XII - Thoracic Trauma
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XIV - Congenital, Structural, and Inflammatory Diseases of the Lung > Chapter 82 - Congenital Vascular Lesions of the Lungs
Chapter 82
Congenital Vascular Lesions of the Lungs
Michael J. Liptay
Michael B. Ujiki
The incidence of congenital vascular malformations of the lung is low despite the complex embryologic derivation of the pulmonary vasculature (see Chapter 2). Some of the anomalies can be associated with significant disability and still be amenable to appropriate surgical management. A classification of congenital abnormalities that affect the main branches of the pulmonary vessels and their tributaries was suggested by Ellis and associates (1964) and has been modified for completeness (Table 82-1). Although arbitrary, such a classification encourages more accurate diagnostic delineations of such defects, which may lead to eventual surgical repair in many.
AGENESIS AND STENOSIS OF THE PULMONARY ARTERY
Agenesis and stenosis of pulmonary arteries are rare but provide unusual challenges in diagnosis and management. Most affected persons with these defects die at an early age from right-sided ventricular failure and hypertension in the pulmonary artery of the unaffected side. A few patients are asymptomatic or are hampered by some dyspnea and recurrent pulmonary infections. The natural history of the anomaly is not well understood. Gregg (1941) reported that these anomalies are associated with maternal rubella.
In this abnormality, the affected lung has a small volume and decreased markings (the hyperlucent lung syndrome) (Fig. 82-1). The thoracic cage is contracted and ventilation is reduced. Ventilation-perfusion scans demonstrate vascular hypoperfusion, and decreased ventilation and obstructive bronchiolar disease can be demonstrated with bronchographic contrast material. Angiocardiography delineates the pulmonary artery anomaly. Stenosis of the pulmonary artery may occur at its origin (Fig. 82-2) or at isolated, multiple peripheral sites. In agenesis of the pulmonary artery, an anomalous artery from the aorta (Fig. 82-3) usually supplies the involved lung, but dilated bronchial arteries may be the main source of the blood that maintains some viability of the affected lung. Kochiadakis (2002) described a patient with an anomalous collateral from a coronary artery that was not associated with coronary ischemia.
Surgical treatment has been reported uncommonly, and although McGoon and Kincaid (1964) reported repair of multiple peripheral areas of stenosis, most have been at the origin of either main-stem artery. The success of patch arterioplasty or graft replacement of absent or stenosed pulmonary arteries depends largely on the extent of the vascular anomaly and the degree of bronchial and parenchymal lung damage secondary to infection and fibrosis. Dilatation by balloons has proved to be successful in certain cases of isolated, discrete stenosis of pulmonary arteries. Rocchini and associates (1984) reported that balloon dilation may be the procedure of choice for multiple stenotic lesions. Successful dilatation, defined as greater than 50% increase in vessel diameter or a decrease in the right ventricular to aortic pressure gradient of at least 50%, occurs in up to 70% of patients. Complications include restenosis, which occurs 20% of the time, transient pulmonary edema, and, rarely, rupture of the pulmonary artery. The mortality rate associated with this procedure is 1% to 2%. Recently, stenting of the affected artery has been described in an effort to decrease the restenosis rates. Large sheaths are needed, which can limit
P.1143
their use to larger patients. In small patients, as well as situations when there is limited vascular access, stent placement in the operating room may be beneficial. Some have argued that reexpansion of the stents as the patient grows can be problematic, but Ungerleider and associates (2001) found in a retrospective study that reexpansion is possible and successful. Antiplatelet therapy is recommended in order to limit restenosis secondary to intimal proliferation and thrombosis. In older patients with unilateral agenesis of the pulmonary artery, persistent respiratory infection is the
P.1144
most prominent complaint, but the clinical course is often benign and frequently misdiagnosed. Pneumonectomy is usually required, and care to identify and control any systemic artery to the lung is mandatory. Canver and colleagues (1991) reported the necessity of a pneumonectomy in a neonate with agenesis of the right pulmonary artery who developed an uncontrollable necrotizing bronchopneumonia in the involved lung. This, as these researchers noted, is an unusual complication in the infant or child with this disorder.
Table 82-1. Classification of Congenital Vascular Lesions of the Lung | ||
|---|---|---|
|
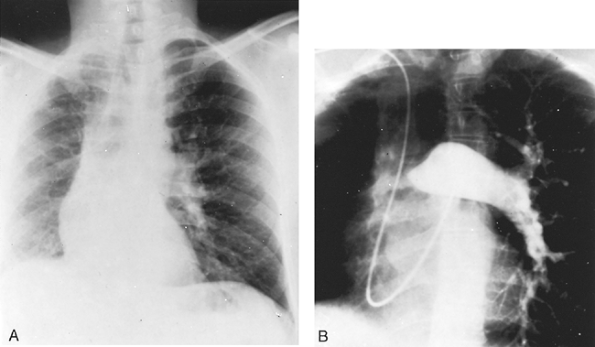 |
Fig. 82-1. Sawyer-James syndrome. A. Posteroanterior chest radiograph demonstrating small right lung with decreased vascular markings. B. Pulmonary angiogram in a patient with agenesis of the right pulmonary artery and minimal vascular markings (hyperlucent lung syndrome). |
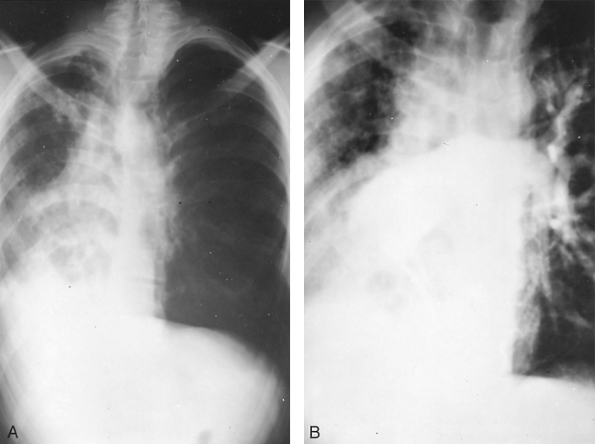 |
Fig. 82-2. Posteroanterior chest radiograph of young adult man with severe pulmonary infection in the right lung with marked shift of the mediastinum and heart into the right hemithorax. B. Angiogram revealing agenesis of the right pulmonary artery. |
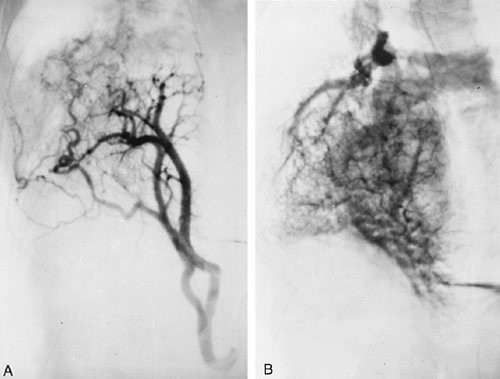 |
Fig. 82-3. This aortogram of the patient shown in Fig. 82-2 demonstrates a large ectopic systemic artery that supplies the lower portion of the right lung and that arises from the subdiaphragmatic portion of the aorta. B. Delayed radiograph showing venous drainage through normal right pulmonary veins. |
PULMONARY ARTERIOVENOUS FISTULAE
Pulmonary arteriovenous fistulae are congenital malformations that result from errant capillary development, with incomplete formation or disintegration of the vascular septa that normally divide the primitive connections between the venous and arterial plexuses. Tobin (1966) verified that some pulmonary arteriovenous shunting exists in normal lungs. This shunting may be hemodynamically important in pathologic conditions associated with venous or arterial pulmonary hypertension, portal cirrhosis, and obstructive lung disease.
