III - Thoracic Imaging
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section III - Thoracic Imaging > Chapter 12 - Positron Emission Tomography Imaging of Thoracic Neoplasms
function show_scrollbar() {}
Chapter 12
Positron Emission Tomography Imaging of Thoracic Neoplasms
Richard L. Wahl
Positron emission tomography (PET) is assuming an increasingly important role in the management of thoracic neoplasms. This technology, long only a research technique, has firmly moved into the mainstream of medical imaging in the last decade of the 20th century and the first years of the 21st century, and has gained an important role in a broad range of cancer applications, as noted in the publication of Cohade and the author (2003). PET differs from anatomic imaging techniques in that it predominantly images physiology. The physiology imaged depends on the choice of the radiopharmaceutical injected, but in clinical practice, as pointed out by Brown and colleagues (1999), PET currently focuses on the use of a radiolabeled glucose analog, [18F]fluorodeoxy-D-glucose (FDG), which targets the increased glucose metabolism present in most cancers of the thorax. The anatomic resolution of PET is less satisfactory than the resolution of computed tomography (CT) or magnetic resonance (MR) imaging. However, the anatomic limitations of PET imaging are being addressed by the combination of PET scanners with CT scanners into PET/CT scanners that are undergoing greater application, as reported by Cohade and the author (2003). Software fusion also allows the combination of PET images with CT or MR images to improve imaging specificity, according to Skalski and associates (2002), but is less commonly applied at present than hardware-fused images using combined PET/CT scanners.
The limitations of conventional imaging for detecting and characterizing thoracic neoplasms include the following: (a) anatomic images display masses but do not generally accurately characterize what is inside the mass, except to reveal whether it is water density, calcium, fat, or air and whether the blood vessels are leaky to contrast (i.e., determines contrast enhancement); (b) anatomic methods are often insensitive to small foci of tumor metastases such as in lymph nodes, although size may be a modest predictor of the presence or absence of tumor in lymph nodes; (c) anatomic methods are incapable of predicting when tumors will be responsive to a given treatment, and anatomic images are slow to change in the face of effective therapy; and finally (d) anatomic imaging methods, as I (2003) have stressed, may be difficult to interpret in the postoperative or postradiation therapy setting when it is important to separate scar from viable tumor. PET in clinical practice is generally based on the imaging of metabolic alterations located within cancers and can address most, if not all, of these issues.
MECHANISM OF FDG UPTAKE
The most common alteration of malignant cells currently targeted in clinical practice in PET imaging is their increased use of glucose. This is traced by the radiotracer FDG. This particular compound is transported into cancer cells by glucose transporters (most notably Glut 1, which is overexpressed in cancers), phosphorylated by hexokinase within cancer cells, and then trapped within cancer cells as FDG-6-phosphate. Brown and coinvestigators (1999) have shown that cancer cells typically have high levels of glycolytic activity, in part due to the increased presence of glucose transporter molecules on the cell surface and their increased levels of hexokinase compared with normal tissue. However, glucose use is not a completely specific phenomenon, and a variety of benign processes (e.g., tuberculosis, sarcoidosis, infectious processes) may have varying levels of elevated glucose use. Thus, FDG PET scanning, although an excellent targeting mechanism for cancers, is not a completely specific method, as I (2003), among others, have observed.
A discussion of the physics, instrumentation, and techniques of PET imaging is beyond the scope of this chapter; however, full textbooks exist on this subject, such as the one published by me and Buchanan (2002). Nonetheless, a few salient points may be briefly noted. PET imaging is
P.200
somewhat similar in concept to most nuclear medicine techniques, such as bone scanning. In nuclear imaging, a radiotracer is injected intravenously that will hone to a specific tissue or organ based on its physical properties. As an example, in the commonly used bone scan, remodeling bone accumulates a technetium 99m (99mTC) medronate methylene diphosphonate (MDP) analog, which can then be imaged using a special camera found in nuclear medicine departments, called a gamma camera, that detects the gamma rays emitted from the patient by the 99mTC. The principle for PET is similar, in that a radioactive tracer that preferentially accumulates in tumor tissue due to altered tumor metabolism is used. Rather than a gamma ray emitting isotope, the radiotracer used is a positron emitter. The positron is a positively charged electron, which is essentially antimatter. The positron travels a short distance in tissue and then encounters an electron. The two combine and then annihilate, giving off two 511-KeV photons, which travel in opposite directions. These photons can then be detected by the PET scanner. The PET scanner and its computers can determine the line on which the disintegration occurred, and then, with multiple decays and measurements, determine the point of origin of the decays using reconstruction methods like those used to generate CT scans. The term PET imaging is actually somewhat of a misnomer because the process does not really image the positrons with a PET scanner; rather, the images obtained are of 511-KeV photons from annihilation of the positron. Several important points need to be considered: PET imaging is currently able to detect many lesions smaller than those detectable by CT because small lesions with high metabolic activity, and thus high radioactivity concentrations, can be seen with a highly sensitive PET scanner but appear normal on CT. Also, PET in its current form is not equivalent to a microscopic examination and typically does not generally detect lesions smaller than 3 mm. Indeed, many lesions that are 3 to 7 or 8 mm in diameter escape detection. Lesion detectability with PET is not simply due to lesion size, but rather is a function of lesion size, lesion location, body size, tumor metabolic activity, scanner performance, and the duration of the scan acquisition. Patient motion can also affect detection by blurring the image signal. Nonetheless, PET is able to detect lesions that are typically not detected using anatomic methods, hence its higher sensitivity than anatomic imaging in a wide array of neoplasms.
Patient Preparation for FDG PET Scanning
Several points regarding patient preparation for FDG PET scanning should be considered. In general, for tumor imaging, patients are evaluated in the fasting state, typically after fasting 4 or more hours. This is designed to lower serum insulin and glucose levels and to minimize cardiac uptake of FDG. Patients who have recently eaten can have altered distribution of FDG, and their PET scans may show increased targeting to muscle and other tissues that can be confusing. Patients with severely altered glucose metabolism may not be ideal candidates for FDG PET scanning, but patients with reasonably well controlled diabetes can be imaged with FDG PET. Increased serum glucose levels can reduce tumor targeting and may, in some cases, make tumor detection extremely difficult. Lung tumors typically have high-enough FDG uptake that they will be detected even if glucose levels are elevated, but many institutions will not perform PET scans if the fasting glucose level is above 200 mg/dL. Patients with such glucose levels may need insulin therapy to optimize their tumor visualization, but caution must be taken because FDG administration just after insulin has been given may be less accurate than PET performed in the fasting state. This area is under study.
Conduct of the PET Scanning
Typically, patients who have FDG PET scans are injected with the radiotracer and then undergo imaging 45 minutes to 1 hour after injection. Scans can take from 20 to 60 minutes to acquire. Technologies continue to improve, and PET scanning durations are shortening. However, a PET scan takes significantly longer than a CT scan with a multidetector unit, and the patient must lie relatively still in the scanner for moderate periods of time. Patients breathe freely during the PET study, and respiratory motion can degrade visualization of small lesions in the lung and upper abdomen, where respiration effects may alter lesion location during the scan. Attempts to gate for the respiratory cycle are under study by Nehmeh and colleagues (2003), among others, but are not routine at this time. Such methods potentially may lead to detectability of smaller lesions than ungated PET images but may lengthen the acquisition times.
Evaluation of the PET Scan
PET is an inherently quantitative technique, and this ability can be used to determine the absolute uptake of FDG or other tracers in the body The standardized uptake value (SUV) is a parameter that is sometimes used to describe lesion uptake. In general, the higher the SUV, the more likely it is that a lesion is malignant. Malignant lesions of the thorax have very high glucose metabolism levels, in general. SUV is discussed in more detail in the section Solitary Pulmonary Nodules, later in this chapter.
For PET scanning to be an accurate quantitative parameter, measurement of body thickness and thus photon attenuation must be performed. Body thickness is usually measured by a so-called transmission scan, which is a low- or high-quality CT scan of the chest and abdomen. The duration of time to acquire this scan can vary. With modern PET/CT scanners, the transmission imaging may take only 20 to 30 seconds. With older PET scanners, transmission measurements can take 15
P.201
to 30 minutes and are performed with a high-energy source providing only limited anatomic resolution. Obviously, shortening the transmission scan is an important tool to shortening the duration of the study. Shorter study durations result in more cost-effective studies in general because more scans can be done in a given amount of time.
PET scanners are typically relatively expensive devices and cost between $1 million and $2.5 million. Instruments at the upper end of the price range may well include an integrated CT scanner, which allows more precise registration of the PET images to anatomic structures. Use of PET/CT scanners is growing rapidly, and for most manufacturers, PET/CT scanners are now the dominant proportion of their PET scanner sales. This technology is in rapid evolution, but the ability to identify tumors using the sensitivity of PET and to locate them precisely using CT is a potent technology that is meeting rapid acceptance in many major medical centers.
Acquisition of an Appropriate PET Scan
PET scans can be performed in a variety of ways, but the most common approach for thoracic neoplasms is for the scan to extend from the midneck through the proximal thighs. In this way, the supraclavicular and cervical lymph nodes; the entire thorax, liver, adrenals, abdomen, and proximal femoral bones are evaluated. A rather thorough assessment of the entire body and not just of the thorax is achieved. Patterns of imaging may vary in different practices, but the so-called whole-body PET study is commonly performed as just described. It should be realized that the whole-body study often does not include the brain and often does not include the entirety of the lower extremities. This differs in various institutions performing the study, but because of time constraints, the lower extremities are often excluded from the field of view. This is important to consider when contemplating using PET to replace bone scanning, a study in which the entire skeleton is adequately imaged. The uses of PET in lung cancers and esophageal cancers are discussed separately.
USE OF FDG PET IN THORACIC NEOPLASMS
Lung Cancer
Solitary Pulmonary Nodules
Solitary pulmonary nodules (SPNs) are reasonably common and typically represent pulmonary parenchymal lesions between 1 and 3 cm in diameter. These lesions are typically not calcified, and their age is indeterminate. Although some level of characterization of risk for neoplasm can be achieved by measuring lesion size, patient age, smoking history, and characteristic of the lesions and density on CT, it is in fact quite difficult to assign precisely a definitive diagnosis in an individual patient. Thus, these SPNs carry with them an intermediate likelihood of containing cancer, typically ranging from 20% to 80%.
In the past, SPNs represented about one third of newly diagnosed lung cancers, but with the greater use of screening programs, SPNs in some centers now represent a larger fraction of the patient population with newly diagnosed lung cancer. Accurate diagnosis and intervention in these patients, according to Chang and Sugarbaker (2003), is quite important because these tumors represent the most curable of lung cancers. Historically, assessment of SPNs has relied on CT with sequential CT scans, fiberoptic bronchoscopy, needle aspiration biopsy, or excision. Needle aspiration biopsy, although popular in some institutions, has a sampling error problem that may result in false-negative exams, and there is a risk for pneumothorax. This technology is being performed less frequently as noninvasive techniques gain favor.
