15. Baclofen - preclinical data
Editors: Spanagel, Rainer; Mann, Karl F.
Title: Drugs for Relapse Prevention of Alcoholism, 1st Edition
Copyright 2005 Springer
> Table of Contents > Baclofen: preclinical data
Baclofen: preclinical data
Mauro A.M. Carai1
Roberta Agabio1
Giovanni Addolorato2
Gian L. Gessa1, 3
Giancarlo Colombo3
1 Department of Neuroscience, University of Cagliari, Cagliari, Italy
2 Institute of Internal Medicine, Catholic University, Rome, Italy
3 C.N.R. Institute of Neuroscience, Section of Cagliari, Viale Diaz 182, I-09126 Cagliari, Italy
Introduction
Alcohol interacts, as a receptor modulator capable of selectively altering specific neurochemical processes, with multiple brain receptor systems. The behavioral consequences of alcohol ingestion are thought to be the summation of its effects at these receptor systems. The contribution of each receptor system to the behavioral effects of alcohol varies as the alcohol dose/concentration is increased, providing a neurochemical basis for the dose-dependent nature of alcohol effects. Accordingly, it may be conceived that at a given alcohol dose/concentration, a specific receptor system is more sensitive to alcohol than others, thus resulting in a more prominent contribution to a particular behavioral effect of alcohol.
Recent experimental results (reviewed in the present chapter) as well as preliminary clinical data (reviewed in another chapter included in this book [1]) suggest that the GABAB receptor may be considered a novel player among the receptor systems controlling different aspects of alcohol drinking behavior, including alcohol consumption, alcohol relapse and alcohol appetitive properties. These preclinical and clinical findings feature GABAB receptor agonists as promising agents in the pharmacotherapy of alcoholism.
Effect of GABAB receptor agonists on alcohol intake and alcohol motivational properties in rats
Previous studies on the ability of GABAB receptor agonists to alter alcohol intake (under the non-operant, 2-bottle choice regimen) and alcohol self-administration (under operant procedures) have yielded mixed results. Indeed, it has been reported that the prototype GABAB receptor agonist, baclofen, reduces [2, 3], produces no change on [4], or even stimulates [5, 6] voluntary alcohol intake in rats, when given a choice between two bottles containing an alcohol solution and water, respectively. In the self-administration studies (where rats were trained to press a lever to gain access to alcohol), and depending on the drug
P.164
dose or the experimental procedure used, baclofen has been found to stimulate [7] or decrease [7, 8 and 9] operant responding for alcohol in rats. Furthermore, when observed, the inhibition of alcohol-motivated lever pressing was not selective, being accompanied by a proportional decrease in lever pressing for an alternative reinforcer such as sucrose [8]. In a study using the sipper tube model of alcohol access, which permits some separation between the appetitive and consummatory aspects of alcohol self-administration, baclofen decreased alcohol-seeking behavior (measured as the time spent in achieving the response requirement to gain access to the alcohol solution), while it increased alcohol-consummatory behavior (the amount of alcohol actually consumed) in rats [10].
More consistent results have been obtained in Sardinian alcohol-preferring (sP) rats, one of the few rat lines selectively bred worldwide for high alcohol preference and consumption. Indeed, recent work (as reviewed below) has demonstrated that the pharmacological activation of the GABAB receptor in sP rats resulted in the suppression of a) acquisition and maintenance of alcohol drinking behavior, b) alcohol motivational properties, and c) alcohol relapse-like drinking.
In the acquisition study [11], the GABAB receptor agonists, baclofen and CGP 44532, were administered to alcohol-naive sP rats, i.e., rats which had never consumed alcohol before the start of the experiment. Adult, male sP rats were singly housed and injected intraperitoneally with either baclofen (0, 1 and 3 mg/kg) or CGP 44532 (0, 0.1, 0.3 and 1 mg/kg) once a day, for a total of 10 consecutive days. Alcohol (10%, v/v) and water were offered under the standard, homecage 2-bottle alcohol versus water choice with unlimited access for 24 h/day, immediately following the first injection of baclofen or CGP 44532. Food was available ad libitum.