Classification
A review of the embryology of the pulmonary vascular system indicates that pulmonary arteriovenous abnormalities may occur as isolated or combined lesions at the arterial, capillary, or venous level. They tend to increase in number and size over time. Anabtawi and colleagues (1965) presented an anatomic classification based on the size and position of arteriovenous communications (Table 82-2). The classification according to the blood supply has both hemodynamic and prognostic importance because the fistulae supplied by systemic arteries have the same hemodynamic consequences as arteriovenous fistulae of the general circulation have. Also, as Dines and associates (1974) noted, it is important to divide the entire group into those associated with Rendu-Osler-Weber disease (ROWD), also called hereditary hemorrhagic telangiectasis (HHT), and those not associated with it, because this stratification has important prognostic value. Patients with cutaneous telangiectasis (ROWD) more often have multiple pulmonary fistulae, a predictable progression of symptoms, and a higher
P.1145
rate of complications. Anywhere between 56% to 87% of patients with pulmonary arteriovenous fistulae have ROWD, and 20% of patients with ROWD develop fistulae.
Table 82-2. Classification of Pulmonary Arteriovenous Malformations | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Patients with HHT have a high incidence of multiple organs involved with telangiectases: the skin, and mucous membranes of the nose, lungs, brain, gastrointestinal tract, and liver. The organ involvement occurs in various combinations, and the sites of involvement appear to be governed by the underlying genetic defect. At present, at least three different phenotypes have been identified. Type I is related to a defect at chromosome 9q33 34 that relates to protein product endoglin, transforming growth factor- (TGF- ) receptor, and mutation and is most often associated with pulmonary arteriovenous malformations. This specific phenotype has been described by Heutink and associates (1994). Type II, described by Porteus (1994) and McAllister (1994) and their co-workers, is related to chromosome 3q22, which also codes for a TGF- II receptor and is associated, although less frequently, with pulmonary arteriovenous fistulae. Type III is related to chromosome 12q and was described by Vincent and colleagues (1995). Recently, it was discovered that this gene codes for activin receptorlike kinase 1 which is a type I serine-threonine kinase receptor that can bind TGF- in the presence of its type II receptor and is expressed on endothelial cells. This latter phenotype does not appear to be associated with pulmonary arteriovenous malformations.
Pulmonary Arteriovenous Fistulae with Pulmonary Arterial Supply
Clinical Aspects
Congenital pulmonary arteriovenous fistulae occur more frequently in women and are transmitted as a dominant gene with incomplete penetrance. Hodgson and Kaye (1963) reported a family that had HHT; 6.4% of those surveyed radiographically had pulmonary arteriovenous fistulae. Jeresaty and associates (1966) reported that symptoms began in childhood and consisted of dyspnea and easy fatigability in 25% to 50% of patients. Most are diagnosed in the third and fourth decades. In patients with HHT, epistaxis is an early sign and may begin in adolescence. It eventually is observed in up to 85% of the afflicted patients. Other frequent extrapulmonary symptoms and signs are headaches in 43%, transient ischemic attacks in 57%, and cerebral vascular accidents in 18%, as noted by Goodenberger (1998). Less commonly, patients may present with chest pain, cough, tinnitus, vertigo, and ocular symptoms. In the most recent Mayo Clinic review by Swanson and colleagues (1999) of 93 patients, 84% of patients had symptoms related to pulmonary arteriovenous fistulae or underlying HHT. Symptoms appear to correlate best with lesion size, with those less than 2 cm in diameter usually asymptomatic. Some series have found that patients with multiple, rather than single, lesions have a higher incidence of symptoms.
Exertional dyspnea, palpitations, and easy fatigability are the most frequent symptoms, usually appearing in the third or fourth decade of life and present in approximately 50% of patients. Hemoptysis is common when the fistula is associated with cutaneous telangiectasis (ROWD). Cutaneous telangiectases are the most common physical finding in patients, and are usually located on the face, mouth, chest, and upper extremities. A continuous bruit is present over the lesion in 75% of patients who have associated hereditary telangiectasis and in 38% of those who are without such an association. The typical bruit has a rough, humming, continuous sound that is accentuated in systole and with deep inspiration; the diastolic accentuation is more subtle. The audibility of the bruit can change with position. It may be associated with mitral valve prolapse in these patients.
The classic triad of cyanosis, polycythemia, and clubbing of the fingers or toes has been noted in approximately 20% of the patients. The presence of cyanosis indicates that at least 25% to 30% of the blood in the lesser circulation is being shunted from the right to the left side of the heart through the fistula. Platypnea is also seen occasionally and is thought to be related to a decrease in blood flow through the fistula in the dependent portions of the lung while in the supine position.
Physiologic Findings
The partial pressure of oxygen (Po2) and oxygen saturation are decreased. Swanson and colleagues (1999) found that the mean Po2 measured on room air was 56 mm Hg. Intracardiac and intravascular pressure proximal to the fistula are normal. As a result, the cardiac output is usually normal, although it may be increased at times. The systemic blood pressure and the electrocardiographic findings are also normal. The heart is usually not enlarged. Blood volume study results reveal an increased red cell mass in most cases. The plasma volume remains normal.
Radiographic Features
In one half to two thirds of the patients, the fistulae are single. The remainder are multiple and may be bilateral in 8% to 10% of the patients. Radiographs of the chest have some abnormality 98% of the time and show the fistulae as lobulated, fairly well-defined densities connected to the hilar structures by broad linear shadows because of the dilated vascular connections (Fig. 82-4). Eighty percent of the lesions are subpleural or superficial. Usually, fistulae are seen more frequently in the lower than in the upper lobes. A solitary lesion initially may be interpreted as a solitary pulmonary nodule, but the characteristic lobulation (the Mickey Mouse sign; Sider L, personal communication, 1993) (Fig. 82-5) should alert one to the true nature of the lesion. On fluoroscopy, they may be seen to pulsate or
P.1146
become smaller with the Valsalva maneuver. Also on fluoroscopy, because of the increased blood flow, there may be an increased amplitude of pulsation of the hilar vessels. Computed tomographic (CT) scanning usually demonstrates the lesion sufficiently well to be diagnostic (Fig. 82-6). Remy and associates (1992) reported that CT scanning enabled identification of 98.2% of 109 pulmonary arteriovenous fistulae in 40 patients versus only 59.6% identified by angiography (Fig. 82-7). Angiography, however, is more reliable in the analysis of the angioarchitecture and is a necessary follow-up of the CT scan in those patients who are to undergo interventional management of the fistula. Helical CT scanning is also an excellent diagnostic tool and may eventually obviate the need for angiography.
