XXII - Trauma to the Esophagus
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXVI - Noninvasive Investigations > Chapter 160 - Mediastinal Tumor Markers
function show_scrollbar() {}
Chapter 160
Mediastinal Tumor Markers
Philip G. Robinson
Introduction to Tumor Markers
The purpose of this chapter is to discuss the tumor markers, particularly the serum ones, of mediastinal tumors. Mediastinal tumors are a diverse group of tumors that share an anatomic location in the thorax. Although Yoneda and colleagues (2001) divide the mediastinum into three compartments based on the lateral chest radiograph: anterior, middle (visceral), and posterior, the boundaries they describe (although they are frequently used radiographically) do not correspond to the anatomic divisions described by Shields (1991) and that were used in this text (see Chapter 154). I will use the latter divisions in this chapter. The anterior mediastinum extends from the posterior sternum to the anterior aspect of the heart and anterior borders of the great vessels as they pass up to the thoracic inlet. The posterior mediastinum extends from the anterior border of the ventral longitudinal ligament of the thoracic vertebral column to the posterior chest wall and includes the paravertebral sulci. The middle mediastinum is the area between the anterior and posterior areas and includes the heart, great vessels, trachea, and esophagus. The tumors and tumor markers discussed in this chapter arise mostly in the anterior and posterior mediastinal compartments (Table 160-1). This chapter discusses the tumor markers of malignant germ cell tumors, thymomas, thymic carcinomas and carcinoids, neurogenic tumors, paragangliomas, and parathyroid tumors.
A tumor marker is a biological property of a neoplasm that indicates its presence. In most instances, tumor markers are not specific enough to establish a diagnosis. They are useful in: (a) supporting a diagnosis, (b) determining response to therapy, (c) detecting a relapse, and (d) screening to detect a neoplasm at an early stage. They may be the expression of new gene products, altered amounts of normal gene products, alterations in chromosomal DNA, or one of many other structural or functional cellular properties. Tumor markers generally fall into one of three categories: (a) tumor product, such as -human chorionic gonadotropin ( -HCG); (b) cytogenetic alteration; and (c) molecular markers, such as an oncogene. Tumor markers are usually hormones, enzymes, intracellular proteins, or cell membrane antigens that can be detected or measured in serum, plasma, urine, or other body fluids. Until recently, the expression of new components by neoplasms has been the easiest way to study differences between normal and neoplastic tissues. The term tumor marker is often taken to mean the differences in antigenic expression between the neoplasm and the normal tissues, but it should not be limited to this definition. For instance, in acute lymphocytic leukemia, the neoplastic cells contain surface proteins that appear in the normal development of lymphocytes, but are inappropriate in the clinical context. The ideal tumor marker would be 100% specific and 100% sensitive. In addition, it would indicate the degree of tumor burden, have prognostic value, be reproducible, and be easily measured in a cost-effective manner. To date, no tumor marker meets these criteria. The tumor marker that most closely approaches the ideal marker is the Bence-Jones protein, either the or light chains of immunoglobulins, which can be found in the urine of some patients with multiple myeloma.
Tumor markers can be detected in serum or urine, or in tissue by immunologic or cytogenetic methods. Serum or urine tumor markers include such markers as -HCG, -fetoprotein (AFP), catecholamines and their degradation products, chromogranin, and parathyroid hormone (PTH). Tissue tumor markers are tissue antigens, such as proteins or hormones, detected by immunologic techniques in either fresh, frozen, or formalin-fixed, paraffin-embedded tissue sections. The technique consists of a primary reaction of an antibody to a tissue antigen and a subsequent reaction to visualize the antibody antigen complex. The two most widely used methods are the peroxidase antiperoxidase immune complex method and the avidin biotin complex technique (Figs. 160-1 and 160-2).
The mediastinal tumors with serum or urine tumor markers are shown in Table 160-2. The tumor markers that are useful in immunohistochemical staining of tissue sections are shown in Table 160-3. For most of the mediastinal tumors, the serum markers are identical to the tissue markers. Thymic carcinoid tumors and thymic small cell carcinomas
P.2426
are extremely rare, and they would be expected to have the same tumor markers as other tumors derived from the foregut Kulchitsky's (enterochromaffin) cells. The tumor markers for mediastinal lymphomas are discussed in Chapter 183.
Table 160-1. Mediastinal Tumors in the Anterior, Middle (Visceral), and Posterior Compartments that May Have Tumor Markers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
Tumor Markers of Malignant Germ Cell Tumors
This section explains the incidence and different types of germ cell tumors and then it discusses germ cell tumor markers. Malignant mediastinal germ cell tumors are uncommon, and their exact incidence is difficult to determine. According to Gonzalez-Crussi (1982), however, the mediastinum is the second most common site after the gonads for malignant germ cell tumors. Germ cell tumors also occur in the sacrococcygeal region, the orbit of the eye, the head (extracranial), the brain, and other sites. Economou and co-workers (1982) reviewed the files of Johns Hopkins Hospital from 1949 to 1980 and the University of California at Los Angeles files from 1955 to 1980 for cases of malignant mediastinal germ cell tumors. They found 28 cases, of which 11 were pure seminomas and 17 were nonseminomatous malignant germ cell tumors. In view of the population and referral bases of these two institutions, this small number of cases indicates that these tumors are extremely uncommon. Moran and Suster (1997a) found 322 cases of primary mediastinal germ cell tumors in the files of the Armed Forces Institute of Pathology, Department of Pulmonary and Mediastinal Pathology, in Washington, DC, and Mount Sinai Medical Center of Greater Miami, Florida, from 1960 to 1994. Two of the patients were women and the remaining 320 were men. The two women had teratomatous lesions with malignant components. The analysis of the tumors yielded 37% seminomas, 16% yolk sac tumors (endodermal sinus tumor), 2% embryonal carcinomas, 2.5% choriocarcinomas, 3% combined nonteratomatous germ cell tumors, and 40% teratomatous tumors. Of the teratomatous tumors, 63% were mature teratomas (benign tumors), 33% were teratomas with additional malignant components, and 4% were immature teratomas. According to Weidner (1999), the literature suggests that mediastinal germ cell tumors are uncommon, but occur in approximately the following distribution: mature cystic teratoma 44%, mixed germ cell tumor 23%, seminomas 18%, immature teratoma 6%, embryonal carcinoma 5%, yolk sac tumor 2%, and choriocarcinoma 2%. The mediastinal seminomas develop mostly in men (97%) and rarely in women.
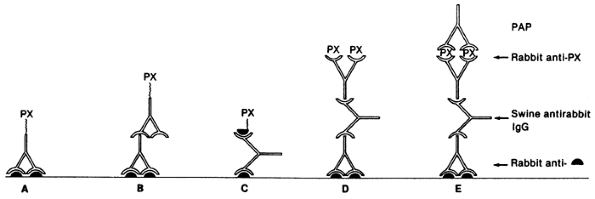 |
Fig. 160-1. Immunoperoxidase procedures. A. Peroxidase (PX) antibody conjugate, direct. B. Peroxidase antibody conjugate, indirect. C. Labeled antigen method. D. Enzyme bridge procedure. E. Peroxidase-antiperoxidase (PAP) immune complex method. Solid semicircle indicates antigen. From Falini B, Taylor CR: New developments in immunoperoxidase techniques and their application. Arch Pathol Lab Med 107:105, 1983. With permission. |
Benign and malignant germ cell tumors are postulated to occur in the mediastinum either because some of the germ cells did not migrate properly to the genital ridges or possibly because a focus of pluripotential embryonic cells escaped the influence of their primary organizer during embryonic
P.2427
development. Another hypothesis is that they represent metastatic testicular lesions and the primary lesion has regressed. Benign and malignant germ cell tumors occur in both men and women, usually in the first three decades of life. The lesions are located in the anterior compartment of the mediastinum. Lewis and colleagues (1983) found that benign germ cell tumors (teratomas) of the mediastinum occur with approximately equal frequency in men (44%) and women (56%). In contrast, Knapp and co-workers (1985) found that malignant germ cell tumors occur at approximately the same age as the benign ones, but they occur more often in men (86%) than in women (14%). Nichols and colleagues (1987) demonstrated that nonseminomatous malignant germ cell tumors occurred with a high frequency in patients with Klinefelter's syndrome. Nichols and associates (1985) also pointed out the high occurrence of acute megakaryocytic leukemia in men with malignant mediastinal germ cell tumors. DeBlois (1995) noted the association with other hematologic malignancies, such as acute nonlymphocytic leukemia, refractory thrombocytopenia, hemophagocytic syndrome, malignant histiocytosis, and systemic mastocytosis.
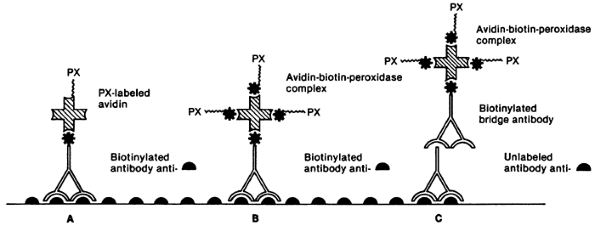 |
Fig. 160-2. Biotin-avidin immunoenzymatic techniques. A. Biotinylated primary antibody method. B. Biotinylated peroxidase method. C. Avidin-biotin-peroxidase complex method. Solid semicircle indicates antigen; PX, peroxidase; *, biotin; shaded open cross, avidin. From Falini B, Taylor CR: New developments in immunoperoxidase techniques and their application. Arch Pathol Lab Med 107:105, 1983. With permission. |
In the past, the testicular germ cell tumor classification was used to classify the mediastinal germ cell tumors. The World Health Organization (WHO) classification by Mostofi and Sobin (1977) is probably the most widely used in the United States; however, other schemes, reported by Dixon and Moore (1953), Mostofi and Price (1973), and the British Testicular Germ Cell Tumor Classification (1976) by Pugh and Cameron, are in use. More recently, Moran and Suster (1997a) proposed a classification specifically for mediastinal germ cell tumors. These various classification schemes are compared in Table 160-4. Occasionally, the term teratocarcinoma is used to describe a teratoma with a focus of embryonal carcinoma, but it also has been applied to primary carcinomas arising in teratomas. The term should not be used in this manner, rather the components of germ cell tumor should be named.
