6. Naltrexone - clinical data
Editors: Spanagel, Rainer; Mann, Karl F.
Title: Drugs for Relapse Prevention of Alcoholism, 1st Edition
Copyright 2005 Springer
> Table of Contents > Naltrexone: clinical data
Naltrexone: clinical data
Charles P. O'Brien
Helen M. Pettinati
David W. Oslin
University of Pennsylvania, Department of Psychiatry/Philadelphia Veterans Affairs Medical Center, Treatment Research Center, 3900 Chestnut Street, Philadelphia, PA 19104-6178, USA
History
Most medications used in the treatment of psychiatric disorders were discovered by clinical serendipity rather than as laboratory models translated to clinical use. Naltrexone took a different path. It was the subject of intense study during the 1970s as scientists scrambled to understand the functions of the newly discovered endogenous opioid system by specifically blocking opiate receptors with naloxone or naltrexone. Both drugs had been developed to reverse or block the effects of externally administered opiates such as heroin, but they could also block internally produced opioids that act at the same receptors. Altshuler presented an interesting study at the 1979 meeting of the College on Problems of Drug Dependence [1] showing that naltrexone blocked the self-administration of alcohol in rhesus monkeys. Interestingly, only 8 of 22 monkeys spontaneously self-administered intravenous alcohol, but these alcohol-preferring monkeys consistently decreased their alcohol taking in a dose-dependent manner when treated with naltrexone. This study was consistent with the hypothesis that alcohol may release endogenous opioid peptides that activate opiate receptors and contribute significantly to the reinforcement produced by alcohol. The fact that alcohol was spontaneously self-administered by only a minority of monkeys suggested genetic variation in this phenomenon.
There were other studies beginning in the early 1980s that further contributed to the development of a hypothesis concerning a linkage between endogenous opioids and alcohol reinforcement and perhaps alcoholism and these are reviewed in more detail elsewhere in this volume (Chapter by Cowen). The view that condensation products of dopamine and alcohol-derived aldehyde (tetrahydroisoquinolines) might be causing opiate effects was never supported by data and did not play a role in motivating the first clinical study. By 1983, there were sufficient clues in the animal literature for one of us (CO'B) to request and receive permission to add alcoholism studies to his Investigational New Drug Licence (IND) for naltrexone. The initial open label experience treating alcoholics with naltrexone was encouraging; thus a formal protocol was submitted to the Philadelphia Veteran's Administration (VA)
P.60
Medical Center human subjects review board. Initial efforts to obtain grant funding from the manufacturer or from the National Institutes of Health (NIH) were unsuccessful. Thus the formal study began with some support from a VA Medical Research Center Grant (CO'B, PI) and a postdoctoral fellowship program. Conducting the study proved difficult because clinical staff at the time resisted the concept of a double-blind medication trial among alcoholics who were also engaged in an intensive 12-step recovery process. Few patients were enrolled until Joseph Volpicelli completed psychiatric training in 1985 and joined the center as a new, enthusiastic post-doctoral fellow. Recruitment of patients immediately increased and the first formal study was completed.
The first naltrexone study in alcoholics
Several unusual aspects of our first study should be mentioned. The patients were all male veterans engaged in a day hospital rehabilitation program at the Philadelphia VA Medical Center. Thus they came to the hospital five days per week for the first month totaling about 25 h per week of time in the clinic. Although they averaged about 20 years of heavy drinking, they were considered moderately well motivated because they were accepted for this intensive program. The dose of 50 mg naltrexone per day was arbitrarily chosen because this was the dose that we were using since 1973 for the prevention of relapse to heroin addiction. We had no idea whether the same dose of naltrexone that blocked the average dose of heroin could work on endogenous opioids theoretically released by alcohol. The major outcome measure, relapse to clinically significant drinking, was carefully chosen and defined. This was important because many prior studies of alcoholism counted a single drink as a relapse. If we had defined relapse as a single drink, there would have been no significant difference between naltrexone and placebo. In this study relapse was defined as 5 or more drinking days within one week, five or more drinks on a single drinking occasion or coming to the clinic with a blood alcohol concentration above 100 mg/dl. Over the 3-month course of the study, 54% of those patients in the intensive day hospital program who received placebo met criteria for relapse. Only 23% of those assigned to the naltrexone group relapsed (Fig. 1). Craving for alcohol was significantly reduced in the patients randomized to naltrexone and liver enzyme levels were lower but not significantly different (P = . 08) than levels in control patients. These results were published in a preliminary version [2] and in a complete version [3].