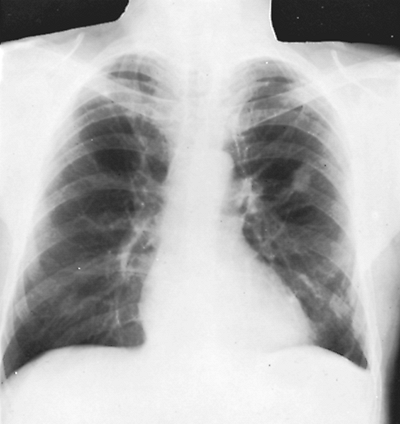 |
Fig. 82-4. Arteriovenous fistula in the left upper lobe with vascular connections to left hilus. |
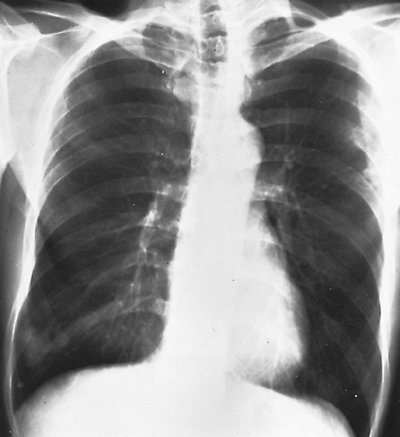 |
Fig. 82-5. Posteroanterior radiograph of the chest revealing a lobulated mass in the lower right lung field. The mass has a Mickey Mouse configuration. The arteriovenous malformation was confirmed on CT examination. |
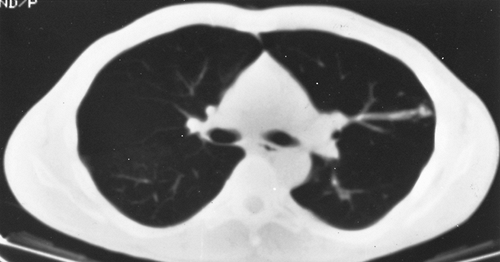 |
Fig. 82-6. Contrast-enhanced CT scan of the same patient as shown in Fig. 82-5 showing a different level from the mass seen in the radiograph reveals several additional arteriovenous malformations in the left lung. The vascular connection to the venous system is clearly demarcated. |
Additional Diagnostic Studies
Burke and Raffin (1986) suggested the use of noninvasive studies before the use of pulmonary angiography in patients suspected of having pulmonary arteriovenous fistulae. These studies include contrast echocardiography, described by Shub and associates (1976), and perfusion lung scintigraphy, reported by Lewis and colleagues (1978). A negative result excludes the presence of a right-to-left shunt and thus excludes the presence of a fistula. A positive result confirms the suspected diagnosis without risk to the patient. Contrast echocardiography has a sensitivity that approaches
P.1147
100%, but warrants additional evaluation. In addition, perfusion lung scintigraphy provides a quantitative estimation of the magnitude of the shunt. With the reliability of CT scans, as noted by Remy and associates (1992), it would appear that neither of these examinations is necessary nor would they supplant the use of contrast angiography in patients who are candidates for invasive therapy.
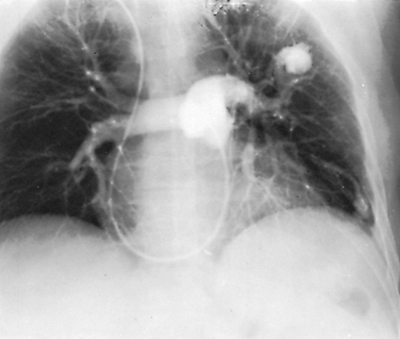 |
Fig. 82-7. Angiocardiogram delineating multiple arteriovenous fistulae: a large fistula in left upper lobe and an additional fistula in the left lower lobe. |
Magnetic resonance (MR) imaging is another potential modality, but randomized controlled studies are needed before it can be considered a regular tool in the diagnosis of pulmonary arteriovenous fistulae. MR angiography may be promising, but it remains an expensive alternative with limited availability.
Complications
Stringer and associates (1955) reported 30 instances of serious complications or death among 140 patients with pulmonary arteriovenous fistulae. Dalton and co-workers (1967) recorded nine instances of intrapleural rupture. Others, including Iwabuchi and colleagues (1993), have noted the occurrence of this potentially fatal complication. Takenaka and associates (1996) reported the successful resection of the offending fistula in a patient who developed a massive left-sided hemothorax. In addition, these researchers reviewed eight additional successful resections of ruptured fistulae reported in the Japanese literature. Massive hemoptysis is uncommon. White (1984) noted that paradoxic embolization and stroke as the result of a bland embolus to the brain may occur. Dines and associates (1974) observed a stroke to have occurred in 10% of all untreated patients followed for 4 to 10 years in whom a pulmonary arteriovenous fistula was seen on the radiograph or if there was hypoxemia on room air. Cerebral abscesses also have been reported, but none of 96 patients reported by Dines and colleagues (1983) had cerebral abscess or intrapleural rupture. The report of Puskas and colleagues (1994) noted the occurrence of a brain abscess in several of their patients who were untreated or who had a failed previous balloon occlusion of a pulmonary arteriovenous malformation. Yeung and associates (1995) have observed that neurologic manifestations, especially in those patients with ROWD, occur in 4% to 12% of the cases. In Swanson and colleagues' (1999) series, neurologic events, including transient ischemic attacks, strokes, brain abscesses, and seizures, were the most prominent complication and occurred in 37% of patients. Strokes were the most common (18%), followed by transient ischemic attacks (12%), abscesses (5%), and seizures (5%).
Treatment
Most patients with one or more pulmonary arteriovenous fistulae are candidates for resection or obliteration of the lesions. Only those few who have a small lesion (10 to 15 mm in diameter) and who are asymptomatic with a minimal shunt may be observed. In this subset, however, the risk of paradoxic embolization is present, as previously noted. Many believe that all lesions with a feeding artery of at least 3 mm should be addressed due to the high risk for paradoxic embolization and neurologic complications. In patients with ROWD, treatment is indicated in all patients, if at all possible, because the complication rate is highest in this group. Interventional therapy is likewise indicated in all symptomatic patients and even in those whose symptoms are minimal if the lesion can be identified on the standard chest radiographs or CT scans or if hypoxemia is present when the patient breathes room air.
Single lesions are best managed by conservative surgical excision. Some multiple lesions also may be treated by excision, but most patients with multiple or bilateral lesions are best managed by radiographically guided embolization.
