complications have occurred, including esophageal perforation and death. Pending further controlled clinical studies, endoluminal therapies are not recommended for routine clinical practice.
Armstrong D et al: Heartburn dominant, uninvestigated dyspepsia: a comparison of PPI-start and H2-RA-start management strategies in primary care the CADET-HR study. Aliment Pharmacol Ther 2005;21:1189.
Barlow WJ et al: The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology 2005; 128:771.
Behm BW et al: Endoluminal therapies for gastroesophageal reflux disease. J Clin Gastroenterol 2004;38:209.
Boyce HW: Dilation of difficult benign esophageal strictures. Am J Gastroenterol 2005;100:744.
Charbel S et al: The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol 2005; 100:283.
Dean BB et al: Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol 2004; 2:656.
Fass R et al: Systematic review: proton-pump inhibitor failure in the gastro-oesophageal reflux disease where next? Aliment Pharmacol Ther 2005;22:79.
Inadomi JM et al: PPI use in the OTC era: who to treat, with what, and for how long? Clin Gastroenterol Hepatol 2005; 3:208.
Johnson D et al: Heartburn severity underestimates erosive esophagitis severity in elderly patients with gastroesophageal reflux disease. Gastroenterology 2004;126:660.
Larghi A et al: EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett's esophagus. Gastrointest Endosc 2005;62:16.
Lee TJ et al: Systematic review: is there excessive use of proton pump inhibitors in gastro-oesophageal reflux disease? Aliment Pharmacol Ther 2004;20:1241.
Peters FP et al: Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett's esophagus. Gastrointest Endosc 2005;61:506.
Sampliner R: Endoscopic ablative therapy for Barrett's esophagus: current status. Gastrointest Endosc 2004;59:66.
Shaheen N: Advances in Barrett's esophagus and esophageal adenocarcinoma. Gastroenterology 2005;128:1554.
Shaheen N: Raising the bar in studies of endoscopic anti-reflux procedures. Gastroenterology 2005;128:779.
Spechler S: The management of patients who have failed antireflux surgery. Am J Gastroenterol 2004;99:552.
Spechler S: Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol 2005;100: 927.
Tack J et al: Gastroesophageal reflux disease poorly responsive to single-dose proton pump inhibitors in patients without Barrett's esophagus: acid reflux, bile reflux, or both? Am J Gastroenterol 2004;99:981.
Tytgat GN: Management of mild and severe gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2003;17(Suppl 2): 52.
Wolfsen HC: Present status of photodynamic therapy for high-grade dysplasia in Barrett's esophagus. J Clin Gastroenterol 2005;39:189.
Infectious Esophagitis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Immunosuppressed patient.
Odynophagia, dysphagia, and chest pain.
Endoscopy with biopsy establishes diagnosis.
General Considerations
Infectious esophagitis occurs most commonly in immunosuppressed patients. Patients with AIDS, solid organ transplants, leukemia, lymphoma, and those receiving immunosuppressive drugs are at particular risk for opportunistic infections. Candida albicans, herpes simplex, and CMV are the most common pathogens. Candida infection may occur also in patients who have uncontrolled diabetes and those being treated with systemic corticosteroids, radiation therapy, or systemic antibiotic therapy. Herpes simplex can affect normal hosts, in which case the infection is generally self-limited.
Clinical Findings
A. Symptoms and Signs
The most common symptoms are odynophagia and dysphagia. Substernal chest pain occurs in some patients. Patients with candidal esophagitis are sometimes asymptomatic. Oral thrush is present in only 75% of patients with candidal esophagitis and 25 50% of patients with viral esophagitis and is therefore an unreliable indicator of the cause of esophageal infection. Patients with esophageal CMV infection may have infection at other sites such as the colon and retina. Oral ulcers (herpes labialis) are often associated with herpes simplex esophagitis.
B. Special Examinations
Treatment may be empiric. For diagnostic certainty, endoscopy with biopsy and brushings (for microbiologic and histopathologic analysis) is preferred because of its high diagnostic accuracy. The endoscopic signs of candidal esophagitis are diffuse, linear, yellow-white plaques adherent to the mucosa. CMV esophagitis is characterized by one to several large, shallow, superficial ulcerations. Herpes esophagitis results in multiple small, deep ulcerations.
Treatment
A. Candidal Esophagitis
Initial episodes of oropharyngeal candidiasis may be treated with clotrimazole troches (10 mg dissolved in mouth five times daily) or nystatin suspension (500,000 units five times daily), but systemic therapy is required for
P.584
esophageal candidiasis. An empiric trial of antifungal therapy is often administered without performing diagnostic endoscopy. Initial therapy is generally with fluconazole, 100 mg/d orally for 14 21 days. Patients not responding to empiric therapy within 7 14 days should undergo endoscopy with brushings, biopsy, and culture to distinguish resistant fungal infection from other infections (eg, CMV, herpes). Esophageal candidiasis not responding to fluconazole therapy may be treated with itraconazole suspension (not capsules), 200 mg/d orally, or voriconazole, 200 mg twice daily. Refractory infection may be treated intravenously with caspofungin, 50 mg daily or amphotericin B, 0.3 0.7 mg/kg/d.
B. Cytomegalovirus Esophagitis
In patients with HIV infection, immune restoration with highly active antiretroviral therapy (HAART) is the most effective means of controlling CMV disease. Initial therapy is with ganciclovir, 5 mg/kg intravenously every 12 hours for 3 6 weeks. Neutropenia is a frequent dose-limiting side effect. Once resolution of symptoms occurs, it may be possible to complete the course of therapy with oral valganciclovir, 900 mg once daily. Patients who either do not respond to or cannot tolerate ganciclovir are treated acutely with foscarnet, 90 mg/kg intravenously every 12 hours for 3 6 weeks. The principal toxicity is renal failure, hypocalcemia, and hypomagnesemia.
C. Herpetic Esophagitis
Immunocompetent patients may be treated symptomatically and generally do not require specific antiviral therapy. Immunosuppressed patients may be treated with oral acyclovir, 400 mg orally five times daily, or 250 mg/m2 intravenously every 8 12 hours, usually for 7 10 days. Famciclovir, 250 mg three times daily, and valacyclovir, 1 g twice daily, are effective but significantly more expensive than generic acyclovir. Nonresponders require therapy with foscarnet, 40 mg/kg intravenously every 8 hours for 21 days.
Prognosis
Most patients with infectious esophagitis can be effectively treated with complete symptom resolution. Depending on the patient's underlying immunodeficiency, relapse of symptoms off therapy can raise difficulties. Chronic suppressive therapy is sometimes required.
Bobak DA: Gastrointestinal infections caused by cytomegalovirus. Curr Infect Dis Rep 2003;5:101.
Pappas PG et al: Guidelines for the treatment of candidasis. Clin Infect Dis 2004;38:161.
Ramanathan J et al: Herpes simplex esophagitis in the immunocompetent host: an overview. Am J Gastroenterol 2000; 95: 2171.
Wilcox CM et al: Prospective comparison of brush cytology, viral culture, and histology for the diagnosis of ulcerative esophagitis in AIDS. Clin Gastroenterol Hepatol 2004;2:564.
Pill-Induced Esophagitis
A number of different medications may injure the esophagus, presumably through direct, prolonged mucosal contact. The most commonly implicated are the NSAIDs, potassium chloride pills, quinidine, zalcitabine, zidovudine, alendronate and risedronate, emepronium bromide, iron, vitamin C, and antibiotics (doxycycline, tetracycline, clindamycin, trimethoprim-sulfamethoxazole). Because injury is most likely to occur if pills are swallowed without water or while supine, hospitalized or bed-bound patients are at greater risk. Symptoms include severe retrosternal chest pain, odynophagia, and dysphagia, often beginning several hours after taking a pill. These may occur suddenly and persist for days. Some patients (especially the elderly) have relatively little pain, presenting with dysphagia. Endoscopy may reveal one to several discrete ulcers that may be shallow or deep. Chronic injury may result in severe esophagitis with stricture, hemorrhage, or perforation. Healing occurs rapidly when the offending agent is eliminated. To prevent pill-induced damage, patients should take pills with 4 oz of water and remain upright for 30 minutes after ingestion. Known offending agents should not be given to patients with esophageal dysmotility, dysphagia, or strictures.
Abid S et al: Pill-induced esophageal injury: endoscopic features and clinical outcomes. Endoscopy 2005;37:740.
Winstead NS et al: Pill esophagitis. Curr Treat Options Gastroenterol 2004;7:71.
Caustic Esophageal Injury
Caustic esophageal injury occurs from accidental (usually children) or deliberate (suicidal) ingestion of liquid or crystalline alkali (drain cleaners, etc) or acid. Ingestion is followed almost immediately by severe burning and varying degrees of chest pain, gagging, dysphagia, and drooling. Aspiration results in stridor and wheezing. Initial examination should be directed to circulatory status and to prompt assessment of airway patency, including laryngoscopy. Subsequently, chest and abdominal radiographs are obtained looking for pneumonitis or free perforation. Initial treatment is supportive, with intravenous fluids and analgesics. Nasogastric lavage and oral antidotes may be dangerous and should generally not be administered. Most patients may be managed medically. Endoscopy is usually performed within the first 24 hours to assess the extent of injury. Many patients are discovered to have no mucosal injury to the esophagus or stomach, allowing prompt discharge and psychiatric referral. Patients with evidence of mild damage (edema, erythema, exudates or superficial ulcers) recover quickly, have low risk of developing stricture, and may be advanced from liquids to a regular diet over 24 48 hours. Nasoenteric feedings should be initiated after 24 48 hours and subsequent oral feedings when the patient is tolerating oral secretions. Patients
P.585
with signs of severe injury deep or circumferential ulcers or necrosis (black discoloration) have a high risk (up to 65%) of acute complications, including perforation with mediastinitis or peritonitis, bleeding, stricture, or esophageal-tracheal fistulas. These patients must be kept fasting and monitored closely for signs of deterioration that warrant emergency surgery with possible esophagectomy and colonic or jejunal interposition. A nasoenteric feeding tube is placed after 24 hours. Oral feedings of liquids may be initiated after 2 3 days if the patient is able to tolerate secretions. Neither corticosteroids nor antibiotics are recommended. Esophageal strictures develop in up to 70% of patients with serious esophageal injury weeks to months after the initial injury, requiring recurrent dilations. Endoscopic injection of intralesional corticosteroids (triamcinolone 40 mg) increases the interval between dilations. The risk of esophageal squamous carcinoma is 2 3%, warranting endoscopic surveillance 15 20 years after the caustic ingestion.
Ramasamy K et al: Corrosive ingestion in adults. J Clin Gastroenterol 2003;37:119.
Poley JW et al: Ingestion of acid and alkaline agents: outcome and prognostic value of early upper endoscopy. Gastrointest Endosc 2004;60:372.
Benign Esophageal Lesions
1. Mallory-Weiss Syndrome (Mucosal Laceration of Gastroesophageal Junction)
![]() Essentials of Diagnosis
Essentials of Diagnosis
Hematemesis; usually self-limited.
Prior history of vomiting, retching in 50%.
Endoscopy establishes diagnosis.
General Considerations
Mallory-Weiss syndrome is characterized by a nonpenetrating mucosal tear at the gastroesophageal junction that is hypothesized to arise from events that suddenly raise transabdominal pressure, such as lifting, retching, or vomiting. Alcoholism is a strong predisposing factor. Mallory-Weiss tears are responsible for approximately 5% of cases of upper gastrointestinal bleeding.
Clinical Findings
A. Symptoms and Signs
Patients usually present with hematemesis with or without melena. A history of retching, vomiting, or straining is obtained in about 50% of cases.
B. Special Examinations
As with other causes of upper gastrointestinal hemorrhage, upper endoscopy should be performed after the patient has been appropriately resuscitated. The diagnosis is established by identification of a 0.5- to 4-cm linear mucosal tear usually located either at the gastroesophageal junction or, more commonly, just below the junction in the gastric mucosa.
Differential Diagnosis
At endoscopy, other potential causes of upper gastrointestinal hemorrhage are found in over 35% of patients with Mallory-Weiss tears, including peptic ulcer disease, erosive gastritis, arteriovenous malformations, and esophageal varices. Patients with underlying portal hypertension are at higher risk of continued or recurrent bleeding.
Treatment
Patients are initially treated as needed with fluid resuscitation and blood transfusions. Most patients stop bleeding spontaneously and require no therapy. Endoscopic hemostatic therapy is employed in patients who have continuing active bleeding. Injection with epinephrine (1:10,000), cautery with a bipolar or heater probe coagulation device, or mechanical compression of the artery by application of an endoclip or band is effective in 90 95% of cases. Angiographic arterial embolization or operative intervention is required in patients who fail endoscopic therapy.
Park CH et al: A prospective, randomized trial of endoscopic band ligation vs. epinephrine injection for actively bleeding Mallory-Weiss syndrome. Gastrointest Endosc 2004;60: 22.
2. Esophageal Webs & Rings
Esophageal webs are thin, diaphragm-like membranes of squamous mucosa that typically occur in the mid or upper esophagus and may be multiple. They may be congenital but also occur with graft-versus-host disease, pemphigoid, epidermolysis bullosa, pemphigus vulgaris, and, rarely, in association with iron deficiency anemia (Plummer-Vinson syndrome). Esophageal Schatzki rings are smooth, circumferential, thin (< 4 mm in thickness) mucosal structures located in the distal esophagus at the squamocolumnar junction. Their pathogenesis is controversial. They are associated in nearly all cases with a hiatal hernia, and reflux symptoms are common, suggesting that acid gastroesophageal reflux may be contributory in many cases. Most webs and rings are over 20 mm in diameter and are asymptomatic. Solid food dysphagia most often occurs with rings less than 13 mm in diameter. Characteristically, dysphagia is intermittent and not progressive. Large poorly chewed food boluses such as beefsteak are most likely to cause symptoms. Obstructing
P.586
boluses may pass by drinking extra liquids or after regurgitation. In some cases, an impacted bolus must be extracted endoscopically. Esophageal webs and rings are best visualized using a barium esophagogram with full esophageal distention. Endoscopy is less sensitive than barium esophagography.
The majority of symptomatic patients with a single ring or web can be effectively treated with the passage of a large (> 16-mm-diameter) bougie dilator to disrupt the lesion. A single dilation may suffice, but repeat dilations are required in many patients. Patients who have heartburn or who require repeated dilation should receive acid suppressive therapy with a chronic proton pump inhibitor.
Eosinophilic esophagitis is an entity that previously was recognized in children but is increasingly identified in young or middle-aged adults with dysphagia and food impactions. Barium swallowing studies may demonstrate a small-caliber esophagus, long and tapered strictures, or multiple concentric rings. Endoscopic appearances include multiple fine concentric rings, vertical furrowing, and whitish papules. Mucosal biopsy demonstrates multiple mucosal eosinophils. Optimal treatment is uncertain, but dietary restrictions and topical corticosteroids (fluticasone 220 mcg/puff swallow four puffs taken after inspiration, without a spacer twice daily after meals) are reported to be beneficial in uncontrolled trials. Graduated dilation of strictures should be conducted cautiously because there is an increased risk of perforation and postprocedural chest pain.
Arora AS et al: Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol 2004;2:523.
Desai TK et al: Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005;61:795.
Ibrahim A et al: Schatzki's ring: to cut or break an unresolved problem. Dig Dis Sci 2004;49:379.
Sgouros SN et al: Long-term acid suppressive therapy may prevent the relapse of esophageal (Schatzki's) rings: a prospective, randomized, placebo-controlled study. Am J Gastroenterol 2005;100:1929.
3. Esophageal Diverticula
Zenker's Diverticulum
Zenker's diverticulum is a protrusion of pharyngeal mucosa that develops at the pharyngoesophageal junction between the inferior pharyngeal constrictor and the cricopharyngeus. The cause is believed to be loss of elasticity of the upper esophageal sphincter, resulting in restricted opening during swallowing. Symptoms of dysphagia and regurgitation tend to develop insidiously over years in older patients. Initial symptoms include vague oropharyngeal dysphagia with coughing or throat discomfort. As the diverticulum enlarges and retains food, patients may note halitosis, spontaneous regurgitation of undigested food, nocturnal choking, gurgling in the throat, or a protrusion in the neck. Complications include aspiration pneumonia, bronchiectasis, and lung abscess. The diagnosis is best established by a barium esophagogram.
Symptomatic patients require upper esophageal myotomy and, in most cases, surgical diverticulectomy. An intraluminal approach has been developed recently in which the septum between the esophagus and diverticulum is incised. Significant improvement occurs in over 90% of patients treated surgically. Small asymptomatic diverticula may be observed.
Altman JI et al: Fiberoptic endoscopic-assisted diverticulotomy: a novel technique for the management of Zenker's divertidulum. Ann Otol Rhinol Laryngol 2005;114:347.
Evrard S et al: Zenker's diverticulum: a new endoscopic treatment with a soft diverticulosope. Gastrointest Endosc 2003;58: 116.
Van Overbeek JJ: Pathogenesis and methods of treatment of Zenker's diverticulum. Ann Otol Rhinol Laryngol 2003;112:583.
Esophageal Diverticula
Diverticula may occur in the mid or distal esophagus. These may arise secondary to motility disorders (diffuse esophageal spasm, achalasia) or may develop above esophageal strictures. Diverticula are seldom symptomatic. For patients with severe symptoms or pulmonary complications, surgical myotomy with or without diverticulectomy is the optimal treatment.
Cassivi SD et al: Diverticula of the esophagus. Surg Clin North Am 2005;85:494.
Michael H et al: Treatment of epiphrenic and mid-esophageal diverticula. Curr Treat Options Gastroenterol 2004;7:41.
4. Benign Esophageal Neoplasms
Benign tumors of the esophagus are quite rare. They are submucosal, the most common being leiomyoma. Most are asymptomatic and picked up incidentally on endoscopy or barium esophagography. Larger lesions can cause dysphagia, pain, and rarely ulceration with bleeding. The risk of malignant transformation is low. Therefore, the major clinical importance of these lesions is to distinguish them from malignant neoplasms. At endoscopy, a smooth, sessile nodule is observed with normal overlying mucosa. Because the lesion is submucosal, endoscopic biopsies are generally nonrevealing. Endoscopic ultrasonography is extremely helpful to confirm the submucosal origin of the tumor and to help distinguish benign leiomyomas from malignant leiomyosarcomas. Surgical (or in selected cases endoscopic) resection is indicated for lesions that are symptomatic, ulcerated, or increasing in size.
Lee LS et al: Current management of esophageal leiomyoma. J Am Coll Surg 2004;198:136.
P.587
5. Esophageal Varices
![]() Essentials of Diagnosis
Essentials of Diagnosis
Develop secondary to portal hypertension.
Found in 50% of patients with cirrhosis.
One-third of patients with varices develop upper gastrointestinal bleeding.
Diagnosis established by upper endoscopy.
General Considerations
Esophageal varices are dilated submucosal veins that develop in patients with underlying portal hypertension and may result in serious upper gastrointestinal bleeding. The causes of portal hypertension are discussed in Chapter 15. Under normal circumstances, there is a 2 6 mm Hg pressure gradient between the portal vein and the inferior vena cava. When the gradient exceeds 12 mm Hg, significant portal hypertension exists. Esophageal varices are the most common cause of important gastrointestinal bleeding due to portal hypertension, though gastric varices and, rarely, intestinal varices may also bleed. Bleeding from esophageal varices most commonly occurs in the distal 5 cm of the esophagus.
The most common cause of portal hypertension is cirrhosis. Although approximately 50% of patients with cirrhosis have esophageal varices, only one-third of patients with varices develop serious bleeding from the varices. Bleeding esophageal varices have a higher morbidity and mortality rate than any other source of upper gastrointestinal bleeding. In the absence of treatment, the acute mortality from variceal hemorrhage is 30 50%; however, with current therapies, the mortality has been reduced to 20%.
A number of factors have been identified that may portend an increased risk of bleeding from esophageal varices. The most important are (1) the size of the varices; (2) the presence at endoscopy of red color signs on the varix (wale markings, hematocystic spots, or red spots); (3) the severity of liver disease (as assessed by Child scoring); and (4) active alcohol abuse alcoholic cirrhotics who continue to drink have an extremely high risk of bleeding. The risk of bleeding correlates poorly with the absolute portosystemic pressure gradient, though bleeding almost never occurs with a gradient under 12 mm Hg.
Clinical Findings
A. Symptoms and Signs
Patients with bleeding esophageal varices present with symptoms and signs of acute gastrointestinal hemorrhage. (See Acute Upper Gastrointestinal Bleeding, above.) In some cases, there may be preceding retching or dyspepsia attributable to alcoholic gastritis or withdrawal. Varices per se do not cause symptoms of dyspepsia, dysphagia, or retching. Variceal bleeding usually is severe, resulting in hypovolemia manifested by postural vital signs or shock. Twenty percent of patients with chronic liver disease who develop bleeding despite findings such as spider angiomas, asterixis, and ascites do so from some other source.
B. Laboratory Findings
These are identical to those listed above in the section on acute upper gastrointestinal tract bleeding.
Initial Management
A. Acute Resuscitation
The initial management of patients with acute upper gastrointestinal bleeding is also discussed in the section on acute upper gastrointestinal bleeding (see above). Variceal hemorrhage is life-threatening; rapid assessment and resuscitation with fluids or blood products are essential. Overtransfusion should be avoided as it leads to increased central and portal venous pressures, increasing the risk of rebleeding. Many patients with bleeding esophageal varices have coagulopathy due to underlying cirrhosis; fresh frozen plasma (20 mL/kg loading dose, then 10 mg/kg every 6 hours) or platelets should be administered to patients with INRs > 1.8 2.0 or with platelet counts < 50,000/mcL in the presence of active bleeding. Patients with advanced liver disease are at high risk for poor outcome regardless of the bleeding source and should be transferred to an ICU. In 50% of cases, variceal hemorrhage stops spontaneously; without therapy, over half of these will rebleed within 1 week. The in-hospital mortality rate associated with bleeding esophageal varices is 15%.
B. Emergent Endoscopy
Emergent endoscopy is performed after the patient's hemodynamic status has been appropriately stabilized (usually within 2 12 hours). In patients with active bleeding, endotracheal intubation is commonly performed to protect against aspiration during endoscopy. An endoscopic examination is performed to exclude other or associated causes of upper gastrointestinal bleeding such as Mallory-Weiss tears, peptic ulcer disease, and portal hypertensive gastropathy. In many patients, variceal bleeding has stopped spontaneously by the time of endoscopy, and the diagnosis of variceal bleeding is made presumptively. Acute endoscopic treatment of the varices is performed with either banding or sclerotherapy. These techniques arrest active bleeding in 80 90% of patients and reduce by 50% (from 70%) the chance of early recurrent bleeding, but their impact on in-hospital mortality is less clear.
If banding is chosen, repeat sessions are scheduled at intervals of 1 3 weeks until the varices are obliterated or reduced to a small size. Banding achieves lower
P.588
rates of rebleeding, complications, and death than sclerotherapy and should be considered the endoscopic treatment of choice.
Sclerotherapy is still preferred by some endoscopists in the actively bleeding patient (in whom visualization for banding may be difficult). Sclerotherapy is performed by injecting the variceal trunks with a sclerosing agent (eg, ethanolamine, tetradecyl sulfate). Complications occur in 20 30% of patients and include chest pain, fever, bacteremia, esophageal ulceration, stricture, and perforation. After initial treatment, band ligation therapy should be performed.
C. Pharmacologic Therapy
1. Antibiotic prophylaxis
Cirrhotic patients admitted with upper gastrointestinal bleeding have a greater than 50% chance of developing a severe bacterial infection during hospitalization such as bacterial peritonitis, pneumonia, or urinary tract infection with or without sepsis. Most organisms causing infections are of gut origin. Prophylactic administration of antibiotics may reduce the risk of serious infection to 10 20% as well as hospital mortality. Although the optimal choice of antibiotic is uncertain, initial treatment with an intravenous quinolone antibiotic (ciprofloxacin 400 mg every 12 hours, levofloxacin 500 mg every 24 hours, or gatifloxacin 400 mg every 24 hours) followed after stabilization by an oral or nasogastric quinolone for 7 10 days may be recommended.
2. Vasoactive drugs
Somatostatin and octreotide infusions reduce portal pressures in ways that are poorly understood. Somatostatin (250 mcg/h) not available in the United States or octreotide (50 mcg intravenous bolus followed by 50 mcg/h) reduces splanchnic and hepatic blood flow and portal pressures in cirrhotic patients. Both agents appear to provide acute control of variceal bleeding in up to 80% of patients although neither has been shown to reduce mortality. Data about the absolute efficacy of both are conflicting, but they may be comparable in efficacy to endoscopic therapy. Combined treatment with octreotide or somatostatin infusion and endoscopic therapy (band ligation or sclerotherapy) is superior to either modality alone in controlling acute bleeding and early rebleeding, and it may improve survival. In patients with advanced liver disease and upper gastrointestinal hemorrhage, it is reasonable to initiate therapy with octreotide or somatostatin on admission and continue for 5 days. If bleeding is determined by endoscopy not to be secondary to portal hypertension, the infusion can be discontinued.
Terlipressin (not available in the United States) is a synthetic vasopressin analog that causes a significant and sustained reduction in portal and variceal pressures while preserving renal perfusion. It is superior to placebo in the control of acute variceal hemorrhage and reduces mortality by 34%. Where available, terlipressin may be preferred to somatostatin or octreotide. Terlipressin is contraindicated in patients with significant coronary, cerebral, or peripheral vascular disease.
3. Vitamin K
In cirrhotic patients with an abnormal prothrombin time, vitamin K (10 mg) should be administered subcutaneously.
4. Lactulose
Encephalopathy may complicate an episode of gastrointestinal bleeding in patients with severe liver disease. In patients with encephalopathy, lactulose should be administered in a dosage of 30 45 mL/h orally until evacuation occurs, then reduced to 15 45 mL/h every 8 12 hours as needed to promote two or three bowel movements daily. (See Chapter 15.)
D. Balloon Tube Tamponade
Mechanical tamponade with specially designed nasogastric tubes containing large gastric and esophageal balloons (Minnesota or Sengstaken-Blakemore tubes) provides initial control of active variceal hemorrhage in 60 90% of patients; rebleeding occurs in 50%. The gastric balloon is inflated first, followed by the esophageal balloon if bleeding continues. After balloon inflation, tension is applied to the tube to directly tamponade the varices. Complications of prolonged balloon inflation include esophageal and oral ulcerations, perforation, aspiration, and airway obstruction (due to a misplaced balloon). Endotracheal intubation is recommended before placement. Given its high rate of complications, mechanical tamponade is used as a temporizing measure only in patients with bleeding that cannot be controlled with pharmacologic or endoscopic techniques until more definitive decompressive therapy (eg, TIPS; see below) can be provided.
E. Portal Decompressive Procedures
In patients with variceal bleeding that cannot be controlled with pharmacologic or endoscopic therapy, emergency portal decompression may be considered.
1. Transvenous intrahepatic portosystemic shunts
Over a wire that is passed through the catheter inserted in the jugular vein, an expandable wire mesh stent (8 12 mm in diameter) is passed through the liver parenchyma, creating a portosystemic shunt from the portal vein to the hepatic vein. TIPS can control acute hemorrhage in over 90% of patients actively bleeding from gastric or esophageal varices. However, when TIPS is performed in the actively bleeding patient, the mortality approaches 40%, especially in patients requiring ventilatory support or blood pressure support and patients with renal insufficiency, bilirubin > 3.0 mg/dL, or encephalopathy. Therefore, TIPS should be considered in the 5 10% of patients with acute variceal bleeding that cannot be controlled with pharmacologic and endoscopic therapy, but it may not be warranted in patients with a poor prognosis.
2. Emergency portosystemic shunt surgery
Emergency portosystemic shunt surgery is associated with a 40 60% mortality rate. At centers where TIPS is available, that procedure has become the preferred means of providing emergency portal decompression.
P.589
Prevention of Rebleeding
Once the initial bleeding episode has been controlled, the risk of rebleeding is 50 70% without further therapy. The highest incidence of rebleeding is in the first 6 weeks. Several options are available to decrease the likelihood of rebleeding, though their relative merits are controversial. Use of these approaches varies in different medical centers.
A. Endoscopic Techniques
Long-term treatment with band ligation reduces the incidence of rebleeding to 20 50%; the mortality rate may also be reduced. Band ligation of the varix appears to be equal or superior to sclerotherapy in preventing rebleeding and is associated with a significantly lower complication rate. In most patients, four to six treatment sessions are needed to eradicate the varices.
B. -Blockers and Nitrates
Nonselective -adrenergic blockers (propranolol, nadolol) are effective in reducing the incidence of rebleeding from esophageal varices and portal hypertensive gastropathy compared with placebo. Some but not all studies demonstrate a lower incidence of rebleeding with band ligation than with -adrenergic blockers. Thus, the choice between -blockers and endoscopic therapy to prevent recurrent variceal bleeding is not established and depends on local experience. Compliant patients with well-compensated liver disease may be optimal candidates for therapy with -blockers alone. Most others may be offered endoscopic variceal banding where available. A combination of band ligation plus -blockers further reduces the rate of recurrent bleeding compared with ligation alone (14% vs 38%). Therefore, patients without contraindications to -blockers may be given propranolol, 20 40 mg twice daily, long-acting propranolol, 60 80 mg once daily, or nadolol, 40 mg once daily, increasing the dosage every 1 2 weeks until the heart rate falls by 25% or reaches 55 beats/min, provided the systolic blood pressure remains above 90 mm Hg and the patient has no side effects. The average dosage of propranolol is 120 mg daily and for nadolol, 80 mg daily. One-third of patients with cirrhosis are intolerant of -blockers, experiencing fatigue or hypotension. Drug administration at bedtime may reduce the frequency and severity of side effects.
C. Transvenous Intrahepatic Portosystemic Shunt
TIPS has resulted in a significant reduction in recurrent bleeding compared with endoscopic sclerotherapy or band ligation either alone or in combination with -blocker therapy. At 1 year, rebleeding rates in patients treated with TIPS versus various endoscopic therapies average 20% and 40%, respectively. However, TIPS was also associated with a higher incidence of encephalopathy (35% versus 15%) and did not result in a decrease in mortality. Another limitation of TIPS is that stenosis and thrombosis of the stents occur in the majority of patients over time with a consequent risk of rebleeding. Therefore, periodic monitoring with Doppler ultrasonography or hepatic venography is required. Stent patency usually can be maintained by balloon angioplasty or additional stent placement. Given these problems, TIPS should be reserved for patients who have recurrent (two or more) episodes of variceal bleeding that have failed endoscopic or pharmacologic therapies. TIPS is also useful in patients with recurrent bleeding from gastric varices or portal hypertensive gastropathy (for which endoscopic therapies cannot be used). TIPS is likewise considered in patients who are noncompliant with other therapies or who live in remote locations (without access to emergency care).
D. Surgical Portosystemic Shunts
Shunt surgery has a significantly lower rate of rebleeding compared with endoscopic therapy but also a higher incidence of encephalopathy. With the advent and widespread adoption of TIPS, surgical shunts are seldom performed.
E. Liver Transplantation
Candidacy for orthotopic liver transplantation should be assessed in all patients with chronic liver disease and bleeding due to portal hypertension. Transplant candidates should be treated with band ligation or TIPS to control bleeding pretransplant.
Prevention of First Episodes of Variceal Bleeding
Because of the high mortality rate associated with variceal hemorrhage, prevention of the initial bleeding episode is desirable. Therefore, patients with cirrhosis should undergo diagnostic endoscopy to determine whether varices are present. The risk of bleeding in patients with small varices (< 5 mm) is 5% per year and with large varices is 15 20% per year. Either nonselective -adrenergic blockers (nadolol, propranolol) or prophylactic band ligation decrease the absolute risk of variceal bleeding by approximately 10% per year and reduce mortality by almost 5%. In meta-analysis of comparative studies of banding versus -blockers, banding appears to reduce the risk of variceal bleeding slightly but not the risk of mortality. However, because endoscopy is invasive and more expensive, a trial of -blockers is recommended as initial therapy for compliant patients with large varices or medium varices with red color markings. For patients with small or absent varices, no prophylaxis is necessary but endoscopy should be repeated in 1 2 years. For patients with contraindications to or intolerance of -blockers, prophylactic treatment with band ligation should be considered.
P.590
Boyer T et al; American Association for the Study of Liver Diseases: The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology 2005;41:386.
De Franchis R: Incidental esophageal varices. Gastroenterology 2004;126:1860.
De la Pena J et al: Variceal ligation plus nadolol compared with ligation for prophylaxis of variceal rebleeding: a multicenter trial. Hepatology 2005;41:572.
Gotzsche PC et al: Somatostatin analogues for acute bleeding oesophageal varices. Cochrane Database Syst Rev 2005;(1): CD000193.
Ioannou G et al: Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev 2003;(1):CD002147.
Jutabha R et al: Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005; 128:870.
Kamath PS: Esophageal variceal bleeding: primary prophylaxis. Clin Gastroenterol Hepatol 2005;3:90.
Khuroo MS et al: Meta-analysis: endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleeding. Aliment Pharmacol Ther 2005;21:347.
Mihas AA et al: Recurrent variceal bleeding despite endoscopic and medical therapy. Gastroenterology 2004;127:621.
Nevens F: Review article: a critical comparison of drug therapies in currently used therapeutic strategies for variceal hemorrhage. Aliment Pharmacol Ther 2004;20(Suppl 3):18.
Qureshi W et al; Standards of Practice Committee: ASGE Guideline: the role of endoscopy in the management of variceal hemorrhage, updated July 2005. Gastrointest Endosc 2005; 62:651.
Sarin SK et al: Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005;100: 797.
Esophageal Cancer
![]() Essentials of Diagnosis
Essentials of Diagnosis
Progressive solid food dysphagia.
Weight loss common.
Endoscopy with biopsy establishes diagnosis.
General Considerations
Esophageal cancer usually develops in persons between 50 and 70 years of age. The overall ratio of men to women is 3:1. There are two histologic types: squamous cell carcinoma and adenocarcinoma. In the United States, squamous cell cancer is much more common in blacks than in whites. Chronic alcohol and tobacco use are strongly associated with an increased risk of squamous cell carcinoma. The risk of squamous cell cancer is also increased in patients with tylosis (a rare disease transmitted by autosomal dominant inheritance and manifested by hyperkeratosis of the palms and soles), achalasia, caustic-induced esophageal stricture, and other head and neck cancers. Squamous cell cancer has a high incidence in certain regions of China and Southeast Asia. Half of all cases occur in the distal third of the esophagus. Adenocarcinoma is more common in whites. It is increasing dramatically in incidence and now is as common as squamous carcinoma. The majority of adenocarcinomas develop as a complication of Barrett's metaplasia due to chronic gastroesophageal reflux. Thus, most adenocarcinomas arise in the distal third of the esophagus. Obesity also is strongly associated with adenocarcinoma, even after controlling for gastroesophageal reflux; however, no causal relationship has been convincingly shown.
Clinical Findings
A. Symptoms and Signs
Most patients with esophageal cancer present with advanced, incurable disease. Over 90% have solid food dysphagia, which progresses over weeks to months. Odynophagia is sometimes present. Significant weight loss is common. Local tumor extension into the tracheobronchial tree may result in a tracheoesophageal fistula, characterized by coughing on swallowing or pneumonia. Chest or back pain suggests mediastinal extension. Recurrent laryngeal involvement may produce hoarseness. Physical examination is often unrevealing. The presence of supraclavicular or cervical lymphadenopathy or of hepatomegaly implies metastatic disease.
B. Laboratory Findings
Laboratory findings are nonspecific. Anemia related to chronic disease or occult blood loss is common. Elevated aminotransferase or alkaline phosphatase concentrations suggest hepatic or bony metastases. Hypoalbuminemia may result from malnutrition.
C. Imaging
Chest x-rays may show adenopathy, a widened mediastinum, pulmonary or bony metastases, or signs of tracheoesophageal fistula such as pneumonia. A barium esophagogram is obtained as the first study to evaluate dysphagia. The appearance of a polypoid, infiltrative, or ulcerative lesion is suggestive of carcinoma and requires endoscopic evaluation. However, even lesions felt to be benign by radiography warrant endoscopic evaluation.
D. Upper Endoscopy
Endoscopy with biopsy establishes the diagnosis of esophageal carcinoma with a high degree of reliability. In some cases, significant submucosal spread of the tumor may yield nondiagnostic mucosal biopsies. Repeated biopsy may be necessary.
P.591
Differential Diagnosis
Esophageal carcinoma must be distinguished from other causes of progressive dysphagia, including peptic stricture, achalasia, and adenocarcinoma of the gastric cardia with esophageal involvement. Benign-appearing peptic strictures should be biopsied at presentation to exclude occult malignancy.
Staging of Disease
After confirmation of the diagnosis of esophageal carcinoma, the stage of the disease should be determined since doing so influences the choice of therapy. Patients should undergo evaluation with CT of the chest and liver to look for evidence of pulmonary or hepatic metastases, lymphadenopathy, and local tumor extension. If there is no evidence of distant metastases or extensive local spread on CT, endoscopic ultrasonography with guided fine-needle aspiration (FNA) biopsy of lymph nodes should be performed, which is superior to CT in demonstrating the level of local mediastinal extension and local lymph node involvement. Positron emission tomography imaging with fluorodeoxyglucose is used increasingly to look for regional or distant spread in patients thought to have localized disease after other diagnostic studies. Bronchoscopy is sometimes required in proximal esophageal cancer to exclude tracheobronchial extension. Apart from distant metastasis, the two most important predictors of poor survival are lymph node involvement and adjacent mediastinal spread.
Stages are determined by the TNM classification as set forth in the accompanying box.
Treatment
The approach to esophageal cancer depends on the tumor stage, patient preference and functional status, and the expertise of the attending surgeons, oncologists, gastroenterologists, and radiotherapists. There is no consensus about the optimal treatment approach. It is helpful, however, to classify patients into two general categories.
Staging Criteria for Esophageal Cancer
Primary Tumor (T)
T1: Invasion of lamina propria or submucosa
T2: Invasion of muscularis propria
T3: Invasion of adventitia
T4: Invasion of adjacent structures
Regional Lymph Nodes
N0: No regional lymph node involvement
N1: Regional lymph node involvement
Distant Metastasis (M)
M0: No metastasis
M1: Distant metastasis
M1a: Cervical or celiac lymph nodes
M1b: Other distant metastasis
Based upon these parameters, the tumor is classified as:
Stage I: T1, N0, M0
Stage IIA: T2 or T3, and N0, M0
Stage IIB: T1 or T2, and N1, M0
Stage IIIA: T3, N1, M0
Stage IIIB: T4, Any N, M0
Stage IVA: Any T, Any N, M1a
Stage IVB: Any T, Any N, M1b
A. Palliative Therapy
Patients with extensive local tumor spread (T4) or distant metastases (M1) are incurable, ie, patients with stage IIIB and stage IV tumors. Surgery is not warranted in these patients. The goal is to provide relief from dysphagia and pain, and the optimal palliative approach depends on the patient's expected survival, patient preference, and local institutional experience. None of the modalities prolongs survival, and many patients may prefer concerted efforts at pain relief and care directed at symptom management (see Chapter 5).
1. Radiation or chemoradiation therapy
Combined radiation therapy and chemotherapy may achieve palliation in two-thirds of patients but is associated with significant side effects. It should be considered for patients with a good functional status without other significant medical problems. Radiation therapy alone may afford significant short-term relief of pain and dysphagia and may be suitable for patients with poor functional status or underlying medical problems. During therapy, esophagitis may lead to worsening of dysphagia and odynophagia.
2. Local antitumor therapy
Patients with advanced esophageal cancer may have a poor functional and nutritional status and an average survival of less than 12 weeks from diagnosis. Radiation or chemoradiation is poorly tolerated. Rapid palliation of dysphagia may be achieved by peroral placement of permanent expandable wire stents, application of endoscopic laser therapy, or photodynamic therapy. Although dysphagia and quality of life are improved significantly, patients can seldom eat normally. Complications of stents occur in 20 40% and include perforation, migration, and tumor ingrowth. These are most suitable for patients with a short life expectancy, patients with tracheoesophageal fistula, patients who have failed radiation therapy, or patients in locations where optimal surgical or radiation modalities are not available. Laser therapy (Nd:YAG laser) maintains luminal patency in up to 90% of patients but involves multiple treatment sessions and can be difficult to administer. Photodynamic
P.592
therapy has been shown to be superior to laser therapy. A photosensitizing agent (porfimer sodium) in combination with a low-power 630-nm laser results in significant tumor necrosis. Side effects include sun sensitivity of the skin for 4 6 weeks and the development of esophageal stricture. Both laser therapy and photodynamic therapy require expensive equipment that is not available at many institutions.
B. Curable Disease
For a discussion of the treatment options for early-stage adenocarcinoma arising in Barrett's esophagus that appears on EUS to be limited to the mucosa, see the section on Barrett's Esophagus. For all other potentially curable esophageal cancers (stage I, II, or IIIA), the approach to therapy depends on institutional experience.
1. Surgery with or without neoadjuvant chemoradiation therapy
There is controversy over the optimal surgical approach. The procedure with the lowest morbidity is transhiatal esophagectomy with anastomosis of the stomach to the cervical esophagus; however, this approach does not involve sampling or removal of mediastinal lymph nodes. Alternatively, many surgeons recommend extended en bloc transthoracic excision of the esophagus extended dissection of lymph nodes in the mediastinum and upper abdomen. In a randomized controlled trial comparing these two approaches, en bloc resection was associated with higher perioperative morbidity but a nonsignificant trend toward improved 5-year survival (39% vs 27%). Transhiatal resection may be more appropriate for patients who are elderly or have comorbid illness and en bloc resection may be more appropriate in younger or healthier patients with a better prognosis.
Patients with stage I and stage IIA cancer have high cure rates with surgery alone and require no other radiation or chemotherapy. Lymph node spread is the most important preditor of survival. If lymph node metastases have occurred (stage IIB and stage IIIA), the rate of cure with surgery alone is reduced to less than 10%. Trials of adjuvant (postoperative) or neoadjuvant (preoperative) radiation therapy or chemotherapy have not demonstrated benefits over surgery alone. However, a combination radiation therapy and chemotherapy (cisplatin and fluorouracil) given before surgery results in complete pathologic remission (no evidence of tumor at the time of surgery) in up to 25% of patients. A meta-analysis of trials comparing neoadjuvant chemoradiation plus surgery to surgery alone concluded that neoadjuvant therapy decreased local and regional tumor recurrence and increased 3-year survival. However, there is significant morbidity associated with chemoradiation therapy. Therefore, multimodal treatment (neoadjuvant chemoradiation plus surgery) remains controversial but may be considered in young patients without other comorbid illnesses, preferably in the context of a clinical trial.
2. Chemotherapy plus radiation therapy
Combined therapy with chemotherapy and radiation therapy is superior to radiation therapy alone and has achieved overall survival rates that equal or exceed those of historical surgical cohorts, though there have been no trials specifically comparing these approaches. However, severe side effects occur commonly with combined therapy. The most promising chemotherapeutic agents have been cisplatin and fluorouracil. Chemoradiation therapy alone should be considered in patients with localized disease (stage II or IIIA) who are poor surgical candidates due to serious medical illness or poor functional status (ECOG score > 2).
Prognosis
The overall 5-year survival rate of esophageal carcinoma is less than 15%. Despite improvements in surgical mortality and increased surgical resectability rates, the prognosis of this disease has not changed for years, in part because most patients present with advanced disease. This suggests that surgical approaches alone are inadequate for most patients. For those patients whose disease progresses despite chemotherapy, meticulous efforts at palliative care are essential (see Chapter 5).
Enzinger PC et al: Esophageal cancer. N Engl J Med 2003; 349:2241.
Fiorica F et al: Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut 2004; 53:925.
Hulscher JB et al: Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662.
Malthaner RA et al: Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a clinical practice guideline. BMC Cancer 2004;4:67.
National Cancer Institute Esophageal Cancer home page: http://www.cancer.gov/cancerinfo/types/esophageal.
Vazquez-Sequeiros E et al: Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology 2003;125:1626.
Wang KK et al; American Gastroenterological Association: American Gastroenterological Association medical position statement: Role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology 2005;128:1468.
Wang KK et al: American Gastroenterological Association Technical Review on the role of the gastroenterologist in the management of esophageal carcinoma. Gastroenterology 2005;128:1471.
Weber WA et al: Imaging of esophageal and gastric cancer. Semin Oncol 2004;31:530.
Esophageal Motility Disorders
1. Achalasia
![]() Essentials of Diagnosis
Essentials of Diagnosis
Gradual, progressive dysphagia for solids and liquids.
Regurgitation of undigested food.
Barium esophagogram with bird's beak distal esophagus.
Esophageal manometry confirms diagnosis.
P.593
General Considerations
Achalasia is an idiopathic motility disorder characterized by loss of peristalsis in the distal two-thirds (smooth muscle) of the esophagus and impaired relaxation of the lower esophageal sphincter. There appears to be denervation of the esophagus resulting primarily from loss of nitric oxide-producing inhibitory neurons in the myenteric plexus. The cause of the neuronal degeneration is unknown.
