136 - Minimally Invasive Esophageal Surgery
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Mediastinum > Section XXVI - Noninvasive Investigations > Chapter 159 - Radionuclide Studies of the Mediastinum
function show_scrollbar() {}
Chapter 159
Radionuclide Studies of the Mediastinum
William G. Spies
Until recently, the role of radionuclide imaging in the chest was primarily related to evaluation of disorders of the heart and lungs, particularly the evaluation of myocardial perfusion and ventricular function, the use of ventilation-perfusion scintigraphy in the detection of pulmonary embolism, and the diagnosis of inflammatory and neoplastic disorders of the lungs and bony thorax using traditional single-photon radionuclides. In the course of pulmonary scintigraphy, findings may indirectly identify the presence of pathology in the mediastinum, such as obstructive airways disease on ventilation studies, or the presence of mediastinal lesions producing secondary effects on pulmonary ventilation, perfusion, or both, including mass lesions and fibrosing mediastinitis.
Gallium imaging of the chest has been used in the detection of inflammatory lesions in the lungs and mediastinum and in the detection and staging of certain intrathoracic neoplasms, notably bronchial carcinoma and lymphoma. Focal mediastinal adenopathy is also an important finding in patients with acquired immunodeficiency syndrome (AIDS) presenting with fever, respiratory symptoms, or both. Indium 111 labeled leukocytes are useful also in the detection of inflammatory processes, including mediastinitis.
With the advent of regulatory agency approval and established literature supporting its use, the past several years have seen a dramatic growth in the clinical use of positron emission tomographic (PET) imaging using the radiopharmaceutical fluorine 18 2-fluoro-2-deoxy-D-glucose (FDG) for the detection and staging of a number of common malignancies. Tumors for which this modality has proven to be efficacious include some of the most important malignancies of the chest and mediastinum, including non small cell lung carcinoma (NSCLC), lymphoma, esophageal carcinoma, carcinoma of the head and neck, and, more recently, breast carcinoma and well-differentiated thyroid carcinoma. PET imaging has been shown to be superior to gallium scintigraphy in the diagnosis, staging, and restaging of NSCLC and lymphoma, and has largely replaced it for these purposes in many centers. Furthermore, PET imaging has also been shown to be superior even to computed tomographic (CT) scanning for the staging, assessment of the response to therapy and detection of recurrences in patients with these tumors. This imaging modality, which was once limited to a few highly specialized research centers, is becoming widely clinically available at much lower expense. In addition, some manufacturers have developed hybrid imaging units capable of performing both PET and CT studies sequentially during a single imaging session.
Radionuclide lymphoscintigraphy was used for a number of years to evaluate for the presence of tumor involvement in the otherwise inaccessible internal mammary lymph nodes, which are a frequent site of metastatic disease in breast carcinoma. More recently, lymphoscintigraphy has been used preoperatively and intraoperatively using specialized probe detectors to localize the sentinel lymph node(s) in patients with breast carcinoma or medium-thickness malignant melanomas. Presently, preoperative lymphoscintigraphy with sentinel lymph node mapping, coupled with intraoperative probe localization, biopsy, and immunohistochemical analysis, has rapidly become the standard of care for patients with operable breast carcinoma.
Peptide radionuclide imaging agents have been developed and evaluated for the detection of specific tumors. The most widely studied agent of this type, a somatostatin analogue of octreotide, is currently routinely used clinically for the detection and follow-up of carcinoid tumor, islet cell carcinoma of the pancreas, medullary carcinoma of the thyroid, and other related neuroendocrine tumors. Some of these agents can also be modified or used directly for radionuclide therapy of these tumors.
The compound metaiodobenzylguanidine (MIBG) labeled with iodine 131 or 123 is a norepinephrine analogue that has specific affinity for adrenal medullary tumors, such as pheochromocytomas, paraganglionomas, and neuroblastomas. This agent can be used in conjunction with laboratory data and CT scanning or magnetic resonance (MR) imaging to detect and localize these tumors, especially extraadrenal pheochromocytomas, which may be located in the mediastinum, retroperitoneum, or pelvis.
A number of murine, chimeric, and even human monoclonal antibodies directed against human neoplasms, including
P.2397
bronchial carcinoma, lymphoma, colorectal carcinoma, ovarian carcinoma, and prostate carcinoma, have been evaluated in the detection and staging of these lesions. Some of these agents have also been radiolabeled with high energy beta-emitting radionuclides and evaluated for use as immunoradiotherapeutic agents as well. At the present time, only one monoclonal antibody has been approved by the U.S. Food and Drug Administration (FDA) for routine clinical use in the treatment of refractory non-Hodgkin's lymphoma, and another related agent is currently undergoing the approval process.
Thyroid scintigraphy is a sensitive and specific technique for establishing the presence of functioning thyroid tissue within mediastinal masses. This approach is valuable in the differential diagnosis of superior mediastinal masses suspected to represent substernal goiters and in the detection of functioning metastases in patients with well-differentiated thyroid carcinoma. In addition, the iodine radioisotope (131I) is used therapeutically to ablate residual normal thyroid tissue and metastatic disease in thyroid carcinoma, once the presence of functioning thyroid tissue is established by 131I whole-body imaging. FDG PET imaging may be useful in the detection of occult mediastinal metastases in patients with elevated serum thyroglobulin levels but negative 131I whole-body scans, and has recently received governmental approval for this indication. Parathyroid scintigraphy using technetium 99m (99mTc) sestamibi is a sensitive method for the detection of parathyroid adenomas in the neck and superior mediastinum.
Functional imaging of the esophagus using orally administered radiopharmaceuticals can be used to detect and quantify the presence of gastroesophageal reflux and to quantitatively evaluate esophageal motility, as is discussed in detail in Chapter 125.
Radionuclide angiography of the chest is most often used in first-pass cardiac studies, for evaluation of right and left ventricular function, but also can help establish the presence of vascular lesions of the great vessels or mediastinum or the presence of superior vena caval obstruction or other vascular abnormalities.
Apart from the direct evaluation of mediastinal lesions, nuclear medicine procedures also are useful in the diagnosis of metastatic lesions from primary mediastinal tumors. In this regard, PET imaging and bone, gallium, and octreotide scintigraphy are currently the most important modalities, with monoclonal antibodies also used in special circumstances.
Ventilation-Perfusion Scintigraphy
Radionuclide imaging of the lungs remains one of the most commonly performed nuclear medicine procedure of the chest. Although spiral CT imaging of the chest has emerged as a viable alternative modality for the evaluation of patients with suspected pulmonary thromboembolism, ventilation-perfusion (V/Q) lung scintigraphy remains an important screening modality in the evaluation of these patients, and this application is by far the most important indication for this procedure. This subject is discussed in greater detail in Chapter 13. However, V/Q imaging can also provide useful information regarding certain types of mediastinal pathology. Ventilation imaging is performed using either radioactive gases, such as xenon 133 or krypton 81m or using fine, uniform aerosols labeled with technetium 99m, which are inhaled by the patient. Alderson and colleagues (1974, 1976, 1980) have shown that these studies are extremely sensitive for the detection of obstructive airways disease, nearly twice as sensitive as routine chest radiographs. The 133Xe study is performed by having the patient slowly inhale as a 10- to 20-mCi (370- to 740-mBq) dose of the radioactive gas is injected into a breathing apparatus similar to a standard spirometer. The patient holds his or her breath as long as possible while a posterior single breath image is acquired, which reflects regional ventilation. The patient then breathes a 133Xe/air mixture in a closed system for 3 to 5 minutes to allow equilibration of the gas in the airways to occur. During or at the conclusion of this period, an equilibrium wash-in image or images are obtained, which reflect total ventilated lung volume. Finally, the patient exhales the radioactive gas and breathes room air while additional images are obtained every 30 to 60 seconds, constituting the wash-out phase. This latter phase is the most sensitive for detection of obstructive airways disease, and cannot be obtained with either 81mKr or 99mTc aerosols. In 133Xe imaging (unlike the other ventilation imaging agents), all images are acquired in the posterior projection, with the exception that posterior oblique views are often obtained during the wash-out phase if possible, to improve the localization of abnormalities in the anteroposterior plane.
In addition to diffuse involvement in emphysema, asthma, or chronic bronchitis, the presence of endobronchial masses, foreign bodies, or mucus plugging can be detected by these studies, particularly using 133Xe imaging. The most significant finding in such cases is the presence of decreased uptake of the gas on the single breath study, with prolonged retention of the tracer in the affected lung during the wash-out phase, when the patient is breathing room air. Whole-lung hypoventilation is particularly suggestive of a central mass, foreign body, or mucus plug resulting in a ball-valve type of airway obstruction, and often results in a secondary global decrease in perfusion to the affected lung via reflex vasoconstriction, as demonstrated in Fig. 159-1. With aerosol ventilation agents, areas of obstructive airways disease present as focal defects in ventilation. In severe cases of chronic obstructive airways disease, clumping of the radioaerosol in the central airways may also be observed.
Pulmonary perfusion imaging is performed by intravenous injection of 99mTc-labeled particles, such as macroaggregated albumin (MAA), which are trapped in the pulmonary capillary system in proportion to regional pulmonary blood flow. Although pulmonary embolism is again the most important cause of perfusion defects, perfusion abnormalities are also
P.2398
commonly associated with the presence of mediastinal lesions, which produce hypoperfusion as the result of compression or invasion of pulmonary arterial or venous branches. In combination with the ventilation study, it can be determined whether the perfusion defect is the primary abnormality (producing V/Q mismatch) or whether there is secondary reflex vasoconstriction and shunting of blood flow away from a site of ventilatory deficit (matching abnormalities). Important mediastinal lesions producing such changes in pulmonary blood flow include mediastinal masses due to lung carcinoma or
P.2399
other tumors and fibrosing mediastinitis. The finding of lobar or whole-lung V/Q mismatch is suggestive of these disorders, although these findings may also occur in other situations, as discussed by Datz (1980), including massive unilateral pulmonary embolism and other primary vascular lesions, such as arteritis or prior radiation therapy, as shown in Fig. 159-2. Thus, a mediastinal mass lesion, depending on its location, may produce either V/Q match or mismatch.
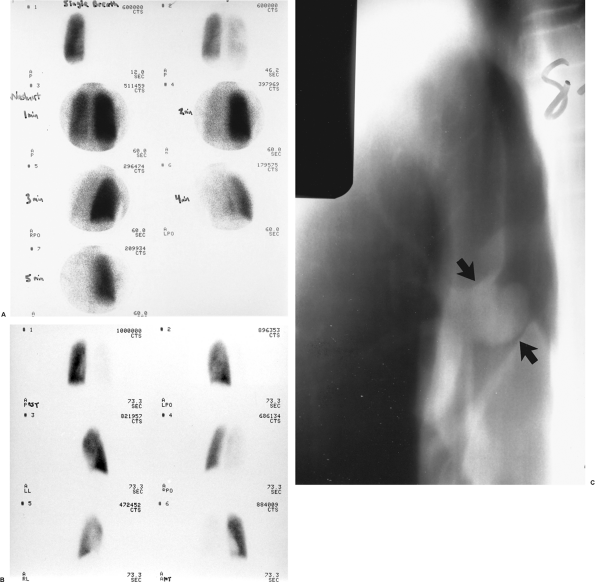 |
Fig. 159-1. Whole-lung ventilation-perfusion match secondary to airway obstruction. The patient is a 16-year-old boy with shortness of breath and hemoptysis. A. Single breath (upper left), equilibrium wash-in (upper right), and five serial 60-second wash-out images from a xenon 133 ventilation study. All images are in the posterior projection except for the 3- and 4-minute wash-out views, which are right posterior oblique and left posterior oblique, respectively (see text for details). There is complete absence of ventilation to the right lung on the single-breath images, which show minimal but greatly diminished ventilation on the equilibrium wash-in image and marked, diffuse 133Xe retention on the wash-out study (normal wash-out is symmetric and complete by 3 4 minutes). B. Perfusion images (clockwise from upper left) in the posterior, left posterior oblique, right posterior oblique, anterior, right lateral, and left lateral projections. There is near-complete nonperfusion of the entire right lung, matching the ventilatory abnormality noted above. C. Anteroposterior tomogram of the right bronchus, demonstrating a large soft tissue mass nearly completely obstructing the right mainstem bronchus (arrows), resulting in a ball-valve obstruction and accounting for the matching ventilation and perfusion abnormalities. The patient proved to have a bronchial carcinoid tumor at surgery. |
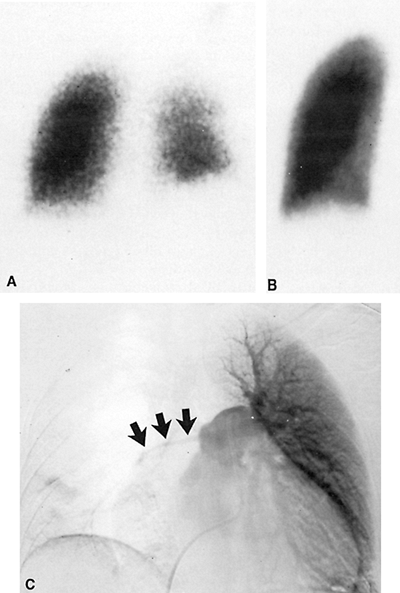 |
Fig. 159-2. Whole-lung ventilation-perfusion mismatch. The patient is a 28-year-old woman with known Takayasu's arteritis, presenting with acute chest pain and dyspnea. A. Posterior krypton 81m ventilation image demonstrating asymmetry in ventilation, with decreased ventilation and apparent decreased lung volume on the right (also the reader's right) but definite ventilation to both lungs. B. Posterior perfusion image demonstrating complete absence of perfusion to the right lung and normal perfusion on the left. C. Anteroposterior subtraction image from a pulmonary angiogram following contrast injection into the main pulmonary artery. There is severe stenosis of the right main pulmonary artery (arrows) secondary to severe vasculitis. Other views demonstrated multiple areas of focal aneurysmal dilatation of the proximal left pulmonary artery branches. No pulmonary emboli were identified. These scintigraphic findings are more commonly seen in bronchogenic carcinoma and fibrosing mediastinitis. C from Spies WG, et al: Ventilation-perfusion scintigraphy in suspected pulmonary embolism: correlation with pulmonary angiography and refinement of criteria for interpretation. Radiology 159:383, 1986. With permission. |
The radiopharmaceutical uptake in ventilation-perfusion imaging is easily quantitated using a digital computer interfaced to the nuclear medicine gamma camera used to acquire the study. White et al. (1969) demonstrated perfusion defects associated with bronchial carcinoma, and determined that the size of the perfusion defect correlated with the extent of the lesion. They found that if the perfusion to the ipsilateral lung was less than 33% of total pulmonary perfusion, then the lesion was nearly always unresectable. Quantitation of both perfusion and ventilation allows calculation of regional V/Q ratios. Comparison of these ratios to normal values can also help predict resectability of tumors, although this analysis is not commonly performed because of relatively low sensitivity and because of the availability of more accurate staging of lung carcinoma by CT and, more recently, PET imaging. However, quantitative V/Q imaging is now performed to predict postoperative pulmonary function following contemplated pneumonectomy, as described by Kristersson (1974), Olsen (1975), and Boysen (1977) and their associates. Assessment of postresection pulmonary reserve is an important aspect of the preoperative evaluation of patients considered for lung resection. Significant disability can result from chronic ventilatory insufficiency if the postoperative forced expiratory volume in 1 second (FEV1) is less than 0.8 L, as reported by Olsen and associates (1975) and by Williams and Brenowitz (1976). Quantitative V/Q scintigraphy can accurately predict the postoperative pulmonary function in these patients, as reported by Wernly and co-workers (1980), and surgery is usually not performed if the predicted FEV1 is less than 0.8 L, as described by Block and Olsen (1976). This technique has also been used to monitor pulmonary function following pulmonary transplantation, as described by the Toronto Lung Transplant Group (1986), and to assess patients preoperatively prior to lung reduction surgery, as described by Wang and co-workers (1997).