In vehicle-treated rats, acquisition of alcohol drinking behavior had a rapid onset, reaching an average daily intake of 5-6 g/kg (i.e., the amount of alcohol ordinarily consumed on a daily basis by sP rats) within 4-7 days. In contrast, daily alcohol intake was dose-dependently suppressed in baclofen- and CGP 44532-dosed rats throughout the 10-day treatment period. Interestingly, in the rat groups treated with the highest dose of each drug (3 mg/kg baclofen and 1 mg/kg CGP 44532), daily alcohol intake was on average lower than 1.5 g/kg. Reduction in alcohol intake, induced by both GABAB receptor agonists, was associated with a full compensatory increase in daily water intake, so that the total daily fluid intake (i.e., the sum of alcohol solution and water consumed) remained unchanged. Daily food intake (a variable usually recorded in pharmacological studies on alcohol intake as a signal of animal malaise or nonselectivity of drug action) tended to be higher in the rat groups treated with baclofen or CGP 44532, as compared to the vehicle-treated groups. This is likely to be due to the fact that in the control group, part of the total calorie intake was provided by alcohol. On completion of the treatment, daily alcohol intake progressively increased in the 3 mg/kg baclofen-treated group as well as in the 1 mg/kg CGP 44532-treated group, reaching control values after 10-14 days. Water intake diminished consistently.
P.165
A separate experiment [12] investigated the effects of baclofen on voluntary alcohol intake in alcohol-experienced sP rats, i.e., rats in which the consumption of pharmacologically relevant doses of alcohol was already established before baclofen administration. Alcohol-experienced rats are thought to represent a model of the maintenance or active drinking phase of human alcoholism. In this study, adult male sP rats were singly housed and exposed to alcohol for approximately 2 months before starting with drug treatment. Alcohol (10%, v/v) was offered under the 2-bottle alcohol versus water regimen with unlimited access for 24 h/day. Food was readily available. Alcohol intake averaged 6 g/kg/day across the 2-month period of alcohol exposure, which preceded the start of the experiment. Baclofen was injected intraperitoneally at doses of 0, 2.5, 5 and 10 mg/kg, once a day for a total of 14 consecutive days.
Alcohol intake in vehicle-treated rats averaged between 5.5 and 7 g/kg throughout the 14-day treatment period, whereas those animals treated with baclofen experienced a dose-dependent reduction of up to 40-50% in daily alcohol consumption. However, tolerance to the reducing effect of baclofen on alcohol intake progressively developed on continuing treatment. An increase in daily water intake fully compensated the reduction in alcohol consumption, with the total daily fluid intake remaining virtually unchanged. Food intake was significantly altered only by treatment with 10 mg/kg baclofen. This effect, however, was present only during the first half of the treatment period.
The results of the acquisition and maintenance experiments suggest that stimulation of the GABAB receptor by baclofen and/or CGP 44532 results in a virtually complete blockade of the disclosure and experience of those effects of alcohol that sustain alcohol drinking behavior, which is otherwise a phenomenon with a rapid onset and stable maintenance in sP rats, as indicated in control rats by the constant daily intake of pharmacologically relevant amounts of alcohol from the very beginning of alcohol exposure.
The above-mentioned experiments, performed with the 2-bottle choice paradigm, focused on the effect of baclofen and CGP 44532 on some consummatory aspects of alcohol ingestive behavior in sP rats. Subsequently, we extended to the appetitive, or motivational, properties of alcohol the investigation on the anti-alcohol effect of GABAB receptor agonists. Specifically, we evaluated the effect of baclofen on the extinction responding for alcohol, defined as the maximal amount of work that a rat trained to lever-press for alcohol is willing to perform to obtain alcohol [13]. Extinction responding has been proposed to represent an index of the appetitive strength of alcohol [14, 15]: the more the rat works on the lever, the stronger its motivation to gain access to alcohol. Recent work [16] has shown that sP rats trained to lever-press for alcohol displayed a) high values of extinction responding for alcohol, and b) a high and positive correlation between extinction responding and alcohol self-administration of the preceding session. Thus it is confirmed that alcohol possesses strong motivational capacities in sP rats, highlighting the suitability of this rat line for the planned study.