Surgical Therapy
Surgical excision of an isolated, single pulmonary arteriovenous fistula is successful or minimal mortality; there is morbidity and little chance of recurrence of the lesion. It is indicated in patients with persistent hemorrhage secondary to intrapleural rupture or hemoptysis despite embolization, in patients with fistulae of large vessels where embolization could lead to paradoxical emboli, and in patients who cannot be treated with embolization (i.e., severe contrast allergy). Because most fistulae are located subpleurally, they can be removed with conservative local resections. Schroder and associates (2001) describe fistulectomy as an alternative to segmentectomy, which preserves lung parenchyma. They found that fistulectomy is possible due to the fact that the fistulae can be freely dissected from the surrounding tissue since most are subpleural and there is usually connective tissue between the fistula and normal lung tissue, giving an excellent plane of dissection. In difficult cases in which the feeding vessel is not definitely localized preoperatively, Almeida and colleagues (1998) suggest the use of transesophageal echocardiography with agitated blood and saline contrast during the procedure in order to assist in localizing the vessel. Puskas and associates (1994) reported the successful excision of a solitary arteriovenous fistula in nine patients; one patient had staged excision of bilateral lesions. All patients were relieved of dyspnea, and no recurrences or neurologic complications occurred in the follow-up of these patients. As noted, multiple fistulae are occasionally suitable for resection. Patients with symptoms and one or more enlarging fistulae are candidates for operation. Dines and associates (1974) suggested that patients with a single fistula and HHT should undergo surgery because enlargement, symptoms, and complications are more frequent. Sperling and colleagues (1977) reported, however, that the combination of pulmonary hypertension and arteriovenous fistulae is a contraindication for surgical excision.
P.1148
Embolic Obliteration
Selective radiographically guided embolization of multiple pulmonary arteriovenous fistulae unsuitable for surgical resection has proved to be a valuable therapeutic modality. The patients with multiple lesions are frequently severely disabled by the presence of a large right-to-left shunt, and occlusion of the multiple fistulae permits a return to a near-normal status.
Taylor and associates (1978) first reported therapeutic embolization of the fistula by pulmonary arterial catheterization and the use of small steel guidewires with 3-cm wool tails. Tadavarthy and colleagues (1975) had described the use of a polyvinyl alcohol sponge [Ivalon, (Ivalon, Inc., San Diego, CA)] as an embolic agent, but its use has been infrequent in the management of pulmonary arteriovenous fistulae. The popular materials used at present are detachable silicone balloons devised by White and co-workers (1980) (Fig. 82-8) and stainless-steel coils introduced by Gianturco and associates (1975).
The occluding element is placed precisely into the feeding pulmonary arterial branch beyond all of the artery's normal branches to the lung. Proper placement is guided by video monitoring, digital subtraction angiography, or both.
P.1149
White (1983) and colleagues described his technique and pointed out that approximately 79% of the fistulae have a single feeding artery and a single draining vein, so occlusion is relatively easy to achieve; the remaining 21% have two or more feeding and draining branches, which makes the embolization process more difficult. Anderson and colleagues (1979) and Hatfield and Fried (1981) described the successful use of the Gianturco miniature stainless-steel coil in patients with multiple fistulae. These coils (Fig. 82-9) cause clotting and obstruction of the fistula and can be seen on the postprocedure chest film (Fig. 82-10). Hartnell and Allison (1989) have detailed the technique of coil embolization in the management of these lesions.
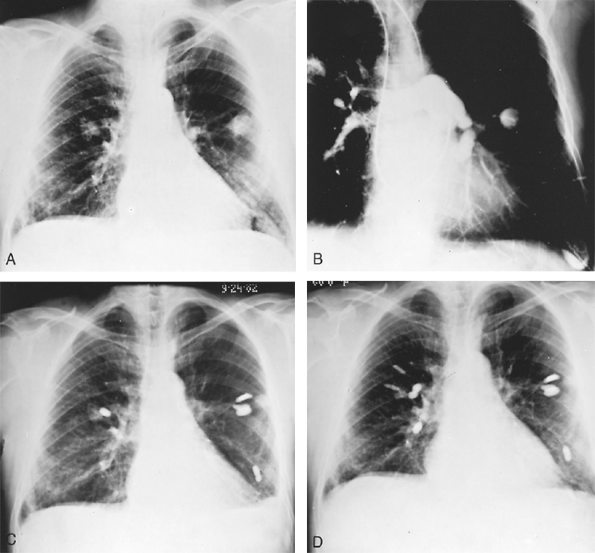 |
Fig. 82-8. A. Posteroanterior radiograph of a 64-year-old white man with multiple bilateral pulmonary infiltrates, frequent nosebleeds, a Po2 of 52 mm Hg, and increasing shortness of breath. B. Pulmonary angiogram revealing multiple bilateral pulmonary arterial venous fistulae. C. Posteroanterior radiograph in August 1982 after balloon embolization of a number of the fistulae by R.I. White, Jr., of Johns Hopkins University, with moderate symptomatic improvement. D. Posteroanterior radiograph in March 1983 following balloon embolization of remaining arteriovenous fistulae, with complete relief of previous dyspnea and return of oxygen saturation and Po2 levels to normal on room air. Courtesy of R.E. Otto. |
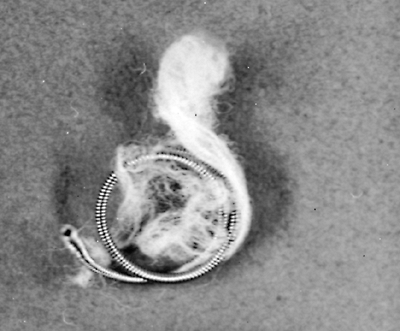 |
Fig. 82-9. Stainless-steel coil that can be inserted via cardiac catheter to occlude and thrombose a pulmonary arteriovenous fistula. |
With proper technique and experience, the results are gratifying. With permanent occlusion of the fistulae by either of the occluding elements, the hypoxemia is corrected. White and colleagues (1988) reported persistent relief of hypoxemia and only minimal growth of small remaining arteriovenous malformations after 5 years of follow-up. The low recurrence rates were also noted by Remy and associates (1992). Chilvers and colleagues (1990), however,
P.1150
pointed out that blood flow through small residual, nonoccluded malformations could account for the persistence of right-to-left shunts after occlusion of the malformations, with larger feed vessels identified by angiographic study. Minimal pulmonary infarction may occur postocclusion, and chest pain may be troublesome early. Haitjema and associates (1995) reported that in a series of embolizations in 32 patients, postembolization pulmonary infarction or pleurisy occurred in 11% of the patients and occasional systemic embolism or cardiac arrhythmias were observed. More important, late recanalization occurred in 5% to 10% of the patients. Late neurologic complications have been noted by Puskas and colleagues (1994) in some patients who have undergone previous balloon occlusion. Of interest was these authors' observation of the disappearance of the occluding balloon in several patients. The fate of the previously present occluding balloon remained unknown, even in the patients in whom the arteriovenous malformation was subsequently removed surgically. Despite these criticisms raised by the aforementioned reports, Lee and associates (1997), of White's group, reiterated their strong support for the use of embolization for the management of pulmonary arteriovenous malformations; not only those with small feeding vessels but those with large ones as well. They reported that in the treatment of 221 consecutive patients with pulmonary arteriovenous malformations, 45 patients with 52 lesions resupplied by feeding arteries 8 mm in diameter or larger were managed successfully. Thirty-eight patients had 44 pulmonary arteriovenous malformations cured by the first attempt at embolization by either a balloon, a coil, or both. The researchers agreed that complications are seen: pleurisy in 31%, angina in 2%, and paradoxical embolization of a device during deployment in 4%; all of these events were self-limiting. Seven patients (11%) had persistence of the shunt because of recanalization or later ingrowth of an accessory artery; these patients were managed by a second or third procedure. The researchers reported that a latex balloon usually deflates within a month because of slow leakage but that the deflated balloon remains in place and the fistula does not recur because thrombosis of the feeding vessel usually occurs within 20 days. However, White (personal communication, 1998) no longer uses the latex balloon but only uses the silicone variety. When filled with isoosmotic solution, the silicone balloon remains inflated for at least 2 years and at times as long as 3 years (Fig. 82-11). White and Pollak (1994) did not observe the occurrence of a brain abscess or stroke in any of their patients. Swanson and associates (1999), reported that 48
P.1151
patients treated with embolization required 78 embolization sessions with more than 200 lesions occluded. Recanalization occurred in 2 patients requiring reembolization. Complications from embolization included pleuritic chest pain in 12%, and 1 patient (1%) developed right-sided hemiparesis. Overall, 91% showed improved symptoms. Other described complications include bleeding, paradoxic embolization of clots or agents used for therapy, infection, pulmonary infarction, pulmonary hypertension, air embolism, and deep venous thrombosis.