Gallium and Indium Leukocyte Scintigraphy
Gallium 67 citrate is an iron analogue used for evaluation of both inflammatory and neoplastic lesions. Gallium 67 is a cyclotron-produced radionuclide with a physical half-life of 78 hours and several gamma photopeaks for imaging. Intravenously injected gallium is largely bound to serum transferrin in the blood, and is taken up by the liver, bone, kidneys, colon, spleen, salivary glands, and lacrimal glands. Approximately 10% to 25% of the injected dose is excreted by the kidneys during the first 24 hours and subsequently by the colon. In children, there is significant uptake by the normal thymus gland, making evaluation of the mediastinum difficult.
Gallium is actively accumulated in a variety of infectious and noninfectious inflammatory lesions and in certain tumors. The mechanisms of gallium uptake have been widely discussed and debated, as reviewed by Hoffer (1980a) and Palestro (1994). Gallium scintigraphy for detection of inflammatory processes usually includes imaging at 24 hours
P.2400
or even earlier, in order to rapidly diagnose acute infections. Further delayed images are also commonly obtained, to confirm the findings on the early images. Tumor imaging is more often performed at 48 to 72 hours or even later, when target/background ratios in lesions typically become higher. While doses as low as 3 to 5 mCi (111 185 mBq) of 67Ga citrate have been used, it is common to administer higher doses, in the range of 8 to 10 mCi (296 370 mBq).
Gallium scintigraphy is a sensitive method for the detection of mediastinal pathology, especially the presence of mediastinitis or lymphadenopathy. This evaluation is especially useful in patients with AIDS presenting with fever or respiratory symptoms, as reported by Kramer (1978), Bitran (1987), and Mehta (1987) and their associates. In these patients, the presence of mediastinal or hilar adenopathy often is due to mycobacterial infection, commonly by atypical mycobacteria such as Mycobacterium avium intracellulare (MAI) (Fig. 159-3). Other nonneoplastic causes of mediastinal adenopathy include viral or bacterial infections and other types of granulomatous disease, such as sarcoidosis.
Neoplasms of the mediastinum with high avidity for gallium include bronchial carcinoma, lymphoma, and metastases from mesothelioma, testicular tumors, and melanoma. Gallium imaging has proven to be particularly useful in the follow-up of these patients, since the gallium activity will resolve as the lesion is effectively treated, even though CT scans may still demonstrate residual soft tissue densities due to fibrosis at sites of prior adenopathy (Fig. 159-4). Furthermore, serial gallium imaging may provide useful prognostic data in patients undergoing chemotherapy or radiation therapy. Janicek and associates (1997) found that early restaging gallium scans during high-dose cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy permitted the differentiation between patients with aggressive non-Hodgkin's lymphoma likely to have prolonged disease-free intervals from those who will likely fail to respond, and are thus candidates for alternative treatment. Front and co-workers (1999) recently found similar results using gallium scintigraphy in patients with Hodgkin's disease (Hodgkin's lymphoma) undergoing chemotherapy. There was a statistically significant difference in the failure-free survival between patients with positive versus negative gallium scan results after one cycle of chemotherapy (p <0.002), whereas there was no significant difference noted between patients with positive versus negative CT scan results at midtreatment. Furthermore, 92% of patients with negative gallium scan results after one cycle and 82% with negative scan results at midtreatment remained in complete response. Front and associates (1997) also indicated that more than 50% of patients with lymphoma who achieve complete responses to therapy have residual soft tissue masses on palpation, radiographs, or CT. Furthermore, gallium imaging is a sensitive means of detecting recurrences early. In AIDS, mediastinal or hilar adenopathy on gallium images is usually due to lymphoma if no infectious process is present. Kaposi's sarcoma is not a gallium-avid tumor, and therefore should be suspected in AIDS patients with soft tissue chest masses on the chest radiograph that do not show increased gallium uptake. With respect to bronchial carcinoma, Hoffer (1980b) reviewed data from several other studies, indicating a sensitivity of 80% to 90% for all cell types, although some have reported a somewhat lower sensitivity in adenocarcinoma. Uptake by mediastinal adenopathy may not be of the same magnitude as uptake by a primary parenchymal lesion, which could have adverse implications with regard to staging of NSCLC with gallium imaging. In addition, there is a potential for false-positive findings in patients with inflammatory lesions. At present, PET imaging has proven to be superior both to gallium scintigraphy and CT in the staging and follow-up of NSCLC, lymphoma, and other tumors, as will be discussed, and is rapidly replacing gallium scintigraphy in these clinical settings, where available.
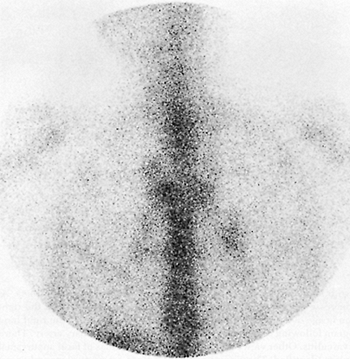 |
Fig. 159-3. Mediastinal and bilateral hilar adenopathy on a gallium study. The patient is a 36-year-old man with AIDS presenting with nonproductive cough and fever. Seventy-two-hour anterior gallium image of the chest showing bilateral hilar and right paratracheal lymphadenopathy. The patient was diagnosed as having Mycobacterium avium intracellulare infection, also involving the liver and bone marrow. From Spies WG: Radionuclide studies of the lung. In Shields TW (ed): General Thoracic Surgery. 3rd Ed. Philadelphia: Lea&Febiger, 1989. With permission. |
The use of single-photon emission computed tomography (SPECT) imaging can significantly enhance the detection of mediastinal and hilar adenopathy in gallium imaging. This modification of the study is performed using a gamma camera that is fitted with a specialized gantry or counterbalance mechanism that permits the camera to rotate a full 360 degrees around the patient. The acquisition of data at multiple angles
P.2401
during rotation around the patient allows the computer to reconstruct tomographic images in a fashion completely analogous to conventional x-ray CT, except that transaxial, sagittal, and coronal images can all be obtained with a single rotation, and reorientation of the images in different planes is also readily achieved. Tomographic images blur data from planes above and below the plane of interest, resulting in improved image contrast and thus improved detection of focal pathology. Although more commonly used in cardiac, brain, and bone imaging, SPECT can be undertaken in conjunction with any nuclear medicine procedure performed with a rotating gamma camera, and is often used as an adjunct to conventional planar gallium imaging. Tumeh and associates (1987) demonstrated significant gains in both sensitivity and specificity when using SPECT imaging compared with planar gallium imaging in patients with lymphoma. The SPECT studies were able to identify additional sites of involvement in the chest and abdomen, and to exclude abnormalities in sites that were equivocally abnormal on the planar images. In a study of gallium scintigraphy in the restaging of patients with aggressive non-Hodgkin's lymphoma undergoing high-dose CHOP chemotherapy, Janicek and associates (1997) found that SPECT imaging provided similar information at the time of diagnosis as planar imaging in patients with bulky, gallium-positive tumors, but also provided confirmatory information in treated lesions that were of lower intensity or located in proximity to normal anatomic structures.
 |
Fig. 159-4. Hodgkin's disease (Hodgkin's lymphoma) on a gallium study, before and after therapy. The patient is a 24-year-old woman with a new anterior mediastinal mass on chest radiography. A. Seventy-two-hour anterior image of the chest demonstrating a large mass with increased gallium uptake involving the mediastinum, with additional foci of adenopathy in the right anterior cervical region and both infraclavicular regions. The tissue diagnosis was Hodgkin's disease. B. Repeat study 7 months later, following chemotherapy, revealing complete interval resolution of the adenopathy. Clinically, the patient was in complete remission. |
In addition to gallium imaging, autologous leukocytes can be labeled with In-111 oxine and reinjected for imaging, as reviewed by Coleman (1982), Marcus (1984), and Datz (1994). This radiopharmaceutical is more specific for infection than gallium, although rarely it is taken up by neoplasms. Normal uptake is seen in the spleen, bone marrow, and liver. Indium 111 white blood cell (WBC) imaging may be preferred over gallium in certain clinical settings, but both are useful for evaluation of suspected mediastinal infections. It should be noted that uptake in the lungs is normal early after injection of approximately 500 Ci (18.5 MBq) 111In WBCs, and imaging is therefore usually performed at 18 to 24 hours. Partly for this reason, Fineman and co-workers (1987) and most other investigators favor the use of gallium over indium leukocytes when pulmonary parenchymal infections are suspected, as in AIDS patients. Unlike gallium, delayed imaging beyond 24 hours is rarely necessary in indium leukocyte scintigraphy. Gallium is probably also more useful in suspected chronic infections, since the majority of labeled leukocytes in 111In WBC imaging are neutrophils; thus, the sensitivity for chronic infection may be less. Other radiopharmaceuticals have also been developed for the detection of sites of infection, including 99mTc hexamethylpropylene amine oxime (HMPAO)-labeled leukocytes, monoclonal antigranulocyte and polyclonal immunoglobulin G nonspecific antibodies, chemotactic peptide agents, liposomes, nanocolloids, and others, as reviewed by Peters (1994) and by Becker (1994), Corstens (1993), and Fischman (1994) and their co-workers. Of these, only the 99mTc-labeled leukocytes have achieved widespread clinical use to date. As further discussed, FDG PET imaging has emerged as a powerful tool in oncologic imaging. Some of the most common false-positive findings on PET imaging are related to infectious and noninfectious inflammatory processes that are associated with increased glucose metabolism, including sarcoidosis, tuberculosis, abscesses, and other infections. As a result, FDG PET imaging may also play a potential role in the evaluation of suspected inflammatory processes, such as enterocolitis,
P.2402
musculoskeletal infections, cerebral infections and others, as described by Kresnik (2002), Temmerman (2001), DeWinter (2001), Stumpe (2000), Sugawara (1998), and Meyer (1994) and their co-workers. This topic has also recently been reviewed by Alavi and associates (2002) and by Zhuang and Alavi (2002). However, at present, this application of PET imaging remains primarily investigational.
Positron Emission Tomographic Imaging
The development of a growing body of literature supporting its use, coupled with recent regulatory and reimbursement approval breakthroughs, has led to PET imaging rapidly becoming a widely used diagnostic modality for the evaluation of patients with a variety of neoplasms. PET has long been available as a research tool in the evaluation of brain and cardiac metabolism, often using ultra-short-lived positron-emitting radionuclides such as carbon 11, nitrogen 13, and oxygen 15. Although these agents permit labeling of biologically important molecules that serve as excellent tracers of normal and abnormal metabolic functions, their physical nature (half-lives in the range of 2 20 minutes) demands that they be prepared locally for immediate use. This preparation requires the availability of an on-site medical cyclotron and equipment required to radiolabel the necessary compounds with these isotopes, which involves complex and expensive instrumentation. In addition, several dedicated personnel are required to operate and maintain this equipment, further adding to the cost. These considerations long relegated PET imaging to the realm of the research laboratory, and kept it beyond the reach of all but the most specialized medical centers. However, there has been a growing body of research developing over the past several years using another radiopharmaceutical for PET imaging: FDG. FDG is also a positron-emitting radiopharmaceutical, but it has the distinct advantage of a longer half-life of 1.83 hours (~110 minutes), permitting it to be transported over short distances within a metropolitan area. Thus, a private or commercial radiopharmaceutical vendor can operate a cyclotron and distribute the FDG regionally for use at many institutions at a more reasonable cost, obviating the need for a cyclotron at each individual site. FDG has proven to be of even greater value as a tumor imaging agent than as a cardiac or brain imaging agent, because it has been found that many malignant neoplasms exhibit increased glucose metabolism and increased expression of glucose transport proteins relative to normal tissue, and thus exhibit increased uptake of FDG on PET scans, as reviewed by Hoh and colleagues (1997a). Furthermore, FDG PET imaging has been shown to be significantly more sensitive and specific than traditional cross-sectional imaging modalities, such as CT and MR imaging, for the staging and restaging of many tumors. At present, PET imaging using FDG is a major diagnostic modality used in the diagnosis, initial staging, restaging, and detection of recurrences in a variety of neoplastic disorders, including, but not limited to, bronchial carcinoma, lymphoma, colorectal carcinoma, malignant melanoma, esophageal carcinoma, and head and neck carcinoma. It is also approved for use in detecting metastatic disease, recurrence or treatment monitoring in breast carcinoma, but not for the initial diagnosis or staging of breast carcinoma, and for the detection of occult recurrent well-differentiated thyroid carcinoma in patients with negative whole-body 131I scans, but elevated serum thyroglobulin levels. PET imaging has intrinsically superior spatial resolution and sensitivity than conventional single-photon (i.e., gamma ray) radionuclide imaging. Recent developments in PET scanner technology have resulted in reduced cost and improved imaging capability, including the capability of obtaining whole-body images. PET images are typically evaluated on a computer workstation, which permits interactive evaluation of tomographic images in the coronal, transaxial, and sagittal planes, as well as evaluation of rotating cine images of the entire data set, using three-dimensional maximum-intensity projection images (MIPs).