Clinical Findings
A. Symptoms and Signs
There is a steady increase in the incidence of achalasia with age; however, it can be seen in individuals as young as 25 years. Patients complain of the gradual onset of dysphagia for solid foods and, in the majority, of liquids also. Symptoms at presentation may have persisted for months to years. Substernal discomfort or fullness may be noted after eating. Many patients eat more slowly and adopt specific maneuvers such as lifting the neck or throwing the shoulders back to enhance esophageal emptying. Regurgitation of undigested food is common and may occur during meals or up to several hours later. Nocturnal regurgitation can provoke coughing or aspiration. Up to 50% of patients report substernal chest pain that is unrelated to meals or exercise and may last up to hours. Weight loss is common. Physical examination is unhelpful.
B. Imaging
Chest x-rays may show an air-fluid level in the enlarged, fluid-filled esophagus. Barium esophagography discloses characteristic findings, including esophageal dilation, loss of esophageal peristalsis, poor esophageal emptying, and a smooth, symmetric bird's beak tapering of the distal esophagus. Without treatment, the esophagus may become markedly dilated ( sigmoid esophagus ).
C. Special Examinations
After esophagography, endoscopy is always performed to evaluate the distal esophagus and gastroesophageal junction to exclude a distal stricture or a submucosal infiltrating carcinoma. The diagnosis is confirmed by esophageal manometry. The typical manometric features are as follows: (1) Complete absence of peristalsis; swallowing results in simultaneous waves that are usually of low amplitude. (2) Incomplete lower esophageal sphincteric relaxation with swallowing. Whereas the normal sphincter relaxes by over 90%, relaxation with most swallows in patients with achalasia is less than 50%. In many patients, the baseline lower esophageal sphincteric pressure is quite elevated. (3) Intraesophageal pressures are greater than gastric pressures due to a fluid- and food-filled esophagus.
Differential Diagnosis
Chagas' disease is associated with esophageal dysfunction that is indistinguishable from idiopathic achalasia and should be considered in patients from endemic regions (Central and South America); it is becoming more common in the southern United States. Primary or metastatic tumors can invade the gastroesophageal junction, resulting in a picture resembling that of achalasia, called pseudoachalasia. Endoscopic ultrasonography and chest CT may be required to examine the distal esophagus in suspicious cases. Tumors such as small cell lung cancer can cause a paraneoplastic syndrome resembling achalasia due to secretion of antineuronal nuclear antibodies (ANNA-1 or Anti-Hu) that affect the myenteric plexus. Achalasia must be distinguished from other motility disorders such as diffuse esophageal spasm and scleroderma esophagus with a peptic stricture.
Treatment
A. Botulinum Toxin Injection
Endoscopically guided injection of botulinum toxin directly into the lower esophageal sphincter results in a marked reduction in lower esophageal sphincter pressure with initial improvement in symptoms in 65 85% of patients. However, symptom relapse occurs in over 50% of patients within 6 9 months and in all patients within 2 years. Three-fourths of initial responders who relapse have improvement with repeated injections. Because it is inferior to pneumatic dilation therapy and surgery in producing sustained symptomatic relief, this therapy is most appropriate for patients with comorbidities who are poor candidates for more invasive procedures.
B. Pneumatic Dilation
Between 75% and 85% of patients derive good to excellent relief of dysphagia after one to three sessions of pneumatic dilation of the lower esophageal sphincter and between 50% and 70% of patients achieve long-term relief. Perforations occur in less than 3% of dilations and may require operative repair.
C. Surgical Myotomy
A modified Heller cardiomyotomy of the lower esophageal sphincter and cardia results in good to excellent symptomatic improvement in over 85% of patients. Because gastroesophageal reflux may develop in up to 20% of patients after myotomy, most surgeons also perform an antireflux procedure (fundoplication). Myotomy is now performed with a laparoscopic approach
P.594
and is preferred to the open surgical approach. Indeed, the low morbidity of laparoscopic surgery has led some experts to recommend it for initial treatment. In experienced hands, the initial efficacies of pneumatic dilation and laparoscopic myotomy are nearly equivalent. Analysis indicates that pneumatic dilation may be a more cost-effective strategy than either botulinum toxin injection or laparoscopic myotomy. The success of laparoscopic surgery is not compromised by prior therapy with either botulinum injection or pneumatic dilation.
Farhoomand K et al: Predictors of outcome of pneumatic dilation in achalsia. Clin Gastroenterol Hepatol 2004;2:389.
Karamanolis G et al: Long-term outcome of pneumatic dilation in the treatment of achalasia. Am J Gastroenterol 2005;100: 270.
Mikaeli J et al: Pneumatic balloon dilation in achalasia: a prospective comparison of safety and efficacy with different balloon diameters. Aliment Pharmacol Ther 2004;20:431.
Park W et al: Etiology and pathogenesis of achalasia: the current understanding. Am J Gastroenterol 2005;100:1404.
Vela MF et al: Complexities of managing achalasia at a tertiary referral center: use of pneumatic dilation, Heller myotomy, and botulinum toxin injection. Am J Gastroenterol 2004; 99:1029.
2. Other Primary Esophageal Motility Disorders
Abnormalities in esophageal motility may cause dysphagia or chest pain. Dysphagia for liquids as well as solids tends to be intermittent and nonprogressive. Periods of normal swallowing may alternate with periods of dysphagia, which usually is mild though bothersome rarely severe enough to result in significant alterations in lifestyle or weight loss. Dysphagia may be provoked by stress, large boluses of food, or hot or cold liquids. Some patients may experience anterior chest pain that may be confused with angina pectoris but usually is nonexertional. The pain generally is unrelated to eating. (See Chest Pain of Undetermined Origin, below.)
The evaluation of suspected esophageal motility disorders includes barium esophagography, upper endoscopy, and, in some cases, esophageal manometry. Barium esophagography is useful to exclude mechanical obstruction and to evaluate esophageal motility. The presence of simultaneous contractions (spasm), disordered peristalsis, or failed peristalsis supports a diagnosis of esophageal dysmotility. Upper endoscopy also is performed to exclude a mechanical obstruction (as a cause of dysphagia) and to look for evidence of erosive reflux esophagitis (a common cause of chest pain) or eosinophilic esophagitis (confirmed by esophageal biopsy).
The further evaluation of noncardiac chest pain is discussed in a subsequent section. Manometry should not be routinely used for mild to moderate symptoms because the findings seldom influence further medical management. For patients with disabling symptoms of dysphagia, stationary esophageal manometry should be performed. Based on the findings of esophageal manometry, the following conditions may be diagnosed: (1) Diffuse esophageal spasm: normal primary peristalsis with 20% simultaneous contractions of > 30 mm Hg contraction amplitude. (2) Nutcracker esophagus: normal peristalsis but increased duration and high amplitude of distal contractions (> 180 mm Hg). (3) Hypertensive lower esophageal sphincter: elevated lower esophageal sphincter pressure (> 45 mm Hg) with normal peristalsis. (4) Ineffective esophageal motility: > 30% of swallows resulting in failed or interrupted peristalsis or distal esophageal hypocontraction (< 30 mm Hg contraction amplitude).
For patients with mild symptoms, therapy is directed at symptom reduction and reassurance. Patients with dysphagia should be instructed to eat more slowly and take smaller bites of food. In some cases, a warm liquid at the start of a meal may facilitate swallowing. Gastroesophageal reflux should be identified and treated. Treatment of more severe cases with nitrates (isosorbide, 10 20 mg four times daily) or nitroglycerin (0.4 mg sublingually as needed) and calcium channel blockers (nifedipine 10 mg or diltiazem 60 90 mg, 30 45 minutes before meals) may be tried; their efficacy is unproved. Injection of botulinum toxin into the lower esophagus may improve chest pain and dysphagia in over 70% for a limited time. For unclear reasons, dilation with esophageal Maloney bougies provides symptomatic relief in some cases. In debilitated patients, a long surgical myotomy (which may be performed via a thoracoscopic approach) may lead to improvement in 70 80% of cases.
Bajaj JS et al: Esophageal veggie spasms: a food-specific cause of chest distress. Am J Gastroenterol 2004;99:1396.
Miller LS et al: Treatment of chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection into the gastroesophageal junction. Am J Gastroenterol 2002;97:1640.
Richter JE: Oesophageal motility disorders. Lancet 2001;358: 823.
Spechler S et al: Classification of oesophageal motility abnormalities. Gut 2001;49:145.
Tutuian R et al: Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol 2004; 99:1020.
Chest Pain of Undetermined Origin
One-third of patients with chest pain undergo negative cardiac evaluation. Patients with recurrent noncardiac chest pain thus pose a difficult clinical problem. Because coronary artery disease is common and
P.595
can present atypically, it must be excluded prior to evaluation for other causes.
Causes of noncardiac chest pain may include the following.
A. Chest Wall and Thoracic Spine Disease
These are easily diagnosed by history and physical examination.
B. Gastroesophageal Reflux
Up to 50% of patients have increased amounts of gastroesophageal acid reflux or a correlation between acid reflux episodes and chest pain demonstrated on esophageal pH testing. An empiric 8-week trial of acid-suppressive therapy with a high-dose proton pump inhibitor is recommended (eg, omeprazole or rabeprazole, 40 mg twice daily; lansoprazole, 30 60 mg twice daily; or esomeprazole or pantoprazole, 40 mg twice-daily), especially in patients with reflux symptoms. Symptomatic improvement strongly suggests that the pain is due to acid esophageal reflux. The sensitivity and specificity of this treatment are 78% and 80%, respectively, compared with therapy based on results of esophageal pH testing. In patients with persistent symptoms, an ambulatory esophageal pH study while continuing antisecretory therapy is useful to definitively exclude a relationship between acid reflux episodes and chest pain events.
C. Heightened Visceral Sensitivity
Studies suggest that many patients with noncardiac chest pain report pain in response to a variety of minor noxious stimuli such as intraesophageal acid infusion, inflation of balloons within the esophageal lumen, injection of intravenous edrophonium (a cholinergic stimulus), or intracardiac catheter manipulation. Low doses of antidepressants such as trazodone 50 mg or imipramine 50 mg reduce chest pain symptoms and are thought to reduce visceral afferent awareness.
D. Psychological Disorders
A significant number of patients have underlying depression, anxiety, and panic disorder. Patients reporting dyspnea, sweating, tachycardia, suffocation, or fear of dying should be evaluated for panic disorder.
E. Esophageal Dysmotility
Esophageal motility abnormalities such as diffuse esophageal spasm or nutcracker esophagus are rare causes of noncardiac chest pain. In patients with chest pain and dysphagia, barium swallow x-ray should be obtained to look for evidence of achalasia or diffuse esophageal spasm. Stationary or ambulatory manometry is not routinely performed because of low specificity and the unlikelihood of finding a clinically significant disorder, but these procedures may be recommended in patients with frequent symptoms.
Bautista J et al: The effect of an empirical trial of high-dose lansoprazole on symptom response of patients with non-cardiac chest pain a randomized, double-blind, placebo-controlled, crossover trial. Aliment Pharmacol Ther 2004;15: 1123.
Cremonini F et al: Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a meta-analysis. Am J Gastroenterol 2005;100:1226.
Dekel R et al: Assessment of oesophageal motor function in patients with dysphagia or chest pain the Clinical Outcomes Research Initiative experience. Aliment Pharmacol Ther 2003;18:1083.
Eslick GD: Noncardiac chest pain: epidemiology, natural history, health care seeking, and quality of life. Gastroenterol Clin North Am 2004;33:1.
Diseases of the Stomach & Duodenum
Gastritis & Gastropathy
The term gastropathy should be used to denote conditions in which there is epithelial or endothelial damage without inflammation, and gastritis should be used to denote conditions in which there is histologic evidence of inflammation. In clinical practice, the term gastritis is commonly applied to three categories: (1) erosive and hemorrhagic gastritis (gastropathy); (2) nonerosive, nonspecific (histologic) gastritis; and (3) specific types of gastritis, characterized by distinctive histologic and endoscopic features diagnostic of specific disorders.
1. Erosive & Hemorrhagic Gastritis (Gastropathy)
![]() Essentials of Diagnosis
Essentials of Diagnosis
Most commonly seen in alcoholics, critically ill patients, or patients taking NSAIDs.
Often asymptomatic; may cause epigastric pain, nausea, and vomiting.
May cause hematemesis; usually not significant bleeding.
General Considerations
The most common causes of erosive gastropathy are drugs (especially NSAIDs), alcohol, stress due to severe medical or surgical illness, and portal hypertension ( portal gastropathy ). Uncommon causes include caustic ingestion and radiation. Erosive and hemorrhagic gastropathy typically are diagnosed at endoscopy, often being performed because of dyspepsia or upper gastrointestinal bleeding. Endoscopic findings include subepithelial
P.596
hemorrhages, petechiae, and erosions. These lesions are superficial, vary in size and number, and may be focal or diffuse. There usually is no significant inflammation on histologic examination.
Clinical Findings
A. Symptoms and Signs
Erosive gastropathy is usually asymptomatic. Symptoms, when they occur, include anorexia, epigastric pain, nausea, and vomiting. There is poor correlation between symptoms and the number or severity of endoscopic abnormalities. The most common clinical manifestation of erosive gastritis is upper gastrointestinal bleeding, which presents as hematemesis, coffee grounds emesis, or bloody aspirate in a patient receiving nasogastric suction, or as melena. Because erosive gastritis is superficial, hemodynamically significant bleeding is rare.
B. Laboratory Findings
The laboratory findings are nonspecific. The hematocrit is low if significant bleeding has occurred; iron deficiency may be found.
C. Special Examinations
Upper endoscopy is the most sensitive method of diagnosis. Although bleeding from gastritis is usually insignificant, it cannot be distinguished on clinical grounds from more serious lesions such as peptic ulcers or esophageal varices. Hence, endoscopy is generally performed within 24 hours in patients with upper gastrointestinal bleeding to identify the source. An upper gastrointestinal series is sometimes obtained in lieu of endoscopy in patients with hemodynamically insignificant upper gastrointestinal bleeds to exclude serious lesions but is insensitive for the detection of gastritis.
Differential Diagnosis
Epigastric pain may be due to peptic ulcer, gastroesophageal reflux, gastric cancer, biliary tract disease, food poisoning, viral gastroenteritis, and functional dyspepsia. With severe pain, one should consider a perforated or penetrating ulcer, pancreatic disease, esophageal rupture, ruptured aortic aneurysm, gastric volvulus, and myocardial colic. Causes of upper gastrointestinal bleeding include peptic ulcer disease, esophageal varices, Mallory-Weiss tear, and arteriovenous malformations.
Specific Causes & Treatment
A. Stress Gastritis
1. Prophylaxis
Stress-related mucosal erosions and subepithelial hemorrhages develop within 72 hours in the majority of critically ill patients. Clinically overt bleeding occurs in 6%, but clinically important bleeding in less than 3%. Bleeding is associated with a higher mortality rate but is seldom the cause of death. Major risk factors include mechanical ventilation, coagulopathy, trauma, burns, shock, sepsis, central nervous system injury, hepatic or renal failure, and multiorgan failure. The use of enteral nutrition reduces the risk of stress-related bleeding.
Pharmacologic prophylaxis with intravenous H2-receptor antagonists, oral proton pump inhibitors such as omeprazole suspension, or sucralfate in critically ill patients has been shown to reduce the incidence of clinically overt and significant bleeding by 50%. The optimal, cost-effective regimen is unclear. Prophylaxis should be routinely administered upon admission to critically ill patients with risk factors for significant bleeding. Two of the most important risk factors are coagulopathy and respiratory failure with the need for mechanical ventilation for over 48 hours. When these two risk factors are absent, the risk of significant bleeding is only 0.1%.
Although most critically ill patients have normal or decreased acid secretion, numerous studies have shown that maintaining intragastric pH > 4 reduces the incidence of clinically significant stress-related bleeding. Continuous infusions of H2-receptor antagonists provide adequate control of intragastric pH in most patients in the following doses over 24 hours: cimetidine (900 1200 mg), ranitidine (150 mg), or famotidine (20 mg). After 4 hours of infusion, the pH should be checked by nasogastric aspirate and the dose doubled if the pH is under 4.0.
Sucralfate suspension (1 g orally every 4 6 hours) is effective also for the prevention of stress-related bleeding, and a reduction of nosocomial pneumonia had been observed compared with H2-receptor antagonists. However, there is a higher incidence of clinically important upper gastrointestinal bleeding with sucralfate (4%) versus H2-receptor antagonists (2%) with no difference in nosocomial pneumonia in more recent studies. In most ICUs, intravenous H2-receptor antagonists or proton pump inhibitors are preferred because of their ease of administration.
At this time, the optimal agent for the reduction of stress-related mucosal bleeding is uncertain. For patients with nasoenteric tubes, immediate-release omeprazole may be preferred to intravenous H2-receptor antagonists because of lower cost, ease of administration, and comparable efficacy. For patients without a nasogastric tube or with significant ileus, intravenous H2-receptor antagonists may be preferred to intravenous proton pump inhibitors because of lower cost, established dosing regimens, and proven efficacy.
2. Treatment
Once bleeding occurs, patients should receive continuous infusions of a proton pump inhibitor (esomeprazole, lansoprazole, or pantoprazole 80 mg intravenous bolus, followed by 8 mg/h continuous infusion) as well as sucralfate suspension. Endoscopy should be performed in patients with clinically significant bleeding to look for treatable causes, especially stress-related
P.597
peptic ulcers with active bleeding or visible vessels. When bleeding arises from diffuse gastritis, endoscopic hemostasis techniques are not helpful.
B. NSAID Gastritis
Of patients receiving NSAIDs in clinical trials, 25 50% have gastritis and 10 20% have ulcers at endoscopy; however, symptoms of significant dyspepsia develop in about 5%. In population surveys, the rate of dyspepsia is increased 1.5- to 2-fold with NSAID use. However, dyspeptic symptoms correlate poorly with significant mucosal abnormalities or the development of adverse clinical events (ulcer bleeding or perforation). Given the frequency of dyspeptic symptoms in patients taking NSAIDs, it is neither feasible nor desirable to investigate all such cases. Patients with alarm symptoms or signs, such as severe pain, weight loss, vomiting, gastrointestinal bleeding, or anemia, should undergo diagnostic upper endoscopy. For other patients, symptoms may improve with discontinuation of the agent, reduction to the lowest effective dose, or administration with meals. Proton pump inhibitors have demonstrated efficacy in controlled trials for the treatment NSAID-related dyspepsia. Although superiority to H2-receptor antagonists for relief of NSAID-related dyspepsia has not been established, proton pump inhibitors have demonstrated superiority for healing of NSAID-related ulcers in the setting of continued NSAID use. Therefore, an empiric 2 4 week trial of a proton pump inhibitor (omeprazole, rabeprazole, or esomeprazole 20 40 mg/d; lansoprazole, 30 mg/d; pantoprazole, 40 mg/d) is recommended for patients with NSAID-related dyspepsia, especially those in whom continued NSAID treatment is required. If symptoms do not improve, diagnostic upper endoscopy should be conducted.
C. Alcoholic Gastritis
Excessive alcohol consumption may lead to dyspepsia, nausea, emesis, and minor hematemesis a condition sometimes labeled alcoholic gastritis. However, it is not proven that alcohol alone actually causes significant erosive gastritis. Therapy with H2-receptor antagonists, proton pump inhibitors, or sucralfate for 2 4 weeks often is empirically prescribed.
D. Portal Hypertensive Gastropathy
Portal hypertension commonly results in gastric mucosal and submucosal congestion of capillaries and venules, which is correlated with the severity of the portal hypertension and underlying liver disease. Usually asymptomatic, it may cause chronic gastrointestinal bleeding in 10% of patients and, less commonly, clinically significant bleeding with hematemesis. Treatment with propranolol or nadolol reduces the incidence of recurrent acute bleeding by lowering portal pressures. Patients who fail propranolol therapy may be successfully treated with portal decompressive procedures (see section on treatment of esophageal varices).
Conrad SA et al: Randomized, double-blind comparison of immediate-release omeprazole suspension versus intravenous cimetidine for the prevention of upper gastrointestinal bleeding in critically ill patients. Crit Care Med 2005;33: 760.
Gupta S et al: Management of nonsteroidal, anti-inflammatory, drug-associated dyspepsia. Gastroenterology 2005;129:1711.
Hawkey C et al; NASA1 SPACE1 Study Group: Improvement with esomeprazole in patients with upper gastrointestinal symptoms taking non-steroidal antiinflammatory drugs, including selective COX-2 inhibitors. Am J Gastroenterol 2005;100:1028.
Merli M et al: The natural history of portal hypertensive gastropathy in patients with liver cirrhosis and mild portal hypertension. Am J Gastroenterol 2004;99:1959.
Stollman N et al: Pathophysiology and prophylaxis of stress ulcer in intensive care unit patients. J Crit Care 2005;20:35.
2. Nonerosive, Nonspecific Gastritis
The diagnosis of nonerosive gastritis is based on histologic assessment of mucosal biopsies. Endoscopic findings are normal in many cases and do not reliably predict the presence of histologic inflammation. The main types of nonerosive gastritis are those due to H pylori infection, those associated with pernicious anemia, and lymphocytic gastritis. (See Specific Types of Gastritis below.)
Helicobacter pylori Gastritis
H pylori is a spiral gram-negative rod that resides beneath the gastric mucous layer adjacent to gastric epithelial cells. Although not invasive, it causes gastric mucosal inflammation with PMNs and lymphocytes. The mechanisms of injury and inflammation may in part be related to the products of two genes, vacA and cagA.
In developed countries the prevalence of H pylori is rapidly declining. In the United States, the prevalence rises from less than 10% in non-immigrants under age 30 years to over 50% in those over age 60 years. The prevalence is higher in non-whites and immigrants from developing countries and is correlated inversely with socioeconomic status. Transmission is from person to person, mainly during infancy and childhood; however, the mode of transmission is unknown.
Acute infection with H pylori may cause a transient clinical illness characterized by nausea and abdominal pain that may last for several days and is associated with acute histologic gastritis with PMNs. After these symptoms resolve, the majority progress to chronic infection with chronic, diffuse mucosal inflammation characterized by PMNs and lymphocytes. Inflammation may be confined to the superficial gastric epithelium or may extend deeper into the gastric glands, resulting in varying degrees of gland atrophy (atrophic gastritis) and metaplasia of the gastric epithelium to intestinal type epithelium. Eradication of H pylori may be achieved with antibiotics in over 85% of patients
P.598
and leads to resolution of the chronic gastritis (see section on peptic ulcer disease).
Although chronic H pylori infection with gastritis is present in 30 50% of the population, the majority are asymptomatic and suffer no sequelae. H pylori infection is strongly associated with peptic ulcer disease; however, only 15% of people with chronic infection develop a peptic ulcer (see section on peptic ulcer disease). Chronic H pylori gastritis is associated with a 3.5- to 20-fold increased risk of gastric adenocarcinoma and low-grade B cell gastric lymphoma (mucosa-associated lymphoid tissue lymphoma; MALToma). There is little evidence that chronic H pylori-associated gastritis is a cause of dyspeptic symptoms. (See Dyspepsia at the beginning of this chapter.)
Testing is indicated for patients with either active or a past history of documented peptic ulcer disease or gastric MALToma and probably for patients with a family history of gastric carcinoma, especially if they are of Asian descent. Testing and empiric treatment may also be cost-effective in young patients (< 55 years of age) with uncomplicated dyspepsia prior to further medical evaluation. The role of testing and treating H pylori in patients with functional dyspepsia remains controversial (see Dyspepsia, above). Because H pylori is a common organism but infrequently causes disease, screening of the general population is not indicated.
A. Noninvasive Testing for H pylori
Although serologic tests are easily obtained and widely available, most clinical guidelines no longer endorse their use for testing for H pylori infection because they are less accurate than other noninvasive tests that measure active infection. Laboratory-based quantitative serologic ELISA tests have an overall accuracy of only 80%. Qualitative office-based kits using whole blood from a finger-stick have a lower sensitivity (< 75%) but can be performed within 10 minutes at a cost of $10. In comparison, the fecal antigen immunoassay and [13C] urea test have excellent sensitivity and specificity (> 95%) at a cost of less than $60. Although more expensive and cumbersome to perform, these tests of active infection are more cost-effective in most clinical settings because they reduce unnecessary treatment for patients without active infection.
Proton pump inhibitors significantly reduce the sensitivity of urea breath tests and fecal antigen assays (but not serologic tests) and should be discontinued 7 14 days prior to testing.
B. Endoscopic Testing for H pylori
Endoscopy is not indicated to diagnose H pylori infection in most circumstances. However, when it is performed for another reason, gastric biopsy specimens can be obtained for detection of H pylori and tested for active infection by urease production. This simple, inexpensive ($10) test has excellent sensitivity and specificity (90%). In patients with active upper gastrointestinal bleeding or patients taking proton pump inhibitors, histologic assessment for H pylori is preferred. Histologic assessment of biopsies from the gastric antrum and body is more definitive but more expensive ($150-$250) than a rapid urease test. Histologic assessment is also indicated in patients with suspected MALTomas and, possibly, in patients with suspected infection whose rapid urease test is negative. However, serologic testing is the most cost-effective means of confirming H pylori infection in patients with a negative rapid urease test.
Axon A: Helicobacter pylori. What do we still need to know? J Clin Gastroenterol 2006;40:15.
Byzer P et al: Treatment of Helicobacter pylori. Helicobacter 2005;10 (Suppl 1):40.
Gillen D et al: Gastroduodenal disease, Helicobacter pylori, and genetic polymorphisms. Clin Gastroenterol Hepatol 2005; 3:1180.
Moayyedi P et al: Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrance Database Syst Rev 2005;(1):CD002096.
Vakil N et al: Non-invasive tests for the diagnosis of H pylori infection. Rev Gastroenterol Disord 2004;4:1.
Vakil N: Primary and secondary treatment for Helicobacter pylori in the United States. Rev Gastroenterol Disord 2005;5:67.
Pernicious Anemia Gastritis
Pernicious anemia gastritis is an autoimmune disorder involving the fundic glands with resultant achlorhydria and vitamin B12 malabsorption. Of patients with B12 deficiency, less than half have pernicious anemia. The majority have malabsorption secondary to aging or chronic H pylori infection that results in atrophic gastritis, hypochlorhydria, and impaired release of B12 from food. Fundic histology in pernicious anemia is characterized by severe gland atrophy and intestinal metaplasia caused by autoimmune destruction of the gastric fundic mucosa. Parietal cell antibodies directed against the H+-K+-ATPase pump are present in 90% of patients. Inflammation and autoimmune destruction of the acid-secreting parietal cells leads to secondary loss of fundic zymogen cells, which secrete intrinsic factor. Achlorhydria leads to pronounced hypergastrinemia (> 1000 pg/mL) due to loss of acid inhibition of gastrin G cells. Hypergastrinemia may induce hyperplasia of gastric enterochromaffin-like cells that may lead to the development of small, multicentric carcinoid tumors in 5% of patients. Metastatic spread is uncommon in lesions smaller than 2 cm. The risk of adenocarcinoma is increased threefold, with a prevalence of 1 3%. Endoscopy with biopsy is indicated in patients with pernicious anemia at the time of diagnosis. Patients with dysplasia or small carcinoids require periodic endoscopic surveillance. Pernicious anemia is discussed in detail in Chapter 13.
Andres E et al: Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004;171:251.
P.599
3. Specific Types of Gastritis
A number of disorders are associated with specific mucosal histologic features.
Infections
Acute bacterial infection of the gastric submucosa and muscularis with a variety of aerobic or anaerobic organisms produces a rare, rapidly progressive, life-threatening condition known as phlegmonous or necrotizing gastritis, which requires broad-spectrum antibiotic therapy and, in many cases, emergency gastric resection. Viral infection with CMV is commonly seen in patients with AIDS and after bone marrow or solid organ transplantation. Endoscopic findings include thickened gastric folds and ulcerations. Fungal infection with Candida may occur in immunocompromised patients. Larvae of Anisakis marina ingested in raw fish or sushi may become embedded in the gastric mucosa, producing severe abdominal pain. Pain persists for several days until the larvae die. Endoscopic removal of the larvae provides rapid symptomatic relief.
Kim GY et al: Phlegmonous gastritis: case report and review. Gastrointest Endosc 2005;61:168.
Granulomatous Gastritis
Chronic granulomatous inflammation may be caused by a variety of systemic diseases, including Crohn's disease, H pylori infection, tuberculosis, syphilis, fungal infections, or sarcoidosis. These may be asymptomatic or associated with a variety of gastrointestinal complaints.
Maeng L et al: Granulomatous gastritis: a clinicopathological analysis of 18 biopsy cases. Am J Surg Pathol 2004;28:941.
Eosinophilic Gastritis
This is a rare disorder in which eosinophils infiltrate the antrum and sometimes the proximal intestine. Infiltration may involve the mucosa, muscularis, or serosa. Peripheral eosinophilia is prominent. Symptoms include anemia from mucosal blood loss, abdominal pain, early satiety, and postprandial vomiting. Treatment with corticosteroids is beneficial in the majority of patients.
Lymphocytic Gastritis
This is an idiopathic condition characterized by fluctuating abdominal pain, nausea, and vomiting. Endoscopic features include mucosal erosions and a varioliform ( pox-like ) appearance. Biopsies reveal a diffuse lymphocytic gastritis. There is no established effective therapy.
M n trier's Disease (Hypertrophic Gastropathy)
This is an idiopathic entity characterized by giant thickened gastric folds involving predominantly the body of the stomach. Patients complain of nausea, epigastric pain, weight loss, and diarrhea. Because of chronic protein loss, patients may develop severe hypoproteinemia and anasarca. The cause is unknown. Treatment is directed at symptoms. There are case reports of resolution of symptoms and improvement in histologic appearance after H pylori eradication. Dramatic improvement recently has been reported in a small number of patients with cetuximab, an antibody that binds epidermal growth factor receptor (EGFR). Gastric resection is required in severe cases.
Settle SH et al: Chronic treatment of Menetrier's disease with Erbitux: clinical efficacy and insight into pathophysiology. Clin Gastroenterol Hepatol 2005;3:654.
Peptic Ulcer Disease
![]() Essentials of Diagnosis
Essentials of Diagnosis
History of nonspecific epigastric pain present in 80 90% of patients with variable relationship to meals.
Ulcer symptoms characterized by rhythmicity and periodicity.
Ten to 20 percent of patients present with ulcer complications without antecedent symptoms.
Of NSAID-induced ulcers, 30 50% are asymptomatic.
Upper endoscopy with antral biopsy for H pylori is the diagnostic procedure of choice in most patients.
Gastric ulcer biopsy or documentation of complete healing necessary to exclude gastric malignancy.
General Considerations
Peptic ulcer is a break in the gastric or duodenal mucosa that arises when the normal mucosal defensive factors are impaired or are overwhelmed by aggressive luminal factors such as acid and pepsin. By definition, ulcers extend through the muscularis mucosae and are usually over 5 mm in diameter. In the United States, there are about 500,000 new cases per year of peptic ulcer and 4 million ulcer recurrences; the lifetime prevalence of ulcers in the adult population is approximately 10%. Ulcers occur five times more commonly in the duodenum, where over 95% are in the bulb or pyloric channel. In the stomach, benign ulcers are located most commonly in the antrum (60%) and at the junction of the antrum and body on the lesser curvature (25%).
Ulcers occur slightly more commonly in men than in women (1.3:1). Although ulcers can occur in any
P.600
age group, duodenal ulcers most commonly occur in patients between the ages of 30 and 55 years, whereas gastric ulcers are more common in patients between the ages of 55 and 70 years. Ulcers are more common in smokers and in patients taking NSAIDs on a chronic basis (see below). Alcohol, dietary factors, and stress do not appear to cause ulcer disease. The incidence of duodenal ulcer disease has been declining dramatically for the past 30 years, but the incidence of gastric ulcers appears to be increasing as a result of the widespread use of NSAIDs and low-dose aspirin.
Etiology
Three major causes of peptic ulcer disease are now recognized: NSAIDs, chronic H pylori infection, and acid hypersecretory states such as Zollinger-Ellison syndrome. Evidence of H pylori infection or NSAID ingestion should be sought in all patients with peptic ulcer. NSAID- and H pylori-associated ulcers will be considered in the present section; Zollinger-Ellison syndrome will be discussed subsequently. Uncommon causes of ulcer disease include CMV (especially in transplant recipients), systemic mastocytosis, Crohn's disease, lymphoma, and medications (eg, alendronate). Up to 10% of ulcers are idiopathic.
A. H pylori-Associated Ulcers
H pylori appears to be a necessary cofactor for the majority of duodenal and gastric ulcers not associated with NSAIDs. Overall, it is estimated that one in six infected patients will develop ulcer disease. The prevalence of H pylori infection in duodenal ulcer patients is 75 90%. Most H pylori-infected duodenal ulcer patients have infection predominantly in the gastric antrum, which is associated with increased gastric acid secretion and decreased duodenal mucosal bicarbonate secretion. It is hypothesized that increased acid exposure can give rise to small islands of gastric metaplasia in the duodenal bulb. Colonization of these islands by H pylori may lead to duodenitis or duodenal ulcer. The association with gastric ulcers is lower, but H pylori is found in the majority of patients in whom NSAIDs cannot be implicated. H pylori-associated gastric ulcers tend to form at the junction of the gastric body and antrum the site of transition from oxyntic to pyloric epithelium. Most H pylori-infected gastric ulcer patients have infection that predominates in the gastric body and is associated with decreased acid secretion. It is hypothesized that chronic inflammation overwhelms the gastric mucosal defense mechanisms.
The natural history of H pylori-associated peptic ulcer disease is well defined. In the absence of specific antibiotic treatment to eradicate the organism, 85% of patients will have an endoscopically visible recurrence within 1 year. Half of these will be symptomatic. After successful eradication of H pylori with antibiotics, ulcer recurrence rates are reduced dramatically to 5 20% at 1 year. Some of these ulcer recurrences may be due to NSAID use or reinfection with H pylori.
B. NSAID-Induced Ulcers
There is a 10 20% prevalence of gastric ulcers and a 2 5% prevalence of duodenal ulcers in long-term NSAID users. The relative risk of gastric ulcers is increased 40-fold, but the risk of duodenal ulcers is only slightly increased. Users of NSAIDs are at least three times more likely than nonusers to suffer serious gastrointestinal complications from these ulcers such as bleeding, perforation, or death. It is noteworthy that gastric ulcers and duodenal ulcers cause about the same number of complications. Approximately 1 2% of long-term NSAID users will have a major complication within 1 year. The risk of NSAID complications is greater within the first 3 months of therapy and in patients who are older, patients who take higher doses of NSAIDs, as well as patients with a prior history of ulcer disease, concomitant corticosteroid or anticoagulation administration, or serious medical illness. Aspirin is the most ulcerogenic NSAID. Use of even low-dose aspirin (81 162 mg/d) leads to complications in 0.6 1.2% of patients each year. H pylori infection increases the risk of ulcer disease and complications over threefold in patients taking NSAIDs or low-dose aspirin. It is hypothesized that NSAID initiation may potentiate or aggravate ulcer disease in susceptible infected individuals.
Celecoxib (and the other coxibs ) are NSAIDs that preferentially inhibit cyclooxygenase-2 (COX-2) the principal enzyme involved in prostaglandin production at sites of inflammation while providing relative sparing of cyclooxygenase-1 (COX-1), the principal enzyme involved with prostaglandin production in the gastroduodenal mucosa and gastric cytoprotection. Two other NSAIDs etodolac and meloxicam have COX-2/COX-1 selectivity that is similar to celecoxib but have undergone limited study to confirm their safety. The generic etodolac is significantly less expensive than other coxib agents. The incidence of endoscopically visible ulcers after 6 12 weeks of coxib therapy is reduced by approximately 75% compared with nonselective NSAIDs. Of greater clinical importance, the risk of significant clinical events (obstruction, perforation, or severe bleeding) is approximately 0.7% in patients taking coxibs and 1.4% in patients taking nonselective NSAIDs a relative risk reduction of 50%. Concurrent use of low-dose aspirin appears to partially negate the mucosal-sparing effects of coxibs. Among patients taking low-dose aspirin, the risk of a serious clinical event was not significantly different between patients taking coxibs and standard NSAIDs.
A twofold increase in the incidence in cardiovascular complications (myocardial infarction, cerebrovascular infarction, and death) was detected in patients taking rofecoxib and valdecoxib compared with placebo, prompting its voluntary withdrawal from the market by the manufacturers. It is hypothesized that selective inhibition of COX-2 leads to decreased vascular prostacyclin, reduced arterial vasodilation, enhanced
P.601
atherogenesis, and enhanced platelet adhesion. A review by a Food and Drug Administration (FDA) panel concluded that celecoxib, which has less COX-2 selectivity than rofecoxib and valdecoxb, does not have a higher incidence of cardiovascular complications compared with other nonselective NSAIDs. Nonetheless, pending further clinical information, celecoxib should be restricted to short-term use (< 3 months) in patients at increased risk for NSAID-induced complications and deemed to have a low risk of cardiovascular disease.
Clinical Findings
A. Symptoms and Signs
Epigastric pain (dyspepsia), the hallmark of peptic ulcer disease, is present in 80 90% of patients. However, this complaint is not sensitive or specific enough to serve as a reliable diagnostic criterion for peptic ulcer disease. The clinical history cannot accurately distinguish duodenal from gastric ulcers. Less than 25% of patients with dyspepsia have ulcer disease at endoscopy. Twenty percent of patients with ulcer complications such as bleeding have no antecedent symptoms ( silent ulcers ). Nearly 60% of patients with NSAID-related ulcer complications do not have prior symptoms.
Pain is typically well localized to the epigastrium and not severe. It is described as gnawing, dull, aching, or hunger-like. Approximately 50% of patients report relief of pain with food or antacids (especially duodenal ulcers) and a recurrence of pain 2 4 hours later. However, many patients deny any relationship to meals or report worsening of pain. Two-thirds of duodenal ulcers and one-third of gastric ulcers cause nocturnal pain that awakens the patient. A change from a patient's typical rhythmic discomfort to constant or radiating pain may reflect ulcer penetration or perforation. Most patients have symptomatic periods lasting up to several weeks with intervals of months to years in which they are pain free (periodicity).
Nausea and anorexia may occur with gastric ulcers. Significant vomiting and weight loss are unusual with uncomplicated ulcer disease and suggest gastric outlet obstruction or gastric malignancy.
The physical examination is often normal in uncomplicated peptic ulcer disease. Mild, localized epigastric tenderness to deep palpation may be present. FOBT is positive in one-third of patients.
B. Laboratory Findings
Laboratory tests are normal in uncomplicated peptic ulcer disease but are ordered to exclude ulcer complications or confounding disease entities. Anemia may occur with acute blood loss from a bleeding ulcer or less commonly from chronic blood loss. Leukocytosis suggests ulcer penetration or perforation. An elevated serum amylase in a patient with severe epigastric pain suggests ulcer penetration into the pancreas. A fasting serum gastrin level to screen for Zollinger-Ellison syndrome is obtained in some patients (see below). Because acid inhibition may raise serum gastrin levels, H2-receptor antagonists should be withheld for 24 hours and proton pump inhibitors for 1 week before a gastrin level is measured.
C. Endoscopy
Upper endoscopy is the procedure of choice for the diagnosis of duodenal and gastric ulcers. Endoscopy provides better diagnostic accuracy than barium radiography and the ability to biopsy for the presence of malignancy and H pylori infection. Duodenal ulcers are virtually never malignant and do not require biopsy. Three to 5 percent of benign-appearing gastric ulcers prove to be malignant. Hence, biopsies of the ulcer margin are almost always performed. Provided that the gastric ulcer appears benign to the endoscopist and adequate biopsy specimens reveal no evidence of cancer, dysplasia, or atypia, the patient may be followed without further endoscopy. If these conditions are not fulfilled, follow-up endoscopy should be performed 12 weeks after the start of therapy to document complete healing; nonhealing ulcers are suspicious for malignancy.
D. Imaging
Barium upper gastrointestinal series is an acceptable alternative to screening of patients with uncomplicated dyspepsia. However, because it has limited accuracy in distinguishing benign from malignant gastric ulcers, all gastric ulcers in patients younger than 45 years diagnosed by x-ray should be reevaluated with endoscopy after 8 12 weeks of therapy.
E. Testing for H pylori
In patients in whom an ulcer is diagnosed by endoscopy, gastric mucosal biopsies should be obtained both for a rapid urease test and for histologic examination. The specimens for histology are discarded if the urease test is positive.
In patients with a history of peptic ulcer or when an ulcer is diagnosed by upper gastrointestinal series, noninvasive assessment for H pylori with fecal antigen assay or urea breath testing should be done. Proton pump inhibitors may cause false-negative urea breath tests and fecal antigen tests and should be withheld for at least 7 days before testing. Because of its lower sensitivity and specificity, serologic testing should not be performed unless fecal antigen testing or urea breath testing is unavailable.
Differential Diagnosis
Peptic ulcer disease must be distinguished from other causes of epigastric distress (dyspepsia). Over 50% of patients with dyspepsia have no obvious organic explanation for their symptoms and are classified as having functional dyspepsia (see sections above on dyspepsia
P.602
and functional dyspepsia). Atypical gastroesophageal reflux may be manifested by epigastric symptoms. Biliary tract disease is characterized by discrete, intermittent episodes of pain that should not be confused with other causes of dyspepsia. Severe epigastric pain is atypical for peptic ulcer disease unless complicated by a perforation or penetration. Other causes include acute pancreatitis, acute cholecystitis or choledocholithiasis, esophageal rupture, gastric volvulus, and ruptured aortic aneurysm.
Pharmacologic Agents
The pharmacology of several agents that enhance the healing of peptic ulcers is briefly discussed here. They may be divided into three categories: (1) acid-antisecretory agents, (2) mucosal protective agents, and (3) agents that promote healing through eradication of H pylori. Recommendations for their use are provided in subsequent sections.
A. Acid-Antisecretory Agents
1. Proton pump inhibitors
Proton pump inhibitors covalently bind the acid-secreting enzyme H+-K+-ATPase, or proton pump, permanently inactivating it. Restoration of acid secretion requires synthesis of new pumps, which have a half-life of 18 hours. Thus, although these agents have a serum half-life of less than 60 minutes, their duration of action exceeds 24 hours. The available agents omeprazole or rabeprazole 20 mg, lansoprazole 30 mg, esomeprazole or pantoprazole 40 mg inhibit over 90% of 24-hour acid secretion, compared with under 65% for H2-receptor antagonists in standard dosages. Proton pump inhibitors should be administered 30 minutes before meals (usually breakfast).
Each of the four proton pump inhibitors results in over 90% healing of duodenal ulcers after 4 weeks and 90% of gastric ulcers after 8 weeks when given once daily. Compared with H2-receptor antagonists, proton pump inhibitors provide faster pain relief and more rapid ulcer healing. However, nearly equivalent overall healing rates may be achieved with longer courses of H2-receptor antagonists.
The proton pump inhibitors are remarkably safe in short-term therapy. Serum gastrin levels rise significantly (> 500 pg/mL) in 3% of patients receiving long-term therapy, which is associated with the development of gastric enterochromaffin-like cell hyperplasia in humans and gastric carcinoid tumors in rats. Clinical experience with these agents for over 10 years has not detected any significant toxicity in humans. Long-term use may lead to a mild decrease in vitamin B12 and iron absorption of unclear significance. Long-term use is unnecessary in peptic ulcer disease but is frequently required in gastroesophageal reflux disease.
2. H2-receptor antagonists
Although H2-receptor antagonists are effective in the treatment of peptic ulcer disease, proton pump inhibitors are now the preferred agents because of their ease of use and superior efficacy. Four H2-receptor antagonists are available: cimetidine, ranitidine, famotidine, and nizatidine. All four agents effectively inhibit nocturnal acid output, but they are less effective at inhibiting meal-stimulated acid secretion. For uncomplicated peptic ulcers, H2-receptor antagonists may be administered once daily at bedtime as follows: ranitidine and nizatidine 300 mg, famotidine 40 mg, and cimetidine 800 mg. Ulcer symptom relief usually occurs within 2 weeks. Duodenal and gastric ulcer healing rates of 85 90% are obtained within 6 weeks and 8 weeks, respectively. All four agents are well tolerated, and serious adverse effects are rare. Cimetidine is rarely used because it inhibits hepatic cytochrome P-450 metabolism (raising the serum concentration of theophylline, warfarin, lidocaine, and phenytoin) and may cause gynecomastia or impotence. Ranitidine binds P-450 with 10% of the avidity of cimetidine; famotidine and nizatidine have negligible effects.
B. Agents Enhancing Mucosal Defenses
Bismuth, misoprostol, and low doses of aluminum-containing antacids all have been shown to promote ulcer healing through the enhancement of mucosal defensive mechanisms. Given the greater efficacy and safety of antisecretory agents and better compliance of patients, these other agents are no longer used as first-line therapy for active ulcers in most clinical settings. Because of the rapid relief of ulcer symptoms they provide, antacids are commonly used as needed to supplement antisecretory agents during the first few days of treatment. Bismuth has direct antibacterial action against H pylori and may be used in combination with antibiotics for eradication (see below). Misoprostol is a prostaglandin analog that stimulates gastroduodenal mucus and bicarbonate secretion. It is effective as a prophylactic agent in reducing the incidence of gastroduodenal ulcers in patients taking nonselective NSAIDs but must be given three or four times daily and causes diarrhea in 10 20% of patients; however, it is not commonly used for this indication because of the advent of proton pump inhibitors and COX-2 selective NSAIDs.
