70 - Blunt and Penetrating Injuries of the Chest Wall, Pleura, and Lungs
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIV - Congenital, Structural, and Inflammatory Diseases of the Lung > Chapter 83 - Chronic Pulmonary Emboli
Chapter 83
Chronic Pulmonary Emboli
Michael M. Madani
Stuart W. Jamieson
Pulmonary hypertension as the result of chronic pulmonary emboli is a common condition that unfortunately continues to be underdiagnosed. The true incidence of this disease is difficult to determine because most episodes of pulmonary embolism (PE) are clinically silent. Pulmonary thromboendarterectomy (PTE) for the treatment of chronic thromboembolic pulmonary hypertension is an uncommon surgical procedure, but it provides immediate and permanent relief of the pulmonary hypertension associated with sequelae of unresolved pulmonary thromboembolic disease. Although PTE is now performed at many medical centers around the world, its large success is owed to the pioneering efforts of surgeons at University of California at San Diego (UCSD), who over the years have developed and perfected the operation.
The exact incidence of PE remains unknown, but there are some valid estimates. According to Landefeld and colleagues (1988), approximately 75% of autopsy-proven pulmonary emboli are not detected clinically. Dalen and Alpert (1975) calculated that PE results in 630,000 symptomatic episodes in the United States yearly, making it about half as common as acute myocardial infarction, and three times as common as cerebrovascular accidents. However, Goldhaber (1982) and Rubinstein (1988) and their associates have indicated this to be a low estimate, since in 70% to 80% of the patients where the primary cause of death was PE, premortem diagnosis was unsuspected.
The disease is particularly common in hospitalized elderly patients. Of hospitalized patients who develop PE, 12% to 21% will die in the hospital, and another 24% to 39% die within 12 months, as stated by Kniffin and co-workers (1994) and Martin (1993). Thus, approximately 36% to 60% of the patients who survive the initial episode live beyond 12 months, and may present later in life with a wide variety of symptoms. Because complete resolution of the thrombus may depend on adequate anticoagulant therapy, it is likely that many patients in the subgroup with missed diagnosis go on to develop the chronic and progressively debilitating form of chronic pulmonary hypertension. Unrecognized until 1926, and previously considered by many researchers to represent a rare and aberrant outcome of acute PE, chronic thromboembolic pulmonary hypertension actually represents the outcome of untreated or recurrent emboli: organization of the thrombi, incorporation into the wall of the pulmonary artery, occlusion, and recanalization, all often occurring in repeated cycles and eventually leading to pulmonary hypertension and right-sided heart failure.
The prognosis for patients with pulmonary hypertension is poor, and it is worse for those who do not have intracardiac shunts. Thus, patients with primary pulmonary hypertension and those with pulmonary hypertension due to pulmonary emboli fall into a higher risk category than those with Eisenmenger's syndrome and encounter a higher mortality rate. In fact, Goodwin (1963) and Sutton (1977) and their colleagues have shown that once the mean pulmonary pressure in patients with thromboembolic disease reaches 50 mm Hg or more, the 3-year mortality rate approaches 90%.
The mainstay of treatment for patients with chronic thromboembolic pulmonary hypertension is the surgical removal of the disease by means of PTE. Medical management is only palliative, and surgery by means of transplantation is an inappropriate use of resources with less than satisfactory results. Although pulmonary transplantation is still used in some centers as the treatment of choice for these patients, a true assessment of the effectiveness of this therapy should take into account the total mortality once the patient has been accepted and put on the waiting list. Thus, the mortality rate for transplantation (and especially double-lung or heart-lung transplantation) as a therapeutic strategy is much higher than is generally appreciated because of the significant loss of patients awaiting donors. Considering, in addition, the long-term use of antirejection medications with their associated side effects, the higher rates of operative morbidity and mortality, the long waiting time, and inferior prognosis even after transplantation, transplantation is clearly an inferior option to PTE for this disease.
P.1158
PATHOPHYSIOLOGY OF CHRONIC THROMBOEMBOLIC PULMONARY HYPERTENSION
The association of PE and deep vein thrombosis is well known and well documented. Although most individuals with chronic thromboembolic disease are unaware of a past thromboembolic event and give no history of deep vein thrombosis, the origin of most cases is indeed from acute embolic episodes. Exactly why some patients have unresolved emboli is not certain, but a variety of factors, alone or in combination, must play a role.
The volume of the embolic material may simply be overwhelming to the thrombolytic mechanisms; the lytic factors may also be hindered from reaching the embolus by complete occlusion of the major arterial branches. Furthermore, the embolic material itself may be made of substances that are resistant to lysis by normal mechanisms (fat, tumor, or already well-organized fibrous thrombus).
Dibble (1958) indicated that after the clot becomes wedged in the pulmonary artery, one of two processes occurs: (a) organization of the clot proceeds to canalization, producing multiple small endothelialized channels separated by fibrous septa (i.e., bands and webs) or (b) complete fibrous organization of the fibrin clot without canalization may result, leading to a solid mass of dense fibrous connective tissue totally obstructing the arterial lumen.
He pointed out that the fibrous plug is firm and adherent to the arterial wall but can be removed as a unit (at the autopsy table) without disrupting the continuity of the arterial wall.
Apart from the embolic material, a propensity for thrombosis or a hypercoagulable state may be present in a few patients. This abnormality may result in spontaneous thrombosis within the pulmonary vascular bed, encourage embolization, or be responsible for proximal propagation of thrombus after an embolus. Whatever the predisposing factors to residual thrombus within the vessels, the final genesis of the resultant pulmonary vascular hypertension may be complex. With the passage of time, the increased pressure and flow as a result of redirected pulmonary blood flow in the previously normal pulmonary vascular bed can create a vasculopathy in the small precapillary blood vessels similar to that seen in Eisenmenger's syndrome.