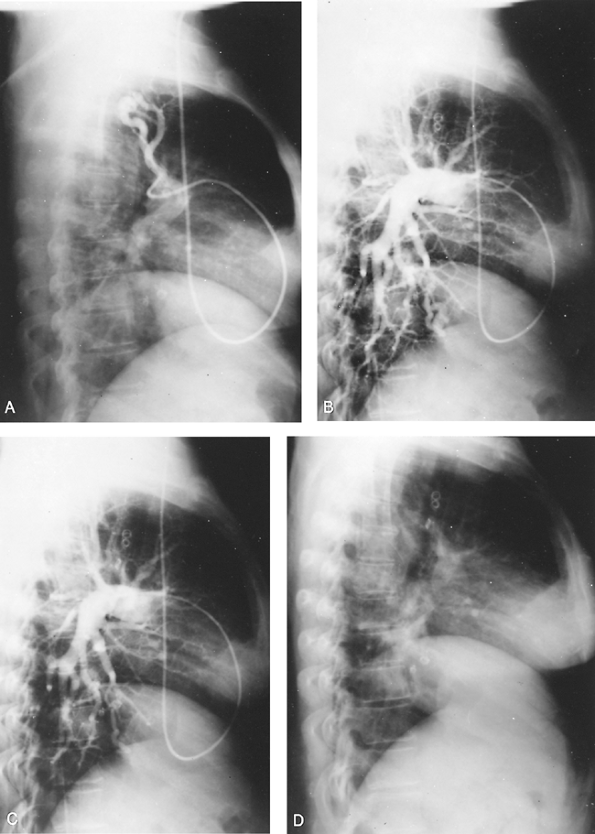 |
Fig. 82-10. A. Lateral view of pulmonary angiogram with selective demonstration of an upper lobe arteriovenous fistula in a patient with multiple fistulae and ROWD. B. The same patient with a nonselective pulmonary angiogram that demonstrates occlusion of the upper lobe fistula by the coil, which is visible on the film, and demonstration of a second fistula in the lower lobe. C. The same patient with both upper and lower lobe fistulae occluded by coils. D. The same patient's lateral chest film demonstrating multiple coils that have been used to occlude fistulae. |
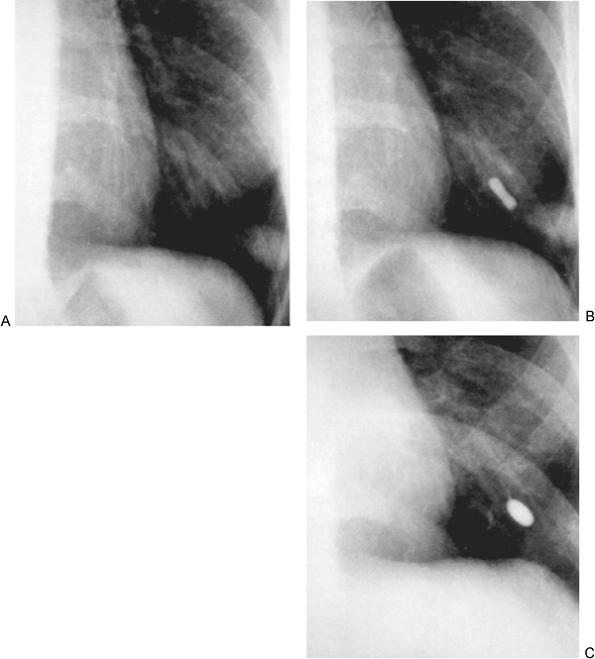 |
Fig. 82-11. Chest radiographs of a 4.5-mm pulmonary arteriovenous malformation. A. Before embolotherapy. B. Immediately after placement of a single detachable silicone balloon. C. Silicone balloon still in place and still inflated 2 years later; the pulmonary arteriovenous malformation had thrombosed and was no longer evident. From White RI, Pollak JS, Wirth JA: Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Intervent Radiol 7:787, 1996. With permission. |
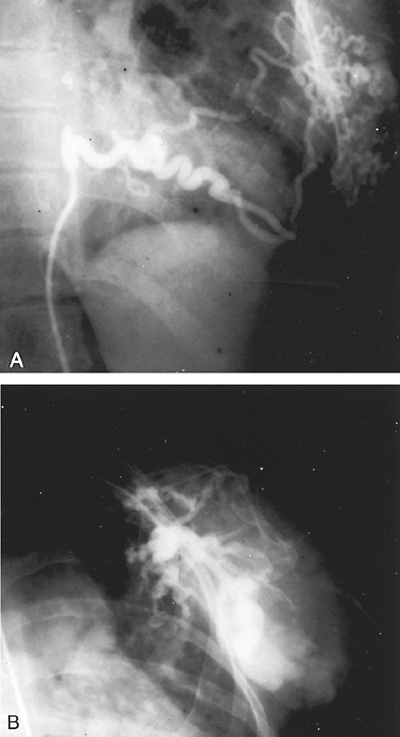 |
Fig. 82-12. A. Selective angiogram of an intercostal artery of a patient with a large systemic arteriovenous fistula of the chest wall. The fistula was draining into the lung. The patient had high-output congestive failure and was refractory to any occlusive therapy or surgical therapy of these multiple communications. B. Venous phase of the arteriovenous fistulae demonstrating drainage into the lung with rapid run-off into the pulmonary venous system. |
P.1152
Systemic Pulmonary Arteriovenous Fistulae
Pulmonary arteriovenous fistulae that have a systemic blood supply are rare. The bronchial arteries, internal mammary arteries, or aorta are the primary and immediate sources of the systemic arterial blood. Resection is indicated, although the extensive collateral circulation in the chest wall may provide formidable technical challenges. Involvement adjacent to the spinal column may preclude complete resection, but partial resection of the systemic arterial sources of blood, in particular, removes the threat of serious hemorrhagic and hemodynamic complications of high-output congestive failure (Fig. 82-12).