These results were initially met with great skepticism and publication of the paper was delayed by negative reviews. Fortunately, Roger Meyer, a member of the University of Pennsylvania Scientific Advisory Board at the time was impressed by the original data and he joined with the team at Yale led by Stephanie O'Malley to attempt a replication. O'Malley et al. [4] studied a sample of DSM III-R defined alcoholics who were recruited by newspaper advertisements for once-weekly therapy using one of two manual guided therapies:
P.61
coping skills/relapse prevention or supportive therapy. They also received either naltrexone 50 mg daily or placebo in a two by two design. The sample consisted of 97 patients who received medication for at least one week. They were 74% male with an average age of 40 years and moderately severe alcoholism. During the three-month trial, naltrexone was found to be clearly superior to placebo on measures of alcohol consumption. There was an interaction between medication and type of therapy with the combination of naltrexone and supportive therapy having the highest abstinence rate. If the patient did any drinking, the coping skills group with naltrexone had a lower relapse rate. As with the prior study at the University of Pennsylvania, the overall naltrexone group had a significantly lower relapse rate and reported less craving for alcohol. At end point, naltrexone-treated patients had significantly lower aspartate aminotransferase (AST) levels (indicative of alcohol-related liver dysfunction), P < .05 with a trend toward lower alanine aminotransferase (ALT) levels (P < .10), a finding consistent with their lower alcohol consumption.
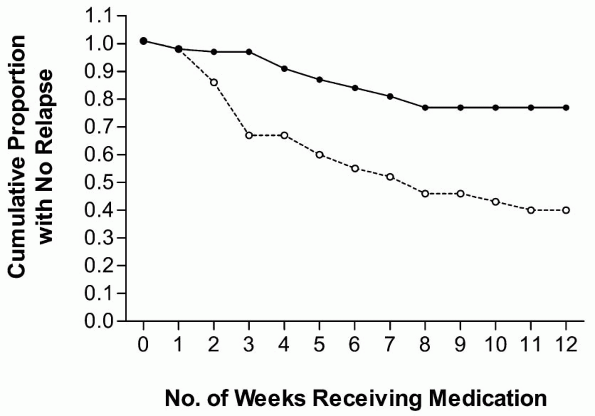 |
Figure 1 Relapse rates (as defined in the text) for the naltrexone hydrochloride- (closed circles) and placebo-treated (open circles) groups across the 12 weeks of study [3]. |
Food and Drug Administration approval
The history of naltrexone's approval by the Food and Drug Administration (FDA) is unusual in that neither of the two above efficacy studies were initiated or funded by the drug's manufacturer. There was no program to obtain FDA approval for alcoholism, but a senior scientist at Dupont, Leonard Cook, was
P.62
scheduled to retire shortly after the studies' publication. One of his retirement awards was a golfing afternoon with the company president. Dr. Cook took this opportunity to point out to the president that one of his medications was effective against alcoholism. This prompted the president to direct his staff to review the studies and initiate the appropriate requests to the FDA. Based on these two studies, the FDA added alcoholism to the indications for naltrexone and since it was then a generic drug, it granted three years of exclusivity to the company. The medication was briefly promoted for the treatment of alcoholism in the United States, but even now few American physicians are aware of this treatment option and fewer still use it regularly. Indeed most prescriptions written in clinical practice are only filled for 30 days with no refills [5]. Clinical researchers, however, have continued to study naltrexone for the treatment of alcoholism and much additional knowledge has been developed.
Clinical trials
Since those first two studies, at least 25 additional controlled clinical trials have been reported, in seven different countries using many different protocols. While 22 of these trials showed a significant benefit for alcoholics randomized to naltrexone, clearly not all patients showed a response. Two of the largest trials showed no benefit for naltrexone over placebo [6, 7]. A third trial was reported as negative based on self reports of drinking, but the group randomized to naltrexone showed significantly lower gamma glutamyl transferase levels, an objective sign of decreased alcohol drinking [8]. The 27 published controlled trials are the subject of an intense review, analyzing their strengths and weaknesses [9]. Most were relatively small sample size (<100 patients per cell) and of relatively short duration (12 weeks). A review of the data from these studies gives some direction to clinicians seeking guidelines for pharmacotherapy of alcoholism.