Factors other than the simple hemodynamic consequences of redirected blood flow are probably also involved in this process. For example, it was shown by Cournad and co-workers (1950) that after a pneumonectomy, 100% of the right ventricular output flows to one lung, yet little increase in pulmonary pressure occurs, even with follow-up of up to 11 years. In patients with thromboembolic disease, however, we frequently detect pulmonary hypertension even when less than 50% of the vascular bed is occluded by thrombus. It thus appears that sympathetic neural connections, hormonal changes, or both might initiate pulmonary hypertension in the initially unaffected pulmonary vascular bed. This process can occur with the initial occlusion either being in the same or the contralateral lung.
Regardless of the cause, the evolution of pulmonary hypertension as a result of changes in the previously unobstructed pulmonary vascular bed is serious because this process may lead to an inoperable situation. Consequently, with our accumulating experience in patients with thrombotic pulmonary hypertension, we have increasingly been inclined toward early operation in order to avoid these changes.
FACTORS PREDISPOSING TO CHRONIC THROMBOEMBOLIC PULMONARY HYPERTENSION
Until recently, it was thought that most patients with chronic thromboembolic pulmonary hypertension did not have a prothrombotic state and that only a small percentage of patients (0.1 to 0.2%) with pulmonary emboli went on to develop the chronic form. However, given the fact that the diagnosis of acute PE is missed in the majority of cases, any prothrombotic state should increase the likelihood of the development of pulmonary hypertension as the result of thromboembolic disease. The most common coagulation abnormality seen is the presence of lupuslike anticoagulant. Auger and colleagues (1995) found a lupus anticoagulant to be present in approximately 11% of patients. Other coagulation abnormalities described include protein C, protein S, and antithrombin III deficiency. Therefore, even though the majority of patients with this disease do not have a coagulation disorder, the incidence of such disorders in these patients is higher than in the general population. Other factors described that have been associated with chronic thromboembolic disease include the presence of malignancy, indwelling venous catheters or pacemaker leads, ventriculoatrial shunts, and atrial septal defect. Rarely emboli from tumor fragments in right atrial myxomas, and other malignancies such as kidney, breast, and stomach, have also been documented to cause chronic pulmonary occlusion.
CLINICAL PRESENTATION
Chronic pulmonary thromboembolism has been described in patients ranging from 15 months to 90 years of age. However, the disorder tends to be most common in patients over 40 years of age. There is no gender difference. In the initial stages of the disease, diagnosis based on the clinical presentation may be difficult, since the clinical picture is insidious. Although at least 90% of the pulmonary thromboembolic material comes from a deep vein thrombosis, less than half of the patients have symptoms of this problem. However, prior symptoms, such as a history of leg
P.1159
swelling, chest pain, or hemoptysis, should be sought in the clinical history.
The most common symptom associated with thromboembolic pulmonary hypertension, as with all other causes of pulmonary hypertension, is exertional dyspnea. The hallmark of the disease is that this dyspnea is out of proportion to any abnormalities found on clinical examination. Syncope, or presyncope (light-headedness during exertion) is another common symptom in pulmonary hypertension. Generally, it occurs in patients with more advanced disease and higher pulmonary arterial pressures.
Nonspecific chest pains occur in approximately 50% of patients with more severe pulmonary hypertension. Peripheral edema, early satiety, and epigastric or right upper quadrant fullness or discomfort may develop as the right heart fails (cor pulmonale). Some patients with chronic pulmonary thromboembolic disease present after a small acute pulmonary embolus that may produce acute symptoms of right heart failure. Sometimes hemoptysis occurs. Thus, a careful history may bring out symptoms of dyspnea on minimal exertion, easy fatigability, diminishing activities, and episodes of angina-like pain or light-headedness.
There are no consistent physical signs in patients with chronic thromboembolism, and the physical examination may be surprisingly unrewarding if right heart failure has not occurred. The physical examination in patients with pulmonary hypertension secondary to thromboembolic disease is similar to that seen in patients with pulmonary hypertension and right-sided heart failure, with no characteristics to distinguish it from primary pulmonary hypertension except for the occasional presence of soft murmurs over the lung fields caused by partial obstruction at the pulmonary arteries, as stated by Moser and co-workers (1965). Patients typically have a prominent right ventricular impulse, a loud second heart sound, a murmur of tricuspid regurgitation, engorged liver and neck veins, peripheral edema, and cyanosis. Rarely do they have stigmata of deep venous disease.
DIAGNOSTIC TESTS
To confirm the diagnosis in patients with chronic pulmonary thromboembolism, a standardized workup is recommended for all patients who present with unexplained pulmonary hypertension. These include blood tests, chest radiography, electrocardiography, pulmonary function tests, echocardiography, radionuclide ventilation-perfusion scan, and pulmonary angiography. Occasionally, other tests such as chest computed tomographic (CT) scanning, magnetic resonance (MR) imaging, or pulmonary angioscopy may be added to the diagnostic evaluation to define accurately the etiology of pulmonary hypertension.
Chest radiography may show hilar fullness caused by enlarged central pulmonary arteries, clear or oligemic lung fields, and right ventricular enlargement. Electrocardiography usually demonstrates right ventricular hypertrophy and strain. Neither of these tests is definitive. Standard two-dimensional echocardiography helps to define the presence and severity of pulmonary hypertension and to exclude certain other causes, such as Eisenmenger's syndrome. Echocardiography is becoming an increasingly useful screening procedure in all forms of cardiovascular disorders, and this disease is no exception. Right-sided heart dimensions, degree of tricuspid insufficiency, presence or absence of a patent foramen ovale, and an estimate of the pulmonary arterial pressure (PAP) can all be readily obtained and used as a baseline for postoperative studies.