PULMONARY ARTERY ANEURYSMS
Aneurysmal dilatation of the pulmonary artery secondary to associated congenital cardiovascular anomalies is not a rare entity, but true aneurysms of this vessel secondary to disintegration of its wall are indeed rare. Aneurysms consequent to long-standing hypertension or high pulmonary blood flow are associated most frequently with patent ductus arteriosus and by definition would be acquired and not congenital. This problem of etiology is not easily solved. Natelson and associates (1970) described aneurysms secondary to cystic medial necrosis of the pulmonary arteries that have caused striking clinicopathologic findings related to pulmonary hypertension, dissection, and rupture of the vessel. Ungaro and associates (1976) classified pulmonary artery aneurysms into specific causes (i.e., tuberculosis, syphilis, trauma) and nonspecific causes (i.e., mycotic, pulmonary hypertension, arteriosclerotic, those related to congenital defects in the vessel wall, and iatrogenic). Most so-called congenital aneurysms of the pulmonary artery, although acquired, are associated with some other congenital anomaly. Because the truly peripheral and often solitary aneurysms rupture in as many as 60% of patients, Ungaro and colleagues (1976) emphasized the necessity of definitive diagnosis and early treatment. Conservative pulmonary resection usually suffices. Some have advocated observation, especially in elderly patients without cardiac malformations, left-to-right shunt, or pulmonary hypertension. Casselman and associates (1997) believe there is not enough literature evidence of possible rupture to support surgery in this group, though they admit many are uncomfortable with observation only.
Significant dilatation of both the right and the left pulmonary arteries may be present with isolated pulmonary valvular insufficiency or may be associated with the tetralogy of Fallot. This anomaly may result in the so-called Mickey Mouse heart, a sign named for its distinctive appearance on a plain chest film. This aneurysmal dilatation can cause airway problems with pressure on one or both main-stem bronchi. Pierce and associates (1970) reported that an emphysematous lobe may be associated with pulmonary artery aneurysm of any etiology. Frequently, correction of the pulmonary valvular insufficiency by conduit replacement may remedy the problem, but resection of the enlarged vessels may be needed to cure the airway problems. Murphy and colleagues (1987) reported successful resection of a peripheral artery aneurysm using cardiopulmonary bypass.
P.1153
PULMONARY VARIX
An isolated pulmonary varix (aneurysmal dilatation) is rare, and fewer than 50 cases have been reported. The cause of these varices is unknown, but a varix may be congenital. Steinberg (1967) reported that an enlarged variceal central portion of a pulmonary vein can be mistaken for either pulmonary or hilar disease on the standard chest radiographs. Batram and Strickland (1971), as well as others, have reconfirmed this impression. The patient with a pulmonary varix most often presents with an asymptomatic perihilar or hilar mass discovered on an incidental chest radiograph. Lobulation of the central mass may be present. The presence of calcification has not been reported. Rarely, complications such as systemic emboli that are caused by the release of clots from within the varix or, even more infrequently, bleeding into the bronchial tree or pleural space that is caused by rupture of the varix may occur. On the standard radiographs, an initially indeterminate perihilar or a hilar mass may be recognized (Fig. 82-13). The diagnosis, if suspected, may be supported by changes in size of the lesion on fluoroscopy when the patient performs Valsalva and M ller maneuvers. The diagnosis is confirmed by angiography, and according to Mannes and associates (1992), five characteristic features are present: (a) the arterial phase is normal without shunting, (b) the varix fills in the venous phase, (c) the varix drains directly into the left atrium, (d) emptying of the varix is delayed, and (e) the varix affects only the proximal portion of the vein. The value of CT scan or magnetic resonance imaging in the diagnosis of this lesion has not been reported. A pulmonary varix requires no treatment. Unfortunately, the nature of the mass often is unrecognized preoperatively, and an unnecessary thoracotomy is performed.
ABNORMAL PULMONARY VENOUS CONNECTION
A complete discussion of anomalous pulmonary venous connections is beyond the scope of this text.
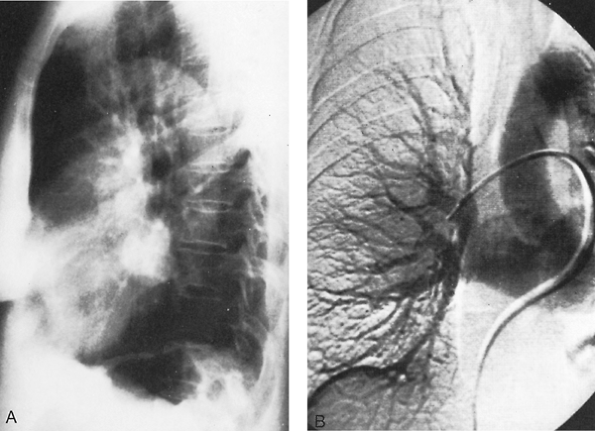 |
Fig. 82-13. A. Lateral radiograph of the chest of an asymptomatic 69-year-old woman with a perihilar mass. CT scan revealed a lobulated mass in the posterior hilum. B. Angiogram revealed filling of large pulmonary varix in the venous phase. From Mannes GPM, van der Jagt E, Postmus PE: An asymptomatic hilar mass. Chest 101:553, 1992. With permission. |
P.1154
Anomalous venous drainage of the right lung into the inferior vena cava (the scimitar syndrome), however, is often associated with bronchial anomalies, hypoplasia, and abnormal systemic arterial supply of the right lung. Although the abnormal left-to-right shunt may be the major cause of clinical problems in most patients with the syndrome, pulmonary symptoms, often of chronic severe pulmonary infection, may be the dominant clinical feature. Thus, consideration of the syndrome is important to the general thoracic surgeon.
Scimitar Syndrome
Scimitar syndrome consists of a constellation of abnormalities, the constant being total or partial anomalous pulmonary venous drainage of the right lung to the inferior vena cava. Neill and associates (1960) emphasized that the gently curved vertical shape of this vein on a chest radiograph resembles the curved Turkish sword or scimitar. Associated findings that may or may not be present are dextroposition of the heart, hypoplasia of the right lung and right pulmonary artery, malformation of the bronchial tree, and anomalous systemic arteries to the right lung from the abdominal aorta or its branches. Trell and colleagues (1971) reported that intracardiac defects are present in up to 40% of these patients.
Symptoms are related to the degree of left-to-right shunting, pulmonary hypertension, parenchymal lung disease, and associated intracardiac lesions. Kiely and co-workers (1967) documented in 70 cases that the more common symptoms were fatigue, dyspnea, decreased exercise tolerance, cough, recurrent respiratory tract infections, and failure to thrive. Chest radiography shows a small right lung, cardiac displacement to the right, and the shadow of the anomalous vein (Fig. 82-14). Electrocardiography reveals right ventricular hypertrophy. Echocardiography is useful in identifying associated intracardiac lesions and the location of the anomalous vein penetrating the diaphragm. Cardiac catheterization and angiocardiography are diagnostic. Mandell (1999) has recorded that coil embolization is sometimes used prior to definitive operative intervention when anomalous arteries to the right lung are present.