Table 160-2. Serum or Urine Markers of Mediastinal Tumors | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Klein (1993) reviewed the tumor markers associated with germ cell tumors and they are (a) AFP (embryonal carcinoma and yolk sac tumor or mixed tumors); (b) placental alkaline phosphatase (PLAP) (seminoma, yolk sac tumor, and embryonal carcinoma); and (c) -HCG (choriocarcinoma). Table 160-5 shows the association between the tumor markers and these tumors. Klein (1993) noted that -HCG also was present in 80% of patients with embryonal carcinoma and in 10% to 25% of patients with pure seminoma. Other serum markers include, lactic acid dehydrogenase
P.2428
P.2429
(LDH), teratocarcinoma mucinlike antigen (TRA-1 60), and CD30.
Table 160-3. Immunohistochemical Staining Markers of Mediastinal Tumors | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Table 160-4. Histologic Classifications of Mediastinal and Testicular Germ Cell Tumors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 160-5. Association of Tumor Markers and Germ Cell Tumors | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
-Fetoprotein
Abelev and co-workers (1963) first described AFP as a serum tumor marker in mouse hepatomas. AFP is a single-chain, oncofetal glycoprotein with a molecular weight of approximately 70,000 daltons. It is synthesized by the liver, yolk sac, and gastrointestinal tract of the fetus. By 1 year of age, AFP decreases to adult levels. It can be elevated in patients with liver disease, especially with liver cell regeneration and with neoplasms such as hepatocellular carcinoma, germ cell tumors, and various other carcinomas. AFP is not present in either pure seminomas or choriocarcinomas. Therefore, its detection in either of these latter two tumors always implies the presence of embryonal or yolk sac elements.
Since the discovery that AFP is present in embryonal carcinoma and yolk sac tumors, many workers have studied its significance. Scardino and co-workers (1977) pointed out the value of AFP in the staging and prognosis of patients with these two types of malignant germ cell tumors of the testis. Kesler and associates (1999) found an elevated AFP level in nonseminomatous germ cell tumors of greater than 1,000 ng/mL after chemotherapy to be a negative predictor of survival. Wright and colleagues (1990) emphasized the association of normalization of serum tumor markers after chemotherapy for malignant germ cell tumor (embryonal carcinoma or yolk sac tumor) with a favorable prognosis (Fig. 160-3). Talerman (1980) and Perlin (1976) and their colleagues have pointed out the usefulness of AFP for following the activity of yolk sac tumors and embryonal carcinomas after they have been treated. AFP levels (and usually a tissue biopsy) are essential in evaluating young adult men with anterior mediastinal masses (Fig. 160-4). The biopsy might show only a teratoma, but an elevated AFP level would indicate that more poorly differentiated elements (yolk sac tumor or embryonal carcinoma) also are present.
Kurman and co-workers (1977) reported the use of immunohistochemical techniques to localize AFP in yolk sac tumors and embryonal carcinomas. They demonstrated that AFP was present in stained tissue sections. AFP is a useful marker for the pathologist in differentiating the various types of malignant germ cell tumors and for the clinician in following the course of a patient's disease. Moran and associates (1997b) immunostained 17 mediastinal yolk sac tumors, three embryonal carcinomas, two combined germ cell tumors containing embryonal carcinoma and yolk sac tumor, and one combined germ cell tumor containing yolk sac tumor and seminoma. The embryonal carcinomas and the yolk sac tumors both stained with low-molecular-weight cytokeratin, but the yolk sac tumors were more focal. Immunostains for AFP were positive in 12 of 17 yolk sac tumors and negative in the three cases of embryonal carcinoma. The researchers thought the negative AFP staining of the embryonal carcinomas was secondary to poor preservation of the tissue. One of the cases of embryonal carcinoma showed strong membrane staining for CD30.
-Human Chorionic Gonadotropin
Human chorionic gonadotropin is a glycoprotein with a molecular weight of approximately 38,000 daltons. It is composed of two dissimilar polypeptide chains, which are designated and . The chain is identical to the polypeptide chains of follicle-stimulating hormone, luteinizing hormone, and thyroid-stimulating hormone, whereas the chain is unique to the human chorionic gonadotropin. -HCG is produced by the syncytiotrophoblasts of the normal placenta. It is thought to have three functions: (a) maintenance of the corpus luteum during the first few weeks of pregnancy, (b) promotion of steroidogenesis of the fetal-placental unit, and (c) stimulation of the secretion of testosterone from the fetal testicle. The hormone is used as a serum marker for pregnancy and trophoblastic disease. However, it may be found in association with other tumors. In 1973, Braunstein and co-workers measured serum HCG levels in 828 patients with nongerm cell tumors and found that only 60 patients had detectable levels of HCG. Nonetheless, the determination of -HCG as a tumor marker in nonseminomatous germ cell tumors is of great value. Perlin and co-workers (1976) pointed out the value of monitoring -HCG to detect early tumor recurrence. Scardino and colleagues (1977) also similarly found that -HCG was a valuable marker in detecting subclinical tumor
P.2430
recurrences. At a National Institutes of Health conference in 1979 moderated by Anderson, Waldmann pointed out that not only was -HCG valuable in detecting tumor recurrences, but it was also valuable in measuring the effectiveness of the therapy the patient received. As previously mentioned, Wright and colleagues (1990) emphasized the association of normalization of serum tumor markers after chemotherapy for a nonseminomatous germ cell tumor of the mediastinum with a favorable prognosis.
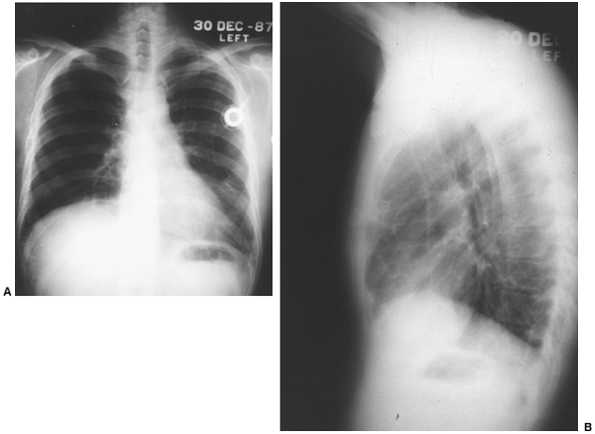 |
Fig. 160-3. A, B. Posteroanterior and lateral chest radiographs of patient after completion of multimodality therapy. Initial chemotherapy resulted in marked reduction in size of tumor and a decrease of the -fetoprotein levels to normal; subsequent resection of residual necrotic tumor was carried out. Patient remains well 2 years after therapy. |
The literature on -HCG levels in mediastinal choriocarcinomas is limited. Knapp and colleagues (1985) described seven patients with anterior mediastinal malignant germ cell tumors who had either pure choriocarcinomas or choriocarcinomas mixed with other malignant germ cell elements. The five patients who had urine pregnancy tests were positive for -HCG. In two other patients, the authors followed the serum -HCG; in these two patients the levels of -HCG increased as the disease progressed, and with a satisfactory therapeutic response, the level was seen to decrease. In a patient with a suspected anterior mediastinal malignant germ cell tumor, a serum -HCG level is essential in determining whether choriocarcinoma is a component of the germ cell tumor. Moran and Suster (1997b) reported a series of eight pure mediastinal choriocarcinomas. The patients were all men and ranged in age from 21 to 63 years. All died within 2 months of diagnosis, emphasizing the poor prognosis of this disease.
Immunohistochemical staining of choriocarcinomas for -HCG is also a valuable diagnostic test. Kurman and co-workers (1977) identified -HCG in the syncytiotrophoblastic cells found in choriocarcinomas and in the syncytiotrophoblastic giant cell that can be seen in embryonal carcinoma and rarely in seminomas or yolk sac tumors. Niehans and co-workers (1988) reported that no major differences existed in
P.2431
immunohistochemical staining of gonadal and extragonadal malignant germ cell tumors. Moran and associates (1997b) immunostained six mediastinal choriocarcinomas. The -HCG immunostained only the multinucleated syncytium-like giant cells. All the tumors stained for low-molecular-weight cytokeratin.
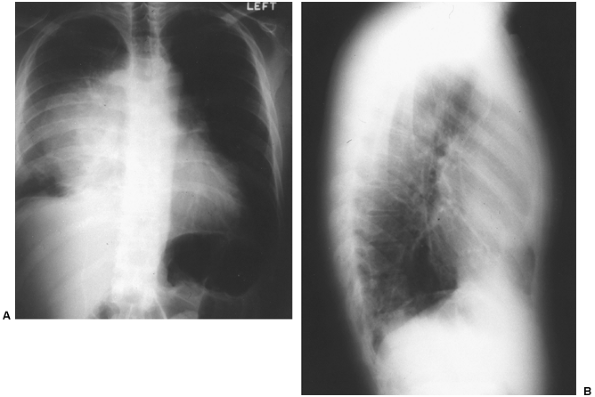 |
Fig. 160-4. A, B. Posteroanterior and lateral radiographs of the chest of a young adult man with a clinically malignant anterior mediastinal tumor. -Fetoprotein levels are significantly elevated. Tissue biopsy confirmed the diagnosis of a malignant nonseminomatous germ cell tumor. |
Placental Alkaline Phosphatase
Alkaline phosphatase is a group of four isoenzymes that come from the liver, bone, placenta, or intestine. The phosphatases are hydrolases of low specificity. These enzymes have a molecular weight of approximately 120,000 daltons and function to catalyze the transfer of a phosphate group from a donor compound to an acceptor compound that contains a hydroxyl group. The placental isoenzyme of alkaline phosphatase is produced by placental syncytiotrophoblast cells by the twelfth week of pregnancy; it is produced also by various malignant tumors, including seminomas.