Some authors have characterized the literature on naltrexone as inconsistent and the effect size as modest. That is a fair characterization if one is comparing naltrexone for alcoholism to penicillin for pneumococcal pneumonia (before the appearance of resistant strains). Perhaps a more appropriate comparison would be to major depression. Both depression and alcoholism are behavioral disorders that are strongly influenced by environmental factors and respond to psychotherapy to a significant degree. One significant difference in the development of these two classes of agents is the funding support for the research. A substantial number of the clinical trials for anti-depressant research are funded by private industry, which has a significant financial reward for favorable research findings. There is concern that this financial or business incentive can lead to suppression of clinical trials that are not favorable to the company. Even with the potential incentive to suppress negative findings, failed trials where the active medication fails to beat placebo are common [10, 11]. No one knows the proportion of trials that fail, but one estimate
P.63
cited by a well-known depression researcher is 50% [12]. Thus the trials of anti-depressants that are published are heavily weighted toward positive trials and any attempt to calculate an effect size based on published trials would give a highly inflated estimate. Naltrexone, in contrast, has a history of development outside of the pharmaceutical industry as described above and investigators tend to publish everything. A second significant difference in the development of medication for addiction is the use of standard behavioral intervention for both the placebo group and active medication group. In essence these trials test the medication as adjunctive treatment and this has a consequence of producing seemingly high placebo response rates. In contrast, depression trials are seldom done in combination with a behavioral intervention. Thus, the naltrexone trials have not been carefully designed to show the drug in the best light.
Calculation of a standard effect size in the case of a medication with this much variability appears to be of dubious value. In a given patient sample, some alcoholics find naltrexone to be a life-changing medication that enables them for the first time to stop compulsive drinking. Others go right on drinking with no apparent effect from the medication. In most patient samples, there is a sufficient number of responders such that there is a difference in favor of naltrexone that meets the 5% significance level or better. Why is there such variability in response to naltrexone among alcoholics? We will begin by discussing heterogeneity in response to alcohol.
Heterogeneity in alcoholism
Alcoholism is well known as a heterogeneous disorder with variability in age of onset, drinking pattern, family history, associated diagnoses and clinical course. There is also a large variation in response to alcohol itself. Gamma-aminobutyric acid (GABA), glutamate, dopamine, serotonin and opioid peptides are involved in alcohol reinforcement [13]. It appears that the endogenous opioid system plays an important role in some but not all individuals. This was first noted in animal studies and was the reason that naltrexone was tested in the first clinical trial. The existing data suggest that there is wide variability in the degree to which alcohol activates endogenous opioids. Naltrexone is a very specific medication that has little affinity for any receptor system except opiate receptors. It will have no effect in alcoholics whose illnesses do not significantly involve the endogenous opioid system.
Both the animal and the human data are consistent with a straightforward hypothesis concerning the mechanism of naltrexone's action in alcoholism: some but not all individuals react to alcohol by an activation of endogenous opioids that have both peripheral and central effects. One central effect is the production of reward or euphoria. Alcohol produces a specific activation of reward systems when self-administered by animals. This is manifested by increased extra-cellular dopamine measured by microdialysis in the nucleus accumbens [14].
P.64
The primary transducer that translates the alcohol signal into release of opioids is still unknown. We have learned from animal models that opioid peptides inhibit GABA inhibitory neurons in the ventral tegmental area (VTA). This inhibition of the inhibitors of dopamine neurons in the VTA results in an activation of these neurons and a release of dopamine in limbic reward pathways where these neurons project. The increase in dopamine in the nucleus accumbens that occurs after alcohol self-administration in rats is blocked by naltrexone pre treatment. The animal also stops taking alcohol, presumably because the reward has been blocked.