The decision for operative intervention must be individualized depending on the degree of symptoms and the findings at cardiac catheterization. For large left-to-right shunts, physiologic correction is preferred. Kirklin and associates (1956) reported that either the anomalous vessel can be anastomosed directly to the left atrium or an intracardiac patch can be used to tunnel the flow from the anomalous vein to the left atrium through an atrial septal defect. Sanger and colleagues (1963) suggested that if inflammatory parenchymal changes are extensive, pulmonary resection or pneumonectomy should be considered. Resection eliminates the left-to-right shunt as well as the lung infection. In all instances, the systemic arterial supply to the lung is divided. Recently, Walles and co-workers (2002) described a simultaneous correction of an adult with scimitar syndrome and coronary artery disease through reconstruction of the anomalous right pulmonary vein and revascularization.
Results depend chiefly on the degree of preoperative pulmonary hypertension and associated cardiac lesions. Older patients with minimal symptoms have an excellent prognosis. Canter and associates (1986) pointed out that infants with cyanosis, failure to thrive, and severe pulmonary hypertension have a high mortality rate.
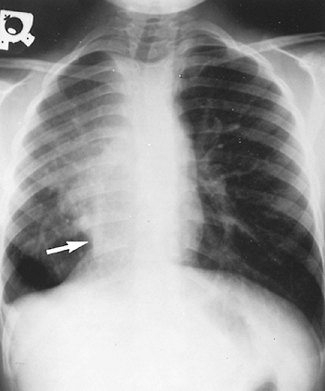 |
Fig. 82-14. Chest radiograph of a child with scimitar syndrome. Arrow points to the anomalous vein, which resembles a Turkish saber. Note the dextroposition of the heart and hypoplasia of the right lung. |
LYMPHANGIECTASIA
Primary pulmonary lymphangiectasia does occur or may be associated with generalized lymphangiectasia. Noonan and co-workers (1970) reported that this anomaly is associated with a syndrome that consists of pulmonary stenosis, atrial septal defect, and mental retardation. This anomaly also has been reported as a familial lesion by Scott-Emuakpor and associates (1981). Chest radiography may show a pattern similar to hyaline membrane disease or may show cystic infiltrates. The patients may develop pleural effusions or chylothorax that require drainage. The only therapy is supportive, and the disease is usually progressive and fatal. Bouchard and associates (2000) did find in a retrospective
P.1155
review, however, that if a patient lives past the neonatal period, survival is possible and improvement in symptoms may be expected.
REFERENCES
Almeida AA, et al: Transesophageal echocardiography in an operation for pulmonary arteriovenous malformations. Ann Thorac Surg 65:267, 1998.
Anabtawi IN, Ellison RG, Ellison LT: Pulmonary arteriovenous aneurysms and fistulas: anatomical variations, embryology, and classification. Ann Thorac Surg 1:277, 1965.
Anderson JH, et al: Mini Gianturco stainless steel coils for transcatheter vascular occlusion. Radiology 132:301, 1979.
Batram C, Strickland B: Pulmonary varices. Br J Radiol 44:927, 1971.
Bouchard S, et al: Pulmonary lymphangiectasias revisited. J Pediatr Surg 35:796, 2000.
Burke CM, Raffin TA: Pulmonary arteriovenous malformations, aneurysms, and reflections. Chest 89:771, 1986.
Canter CE, et al: Scimitar syndrome in childhood. Am J Cardiol 58:652, 1986.
Canver CC, Pigott JD, Mentzer RM Jr: Neonatal pneumonectomy for isolated unilateral pulmonary artery agenesis. Ann Thorac Surg 52:294, 1991.
Casselman F, et al: Pulmonary artery aneurysm: Is surgery always indicated? Acta Cardiol 52:431, 1997.
Chilvers ER, et al: Effect of percutaneous transcatheter embolization on pulmonary function, right-to-left shunt and arterial oxygenation in patients with pulmonary arteriovenous malformations. Am Rev Respir Dis 142:420, 1990.
Dalton ML Jr, et al: Intrapleural rupture of pulmonary arteriovenous aneurysm: report of a case. Chest 52:97, 1967.
Dines DE, Seward JB, Bernatz PE: Pulmonary arteriovenous fistulas. Mayo Clin Proc 58:176, 1983.
Dines DE, et al: Pulmonary arteriovenous fistulas. Mayo Clin Proc 49:460, 1974.
Ellis FH, McGoon DC, Kincaid OW: Congenital vascular malformations of the lungs. Med Clin North Am 48:1069, 1964.
Gianturco C, Anderson JH, Wallace S: Mechanical devices for arterial occlusion. Am J Roentgenol Radium Ther Nucl Med 124:428, 1975.
Goodenberger DM: Pulmonary arteriovenous malformations. In Fishman AP, et al (eds): Fishman's Pulmonary Diseases and Disorders. 3rd Ed. New York: McGraw-Hill, 1998.
Gregg NM: Congenital cataract following German measles in the mother. Trans Ophthalmol Soc Aust 3:35, 1941.
Haitjema TJ, et al: Embolisation of pulmonary arteriovenous malformations: results and follow up in 32 patients. Thorax 50:719, 1995.
Hartnell GG, Allison DJ: Coil embolization in the treatment of pulmonary arteriovenous malformations. J Thorac Imaging 4:81, 1989.
Hatfield DR, Fried AM: Therapeutic embolization of diffuse pulmonary arteriovenous malformations. AJR 137:861, 1981.
Heutink P, et al: Linkage of haemorrhagic telangiectasia to chromosome 9q34 and evidence for locus heterogeneity. J Med Genet 31:933, 1994.
Hodgson CH, Kaye RL: Pulmonary arteriovenous fistula and hereditary hemorrhagic telangiectasia: a review and report of 35 cases of fistula. Chest 43:449, 1963.
Iwabuchi S, et al: Intrapleural rupture of a pulmonary arteriovenous fistula occurring beneath the pleura: report of a case. Surg Today 23:468, 1993.
Jeresaty RM, Knight HF, Hart WE: Pulmonary arteriovenous fistulas in children: report of two cases and review of literature. Am J Dis Child 111:256, 1966.
Kiely B, et al: Syndrome of anomalous venous drainage of the right lung to the inferior vena cava: a review of 67 reported cases and three new cases in children. Am J Cardiol 20:102, 1967.
Kirklin JW, Ellis FH, Wood EH: Treatment of anomalous venous connections in association with interatrial communications. Surgery 39:389, 1956.
Kochiadakis G: Anomalous collateral from coronary artery to affected lung in a case of congenital absence of the left pulmonary artery: effect on coronary circulation. Chest 121:2063, 2002.
Lee DW, et al: Embolotherapy of large pulmonary arteriovenous malformations: long term results. Ann Thorac Surg 64:930, 1997.
Lewis AB, Gates GF, Stanley P: Echocardiography and perfusion scintigraphy in the diagnosis of pulmonary arteriovenous fistula. Chest 73:675, 1978.
Mandell VS: Interventional procedures for congenital heart disease. Radiol Clin North Am 37:429, 1999.
Mannes GP, van der Jagt EJ, Postmus PE: An asymptomatic hilar mass. Chest 101:553, 1992.
McAllister KA, et al: Genetic heterogeneity in hereditary haemorrhagic telangiectasia: possible correlation with clinical phenotype. J Med Genet 31:927, 1994.