The ventilation-perfusion lung scan is an important test in the differential diagnosis of primary as opposed to obstructive pulmonary hypertension. A completely normal lung scan essentially precludes the diagnosis of chronic thromboembolic disease, but even a single ventilation-perfusion mismatch should ellicit suspicion and is distinctly different than the mottled, patchy, or plexiform pattern seen in primary pulmonary hypertension. When a patchy pattern is seen, it may not be necessary to perform pulmonary arteriography to confirm the diagnosis of primary pulmonary hypertension. Any segmental or subsegmental perfusion defect, however, should lead to pulmonary arteriography.
Long considered to be the standard of diagnosis in patients with chronic pulmonary hypertension secondary to thromboembolic disease, as noted by Moser and associates (1992), angiography is the examination that also determines technical operability. When subsegmental or larger perfusion defects are noted on the scan, even when matched with ventilatory defects, pulmonary angiography is appropriate to confirm or rule out thromboembolic disease. As shown in Fig. 83-1 and indicated by Auger and colleagues (1992), organized thrombi appear as unusual filling defects, webs, or bands, or completely thrombosed vessels that may resemble congenital absence of the vessel. Organized material along a vascular wall of a recanalized vessel produces a scalloped or serrated luminal edge. Because of both vessel wall thickening and dilatation of proximal vessels, the contrast-filled lumen may appear relatively normal in diameter. Distal vessels demonstrate the rapid tapering and pruning characteristic of pulmonary hypertension.
Although some risk remains, the benefit of establishing the presence of a treatable cause of the hypertension far outweighs the small risk; pulmonary angiography should be performed whenever there is a possibility that chronic thromboembolism is the etiology of pulmonary hypertension. Historically, angiography in those with pulmonary hypertension has been thought to carry disproportionate risk. We have found that not to be the case, and at our institution pulmonary angiographies are performed daily in these patients with minimal associated risks. Several thousand angiograms in pulmonary hypertensive patients have now been performed at our institution without mortality.
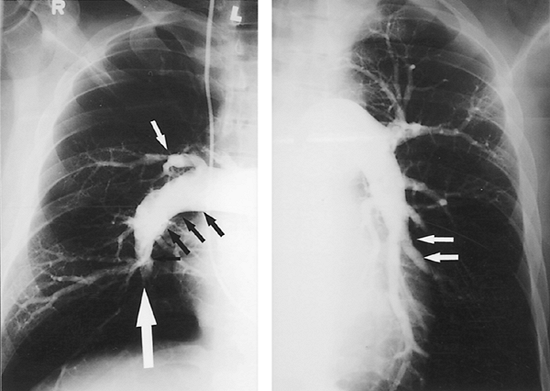 |
Fig. 83-1. Right and left pulmonary angiograms from a patient with chronic thromboembolic pulmonary hypertension. Note the intraluminal filling defects, abrupt pouchlike cut-offs of the branches (large white arrow), multiple fibrous bands (small white arrows), and vascular irregularity (black arrows), usually indicating the presence of a large amount of thromboembolic material. |
P.1160
It is our practice that, in addition to pulmonary angiography, patients over 35 years of age undergo coronary arteriography and other cardiac investigation as necessary. If significant disease is found, additional cardiac surgery is performed at the time of PTE.
In approximately 20% of cases, the differential diagnosis between primary pulmonary hypertension and distal and small vessel pulmonary thromboembolic disease remains unclear. In these patients, pulmonary angioscopy may be helpful. The pulmonary angioscope is a fiberoptic telescope that is placed through a central line into the pulmonary artery. The tip contains a balloon that is then filled with saline and pushed against the vessel wall. A bloodless field can thus be obtained to view the pulmonary artery wall. The classic appearance of chronic pulmonary thromboembolic disease by angioscopy consists of intimal thickening, with intimal irregularity and scarring, and webs across small vessels. These webs are thought to be the residue of resolved occluding thrombi of small vessels, but are important diagnostic findings. The presence of embolic disease, occlusion of vessels, or the presence of thrombotic material is diagnostic.
TREATMENT
Dalen and Alpert (1975) described the natural history of pulmonary hypertension from thromboembolic disease (Fig. 83-2). When the mean pulmonary artery pressure reaches 30 mm Hg, the survival rate at 5 years is 30%; at 50 mm Hg, the 5-year survival rate is less than 10%. The prognosis of chronic thromboembolic pulmonary hypertension is dismal, and nearly all patients die of progressive right heart failure. Medical treatment is limited, and surgical removal of the disease remains the only curative option.
Chronic anticoagulation represents the mainstay of the medical regimen. Anticoagulation is primarily used to prevent future embolic episodes, but it also serves to limit the development of thrombus in regions of low flow within the pulmonary vasculature. Inferior vena caval filters are used routinely to prevent recurrent embolization. If caval filtration and anticoagulation fail to prevent recurrent
P.1161
emboli, immediate thrombolysis may be beneficial, but lytic agents are incapable of altering the chronic component of the disease.