Lange and co-workers (1982) studied serum levels of PLAP in patients with seminomas and nonseminomatous malignant germ cell tumors. They concluded that PLAP was a clinically useful serum marker in patients with seminoma. The International Union Against Cancer Workshop on Immunodiagnosis (1986) noted that PLAP is also a useful serum and immunohistochemical marker in the management of seminomas. Tonik (1983) and Maslow (1983) and their co-workers, however, demonstrated that PLAP also could be elevated in patients who smoke cigarettes. Tucker and co-workers (1985) concurred with this finding and suggested that PLAP measurements might not be as useful in following patients who have seminomas and who also smoke.
Nielsen and colleagues (1990) evaluated PLAP as a serum tumor marker in patients with seminomas who were smokers and nonsmokers. After careful evaluation, they concluded that PLAP was not a useful stand-alone marker for seminoma and it contributed little to the follow-up of these patients. Subsequently, reports have appeared that suggest PLAP may be of some value in patients with seminomas. Nielsen (1990) and Munro (1991) and their co-workers evaluated the combined use of PLAP, LDH, and -HCG as follow-up tumor markers in patients with seminomas. They found that if either the -HCG was greater than 6 U/L-1 or the LDH was greater than 400 U/L-1 and the PLAP was
P.2432
greater than 60 U/L-1, this combination would detect approximately 50% of patients with disease. Koshida and colleagues (1996) found PLAP to be elevated at the time of initial diagnosis in 50% of patients with seminomas and nonseminomatous germ cell tumors. They concluded PLAP might be useful in monitoring these patients. Weissbach and associates (1997) used HCG, LDH, and PLAP to monitor seminomas, before and after orchiectomy, and in follow-up. They concluded that all three markers should be determined in patients with seminomas. PLAP was most likely to be elevated, and it was the most sensitive in detecting metastatic disease. In summary, the most recent reports suggest PLAP in conjunction with other markers may be of value in monitoring seminomas.
Burke and Mostofi (1988) detected PLAP immunohistochemically in the tissue of other malignant germ cell tumors. These authors have published the largest series of benign and malignant germ cell tumors that were immunohistochemically stained with PLAP. They found that 96% of seminomas stained positively for PLAP, but they also found that other benign and malignant germ cell tumors stained with PLAP in varying percentages. The percentage of the other germ cell tumors that stained with PLAP were as follows: embryonal carcinoma 96%, choriocarcinoma 45%, syncytiotrophoblast 43%, yolk sac tumor 25%, mature teratoma 5%, and immature teratoma 4%. The study of Niehans and co-workers (1988) agreed with the results of Burke and Mostofi (1988). The former researchers also noted that extragonadal malignant germ cell tumors did not immunostain significantly differently from those of the gonads. Moran and associates (1997c) immunostained 50 mediastinal seminomas for PLAP and found 80% of the tumors to be strongly positive. The low-molecular-weight cytokeratin CAM 5.2 was positive in 75% of the cases and had focal dot-like positivity. The broad-spectrum cytokeratin was positive in 70% of the cases. In approximately 5% of the cases, HCG was present in individual seminoma cells. No immunostaining was observed for AFP, epithelial membrane antigen, or carcinoembryonic antigen.
Lactic Acid Dehydrogenase
Lactic acid dehydrogenase is an enzyme that helps with the oxidation of lactic acid to pyruvic acid. It has a molecular weight of 138,000 daltons and is composed of four isoenzymes. According to Klein (1993), 80% of patients with advanced malignant germ cell tumors have elevated levels of LDH. The elevation is nonspecific and is seen with all types of malignant germ cell tumors. It is proportional to the amount of tumor present. LDH may be useful as an adjunct tumor marker in monitoring the progression of malignant germ cell tumors. von Eyben and colleagues (2001) found that patients with metastatic testicular germ cell tumor who had an elevated level of isoenzyme 1 had a poorer prognosis than other patients.
Teratocarcinoma Mucinlike Antigen
Teratocarcinoma mucinlike antigen is a new tumor marker for embryonal carcinoma or germ cell tumors with a component of embryonal carcinoma. TRA-1 60 is an antibody that recognizes a high-molecular-weight glycoprotein on the surface of embryonal carcinoma progenitor cells. In 15 patients with embryonal carcinoma in the primary tumor, Marrink and associates (1991) found that TRA-1 60 was elevated more often (76% of cases) than AFP (57% of cases) and in some instances it was the only marker elevated. Gels and colleagues (1997) studied 42 patients with stage I nonseminomatous testicular germ cell tumors. They found the TRA-1 60 level to be elevated in 21 of 42 patients 1 month before recurrence of the tumor. Half of the 21 patients did not have detectable levels of AFP or -HCG. Lajer and colleagues (2002) used TRA-1 60 as a tumor marker in germ cell tumors. They found that TRA-1 60 did not normalize after chemotherapy and concluded that it was not a useful marker.
CD30
CD30 was initially described as a diagnostic marker for Hodgkin's disease and anaplastic lymphoma. Latza and associates (1995) detected soluble CD30 antigen in the sera of eight of eight patients with embryonal carcinoma. They did not detect it in the sera of 8 of 10 patients with other testicular germ cell tumors. They concluded serum levels of CD30 could be a useful test for monitoring patients with embryonal carcinoma. To date, the marker has not gained widespread use.
In 1994, Ferreiro, using immunohistochemical techniques, detected the antigen in 88% of embryonal carcinomas and in 83% of embryonal carcinoma elements in mixed malignant germ cell tumors. None of the seminomatous, yolk sac tumor, or teratomatous components expressed CD30. Suster and colleagues (1998) using immunohistochemistry found CD30 in many embryonal carcinomas, but also in some yolk sac tumors and seminomas. Both reports concluded that CD30 would be a helpful immunohistochemical marker for embryonal carcinoma.
Cytogenetic Markers
In 1983, Atkin and Baker performed karyotypes on 10 seminomas, one combined seminoma-teratoma, and one malignant teratoma. In four of the seminomas, they identified a duplication of the short arm of chromosome 12, which is known as i(12p). Samaniego and colleagues (1990) performed karyotypes on 24 germ cell tumors of all types and found the i(12p) chromosome in 90% of the tumors. They concluded that i(12p) was a highly nonrandom chromosome marker for all types of germ cell tumors. Summersgill and associates (2001)
P.2433
reiterated that i(12p) and the gain of 12p material have been consistently found in germ cell tumors.
Molecular Markers
Alterations in oncogenes and tumor suppressors may play a role in germ cell tumors. Klein (1993) described several, but none of them are specific or consistent enough to serve as useful tumor markers. Schrader and associates (2001) described several molecular markers, such as ribonucleoprotein telomerase and i(12p). They believed that eventually these markers would lead to new classifications and therapies.
Summary of Tumor Markers for Germ Cell Tumors
According to Sturgeon (2002), in the United States, the most important serum markers for germ cell tumors are AFP, HCG, and LDH. In Europe, the European Group on Tumor Markers also recommends serum PLAP because it is elevated in 80% of seminomas. The first three tumor markers are important to measure because pretreatment levels classify metastatic germ cell tumors as having a good, intermediate, or poor prognosis. Posttreatment levels help in assessing the response to chemotherapy. Finally, studies by the International Germ Cell Cancer Collaborative Group confirmed the prognostic value of AFP, HCG, and LDH. These markers are now included in the staging systems of the American Joint Committee on Cancer and the Union International Contre le Cancer. In conclusion, any patient with a suspected germ cell tumor must have serum levels of AFP, HCG, and LDH assessed.
Table 160-6. Staging of Thymomas | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Thymoma and Thymic Carcinoma
The thymus is located in the anterior mediastinum, and its major function is the development and maintenance of cell-mediated immune reactions. It is composed of epithelial cells derived from the endoderm and the ectoderm of the third branchial cleft, and possibly a small part of the fourth branchial cleft, as well as lymphocytes that migrate into the gland.
The most common tumor of the thymus is a thymoma. According to Rosai and Levine (1976), a thymoma should be defined as a histologically benign-appearing neoplasm of the thymic epithelial cells, regardless of the presence or absence of a lymphoid component. The thymoma may be either noninvasive or invasive and can be divided into four stages (Table 160-6) (see Chapter 173). Suster and Rosai (1991) and Shimosato and Mukai (1997) reviewed the classification of thymomas and discussed the thymic carcinoma. The new WHO classification of thymic tumors (A, AB, B1, B2, B3, and C) is discussed in detail in Chapter 173. In contrast to the noninvasive and invasive thymomas, thymic carcinomas are characterized by cytologically malignant epithelial cells. Thymic carcinomas include such lesions as poorly differentiated carcinoma, anaplastic carcinoma, squamous cell carcinoma, lymphoepithelioma-like carcinoma, sarcomatoid carcinoma (carcinosarcoma), clear cell carcinoma, basaloid carcinoma, mucoepidermoid carcinoma, and small cell carcinoma, although the latter tumor is more properly classified as a neuroendocrine tumor.
The thymus produces several hormones. Goldstein and co-workers reviewed the thymic hormones in 1981. The best known ones are thymosin 1, thymic humoral factor, serum thymic factor, thymopoietin, and thymic fraction 5. In neither Gray and Gutowski's (1979) nor Verley and Hollmann's (1985) review of thymomas were thymic hormones used as serum tumor markers.
P.2434
In contrast, many immunohistochemical studies of thymomas have identified a variety of markers. The immunohistochemical markers can be divided into those that react with the thymic epithelial cells and those that react with the lymphocytes. Battifora and colleagues (1980) demonstrated that thymic epithelial cells stain with antibodies to cytokeratin. This reaction is helpful in separating thymomas from lymphomas and seminomas. Kornstein and co-workers (1988) demonstrated that epithelial membrane antigen was present in 37 of 48 thymomas on immunohistochemical staining. These plus additional markers are valuable in differentiating thymomas from other lesions in the anterior mediastinum (Table 160-7). In addition to cytokeratin and epithelial membrane antigen, Hirokawa and colleagues (1988) showed that a high percentage of thymic epithelial cells of thymomas also stained fairly consistently with thymosin 1 and thymosin 3.