P.166
In the baclofen study [13], adult male sP rats were initially trained to lever-press for oral alcohol (15%, v/v) in daily 30-min sessions under a fixed ratio 4 (FR4) schedule (i.e., every 4 consecutive presses of the lever resulted in the presentation of a 0.1 ml drop of alcohol solution). After approximately 20 sessions, all rats displayed a robust and stable lever-pressing behavior, which resulted in a mean alcohol intake of 0.6 g/kg/session, with blood alcohol levels in the range of 40-50 mg%. A separate group of sP rats, trained to lever-press for 3% (w/v) sucrose under identical conditions, was included in the study to assess the specificity of the baclofen action on extinction responding for alcohol. Extinction responding for alcohol or sucrose was defined as the maximal number of lever presses attained by each rat in the absence of alcohol or sucrose reinforcement. More specifically, during extinction sessions, rats were exposed to the operant chamber for 30 min with lever-pressing not resulting in any alcohol or sucrose presentation. On test sessions, baclofen was injected intraperitoneally at doses of 0, 1, 2 and 3 mg/kg. Each dose of baclofen was tested in every single rat of both groups under a latin-square design.
Extinction responding for alcohol and sucrose in saline-treated rats averaged 54.8 + 8.4 and 55.6 + 13.2 (mean + SEM), respectively, suggesting that 15% alcohol and 3% sucrose had comparable motivational properties in sP rats. Pretreatment with baclofen resulted in a dose-dependent suppression of extinction responding for alcohol: lever pressing of the rat groups treated with 1, 2 and 3 mg/kg baclofen was 64, 88 and 98% lower, respectively, than that observed in saline-treated rats. Seven out of eight rats in the 3 mg/kg baclofengroup, in fact, completely avoided pressing the lever. In the sucrose experiment, only the highest dose of baclofen tested significantly affected extinction responding for sucrose; extinction values in the rat groups treated with 1, 2 and 3 mg/kg baclofen were 23, 46 and 99% lower, respectively, than those observed in saline-treated rats. Taken together, these results suggest that baclofen was more potent in reducing the motivation for alcohol than for sucrose.
A separate experiment found that the above doses of baclofen did not affect spontaneous motor activity in sP rats, when tested in an open-field arena, suggesting that the suppressing effects of baclofen on extinction responding were indeed secondary to its ability in reducing the appetitive strength of alcohol and, furthermore, not due to muscle-relaxant or sedative properties of the drug.
These results suggest the involvement of the GABAB receptor in the neural system mediating alcohol reinforcement in sP rats, and that the reducing effects of baclofen on alcohol intake in sP rats may be secondary to its ability in suppressing the motivational properties of alcohol.
In conclusion, there appear to be some discrepancies of present-day data in the literature (indicating mixed effects of baclofen on alcohol intake and self-administration [2, 3, 4, 5, 6, 7, 8, 9 and 10]) and those collected in sP rats (which consistently depict a reducing effect of baclofen and CGP 44532 on alcohol preference, consumption and motivational properties). Differences in the baclofen dose-range, route of baclofen administration, and the procedure of alcohol exposure may account, at least in part, for these discrepancies. However, the strain of rats used in these
P.167
studies may be - to our understanding - an important factor in explaining these differences. Rats of the sP lines apparently possess a genetically determined sensitivity to the reducing effect of GABAB receptor agonists on alcohol intake and reinforcement, which may not be present in other rat strains. This peculiar sensitivity makes sP rats a proper animal model for investigations concerning the role of the GABAB receptor in the control of alcohol-ingestive behavior. Furthermore, the inconsistencies among the data generated with sP and other rat strains are not completely surprising, as a wide heterogeneity has historically been found among the different lines of alcohol-preferring rats with regard to the efficacy of certain drugs in reducing alcohol intake and self-administration. Thus, this replicates, to some extent, the different efficacies of some pharmacotherapies among the different types of alcoholics (e.g. [17, 18 and 19]).