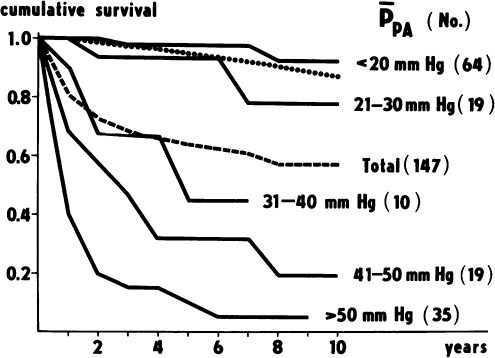 |
Fig. 83-2. Cumulative survival curves according to initial mean pulmonary artery pressure in pulmonary hypertension secondary to thromboembolic disease. From Riedel M, et al. Long-term follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 81:151, 1982. With permission. |
Right ventricular failure is treated with diuretics and vasodilators, and although some improvement may result, the effect is generally transient because the failure is due to a mechanical obstruction and will not resolve until the obstruction is removed. Dash and associates (1980) and Dantzker and Bower (1981) have demonstrated that the prognosis is unaffected by medical therapy, which should be regarded as only supportive. However, because of the bronchial circulation, pulmonary embolization seldom results in tissue necrosis. Surgical endarterectomy therefore will allow distal pulmonary tissue to be used once more in gas exchange. The only other surgical option for these patients is transplantation. However, we consider transplantation to be inappropriate for this disease because of the mortality and morbidity rates of patients on the waiting list, the higher risks of the operation, and the contrasted survival rate (~80% at 1 year at experienced centers for transplantation vs. 95% for pulmonary endarterectomy). Furthermore, pulmonary endarterectomy appears to be permanently curative, and the issues of a continuing risk for rejection and immunosuppression are not present.
Patient Selection
Once the diagnosis of thromboembolic pulmonary hypertension has been confirmed, the decision for an operation is made based on the severity of symptoms and the general condition of the patient. Moser and colleagues (1965) pointed out that there are three major reasons for considering thromboendarterectomy: hemodynamic, alveolorespiratory, and prophylactic. The experience of one of us (SWJ) and Kapelanski (2000) has added another prophylactic goal: the prevention of secondary arteriopathic changes in the remaining patent vessels.
The indications for operation have already been described. The typical patient is severely symptomatic, generally in New York Heart Association (NYHA) class III or IV, has no other life-threatening illness, a pulmonary vascular resistance (PVR) higher than 800 dynes/s/cm5, and documented disease on angiogram. There is no upper limit of PVR level or degree of right ventricular dysfunction that excludes patients from an operation. Many of our patients have PVR levels in excess of 1,000 dynes/s/cm5. In general, the PVR level even in minimally symptomatic individuals exceeds 300 dynes/s/cm5 at rest or during exercise. With the growth of our surgical experience, we also now accept patients for surgery with more distal thromboembolic disease that is probably contributing to, but not entirely responsible for, a patient's symptoms and also patients with advanced right-sided cardiac failure with ascites and hepatic and renal dysfunction that is presumed to be reversible. An inferior vena cava filter is routinely placed several days in advance of the operation.
OPERATION
Principles
There are several guiding principles for this operation, as previously described by one of us (SWJ) (1998) and with Kapelanski (2000). It must be performed bilaterally because, for pulmonary hypertension to be a major factor, both pulmonary arteries have to be substantially involved. The only reasonable approach to both pulmonary arteries is through a median sternotomy incision. Historically, there were many reports of unilateral operation, and occasionally this is still performed through a thoracotomy incision in inexperienced centers. However, the unilateral approach ignores the disease on the contralateral side, subjects the patient to hemodynamic jeopardy during the clamping of the pulmonary artery, and does not allow good visibility because of the continued presence of bronchial blood flow. In addition, collateral channels develop in chronic thrombotic hypertension not only through the bronchial arteries but also from diaphragmatic, intercostal, and pleural vessels. The dissection of the lung in the pleural space via a thoracotomy incision can therefore be extremely bloody. The median sternotomy incision, apart from providing bilateral access, avoids entry into the pleural cavities and allows the ready institution of cardiopulmonary bypass.
Excellent visualization in a bloodless field is required to define an adequate endarterectomy plane and to then follow the pulmonary endarterectomy specimen deep into the subsegmental vessels. Because of the copious bronchial blood flow usually present in these cases, periods of circulatory arrest are necessary to ensure optimal visibility. During the operation cardiopulmonary bypass is essential to ensure cardiovascular stability, and to cool the patient to allow circulatory arrest. Again, there have been sporadic reports of the performance of this operation without circulatory arrest, but it should be emphasized that although endarterectomy is possible without circulatory arrest, a complete endarterectomy is not. We always initiate the procedure without circulatory arrest, and a variable amount of dissection is possible before the circulation is stopped, but never a complete dissection. The circulatory arrest periods are generally limited to 20 minutes, with restoration of flow between each arrest. With experience, the endarterectomy usually can be performed with a single period of circulatory arrest on each side.
The next principle, providing perhaps the most important technical aspect of this operation, is to establish the correct endarterectomy plane. A true endarterectomy in the plane of the media must be accomplished, and it is essential to appreciate that the removal of visible surface thrombus is
P.1162
largely incidental to this operation. Indeed, in most patients, no free thrombus is present, and on initial direct examination, the pulmonary vascular bed may appear normal. The early literature on this procedure describes many cases in which thrombectomy was performed without endarterectomy, and in these cases the pulmonary artery pressures did not improve, often with the resultant death of the patient.
Preoperative and Anesthetic Preparation
The preoperative preparation is common to most other open-heart procedures. Routine monitoring for anesthetic induction includes a surface electrocardiogram, cutaneous oximetry, and radial and pulmonary artery pressures. After anesthetic induction, a femoral artery catheter, in addition to a radial arterial line, is also placed. This provides more accurate measurements during rewarming and on discontinuation of cardiopulmonary bypass because of the peripheral vasoconstriction that occurs after hypothermic circulatory arrest. It is generally removed in the intensive care unit when the two readings correlate.
Electroencephalographic recording is performed to ensure the absence of cerebral activity before circulatory arrest is induced. The patient's head is enveloped in a cooling jacket, and cerebral cooling is begun after the initiation of bypass. Temperature measurements are made of the esophagus, tympanic membrane, urinary catheter, rectum, and blood (through the Swan-Ganz catheter). A transesophageal echocardiogram is also routinely placed by the cardiac anesthesiologist and removed after successful separation from cardiopulmonary bypass.