During the late 1980s, PET imaging was the subject of a series of reports from the Positron Emission Tomography Panel of the Council on Scientific Affairs of the American Medical Association (1988a e). One of these reports (1988c) described the potential impact of PET in the area of oncology, including evaluation of the metabolism and physiology of neoplasms, their effects on adjacent tissues, and development of target sites on tumors, such as monoclonal antibodies and specific growth factors. The unique ability of PET to quantitate these processes will be invaluable in future tumor biology research and in the evaluation of the responses of tumors to therapy.
Subsequent to these early reports, a large body of literature has developed that indicates the superiority of FDG PET imaging in the staging and restaging of several neoplasms, as previously noted, as well as demonstrating its cost effectiveness in this role. PET has been shown to be substantially more accurate in staging many tumors than CT or MR imaging. In the chest, the major lesions for which PET is routinely used and is Center for Medicare and Medicaid Services approved include evaluation of solitary pulmonary nodules and in the diagnosis, staging, and restaging of NSCLC, lymphoma, and esophageal carcinoma. Metastatic disease from other FDG-avid tumors, such as malignant melanoma, colorectal carcinoma, and breast carcinoma, are also readily detected. For example, in a multicenter trial of 89 patients, Lowe and co-workers (1998) found FDG PET imaging to be a highly sensitive technique for the characterization of indeterminate pulmonary nodules identified on CT, with an overall sensitivity and specificity of approximately 90%, using semiquantitation by means of calculating standardized uptake values to compare lesion uptake with surrounding normal tissues. Visual analysis was slightly more sensitive, but less specific. False-positive findings may occur in granulomas or in noninfectious inflammatory processes, such as sarcoidosis. False-negative imaging results may occur with cell
P.2403
types that may be associated with lower levels of metabolic activity, such as bronchoalveolar carcinoma and carcinoid tumors, as reported by Higashi and co-workers (1998) and by Rege and associates (1993), respectively. False-negative imaging results or underestimation of the metabolic activity of lesions may also occur in the case of very small pulmonary nodules, those less than 7 mm in diameter.
With respect to the mediastinum, the lesions most commonly evaluated by PET include NSCLC, lymphoma, and esophageal carcinoma. In the preoperative staging of NSCLC, one of the key factors is determining the presence or absence of mediastinal or hilar tumor involvement. This information affects not only assessment of operability, but also strategies for subsequent treatment and management of the patient. PET has been shown to be superior to CT imaging in this regard, with overall significantly greater sensitivity, specificity, and overall accuracy, as reviewed by Al-Sugair and Coleman (1998) and by Bar-Shalom and colleagues (2000). CT identifies adenopathy on the basis of size criteria, whereas PET relies on the finding of increased tracer uptake, indicative of increased glucose metabolism within the lesion. Major advantages of PET over CT include the ability to detect tumor involvement in lymph nodes that do not meet CT criteria for pathologic enlargement, and the ability to correctly exclude the presence of tumor involvement in enlarged, nonneoplastic nodes identified on CT. Webb and co-workers (1991) reported the overall sensitivity and specificity of CT in the staging of NSCLC to be only 52% and 69%, respectively. By contrast, as reviewed by Al-Sugair and Coleman (1998), numerous studies have shown PET to have sensitivities and specificities for mediastinal lymph node staging in the range of 70% to 90% and 75% to 100%, respectively. Pieterman and associates (2000) reported a sensitivity and specificity of PET in NSCLC staging of mediastinal involvement of 91% and 86%, respectively, compared with values of 75% and 66% for CT, respectively. Overall, PET was 95% sensitive and 83% specific for the detection of mediastinal involvement and distant metastatic disease. They also found PET to be the only procedure that positively correlated with histopathologic findings in mediastinal lymph nodes, and found that the results of the PET study resulted in changes in staging in 62 of 102 patients, raising the stage in 42 and lowering it in 20. Because of the potential for false-positive results secondary to infectious or noninfectious inflammatory processes, such as fungal infection, tuberculosis or sarcoidosis, it is recommended that patients with positive findings in the mediastinum have histologic verification by mediastinoscopy or other methods before surgical management is ruled out. On the other hand, the very high negative predictive value of PET obviates the need for preoperative invasive procedures. Finally, the intrinsic whole-body nature of PET imaging also permits the detection of occult distant metastases, such as involvement of the adrenal glands, liver, or skeleton. PET imaging is also extremely valuable in the follow-up assessment of the response to therapy, again outperforming CT in detecting recurrences and differentiating between posttreatment fibrosis versus residual or recurrent tumor involvement. Examples of FDG PET studies in patients with NSCLC are shown in Fig. 159-5. FDG PET is also sensitive for the detection of small cell lung carcinoma, but because of the typically extensive involvement encountered in small cell carcinoma at initial presentation and its usual poor prognosis, management is often similar for many patients. Therefore, it has been more difficult to date to establish a cost-effective role for PET imaging in this disorder. A more limited role for PET in small cell lung carcinoma may emerge in the future.
Another common neoplasm involving the mediastinum for which FDG PET imaging plays an important role is lymphoma, including both Hodgkin's lymphoma and non-Hodgkin's lymphoma. Whole-body FDG PET imaging has been shown to be an accurate and cost-effective method for staging or restaging lymphoma relative to CT, MR imaging, and other conventional staging modalities, as reported by Hoh and associates (1997b). Kostakoglu and Goldsmith (2000) found that PET imaging is equivalent or superior to CT for initial staging of lymphoma. More importantly, as in the case of NSCLC, PET has proven to be superior to CT in the evaluation of patients with lymphoma for assessment of the response to therapy and for detection of recurrences. Jerusalem and associates (1999) reported that PET found 100% of recurrences in patients with residual soft tissue masses on CT posttreatment, whereas only 26% of PET-negative cases presented with recurrence subsequently. The positive predictive value for recurrence was 100% for PET, as compared with only 42% for CT. PET can also be used for treatment monitoring, since a positive response to therapy may be detected by a decrease in FDG uptake in lesions after as few as one or two cycles of chemotherapy, as reported by Jerusalem and associates (2000). An example of the role of PET in the restaging of lymphoma is shown in Fig. 159-6. PET also has been shown to be at least as accurate, and more likely superior to gallium scintigraphy in this entity, as reported by Willkomm and colleagues (1998) and later by Mahajan and associates (2002). More recently, Bar-Shalom and co-workers (2003) reported similar findings when comparing gallium scintigraphy to the less sensitive coincidence gamma camera positron imaging technique, in which a standard nuclear medicine gamma camera is modified to detect positron decay using coincidence detection circuitry. As in the case of lung carcinoma, false-negative imaging results may occur with small lesions and in certain cell types, for example, the mucosa-associated lymphoid tumor (MALT) variety, as reported by Hoffmann and associates (1999).
Finally, PET imaging has developed an increasing role in the initial staging and follow-up of patients with esophageal carcinoma. In normal studies, faint uptake may be seen in the distal esophagus, which may represent a normal variant, or in some cases may reflect mild inflammatory changes secondary to gastroesophageal reflux. As recently reviewed by Kostakoglu and associates (2000), FDG PET is sensitive for the detection of both squamous cell and adenocarcinoma
P.2404
of the esophagus, except for small (<5 mm) T-1 weighted lesions. PET is approximately 80% sensitive and 95% specific overall in esophageal carcinoma, and detects occult distant metastases missed by CT in approximately 20% of cases, as reported by Block and associates (1997) and by Yeung and co-workers (1999). Neither PET nor CT is sensitive for the staging of mediastinal nodal involvement, although PET is more accurate than CT in this regard. Yoon and associates (2003) recently reported a series of 81 patients who underwent preoperative PET and CT imaging. For the detection of the primary lesions, PET correctly identified the primary lesion in 91% of patients, compared with 80% for CT. For staging of lymph node involvement, PET was 30% sensitive and 90% specific for detection of nodal involvement at the time of preoperative evaluation, compared with only 11% sensitivity for CT. CT was slightly more specific than PET, with 95% specificity, but overall accuracy was nearly equal. With respect to thoracic nodal involvement, PET detected 31% of the positive nodes found at surgery, compared with only 3% for CT. Again, PET is also superior to CT for detection of recurrent disease and in predicting the response to neoadjuvant therapy, as reported by Kelly and colleagues (2001). The response to preoperative chemotherapy and radiation therapy, as judged by PET imaging, may prove to be a useful indicator for the stratification of patients for subsequent medical versus surgical management.
P.2405
Examples of primary and metastatic esophageal carcinoma are shown in Fig. 159-7.
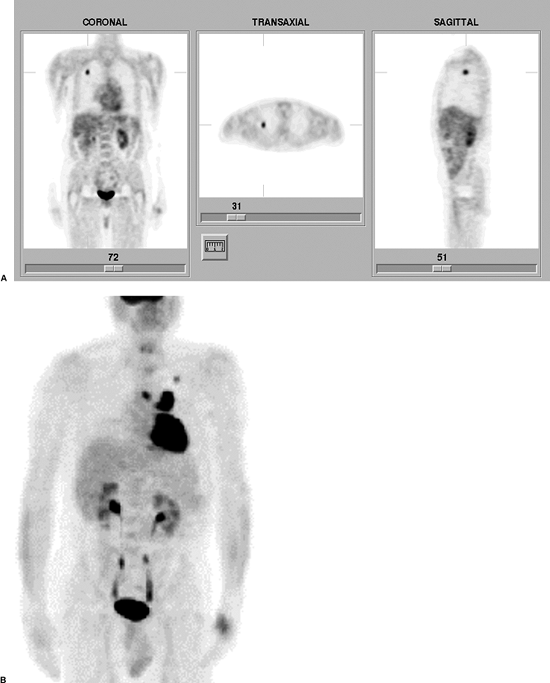 |
Fig. 159-5. FDG PET in NSCLC. A. FDG PET scan in a solitary pulmonary nodule. Coronal, transaxial, and sagittal images from an FDG PET scan demonstrate a focal area of increased tracer uptake in the right upper lobe, corresponding to a 1.1-cm soft tissue nodule in this region on a recent computed tomographic scan of the chest (not shown). The lesion demonstrated an elevated maximum standardized uptake value of 4.3, which is consistent with malignancy. The patient proved to have NSCLC without mediastinal or hilar involvement at surgery. B. Another patient presenting with a focal pulmonary nodule in the left upper lobe on a chest radiograph. A three-dimensional maximum intensity projections FDG PET image demonstrates increased uptake within the pulmonary nodule. In addition, there is markedly increased uptake in bilateral foci of mediastinal adenopathy (left greater than right), consistent with metastatic disease. |
 |
Fig. 159-6. FDG PET in lymphoma (pre- and posttherapy). The patient is a 32-year-old man who presented with recent onset of shortness of breath and chest discomfort. A. A CT image of the chest demonstrates extensive mediastinal adenopathy. Subsequent biopsy revealed nodular sclerosing Hodgkin's disease. B. An FDG PET scan at presentation (maximum intensity projections image) demonstrates extensive mediastinal, right hilar, and left supraclavicular adenopathy, corresponding to the areas of involvement on CT, and consistent with active disease in these sites. The striking uptake in the left lower chest represents normal myocardial activity. C. A repeat CT scan obtained 6 months later, following a course of six cycles of chemotherapy, demonstrates marked interval improvement in mediastinal adenopathy, but with definite residual abnormal soft tissue density in this region. D. A follow-up PET examination obtained at the same time as the follow-up CT demonstrates complete interval resolution of abnormal uptake, with no abnormal foci remaining, consistent with complete remission (see text for further details). |
In the case of breast carcinoma, PET imaging has been used both in the characterization of benign versus malignant lesions as well as in the evaluation of the extent of tumor spread, especially with respect to axillary lymph node involvement, as discussed by Flanagan and associates (1998). Whether or not a negative PET result is sufficient to obviate the need for axillary lymph node dissection is not clearly established at present, and the recommended approach to staging the axillae in breast carcinoma remains lymphoscintigraphy with directed lymph node dissection and immunohistochemical analysis, as reviewed by Bar-Shalom and associates (2000). PET may also permit superior evaluation of the internal mammary nodes, which, as previously discussed, are difficult to evaluate using other existing modalities. PET may also play a role in the follow-up of patients, providing data regarding prognosis and the
P.2406
response to therapy. PET is more sensitive than bone scans for detecting osteolytic skeletal metastases, and can be used to monitor the response to chemohormonal therapy, even early after initiation of treatment, as described by Wahl and associates (1993) and subsequently reviewed by Bar-Shalom and colleagues (2000). Research involving PET imaging using fluorine 18 labeled estrogen analogues holds promise in the assessment of the receptor function of breast tumors, which may also have significant impact on therapeutic decisions.
