13 - Angioplasty and Stenting
Editors: Norris, John W.; Hachinski, Vladimir
Title: Stroke Prevention, 1st Edition
Copyright 2001 Oxford University Press
> Table of Contents > II - Secondary Prevention > 13 - Angioplasty and Stenting
13
Angioplasty and Stenting
Martin M. Brown
The treatment of arterial stenosis by percutaneous transluminal angioplasty (PTA) and stenting to prevent stroke has the attraction of avoiding an invasive surgical incision and the general anesthesia usually used for surgical procedures. PTA and stenting are very widely used in the coronary and lower limb vessels to prevent angina, myocardial infarction, and the consequences of peripheral vascular disease. The acceptance of coronary and peripheral PTA reflects an acceptably low complication rate, similar to that of more invasive surgical procedures.
The pathology of atherosclerosis at these sites is very similar to that found in the carotid and vertebral arteries, yet reluctance persists to recommend PTA for the prevention of stroke because anxiety about risks cerebral embolism. Nevertheless, a number of centers have been using angioplasty and stenting in selected patients with carotid and vertebral stenosis over the last decade, and a large randomized trial comparing carotid surgery with carotid angioplasty, the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), has been completed. Most experience relates to the treatment of internal carotid artery stenosis at the carotid bifurcation, and only limited data are available about angioplasty at other sites relevant to the cerebral circulation.
The results of individual case series and the randomized trial suggest that the risks of angioplasty and stenting are similar to those conventional carotid surgery. Surgery is well-established, while the techniques and devices available for
P.238
angioplasty and stenting are still evolving, so more evidence is required from randomized trials before angioplasty and stenting will become widely used for stroke prevention. However, the techniques already provide a valuable option in experienced centers for the treatment of patients unsuitable surgery.
Disadvantages of Carotid Surgery
Surgical endarterectomy has become the standard treatment for severe symptomatic carotid artery stenosis following the publication of the results European Carotid Surgery Trial (ECST) and the North American Symptomatic Carotid Endarterectomy Trial (NASCET), but significant morbidity and mortality are associated with carotid surgery. The combined stroke and death rate within 30 days of surgery in patients with severe stenosis was 7.5% ECST and 5.8% in NASCET.1,2 Surgery also risks myocardial infarction, deep vein thrombosis, and pulmonary embolism.
The systemic effects of the anesthetic and muscle relaxants the discomforts of intubation and pneumonia are additional potential complications when general anesthesia is used. Some of these risks can be avoided by using local anesthesia, but this is not popular with many surgeons. Neck dissection and retraction needed to reach the carotid artery may injure cranial nerves, and the wound may be complicated by hematoma and infection. These complications affected 10% of patients after surgery in the ECST.3 The incision also injures cutaneous nerves and frequently results in numbness around the scar, which may extend up to the face. Keloid scar formation may be troublesome in some patients. Fortunately, few of these complications lead to long-lasting complaints or permanent disability.
Some patients are not suitable for surgery because of severe ischemic heart disease, recent myocardial infarction, uncontrolled hypertension, or other medical risk factors that are contraindications to the procedure. The risk is also increased in women and in patients with contralateral carotid occlusion.4 In Sundt et al.'s influential study, the presence of major medical risk factors increased risk of surgery sevenfold.5 Patients with major risk factors were therefore excluded from recent trials but may be included in routine practice.
Surgery is also more hazardous or impractical at sites such as the distal internal carotid and vertebral arteries, which are not easily or safely accessible to surgery. In these patients, angioplasty and stenting may provide the only alternatives to medical treatment.
Economically, carotid surgery has the disadvantage of being an expensive procedures requiring operating theater time, intensive postoperative care, and a stay in hospital of up to a week, even in uncomplicated cases. Even if discharged after a few days, patients rarely return to full activities until a month after surgery.
P.239
Advantages of Angioplasty and Stenting
The main advantages of the interventional radiology techniques for treating arterial stenosis are the avoidance of a neck incision and general anesthesia. The discomfort and local neurological complications from an incision in the neck, particularly cranial and superficial nerve injury, are avoided, although hematoma can occur in the groin. Deep vein thrombosis, pneumonia, and myocardial ischemia have not been reported. From the patient's point of view, procedure is minor, and if all goes well, involves no more discomfort than a conventional angiogram. The patient is only required to stay in bed overnight after the procedure and can usually be discharged after 24 hours, resuming normal activities almost immediately.
Angioplasty is usually cheaper than surgery in economic terms, mainly because of shorter hospital stays. However, the cost advantage angioplasty compared to surgery is reduced if expensive devices, such as stents or protection devices, are required or if further treatment is needed because of restenosis.