Operative Technique
A median sternotomy incision is made, and the pericardium is incised longitudinally. A pericardial well is then established. Most patients with chronic thromboembolic pulmonary hypertension have an enlarged right heart, with a tense right atrium and a variable degree of tricuspid regurgitation. There is usually severe right ventricular hypertrophy, and with critical degrees of obstruction, the patient's condition may become unstable with the manipulation of the heart.
Once adequate anticoagulation is achieved, full cardiopulmonary bypass is instituted with high ascending aortic cannulation and two caval cannulae. These cannulae must be inserted into the superior and inferior vena cavae sufficiently to enable subsequent opening of the right atrium. The heart is emptied on bypass, and a temporary pulmonary artery vent is placed in the midline of the main pulmonary artery 1 cm distal to the pulmonic valve. This will mark the beginning of the left pulmonary arteriotomy.
When cardiopulmonary bypass is initiated, surface cooling with both the head jacket and the cooling blanket is begun. The blood is cooled with the pump oxygenator. Cooling generally takes 45 minutes to an hour. When ventricular fibrillation occurs, an additional vent is placed in the left atrium through the right superior pulmonary vein. This prevents atrial and ventricular distention from the large amount of bronchial arterial blood flow that is common in these patients.
The primary surgeon starts the operation on the left side of the patient. During the cooling period, some preliminary dissection can be performed, with full mobilization of the right pulmonary artery from the ascending aorta and superior vena cava, which is also fully mobilized. The approach to the right pulmonary artery is made medial, not lateral, to the superior vena cava (Fig. 83-3A).
All dissection of the pulmonary arteries takes place intrapericardially, and neither pleural cavity should be entered. An incision is then made in the right pulmonary artery from beneath the ascending aorta out under the superior vena cava and entering the lower lobe branch of the pulmonary artery just after the take-off of the middle lobe artery (Fig. 83-3B). It is important that the incision stays in the center of the vessel and continues into the lower, rather than the middle, lobe artery. Any loose thrombus, if present, is now removed. This is necessary to obtain good visualization. It is most important to recognize, however, that first, an embolectomy without subsequent endarterectomy is quite ineffective and, second, in most patients with chronic thromboembolic hypertension, direct examination of the pulmonary vascular bed at operation generally shows no obvious embolic material. Therefore, to the inexperienced or cursory glance, the pulmonary vascular bed may well appear normal even in patients with severe chronic embolic pulmonary hypertension.
If the bronchial circulation is not excessive, the endarterectomy plane can be found during this early dissection (Fig. 83-3C). However, although a small amount of dissection can be performed before the initiation of circulatory arrest, it is unwise to proceed unless perfect visibility is obtained because the development of a correct plane is essential.
When the patient's temperature reaches 20 C, the aorta is cross-clamped and a single dose of cold cardioplegic solution (1 L) is administered. Additional myocardial protection is obtained by the use of a cooling jacket. The entire procedure is now performed with a single aortic cross-clamp period with no further administration of cardioplegic solution. A modified cerebellar retractor is placed between the aorta and superior vena cava (Fig. 83-3B). When blood obscures direct vision of the pulmonary vascular bed, circulatory arrest is initiated, and the patient undergoes exsanguination. All monitoring lines to the patient are turned off to prevent the aspiration of air. Snares are tightened around the cannulae in the superior and inferior vena cavae. It is rare that one 20-minute arrest period for each side is exceeded. Although retrograde cerebral perfusion has been advocated for total circulatory arrest in other procedures, it is not helpful in this
P.1163
operation because it does not allow a completely bloodless field, and with the short arrest times that can be achieved with experience, it is not necessary. Cerebral temperatures, with the use of the cooling jacket to the head, usually reach 15 C.
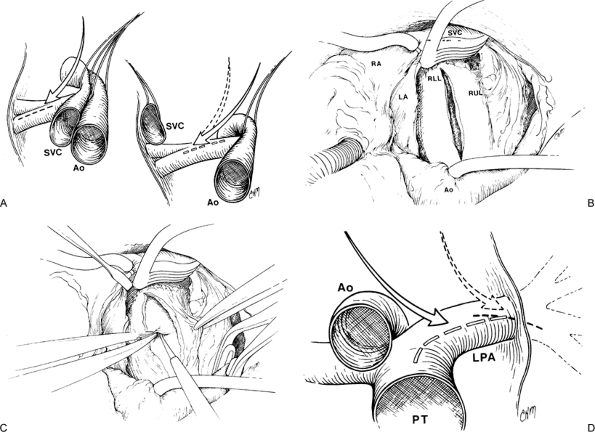 |
Fig. 83-3. Surgical technique of pulmonary thromboendarterectomy. A. On the right side, the surgical approach is medial to the superior vena cava (SVC), between the SVC and aorta (Ao). This provides a direct view down the right pulmonary artery. B. Exposure of the right pulmonary artery and the incision placed between the SVC and aorta (Ao). A cerebellar blunt tip retractor maintains the exposure. C. The endarterectomy plane is begun posteriorly rather than at the cut edges of the arteriotomy. D. The left pulmonary arteriotomy begins at the midpoint of the main pulmonary artery at the site of the incision of the pulmonary artery vent (arrow) and affords better visualization than a more distal approach (dotted arrow). LA, left atrium; LPA, left pulmonary artery; PT, pulmonary trunk; RA, right atrium; RLL, right lower lobe; RUL, right upper lobe. From Jamieson SW, et al: Experience and results of 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg 106:116, 1993. With permission. |
Any residual loose thrombotic debris encountered is now removed. A microtome knife is then used to develop the endarterectomy plane posteriorly (Fig. 83-3C). Dissection in the correct plane is critical because if the plane is too deep the pulmonary artery may be perforated, with fatal results, and if the dissection plane is not deep enough, inadequate amounts of the chronic thromboembolic material will be removed. Several reasons exist for initiating the dissection posteriorly. First, it is desirable to leave a rim of thickened pulmonary artery at the site of the pulmonary artery incision for subsequent closure of the pulmonary arteriotomy. Second, any inadvertent egress posteriorly can be repaired readily, or simply left alone. Finally, when the plane is begun posteriorly and proximally, the specimen starts out quite thin but gets thicker as the surgeon progresses further distally into the arterial branches. This automatically results in development of the correct plane while making it possible to keep the specimen intact. It is quite easy to tell when the plane is too deep because visualization of pinkish tissue indicates that the adventitia has been exposed. A new plane should be begun elsewhere.