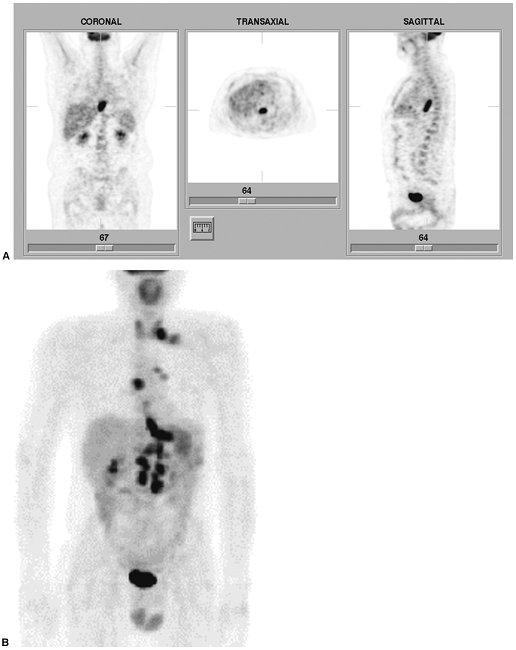 |
Fig. 159-7. FDG PET in esophageal carcinoma. A. FDG PET scan (coronal, transaxial, and sagittal images) in a 55-year-old man with recently diagnosed distal esophageal carcinoma. There is a focal area of intensely increased tracer uptake along the course of the distal esophagus, corresponding to the primary lesion. There is no evidence of metastatic disease. B. Another patient presenting with a newly diagnosed lesion in the distal esophagus, extending to the gastroesophageal junction. The maximum intensity projections image from an FDG PET scan demonstrates increased uptake in the primary lesion, but also evidence of widespread metastatic disease, involving the mediastinum, left hilum, left supraclavicular lymph nodes, retroperitoneal nodes, and liver. Normal bladder activity is noted in the midline inferior pelvis. |
The greater accuracy of PET compared with CT in the staging and follow-up of patients with mediastinal neoplasms can result in significant cost savings, such as eliminating unnecessary surgical procedures, as reviewed by Al-Sugair and Coleman (1998). For example, given the high negative predictive value of PET for staging of mediastinal lymph node involvement in NSCLC, a negative PET result can obviate the need for preoperative mediastinoscopy for staging. Patients with positive mediastinal findings on PET should still undergo follow-up confirmatory biopsy, to prevent denying potentially curative surgery to patients with false-positive PET results secondary to sarcoidosis, tuberculosis, or other inflammatory disorders. Whole-body PET studies can detect metastatic disease that was unsuspected using conventional imaging modalities, and can also correctly characterize some anatomic lesions detected by CT as representing benign lesions. Changes in patient management have been reported to occur in up to
P.2407
41% of patients undergoing whole-body PET imaging, as noted by Al-Sugair and Coleman (1998). In addition, as discussed, PET is playing an increasingly important role in the assessment of the response to therapy, in light of its extreme accuracy in differentiating between soft tissue masses on CT or MR imaging due to recurrent tumor versus postradiation therapy or postchemotherapy fibrosis and scarring. Recurrent tumor demonstrates increased FDG uptake, whereas fibrosis and scar tissue do not.
Prior to the widespread availability of dedicated PET scanners at reasonable cost, attention was directed to the development of instrumentation permitting positron imaging using conventional gamma cameras modified for positron detection. SPECT cameras with thicker sodium iodide crystals and additional circuitry to detect coincidence events (which indicate a positron decay within the patient) permit lower-resolution FDG images to be acquired. The additional cost of including these modifications to a gamma camera is offset by the added flexibility of being able to use one camera for both conventional planar and SPECT as well as positron imaging. However, with the wider availability of dedicated PET scanners, as well as the demonstration that dedicated PET imaging devices are necessary in order to maximize the sensitivity and resolution of positron imaging studies, the use of these modified gamma cameras for positron imaging is now on the wane.
Lymphoscintigraphy
Radionuclide lymphoscintigraphy is an easily performed, noninvasive alternative to contrast lymphangiography for evaluation of regional lymphatics. The study is performed by injecting small radioactive colloids into subcutaneous or intradermal sites, or directly within or adjacent to certain tumor masses. Previously, this technique was most widely used to evaluate the internal mammary lymph node chains in patients with breast carcinoma, both for identification of normal versus abnormal nodes preoperatively and as a guide to radiation therapy treatment planning. The technique and interpretation of internal mammary node breast lymphoscintigraphy has been extensively described by Ege (1976, 1983). The radiopharmaceutical in common usage at that time for lymphoscintigraphy was 99mTc antimony sulfide colloid, consisting of particles in the range of 4 to 12 microns in diameter. This agent is no longer commercially available, and has been replaced by several other agents, including human serum albumin and both filtered and unfiltered sulfur colloid, all labeled with 99mTc. In Ege's studies, injection of 500 Ci (18.5 MBq) was made first in the subcostal region on the side of the breast tumor, just anterior to the posterior rectus sheath. Immediate images of the injection site were obtained, and then 3-hour delayed images were obtained to evaluate nodal uptake. If the study was judged technically adequate, then repeat injection was made on the contralateral side and further delayed images obtained in another 3 hours. Normal studies demonstrate bilateral focal areas of nodal uptake in the parasternal regions. If nodes are involved by tumor, they are either nonvisualized if the lymphatics are completely blocked or there may be enlargement and distortion of the nodes or focal defects. Meyer and Munzenrider (1981) and associates and Collier and co-workers (1983) pointed out that these nodes, which are not accessible to physical examination, are also difficult to visualize either on standard chest radiographs or CT scans. Matsuo (1974)X found 90% true-positive and 100% true-negative lymphoscintigrams in preoperative evaluation of patients with breast carcinoma. Parasternal lymph node involvement in breast carcinoma may occur in one third of patients, is not limited to inner quadrant lesions, and is associated with a significant worsening of prognosis, especially when axillary lymph node involvement is also present. Ege (1976) reported that the prognosis of clinical stage II patients with positive lymphoscintigraphy studies was as poor as stage III patients. Ege and Clark (1980) further found that in 243 patients undergoing lymphoscintigraphy following partial mastectomy, an abnormal result was associated with a threefold increase in the incidence of distant disease and 67% overall incidence of recurrence, compared with 34% in patients with normal studies. With respect to radiation therapy planning, Dionne and colleagues (1983) found that initially planned radiation ports needed to be modified in 20% of cases. Collier and associates (1983) found that ports were altered in 60% of stage I and II patients undergoing adjuvant irradiation. Modifications of radiation ports may include enlargement of the port to include parasternal nodes located lateral to standard ports or the ability to reduce port size if nodes are more centrally located. Furthermore, because of cross-drainage patterns, inclusion of the contralateral nodes may be needed in some cases. This procedure never gained widespread clinical acceptance, and is now rarely performed clinically.
At present, lymphoscintigraphy is most often performed in most nuclear medicine laboratories to evaluate lymphatic drainage patterns in truncal melanomas, to localize the axillary lymph nodes preoperatively or intraoperatively in patients with melanoma and breast carcinoma, and to detect primary and secondary lower extremity lymphatic obstruction. Of these, the pre- and intraoperative localization of the sentinel node has received the most attention recently, as reviewed by Alazraki (1997) and Glass (1999) and their associates. The presence or absence of metastatic disease in the axillary nodes is the most important prognostic indicator in patients with breast carcinoma or melanoma of the upper extremity. It has been demonstrated that axillary lymph node dissections performed in patients with upper extremity melanomas or breast carcinoma can be significantly limited without loss of diagnostic accuracy if certain key lymph nodes are identified and examined histologically. The sentinel node represents the first node (or occasionally, more than one node) that drains the region of the breast or cutaneous lesion. If this node is removed surgically and found to be free of tumor, then a more extensive lymph node dissection
P.2408
is not necessary. In over 90% of cases, a negative sentinel node correctly predicts the absence of other positive axillary nodes, as reported by Glass and associates (1999). Furthermore, the sentinel node itself may be the only positive node present in 38% to 67% of cases. Lymphoscintigraphy studies can accurately identify the sentinel node when injection of the radiopharmaceutical is injected either intradermally surrounding a cutaneous melanoma lesion (or resection site) or subcutaneously or interstitially surrounding the site of a breast neoplasm or lumpectomy site. A recent paper by Kumar and associates (2003) described the results of retrospective analysis of sentinel node lymphoscintigraphy and blue dye studies in 59 patients with multicentric or multifocal breast carcinoma. They found concordance between the blue dye studies and lymphoscintigraphy in 91% of cases, with only 37.5% of patients having only a single sentinel node identified. The negative predictive value of lymphoscintigraphy for sentinel node evaluation was 100% in this study. When imaging is performed, the sentinel node can be localized using the gamma camera's persistence scope and a radioactive hot marker, and the lesion can then be marked with an indelible pen on the patient's skin, typically in two projections that mimic the surgical position. An example of a sentinel node lymphoscintigraphy study is shown in Fig. 159-8. Additionally, scintigraphic probe detectors have been developed that permit the surgeon to intraoperatively map out the location of the sentinel node by assessing the count rate in the axilla as the area is scanned with the highly sensitive and directional probe detector. This method has proven to be comparable with or perhaps superior to the intraoperative blue dye method of nodal localization. Some surgeons prefer to use both modalities simultaneously. At present, considerable controversy remains regarding the relative roles of imaging versus probe measurements in sentinel lymph node localization, but many authorities believe that the inclusion of imaging provides important additional information regarding the number and location of sentinel nodes, as well as indicating the presence of other potential routes of spread, such as internal mammary lymph node involvement in breast carcinoma. In summary, lymphoscintigraphy is an easily performed, noninvasive method, which can significantly improve the accuracy of staging of breast carcinoma and melanoma and contribute to treatment planning.
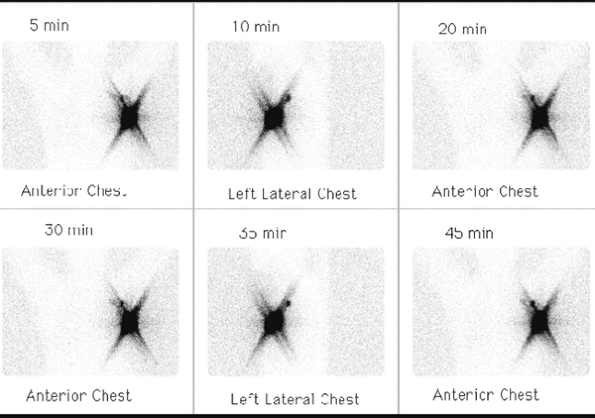 |
Fig. 159-8. Lymphoscintigraphy with sentinel lymph node mapping. Serial images from a lymphoscintigraphy study in a patient with recently diagnosed carcinoma of the left breast demonstrate markedly increased uptake in the region of the injections sites, overlying the patient's left breast mass. The linear star artifact emanating from the region of the injection sites is secondary to the phenomenon of septal penetration artifact, which occurs when high concentrations of activity exist in a small location, allowing some photons to penetrate between the holes of the gamma camera collimator. In addition, there is visualization of a focal area of lymph node uptake superior and posterior to the lesion, representing the sentinel left axillary lymph node (see text for further details). |
Octreotide Imaging
There has been considerable effort given to the development of new peptide imaging agents mentioned previously. Of these agents, the most widely adopted and only FDA-approved radiopharmaceutical to date has been an analogue of the somatostatin receptor agent octreotide. Somatostatin is a naturally occurring peptide hormone first isolated from hypothalamic extracts, as reviewed by Olsen and associates (1995). Acting as an antigrowth hormone factor, this peptide inhibits the synthesis and secretion of various peptide hormones by neuroendocrine tissue, including insulin, gastrin, vasoactive intestinal peptide, and many others. Octreotide is a synthetic somatostatin analogue possessing a longer serum half-life and higher potency. Early imaging studies used a 123I-labeled octreotide. Disadvantages of this agent included the short 13-hour half-life of 123I, a technically difficult labeling procedure, and interference by large amounts of intestinal activity secondary to its rapid hepatic and biliary clearance, as discussed by Krenning and co-workers (1992). The commercially available, FDA-approved agent Octreoscan (Mallinckrodt Inc., Hazelwood, CO) is the 111In-labeled octreotide derivative pentetreotide, which incorporates the compound diethylenetriaminepentaacetic
P.2409
acid (DTPA), which is commonly used in radiopharmaceuticals, being a glomerular filtration imaging agent itself when labeled with 99mTc. This radiopharmaceutical localizes with high affinity in a number of neuroendocrine neoplasms, including carcinoid tumor, islet cell carcinoma of the pancreas, medullary carcinoma of the thyroid, pheochromocytoma, paragangliomas, pituitary tumors, small cell lung carcinoma, and some lymphomas. It has demonstrated sensitivity in the 80% 100% range for the detection of many neuroendocrine tumors, with lower sensitivities reported for insulinomas and medullary carcinoma of the thyroid. An example of an abnormal pentetreotide scan is shown in Fig. 159-9. As is the case for most new tumor imaging agents and even gallium scintigraphy, SPECT imaging has proven to be an essential element of octreotide imaging in order to maximize the sensitivity of the study. A negative octreotide scan result in a patient with large pancreatic or hepatic lesions is strong evidence against a neuroendocrine tumor as the etiology of the lesions. The role of this radiopharmaceutical in the evaluation of patients with Hodgkin's lymphoma and non-Hodgkin's lymphoma relative to gallium imaging and other modalities is far more limited, as reviewed by Goldsmith and co-workers (1995), and as discussed, these patients are now primarily evaluated with FDG PET imaging. Clinical trials are currently underway to evaluate the efficacy of radiolabeled octreotide analogues for radionuclide therapy of neuroendocrine tumors, as reviewed by Boerman and colleagues (2000).