These advantages mean that patients often choose angioplasty over surgery when given the choice, particularly if they have experienced angioplasty at other sites. Clinicians offering angioplasty or stenting to a patient must be satisfied that the complication rates in their unit are not significantly different from those of surgery, or from medical treatment if the patient is unsuitable for surgery.
Techniques of Angioplasty and Stenting
Balloon Angioplasty
Standard balloon angioplasty technique consists of inflation a on the end of a catheter across the stenosis, with access to the vessel through femoral artery in the groin after insertion of a sheath. A standard diagnostic catheter for neurological procedures is then passed through the sheath, up the femoral artery, and into the common carotid artery to take views of the stenosis. The diagnostic catheter is then exchanged for a guiding catheter, and a guidewire carefully placed across and beyond the stenosis. An inflatable balloon catheter is then passed over the guidewire and maneuvered to straddle stenosis. The diameter of the balloon is chosen to match the estimated diameter of the vessel, with the aim of avoiding overdilation.
For lesions at the carotid bifurcation, the dilated balloon is usually 5 6 mm (0.2 0.24 in) in diameter and 2 cm (0.8 long. The ideal catheter has a lowprofile tip and a rapid deflation time. It is inflated across the stenosis up to five times to achieve satisfactory dilation. The inflation pressure should be monitored during the procedure to avoid excessive pressures and overdilation; although there
P.240
is no consensus about the ideal pressure, usually less than two atmospheres is used. Inflation time should be less than 10 seconds. Brief total occlusion time limits the risk of hemodynamic ischemia unless stenosis is so tight that guidewire occludes the vessel for a longer time. This contrasts with carotid endarterectomy, in which, even if shunts are used, it may take several minutes to insert the shunt after internal carotid artery has been clamped (see section on hemodynamic ischemia below).
Excellent anatomical results can be achieved after simple balloon dilation, even when the stenosis is very severe (Fig. 13.1), but fill dilation of the artery may not be achieved because of elastic recoil. Progressive spontaneous dilation the artery over the next few weeks or months may follow initial suboptimal residual
P.241
lumen seen immediately after angioplasty ( remodelling ; Fig. 13.2). In the only angiographic study of this phenomenon after carotid PTA, remodelling was demonstrated in 7 out of 12 cases at one year follow-up.6 Remodelling only occurred when at least 20% reduction in the severity of stenosis was achieved during the angioplasty procedure.
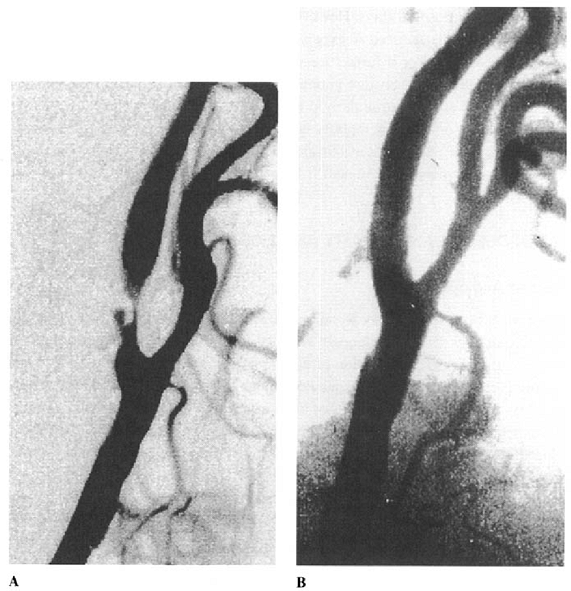 |
FIGURE 13.1. Digital subtraction angiogram showing results of simple balloon angioplasty in a patient with severe ulcerated internal carotid artery stenosis. A: immediately before angioplasty. B: immediately after angioplasty. |
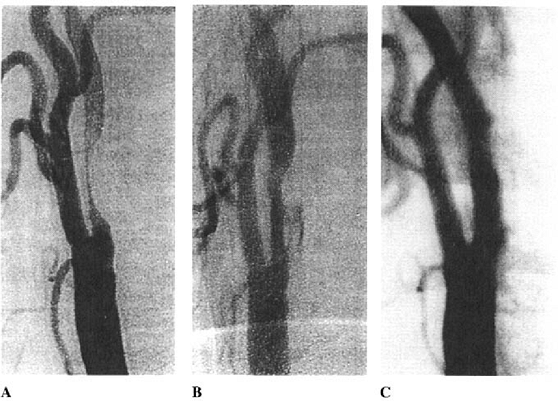 |
FIGURE 13.2. Digital subtraction angiogram showing remodeling of the arterial wall after a simple balloon angioplasty. A: immediately before B: after angioplasty with suboptimal dilation and contrast medium within the atheromatous plaque, indicating plaque fissuring. C: one year after angioplasty demonstrating remodeling and a widely patent lumen. |
Stenting
A stent is a collapsed wire mesh inserted into the artery within a catheter or over a balloon and then expanded at the site of stenosis. Various designs stents are available, including self-expanding and balloon-expandable models. Stents have the advantage of compressing flaps intimal dissection against the arterial wall and so limiting the size of any embolic material released into lumen. Stenting improves the appearance after balloon angioplasty, especially if the initial dilation is inadequate or produces dissection (Fig. 13.3). Initially, Stenting was only used to bail out a poor result after balloon angioplasty, but it is now the technique of choice in most patients. In primary Stenting, no previous balloon dilation
P.242
is needed, but with severe stenosis, predilatation with a balloon may be needed to allow the stent cross stenosis. After deployment of a stent, a balloon catheter can be placed in the stent and inflated if further dilation is required. In the coronary circulation, stenting improves outcome of angioplasty, and, similarly, primary carotid stenting will probably prove safer than simple balloon angioplasty, since closure of the artery is less likely, especially when dissection or plaque rupture occurs because of the procedure.