The endarterectomy is then performed with an eversion technique. It is important that each lobar, segmental, and
P.1164
subsegmental branch is followed and freed individually until it ends in a tail, beyond which there is no further obstruction. Residual material should never be cut free; the entire specimen should tail off and come free spontaneously.
Once the right-sided endarterectomy is completed, circulation is restarted, and the arteriotomy is repaired with a continuous 6 0 polypropylene suture. The hemostatic nature of this closure is aided by the nature of the initial dissection, with the full thickness of the pulmonary artery being preserved immediately adjacent to the incision.
After the completion of the repair of the right arteriotomy, the surgeon moves to the patient's right side. The pulmonary vent catheter is withdrawn, and an arteriotomy is made from the site of the pulmonary vent hole laterally to the pericardial reflection, again avoiding entry into the left pleural space (Fig. 83-3D). Additional lateral dissection does not enhance intraluminal visibility, may endanger the left phrenic nerve, and makes subsequent repair of the left pulmonary artery more difficult. The left-sided dissection is virtually analogous in all respects to that accomplished on the right. The duration of circulatory arrest intervals during the performance of the left-sided dissection is subject to the same restriction as the right.
After the completion of the endarterectomy, cardiopulmonary bypass is reinstituted and warming is commenced. During warming, a 10 C temperature gradient is maintained between the perfusate and body temperature. If the systemic vascular resistance level is high, nitroprusside is administered to promote vasodilatation and warming. The rewarming period generally takes approximately 90 minutes but varies according to the body mass of the patient.
When the left pulmonary arteriotomy has been repaired, the pulmonary artery vent is replaced at the top of the incision. The right atrium is then opened and examined, unless prior to cardiopulmonary bypass, a negative bubble test was confirmed on transesophageal echocardiography. Otherwise, any intraatrial communication (present in ~20% of patients) is closed at this point.
Although tricuspid valve regurgitation is invariable in these patients and is often severe, tricuspid valve repair is not performed. Right ventricular remodeling occurs within a few days, with the return of tricuspid competence. If other cardiac procedures are required, such as coronary artery or mitral or aortic valve surgery, these are conveniently performed during the systemic rewarming period. Myocardial cooling is discontinued once all cardiac procedures have been concluded. The left atrial vent is removed, and the vent site is repaired. All air is removed from the heart, and the aortic cross-clamp is removed.
When the patient has rewarmed, cardiopulmonary bypass is discontinued. Dopamine hydrochloride is routinely administered at renal doses, and other inotropic agents and vasodilators are titrated as necessary to sustain acceptable hemodynamics. The cardiac output is generally high, with a low systemic vascular resistance. Temporary atrial and ventricular epicardial pacing wires are placed.
Despite the duration of extracorporeal circulation, hemostasis is readily achieved, and the administration of platelets or coagulation factors is generally unnecessary. Wound closure is routine. A vigorous diuresis is usual for the next few hours, also a result of the previous systemic hypothermia.
DISEASE CLASSIFICATION
There are four broad types of pulmonary occlusive disease related to thrombus that have been described by one of us (SWJ) (1998) and associates (1993, 2000):
Type I disease, accounting for approximately 30% of cases of thromboembolic pulmonary hypertension (Fig. 83-4), refers to the situation in which major vessel clot is present and readily visible on the opening of the pulmonary arteries. As mentioned earlier, all central thrombotic material has to be completely removed before the endarterectomy.
In Type II disease, accounting for approximately 60% of cases (see Fig. 83-4, as well as color figure), no major vessel thrombus can be appreciated. In these cases only thickened intima can be seen, occasionally with webs, and the endarterectomy plane is raised in the main, lobar, or segmental vessels.
Type III disease, accounting for approximately 10% of cases (Fig. 83-5), presents the most challenging surgical situation. The disease is very distal and confined to the segmental
P.1165
and subsegmental branches. No occlusion of vessels can be seen initially. The endarterectomy plane must be carefully and painstakingly raised in each segmental and subsegmental branch. Type III disease is most often associated with presumed repetitive thrombi from indwelling catheters (such as pacemaker wires) or ventriculoatrial shunts, and sometimes represents burnt out disease, where most of the embolic material has been resorbed.Type IV disease does not represent classic chronic thromboembolic pulmonary hypertension and is inoperable. In this entity there is intrinsic small vessel disease, although secondary thrombus may occur as a result of stasis. Small-vessel disease may be unrelated to thromboembolic events ( primary pulmonary hypertension) or occur in relation to thromboembolic hypertension as a result of a high-flow or high-pressure state in previously unaffected vessels, similar to the generation of Eisenmenger's syndrome. We believe that there may also be sympathetic cross-talk from an affected contralateral side or stenotic areas in the same lung.