A relatively large number of reports have evaluated the utility of PET imaging with FDG of SPNs and have been summarized in several analyses, such as the meta-analysis published by Gould and associates (2001). These assessments are based on the expectation and observation that most lung cancers within SPNs have higher levels of glycolytic metabolic activity than benign pulmonary nodules. A number of reports dating from the early 1990s have shown sensitivity for cancers within pulmonary nodules to be in the 90% to 100% range, with specificities typically in the 60% to high-80% range. The meta-analysis by Gould and associates (2001) of 450 pulmonary nodules reported the mean joint operating sensitivity and specificity for PET to be about 91.2%. Using more typical readings, to optimize sensitivity, a sensitivity of 96.8% for cancers could be achieved with a specificity of about 77.8%. As noted by Gould and associates (2001), most interpreters of PET studies err on the side of higher sensitivity so as to try to achieve at least 95% sensitivity in lesion detection at the expense of slightly lower specificity. There are some data to suggest that the utility of FDG PET will differ depending on the prevalence of inflammatory and granulomatous disease in the population. As an example, in a patient population with a high frequency of histoplasmosis, the positive predictive value of PET was lowered substantially owing to the common occurrence of false-positive scans of nonmalignant, inflammatory pulmonary lesions, as discussed by Croft and colleagues (2002).
In general, SPNs are assessed by PET imaging about 1 hour after FDG injection by visual assessment to determine whether there is increased FDG uptake in the lesion that is greater than the activity in the cardiac blood pool. This is usually done on attenuation-corrected images. It is recognized that very small nodules (<1 cm) may have somewhat less FDG uptake than blood pool activity and yet be malignant. Thus, many investigators tend to report lesions that are smaller than 1 cm as positive even if uptake is slightly
P.202
less than that of the blood pool. The PET techniques are best for characterizing lesions larger than 1 cm. The data in lesions 7 mm to 1 cm in size are relatively scanty. Clearly, in lesions smaller than 7 mm, PET must be used with caution because the negative PET scan is unlikely to have the same high sensitivity and negative predictive value that it does in larger lesions. This is because of the inherent detection limitations of small lesions with current PET technology. CT of the thorax remains necessary for pulmonary nodules smaller than 7 mm. It is also clear that lesions near the lung bases may have somewhat higher background activity around them than lesions in the upper lobes and that the lower lobe lesions may have more motion that can blur the images. Corrections of quantitative SUVs by lesion size are also feasible, and Hickeson and coinvestigators (2002) suggest that this may improve the accuracy of PET in small nodules.
How PET with FDG is used in evaluating pulmonary nodules may vary a bit among various centers. It is clear, however, that if the pretest likelihood of disease is relatively low, the post-test probability of cancer after a negative PET scan can be very low, in the 1% to 2% range. In such patients, only very limited follow-up imaging is necessary if observation is chosen. However, a conservative approach would suggest that some follow-up imaging, possibly anatomic, 6 to 12 months after imaging might be appropriate even when there is a negative PET scan to exclude growth of an FDG-negative tumor. In patients with larger primary lesions, extensive smoking history, and other risk factors, a negative scan may not be sufficiently predictive of the absence of disease to exclude tumor completely. Follow-up imaging may be required with anatomic methods to determine whether a false-negative result has occurred. False-positive results can occur in patients with tuberculosis, granulomatosis, and sometimes other inflammatory processes. False-positive results are part of the known limitations of FDG PET of SPNs, as emphasized by Hickeson and colleagues (2002).
Our group, like others, has found that there is higher FDG background activity in the lung bases and in the posterior portions of the lungs. Small lesions near the lung bases may be somewhat more difficult to detect than larger lesions. Most institutions, although capable of performing SUVs, do not report the SUV, but rather interpret the images qualitatively, that is, whether the lesion has increased glucose metabolism relative to surrounding background tissues. SUVs determinations are quite useful, however, for evaluating lesions after therapy and are so used in some centers.
Quantitative SUVs have been used in PET imaging to try to separate malignant and benign pulmonary lesions. In some studies, SUVs greater than 2.5 are associated with a higher probability of cancer being present than lesions with an SUV of less than 2.5. However, the SUV is affected by a variety of other factors, including time from injection, quality of injected dose, patient body mass, and region-of-interest selections. Pulmonary nodules of this size, however, may have considerably varying levels of uptake. Because the variability of SUV determinations is typically about 10%, lesions with an SUV of 2.3 to 2.7 may in fact have very similar true SUVs, but the difference is largely based on statistical variation. For this reason, it is probable that absolute reliance on SUV will be a mistake in lesion assessments. Thus, most experienced laboratories mainly use qualitative analysis to assess pulmonary nodules. When quantitative SUV is applied, there is evidence presented by Hickeson and associates (2002) that higher accuracy may be achieved by incorporating corrections for partial volume effects based on lesion size on CT.
It is important to compare lesion size in PET images with CT. This should be done with combined PET/CT scanners or with images obtained separately with the two modalities. Pulmonary nodule size is extremely important for accurate interpretation of PET images of the lungs for the presence or absence of lung cancer. There have been some limited direct comparative studies evaluating the accuracy of PET versus aspiration biopsy in SPNs. In these studies, aspiration biopsy was less sensitive than PET for detecting cancer in pulmonary nodules. This lower sensitivity was a result of inadequate sampling by the aspiration biopsy with quite sufficient noninvasive sampling achieved by PET imaging. The effectiveness of FDG PET in SPNs has been demonstrated but is not likely to be cost effective as a method of avoiding a thoracotomy if there is a high prevalence of cancer within the nodules. In general, avoidance of thoracotomy or other major invasive procedures is the mechanism by which PET saves money in patient management. If PET only adds to the number of required procedures and is not a replacement for other procedures, it can add to patient care costs. Nonetheless, there is increasing application of PET in the evaluation of SPNs because of its overall accuracy, in the 90% range.
A limitation of FDG PET in SPNs is the inability of FDG PET to detect a small fraction of primary lung cancers. The lung cancers least likely to be detected by FDG PET, as reported by Marom and associates (2002), include bronchioloalveolar carcinoma (tumors that typically have low cellularity and low glycolytic activity), some neuroendocrine tumors such as carcinoids, and mucinous tumors in which much of the tumor bulk is made of mucin and not viable tumor cells. Other false-negative reports include very small tumors. False-positive reports can occur most commonly in the inflammatory conditions, with tuberculosis being one of the most problematic, especially in areas where tuberculosis is endemic. Some data published by Matthies and coinvestigators (2002) indicate that SPNs that have an increase in FDG uptake between 1 and 3 hours after tracer injection are malignant, whereas those with a decline are more likely benign. Recently, the added value of PET to screening CT programs was demonstrated by Lowe (2003), suggesting that the value of PET in assessing lung nodules found by standard methods might also be applicable for lung nodules detected
P.203
by screening programs. Although most publications have focused on primary lung cancers, Dalrymple-Hay and collaborators (2002) suggest that PET appears to be equally good in detecting metastatic cancers to the lung such as melanoma, albeit with size limitations on its overall performance.
Local and Regional Nodal Staging in Lung Cancer
After lung cancer is diagnosed, whether tumor involves the regional lymph nodes or does not is an important consideration for prognosis and choice of management. CT has been extensively used in the evaluation of locoregional lymph nodes for their involvement with tumor for some time, and, although a useful technique, CT is not particularly sensitive nor specific in determining the presence or absence of metastases to mediastinal lymph nodes. The 1-cm criterion used as a threshold value to separate benign and malignant disease is only a fair predictor of whether mediastinal tumor involvement is present and MR imaging has not, to date, been more accurate, according to Dwamena (1999) and Gould (2003) and their colleagues. This only modest accuracy of CT is related to the limited ability of size alone to determine whether tumor is present or absent in a given structure. Some nodes are enlarged as a result of benign processes, whereas other nodes are of normal size but still involved with tumor.
In 1994, I and my associates reported PET to be significantly more accurate than CT in mediastinal nodal staging in a prospective trial. We compared PET directly to CT to make this conclusion. We also used computer fusion software to combine digitally the CT and PET and showed that this did not significantly increase the accuracy of the PET imaging using existing software. A substantial number of reports have come forth in the past decade indicating PET to be more accurate than CT for mediastinal nodal staging. A meta-analysis by Dwamena and coinvestigators (1999) of 14 studies with a total of 514 PET patients and of 29 CT studies with a total of 2,226 patients revealed a higher sensitivity of 91% for PET versus 79% for CT and a higher specificity of 77% for PET versus 60% for CT in the assessment of mediastinal lymph nodes. Furthermore, the overall accuracy of PET was superior to CT in this analysis; the observed characteristics were clearly and significantly superior for PET over CT scans. It must be realized, however, that these data, although impressive, do not indicate PET to be equivalent to histologic sampling. A more recent meta-analysis by Gould and colleagues (2003) that included 39 studies also showed PET to be more accurate than CT for identifying mediastinal nodal tumor involvement (p < 0.001). For CT, median sensitivity and specificity were 61% (interquartile range, 50% to 71%) and 79% (interquartile range, 66% to 89%), respectively. For FDG PET, median sensitivity and specificity were 85% (interquartile range, 67% to 91%) and 90% (interquartile range, 82% to 96%), respectively. Fourteen studies provided information about the conditional test performance of CT and FDG PET. FDG PET was more sensitive but less specific when CT showed enlarged lymph nodes [median sensitivity, 100% (interquartile range, 90% to 100%); median specificity, 78% (interquartile range, 68% to 100%)] than when CT showed no lymph node enlargement [median sensitivity, 82% (interquartile range, 65% to 100%); median specificity, 93% (interquartile range, 92% to 100%); p = 0.002]. Optimal methods to integrate nodal size with PET findings and risk for cancer continue to evolve.
A review of the literature by Toloza and associates (2003) provided similar results and showed PET to be more accurate than CT or transesophageal endoscopic ultrasound (EUS) studies. Pooled sensitivities and specificities for staging the mediastinum were as follows: (a) for CT scanning: sensitivity, 0.57 [95% confidence interval (CI), 0.49 to 0.66] and specificity, 0.82 (95% CI, 0.77 to 0.86); (b) for PET scanning: sensitivity, 0.84 (95% CI, 0.78 to 0.89) and specificity, 0.89 (95% CI, 0.83 to 0.93); and (c) for EUS: sensitivity, 0.78 (95% CI, 0.61 to 0.89) and specificity, 0.71 (95% CI, 0.56 to 0.82). The EUS technique is still in evolution, but when combined with PET, it may be very useful.