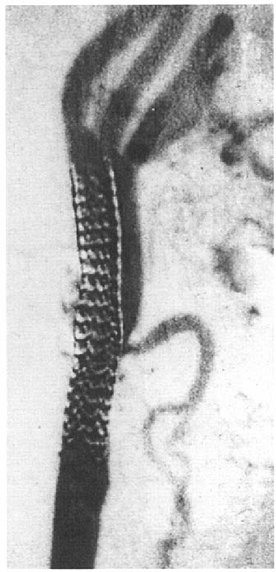 |
FIGURE 13.3. Digital subtraction angiogram showing the results of stent deployment at the carotid bifurcation. |
Primary stenting aims to minimize the adverse consequencs of dissection or plaque rupture, maintain laminar flow, and prevent any free intimal flaps. In the long term, it is possible that restenosis will be less frequent after stenting than after simple balloon angioplasty. Because of these apparent advantages stenting, it is likely that primary stenting will be increasingly used in preference to simple balloon angioplasty, even though there are no clinical trials demonstrating the superiority of the technique.
P.243
Protection Devices
Theron et al. designed a special triple lumen catheter for a carotid angioplasty to prevent cerebral embolism following disruption of plaque.7 After introducing the catheter into the common carotid artery, a balloon is inflated beyond stenosis to occlude the internal carotid artery distally. Next, a balloon dilatation catheter or stent is passed over this catheter and deployed across the stenosis to dilate lumen. After the balloon or stent has been withdrawn, third lumen of the introduction catheter is used to withdraw blood and irrigate with saline proximal to the occlusion and so remove any debris. Theron et al. reported cholesterol crystals up to 200 micrometers long in the aspirate using this technique.7 A technique has also been described in which the carotid artery is occluded by a balloon below the stenosis.8
Theron's technique has the disadvantage of using a large introducer catheter, which may not be appropriate for the treatment of very severe stenosis, and the complexity of the procedure increases the hazards. The prolonged total occlusion time of more than 10 minutes increases the chances hemodynamic ischemia, and the occlusion balloon may add to the risk of thrombosis. However, similar methods are currently undergoing clinical trials.
Carotid Filters
An alternative approach to the triple lumen catheter is to use a filter beyond the stenosis that opens like an umbrella, catching small particles of debris but allowing continuous flow of blood to maintain cerebral perfusion. Afterwards, the umbrella is collapsed, enclosing any debris, and then withdrawn across the dilated stenosis. Clinical experience with such filters is very limited, and their effectiveness remains to be determined.
Antithrombotic Regimes
Because of the risk of thromboembolism during carotid PTA and stenting, patients receive intravenous or intra-arterial heparin at the time of procedure. In early studies, intravenous heparin was continued for at least 24 hours after the procedure, and an antiplatelet agent, often aspirin, was given 24 hours before treatment. Recently, it has been suggested that a combination of ticlopidine or clopidogrel with aspirin may replace heparin. It is possible that these regimes, in turn, will be replaced by more powerful drugs, such as glycoprotein Ilb-IIIa antagonists. In some early series, complete anticoagulation was established with warfarin for some weeks before angioplasty to try to remove any thrombus that
P.244
might be present within the atheromatous plaque. This is not currently recommended as a routine, but anticoagulation for two to four weeks may be a sensible precaution in patients who have had very recent symptoms.
Monitoring
One advantage of carotid PTA and stenting is that the patient remains awake during the procedure, and neurological complications can be easily detected. Simple neurological examinations, blood pressure monitoring, and pulse measurements at regular intervals during the procedure help to ensure that it is proceeding safely. Transcranial Doppler (TCD) to monitor blood flow velocity and to detect embolism in the middle cerebral artery has also been used.