Metaiodobenzylguanidine Imaging
Pheochromocytomas are tumors containing adrenal medullary tissue, and are located in the adrenal glands in approximately 90% of patients. These tumors are initially detected by elevations of biochemical markers, including various catecholamine metabolites, such as vanillylmandelic acid (VMA) and metanephrines. The adrenal lesions are often localized on CT as large soft tissue masses, which may have necrotic centers. In the remaining 10% of cases, the lesion or lesions are extraadrenal, often located along the paravertebral paraaortic or mediastinal regions. These lesions are detectable in some cases by CT or MR imaging, but only if extensive imaging of the entire chest, abdomen, and pelvis is performed. The radiopharmaceutical 131I-MIBG localizes in these tumors as well as related neural tumors, including ganglioneuromas and neuroblastomas in children. This radionuclide study, described by Sisson and associates from the University of Michigan (1981), has the advantage of whole-body imaging, so the lesions can be detected regardless of location, and has been shown by Francis and co-workers from the same group (1983) to be complementary to CT. Lynn and colleagues from the University of Michigan group (1985) later developed an 123I-labeled form of the radiopharmaceutical, which has the advantage of more favorable radiation dosimetry and superior imaging
P.2410
characteristics. Iodine 123 MIBG in their hands was more sensitive than the 131I form, and is also more amenable to the use in conjunction with SPECT imaging. An example of a mediastinal pheochromocytoma is shown in Fig. 159-10. In a total of 400 patients, Shapiro and co-workers (1985) from the same group reported an overall sensitivity of 87.4% and specificity of 98.9% for 131I-MIBG, including patients with primary, sporadic pheochromocytoma; malignant pheochromocytoma and familial pheochromocytoma, with slightly poorer results in the former category. Lindberg and colleagues (1988) suggested that better results may be obtained by using a higher dose of 1 mCi instead of the 0.5-mCi dose advocated by the University of Michigan group, without an unacceptably high radiation dose to the adrenal, a suggestion that has been widely adopted. Thyroid uptake of free radioiodide, which would result in a significant radiation dose to the thyroid, is blocked by administration of iodine solution or perchlorate during the study. An example of a positive imaging result in a mediastinal neuroblastoma in a 2 -year-old child is shown in Fig. 159-11.
 |
Fig. 159-9. Abnormal Octreoscan study. Forty-eight-hour whole-body images in a 33-year-old man with a left perihilar mass on a prior chest radiograph (not shown) and clinically suspected carcinoid tumor of the chest. There is a large focal area of markedly increased activity in the left perihilar region (arrow), which corresponded to the lesion on the prior chest radiograph. This finding supports the diagnosis of carcinoid tumor, although increased octreotide uptake may also be seen in small cell lung carcinoma. This finding would not be present in the case of a squamous cell carcinoma or an adenocarcinoma of the lung. The uptake in the liver, spleen, kidneys, bladder, bowel, and thyroid are normal. |
Monoclonal Antibodies
Murine-produced monoclonal antibodies directed against a wide variety of human tumor antigens have been developed using hybridoma techniques, as discussed by Halpern (1983), Rosen (1985), and Keenan (1985) and their coinvestigators and by Larson (1985). Many of these antibodies have high affinity for human neoplasms, although there is often some overlap in specificity for related tumors. Currently, newer monoclonal antibodies with superior tumor uptake characteristics, including human and chimeric antibodies, have been developed. Monoclonal antibodies have been successfully radiolabeled with a variety of radionuclides, including 131I, 111In, yttrium 90, 99mTc, and others. These radiopharmaceuticals have been evaluated for use in imaging of neoplasms, such as lymphomas, melanoma, and lung, colon, ovarian, prostatic, and breast carcinoma. At present, several monoclonal antibody radiopharmaceuticals have received FDA approval for clinical use as imaging agents, including agents directed against colorectal, ovarian, and prostate carcinoma and anticarcinoembryonic antigen antibodies, as reviewed by Abdel-Nabi and Doerr (1993), as well as by Neal (1993) and Podoloff (1993) and their associates. Examples of monoclonal antibody studies demonstrating abnormalities in the mediastinum and chest are shown in Figs. 159-12, 159-13, 159-14 and 159-15. These studies have the potential advantage of more specific detection of tumor than standard anatomic imaging techniques, such as CT, analogous to the advantages of FDG PET imaging. For example, it is possible to have enlarged, but nonneoplastic lymph nodes detected by CT or, conversely, to have normal-sized, but involved nodes, as often occurs in Hodgkin's lymphoma. Furthermore, monoclonal antibody imaging also allows for whole-body surveys, leading to the potential for detection of occult disease in clinically unsuspected sites.
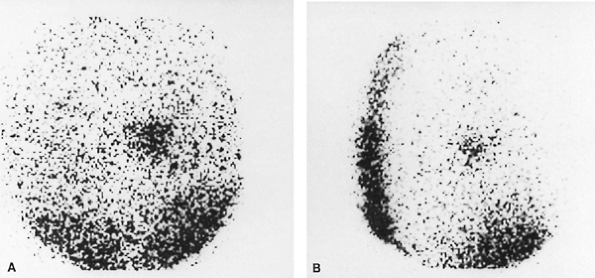 |
Fig. 159-10. Mediastinal pheochromocytoma diagnosed on an 131I MIBG study. The patient had symptoms and biochemical abnormalities suggestive of pheochromocytoma and had undergone a negative laparotomy 3 years earlier, without benefit of preoperative localization. An MIBG study was performed because of continued symptoms and persistent biochemical abnormalities. A. Posterior image of the chest demonstrating a large focal area of increased activity in the mediastinum, slightly to the right of midline. B. Right lateral chest image, localizing the lesion with certainty to the mediastinum. Subsequent to this study, a CT scan of the chest confirmed a lesion in this location, and at surgery a benign pheochromocytoma involving the left atrium was found and removed, with complete resolution of all clinical findings. Case courtesy of Richard L. Wahl, M.D., University of Michigan Medical Center, Ann Arbor, MI. With permission. |
Labeling monoclonal antibodies with high-energy beta- or alpha-emitting radionuclides, such as 131I and 90Y, allows
P.2411
these agents to be used therapeutically, analogous to the use of free 131I for treatment in well-differentiated thyroid carcinoma. Unlabeled monoclonal antibodies are also used for treatment of various neoplasms, most commonly non-Hodgkin's lymphoma. The most commonly used agent of this type is the murine monoclonal antibody rituximab (Rituxan, Genentech, Inc., Vacaville, CA). The addition of radiolabeling with -emitting isotopes to the monoclonal antibody has been shown to enhance the tumoricidal effects of the agent. In general, radioimmunotherapy is associated with far fewer systemic side effects than standard chemotherapy or irradiation, although in some instances the high doses of radioactivity used may require hospitalization of patients for radiation safety considerations, particularly if the radionuclide used also has significant gamma emission, like 131I. These therapeutic agents are associated with clinically significant bone marrow depression or, less often, other toxicity, but these adverse effects are usually self-limited, and do not frequently require further intervention.
At present, most of the therapeutic monoclonal antibody agents being evaluated are directly against non-Hodgkin's lymphoma, as reviewed by Bischof Delaloye (2000). Previous agents of this type, such as the LYM-1 antibody (Oncolym, Peregrine Inc., Tustin, CA) were used with some success during the 1980s and 1990s, as reported by DeNardo and co-workers (1998). Newer agents are directed against the CD-20 cell surface antigen, which is found on over 90% of non-Hodgkin's lymphoma tumor cells. Studies to date have focused on the treatment of patients with refractory disease or those who have undergone transformation to more aggressive forms of non-Hodgkin's lymphoma. Studies demonstrating significant efficacy of these agents in this clinical setting, following standard therapy and even in patients' refractory to unlabeled monoclonal antibody therapy, as reported by Witzig and associates (1999, 2002a, 2002b), have resulted in recent FDA approval of one 90Y-labeled agent, ibritumomab tiuxetan (Zevalin, IDEC Pharmaceuticals Corporation, San Diego, CA). Overall response rates of approximately 70% have been reported, with complete response rates of up to 15% to 30%, lasting for a mean of approximately 7 months. An example of pre- and posttherapy PET studies in a patient with non-Hodgkin's lymphoma treated with Zevalin is shown in Fig. 159-16. Another 131I-labeled agent, tositumomab (Bexxar, Corixa Corporation, Seattle, WA), directed against the same antigen, has demonstrated similar efficacy, and may soon receive approval as well. Hematologic toxicity is the primary form of toxicity noted in these patients, but as mentioned earlier, is usually only moderate in degree and is self-limited. Even patients with mild thrombocytopenia can safely be treated with reduced doses, as reported by Wiseman and colleagues (2002). Future developments in this field of research will focus on the wider application of radioimmunotherapy, the continued development of new antibodies having greater affinity and specificity for specific neoplasms, and perhaps the development of more human or humanized monoclonal antibodies, which can further reduce the problem of the development of human antimouse antibody (HAMA) responses, as described by De Jager and associates (1993). The development of HAMA, when it occurs, interferes with the repeated use of the antibody for imaging or therapy.
 |
Fig. 159-11. Mediastinal neuroblastoma on an 131I MIBG study. The patient is a 2 -year-old child with myoclonus and opisthotonos, treated unsuccessfully for several weeks with anticonvulsives. Laboratory data revealed elevated urinary VMA levels. A. Anteroposterior chest radiograph demonstrating a mediastinal soft tissue mass to the right of midline (arrows). B. Anterior 131I-MIBG image with radioactive markers on both axillae and iliac crests. There is a focal area of increased uptake in the mediastinum, to the right of midline (arrow), corresponding to the radiographic lesion. The lesion proved to be a neuroblastoma. The midline focus of activity in the pelvis represents free 131I within the urinary bladder. Case courtesy of James J. Conway, M.D., Children's Memorial Hospital, Chicago, IL. |
Thyroid and Parathyroid Imaging
Thyroid imaging using 99mTc pertechnetate or iodine radionuclides (123I or 131I) is primarily used for evaluation of thyroid size and morphology, detection and assessment of the functional status of thyroid nodules, evaluation of patients with suspected abnormalities in thyroid function,
P.2412
and detection of metastatic disease in patients with well-differentiated thyroid carcinoma. Iodine radionuclides are trapped and organified by the gland. Technetium 99m pertechnetate is a monovalent anion similar in size to an iodide ion, and is also trapped, but not organified, by the thyroid. Because of the high sensitivity of radionuclide thyroid imaging for the detection of functioning thyroid tissue, it is extremely valuable for the detection of ectopic functioning thyroid tissue. Specifically, thyroid imaging can be used to detect substernal extension of the thyroid presenting as a mediastinal mass or the presence of functioning metastases from well-differentiated thyroid carcinoma.
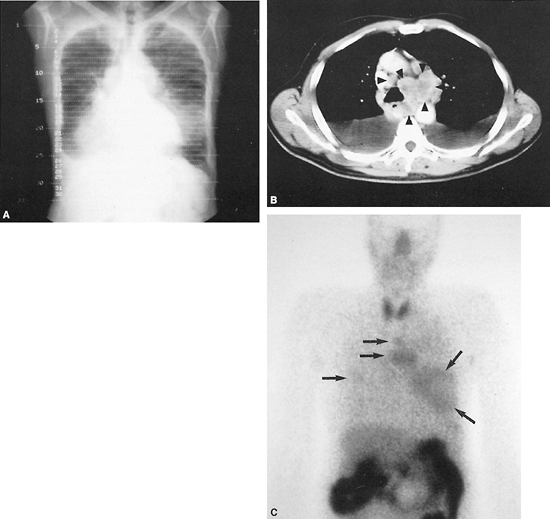 |
Fig. 159-12. Small cell bronchogenic lung monoclonal antibody study. The patient is a 72-year-old man with small cell carcinoma of the lung diagnosed by bronchoscopy. Bone scintigraphy and CT scans of the abdomen and pelvis were negative. A. Pilot radiograph of the chest from a CT scan, demonstrating marked mediastinal widening, bilateral lung masses (the largest of which is located in the left lower lung field), and a right pleural effusion. B. CT scan of the chest at the level of the aortopulmonary window and great vessels, demonstrating extensive mediastinal adenopathy (arrowheads) and bilateral pleural effusions. C. Anterior technetium 99m NRLU-10 (Nexell Therapeutics, Inc., Irvine, CA) monoclonal antibody images of the lower head and neck, chest, and upper abdomen performed approximately 12 hours after administration of the radiolabeled monoclonal antibody. Several foci of tumor uptake are noted in the lung fields and mediastinum bilaterally (arrows), corresponding to the lesions noted on the chest radiograph and CT scan. Subtle foci of uptake are also questioned in both axillary regions. Intense activity in the abdomen represents bowel activity, which is a normal finding on this study. Normal thyroid uptake is also noted, possibly secondary to small amounts of free 99mTc pertechnetate. Case published with permission of the NeoRx Corporation, Seattle, WA, U.S.A. |
The presence of substernal extension of the thyroid may be suggested by the incidental finding of a superior mediastinal mass with tracheal deviation on a routine chest radiograph. Evaluation of substernal extension of the thyroid is now most often performed using 123I sodium iodide. Iodine 131 was previously used for this purpose, but has been largely replaced by 123I because of the latter's much lower radiation dose to the patient and the relatively better spatial resolution of the 123I images. The shortcomings of 131I as a diagnostic imaging agent result from the long 8-day half-life and beta decay of 131I (resulting in a high patient radiation dose and limiting the dose that may be administered) and high 364 keV energy gamma emission, which is not optimal for the nuclear medicine gamma camera, resulting in lower-resolution images. The previously discussed advantage of using 131I rather than 99mTc pertechnetate because of
P.2413
the former's higher energy, allowing better penetration of photons through the sternum, is probably a minor factor that is far less significant than the aforementioned considerations. Iodine 123 has a half-life of only 13 hours and a gamma photon energy of 159 keV, which is readily imaged with a gamma camera. Iodine 123 offers some advantages over 99mTc pertechnetate in this application as well, because it can be imaged anywhere from 4 to 24 hours after administration, and is associated with less interfering background activity in the blood pool and soft tissues. Blood pool activity
P.2414
in the great vessels on pertechnetate images may interfere with the visualization of small foci of thyroid tissue or poorly functioning mediastinal goiters. In addition, 123I may be given orally in capsule form, typically in doses of 200 to 300 Ci (7.4 11.1 MBq), whereas 99mTc pertechnetate must be given intravenously, in doses of 8 to 10 mCi (296 370 MBq). Radionuclide thyroid imaging must be performed prior to CT imaging with iodinated contrast material or any other studies involving administration of contrast material. Even small amounts of nonradioactive iodine released into the bloodstream from iodinated contrast material can flood the extracellular iodide pool, resulting in competitive inhibition of tracer uptake by the thyroid, and thus nonvisualization of thyroid tissue. If the patient has recently received iodinated contrast material or has ingested large amounts of iodine from food or other medications, then radionuclide thyroid imaging must be delayed for at least 4 weeks. An example of substernal extension of the thyroid is shown in Fig. 159-17.