C. H pylori Eradication Therapy
Eradication of H pylori has proved difficult. Combination regimens that use two antibiotics with a proton pump inhibitor or bismuth are required to achieve adequate rates of eradication and to reduce the number of failures due to antibiotic resistance. Resistance develops rapidly to metronidazole and clarithromycin but not to amoxicillin or tetracycline. In the United States, up to 50% of strains are resistant to metronidazole and 7% are resistant to clarithromycin. It is advisable to include amoxicillin in first-line therapy in most patients, reserving metronidazole for penicillin-allergic patients. Recommended regimens are listed in Table 14-11. All currently recommended regimens achieve
P.603
rates of eradication greater than 85% after 7 14 days of treatment. In most centers in the United States, the preferred regimen is with a 10-day course of treatment with a proton pump inhibitor omeprazole or rabeprazole 20 mg twice daily, lansoprazole 30 mg twice daily, pantoprazole 40 mg twice daily, or esomeprazole 40 mg once daily plus amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily. In patients whose infection persists after an initial course of antibiotic therapy, the optimal regimen may be quadruple therapy with a proton pump inhibitor, bismuth subsalicylate, tetracycline, and metronidazole for 14 days (Table 14-11).
Table 14-11. Treatment options for peptic ulcer disease. | ||
|---|---|---|
|
Medical Treatment
Patients should be encouraged to eat balanced meals at regular intervals. There is no justification for bland or restrictive diets. Moderate alcohol intake is not harmful. Smoking retards the rate of ulcer healing and increases the frequency of recurrences and should be discouraged.
A. Treatment of H pylori-Associated Ulcers
1. Treatment of active ulcer
The goals of treatment of active H pylori-associated ulcers are to relieve dyspeptic symptoms, to promote ulcer healing, and to
P.604
eradicate H pylori infection. Uncomplicated H pylori-associated ulcers should be treated for the first 10 days with one of the proton pump inhibitor-based H pylori eradication regimens listed in Table 14-11. An antisecretory agent must be administered for an additional 2 4 weeks (duodenal ulcer) or 4 6 weeks (gastric ulcer) after completion of the antibiotic regimen to ensure complete ulcer healing. A once-daily proton pump inhibitor (omeprazole or rabeprazole 20 mg, lansoprazole 30 mg, pantoprazole or esomeprazole 40 mg) is most convenient, but H2-receptor antagonists may be chosen as less expensive therapy. Confirmation of H pylori eradication in patients with uncomplicated ulcers is not necessary. Confirmation is required in all patients with ulcers complicated by bleeding, perforation, or obstruction.
2. Therapy to prevent recurrence
Successful eradication reduces ulcer recurrences to less than 20% after 1 2 years. Therefore, antisecretory therapy can be discontinued after 4 8 weeks in patients with uncomplicated ulcers and the patient observed for recurrence of symptoms. The most common cause of recurrence after antibiotic therapy is failure to achieve successful eradication, which must be evaluated. Once cure has been achieved, reinfection rates are less than 0.5% per year. Although H pylori eradication has reduced the need for chronic maintenance antisecretory therapy to prevent ulcer recurrences, there remains a subset of patients who require chronic therapy with either a proton pump inhibitor once daily or an H2-receptor antagonist at bedtime. This subset includes patients with H pylori-positive ulcers who have failed recurrent attempts at eradication therapy, patients with a history of H pylori-positive ulcers who have recurrent ulcers despite successful eradication, and patients with idiopathic ulcers (ie, H pylori-negative and not taking NSAIDs). In all patients with recurrent ulcers, NSAID usage (unintentional or surreptitious) and hypersecretory states (including gastrinoma) should be excluded.
B. Treatment of NSAID-Associated Ulcers
1. Treatment of active ulcers
In patients with NSAID-induced ulcers, the offending agent should be discontinued whenever possible. Both gastric and duodenal ulcers respond rapidly to therapy with H2-receptor antagonists or proton pump inhibitors (Table 14-11) once NSAIDs are eliminated. In some patients with severe inflammatory diseases, it may not be feasible to discontinue NSAIDs. These patients should be treated with proton pump inhibitors once daily, which results in ulcer healing rates of approximately 80% at 8 weeks in patients continuing to take NSAIDs. All patients with NSAID-associated ulcers should undergo testing for H pylori infection. Antibiotic eradication therapy should be given if H pylori tests are positive.
2. Prevention of NSAID-induced ulcers
The goal of prophylactic therapy is to prevent ulcer complications, which occur in only 1 2% of NSAID-treated patients per year. Therefore, prophylactic therapy should be reserved for patients at high risk for developing complications. High-risk factors include a history of ulcer disease or complications, concurrent therapy with corticosteroids or anticoagulants, concurrent use of NSAID plus low-dose aspirin, serious underlying medical illness, and age over 60 years. Such patients have a 2 10% chance per year of developing a complicated ulcer from taking a nonselective NSAID. Whenever possible, NSAIDs should be avoided in this high-risk population. If NSAIDs must be given, the following options can be considered. (At this time, the optimal cost-effective approach has not been determined.)
a. Test for and treat H pylori infection
All patients who are started on nonselective NSAIDs should have noninvasive testing for H pylori infection followed by treatment, if positive. Eradication of H pylori reduces the incidence of endoscopic ulcers in acute NSAID users from 26 34% to 7 12% within 12 weeks.
b. Proton pump inhibitor
Treatment with a proton pump inhibitor given once daily (omeprazole or rabeprazole 20 mg, lansoprazole 30 mg, or pantoprazole or esomeprazole 40 mg) appears to be effective in the prevention of NSAID-induced gastric and duodenal ulcers and is approved by the FDA for this indication. For patients taking a daily proton pump inhibitor for another indication (such as gastroesophageal reflux disease), use of a generic nonselective NSAID is an attractive, cost-effective option.
c. Misoprostol
The prostaglandin analog misoprostol is effective in the prevention of NSAID-induced gastric and duodenal ulcers when given at a dosage of 100 200 mcg four times daily. However, misoprostol is less commonly used as a prophylactic agent against NSAID-induced complications than either concurrent therapy with a proton pump inhibitor or COX-2 selective agent because of its high side effect profile and the need for dosing four times daily. With increased restriction on long-term use of COX-2 selective drugs, usage of misoprostol in conjunction with nonselective NSAIDs may increase.
d. COX-2 selective agents
As discussed above, there is an increased risk of major cardiovascular events in patients taking rofecoxib or valdecoxib compared with nonselective NSAIDs or placebo an absolute increase of approximately 1.6% in patients taking rofecoxib, which led to the voluntary withdrawal of these agents from the market. Although celecoxib does not appear to have an increased risk of cardiovascular events compared with nonselective NSAIDs, one long-term polyp prevention study has shown an increased risk of such adverse events with celecoxib compared with placebo. Pending further outcomes data, celecoxib should be restricted to < 90 days of therapy in patients deemed at increased risk for NSAID-induced complications and at low risk for cardiovascular complications. In patients who require concurrent therapy with low-dose aspirin for cardiovascular prophylaxis,
P.605
the gastrointestinal safety of a COX-2 selective agent is partially or completely negated.
e. Multiple NSAID risk factors
Patients with prior ulcer complications or with multiple risk factors are at particularly high risk for NSAID-induced complications (10 20% per year). The optimal cost-effective approach to management of these patients is unknown. All patients should undergo noninvasive testing for H pylori, and if results are positive, treatment should be given. If NSAID therapy is deemed to be clinically required despite the increased risks, treatment options include a COX-2 selective agent (celecoxib, etodolac, or meloxicam) plus a prophylactic agent (proton pump inhibitor or misoprostol) or in patients with increased risk of cardiovascular disease a standard NSAID and a prophylactic agent (double-dose proton pump inhibitor or misoprostol).
D. Refractory Ulcers
Ulcers that are truly refractory to medical therapy are now uncommon. Less than 5% of ulcers are unhealed after 8 weeks of therapy with proton pump inhibitors. Noncompliance is the most common cause of ulcer nonhealing. Cigarettes retard ulcer healing and should be proscribed. NSAID and aspirin use, sometimes surreptitious, are commonly implicated in refractory ulcers and must be stopped. H pylori eradication enhances healing and decreases the high recurrence rates of refractory ulcers. Therefore, evidence of H pylori infection should be sought and the infection treated, if present, in all refractory ulcer patients. Fasting serum gastrin levels should be obtained to exclude gastrinoma with acid hypersecretion (Zollinger-Ellison syndrome). Nonhealing gastric ulcers raise concerns that an undiagnosed gastric malignancy may be masquerading as a benign gastric ulcer. Repeat ulcer biopsies are mandatory after 2 3 months of therapy in all nonhealed gastric ulcers, and they should be followed with serial endoscopies to verify complete healing. Almost all benign refractory ulcers heal within 8 weeks with a proton pump inhibitor twice daily (omeprazole or rabeprazole 20 mg twice daily, lansoprazole 30 mg twice daily). Patients with persistent nonhealing ulcers are referred for surgical therapy after exclusion of NSAID use and persistent H pylori infection.
Chan FK et al: Peptic-ulcer disease. Lancet 2002;360:933.
Fitzgerald GA: Coxibs and cardiovascular disease. N Engl J Med 2004;351:1709.
Ford AD et al: Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol 2004;99:1833.
Gisbert JP et al: Systematic review and meta-analysis: is 1-week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther 2005;21:795.
Gatta L et al: A 10-day levofloxacin-based triple therapy in patients who have failed two eradication courses. Aliment Pharmacol Ther 2005;22:45.
Laine L et al: Ulcer formation with low-dose enteric-coasted aspirin and the effect of COX-2 selective inhibition: a double-blind trial. Gastroenterology 2004;127:395.
Lanas A: Gastrointestinal complications from NSAID therapy. How to reduce the risk of complications. Postgrad Med 2005;117:23.
Vakil N: Primary and secondary treatment for Helicobacter pylori in the United States. Rev Gastroenterol Disord 2005;5:67.
Vergara M et al: Meta-analysis: role of Helicobacter pylori eradication in the prevention of peptic ulcer in NSAID users. Aliment Pharmacol Ther 2005;21:1411.
Zullo A et al: High rate of Helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: a prospective controlled study. Aliment Pharmacol Ther 2005; 21:1419.
Complications of Peptic Ulcer Disease
1. Gastrointestinal Hemorrhage
![]() Essentials of Diagnosis
Essentials of Diagnosis
Coffee grounds emesis, hematemesis, melena, or hematochezia.
Emergent upper endoscopy is diagnostic and therapeutic.
General Considerations
Approximately 50% of all episodes of upper gastrointestinal bleeding are due to peptic ulcer. Clinically significant bleeding occurs in 10% of ulcer patients. About 80% of patients stop bleeding spontaneously and generally have an uneventful recovery; the remaining 20% have more severe bleeding. The overall mortality rate for ulcer bleeding is 6 10%, but it is higher in the elderly, in patients with comorbid medical problems, and in patients with nosocomial bleeding. Mortality is also higher in patients who present with persistent hypotension or shock, bright red blood in the vomitus or nasogastric lavage fluid, or severe coagulopathy.
Clinical Findings
A. Symptoms and Signs
Up to 20% of patients have no antecedent symptoms of pain; this is particularly true of patients receiving NSAIDs. Common presenting signs include melena and hematemesis. Massive upper gastrointestinal bleeding or rapid gastrointestinal transit may result in hematochezia rather than melena; this may be misinterpreted as signifying a lower tract bleeding source. Nasogastric lavage that demonstrates coffee grounds or bright red blood confirms an upper tract source. Recovered nasogastric lavage fluid that is negative for
P.606
blood does not exclude active bleeding from a duodenal ulcer.
B. Laboratory Findings
The hematocrit may fall as a result of bleeding or expansion of the intravascular volume with intravenous fluids. The blood urea nitrogen (BUN) may rise as a result of absorption of blood nitrogen from the small intestine and prerenal azotemia.
Treatment
The assessment and initial management of upper gastrointestinal tract bleeding are discussed elsewhere in this chapter. Specific issues pertaining to peptic ulcer bleeding are described below.
A. Medical Therapy
1. Antisecretory agents
Intravenous proton pump inhibitors or high-dose oral proton pump inhibitors should be administered for 3 days in patients with ulcers whose endoscopic appearance suggests a high risk of rebleeding after endoscopic therapy. Intravenous or high-dose oral proton pump inhibitors have been associated with a reduction in rebleeding, transfusions, the need for further endoscopic therapy, and surgery in the subset of patients with high-risk ulcers, ie, an ulcer with active bleeding, visible vessel, or adherent clot (see below). Intravenous omeprazole (80 mg bolus injection, followed by 8 mg/h continuous infusion for 72 hours) has undergone the most clinical testing but is not available in the United States. After initial successful endoscopic treatment of ulcer hemorrhage, intravenous omeprazole reduces the rebleeding rate from approximately 20% to < 10%. In the United States, intravenous esomeprazole, lansoprazole, and pantoprazole are available and commonly used at comparable dosing (80 mg bolus injection, followed by 8 mg/h) for this indication, although published data confirming their efficacy are unavailable. High-dose oral proton pump inhibitors (omeprazole 40 mg twice daily) also appear to be effective in reducing rebleeding but have not been compared to the intravenous regimen. Many physicians choose to administer a high-dose proton pump inhibitor prior to endoscopy in all patients admitted to the hospital with gastrointestinal bleeding suspected to be due to peptic ulcer, discontinuing therapy after endoscopy in patients with ulcers deemed to be at low risk of rebleeding. Intravenous H2-receptor antagonists have not been demonstrated to be of any benefit in the treatment of acute ulcer bleeding.
2. Long-term prevention of rebleeding
Recurrent ulcer bleeding develops within 3 years in one-third of patients if no specific therapy is given. In patients with bleeding ulcers who are H pylori positive, successful eradication effectively prevents recurrent ulcer bleeding in almost all cases. It is therefore recommended that all patients with bleeding ulcers be tested for H pylori infection and treated if positive. Four to 8 weeks after completion of antibiotic therapy, a urea breath or fecal antigen test for H pylori should be administered or endoscopy performed with biopsy for histologic confirmation of successful eradication. In patients in whom H pylori persists or the small subset of patients whose ulcers are not associated with NSAIDs or H pylori, chronic acid suppression with a bedtime dose of an H2-antagonist (ranitidine 150 mg) or a once-daily proton pump inhibitor should be prescribed to reduce the likelihood of recurrence of bleeding.
B. Endoscopy
Endoscopy is the preferred diagnostic procedure in almost all cases of upper gastrointestinal bleeding because of its high diagnostic accuracy, its ability to predict the likelihood of recurrent bleeding, and its availability for therapeutic intervention in high-risk lesions. Endoscopy should be performed within 12 24 hours in most cases. In cases of severe active bleeding, endoscopy is performed as soon as patients have been appropriately resuscitated and are hemodynamically stable.
On the basis of clinical and endoscopic criteria, it is possible to predict which patients are at a higher risk of rebleeding and therefore to make more rational use of hospital resources. Nonbleeding ulcers under 2 cm in size with a base that is clean have a less than 5% chance of rebleeding. Most young (under age 60), otherwise healthy patients with clean-based ulcers may be safely discharged from the emergency or hospital after endoscopy. Ulcers that have a flat red or black spot have a less than 10% chance of significant rebleeding. Patients who are hemodynamically stable with these findings should be admitted to a hospital ward for 24 72 hours and may begin immediate oral feedings and antiulcer (or anti-H pylori) medication.
By contrast, the risk of rebleeding or continued bleeding in ulcers with a firmly adherent clot is 12 33%, with a nonbleeding visible vessel is 50%, and with active bleeding it is 80 90%. Endoscopic therapy with dilute epinephrine (1:10,000) injection, thermocoagulation (bipolar or heater probes), or application of endoscopic clips (akin to a staple) is the standard of care for such lesions because it reduces the risk of rebleeding, the number of transfusions, and the need for subsequent surgery. Using any of these techniques, successful hemostasis of actively bleeding lesions is achieved in 90%. For actively bleeding ulcers, a combination of epinephrine injection followed by thermocoagulation yields better control of bleeding than either modality alone. Significant rebleeding occurs in 10 20% of cases, of which over 70% can be managed successfully with repeat endoscopic treatment. In limited comparative studies, hemoclipping appears to be as effective as injection and thermocoagulation techniques. Patients with these high-risk lesions should receive a high-dose intravenous or oral proton pump inhibitor regimen for 72 hours. After endoscopic treatment, high-risk patients
P.607
should be monitored in an ICU setting for at least 24 hours and should remain hospitalized for at least 72 hours, when the risk of rebleeding is < 3%.
C. Surgical Treatment
Patients with recurrent bleeding or bleeding that cannot be controlled by endoscopic techniques should be evaluated by a surgeon. However, less than 5% of patients treated with hemostatic therapy require surgery for continued or recurrent bleeding. Overall surgical mortality for emergency ulcer bleeding is less than 6%. The prognosis is poorer for patients over age 60 years, those with serious underlying medical illnesses or chronic renal failure, and those who require more than 10 units of blood transfusion.
2. Ulcer Perforation
Perforations develop in < 5% of ulcer patients, usually from ulcers on the anterior wall of the stomach or duodenum. The incidence of perforations may be increasing, perhaps as a consequence of using NSAIDs or crack cocaine. Perforation results in a chemical peritonitis that causes sudden, severe generalized abdominal pain that prompts most patients to seek immediate attention. Elderly or debilitated patients and those receiving long-term corticosteroid therapy may experience minimal initial symptoms, presenting late with bacterial peritonitis, sepsis, and shock. On physical examination, patients appear ill, with a rigid, quiet abdomen and rebound tenderness. Hypotension develops later after bacterial peritonitis has developed. If hypotension is present early with the onset of pain, other abdominal emergencies should be considered such as a ruptured aortic aneurysm, mesenteric infarction, or acute pancreatitis. Leukocytosis is almost always present. A mildly elevated serum amylase (less than twice normal) is sometimes seen. Upright or decubitus films of the abdomen reveal free intraperitoneal air in 75% of cases, and in most cases this establishes the diagnosis without need for further studies. The absence of free air may lead to a misdiagnosis of pancreatitis, cholecystitis, or appendicitis. Upper gastrointestinal or CT radiography with water-soluble contrast may be useful in this setting. Barium studies are contraindicated in patients with possible perforation.
Traditional surgical dogma held that the majority of patients with perforated ulcers should undergo emergency laparotomy. Closure of the perforation was performed with an omental ( Graham ) patch and, in stable patients, a proximal gastric vagotomy was performed to decrease the chance of ulcer recurrence. This approach is changing as a result of two factors. The first is minimally invasive surgical technique. Laparoscopic perforation closure can be performed in many centers, significantly reducing operative morbidity. Second is the recognition that H pylori infection is associated with most ulcers and ulcer perforations. Postoperative treatment of H pylori reduces the risk of ulcer recurrence, obviating the need for intraoperative vagotomy. The overall mortality rate in patients treated surgically is 5%.
Up to 40% of ulcer perforations seal spontaneously by the adherence of omentum or adjacent organs to the lesion and do not have significant intraperitoneal spillage. Thus, initial nonoperative management may be suitable for patients whose onset of symptoms is less than 12 hours and whose upper gastrointestinal series with water-soluble contrast medium does not demonstrate leakage. Such conservative therapy is most appropriate for patients who are poor operative candidates. Patients should be monitored closely while receiving fluids, nasogastric suction, intravenous proton pump inhibitors, and broad-spectrum antibiotics. If their condition deteriorates over the first 12 hours (as evidenced by increasing pain, rising pulse or temperature, or worsening peritonitis), they should be taken to the operating room.
3. Ulcer Penetration
An ulcer located along the posterior wall of the duodenum or stomach may perforate into contiguous structures such as the pancreas, liver, or biliary tree. Patients complain of a change in the intensity and rhythmicity of their ulcer symptoms. The pain becomes more severe and constant, may radiate to the back, and is unresponsive to antacids or food. Physical examination and laboratory tests are nonspecific. Mild amylase elevations may sometimes occur. Endoscopy and barium x-ray studies confirm the ulceration but are not diagnostic of an actual penetration. Patients should be given intravenous proton pump inhibitors and monitored closely. Those who do not improve should be considered for surgery.
4. Gastric Outlet Obstruction
Gastric outlet obstruction occurs in 2% of patients with ulcer disease and is due to edema or cicatricial narrowing of the pylorus or duodenal bulb. Most patients have a prior known history of ulcer disease. Obstruction is less commonly caused by gastric neoplasms or extrinsic duodenal obstruction by intra-abdominal neoplasms. The most common symptoms are early satiety, vomiting, and weight loss. Early symptoms are epigastric fullness or heaviness after meals. Later, vomiting may develop that typically occurs one to several hours after eating and consists of partially digested food contents. Chronic obstruction may result in a grossly dilated, atonic stomach, severe weight loss, and malnutrition. Patients may develop dehydration, metabolic alkalosis, and hypokalemia. On physical examination, a succussion splash may be heard in the epigastrium. In most cases, nasogastric aspiration will result in evacuation of a large amount (> 200 mL) of foul-smelling fluid, which establishes the diagnosis. More subtle obstruction is diagnosed by a saline load test or by a nuclear gastric emptying study. Patients are treated initially with intravenous isotonic saline and KCl to correct fluid and electrolyte disorders, an intravenous proton pump inhibitor, and nasogastric decompression of the
P.608
stomach. Severely malnourished patients should receive TPN. Upper endoscopy is performed after 24 72 hours to define the nature of the obstruction and to exclude gastric neoplasm. Patients whose symptoms fail to improve within 5 7 days on nasogastric suction require endoscopic or surgical treatment. Upper endoscopy with dilation of the gastric obstruction by hydrostatic balloons passed through the instrument improves symptoms in up to two-thirds of patients and may be attempted in patients with milder symptoms. Surgical treatment with vagotomy and either pyloroplasty or antrectomy is required in patients with inadequate response or symptom relapse after endoscopic dilation.
ASGE guideline: the role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc 2004;60:497.
Barkun A et al: Consensus guidelines for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2003;139:843.
Bardou M et al: Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther 2005;21:677.
Calvet X et al: Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology 2004;126:441.
Hung LC et al: Long-term outcome of Helicobacter pylori-negative idiopathic bleeding ulcers: a prospective cohort study. Gastroenterology 2005;128:1845.
Julapalli VR et al: Appropriate use of intravenous proton pump inhibitors in the management of bleeding peptic ulcer. Dig Dis Sci 2005;50:1185.
Lanas A et al: A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol 2005;100:1685.
Park CH et al: A prospective, randomized trial comparing mechanical methods of hemostasis plus epinephrine injection to epinephrine injection alone for bleeding peptic ulcer. Gastrointest Endosc 2004;60:173.
Ramsoekh D et al: Outcome of peptic ulcer bleeding, nonsteroidal anti-inflammatory drug use, and Helicobacter pylori infection. Clin Gastroenterol Hepatol 2005;3:859.
Saltzman JR et al: Prospective trial of endoscopic clips versus combination therapy in upper GI bleeding (PROTECCT UGI Bleeding). Am J Gastroenterol 2005;100:1503.
Zollinger-Ellison Syndrome (Gastrinoma)
![]() Essentials of Diagnosis
Essentials of Diagnosis
Peptic ulcer disease; may be severe and atypical.
Gastric acid hypersecretion.
Diarrhea common, relieved by nasogastric suction.
Most cases are sporadic; 25% with multiple endocrine neoplasia type 1 (MEN 1).
General Considerations
Zollinger-Ellison syndrome is caused by gastrin-secreting gut neuroendocrine tumors (gastrinomas), which result in hypergastrinemia and acid hypersecretion. Less than 1% of peptic ulcer disease is caused by gastrinomas. Primary gastrinomas may arise in the pancreas (25%), duodenal wall (45%), or lymph nodes (5 15%), and in other locations or of unknown primary in 20%. Approximately 80% arise within the gastrinoma triangle bounded by the porta hepatis, the neck of the pancreas, and the third portion of the duodenum. Most gastrinomas are solitary or multifocal nodules that are potentially resectable. Over two-thirds of gastrinomas are malignant, and one-third have already metastasized to the liver at initial presentation. Approximately 25% of patients have small multicentric gastrinomas associated with MEN 1 that are more difficult to resect.
Clinical Findings
A. Symptoms and Signs
Over 90% of patients with Zollinger-Ellison syndrome develop peptic ulcers. In most cases, the symptoms are indistinguishable from other causes of peptic ulcer disease and therefore may go undetected for years. Ulcers usually are solitary and located in the duodenal bulb, but they may be multiple or occur more distally in the duodenum. Isolated gastric ulcers do not occur. Gastroesophageal reflux symptoms occur often. Diarrhea occurs in one-third of patients, in some cases in the absence of peptic symptoms. Gastric acid hypersecretion can cause direct intestinal mucosal injury and pancreatic enzyme inactivation, resulting in diarrhea, steatorrhea, and weight loss; nasogastric aspiration of stomach acid stops the diarrhea. Screening for Zollinger-Ellison syndrome with fasting gastrin levels should be obtained in patients with ulcers that are refractory to standard therapies, giant ulcers (> 2 cm), ulcers located distal to the duodenal bulb, multiple duodenal ulcers, frequent ulcer recurrences, ulcers associated with diarrhea, ulcers occurring after ulcer surgery, and patients with ulcer complications. Ulcer patients with hypercalcemia or family histories of ulcers (suggesting MEN 1) should also be screened. Finally, patients with peptic ulcers who are H pylori negative and who are not taking NSAIDs should be screened.
B. Laboratory Findings
The most sensitive and specific method for identifying Zollinger-Ellison syndrome is demonstration of an increased fasting serum gastrin concentration (> 150 pg/mL). Levels should be obtained with patients not taking H2-receptor antagonists for 24 hours or proton pump inhibitors for 6 days. The median gastrin level is 500 700 pg/mL, and 60% of patients have levels less than 1000 pg/mL. Hypochlorhydria with increased
P.609
gastric pH is a much more common cause of hypergastrinemia than is gastrinoma. Therefore, a measurement of gastric pH (and, where available, gastric secretory studies) is performed in patients with fasting hypergastrinemia. Most patients have a basal acid output of over 15 mEq/h. A gastric pH of > 3.0 implies hypochlorhydria and excludes gastrinoma. In a patient with a serum gastrin level of > 1000 pg/mL and acid hypersecretion, the diagnosis of Zollinger-Ellison syndrome is established. With lower gastrin levels (150 1000 pg/mL) and acid secretion, a secretin stimulation test is performed to distinguish Zollinger-Ellison syndrome from other causes of hypergastrinemia. Intravenous secretin (2 units/kg) produces a rise in serum gastrin of over 200 pg/mL within 2 30 minutes in 85% of patients with gastrinoma. An elevated serum calcium suggests hyperparathyroidism and MEN 1 syndrome. In all patients with Zollinger-Ellison syndrome, a serum parathyroid hormone (PTH), prolactin, luteinizing hormone-follicle-stimulating hormone (LH-FSH), and growth hormone (GH) level should be obtained to exclude MEN 1.
C. Imaging
Imaging studies are obtained in an attempt to determine whether there is metastatic disease and, if not, to identify the site of the primary tumor. Gastrinomas express somatostatin receptors that bind radiolabeled octreotide. Somatostatin receptor scintigraphy (SRS) with single photon emission computed tomography (SPECT) allows total body imaging for detection of primary gastrinomas in the pancreas and lymph nodes, primary gastrinomas in unusual locations, and metastatic gastrinomas (liver and bone). SRS has a sensitivity (> 80%) for tumor detection that exceeds all other imaging studies combined. If SRS is positive for tumor localization, further imaging studies are not necessary. In patients with negative SRS, endoscopic ultrasonography (EUS) may be useful to detect small gastrinomas in the duodenal wall, pancreas, or peripancreatic lymph nodes. CT and MRI scans are commonly obtained to look for large hepatic metastases and primary lesions, but they have low sensitivity for small lesions. With a combination of SRS and EUS, more than 90% of primary gastrinomas can be localized preoperatively.
Differential Diagnosis
Gastrinomas are one of several gut neuroendocrine tumors that have similar histopathologic features and arise either from the gut or pancreas. These include carcinoid, insulinoma, VIPoma, glucagonoma, and somatostatinoma. These tumors usually are differentiated by the gut peptides that they secrete; however, poorly differentiated neuroendocrine tumors may not secrete any hormones. Patients may present with symptoms caused by tumor metastases (jaundice, hepatomegaly) rather than functional symptoms. Once a diagnosis of a neuroendocrine tumor is established from the liver biopsy, the specific type of tumor can subsequently be determined. Both carcinoids and gastrinomas may be detected incidentally during endoscopy after biopsy of a submucosal nodule and must be distinguished by subsequent studies.
Hypergastrinemia due to gastrinoma must be distinguished from other causes of hypergastrinemia. Atrophic gastritis with decreased acid secretion is detected by gastric secretory analysis. Other conditions associated with hypergastrinemia (eg, gastric outlet obstruction, vagotomy, chronic renal failure) are associated with a negative secretin stimulation test.
Treatment
A. Metastatic Disease
The most important predictor of survival is the presence of hepatic metastases. In patients with multiple hepatic metastases, initial therapy should be directed at controlling hypersecretion. Proton pump inhibitors (omeprazole, esomeprazole, rabeprazole, pantoprazole, or lansoprazole) are given at a dose of 40 120 mg/d, titrated to achieve a basal acid output of < 10 mEq/h. At this level, there is complete symptomatic relief and ulcer healing. In patients with isolated hepatic metastases, surgical resection or cryoablation may decrease the need for antisecretory medications and may prolong survival. Owing to the slow growth of these tumors, 30% of patients with hepatic metastases have a survival of 10 years.
B. Localized Disease
Cure can be achieved only if the gastrinoma can be resected before hepatic metastatic spread has occurred. Lymph node metastases do not adversely affect prognosis. Laparotomy should be considered in all patients in whom preoperative studies fail to demonstrate hepatic or other distant metastases. A combination of preoperative studies, duodenotomy with careful duodenal inspection, and intraoperative palpation and sonography allows successful localization and resection in the majority of cases. The 15-year survival of patients who do not have liver metastases at initial presentation is over 95%. The role of surgery in patients with MEN 1 is controversial. Surgical cure in patients with MEN 1 rarely occurs, and long-term survival is common in the absence of surgery. Some experts recommend surgery only in patients with MEN 1 whose tumors are larger than 2 cm, in whom the risk of hepatic metastases is increased.
Norton J et al: Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg 2004;240:757.
Benign Tumors of the Stomach
Gastric epithelial polyps are usually detected incidentally at endoscopy. The majority are fundic gland polyps or hyperplastic polyps, which are small, single or
P.610
multiple, have no malignant potential, and do not require removal or endoscopic surveillance. Adenomatous polyps account for 10 20% of gastric polyps. They are usually solitary lesions. In rare instances they ulcerate, causing chronic blood loss. Because of their premalignant potential, endoscopic removal is indicated. Annual endoscopic surveillance is recommended to screen for further polyp development. Submucosal gastric polypoid lesions include benign gastric stromal tumors (commonly misclassified as leiomyomas) and pancreatic rests.
Burt R: Gastric fundic gland polyps. Gastroenterology 2003; 125:1462.
Malignant Tumors of the Stomach
1. Gastric Adenocarcinoma
![]() Essentials of Diagnosis
Essentials of Diagnosis
Dyspeptic symptoms with weight loss in patients over age 40 years.
Iron deficiency anemia; occult blood in stools.
Abnormality detected on upper gastrointestinal series or endoscopy.
General Considerations
Although gastric adenocarcinoma is the most common cancer (other than skin cancer) worldwide, its incidence in the United States has declined by two-thirds over the last 30 years to 20,000 cases annually. Gastric cancer is uncommon in persons younger than 40 years; the mean age at diagnosis is 63 years. Men are affected twice as often as women. The incidence is higher in Latinos, African-Americans, and Asian-Americans. Certain regions such as Chile, Colombia, Central America, and Japan have rates as high as 80 per 100,000 population. Although most gastric cancers arise in the antrum, the incidence of proximal tumors of the cardia and fundus is increasing dramatically.
Chronic H pylori gastritis is a strong risk factor for gastric carcinoma of the distal (but not proximal) stomach, increasing the relative risk 3.5- to 20-fold. It is estimated that 60 90% of cases of distal gastric carcinoma may be attributable to H pylori. Patients infected with a virulent strain of H pylori (CagA-positive) and patients with gastritis associated with atrophy or intestinal metaplasia are at increased risk. During a mean of 7.5 years of follow-up, gastric cancer developed in approximately 1.4 2.9% of patients with chronic H pylori gastritis. Prospective controlled studies have not demonstrated a reduction in gastric cancer incidence after H pylori eradication, although a decreased incidence has been observed in the subset of infected patients without precancerous lesions (atrophy or intestinal metaplasia) prior to therapy. Because of its unproven efficacy and cost-effectiveness, screening for H pylori infection and treating it to prevent gastric cancer is not recommended for asymptomatic adults in the general population but may be considered in patients who have immigrated from regions with a high incidence of gastric cancer or who have a family history of gastric cancer. Other risk factors for gastric cancer include pernicious anemia and a history of partial gastric resection more than 15 years previously.
Gastric cancer may occur in a variety of morphologic types: (1) polypoid or fungating intraluminal masses; (2) ulcerating masses; (3) diffusely spreading (linitis plastica), in which the tumor spreads through the submucosa, resulting in a rigid, atonic stomach with thickened folds (prognosis dismal); and (4) superficially spreading or early gastric cancer confined to the mucosa or submucosa (with or without lymph node metastases) and associated with an excellent prognosis.
Clinical Findings
A. Symptoms and Signs
Gastric carcinoma is generally asymptomatic until the disease is quite advanced. Symptoms are nonspecific and are determined in part by the location of the tumor. Dyspepsia, vague epigastric pain, anorexia, early satiety, and weight loss are the presenting symptoms in most patients. Patients may derive initial symptomatic relief from over-the-counter remedies, further delaying diagnosis. Ulcerating lesions can lead to acute gastrointestinal bleeding with hematemesis or melena. Pyloric obstruction results in postprandial vomiting. Lower esophageal obstruction causes progressive dysphagia. Physical examination is rarely helpful. A gastric mass is palpated in less than 20% of patients. Signs of metastatic spread include a left supraclavicular lymph node (Virchow's node), an umbilical nodule (Sister Mary Joseph nodule), a rigid rectal shelf (Blumer's shelf), and ovarian metastases (Krukenberg tumor). Guaiac-positive stools may be detectable.
B. Laboratory Findings
Iron deficiency anemia due to chronic blood loss or anemia of chronic disease is common. Liver function test abnormalities may be present if there is metastatic liver spread. Other tumor markers are of no value.
C. Endoscopy
Upper endoscopy should be obtained in all patients over age 55 years with new onset of epigastric symptoms (dyspepsia) and in anyone with dyspepsia that is persistent or fails to respond to a short trial of antisecretory therapy. Endoscopy with cytologic brushings and biopsies of suspicious lesions is highly sensitive for
P.611
detecting gastric carcinoma. It can be difficult to obtain adequate biopsy specimens in linitis plastica lesions. Because of the high incidence of gastric carcinoma in Japan, screening upper endoscopy is performed to detect early gastric carcinoma. Approximately 40% of tumors detected by screening are early, with a 5-year survival rate of almost 90%. Screening programs are not recommended in the United States.
D. Imaging
A barium upper gastrointestinal series is an acceptable alternative when endoscopy is not readily available but may not detect small or superficial lesions and cannot reliably distinguish benign from malignant ulcerations. Any abnormalities detected with this procedure require endoscopic confirmation.
Once a gastric cancer is diagnosed, preoperative evaluation with abdominal CT and EUS is indicated to delineate the local extent of the primary tumor as well as nodal or distant metastases. Abdominal CT is valuable in identifying distant metastases and direct invasion of adjacent structures. Endoscopic ultrasound imaging is superior to CT in determining the depth of tumor penetration and nodal metastases.
E. Staging
Staging is defined according to the TNM system, in which T1 tumors invade the lamina propria (T1a) or submucosa (T1b), T2 invade the muscularis propria, T3 penetrate the serosa, and T4 invade adjacent structures. Nodes are graded as N0 if there is no involvement, N1 if there are metastases to perigastric nodes, and N2 if regional lymph nodes are involved. M1 signifies the presence of metastatic disease. The stages are defined in the accompanying box.
Staging Criteria for Gastric Adenocarcinoma
Stage I: T1N0, T1N1, T2N0, all M0
Stage II: T1N2, T2N1, T3N0, all M0
Stage III: T2N2, T2N1, T4N0, all M0
Stage IV: T4N2M0, any M1
Differential Diagnosis
Ulcerating gastric adenocarcinomas are distinguished from benign gastric ulcers by biopsies. Approximately 3% of gastric ulcers initially believed to be benign later prove to be malignant. To exclude malignancy, all gastric ulcers identified at endoscopy should be biopsied. Ulcers that are suspicious for malignancy to the endoscopist or that have atypia or dysplasia on histologic examination warrant repeat endoscopy in 2 3 months to verify healing and exclude malignancy. Nonhealing ulcers should be considered for resection. Infiltrative carcinoma with thickened gastric folds must be distinguished from lymphoma and other hypertrophic gastropathies such as M n trier's disease.
Treatment
A. Curative Surgical Resection
After preoperative staging, about two-thirds of patients will be found to have localized disease (ie, stages I-III). In Japan and some centers in other countries, endoscopic mucosal resection is performed in selected patients with small (< 3 cm), early (intramucosal or T1aN0) gastric cancers after careful staging. For all other patients, surgical resection is the only therapy with curative potential. At surgery, approximately 25% of these patients will be found to have locally unresectable tumors or peritoneal, hepatic, or distant lymph node metastases for which curative surgical resection is not warranted (see below). The remaining patients with confirmed localized disease should undergo radical surgical resection with curative intent. For adenocarcinoma localized to the distal two-thirds of the stomach, a subtotal distal gastrectomy should be performed. For proximal gastric cancer or diffusely infiltrating disease, total gastrectomy is necessary. Although lymph node dissection should be performed for curative resections, there has been ongoing debate about whether an extended (perigastric and regional) lymph node dissection or a limited (perigastric) dissection is needed. However, there appears to be greater short-term morbidity and no long-term survival advantage for extended lymph node dissection. Neither preoperative nor postoperative (adjuvant) chemotherapy or radiochemotherapy appear to confer a survival benefit in most studies in patients who have undergone curative resection with careful nodal dissection. Nevertheless, adjuvant treatment may be considered in patients with stage III cancer, preferably as part of a clinical trial.
B. Palliative Modalities
Many patients will be found either preoperatively or at the time of surgical exploration to have advanced disease that is not amenable to curative surgery due to peritoneal or distant metastases or local invasion of other organs. In many of these cases, palliative resection of the tumor nonetheless may be indicated. Such resection removes the risk of bleeding and obstruction, leads to improved quality of life, and improves survival. For patients with unresectable disease, gastrojejunostomy may be indicated to prevent obstruction. Bleeding or obstruction from unresected tumors may be treated with endoscopic laser or stent therapy, radiation therapy, or angiographic embolization. Although chemotherapy has not been shown to prolong life, single-agent or combination therapies with fluorouracil, doxorubicin, and cisplatin or mitomycin may provide palliation in up to 30%.
P.612
Prognosis
The long-term survival of gastric carcinoma is less than 15%. However, 5-year survival in patients who undergo successful curative resection is over 45%. Survival is related to tumor stage, location, and histologic features. Stage I and stage II tumors resected for cure have a greater than 50% long-term survival. Patients with stage III tumors have a poor prognosis (< 20% long-term survival) and should be considered for enrollment in clinical trials. Tumors of the diffuse and signet ring type have a worse prognosis than the intestinal type. Tumors of the proximal stomach (fundus and cardia) carry a far worse prognosis than distal lesions. Even with apparently localized disease, proximal tumors have a 5-year survival of less than 15%. For those whose disease progresses despite therapy, meticulous efforts at palliative care are essential (see Chapter 5).
Dicken BJ et al: Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005;241:27.
Genta RM: Screening for gastric cancer: does it make sense? Aliment Pharmacol Ther 2004;20(Suppl 2):42.
Hohenberger P et al: Gastric cancer. Lancet 2003;362:305.
Macdonald JS: Adjuvant therapy for gastric cancer. Semin Oncol 2003;30(suppl 11):19.
McCulloch P et al: Extended versus limited lymph node dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev 2004;(4):CD001964.
Melfertheiner P et al: Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol 2005;100:2100.
2. Lymphoma
Lymphoma is the second most common gastric malignancy, accounting for 3 6% of gastric cancers. More than 95% of these are non-Hodgkin's B cell lymphomas. Gastric lymphomas may be primary (arising from the gastric mucosa) or may represent a site of secondary involvement in patients with nodal lymphomas. About 60% of primary gastric lymphomas are believed to arise from mucosa-associated lymphoid tissue (MALT). Distinguishing advanced primary gastric lymphoma with adjacent nodal spread from advanced nodal lymphoma with secondary gastric spread can be problematic. Because the prognosis and treatment of primary and secondary gastric lymphomas are entirely different, the distinction is important. B cells of nodal origin may be distinguished from those derived from MALT (CD19 and CD20 positive).
Infection with H pylori may be an important risk factor for the development of primary gastric lymphoma. Chronic infection with H pylori causes an intense lymphocytic inflammatory response that may lead to the development of lymphoid follicles. Over 85% of low-grade primary gastric lymphomas and 40% of high-grade lymphomas are associated with H pylori infection. The risk of developing lymphoma is increased sevenfold in patients with chronic H pylori infection. It is hypothesized that chronic antigenic stimulation may result in a monoclonal lymphoproliferation that may culminate in a low-grade MALT lymphoma. At present, the relationship between high-grade primary lymphomas, MALT, and H pylori infection is unclear.
The clinical presentation and endoscopic appearance of gastric lymphoma are similar to those of adenocarcinoma. Most patients have abdominal pain, weight loss, or bleeding. Night sweats are absent in primary lymphoma. At endoscopy, lymphoma may appear as an ulcer, mass, or diffusely infiltrating lesion. The diagnosis is established with endoscopic biopsy. All patients should undergo staging with abdominal and chest CT. EUS is the most sensitive test for determining the presence of perigastric lymphadenopathy.
Nodal lymphomas with secondary gastrointestinal involvement usually present at an advanced stage with widely disseminated disease and are seldom curable. Their treatment is addressed in Chapter 13. By contrast, primary low-grade gastric lymphomas usually are localized to the stomach wall (stage IE) or adjacent lymph nodes (stage IIE) and have an excellent prognosis. Patients with primary low-grade gastric MALT-lymphoma should be tested for H pylori infection and treated if positive. Where available, EUS should be performed to accurately determine tumor stage. Complete lymphoma regression after successful H pylori eradication occurs in 75% of cases of stage IE low-grade lymphoma. Remission may take as long as a year. Patients with stage IE or IIE low-grade lymphomas who either are not infected with H pylori or fail to respond to eradication therapy can be treated successfully with surgical resection, local radiation therapy, or combination therapy. Stage IE or IIE high-grade lymphomas may be treated with resection and CHOP chemotherapy. Stage III and stage IV primary lymphomas are treated with combination chemotherapy. Because of a low risk of perforation with either radiation therapy or chemotherapy, surgical resection is no longer recommended. The long-term survival of primary gastric lymphoma for stage I is over 85% and for stage II is 35 65%.
Al-Akwaa AM et al: Primary gastric lymphoma. World J Gastroenterol 2004;10:5.
Bierman PJ: Gastrointestinal lymphoma. Curr Treat Options Oncol 2003;4:421.
Farinha P et al: Helicobacter pylori and MALT lymphoma. Gastroenterology 2005;128:1579.
Levy M et al: Conservative treatment of primary gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue: predictive factors of response and outcome. Am J Gastroenterol 2002;97:292.
3. Carcinoid Tumors
Gastric carcinoids are rare neuroendocrine tumors that make up less than 1% of gastric neoplasms. They may
P.613
occur sporadically or secondary to chronic hypergastrinemia that results in hyperplasia and transformation of enterochromaffin cells in the gastric fundus. Sporadic carcinoids account for 20% of gastric carcinoids. Most are solitary, over 2 cm in size, and have a strong propensity for metastatic spread. Most sporadic carcinoids already have carcinoid syndrome and hepatic or pulmonary metastatic involvement at initial presentation. Localized sporadic carcinoids should be treated with radical gastrectomy.
The majority of carcinoids caused by hypergastrinemia occur in association with either pernicious anemia (75%) or Zollinger-Ellison syndrome (5%). Carcinoids associated with Zollinger-Ellison syndrome occur almost exclusively in patients with MEN 1, in which loss of 11q13 has been reported. Carcinoids caused by hypergastrinemia tend to be multicentric, less than 1 cm in size, and have a low potential for metastatic spread or development of carcinoid syndrome. Small lesions may be successfully treated with endoscopic resection followed by periodic endoscopic surveillance. Antrectomy reduces serum gastrin levels and may lead to regression of small tumors. Patients with large or multiple carcinoids should undergo surgical tumor resection.