Mechanisms of Arterial Dilation
The increase in arterial diameter achieved by angioplasty results from an in diameter of the whole vessel, moving the walls outward,9 and compression or redistribution of the atheromatous plaque does not occur. Experimental studies in animal models have shown that balloon inflation denudes the endothelium, splits atheromatous plaque so that it dehisces from the underlying media, and stretches the media and adventitia. Splitting of the atheromatous plaque appears to be essential for successful angioplasty and is the only way that concentric plaque can be dilated.
The arterial wall injury caused by angioplasty results in the stimulation of fibroblasts and smooth muscle cell replication, this process continues throughout the following few weeks and possibly months. The process of repair may lead to remodelling of the artery, with an increase in diameter. Early absorption of hematoma within the wall and passive stretching of the artery over time may also contribute to an increase in diameter of the artery after the initial dilation. If the repair process is excessive, then restenosis may occur. Histological examination in one case of restenosis showed excessive smooth muscle proliferation narrowing of the lumen.10 It is likely that similar pathological changes occur after stenting.
Complications
Splitting the atheromatous plaque, which is often required for successful dilation, produces mechanical complications (Table 13.1). Some intimal dissection is inevitable, usually localized to the area of plaque, but inadvertent subintimal insertion of the guidewire or catheter may produce extensive dissection, causing occlusion or pseudoaneurysm formation.
TABLE 13.1. Complications of Angioplasty and Stenting | ||
|---|---|---|
|
P.245
Irritation of the wall artery by guidewire or catheter causes arterial spasm, but this is usually symptomatic only if severe enough to result in thrombus formation. Vessel rupture is very rare but easily recognized by the sudden onset of severe pain in the neck associated with extravasation contrast media outside the vessel. Balloon inflation or stent deployment at the carotid bifurcation frequently results in stimulation of the carotid sinus, leading to bradycardia and occasionally to brief periods of asystole. To prevent this complication, all patients should be pretreated with atropine to reduce the consequences of receptor stimulation. Hypotension may occasionally be troublesome for 48 hours after the procedure.
P.246
The major risks are cerebral embolism and carotid occlusion, occurring mainly in patients with unstable and very stenosed plaques. This present with crescendo transient ischemic attacks. Hemodynamic ischemia during the procedure may be responsible for a brief transient attack during balloon inflation, but this rarely leads to stroke.
Occlusion causes major stroke in about 50% of patients. Acute occlusion of the artery may follow hemorrhage into the plaque or be secondary to dissection after angioplasty. It is probable that this complication occurs less often after primary stenting than after simple balloon angioplasty. If occlusion does occur, options for management include immediate thrombolysis with or without stenting, emergency surgical endarterectomy, or conservative management with anticoagulation.
About half of patients experience some brief discomfort in the neck at site of angioplasty or stenting at the time of procedure, and occasionally this radiates to the eye and forehead or scapula (carotidynia). This pain is usually short lived and lasts only a few seconds during balloon inflation but occasionally may last up to 48 hours.
Despite the long list of potential complications, most tolerate angioplasty and stenting with little discomfort. Groin hematoma may cause problems, particularly if the stent requires a large introducer sheath, but this complication has been considerably reduced by devices that seal the artery and compress groin after the procedure.
Occasional angiographic complications occur, including stroke from catheter or guidewire dislodgment of atheroma and thrombus in the aortic arch or major vessel on route to the site of stenosis. Delayed stroke may occur afterward due to either thrombosis on the stent or damaged intima, as a sequel to dissection. Cerebral hemorrhage unrelated to anticoagulation may occur following successful angioplasty or stenting due to the reperfusion syndrome.11,12 This occurs after treatment of very severe stenosis with subsequent marked increase in the velocities of flow in the ipsilateral carotid and middle cerebral arteries. Edema or hemorrhage of the ipsilateral cerebral hemisphere may also occur due to failure of autoregulation within the reperfused microcirculation. These delayed complications are unlikely to occur more than 10 days after the procedure.
Among the few long-term complications of angioplasty and stenting, main concern is restenosis. Approximately 20% of carotid arteries treated by simple balloon angioplasty will have some degree of restenosis at one year by ultrasound examination.13,14 However, few of those with restenosis become symptomatic, at least in the short term, because restenosis these cases is due to smooth muscle proliferation and not atheromatous plaque. Restenosis is not, therefore, an indication for intervention, unless the patient is symptomatic. Symptoms are more likely to be caused by hemodynamic, rather than embolic, mechanisms, especially if associated with contralateral carotid occlusion.10
P.247
The restenosis rate of stenting is as yet unknown. Collapse the stent may produce severe stenosis, possibly due to pressure on the neck deforming it, and new stents are being designed to avoid this complication.
Unlike surgery, with angioplasty and stenting the athermatous plaque remains, with its potential for recurrent ulceration and further growth, although the endothelial proliferative following the procedure may prevent this. There is insufficient long-term follow-up of patients treated by angioplasty or stenting beyond a few years, but available evidence suggests that, in the long term, recurrence of symptoms is unusual and probably no more frequent than after carotid surgery.