Among all the immunohistochemical stains, cytokeratin is probably the most helpful in differentiating a thymoma from a lymphoma. The lymphocytes present in a thymoma are not considered neoplastic. Rosai (1987) emphasized that thymic lymphocytes have the phenotype of T cells. The thymic lymphocytes show an entire range of differentiation from a prothymocyte to peripheral T cell. At present, the phenotyping of lymphocytes in a thymoma does not appear to have any diagnostic or prognostic significance.
Thymic carcinomas are immunohistochemically positive for CD5 on a fairly consistent basis. Hishima and co-workers (1994) described the immunostaining with CD5 of thymic carcinomas, atypical thymomas (B3 with features intermediate between invasive thymoma and thymic carcinoma), and thymomas. All seven of the thymic carcinomas and two of the five atypical thymomas expressed CD5, but none of the 11 typical thymomas did. They did not observe CD5 expression in non small cell carcinomas of the lung (NSCLC), breast, esophagus, stomach, colon, and cervix. Subsequently, Dorfman and associates (1997) immunostained a variety of thymic neoplasms with CD5. Most of the atypical thymomas (type B3) and thymic carcinomas (type C), but not the small cell carcinomas or some of the lymphoepitheliallike thymic carcinomas, were positive for CD5. Kornstein and Rosai (1998) questioned the validity of the CD5-positive results. They performed immunostaining with two clones of antibody to CD5. One clone stained 30% of the thymic carcinomas and no other carcinomas, whereas the other clone stained 62% of the thymic carcinomas as well as several other types of malignant neoplasms. More recently, Pomplun and associates (2002) showed that CD5 stained three of six thymic carcinomas. They concluded that it might be useful in conjunction with other markers in separating thymic from pulmonary neoplasms.
In summary, no serum marker exists for thymomas or thymic carcinoma. Patients with myasthenia gravis may have thymomas. These patients can have antititin antibodies and acetylcholine receptor autoantibodies, but they do not correlate with the presence of a thymoma. With regard to tissue immunohistochemical markers, keratins are useful in identifying thymomas.
Table 160-7. Immunohistologic Findings in Thymoma and Its Differential Diagnostic Alternatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Introduction to Mediastinal Neuroendocrine Tumors and Their Serum Markers
In addition to thymic and germ cell neoplasms, the mediastinum is host to a variety of neuroendocrine tumors that have specific and nonspecific markers. The neuroendocrine family of tumors includes neural, neuroendocrine, and endocrine tumors. In the review of mediastinal tumors by Hoffman and colleagues (1993), these tumors include: (a) neural tumors (neuroblastomas, ganglioneuroblastomas, and paragangliomas), (b) neuroendocrine tumors (parathyroid tumors and thymic carcinoids), and (c) endocrine tumors (thyroid lesions). The specific serum markers for these tumors are the hormones they produce. The nonspecific markers indicate whether the tissue is of neural or neuroendocrine differentiation. These nonspecific serum markers include chromogranin and neuron-specific enolase (NSE). After the
P.2435
neoplasms have been resected, immunohistochemical stains can be performed for these same serum markers. The most useful immunohistochemical markers are chromogranin and synaptophysin. The neural tumors, such as pheochromocytoma, do not stain with cytokeratin, but the neuroendocrine ones, such as carcinoids, do. Table 160-8 shows the association between these tumors and their markers.
Thymic Carcinoid Tumors and Small Cell Carcinomas
Carcinoid tumors, atypical carcinoids, and small cell carcinomas, all of which are extremely rare, are considered together, because as Rosai and co-workers (1976) as well as Shimosato and Mukai (1997) emphasized, all these neoplasms represent different degrees of differentiation of tumors derived from Kulchitsky's cells, which have also been called enterochromaffin, argentaffin, argyrophil, or basal cells. These cells are neuroendocrine cells that are distributed throughout the body and are especially present in those organs derived from the primitive digestive system.
Pearse (1974) investigated this group of dispersed neuroendocrine cells. He renamed these cells the amine precursor uptake and decarboxylation (APUD) system, and he provided evidence that these cells were of neural origin, hence the term neuroendocrine. Erlandson and Nesland (1994) reviewed the subject of the APUD system. Through embryologic experiments, it became apparent that only a portion of the cells that make up the APUD system are derived from the neural crest. Pearse (1974) eventually divided the APUD cells into the following three groups: (a) cells derived from the neural crest, such as C cells, ganglion cells, and melanocytes; (b) cells derived from the neuroectoderm, such as the pituitary; and (c) cells derived from the neuroendocrine programmed epiblast, such as gastroenteropancreatic and bronchopulmonary endocrine cells and placental endocrine cells.
The following section discusses the general properties of thymic carcinoids and specific neuroendocrine tumor markers. Then, the chapter discusses thymic small cell carcinoma and small cell carcinoma tumor markers.
Table 160-8. Association of Tumor Markers and Other Mediastinal Tumors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Thymic Carcinoids
Thymic carcinoids are uncommon tumors, with most being atypical carcinoids. Atypical carcinoids, in contrast to the usual carcinoids, have a higher degree of cytologic anaplasia, smaller cells, and areas of necrosis. In the series of thymic carcinoids described by de Montpr ville and associates (1996) all 14 were atypical carcinoids. Wick and colleagues (1980, 1982) initially reviewed seven cases and later 15 cases of carcinoids of the thymus and noted their ectopic production of adrenocorticotropic hormone (ACTH) in approximately 50% of the cases. Klemm and Moran (1999) reviewed the topic of thymic carcinoids and proposed a new classification. They found that these tumors account for less than 5% of anterior mediastinal tumors, and most (82%) of these tumors behave in an aggressive fashion. Wick and Rosai (1991) also reviewed the subject of mediastinal neuroendocrine neoplasms. They concluded that thymic carcinoids are: (a) capable of producing ectopic hormones, particularly ACTH, which resulted in Cushing's syndrome; (b) associated with multiple endocrine neoplasia type I; and (c) are generally aggressive. Carcinoid tumors also are known to produce various peptides, such as gastrin, calcitonin, ACTH, antidiuretic hormone, and other peptides.
Serotonin, commonly produced by the carcinoids of the gastrointestinal tract and ovary, is only rarely produced by carcinoids of the lung or thymus. According to Wick and Scheithauer (1984), foregut carcinoids, such as a thymic carcinoid, lack the enzyme aromatic amino acid decarboxylase, and they cannot synthesize serotonin (5-hydroxytryptamine). Thus, the carcinoid syndrome of diarrhea or facial flushing is not seen in patients with thymic carcinoids. This inability to synthesize serotonin means that the breakdown product of serotonin, 5-hydroxyindoleacetic acid, is not excreted in the urine, and serotonin or its by-products cannot be used as serum markers in patients with thymic carcinoids.
Adrenocorticotropic Hormone
The most useful marker is ACTH, which is produced in excessive quantities in 30% to 35% of patients with these
P.2436
tumors. The increase in ACTH results in Cushing's syndrome. With regard to the immunohistochemical markers, tumors producing ACTH would stain for that hormone. Moran and Suster (2000) immunostained six cases of thymic neuroendocrine carcinoma. All cases stained with CAM 5.2, a low-molecular-weight cytokeratin. Four cases stained with synaptophysin and chromogranin and three stained for Leu-7.
Neuron-Specific Enolase
Enolase is one of the enzymes involved in glycolysis. It is an isoenzyme composed of two combined subunits, of which there are three known subunit chains: , , and . Neuron-specific enolase (NSE) is composed of two subunits and is found in neurons. Tapia and colleagues (1981) showed that NSE was produced by neuroendocrine tumors. Wick and associates (1983) immunostained thymic carcinoids, and all were positive for NSE. de Montpr ville and colleagues (1996) reported 14 thymic carcinoids, all of which immunostained for NSE. As NSE became more widely used, it was found to be in a variety of tissues apart from neural ones and consequently is not as specific as one would like. Haimoto and colleagues (1985) found that NSE could be immunohistochemically localized to a variety of tissues other than nervous and neuroendocrine ones. They found it expressed by smooth muscle cells, epithelial cells of the loop of Henle, and a variety of hematopoietic cells.
Baudin and colleagues (1998) discussed the use of serum NSE as a marker of neuroendocrine tumors. They found the serum NSE to be elevated in 27 of 59 (46%) of foregut carcinoids. They did not evaluate any thymic carcinoids. They found that serum NSE was not as sensitive as serum chromogranin A and that elevated NSE levels were associated with poor tumor differentiation rather than tumor burden. They concluded that serum chromogranin A was more sensitive than NSE and that it correlated with tumor burden.
Serum NSE has been used by Ariyoshi (1983), Akoun (1985), and Carney (1982) and their co-workers as a serum marker for small cell lung carcinoma. In view of these studies, NSE could probably be used as both a serum marker and an immunohistochemical tissue marker for thymic carcinoids. NSE is potentially a useful marker, but reports have questioned its specificity as a neuroendocrine marker. Pinson and co-workers (1997) evaluated the use of serum NSE in small cell lung cancers. They concluded that the serum levels were of doubtful usefulness in the diagnosis and follow-up of small cell lung cancers.
Chromogranin
Chromogranin belongs to a family of acidic proteins. Three major chromogranin proteins have been identified, which have been designated A, B, and C with respective molecular weights of 75,000, 100,000, and 86,000 daltons. These soluble proteins were first isolated from the catecholamine-containing vesicles of the bovine adrenal medulla. The function of the chromogranins in the secretory granules of the neuroendocrine tissues is not completely known. Hagn and co-workers (1986) demonstrated chromogranin in the adrenal medulla and other endocrine tissues. Lloyd and associates (1988) also showed the distribution of chromogranin in normal endocrine tissue, and in addition demonstrated its presence in two pulmonary carcinoid tumors. Chromogranin is considered the most specific of the general markers, but it is not very sensitive. The intensity of its expression depends on the number of secretory granules in the cells. For instance, small cell carcinomas, which have few cytoplasmic neuroendocrine granules, do not stain at all or only slightly with chromogranin.
Herbert (1987) and Kornstein (1988) and their associates identified chromogranin in a number of thymic carcinoids (see Chapter 173). de Montpr ville and associates (1996) reported 8 of 14 thymic carcinoids that immunostained for chromogranin.