In human subjects, endogenous opioids in the brain cannot be measured directly. Gianoulakis [15] has studied plasma beta endorphin (BE) and has shown a low baseline level in both abstinent alcoholics and in non-alcoholics with a high risk of developing alcoholism because of a strong family history of the disorder. This finding is supported by the report of Genazzani [16] showing BE levels in the cerebrospinal fluid three-fold lower in alcoholics compared to controls. The high-risk subjects showed a dose-related BE response to alcohol in the laboratory (Fig. 2), an effect not seen in the low-risk subjects [17]. In animals, both the central and pituitary endogenous opioid response to alcohol has been observed, but only the pituitary response has been verified in humans. Another study [18] showed that alcohol produces more simulation (high) in non-alcoholics with a family history of alcoholism than in subjects with no history of alcohol in the family. Pre-treatment with naltrexone blocks this high during laboratory administration of alcohol under double-blind conditions. This finding is consistent with patient reports during double-blind
P.65
clinical trials stating that the expected high from drinking alcohol is diminished when the patient is receiving naltrexone but not placebo [19]. There are additional data from human laboratory studies showing that pretreatment with naltrexone lessens desire for alcohol and quantity consumed [20, 21].
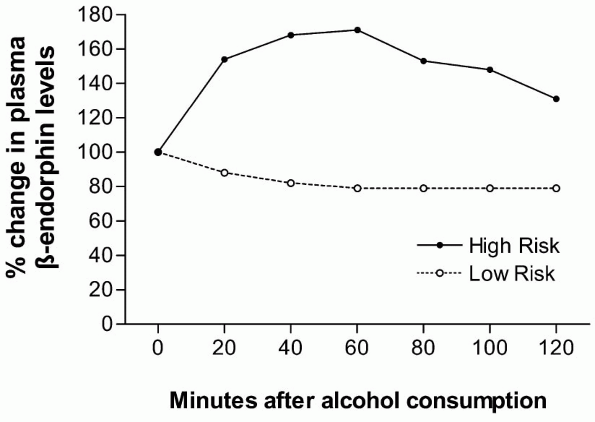 |
Figure 2. Change in -endorphin levels after alcohol consumption [17]. |
Adherence to the medication regimen
A major variable in any clinical trial involving medication is the degree to which the patients actually take the medication. Obviously the medication cannot be expected to help if patients do not take it regularly. Most patients beginning treatment for alcoholism are somewhat ambivalent about their motivation to abstain from all alcohol. Thus missed doses are common. Several of the studies reported as showing a significant benefit to the patients randomized to naltrexone would not be positive on a strict intent to treat analysis [22, 23]. Of course, any system to measure adherence must be applicable to both drug and placebo groups.
Another factor is medication side-effects. In one of the negative clinical trials, adverse subjective effects seemed to play a major role [6]. Indeed, up to 10% of alcoholics may report nausea and this may be related to a kind of endogenous opioid precipitated withdrawal. It is well known that naltrexone precipitates withdrawal in opiate addicts even days after the last dose of an opiate. Alcohol causes the release of endogenous opioids that may be displaced by naltrexone due to its high affinity for opiate receptors. There is some evidence that the more recent the last alcohol intake and the higher the dose of alcohol, the more likely that nausea will occur with naltrexone ingestion [24]. There is also evidence that there may be an inherited sensitivity to opiate antagonists, perhaps due to differences in baseline tone of the endogenous opiate system. Non-alcoholic family members of alcoholics show increased sensitivity to naloxone as measured by cortisol response [25].
A major recent advance in the use of naltrexone has been the development of a depot preparation that provides effective blood levels for 30-40 days. Three such products are currently in clinical trials and only preliminary results have been published [26, 27]. The largest trial was just completed involving 624 subjects treated for 12 weeks [28]. It showed a highly significant effect in male alcoholics (48% reduction, p < .0001) but no benefit for females. Noteworthy was the minimal rate of side-effects. The slow release formulation gives long lasting blood levels but no peak effect as is seen within the first 2 h of an oral dose. Thus few patients complained of nausea and less than 2% had an injection site reaction. It is hoped that a depot formulation will soon be approved for general clinical use. With this advance, the problem of adherence should be greatly improved.
P.66
Pharmacology of naltrexone
Naltrexone was developed in the early 1970s as a treatment for heroin addiction. The early clinical studies showed that it was a competitive antagonist of heroin and other opioids. As information about the opiate receptor system was developed, it was found that naltrexone had highest affinity for receptors, but also affinity for and receptors (Tab. 1) [29]. This broad-spectrum effect on opiate receptors may be important to its effect on alcohol drinking. Using a rat model of drinking, Stromberg [30] showed that specific antagonists at and receptors were not as effective individually as naltrexone, which acts on all three types of opiate receptors.