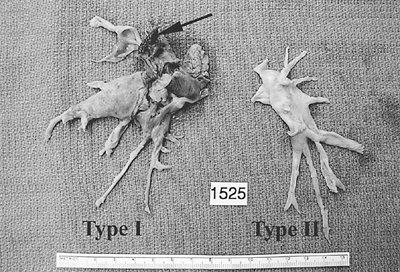 |
Fig. 83-4. Surgical specimen removed from right and left sides of the patient whose angiogram is shown in Fig. 83-1. Arrow points to the presence of some fresh proximal thrombus, making the surgical classification for this patient type I. It is not uncommon for more material to be removed at operation than would be suggested by the angiogram. The ruler measures 15 cm. |
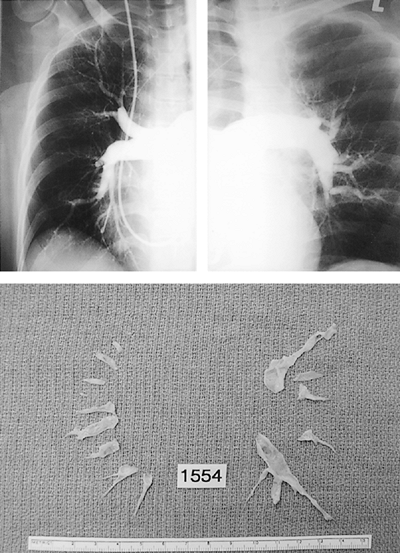 |
Fig. 83-5. Right and left pulmonary angiograms as well as the surgical specimen retrieved from the same patient. In this scenario the plane is raised at each segmental level to ensure complete endarterectomy and retrieval of any material to the tail end. The surgical classification is type III. |
POSTOPERATIVE CARE
Meticulous postoperative management is essential to the success of this operation. All patients are mechanically ventilated at least overnight, and a forced diuresis is maintained with the goal of reaching the patient's preoperative weight within 24 hours. The electrocardiogram, systemic and pulmonary arterial and central venous pressures, temperature, urine output, arterial oxygen saturation, chest tube drainage, and fluid balance are monitored. A pulse oximeter is used to continuously monitor peripheral oxygen saturation. Management of cardiac arrhythmias and output and treatment of wound bleeding are identical to other open-heart operations. Although we used to believe that prolonged sedation and ventilation was beneficial and led to less pulmonary edema, subsequent experience has shown this not to be so. Extubation should be performed on the first postoperative day, if possible. Postoperative venous thrombosis prophylaxis with intermittent pneumatic compression devices is used, and the use of subcutaneous heparin is begun on the evening of surgery.
Anticoagulation with warfarin is begun as soon as the pacing wires and mediastinal drainage tubes are removed, with a target international normalized ratio of 2.5 to 3.
COMPLICATIONS
Patients are, of course, subject to all complications associated with open heart and major lung surgery (arrhythmias, atelectasis, wound infection, pneumonia, mediastinal bleeding, etc.). Neurologic disorders related to deep hypothermia, specifically delirium, used to be considered a major complication of this operation affecting a large number of these patients, as reported by Wragg and associates (1988). However, with the more expeditious operation that has come with increased experience, and the use of the cooling jacket to the head, postoperative confusion is now encountered no more commonly than with ordinary open heart surgery.
There are two complications specific to this operation: the reperfusion pulmonary response and persistent pulmonary hypertension. Reperfusion injury is defined as a radiologic opacity seen in the lungs within 72 hours of pulmonary endarterectomy. This unfortunately loose definition may therefore encompass many causes, such as fluid overload and infection. True reperfusion injury, manifested by increased capillary permeability that directly adversely impacts the clinical course of the patient, now occurs in less than 10% of patients. One common cause of the reperfusion pulmonary edema is persistent high pulmonary artery pressures after operation when a thorough endarterectomy has been performed in certain areas, but a large part of the pulmonary vascular bed remains affected by type IV change. However, the reperfusion phenomenon is occasionally
P.1166
encountered in patients after a seemingly technically perfect operation with complete resolution of high pulmonary artery pressures. In these cases the response may be one of reactive hyperemia, after the revascularization of segments of the pulmonary arterial bed that have long experienced no flow. Fortunately, the incidence of this complication is now rare, probably as a result of the more complete and expeditious removal of the endarterectomy specimen that has come with the large experience over the past few years.
RESULTS
More than 1,550 pulmonary thromboembolism operations have been performed at the UCSD Medical Center since 1970. Most of these cases (>1,350) have been completed since 1990, when the surgical procedure was modified as described earlier in this chapter. The mean patient age in the last 1,300 patients was 52 years, with a range of 8 to 85 years. In nearly one third of these cases, at least one additional cardiac procedure was performed at the time of operation. Most commonly, the adjunct procedure was closure of a persistent foramen ovale or atrial septal defect (26%) or coronary artery bypass grafting (8%).
Hemodynamic Results
A reduction in pulmonary pressures and resistance to normal levels, and a corresponding improvement in pulmonary blood flow and cardiac output, are generally immediate and sustained. In general, these changes can be assumed to be permanent. Archibald and colleagues (1999) have shown that, whereas before the operation more than 95% of the patients are in NYHA functional class III or IV, at 1 year after the operation 95% of patients remain in NYHA functional class I or II. Detailed echocardiographic studies have also shown normalization of the right ventricle. Tricuspid valve function returns to normal within a few days as a result of restoration of tricuspid annular geometry after the remodeling of the right ventricle, and tricuspid repair is not therefore part of the operation.