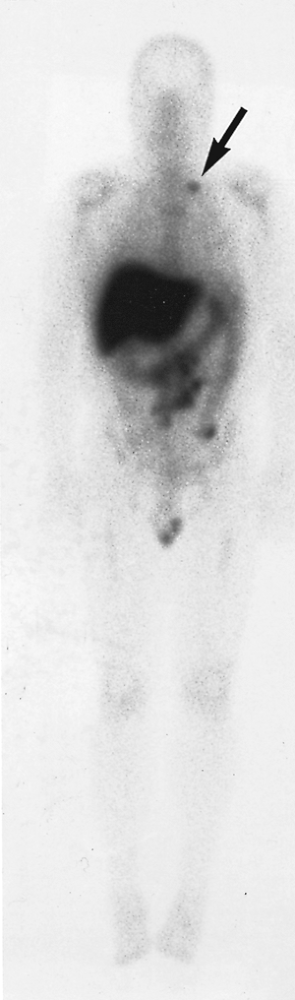 |
Fig. 159-13. Abnormal 111In satumomab pendetide (Oncoscint, CYTOGEN Corporation, Princeton, NJ) monoclonal antibody study. A 72-hour whole-body image in a 60-year-old man who is 8 years postresection of a rectal carcinoma. The patient previously underwent both chemotherapy and radiation therapy, and presented with obstructive symptoms and suspected recurrence. There is a large focal area of abnormal uptake in the retroperitoneal and mesenteric region, representing recurrent colon carcinoma. In addition, there is a focal area of increased activity in the left supraclavicular region (arrow), representing an unsuspected lymph node metastasis. Activity in the liver, colon, bone marrow, and testes represent normal sites of tracer localization. |
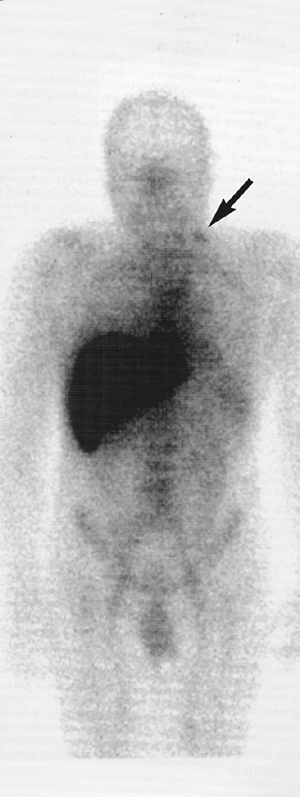 |
Fig. 159-14. Abnormal 111In capromab pendetide (Prostascint, CYTOGEN Corporation, Princeton, NJ) monoclonal antibody study. Five-day whole-body images in a 69-year-old man approximately 3 years after radical prostatectomy for prostate carcinoma. The patient received no additional treatment and presented with a mild interval increase in the serum prostate-specific antigen level from a prior value of 0.1 ng/mL 1 year earlier to 0.4 ng/mL at the time of this study. There is a small focal area of increased activity in the left supraclavicular region (arrow), representing a focal lymph node metastasis. Single-photon emission computed tomographic images of the pelvis (not shown) also demonstrated findings suspicious for local recurrence of prostate carcinoma in the prostate bed. As in the case in Fig. 159-13, the activity in the liver and bone marrow and the activity noted on this study in the blood pool and spleen represent normal findings. |
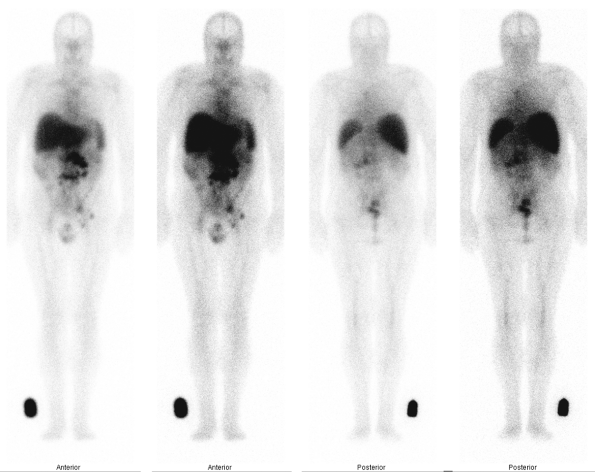 |
Fig. 159-15. Indium 111 ibritumomab tiuxetan (Zevalin) monoclonal antibody scan in non-Hodgkin's lymphoma. The patient is a 61-year-old man with a history of non-Hodgkin's lymphoma, status 4 years post-chemotherapy, presenting with clinical evidence of recurrence. Seventy-two-hour anterior and posterior whole-body 111In-Zevalin images demonstrate multiple foci of abnormal lymph node uptake in the retroperitoneal, mesenteric, left iliac, and left inguinal regions. There is no involvement above the diaphragm in this patient. The activity in the midline and upper chest represents normal, residual blood pool activity in the heart and great vessels. Normal activity is also noted in the liver, spleen, bone marrow, and testes. Based on these findings, the patient is a potential candidate for follow-up radioimmunotherapy with the Y-90 formulation of the monoclonal antibody, provided that he fulfills other hematologic and clinical criteria. |
In the assessment of patients with well-differentiated thyroid carcinoma, whole-body imaging is performed using 2 to 10 mCi (74 370 MBq) of 131I administered orally. The best dose to use for diagnostic 131I whole-body imaging is controversial, as discussed by Reynolds and Robbins (1997).
P.2415
Higher doses of 5 to 10 mCi (185 370 MBq) are associated with higher sensitivity for the detection of metastatic disease, but the higher doses may also produce a stunning phenomenon, resulting in lower uptake by lesions after subsequent therapeutic administration of 131I, creating the potential for less effective ablation of thyroid carcinoma metastases. Lower doses of 1 to 2 mCi (37 74 MBq) avoid the problem of stunning, but also result in poorer image quality and decreased sensitivity for the detection of metastases. This study is appropriately performed only in patients who have already undergone subtotal or total thyroidectomy. Beirwaltes (1978) and others suggested that this study be performed 4 to 6 weeks after thyroidectomy without thyroid hormone supplementation or after withdrawal of thyroid supplements for approximately 6 weeks in order to obtain maximal endogenous thyroid-stimulating hormone (TSH) stimulation of uptake by functioning metastases. It has become a widespread practice to switch patients to the shorter-acting 3,5,5 -triiodothyronine (Cytomel, Jones Pharma, St. Louis, MO) 6 weeks before imaging, and withdrawing the hormone at 2 to 3 weeks before the study, permitting the patient to suffer a shorter period of symptomatic hypothyroidism. Obtaining serum TSH and thyroglobulin levels prior to imaging is highly recommended, with a TSH level of greater than 30 IU/mL indicative of adequate stimulation. Recently, the FDA approved the use of a new form of recombinant human TSH (Thyrogen, Genzyme Corporation, Cambridge, MA, U.S.A.), which may be administered to patients for 2 or 3 days prior to imaging, without undergoing thyroid hormone withdrawal. This approach has been shown to be a less sensitive but acceptable alternative for diagnostic imaging in patients with low clinical risk for recurrent disease or those unable to tolerate hormone withdrawal, as described in the Thyrogen package insert (1998). It is best used in patients who have already undergone at least one negative hormone-withdrawal scan. The use of Thyrogen is probably not advisable for first postoperative evaluations or evaluation of patients at high risk for recurrence, since it has been shown to be less sensitive than hormone withdrawal for detecting lesions. Furthermore, the use of recombinant TSH is definitely not recommended for the preparation of patients for high-dose radioiodine therapy. In addition, serum pregnancy tests must be performed in women of childbearing age to preclude the possibility of administering the dose during pregnancy, which could have adverse effects on the fetus, especially if
P.2416
P.2417
given after function of the fetal thyroid has begun. Women who are breast-feeding infants, or even those still lactating after cessation of breast-feeding, should not receive 131I for evaluation of thyroid carcinoma because of unacceptably high radiation exposure to the fetus and maternal breasts, respectively. In selected cases, other radiopharmaceuticals may be used to evaluate specific patients, such as FDG, thallium, 99mTc sestamibi, and, in the case of medullary carcinoma of the thyroid (which does not accumulate 131I), 111In octreotide, which was discussed earlier in this chapter.
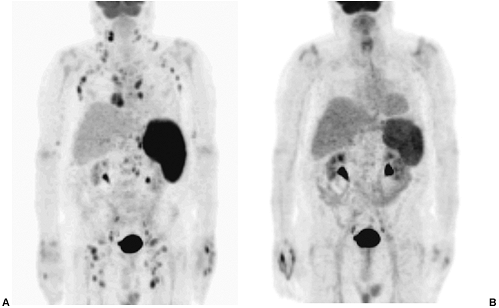 |
Fig. 159-16. Non-Hodgkin's lymphoma FDG PET scans before and after Zevalin treatment. A 64-year-old man with a several-year history of non-Hodgkin's lymphoma, originally arising in the eye, presented with clinical evidence of recurrent adenopathy and splenomegaly, following multiple courses of chemotherapy and autologous bone marrow transplantation. A. An FDG PET maximum intensity projection image demonstrates widespread foci of lymphadenopathy on both sides of the diaphragm, involving the neck, axillae, mediastinum, retroperitoneal, iliac, and inguinal lymph node regions. Marked splenomegaly with diffusely increased splenic uptake is also evident. B. Repeat PET imaging following radioimmunotherapy with Zevalin, demonstrating complete interval resolution of adenopathy and improvement in splenomegaly and increased splenic tracer uptake. |
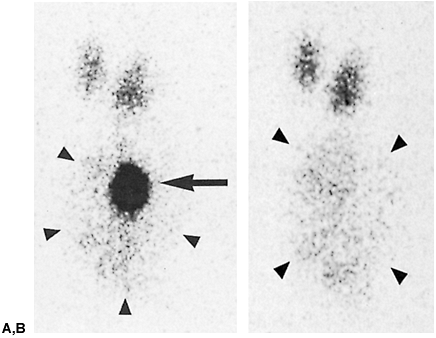 |
Fig. 159-17. Substernal goiter. The patient is a 73-year-old woman with deviation of the trachea on a chest radiograph, associated with a soft tissue density in the mediastinum. A. Iodine 131 anterior pinhole image of the neck and upper mediastinum, with a radioactive hot marker at the sternal notch (arrow). Note the activity in thyroid tissue inferior to the normal thyroid gland, extending below the marker (arrowheads), indicating substernal extension of the thyroid. B. Same view as in A, with the sternal notch marker removed to demonstrate the substernal thyroid tissue (arrowheads) to better advantage. |
Although metastases from thyroid carcinoma are most often found in cervical lymph nodes, the lungs, or skeleton, mediastinal metastases can also occur and can be detected by whole-body 131I imaging, as demonstrated in Fig. 159-18. Metastatic lesions and normal thyroid remnants, which may interfere with the future detection of recurrences, may be ablated with larger, therapeutic doses of 131I, usually given in doses of 100 to 200 mCi (3,700 7,400 mBq) or even higher, if dosimetry estimates are performed. Patients receiving therapeutic doses of greater than 30 mCi (1,110 MBq) of 131I are usually hospitalized, although new Nuclear Regulatory Commission (NRC) regulations permit outpatient therapies in certain locations, under specific conditions. All patients should undergo posttherapy whole-body 131I scans, which are typically performed 5 to 7 days after the therapy. Because of the higher amount of radioactivity present, these posttherapy studies often demonstrate additional findings or better delineation of abnormalities than the diagnostic study, as described by me and my colleagues (1989). Follow-up studies performed annually can be used to assess the response to therapy and detect and treat recurrences. In patients without evidence of recurrence, the follow-up interval may be increased to every 2 to 5 years. Suppressive doses of thyroid hormone are administered between studies, and serial thyroglobulin levels are also monitored. Recently, there has been growing support for 131I treatment of patients with normal whole-body 131I diagnostic studies but elevated serum thyroglobulin levels, as reviewed by Reynolds and Robbins (1997). In many such cases, functioning metastases may be visible on follow-up posttherapy scans in these patients. Alternatively, such patients may first undergo FDG PET imaging to assess for metastases without significant iodine uptake but having increased glucose metabolism, as reported by Chung (1999), Grunwald (1999), and Alnafisi (2000) and their colleagues. An example of the utility of this approach is demonstrated in Fig. 159-19. Lesions identified by this technique may be treated either with 131I or surgically. In general, the more well-differentiated lesions are more likely to be detected with 131I imaging, whereas less well-differentiated lesions may be better detected with FDG PET.