Modlin IM et al: Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717.
4. Mesenchymal Tumors
Gastrointestinal mesenchymal tumors occur throughout the gastrointestinal tract, but approximately two-thirds occur in the stomach. These tumors (which include stromal tumors, leiomyomas, and schwannomas) derive from mesenchymal stem cells and have an epithelioid or spindle cell histologic pattern, resembling smooth muscle. The most common stromal tumors are gastrointestinal stromal tumors ( GIST ), which appear to originate from interstitial cells of Cajal, and which have a mutation in the protooncogene c-kit tyrosine kinase that leads to constitutive activation. Thus, stromal tumors stain positively for CD117 (part of the c-kit protein); most also stain positively for CD34. Other mesenchymal tumors such as leiomyomas, which derive from smooth muscle cells, stain negative for CD117. Mesenchymal tumors may grow quite large before causing symptoms, mainly acute or chronic bleeding due to central ulceration within the tumor. At endoscopy, they appear as a submucosal mass that may have central umbilication or ulceration. EUS (possibly with guided FNA biopsy) is the optimal study for diagnosing mesenchymal tumors and distinguishing them from other submucosal lesions. However, it is difficult to distinguish with certainty benign from malignant (sarcoma) tumors by EUS appearance, FNA, or even by histologic specimens obtained at surgery. Lesions that are smaller than 3 cm, have a smooth border, and have a homogeneous echo pattern on EUS are more likely benign.
Surgery is recommended for all patients with tumors that are symptomatic, 3 cm, are increasing in size, or have an EUS appearance suspicious for malignancy. The management of asymptomatic benign-appearing lesions 1 3 cm in size is problematic. Because of a low risk of malignancy, surgical resection should be considered in younger, otherwise healthy patients; however, other patients may be followed up with serial EUS examinations or, in selected cases, endoscopic resections. There is a high risk of metastasis or recurrence when tumors are larger than 5 cm, have an irregular border or cystic spaces, and have increased mitotic activity (more than five mitoses per high-power field). These high-risk tumors have a 50% 5-year survival rate after complete surgical resection. Metastatic tumors are aggressive and carry a poor prognosis. The tyrosine kinase inhibitor imatinib induces partial response and clinical improvement in up to half of patients with metastatic disease.
Davila R et al: GI stromal tumors. Gastrointest Endosc 2003;58:80.
Hwang JH et al: The incidental upper gastrointestinal subepithelial mass. Gastroenterology 2004;126:301.
Lograno R et al: Recent advances in cell biology, diagnosis, and therapy of gastrointestinal stromal tumor (GIST). Cancer Cell Biol 2004;3:251.
Van Glabbeke M et al: Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 2005;20:5795.
Diseases of the Small Intestine
Malabsorption
The term malabsorption denotes disorders in which there is a disruption of digestion and nutrient absorption. The clinical and laboratory manifestations of malabsorption are summarized in Table 14-12.
Table 14-12. Clinical and laboratory manifestations of malabsorption. | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Normal Digestion
Normal digestion and absorption may be divided into three phases.
A. Intraluminal Phase
Dietary fats, proteins, and carbohydrates are hydrolyzed and solubilized by pancreatic and biliary secretions. Fats are broken down by pancreatic lipase to monoglycerides and fatty acids that form micelles with bile salts. Micelles are important for the solubilization and absorption of fat-soluble vitamins (A, D, E, K). Proteins are hydrolyzed by pancreatic proteases to di- and tripeptides and amino acids.
P.614
Impaired intraluminal digestion may be caused by insufficient intraluminal concentrations of pancreatic enzymes or bile salts. These conditions will not be covered in detail here (see Chapter 15).
Pancreatic insufficiency may be caused by chronic pancreatitis, cystic fibrosis, or pancreatic cancer. Pancreatic enzymes may also be inactivated within the intestinal lumen by acid hypersecretion (Zollinger-Ellison syndrome). Significant pancreatic enzyme insufficiency generally results in significant steatorrhea (due to malabsorption of triglycerides) often more than 20 40 g/24 h resulting in weight loss, gaseous distention and flatulence, and large, greasy, foul-smelling stools. The digestion of proteins and carbohydrates is affected to a far lesser degree and is generally not clinically significant. Because micellar function and intestinal absorption are normal, signs of other nutrient or vitamin deficiencies are rare.
Decreased bile salt concentrations may be due to biliary obstruction or cholestatic liver diseases. Because bile salts are resorbed in the terminal ileum, resection or disease of this area (eg, Crohn's disease) can lead to insufficient intraluminal bile salts. Finally, destruction or loss of bile salts may be caused by bacterial overgrowth, massive acid hypersecretion, or medications that bind bile salts (eg, cholestyramine). (Bacterial overgrowth is discussed below.)
Insufficient concentrations of intraluminal bile salts lead to mild steatorrhea (due to malabsorption of fatty acids and monoglycerides), though generally less than 20 g/d. Weight loss is minimal. Impaired absorption of fat-soluble vitamins (A, D, E, K) is common, resulting in bleeding tendencies, osteoporosis, and hypocalcemia (Table 14-12). Other nutrient absorption is intact. Intestinal loss of bile salts into the colon may cause a watery secretory diarrhea.
B. Mucosal Phase
The mucosal phase requires a sufficient surface area of intact small intestinal epithelium. Brush border enzymes are important in the hydrolysis of disaccharides and di- and tripeptides. Malabsorption of specific nutrients may occur as a result of deficiency in an isolated brush border enzyme. With the exception of lactase deficiency, these are rare congenital disorders that are evident in childhood. Malabsorption due to primary mucosal diseases, extensive intestinal resections (short bowel syndrome), or lymphoma is discussed below. These disorders result in malabsorption of all nutrients: fats, proteins, and amino acids. Depending on the severity of malabsorption, patients may manifest a number of symptoms and signs, as outlined in Table 14-12.
C. Absorptive Phase
Obstruction of the lymphatic system results in impaired absorption of chylomicrons and lipoproteins.
P.615
This may lead to steatorrhea and significant enteric protein losses or protein-losing enteropathy, discussed below.
1. Celiac Disease
![]() Essentials of Diagnosis
Essentials of Diagnosis
Typical symptoms: weight loss, chronic diarrhea, abdominal distention, growth retardation.
Atypical symptoms: dermatitis herpetiformis, iron deficiency anemia, osteoporosis.
Abnormal serologic test results.
Abnormal small bowel biopsy.
Clinical improvement on gluten-free diet.
General Considerations
Also known as gluten enteropathy or celiac sprue, celiac disease is characterized by diffuse damage to the proximal small intestinal mucosa that results in malabsorption of most nutrients. Although typical symptoms may manifest between 6 months and 24 months of age after the introduction of weaning foods, the majority of cases present with atypical symptoms in childhood or adulthood. Population screening with serologic tests suggests that the disease is present in 1:100 whites of Northern European ancestry, in whom a clinical diagnosis of celiac disease is made in only 10%, suggesting that most cases are undiagnosed or asymptomatic. Celiac disease only develops in people with the HLA-DQ2 (80%) or -DQ8 (20%) class II molecules, which are present in up to 50% of the population. Although the precise mechanism of damage is unknown, it is clear that removal of gluten from the diet results in resolution of symptoms and intestinal healing in most patients. Glutens are storage proteins that are present in certain grains such as wheat, rye, and barley but not oats, rice, or corn. It is hypothesized that in a small number of genetically susceptible dietary gluten stimulates an inappropriate T cell-mediated autoimmune response in the intestinal submucosa that results in destruction of mucosal enterocytes. One target of this autoimmune response is tissue transglutaminase (tTG), an enzyme that modifies a component of gluten (gliadin) to a form that more strongly stimulates T cells.
Clinical Findings
The most important step in diagnosing celiac disease is to consider the diagnosis. Symptoms are present for more than 10 years in most adults before the correct diagnosis is established. Because of its protean manifestations, celiac disease is grossly underdiagnosed in the adult population.
A. Symptoms and Signs
The gastrointestinal symptoms and signs of celiac disease depend on the length of small intestine involved and the patient's age when the disease presents. Typical symptoms of malabsorption, including diarrhea, steatorrhea, weight loss, abdominal distention, weakness, muscle wasting, or growth retardation, more commonly present in infants (< 2 years). Older children and adults are less likely to manifest signs of serious malabsorption. They may report chronic diarrhea, dyspepsia, or flatulence due to colonic bacterial digestion of malabsorbed nutrients, but the severity of weight loss is variable. Many adults have minimal or no gastrointestinal symptoms but present with extraintestinal manifestations, including fatigue, depression, anemia, osteoporosis, short stature, delayed puberty, amenorrhea or reduced fertility, dental enamel hypoplasia, or neurologic symptoms (epilepsy, peripheral neuropathy, ataxia). Approximately 40% of patients with positive serologic tests consistent with sprue have no symptoms of disease; the natural history of these patients with silent sprue is unclear.
Physical examination may be normal in mild cases or may reveal signs of malabsorption such as loss of muscle mass or subcutaneous fat, pallor due to anemia, easy bruising due to vitamin K deficiency, hyperkeratosis due to vitamin A deficiency, bone pain due to osteomalacia, or neurologic signs (peripheral neuropathy, ataxia) due to vitamin B12 or vitamin E deficiency. Abdominal examination may reveal distention with hyperactive bowel sounds.
Dermatitis herpetiformis is regarded as a cutaneous variant of celiac disease. It is a characteristic skin rash consisting of pruritic papulovesicles over the extensor surfaces of the extremities and over the trunk, scalp, and neck. Dermatitis herpetiformis occurs in less than 10% of patients with celiac disease; however, almost all patients who present with dermatitis herpetiformis have evidence of celiac disease on intestinal mucosal biopsy, though it may not be clinically evident.
B. Laboratory Findings
1. Routine laboratory tests
Depending on the severity of illness and the extent of intestinal involvement, nonspecific laboratory abnormalities may be present that may raise the suspicion of malabsorption and celiac disease. Limited proximal involvement may result only in microcytic anemia due to iron deficiency. More than 10% of adults with iron deficiency not due to gastrointestinal blood loss may have undiagnosed celiac disease. More extensive involvement results in a megaloblastic anemia due to folate or vitamin B12 deficiency. Low serum calcium or elevated alkaline phosphatase may reflect impaired calcium or vitamin D absorption with osteomalacia or osteoporosis. Dual-energy x-ray densitometry scanning is recommended for all patients with sprue to screen for osteoporosis. Elevations of prothrombin time, or
P.616
decreased vitamin A or D levels reflect impaired fat-soluble vitamin absorption. A low serum albumin may reflect small intestine protein loss or poor nutrition. Severe diarrhea may result in a nonanion gap acidosis and hypokalemia. Mild elevations of aminotransferases are found in up to 40%.
2. Serologic tests
Serologic tests should be performed in all patients in whom there is a suspicion of celiac disease. The two tests with the highest diagnostic accuracy are the IgA endomysial antibody and IgA tTG antibody tests, both of which have a 90% sensitivity and 95% specificity for the diagnosis of celiac disease. A negative test reliably excludes the diagnosis of celiac disease. Antigliadin antibodies are no longer recommended because of their lower sensitivity and specificity. Because up to 3% of patients with celiac disease have IgA deficiency, an IgA level should be obtained. For the subset of patients with IgA deficiency, IgG tTG or endomysial antibodies can be obtained. Levels of all antibodies become undetectable after 6 12 months of dietary gluten withdrawal and may be used to monitor dietary compliance, especially in patients whose symptoms fail to resolve after institution of a gluten-free diet.
C. Mucosal Biopsy
Endoscopic mucosal biopsy of the distal duodenum or proximal jejunum is the standard method for confirmation of the diagnosis in patients with a positive serologic test for celiac disease. Rarely, mucosal biopsy may be pursued in patients with negative serologies when symptoms and laboratory studies are suggestive of celiac disease. At endoscopy, atrophy or scalloping of the duodenal folds may be observed. Histology reveals loss or blunting of intestinal villi, hypertrophy of the intestinal crypts, and extensive infiltration of the lamina propria with lymphocytes and plasma cells. An adequate normal biopsy excludes the diagnosis. Reversion of these abnormalities on repeat biopsy after a patient is placed on a gluten-free diet establishes the diagnosis. However, if a patient with a compatible biopsy demonstrates prompt clinical improvement on a gluten-free diet and a decrease in antigliadin antibodies, a repeat biopsy is unnecessary.
Differential Diagnosis
Many patients with chronic diarrhea or flatulence are erroneously diagnosed as having irritable bowel syndrome. Celiac sprue must be distinguished from other causes of malabsorption, as outlined above. Severe panmalabsorption of multiple nutrients is almost always caused by mucosal disease. Other causes of steatorrhea include pancreatic insufficiency, reduced bile salts, bacterial overgrowth, or lymphatic obstruction. The histologic appearance of celiac sprue may resemble other mucosal diseases such as tropical sprue, bacterial overgrowth, cow's milk intolerance, viral gastroenteritis, eosinophilic gastroenteritis, and mucosal damage caused by acid hypersecretion associated with gastrinoma. Documentation of clinical response to gluten withdrawal therefore is essential to the diagnosis.
Treatment
Removal of all gluten from the diet is essential to therapy all wheat, rye, and barley must be eliminated. Although oats appear to be safe, commercial products may be contaminated with wheat or barley during processing. Because of the pervasive use of gluten products in manufactured foods and additives, in medications, and by restaurants, it is imperative that patients and their families confer with a knowledgeable dietitian to comply satisfactorily with this lifelong diet. Several excellent dietary guides and patient support groups are available. Most patients with celiac disease also have lactose intolerance either temporarily or permanently and should avoid dairy products until the intestinal symptoms have improved on the gluten-free diet. Dietary supplements (folate, iron, calcium, and vitamins A, B12, D, and E) should be provided in the initial stages of therapy but usually are not required long-term with a gluten-free diet. Patients with confirmed osteoporosis may require long-term calcium, vitamin D, and bisphosphonate therapy.
Improvement in symptoms should be evident within a few weeks on the gluten-free diet. The most common reason for treatment failure is incomplete removal of gluten.
Prognosis & Complications
If appropriately diagnosed and treated, patients with celiac disease have an excellent prognosis. Celiac disease may be associated with other autoimmune disorders, including Addison's disease, Graves' disease, type 1 diabetes mellitus, myasthenia gravis, scleroderma, Sj gren's syndrome, atrophic gastritis, and pancreatic insufficiency. In some patients, celiac disease may evolve and become refractory to the gluten-free diet. The most common cause is intentional or unintentional dietary noncompliance, which may be suggested by positive serologic tests. Celiac disease that is truly refractory to gluten withdrawal generally carries a poor prognosis. It may be caused by the development of ulcerative jejunitis or rarely of enteropathy associated T cell lymphoma. These conditions should be considered in patients previously responsive to the gluten-free diet in whom new weight loss, abdominal pain, and malabsorption develop. Many other patients with refractory symptoms have a cryptic intestinal lymphoma, ie, a monoclonal expansion of the intraepithelial T lymphocyte that may or may not progress. Patients with refractory sprue who do not have intestinal T cell lymphoma or ulcerative jejunitis may respond to corticosteroids or immunosuppression with azathioprine or cyclosporine.
Celiac Disease Foundation, 13251 Ventura Blvd, Suite #1, Studio City, CA 91604 1838; http://www.celiac.org
Celiac Disease and Gluten-Free Support Page: http://www.celiac.com
Chand N et al: Celiac disease. Current concepts in diagnosis and treatment. J Clin Gastroenterol 2006;40:3.
National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28 30, 2004: Gastroenterology 2005;128:(4 Suppl 1):S1.
P.617
2. Whipple's Disease
![]() Essentials of Diagnosis
Essentials of Diagnosis
Multisystemic disease.
Fever, lymphadenopathy, arthralgias.
Weight loss, malabsorption, chronic diarrhea.
Duodenal biopsy with periodic acid-Schiff (PAS)-positive macrophages with characteristic bacillus.
General Considerations
Whipple's disease is a rare multisystemic illness caused by infection with the bacillus Tropheryma whippelii. It may occur at any age but most commonly affects white men in the fourth to sixth decades. The source of infection is unknown, but no cases of human-to-human spread have been documented.
Clinical Findings
A. Symptoms and Signs
The clinical manifestations are protean. Arthralgias or a migratory, nondeforming arthritis occurs in 80% and is typically the first symptom experienced. Gastrointestinal symptoms occur in approximately 75% of cases. They include abdominal pain, diarrhea, and some degree of malabsorption with distention, flatulence, and steatorrhea. Weight loss is the most common presenting symptom seen in almost all patients. Loss of protein due to intestinal or lymphatic involvement may result in protein-losing enteropathy with hypoalbuminemia and edema. In the absence of gastrointestinal symptoms, the diagnosis often is delayed for several years. Intermittent low-grade fever occurs in over 50% of cases. Chronic cough is common. There may be generalized lymphadenopathy that resembles sarcoidosis. Myocardial or valvular involvement may lead to congestive failure or valvular regurgitation. Ocular findings include uveitis, vitreitis, keratitis, retinitis, and retinal hemorrhages. Central nervous system involvement in approximately 10% of cases is manifested by a variety of findings such as dementia, lethargy, coma, seizures, myoclonus, or hypothalamic signs. Cranial nerve findings include ophthalmoplegia or nystagmus.
Physical examination may reveal hypotension (a late finding), low-grade fever, and evidence of malabsorption (see Table 14-12). Lymphadenopathy is present in 50%. Heart murmurs due to valvular involvement may be evident. Peripheral joints may be enlarged or warm, and peripheral edema may be present. Neurologic findings are cited above. Hyperpigmentation on sun-exposed areas is evident in up to 40%.
B. Laboratory Findings
If significant malabsorption is present, patients may have laboratory abnormalities as outlined in Table 14-12. There may be steatorrhea.
C. Histologic Evaluation
In most cases, the diagnosis of Whipple's disease is established by endoscopic biopsy of the duodenum with histologic evaluation, which demonstrates infiltration of the lamina propria with PAS-positive macrophages that contain gram-positive bacilli (which are not acid-fast) and dilation of the lacteals. The Whipple bacillus has a characteristic trimellar wall appearance on electron microscopy. In some patients who present with nongastrointestinal symptoms, the duodenal biopsy may be normal, and biopsy of other involved organs or lymph nodes may be necessary. Because the PAS stain is less sensitive and specific for extraintestinal Whipple's disease, polymerase chain reaction (PCR) is used to confirm the diagnosis by demonstrating the presence of 16S ribosomal RNA of T whippelii in blood, cerebrospinal fluid, vitreous fluid, synovial fluid, or cardiac valves. The sensitivity of PCR is 97% and the specificity 100%.
Differential Diagnosis
Whipple's disease should be considered in patients who present with signs of malabsorption, fever of unknown origin, lymphadenopathy, seronegative arthritis, culture-negative endocarditis, or multisystemic disease. Small bowel biopsy readily distinguishes Whipple's disease from other mucosal malabsorptive disorders, such as celiac sprue. Patients with AIDS and infection of the small intestine with Mycobacterium avium complex (MAC) may have a similar clinical and histologic picture; although both conditions are characterized by PAS-positive macrophages, they may be distinguished by the acid-fast stain, which is positive for MAC and negative for the Whipple bacillus. Other conditions that may be confused with Whipple's disease include sarcoidosis, Reiter's syndrome, familial Mediterranean fever, systemic vasculitides, Beh et's disease, intestinal lymphoma, and subacute infective endocarditis.
Treatment
Antibiotic therapy results in a dramatic clinical improvement within several weeks, even in some patients with neurologic involvement. The optimal regimen is unknown. Complete clinical response usually is evident within 1 3 months; however, relapse may occur in up to one-third of patients after discontinuation of treatment. Therefore, prolonged treatment for at least 1 year is required. Drugs that cross the blood-brain barrier are preferred. In severely ill patients, treatment should be initiated with intravenous ceftriaxone (2 g daily) for 2 weeks. Thereafter, trimethoprim-sulfamethoxazole (one double-strength tablet twice daily
P.618
for 1 year) is recommended as first-line therapy. In patients allergic to sulfonamides or resistant to therapy, long-term treatment with cephalosporins or fluoroquinolones and interferon- has been proposed. After treatment, repeat duodenal biopsies may be obtained at 6 and 12 months for histologic evaluation. The absence of PAS-positive material predicts a low likelihood of clinical relapse.
Prognosis
If untreated, the disease is fatal. Because some neurologic signs may be permanent, the goal of treatment is to prevent this progression. Patients must be followed closely after treatment for signs of symptom recurrence.
Bai JC: Whipple's disease. Clin Gastroenterol Hepatol 2004; 2:849.
Marth T et al: Whipple's disease. Lancet 2003;361:239.
3. Bacterial Overgrowth
The small intestine normally contains a small number of bacteria. Bacterial overgrowth in the small intestine of whatever cause may result in malabsorption via a number of mechanisms. Bacterial deconjugation of bile salts may lead to inadequate micelle formation, resulting in decreased fat absorption with steatorrhea. Microbial uptake of specific nutrients reduces absorption of vitamin B12 and carbohydrates. Bacterial proliferation also causes direct damage to intestinal epithelial cells and the brush border, further impairing absorption of proteins and carbohydrates. Passage of the malabsorbed bile acids and carbohydrates into the colon leads to an osmotic and secretory diarrhea.
Causes of bacterial overgrowth include (1) gastric achlorhydria (especially if other predisposing conditions are present); (2) anatomic abnormalities of the small intestine with stagnation (afferent limb of Billroth II gastrojejunostomy, small intestine diverticula, obstruction, blind loop, radiation enteritis); (3) small intestine motility disorders (scleroderma, diabetic enteropathy, chronic intestinal pseudo-obstruction); (4) gastrocolic or coloenteric fistula (Crohn's disease, malignancy, surgical resection); and (5) miscellaneous disorders (AIDS, chronic pancreatitis). Bacterial overgrowth is an important cause of malabsorption in the elderly, perhaps because of decreased gastric acidity or impaired intestinal motility.
Clinical Findings
Many patients with bacterial overgrowth are asymptomatic. Patients with severe overgrowth have symptoms and signs of malabsorption, including distention, weight loss, and steatorrhea (Table 14-12). Watery diarrhea is common. Megaloblastic anemia or neurologic signs due to vitamin B12 deficiency are common findings and may be manifest at presentation. In patients with vitamin B12 deficiency, the Schilling test is diagnostic of bacterial overgrowth if it is abnormal in phase I and II (without and with intrinsic factor) but normalizes after a course of antibiotics. Qualitative or quantitative fecal fat assessment typically is abnormal. D-Xylose absorption is also abnormal due to bacterial uptake of the carbohydrate.
Bacterial overgrowth should be considered in any patient with diarrhea, steatorrhea, weight loss, or macrocytic anemia, especially if the patient has a predisposing cause (such as prior gastrointestinal surgery). A stool collection should be obtained to corroborate the presence of steatorrhea. A small bowel barium radiography study should be obtained to look for mechanical factors predisposing to intestinal stasis. A small intestinal biopsy may be necessary to exclude other mucosal malabsorptive conditions and to detect intestinal inflammation, commonly present with symptomatic bacterial overgrowth. A specific diagnosis can be established firmly only by an aspirate and culture of proximal jejunal secretion that demonstrates over 105 organisms/mL. However, this is an invasive and laborious test that requires careful collection and culturing techniques and therefore is not available in many clinical settings. Noninvasive breath tests have been developed that are easier to perform and have a sensitivity of 60 90% compared with jejunal cultures. The [14C]xylose breath test is the most reliable. In this test, bacterial uptake and degradation of the isotope leads to the release of 14CO2, which can be measured in exhaled breath. Breath hydrogen tests with glucose or lactulose as substrate are commonly done because of their ease of use, but they have lower sensitivity (< 65%) and specificity (< 85%).
Owing to the lack of an optimal test for bacterial overgrowth, many clinicians use an empiric antibiotic trial as a diagnostic and therapeutic maneuver in patients with predisposing conditions for bacterial overgrowth who develop unexplained diarrhea or steatorrhea.
Treatment
Where possible, the anatomic defect that has potentiated bacterial overgrowth should be corrected. Otherwise, treatment as follows for 1 2 weeks with broad-spectrum antibiotics effective against enteric aerobes and anaerobes usually leads to dramatic improvement: twice daily ciprofloxacin 500 mg, norfloxacin 400 mg, or amoxicillin clavulanate 875 mg, or a combination of metronidazole 250 mg three times daily plus either trimethoprim-sulfamethoxazole (one double-strength tablet) twice daily or cephalexin 250 mg four times daily. Rifaximin 400 mg three times daily is a nonabsorbable antibiotic that also appears to be effective but has fewer side effects than the other systemically absorbed antibiotics.
In patients in whom symptoms recur off antibiotics, cyclic therapy (eg, 1 week out of 4) may be sufficient. Continuous antibiotics should be avoided, if possible, to avoid development of bacterial antibiotic resistance.
In patients with severe intestinal dysmotility, treatment with small doses of octreotide may prove to be of benefit.
P.619
Lauritano EC et al: Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005;22:31.
Romagnuolo J et al: Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol 2002;97:1113.
Singh VV et al: Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep 2003; 5:365.
4. Short Bowel Syndrome
Short bowel syndrome is the malabsorptive condition that arises secondary to removal of significant segments of the small intestine. The most common causes in adults are Crohn's disease, mesenteric infarction, radiation enteritis, volvulus, tumor resection, and trauma. The type and degree of malabsorption depend on the length and site of the resection and the degree of adaptation of the remaining bowel.
Terminal Ileal Resection
Resection of the terminal ileum results in malabsorption of bile salts and vitamin B12, which are normally absorbed in this region. Patients with low serum vitamin B12 levels, an abnormal Schilling test, or resection of over 50 cm of ileum require monthly intramuscular vitamin B12 injections. In patients with less than 100 cm of ileal resection, bile salt malabsorption stimulates fluid secretion from the colon, resulting in watery diarrhea. This may be treated with bile salt binding resins (cholestyramine, 2 4 g three times daily with meals). Resection of over 100 cm of ileum leads to a reduction in the bile salt pool that results in steatorrhea and malabsorption of fat-soluble vitamins. Treatment is with a low-fat diet and vitamins supplemented with medium-chain triglycerides, which do not require micellar solubilization. Unabsorbed fatty acids bind with calcium, reducing its absorption and enhancing the absorption of oxalate. Oxalate kidney stones may develop. Calcium supplements should be administered to bind oxalate and increase serum calcium. Cholesterol gallstones due to decreased bile salts are common also. In patients with resection of the ileocolonic valve, bacterial overgrowth may occur in the small intestine, further complicating malabsorption (as outlined above).
Extensive Small Bowel Resection
Resection of 40 50% of the total length of small intestine usually is well tolerated. A more massive resection may result in short-bowel syndrome, characterized by weight loss and diarrhea due to nutrient, water, and electrolyte malabsorption. After resection, the remaining small intestine has a remarkable ability to adapt, gradually increasing its absorptive capacity up to fourfold over 1 year. The colon also plays an important role in absorption of fluids, electrolytes, and digestion of complex carbohydrates (through bacterial fermentation to short-chain fatty acids) after small bowel resection. If the colon is preserved, 100 cm of proximal jejunum may be sufficient to maintain adequate oral nutrition with a low-fat, high complex-carbohydrate diet, though fluid and electrolyte losses may still be significant. In patients in whom the colon has been removed, at least 200 cm of proximal jejunum is typically required to maintain oral nutrition. Duodenal resection may result in folate, iron, or calcium malabsorption. Levels of other minerals such as zinc, selenium, and magnesium should be monitored. Parenteral vitamin supplementation may be necessary. Antidiarrheal agents (loperamide, 2 4 mg three times daily) slow transit and reduce diarrheal volume. Octreotide reduces intestinal transit time and fluid and electrolyte secretion. Gastric hypersecretion usually complicates intestinal resection and should be treated with proton pump inhibitors.
Patients with less than 100 200 cm of proximal jejunum remaining almost always require parenteral nutrition. Of patients who do, the estimated annual mortality rate is 2 5% per year. Death is most commonly due to TPN-induced liver disease, sepsis, or loss of venous access. Small intestine transplantation is now being performed with reported 5-year graft survival rates of 40%. Currently, it is performed chiefly in patients who develop serious problems due to parenteral nutrition.
Buchman AL: Short-bowel syndrome. Clin Gastroenterol Hepatol 2005;3:1066.
Buchman AL et al: AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124:1111.
DiBaise JK et al: Intestinal rehabilitation and short bowel syndrome: Part 2. Am J Gastroenterol 2004;99:1823.
5. Lactase Deficiency
Lactase is a brush border enzyme that hydrolyzes the disaccharide lactose into glucose and galactose. The concentration of lactase enzyme levels is high at birth but declines steadily in most people of non-European ancestry during childhood and adolescence and into adulthood. Thus, approximately 50 million people in the United States have partial to complete lactose intolerance. As many as 90% of Asian-Americans, 70% of African-Americans, 95% of Native Americans, 50% of Mexican-Americans, and 60% of Jewish Americans are lactose intolerant compared with less than 25% of white adults. Lactase deficiency may also arise secondary to other gastrointestinal disorders that affect the proximal small intestinal mucosa. These include Crohn's disease, sprue, viral gastroenteritis, giardiasis, short bowel syndrome, and malnutrition. Malabsorbed lactose is fermented by intestinal bacteria, producing gas and organic acids. The nonmetabolized lactose and organic acids result in an increased stool osmotic load with an obligatory fluid loss.
P.620
Clinical Findings
A. Symptoms and Signs
Patients have great variability in clinical symptoms, depending both on the severity of lactase deficiency and the amount of lactose ingested. Because of the nonspecific nature of these symptoms, there is a tendency for both lactose-intolerant and lactose-tolerant individuals to mistakenly attribute a variety of abdominal symptoms to lactose intolerance. Most patients with lactose intolerance can drink one or two 8 oz glasses of milk daily without symptoms if taken with food at wide intervals, though rare patients have almost complete intolerance. With mild to moderate amounts of lactose malabsorption, patients may experience bloating, abdominal cramps, and flatulence. With higher lactose ingestions, an osmotic diarrhea will result. Isolated lactase deficiency does not result in other signs of malabsorption or weight loss. If these findings are present, other gastrointestinal disorders should be pursued. Diarrheal specimens reveal an increased osmotic gap and a pH of less than 6.0.
B. Laboratory Findings
The most widely available test for the diagnosis of lactase deficiency is the hydrogen breath test. After ingestion of 50 g of lactose, a rise in breath hydrogen of greater than 20 ppm within 90 minutes is a positive test, indicative of bacterial carbohydrate metabolism. In clinical practice, many physicians prescribe an empiric trial of a lactose-free diet for 2 weeks. Resolution of symptoms (bloating, flatulence, diarrhea) is highly suggestive of lactase deficiency (though a placebo response cannot be excluded) and may be confirmed, if necessary, with a breath hydrogen study.
Differential Diagnosis
The symptoms of late-onset lactose intolerance are nonspecific and may mimic a number of gastrointestinal disorders, such as inflammatory bowel disease, mucosal malabsorptive disorders, irritable bowel syndrome, and pancreatic insufficiency. Furthermore, lactase deficiency frequently develops secondary to other gastrointestinal disorders (as listed above). Concomitant lactase deficiency should always be considered in these gastrointestinal disorders.
Treatment
The goal of treatment in patients with isolated lactase deficiency is achieving patient comfort. Patients usually find their threshold of intake at which symptoms will occur. Foods that are high in lactose include milk (12 g/cup), ice cream (9 g/cup), and cottage cheese (8 g/cup). Aged cheeses have a lower lactose content (0.5 g/oz). Unpasteurized yogurt contains bacteria that produce lactase and is generally well tolerated.
Many patients will choose simply to restrict or eliminate milk products. By spreading dairy product intake throughout the day in quantities of less than 12 g of lactose (one cup of milk), most patients can take dairy products without symptoms and do not require lactase supplements. Calcium supplementation should be considered in susceptible patients to prevent osteoporosis. Most food markets provide milk that has been pretreated with lactase, rendering it 70 100% lactose free. Lactase enzyme replacement is commercially available as a nonprescription formulation (Lactaid). Caplets of lactase may be taken with milk products, improving lactose absorption and eliminating symptoms. The number of caplets ingested depends on the degree of lactose intolerance.
Mathews SB et al: Systemic lactose intolerance: a new perspective on an old problem. Postgrad Med J 2005;81:167.
Swagerty DL Jr et al: Lactose intolerance. Am Fam Physician 2002;65:1845.
Intestinal Motility Disorders
1. Acute Paralytic Ileus
![]() Essentials of Diagnosis
Essentials of Diagnosis
Precipitating factors: surgery, peritonitis, electrolyte abnormalities, medications, severe medical illness.
Nausea, vomiting, obstipation, distention.
Minimal abdominal tenderness; decreased bowel sounds.
Plain abdominal radiography with gas and fluid distention in small and large bowel.
General Considerations
Ileus is a condition in which there is neurogenic failure or loss of peristalsis in the intestine in the absence of any mechanical obstruction. It is commonly seen in hospitalized patients as a result of (1) intra-abdominal processes such as recent gastrointestinal or abdominal surgery or peritoneal irritation (peritonitis, pancreatitis, ruptured viscus, hemorrhage); (2) severe medical illness such as pneumonia, respiratory failure requiring intubation, sepsis or severe infections, uremia, diabetic ketoacidosis, and electrolyte abnormalities (hypokalemia, hypercalcemia, hypomagnesemia, hypophosphatemia); and (3) medications that affect intestinal motility (opioids, anticholinergics, phenothiazines). Following surgery, small intestinal motility usually normalizes first (often within hours), followed by the stomach (24 48 hours), and the colon (48 72 hours).
Clinical Findings
A. Symptoms and Signs
Patients who are conscious report mild diffuse, continuous abdominal discomfort with nausea and vomiting.
P.621
Generalized abdominal distention is present with minimal abdominal tenderness but no signs of peritoneal irritation (unless due to the primary disease). Bowel sounds are diminished to absent.
B. Laboratory Findings
The laboratory abnormalities are attributable to the underlying condition. Serum electrolytes, including potassium, magnesium, phosphorus, and calcium, should be obtained to exclude abnormalities as contributing factors.
C. Imaging
Plain film radiography of the abdomen demonstrates distended gas-filled loops of small and large intestine. Air-fluid levels may be seen. Under some circumstances, it may be difficult to distinguish ileus from partial small bowel obstruction. A limited barium small bowel series or a CT scan may be useful in such instances to exclude mechanical obstruction, especially in postoperative patients.
Differential Diagnosis
Ileus must be distinguished from mechanical obstruction of the small bowel or proximal colon. Pain from small bowel mechanical obstruction is usually intermittent, cramping, and associated initially with profuse vomiting. Acute gastroenteritis, acute appendicitis, and acute pancreatitis may all present with ileus.
Treatment
The primary medical or surgical illness that has precipitated adynamic ileus should be treated. Most cases of ileus respond to restriction of oral intake with gradual liberalization of diet as bowel function returns. Severe or prolonged ileus requires nasogastric suction and parenteral administration of fluids and electrolytes.
Behm B et al: Postoperative ileus: etiologies and interventions. Clin Gastroenterol Hepatol 2003;1:71.
Luckey A et al: Mechanisms and treatment of postoperative ileus. Arch Surg 2003;138:206.
Taguchi A et al: Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med 2001;345:935.
2. Acute Colonic Pseudo-obstruction (Ogilvie's Syndrome)
![]() Essentials of Diagnosis
Essentials of Diagnosis
Severe abdominal distention.
Arises in postoperative state or with severe medical illness.
May be precipitated by electrolyte imbalances, medications.
Absent to mild abdominal pain; minimal tenderness.
Massive dilation of cecum or right colon.
General Considerations
Spontaneous massive dilation of the cecum and proximal colon may occur in a number of different settings in hospitalized patients. Progressive cecal dilation may lead to spontaneous perforation with dire consequences. The risk of perforation correlates poorly with absolute cecal size and duration of colonic distention. Early detection and management are important to reduce morbidity and mortality. Colonic pseudo-obstruction is most commonly detected in postsurgical patients (mean 3 5 days), after trauma, and in medical patients with respiratory failure, metabolic imbalance, malignancy, myocardial infarction, congestive heart failure, pancreatitis, or a recent neurologic event (stroke, subarachnoid hemorrhage, trauma). Liberal use of opioids or anticholinergic agents may precipitate colonic pseudo-obstruction in susceptible patients. It may also occur as a manifestation of colonic ischemia. The etiology of colonic pseudo-obstruction is unknown, but either an increase in gut sympathetic activity or a decrease in sacral parasympathetic activity of the distal colon, or both, is hypothesized to impair colonic motility.
Clinical Findings
A. Symptoms and Signs
Many patients are on ventilatory support or are unable to report symptoms due to altered mental status. Abdominal distention is frequently noted by the clinician as the first sign, often leading to a plain film radiograph that demonstrates colonic dilation. Some patients are asymptomatic, although most report constant but mild abdominal pain. Nausea and vomiting may be present. Bowel movements may be absent, but up to 40% of patients continue to pass flatus or stool. Abdominal tenderness with some degree of guarding or rebound tenderness may be detected; however, signs of peritonitis are absent unless perforation has occurred. Bowel sounds may be normal or decreased.
B. Laboratory Findings
Laboratory findings reflect the underlying medical or surgical problems. Serum sodium, potassium, magnesium, phosphorus, and calcium should be obtained. Significant fever or leukocytosis raises concern for colonic ischemia or perforation.
C. Imaging
Radiographs demonstrate colonic dilation, usually confined to the cecum and proximal colon. The upper limits of normal for cecal size is 9 cm. A cecal diameter
P.622
greater than 10 12 cm is associated with an increased risk of colonic perforation. Varying amounts of small intestinal dilation and air-fluid levels due to adynamic ileus may be seen. Because the dilated appearance of the colon may raise concern that there is a distal colonic mechanical obstruction due to malignancy, volvulus, or fecal impaction, a CT scan or water-soluble (diatrizoate meglumine) enema may sometimes be performed.
Differential Diagnosis
Colonic pseudo-obstruction should be distinguished from distal colonic mechanical obstruction (as above) and toxic megacolon, which is acute dilation of the colon due to inflammation (inflammatory bowel disease) or infection (C difficile-associated colitis, CMV). Patients with toxic megacolon manifest fever; dehydration; significant abdominal pain; leukocytosis; and diarrhea, which is often bloody.
Treatment
Conservative treatment is the appropriate first step for patients with no or minimal abdominal tenderness, no fever, no leukocytosis, and a cecal diameter less than 12 cm. The underlying illness is treated appropriately. A nasogastric tube and a rectal tube should be placed. Patients should be ambulated or periodically rolled from side to side and to the knee-chest position in an effort to promote expulsion of colonic gas. All drugs that reduce intestinal motility, such as opioids, anticholinergics, and calcium channel blockers, are discontinued if possible. Enemas may be administered judiciously if large amounts of stool are evident on radiography. Oral laxatives are not helpful and may cause perforation, pain, or electrolyte abnormalities.
Conservative treatment is successful in over 80% of cases within 1 2 days. Patients must be watched for signs of worsening distention or abdominal tenderness. Cecal size should be assessed by abdominal radiographs every 12 hours. Intervention should be considered in patients with any of the following: (1) no improvement or clinical deterioration after 24 48 hours of conservative therapy; (2) cecal dilation > 10 cm for a prolonged period (> 3 4 days); (3) patients with cecal dilation > 12 cm. Neostigmine injection should be given unless contraindicated. A single dose (2 mg intravenously) results in rapid (within 30 minutes) colonic decompression in 75 90% of patients. Cardiac monitoring during neostigmine infusion is indicated for possible bradycardia that may require atropine administration. Colonoscopic decompression is indicated in patients who fail to respond to neostigmine. Colonic decompression with aspiration of air or placement of a decompression tube is successful in 70% of patients. However, the procedure is technically difficult in an unprepared bowel and has been associated with perforations in the distended colon. Dilation recurs in up to 50% of patients. In patients in whom colonoscopy is unsuccessful, a tube cecostomy can be created through a small laparotomy or with percutaneous radiologically guided placement.
Prognosis
In most cases, the prognosis is related to the underlying illness. The risk of perforation or ischemia is increased with cecal diameter > 12 cm and when distention has been present for more than 6 days. With aggressive therapy, the development of perforation is unusual.
Kahi CJ et al: Bowel obstruction and pseudo-obstruction. Gastroenterol Clin North Am 2003;32:1229.
Saunders MD et al: Systematic review: acute colonic pseudo-obstruction. Aliment Pharmcol Ther 2005;22:917.
3. Chronic Intestinal Pseudo-obstruction & Gastroparesis
Gastroparesis and chronic intestinal pseudo-obstruction are chronic conditions characterized by intermittent, waxing and waning symptoms and signs of gastric or intestinal obstruction in the absence of any mechanical lesions to account for the findings. They are caused by a heterogeneous group of endocrine disorders (diabetes mellitus, hypothyroidism, cortisol deficiency), postsurgical conditions (vagotomy, partial gastric resection, fundoplication, gastric bypass, Whipple procedure), neurologic conditions (Parkinson's disease, muscular and myotonic dystrophy, autonomic dysfunction, multiple sclerosis, postpolio syndrome, porphyria), rheumatologic syndromes (progressive systemic sclerosis), infections (postviral, Chagas' disease), amyloidosis, paraneoplastic syndromes, medications, and eating disorders (anorexia); a cause may not always be identified. Gastric involvement leads to chronic or intermittent symptoms of gastroparesis with early satiety, nausea, and postprandial vomiting (1 3 hours after meals).
Patients with predominantly small bowel involvement may have abdominal distention, vomiting, diarrhea, and varying degrees of malnutrition. Abdominal pain is not common and should prompt investigation for structural causes of obstruction. Bacterial overgrowth in the stagnant intestine may result in malabsorption. Colonic involvement may result in constipation or alternating diarrhea and constipation.
Plain film radiography may demonstrate dilation of the esophagus, stomach, small intestine, or colon resembling ileus or mechanical obstruction. Mechanical obstruction of the stomach, small intestine, or colon is much more common than gastroparesis or intestinal pseudo-obstruction and must be excluded with endoscopy or barium radiography (upper gastrointestinal series with small bowel follow-through), especially in patients with prior surgery, recent onset of symptoms, or abdominal pain. In cases of unclear origin, studies
P.623
based on the clinical picture are obtained to exclude underlying systemic disease. Gastric scintigraphy with a low-fat solid meal is the optimal means for assessing gastric emptying. Gastric retention of 60% after 2 hours or more than 10% after 4 hours is abnormal. Small bowel manometry is useful for distinguishing visceral from myopathic disorders and for excluding cases of mechanical obstruction that are otherwise difficult to diagnose by endoscopy or radiographic studies.
There is no specific therapy for gastroparesis or pseudo-obstruction. Acute exacerbations are treated with nasogastric suction and intravenous fluids. Long-term treatment is directed at maintaining nutrition. Patients should eat small, frequent meals that are low in fiber, milk, gas-forming foods, and fat. Some patients may require liquid enteral supplements. Agents that reduce gastrointestinal motility (opioids, anticholinergics) should be avoided. In diabetics, glucose levels should be maintained below 200 mg/dL, as hyperglycemia may slow gastric emptying even in the absence of diabetic neuropathy. Metoclopramide (5 20 mg orally or 5 10 mg intravenously or subcutaneously four times daily) and erythromycin (50 125 mg orally three times daily) before meals are of benefit in treatment of gastroparesis but not small bowel dysmotility. The 5-HT4-receptor agonist, tegaserod 6 12 mg twice daily, enhances gastric emptying and colonic motility but has undergone limited testing for gastroparesis or chronic intestinal pseudo-obstruction. Unblinded studies in small numbers of patients with diabetic or idiopathic gastroparesis report improvement in symptoms and gastric emptying after injection of botulinum toxin into the pylorus, which is hypothesized to reduce pyloric spasm. Gastric pacing with internally implanted neurostimulators has shown benefit in small studies of patients with severe gastroparesis. Bacterial overgrowth should be treated with intermittent antibiotics (see above). Patients with predominant small bowel distention may require a venting gastrostomy to relieve distress. Some patients may require placement of a jejunostomy for long-term enteral nutrition. Patients unable to maintain adequate enteral nutrition require TPN or small bowel transplantation. Difficult cases should be referred to centers with expertise in this area.
Abell T et al: Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 2003;125:421.
Friedenberg FK et al: Management of delayed gastric empyting. Clin Gastroenterol Hepatol 2005;3:642.
Jones M et al: A systematic review of surgical therapy for gastroparesis. Am J Gastroenterol 2003;98:2122.
Maganti K et al: Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Gastroenterology 2003;98: 259.
Panganamamula KV et al: Chronic intestinal pseudo-obstruction. Curr Treat Options Gastroenterol 2005;8:3.
Parkman H et al: American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology 2004;127:1589.