McGoon DC, Kincaid OW: Stenosis of branches of the pulmonary artery: surgical repair. Med Clin North Am 48:1083, 1964.
Murphy JP, et al: Peripheral pulmonary artery aneurysm in a patient with limited respiratory reserve: controlled resection using cardiopulmonary bypass. Ann Thorac Surg 43:323, 1987.
Natelson EA, Watts HD, Fred HL: Cystic medionecrosis of the pulmonary arteries. Chest 57:333, 1970.
Neill CA, et al: The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage: Scimitar syndrome. Bull Johns Hopkins Hospital 107:1, 1960.
Noonan JA, Walters LR, Reeves JT: Congenital pulmonary lymphangiectasis. Am J Dis Child 120:314, 1970.
Pierce WS, et al: Concomitant congenital heart disease and lobar emphysema in infants: incidence, diagnosis, and operative management. Ann Surg 172:951, 1970.
Porteus ME, et al: Genetic heterogeneity in hereditary haemorrhagic telangiectasia. J Med Genet 31:925, 1994.
Puskas JD, et al: Pulmonary arteriovenous malformations: therapeutic options. Ann Thorac Surg 56:253, 1994.
Remy J, et al: Pulmonary arteriovenous malformations: evaluation with CT of the chest before and after treatment. Radiology 182:809, 1992.
Rocchini AP, et al: Use of balloon angioplasty to treat peripheral pulmonary stenosis. Am J Cardiol 54:1069, 1984.
Sanger PW, Taylor FH, Robicsek F: The scimitar syndrome, diagnosis and treatment. Arch Surg 86:84, 1963.
Schroder C, et al: Fistulectomy as an alternative to segmentectomy for pulmonary arteriovenous fistula. J Thorac Cardiovasc Surg 122:386, 2001.
Scott-Emuakpor AB, et al: Familial occurrence of congenital pulmonary lymphangiectasis. Genetic implications. Am J Dis Child 135:532, 1981.
Shub C, et al: Detecting intrapulmonary right-to-left shunt with contrast echocardiography: observations in a patient with diffuse pulmonary arteriovenous fistulas. Mayo Clin Proc 51:81, 1976.
Sperling DC, et al: Pulmonary arteriovenous fistulas with pulmonary hypertension. Chest 71:753, 1977.
Steinberg I: Pulmonary varices mistaken for pulmonary and hilar disease. Am J Roentgenol Radium Ther Nucl Med 101:947, 1967.
Stringer CJ, et al: Pulmonary arteriovenous fistula. Am J Surg 89:1054, 1955.
Swanson KL, Prakash UB, Stanson AW: Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982 1997. Mayo Clin Proc 74:671, 1999.
Tadavarthy SM, Moller JH, Amplatz K: Polyvinyl alcohol (Ivalon) a new embolic material. Am J Roentgenol Radium Ther Nucl Med 125: 609, 1975.
Takenaka K, et al: Intrapleural rupture of pulmonary arteriovenous fistula: a case report. J Jpn Assoc Chest Surg 10:479, 1996.
Taylor BG, et al: Therapeutic embolization of the pulmonary artery in pulmonary arteriovenous fistula. Am J Med 64:360, 1978.
Tobin CE. Arteriovenous shunts in the peripheral pulmonary circulation in the human lung. Thorax 21:197, 1966.
Trell E, et al: The scimitar syndrome with particular reference to its pathogenesis. Z Kreislaufforsch 60:880, 1971.
Ungaro R, et al: Solitary peripheral pulmonary artery aneurysms: pathogenesis and surgical treatment. J Thorac Cardiovasc Surg 71:566, 1976.
Ungerleider R, et al: Intraoperative stents to rehabilitate severely stenotic vessels. Ann Thorac Surg 71:476, 2001.
Vincent P, et al: A third locus for hereditary haemorrhagic telangiectasia maps to chromosome 12q. Hum Mol Genet 4:945, 1995.
Walles T, et al: Combined correction of an adult scimitar syndrome and coronary artery bypass grafting. Ann Thorac Surg 73:640, 2002.
White RI, Pollak JS, Wirth JA: Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Intervent Radiol 7: 787, 1996.
P.1156
White RI Jr: Embolotherapy in vascular disease. AJR 142:27, 1984.
White RI Jr, Pollak JS: Pulmonary arteriovenous malformations: options for management [Letter]. Ann Thorac Surg 57:519, 1994.
White RI Jr, et al: Detachable silicone balloons: results of experimental study and clinical investigations in hereditary hemorrhagic telangiectasia. Ann Radiol (Paris) 23:338, 1980.
White RI Jr, et al: Angioarchitecture of pulmonary arteriovenous malformations: an important consideration before embolotherapy. AJR 140: 681, 1983.
White RI Jr, et al: Pulmonary arteriovenous malformations: technique and long-term outcome of embolotherapy. Radiology 169:663, 1988.
Yeung M, et al: Transcranial Doppler ultrasonography and transesophageal echocardiography in the investigation of pulmonary arteriovenous malformation in a patient with hereditary hemorrhagic telangiectasia presenting with stroke. Stroke 26:1941, 1995.
Reading References
Berg J, Gallione T: The activin receptor like kinase 1 gene: genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet 61:60, 1997.
Bosher LH Jr, Blaki DA, Byrd BR: An analysis of the pathologic anatomy of pulmonary arteriovenous aneurysms with particular reference to the applicability of local excision. Surgery 45:91, 1959.
Good CA: Certain vascular abnormalities of lungs. Hickey Lecture. AJR 85:1009, 1961.
Gossage J, Kanj G: Pulmonary arteriovenous malformations: a state of the art review. Am J Resp Crit Care Med 158:643, 1998.
Hepburn J, Dauphinee JA: Successful removal of hemangioma of the lung followed by the disappearance of polycythemia. Am J Med Sci 204:681, 1942.
Hipona FA, Jamshidi A: Observations on the natural history of varicosity of pulmonary veins. Circulation 35:471, 1967.
Kaufman SL: Intrathoracic interventional vascular techniques in congenital cardiovascular disease. J Thorac Imaging 2:1, 1987.
Kiphart RJ, et al: Systemic-pulmonary arteriovenous fistula of the chest wall and lung: a report of a case review of the literature. J Thorac Cardiovasc Surg 54:113, 1967.
Pihkala J, et al: Interventional cardiac catheterization. Pediatr Clin North Am 46:441, 1999.
Remy-Jardin M, Remy J: Comparison of vertical and oblique CT in evaluation of bronchial tree. J Comput Assist Tomogr 12:956, 1988.
Robin ED, et al: Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med 294:941, 1976.
Smith HL, Horton BT: Arteriovenous fistula of the lung associated with polycythemia vera: report of a case in which the diagnosis was made clinically. Am Heart J 18:589, 1939.
White RI Jr, Pollak JS, Wirth JA: Pulmonary arteriovenous malformations: diagnosis and transcatheter embolotherapy. J Vasc Intervent Radiol 7: 787, 1996.
Wolfe WG, Anderson RW, Sealy WC: Hyperlucent lung. Pathophysiology and surgical management. Ann Thorac Surg 18:172, 1974.
EAN: 2147483647
Pages: 203