Although sensitivities of 85% to 90% are encouraging, this means that about 10% to 15% of nodal metastases from lung cancer in the mediastinum will not be detected by PET. Further, the specificity of 80% to 90% for PET indicates that a moderate number of false-positive results occur. My impression, and that of many in my field, is that the specificity of PET can differ based on the prevalence of inflammatory disease in the patient population. In patient populations with extensive tuberculosis, PET may be less specific than in developed countries where infectious diseases are well controlled. Inflammatory disease such as histoplasmosis and sarcoidosis can have false-positive results as well, causing the performance of PET to vary based on geographic locale and endogenous prevalence of granulomatous disease. Nonetheless, the sensitivity of PET is high, and the specificity is reasonably high, but a negative PET does not categorically exclude nodal metastases in the mediastinum, and a positive PET does not categorically prove that tumor is present.
There is some limited recent evidence to suggest that the quantitative assessment of FDG uptake levels in mediastinal lymph nodes has some predictive value for the presence or absence of mediastinal metastases. In a study in Korea where there is a relatively high frequency of granulomatous disease, SUVs of more than 3.4 were found to be a good separator between malignant and benign mediastinal disease, with an accuracy of about 85% (greater than that of CT) according to Kang and co-workers (2004). My practice is to recommend the most advanced site of apparent metastatic disease identified by PET be confirmed histologically to avoid erroneously placing a patient into a nonsurgical group who in fact would otherwise be potentially curable with surgical intervention.
PET/CT technology is reasonably new but has been shown to be more accurate than PET alone or PET visually
P.204
compared (but not fused) to a concurrent CT in staging the mediastinum and in assessing T stage. In the study by Lardinois and associates (2003), integrated PET/CT provided additional information in 20 of 49 patients (41%) beyond that provided by conventional visual correlation of PET and CT. Integrated PET/CT had better diagnostic accuracy than the other imaging methods. Tumor staging was significantly more accurate with integrated PET/CT than with CT alone (p = 0.001), PET alone (p < 0.001), or visual correlation of PET and CT (p = 0.013); lymph node staging was also significantly more accurate with integrated PET/CT than with PET alone (p = 0.013). In metastasis staging, integrated PET/CT increased the diagnostic certainty in two of eight patients reported by Lardinois and colleagues (2003).
Similar results were reported in a study by Antoch and associates (2003) of 27 patients with non small cell lung cancer (NSCLC) using PET/CT in which overall tumor stage was correctly classified as 0 to IV with CT in 19 patients, with PET in 20 patients, and with PET/CT in 26 patients. Differences in the accuracy of overall tumor staging between PET/CT and CT (p = 0.008) and between PET/CT and PET (p = 0.031) were significant. Primary tumor stage was correctly determined in more patients with PET/CT than with either PET alone or CT alone. Accuracy of PET/CT for regional nodal staging was 93%, for PET 89%, and for CT 63%. Clearly, PET is a very accurate method in lung cancer assessment, but it appears that the fused PET/CT study adds incremental value and some modest improvement in accuracy.
Thus, in many institutions, PET or PET/CT is being performed at the initial assessment of lung cancers to determine whether the patient should have surgery. In many centers, patients with a negative CT scan of the mediastinum and a negative PET scan will proceed directly to surgery and will not have a mediastinoscopy performed. In general, if the PET scan is positive, regardless of the CT, tissue sampling is in order. Obviously, positive PET and CT scans in the mediastinum are considered very suspicious for metastatic disease; however, confirmation by tissue is again recommended by Silvestri and co-workers (2003) in such cases to avoid the infrequent situation when increased tracer uptake in the mediastinum represents a false-positive finding. In general, a false-negative PET in the mediastinum is associated with low tumor burden in lymph nodes, and it is probable, though not proven, that such findings are associated with a better prognosis than more bulky metastatic involvement.
Staging Distant Metastatic Disease
Lung cancer with mediastinal nodal metastases is only infrequently cured. Lung cancer with distant metastases is not surgically curable (with rare exceptions; see Chapter 106), and if distant metastases are identified when the patient presents, surgery for cure will typically be canceled. The frequency of distant metastases at disease presentation is reasonably common at 10% to 20%, and PET has assumed an increasingly important role in staging for metastatic lung cancer outside of the thorax. Several studies, such as that of Lowe (2003) and that of Mac Manus and Hicks (2003), have shown that PET can detect distant metastases following normal standard staging in 5% to 20% of patients, depending on the stage of the primary tumor and intensity of the conventional staging evaluation. Further, Stroobants and colleagues (2003) have noted that PET can offer confidence that equivocal lesions seen on standard staging methods are indeed benign in a significant fraction of cases.
Areas of particular strength for PET beyond lymph nodal assessments include: (a) the evaluation of the adrenal gland, where the accuracy of PET has been reported by Gupta and associates (2001) to be about 90%; and (b) in assessing the presence of metastatic disease in the bones, where the predominantly osteolytic lesions of lung cancer are well visualized on PET and where false-positive findings appear to occur with lower frequency than occur on bone scans (such as in degenerative change that are often positive on bone scan but negative on PET). The bone scan remains a useful test, however. A recent comparative study of PET with FDG and bone scan revealed a sensitivity, specificity, and positive and negative predictive value of 81%, 78%, 34%, and 93%, respectively, for bone scan and for FDG PET [73% (p = 0.81), 88% (p = 0.03), 46% (p = 0.5,) and 97% (p = 0.04), respectively]. Gayed and co-workers (2003) reported that FDG PET scans demonstrated significantly higher specificity and negative predictive values than bone scans in evaluating bony metastases from lung cancer. Nonetheless, because some bone metastases are not detected by FDG PET alone, it is probably not appropriate to eliminate the bone scan from the lung cancer staging workup until more data are available.
PET with FDG has also shown promise in characterizing pleural effusions as malignant or benign, based on the intensity of FDG uptake. Gupta and associates (2002) suggested that those effusions with intense FDG uptake are much more likely to be malignant than those with modest or faint FDG uptake. Another area in which PET with FDG is particularly helpful is in the assessment of liver metastases. PET with FDG appears to be less likely to have false-positive results in the liver than CT. A study from Duke University, reported by Marom and colleagues (1999), in which both PET and CT were performed of 100 lung cancer patients showed that PET detected half as many liver lesions as CT, but all the hepatic lesions detected by PET were malignant, whereas half of the lesions detected by CT were not malignant. Thus, PET had fewer false-positive results. Meta-analysis of PET in lung cancer for evaluation of liver lesions indicated PET to be more sensitive than CT in the evaluation of metastases. Nonetheless, CT of the liver remains a useful technique, and some lesions will not be detected on PET, although they will be infrequent, at least
P.205
based on the experience of Kinkel and coinvestigators (2002) in a broad array of cancers metastatic to the liver.
As indicated earlier in this chapter, brain imaging often is not performed as part of the PET scan, and most whole-body protocols exclude the brain from the imaging field. This is because there is intense FDG uptake within the normal brain that can overlap with the intensity of FDG uptake in brain tumors. Thus, a significant fraction of brain metastases from lung cancer will not detected by PET imaging, owing to the intense metabolic activity in normal brain. If brain metastases are strongly suspected, it is important to perform anatomic imaging with contrast enhancement to rule out metastases. In a study from Vanderbilt University, screening for cerebral lesions in patients with body malignancy had little clinical impact. Unsuspected cerebral or skull metastases were detected in only 0.4% of patients (4 of 1,026) in a study reported by Ludwig and colleagues (2002). Thus, most PET protocols for brain imaging do not include imaging of the brain.
Better staging accuracy should result in better decisions in individual patient management. Few studies have been done to prove this with any particular imaging method. However, a randomized study has been published by van Tinteren and associates (2002) comparing the frequency of futile thoracotomies in apparent newly diagnosed lung cancer patients who underwent PET scanning, compared with those who did not undergo PET for staging. Ninety-six patients were randomly assigned to a conventional workup lacking PET and 92 to a conventional workup including PET. Two patients in the conventional workup plus PET group did not undergo PET. Eighteen patients in the conventional workup group and 32 in the conventional workup plus PET group did not have thoracotomy. In the conventional workup group, 39 (41%) patients had a futile thoracotomy, compared with 19 (21%) in the conventional workup plus PET group (relative reduction, 51%; 95% CI, 32% to 80%; p = 0.003). In this study, the addition of PET to the conventional workup prevented unnecessary surgery in one of five patients with suspected NSCLC. This reduction in the number of futile thoracotomies would be expected to be cost-effective in most health care systems. Elimination of surgery in patients with unsuspected distant metastases is appropriate, and for this reason, in many centers, a very large fraction of primary lung cancer patients receive PET scans as a part of the presurgical workup.
Most NSCLCs, of both squamous and adenocarcinoma histology, have similar levels of FDG uptake, although it must be cautioned, as noted by Marom and colleagues (2002), that bronchioloalveolar carcinoma generally has lower FDG uptake than the other types of NSCLC, as do carcinoid tumors. Untreated small cell lung carcinomas (SCLCs) often have very intense FDG uptake, often associated with a large mass lesion. Although the data for SCLC are less comprehensive than for NSCLC, FDG uptake in SCLC, as reported by Zhao and associates (2002), is sufficient to allow visualization of active disease in most tissues, including pleural recurrences and in the bone marrow. For this reason, SCLC imaging with FDG increasingly is being applied. Because some of the decisions in patients with SCLC usually involve determining whether radiation therapy is required, knowing the precise extent of disease and whether it would extend beyond the radiation ports is important. My group is seeing increased use of PET for this clinical indication. Data available to date indicate that virtually all untreated SCLC can be imaged with FDG PET and that the intensity of FDG uptake, according to Pandit and coinvestigators (2003), is associated with prognosis, with those lesions having the highest uptake having a less poorer prognosis than those not intensely FDG avid. The role of PET in outcome and management of SCLC is in evolution.
Technical Considerations in PET Imaging in the Thorax
Although the aforementioned body of data supports the use of PET with FDG to diagnose and to stage lung cancer, there are differences in performance based on the type of imaging cameras used. As indicated earlier, PET/CT devices, although early in their technical evolution, appear to be modestly superior to PET alone for assessing primary and metastatic lung cancers, as discussed by Lardinois (2003) and Antoch (2003) and their associates. Not all PET scanners are equivalent, either, and the data supporting the use of PET in cancer imaging have predominantly been derived using dedicated PET cameras. A less sensitive form of PET detection, dual-head coincidence imaging (DHCD), involves the use of a dual-head gamma camera for detection of annihilation photons from PET. A gamma camera is the device used for bone scanning, as an example. These devices are far less expensive per unit than dedicated PET scanners. The devices are also less able to detect the energetic photons resulting from positron annihilation and thus less able to detect small tumors in comparison to dedicated PET cameras. For lesions smaller than 1.5 cm, DHCD systems are quite insensitive to the metabolic activity of such small lung cancers.