Talley NJ: Diabetic gastropathy and prokinetics. Am J Gastroenterol 2003;98:264.
Tumors of the Small Intestine
Benign and malignant tumors of the small intestine are rare. They often cause no symptoms or signs. However, they may cause acute gastrointestinal bleeding with hematochezia or melena or chronic gastrointestinal blood loss resulting in fatigue and iron deficiency anemia. Small bowel tumors may cause obstruction due to luminal narrowing or intussusception of a polypoid mass. Small bowel tumors usually are identified by barium radiographic studies, either enteroclysis or a small bowel series. Visualization and biopsy of duodenal and proximal jejunal mass lesions are performed with a long upper endoscope known as an enteroscope.
1. Benign Tumors of Small Intestine
Benign polyps may be symptomatic or may be incidental findings detected on endoscopy or radiographic study. Most occur singly, and the presence of multiple polyps is suggestive of hereditary polyposis syndrome (discussed under Diseases of the Colon and Rectum). With the exception of lipomas, surgical or endoscopic excision usually is recommended. Adenomatous polyps are the most common benign mucosal tumor. The majority are asymptomatic, though acute or chronic bleeding may occur. Because malignant transformation does occur, endoscopic or surgical resection is warranted. Villous adenomas occur most commonly in the periampullary region of the duodenum (especially in patients with familial adenomatous polyposis) and carry a high risk for development of invasive cancer. Periodic endoscopic surveillance to detect early ampullary neoplasms is recommended in patients with familial adenomatous polyposis. Evaluation with endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound is required to exclude invasive carcinoma. Ampullary adenomas may be removed by endoscopic or surgical techniques. Lipomas occur commonly in the ileum. Most are asymptomatic and identified incidentally at endoscopy or radiography; however, they rarely may cause obstruction with intussusception. Benign stromal tumors (formerly called leiomyomas) are found at all levels of the intestine. These submucosal mesenchymal lesions may be intraluminal, intramural, or extraluminal. Although most are asymptomatic, they may ulcerate and cause acute or chronic bleeding or obstruction. It is difficult to distinguish benign from malignant stromal tumors (leiomyosarcomas) except by excision.
Catalano M et al: Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc 2004;59:225.
P.624
2. Malignant Tumors of Small Intestine
Malignant tumors of the small intestine are extremely rare, accounting for less than 2% of all gastrointestinal malignancies. They may present with anemia, bleeding, obstruction, or evidence of metastatic disease.
Adenocarcinoma
These are aggressive tumors that occur most commonly in the duodenum or proximal jejunum. The ampulla of Vater is the most common site of small bowel carcinoma, and the incidence of ampullary carcinoma is increased more than 200-fold in patients with familial adenomatous polyposis. Ampullary carcinoma may present with jaundice due to bile duct obstruction or bleeding. Surgical resection of early lesions is curative in up to 40% of patients. Periodic endoscopic surveillance to detect early ampullary neoplasms therefore is recommended in patients with this disorder. Nonampullary adenocarcinoma of the small intestine accounts for 30 40% of small bowel cancers. Most cases present with symptoms of obstruction, acute or chronic bleeding, or weight loss. Eighty percent of small bowel cancers have already metastasized at the time of diagnosis. Resection is recommended for control of symptoms. Patients with Crohn's disease have an increased risk of small intestine adenocarcinoma, most commonly in the ileum, which may be difficult to distinguish from disease-related fibrous strictures.
Lymphoma
Gastrointestinal lymphomas may arise in the gastrointestinal tract or involve it secondarily with disseminated disease. In Western countries, primary gastrointestinal lymphomas account for 5% of lymphomas and 20% of small bowel malignancies. They occur most commonly in the distal small intestine. The majority are non-Hodgkin's intermediate or high-grade B cell lymphomas. However, T cell lymphomas may arise in patients with celiac sprue. In the Middle East, lymphomas may arise also in the setting of immunoproliferative small intestinal disease. In this condition, there is diffuse lymphoplasmacytic infiltration with IgA-secreting B lymphocytes of the mucosa and submucosa that results in weight loss, diarrhea, and malabsorption, which may lead to lymphomatous transformation. A characteristic feature of the disease is the presence of heavy chains in the serum in 70% produced by clones of IgA plasma cells. The early, premalignant phase may respond to antibiotic therapy for H pylori alone (Table 14-11).
Presenting symptoms or signs of primary lymphoma include abdominal pain, weight loss, nausea and vomiting, distention, anemia, and occult blood in the stool. Fevers are unusual. Protein-losing enteropathy may result in hypoalbuminemia, but other signs of malabsorption are unusual. Barium radiography helps to localize the site of the lesion. The diagnosis requires endoscopic, percutaneous, or laparoscopic biopsy. To determine tumor stage, patients must undergo chest and abdominal CT, bone marrow biopsy, and, in some cases, lymphangiography.
Treatment depends on the stage of disease. Resection of primary intestinal lymphoma, if feasible, is usually recommended. Even in cases of stage III or stage IV disease, surgical debulking may improve survival. In patients with limited disease (stage IE) in whom resection is performed, the role of adjuvant chemotherapy is unclear. Most patients with more extensive disease are treated with systemic chemotherapy with or without radiation therapy (see Chapter 40).
Carcinoid Tumors
Gastrointestinal carcinoids are slow growing neuroendocrine tumors that may arise anywhere in the gastrointestinal tract but most commonly occur in the small intestine (30%), rectum (12%), colon (8%), appendix (8%), and stomach (10 30%; see above). Carcinoids have intracytoplasmic electron-dense secretory granules that may contain a variety of hormones, including serotonin, somatostatin, gastrin, and substance P (which may or may not be secreted), and usually display immunoreactivity to chromogranin A. Although many carcinoids behave in an indolent fashion, the overall 5-year survival rate for patients with carcinoids is 50%, suggesting that most are malignant. The risk of metastatic spread is closely related to tumor size and tumor location. Many small carcinoids are detected incidentally at endoscopy or autopsy. It is not possible by histologic examination to distinguish benign from malignant disease. The best indicator of prognosis is evidence of invasive growth and the presence of regional or distant metastasis.
Rectal carcinoids are usually detected incidentally as submucosal nodules during proctoscopic examination. Similarly, appendiceal carcinoids are identified in 0.3% of appendectomies. Almost 80% of these tumors are less than 1 cm in size, and 90% are less than 2 cm. Rectal carcinoids less than 1 cm and appendiceal carcinoids less than 2 cm virtually never metastasize and are treated effectively with local excision or simple appendectomy. Tumors larger than 2 cm are associated with the development of metastasis in over 20% of appendiceal carcinoids and 10% of rectal carcinoids. Hence, in younger patients who are good operative risks, a more extensive cancer resection operation is warranted.
Carcinoids account for up to one-third of small intestinal tumors. Approximately two-thirds of carcinoids of the duodenum secrete gastrin (gastrinomas) (see Zollinger-Ellison Syndrome). Small intestinal carcinoids most commonly arise in the ileum. Up to one-third are multicentric. Although 60% are less than 2 cm in size, even these small carcinoids may metastasize. Almost all tumors over 2 cm are associated with metastasis. Most smaller lesions are asymptomatic and difficult to detect by endoscopy or imaging studies. Therefore,
P.625
most carcinoids present at an advanced and incurable stage. Through local extension or metastasis to mesenteric lymph nodes, carcinoids engender a fibroblastic reaction with contraction and kinking of the bowel. This may lead to symptoms of partial small bowel obstruction with intermittent abdominal pain or obstruction. Small bowel barium studies may reveal kinking, but because the lesion is extraluminal the diagnosis may be overlooked for several years. Encasement of the mesenteric vessels can lead to bowel infarction. Carcinoid involvement of the heart (resulting in right-sided valvular lesions) is a late manifestation of metastatic disease. Carcinoid syndrome occurs in less than 10% of patients (see Chapter 40) and only in patients with hepatic metastasis. It is caused by tumor secretion of hormonal mediators that cause cramps, flushing, diarrhea, cyanosis, or bronchospasm. Almost all patients with carcinoid syndrome have obvious signs of cancer with liver metastasis on abdominal imaging.
Plasma chromogranin A (CgA) is the most sensitive screening test for small intestine carcinoids, although its sensitivity for small, localized tumors is unknown. CgA is elevated in almost 90% of patients with advanced small bowel carcinoid. Urinary 5-HIAA or platelet serotonin levels are also elevated in patients with metastatic carcinoid; however, these tests are less sensitive than CgA. Abdominal CT may demonstrate a mesenteric mass with tethering of the bowel, lymphadenopathy, and hepatic metastasis. Somatostatin receptor scintigraphy, which is positive in over 90% of patients with metastatic carcinoid, is superior to all other imaging modalities for the diagnosis of primary and metastatic lesions.
Small intestinal carcinoids are extremely indolent tumors with slow spread. Patients with disease confined to the small intestine should have local excision, for which the cure rate exceeds 85%. In patients with resectable disease who have lymph node involvement, the 5-year disease-free survival is 80%; however, by 25 years, less than 25% remain disease free. Even patients with hepatic metastases may have an indolent course with a median survival of 3 years. In patients with advanced disease, therapy should be deferred until the patient is symptomatic. Surgery should be directed toward palliation of obstructive symptoms. In patients with carcinoid syndrome or diarrhea, resection of hepatic metastases may provide dramatic improvement. The somatostatin analog octreotide (150 500 mcg subcutaneously three times daily) inhibits hormone secretion from the carcinoid tumor, resulting in dramatic relief of diarrhea and symptoms of carcinoid syndrome in 90% of patients for a median period of 1 year. Thereafter, many patients escape from octreotide control. Hepatic artery occlusion and chemotherapy may provide symptomatic improvement in some patients with hepatic metastases.
Sarcoma
Most small intestine sarcomas arise from stromal tumors that stain positive for CD117; a minority arise from smooth muscle tumors (leiomyosarcomas) (see Malignant Tumors of the Stomach: Mesenchymal Tumors). Tumors may grow quite large before causing symptoms due to obstruction, intussusception, or bleeding due to central ulceration within the tumor. Because many of these tumors cannot be reached by endoscopy for biopsy, surgery should be performed in patients with symptomatic tumors and asymptomatic tumors larger than 3 cm (in which the risk of malignancy is increased).
Kaposi's sarcoma was at one time a common complication in AIDS, but the incidence is declining with highly active antiretroviral therapy (HAART). It is strongly associated with infection with human herpesvirus 8. Lesions may be present anywhere in the intestinal tract. Visceral involvement usually is associated with cutaneous disease. Most lesions are clinically silent; however, large lesions may be symptomatic. Lesions of the gingiva, palate, and hypopharynx can lead to painful mastication and dysphagia. Lesions of the stomach or small intestine may lead to bleeding, obstruction, or even perforation. The diagnosis may be confirmed at endoscopy by the characteristic visual appearance and by biopsy. Oral complications can be treated with the CO2 laser or radiation. Limited bleeding or obstructing lesions in the stomach or anus can be treated with the YAG laser or radiation therapy. Interferon- induces regression in up to one-third of patients who have a CD4 cell count of > 200/mcL. Widespread involvement may be best treated by systemic chemotherapy using combinations of vincristine, bleomycin, or doxorubicin, to which the tumor is very responsive.
Bierman PJ: Gastrointestinal lymphoma. Curr Treat Options Oncol 2003;4:421.
Debaja BS et al: Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 2004;101:518.
Modlin IM et al: Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717.
Torres M et al: Malignant tumors of the small intestine. J Clin Gastroenterol 2003;37:372.
Appendicitis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Early: periumbilical pain; later: right lower quadrant pain and tenderness.
Anorexia, nausea and vomiting, obstipation.
Tenderness or localized rigidity at McBurney's point.
Low-grade fever and leukocytosis.
General Considerations
Appendicitis is the most common abdominal surgical emergency, affecting approximately 10% of the population. It occurs most commonly between the ages of 10
P.626
and 30 years. It is initiated by obstruction of the appendix by a fecalith, inflammation, foreign body, or neoplasm. Obstruction leads to increased intraluminal pressure, venous congestion, infection, and thrombosis of intramural vessels. If untreated, gangrene and perforation develop within 36 hours.
Clinical Findings
A. Symptoms and Signs
Appendicitis usually begins with vague, often colicky periumbilical or epigastric pain. Within 12 hours the pain shifts to the right lower quadrant, manifested as a steady ache that is worsened by walking or coughing. Almost all patients have nausea with one or two episodes of vomiting. Protracted vomiting or vomiting that begins before the onset of pain suggests another diagnosis. A sense of constipation is typical, and some patients administer cathartics in an effort to relieve their symptoms though some report diarrhea. Low-grade fever (< 38 C) is typical; high fever or rigors suggest another diagnosis or appendiceal perforation.
On physical examination, localized tenderness with guarding in the right lower quadrant can be elicited with gentle palpation with one finger. When asked to cough, patients may be able to precisely localize the painful area, a sign of peritoneal irritation. Light percussion may also elicit pain. Although rebound tenderness is also present, it is unnecessary to elicit this finding if the above signs are present. The psoas sign (pain on passive extension of the right hip) and the obturator sign (pain with passive flexion and internal rotation of the right hip) are indicative of adjacent inflammation and strongly suggestive of appendicitis.
B. Laboratory Findings
Moderate leukocytosis (10,000 20,000/mcL) with neutrophilia is common. Microscopic hematuria and pyuria are present in 25% of patients.
C. Imaging
Both abdominal ultrasound and CT scanning are useful in diagnosing appendicitis as well as excluding other diseases presenting with similar symptoms, including adnexal disease in younger women. However, CT scanning appears to be more accurate (sensitivity 94%, specificity 95%, positive likelihood ratio 13.3, negative likelihood ratio 0.09). Abdominal CT scanning is also useful in cases of suspected appendiceal perforation to diagnose a periappendiceal abscess. In patients in whom there is a clinically high suspicion of appendicitis, some surgeons feel that preoperative diagnostic imaging is unnecessary. However, studies suggest that even in this group, imaging studies suggest an alternative diagnosis in up to 15%.
Atypical Presentations of Appendicitis
Owing to the variable location of the appendix, there are a number of atypical presentations. Because the retrocecal appendix does not touch the anterior abdominal wall, the pain remains less intense and poorly localized; abdominal tenderness is minimal and may be elicited in the right flank. The psoas sign may be positive. With pelvic appendicitis there is pain in the lower abdomen, often on the left, with an urge to urinate or defecate. Abdominal tenderness is absent, but tenderness is evident on pelvic or rectal examination; the obturator sign may be present. In the elderly, the diagnosis of appendicitis is often delayed because patients present with minimal, vague symptoms and mild abdominal tenderness. Appendicitis in pregnancy may present with pain in the right lower quadrant, periumbilical area, or right subcostal area owing to displacement of the appendix by the uterus.
Differential Diagnosis
Given its frequency and myriad presentations, appendicitis should be considered in the differential diagnosis of all patients with abdominal pain. It is difficult to reliably diagnose the disease in some cases. A several-hour period of close observation with reassessment usually clarifies the diagnosis. Absence of the classic migration of pain (from the epigastrium to the right lower abdomen), right lower quadrant pain, fever, or guarding makes appendicitis less likely. Ten to 20 percent of patients with suspected appendicitis have either a negative examination at laparotomy or an alternative surgical diagnosis. The widespread use of ultrasonography and CT has reduced the number of incorrect diagnoses. Still, in some cases diagnostic laparotomy or laparoscopy is required. The most common causes of diagnostic confusion are gastroenteritis and gynecologic disorders. Viral gastroenteritis presents with nausea, vomiting, low-grade fever, and diarrhea and can be difficult to distinguish from appendicitis. The onset of vomiting before pain makes appendicitis less likely. As a rule, the pain of gastroenteritis is more generalized and the tenderness less well localized. Acute salpingitis or tubo-ovarian abscess should be considered in young, sexually active women with fever and bilateral abdominal or pelvic tenderness. A twisted ovarian cyst may also cause sudden severe pain. The sudden onset of lower abdominal pain in the middle of the menstrual cycle suggests mittelschmerz. Sudden severe abdominal pain with diffuse pelvic tenderness and shock suggests a ruptured ectopic pregnancy. A positive pregnancy test and pelvic ultrasonography are diagnostic. Retrocecal or retroileal appendicitis (often associated with pyuria or hematuria) may be confused with ureteral colic or pyelonephritis. Other conditions that may resemble appendicitis are diverticulitis, Meckel's diverticulitis, carcinoid of the appendix, perforated colonic cancer, Crohn's ileitis, perforated peptic ulcer, cholecystitis, and mesenteric adenitis. It is virtually impossible to distinguish appendicitis from Meckel's diverticulitis, but both require surgical treatment.
Complications
Perforation occurs in 20% of patients and should be suspected in patients with pain persisting for over 36
P.627
hours, high fever, diffuse abdominal tenderness or peritoneal findings, a palpable abdominal mass, or marked leukocytosis. Localized perforation results in a contained abscess, usually in the pelvis. A free perforation leads to suppurative peritonitis with toxicity. Septic thrombophlebitis (pylephlebitis) of the portal venous system is rare and suggested by high fever, chills, bacteremia, and jaundice.
Treatment
The treatment of uncomplicated appendicitis is surgical appendectomy. This may be performed through a laparotomy or by laparoscopy. Prior to surgery, patients should be given systemic antibiotics, which reduce the incidence of postoperative wound infections. Emergency appendectomy is also required in patients with perforated appendicitis with generalized peritonitis.
The optimal treatment of stable patients with perforated appendicitis and a contained abscess is controversial. Surgery in this setting can be difficult. Many recommend percutaneous CT-guided drainage of the abscess with intravenous fluids and antibiotics to allow the inflammation to subside. An interval appendectomy may be performed after 6 weeks to prevent recurrent appendicitis.
Prognosis
The mortality rate from uncomplicated appendicitis is extremely low. Even with perforated appendicitis, the mortality rate in most groups is only 0.2%, though it approaches 15% in the elderly.
Rettenbacher T et al: Appendicitis: should diagnostic imaging be performed if the clinical presentation is highly suggestive of the disease? Gastroenterology 2002;123:992.
Sauerland S et al: Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2004;(4):CD0001546.
Terasawa T et al: Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 2004;141:537.
Intestinal Tuberculosis
Intestinal tuberculosis is common in underdeveloped countries. Previously rare in the United States, its incidence has been rising in immigrant groups and patients with AIDS. It is caused by both Mycobacterium tuberculosis and M bovis. Active pulmonary disease is present in less than 50% of patients. The most frequent site of involvement is the ileocecal region; however, any region of the gastrointestinal tract may be involved. Intestinal tuberculosis may cause mucosal ulcerations or scarring and fibrosis with narrowing of the lumen. Patients may be without symptoms or complain of chronic abdominal pain, obstructive symptoms, weight loss, and diarrhea. An abdominal mass may be palpable. Complications include intestinal obstruction, hemorrhage, and fistula formation. The purified protein derivative (PPD) skin test may be negative, especially in patients with weight loss or AIDS. Barium radiography may demonstrate mucosal ulcerations, thickening, or stricture formation. Colonoscopy may demonstrate an ulcerated mass, multiple ulcers with steep edges and adjacent small sessile polyps, small ulcers or erosions, or small diverticula, most commonly in the ileocecal region. The differential diagnosis includes Crohn's disease, carcinoma, and intestinal amebiasis. The diagnosis is established by either endoscopic or surgical biopsy revealing acid-fast bacilli, caseating granuloma, or positive cultures from the organism. Detection of tubercle bacilli in biopsy specimens by PCR is now the most sensitive means of diagnosis.
Treatment with standard antituberculous regimens is effective.
Sato S et al: Colonoscopy in the diagnosis of intestinal tuberculosis. Gastrointest Endosc 2004;59:362.
Villanueva Saenz E et al: Colonic tuberculosis. Dig Dis Sci 2002; 47:2045.
Protein-Losing Enteropathy
Protein-losing enteropathy comprises a number of conditions that result in excessive loss of serum proteins into the gastrointestinal tract. The essential diagnostic features are hypoalbuminemia and an elevated fecal 1-antitrypsin level.
The normal intact gut epithelium prevents the loss of serum proteins. Proteins may be lost through one of three mechanisms: (1) mucosal disease with ulceration, resulting in the loss of proteins across the disrupted mucosal surface; (2) lymphatic obstruction, resulting in the loss of protein-rich chylous fluid from mucosal lacteals; and (3) idiopathic change in permeability of mucosal capillaries and conductance of interstitium, resulting in weeping of protein-rich fluid from the mucosal surface (Table 14-13).
Hypoalbuminemia is the sine qua non of protein-losing enteropathy. However, a number of other serum proteins such as 1-antitrypsin also are lost from the gut epithelium. In protein-losing enteropathy caused by lymphatic obstruction, loss of lymphatic fluid commonly results in lymphocytopenia (< 1000/mcL), hypoglobulinemia, and hypocholesterolemia.
In most cases, protein-losing enteropathy is recognized as a sequela of a known gastrointestinal disorder. In patients in whom the cause is unclear, evaluation is indicated and is guided by the clinical suspicion. Protein-losing enteropathy must be distinguished from other causes of hypoalbuminemia, which include liver disease and nephrotic syndrome; and from congestive heart failure. Protein-losing enteropathy is confirmed by determining the gut 1-antitrypsin clearance (24-hour volume of feces stool concentration of 1-antitrypsin serum 1-antitrypsin
P.628
concentration). A clearance of more than 13 mL/24 h is abnormal.
Table 14-13. Causes of protein-losing enteropathy. | |
|---|---|
|
Laboratory evaluation of protein-losing enteropathy includes serum protein electrophoresis, lymphocyte count, and serum cholesterol to look for evidence of lymphatic obstruction. Serum ANA and C3 levels are useful to screen for autoimmune disorders. Stool samples should be examined for ova and parasites. Evidence of malabsorption is evaluated by means of a stool qualitative fecal fat determination. Intestinal imaging is performed with an upper endoscopy with small bowel biopsy and a small bowel barium series. Colonic diseases are excluded with barium enema or colonoscopy. A CT scan of the abdomen is performed to look for evidence of neoplasms or lymphatic obstruction. Rarely, lymphangiography is helpful. In some situations, laparotomy with full-thickness intestinal biopsy is required to establish a diagnosis.
Treatment is directed at the underlying cause. Patients with lymphatic obstruction benefit from low-fat diets supplemented with medium-chain triglycerides. Case reports suggest that octreotide may lead to symptomatic and nutritional improvement in some patients.
Landzberg BR et al: Protein-losing enteropathy and gastropathy. Curr Treat Options Gastroenterol 2001;4:39.
Diseases of the Colon & Rectum
Irritable Bowel Syndrome
![]() Essentials of Diagnosis
Essentials of Diagnosis
Chronic functional disorder characterized by abdominal pain or discomfort with alterations in bowel habits.
Symptoms usually begin in late teens to early twenties.
Limited evaluation to exclude organic causes of symptoms.
General Considerations
The functional gastrointestinal disorders are characterized by a variable combination of chronic or recurrent gastrointestinal symptoms not explicable by the presence of structural or biochemical abnormalities. Several clinical entities are included under this broad rubric, including chest pain of unclear origin (noncardiac chest pain), functional dyspepsia, and biliary dyskinesia (sphincter of Oddi dysfunction). There is a large overlap among these entities. For example, over 50% of patients with noncardiac chest pain and over one-third with functional dyspepsia also have symptoms compatible with irritable bowel syndrome. In none of these disorders is there a definitive diagnostic study. Rather, the diagnosis is a subjective one based on the presence of a compatible profile and the exclusion of similar disorders.
Irritable bowel syndrome can be defined, therefore, as an idiopathic clinical entity characterized by some combination of chronic (more than 3 months) lower abdominal symptoms and bowel complaints that may be continuous or intermittent. Consensus definition of irritable bowel syndrome is abdominal discomfort or pain that has two of the following three features: (1) relieved with defecation, (2) onset associated with a change in frequency of stool, or (3) onset associated with a change in form (appearance) of stool. Other symptoms supporting the diagnosis include abnormal stool frequency (more than three bowel movements per day or fewer than three per week); abnormal stool form (lumpy or hard; loose or watery); abnormal stool passage (straining, urgency, or feeling of incomplete evacuation); passage of mucus; and bloating or a feeling of abdominal distention.
Patients may have other somatic or psychological complaints such as dyspepsia, heartburn, chest pain, headaches, fatigue, myalgias, urologic dysfunction, gynecologic symptoms, anxiety, or depression.
P.629
The disorder is a common problem presenting to both gastroenterologists and primary care physicians. Up to 20% of the adult population have symptoms compatible with the diagnosis, but most never seek medical attention. Approximately two-thirds of patients with irritable bowel syndrome are women.
Pathogenesis
A number of pathophysiologic mechanisms have been identified and may have varying importance in different individuals.
A. Abnormal Motility
A variety of abnormal myoelectrical and motor abnormalities have been identified in the colon and small intestine. In some cases, these are temporally correlated with episodes of abdominal pain or emotional stress. Whether they represent a primary motility disorder or are secondary to psychosocial stress is debated. Differences between patients with constipation-predominant and diarrhea-predominant syndromes are reported.
B. Visceral Hypersensitivity
Patients often have a lower visceral pain threshold, reporting abdominal pain at lower volumes of colonic gas insufflation or colonic balloon inflation than controls. Although many patients complain of bloating and distention, their absolute intestinal gas volume is normal. Many patients report rectal urgency despite small rectal volumes of stool.
C. Enteric Infection
Symptoms compatible with irritable bowel syndrome develop in up to 30% of patients after an episode of bacterial gastroenteritis. Women and patients with increased life stressors at the onset of gastroenteritis appear to be at increased risk for developing postinfectious irritable bowel syndrome. Increased inflammatory cells have been found in the mucosa, submucosa, and muscularis of some patients with irritable bowel syndrome, but their importance is unclear. Chronic inflammation is postulated by some investigators to contribute to alterations in motility or visceral hypersensitivity. Some recent investigations report an increase in breath hydrogen excretion after lactulose ingestion in up to 80% of patients with irritable bowel syndrome, believed to be suggestive of small intestinal bacterial overgrowth, although other investigators have not confirmed these findings. It is hypothesized that bacterial overgrowth may lead to alterations in immune alterations that affect motility or visceral sensitivity. In addition, bacterial degradation of carbohydrates in the small intestine could cause increased postprandial gas, bloating and distention, which may improve after antibiotic treatment. However, other studies dispute these findings.
D. Psychosocial Abnormalities
More than 50% of patients with irritable bowel who seek medical attention have underlying depression, anxiety, or somatization. By contrast, those who do not seek medical attention are similar psychologically to normal individuals. Psychological abnormalities may influence how the patient perceives or reacts to illness and minor visceral sensations. Chronic stress may alter intestinal motility or modulate pathways that affect central and spinal processing of visceral afferent sensation.
Clinical Findings
A. Symptoms and Signs
Irritable bowel is a chronic condition. Symptoms usually begin in the late teens to twenties. Symptoms should be present for at least 3 months before the diagnosis can be considered. The diagnosis is established in the presence of compatible symptoms and the judicious use of tests to exclude organic disease.
Abdominal pain usually is intermittent, crampy, and in the lower abdominal region. As previously stated, the onset of pain typically is associated with a change in stool frequency or form and commonly is relieved by defecation. It does not usually occur at night or interfere with sleep. Patients with irritable bowel syndrome may be classified into one of three categories: those who have predominant problems with constipation, diarrhea, or alternating constipation and diarrhea. It is important to clarify what the patient means by these complaints. Patients with irritable bowel and constipation report infrequent bowel movements (less than three per week), hard or lumpy stools, or straining. Patients with irritable bowel syndrome with diarrhea refer to loose or watery stools, frequent stools (more than three per day), urgency, or fecal incontinence. Many patients report that they have a firm stool in the morning followed by progressively looser movements. Mucus is commonly seen. Complaints of visible distention and bloating are common, though these are not always clinically evident.
The patient should be asked about alarm symptoms that suggest a diagnosis other than irritable bowel syndrome and warrant further investigation. The acute onset of symptoms raises the likelihood of organic disease, especially in patients aged > 40 50 years. Nocturnal diarrhea, severe constipation or diarrhea, hematochezia, weight loss, and fever are incompatible with a diagnosis of irritable bowel syndrome and warrant investigation for underlying disease. Patients who have a family history of cancer, inflammatory bowel disease, or celiac disease should undergo additional evaluation.
A physical examination should be performed to look for evidence of organic disease and to allay the patient's anxieties. The physical examination usually is normal. Abdominal tenderness, especially in the lower abdomen, is common but not pronounced. A new
P.630
onset of symptoms in a patient over age 40 years warrants further examination.
B. Laboratory Findings and Special Examinations
In patients whose symptoms fulfill the diagnostic criteria for irritable bowel syndrome and who have no other alarm symptoms, evidence-based consensus guidelines do not support further diagnostic testing, as the likelihood of serious organic diseases does not appear to be increased. Although the vague nature of symptoms and patient anxiety may prompt clinicians to consider a variety of diagnostic studies, overtesting should be avoided. A complete blood count, chemistry panel, and serum albumin should be obtained in most patients. However, erythrocyte sedimentation rate, thyroid function tests, stool occult blood test, stool for ova and parasites, sigmoidoscopy or colonoscopy, or barium enema in patients with symptoms of irritable bowel syndrome are not recommended for patients without alarm symptoms or abnormal hematology or chemistry studies. In patients with diarrhea, serologic tests for celiac disease may be performed. In all patients age 50 years or older who have not had a previous evaluation, barium enema or colonoscopy should be considered to exclude malignancy. Pending further clinical studies, routine testing for bacterial overgrowth cannot be recommended.
Differential Diagnosis
A number of disorders may present with similar symptoms. Examples include colonic neoplasia, inflammatory bowel disease (ulcerative colitis, Crohn's disease, microscopic colitis), hyperthyroidism or hypothyroidism, parasites, malabsorption (especially celiac disease, bacterial overgrowth, lactase deficiency), causes of chronic secretory diarrhea (carcinoid), and endometriosis. Psychiatric disorders such as depression, panic disorder, and anxiety must be considered as well. Women with refractory symptoms have an increased incidence of prior sexual and physical abuse. These diagnoses should be excluded in patients with presumed irritable bowel syndrome who do not improve within 2 4 weeks of empiric treatment or in whom subsequent alarm symptoms develop.
Treatment
A. General Measures
As with other functional disorders, the most important interventions the physician can offer are reassurance, education, and support. An ongoing therapeutic relationship may be the most important factor in successful management of this disorder. This includes identifying and responding to the patient's concerns, careful explanation of the pathophysiology and natural history of the disorder, setting realistic treatment goals, and involving the patient in the treatment process. Because irritable bowel symptoms are chronic, the patient's reasons for seeking consultation at this time should be determined. These may include major life events or recent psychosocial stressors, dietary or medication changes, concerns about serious underlying disease, or reduced quality of life and impairment of daily activities. In discussing with the patient the importance of the mind-gut interaction, it may be helpful to explain that alterations in visceral motility and sensitivity may be exacerbated by environmental, social, or psychological factors such as foods, medications, hormones, and stress. Symptoms such as pain, bloating, and altered bowel habits may lead to anxiety and distress, which in turn may further exacerbate bowel disturbances due to disordered communication between the gut and the central nervous system. A symptom diary in which patients record the time and severity of symptoms, food intake, and life events may help uncover aggravating dietary or psychosocial factors. Physicians will earn the confidence of their patients by being nonjudgmental and attentive. Fears that the symptoms will progress, require surgery, or degenerate into serious illness should be allayed. The patient should understand that irritable bowel syndrome is a chronic disorder characterized by periods of exacerbation and quiescence. The physician can help but cannot cure such a disorder. The emphasis should be shifted from finding the cause of the symptoms to finding a way to cope with them. Physicians must resist the temptation to chase chronic complaints with new or repeated diagnostic studies.
B. Dietary Therapy
Patients commonly report dietary intolerances, although a role for dietary triggers in irritable bowel syndrome has never been convincingly demonstrated. Fatty foods and caffeine are poorly tolerated by many patients with irritable bowel syndrome. In patients with diarrhea, bloating, and flatulence, lactose intolerance should be excluded with a breath hydrogen test or a trial of a lactose-free diet. Malabsorption of dietary fructose or sorbitol (contained in fruits and artificially sweetened foods) may exacerbate bloating, flatulence, and diarrhea. A variety of foods are flatulogenic, producing pain and distention in some patients. These include brown beans, Brussels sprouts, cabbage, cauliflower, raw onions, grapes, plums, raisins, coffee, garlic, red wine, and beer.
A high-fiber diet appears to be of little value in patients with irritable bowel syndrome. Many patients report little change in bowel frequency but increased gas and distention with high-fiber diets or fiber supplementation.
C. Pharmacologic Measures
More than two-thirds of patients with irritable bowel syndrome have mild symptoms that respond readily to education, reassurance, and dietary interventions. Drug therapy should be reserved for patients with moderate to severe symptoms that do not respond to
P.631
conservative measures. These agents should be viewed as being adjunctive rather than curative. Given the wide spectrum of symptoms, no single agent is expected to provide relief in all or even most patients. Nevertheless, therapy targeted at the specific dominant symptom (pain, constipation, or diarrhea) may be beneficial.
1. Antispasmodic agents
Anticholinergic agents are used by some practitioners for treatment of acute episodes of pain or bloating despite a lack of well-designed trials demonstrating efficacy. Available agents include hyoscyamine, 0.125 mg orally (or sublingually as needed) or sustained-release, 0.037 mg or 0.75 mg orally twice daily; dicyclomine, 10 20 mg orally; or methscopolamine 2.5 5 mg orally before meals and at bedtime. Anticholinergic side effects are common, including urinary retention, constipation, tachycardia, and dry mouth. Hence, these agents should be used with caution in the elderly and in patients with constipation.
2. Antidiarrheal agents
Loperamide (2 mg orally three or four times daily) is effective for the treatment of patients with diarrhea, reducing stool frequency, liquidity, and urgency. It may best be used prophylactically in situations in which diarrhea is anticipated (such as stressful situations) or would be inconvenient (social engagements).
3. Anticonstipation agents
Treatment with osmotic laxatives (milk of magnesia or polyethylene glycol) may increase stool frequency, improve stool consistency, and reduce straining. Lactulose or sorbitol produces increased flatus and distention, which are poorly tolerated in patients with irritable bowel syndrome. Patients with intractable constipation should undergo further assessment for slow colonic transit and pelvic floor dysfunction (see Constipation, above).
4. Psychotropic agents
Patients with predominant symptoms of pain or bloating may benefit from low doses of tricyclic antidepressants, which are believed to have effects on motility, visceral sensitivity, and central pain perception that are independent of their psychotropic effects. Because of their anticholinergic effects, these agents may be more useful in patients with diarrhea-predominant than constipation-predominant symptoms. Nortriptyline, desipramine, or imipramine, may be started at a low dosage of 10 mg at bedtime and increased gradually to 50 150 mg as tolerated. Response rates do not correlate with dosage, and many patients respond to doses of 50 mg daily. Side effects are common, and lack of efficacy with one agent does not preclude benefit from another. Improvement should be evident within 4 weeks. The serotonin reuptake inhibitors (sertraline, 50 150 mg daily; paroxetine 10 20 mg daily; or fluoxetine, 20 40 mg daily) may lead to improvement in overall sense of well-being but have little impact on abdominal pain or bowel symptoms. Anxiolytics should not be used chronically in irritable bowel syndrome because of their habituation potential. Patients with major depression or anxiety disorders should be identified and treated with therapeutic doses of appropriate agents.
5. Serotonin receptor agonists and antagonists
Serotonin is an important mediator of gastrointestinal motility and sensation. Two agents approved for treatment of irritable bowel syndrome that modulate serotonin pathways are tegaserod and alosetron. Although both of these agents have some effect on visceral sensation, they have very different effects on intestinal motility.
Tegaserod is a selective partial 5-HT4-receptor agonist that has been approved recently for treatment of women who have irritable bowel syndrome with predominant constipation. Through stimulation of neurons in the intestinal mucosa, tegaserod stimulates intestinal peristalsis, inhibits visceral afferents involved in pain sensation, and stimulates colonic chloride and water secretion. In humans it acts as a prokinetic agent, accelerating gastric emptying and small and large bowel transit. Tegaserod (6 mg twice daily) results in modest but significant improvement compared with placebo in global irritable bowel symptoms in women. Efficacy in men has not been established. Tegaserod leads to an increase in stool frequency, improvement of stool consistency, and a reduction in abdominal pain and bloating. Symptom improvement is evident within 1 week in responsive patients. Diarrhea occurs in about 10% but usually resolves with continued therapy. The drug is otherwise extremely well tolerated and without significant drug interactions. For patients who demonstrate symptomatic improvement, it may be continued for 4 12 weeks and used intermittently for symptomatic flares.
Alosetron is a 5-HT3 antagonist that has been approved for the treatment of women with severe irritable bowel syndrome with predominant diarrhea. It appears to alter visceral sensation through blockade of peripheral 5-HT3-receptors on enteric afferent neurons as well as central receptors. It also inhibits enteric cholinergic motor neurons, resulting in inhibition of colonic motility. In humans, it increases the pain threshold in response to intestinal distention and slows colonic transit time. Alosetron (1 mg twice daily) results in improvement in 50 60% of women compared with 30 40% treated with placebo. Alosetron reduces symptoms of pain, discomfort, cramps, urgency, and diarrhea. Efficacy in men has not been demonstrated. In contrast to the excellent safety profile of other 5-HT3 antagonists (eg, ondansetron), alosetron may cause constipation in 30% of patients, frequently requiring discontinuation. Severe constipation (requiring hospitalization) and ischemic colitis may occur in 4:1000 patients. Given the seriousness of these side effects, alosetron is restricted to women with severe irritable bowel syndrome with diarrhea who have failed conventional therapies and who have been educated about the relative risks and benefits of the agent. It should not be used in patients with constipation.
P.632
6. Antibiotics
Controlled clinical trials from some centers report symptoms in up to half of patients with irritable bowel syndrome who were given antibiotics for presumed small intestinal bacterial overgrowth. Pending further clinical investigation, routine testing and treatment for small intestinal bacterial overgrowth cannot be recommended.
7. Probiotics
Small controlled clinical trials report improved symptoms in some patients treated with one probiotic, Bifidobacterium infantis, but not with another probiotic, Lactobacillus salivarius, or placebo. Results of further clinical trials are awaited before probiotic therapy can be recommended for irritable bowel syndrome.
D. Psychological Therapies
Cognitive-behavioral therapies, relaxation techniques, and hypnotherapy appear to be beneficial in some patients. Patients with underlying psychological abnormalities may benefit from evaluation by a psychiatrist or psychologist. Patients with severe disability should be referred to a pain treatment center.
Prognosis
The majority of patients with irritable bowel syndrome learn to cope with their symptoms and lead productive lives.
Chey WD et al: Long-term safety and efficacy of alosetron in women with severe diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol 2004;99:2195.
Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol 2002;97(11 Suppl):S1.
Halpert A et al: Clinical response to tricyclic antidepressants in functional bowel disorders is not related to dosage. Am J Gastroenterol 2005;100:664.
Lesbros-Pantoflickova D et al: Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004; 20:1253.
Lin HC: Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA 2004;292: 852.
Muller-Lissner S et al: Tegaserod is effective in the initial and retreatment of irritable bowel syndrome with constipation. Aliment Pharmacol Ther 2005;21:11.
O'Mahony L et al: Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005;128:541.
Spiller RC: Infection, immune function, and functional gut disorders. Clin Gastroenterol Hepatol 2004;2:445.
Walters B et al: Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol 2005;100:1566.
Whorwell PJ: Review article: the history of hypnotherapy and its role in the irritable bowel syndrome. Aliment Pharmacol Ther 2005;22:1061.
Antibiotic-Associated Colitis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Most cases of antibiotic-associated diarrhea are not attributable to C difficile and are usually mild and self-limited.
Symptoms of antibiotic-associated colitis vary from mild to fulminant; almost all colitis is attributable to C difficile.
Diagnosis in mild to moderate cases established by stool toxin assay.
Flexible sigmoidoscopy provides most rapid diagnosis in severe cases.
General Considerations
Antibiotic-associated diarrhea is a common clinical occurrence. Characteristically, the diarrhea occurs during the period of antibiotic exposure, is dose related, and resolves spontaneously after discontinuation of the antibiotic. In most cases, this diarrhea is mild, self-limited, and does not require any specific laboratory evaluation or treatment. Stool examination usually reveals no fecal leukocytes, and stool cultures reveal no pathogens. Although C difficile is identified in the stool of 15 25% of cases of antibiotic-associated diarrhea, it is also identified in 5 10% of patients treated with antibiotics who do not have diarrhea. Most cases of antibiotic-associated diarrhea are due to changes in colonic bacterial fermentation of carbohydrates and are not due to C difficile.
Antibiotic-associated colitis is a significant clinical problem almost always caused by C difficile infection. Hospitalized patients are most susceptible, especially those who are severely ill or malnourished or who are receiving chemotherapy. C difficile-colitis is the major cause of diarrhea in patients hospitalized for more than 3 days, affecting 22 patients of every 1000. This anaerobic bacterium colonizes the colon of 3% of healthy adults. However, it is acquired in approximately 20% of hospitalized patients, most of whom have received antibiotics that disrupt the normal bowel flora and thus allow the bacterium to flourish. Although almost all antibiotics have been implicated, colitis most commonly develops after use of ampicillin, clindamycin, third-generation cephalosporins, and flouroquinolones. C difficile-colitis will develop in approximately one-third of infected patients. Symptoms usually begin during or shortly after antibiotic therapy but may be delayed for up to 8 weeks. All patients with acute diarrhea should be asked about recent antibiotic exposure. Patients who are elderly, debilitated, immunocompromised, receiving multiple antibiotics or prolonged (> 10 days) antibiotic therapy, or receiving enteral tube feedings have a higher risk of acquiring
P.633
C difficile and developing C difficile-associated diarrhea. The organism is spread by fecal-oral transmission. It is found throughout hospitals in patient rooms and bathrooms and is readily transmitted from patient to patient by hospital personnel. Fastidious hand washing and use of disposable gloves are helpful in minimizing transmission.
The incidence and severity of C dfficile-colitis in hospitalized patients appear to be increasing, which is attributable to the emergence of a more virulent strain of C difficile (BI/NAP1) that contains an 18-base pair deletion of the tcdC gene, resulting in higher toxin production. This toxin-gene variant strain has been associated with several hospital outbreaks. The 30-day mortality attributed to infection with this strain is 7% but is 14% in the elderly.
Clinical Findings
A. Symptoms and Signs
Most patients report mild to moderate greenish, foul-smelling watery diarrhea with lower abdominal cramps. Physical examination is normal or reveals mild left lower quadrant tenderness. With more serious illness, there is abdominal pain and profuse watery diarrhea with up to 30 stools per day. The stools may have mucus but seldom gross blood. There may be fever up to 40 C, abdominal tenderness, and leukocytosis as high as 50,000/mcL. C difficile-colitis should be considered in all hospitalized patients with unexplained leukocytosis. In most patients, colitis is most severe in the distal colon and rectum. When colitis is more severe in the right side of the colon, there may be little or no diarrhea. In such cases, fever, abdominal distention, pain and tenderness, and leukocytosis suggest the presence of infection.
B. Special Examinations
1. Stool studies
Pathogenic strains of C difficile produce two toxins: toxin A is an enterotoxin and toxin B is a cytotoxin. In most patients, the diagnosis of antibiotic-associated colitis is established by the demonstration of C difficile toxins in the stool. A cytotoxicity assay (toxin B) performed in cell cultures has a specificity of 90% and a sensitivity of 95%. This is the definitive test, but it is expensive and results are not available for 24 48 hours. Rapid enzyme immunoassays (EIA) (2 4 hours) for toxins A and B are now widely used and have an 80 90% sensitivity with a single stool specimen but > 90% with two specimens. Some toxic strains of C difficile do not produce a functional toxin A (and therefore have a negative EIA); therefore, testing for toxin A and B is preferred. When C difficile is suspected but the EIA is negative, a cytotoxicity assay should be performed. Culture for C difficile is the most sensitive test, but 25% of isolates are not pathogenic. Because it is slower (2 3 days), more costly, and less specific than toxin assays, it is not used in most clinical settings. Fecal leukocytes are present in only 50% of patients with colitis.
2. Flexible sigmoidoscopy
Flexible sigmoidoscopy is performed in patients with more severe symptomatology when a rapid diagnosis is desired so that therapy can be initiated. In patients with mild to moderate symptoms, there may be no abnormalities or only patchy or diffuse, nonspecific colitis indistinguishable from other causes. In patients with severe illness, true pseudomembranous colitis is seen. This has a characteristic appearance, with yellow adherent plaques 2 10 mm in diameter scattered over the colonic mucosa interspersed with hyperemic mucosa. Biopsies reveal epithelial ulceration with a classic volcano exudate of fibrin and neutrophils. In 10% of cases, pseudomembranous colitis is confined to the proximal colon and may be missed at sigmoidoscopy.