Effect of GABAB receptor agonists on relapse-like behaviors in rats
With a deeper focus on the scope of the present book, we report here the results of a series of experiments designed to investigate the effects of baclofen and CGP 44532 on the so-called alcohol deprivation effect (ADE), i.e., the transient increase in alcohol intake which occurs in several animal species following a period of abstinence from alcohol. This phenomenon has been proposed to model the loss of control over alcohol and the episodes of alcohol relapse seen in human alcoholics (see [20, 21]). Rats of the sP line appear to constitute a proper animal model for pharmacological investigations of ADE, as they have been found to display a pronounced ADE during the first hour of re-access to alcohol following its deprivation [22, 23]. Furthermore, ADE in sP rats has been found to be reduced with naltrexone (this laboratory, unpublished observations), a drug reported to possess some efficacy in reducing the likelihood of relapse in alcoholics (see [24]), providing evidence for the predictive value of this animal model with regard to human pathology.
In experiments carried out to test the effect of baclofen [25] and CGP 44532 on ADE, individually housed adult male sP rats were initially offered alcohol (10%, v/v) and water under the standard 2-bottle choice with unlimited access for 8 consecutive weeks. Subsequently, rats were divided into 2 groups (matched for alcohol intake over the last 7 days): one group was deprived of alcohol for 14 consecutive days, during which water was the sole fluid available (alcohol-deprived rats); the second group continued to have unlimited access to alcohol and water (alcohol-nondeprived rats), consuming an average of approximately 6 g/kg/day alcohol. At the end of the deprivation phase, 30 min before lights off, rats of both groups (alcohol-deprived and -nondeprived) were further divided into 4 subgroups (n = 7-8 in both experiments) and acutely injected with 0, 1, 1.7 and 3 mg/kg baclofen, or with 0, 0.03, 0.1 and 0.17 mg/kg CGP 44532. Both drugs were injected intraperitoneally. Alcohol was re-presented at lights off and its consumption was recorded 60 min later (previous studies indicated that 60 min after alcohol representation
P.168
is the time interval during which ADE is maximal in sP rats [22, 23]). Standard rat chow was available throughout the studies.
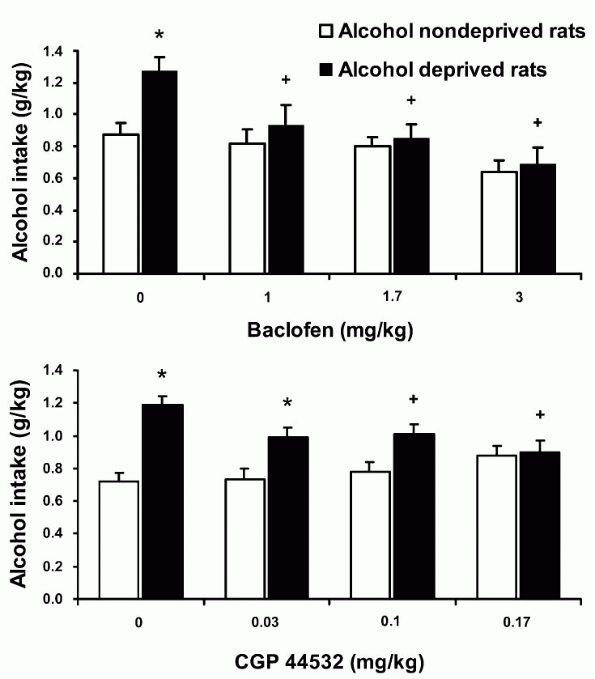 |
Figure 1. Suppressing effect of the GABAB receptor agonists, baclofen (top) and CGP 44532 (bottom), on the alcohol deprivation effect (ADE) in sP rats given alcohol under the 2-bottle choice regimen. Each bar is the mean + SEM of n = 7-8. *: P < 0.05 with respect to saline-treated alcohol-nondeprived rats; +: P < 0.05 with respect to saline-treated alcohol-deprived rats (Newman-Keuls test). Top panel reprinted from Drug Alcohol Dep 70: 105-108, 2003, with permission from Elsevier. |
In both experiments, alcohol intake was 50-80% higher in saline-treated alcohol-deprived rats than in saline-treated alcohol-nondeprived rats (Fig. 1); this increase in alcohol intake was indicative of the development of a marked ADE. This increase in alcohol intake was eliminated by baclofen and CGP 44532. Indeed, all doses of baclofen (Fig. 1, top panel) and the two highest doses of CGP 44532 (Fig. 1, bottom panel) resulted in a virtually complete suppression of the extra intake of alcohol produced by alcohol deprivation. Importantly, no dose of baclofen or CGP 44532 affected water and food intake, tending to exclude that the action of baclofen and CGP 44532 on ADE was due to their muscle-relaxant and sedative effects. Accordingly, complementary experiments on motor activity found that the doses of baclofen and CGP 44532 that suppressed ADE neither affected the time spent moving, nor the distance
P.169
traveled, nor the number of rearings (measures of horizontal and vertical motor activities in rodents) in alcohol-consuming sP rats tested in an open-field arena.