Results in Case Series
Small case series of patients with athermatous carotid stenosis treated by balloon angioplasty first appeared nearly 20 years ago, but the procedure was limited to a small number of centers. Even in 1992, a review all the published cases reported a total of only 123 patients with atheromatous internal carotid artery stenosis.15 It was not until the 1990s that larger series were published8-16,17,18,19,20,21,22,23,24 (see Table 13.2). Stroke and death rates from the procedure were similar to those of carotid
P.248
surgery. At present, little evidence indicates that stents are superior to balloon angioplasty alone, with the possible exception of the study by Theron et al.19 In a survey of 24 centers throughout the world up to 1998, a total 2048 endovascular carotid stent procedures were reported, with a stroke and death rate of 5.8% within 30 days.25 However, few case reports have been published that provide adequate information about long-term follow-up beyond 6 to 12 months. In the short term, very few recurrent strokes are described, suggesting that angiography and stenting are effective at preventing stroke after a successful procedure.
TABLE 13.2. Selected Series of Patients Treated by Carotid Angioplasty and/or Stenting | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
A number of potential biases are inherent in these data. Patients with carotid bifurcation lesions are likely to be highly selected, leading a false underestimation of the risks. At least 40% of the cases in stent studies were asymptomatic, while many had restenosis after carotid endarterectomy. Few studies adequately describe the degree of stenosis, and some patients may have had relatively mild narrowing. Also, case studies with poor results are unlikely to be reported. Conversely, patients referred for angioplasty and stenting because surgery was contraindicated may have above-average risks.24
Most reports of these procedures concern carotid bifurcation due to stenosis, either from atherosclerosis or restenosis following previous carotid endarterectomy. Other forms of carotid artery stenosis treated by PTA include fibromuscular dysplasia,26 Takayasu's arteritis,'27 common carotid, external carotid artery stenosis, and distal internal carotid lesions too high in the neck to be surgically accessible.28,29,30 Treatment of intracranial stenosis in the distal carotid, distal vertebral, basilar, and middle cerebral arteries is usually considered too hazardous, but has been attempted.30,31,32,33,34
Vertebral artery stenosis, particularly at the origin of vertebral artery from the arch of the aorta, is relatively easy to treat by angioplasty or stenting. However, only a small number of case series have been reported in the literature, all with very low complication rates.35 The limited number of cases presumably reflects the relative rarity of vertebrobasilar symptoms in comparison to carotid disease and the limited value of noninvasive investigations, such as ultrasound and magnetic resonance angiography, in detecting vertebral artery stenosis.36 A larger series of patients with subclavian stenosis, sometimes associated with subclavian steal, have been treated successfully by angioplasty or stenting with a very low complication rate, but neurological details are almost universally lacking from these reports.
Randomized Trials
Only two randomized trials of angioplasty or stenting for stroke prevention have been reported. The first was a single-center study from Leicester, England, but the trial was stopped after only 17 patients had been treated because 5 of 7 treated
P.249
by stenting had a stroke at the time of procedure.37 These poor results may reflect poor radiologic technique. The second trial was the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS),38 which compared carotid and vertebral PTA to conventional treatment in 560 patients Australia, Canada, the United States, and other countries in Europe between 1992 1997. The published analysis was restricted to those with carotid stenosis suitable for surgery, randomized between PTA (balloon angioplasty or stenting, 251 patients) and carotid surgery (253 patients). Almost all the patients were recently symptomatic, and baseline variables were well matched (Table 13.3).
These patients had a high incidence of vascular risk factors and twice the prevalence of ischemic heart disease those in the European Carotid Surgery Trial, and most patients had severe carotid stenosis. Because stents were introduced only during the last few years of the study, only 22% of patients received them; most patients were treated by balloon angioplasty. The rate of disabling stoke was virtually identical, at 5.9% in the surgical group and 6.0% angioplasty group, and, similarly, the rate of all strokes or death within 30 days treatment was 9.9% in the surgical group and 10% in the angioplasty group (Table 13.4). CAVATAS counted only strokes lasting more than 7 days in order to match the criterion used in the European Carotid Surgery Trial and to avoid bias related to the fact that the majority of PTAs were carried out under local anaesthetic on neurological wards, while surgery was mostly carried out under general anesthesia on surgical wards without immediate neurological assessment.
PTA was found safer than surgery for minor morbidity, including cranial or peripheral nerve palsy (9% in the surgical group and none in the PTA group), as well as major adverse events, such myocardial infarction and angina (2% in the surgical group and none in the PTA group). Hematomas prolonging hospital stay were more common in the surgical patients (7% compared to 2%).