3. Imaging studies
Abdominal radiographs are obtained in patients with fulminant symptoms to look for evidence of toxic dilation or megacolon but are of no value in mild disease. Mucosal edema or thumbprinting may be evident. Abdominal CT scan may be very useful in detecting colonic edema, especially in patients with predominantly right-sided colitis or abdominal pain without significant diarrhea (in whom the diagnosis may be unsuspected). CT scanning is also useful in the evaluation of possible complications.
Differential Diagnosis
In the hospitalized patient in whom acute diarrhea develops after admission, the differential diagnosis includes simple antibiotic-associated diarrhea (not related to C difficile), enteral feedings, medications, and ischemic colitis. Other infectious causes are unusual in hospitalized patients in whom diarrhea develops more than 72 hours after admission, and it is not cost-effective to obtain stool cultures unless tests for C difficile are negative. Rarely, other organisms (staphylococci, Clostridium perfringens) have been associated with pseudomembranous colitis.
Complications
Fulminant disease may result in dehydration, electrolyte imbalance, toxic megacolon, perforation, and death. Chronic untreated colitis may result in weight loss and protein-losing enteropathy.
Treatment
A. Immediate Treatment
If possible, antibiotic therapy should be discontinued. In patients with mild symptoms, doing so may result in prompt resolution of symptoms without specific treatment. If diarrhea is severe or persistent, specific therapy is warranted. The drug of choice is metronidazole, 500 mg orally three times daily. The duration of therapy is usually 10 14 days. However, in patients requiring long-term systemic antibiotics, it may be appropriate to continue metronidazole therapy until the
P.634
antibiotics can be discontinued. Vancomycin, 125 mg orally four times daily, is as effective as metronidazole but significantly more expensive, and it promotes the emergence of vancomycin-resistant nosocomial infections. Therefore, metronidazole is the preferred first-line therapy in most patients. Vancomycin should be reserved for patients who are intolerant of metronidazole, pregnant women, and children. Symptomatic improvement occurs in most patients within 72 hours. For patients with severe disease who do not respond rapidly to initial metronidazole therapy, therapy should be switched to vancomycin, 125 mg orally four times daily, escalating the dose to 500 mg four times daily if diarrhea and leukocytosis fail to improve. In patients who are unable to take oral medications and those with toxic megacolon, intravenous metronidazole, 500 750 mg every 6 hours, should be given sometimes supplemented by oral vancomycin administered per nasoenteric tube or enema. Intravenous vancomycin does not penetrate the bowel and should not be used. Total abdominal colectomy may be required in patients with toxic megacolon, perforation, sepsis, or hemorrhage.
B. Treatment of Relapse
Up to 20% of patients have a relapse of diarrhea from C difficile within 1 or 2 weeks after stopping initial therapy. This may be due to reinfection or failure to eradicate the organism. Most relapses respond promptly to a second course of metronidazole therapy. Some patients have recurrent relapses that can be difficult to treat. The optimal treatment regimen for recurrent relapses is unknown. Many authorities recommend a 6-week tapering regimen of vancomycin (125 mg orally four times daily for 7 days; twice daily for 7 days; once daily for 7 days; every other day for 7 days; and every third day for 2 weeks). Controlled trials show that oral administration of a live yeast, Saccharomyces boulardii, 500 mg twice daily, reduces the incidence of relapse by 50%. Probiotic therapy with this agent is recommended as adjunctive therapy in patients with relapsing disease.
Bartlett JG: Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002;346:334.
Bricker E et al: Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database Syst Rev 2005;(1):CD004610.
Loo VG et al: A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005;353:2442.
McDonald LC et al: An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353:2433.
Morelli M et al: Clinical application of the polymerase chain reaction to diagnose Clostridium difficile in hospitalized patients with diarrhea. Clin Gastroenterol Hepatol 2004; 2:669.
Szajewska H et al: Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea. Aliment Pharmacol Ther 2005;22:365.
Inflammatory Bowel Disease
The term inflammatory bowel disease includes ulcerative colitis and Crohn's disease. Ulcerative colitis is a chronic, recurrent disease characterized by diffuse mucosal inflammation involving only the colon. Ulcerative colitis invariably involves the rectum and may extend proximally in a continuous fashion to involve part or all of the colon. Crohn's disease is a chronic, recurrent disease characterized by patchy transmural inflammation involving any segment of the gastrointestinal tract from the mouth to the anus.
Crohn's disease and ulcerative colitis may be associated in 25% of patients with a number of extraintestinal manifestations, including oligoarticular or polyarticular nondeforming peripheral arthritis, spondylitis or sacroiliitis, episcleritis or uveitis, erythema nodosum, pyoderma gangrenosum, sclerosing cholangitis, and thromboembolic events.
Drug Therapies for Inflammatory Bowel Disease
Although ulcerative colitis and Crohn's disease appear to be distinct entities, the same pharmacologic agents are used to treat both. Despite extensive research, there are still no specific therapies for these diseases. The mainstays of therapy are 5-aminosalicylic acid derivatives, corticosteroids, immunomodulating agents (such as mercaptopurine or azathioprine), methotrexate, and infliximab.
A. 5-Aminosalicylic Acid (5-ASA)
5-ASA is a topically active agent that has a variety of anti-inflammatory effects. It is used in the active treatment of ulcerative colitis and Crohn's disease and during disease inactivity to maintain remission. It is readily absorbed from the small intestine but demonstrates minimal colonic absorption. A number of oral and topical compounds have been designed to target delivery of 5-ASA to the colon or small intestine while minimizing absorption. Commonly used formulations of 5-ASA are sulfasalazine, mesalamine, and azo compounds. Side effects of these compounds are uncommon but include nausea, rash, diarrhea, pancreatitis, and acute interstitial nephritis.
1. Oral mesalamine agents
These 5-ASA agents are coated in various pH-sensitive resins (Asacol) or packaged in timed-release capsules (Pentasa). Mesalamine tablets dissolve at pH 7.0, releasing 5-ASA in the terminal small bowel and proximal colon. Pentasa releases 5-ASA slowly throughout the small intestine and colon.
2. Azo Compounds
Sulfasalazine, balsalazide and olsalazine contain 5-ASA linked by an azo bond that requires cleavage by colonic bacterial azoreductases to release 5-ASA. Absorption of these drugs from the small intestine is negligible. After release within the colon, the 5-ASA works topically and is largely unabsorbed.
P.635
Olsalazine contains two 5-ASA molecules connected by the azo bond. Balsalazide contains 5-ASA linked to an inert carrier (4-aminobenzoyl- -alanine).
Sulfasalazine contains 5-ASA linked to a sulfapyridine moiety. It is unclear whether the sulfapyridine group has any anti-inflammatory effects. One gram of sulfasalazine contains 400 mg of 5-ASA. The sulfapyridine group, however, is absorbed and may cause side effects in 15 30% of patients much higher than with other 5-ASA compounds. Dose-related side effects include nausea, headaches, leukopenia, oligospermia, and impaired folate metabolism. Allergic and idiosyncratic side effects are fever, rash, hemolytic anemia, neutropenia, worsened colitis, hepatitis, pancreatitis, and pneumonitis. Despite its side effects, sulfasalazine continues to be used because it is significantly less expensive than other 5-ASA agents. It should always be administered in conjunction with folate. Eighty percent of patients intolerant of sulfasalazine can tolerate mesalamine.
3. Topical mesalamine
5-ASA is provided in the form of suppositories (Canasa; 500 mg) and enemas (Rowasa; 4 g/60 mL). These formulations can deliver much higher concentrations of 5-ASA to the distal colon than oral compounds. Side effects are uncommon.
B. Corticosteroids
A variety of intravenous, oral, and topical corticosteroid formulations have been used in inflammatory bowel disease. They have utility in the short-term treatment of moderate to severe disease. However, long-term use is associated with serious, potentially irreversible side effects and is to be avoided. The agents, route of administration, duration of use, and tapering regimens used are based more on personal bias and experience than on data from rigorous clinical trials. The most commonly used intravenous formulations have been hydrocortisone or methylprednisolone, which are given by continuous infusion or every 6 hours. Oral formulations are prednisone or methylprednisolone. Adverse events commonly occur during systemic corticosteroid therapy, including mood changes, insomnia, hypertension, weight gain, edema, elevated serum glucose levels, and moon facies. Topical preparations are provided as hydrocortisone suppositories (100 mg), foam (90 mg), and enemas (100 mg). Budesonide is an oral glucocorticoid with high topical anti-inflammatory activity but low systemic activity due to high first-pass hepatic metabolism. A controlled-release formulation is available (Entocort) that targets delivery to the terminal ileum and colon. It produces less suppression of the hypothalamic-pituitary-adrenal axis and fewer steroid-related side effects than hydrocortisone or prednisone.
C. Mercaptopurine, Azathioprine, or Methotrexate
Mercaptopurine and azathioprine are thiopurine drugs that are used in many patients with refractory Crohn's disease and, increasingly, in patients with ulcerative colitis. Azathioprine is converted in vivo to mercaptopurine. It is believed that the active metabolite of mercaptopurine is 6-thioguanine. Monitoring of 6-thioguanine levels is performed in some research settings but is of unproven value in the management of most patients. Allergic and nonallergic side effects of mercaptopurine and azathioprine occur in 10% of patients, including pancreatitis, bone marrow suppression, infections, hepatitis or cholestatic jaundice and, potentially, a higher risk of neoplasm.
Three competing enzymes are involved in the metabolism of mercaptopurine to its active (6-thioguanine) and inactive metabolites. About 1 person in 300 has a homozygous mutation of one of the enzymes that metabolizes thiopurine methyltransferase (TPMT), placing them at risk for profound immunosuppression; 1 person in 9 is heterozygous for TPMT, resulting in intermediate enzyme activity. Measurement of TPMT functional activity is recommended prior to initiation of therapy. Treatment should be withheld in patients with absent TPMT activity and increased slowly in those with intermediate activity. For patients with normal TPMT activity, the recommended dose of mercaptopurine is 1.5 mg/kg or of azathioprine is 2.5 mg/kg daily. Some clinicians prefer to initiate therapy with either drug at 50 mg/d for 2 weeks before increasing to the full recommended dose. For patients with intermediate activity and for patients in whom TPMT measurement is not available, both drugs should be started at 25 mg/d and increased by 25 mg every 2 weeks while monitoring for myelosuppression. For all patients, a complete blood count, liver chemistries, and amylase should be obtained every 2 weeks for 3 months, then every 3 months for the duration of therapy. If the white blood count falls below 3500 4000/mcL, the medication should be held for at least 1 week before reducing the daily dose by 25 50 mg.
Methotrexate is increasingly used in the treatment of patients with inflammatory bowel disease, especially patients with Crohn's disease who are intolerant of mercaptopurine. Methotrexate is an analog of dihydrofolic acid. Although at high doses it interferes with cell proliferation through inhibition of nucleic acid metabolism, at low doses it has anti-inflammatory properties, including inhibition of expression of tumor necrosis factor- (TNF- ) in monocytes and macrophages. Methotrexate may be given intramuscularly, subcutaneously, or orally. Side effects of methotrexate include nausea, vomiting, diarrhea, alopecia, stomatitis, infections, bone marrow suppression, hepatitis, hepatic fibrosis, and life-threatening pneumonitis. A complete blood count and liver chemistries should be monitored every 1 3 months. Folate supplementation (1 mg/d) should be administered.
D. Anti-TNF Therapy
A dysregulation of the TH1 T cell response is present in inflammatory bowel disease, especially Crohn's disease. One of the key proinflammatory cytokines in the
P.636
TH1 response is TNF- . Several antibodies to TNF currently are available or in clinical testing for the treatment of inflammatory bowel disease. Infliximab is an immunomodulating agent that represents a major advance in the treatment of patients with moderate to severe Crohn's disease and ulcerative colitis who are unresponsive to conventional therapy. Infliximab is a chimeric (human/mouse) IgG monoclonal antibody that binds with high specificity to membrane-associated TNF- on monocytes and activated T lymphocytes, promoting apoptosis and cell death. Infliximab is administered by intravenous infusion. The half-life of infliximab after intravenous infusion is 8 10 days; however, therapeutic serum concentrations persist for approximately 8 weeks. A three-dose regimen of 5 mg/kg administered at 0, 2, and 6 weeks is recommended for acute induction, followed by infusions every 8 weeks for maintance therapy. Acute infusion reactions occur in 4 6% of infusions but occur less commonly in patients receiving concomitant immunomodulators (ie, azathioprine or methotrexate). Most are mild or moderate (nausea; headache; dizziness; urticaria; diaphoresis; or mild cardiopulmonary symptoms that include chest tightness, dyspnea, or palpitations) and can be treated by slowing the infusion rate and administering acetaminophen and diphenhydramine. Severe reactions (hypotension, severe shortness of breath, rigors, severe chest discomfort) occur in 1% and may require oxygen, diphenhydramine, hydrocortisone, and epinephrine. Delayed serum sickness-like reactions occur in 1%. With repeated, intermittent intravenous injections, antibodies to infliximab (ATI) develop in up to 40% of patients, which are associated with a shortened duration or loss of response and increased risk of acute infusion reactions. Giving infliximab in a regularly scheduled maintenance therapy (eg, every 8 weeks), concomitant use of infliximab with other immunomodulating agents (azathioprine, mercaptopurine, or methotrexate), or preinfusion treatment with corticosteroids (intravenous hydrocortisone 200 mg) significantly reduces the development of ATI.
Serious infections may occur in up to 5% of patients, including sepsis, pneumonia, abscess, and cellulitis. Patients treated with infliximab are at increased risk for the development of disseminated tuberculosis as well as other opportunistic infections (Pneumocystis jiroveci, listeriosis, histoplasmosis, aspergillosis, varicella). Prior to use of infliximab, patients should be screened for latent tuberculosis with PPD testing and a chest radiograph. Autoantibodies including anti-DNA antibody occur in a small number of patients; however, the development of drug-induced lupus is rare. Infliximab may cause severe hepatic reactions leading to acute hepatic failure; liver enzymes should be monitored routinely during therapy. It is speculated but unproven that infliximab may increase the risk of lymphoproliferative malignancies. Rare cases of multiple sclerosis have been reported. Infliximab may worsen congestive heart failure in patients with cardiac disease.
Although infliximab is currently the only anti-TNF agent approved in the United States for treatment of patients with inflammatory bowel disease, other agents that are commercially available or may soon be available have demonstrated efficacy in the treatment of Crohn's disease. These include adalimumab (a fully humanized IgG antibody that is approved for the treatment of rheumatoid arthritis) and certolizumab (a polyethlene glycolated Fab' fragment of humanized anti-TNF), both of which are administered by subcutaneous injection. It is unknown whether these agents will have similar efficacy to infliximab with reduced complications related to antibody formation.
Social Support for Patients with Inflammatory Bowel Disease
Inflammatory bowel disease is a lifelong illness that can have profound emotional and social impacts on the individual. Patients should be encouraged to become involved in the Crohn's and Colitis Foundation of America (CCFA). National headquarters may be contacted at 444 Park Avenue South, 11th Floor, New York, NY 10016 7374; phone 212 685-3440. Internet address: http://www.ccfa.org.
1. Crohn's Disease
![]() Essentials of Diagnosis
Essentials of Diagnosis
Insidious onset.
Intermittent bouts of low-grade fever, diarrhea, and right lower quadrant pain.
Right lower quadrant mass and tenderness.
Perianal disease with abscess, fistulas.
Radiographic evidence of ulceration, stricturing, or fistulas of the small intestine or colon.
General Considerations
One-third of cases of Crohn's disease involve the small bowel only, most commonly the terminal ileum (ileitis). Half of all cases involve the small bowel and colon, most often the terminal ileum and adjacent proximal ascending colon (ileocolitis). In 20% of cases, the colon alone is affected. One-third of patients have associated perianal disease (fistulas, fissures, abscesses). A small number of patients have involvement of the mouth (aphthous ulcers) or upper intestinal tract. Unlike ulcerative colitis, Crohn's disease is a transmural process that can result in mucosal inflammation and ulceration, stricturing, fistula development, and abscess formation. Cigarette smoking is strongly associated with the development of Crohn's disease, resistance to medical therapy, and early disease relapse.
P.637
Clinical Findings
A. Symptoms and Signs
Because of the variable location of involvement and severity of inflammation, Crohn's disease may present with a variety of symptoms and signs. In eliciting the history, the clinician should take particular note of fevers, the patient's general sense of well-being, the presence of abdominal pain, the number of liquid bowel movements per day, and prior surgical resections. Physical examination should focus on the patient's temperature, weight, and nutritional status, the presence of abdominal tenderness or an abdominal mass, rectal examination, and extraintestinal manifestations. Most commonly, there is one or a combination of the following clinical constellations.
1. Chronic inflammatory disease
This is the most common presentation and is often seen in patients with ileitis or ileocolitis. Patients report low-grade fever, malaise, weight loss, and loss of energy. There may be diarrhea, which is nonbloody and often intermittent. Cramping or steady right lower quadrant or periumbilical pain is present. Physical examination reveals focal tenderness, usually in the right lower quadrant. A palpable, tender mass that represents thickened or matted loops of inflamed intestine may be present in the lower abdomen.
2. Intestinal obstruction
Narrowing of the small bowel may occur as a result of inflammation, spasm, or fibrotic stenosis. Patients report postprandial bloating, cramping pains, and loud borborygmi. This sometimes occurs in patients with active inflammatory symptoms (as above). More commonly, however, it occurs later in the disease from chronic fibrosis without other systemic symptoms or signs of inflammation.
3. Fistulization with or without infection
A subset of patients develops sinus tracts that penetrate through the bowel and form fistulas to a number of locations. Fistulas to the mesentery are usually asymptomatic but can result in intra-abdominal or retroperitoneal abscesses manifested by fevers, chills, a tender abdominal mass, and leukocytosis. Fistulas from the colon to the small intestine or stomach can result in bacterial overgrowth with diarrhea, weight loss, and malnutrition. Fistulas to the bladder or vagina produce recurrent infections. Enterocutaneous fistulas usually occur at the site of surgical scars.
4. Perianal disease
One-third of patients with either large or small bowel involvement develop perianal disease manifested by anal fissures, perianal abscesses, and fistulas.
5. Extraintestinal manifestations
The extracolonic manifestations (described in the section on ulcerative colitis) may also be seen with Crohn's disease, particularly Crohn's colitis. Other problems may also arise. Oral aphthous lesions are common. There is an increased prevalence of gallstones due to malabsorption of bile salts from the terminal ileum. Nephrolithiasis with urate or calcium oxalate stones may occur.
B. Laboratory Findings
There is a poor correlation between laboratory studies and the patient's clinical picture. Laboratory values may reflect inflammatory activity or nutritional complications of disease. A complete blood count and serum albumin should be obtained in all patients. Anemia may reflect chronic inflammation, mucosal blood loss, iron deficiency, or vitamin B12 malabsorption secondary to terminal ileal inflammation or resection. Leukocytosis may reflect inflammation or abscess formation or may be secondary to corticosteroid therapy. Hypoalbuminemia may be due to intestinal protein loss (protein-losing enteropathy), malabsorption, bacterial overgrowth, or chronic inflammation. The sedimentation rate or C-reactive protein level is elevated in many patients during active inflammation. Stool specimens are sent for examination for routine pathogens, ova and parasites, leukocytes, fat, and C difficile toxin.
C. Special Diagnostic Studies
In most patients, the initial diagnosis of Crohn's disease is based on a compatible clinical picture with supporting endoscopic and radiographic findings. An upper gastrointestinal series with small bowel follow-through or enteroclysis is obtained in all patients with suspected small bowel involvement. Suggestive findings include ulcerations, strictures, and fistulas. Capsule imaging may help to establish a diagnosis in patients in whom the clinical suspicion for small bowel involvement is high but radiographs are normal or nondiagnostic. To evaluate the colon, a barium enema or colonoscopy is obtained. Colonoscopy offers the advantage of obtaining mucosal biopsies of the colon or terminal ileum. Typical endoscopic findings include aphthoid ulcers, linear or stellate ulcers, strictures, and segmental involvement with areas of normal-appearing mucosa adjacent to inflamed mucosa. In 10% of cases, it may be difficult to distinguish ulcerative colitis from Crohn's disease. Granulomas on biopsy are present in less than 25% of patients but are highly suggestive of Crohn's disease. When the diagnosis remains uncertain, two serologic tests may be useful in further distinguishing these two diseases. Antineutrophil cytoplasmic antibodies with perinuclear staining (p-ANCA) are found in 50 70% of patients with ulcerative colitis and 5 10% of patients with Crohn's disease. Antibodies to the yeast Saccharomyces cerevisiae (ASCA) are found in 60 70% of patients with Crohn's disease and 10 15% of patients with ulcerative colitis. The accuracy and predictive value of these tests in patients with indeterminate colitis remain uncertain.
Complications
A. Abscess
The presence of a tender abdominal mass with fever and leukocytosis suggests an abscess. Emergent CT of
P.638
the abdomen is necessary to confirm the diagnosis. Patients should be given broad-spectrum antibiotics and, if malnourished, maintained on TPN. Percutaneous drainage or surgery is usually required.
B. Obstruction
Small bowel obstruction may develop secondary to active inflammation or chronic fibrotic stricturing and is often acutely precipitated by dietary indiscretion. Patients should be given intravenous fluids with nasogastric suction for several days. Systemic corticosteroids are indicated in patients with symptoms or signs of active inflammation but are unhelpful in patients with inactive, fixed disease. Patients unimproved on medical management require surgical resection of the stenotic area or stricturoplasty.
C. Fistulas
The majority of enteromesenteric and enteroenteric fistulas are asymptomatic and require no specific therapy. Large abscesses associated with fistulas require percutaneous or surgical drainage. Medical therapy is effective in a subset of patients and is usually tried before surgery. Although fistulas may close temporarily in response to TPN or oral elemental diets, they recur when oral feedings are resumed. Azathioprine or mercaptopurine heals fistulas in 30 40% of patients but requires 3 6 months. Infliximab is the most effective medical therapy for chronic fistulizing Crohn's disease. Infliximab injections (5 mg/kg) given at 0, 2, and 6 weeks result in complete closure of fistulas (both perianal and abdominal) in up to 50% of patients and improvement in up to 75%. Although cyclosporine has demonstrated some value for fistulous disease, high relapse rates and the risk of toxicity have limited its use. Surgical therapy is required for symptomatic fistulas that do not respond to medical therapy. Fistulas that arise above (proximal to) areas of intestinal stricturing commonly require surgical treatment.
D. Perianal Disease
Patients with fissures, fistulas, and skin tags commonly have perianal discomfort. Severe pain should suggest a perianal abscess, which usually requires simple incision and drainage. Pelvic MRI and endoscopic ultrasonography are the best studies for evaluating perianal fistulas and abscesses. Deep abscesses require drainage with noncutting drains, which allow drainage and improve comfort while medical treatment is initiated. In small series, closure of the internal opening with a suture followed by instillation of fibrin glue into the external fistula opening achieves successful closure in two-thirds of patients. Where possible, surgical fistulotomy should be avoided in the presence of active Crohn's disease because of the risk of poor wound healing. The medical treatment of perianal fistulas can be difficult. Metronidazole, 250 mg three times daily, and ciprofloxacin, 500 mg twice daily, are commonly prescribed based on efficacy in uncontrolled studies. Aminosalicylates and corticosteroids are of no benefit. Patients unresponsive to antibiotics may be treated with mercaptopurine or azathioprine, which results in symptomatic improvement in up to two-thirds of patients but fistula closure in less than one-third. Persistent perianal fistulas are treated with intravenous infliximab, 5 mg/kg, given at 0, 2, and 6 weeks followed by either maintenance therapy with infliximab every 8 weeks or mercaptopurine or azathioprine.
E. Carcinoma
Patients with extensive colonic Crohn's disease are at increased risk of developing colon carcinoma. Screening colonoscopy to detect dysplasia or cancer is recommended by most authorities for patients with a history of 8 or more years of Crohn's colitis. Patients with Crohn's disease have an increased risk of lymphoma and of small bowel adenocarcinoma; however, both are rare.
F. Hemorrhage
Unlike ulcerative colitis, severe hemorrhage is unusual in Crohn's disease.
G. Malabsorption
Malabsorption may arise from bacterial overgrowth in patients with enterocolonic fistulas, strictures, and stasis resulting in bacterial overgrowth, extensive jejunal inflammation, and prior surgical resections.
Differential Diagnosis
Chronic cramping abdominal pain and diarrhea are typical of both irritable bowel syndrome and Crohn's disease, but x-ray examinations are normal in the former. Acute fever and right lower quadrant pain may resemble appendicitis or Yersinia enterocolitica enteritis. Intestinal lymphoma causes fever, pain, weight loss, and abnormal small bowel radiographs that may mimic Crohn's disease. Patients with undiagnosed AIDS may present with fever and diarrhea. Segmental colitis may be caused by tuberculosis, E histolytica, Chlamydia, or ischemic colitis. C difficile or CMV infection may develop in patients with inflammatory bowel disease, mimicking disease recurrence. Diverticulitis with abscess formation may be difficult to distinguish acutely from Crohn's disease. NSAIDs may exacerbate inflammatory bowel disease and may also cause NSAID-induced colitis characterized by small bowel or colonic ulcers, erosion, or strictures that tend to be most severe in the terminal ileum and right colon.
Treatment of Active Disease
Crohn's disease is a chronic lifelong illness characterized by exacerbations and periods of remission. As no specific therapy exists, current treatment is directed toward symptomatic improvement and controlling the disease process. The treatment must address the specific problems of the individual patient. All patients
P.639
with Crohn's disease should be counseled to discontinue cigarettes.
A. Nutrition
1. Diet
Patients should eat a well-balanced diet with as few restrictions as possible. Because lactose intolerance is common, a trial off dairy products is warranted if flatulence or diarrhea is a prominent complaint. Patients with mainly colonic involvement benefit from fiber supplementation. Conversely, patients with obstructive symptoms should be placed on a low-roughage diet, ie, no raw fruits or vegetables, popcorn, nuts, etc. Resection of more than 100 cm of terminal ileum results in fat malabsorption for which a low-fat diet is recommended. Parenteral vitamin B12 (100 mcg intramuscularly per month) commonly is needed for patients with previous ileal resection or extensive terminal ileal disease.
2. Enteral therapy
Supplemental enteral therapy via nasogastric tube may be required for children and adolescents with poor intake and growth retardation.
3. Total parenteral nutrition
TPN is used short term in patients with active disease and progressive weight loss or those awaiting surgery who have malnutrition but cannot tolerate enteral feedings because of high-grade obstruction, high-output fistulas, severe diarrhea, or abdominal pain. It is required long term in a small subset of patients with extensive intestinal resections resulting in short bowel syndrome with malnutrition.
B. Symptomatic Medications
There are several potential mechanisms by which diarrhea may occur in Crohn's disease in addition to active Crohn's disease. A rational empiric treatment approach often yields therapeutic improvement that may obviate the need for corticosteroids or immunosuppressive agents. Involvement of the terminal ileum with Crohn's disease or prior ileal resection may lead to reduced absorption of bile acids that may induce secretory diarrhea from the colon. This diarrhea commonly responds to cholestyramine 2 4 g or colestipol 5 g two or three times daily before meals to bind the malabsorbed bile salts. Patients with extensive ileal disease or more than 100 cm of ileal resection have such severe bile salt malabsorption that steatorrhea may arise. Such patients may benefit from a low-fat diet; bile salt-binding agents will exacerbate the diarrhea and should not be given. Patients with Crohn's disease are at risk for the development of small intestinal bacterial overgrowth due to enteral fistulas, ileal resection, and impaired motility and may benefit from a course of broad-spectrum antibiotics (see Bacterial Overgrowth, above). Other causes of diarrhea include lactase deficiency and short bowel syndrome (described in other sections). Use of antidiarrheals may provide benefit in some patients. Loperamide (2 4 mg), diphenoxylate with atropine (one tablet), or tincture of opium (5 15 drops) may be given as needed up to four times daily. Because of the risk of toxic megacolon, these drugs should not be used in patients with active severe colitis.
C. Specific Drug Therapy
1. 5-Aminosalicylic acid agents
Mesalamine (Asacol 2.4 4.8 g/d; Pentasa 4 g/d) traditionally has been used as initial therapy for the treatment of mild to moderately active colonic and ileocolonic Crohn's disease. However, the efficacy of 5-ASA agents recently has been called into question. In early studies, remission rates of over 40% were reported in patients with mild to moderate disease compared with 20 30% in patients treated with placebo. However, recent meta-analyses of published and unpublished trial data suggest that mesalamine is of little or no value in the treatment of Crohn's disease, especially for disease involving the small intestine. Mesalamine or sulfasalazine (3 4 g/d) may be of benefit for disease confined to the colon. 5-ASA agents also appear to have little or no efficacy for maintenance of remission or for prevention of postoperative recurrences.
2. Antibiotics
Antibiotics also are widely used by clinicians for the treatment of active Crohn's disease, although meta-analyses of controlled trials suggest that they have little or no efficacy. It is hypothesized that antibiotics may reduce inflammation through alteration of gut flora, reduction of bacterial overgrowth, or treatment of microperforations. Metronidazole (10 mg/kg/d) or ciprofloxacin (500 mg twice daily) commonly are administered for 6 12 weeks.
3. Corticosteroids
Corticosteroids dramatically suppress the acute clinical symptoms or signs in most patients with both small and large bowel disease; however, they do not alter the underlying disease. An ileal-release budesonide preparation (Entocort), 9 mg once daily for 8 16 weeks, induces remission in 50 70% of patients with mild to moderate Crohn's disease involving the terminal ileum or ascending colon. After initial treatment, budesonide is tapered over 2 4 weeks in 3 mg increments. In some patients, low-dose budesonide (6 mg/d) may be used for up to 1 year to maintain remission. Budesonide is superior to mesalamine but somewhat less effective than prednisone. However, because budesonide has markedly reduced acute and chronic steroid-related adverse effects, including smaller reductions of bone mineral density, it is preferred to other systemic corticosteroids for the treatment of mild to moderate Crohn's disease involving the terminal ileum or ascending colon.
Prednisone or methylprednisolone, 40 60 mg/d, is generally administered to patients with Crohn's disease that is severe, that involves the distal colon or proximal small intestine, or that has failed treatment with budesonide. Remission or significant improvement occurs in 60 90% of patients after 8 16 weeks of therapy. After improvement at 2 weeks, tapering proceeds at 5 mg/wk until a dosage of 20 mg/d is being given. Thereafter, slow tapering by 2.5 mg/wk is recommended. Approximately 20% of patients cannot be completely withdrawn
P.640
from corticosteroids without experiencing a symptomatic flare-up. Furthermore, 75% of patients who achieve initial remission on corticosteroids will experience a relapse within 1 year. Use of long-term low corticosteroid doses (2.5 10 mg/d) should be avoided, because of associated complications including aseptic necrosis of the hips, osteoporosis, cataracts, diabetes, and hypertension. Calcium and vitamin D supplementation should be administered to all patients receiving chronic corticosteroid therapy. Bone densitometry should be considered in patients with inflammatory bowel disease with other risk factors for osteoporosis and in all patients requiring long-term corticosteroids. Bisphosphonate therapy is recommended for patients with proven osteoporosis and those requiring > 3 months of corticosteroid therapy to reduce bone loss. Patients requiring long-term corticosteroid treatment should be given immunomodulatory drugs (as described below) in an effort to wean them from corticosteroids.
Patients with persisting symptoms despite oral corticosteroids or those with high fever, persistent vomiting, evidence of intestinal obstruction, severe weight loss, severe abdominal tenderness, or suspicion of an abscess should be hospitalized. In patients with a tender, palpable inflammatory abdominal mass, CT scan of the abdomen should be obtained prior to administering corticosteroids to rule out an abscess. If no abscess is identified, parenteral corticosteroids should be administered (as described for ulcerative colitis).
4. Immunomodulating drugs: Azathioprine, mercaptopurine, or methotrexate
Immunomodulating agents are used in approximately one-third of patients with Crohn's disease who have not responded to corticosteroids or who require repeated courses of long-term corticosteroids to control symptoms. These agents permit elimination or reduction of corticosteroids in over 75% and fistula closure in 30% of patients. In the United States, mercaptopurine or azathioprine are more commonly used than methotrexate. The mean time to symptomatic response is 2 4 months, so these agents are not useful for acute exacerbations. Meta-analyses of controlled trials suggest that patients with Crohn's disease treated with immunomodulating agents are three times as likely to achieve remission and 2.25 times as likely to maintain remission than those treated with corticosteroids. Once patients achieve remission, immunomodulating drugs reduce the 3-year relapse rate from over 60% to less than 25%. Methotrexate (25 mg intramuscularly or subcutaneously weekly for 12 weeks, followed by 12.5 15 mg once weekly, orally or subcutaneously) is increasingly used in patients who are unresponsive to or intolerant of mercaptopurine or azathioprine. Because oral absorption may be erratic, parenteral administration is more commonly recommended. Other immunosuppressive agents have been investigated in the treatment of Crohn's disease, including cyclosporine and thalidomide; however, efficacy has been modest and toxicity greater than with the thiopurines.
5. Immunomodulating drugs: Anti-TNF therapies
Infliximab is used for the treatment of patients with active moderate to severe Crohn's disease with an inadequate response to corticosteroids or other immunomodulators (azathioprine or mercaptopurine), symptomatic flares when corticosteroids are tapered, severe illness requiring hospitalization, or fistulizing disease. It is also used instead of corticosteroids to promote rapid initial improvement while other immunosuppressives (azathioprine, mercaptopurine) that take weeks to months to achieve therapeutic effect are being initiated. A three-dose regimen of 5 mg/kg administered at 0, 2, and 6 weeks is recommended for acute induction. Improvement occurs in two-thirds of patients and remission in one-third. After initial clinical response, symptom relapse occurs in > 80% of patients within 1 year in the absence of further maintenance therapy. Options for maintenance therapy include long-term continuation of other immunosuppressive agents (mercaptopurine, azathioprine, or methotrexate) with need for subsequent infliximab determined by the clinical course or systematic maintenance therapy with infliximab 5 mg/kg every 8 weeks (with or without concomitant immunomodulating agents). A gradual or complete loss of efficacy occurs over time in some patients, necessitating increased dosing (10 mg/kg) or decreased dosing intervals (every 6 weeks), or both, which may be partly attributable to development of ATI. Systematic maintenance therapy is associated with an increased likelihood of sustained response and a lower likelihood of developing ATI. For these reasons, maintenance therapy with infliximab is increasingly used. Concomitant therapy with low doses of other immunomodulating agents (mercaptopurine 50 mg/d, azathioprine 100 mg/d, or methotrexate 7.5 mg/wk) may also reduce development of ATI.
Adalimumab is a fully human anti-TNF monoclonal antibody that is as efficacious as infliximab for the treatment of rheumatoid arthritis. Although controlled studies of adalimumab in Crohn's disease are currently ongoing, open-label studies have demonstrated efficacy in patients who have intolerance or loss of responsiveness to infliximab.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) (sargramostim) is a myeloid growth factor that may augment the innate intestinal immune system. Recent controlled studies suggest that sargramostim may have significant benefit in a subset of patients. The role of this agent, which is safer than anti-TNF therapy, in Crohn's disease is undergoing further testing.
Indications for Surgery
Over 50% of patients will require at least one surgical procedure. The main indications for surgery are intractability to medical therapy, intra-abdominal abscess, massive bleeding, symptomatic refractory internal or perianal fistulas, and intestinal obstruction. Patients
P.641
with chronic obstructive symptoms due to a short segment of ileal stenosis are best treated with resection or stricturoplasty (rather than chronic medical therapy), which promotes rapid return of well-being and elimination of corticosteroids. After surgery, clinical recurrence occurs in 20% of patients within 1 year and 80% within 10 15 years. Postsurgical prophylaxis with mesalamine commonly does not appear to reduce the risk of clinical recurrence. A recent randomized controlled trial reported that ornidazole (a nitroimidazole antibiotic with a superior safety profile) reduced the postoperative clinical recurrence rate at 1 year from 38% in the placebo group to 8% in the ornidazole group. Long-term immunosuppressive therapy with mercaptopurine (50 mg daily) may be effective than placebo in preventing clinical and endoscopic recurrence after ileocolic resection and is recommended for high-risk patients.
Prognosis
With proper medical and surgical treatment, the majority of patients are able to cope with this chronic disease and its complications and lead productive lives. Few patients die as a direct consequence of the disease.
Colombel JF et al: The safety profile of infliximab in patients with Crohn's disease: the Mayo Clinic experience of 500 patients. Gastroenterology 2004;126:19.
Hanauer S et al: Oral pentasa in the treatment of active Crohn's disease: a meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol 2004;2:379.
Hanauer S et al: Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology 2004;127:723.
Korzenik JR et al: Sargramostim for active Crohn's disease. N Engl J Med 2005;352:2193.
Lichtenstein GR: Infliximab: lifetime use for maintenance is appropriate in Crohn's Disease. PRO: maintenance therapy is superior to episodic therapy. Am J Gastroenterol 2005; 100:1433.
Loftus EV: Infliximab: lifetime use for maintenance is appropriate in Crohn's Disease. CON: lifetime use is an awfully long time. Am J Gastroenterol 2005;100:1435.
Papadakis KA et al: Safety and efficacy of adalimumab (D2E7) in Crohn's disease patients with an attenuated response to infliximab. Am J Gastroenterol 2005;100:75.
Rutgeerts P et al: Comparison of scheduled and episodic treatment strategies of infliximab for Crohn's disease. Gastroenterology 2004;126:402.
Rutgeerts P et al: Ornidazole for prophylaxis of postoperative Crohn's disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology 2005;128:856.
Sandborn WJ et al: Budesonide for maintenance of remission in patients with Crohn's disease in medically induced remission: a predetermined pooled analysis of four randomized, double-blind, placebo-controlled trials. Am J Gastroenterol 2005;100:1780.
Sands B et al: Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004;350:876.
Siegel CA et al: Review article: practical management of inflammatory bowel disease patients taking immunomodulators. Aliment Pharmacol Ther 2005;22:1.
2. Ulcerative Colitis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Bloody diarrhea.
Lower abdominal cramps and fecal urgency.
Anemia, low serum albumin.
Negative stool cultures.
Sigmoidoscopy is the key to diagnosis.
General Considerations
Ulcerative colitis is an idiopathic inflammatory condition that involves the mucosal surface of the colon, resulting in diffuse friability and erosions with bleeding. Approximately one-third of patients have disease confined to the rectosigmoid region (proctosigmoiditis); one-third have disease that extends to the splenic flexure (left-sided colitis); and one-third have disease that extends more proximally (extensive colitis). There is some correlation between disease extent and symptom severity. In the majority of patients, the extent of colonic involvement does not progress over time. In most patients, the disease is characterized by periods of symptomatic flare-ups and remissions. Ulcerative colitis is more common in nonsmokers and former smokers. Disease severity may be lower in active smokers and may worsen in patients who stop smoking. Appendectomy before the age of 20 years for acute appendicitis is associated with a reduced risk of developing ulcerative colitis.
Clinical Findings
A. Symptoms and Signs
The clinical profile in ulcerative colitis is highly variable. Bloody diarrhea is the hallmark. On the basis of several clinical and laboratory parameters, it is clinically useful to classify patients as having mild, moderate, or severe disease (Table 14-14). Patients should be asked about stool frequency, the presence and amount of rectal bleeding, cramps, abdominal pain, fecal urgency, and tenesmus. Physical examination should focus on the patient's volume status as determined by orthostatic blood pressure and pulse measurements and by nutritional status. On abdominal examination, the clinician should look for tenderness and evidence of peritoneal inflammation. Red blood may be present on digital rectal examination.
1. Mild to moderate disease
Patients with mild disease have a gradual onset of infrequent diarrhea (less than five movements per day) with intermittent rectal bleeding and mucus. Stools may be formed too loose in consistency. Because of rectal inflammation, there is fecal urgency and tenesmus. Left lower quadrant
P.642
cramps relieved by defecation are common, but there is no significant abdominal tenderness. Patients with moderate disease have more severe diarrhea with frequent bleeding. Abdominal pain and tenderness may be present but are not severe. There may be mild fever, anemia, and hypoalbuminemia.
Table 14-14. Ulcerative colitis: Assessment of disease activity. | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
2. Severe disease
Patients with severe disease have more than six to ten bloody bowel movements per day, resulting in severe anemia, hypovolemia, and impaired nutrition with hypoalbuminemia. Abdominal pain and tenderness are present. Fulminant colitis is a subset of severe disease characterized by rapidly worsening symptoms with signs of toxicity.
B. Laboratory Findings
The degree of abnormality of the hematocrit, sedimentation rate, and serum albumin reflects disease severity.
C. Endoscopy
In acute colitis, the diagnosis is readily established by sigmoidoscopy. The mucosal appearance is characterized by edema, friability, mucopus, and erosions. Colonoscopy should not be performed in patients with severe disease because of the risk of perforation. After patients have demonstrated improvement on therapy, colonoscopy is sometimes performed to determine the extent of disease, which will dictate the need for subsequent cancer surveillance.
D. Imaging
Plain abdominal radiographs are obtained in patients with severe colitis to look for significant colonic dilation. Barium enemas are of little utility in the evaluation of acute ulcerative colitis and may precipitate toxic megacolon in patients with severe disease.
Differential Diagnosis
The initial presentation of ulcerative colitis is indistinguishable from other causes of colitis, clinically as well as endoscopically. Thus, the diagnosis of idiopathic ulcerative colitis is reached after excluding other known causes of colitis. Infectious colitis should be excluded by sending stool specimens for routine bacterial cultures (to exclude Salmonella, Shigella, and Campylobacter), ova and parasites (to exclude amebiasis), and stool toxin assay for C difficile. Mucosal biopsy can distinguish amebic colitis from ulcerative colitis. Enteroinvasive E coli and E coli O157:H7 will not be detected on routine bacterial cultures. CMV colitis occurs in immunocompromised patients (especially those with AIDS) and is diagnosed on mucosal biopsy. Gonorrhea, chlamydial infection, herpes, and syphilis are considerations in sexually active patients with proctitis. In elderly patients with cardiovascular disease, ischemic colitis may involve the rectosigmoid. A history of radiation to the pelvic region can result in proctitis months to years later. Crohn's disease involving the colon but not the small intestine may be confused with ulcerative colitis. In 10% of patients, a distinction between Crohn's disease and ulcerative colitis may not be possible. The utility of ANCA and ASCA antibodies in these difficult patients is discussed in the section on Crohn's disease.
Treatment
There are two main treatment objectives: (1) to terminate the acute, symptomatic attack and (2) to prevent recurrence of attacks. The treatment of acute ulcerative colitis depends on the extent of colonic involvement and the severity of illness.
Patients with mild to moderate disease should eat a regular diet but limit their intake of caffeine and gas-producing vegetables. Fiber supplements decrease diarrhea and rectal symptoms (psyllium, 3.4 g twice daily; methylcellulose, 2 g twice daily; bran powder, 1 tbsp twice daily). Antidiarrheal agents should not be given in the acute phase of illness but are safe and helpful in patients with mild chronic symptoms. Loperamide (2 mg), diphenoxylate with atropine (one tablet), or tincture of opium (8 15 drops) may be given up to four times daily. Such remedies are particularly useful at nighttime and when taken prophylactically for occasions when patients may not have reliable access to toilet facilities.
A. Distal Colitis
Patients with disease confined to the rectum or rectosigmoid region generally have mild but distressing symptoms. Treatment of acute disease is best approached with topical agents. Topical mesalamine is the drug of choice and is superior to topical corticosteroids. Mesalamine is administered as a suppository, 500 mg twice daily for proctitis, and as an enema, 4 g at bedtime for proctosigmoiditis, for 3 12 weeks, with 75% of patients improving (Table 14-15). Topical corticosteroids are a less expensive alternative to mesalamine but are also less effective. Hydrocortisone suppository or foam is prescribed for proctitis and hydrocortisone
P.643
enema (80 100 mg) for proctosigmoiditis. Systemic effects from short-term use are very slight. For patients with distal disease who fail to improve with once daily topical therapy, the following options may be considered: (1) increase the same topical agent to twice daily, (2) combination therapy with a 5-ASA enema at bedtime and a corticosteroid enema or foam in the morning, and (3) a combination of a topical agent with an oral 5-ASA agent.
Table 14-15. Treatment of ulcerative colitis. | |
|---|---|
|
Patients whose acute symptoms resolve rapidly with acute therapy may have prolonged periods of remission that are treated successfully with intermittent courses of therapy. Patients with early or frequent relapse should be treated with maintenance therapy with mesalamine suppositories (500 mg daily) or with oral 5-ASA agents (see below).
B. Mild to Moderate Colitis
1. 5-ASA Agents
Disease extending above the sigmoid colon is best treated with mesalamine or balsalazide, which result in symptomatic improvement in 50 75% of patients. Most patients improve within 3 6 weeks, though some require 2 3 months. Mesalamine, 0.8 1.6 g (Asacol) three times daily, or 1 g four times daily (Pentasa), and balsalazide, 2.25 g three times daily, are approved for active disease (Table 14-15). In a recent large multicenter study, 72% of patients treated with mesalamine 4.8 g/d achieved clinical response or remission compared with 59% treated with 2.4 g/d. Sulfasalazine is comparable in efficacy to mesalamine and because of its low cost is still commonly used as a first-line agent by many providers, though it is associated with greater side effects. To minimize side effects, sulfasalazine is begun at a dosage of 500 mg twice daily and increased gradually over 1 2 weeks to 2 g twice daily (Table 14-15). Total doses of 5 6 g/d may have greater efficacy but are poorly tolerated. Folic acid, 1 mg/d, should be administered to all patients taking sulfasalazine.