O'Connor and Deftos (1986) found elevated levels of chromogranin A in the serum of patients with a variety of endocrine tumors. Serum levels of chromogranin A are potentially helpful in managing patients with endocrine tumors. Nobels and colleagues (1997) found serum levels of chromogranin A to be elevated in 50% of patients with neuroendocrine tumors. It was most frequently elevated in patients with gastrinomas, pheochromocytomas, and carcinoid tumors. More recently, Seregni (2001) and Baudin (1998) and their colleagues concluded that chromogranin A is a better serum marker for following and for screening patients with neuroendocrine tumors, such as carcinoids and pheochromocytomas, than is NSE.
Synaptophysin
Synaptophysin is another general neuroendocrine marker. It is a glycosylated transmembrane protein with a molecular weight of approximately 38,000 daltons and is an integral membrane protein. It was isolated from the presynaptic vesicles of neurons. Wiedenmann and Franke (1985) demonstrated the protein by immunofluorescent microscopy and biochemical techniques in the neurons and paraganglia of a variety of mammalian species. It is considered to be a highly sensitive and a fairly specific neuroendocrine marker. Gould (1987) and Lee (1987) and their associates, as well as Miettinen (1987), have all demonstrated that synaptophysin is present in pulmonary carcinoids. Synaptophysin has not been demonstrated in thymic carcinoids, but in all probability it would be present. It does not yet appear to have been used as a serum marker for neuroendocrine tumors in the way that chromogranin and NSE have been used.
P.2437
Bombesin
Bombesin is a marker for neuroendocrine tumors, such as a thymic carcinoid. Bombesin is a 14 amino acid peptide that was originally isolated from the skin of the frog Bombina bombina. Its mammalian analogue appears to be gastrin-releasing peptide. Bombesin's wide range of actions include a constrictor effect on bronchiolar smooth muscle. Moody and associates (1981) first reported that small cell lung carcinomas contain high levels of bombesin. Said (1985) and Bostwick (1984) and their associates have demonstrated that pulmonary carcinoids stain immunohistochemically for bombesin. Bombesin has not been demonstrated in thymic carcinoid tumors, but it is most likely present. Bombesin does not appear to have been used as a serum marker for neuroendocrine neoplasms.
Leu-7 (CD57)
Leu-7 is an antibody that reacts with cell surface antigens of natural killer lymphocytes and by chance also reacts with neuroendocrine cells. Tischler and co-workers (1986) described the reaction of this antibody with the cells of the adrenal medulla, most pheochromocytomas, and smaller percentages of pancreatic islet cells, anterior pituitary cells, and normal enteric endocrine cells. It is possible that with further testing this antibody might be a useful marker for foregut carcinoid tumors. Wick (2000) reviewed neuroendocrine markers and noted that because of the imperfect specificity of Leu-7, it could not be used as a stand-alone marker.
Other Neuroendocrine Markers
Capella and associates (1994), in a review of neuroendocrine tumors, mentioned protein gene product 9.5 and 7B2 as general neuroendocrine markers. Protein gene product 9.5 is a soluble protein originally extracted from brain tissue. Ermisch and Schwechheimer (1995) found that protein gene product 9.5 was a sensitive marker for tumors with neuronal and neuroendocrine differentiation. The 7B2 marker is a 179 amino acid protein extracted from the pig and human pituitary. It is localized to several tissues, including the pituitary, thyroid, and endocrine pancreas. Its value is still being investigated. Neither has been used as a serum marker. They have both been studied immunohistochemically in neoplasms.
Another general neuroendocrine marker is the family of neuroendocrine-specific protein (NSP) reticulon. These are endoplasmic reticulum associated protein complexes. Senden and colleagues (1997) studied two members of this family, NSP-A and NSP-C. They found that pulmonary carcinoids, some atypical carcinoids, and small cell lung cancers immunostained with these markers. They concluded that these two markers were valuable in determining neuroendocrine differentiation.
Small Cell Carcinoma of the Thymus
Small cell carcinoma of the thymus is exceedingly rare. According to Rosai and associates (1976), a small cell carcinoma represents the poorly differentiated end of the spectrum of neuroendocrine neoplasms. They reported four cases of thymic small cell carcinomas. Duguid and Kennedy (1930) reported two cases of thymic small cell carcinomas. In all six cases, autopsies were performed and primary pulmonary neoplasms were not found. Wick and Scheithauer (1982) reported a case of thymic small cell carcinoma, but they did not have autopsy confirmation of its being a thymic primary lesion. The tumor markers for pulmonary small cell carcinomas are similar to bronchial carcinoid tumors and include the hormones calcitonin, ACTH, gastrin, somatostatin, and other peptides, as well as neuroendocrine markers, such as NSE, chromogranins, synaptophysin, and bombesin.
Akoun (1985), Carney (1982), and Ariyoshi (1983) and their associates have demonstrated that NSE can be used as a serum marker for small cell carcinoma of the lung. This observation has not been made in thymic small cell carcinomas, but it would probably also apply to them. In 1996, Jorgensen and associates found that pretreatment levels of NSE were a prognostic factor in small cell lung cancer, with higher levels associated with a poorer prognosis. They also found the stage of the disease and the patient's performance status to be prognostic factors. Subsequently, Fizazi and co-workers (1998) investigated the serum NSE on day 28 following chemotherapy. They found the serum NSE to be an independent factor for complete response and survival. They also pointed out that if the serum NSE did not normalize on day 28, other regimens could be instituted. Another marker for small cell lung carcinoma is pro gastrin-releasing peptide (ProGRP). Shibayama and colleagues (2001) found serum ProGRP to be more sensitive for the diagnosis of small cell carcinoma than serum NSE. Although these descriptions are for lung small cell carcinomas, they probably may apply to thymic small cell carcinomas.
Sobol and associates (1986) and O'Connor and Deftos (1986) measured serum chromogranin A levels and found them elevated in small cell carcinomas of the lungs. Giovanella and colleagues (2001) found that serum chromogranin A had a better diagnostic sensitivity than NSE in small cell lung carcinoma (61% versus 57%), but serum NSE reflected the extent of the disease more accurately. These results would also probably apply to thymic small cell carcinomas.
Wick (2000) recently reviewed the immunostaining of small cell carcinomas. The antikeratin antibody CAM 5.2 produces a distinctive staining of small cell carcinoma. They have a punctate of globoid perinuclear zone of staining.
P.2438
Other neuroendocrine markers, such as chromogranin A, synaptophysin, NSE, CD56, and protein gene product 9.5 stain small cell carcinomas. Another marker is neural cell adhesion molecule (NCAM, CD56). The adhesion molecule plays a role in the cohesion of cells in the peripheral and central nervous systems, and it is present in neuroendocrine and neuroectodermal tumors. In summary, the most important immunostain for a small cell carcinoma is probably the keratin stain CAM 5.2. The positive reaction, in conjunction with the histology, shows that the tumor is of epithelial origin rather than of stromal or lymphocytic origin.
Neurogenic Mediastinal Tumors
Neurogenic mediastinal tumors can occur in any of the mediastinal compartments, but usually they occur in the paravertebral sulci of the posterior mediastinal compartment. These neurogenic tumors include: neurilemomas (schwannomas; melanotic schwannomas; neurofibromas; and neurogenic sarcomas, which are malignant schwannomas of nerve sheath origin); ganglioneuromas; ganglioneuroblastomas; neuroblastomas; infrequently, pheochromocytomas; and, rarely, pigmented malignant tumors of possible ganglion cell origin (see Chapter 189).
Nerve Sheath Tumors and Ganglioneuromas
Neurilemomas, neurofibromas, and ganglioneuromas are benign tumors. None of these three tumors, or the malignant schwannomas, have any known serum tumor markers. The examination of hematoxylin and eosin stained tissue sections are usually diagnostic for neurilemomas and neurofibromas. A malignant schwannoma may be difficult to differentiate from other sarcomas, such as a fibrosarcoma. In this instance, an immunohistochemical stain for S-100 protein is helpful. Schwann cells stain positively for S-100 protein, as do the cells of a malignant melanoma, but fibrosarcomas or other spindle cell tumors of stromal origin do not stain for S-100. The S-100 protein immunohistochemical technique is also helpful in separating benign tumors from one another. For instance, a neurilemoma has a positive S-100 protein reaction, and a leiomyoma has a negative S-100 protein reaction.
Malignant Ganglionic Tumors
Neuroblastomas and ganglioneuroblastomas are malignant neoplasms of the peripheral sympathetic nervous system. According to Weiss and Goldblum (2001), most of these tumors are diagnosed by the age of 5 years. They are usually found in the adrenal medulla but also are seen in the thorax, most often in a paravertebral sulcus, although Argani and colleagues (1997) reported three anterior mediastinal neuroblastomas in patients ranging in age from 67 to 80 years. One of their patients had the syndrome of inappropriate antidiuretic hormone. In their review of the literature, they found five thymic neuroblastomas, and three of those had inappropriate secretion of antidiuretic hormone. Matthay (1997) reviewed the biology and therapy of neuroblastomas.
Catecholamine Levels
The malignant ganglionic tumors may produce elevations of catecholamines (i.e., epinephrine and norepinephrine) in the serum and urine. Vanillylmandelic acid (VMA) is the major end product of catecholamine metabolism. The most common abnormal laboratory finding in patients with a neuroblastoma is an elevated VMA level. Because of the poor differentiation of some neuroblastomas, they lack the -hydrolase to convert dopamine to norepinephrine. This results in increased serum and urine levels of homovanillic acid (HVA). Gitlow and associates (1973) found a significant correlation between low initial levels of HVA, and a good prognosis. Lang and co-workers (1978) found that the prognosis of disseminated neuroblastoma correlated with the urinary VMA to HVA ratio, but not with the absolute levels of HVA.
Serum Ferritin Levels
Serum ferritin is a marker for neuroblastomas and ganglioneuroblastomas. Hann and co-workers (1981) pointed out that children with stage IV-S neuroblastoma had lower serum ferritin levels than those with stage IV. Stage IV-S has a more favorable prognosis than stage IV (see Chapter 189). In 1984, Hann and associates demonstrated that human neuroblastoma transplanted to mice produced human liver type ferritin in their serum. Matthay (1997) pointed out that patients with levels of serum ferritin below 143 ng/mL had a much better rate of survival than did those with levels greater than 143 ng/mL.