TABLE 13.3. Baseline Variables in Patients with Carotid Stenosis Fit for Surgery, Randomized in CAVATAS (n = 504) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
TABLE 13.4. Major Outcome Events in Patients with Carotid Stenosis Fit for Surgery Randomized in CAVATAS, Analyzed by Intention to Treat. (Values are percentages.) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
P.250
Long-term follow-up in CAVATAS showed no difference stroke rate for up to three years after treatment. Survival analysis showed no difference in any major outcome events between the two groups, and the survival curves for ipsilateral stroke showed that both treatments were equally effective at preventing stroke over the time course of the studies, with virtually no ipsilateral strokes after postoperative period (Fig. 13.4).
At a mean follow-up of 12 months, 19% of PTA patients had >70% stenosis or occlusion by ultrasound criteria, compared to only 5% of surgical patients, but
P.251
this restenosis was rarely symptomatic. Some patients initially randomized to angioplasty but in whom the procedure was technically unsuccessful then went on to carotid surgery.
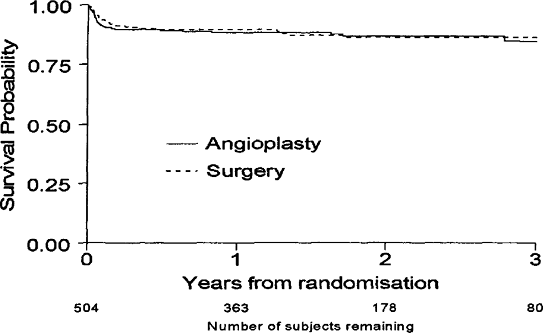 |
FIGURE 13.4. Kaplan-Meier survival curves in patients with carotid stenosis fit for surgery randomized in CAVATAS showing rate of ipsilateral stroke (>7 days duration). There is no significant difference between the curves. |
Little difference in quality of life resulted after these two procedures. Also, the procedural costs of these two techniques were similar, but angioplasty was more cost beneficial due to the shorter length of hospital stay.39 The use of more expensive radiologic devices or shortening the length of surgical stay might abolish the cost advantages of stenting.
A relatively high rate of stroke or death occurred in the CAVATAS study, and although it was similar to the data of ECST trial, included patients with higher surgical risks than average, especially those with ischemic heart disease. There was some evidence in CAVATAS that stenting safer than balloon angioplasty, but the numbers were small and further studies of stenting are needed.
The 30-day risk of stroke or death in CAVATAS was 10%, and the wide confidence limits of 5% 15% emphasize the imprecision the findings. Although no evident difference was seen between surgery and stenting, with larger numbers a clinically important difference might emerge. Further trials of the procedure are therefore needed.
Hemodynamic and Embolic Consequences
Cerebral hemodynamics evaluations using transcranial Doppler (TCD) indicated a variety of effects following carotid stenting, and one study showed a 30% increase in CO2 reactivity the ipsilateral hemisphere, indicating improved vasodilator capacity secondary to improved perfusion pressure.40 Severe reductions in middle cerebral artery blood flow at the time of PTA may be associated with hemodynamic symptoms.41 This improvement was gradual over the first four weeks, presumably reflecting the process of remodelling occurring in the days after angioplasty.
Microembolic signals detected by TCD were frequent during carotid angioplasty,42,43 although the cause of these signals is uncertain, varying from particulate matter to air bubbles. Numerous high-intensity signals occur during routine carotid angiography as a result of small bubbles in the contrast medium, and many of the embolic signals during carotid angioplasty are likely to represent similar air bubbles.44
More embolic signals are seen during and immediately after carotid angioplasty than after carotid surgery.43 (Table 13.5). Although the duration of occlusion and reduced blood flow in the middle cerebral artery to <30% were greater during surgery, no correlation was found between either this, the number of emboli, or clinical complications.
Microemboli are usually asymptomatic, and the duration of the signals suggests that most are very small and will only occlude the smallest capillaries,
P.252
though they potentially could produce subclinical cerebral damage. However, in one study, no significant differences were detected in neuropsychological outcome after carotid surgery compared to angioplasty.44
TABLE 13.5. Randomized Comparison of Embolic Signals and Ischemic Time During Carotid Surgery and Percutaneous Transluminal Angioplasty Detected by Transcranial Doppler of the Ipsilateral Middle Cerebral Artery (Adapted from ref. 43.) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
The Future of Angioplasty and Stenting
The risks of PTA and stenting are similar to those carotid surgery but avoid the problems of surgical incision and anesthesia. Before this technique becomes accepted as routine treatment, however, data from randomized clinical trials are essential. Currently, several such trials are in progress or planned, and advances in technology, particularly the development of filter and other protection devices, are likely to improve the safety and applicability of PTA and stenting. Other potential advances in balloon technology include the local delivery of anticoagulant agents to reduce the chances of thrombosis and other drugs that may inhibit smooth muscle proliferation and limit restenosis.