When one of our group directly evaluated an early-generation dual-head coincidence camera versus dedicated PET imaging, we observed a major decline in lesion detection capability for lesions less than 3 cm in diameter for the coincidence cameras, with particular problems in detecting abdominal lesions, reported by Shreve and colleagues (1998). In a more recent comparative study by Delahaye and coinvestigators (2003), DHCD successfully imaged 105 of 145 lesions considered as pathologic on PET (73%; p = 0.01), with a concurrence of 89% (NS) in lesions larger than 1.5 cm, and only 17% (p = 0.03) in those 1 cm or smaller. Thus, although the DHCD technology may provide some useful information in larger lung lesions, Delahaye and associates (2003) described that it is substantially less accurate than dedicated PET and is generally not considered an appropriate diagnostic tool for lung cancer, this is
P.206
especially true when small lesions are present (as they often are). DHCD systems are widely distributed, however, and may be the only system available in some settings. One must be very aware of the type of system being used in the imaging center performing the PET scans. It is possible that improvements in PET imaging of smaller pulmonary nodules may result from gating the acquisition to the respiratory cycle, as suggested by Nehmeh and colleagues (2003).
 |
Fig. 12-1. FDG PET scan demonstrating disseminated metastatic lung cancer including bone metastases. B. Primary lung cancer after treatment in left hilar region with bone metastasis seen clearly on PET/CT. Same patient as part A. |
Although PET with proper instrumentation is an effective technique for staging lung cancer, it cannot be overemphasized that proof, by biopsy, of the most advanced site of metastatic disease (not just that in the mediastinum) is important so as not to deny patients the chance for curative therapy. This has been emphasized in recent procedure guidelines, such as the one prepared by Silvestri and collaborators (2003) for the American College of Chest Physicians. Examples of PET imaging of primary and metastatic lung cancer are shown in Figures 12-1 and 12-2. A false-positive PET scan is shown in Figure 12-3.
PET and Planning Radiation Therapy
Patients with inoperable lung cancers may benefit substantially from external-beam radiation therapy. The anatomic volume of tumor from CT and the metabolically active volume of tumor defined by PET are often not identical. A collapsed lung can exist distal to a large central primary
P.207
or metastatic tumor, but the two processes may be indistinguishable based on anatomic imaging alone. Separating metabolically active tumor from metabolically inactive collapsed opacified lung is feasible using PET imaging, which selectively demonstrates tumor. Defining the precise extent of tumor is feasible using PET and is important as in radiation therapy; appropriate irradiation of the tumor, but with the sparing of irradiation of normal tissues, is desirable to achieve maximum efficacy and safety.
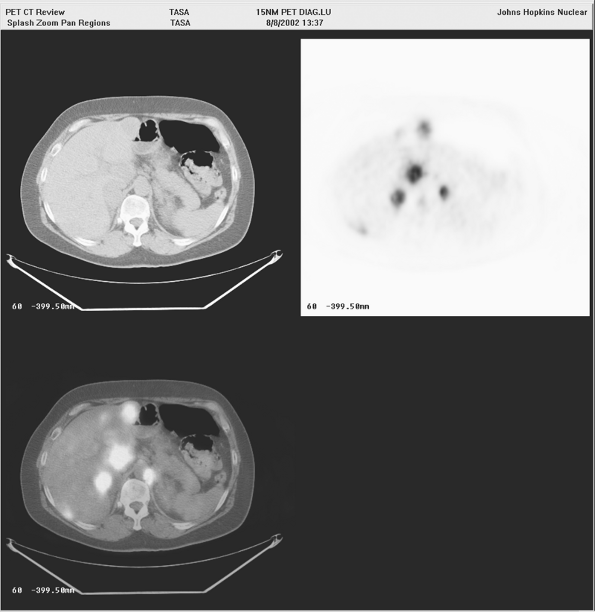 |
Fig. 12-2. A. Metastatic lung cancer to the liver and adrenal glands seen clearly on PET/CT. B. Collapsed lung in patient with history of lung cancer and lung metastases. Note difference in PET/CT appearance between collapsed lung (not FDG avid) and tumor (FDG avid). |
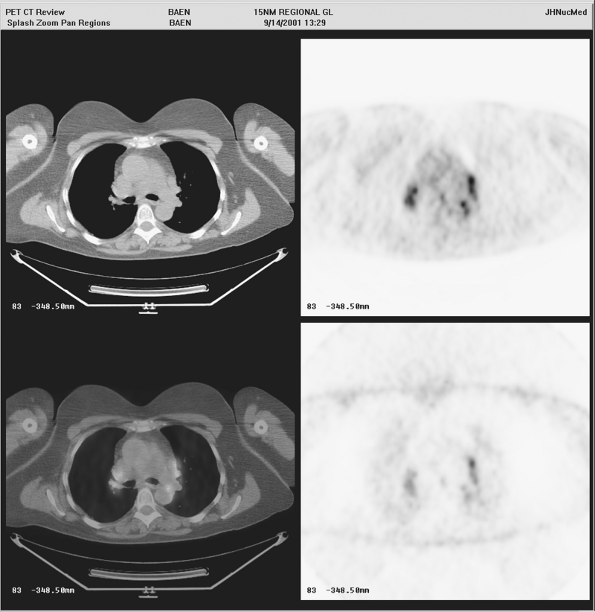 |
Fig. 12-3. Intense FDG uptake into mediastinal lymph nodes is seen, which is not due to tumor but rather is a result of sarcoidosis. |
PET can be used in several ways to plan radiation therapy. First, visual examination of the PET images can be performed to define the exact location of the primary tumor and of any regional or systemic metastases. The visual assessment of the primary tumor volume can be directly compared to the apparent tumor volume seen on CT. Mac Manus and Hicks (2003) have reported changes in the radiation treatment plans in about 25% of cases using visually apparent findings from PET. More recently, construction of treatment plans using the PET data registered with the CT volume has been shown feasible, with importation of the PET data into the radiation treatment planning systems. Mah and co-workers (2002) have discussed the impact of changes in gross tumor volume with both increases and decreases in port sizes, as well as changes in the intent of the treatment (e.g., from palliative to curative intent), that are quite frequent in comparing the PET and CT plans. Most of the time, these changes in treatment plans were appropriate. For optimal definition of treatment plans, use of a flat bed table on both the PET and CT studies is probably important because radiation therapy is administered to patients on a flat table, but most PET scanners normally have a curved tabletop. Such plans, as emphasized by Lee and associates (2003), can result in positioning the patient as closely as possible into the planned treatment position for both the PET (or PET/CT), and the radiation treatment planning is essential. Optimal plans for radiation therapy include limiting radiation dose delivery to normal lung and key structures such as the esophagus, while maximizing radiation dose delivery to tumor. Recently, a special radioactive marker for the location of the esophagus suitable for PET imaging and for fusion to CT has been developed by Thomas and coinvestigators (2003).
The use of PET/CT methods to plan radiation therapy has recently been explored. Plans based on PET/CT can both increase and decrease radiation therapy treatment volumes compared with CT alone. PET approaches appear to be more reproducible across multiple examiners than anatomic approaches, as recorded by Ciernik and colleagues (2003). Studies comparing radiation treatment outcomes based on PET imaging are not yet available, but the expectation would be that comparable tumor control efficacy with reduced normal tissue toxicity could result from the PET approach. Although there is increasing acceptance of PET for planning lung cancer radiation therapy, the literature is limited at the present time. An exciting area of opportunity is the phenotyping of tumors with a variety of tracers to determine their biological phenotype and potential for response to a specific type and course of radiation therapy.
FDG PET for Monitoring Treatment of Lung Cancer
PET is commonly used in the initial assessment of the stage of lung cancer, and it is increasingly being applied to follow patients who have been treated. Effective chemotherapy can result in relatively rapid decrements in FDG uptake within responding tumors. PET has been evaluated in both neoadjuvant therapy and in assessing metastatic disease response to treatment. Cerfolio and coinvestigators (2003) report that for neoadjuvant therapy, PET is a significantly more accurate predictor of residual tumor and of response than is CT (p < 0.005). A study of 57 patients with metastatic lung cancer assessed by PET before and after one cycle of platinum-based therapy showed a close correlation between metabolic response of a decline in SUV of at least 20% and the best response to therapy (p < 0.0001; sensitivity and specificity for prediction of best response, 95% and 74%, respectively). More responders were identified by PET than by CT. Median time to progression and overall survival were significantly longer for metabolic responders than for metabolic nonresponders (163 vs. 54 days and 252 days vs. 151 days, respectively). In NSCLC, reduction of metabolic activity after one cycle of chemotherapy is closely correlated with final outcome of therapy. Weber and associates (2003) suggest that using metabolic response as
P.208
an end point may shorten the duration of phase II studies evaluating new cytotoxic drugs and may decrease the morbidity and costs of therapy in nonresponding patients. Thus, follow-up studies after one or more cycles of treatment may be quite useful in determining whether chemotherapy has been effective. In general, persistent FDG uptake in lesions after chemotherapy indicates persistent residual tumor, although this remains under study, and results have been somewhat variable depending on the mode of treatment.
Changes in tumor FDG uptake after radiation therapy can be more gradual, and assessment several months after treatment generally is quite accurate using PET techniques. Patz and colleagues (1994) have found that intense FDG uptake in lesions after therapy with SUVs of more than 2.5 have been associated with recurrent or persistent tumor with reasonably high accuracy. Early assessments during and very soon after radiation therapy may encounter radiation-induced changes and slow tumor response that can confuse interpretation. Thus, my group is usually more cautious in applying PET in the immediate postradiation period, and we prefer to wait several months until assessment is made. Chemotherapy generally can be assessed more rapidly. Fewer studies exist detailing the early posttreatment changes induced by radiation treatment than by chemotherapy.
Recently, a study conducted by Mac Manus and coinvestigators (2003) evaluated the prognostic significance of PET for lung cancer outcomes in 73 patients (of whom 63 had chemotherapy and 10 had radiation therapy). The prognostic significance of PET versus CT was compared using scans performed about 2.5 months after treatment. PET was found to be much more predictive of survival than was CT.
In a study of mediastinal nodal histopathology following a variety of initial therapies of lung cancer, most notably chemotherapy or chemotherapy and radiation therapy, PET was very accurate at characterizing whether residual tumor was present in the treated primary lung lesion, but substantially less accurate at characterizing the histopathologic status of treated regional lymph nodes. Thus, although it seems clear that rapid declines in tracer uptake following treatment are highly predictive of a good response, Akhurst and associates (2002) found that the PET scan with FDG is not able to assess consistently mediastinal nodal tumor involvement in isolation after therapy. This may be related to underlying or concomitant inflammatory disease, which may have residual glycolytic activity.