Operative Morbidity and Mortality
In our cumulative experience at UCSD, severe reperfusion injury has been the single most frequent complication, occurring in 10% of patients. Some of these patients did not survive, and other patients required prolonged mechanical ventilatory support. A few patients were salvaged only by the use of extracorporeal support and blood carbon dioxide removal. As described earlier, this complication is now rare. Neurologic complications from circulatory arrest appear to have been eliminated, and perioperative confusion and stroke are now no more frequent than with conventional open-heart surgery. Early postoperative hemorrhage required reexploration in 2.5% of patients, and only 50% of patients required intra- or postoperative blood transfusion. Despite the prolonged operation, wound infections are relatively infrequent. Only 1.8% experienced the development of sternal wound complications, including sterile dehiscence or mediastinitis.
In our experience, the overall mortality rate (30 days or in-hospital if the hospital course is prolonged) was 9% for the entire patient group, which encompasses a time span of 30 years. The mortality rate was 9.4% in 1989 and has been 4.4% (22 patients) in the last 500 patients. We generally quote an operative risk of approximately 5%, but some patients predictably fall within a much higher risk group. With our increasing experience and many referrals, we continue to accept some patients who, in retrospect, were unsuitable candidates for the procedure (type IV disease). We also accept patients in whom we know that the entire degree of pulmonary hypertension cannot be explained by the occlusive disease detected by angiography but feel that they will be benefited by operation, albeit at higher risk.
CONCLUSION
Chronic thromboembolic pulmonary hypertension is a condition that is underrecognized and carries a poor prognosis. Medical therapy is palliative, ineffective in prolonging life, and only transiently improves the symptoms. The only therapeutic alternative to PTE is lung transplantation, which is considered inappropriate for obvious reasons. The advantages of thromboendarterectomy include a lower operative mortality rate and excellent long-term results without the risks associated with chronic immunosuppression and chronic allograft rejection. The mortality rate for thromboendarterectomy at our institution is now in the range of 4.5%, with sustained benefit. These results are clearly superior to those for transplantation both in the short and long term.
Successive improvements in operative technique developed over the past 4 decades now allow pulmonary endarterectomy to be offered to patients with an acceptable mortality rate and excellent anticipation of clinical improvement. With this growing experience, it has also become clear that unilateral operation is obsolete and that circulatory arrest is essential. Improved awareness of both the prevalence of this condition and the possibility of a complete surgical cure should provide more patients the opportunity for relief from this devastating and ultimately fatal disease.
REFERENCES
Archibald CJ, et al: Long-term outcome after pulmonary thromboendarterectomy. Am J Respir Crit Care Med 160:523, 1999.
P.1167
Auger WR, Permpikul P, Moser KM: Lupus anticoagulant, heparin use, and thrombocytopenia in patients with chronic thromboembolic pulmonary hypertension: a preliminary report. Am J Med 99:392, 1995.
Auger WR, et al: Chronic major-vessel thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology 182:393, 1992.
Cournad A, et al: Pulmonary circulation in the alveolar ventilation perfusion relationship after pneumonectomy. J Thorac Surg 19:80, 1950.
Dalen JE, Alpert JS: Natural history of pulmonary embolism. Prog Cardiovasc Dis 17:259, 1975.
Dantzker DR, Bower JS: Partial reversibility of chronic pulmonary hypertension caused by pulmonary thromboembolic disease. Am Rev Respir Dis 124:129, 1981.
Dash H, Ballentine N, Zelis R: Vasodilators ineffective in secondary pulmonary hypertension. N Engl J Med 303:1062, 1980.
Dibble JH: Organization and canalization in arterial thrombosis. J Pathol Bacteriol 75:1 4, 1958.
Goldhaber SZ, et al: Factors associated with correct antemortem diagnosis of major pulmonary embolism. Am J Med 73:822, 1982.
Goodwin JF, Harrison CV, Wilcken DEL: Obliterative pulmonary hypertension and thromboembolism. BMJ 1:701, 1963.
Jamieson SW: Pulmonary thromboendarterectomy. In Franco KL, Putnam JB (eds): Advanced Therapy in Thoracic Surgery. Hamilton, Ontario, Canada: BC Decker, 1998, p. 310.
Jamieson SW, Kapelanski DP: Pulmonary endarterectomy. Curr Probl Surg 37:165, 2000.
Jamieson SW, et al: Experience and results of 150 pulmonary thromboendarterectomy operations over a 29-month period. J Thorac Cardiovasc Surg 106:116, 1993.
Kniffin WD Jr, et al: The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med 154:861, 1994.
Landefeld CS, et al: Diagnostic yield of the autopsy in a university hospital and a community hospital. N Engl J Med 318:1249, 1988.
Martin M. PHLECO. A multicenter study of the fate of 1647 hospital patients treated conservatively without fibrinolysis and surgery. Clin Invest 71:471, 1993.
Moser KM, et al: Chronic, massive thrombotic obstruction of the pulmonary arteries: analysis of four operated cases. Circulation 32:377, 1965.
Moser KM, et al: Chronic thromboembolic pulmonary hypertension: clinical picture and surgical treatment. Eur Respir J 5:334, 1992.
Riedel M, et al. Long-term follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 81:151, 1982.
Rubinstein I, Murray D, Hoffstein V: Fatal pulmonary emboli in hospitalized patients: an autopsy study. Arch Intern Med 148:1425, 1988.
Sutton GC, Hall RJ, Kerr IH: Clinical course and late prognosis of treated subacute massive, acute minor, and chronic pulmonary thromboembolism. Br Heart J 39:1135, 1977.
Wragg RE, et al: Operative predictors of delirium after pulmonary thromboendarterectomy. A model for postcardiotomy syndrome? J Thorac Cardiovasc Surg 96:524, 1988.
READING REFERENCE
Levinson RM, Shure D, Moser KM: Reperfusion pulmonary edema after pulmonary artery thromboendarterectomy. Am Rev Respir Dis 134: 1241, 1986.
EAN: 2147483647
Pages: 203