2. Corticosteroids
Patients with mild to moderate disease who fail to improve after 2 3 weeks of 5-ASA therapy should have the addition of corticosteroid therapy. Topical therapy with hydrocortisone foam or enemas (80 100 mg once or twice daily) or 5-ASA enemas (4 g once daily) may be tried first. Patients who do not improve after 2 more weeks require systemic corticosteroid therapy. Prednisone and methylprednisolone are most commonly used. Depending on the severity of illness, the initial oral dose of prednisone is 40 60 mg daily. Rapid improvement is observed in most cases. It is usually possible to begin to taper prednisone after 2 weeks. Tapering of prednisone should proceed by no more than 5 mg/wk. After tapering to 15 mg/d, slower tapering is sometimes required. Complete tapering without symptomatic flare-ups is possible in the majority of patients.
3. Immunomodulating agents
A subset of patients either does not respond to aminosalicylates or corticosteroids or has symptomatic flares during attempts at corticosteroid tapering. Although surgical resection is traditionally recommended for patients with refractory disease, some patients may wish to avoid surgery and others have moderately severe disease for which surgery might not otherwise be warranted. Immunomodulating agents are increasingly used for the treatment of ulcerative colitis; however, the risks of these drugs from chronic immunosuppression must be weighed against the certainty of cure with surgical resection. Limited trials suggest mercaptopurine or azathioprine is of benefit in 60% of patients, allowing tapering of corticosteroids and maintenance of remission. There is less evidence that methotrexate is effective.
Based on the results of two large multicenter studies (ACT 1 and ACT 2), infliximab was recently approved in the United States for the treatment of patients with moderate to severe ulcerative colitis who have had an inadequate response to conventional therapies (oral corticosteroids, mercaptopurine or azathioprine, and mesalamine). Following a three-dose regimen of 5 mg/kg administered at 0, 2, and 6 weeks, clinical response occurred in 65% and clinical remission in 26 34%.
C. Severe Colitis
About 10 15% of patients with ulcerative colitis have a more severe course. Because they may progress to fulminant colitis or toxic megacolon, hospitalization is generally required.
1. General measures
Discontinue all oral intake for 24 48 hours or until the patient demonstrates clinical improvement. TPN is indicated only in patients with poor nutritional status or if feedings cannot be reinstituted within 7 10 days. All opioid or anticholinergic agents should be discontinued. Restore circulating
P.644
volume with fluids, correct electrolyte abnormalities, and consider transfusion for significant anemia (hematocrit < 25 28%). Abdominal examinations should be repeated to look for evidence of worsening distention or pain. A plain abdominal radiograph should be ordered on admission to look for evidence of colonic dilation. Send stools for bacterial (including C difficile) culture and examination for ova and parasites. Surgical consultation should be sought for all patients with severe disease.
2. Corticosteroid therapy
Methylprednisolone, 48 64 mg, or hydrocortisone, 300 mg, is administered in four divided doses or by continuous infusion over 24 hours. Higher or pulse doses are of no benefit. Hydrocortisone enemas (100 mg) may also be administered twice daily for treatment of urgency or tenesmus. In patients who have not previously received corticosteroids, administration of ACTH, 120 units/24 h, may be superior to corticosteroids. Approximately 50 75% of patients achieve remission with systemic corticosteroids within 7 10 days. Once symptomatic improvement has occurred, oral fluids are reinstituted. If fluids are well tolerated, intravenous corticosteroids are discontinued and the patient is started on oral prednisone (as described for moderate disease).
3. Anti-TNF therapies
A single infusion of infliximab, 5 mg/kg, has been shown in recent controlled and uncontrolled studies to be effective in treating severe to fulminant colitis in patients who did not improve within 4 7 days of intravenous corticosteroid therapy. In a controlled study of patients hospitalized for ulcerative colitis, colectomy was required within 3 months in 69% who received placebo therapy, compared with 47% who received infliximab. Although further studies are needed, infliximab therapy should be considered in patients with severe ulcerative colitis who have not improved with intravenous corticosteroid therapy.
4. Cyclosporine
Intravenous cyclosporine (2 4 mg/kg/d as a continuous infusion) benefits 60 75% of patients with severe colitis who have not improved after 7 10 days of corticosteroids. In patients with severe steroid-resistant colitis who are reluctant to undergo colectomy, intravenous cyclosporine may be considered as a bridge therapy while mercaptopurine or azathioprine therapy (which take 2 4 months for full efficacy) is initiated. Up to two-thirds of responders may be maintained in remission with a combination of oral cyclosporine for 3 months and long-term therapy with mercaptopurine or azathioprine. The relative role of infliximab versus cyclosporine in the treatment of severe colitis requires further clinical study.
4. Surgical therapy
Patients with severe disease who fail to improve after 7 10 days of corticosteroid, infliximab or cyclosporine therapy are unlikely to respond to further medical therapy, and surgery is recommended.
D. Fulminant Colitis and Toxic Megacolon
A subset of patients with severe disease has a more fulminant course with rapid progression of symptoms over 1 2 weeks and signs of severe toxicity. These patients appear quite ill, with fever, prominent hypovolemia, hemorrhage requiring transfusion, and abdominal distention with tenderness. They are at a higher risk of perforation or development of toxic megacolon and must be followed closely. Broad-spectrum antibiotics should be administered to cover anaerobes and gram-negative bacteria.
Toxic megacolon develops in less than 2% of cases of ulcerative colitis. It is characterized by colonic dilation of more than 6 cm on plain films with signs of toxicity. In addition to the therapies outlined above, nasogastric suction should be initiated. Patients should be instructed to roll from side to side and onto the abdomen in an effort to decompress the distended colon. Serial abdominal plain films should be obtained to look for worsening dilation or ischemia. Patients with fulminant disease or toxic megacolon who worsen or fail to improve within 48 72 hours should undergo surgery to prevent perforation. If the operation is performed before perforation, the mortality rate should be low.
Maintenance of Remission
Without long-term therapy, 75% of patients who initially go into remission on medical therapy will experience a symptomatic relapse within 1 year. Long-term maintenance therapy with sulfasalazine, 1 1.5 g twice daily; olsalazine, 500 mg twice daily; and mesalamine, 800 mg three times daily or 500 mg four times daily, has been shown to reduce relapse rates to less than 33%. Uncontrolled studies suggest that the immunomodulators mercaptopurine or azathioprine may be useful in patients with frequent disease relapses (more than two per year) to maintain remission. The role of long-term infliximab therapy in the maintenance of remission is unclear. In the ACT 1 and 2 studies, initial induction therapy with infliximab 0, 2, and 6 weeks, infliximab maintenance infusions were administered every 8 weeks for 30 54 weeks. At both 8 weeks and the end of the study (30 or 54 weeks), 40% of patients had a sustained clinical response and 20% were in clinical remission, a modest but impressive response in patients with more refractory disease. In considering long-term infliximab therapy, patients and providers need to weigh the long-term risks of immunosuppression against colectomy.
Risk of Colon Cancer
In patients with ulcerative colitis with disease proximal to the sigmoid colon, there is a markedly increased risk of developing colon carcinoma. In patients who have had colitis for more than 10 years, the risk of developing colon cancer increases approximately 0.5 1% per year.
P.645
Meta-analysis of observational studies suggests that the risk of colon cancer is reduced by 50% in patients treated with long-term 5-ASA therapy. Ingestion of folic acid, 1 mg/d, also is associated with a decreased risk of cancer development. Colonoscopies are recommended every 1 2 years in patients with extensive colitis, beginning 8 10 years after diagnosis. At colonoscopy, multiple (at least 32) random mucosal biopsies are taken throughout the colon at 10-cm intervals as well as biopsies of mass lesions to look for dysplasia or carcinoma. Because of the relatively high incidence of concomitant carcinoma in patients with dysplasia (either low or high grade) in flat mucosa or mass lesions, colectomy is recommended. When low-grade dysplasia is detected only in flat mucosa, some authorities recommend a repeat colonoscopy in 3 6 months to confirm the presence of dysplasia before proceeding with colectomy.
Surgery in Ulcerative Colitis
Surgery is required in 25% of patients. Severe hemorrhage, perforation, and documented carcinoma are absolute indications for surgery. Surgery is indicated also in patients with fulminant colitis or toxic megacolon that does not improve within 48 72 hours, in patients with dysplasia on surveillance colonoscopy, and in patients with refractory disease requiring long-term corticosteroids to control symptoms.
Although total proctocolectomy (with placement of an ileostomy) provides complete cure of the disease, most patients seek to avoid it out of concern for the impact it may have on their bowel function, their self-image, and their social interactions. After complete colectomy, patients may have a standard ileostomy with an external appliance, a continent ileostomy, or an internal ileal pouch that is anastomosed to the anal canal (ileal pouch-anal anastomosis). The latter maintains intestinal continuity, thereby obviating an ostomy. Under optimal circumstances, patients have five to seven loose bowel movements per day without incontinence. Endoscopic or histologic inflammation in the ileal pouch ( pouchitis ) develops in over 25% of patients, resulting in increased stool frequency, fecal urgency, cramping, and bleeding, but usually resolves with a 2-week course of metronidazole (10 mg/kg/d) or ciprofloxacin (500 mg twice daily). Probiotics containing nonpathogenic strains of lactobacilli, bifidobacteria, and streptococci (VSL#3) are effective in the maintenance of remission in patients with recurrent pouchitis. Bismuth subsalicylate (Pepto Bismol, 262 mg, two tablets four times daily) has demonstrated benefit in some series. Some clinicians report that topical corticosteroids or oral budesonide 9 mg/d are of benefit. Refractory cases of proctitis can be disabling and may require conversion to a standard ileostomy.
Prognosis
Ulcerative colitis is a lifelong disease characterized by exacerbations and remissions. For most patients, the disease is readily controlled by medical therapy without need for surgery. The majority never require hospitalization. A subset of patients with more severe disease will require surgery, which results in complete cure of the disease. Properly managed, most patients with ulcerative colitis lead close to normal productive lives.
Aberra FN et al: Review article: monitoring of immunomodulators in inflammatory bowel disease. Aliment Pharmacol Ther 2005;21:307.
Bebb JR et al: Systematic review: how effective are the usual treatments for ulcerative colitis? Aliment Pharmacol Ther 2004; 20:143.
Bernstein CN: Ulcerative colitis with low-grade dysplasia. Gastroenterology 2004;127:950.
Gan SI et al: A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol 2003;98:2363.
Hanauer S: Medical therapy for ulcerative colitis 2004. Gastroenterology 2004;126:1582.
Hanauer SB et al: Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II Trial. Am J Gastroenterol 2005;100:2478.
Itzkowitz SH et al; Crohn's and Colitis Foundation of America Colon Cancer in IBD Study Group: Consensus Conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflam Bowel Dis 2005;11:314.
Jarnerot G et al: Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805.
Kornbluth A et al: Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2004;99:1371.
Probert CS et al: Infliximab in moderately severe glucocorticoid resistant ulcerative colitis: a randomized controlled trial. Gut 2003;52:998.
Rutgeerts P et al: Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462.
Sandborn WJ et al: Clinical management of pouchitis. Gastroenterology 2005;127:1809.
Sartor RB: Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620.
Velayos FS et al: Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2005;100: 1345.
3. Microscopic Colitis
Microscopic colitis is an idiopathic condition that is increasing in incidence in which patients have chronic or intermittent watery diarrhea with normal-appearing mucosa at endoscopy. A more severe illness characterized by abdominal pain, fatigue, dehydration, and weight loss may develop in a subset of patients. There appear to be two subtypes lymphocytic colitis and collagenous
P.646
colitis. In both, histologic evaluation of mucosal biopsies reveals chronic inflammation (lymphocytes, plasma cells) in the lamina propria and increased intraepithelial lymphocytes. Collagenous colitis is further characterized by the presence of a thickened band (> 10 mcm) of subepithelial collagen. Both forms occur more commonly in women, especially in the fifth to sixth decades. Symptoms tend to be chronic or recurrent but may remit in most patients after several years. Long-term NSAID therapy has been implicated as a causative factor in up to 50% of cases. Microscopic colitis occurs in up to one-third of patients with celiac sprue and should be considered in patients with continued diarrhea after institution of a gluten-free diet. Mild disease may be treated with antidiarrheal agents (loperamide, cholestyramine). NSAIDs should be discontinued. Treatment with 5-ASAs (sulfasalazine, mesalamine) is reported to be effective in uncontrolled studies. Although a controlled trial demonstrated efficacy for bismuth subsalicylate (two tablets four times daily) for 2 months, reported clinical experience has reported only modest benefit. Delayed release budesonide (Entocort) 9 mg/d for 6 8 weeks has been shown in several prospective controlled studies to induce clinical remission in more than 60 80% of patients and is well tolerated; however, clinical relapse is common after cessation of therapy. Patients who do not respond to bismuth or budesonide may be treated with systemic corticosteroids or, rarely, azathioprine. After entering remission, clinical relapse occurs in 20 30% of patients within 3 years.
Chande N et al: Interventions for treating collagenous colitis. Cochrane Database Syst Rev 2005;(4):CD003575.
Fernandez-Banares F et al: Collagenous and lymphocytic colitis: evaluation of clinical and histological features, response to treatment, and long-term follow up. Am J Gastroenterol 2003;98:340.
Feyen B et al: Meta-analysis: budesonide treatment for collagenous colitis. Aliment Pharmacol Ther 2004;20:745.
Miehlke S et al: Long-term follow-up of collagenous colitis after induction of clinical remission with budesonide. Aliment Pharmacol Ther 2005;22:1115.
Diverticular Disease of the Colon
Colonic diverticulosis increases with age, ranging from 5% in those under age 40, to 30% at age 60, to more than 50% over age 80 years in Western societies. In contrast, it is very uncommon in developing countries with much lower life expectancies. Most are asymptomatic, discovered incidentally at endoscopy or on barium enema. Complications in one-third include lower gastrointestinal bleeding and diverticulitis.
Colonic diverticula may vary in size from a few millimeters to several centimeters and in number from one to several dozen. Almost all patients with diverticulosis have involvement in the sigmoid colon; however, only 15% have proximal colonic disease.
In most patients, diverticulosis is believed to arise after many years of a diet deficient in fiber. The undistended, contracted segments of colon have higher intraluminal pressures. Over time, the contracted colonic musculature, working against greater pressures to move small, hard stools, develops hypertrophy, thickening, rigidity, and fibrosis. Diverticula may develop more commonly in the sigmoid because intraluminal pressures are highest in this region. The extent to which abnormal motility and hereditary factors contribute to diverticular disease is unknown. Patients with diffuse diverticulosis may have an inherent weakness in the colonic wall. Patients with abnormal connective tissue are also disposed to development of diverticulosis, including Ehlers-Danlos syndrome, Marfan's syndrome, and scleroderma.
1. Uncomplicated Diverticulosis
More than two-thirds of patients with diverticulosis have uncomplicated disease and no specific symptoms. In some, diverticulosis may be an incidental finding detected during colonoscopic examination or barium enema examination. Some patients have nonspecific complaints of chronic constipation, abdominal pain, or fluctuating bowel habits. It is unclear whether these symptoms are due to alterations in the colonic musculature or underlying irritable bowel syndrome. Physical examination is usually normal but may reveal mild left lower quadrant tenderness with a thickened, palpable sigmoid and descending colon. Screening laboratory studies should be normal in uncomplicated diverticulosis.
There is no reason to perform imaging studies for the purpose of diagnosing uncomplicated disease. Diverticula are best seen on barium enema. Involved segments of colon may also be narrowed and deformed. Colonoscopy is a less sensitive means of detecting diverticula.
Asymptomatic patients in whom diverticulosis is discovered and patients with a history of complicated disease (see below) should be treated with a high-fiber diet or fiber supplements (bran powder, 1 2 tbsp twice daily; psyllium or methylcellulose) (see section on constipation). Retrospective studies suggest that such treatment may decrease the likelihood of subsequent complications.
2. Diverticulitis
![]() Essentials of Diagnosis
Essentials of Diagnosis
Acute abdominal pain and fever.
Left lower abdominal tenderness and mass.
Leukocytosis.
P.647
Clinical Findings
A. Symptoms and Signs
Perforation of a colonic diverticulum results in an intra-abdominal infection that may vary from microperforation (most common) with localized paracolic inflammation to macroperforation with either abscess or generalized peritonitis. Thus, there is a range from mild to severe disease. Most patients with localized inflammation or infection report mild to moderate aching abdominal pain, usually in the left lower quadrant. Constipation or loose stools may be present. Nausea and vomiting are frequent. In many cases, symptoms are so mild that the patient may not seek medical attention until several days after onset. Physical findings include a low-grade fever, left lower quadrant tenderness, and a palpable mass. Stool occult blood is common, but hematochezia is rare. Leukocytosis is mild to moderate. Patients with free perforation present with a more dramatic picture of generalized abdominal pain and peritoneal signs.
B. Imaging
Plain abdominal films are obtained in all patients to look for evidence of free abdominal air (signifying free perforation), ileus, and small or large bowel obstruction. In patients with mild symptoms and a presumptive diagnosis of diverticulitis, empiric medical therapy is started without further imaging in the acute phase. Patients who respond to acute medical management should undergo complete colonic evaluation with colonoscopy or barium enema after resolution of clinical symptoms to corroborate the diagnosis or exclude other disorders such as colonic neoplasms. In patients who do not improve rapidly after 2 4 days of empiric therapy and in those with severe disease, CT scan of the abdomen is obtained to look for evidence of diverticulitis, including colonic diverticula and wall thickening, pericolic fat infiltration, abscess formation, or extraluminal air or contrast. Endoscopy and barium enema are contraindicated during the initial stages of an acute attack because of the risk of free perforation, though sigmoidoscopy with minimal air insufflation is sometimes required to exclude other diagnoses.
Differential Diagnosis
Localized diverticulitis must be distinguished from perforated colonic carcinoma, Crohn's disease, appendicitis, ischemic colitis, C difficile-associated colitis, and gynecologic disorders (ectopic pregnancy, ovarian cyst or torsion).
Complications
Fistula formation may involve the bladder, ureter, vagina, uterus, bowel, and abdominal wall. Diverticulitis may result in stricturing of the colon with partial or complete obstruction.
Treatment
A. Medical Management
Most patients can be managed with conservative measures. Patients with mild symptoms and no peritoneal signs may be managed initially as outpatients on a clear liquid diet and broad-spectrum oral antibiotics with anaerobic activity. Reasonable regimens include amoxicillin and clavulanate potassium (875 mg/125 mg) twice daily; or metronidazole, 500 mg three times daily; plus either ciprofloxacin, 500 mg twice daily, or trimethoprim-sulfamethoxazole, 160/800 mg twice daily orally, for 7 10 days or until the patient is afebrile for 3 5 days. Symptomatic improvement usually occurs within 3 days, at which time the diet may be advanced. Patients with increasing pain, fever, or inability to tolerate oral fluids require hospitalization. Patients with severe diverticulitis (high fevers, leukocytosis, or peritoneal signs) and patients who are elderly or immunosuppressed or who have serious comorbid disease require hospitalization acutely. Patients should be given nothing by mouth and should receive intravenous fluids. If ileus is present, a nasogastric tube should be placed. Intravenous antibiotics should be given to cover anaerobic and gram-negative bacteria. Single-agent therapy with either a second-generation cephalosporin (eg, cefoxitin), piperacillin-tazobactam, or ticarcillin clavulanate appears to be as effective as combination therapy (eg, metronidazole or clindamycin plus an aminoglycoside or third-generation cephalosporin [eg, ceftazidime, cefotaxime]). Symptomatic improvement should be evident within 2 3 days. The antibiotics should be continued for 7 10 days, after which time elective evaluation with colonoscopy or barium enema should be performed.
B. Surgical Management
Approximately 20 30% of patients with diverticulitis will require surgical management. Surgical consultation should be obtained on all patients with severe disease or those who fail to improve after 72 hours of medical management. Indications for emergent surgical management include free peritonitis and large abscesses. Patients with fistulas or colonic obstruction due to chronic disease will require elective surgery.
Patients with a localized abdominal abscess can be treated acutely with a percutaneous catheter drain placed by an interventional radiologist. This permits control of the infection and resolution of the immediate infectious inflammatory process. In this manner, a subsequent single-stage elective surgical operation can be performed in which the diseased segment of colon is removed and primary colonic anastomosis performed. In patients in whom catheter drainage is not possible or helpful or in cases requiring emergency surgery, it is necessary to perform surgery in two stages. In the first stage, the diseased colon is resected and the proximal colon brought out to form a temporary colostomy. The distal colonic stump is either
P.648
closed (forming a Hartmann pouch) or exteriorized as a mucous fistula. Weeks later, after inflammation and infection have completely subsided, the colon can be reconnected electively.
Prognosis
Diverticulitis recurs in one-third of patients treated with medical management. Recurrent attacks warrant elective surgical resection, which carries a lower morbidity and mortality risk than emergency surgery.
Biondo S et al: Acute colonic diverticulitis in patients under 50 years of age. Br J Surg 2002;89:1137.
Kircher MF et al: Frequency, sensitivity, and specificity of individual signs of diverticulitis on thin-section helical CT with colonic contrast material: experience with 312 cases. AJR Am J Roentgenol 2002;178:1313.
Mizuki A et al: The outpatient management of patients with acute mild-to-moderate colonic diverticulitis. Aliment Pharmacol Ther 2005;21:889.
Stollman N et al: Diverticular disease of the colon. Lancet 2004;363:631.
3. Diverticular Bleeding
Half of all cases of acute lower gastrointestinal bleeding are attributable to diverticulosis. For a full discussion, see the section on Acute Lower Gastrointestinal Bleeding.
Polyps of the Colon & Small Intestine
Polyps are discrete mass lesions that protrude into the intestinal lumen. Although most commonly sporadic, they may be inherited as part of a familial polyposis syndrome. Polyps may be divided into three major pathologic groups: mucosal neoplastic (adenomatous) polyps, mucosal nonneoplastic polyps (hyperplastic, juvenile polyps, hamartomas, inflammatory polyps), and submucosal lesions (lipomas, lymphoid aggregates, carcinoids, pneumatosis cystoides intestinalis). The nonneoplastic mucosal polyps have no malignant potential and usually are discovered incidentally at colonoscopy or barium enema. Only the adenomatous polyps have significant clinical implications and will be considered further here. Of polyps removed at colonoscopy, over 70% are adenomatous; most of the remainder are hyperplastic. Hyperplastic polyps are generally small (< 5 mm) and of no consequence. Their only importance is that they cannot be reliably distinguished from adenomatous lesions except by biopsy.
Nonfamilial Adenomatous Polyps
Histologically, adenomas are classified as tubular, villous, or tubulovillous. They may be flat, sessile or pedunculated (containing a stalk). They are present in 35% of adults over 50 years of age. Their significance is that over 95% of cases of adenocarcinoma of the colon are believed to arise from adenomas. It is proposed that there is an adenoma carcinoma sequence whereby colorectal cancer develops through a continuous process from normal mucosa to adenoma to carcinoma. Most adenomas are small (< 1 cm) and have a low risk of becoming malignant; fewer than 5% of these enlarge with time. Adenomas are classified as advanced if they are 1 cm, or contain villous features or high-grade dysplasia. Advanced adenomas are believed to have a higher risk of harboring or progressing to malignancy. It has been estimated from longitudinal studies that it takes an average of 5 years for a medium-sized polyp to develop from normal-appearing mucosa and 10 years for a gross cancer to arise. In an asymptomatic population of male veterans over age 50 years undergoing screening with colonoscopy, invasive cancer was detected in 1%, adenomas containing high-grade dysplasia in 1.7%, and other adenomas 1 cm in size in 7.2%. The role of aspirin and NSAIDs for the chemoprevention of adenomatous polyps is discussed in the section on Colorectal Cancer.
Clinical Findings
A. Symptoms and Signs
Most patients with adenomatous polyps are completely asymptomatic. Chronic occult blood loss may lead to iron deficiency anemia. Large polyps may ulcerate, resulting in intermittent hematochezia.
B. Fecal Occult Blood or Multitarget DNA Tests
FOBT and, more recently, fecal DNA tests are available as part of colorectal cancer screening programs (see Colorectal Cancer: Screening for Colorectal Neoplasms). Unfortunately, both tests detect less than 20% of advanced adenomas.
C. Radiologic Tests
Polyps are identified by means of barium enema examinations or CT colonography. Barium enema examinations (either single- or double-contrast) as currently performed detect < 50% of colorectal polyps 1 cm in size. CT colonography ( virtual colonoscopy ) uses data from helical CT imaging with computer-enabled luminal image reconstruction to generate two-dimensional and three-dimensional images of the colon. Using optimal imaging software with multidetector helical CT scanners, several studies report a sensitivity of > 90% for the detection of clinically significant neoplasms (polyps > 10 mm). Other studies report sensitivities of only 50%, especially when performed for colorectal screening in asymptomatic patients. Pending further validation, CT colonography is not yet recommended for routine colorectal cancer screening, but may be best suited for patients with significant cardiac or pulmonary comorbidities for whom colonoscopy is deemed unsafe or in clinical settings in which endoscopic expertise is not readily available.
P.649
D. Endoscopic Tests
Flexible sigmoidoscopy is commonly performed as part of colorectal screening programs. Approximately one-half to two-thirds of colonic adenomas are within the reach of a flexible sigmoidoscope. Polyps are seen in 10 20% of patients undergoing screening sigmoidoscopy, and polyps less than 8 mm in size should be removed by excisional biopsy. Patients with hyperplastic polyps require no further evaluation. Patients with advanced neoplasms (as defined above) have an increased risk (12%) of harboring other advanced neoplasms in the proximal colon and should undergo colonoscopy. At present, the management of patients with small adenomas without villous features or high-grade dysplasia is controversial. Opinions are conflicting about whether diminutive adenomas found on sigmoidoscopy are predictive of finding an increased prevalence of advanced neoplasms (> 1 cm or containing villous features or high-grade dysplasia) in the colon proximal to the splenic flexure during colonoscopy. In patients in whom no adenomas have been detected in the distal colon, the prevalence of advanced proximal adenomas is 2 5%; among patients with a distal adenoma less than 10 mm in size, the prevalence of advanced proximal neoplasia is 5 7%. Some physicians have not routinely performed colonoscopy in patients found to have a single small (< 5 8 mm) polyp in the distal colon on screening sigmoidoscopy. However, several professional guidelines recommend colonoscopy for all patients with adenomas found in the distal colon at sigmoidoscopy irrespective of size.
Colonoscopy allows evaluation of the entire colon and is the best means of detecting and removing adenomatous polyps. It should be performed in all patients who have positive FOBT or DNA tests or iron deficiency anemia (see Occult & Obscure Gastrointestinal Bleeding, above), as the prevalence of colonic neoplasms is increased in these patients. Colonoscopy should also be performed in patients with polyps detected on radiologic imaging studies (barium enema or CT colonography) or adenomas detected on flexible sigmoidoscopy to remove these polyps and to fully evaluate the entire colon.
Treatment
A. Colonoscopic Polypectomy
Most adenomatous polyps are amenable to colonoscopic removal with biopsy forceps or snare cautery. Large sessile polyps (> 2 3 cm) may be removed in piecemeal fashion or may require primary surgical resection. Complications after colonoscopic polypectomy include perforation in 0.2% and clinically significant bleeding in 0.3 1% of patients.
A malignant polyp is an adenoma that appears grossly benign at endoscopy but on histologic assessment is found to contain cancer that has penetrated through the muscularis mucosae into the submucosa. Malignant polyps may be considered to be adequately treated by polypectomy alone if (1) the polyp is completely excised and submitted for pathologic examination, (2) it is well differentiated, (3) the margin is not involved, and (4) there is no tumor budding vascular invasion. The risk of residual cancer or nodal metastasis with favorable histologic features is < 1%. The excision site of these favorable malignant polyps should be checked in 3 months for residual tissue. In patients with malignant polyps that have unfavorable histologic features, cancer resection is advisable if the patient is a good operative candidate.
B. Postpolypectomy Surveillance
Adenomas can be found in 30 40% of patients when another colonoscopy is performed within 3 5 years after the initial examination. Periodic colonoscopic surveillance is therefore recommended to detect these metachronous adenomas, which either may be new or may have been overlooked during the initial examination. Most of these adenomas are small, without high-risk features and of little immediate clinical significance. The probability of detecting advanced neoplasms at surveillance colonoscopy is increased significantly if the polyps found on initial (index) colonoscopy were advanced ( 1 cm, villous features, or high-grade dysplasia) or multiple (more than two polyps) or if the patient's family includes a first-degree member with colorectal cancer. (See discussion below under Colorectal Cancer.) In these higher-risk patients, repeat or surveillance colonoscopy should be performed 3 years after the initial colonoscopy and polypectomy. For patients with adenomatous polyps who had low-risk features on initial colonoscopy and polypectomy (ie, one or two polyps less than 10 mm in size without villous features or high-grade dysplasia) or for higher-risk patients whose follow-up surveillance colonoscopy is negative for further polyps after 3 years, repeat colonoscopy to check for metachronous adenomas should be performed in 5 years.
Imperiale TF et al: Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Ann Intern Med 2003;139:959.
Imperiale T et al: Fecal DNA versus fecal occult blood for colorectal cancer screening in an average-risk population. N Engl J Med 2004;351:2704.
Pickhardt P et al: Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349:2191.
Rockey DC et al: Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005;365:305.
Ueno H et al: Risk factors for an adverse outcome in early invasive colorectal adenocarcinoma. Gastroenterology 2004; 127:385.
Van Dam J et al: AGA future trends report: CT colonography. Gastroenterology 2004;127:970.
P.650
Hereditary Colorectal Cancer & Polyposis Syndromes
Up to 4% of all colorectal cancers are caused by germline genetic mutations that impose on carriers a high lifetime risk of developing colorectal cancer. Because the diagnosis of these disorders has important implications for treatment of affected members and for screening of family members, it is important to consider these disorders in patients with a family history of colorectal cancer that has affected more than one family member, those with a personal or family history of colorectal cancer developing at an early age ( 50 years), those with a personal or family history of multiple polyps (> 20), and those with a personal or family history of multiple extracolonic malignancies.
1. Familial Adenomatous Polyposis
Familial adenomatous polyposis (FAP) is a syndrome affecting 1:10,000 people and accounts for approximately 0.5% of colorectal cancer. The classic form of FAP is characterized by the development of hundreds to thousands of colonic adenomatous polyps and a variety of extracolonic manifestations. It is caused by an autosomally dominant inherited mutation in the adenomatous polyposis coli (APC) gene on chromosome 5q21 that leads to frameshifts or premature stop codons, most of which result in truncation of the APC gene product, a protein important in the regulation of cell adhesion and apoptosis. More than 300 different mutations have been reported. The location of the mutation affects the number of polyps formed and the type of extracolonic features seen. An attenuated variant of FAP has been recognized; an average of only 25 polyps (range of 0 500) develop in affected family members due to mutations at the 3' or 5' end of the APC gene. Recently, mutations in the MYH gene have been identified in patients with FAP who do not have mutations in the FAP gene. MYH is a recessive gene involved with base excision repair. FAP due to MYH mutation is inherited in an autosomal recessive fashion, hence a family history of colorectal cancer may not be evident.
Colorectal polyps develop by a mean age of 15 years and cancer at 40 years. Unless prophylactic colectomy is performed, colorectal cancer is inevitable by age 50 years. In attenuated FAP, the mean age for development of cancer is about 56 years.
Adenomatous polyps of the duodenum and periampullary area develop in over 90% of patients, resulting in a 5% lifetime risk of adenocarcinoma. Adenomas occur less frequently in the gastric antrum and small bowel and in those locations have a lower risk of malignant transformation. Gastric fundus gland polyps occur in over 50% but have an extremely low (0.6%) malignant potential.
Some patients with FAP develop a variety of other benign extraintestinal manifestations, including soft tissue tumors of the skin, desmoid tumors, osteomas, and congenital hypertrophy of the retinal pigment. They may also develop malignancies of the central nervous system (Turcot's syndrome) and tumors of the thyroid and liver (hepatoblastomas). These extraintestinal manifestations vary among families, depending in part on the type or site of mutation in the APC gene.
Genetic counseling and testing should be offered to patients with a diagnosis of FAP established by endoscopy and to first-degree family members of patients with the disease; testing should be done also to confirm a diagnosis of attenuated disease in patients with 20 or more adenomas. Genetic testing is best performed by sequencing the APC gene to identify disease-associated mutations, which are identified in approximately 90% of cases of typical FAP. Mutational assessment of MYH should be considered in patients with negative test results. First-degree relatives of patients with FAP should undergo genetic screening after age 10 years. A negative result can be considered to be a true negative only if an affected family member has a positive test result. If the assay cannot be done or is not informative, family members at risk should undergo yearly sigmoidoscopy beginning at 12 years of age. Once the diagnosis has been established, complete proctocolectomy with ileoanal anastomosis or colectomy with ileorectal anastomosis is recommended, usually before age 20 years. Ileorectal anastomosis affords superior bowel function but has a 10% risk of development of rectal cancer, and for that reason frequent sigmoidoscopy with fulguration of polyps is required. Sulindac and COX-2 selective agents (celecoxib) have been shown to decrease the number and size of polyps in the rectal stump but not the duodenum. Upper endoscopic evaluation of the stomach, duodenum, and periampullary area should be performed every 1 3 years to look for adenomas or carcinoma. Large (> 2 cm) periampullary adenomas require surgical resection.
Burt R et al: Genetic testing for inherited colon cancer. Gastroenterology 2005;128:1696.
Cruz-Correa M et al: Familial adenomatous polyposis. Gastrointest Endosc 2003;58:885.
Wang L et al: MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology 2004;127:9.
2. Hamartomatous Polyposis Syndromes
Hamartomatous polyposis syndromes are rare and account for less than 0.1% of colorectal cancers.
Peutz-Jeghers syndrome is an autosomal dominant condition characterized by hamartomatous polyps throughout the gastrointestinal tract (most notably in the small intestine) as well as mucocutaneous pigmented macules on the lips, buccal mucosa, and skin. The hamartomas may become large, leading to bleeding, intussusception, or obstruction. Although hamartomas are not malignant, gastrointestinal malignancies
P.651
(stomach, small bowel, and colon) develop in 40%, breast cancer in 50%, as well as a host of other malignancies of nonintestinal organs (gonads, pancreas). The defect has been localized to the serine threonine kinase 11 gene, and genetic testing is available.
Familial juvenile polyposis is also autosomal dominant and is characterized by several (more than ten) juvenile hamartomatous polyps located most commonly in the colon. There is an increased risk (up to 50%) of adenocarcinoma due to synchronous adenomatous polyps or mixed hamartomatous-adenomatous polyps. Genetic defects have been identified to loci on 18q and 10q (MADH4 and BMPR1A). Genetic testing is available.
PTEN multiple hamartoma syndrome (Cowden disease) is characterized by hamartomatous polyps and lipomas throughout the gastrointestinal tract, trichilemmomas, and cerebellar lesions. An increased rate of malignancy is demonstrated in the thyroid, breast, and urogenital tract.
Schreibman IR et al: The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol 2005; 100:476.
3. Hereditary Nonpolyposis Colorectal Cancer (HNPCC)
HNPCC is an autosomal dominant condition in which there is a markedly increased risk of developing colorectal cancer as well as a host of other cancers, including endometrial, ovarian, renal or vesical, hepatobiliary, gastric, and small intestinal cancers. It is estimated to account for up to 3% of all colorectal cancers. Affected individuals have a 60 80% lifetime risk of developing colorectal carcinoma and an over 30% lifetime risk of endometrial cancer. Unlike individuals with familial adenomatous polyposis, patients with HNPCC develop only a few adenomas, which may be flat and more often contain villous features or high-grade dysplasia. In contrast to the traditional polyp cancer progression (which may take over 10 years), these polyps are believed to undergo rapid transformation from normal tissue adenoma cancer. It has been believed that HNPCC tends to develop at an earlier age than sporadic colorectal cancers (mean age: 44 years); however, in a recent study of HNPCC families, the median age at diagnosis of colorectal cancer (excluding probands) was 61 years very similar to sporadic cancer. Compared with patients with sporadic tumors of similar pathologic stage, those with HNPCC tumors have improved survival. Synchronous or metachronous cancers occur within 10 years in up to 45% of patients.
HNPCC is caused by a defect in one of several genes that are important in the detection and repair of DNA base-pair mismatches: MLH1, MSH2, MSH6, and PMS2. Germline mutations in MLH1 and MSH2 account for more than 90% of the known mutations in families with HNPCC. Mutations in any of these mismatch repair genes result in a characteristic phenotypic abnormality known as microsatellite instability. In over 95% of cancers in patients with HNPCC, microsatellite instability is readily demonstrated by expansion or contraction of DNA microsatellites (short, repeated DNA sequences). Microsatellite instability also occurs in 15% of sporadic colorectal cancers, usually due to aberrant methylation of the MLH1 promoter resulting in decreased gene expression.
A thorough family cancer history is essential to identify families that may be affected with HNPCC so that appropriate genetic and colonoscopic screening can be offered. Owing to the limitations of genetic testing for HNPCC and the medical, psychological, and social implications that such testing may have, families with suspected HNPCC should be evaluated first by a genetic counselor and should give informed consent in writing before genetic testing is performed. Patients whose families meet any of the revised Bethesda criteria have an increased likelihood of harboring a germline mutation in one of the mismatch repair genes and should be considered for genetic testing: (1) colorectal cancer under age 50; (2) synchronous or metachronous colorectal or HNPCC-associated tumor regardless of age (endometrial, stomach, ovary, pancreas, ureter and renal pelvis, biliary tract, brain); (3) colorectal cancer with one or more first-degree relatives with colorectal or HNPCC-related cancer, with one of the cancers occurring before age 50; (4) colorectal cancer with two or more second-degree relatives with colorectal or HNPCC cancer, regardless of age. These criteria will identify > 90% of mutation-positive HNPCC families. Tumor tissues of affected individuals or family members meeting the revised Bethesda criteria should undergo immunohistochemical staining for MSH2, MLH1, and PMS2 (commercially available assays) or tested for microsatellite instability. Individuals whose tumors have normal immunohistochemical staining or do not have microsatellite instability are unlikely to have germline mutations in mismatch repair genes, do not require further genetic testing, and do not require intensive cancer surveillance. Germline testing for gene mutations is positive in > 90% of individuals whose tumors show absent histochemical staining of one of the mismatch repair genes and in 50% of those whose tumors have a high level of microsatellite instability. Germline testing is also warranted in families with a strong history consistent with HNPCC when tumors from affected members are unavailable for assessment. If a mutation is detected in a patient with cancer in one of the known mismatch genes, genetic testing of other first-degree family members is indicated.
If genetic testing documents an HNPCC gene mutation, affected relatives should be screened with colonoscopy every 1 2 years beginning at age 25 (or at age 5 years younger than the age at diagnosis of the youngest affected family member). If cancer is found, subtotal colectomy with ileorectal anastomosis (followed by annual surveillance of the rectal stump)
P.652
should be performed. Upper endoscopy should be performed every 2 3 years to screen for gastric cancer. Women should undergo screening for endometrial and ovarian cancer beginning at age 25 35 years with pelvic examination, CA-125 assay, endometrial aspiration, and transvaginal ultrasound. Prophylactic hysterectomy and oophorectomy may be considered, especially in women of postchildbearing age. Similarly, consideration should be given for increased cancer surveillance in family members in proven or suspected HNPCC families who do not wish to undergo germline testing.
Hampel H et al: Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005; 352: 1851.
Hampel H et al: Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: later age of onset. Gastroenterology 2005; 129:415.
Lindor NM et al: Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005;293:1979.
Terdiman JP: Colorectal cancer at a young age. Gastroenterology 2005;128:1067.
Colorectal Cancer
![]() Essentials of Diagnosis
Essentials of Diagnosis
Symptoms or signs depend on tumor location.
Proximal colon: fecal occult blood, anemia.
Distal colon: change in bowel habits, hematochezia.
Characteristic findings on barium enema or CT colonography.
Diagnosis established with colonoscopy.
General Considerations
Colorectal cancer is the second leading cause of death due to malignancy in the United States. Approximately 6% of Americans will develop colorectal cancer and 40% of those will die of the disease. An estimated 145,000 new cases and 55,000 deaths occur annually. Colorectal cancers are almost all adenocarcinomas, which tend to form bulky exophytic masses or annular constricting lesions. Slightly less than 50% of cancers are located distal to the splenic flexure (within the descending colon or rectosigmoid region), where they are within reach of detection by flexible sigmoidoscopy. There appears to be a gradual increase in incidence of cancers in the cecum and ascending colon, and over half of cancers now develop proximal to the splenic flexure. It is currently believed that the majority of colorectal cancers arise from malignant transformation of an adenomatous polyp. Approximately 85% of sporadic colorectal cancers have loss of function of one or more tumor suppressor genes (eg, p53, APC, or DCC) due to a combination of spontaneous mutation of one allele combined with chromosomal instability that leads to deletion and loss of heterozygosity of the other allele (eg, 5q, 17q, or 18p deletion). Approximately 15% of colorectal cancers have microsatellite instability, suggesting inactivation of mismatch repair genes that is most commonly acquired but may be an indicator of unrecognized HNPCC (see above).
Risk Factors
A number of factors increase the risk of developing colorectal cancer. Recognition of these has impact on screening strategies. However, 75% of all cases occur in people with no known predisposing factors.
A. Age
The incidence of colorectal cancer rises sharply after age 45 years, and 90% of cases occur in persons over the age of 50 years.
B. Family History of Neoplasia
A family history of colorectal cancer is present in 20% of patients with colon cancer. Hereditary factors are believed to contribute to 20 30% of colorectal cancers; however, the genes responsible for most of these cases have not yet been identified. Up to 5% of colorectal cancers are caused by inherited germline mutations resulting in polyposis syndromes or HNPCC (reviewed elsewhere in this chapter). Approximately 6% of the Ashkenazic Jewish population has a missense mutation in the APC gene (APC I1307K) that confers a modestly increased lifetime risk of developing colorectal cancer (OR 1.4 1.9) but phenotypically resembles sporadic colorectal cancer rather than FAP. Genetic screening is available, and patients harboring the mutation merit intensive screening.
A family history of colorectal cancer or adenomatous polyps is one of the most important risk factors for colorectal cancer. The risk of colon cancer is proportionate to the number and age of affected first-degree family members with colon cancer. People with one first-degree family member with colorectal cancer have an increased risk approximately 2 times that of the general population. However, the relative risk is 3.8 times if the family member's cancer was diagnosed at < 45 years of age, 2.2 if diagnosed at 45 59 years of age, and only 1.8 if diagnosed at > 59 years of age. Patients with two first-degree relatives have a fourfold or 25 30% lifetime risk of developing colon cancer. First-degree relatives of patients with adenomas also are at increased risk for colorectal neoplasia, especially if the adenoma was detected before age 60 years. Cancers arise at an earlier age in patients with a positive family history, meriting screening at an earlier age. The risk of a 40-year-old person with a positive family history is comparable to that of an average-risk 50-year-old person.
P.653
C. Inflammatory Bowel Disease
The risk of adenocarcinoma of the colon begins to rise 7 10 years after disease onset in patients with ulcerative colitis and Crohn's colitis. The cumulative risk approaches 5 10% after 20 years and 20% after 30 years. Chronic treatment with 5-ASA agents and folate is associated with a lower risk of cancer in patients with ulcerative colitis.
D. Dietary Factors and Chemoprevention
In epidemiologic studies, diets rich in fats and red meat are associated with an increased risk of colorectal adenomas and cancer, whereas diets high in fruits, vegetables, and fiber are associated with a decreased risk. However, two prospective, randomized controlled trials failed to demonstrate a risk reduction in the recurrence of adenomas after treatment with a diet low in fat and high in fiber, fruits, and vegetables or with fiber supplementation over a 3- to 4-year period. Both calcium carbonate (3 g/d) and folate therapy have been shown in prospective trials to yield a modest but significant reduction in the relative risk of developing colorectal neoplasia. In women, hormone replacement therapy may also be beneficial. The antioxidant vitamins A, C, and E have not been shown to be of benefit in prospective controlled studies.
Cohort and case-control studies show that prolonged regular use of aspirin (at least 325 mg twice weekly) and NSAIDs is associated with a 30 50% decrease in the incidence of colorectal cancer and adenomas. Several prospective, blinded clinical trials have shown that daily low-dose aspirin (80 325 mg) reduces the number of recurrent adenomas at 1 3 years in patients with a history of colorectal adenomas or cancer. Because chronic aspirin use is associated with a low incidence of serious complications (gastrointestinal hemorrhage, stroke), it should not be prescribed as a chemopreventive agent in people without polyps or with small adenomas unless there are other medical indications. Chronic administration of these agents may be considered in patients with a personal or family history of colorectal cancer or advanced adenomas; however, they do not obviate the need for screening and surveillance.