Prognostic Information in Lung Cancer
Although results have been somewhat variable, in general, high FDG uptake is associated with larger, more aggressive tumors. These tumors usually have a poorer prognosis than smaller, less glycolytically active tumors. However, in one of our earlier studies reported by Sugawara and colleagues (1999), we were unable to show in a small patient group that lesion FDG uptake was predictive of outcome. It is quite clear that many patients with lung cancer have an unfavorable prognosis regardless of their scan findings and that the stage by imaging is a critical determinant of outcome. Nonetheless, based on the available data, for patients of a given stage, patients with lower FDG uptake generally have poorer outcomes than those with more metabolically active tumors, as noted by the studies of both Higashi (2002) and Jeong (2002) and their co-workers.
Cost-Effectiveness of PET Imaging
PET is an accurate technique based on the available data in a variety of settings. It has been shown to be cost-effective in several studies. However, one of the most persuasive studies was the previously described PLUS study reported by Verboom and associates (2003), in which before invasive staging or thoracotomy, 188 patients with (suspected) NSCLC were randomly assigned to conventional workup (CWU) plus whole-body PET or to CWU alone. Preoperative staging was followed by 1 year of follow-up. In the CWU-only group, 41% of the patients underwent a futile thoracotomy, whereas in the CWU plus PET group, only 21% of the thoracotomies were considered futile (p = 0.003). Verboom and colleagues (2003) found that the average cost per patient in the CWU only group was 9,573 euros, and in the CWU plus PET group, 8,284 euros. The major savings associated with PET were reductions in the costs of unnecessary surgery and in hospital days recovering from surgery. The addition of PET to CWU prevented futile surgery in one of five patients with suspected NSCLC. Thus, in this large randomized study, PET was cost-effective in the initial management of suspected lung cancer. This cost savings indicates that medical care costs are driven not just by imaging costs but by the costs associated with management based on inaccurate staging data.
Cost efficacy can vary across societies depending on their medical cost structures and patient characteristics. Of note is that a cost-effectiveness study reported by Tsushima and associates (2003) of PET and CT versus CT alone in the management of SPNs showed that the PET plus CT strategy was more accurate and cost effective in a Japanese population (cost savings of the PET approach about $600 per case). Cost-effectiveness data for PET in radiation therapy planning and in planning chemotherapy lag, but are expected to move in a similar direction as in the surgical data. Cost effectiveness can only be achieved when major changes in management result from the use of PET. This is the case in lung cancer, as recorded by Seltzer and colleagues (2002), in which, about 40% of the time, management is changed by the use of PET.
Alternative Radiotracers for PET Imaging of Lung Cancer
Although FDG is the predominant radiotracer used in lung cancer imaging, elevated rates of glycolysis are only one of the many functional alterations present in lung cancer
P.209
that distinguish lung cancer from normal tissues. Untreated lung cancers are characterized by increased rates of membrane transport of amino acids, increased rates of protein synthesis, increased rates of DNA synthesis, increased rates of membrane synthesis, and a relatively high frequency of hypoxia. Each of these processes can be imaged with PET tracers. As an example, some of the first imaging of lung cancer was achieved with the radiolabeled amino acid l-methionine labeled with carbon 11 (11C). A limitation to its routine use is the short half-life of 11C (20 minutes), but for evaluating the thorax, it has characteristics comparable to those of FDG. 11C tyrosine has also been used by Pieterman and coinvestigators (2002a) for imaging of lung cancer. DNA synthetic rates are increased in many lung cancers, and both 11C thymidine and 18F fluorothymidine (18F FLT) have been used by Vesselle and associates (2002) to image lung cancer. 18F FLT has considerably lower metabolism than 11C thymidine and lower uptake in tumors than FDG in general, but it appears to be better able to separate tumor from inflammation, according to Buck and colleagues (2003), but some slowly proliferating tumors may not be detectable with FLT scanning for this reason. Although the proliferation rate of tumors may be more predictive of outcome than the glycolytic rate, this warrants further study, as noted by the aforementioned authors. Other tracers, such as 11C choline, fluorocholine, and 11C acetate, are not commonly applied in lung cancer imaging at this time; however, recent reports by Hara (2003) and Pieterman (2002b) and their coinvestigators suggest that 11C choline may be better able to separate primary lung cancers from infections than FDG, with 11C choline having lower uptake in inflammatory processes than FDG, but with both having high uptake in primary lung cancers. Recently, 11C acetate was shown by Higashi and co-workers (2004) to be generally inferior to FDG for lung cancer detection, except possibly in some bronchioloalveolar carcinomas. Another possible target for tumor imaging is tumor hypoxia. A variety of radiopharmaceuticals have been developed for this purpose, but they are still in the evaluative phase. A recent study of copper 60 (60Cu) ATSM (a copper-labeled hypoxia-targeting agent) conducted by Dehdashti and associates (2003) showed that those tumors with higher tumor-to-muscle uptake ratios of 60Cu ATSM were least likely to respond to therapy. Thus, imaging lung cancer is feasible with a wide array of radiotracers, which image a broad array of physiologic processes.
Summary: Lung Cancer
PET with FDG has assumed an important role at several phases in the management of lung cancer. In the diagnostic setting, this method can help characterize SPNs as malignant or benign noninvasively. For staging, both locoregional and systemic staging can be achieved successfully. For assessing treatment response, PET is also a useful early predictor of response in chemotherapy and has prognostic value. PET is also useful in follow-up assessments and has promise in individualizing therapy, both diagnostic and therapeutic, of lung cancer.
Mesothelioma
Mesotheliomas are commonly associated with exposure to asbestos. Gerbaudo and co-workers (2002) have shown that untreated tumors have intense FDG uptake in most instances. PET is increasingly being used to determine the extent of mesothelioma and to determine whether there is systemic involvement. In patients with thoracic-only disease, aggressive surgical procedures, such as extended pleuropneumonectomy, are being used in some instances to remove all of the lung and pleura in selected patients with mesothelioma in an effort to achieve better survival (see Chapters 65 and 66). Although it is still unclear whether this aggressive surgical technique improves survival, it is clear that knowing the precise location of tumor and whether it is in the operative field is important for identifying candidates for this surgical procedure. Follow-up of patients with mesothelioma is also challenging because the lungs and pleural surfaces are often extremely distorted and postsurgical changes are substantial. I have seen increased application of PET in the diagnosis and follow-up of mesothelioma at our institution.
Esophageal Cancer
Esophageal carcinoma is a relatively uncommon cancer, with an estimated 13,500 new cases expected (10,000 men, 3,500 women) in the United States in 2003 according to ACS Cancer Statistics (2003). The cancer is highly lethal, with an estimated death rate of 12,500 patients per year, with 9,600 men and 3,000 women dying of the disease. This very high mortality rate is consistent with a very low 5-year survival rate after diagnosis of about 12% for all stages. Although the 5-year survival rate is poor, it has improved slightly, but significantly (p < 0.05), from 5% to 7% in 1974 to 1976 to 12% in 1989 to 1996, presumably owing to improved diagnosis and treatment. However, some of this improvement may simply be due to the detection of earlier-stage disease by fiberoptic endoscopy, as noted in the ACS Cancer Statistics (2003) and by Orringer (2001) and Rice (2000). Most esophageal cancers diagnosed in the world are of squamous cell histology. However, in the Western world, there has been a substantial increase in the incidence and prevalence of adenocarcinoma of the esophagus, especially those arising at the esophagogastric junction, as noted by the aforementioned authors.
Most, if not all, adenocarcinomas of the distal esophagus arise from areas with specialized intestinal metaplasia, which often develop as a consequence of gastroesophageal reflux disease (GERD). In some cases, it can be difficult to
P.210
determine whether tumors at the gastroesophageal junction arose from the esophagus or the stomach itself, but there are some data to suggest that imaging characteristics of tumors arising below the esophageal gastric junction differ from those arising above this area. A major effort is now being placed on the prevention of GERD and prevention and treatment of Barrett's esophagus in an ongoing attempt to reduce the increase in the rate of adenocarcinoma of the esophagus in the Western world. Risk factors for esophageal carcinoma in addition to GERD include achalasia, Chagas' disease, and lye ingestion.
When gastric carcinomas occur in the fundus, they can be difficult to distinguish from esophageal carcinomas. These occur in an additional 21,700 patients per year in the United States, as noted in the ACS Cancer Statistics report (2003). The most common sites of metastatic disease are locoregional lymph nodes immediately adjoining the esophagus as well as the lymph nodes in the upper abdomen and in the cervical region (see Chapter 124). Distant metastases involve the liver, lungs, and other organs, including bone.
Decisions about primary therapy of esophageal cancer are based on knowledge of the extent of the primary tumor. If surgery is planned, it is obviously best to remove all known cancer. Surgery for esophageal carcinoma is a major procedure and carries risks. Determining whether aggressive curative intent surgery or surgery for palliation only is appropriate is a key decision.
The stage at presentation is important both to prognosis and to rational management decisions. Staging of esophageal carcinoma is commonly tabulated using the American Joint Committee on Cancer (AJCC) TNM staging system, discussed in Chapters 124 and 136 as well as elsewhere in this text. Survival is dependent on stage, and although poor in all stages, is much better for localized and locoregional disease than for disseminated esophageal cancers (25%, 12%, and 2% 5-year survival rates, respectively). A variety of methods have been used to stage esophageal cancer, including EUS, CT, and more recently, PET imaging using FDG. Imaging is key to defining the stage initially and to planning management, as outlined by Rice (2000) and Messman and Schjlottmann (2001).
The management of esophageal cancer continues to be in evolution. Traditionally, surgery has been the first choice for curative treatment, but the very low historical survival rates of esophageal cancer to surgery alone, 5% or less for 5 years in many instances, showed that additional effective therapy was needed. Esophageal cancers are quite responsive to chemotherapy and external-beam radiation therapy. Thus, combined treatment methods are increasingly common. Results with combined treatments have been somewhat mixed and have recently been reviewed by Geh and associates (2001) and Law and Wong (2001).
In many centers, aggressive chemotherapy, often neoadjuvant (to reduce tumor burden), has been performed before surgery, and assessing response to such therapy is of key importance. In at least some series, 5-year survival rates in excess of 50% have been reported in patients in whom a complete response is seen after neoadjuvant therapy. PET has a growing role in assessing this form of treatment.
Primary Tumor Detection
Nearly all localized primary esophageal carcinomas are detected first by clinical symptoms of dysphagia or in the context of endoscopy performed as part of evaluation of the upper gastrointestinal (GI) tract for gastroesophageal reflux. PET has not been used as a primary screening method for esophageal carcinoma. Both untreated primary adenocarcinomas and squamous cell carcinomas appear to have similar levels of FDG uptake on PET.
The precursor lesion to many cases of adenocarcinoma of the esophagus, Barrett's esophagus, generally has a low total volume, and thus would not be expected to be detected using whole-body PET imaging devices, although on rare occasions, it can be, as reported by Bakheet and colleagues (1999). Also, these authors have reported that, similarly, some inflammation of the distal esophagus can have mild increased FDG uptake. Several studies by Flanagan (1997), Kole (1998), and Skehan (2000) and their associates have shown that most primary esophageal cancers, which are first diagnosed by other methods, are detectable by FDG PET, with sensitivities in the 90% to 100% range. According to Menzel and coinvestigators (2003), FDG uptake seems to be quite comparable across both squamous and adenocarcinoma histologies. When PET has been reported to be falsely negative in esophageal cancers, it has usually been in lesions with small tumor volumes, such as stage IA primary lesions.