Naltrexone also has a long duration of activity at brain receptor sites. Although the plasma half-life of naltrexone and its active metabolite Beta naltrexol is only 10-12 h, two studies using C11 carfentanil showed that one 50 mg dose blocks brain receptors for 48-72 h [31, 32]. This duration of action is an advantage in that one or two missed doses would not necessarily leave the patient unprotected. Of course the availability of a depot preparation will add much more consistency to the long-term use of naltrexone.
Table 1. Naltrexone affinity at opioid receptor subtypes | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Anti-craving effect
The first two clinical trials of naltrexone in the treatment of alcoholism measured craving for alcohol. Patients randomly assigned to naltrexone reported significantly less craving and this predicted less alcohol use and a lower likelihood of relapse. Other studies that measured craving as a secondary outcome measure also reported a similar effect. It is not clear how naltrexone could have this effect. Craving is a subjective phenomenon which is also controversial [33] Some have argued that craving has no meaning, but it is at least very interesting that so often in these double-blind trials, craving reduction predicted decreased alcohol consumption.
P.67
Animal models have shown that placing a rat in a chamber where the animal has previously self-administered alcohol results in a conditioned increase in dopamine (DA) before any alcohol has been received. Could this be a model of craving? While naltrexone in this model has been found to block the DA increase seen after alcohol ingestion, it did not block the conditioned DA increase seen prior to alcohol availability.
Naltrexone has been reported to reduce craving elicited by cues that have been previously associated with alcohol during a clinical trial [34]. In a laboratory study, O'Malley [35] elicited craving by a priming dose of alcohol and then offered alcoholics additional drinks. Those alcoholics pre treated with naltrexone showed less craving and consumed fewer drinks than those pre treated with placebo. The naltrexone group also showed more activation of the hypothalamo-pituitary-adrenal axis. Naltrexone elevated cortisol in the baseline period and higher cortical levels were significantly correlated with lower craving throughout the experiment.
Psychotherapy
One of the major variables across studies was the type of psychosocial intervention used in conjunction with naltrexone. Only one study referred patients to primary care physicians with no psychotherapy or counseling [36]. The first study [2, 3] consisted of patients in an intensive outpatient program or day hospital. Abstinence was the goal and 12-step groups were a part of treatment, but the program took pains to avoid having patients feel guilty over slips. In the second study [4], patients received either coping skills or supportive therapy and there was an interaction. Supportive therapy with naltrexone had the highest abstinence rate, but of those who drank, coping skills with naltrexone had the lowest relapse rate.
In reviewing all of the studies, it appears that tolerance of some drinking is an important aspect of counseling. Those patients randomized to naltrexone may remain completely abstinent, but commonly some drinking occurs. Since the patients perceive less reward, they are less likely to continue drinking heavily and thus they slip without a relapse. If this behavior is regarded in a strongly negative manner by the therapist as might occur in a strict 12-step program, the patient is more likely to become discouraged or even drop out of treatment. Overall, the best results seem to occur in programs where therapy is supportive and oriented toward medication adherence [37].
Genetics
Alcoholism has long been known to have a strong heredity component. In addition to the elegant adoption studies over the past 30 years, there are more recent studies dealing with non-alcoholic relatives of alcoholics that provide
P.68
relevant information concerning the effects of naltrexone. The studies of Gianoulakis and colleagues reviewed above demonstrate differences in sensitivity of the plasma BE response to graded doses of alcohol. Blood alcohol levels were similar between high-risk and low-risk subjects, but the BE response to the same alcohol blood level was much greater in the high-risk subjects [15]. In a similar vein, King [18] found that high-risk subjects were more likely to report stimulation (euphoria) than low-risk subjects with similar alcohol blood levels in the laboratory and this stimulation was attenuated by naltrexone pretreatment, but not placebo. Family history has also been found to be a predictor of clinical response to naltrexone in alcoholics [38, 39]
More recently, the A+118G polymorphism of the opiate receptor gene was examined in alcoholics who had participated in naltrexone clinical trials [40]. In subjects of European descent, individuals with one or two copies of the Asp40 allele treated with naltrexone had significantly lower rates of relapse (p = 0.045) and a longer time to return to heavy drinking (p = 0.040) than those homozygous for the Asn40 allele. There were no differences in overall abstinence rates (p = 0.668), nor were there differences in relapse rates between patients of the two genotypes among those assigned to placebo. The A+118G polymorphism is of interest because functional differences have been demonstrated both in vitro and in vivo. Bond and colleagues showed that, in cell culture, mu-opioid receptors encoded by the Asp40 variant bind beta-endorphin and activate G- protein coupled protein potassium ion channels with three times greater potency than receptors encoded by the Asn40 variant. Both Wand et al. and Hernandez-Avila et al. [41, 42] found that individuals with one or two copies of the Asp40 allele had altered hypothalamic-pituitary-adrenal (HPA) axis activation induced by naloxone, while Smolka et al. [43] showed that individuals with the Asp40 variant display greater dopaminergic sensitivity during acute alcohol withdrawal. The Asp40 allele has also been shown to have a dose response association with frequency of drinking among alcohol-dependent patients such that those homozygous for the Asp40 allele drink more often than those heterozygous or homozygous for the Asn40 allele [44].