As demonstrated for several other alcohol-related behavioral responses, multiple receptor systems are likely to be involved in the expression of ADE. Indeed, drugs acting at the glutamate [26, 27, 28, 29, 30 and 31], opioid [32, 33 and 34], dopamine [35], GABAA/benzodiazepine [36], and cannabinoid [37] receptors have also been reported to modulate ADE in rats and mice. The results of the present study include the GABAB receptor in the neural substrate mediating ADE.
Finally, because of the predictive validity of ADE as an experimental model of alcohol relapse, the results of the present study suggest that baclofen may possess some efficacy in preventing relapse in human alcoholics. The results of preliminary, clinical surveys, reviewed by Addolorato and colleagues [1], apparently support this hypothesis, suggesting that baclofen may constitute a novel medication for alcoholism.
References
1 Addolorato G, Abenavoli L, Leggio L, De Lorenzi G, Ferrulli A, Caputo F, Agabio R, Gessa GL, Colombo G, Gasbarrini G (2004) Baclofen: clinical data. In: R Spanagel, K Mann (eds): Drugs for Relapse Prevention of Alcoholism. Birkh user, Basel, 171-180
2 Daoust M, Saligaut C, Lhuintre JP, Moore N, Flipo JL, Boismare F (1987) GABA transmission, but not benzodiazepine receptor stimulation, modulates ethanol intake by rats. Alcohol 4: 469-472
3 Perfumi M, Santoni M, Ciccocioppo R, Massi M (2002) Blockade of -aminobutyric acid receptors does not modify the inhibition of ethanol intake induced by Hypericum perforatum in rats. Alcohol Alcoholism 37: 540-546
4 Tomkins DM, Fletcher PJ (1996) Evidence that GABAA but not GABAB receptor activation in the dorsal raphe nucleus modulates ethanol intake in Wistar rats. Behav Pharmacol 7: 85-93
5 Smith BR, Robidoux J, Amit Z (1992) GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcoholism 27: 227-231
6 Smith BR, Boyle AEL, Amit Z (1999) The effects of GABAB agonist baclofen on the temporal and structural characteristics of ethanol intake. Alcohol 17: 231-240
7 Petry NM (1997) Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol 5: 183-194
8 Anstrom KK, Cromwell HC, Markowski T, Woodward DJ (2003) Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res 27: 900-908
9 Janak PH, Gill TM (2003) Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol 30: 1-7
10 Czachowski CL, Legg BH, Stansfield KH (2002) Effects of the GABA(B) agonist, baclofen, on ethanol- and sucrose-seeking and self-administration. Alcohol Clin Exp Res 26: 115A
11 Colombo G, Serra S, Brunetti G, Atzori G, Pani M, Vacca G, Addolorato G, Froestl W, Carai MAM, Gessa GL (2002) The GABAB receptor agonists baclofen and CGP 44532 prevent acquisition of alcohol drinking behaviour in alcohol-preferring rats. Alcohol Alcoholism 37: 499-503
12 Colombo G, Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL (2000) Ability of baclofen in reducing alcohol intake and withdrawal severity: I - Preclinical evidence. Alcohol Clin Exp Res 24: 58-66
13 Colombo G, Vacca G, Serra S, Brunetti G, Carai MAM, Gessa GL (2003) Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology 167: 221-224
14 Samson HH, Chappell A, Czachowski C, Sharpe A (2001) Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol 24: 205-209
15 Samson HH, Czachowski C, Chappell A, Legg B (2003) Measuring the appetitive strength of ethanol: use of an extinction trial procedure. Alcohol 31: 77-86
P.170
16 Vacca G, Serra S, Brunetti G, Carai MAM, Samson HH, Gessa GL, Colombo G (2002) Operant self-administration of ethanol in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res 26: 1678-1685