Tumors at the gastroesophageal junction are frequent and are most commonly adenocarcinomas. PET with FDG was studied in 52 patients by Ott and co-workers (2003) with untreated, histologically proven, locally advanced adenocarcinoma of the esophagogastric junction (AEG) (distal esophagus, type I: n = 31; cardia, type II: n = 21). None of the tumors had been previously treated. There was no correlation between FDG uptake and clinical stage, grade, Lauren classification, or survival. All AEG I tumors were visualized by FDG-PET with high contrast, whereas FDG uptake by five AEG II tumors (24%) did not differ from background activity. In a quantitative analysis, mean FDG uptake of AEG I tumors was 1.6 times higher than that of AEG II tumors (p = 0.0005). It is not clear what these findings mean biologically, but for PET imaging, it is clear that tumors of the cardia may be more difficult to detect, possibly related to the higher background in the stomach as well as the apparently lower tracer uptake in the primary lesion in the results reported by Ott and colleagues (2003). Thus, most primary esophageal cancers located in the esophagus presenting clinically and first detected by endoscopy will be detected at PET imaging. This does not imply PET is useful as a screening method for esophageal cancer, however, and it has not been rigorously studied in such a setting.
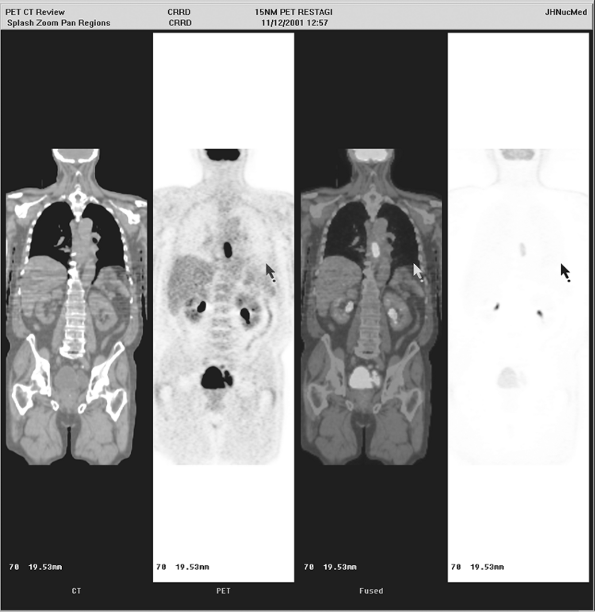 |
Fig. 12-4. FDG PET showing small primary esophageal carcinoma in the distal esophagus. |
P.211
Diagnostic challenges with PET in esophageal cancer can include determining whether there is abnormal or physiologic uptake at the gastroesophageal junction. There can be some uptake in this location normally, so that detecting small esophageal cancers could be problematic. However, it is probable that early, low-volume esophageal cancer would be much more easily detected by direct visualization using an endoscope than by PET. An example of PET visualization of a primary esophageal carcinoma lesion is shown in Figures 12-4 and 12-5A.
Locoregional Nodal Metastasis Detection in Esophageal Carcinoma
With PET, the detection of nodal metastases is dependent on the volume of tumor in the metastasis, the intensity of tracer uptake in the lesion, the background tracer activity (as well as the injected dose of radiotracer and the performance and resolution of the scanner), and the interpretation criteria. CT detects nodal metastases based on their size, and what is a positive or negative node by CT differs based on the observer's interpretation. The larger the size of a node, the more likely it generally is to represent a node involved by cancer; however, the optimal size cutoff for positive or negative nodes is not clear. Both PET and CT can fail to detect small metastases of esophageal cancer in locoregional lymph nodes. Lesions smaller than 5 mm in size are commonly not detected on PET with FDG, which is consistent with some of the detection challenges seen with current FDG PET technology. To achieve high sensitivity with CT, small lymph nodes must be called positive. For example, 5-mm and larger nodes may be called abnormal in some CT studies, whereas others may choose a 10-mm size cutoff. The smaller the node size cutoff, the more likely that cancer will be detected, but at the price of a lower specificity. Thus, there is a considerable range in the sensitivity and specificity of PET for assessing tumor involvement in
P.212
regional lymph nodes and an even greater range in accuracy for CT.
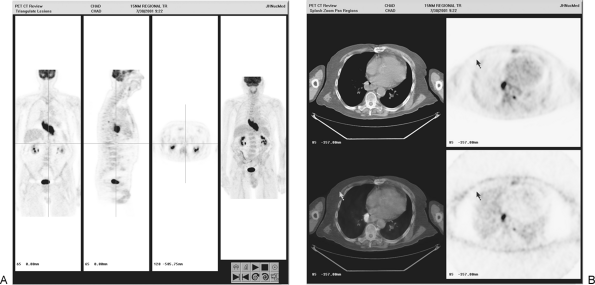 |
Fig. 12-5. Primary tumor clearly delineated on PET-only image. B. Transverse PET/CT images show primary tumor and small locoregional retroaortic nodal metastasis of esophageal cancer. |
In general, PET is not exceptionally sensitive but has high specificity for the detection of locoregional lymph node metastases (typical sensitivities of 22% to 76%, but with specificities of about 90%) (see Fig. 12-5B). By contrast, reported CT sensitivity and specificity pairs range from (18% and 78% to 87% and 14%), meaning that CT can be either very insensitive and reasonably specific (although not as specific as PET) or very sensitive and extremely nonspecific, depending on how the reader operates on the ROC curve. When directly compared, in several studies, PET is in general an equivalent or more accurate method for nodal staging than is CT, but both are somewhat limited in accuracy as compared with histologic assessment of lymph nodes in the reports of Flanagan (1997) and Kole (1998) and their associates, among many other authors.
EUS appears to be more sensitive than either PET or CT for regional nodal staging and is reasonably specific according to Flamen (2000a), Lerut (2000), Meltzer (2000), and Choi (2000) and their associates. EUS is better at assessing tumor size and depth of invasion, and there are no data showing that PET is accurate in evaluating the primary tumor stage of esophageal cancers. EUS has a significant learning curve and may not be routinely available. Further, EUS may be technically impossible if there is an esophageal stricture due to cancer because it may be impossible to pass the scope beyond a tumor-induced stricture, as noted by Rice (2000) and Law and Wong (2001), as well as by Flamen (2000a) and Rasanen (2003) and their colleagues.
There is some interest in using minimally invasive surgical techniques for staging esophageal cancer (see Chapter 136). These are likely to be more sensitive than imaging but are clearly more invasive, and their success is dependent on sampling the proper tissues.
Systemic Metastases
In most clinical studies, PET has been more accurate than other conventional diagnostic methods in detecting systemic metastases. Reported sensitivities of PET in larger peer-reviewed manuscripts have been in the 70% to 100% range, whereas those of CT have been lower, at 29% to 68%. The specificity of PET is high, typically in the mid-90% range, whereas that of CT is in the 74% to 81% range. A review of the literature, including abstracts with limited data and more heterogeneous patient groups, showed comparable results in large numbers of patients in many of the aforementioned reports as well as those by Block (1997), Luketich (1997, 1999), Rankin (1998), Yeung (1999), Junginger (2002), and Yoon (2003) and their associates. It is quite clear that PET with FDG is the most sensitive noninvasive method for detecting systemic metastatic esophageal carcinoma. A recent study from Finland, prospectively performed and reported by Rasanen and colleagues (2003), generally supported these observations, but had somewhat lower overall performance for PET in 42 patients with adenocarcinoma of the esophagus or the esophagogastric junction in whom surgery was performed and in whom CT, PET, and EUS were performed. Diagnostic sensitivity for the primary tumor was 83% for PET and 67% for CT; for local peritumoral lymph node metastasis, it was 37% for PET and 89% for EUS; and for distant metastasis, it was 47% for PET and 33% for CT, the latter figures considerably lower than reported in most other series. Diagnostic specificity for local lymph node metastasis was 100% with PET and 54% with EUS, and for distant metastasis, it was 89% for PET and 96% for CT. Accuracy for locoregional lymph node metastasis was 63% for PET, 66% for CT, and 75% for EUS; for distant metastasis, it was 74% with PET and 74% with CT. Of the 10 patients who were considered inoperable during surgery, PET identified 7 and CT 4. PET showed false-positive lymph nodes at the jugulum in 3 patients, again emphasizing the need for careful histologic proof of the first site of metastases in patients. Another study by Junginger and coinvestigators (2002) in 30 patients from Germany did not show improvements in overall staging accuracy for PET versus CT for metastases of esophageal cancer, with PET being more specific, but CT more sensitive, with overall comparable accuracy rates.
In areas of the body or geographies where there is substantial inflammatory disease, the performance of PET can suffer as well as that of CT in characterizing nodal metastases. Thus, in a prospective study of PET and CT in 81 patients with squamous cell carcinoma of the esophagus who underwent surgery and nodal assessments, the accuracy rates of CT and FDG PET for depiction of metastasis to lymph nodes for depiction of malignant nodal groups in each lymph node group: sensitivity, specificity, and accuracy rates, respectively, of CT were 11% (11 of 96 nodal groups), 95% (553 of 581), and 83% (564 of 677), whereas those of FDG PET were 30% (29 of 96), 90% (525 of 581), and 82% (554 of 677) (p values: < 0.001, 0.009, and 0.382, respectively) in the report of Couper and colleagues (1998). With PET, the most common false-positive results were in the hilar or mediastinal lymph nodes. Both methods had low sensitivity for nodal metastases.
Thus, whereas PET is of greatest use in finding distant metastatic disease, it remains an imperfect technique, although with generally favorable performance characteristics versus CT. For locoregional nodal disease along the course of the esophagus, PET is generally inferior to EUS. It has not been carefully studied, but available data suggest, as in lung cancer, that PET is not superior to CT or MR imaging in the detection of brain metastases. The author recommends the use of anatomic imaging to assess the brain if, after the PET study, a concern regarding the presence of brain metastases remains. It is also likely that very small (a few millimeters) esophageal metastases is better detected by CT than by PET. This would seem probable based on the known physical performance characteristics of PET for small lesions using current PET devices.
P.213
The relative performance of PET versus bone scans in the detection of bone metastases is only reported to a limited extent. Given the relatively high uptake of FDG into esophageal cancers, it is likely that esophageal cancer will be detected well if it is metastatic, even if in bone.