Medication side effects
The most common side-effect of naltrexone is nausea and sometimes vomiting. There is some evidence that this is related to quantity and recency of alcohol consumption as mentioned above. Beyond nausea, there is great concern over the possibility of hepatic toxicity. When alcoholism was added to the indications for naltrexone in 1995, the FDA was concerned about a report of increased liver enzymes reported in a trial of naltrexone in the treatment of obesity. The patients were receiving 350 mg daily or seven times the normal daily dose. The increase in enzymes was reversible and did not result in liver damage, but it caused the inclusion of a black box warning about potential
P.69
liver toxicity. Subsequently, naltrexone has not been found to produce hepatic toxicity in clinical trials of alcoholism or heroin addiction. Actually the converse is often true. Naltrexone produces a decrease in alcohol consumption and with decreased alcohol, a true hepatic toxin, the liver profile improves.
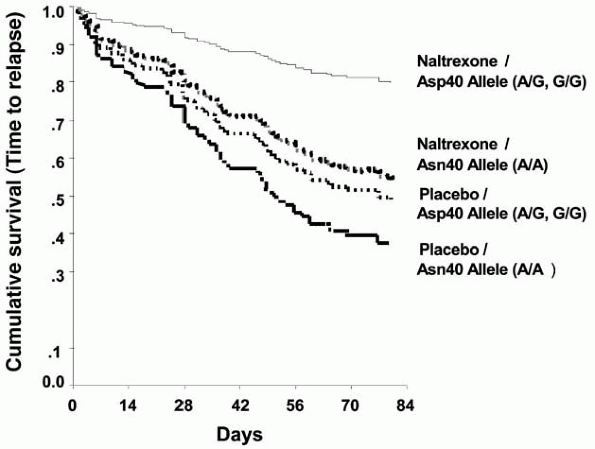 |
Figure 3. Survival analyses for time to relapse in subjects with one or two copies of the Asp40 allele vs those homozygous for the Asn40 allele by medication group. |
Selection of patients
Faced with the treatment of a patient with alcoholism, a clinician must consider whether a medication should be included in the treatment plan. Of course detoxification is the first goal, but a long-term plan for prevention of relapse is essential. Ideally there should be a psychiatric evaluation that includes assessment of need for treatment in all the domains of the Addiction Severity Index [45]. If a co-existing disorder is present such as depression, appropriate medication should be prescribed. For specific relapse prevention, medication in conjunction with psychotherapy should always be considered. Alcoholism is a potentially fatal disease and it makes no sense to withhold effective therapy. The choices in the United States at present are naltrexone, disulfiram and the recently approved acamprosate. Depending on the circumstances and willingness of the patient, disulfiram may be ideal. Many patients, however, are unwilling to accept disulfiram. Naltrexone is generally more acceptable and has the advantage of possibly reducing craving for alcohol. Furthermore, if the
P.70
patient does drink, the pleasant effects of the alcohol may be less, but there will be no adverse consequences of drinking such as those seen in patients receiving disulfiram. Beginning in July 2004, acamprosate became another possibility and more studies comparing the two medications are needed [46]. There is preliminary evidence [47] that specific types of alcoholics are likely to respond better to naltrexone or to acamprosate and thus prospective studies of the two medications involving well-characterized alcoholics are in order.