E. Race
The incidence of colon adenocarcinoma is higher in blacks than in whites. It is unclear whether this is due to genetic or socioeconomic factors (eg, diet or reduced access to screening).
Clinical Findings
A. Symptoms and Signs
Adenocarcinomas grow slowly and may be present for several years before symptoms appear. However, asymptomatic tumors may still be detected by the presence of fecal occult blood (see Colorectal Cancer Screening, below). Symptoms depend on the location of the carcinoma. Chronic blood loss from right-sided colonic cancers may cause iron deficiency anemia, manifested by fatigue and weakness. Obstruction, however, is uncommon because of the large diameter of the right colon and the liquid consistency of the fecal material. Lesions of the left colon often involve the bowel circumferentially. Because the left colon has a smaller diameter and the fecal matter is solid, obstructive symptoms may develop with colicky abdominal pain and a change in bowel habits. Constipation may alternate with periods of increased frequency and loose stools. The stool may be streaked with blood, though marked bleeding is unusual. With rectal cancers, patients note tenesmus, urgency, and recurrent hematochezia. Weight loss is uncommon. Physical examination is usually normal except in advanced disease. A mass may be palpable in the abdomen. The liver should be examined for hepatomegaly, suggesting metastatic spread. For cancers of the distal rectum, digital examination is necessary to determine whether there is extension into the anal sphincter or fixation, suggesting extension to the pelvic floor.
B. Laboratory Findings
A complete blood count is obtained to look for evidence of anemia. Elevated liver function tests are suspicious for metastatic disease. Carcinoembryonic antigen (CEA) should be measured in all patients with proved colorectal cancer. A preoperative CEA level > 5 ng/mL is a poor prognostic indicator. After complete surgical resection, CEA levels should normalize; persistently elevated levels suggest the presence of persistent disease and warrant further evaluation.
C. Inspection of the Colon
Cancers may be detected with a high degree of reliability with barium enema, CT colonography ( virtual colonoscopy ), or colonoscopy. Colonoscopy is the diagnostic procedure of choice in patients with a clinical history suggestive of colon cancer or in patients with an abnormality suspicious for cancer detected on radiographic imaging. Colonoscopy permits biopsy for pathologic confirmation of malignancy. In patients in whom colonoscopy is unable to reach the cecum (< 5% of cases) or when a nearly obstructing tumor precludes passage of the colonoscope, barium enema or CT colonography examination should be performed.
D. Imaging
Clinicians obtain an abdominal and pelvic CT scan to assist in preoperative staging, although it is unclear that it alters surgical management in most cases. CT scans may demonstrate distal metastases but is less accurate in the determination of the level of local tumor extension (T stage) or lymphatic spread (N stage). Intraoperative assessment of the liver by direct palpation and ultrasonography is more accurate than CT scanning for the detection of hepatic metastases. For rectal cancer, pelvic MRI or endorectal ultrasonography provides important accurate information
P.654
about the depth of penetration of the cancer through the rectal wall and pararectal lymph nodes that may guide preoperative (neoadjuvant) chemoradiotherapy and operative management. A chest CT scan should also be obtained because the systemic blood supply of the distal rectum may promote distal tumor metastasis outside the abdomen. Positron emission tomography (PET) is useful to determine recurrent colorectal cancer but currently is not used for staging of primary tumors.
Table 14-16. Staging of colorectal cancer. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Differential Diagnosis
The nonspecific symptoms of colon cancer may be confused with those of irritable bowel syndrome, diverticular disease, ischemic colitis, inflammatory bowel disease, infectious colitis, and hemorrhoids. Neoplasm must be excluded in any patient over age 40 years who reports a change in bowel habits or hematochezia or who has an unexplained iron deficiency anemia or occult blood in the stools.
Staging
Determination of the stage of colorectal cancer is important not only because it correlates with the patient's long-term survival but also because it is used to determine which patients should receive adjuvant therapy (Table 14-16). Although the Dukes classification has been widely employed in the past, the TNM system is now more commonly used.
Treatment
Resection of the primary colonic or rectal cancer is the treatment of choice for almost all patients who have resectable lesions and can tolerate general anesthesia. Multiple studies demonstrate that minimally invasive, laparoscopically assisted colectomy results in similar outcomes and rates of recurrence to open colectomy. Regional lymph node dissection should be performed to determine staging, which guides decisions about adjuvant therapy. Even patients with extensive metastatic disease may benefit from resection of the colonic tumor to reduce the likelihood of intestinal obstruction or serious bleeding.
For rectal carcinoma, the operative approach depends on the level of the tumor above the anal verge, the size and depth of penetration, and the patient's overall condition. Clinical staging by endorectal ultrasound or MRI with endorectal coil is important in guiding the clinical approach. In carefully selected patients with small, mobile (< 4 cm), well-differentiated T1 or T2 rectal tumors that are less than 7.5 cm from the anal verge and that appear on endosonography to be localized to the rectal wall, transanal excision may be performed. This approach avoids laparotomy and spares the rectum and anal sphincter, preserving normal bowel continence. All other patients will require either a low anterior resection with a colorectal or coloanal anastomosis or an abdominoperineal resection with a colostomy, depending on how far above the anal verge the tumor is located and the extent of local tumor spread. Careful dissection of the entire mesorectum at the time of surgery has been shown to reduce local recurrence to 8%. With improvements in surgical stapling techniques, it is possible to perform low anterior resection provided there is a margin of at least 2 cm of normal tissue below the tumor. Although low resections obviate a colostomy, they are associated
P.655
with increased immediate postsurgical complications (leak, dehiscence, stricture) and defecatory complaints (increased stool frequency, defecatory problems, and incontinence). With unresectable rectal cancer, the patient may be palliated with a diverting colostomy, laser fulguration, or placement of an expandable wire stent.
A. Adjuvant Therapy for Colon Cancer
Adjuvant chemotherapy and radiotherapy have been demonstrated to improve overall and tumor-free survival in selected patients with colon cancer.
1. Stage I
Because of the excellent 5-year survival rate (90 100%), no adjuvant therapy is recommended.
2. Stage II (node-negative disease)
The expected 5-year survival rate is 80%. A benefit from adjuvant chemotherapy has not been demonstrated in most controlled trials for stage II colon cancer (see discussion for Stage III disease). Patients with advanced local stage II disease (perforation, T4) may be considered for study protocols looking at the role of adjuvant chemotherapy for control of local recurrence.
3. Stage III (node-positive) disease
With surgical resection alone, the expected 5-year survival rate is 30 50%. Postoperative adjuvant chemotherapy significantly increases disease-free survival as well as overall survival and is recommended for all patients. In stage III colorectal cancer, patients treated for 6 months with intravenous 5-fluorouracil (5-FU) (bolus or continuous infusion) and leucovorin (folinic acid) who have one to three involved nodes have a 5-year survival of 65%, and those with more than three involved nodes have a 5-year survival of up to 40%. Therefore, until recently, adjuvant therapy with 5-FU and leucovorin was most widely used for stage III disease. However, in a recent multicenter trial, monotherapy with an oral 5-FU analog capecitabine yielded a similar rate of disease-free survival with a lower rate of serious side effects compared with intravenous 5-FU and leucovorin. Furthermore, a large, well-designed study (MOSAIC trial) of adjuvant therapy for stage II and III colon cancer reported at 4 years a higher rate of disease-free survival for patients treated with a combination of oxaliplatin, fluorouracil, and leucovorin (FOLFOX) (76%) than with fluorouracil and leucovorin (FL) alone (69%). Benefit was higher for stage III than stage II cancers. The addition of oxaliplatin was associated with an increased incidence of neutropenia and sensory neuropathy. Although further study is needed, combined adjuvant therapy with oxaliplatin should be considered for stage III disease in optimal candidates.
4. Stage IV (metastatic disease)
Approximately 20% of patients have metastatic disease at the time of initial diagnosis, and another 30% eventually develop metastasis. The long-term survival of these patients is only 5%, and the median survival is only 6 months in the absence of other treatment. Resection of isolated (one to three) liver or lung metastases may result in long-term (over 5 years) survival in 35 55% of cases. For those with unresectable hepatic metastases, local ablative techniques (cryosurgery, radiofrequency or microwave coagulation, embolization, hepatic intra-arterial chemotherapy) may provide long-term tumor control. Approximately 20% of patients with metastatic disease respond to chemotherapy regimens containing intravenous 5-FU and folinic acid (leucovorin), prolonging median survival to about 11 months. The oral 5-FU agent capecitabine may be preferred in patients with advanced disease who desire the convenience of oral rather than intravenous therapy. However, the addition of either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI; IFL) to 5-FU and folinic acid provides further improvement in tumor response rate (40%) and median survival (14 16 months). Currently, oxaliplatin-based regimens are commonly used as first-line therapy for metastatic disease, because irinotecan-based regimens are associated with greater toxicity (especially diarrhea and febrile neutropenia). Patients progressing with one regimen may respond to the alternative regimen, prolonging mean survival to > 20 months. Cetuximab, a monoclonal antibody to EGFR, has demonstrated improvement in tumor response rates and median survival when used in combination with irinotecan-based chemotherapeutic regimens, including tumors with resistance to irinotecan. Bevacizumab is a monoclonal antibody to vascular endothelial growth factor that is inactive as a single-agent against colorectal cancer but leads to further prolongation of mean survival by 5 months when used in combination with either irinotecan-based (IFL) or oxaliplatin-based (FOLFOX) regimens. The mean survival in patients treated with bevacizumab plus these regimens exceeds 20 months. However, bevacizumab may cause serious thromboembolic events (including stroke and myocardial infarction) in 5% of patients. Currently, the role of these effective but very expensive regimens as first-line or second-line therapy for metastatic disease is unclear.
B. Adjuvant Therapy for Rectal Cancer
Compared with colon cancer, rectal cancer has lower long-term survival rates and significantly higher rates of local tumor recurrence (25%) due to the difficulty of achieving adequate surgical resection margins. Combination therapy with fluorouracil and radiation has been shown to improve the disease-free survival rate and to decrease pelvic recurrence and is recommended for all patients with stage II and stage III rectal cancers. It has long been controversial whether chemoradiation should be administered preoperatively ( neoadjuvant ) or postoperatively ( adjuvant ). Neoadjuvant therapy may decrease the size of the tumor before surgery (tumor downstaging), allowing more patients to undergo curative resection with sphincter preservation rather than abdominoperineal resection. When initial imaging studies suggest stage I disease, surgery may be performed first, followed by postoperative chemoradiation in patients found at surgery to have more advanced (stage II or III) disease. A recent,
P.656
large, randomized controlled trial reported that preoperative therapy led to better patient treatment compliance, reduced local recurrence and toxicity, and a higher number of sphinter-preserving resections. Therefore, preoperative chemoradiation increasingly is recommended for patients with distal rectal cancers that are determined to be stage II or III by endorectal ultrasound or MRI.
Follow-Up after Surgery
Patients who have undergone resections for cure are followed closely to look for evidence of symptomatic or asymptomatic tumor recurrence that may be amenable to curative resection in a small number of patients. The optimal cost-effective strategy is not clear and professional guidelines provide different recommendations. Two randomized trials reported that intense follow-up with yearly colonoscopy, abdominal CT, and chest radiography did not improve overall outcome compared with most standard follow-up protocols. In the absence of consensus guidelines, the following may be recommended. Patients should be evaluated every 3 6 months for 3 5 years with history, physical examination, and CEA determinations. All patients should undergo colonoscopy either preoperatively or within 3 6 months postoperatively to exclude other synchronous colorectal neoplasms. Thereafter, surveillance colonoscopy should be performed every 3 5 years to look for metachronous polyps or cancer. Because of the high incidence of local tumor recurrence in patients with rectal cancer, sigmoidoscopy should be performed every 6 12 months for 3 years. New onset of symptoms or a rising CEA warrants investigation with chest and abdominal CT to look for recurrent or metastatic disease that may be amenable to therapy. For patients with a rising CEA with unrevealing CT imaging, a PET scan is more sensitive for the detection of occult metastatic disease.
Prognosis
The stage of disease at presentation is the most important determinant of long-term survival: stage I, > 90%; stage II, 70 80%; stage III with fewer than four positive lymph nodes, 67%; stage III with more than four positive lymph nodes, 33%; and stage IV, 5 7%. For each stage, rectal cancers have a worse prognosis. For those patients whose disease progresses despite therapy, meticulous efforts at palliative care are essential (see Chapter 5).
Screening for Colorectal Neoplasms
Colorectal cancer is ideal for screening because it is a common disease affecting 6% of men and women that is fatal in almost 50% of cases yet is curable if detected at an earlier stage. Furthermore, the vast majority of cases arise from benign adenomas that progress over many years to cancer, and removal of adenomas has been shown to prevent the vast majority of cancers. Colorectal cancer screening has now been endorsed by the United States Preventive Services Task Force, the Agency for Health Care Policy and Research, the American Cancer Society, and every professional gastroenterology and colorectal surgery society. Although there is continued debate about the optimal cost-effective means of providing population screening, there is unanimous consent that screening of some kind should be offered to every patient over the age of 50 years. Several analyses suggest that the cost of screening is approximately $25,000 per year of life saved for all recommended screening strategies, which is well below the range of $40,000-$50,000 per year generally considered to be cost-effective.
A number of options for screening are available and reimbursed by third-party payers and by Medicare. The recommendations of a multidisciplinary consensus panel are listed in Table 14-17 for patients at average risk of developing colorectal cancer. Patients with first-degree relatives with colorectal cancer are at increased risk, and for these individuals more intensive screening recommendations are recommended. Recommendations for screening in families with inherited cancer syndromes or inflammatory bowel disease are provided in separate sections. (See Hereditary Colorectal Cancer and Polyposis Syndromes; Inflammatory Bowel Disease.) The advantages and disadvantages of
P.657
the various options are discussed below. It is important for primary care providers to understand the relative merits of various options and to discuss them with their patients. Despite growing awareness of the importance of screening on the part of medical professionals and the public, less than 50% of patients have undergone screening of any kind. Discussion and encouragement by the primary care provider are the most important factors in achieving patient compliance with screening programs. The potential for harm from screening must be weighed against the likelihood of benefit, especially in elderly patients with comorbid illnesses and shorter life expectancy.
Table 14-17. Recommendations for colorectal cancer screening.1 | ||
|---|---|---|
|
A. Fecal Occult Blood Test or Multitarget DNA Assay
Most colorectal cancers and some large adenomas result in increased chronic blood loss that may be detectable. A variety of tests have been developed that have varying sensitivities for fecal occult blood, some of which are in clinical testing. A guaiac-based test (Hemoccult II) has undergone the most extensive testing and has had the greatest clinical use. Two slides must be prepared from three consecutive bowel movements. To reduce the likelihood of false-positive tests, patients should abstain from aspirin (in doses greater than 325 mg/d), NSAIDs, red meat, poultry, fish, and vegetables with peroxide activity (turnips, horseradish) for 72 hours. Vitamin C may cause a false-negative test. Slides should be developed within 7 days after preparation. The World Health Organization recently endorsed another guaiac test for FOBT, Hemoccult Sensa, because it has higher sensitivity than the Hemoccult II test; however, data from large clinical trials with this test are lacking.
When FOBT is administered to the general population as part of a screening program, 1 5% of tests are positive. Patients with positive tests should undergo colonoscopy accompanied by removal of any polyps identified. If colonoscopy reveals no colorectal neoplasm, further screening for colorectal cancer can be deferred for 10 years. Of those with positive tests, 5 18% have colorectal cancer, more likely to be at an earlier stage (Dukes A or B). Adenomatous polyps are identified in 25 50% of patients with positive tests. Finding these polyps is somewhat fortuitous since most are less than 1 cm in size and unlikely to cause occult bleeding. The estimated sensitivity of a guaiac-based FOBT for colorectal cancer is only 30 50%, but 65% with immunochemical tests. Sensitivity may be increased in patients who are compliant with annual testing. In several large prospective studies, FOBT has been demonstrated to reduce mortality from colorectal cancer by 15 33%. Higher risk reductions are obtained with annual versus biennial testing.
A fecal DNA assay (Pre-Gen Plus) is commercially available for screening for colorectal neoplasia. The test analyzes fecal DNA for point mutations in the APC, K-ras, and p53 genes, microsatellite instability, and a marker of abnormal apoptosis. In studies conducted in nonscreening populations, the average sensitivity and specificity for detection of advanced adenomas and cancer are approximately 67% and 97%, respectively. However, in a recent study performed in a large asymptomatic screening population, the fecal DNA panel detected only 41% of cancers and only 18% of adenomas containing high-grade dysplasia and only 18% of all advanced neoplasms (cancers or adenomas that were 1 cm or that contained villous features or high-grade dysplasia). The FOBT (Hemoccult II) performed even worse, detecting only 14% of invasive cancers and 10% of all advanced neoplasms.
The low sensitivity of FOBT and DNA tests for advanced neoplasia makes them a less attractive choice for population-based screening than endoscopic or radiographic tests. At present, they are most suitable in settings where health care resources are limited or in patients who desire a noninvasive method of screening.
B. Flexible Sigmoidoscopy
Use of a 60-cm flexible sigmoidoscope permits visualization of the rectosigmoid and descending colon. It requires no sedation and in many centers is performed by a nurse specialist or physician's assistant. Adenomatous polyps are identified in 10 20% and colorectal cancers in 1% of patients. Polyps less than 5 8 mm in diameter should be removed by biopsy to determine the histology. All patients found to have a high-risk adenomatous polyp (> 1 cm, villous features, or high-grade dysplasia) or more than two diminutive adenomatous polyps should undergo pancolonoscopy to look for synchronous neoplasms in the proximal colon. As discussed previously, there are conflicting opinions about whether patients found to have one or two diminutive adenomas on flexible sigmoidoscopy require colonoscopy (See Adenomatous Polyps, above.) Up to 80% of cases of advanced neoplasia will be detected in flexible sigmoidoscopy screening programs provided that all patients in whom an adenoma is identified at sigmoidoscopy undergo subsequent colonoscopy. The risk of serious complications (perforation) associated with flexible sigmoidoscopy is less than 1:10,000 patients.
C. Screening Colonoscopy
Colonoscopy permits examination of the entire colon. Approximately 50% of advanced neoplasms (cancer, adenomas 1 cm, polyps with villous histology, or high-grade dysplasia) are proximal to the splenic flexure, ie, above the reach of a flexible sigmoidoscopic examination. Up to 50 60% of such patients do not have an adenomatous polyp distal to the splenic flexure at sigmoidoscopy. Therefore, screening programs that use flexible sigmoidoscopy will not identify approximately 20 30% of patients with advanced colonic neoplasia. For this reason, screening colonoscopy is the preferred screening test in patients deemed to be at higher risk due to a positive family history of colorectal cancer. Colonoscopy has been advocated by the American College of Gastroenterology as the preferred screening modality
P.658
in average-risk patients as well. In a population of asymptomatic veterans between 50 and 75 years of age undergoing screening colonoscopy, the prevalence of advanced adenomas or cancer was 10.7%. To alleviate discomfort, intravenous sedation is used for most patients. The incidence of serious complications after colonoscopy (perforation, bleeding, cardiopulmonary events) is 0.3%. Although colonoscopy is believed to be the most sensitive test for detecting adenomas and cancer, it is not infallible. In several studies, the rate of colorectal cancer within 3 years of a screening colonoscopy was 0.7 0.9%, ie, 1 in 110 patients. Even skilled endoscopists may overlook polyps that are small, flat, or located behind folds. Furthermore, some precancerous polyps may undergo rapid progression to cancer.
D. Double Contrast Barium Enema
Like colonoscopy, barium enema permits examination of the entire colon. Barium enema has been an attractive screening technique because it was widely available, relatively inexpensive, and safe. However, it is being rapidly supplanted by colonoscopy and CT colonography. A recent multicenter trial demonstrated that the sensitivity of barium enema is only 50% for polyps 1 cm and 55 85% for early-stage cancers when compared with colonoscopy. At present, barium enema may be recommended when screening of the entire colon is desired and the patient is unable or unwilling to undergo colonoscopy, and expertise in CT colonography is unavailable.
E. CT Colonography (Virtual Colonoscopy)
Using computer-assisted image reconstruction and rapid helical CT, two- and three-dimensional views can be generated of the colon lumen that simulate the view of colonoscopy. This technique is performed rapidly, requires no sedation or intravenous contrast, and has minimal risk except for radiation exposure. At this time, it requires a similar bowel cleansing regimen as colonoscopy as well as the insufflation of air into the colon through a rectal tube, which may be associated with discomfort. In several recent large studies, the accuracy of virtual colonoscopy compared with colonoscopy has been assessed for the screening of colorectal neoplasia in asymptomatic screening populations. In several studies, the sensitivity of virtual colonoscopy for the detection of polyps larger than 8 10 mm is > 90%; however, other studies report sensitivity of only 50 60%. Abnormalities identified on CT imaging require assessment by colonoscopy. Although most professional guidelines do not yet endorse virtual colonoscopy for routine colorectal screening, it is a reasonable alternative for patients in whom screening of the entire colon is desired but who are unable or unwilling to undergo colonoscopy.
Callery MP: Combined modality therapy for rectal cancer. Gastroenterology 2005;128:1516.
Colon Cancer National Cancer Institute Cancer Net: http://www.cancernet.nci.nih.gov.
Hurwitz H et al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335.
Imperiale T et al: Fecal DNA versus fecal occult blood for colorectal cancer screening in an average-risk population. N Engl J Med 2004;351:2704.
Meyerhardt JA et al: Systematic therapy for colorectal cancer. N Engl J Med 2005;352:476.
Minsky BD: Adjuvant therapy for rectal cancer the transatlantic view. Colorectal Dis 2003;5:416.
Morikawa T et al: A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 2005;129:422.
Nelson H et al: A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350: 2050.
Nicholson FB et al: The role of CT colography in colorectal cancer screening. Am J Gastroenterol 2005;100:2316.
Ouyang DL et al: Noninvasive testing for colorectal cancer: a review. Am J Gastroenterol 2005;100:1393.
Pfister DG et al: Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med 2004;350:2375.
Rockey DC et al: Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005;365:305.
Schoenfeld P et al: Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med 2005;352:2061.
Twelves C et al: Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696.
Van Dam J et al: AGA future trends report: CT colonography. Gastroenterology 2004;127:970.
Walsh JM et al: Colorectal cancer screening: clinical applications. JAMA 2003;289:1297.
Weitz J et al: Colorectal cancer. Lancet 2005;365:153.
Anorectal Diseases
Hemorrhoids
![]() Essentials of Diagnosis
Essentials of Diagnosis
Bright red blood per rectum.
Protrusion, discomfort.
Characteristic findings on external anal inspection and anoscopic examination.
General Considerations
Internal hemorrhoids are subepithelial vascular cushions consisting of connective tissue, smooth muscle fibers, and arteriovenous communications between terminal branches of the superior rectal artery and rectal veins. They are a normal anatomic entity, occurring in all adults, that contribute to normal anal pressures and ensure
P.659
a water-tight closure of the anal canal. They commonly occur in three primary locations right anterior, right posterior, and left lateral. External hemorrhoids arise from the inferior hemorrhoidal veins located below the dentate line and are covered with squamous epithelium of the anal canal or perianal region.
Hemorrhoids may become symptomatic as a result of activities that increase venous pressure, resulting in distention and engorgement. Straining at stool, constipation, prolonged sitting, pregnancy, obesity, and low-fiber diets all may contribute. With time, redundancy and enlargement of the venous cushions may develop and result in bleeding or protrusion.
Clinical Findings
A. Symptoms and Signs
Patients often attribute a variety of perianal complaints to hemorrhoids. However, the principal problems attributable to internal hemorrhoids are bleeding, prolapse, and mucoid discharge. Bleeding is manifested by bright red blood that may range from streaks of blood visible on toilet paper or stool to bright red blood that drips into the toilet bowl after a bowel movement. Rarely is bleeding severe enough to result in anemia. Initially, internal hemorrhoids are confined to the anal canal (stage I). Over time, the internal hemorrhoids may gradually enlarge and protrude from the anal opening. At first, this mucosal prolapse occurs during straining and reduces spontaneously (stage II). With progression over time, the prolapsed hemorrhoids may require manual reduction after bowel movements (stage III) or may remain chronically protruding (stage IV). Chronically prolapsed hemorrhoids may result in a sense of fullness or discomfort and mucoid perianal discharge, resulting in irritation and soiling of underclothes. Pain is unusual with internal hemorrhoids, occurring only when there is extensive inflammation and thrombosis of irreducible tissue or with thrombosis of an external hemorrhoid (see below).
B. Examination
External hemorrhoids are readily visible on perianal inspection. Nonprolapsed internal hemorrhoids are not visible but may protrude through the anus with gentle straining while the physician spreads the buttocks. Prolapsed hemorrhoids are visible as protuberant purple nodules covered by mucosa. The perianal region should also be examined for other signs of disease such as fistulas, fissures, skin tags, or dermatitis. On digital examination, uncomplicated internal hemorrhoids are neither palpable nor painful. Anoscopic evaluation, best performed in the prone jackknife position, provides optimal visualization of internal hemorrhoids.
Differential Diagnosis
Small volume rectal bleeding may be caused by anal fissure or fistula, neoplasms of the distal colon or rectum, ulcerative colitis or Crohn's colitis, infectious proctitis, or rectal ulcers. Rectal prolapse, in which a full thickness of rectum protrudes concentrically from the anus, is readily distinguished from mucosal hemorrhoidal prolapse. Proctosigmoidoscopy should be performed in all patients with hematochezia to exclude disease in the rectum or sigmoid colon that could be misinterpreted in the presence of hemorrhoidal bleeding. Patients with iron deficiency anemia should undergo colonoscopy or barium enema to exclude disease proximal to the sigmoid colon.
Treatment
A. Conservative Measures
Most patients with early (stage I and stage II) disease can be managed with conservative treatment. To decrease straining with defecation, patients should be given instructions for a high-fiber diet and told to increase fluid intake with meals. Dietary fiber may be supplemented with bran powder (1 2 tbsp twice daily added to food or in 8 oz of liquid) or with commercial psyllium bulk laxatives (eg, Metamucil, Citrucel). Suppositories and rectal ointments have no demonstrated utility in the management of mild disease. Mucoid discharge may be treated effectively by the local application of a cotton ball tucked next to the anal opening after bowel movements. For edematous, prolapsed hemorrhoids, gentle manual reduction may be supplemented by suppositories (eg, Anusol with or without hydrocortisone) or topical pads containing witch hazel (eg, Tucks) that have anesthetic and astringent properties and by warm sitz baths.
B. Medical Treatment
Patients with stage I, stage II, and stage III hemorrhoids and recurrent bleeding despite conservative measures may be treated without anesthesia with injection sclerotherapy, rubber band ligation, or application of electrocoagulation (bipolar cautery or infrared photocoagulation). The choice of therapy is dictated by operator preference, but rubber band ligation increasingly is preferred due to its ease of use and high rate of efficacy. Major complications occur in < 2%, including pelvic sepsis, pelvic abscess, urinary retention, and bleeding. Recurrence is common unless patients alter their dietary habits.
C. Surgical Treatment
Surgical excision (hemorrhoidectomy) is reserved for < 5 10% of patients with chronic severe bleeding due to stage III or stage IV hemorrhoids or patients with acute thrombosed stage IV hemorrhoids. Complications of surgical hemorrhoidectomy include postoperative pain (which may persist for 2 4 weeks) and impaired continence.
Thrombosed External Hemorrhoid
Thrombosis of the external hemorrhoidal plexus results in a perianal hematoma. It most commonly occurs in
P.660
otherwise healthy young adults and may be precipitated by coughing, heavy lifting, or straining at stool. The condition is characterized by the relatively acute onset of an exquisitely painful, tense and bluish perianal nodule covered with skin that may be up to several centimeters in size. Pain is most severe within the first few hours but gradually eases over 2 3 days as edema subsides. Symptoms may be relieved with warm sitz baths, analgesics, and ointments. If the patient is evaluated in the first 24 48 hours, removal of the clot may hasten symptomatic relief. With the patient in the lateral position, the skin around and over the lump is injected subcutaneously with 1% lidocaine using a tuberculin syringe with a 30-gauge needle. An ellipse of skin is then excised and the clot evacuated. A dry gauze dressing is applied for 12 24 hours, and daily sitz baths are then begun.
Madoff RD et al: American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology 2004;126:1463.
Nisar PJ et al: Managing haemorrhoids. BMJ 2003;327:847.
Wehrmann T et al: Hemorrhoidal elastic band ligation with flexible videoendoscopes: a prospective, randomized comparison with the conventional technique that uses rigid proctoscopes. Gastrointest Endosc 2004;60:191.
Anorectal Infections
A number of organisms can cause inflammation of the anal and rectal mucosa. Proctitis is defined as inflammation of the distal 15 cm of rectum and is characterized by anorectal discomfort, tenesmus, constipation, and discharge. Most cases of proctitis are sexually transmitted, especially by anal-receptive intercourse. Proctocolitis implies inflammation that extends above the rectum to the sigmoid colon or more proximally and is caused by entirely different organisms such as Campylobacter, E histolytica, Shigella, and enteroinvasive E coli. These organisms are discussed earlier in the section on diarrhea. Symptoms include frequent, small-volume, bloody or watery diarrhea, urgency, cramps, and tenesmus.
Etiology & Management
Several organisms may cause infectious proctitis.
A. Neisseria gonorrhoeae
Gonorrhea may cause itching, burning, tenesmus, and a mucopurulent discharge. Blind swabs of the anal canal have a sensitivity of less than 60%. Swabs should be taken for Gram staining and culture during anoscopy, expressing mucopus from the anal crypts. Cultures should also be taken from the urethra and pharynx in men and from the cervix in women. Complications of untreated infections include strictures, fissures, fistulas, and perirectal abscesses.
B. Treponema pallidum
Anal syphilis may be asymptomatic or may lead to perianal pain and discharge. With primary syphilis, the chancre may be at the anal margin or within the anal canal and may mimic a fissure, fistula, or ulcer. Proctitis or inguinal lymphadenopathy may be present. With secondary syphilis, condylomata lata (pale-brown, flat verrucous lesions) may be seen, with secretion of foul-smelling mucus. The diagnosis is established with dark-field microscopy of scrapings from the chancre or condylomas. The VDRL test is positive in 75% of primary cases and in 99% of secondary cases.
C. Chlamydia trachomatis
Chlamydial infection may cause proctitis similar to gonorrheal proctitis or may cause lymphogranuloma venereum, characterized by proctocolitis with fever and bloody diarrhea, painful perianal ulcerations, anorectal strictures and fistulas, and inguinal adenopathy (buboes). The diagnosis is established by serology and culture of rectal discharge or rectal biopsy.
D. Herpes Simplex Type 2
Herpes simplex virus is a common cause of anorectal infection. Symptoms occur 4 21 days after exposure and include severe pain, itching, constipation, tenesmus, urinary retention, and radicular pain from involvement of lumbar or sacral nerve roots. Small vesicles or ulcers may be seen in the perianal area or anal canal. Sigmoidoscopy is not usually necessary but may reveal vesicular or ulcerative lesions in the distal rectum. Diagnosis is established by viral culture or antigen detection assays of vesicular fluid. Symptoms resolve within 2 weeks, but viral shedding may continue for several weeks. Patients may remain asymptomatic with or without viral shedding or may have recurrent mild relapses. Treatment of acute infection with acyclovir, 400 mg orally five times daily for 5 10 days, has been shown to reduce the duration of symptoms and viral shedding. Patients with AIDS and recurrent relapses may benefit from chronic suppressive therapy (see Chapter 32).
E. Condylomata Acuminata
Condylomata acuminata are a significant cause of anorectal symptoms. Caused by the human papillomavirus (HPV), they are seen in up to 25% of homosexual men. HIV-positive individuals with condylomas have a higher relapse rate after therapy and a higher rate of progression to high-grade dysplasia or anal cancer. The warts are located on the perianal skin and extend within the anal canal up to 2 cm above the dentate line. Patients may have no symptoms or may report itching, bleeding, and pain. The warts may form a confluent mass that may obscure the anal opening. Treatment can be difficult. Sexual partners should also be examined and treated. Topical application of podophyllum resin, imiquimod cream 5%, or podofilox 0.5% gel is effective for small external perianal lesions. Anal canal lesions require topical application of bichloroacetic acid, cryotherapy, or electrocautery. Refractory or large lesions may be treated with local injection
P.661
of interferon- beneath the lesions or surgical excision. HIV-positive individuals with condylomas who have detectable serum HIV RNA levels should have anoscopic surveillance every 3 6 months.
Mabey D et al: Lymphogranuloma venereum. Sex Transm Infect 2002;78:90.
Piketty C et al: High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med 2003;138:453.
Vukasin P: Anal condyloma and HIV-associated anal disease. Surg Clin North Am 2002;82:1199.
Workowski KA et al: Sexually transmitted diseases treatment guidelines 2002. Centers for Disease Control and Prevention. MMWR Recomm Rep 2002;51(RR-6):1. (Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5106a1.htm.)
Rectal Prolapse & Solitary Rectal Ulcer Syndrome
Rectal prolapse is protrusion through the anus of some or all of the layers of the rectum. Hemorrhoidal (mucosal) prolapse is common (see discussion under Hemorrhoids). Full thickness is uncommon and is usually caused by surgical or traumatic injuries or from chronic, excessive straining at stool in conjunction with weakening of pelvic support structures, especially in patients who are elderly, psychotic, or paraplegic. Although prolapse initially reduces spontaneously after defecation, with time the rectal mucosa becomes chronically prolapsed, resulting in mucous discharge, bleeding, incontinence, and sphincteric damage. Patients with complete prolapse require surgical correction.
The term solitary rectal ulcer syndrome is a misnomer. The syndrome is characterized by anal pain, excessive straining at stool, and passage of mucus and blood. It is most commonly seen in young adults, especially women. Proctoscopic examination reveals either shallow ulcerations (single or multiple) or a nodular mass located anteriorly 6 10 cm above the anal verge. Biopsy is diagnostic. The disorder may be caused by rectal intussusception or prolapse with straining. Treatment is directed at decreasing straining through education of the patient, use of bulking agents, and surgical treatment of prolapse, when present.
Purkayastha S et al: A comparison of open vs. laparoscopic abdominal rectopexy for full-thickness rectal prolapse: a meta-analysis. Dis Colon Rectum 2005;48:1930.
Sharara AI et al: Solitary rectal ulcer syndrome: endoscopic spectrum and review of the literature. Gastrointest Endosc 2005;62:755.
Fecal Incontinence
There are five general requirements for bowel continence: (1) solid or semisolid stool (even healthy young adults have difficulty maintaining continence with liquid rectal contents); (2) a distensible rectal reservoir (as sigmoid contents empty into the rectum, the vault must expand to accommodate); (3) a sensation of rectal fullness (if the patient cannot sense this, overflow may occur before the patient can take appropriate action); (4) intact pelvic nerves and muscles; and (5) the ability to reach a toilet in a timely fashion.
Minor Incontinence
Many patients complain of inability to control flatus or slight soilage of undergarments that tends to occur after bowel movements or with straining or coughing. This may be due to local anal problems such as hemorrhoids and skin tags that make it difficult to form a tight anal seal, especially if stools are somewhat loose. Patients should be treated with fiber supplements to provide greater stool bulk. Coffee and other caffeinated beverages should be eliminated. The perianal skin should be cleansed with moist, lanolin-coated tissue (baby wipes) to reduce excoriation and infection. After wiping, loose application of a cotton ball near the anal opening may absorb small amounts of fecal leakage. Prolapsing hemorrhoids may be treated with band ligation or surgical hemorrhoidectomy. Control of flatus and seepage may be improved by Kegel perineal exercises. Conditions such as ulcerative proctitis that cause tenesmus and urgency, chronic diarrheal conditions, and irritable bowel syndrome may result in difficulty in maintaining complete continence, especially if a toilet is not readily available. Loperamide may be helpful to reduce urge incontinence in patients with loose stools and may be taken in anticipation of situations in which a toilet may not be readily available. The elderly may require more time or assistance to reach a toilet, which may lead to incontinence. Scheduled toileting and the availability of a bedside commode are helpful. Elderly patients with chronic constipation may develop stool impaction leading to overflow incontinence.
Major Incontinence
Complete uncontrolled loss of stool reflects a significant problem with central perception or neuromuscular function. Incontinence that occurs without awareness suggests a loss of central awareness (eg, dementia, cerebrovascular accident, multiple sclerosis) or peripheral nerve injury (eg, spinal cord injury, cauda equina syndrome, pudendal nerve damage due to obstetric trauma or pelvic floor prolapse, aging, or diabetes mellitus). Incontinence that occurs despite awareness and active efforts to retain stool suggests sphincteric damage, which may be caused by traumatic childbirth (especially forceps delivery), episiotomy, prolapse, prior anal surgery, and physical trauma.
Physical examination should include careful inspection of the perianal area for hemorrhoids, rectal prolapse, fissures, fistulas, and either gaping or a keyhole defect of the anal sphincter (indicating severe sphincteric injury). The perianal skin should be stimulated
P.662
to confirm an intact anocutaneous reflex. Digital examination during relaxation gives valuable information about resting tone (due mainly to the internal sphincter) and contraction of the external sphincter and pelvic floor during squeezing. It also excludes fecal impaction. Anoscopy is required to evaluate for hemorrhoids, fissures, and fistulas. Proctosigmoidoscopy is useful to exclude rectal carcinoma or proctitis. Anal ultrasonography or pelvic MRI is the most reliable test for definition of anatomic defects in the external and internal anal sphincters. Anal manometry may also be useful to define the severity of weakness, to assess sensation, and to predict response to biofeedback training. In special circumstances, surface electromyography is useful to document sphincteric denervation and proctography to document perineal descent or rectal intussusception.
Patients who are incontinent only of loose or liquid stools are treated with bulking agents and antidiarrheal drugs (eg, loperamide, 2 mg before meals and prophylactically before social engagements, shopping trips, etc). Patients with incontinence of solid stool benefit from scheduled toilet use after glycerin suppositories or tap water enemas. Biofeedback training with anal sphincteric strengthening (Kegel) exercises (alternating 5-second squeeze and 10-second rest for 10 minutes twice daily) is helpful in motivated patients to lower the threshold for awareness of rectal filling or to improve anal sphincter squeeze function or both. Operative management is seldom needed but should be considered in patients with major incontinence due to prior injury to the anal sphincter who have failed medical therapy.
Madoff RD et al: Faecal incontinence in adults. Lancet 2004; 364:621.
Rao SS et al: Diagnosis and management of fecal incontinence. American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol 2004;99:1585.
Schnelle JF et al: Urinary and fecal incontinence in nursing homes. Gastroenterology 2004;126(1 Suppl 1):S41.
Other Anal Conditions
Anal Fissures
Anal fissures are linear or rocket-shaped ulcers that are usually less than 5 mm in length. Most fissures are believed to arise from trauma to the anal canal during defecation, perhaps caused by straining, constipation, or high internal sphincter tone. They occur most commonly in the posterior midline, but 10% occur anteriorly. Fissures that occur off the midline should raise suspicion for Crohn's disease, HIV/AIDS, tuberculosis, syphilis, or anal carcinoma. Patients complain of severe, tearing pain during defecation followed by throbbing discomfort that may lead to constipation due to fear of recurrent pain. There may be mild associated hematochezia, with blood on the stool or toilet paper. Anal fissures are confirmed by visual inspection of the anal verge while gently separating the buttocks. Acute fissures look like cracks in the epithelium. Chronic fissures result in fibrosis and the development of a skin tag at the outermost edge (sentinel pile). Digital and anoscopic examinations may cause severe pain and may not be possible. Medical management is directed at promoting effortless, painless bowel movements. Fiber supplements and sitz baths should be prescribed. Suppositories are of no benefit. Topical 0.2 0.4% nitroglycerin ointment (1 cm of ointment) applied twice daily just inside the anus with the tip of a finger for 6 8 weeks results in healing in 50 80% of patients with chronic anal fissure; however, headaches occur in up to 40%. Injection of botulinum toxin (20 units) into the internal anal sphincter has been shown to cause healing in over 80% of patients with chronic anal fissure. Fissures may recur in up to 40% of patients treated with either nitrates or botulinum toxin. Chronic or recurrent fissures benefit from lateral internal sphincterotomy; however, minor incontinence may complicate this procedure.
Arroyo A et al: Surgical versus chemical (botulinum toxin) sphincterotomy for chronic anal fissure: long term results from a prospective randomized clinical and manometric study. Am J Surg 2005;189:429.
Madoff RD et al: AGA technical review on the diagnosis and care of patients with anal fissure. Gastroenterology 2003;124: 235.
Utzig MJ et al: Concepts in the pathogenesis and treatment of chronic anal fissure a review of the literature. Am J Gastroenterol 2003;98:968.
Perianal Abscess & Fistula
The anal glands located at the base of the anal crypts at the dentate line may become infected, leading to abscess formation. Other causes of abscess include anal fissure and Crohn's disease. Abscesses may extend upward or downward through the intersphincteric plane. Symptoms of perianal abscess are throbbing, continuous perianal pain. Erythema, fluctuance, and swelling may be found in the perianal region on external examination or in the ischiorectal fossa on digital rectal examination. Perianal abscesses are treated with local incision and drainage, while ischiorectal abscesses require drainage in the operating room. After drainage of an abscess, most patients are found to have a fistula in ano.
Fistula in ano most often arises in an anal crypt and is usually preceded by an anal abscess. In patients with fistulas that connect to the rectum, other disorders such as Crohn's disease, lymphogranuloma venereum, rectal tuberculosis, and cancer should be considered. Fistulas are associated with purulent discharge that may lead to itching, tenderness, and pain. The treatment of Crohn's-related fistula is discussed elsewhere in this chapter. Treatment of idiopathic fistula in ano is by surgical incision or excision under anesthesia. Care must be taken to preserve the anal sphincters.
P.663
Pruritus Ani
Pruritus ani is characterized by perianal itching and discomfort. It may be caused by poor anal hygiene associated with fistulas, fissures, prolapsed hemorrhoids, skin tags, and minor incontinence. Conversely, overzealous cleansing with soaps may contribute to local irritation or contact dermatitis. Contact dermatitis, atopic dermatitis, bacterial infections (staphylococcus or streptococcus), parasites (pinworms, scabies), candidal infection (especially in diabetics), sexually transmitted disease (condylomata acuminata, herpes, syphilis, molluscum contagiosum), and other skin conditions (psoriasis, Paget's) must be excluded. In patients with idiopathic pruritus ani, examination may reveal erythema, excoriations, or lichenified, eczematous skin. Education is vital to successful therapy. Spicy foods, coffee, chocolate, and tomatoes may cause irritation and should be eliminated. After bowel movements, the perianal area should be cleansed with nonscented wipes premoistened with lanolin followed by gentle drying. A piece of cotton ball should be tucked next to the anal opening to absorb perspiration or fecal seepage. Anal ointments and lotions may exacerbate the condition and should be avoided. A short course of high-potency topical corticosteroid may be tried, although efficacy has not been demonstrated. Diluted capsaicin cream (0.006%) led to symptomatic relief in 75% of patients in a recent double-blind crossover study.
Oztas MO et al: Idiopathic perianal pruritus: washing compared with topical corticosteroids. Postgrad Med J 2004;80:295.
Weichert GE: An approach to the treatment of anogenital pruritus. Dermatol Ther 2004;17:129.
Carcinoma of the Anus
These tumors are relatively rare, comprising only 1 2% of all cancers of the anus and large intestine. Squamous cancers make up the majority of anal cancers. Squamous cancer involving the anal canal may be subclassified as transitional and cloacogenic carcinoma; however, these are treated similarly. Anal cancer is increased among people practicing receptive anal intercourse and those with a history of other sexually transmitted diseases. In over 80% of cases, HPV may be detected, suggesting that this virus may be a causal factor. Anal cancer is increased in HIV-infected individuals. Combined HIV and HPV infection markedly increases the risk of anal carcinoma. Bleeding, pain, and local tumor are the commonest symptoms. The lesion is often confused with hemorrhoids or other common anal disorders. These tumors tend to become annular, invade the sphincter, and spread upward via the lymphatics into the perirectal mesenteric lymphatic nodes.
Treatment depends on the tumor stage. MR scan and endoluminal ultrasound assist in determining the depth of penetration and local spread. Small (< 3 cm) superficial lesions of the perianal skin may be treated with wide local excision. Squamous cancer of the anal canal and large perianal tumors invading the sphincter or rectum are treated with combined-modality therapy that includes external radiation with simultaneous chemotherapy (fluorouracil and either mitomycin or cisplatin). Local control is achieved in 80% of patients. Radical surgery (abdominoperineal resection) is reserved for patients who fail chemotherapy and radiation therapy. The 5-year survival rate is 60 70% for localized tumors and over 25% for metastatic (stage IV) disease.
Anal Cancer (PDQ) Treatment National Cancer Institute Cancer Net: http://www.cancernet.nci.nih.gov.
Moore HG et al: Anal neoplasms. Surg Clin North Am 2002;82:1233.
Welton ML et al: The etiology and epidemiology of anal cancer. Surg Oncol Clin N Am 2004;13:263.
EAN: 2147483647
Pages: 49