Cautions
PET is currently approved by Medicare in the United States for a number of applications in esophageal cancer, including staging and monitoring of treatment response. Most of the literature to date is based on the use of dedicated, two-dimensional, high-density and sensitivity detector PET cameras. Some early experience in PET imaging of esophageal cancer was based on the use of three-dimensional partial-ring detector PET systems. Such devices are somewhat more prone to scattered events, which can lower target-to-background ratios unless accurate computer corrections are put in place. It is of interest that the only paper in which PET fared worse than CT in sensitivity was obtained using such a device.
Little data exist to demonstrate that coincidence imaging with dual-head gamma cameras is an effective method for detecting small metastases of esophageal carcinoma. Medicare specifically excludes hybrid gamma cameras from the list of devices approved for reimbursement, and these devices are not used much in the United States for detection of esophageal cancer with FDG. Our initial data using two-dimensional high-density and sensitivity detector PET with CT fusion device has been encouraging, and a representative image is included from our system (see Fig. 12-4).
Detection of Recurrence
Esophageal cancer commonly recurs; therefore, detection of recurrence by imaging is an important clinical question. This has been addressed by Flamen and colleagues (2000b), who evaluated 33 patients with 40 recurrent sites. PET was not as reliable in characterizing anastomotic recurrences as conventional methods, which included EUS. PET tended to have false-positive results in patients with benign strictures after dilation, although PET was very sensitive (sensitivity, specificity, and accuracy rates of PET were 100%, 57%, and 74%, respectively, compared with 100%, 93%, and 96%, respectively, for CT). For systemic metastases, PET was very reliable, with sensitivity, specificity, and accuracy rates for PET of 94%, 82%, and 87%, respectively, compared with 81%, 82%, and 81%, respectively, for CT (p value not statistically significant). Although the accuracy rates in these patients were comparable, PET provided additional information in about 27% of patients according to the study of Flamen and colleagues (2000b). Thus, PET appears to add incremental value to CT, but in this setting, it is not clearly proven superior to CT. It seems the combination of tests would be most rational for recurrence detection based on available data.
Assessing Response to Therapy
Esophageal carcinomas, although traditionally treated by surgery, are increasingly treated with neoadjuvant chemotherapy, and often also include radiation therapy. In some studies, if a pathologic complete remission is achieved after combined chemoradiation therapy, 5-year survival rates of up to 60% have been reported.
It has been suggested that it might be possible to eliminate the need for surgery if it were possible, through imaging, to determine that there was no residual tumor present. Determining whether the treatment is effective early after it is started [and likely to lead to a pathologic complete response (CR)] or has achieved a pathologic complete response after treatment is completed can help determine the next step in treatment and also may reflect prognosis.
There have been several reports showing that FDG PET can measure response of a variety of cancers to therapy. In breast cancer, early declines in FDG uptake in cancers antedate changes in tumor size and can predict response and outcome. In a study by Couper and associates (1998), pretreatment and posttreatment PET scans in 13 patients with esophageal carcinoma showed a change in FDG uptake paralleling the change in tumor size on CT. The largest reductions in FDG uptake were seen in patients who achieved a CR, whereas a mild increase in FDG uptake was seen during therapy in two patients with progressive disease.
In a similar study by Weber and colleagues (2001), 27 patients with histopathologically proven squamous cell carcinoma of the esophagus, located at or above the tracheal bifurcation, underwent neoadjuvant therapy consisting of external-beam radiation therapy and 5-fluorouracil as a continuous infusion. FDG PET was performed before and 3 weeks after the end of radiation therapy, and chemotherapy was performed before operation. Quantitative measurements of tumor FDG uptake were correlated with histopathologic response and patient survival. After neoadjuvant therapy, 24 patients underwent surgery. Histopathologic evaluation revealed less than 10% viable tumor cells in 13 patients (responders) and more than 10% viable tumor cells in 11 patients (nonresponders). In responders, FDG uptake decreased by 72% 11%; in nonresponders, it decreased by only 42% 22%. At a threshold of 52% decrease of FDG uptake compared with baseline, the sensitivity to detect response was 100%, with a corresponding specificity of 55%. The positive and negative predictive values were 72% and 100%. Nonresponders to PET scanning had a significantly worse survival after resection than responders. These data are promising, but the relatively low positive predictive value for response is consistent with the limitation of PET in detecting small amounts of residual cancer noninvasively.
Difficulties in detecting small tumor volumes are compounded by further decreases in FDG uptake with treatment, which further reduce the detectable signal. Nonetheless, PET performed quite well in this setting.
P.214
Ideally, PET could be performed soon after treatment is started in an effort to determine longer-term response. PET scans before and 14 days after the start of a platinum-based chemotherapy for esophageal carcinoma were performed by Weber and colleagues (2001) in 40 patients, with response assessed after 3 months of therapy by EUS and histology. Responding tumors had a decrease in tracer uptake of 54% 17%, whereas nonresponding tumors showed only a minimal decline in tracer uptake of 15% 21%.
A cutoff value of a 35% decline was about 95% effective in separating tumors that ultimately responded from those that did not. Patients with greater declines in tumor FDG uptake were more likely to achieve a histologic complete response, as well. The patients with a larger decline in FDG uptake also had significantly longer times to tumor progression or recurrence than those patients with only minimal decreases in tracer uptake with treatment.
Radiation therapy also plays an important role in the management of esophageal carcinoma in some settings. PET has been examined as a tool to evaluate the response of esophageal cancers to monotherapy with external-beam gamma irradiation. PET with FDG showed significant differences in the average median SUV between the controls and the treated patients (8.1 1.6 vs. 3.3 1.1); it also revealed differences between the poor responders and good responders (4.1 0.8 vs. 2.7 0.9) as assessed by ultrasound, but could not seem to detect those patients who were destined for recurrence. These data, presented by Nakamura and associates (2002), however, show that responses to radiation are substantial, as assessed by SUV during the course of the treatment, but do not indicate that residual tracer uptake is invariably indicative of residual tumor.
Economic Implications and Implications for Quality of Life
A comprehensive review by Gambhir and co-workers (2001) of the PET literature in esophageal cancer, including abstracts, suggested that PET changed management in 14% to 20% of cases. This is often in the form of finding disseminated metastatic disease not detected by other methods but can also include clarifying that a positive abnormality on CT is in fact not positive on PET for metastases, and thus not likely to represent a metastasis.
The cost efficacy of PET in esophageal cancer has not been extensively studied, but like in lung cancer, reductions in cost are likely to be achieved through avoidance of unnecessary surgical procedures. Recently, a variety of management strategies were compared for the initial assessment of esophageal carcinoma. Wallace and co-workers (2002) reported that the most effective strategy was PET imaging plus EUS; however, it was about $60,000 per quality-adjusted life years (QALYs) more expensive than CT plus EUS methods. However, based on typical valuation of QALYs, the PET-based strategy was considered the preferable one, even though in this analysis, it did not result in cost savings.
Other Tracers for PET in Esophageal Cancer
PET has been studied most extensively with FDG as the tracer, and the results have been good. Only modest work to assess esophageal cancer has been performed with other PET tracers.
PET with methyl-11C choline and 2-FDG were evaluated in 33 patients with biopsy-proven esophageal carcinoma (16 patients with tumors classified as T1 and 17 patients with tumors classified as T2 to T4. 11C choline PET was more effective than FDG PET and CT in detecting very small metastases localized in the mediastinum. It was ineffective, however, in detecting metastases localized in the upper abdomen, because of the normal uptake of 11C choline in the liver.
FDG PET was superior to CT in detecting metastases in the mediastinum and the upper abdomen, whereas 11C choline PET was superior to FDG PET in detecting metastases in the mediastinum. These data were preliminary, and Kobori and associates (1999) believed PET with both tracers might be the most accurate approach for diagnosing esophageal carcinoma. A follow-up paper by Jager and co-workers (2001) evaluated 18 patients with esophageal carcinoma with both tracers.
PET results were compared with surgical and histopathologic findings. FDG-PET was able to detect all (100%) of the 16 malignant primary lesions, whereas 11C choline PET detected 73%. On a lesion basis, FDG-PET detected 10 of 12 nonregional metastases (sensitivity, 83%), whereas 11C choline PET detected 5 of 12 (sensitivity, 42%). SUVs were significantly higher for FDG (FDG, 6.6 3.5; 11C choline PET, 5.5 2.5; p= 0.04). Jager and associates (2001) concluded that 11C choline PET was able to visualize esophageal carcinoma and its metastases, but appears to be inferior to FDG PET. The aforementioned authors believed choline uptake in the liver, stomach wall, and upper abdomen made FDG the superior agent. Thus, at present, FDG is the preferred PET agent for imaging esophageal carcinoma.
Summary, Esophageal Cancer
PET with FDG is an accurate method for noninvasive detection of primary esophageal cancers, but endoscopy and EUS are more reliable methods for characterizing primary lesion size and local invasion. FDG PET is generally more specific than CT and somewhat superior to CT in accuracy but can fail to detect small nodal metastases in many instances. EUS in skilled hands may be superior for assessing locoregional metastases to lymph nodes. FDG PET is generally found to superior to other imaging methods for detecting systemic metastatic disease and is recommended as part of the initial staging algorithm in esophageal cancer.
P.215
Data to date on assessment of treatment response suggest PET provides an early and accurate evaluation of the efficacy of the therapy. PET is incapable of detecting residual microscopic disease at the conclusion of treatment owing to resolution limitations. Tracers other than FDG, although of interest, have not demonstrated clear superiority to FDG in imaging this tumor.
REFERENCES
ACS Cancer Statistics, 2003. [Online.] Available: http://www.cancer.org.
Akhurst T, et al: An initial experience with FDG-PET in the imaging of residual disease after induction therapy for lung cancer. Ann Thorac Surg 73:259, 2002.
Antoch G, et al: Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology 229:526, 2003.
Bakheet SM, et al: F-18 FDG uptake in benign esophageal disease. Clin Nucl Med 24:995, 1999.
Block MI, et al: Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 64:770, 1997.
Brown RS, et al: Glucose transporters and FDG uptake in untreated primary human non-small cell lung cancer. J Nucl Med 40:556, 1999.
Buck AK, et al: Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med 44:1426, 2003.
Cerfolio RJ, et al: Positron emission tomography scanning with 2-fluoro-2-deoxy-d-glucose as a predictor of response of neoadjuvant treatment for non-small cell carcinoma. J Thorac Cardiovasc Surg 125:938, 2003.
Chang MY, Sugarbaker DJ: Surgery for early stage non-small cell lung cancer. Semin Surg Oncol 21:74, 2003.
Choi JY, et al: Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med 41:808, 2000.
Ciernik IF, et al: Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys 57:853, 2003.
Cohade C, Wahl RL: Applications of positron emission tomography/computed tomography image fusion in clinical positron emission tomography: clinical use, interpretation methods, diagnostic improvements. Semin Nucl Med 33:228, 2003.
Couper GW, et al: Detection of response to chemotherapy using positron emission tomography in patients with oesophageal and gastric cancer. Br J Surg 85:1403, 1998.