17 Kranzler HR, Burleson JA, Brown J, Babor TF (1996) Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res 20: 1534-1541
18 Johnson BA, Ait-Daoud N, Prihoda TJ (2000) Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypotheses to preliminary clinical evidence. Alcohol Clin Exp Res 24: 737-742
19 Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnann A (2000) Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res 24: 1041-1049
20 Boening JA-L, Lesch OM, Spanagel R, Wolffgramm J, Narita M, Sinclair D, Mason BJ, Wiesbeck GA (2001) Pharmacological relapse prevention in alcohol dependence: from animal models to clinical trials. Alcohol Clin Exp Res 25: 127S-131S
21 McBride WJ, Le A-D, Noronha A (2002) Central nervous system mechanisms in alcohol relapse. Alcohol Clin Exp Res 26: 280-286
22 Agabio R, Carai MAM, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G (2000) Development of short-lasting alcohol deprivation effect (ADE) in Sardinian alcohol-preferring rats. Alcohol 21: 59-62
23 Serra S, Brunetti G, Vacca G, Lobina C, Carai MAM, Gessa GL, Colombo G (2003) Stable preference for high ethanol concentrations after alcohol deprivation in Sardinian alcohol-preferring (sP) rats. Alcohol 29: 101-108
24 Streeton C, Whelan G (2001) Naltrexone, a relapse prevention maintenance treatment of alcohol dependence: a meta-analysis of randomized controlled trials. Alcohol Alcoholism 36: 544-552
25 Colombo G, Serra S, Brunetti G, Vacca G, Carai MAM, Gessa GL (2003) Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Dep 70: 105-108
26 Salimov RM, Salimova NB (1993) L-Glutamate abolishes differential responses to alcohol deprivation in mice. Alcohol 10: 251-257
27 H lter SM, Danysz W, Spanagel R (1996) Evidence for alcohol anti-craving properties of memantine. Eur J Pharmacol 314: R1-R2
28 Spanagel R, H lter SM, Allingham K, Landgraf R, Zieglg nsberger W (1996) Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol 305: 39-44
29 H lter SM, Landgraf R, Zieglg nsberger W, Spanagel R (1997) Time course of acamprosate action on operant ethanol self-administration after ethanol deprivation. Alcohol Clin Exp Res 21: 862-868
30 Heyser CJ, Schulteis G, Durbin P, Koob GF (1998) Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology 18: 125-133
31 H lter SM, Danysz W, Spanagel R (2000) Novel uncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonist MRZ 2/579 suppresses ethanol intake in long-term ethanol-experienced rats and generalizes to ethanol cue in drug discrimination procedure. J Pharmacol Exp Ther 292: 545-552
32 Cowen MS, Rezvani AH, Jarrott B, Lawrence AJ (1999) Ethanol consumption by Fawn-Hooded rats following abstinence: effect of naltrexone and changes in -opioid receptor density. Alcohol Clin Exp Res 23: 1008-1014
33 H lter SM, Spanagel R (1999) Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacology 145: 360-369
34 H lter SM, Henniger MSH, Lipkowski AW, Spanagel R (2000) Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology 153: 93-102
35 Salimov RM, Salimova NB, Shvets LN, Maisky AI (2000) Hapoleridol administered subchronically reduces the alcohol-deprivation effect in mice. Alcohol 20: 61-68
36 Schmitt U, Waldhofer S, Weigelt T, Hiemke C (2002) Free-choice ethanol consumption under the influence of GABAergic drugs in rats. Alcohol Clin Exp Res 26: 457-462
37 Carai MAM, Lobina C, Gessa GL, Colombo G (2004) Cannabinoid receptor antagonists: a perspective. In: R Spanagel, K Mann (eds): Drugs for Relapse Prevention of Alcoholism. Birkh user, Basel, 181-187
EAN: 2147483647
Pages: 26