17. Yeast prions show unusual inheritance
19.17 Yeast prions show unusual inheritance |
A striking epigenetic effect is found in yeast, where two different states can be inherited that map to a single genetic locus, although the sequence of the gene is the same in both states. The two different states are [psi V] and [PSI+] . A switch in condition occurs at a low frequency as the result of a spontaneous transition between the states (for review see Wickner, 1996; Lindquist, 1997).
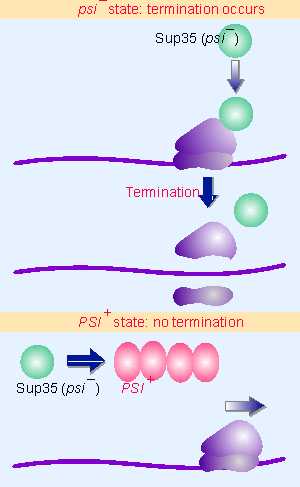 |
Figure 19.55 The state of the Sup35 protein determines whether termination of translation occurs. |
The psi genotype maps to the locus sup35, which codes for a translation termination factor. Figure 19.55 summarizes the effects of the Sup35 protein in yeast. In wild-type cells, which are characterized as [psi V], the gene is active, and Sup35 protein terminates protein synthesis. In cells of the mutant [PSI+] type, the factor does not function, causing a failure to terminate protein synthesis properly. (This was originally detected by the lethal effects of the enhanced efficiency of suppressors of ochre codons in [PSI+] strains.)
[PSI+] strains have unusual genetic properties. When a [psi V] strain is crossed with a [PSI+] strain, all of the progeny are [PSI+]. This is a pattern of inheritance that would be expected of an extrachromosomal agent, but the [PSI+] trait cannot be mapped to any such nucleic acid. The [PSI+] trait is metastable, which means that, although it is inherited by most progeny, it is lost at a higher rate than is consistent with mutation. Similar behavior is shown also by the locus URE2, which codes for a protein required for nitrogen-mediated repression of certain catabolic enzymes. When a yeast strain is converted into an alternative state, called [URE3], the Ure2 protein is no longer functional (Wickner, 1994).
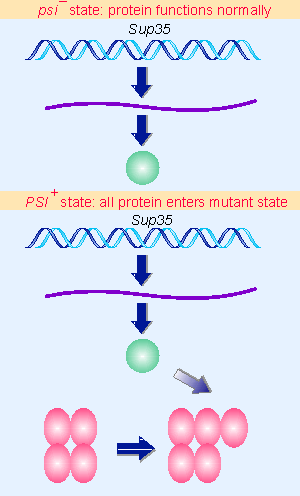 |
Figure 19.56 Newly synthesized Sup35 protein is converted into the [PSI+] state by the presence of pre-existing [PSI+] protein. |
The [PSI+] state is determined by the conformation of the Sup35 protein. In a wild-type [psi V] cell, the protein displays its normal function. But in a [PSI+] cell, the protein is present in an alternative conformation in which its normal function has been lost. To explain the unilateral dominance of [PSI+] over [psi V] in genetic crosses, we must suppose that the presence of protein in the [PSI+] state causes all the protein in the cell to enter this state. This requires an interaction between the [PSI+] protein and newly synthesized protein, probably reflecting the generation of an oligomeric state in which the [PSI+] protein has a nucleating role, as illustrated in Figure 19.56 (for review see Serio and Lindquist, 1999).
A common feature in both the Sup35 and Ure2 proteins is that each consists of two domains that function independently. The C-terminal domain is sufficient for the activity of the protein. The N-terminal domain is sufficient for formation of the structures that make the protein inactive. So yeast in which the N-terminal domain of Sup35 has been deleted cannot acquire the [PSI+] state; and the presence of an [PSI+] N-terminal domain is sufficient to maintain Sup35 protein in the [PSI+] condition (Masison and Wickner, 1995).
Loss of function in the [PSI+] state is due to the sequestration of the protein in an oligomeric complex. Sup35 protein in [PSI+] cells is clustered in discrete foci, whereas the protein in [psi V] cells is diffused in the cytosol. Sup35 protein from [PSI+] cells forms amyloid fibers in vitro Xthese have a characteristic high content of β sheet structures (Glover et al., 1997).
The involvement of protein conformation (rather than covalent modification) is suggested by the effects of conditions that affect protein structure. Denaturing treatments cause loss of the [PSI+] state. And in particular, the chaperone Hsp104 is involved in inheritance of [PSI+]. Its effects are paradoxical. Deletion of HSP104 prevents maintenance of the [PSI+] state. And overexpression of Hsp104 also causes loss of the [PSI+] state. This suggests that Hsp104 is required for some change in the structure of Sup35 that is necessary for acquisition of the [PSI+] state, but that must be transitory (Chernoff et al., 1995; for review see Horwich and Weissman, 1997).
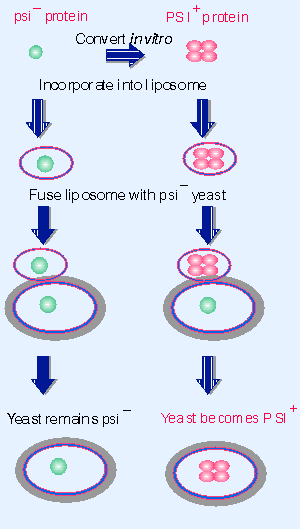 |
Figure 19.57 Purified protein can convert the[[psi-] state of yeast to [PSI+]. |
Using the ability of Sup35 to form the inactive structure in vitro, it is possible to provide biochemical proof for the role of the protein. Figure 19.57 illustrates a striking experiment in which the protein was converted to the inactive form in vitro, put into liposomes (when in effect the protein is surrounded by an articifial membrane), and then introduced directly into cells by fusing the liposomes with [psi V] yeast (Sparrer et al., 2000). The yeast cells were convertd to [PSI+]! This experiment refutes all of the objections to concluding that the protein has the ability to confer the epigenetic state. Experiments in which cells are mated, or in which extracts are taken from one cell to treat another cell, always are susceptible to the possibility that a nucleic acid has been transferred. But when the protein by itself does not convert target cells, but protein converted to the inactive state can do so, the only difference is the treatment of the protein Xwhich must therefore be responsible for the conversion.
This section updated 8-23-2000
| Reviews | |
| Horwich, A. L. and Weissman, J. S. (1997). Deadly conformations: protein misfolding in prion disease. Cell 89, 499-510. | |
| Lindquist, S. (1997). Mad cows meet psi-chotic yeast: the expansion of the prion hypothesis. Cell 89, 495-498. | |
| Serio, T. R. and Lindquist, S. L. (1999). [PSI+]: an epigenetic modulator of translation termination efficiency.. Ann. Rev. Cell Dev. Biol. 15, 661-703. | |
| Wickner, R. B. (1996). Prions and RNA viruses of S. cerevisiae. Ann. Rev. Genet. 30, 109-139. | |
| Research | |
| Chernoff, Y. O. et al. (1995). Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [PSI+]. Science 268, 880-884. | |
| Glover, J. R. et al. (1997). Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89, 811-819. | |
| Masison, D. C. and Wickner, R. B. (1995). Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science 270, 93-95. | |
| Sparrer, H. E. , Santoso, A. , Szoka FC, J. r. , and Weissman, J. S. (2000). Evidence for the prion hypothesis: induction of the yeast . Science 289, 595-599. | |
| Wickner, R. B. (1994). [URE3] as an altered URE2 protein: evidence for a prion analog in S. cerevisiae. Science 264, 566-569. | |