Neuron-Specific Enolase Levels
Ishiguro and co-workers (1983) measured serum levels of NSE in children with neuroblastomas. They concluded that elevated levels of NSE correlated with a poor prognosis, and that during therapy the serum levels correlated with the course of the disease. Massaron and associates (1998) also found a correlation between NSE levels, stage, and outcome. They concluded that NSE was a useful marker for confirming the diagnosis, monitoring treatment, and detecting recurrent disease. Seregni and colleagues (2001) found the specificity of serum NSE to be 50% and the sensitivity to be close to 90%. These figures were independent
P.2439
of tumor stage. Finally, serum NSE decreased in response to treatment.
Chromogranin Levels
Hsiao and associates (1990) found serum chromogranin A levels to be a useful diagnostic tool for neuroblastoma. With a level of less than 190 ng/mL at the time of diagnosis, patients had a better rate of survival (69%) than did those with a higher level (30%). The researchers concluded that chromogranin A (a) is a sensitive and specific diagnostic tool, (b) correlates with the tumor burden, and (c) is a useful predictor of survival. Seregni and colleagues (2001) found a specificity of 83% for chromogranin A. The sensitivity depended on the stage of the tumor, with 50% for stages I and II, 60% for stage III, and 100% for stage IV. Finally, serum chromogranin A decreased in response to therapy.
Cytogenetic Markers
Matthay (1997) pointed out that deletion of the short arm of chromosome 1 is the most consistently reported abnormality in neuroblastomas. This deletion is usually found in patients with advanced disease and a poor prognosis. Bown (2001) reviewed the cytogenetics of neuroblastomas and pointed out that the loss of a distal 1p and the gain of a distal 17q is a recurring feature of neuroblastomas.
Molecular Markers
Matthay (1997) pointed out that the amplification of MYCN correlates closely with outcome. MYCN, a protooncogene, is derived from the C-myc viral oncogene. In general, the more this gene is amplified, the poorer the survival. Combaret and associates (2002) used polymerase chain reaction (PCR) to search for MYCN DNA in the peripheral blood of neuroblastoma patients. They concluded that circulating MYCN DNA is a valuable prognostic marker in patients with neuroblastoma.
Immunohistochemical Markers
As for immunohistochemical markers, Hachitanda and associates (1989) demonstrated that ganglioneuroblastomas and neuroblastomas stain positively for NSE, chromogranin, and synaptophysin. The chromogranin and synaptophysin stained the majority of the tumors, but the NSE stained all of them.
Pheochromocytomas
Mediastinal pheochromocytomas are rare. In 1970, McNeill and associates reviewed 23 cases of thoracic pheochromocytomas. In 1991, Wick and Rosai reviewed mediastinal neuroendocrine neoplasms, and in 1993, Moran and colleagues reviewed 16 mediastinal paragangliomas. Pheochromocytomas produce elevated levels of catecholamines in the serum and urine. These catecholamines include epinephrine, norepinephrine, dopamine, metanephrine, normetanephrine, VMA, and HVA, and they can be measured in the urine and serum. According to Plouin and associates (1981), the urine catecholamines, metanephrines, and VMA were better than the plasma levels in detecting pheochromocytomas.
The enzyme phenylethanolamine-N-methyltransferase converts norepinephrine to epinephrine. Ciaranella (1978) noted that this enzyme requires levels of cortisol found only in the proximity of the adrenal cortex. Therefore, extraadrenal pheochromocytomas secrete only norepinephrine and its metabolite. In a series of eight middle mediastinal pheochromocytomas reported by Shapiro and colleagues (1984), the biochemical markers were in keeping with the extraadrenal location of the pheochromocytomas. Plasma norepinephrine and urinary excretion rates for norepinephrine, normetanephrine, and VMA were all markedly elevated, whereas plasma epinephrine concentrations were normal in five of eight cases, as was the urine epinephrine excretion rate. The urine metanephrine excretion rate was normal in all cases.
O'Connor and Deftos (1986) demonstrated that pheochromocytomas produced elevated serum levels of chromogranin A. Hsiao and associates (1991) and Canale and Bravo (1994) evaluated the use of serum chromogranin A in patients with pheochromocytomas. Both groups found that chromogranin A had poor diagnostic specificity in patients with impaired renal function. Both groups believed that chromogranin A was a useful marker for the detection of pheochromocytoma. Canale and Bravo (1994) concluded that when serum chromogranin A was combined with plasma catecholamines, it provided a lower sensitivity, but excellent specificity, accuracy, and positive predictive value in the diagnosis of pheochromocytoma. In contrast, Seregni (2001) found that only 40% of 10 patients had an elevated serum chromogranin A. They concluded that serum chromogranin A was not a reliable marker.
Immunohistochemically, Lloyd and colleagues (1984) detected norepinephrine, epinephrine, and chromogranin in the cells of all 25 pheochromocytomas they examined immunohistochemically. They also demonstrated NSE in 26 of 26 pheochromocytomas. Moran and associates (1993) performed immunohistochemical staining on mediastinal paragangliomas. In 10 of 10 cases, the tumor cells stained with chromogranin. In 9 of 10 cases, the tumor-supporting cells, the sustentacular cells, stained with S-100 protein. The tumor cells also immunostained with several other general neuroendocrine markers. In a later study Moran and associates (1997a) examined 23 spinal paragangliomas by immunohistochemistry. NSE stained 100% of the cases, with chromogranin and synaptophysis staining 91% cases. The sustentacular cells stained with S-100 protein in 95% of the cases.
P.2440
Parathyroid Adenomas
The parathyroid glands are composed of four separate glands that are derived from the third and fourth branchial clefts. The two glands from the third cleft (III) are adherent to the thymus, whereas the two glands from the fourth cleft (IV) are adherent to the lower portions of thyroid lobes. During the course of migration from the branchial clefts, the parathyroids from the third cleft may migrate too far and end up in the anterior mediastinum, near the thymus (see Chapters 156 and 193). Also during migration, the parathyroids from the fourth cleft may migrate into the visceral compartment. Gurbuz and Peetz (1996) described a mediastinal parathyroid cyst that resulted in hypercalcemic crisis. A detailed review of parathyroid cysts is presented in Chapter 197.
The parathyroids produce hormones (PTH), which regulates serum calcium. A primary increase in PTH production can result from an adenoma, hyperplasia, a functioning parathyroid cyst, or carcinoma of the parathyroid glands. A secondary increase in PTH can be caused by a neoplasm, thyrotoxicosis, thiazide diuretics, lithium, vitamins A and D, sarcoidosis, and various other conditions.
Radioimmunoassays are available for measuring serum PTH levels. Martin and co-workers (1979) pointed out that once PTH is secreted, it is rapidly metabolized into a biologically active amino N-terminal fragment and an inactive carboxy C-terminal fragment. The N-terminal fragment has a half-life of 4 minutes, whereas the C-terminal fragment has a half-life of 30 to 60 minutes. Most assays have been directed against the C-terminal fragment. This fragment is excreted primarily by glomerular filtration and may be elevated in renal failure. Serum PTH is a useful hormonal marker in evaluating elevated levels of serum calcium. Because parathyroid adenomas contain chromogranin, serum chromogranin levels may be of value in parathyroid tumors.
Immunohistochemical staining of parathyroid lesions with an antibody to PTH is possible. The various precursor and metabolized forms of the hormone may make it difficult to achieve a good stain. The immunohistochemical stains may be helpful in differentiating an intrathyroidal lesion or a metastatic parathyroid carcinoma. Parathyroid neoplasms also immunostain with cytokeratin, chromogranin, and synaptophysin.
Summary
In summary, this chapter focused on the serum markers for mediastinal tumors. The mediastinal tumors that produce serum markers are the germ cell, neuroendocrine, and parathyroid tumors. The germ cell tumors are in the anterior mediastinum and include teratomas, serminomas, yolk sac tumors, embryonal carcinomas, and choriocarcinomas. Most commonly, the germ cell tumors are mixed. AFP is the serum marker for yolk sac tumors and embryonal carcinomas. -HCG is the serum marker for choriocarcinoma. PLAP is associated with seminoma. Thymomas do not have a specific serum marker. In contrast, thymic neuroendocrine lesions, carcinoids, and small cell carcinomas may show elevated serum levels of chromogranin and NSE. These are generic serum markers for neuroendocrine tumors. Parathyroid tumors will show an elevated level of PTH and probably elevated levels of neuroendocrine markers, such as chromogranin and NSE.
The posterior mediastinum may give rise to such other neuroendocrine tumors as pheochromocytomas, neuroblastomas, and ganglioneuroblastomas. These tumors produce elevations of the nonspecific serum markers (chromogranin and NSE) and elevate the levels of serum and urine catecholamines.
REFERENCES
Abelev GI, et al: Production of embryonal -globulin by transplantable mouse hepatomas. Transplantation 1: 174, 1963.
Akoun GM, et al: Serum neuron-specific enolase. A marker for disease extent and response to therapy for small-cell lung cancer. Chest 87:39, 1985.
Anderson T, et al: Testicular germ-cell neoplasms: recent advances in diagnosis and therapy. Ann Intern Med 90:373, 1979.
Argani P, Erlandson RA, Rosai J: Thymic neuroblastoma in adults: report of three cases with special emphasis on its association with the syndrome of inappropriate secretion of antidiuretic hormone. Am J Clin Pathol 108:537, 1997.
Ariyoshi Y, et al: Evaluation of serum neuron-specific enolase as a tumor marker for carcinoma of the lung. Gann 74:219, 1983.
Atkin NB, Baker MC: i(12p): specific chromosomal marker in seminoma and malignant teratoma of the testis? Cancer Genet Cytogenet 10:199, 1983.
Battifora H, et al: The use of antikeratin antiserum as a diagnostic tool: thymoma versus lymphoma. Hum Pathol 11:635, 1980.
Baudin E, et al: Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumors. Br J Cancer 78:1102, 1998.