Parathyroid imaging is useful in the preoperative evaluation of patients with biochemical evidence of primary or secondary hyperparathyroidism. Eighty-five percent of patients with primary hyperparathyroidism have a solitary parathyroid adenoma and 10% to 15% have hyperplasia of all four glands. While in experienced hands, there is a 90% to 95% cure rate without any preoperative localization procedure, there remains a 5% to 10% recurrence rate, primarily due to ectopic lesions not located in the thyroid bed. Furthermore, reexploration is technically difficult and is associated with higher morbidity and a poorer cure rate. The goals of preoperative localization include decreasing operative time, allowing unilateral neck dissection in some cases, and minimizing the frequency of reexploration. Noninvasive parathyroid localization techniques include high-resolution ultrasonography, CT, MR imaging, and radionuclide imaging. Many authorities believe that ultrasonography and radionuclide imaging may at present be the best screening techniques for most patients, although both CT and MR imaging may play a role as well. Ultrasonography in particular is less likely to detect lesions in the upper mediastinum, where imaging is limited by absorption or reflection of the sound waves by air in lung tissue and the bones of the sternum.
Previously, radionuclide parathyroid imaging was performed using a dual-isotope digital subtraction technique. Technetium 99m pertechnetate is taken up by thyroid tissue, as previously discussed. Thallium 201 chloride is primarily used as a myocardial perfusion imaging agent. Thallium is also accumulated both by thyroid and parathyroid tissue. Several variations in technique were described for use in dual-isotope radionuclide parathyroid scintigraphy, as described by Young and associates (1983), Winzelberg and Hydovitz (1985), Gimlette and Taylor (1985), Basarab and co-workers (1985), and Okerlund (1988), all based on the same basic principle. Both radionuclides were given sequentially to the patient, and the image data acquired
P.2418
on a digital computer. The patient was immobilized with sandbags to prevent motion between the two sets of images. Images of both the neck and the upper chest were obtained, so that mediastinal lesions could be visualized. The images were then normalized, and the pertechnetate image subtracted from the 201Tl image. Essentially all thyroid activity was removed from the resulting subtraction image, which therefore demonstrated only abnormal parathyroid activity (Fig. 159-20). Problems associated with this technique include difficulty in correcting for patient motion, cross-talk between the 99mTc and 201Tl gamma photon energies and difficulty in performing the image normalization and subtraction manipulations. As a result, the overall sensitivity of dual-isotope parathyroid scintigraphy was only about 70% to 80%, but comparable with that of the other competing modalities.
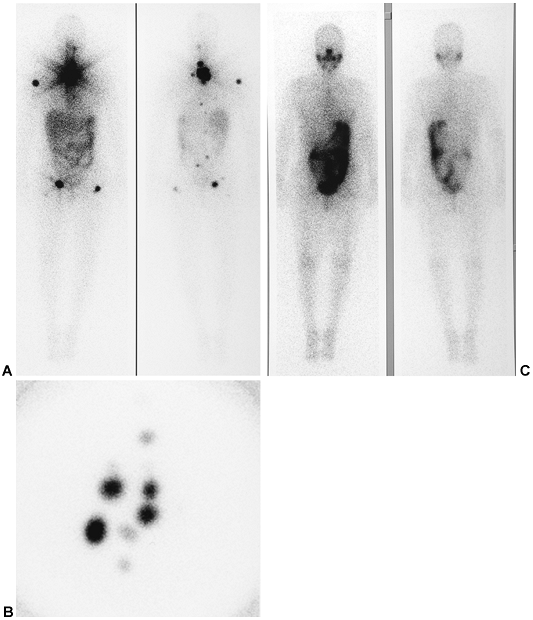 |
Fig. 159-18. Whole-body 131I scan with mediastinal metastases. A. Forty-eight-hour anterior and posterior whole-body 131I images obtained approximately 1 month following subtotal thyroidectomy for well-differentiated papillary carcinoma of the thyroid demonstrate multiple adjacent foci of intensely increased tracer uptake in the thyroid bed, extending superiorly into the neck and inferiorly into the mediastinum. Additional foci of uptake consistent with skeletal metastases are noted in the proximal right humerus and in both proximal femora. Subtle foci of increased uptake in the thoracolumbar spine and pelvis are also consistent with skeletal metastases. The areas of increased activity in the liver and bowel represent normal sites of localization. B. A magnified pinhole image of the neck from the same study as in A demonstrates the striking activity in the neck to represent numerous focal lesions, consistent with residual thyroid carcinoma in the thyroid bed, cervical lymph nodes, and superior mediastinum. C. Repeat anterior and posterior whole-body 131I images obtained 1 year following administration of a therapeutic dose of 150 mCi (5,550 MBq) of 131I sodium iodide demonstrate complete interval ablation of all of the above foci of thyroid carcinoma, with no new sites of involvement evident. Uptake within the salivary glands, bowel, and urinary tract constitute normal sites of localization. |
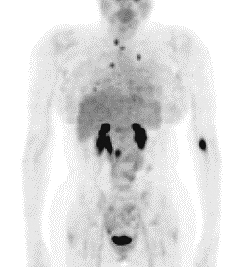 |
Fig. 159-19. FDG PET scan in well-differentiated thyroid carcinoma. A patient underwent total thyroidectomy for papillary carcinoma of the thyroid, followed by 131I therapy. A follow-up 131I whole-body scan obtained a year later (not shown) was normal, without evidence of recurrent disease, but the patient presented with a markedly elevated serum thyroglobulin level of 1,600 ng/mL (normal <60 ng/mL in patients with intact thyroid glands, and less in patients having previously undergone total thyroidectomy), obtained during the period of thyroid hormone withdrawal. An FDG PET maximum intensity projection image demonstrates multiple foci of increased FDG uptake in the mediastinum and bilateral hilar regions, as well as more subtle foci in the lower lung fields bilaterally, right greater than left. The findings are consistent with metastatic disease in these sites not associated with significant 131I uptake. |
Dual-isotope radionuclide parathyroid imaging has been replaced by a simpler and more accurate single-isotope method using 99mTc sestamibi (methoxyisobutylisonitrile), another commonly used myocardial perfusion imaging agent. Sestamibi is taken up by thyroid and parathyroid tissue, but demonstrates differential washout. Thus, immediate static images demonstrate uptake in normal thyroid tissue and abnormal parathyroid tissue (i.e., adenomas or hyperplastic glands), but the normal thyroid activity washes out over time more rapidly than the parathyroid uptake. Three-hour delayed images demonstrate clear visualization of parathyroid adenomas, without the need for subtraction, reregistration or dual-isotope cross-talk (downscatter) correction (Fig. 159-21). In addition, the more favorable imaging characteristics of 99mTc compared with thallium also result in improved lesion visibility. Normal parathyroid glands are not usually visualized. Some authorities prefer to use 123I subtraction even with sestamibi parathyroid imaging, but this step is often unnecessary.
Using either technique, in the presence of a parathyroid adenoma or diffuse parathyroid hyperplasia, a focal hot spot or spots was seen. Although these lesions are most often found in the region of the thyroid bed, ectopic parathyroid tissue may occur in the neck or superior mediastinum, since the parathyroids embryologically descend into the neck and are variably located, especially the inferior parathyroids (see Fig. 159-20). As discussed, such mediastinal foci of tumor may be difficult to localize preoperatively using other modalities, particularly ultrasonography, and especially in patients with recurrent hyperparathyroidism after a prior neck exploration. Radionuclide parathyroid scintigraphy has been reported to have a sensitivity in the 80% to 90% range, with better results reported since the use of the single isotope sestamibi method has become widespread. Sensitivity is related to the size of the lesion, with poorer sensitivity for lesions of less than 500 mg and for hyperplastic glands, and with virtually 100% detection of lesions of greater than 1,500 mg. Specificity is lower in patients without definite biochemical evidence of hyperparathyroidism. A common cause of false-positive imaging results is focal thyroid pathology, such as thyroid adenomas or less often carcinomas, which also accumulate thallium. Like all of the other methods, radionuclide parathyroid scintigraphy is also less sensitive for detection of parathyroid hyperplasia, which is more common in secondary hyperparathyroidism, than it is for adenomas. Recently, the use of the intraoperative probe techniques developed for intraoperative lymphoscintigraphy studies, as discussed, have been applied to parathyroid scintigraphy as well, resulting in shorter, less invasive surgical procedures in these patients.
Esophageal Scintigraphy
Nuclear medicine procedures of the upper gastrointestinal tract are primarily directed toward evaluation of esophageal transit time and gastric emptying and detection of gastroesophageal reflux. Technetium 99m sulfur colloid, a radiocolloid given intravenously for liver-spleen scintigraphy, can be given orally in water to visualize the esophagus and stomach. In the case of gastric emptying, a solid-phase marker using 99mTc sulfur colloid labeled eggs is more often used. Radionuclide techniques are sensitive, noninvasive methods for evaluating these functions, and provide the only truly quantitative data available.
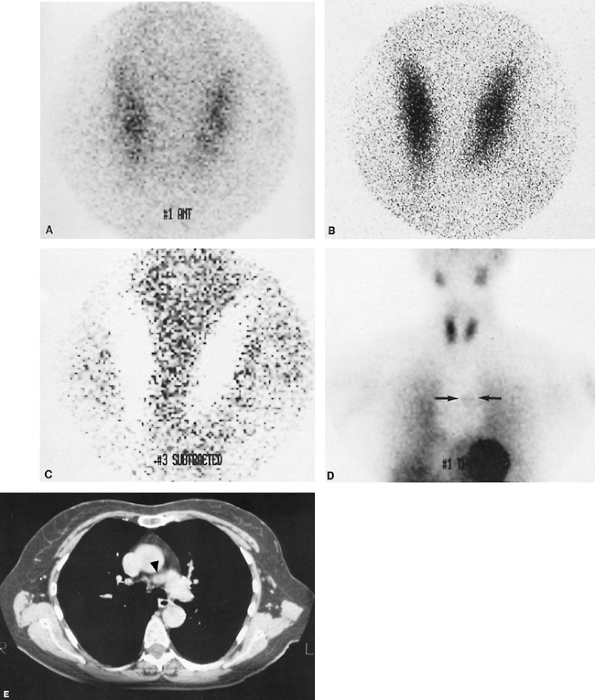 |
Fig. 159-20. Mediastinal parathyroid adenoma. The patient is a 61-year-old woman with severe hypercalcemia. Ultrasound examination of the neck revealed no evidence of a parathyroid adenoma. A. Anterior thallium 201 pinhole image of the neck, demonstrating uptake in a normal-appearing thyroid and no abnormal foci of activity in the neck. B. Anterior technetium 99m pertechnetate pinhole image of the neck, similarly demonstrating a normal thyroid. C. Digital 201Tl/99mTc subtraction image demonstrating good subtraction of the thyroid (appearing white) and no abnormal foci of activity in the neck. D. Anterior 201Tl parallel hole image of the neck and chest, demonstrating a subtle focus of increased activity in the mediastinum (arrows). E. Computed tomographic image of the mediastinum, demonstrating a focal soft tissue nodule in the aortopulmonary window (arrowhead). At surgery, a 1.4 1.0 0.5 cm parathyroid adenoma adherent to the left recurrent laryngeal nerve was found and resected. Case courtesy of Gary L. Dillehay, M.D., Loyola University Medical Center, Maywood, IL. |
P.2419
Radionuclide Angiography
Radionuclide angiography is a simple, noninvasive technique for evaluating the vascular anatomy and blood flow of organs, body regions, or masses, in conjunction with other standard radionuclide studies, as reviewed by Muroff and Freedman (1976). In the case of the mediastinum, the anatomy and blood flow patterns of the great vessels can readily be evaluated, although spatial resolution is limited. The loss in resolution due to the low photon flux obtained during a rapid sequence of dynamic images is partially offset by the large concentration of radioactivity in the bolus.
P.2420
Virtually any intravenously injected 99mTc-labeled radiopharmaceutical can be used, although particulate agents are not used, because they are rapidly trapped by the lungs, liver, spleen, or bone marrow, depending on the particle size. In the chest, 99mTc DTPA is most often used, unless the study is being performed in conjunction with another radionuclide examination, such as a bone scan or gated blood pool cardiac study.
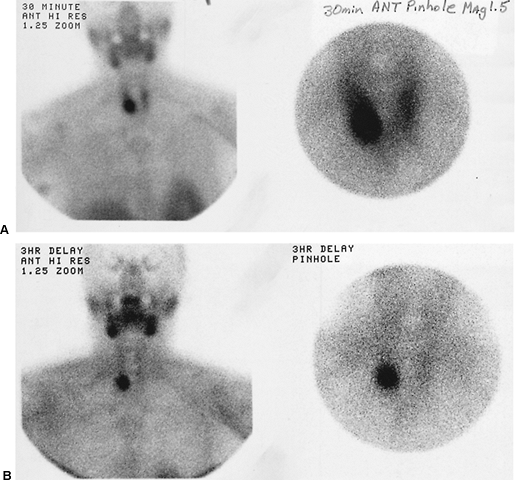 |
Fig. 159-21. Parathyroid adenoma on a technetium 99m sestamibi study. A. Parallel hole collimator image of the entire neck and chest (left) and magnified pinhole image of the neck (right) obtained 30 minutes following intravenous administration of 99mTc sestamibi. There is diffuse activity in the thyroid, with a more focal area of uptake overlying the right lower pole region. Normal sestamibi uptake is also noted in the salivary glands, oropharynx, and myocardium. B. Three-hour delayed images (same views as in Fig. 159-7A) demonstrating marked clearance of activity from the thyroid, with persistent focal uptake in the right lower pole region, representing a parathyroid adenoma. |
This examination can be used to detect suspected aneurysms or vascular malformations or to assess the vascularity of masses, such as hemangiomas. However, the most common indication for the study is the detection of superior vena caval obstruction or thrombosis of the subclavian artery or other vessels. An example of superior vena caval obstruction is shown in Fig. 159-22. These data may be quantitated using the computer. Radionuclide angiography can preclude the need for other, more invasive studies, such as contrast arteriography or venography. As noted, its major limitation is the relatively poor spatial resolution. For example, this study would not be sensitive for detecting a subtle aortic dissection, which might be demonstrated on a dynamic spiral CT study. Occasionally studies may be technically inadequate due to poor bolus geometry or poor patient positioning.