Until the results of further clinical trials are available, interventional treatment should be restricted to specialised centers with demonstrated low complication rates and to the discipline of clinical trials. Despite understandable opposition from some vascular surgeons, it is likely that cerebrovascular PTA will eventually join coronary and peripheral PTA as a major first-line treatment for atheromatous cerebrovascular disease.
References
1. European Carotid Surgery Trialists Collaboration Group. MRC Surgery Trial: Interim results for symptomatic patients with severe (70 99%) or with mild (0 29%) carotid stenosis. Lancet 1991;337:1235 1243.
P.253
2. North American Symptomatic Carotid Endartectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445 53.
3. Rothwell P. Morbidity and mortality of carotid endarterectomy in the European Carotid Surgery Trial. Cerebrovascular Diseases 1995;4:226.
4. Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy. BMJ 1997;315:1591 1597.
5. Sundt TM, Sandok BA, Whisnant JP. Carotid endarterectomy: Complications and preoperative assessment of risk. Mayo Clin Proc 1975;50:301 306.
6. F Crawley, A Clifton, H Markus, M M Brown. Delayed improvement in carotid artery diameter after carotid angioplasty. Stroke 1997;28:575 579.
7. Theron J, Coutheouz P, Alachkar F, Bouvard G, Maiza D. New triple coaxial catheter system for carotid angioplasty with cerebral protection. American Journal of Neuroradiology 1990; 11:869 874.
8. Kachel-R. Results of balloon angioplasty in the carotid arteries. J Endovasc Surg 1996;3:22 30.
9. Castaneda-Zuniga WR, Formanek A, Tadavarthy M, et al. The mechanisms of balloon angioplasty. Radiology 1980; 135:565 571.
10. F Crawley, A Clifton RS Taylor, MM Brown. Symptomatic restenosis after carotid percutaneous transluminal angioplasty. Lancet 1998;352:708 709.
11. Schoser BG, Heesen C, Eckert B, Thie A. Cerebral hyperperfusion injury after percutaneous transluminal angioplasty of extracranial arteries. J Neurol 1997;244:101 104.
12. McCabe DJH, Brown MM, Clifton A. Fatal cerebral reperfusion hemorrhage following carotid stenting. Stroke 1999;30:2483 2486.
13. Madrid A, Gil-Peralta Gonzalez-Marcos JR, Otero Crespo P. Restenosis and remodeling after percutaneous transluminal carotid angioplasty. Rev Neurol 1998;27: 649 652.
14. Schoser BG, Becker VU, Eckert B, Zeumer H, Thie A. Clinical and ultrasonic longterm results of percutaneous transluminal carotid angioplasty: A prospective followup of 30 carotid angioplasties. Cerebrovascular Diseases 1998;8:38 41.
15. Brown MM. Balloon angioplasty for Cerebrovascular disease. Neurol Res 1992; 14(suppl): 159 173.
16. Munari LM, Belloni G, Perretti A, Gatti Moschini L, Porta M. Carotid percutaneous angioplasty. Neurol Res 1992; 14(suppl): 156 158.
17. Eckert B, Zanella FE, Thie A, Steinmetz J, Zeumer H. Angioplasty of the internal carotid artery: Results, complications and follow-up in 61 cases. Cerebrovascular Diseases 1996;6:97 105.
18. Gil-Peralta A, Mayol Marcos JR, Gonzalez Ruano J, Boza F, Duran F. Percutaneous transluminal angioplasty of the symptomatic atherosclerotic carotid arteries: Results, complications, and follow-up. Stroke 1996;27:2271 2273.
19. Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: Treatment with protected balloon angioplasty and stent placement. Radiology 1996; 201:27 36.
20. Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: Initial experience in 110 patients. J Endovasc Surg 1996;3:42 62.
21. Wholey MH, Wholey Jarmolowski CR, Eles G, Levy D, Buecthel J. Endovascular stents for carotid artery occlusive disease. J Endovasc Surg 1997;4:326 338.
P.254
22. Waigant J, Gross CM, Uhlich F, Kramer Tamaschke C, Vogel P, Luft FC, Dietz R. Elective stenting of carotid artery stenosis in patients with severe coronary artery disease. Eur Heart J 1998;19:1365 1370.
23. Henry M, Amor Masson I, Tzvetanov K, Chati Z, Khanna N. Angioplasty and stenting of the extracranial carotid arteries. J Endovasc Surg 1998;5: 293 304.
24. Mathur A, Roubin GS, Iyer SS, Piamsonboon C, Liu MW; Gomez CR, Yadav JS, Chastain HD, Fox LM, Dean LS, Vitek JJ. Predictors of stroke complicating carotid artery stenting. Circulation 1998;97:1239 1245.