Based on clinical experience and evidence from clinical trials, naltrexone seems to have greater efficacy in those with a family history of alcoholism. Additional studies involving genotyping of alcoholics in clinical trials are in order to determine whether the Oslin study described above can be replicated and extended so that more precise patient selection can be based on genotype. A large trial of depot naltrexone [28] found a strong gender effect with naltrexone being highly effective in males but not in females. Other selection factors seem to relate to medication adherence. Older alcoholics were more likely to take medication regularly, come to appointments and show a strong drug/placebo difference [48].
References
1 Altshuler HL Phillips PA, Feinhandler DA (1980) Alternation of enthanol self-administration by naltrexone. Life Sciences 26: 679-688
2 Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP (1992) Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry 49: 876-880
3 Volpicelli JR, O'Brien CP, Alerman AI, Hayashida M (1990) Naltrexone and the treatment of alcohol dependence: initial observations. In: LB Reid (ed.): Opioids, Bulimia, Alcohol Abuse and Alcoholism, Springer-Verlag, New York, 195-214
4 O'Malley SS, Jaffe, AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B (1992) Naltrexone and coping skills therapy for alcohol dependence: a control study. Arch Gen Psychiat 49: 881-887
5 Harris KM, DeVries A, Dimidjian K (2004) Datapoints: Trends in naltrexone use among members of a large private health plan. Psychiatric Services 55(3): 221
6 Kranzler HR, Modesto-Lowe V, Van Kirk J (2000) Naltrexone versus nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology 22(5): 493-503
7 Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA, Veterans Affairs Naltrexone Cooperative Study 425 Group (2001) Naltrexone in the treatment of alcohol dependence. N Engl J Med 345(24): 1734-1739
8 Gastpar M, Bonnet U, Boning J. Mann K. Schmidt LG. Soyka M. Wetterling T. Kielstein V. Labriola D. Croop R (2002) Lack of efficacy of naltrexone in the prevention of alcohol relapse: results from a German multicenter study. J Clin Psychopharmacol 22(6): 592-598
9 Pettinanti HM, Oslin D, O'Brien CP (in preparation) A review of all naltrexone clinical trials for alcoholism
10 Robinson DS, Rickels K (2000) Concerns about clinical drug trials. Journal of Clinical Psychopharmacology 20(6): 593-596
11 Chan A-W, Hrobjartsson A, Haahr M, Gotzsche PC, Altman DG (2004) Empirical evidence for selective reporting of outcomes in randomized trials. JAMA 291(20): 2457-2465
12 Thase M (2003) Issues and Ethics. Presented at the Annual American College of Neuropsychopharmacology Annual Meeting, San Juan, Puerto Rico
13 Koob GF, Rassnick S, Heinrichs S, Weiss F (1994) Alcohol, the reward system and dependence. Exs 71: 103-114
P.71
14 Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. The Journal of Neuroscience 18(24): 10663-10671
15 Gianoulakis C (1996) Implications of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol and Alcoholism 31(1): 33-42
16 Genazzani AR, Nappi G, Facchinetti F, Mazzella GL, Parrini D, Sinforiani E, Petraglia F, Savoldi F (1982) Central deficiency of beta-endorphin in alcohol addicts. J Clin Endocrinol Metab 55(3): 583-586
17 Gianoulakis C (1990) Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol 180: 21-29
18 King A, Volpicelli J, Frazer A, O'Brien CP (1997) Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology 129: 15-22
19 Volpicelli JR, Clay KL, Watson NT, O'Brien CP (1995) Naltrexone in the treatment of alcoholism: predicting response to naltrexone. Journal of Clinical Psychiatry 56: 39-44
20 Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H (1994) Naltrexone-induced alterations in human ethanol intoxication. American Journal of Psychiatry 151(10): 1463-1467
21 Davidson D, Palfai T, Bird C, Swift R (1999) Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res 23(2): 195-203
22 Volpicelli JR, Rhines KC, Rhines, JS, Volpicelli LA, Alterman, AI, O'Brien CP (1997) Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry 54(8): 737-742
23 Chick J., Anton R, Checinski K. Croop R. Drummond DC. Farmer R. Labriola D. Marshall J. Moncrieff J. Morgan MY. Peters T. Ritson B. (2000) A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol and Alcoholism 35(6): 587-593