Bostwick DG, et al: Gastrin-releasing peptide, a mammalian analog of bombesin, is present in human neuroendocrine lung tumors. Am J Pathol 117: 195, 1984.
Bown N: Neuroblastoma tumour genetics: clinical and biological aspects. J Clin Pathol 54:897, 2001.
Braunstein GD, et al: Ectopic production of human chorionic gonadotropin by neoplasms. Ann Intern Med 78:39, 1973.
Burke AP, Mostofi FK: Placental alkaline phosphatase immunohistochemistry of intratubular malignant germ cells and associated testicular germ cell tumors. Hum Pathol 19:663, 1988.
Canale MP, Bravo EL: Diagnostic specificity of serum chromogranin-A for pheochromocytoma in patients with renal dysfunction. J Clin Endocrinol Metab 78:1139, 1994.
Capella C, et al: Revised classification of neuroendocrine tumors of the lung, pancreas and gut. Digestion 55(suppl 3):11, 1994.
Carney DN, et al: Serum neuron-specific enolase: a marker for disease extent and response to therapy of small-cell lung cancer. Lancet 1:583, 1982.
Ciaranella RD: Regulation of phenol phenylethanolamine-methyltransferase. Biochem Pharmacol 27:1895, 1978.
Combaret V, et al: Circulating MYCN DNA as a tumor-specific marker in neuroblastoma patients. Cancer Res 62:3646, 2002.
DeBlois GG: Germ cell tumors. In Kornstein MJ (ed): Pathology of the Thymus and Mediastinum. Philadelphia: WB Saunders, 1995, p. 172.
de Montpr ville VT, Macchiarini P, Dulmet E: Thymic neuroendocrine carcinoma (carcinoid): a clinicopathologic study of fourteen cases. J Thorac Cardiovasc Surg 111:134, 1996.
Dixon FJ, Moore RA: Testicular tumors. A clinicopathological study. Cancer 6:427, 1953.
Dorfman DM, et al: Thymic carcinomas, but not thymomas and carcinomas of other sites, show CD 5 immunoreactivity. Am J Surg Pathol 21:936, 1997.
P.2441
Duguid JB, Kennedy AM: Oatcell tumours of mediastinal glands. J Pathol Bacteriol 33:93, 1930.
Economou JS, et al: Management of primary germ cell tumors of the mediastinum. J Thorac Cardiovasc Surg 83:643, 1982.
Ermisch B, Schwechheimer K: Protein gene product (PGP) 9.5 in diagnostic (neuro) oncology. An immunomorphological study. Clin Neuropathol 14:130, 1995.
Erlandson RA, Nesland JM: Tumors of the endocrine/neuroendocrine system: an overview. Ultrastruct Pathol 18:149, 1994.
Falini B, Taylor CR: New developments in immunoperoxidase techniques and their application. Arch Pathol Lab Med 107:105, 1983.
Ferreiro JA: Ber-H2 expression in testicular germ cell tumors. Hum Pathol 25:522, 1994.
Fizazi K, et al: Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: an early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer 82:1049, 1998.
Gels ME, et al: Importance of a new tumor marker TRA-1 60 in the follow-up of patients with clinical stage I nonseminomatous testicular germ cell tumors. Ann Surg Oncol 4:321, 1997.
Giovanella L, et al: Immunoradiometric assay of chromogranin A in the diagnosis of small cell lung cancer: comparative evaluation with neuron-specific enolase. Int J Biol Markers 16:50, 2001.
Gitlow SE, et al: Biochemical and histologic determinants in the prognosis of neuroblastoma. Cancer 32:898, 1973.
Goldstein AL, et al: Current status of thymosin and other hormones of the thymus gland. Recent Prog Horm Res 37:369, 1981.
Gonzalez-Crussi F: Extragonadal teratomas. In Atlas of Tumor Pathology. Second Series, Fascicle 18. Washington, DC: Armed Forces Institute of Pathology, 1982.
Gould VE, et al: Synaptophysin expression in neuroendocrine neoplasms as determined by immunocytochemistry. Am J Pathol 126:243, 1987.
Gray GF, Gutowski WT: Thymoma, a clinicopathologic study of 54 cases. Am J Surg Pathol 3:235, 1979.
Gurbuz AT, Peetz ME: Giant mediastinal parathyroid cyst: an unusual cause of hypercalcemic crisis case report and review of the literature. Surgery 120:795, 1996.
Hachitanda Y, Tsuneyoshi M, Enjoji M: Expression of panneuroendocrine proteins in 53 neuroblastic tumors. Arch Pathol Lab Med 113:381, 1989.
Hagn C, et al: Chromogranin A, B and C in human adrenal medulla and endocrine tissues. Lab Invest 55:405, 1986.
Haimoto H, et al: Immunohistochemical localization of -enolase in normal human tissues other than nervous and neuroendocrine tissues. Lab Invest 52: 257, 1985.
Hann H, et al: Biological differences between neuroblastoma stages IV-S and IV. Measurement of serum ferritin and Erosette inhibition in 30 children. N Engl J Med 305:425, 1981.
Hann H, Stahlhut MW, Millman I: Human ferritins present in the sera of nude mice transplanted with human neuroblastoma or hepatocellular carcinoma. Cancer Res 44:3898, 1984.
Herbert WM, et al: Carcinoid tumors of the thymus. An immunohistochemical study. Cancer 60:2465, 1987.
Hirokawa K, et al: Immunohistochemical studies in human thymomas. Localization of thymosin and various cell markers. Virchows Arch [B] 55:371, 1988.
Hishima T, et al: CD 5 expression in thymic carcinoma. Am J Pathol 145:268, 1994.
Hoffman OA, et al: Primary mediastinal neoplasms (other than thymoma). Mayo Clin Proc 68:880, 1993.
Hsiao RJ, et al: Chromogranin A in children with neuroblastoma. Serum concentration parallels disease stage and predicts survival. J Clin Invest 85:1555, 1990.
Hsiao RJ, et al: Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine 70:33, 1991.
International Union Against Cancer Report: Workshop on immunodiagnosis. Cancer Res 46:3744, 1986.
Ishiguro Y, et al: Nervous system-specific enolase in serum as a marker for neuroblastoma. Pediatrics 72:696, 1983.
Jorgensen LG, et al: Serum neuron-specific enolase (S-NSE) and the prognosis in small-cell lung cancer (SCLC): a combined multivariable analysis on data from nine centres. Br J Cancer 74:463, 1996.
Kesler KA, et al: Primary mediastinal nonseminomatous germ cell tumors: the influence of post chemotherapy pathology on long term survival after surgery. J Thorac Cardiovasc Surg 118:692, 1999.
Klein EA: Tumor markers in testis cancer. Urol Clin North Am 20:67, 1993.
Klemm KM, Moran CA. Primary neuroendocrine carcinomas of the thymus. Semin Diagn Pathol 16:32, 1999.
Knapp RH, et al: Malignant germ cell tumors of the mediastinum. J Thorac Cardiovasc Surg 89:82, 1985.
Kornstein MJ, et al: Cortic versus medullary thymoma. A useful morphologic classification? Hum Pathol 19:1335, 1988.
Kornstein MJ, Rosai J: CD 5 labeling of thymic carcinomas and other nonlymphoid neoplasms. Am J Clin Pathol 109:722, 1998.
Koshida K, et al: Significance of placental alkaline phosphatase (PLAP) in the monitoring of patients with seminoma. Br J Urol 77:138, 1996.
Kurman RJ, et al: Cellular localization of alpha-fetoprotein and human chorionic gonadotropin in germ cell tumors of the testis using an indirect immunoperoxidase technique. Cancer 40:2136, 1977.
Lajer H, et al: Clinical use of serum TRA-1 60 as tumor marker in patients with germ cell cancer. Int J Cancer 100:244, 2002.
Lang WE, et al: Initial urinary catecholamine metabolite concentrations and prognosis in neuroblastoma. Pediatrics 62:77, 1978.
Lange PH, et al: Placental alkaline phosphatase as a tumor marker for seminoma. Cancer Res 42:3244, 1982.
Latza U, et al: CD30 antigen in embryonal carcinoma and embryogenesis and release of the soluble molecule. Am J Pathol 146:463, 1995.
Lee I, et al: Synaptophysin expressed in the bronchopulmonary tract: neuroendocrine cells, neuroepithelial bodies, and neuroendocrine neoplasms. Differentiation 34: 115, 1987.
Lewis BD, et al: Benign teratomas of the mediastinum. J Thorac Cardiovasc Surg 86:727, 1983.
Lewis JE, et al: Thymoma: a clinicopathologic review. Cancer 60:2727, 1987.
Lloyd RV, et al: An immunohistochemical study of pheochromocytomas. Arch Pathol Lab Med 108:541, 1984.
Lloyd RV, et al: Distribution of chromogranin A and secretogranin I (chromogranin B) in neuroendocrine cells and tumors. Am J Pathol 130: 296, 1988.
Marrink J, et al: TRA-1 60: a new serum marker in patients with germ-cell-tumors. Int J Cancer 49:368, 1991.
Martin KJ, et al: The peripheral metabolism of parathyroid hormone. N Engl J Med 301:1092, 1979.
Masaoka A, et al: Follow-up study of thymomas with special reference to their clinical stages. Cancer 48:2485, 1981.
Maslow WC, et al: Sensitive fluorometry of heat-stable alkaline phosphatase (Regan enzyme) activity in serum from smokers and nonsmokers. Clin Chem 29:260, 1983.
Massaron S, et al: Neuron-specific enolase evaluation in patients with neuroblastoma. Tumour Biol 19:261, 1998.
Matthay KK: Neuroblastoma: biology and therapy. Oncology 11:1857, 1997.
McNeill AD, Gropen BM, Nelville AM: Intrathoracic phaeochromocytoma. Br J Surg 57:457, 1970.
Miettinen M: Synaptophysin and neurofilament proteins as markers for neuroendocrine tumors. Arch Pathol Lab Med 111:813, 1987.
Moody TW, et al: High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science 214:1246, 1981.
Moran CA, Suster S: Primary germ cell tumors of the mediastinum. I. Analysis of 322 cases with special emphasis on teratomatous lesions and proposal for histopathologic classification and clinical staging. Cancer 80:681, 1997a.