25. Wholey MH, Wholey M, Bergeron P, Diethrich EB, Henry Laborde JC, Mathias K, Myla S, Roubin GS, Shawl F, Theron JG, Yadav JS, Dorros G, Guimaraens J, Higashida R, Kumar V, Leon M, Lim M, Londero H, Mesa J, Ramee S, Rodriguez A, Rosenfield K, Teitelbaum G, Vozzi C. Current global status of carotid artery stent placement. Cathet Cardiovasc Diagn 1998;44:l-6.
26. Mathias KD. Percutaneous transluminal angioplasty in supra-aorta artery disease. In: Roubin GS, ed. Internventional Cardiovascular Medicine: Principles and Practice. New York: Churchill Livingstone, 1994:745 775.
27. Tsai FY, Matovich V, Hieshima G, et al. Percutaneuous transluminal angioplasty of the carotid artery. Am J Neuroradiol 1986;7:349 358.
28. Rostmily RC, Mayberg MR, Eskridge JM, Goodkin R, Winn HR. Resolution of petrous internal carotid artery stenosis after transluminal angioplasty: Case report. Neurosurg 1992;76:520 523.
29. O'Leary DH, Clouse ME. Percutaneous transluminal angioplasty of the cavernous carotid artery for recurrent ischemia. Am J Neuroradiol 1994;5:644.
30. Tsai FY, Higashida R, Meoli C. Percutaneous transluminal angioplasty of extracranial and intracranial arterial stenosis in the head neck. Interventional Neuroradiology 1992 2:311 384.
31. Mori T, Mori K, Fukuoka M, Arisawa Honda S. Percutaneous transluminal cerebral angioplasty: Serial angiographic follow-up after successful dilatation. Neuroradiology 1997;39:111 116.
32. Takis C, Kwan ES, Pessin MS, Jacobs DH, Caplan LR. Intracranial angioplasty: Experience and complications. Am J Neuroradiol 1997;18:1661 1668.
33. Yokote H, Terada T, Ryujin K, Konoshita Y, Tsuura M, Nakai E, Kamei I, Moriwaki H, Hayashi S, Itakura T. Percutaneous transluminal angioplasty for intracranial arteriosclerotic lesions. Neuroradiology. 1998;40:590 596.
34. Eckard DA, Zarnow DM, McPherson CM, Siegel EL, VR, Batnitzky S, Hermreck A. Intracranial internal carotid artery angioplasty: Technique with clinical and radiographic results and follow-up. Am J Roentgenol 1999; 172:703 707.
35. Higashida RT, Tsai FY, Halbach V, et al. Transluminal angioplasty for atherosclerotic disease for the vertebral and basilar arteries. J Neurosurg 1993;78:192 198.
36. Crawley F, Clifton A, Brown MM. Treatable lesions demonstrated on vertebral angiography for posterior circulation ischemic events. Br J Radiol 1998;71:1266 1270.
37. Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith M, Lennard N, Lloyd AJ, London NJ, Bell PR. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: A stopped trial. Vase Surg 1998;28:326 334.
38. The CAVATAS Investigators. Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): results in patients with carotid artery stenosis randomised between surgery and angioplasty. Submitted to Lancet
P.255
39. Davies A, Buxton M, Brown MM. An economic evaluation of the cost effectiveness of carotid angioplasty and stenting compared with surgery in patients with stenosis randomised in The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS). Submitted for publication.
40. Markus HS, Clifton A, Brown MM. Carotid Angioplasty: Haemodynamic and embolic consequences. Cerebrovascular Diseases 1994;4:259.
41. Eckert B, Thie A, Valdueza J, Zanella F, Zeumer H. Transcranial Doppler sonographic monitoring during percutaneous transluminal angioplasty of the internal carotid artery. Neuroradiology 1997;39:229 234.
42. Markus HS, Clifton A, Buckenham T, Brown MM. Carotid angioplasty: Detection of embolic signals during and after the procedure. Stroke 1994;25:2403 2406.
43. Crawley F, Clifton A, Buckenham T, Loosemore Taylor RS, Brown MM. Comparison of hemodynamic cerebral ischemia and microembolic signals detected during carotid endarterectomy and angioplasty. Stroke 1997;28:2460 2464.
44. Crawley F, Stygall J, Lunn S, Harrison M, Brown MM, Newman S. Comparison of microembolism detected by transcranial Doppler and neuropsychological sequelae of carotid surgery and percutaneous transluminal angioplasty. Stroke 2000;31:1329 1334.
EAN: 2147483647
Pages: 23
- ERP Systems Impact on Organizations
- Context Management of ERP Processes in Virtual Communities
- Intrinsic and Contextual Data Quality: The Effect of Media and Personal Involvement
- A Hybrid Clustering Technique to Improve Patient Data Quality
- Development of Interactive Web Sites to Enhance Police/Community Relations