24 O'Malley SS, Krishnan-Sarin S, Farren C, O'Connor PG (2000) Naltrexone-induced nausea in patients treated for alcohol dependence: clinical predictors and evidence for opioid-mediated effects. J Clin Psychopharmacol 20(1): 69-76
25 Wand GS, Mangold, D, El Deiry S, McCaul ME, Hoover D (1998) Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry 55(12): 1114-1119
26 Kranzler HR, Modesto V, Nuwayser ES (1998) A sustained-release naltrexone preparation for treatment of alcohol dependence. Alcoholism: Clinical and Experimental Research 22(5): 1074-1079
27 Kranzler HR, Wesson DR, Billot L for the Drug Abuse Sciences Naltrexone Depot Study Group (2004) Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcoholism: Clinical and Experiment Research 28(7): 1051-1059
28 Silverman B (2004) Clinical Trial of Depot Naltrexone. Presented at the American Psychiatry Association Annual Meeting, New York City, New York
29 Schmidt WK, Tam SW, Shotzberger GS, Smith DH Jr, Clark R, Vernier VG (1985) Nalbuphine. Drug Alcohol Depend 14(3-4): 339-362
30 Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP (1998) A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol 15(4): 281-289
31 Lee MC, Wagner HN, Tanada S, Frost JJ, Bice AN, Dannals RF (1988) Duration of occupancy of opiate receptors by naltrexone. J Nuclear Med 29(7): 1207-1211
32 McCaul ME, Kim WK Wand GS, Bencherif B, Dannals RF, Frost JJ (2004) PET Measurement of - and d-opioid receptor availability before and during naltrexone treatment in alcohol dependent subjects (poster). Presented at the Winter Conference on Brain Research, Copper Mountain, Colorado
33 Tiffany ST (1999) Cognitive concepts of craving. Alcohol Res Health 23(3): 215-224
34 Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB (1999) Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res 23(8): 1386-1394
35 O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ (2002) Naltrexone decreases craving and alcohol self-administration in alcohol dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology 160(1): 19-29
36 Latt NC, Jurd S, Houseman J, Wutzke SE (2002) Naltrexone in alcohol dependence: a randomized controlled trial of effectiveness in a standard clinical setting. The Medical Journal of Australia 176(11): 530-534
P.72
37 Pettinati HM, Volpicelli JR, Pierce JD, O'Brien CP, Pierce JD Jr, (2000) Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis 19(1): 71-83
38 Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O'Malley SS (1996) Naltrexone, relapse prevention, and supportive therapy with alcoholics: an analysis of patient treatment matching. J Consult Clin Psychol 64(5): 1044-1053
39 Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR (2001) Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict 10(3): 258-268
40 Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP (2003) A functional polymorphism of the -opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 28: 1546-1552
41 Wand GS, Mangold E, El Deiry S, McCaul ME, Hoover D (1998) Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry 55: 1114-1119
42 Hernandez-Avila C, Wand G, Luo X, Gelernter J, Kranzler H (2003) Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1). Am J Med Genet 118B: 60-65
43 Smolka M. Sander T, Schmidt L, Samochowiec J, Rommelspacher H, Gschiedel N, Wendel B. Hoehe MR (1999) Mu-opioid receptor variants and dopaminergic sensitivity in alcohol withdrawal. Psychoneuroendocrinology 24: 629-638
44 Kim SG, Kim C-M, Kang D-H, Kim J-K, Byun W-T, Kim S-Y, Park J-M, Kim M-J, Oslin DW (2004) Association of functional opioid receptor genotypes with alcohol dependence in Koreans. Alcohol Clin Exp Res 28(7): 986-990.
45 McLellan AT, Luborsky L, Woody GE, O'Brien CP (1980) An improved diagnostic evaluation instrument for substance abuse patients: The Addiction severity Index. J Nervous and Mental Disease 168: 26-33
46 Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Widemann K (2003) Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry 60(1): 92-99
47 Lesch, OM (2004) How to translate the biological basic glutamate mechanisms in preclinical research to human trials and therapy approaches. Presented at the European Winter Conference on Brain Research, Les Arcs, France
48 Oslin DW, Pettinati H, Volpicelli JR (2002) Alcoholism treatment adherence: older age predicts better adherence and drinking outcomes. Am J Geriatr Psychiatry 19(6); 740-747
EAN: 2147483647
Pages: 26