Moran CA, Suster S: Primary mediastinal choriocarcinomas: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 21:1007, 1997b.
Moran CA, Suster S: Thymic neuroendocrine carcinomas with combined features ranging from well-differentiated (carcinoid) to small cell carcinoma. Am J Clin Pathol 113:345, 2000.
Moran CA, Rush W, Mena H. Primary spinal paragangliomas: a clinicopathological and immunohistochemical study of 30 cases. Histopathology 31:167. 1997a.
Moran CA, et al: Mediastinal paragangliomas. A clinicopathologic and immunohistochemical study of 16 cases. Cancer 72:2358, 1993.
Moran CA, et al: Primary germ cell tumors of the mediastinum. III. Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ cell tumors of the mediastinum a clinicopathologic and immunohistochemical study of 64 cases. Cancer 80:699, 1997b.
Moran CA, et al: Primary germ cell tumors of the mediastinum. II. Mediastinal seminomas a clinicopathologic and immunohistochemical study of 120 cases. Cancer 80:691, 1997c.
Mostofi FK, Price EB Jr: Tumors of the male genital system. In Atlas of Tumor Pathology, Second Series, Fascicle 8. Washington, DC: Armed Forces Institute of Pathology, 1973.
P.2442
Mostofi FK, Sobin LH: Histological typing of testis tumours. In International Histologic Classification of Tumours, No. 16. Geneva: World Health Organization, 1977.
Munro AJ, et al: An assessment of combined tumor markers in patients with seminoma: placental alkaline phosphatase (PLAP), lactate dehydrogenase (LD) and -human chorionic gonadotropin ( -HCG). Br J Cancer 64:537, 1991.
Nichols CR, et al: Hematologic malignancies associated with primary mediastinal germ-cell tumors. Ann Intern Med 102:603, 1985.
Nichols CR, et al: Klinefelter's syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol 5:1290, 1987.
Niehans GA, et al: Immunohistochemistry of germ cell and trophoblastic neoplasms. Cancer 62:1113, 1988.
Nielsen OS, et al: Is placental alkaline phosphatase (PLAP) a useful marker for seminoma? Eur J Cancer 26:1049, 1990.
Nobels FRE, et al: Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab 82:2622, 1997.
O'Connor DT, Deftos LJ: Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med 314:1145, 1986.
Pearse AGE: The APUD cell concept and its implications in pathology. Pathol Annu 9:27, 1974.
Perlin E, et al: The value of serial measurement of both human chorionic gonadotropin and alpha-fetoprotein for monitoring germinal cell tumors. Cancer 37:215, 1976.
Peterson RO. Urologic Pathology. Philadelphia: JB Lippincott, 1986 Pinson P, et al: Serum neuron-specific enolase as a marker in the diagnosis and follow-up of small cell lung cancer. Respiration 64:102, 1997.
Plouin PF, et al: Biochemical tests for diagnosis of pheochromocytoma: urinary versus plasma determinations. BMJ 282:853, 1981.
Pomplun S, et al: Immunohistochemical markers in the differentiation of thymic and pulmonary neoplasms. Histopathology 40:152, 2002.
Pugh RCB, Cameron KM: Teratoma. In Pugh RCB, ed. Pathology of the Testis. Oxford: Blackwell Scientific, 1976, pp. 199 244.
Rosai J: The pathology of thymic neoplasia. In Costan BW, Dorfman RF, Kaufman N (eds): International Academy of Pathology Monograph: Malignant Lymphoma. Baltimore: Williams&Wilkins, 1987.
Rosai J, et al: Carcinoid tumors and oat cell carcinomas of the thymus. Pathol Annu 11:201, 1976.
Rosai J, Levine GD: Tumors of the thymus. In Atlas of Tumor Pathology. Second Series, Fascicle 13. Washington, DC: Armed Forces Institute of Pathology, 1976.
Roszi J, Sobin LH: Histological typing of tumors of the thymus. In World Health Organization, International Histological Classification of Tumors. 2nd Ed. New York: Springer, 1999, p. 9.
Said JW, et al: Immunoreactive neuron-specific enolase, bombesin, and chromogranin as markers for neuroendocrine lung tumors. Hum Pathol 16: 236, 1985.
Samaniego F, et al: Cytogenetic and molecular analysis of human male germ cell tumors: chromosome 12 abnormalities and gene amplification. Genes Chromosomes Cancer 1:289, 1990.
Scardino PT, et al: The value of serum tumor markers in staging and prognosis of germ cell tumors of the testis. J Urol 118:994, 1977.
Schrader M, et al: Molecular markers in testicular germ cell tumors object of clinical research or close to becoming clinical tools? Onkologie 24:144, 2001.
Senden NHM, et al: A comparison of NSP-reticulons with conventional neuroendocrine markers in immunophenotyping of lung cancers. J Pathol 182:13, 1997.
Seregni E, et al: Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol Suppl 2 12:569, 2001.
Shapiro B, et al: The location of middle mediastinal pheochromocytomas. J Thorac Cardiovasc Surg 87:814, 1984.
Shibayama T, et al: Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small cell lung cancer (SCLC). Lung Cancer 32:61, 2001.
Shields TW: The mediastinum and its compartments. In Shields TW (ed): Mediastinal Surgery. Philadelphia: Lea&Febiger, 1991, p. 3.
Shimosato Y, Mukai K: Tumors of the mediastinum. In Atlas of Tumor Pathology. Fascicle 21, 3rd Series. Washington, DC: Armed Forces Institute of Pathology, 1997.
Sobol RE, et al: Elevated serum chromogranin A concentrations in small-cell lung carcinoma. Ann Intern Med 105:698, 1986.
Sturgen C: Practice guidelines for tumor marker use in the clinic. Clin Chem 48:1151, 2002.
Summersgill BM, et al: Definition of chromosome abberations in testicular germ cell tumor cell lines by 24-color karyotyping and complementary molecular cytogenetic analysis. Cancer Genet Cytogenet 128:120, 2001.
Suster S, Rosai J: Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 67:1025, 1991.
Suster S, et al: Germ cell tumors of the mediastinum and testis: a comparative immunohistochemical study in 120 cases. Hum Pathol 29:737, 1998.
Talerman A, Haije WG, Bagerman L: Serum alpha-fetoprotein (AFP) in patients with germ cell tumors of the gonads and extragonadal sites: correlation between endodermal sinus (yolk sac) tumor and raised serum AFP. Cancer 46:380, 1980.
Tapia FJ, et al: Neuron-specific enolase is produced by neuroendocrine tumors. Lancet 1:808, 1981.
Tischler AS, et al: Anti-lymphocyte antibody Leu-7 (HNK-1) recognizes a constituent of neuroendocrine granule matrix. J Histochem Cytochem 34:1213, 1986.
Tonik SE, et al: Elevation of serum placental alkaline phosphatase levels in cigarette smokers. Int J Cancer 31:51, 1983.
Tucker DF, et al: Serum marker potential of placental alkaline phosphatase-like activity in testicular germ cell tumours evaluated by HI7E2 monoclonal antibody assay. Br J Cancer 51:631, 1985.
Verley JM, Hollmann KH: Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 55:1074, 1985,
von Eyben FE, et al: Serum lactate dehydrogenase isoenzyme 1 and prediction of death in patients with metastatic testicular germ cell tumors. Clin Chem Lab Med 39:38, 2001.
Weidner N: Germ-cell tumors of the mediastinum. Semin Diagn Pathol 16:42, 1999.
Weiss SW, Goldblum Jr: Enzinger and Weiss's Soft Tissue Tumors. 4th Ed. St. Louis: CV Mosby, 2001.
Weissbach L, et al: The value of tumor markers in testicular seminomas. Results of a prospective multicenter study. Eur Urol 32:16, 1997.
Wick MR: Immunohistology of neuroendocrine and neuroectodermal tumors. Semin Diagn Pathol 17:194, 2000.
Wick MR, Rosai J: Neuroendocrine neoplasms of the mediastinum. Semin Diagn Pathol 8:35, 1991.
Wick MR, Scheithauer BW: Oat-cell carcinoma of the thymus. Cancer 49:1652, 1982.
Wick MR, Scheithauer BW: Thymic carcinoid. a histologic, immunohistochemical and ultrastructural study of 12 cases. Cancer 53:475, 1984.
Wick MR, Scheithauer BW, Kovacs K: Neuron-specific enolase in neuroendocrine tumors of the thymus, bronchus, and skin. Am J Clin Pathol 79:703, 1983.
Wick MR, et al: Carcinoid tumor of the thymus: a clinicopathologic report of seven cases with a review of the literature. Mayo Clin Proc 55:246, 1980.
Wick MR, et al: Primary mediastinal carcinoid tumors. Am J Surg Pathol 6:195, 1982.
Wiedenmann B, Franke WW: Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41:1017, 1985.
Wright CD, et al: Primary mediastinal nonseminomatous germ cell tumors: results of a multimodality approach. J Thorac Cardiovasc Surg 99:210, 1990.
Wright CD, Mathisen DJ: Mediastinal tumors: diagnosis and treatment. World J Surg 25:204, 2001.
Yoneda KY, Louie S, Shelton DK: Mediastinal tumors. Curr Opin Pulm Med 7:226, 2001.
EAN: 2147483647
Pages: 203
- Integration Strategies and Tactics for Information Technology Governance
- A View on Knowledge Management: Utilizing a Balanced Scorecard Methodology for Analyzing Knowledge Metrics
- Measuring ROI in E-Commerce Applications: Analysis to Action
- Governance in IT Outsourcing Partnerships
- Governance Structures for IT in the Health Care Industry
- Article 100 Definitions
- Article 410: Luminaires (Lighting Fixtures), Lampholders, and Lamps
- Article 411: Lighting Systems Operating at 30 Volts or Less
- Example D3(a) Industrial Feeders in a Common Raceway
- Example No. D10 Feeder Ampacity Determination for Adjustable-Speed Drive Control [See 215.2, 430.24, 620.13, 620.14, 620.61, Tables 430.22(E), and 620.14]