Croft DR, et al: FDG-PET imaging and the diagnosis of non-small cell lung cancer in a region of high histoplasmosis prevalence. Lung Cancer 36:297, 2002.
Dalrymple-Hay MJ, et al: Pulmonary metastatic melanoma: the survival benefit associated with positron emission tomography scanning. Eur J Cardiothorac Surg 21:611, 2002.
Dehdashti F, et al: In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging 30:844, 2003.
Delahaye N, et al: Comparative impact of standard approach, FDG PET and FDG dual-head coincidence gamma camera imaging in preoperative staging of patients with non-small-cell lung cancer. Nucl Med Commun 24:1215, 2003.
Dwamena BA, et al: Metastases from non-small cell lung cancer: mediastinal staging in the 1990s: meta-analytic comparison of PET and CT. Radiology 213:530, 1999.
Flamen P, et al: Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 18:3202, 2000a.
Flamen P, et al: The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. J Thorac Cardiovasc Surg 120:1085, 2000b.
Flanagan FL, et al: Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. AJR Am J Roentgenol 168: 417, 1997.
Gambhir S, et al: A tabulated summary of the FDG PET literature. J Nucl Med 42(5 Suppl):1S, 2001.
Gayed I, et al: Comparison of bone and 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in the evaluation of bony metastases in lung cancer. Mol Imaging Biol 5:26, 2003.
Geh JI, Crellin AM, Glynne-Jones R: Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg 88:338, 2001.
Gerbaudo VH, et al: Assessment of malignant pleural mesothelioma with (18)F-FDG dual-head gamma-camera coincidence imaging: comparison with histopathology. J Nucl Med 43:1144, 2002.
Gould MK, et al: Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 285: 914, 2001.
Gould MK, et al: Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 139:879, 2003.
Gupta NC, et al: Clinical utility of PET-FDG imaging in differentiation of benign from malignant adrenal masses in lung cancer. Clin Lung Cancer 3:59, 2001.
Gupta NC, et al: Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest 122:1918, 2002.
Hara T, et al: Uptake rates of 18F-fluorodeoxyglucose and 11C-choline in lung cancer and pulmonary tuberculosis: a positron emission tomography study. Chest 124:893, 2003.
Hickeson M, et al: Use of a corrected standardized uptake value based on the lesion size on CT permits accurate characterization of lung nodules on FDG-PET. Eur J Nucl Med Mol Imaging 29:1639, 2002.
Higashi K, et al: 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 43:39, 2002.
Higashi K, et al: 11C-acetate PET imaging of lung cancer: comparison with (18)F-FDG PET and (99m)Tc-MIBI SPET. Eur J Nucl Med Mol Imaging 31:13, 2004.
Jager PL, et al: Carbon-11 choline or FDG-PET for staging of oesophageal cancer? Eur J Nucl Med 28:1845, 2001.
Jeong HJ, et al: Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun 23:865, 2002.
Junginger T, et al: Positron emission tomography for the preoperative staging of esophageal carcinoma. Dtsch Med Wochenschr 127:1935, 2002.
Kang WJ, et al: Differentiation of mediastinal FDG uptake observed in patients with non-thoracic tumours. Eur J Nucl Med Mol Imaging 21:202, 2004.
Kinkel K, et al: Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology 224:748, 2002.
Kobori O, et al: Positron emission tomography of esophageal carcinoma using (11)C-choline and (18)F-fluorodeoxyglucose: a novel method of preoperative lymph node staging. Cancer 86:1638, 1999.
Kole AC, et al: Positron emission tomography for staging of oesophageal and gastroesophageal malignancy. Br J Cancer 78:521, 1998.
Lardinois D, et al: Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 348:2500, 2003.
Law S, Wong J: What is appropriate treatment for carcinoma of the thoracic esophagus? World J Surg 25:189, 2001.
Lee SW, et al: Stereotactic body frame based fractionated radiosurgery on consecutive days for primary or metastatic tumors in the lung. Lung Cancer 40:309, 2003.
Lerut T, et al: Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: a prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg 232:743, 2000.
Lowe VJ: Positron emission tomography scanning in lung cancer. Respir Care Clin N Am 9:119, 2003.
Ludwig V, et al: Cerebral lesions incidentally detected on 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography images of patients evaluated for body malignancies. Mol Imaging Biol 4:359, 2002.
Luketich JD, et al: Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg 64:765, 1997.
Luketich JD, et al: Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg 68:1133, 1999.
P.216
Mac Manus MP, Hicks RJ: PET scanning in lung cancer: current status and future directions. Semin Surg Oncol 21:149, 2003.
Mac Manus MP, et al: Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol 21:1285, 2003.
Mah K, et al: The impact of (18)FDG-PET on target and critical organs in CT-based treatment planning of patients with poorly defined non-small-cell lung carcinoma: a prospective study. Int J Radiat Oncol Biol Phys 52:339, 2002.
Marom EM, et al: Staging non-small cell lung cancer with whole-body PET. Radiology 212:803, 1999.
Marom EM, et al: T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose PET. Radiology 223:453, 2002.
Matthies A, et al: Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 43:871, 2002.
Meltzer CC, et al: Whole-body FDG positron emission tomographic imaging for staging esophageal cancer comparison with computed tomography. Clin Nucl Med 25:882, 2000.
Menzel CH, et al: [18F-Deoxyglucose PET for the staging of oesophageal cancer: influence of histopathological subtype and tumour grading]. Nuklearmedizin 42:90, 2003.
Messman J, Schjlottmann K: Role of endoscopy in the staging of esophageal and gastric cancer. Semin Surg Oncol 20:78, 2001.
Nakamura R, et al: Failure in presumption of residual disease by quantification of FDG uptake in esophageal squamous cell carcinoma immediately after radiotherapy. Radiat Med 20:181, 2002.
Nehmeh SA, et al: Reduction of respiratory motion artifacts in PET imaging of lung cancer by respiratory correlated dynamic PET: methodology and comparison with respiratory gated PET. J Nucl Med 44: 1644, 2003.
Orringer MS: Esophagus: tumors, injuries, and miscellaneous conditions of the esophagus. In Greenfield LJ, et al (eds.): Surgery: Scientific Principles and Practice. 3rd Ed. Philadelphia: Lippincott Williams Wilkins, 2001. Chapter 20.
Ott K, et al: Fluorodeoxyglucose-positron emission tomography in adenocarcinomas of the distal esophagus and cardia. World J Surg 27:1035, 2003.
Pandit N, et al: Prognostic value of [18F]FDG-PET imaging in small cell lung cancer. Eur J Nucl Med Mol Imaging 30:78, 2003.
Patz EF Jr, et al: Persistent or recurrent bronchogenic carcinoma: detection with PET and 2-[F-18]-2-deoxy-D-glucose. Radiology 191:379, 1994.
Pieterman R, et al: Visualisation and assessment of the protein synthesis rate of lung cancer using carbon-11 tyrosine and positron emission tomography. Eur J Nucl Med Mol Imaging 29:243, 2002a.
Pieterman RM, et al: Comparison of (11)C-choline and (18)F-FDG PET in primary diagnosis and staging of patients with thoracic cancer. J Nucl Med 43:167, 2002b.
Rankin SC, et al: Computed tomography and positron emission tomography in the pre-operative staging of oesophageal carcinoma. Clin Radiol 53:659, 1998.
Rasanen JV, et al: Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol 10:954, 2003.
Rice TW: Clinical staging of esophageal carcinoma: CT, EUS, and PET. Chest Surg Clin N Am 10:471, 2000.
Seltzer MA, et al: The impact of PET on the management of lung cancer: the referring physician's perspective. J Nucl Med 43:752, 2002.
Shreve PD, et al: Oncologic diagnosis with 2-[f-18]fluoro-2-deoxy-D-glucose imaging: dual-head coincidence gamma camera versus positron emission tomographic scanner. Radiology 207:431, 1998.
Silvestri GA, et al, for the American College of Chest Physicians: The noninvasive staging of non-small cell lung cancer: the guidelines. Chest 123(1 Suppl):147S, 2003.
Skalski J, Wahl RL, Meyer CR: Comparison of mutual information-based warping accuracy for fusing body CT and PET by 2 methods: CT mapped onto PET emission scan versus CT mapped onto PET transmission scan. J Nucl Med 43:1184, 2002.
Skehan SJ, et al: Imaging features of primary and recurrent esophageal cancer at FDG PET. Radiographics 20:713, 2000.
Stroobants SG, et al: Additional value of whole-body fluorodeoxyglucose positron emission tomography in the detection of distant metastases of non-small-cell lung cancer. Clin Lung Cancer 4:242, 2003.
Sugawara Y, et al: Does the FDG uptake of primary non-small cell lung cancer predict prognosis? a work in progress. Clin Positron Imaging 2:111, 1999.
Thomas CT, et al: A positron-emitting internal marker for identification of normal tissues by positron emission tomography: phantom studies and validation in patients. Mol Imaging Biol 4:79, 2003.
Toloza EM, Harpole L, McCrory DC: Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 123(1 Suppl): 137S, 2003.
Tsushima Y, Aoki J, Endo K: Whether and under what conditions FDG-PET might be cost-effective in evaluating solitary pulmonary nodules depicted on lung cancer screening in Japan. Nippon Igaku Hoshasen Gakkai Zasshi 63:390, 2003.
van Tinteren H, et al: Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 359:1388, 2002.
Verboom P, et al: PLUS study group. Cost-effectiveness of FDG-PET in staging non-small cell lung cancer: the PLUS study. Eur J Nucl Med Mol Imaging 30:1444, 2003.
Vesselle H, et al: In vivo validation of 3 deoxy-3 -[(18)F]fluorothymidine ([(18)F]FLT) as a proliferation imaging tracer in humans: correlation of [(18)F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 8:3315, 2002.
Wahl RL: Anatomolecular imaging with 2-deoxy-2-[18F]fluoro-D-glucose: bench to outpatient center. Mol Imaging Biol 5:456, 2003.
Wahl RL, Buchanan JW (eds): Principles and Practice of Positron Emission Tomography. Philadelphia: Lippincott Williams Wilkins, 2002.
Wahl RL, et al: Staging of mediastinal non-small cell lung cancer with FDG PET, CT, and fusion images: preliminary prospective evaluation. Radiology 191:371, 1994.
Wallace MB, et al: An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg 74:1026, 2002.
Weber WA, et al: Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19:3058, 2001.
Weber WA, et al: Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 21:2651, 2003.
Yeung H, et al: FDG PET in esophageal cancer: incremental value over computed tomography. Clin Positron Imaging 2:255, 1999.
Yoon YC, et al: Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology 227:764, 2003.
Zhao DS, et al: 18F-fluorodeoxyglucose positron emission tomography in small-cell lung cancer. Semin Nucl Med 32:272, 2002.
EAN: 2147483647
Pages: 203