Other Studies
In addition to direct evaluation of the mediastinum, nuclear medicine procedures may also play a role in the evaluation of the spread of primary mediastinal pathology to other sites, such as metastatic disease from primary lung carcinoma, lymphoma, or esophageal carcinoma in the mediastinum, or spread of infection from the mediastinum. The role of gallium and indium leukocyte scintigraphy in these entities has already been discussed. In the near future, FDG PET imaging may also play a role in such cases, as discussed by Stumpe and co-workers (2000), Zhuang and Alavi (2002), and Alavi and associates (2002). With respect to the staging of intrathoracic neoplasms, radionuclide bone imaging with 99mTc diphosphonate radiopharmaceuticals remains the most important procedure. Bone scintigrams are sensitive indicators of metastatic disease, demonstrate findings long before radiographic results become positive, and provide a unique opportunity to evaluate the entire skeleton at once. MR imaging of regions of the skeleton may also play a role in the detection of metastatic disease, especially in the spine, but the bone scan remains the overall best screening modality for this purpose. While most widely studied in breast and prostatic carcinoma, radionuclide bone imaging can play an important role in the staging and follow-up of patients with lung carcinoma, as reviewed by McNeil (1984). PET imaging using 18F sodium fluoride is also available as a potentially powerful whole-body imaging technique for the detection of metastases and other focal skeletal pathology. Fluorine 18 fluoride bone scans were first employed years ago using conventional radionuclide imaging instruments, but now can be obtained using
P.2421
state-of-the-art PET scanners, as reviewed by Hawkins and associates (2003). Having the advantages of PET imaging described earlier in this chapter, including high resolution, an intrinsic tomographic nature, and high sensitivity for disease detection, 18F fluoride bone scintigraphy has the potential to achieve more widespread use in the near future, as suggested by the earlier work of Hoh and associates (1993). Liver-spleen scintigraphy has been supplanted by techniques having higher spatial resolution and specificity, such as CT, MR imaging, and ultrasonography. It continues to play an ancillary role in the characterization of some intrahepatic masses, such as focal nodular hyperplasia, as well as in the evaluation of patients with known or suspected hepatocellular disease or suspected hypersplenism or accessory splenic tissue.
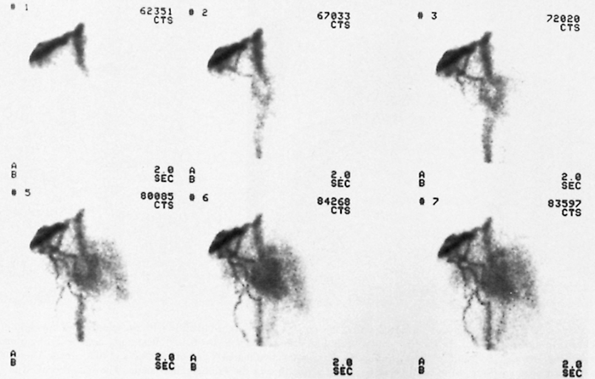 |
Fig. 159-22. Radionuclide angiogram in superior vena caval obstruction. The patient is a 65-year-old man with poorly differentiated adenocarcinoma of the lung, with brain and liver metastases and clinical signs suggestive of superior vena caval obstruction. Selected anterior images of the chest from a radionuclide angiogram performed after injection of technetium 99m diethylenetriaminepentaacetic acid in a right antecubital vein demonstrate high-grade partial obstruction of the superior vena cava, with marked flow in dilated collateral veins in the right axilla and anterior chest wall. Mild reflux into the right internal jugular vein and early visualization of the inferior vena cava via collateral flow are also noted. A small amount of activity is directly entering the superior vena cava and right heart. From Spies WG: Radionuclide studies of the lung. In Shields TW (ed): General Thoracic Surgery. 3rd Ed. Philadelphia: Lea&Febiger, 1989. With permission. |
Future Developments
In this era of superb spatial resolution and three-dimensional digital image display provided by the various anatomic imaging modalities, such as CT and MR imaging, the thrust of nuclear medicine research has appropriately returned to the development of improved methods for the evaluation of human physiology and pathophysiology. Recent innovations in nuclear medicine techniques have largely involved the development of new radiopharmaceuticals that are better suited to specific clinical indications and improvements in instrumentation and data processing. The goals in developing new radiopharmaceuticals are to design agents that provide better image quality; more specific localization in various lesions and regions of interest; lower radiation exposure to patients, technologists, and physicians; easier preparation; lower cost; wider availability; and a minimum of side effects. With respect to mediastinal pathology, the major developments in these areas relate to FDG as a PET imaging agent, the second generation of monoclonal antibodies and the peptide receptor imaging agents, and the development of radioimmunotherapy and other forms of targeted radionuclide therapy using unsealed sources. In addition, the rapid growth of PET imaging in clinical oncology has spurred research aimed at developing newer PET radiopharmaceuticals directed against specific lesions not adequately addressed by FDG. Examples of such agents are 18F-labeled fluorocholine and 11C acetate, both being evaluated for the detection and follow-up of prostate carcinoma. Other PET radiopharmaceuticals labeled with 18F and other radionuclides, such as 11C, and agents consisting of radiolabeled compounds targeting other physiologic functions, such as protein synthesis, hormone
P.2422
receptors, and DNA synthesis, are also being developed and evaluated. Specifically, the clinical role of PET imaging in the diagnosis, staging, and follow-up of primary and secondary tumors in the mediastinum is likely to be further expanded as additional data corroborating its use and increased reimbursement become available. PET imaging is rapidly becoming more widely available at both academic and clinical imaging facilities, and is becoming more easily performed at a more competitive cost. Both private and commercial ventures have greatly expanded the availability and affordability of FDG as a routine clinical radiopharmaceutical. At present, the metabolic and pathophysiologic information provided by PET imaging is not presently obtainable by any other imaging modality, although research in MR spectroscopy holds some promise for making inroads into this area in the future.
Current developments in nuclear medicine instrumentation are directed primarily toward the design of improved, more efficient, and more cost-effective dedicated PET scanners. These developments include efforts to further improve system resolution and performance, improvements in image processing and display, and inclusion of newer imaging crystals, such as lutetium orthosilicate and gadolinium orthosilicate, which permit improved sensitivity and reduced imaging times. The rapid acceptance and proliferation of PET technology are facilitating a reduction in the cost of dedicated PET systems as well. In addition, considerable development of hybrid imaging devices, capable of performing both PET and CT imaging or SPECT and CT imaging on the same device, during a single imaging session, are now available. In the near future, units combining PET and MR imaging may also appear. These units are, not surprisingly, significantly more expensive than standard dedicated PET units, and because of their complexity, more prone to down time. Their use also raises other technical and logistical concerns. Nevertheless, although the desirability of such devices is currently being hotly debated, there is no question that they have already gained significant acceptance by the medical imaging community, and may be particularly useful in certain clinical settings, such as radiation therapy planning and in the assessment of patients with head and neck carcinomas. Further refinement of conventional radionuclide SPECT imaging systems and more advanced image acquisition and processing hardware and software are also under development.
The ability to share data between different imaging modalities and with referring physicians is rapidly becoming the standard of care. One area in which this capability is being extensively used is in the implementation of picture archiving and communication systems (PACS), whereby all-digital or filmless imaging departments are rapidly proliferating. In PACS, all imaging studies are available for evaluation and comparison online to radiologists, nuclear medicine specialists, surgeons, and other clinicians at multiple sites, including Internet or local area network access or dedicated teleradiology systems. Digital images are now stored in standardized formats, allowing portability to other systems, such as the digital imaging and communications in medicine standard (DICOM). Furthermore, it is possible to perform image fusion of digital images from different modalities, permitting scintigraphic data to be viewed superimposed on anatomic CT or MR imaging data in a single composite image. Such fusion allows for improved lesion localization, especially for less trained observers, and can obviate the need for a much costlier hybrid imaging system in many cases. For example, a hypermetabolic mediastinal lymph node seen on an FDG PET study can be superimposed on the corresponding soft tissue density on a CT image.
Most new gamma camera designs incorporate the use of multiple detector systems, typically with changeable geometry and oversized rectangular detectors, permitting improved and more rapid SPECT and whole-body imaging. The rapid proliferation and technical advances in microcomputer and imaging workstation technologies over the past several years have provided greatly improved and much faster techniques for digital image acquisition, processing, and display of nuclear medicine image data. While not specifically aimed toward applications related to the mediastinum, these areas of research and development within nuclear medicine will doubtless have significant beneficial future impact on the noninvasive evaluation of mediastinal pathology in the near future.
REFERENCES
Abdel-Nabi H, Doerr RJ: Clinical applications of indium-111 labeled monoclonal antibody imaging in colorectal cancer patients. Semin Nucl Med 23:99, 1993.
Alavi A, et al: Positron emission tomography imaging in nonmalignant thoracic disorders. Semin Nucl Med 32:293, 2002.
Alazraki NP, et al: Lymphoscintigraphy, the sentinel node concept, and the intraoperative gamma probe in melanoma, breast cancer, and other potential cancers. Semin Nucl Med 27:55, 1997.
Alderson PO, Line BR: Scintigraphic evaluation of regional pulmonary ventilation. Semin Nucl Med 10:218, 1980.
Alderson PO, Secker-Walker RH, Forrest JV: Detection of obstructive pulmonary disease. Relative sensitivity of ventilation-perfusion studies and chest radiography. Radiology 111:643, 1974.
Alderson PO, et al: The role of 133Xe ventilation studies in the scintigraphic detection of pulmonary embolism. Radiology 120:633, 1976.
Alnafisi NS, et al: FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. J Nucl Med 41:1010, 2000.
Al-Sugair A, Coleman RE: Applications of PET in lung cancer. Semin Nucl Med 28:303, 1998.
Bar-Shalom R, Valdivia AY, Blaufox MD: PET imaging in oncology. Semin Nucl Med 30:150, 2000.
Bar-Shalom R, et al: Camera-based FDG PET and 67Ga SPECT in evaluation of lymphoma: comparative study. Radiology 227:353, 2003.
Basarab RM, Manni A, Harrison TS: Dual isotope subtraction parathyroid scintigraphy in the preoperative evaluation of suspected hyperparathyroidism. Clin Nucl Med 10:300, 1985.
Becker W, Goldenberg DM, Wolf F: The use of monoclonal antibodies and antibody fragments in the imaging of infectious lesions. Semin Nucl Med 24:142, 1994.
Beirwaltes WH: The treatment of thyroid carcinoma with radioactive iodine. Semin Nucl Med 8:79, 1978.
Bischof Delaloye A: Radioimmunoimaging and radioimmunotherapy: will these be routine procedures? Semin Nucl Med 30:186, 2000.
P.2423
Bitran J, et al: Patterns of gallium-67 scintigraphy in patients with acquired immunodeficiency syndrome and the AIDS related complex. J Nucl Med 28:1103, 1987.
Block AJ, Olsen GN: Preoperative pulmonary function testing. JAMA 235:257, 1976.
Block MI, et al: Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 64:770, 1997.
Boerman OC, Oyen JG, Corstens FHM: Radio-labeled receptor-binding peptides: a new class of radiopharmaceutical. Semin Nucl Med 30:195, 2000.
Boysen PG, et al: Prospective evaluation for pneumonectomy using the 99m technetium quantitative perfusion lung scan. Chest 72:422, 1977.
Chung J-K, et al: Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med 40:986, 1999.
Coleman RE: Radiolabeled leukocytes. In Freeman LM, Weissmann HS (eds): Nuclear Medicine Annual 1982. New York: Raven, 1982.
Collier BD, et al: Internal mammary lymphoscintigraphy in patients with breast cancer. Correlation with computed tomography and impact on radiation therapy planning. Radiology 147:845, 1983.
Corstens FHM, Oyen WJG, Becker WS: Radioimmunoconjugates in the detection of infection and inflammation. Semin Nucl Med 23:148, 1993.
Council on Scientific Affairs: Instrumentation in positron emission tomography. JAMA 259:1531, 1988a.
Council on Scientific Affairs: Cyclotrons and radiopharmaceuticals in positron emission tomography. JAMA 259:1854, 1988b.
Council on Scientific Affairs: Positron emission tomography in oncology. JAMA 259:2126, 1988c.
Council on Scientific Affairs: Application of positron emission tomography in the heart. JAMA 259:2438, 1988d.
Council on Scientific Affairs: Positron emission tomography a new approach to brain chemistry. JAMA 260:2704, 1988e.
Datz FL. Gamuts: ventilation-perfusion mismatch: lung imaging. Semin Nucl Med 10:193, 1980.
Datz FL. Indium-111 labeled leukocytes for the detection of infection: current status. Semin Nucl Med 24:92, 1994.
De Jager R, et al: Current status of cancer immunodetection with radiolabeled human monoclonal antibodies. Semin Nucl Med 23:165, 1993.
DeNardo SJ, et al: Treatment of B cell malignancies with 131I Lym-1 monoclonal antibodies. Int J Cancer Suppl 3:96, 1998.
DeWinter F, et al: Fluorine-18 fluorodeoxyglucose-positron emission tomography: a highly accurate imaging modality for the diagnosis of chronic musculoskeletal infection. J Bone Joint Surg [Am] 83:651, 2001.
Dionne L, Friede J, Blais R: Internal mammary lymphoscintigraphy in breast carcinoma a surgeon's perspective. Semin Nucl Med 13:35, 1983.
Ege GN: Internal mammary lymphoscintigraphy. The rationale, technique, interpretation and clinical application: a review based on 848 cases. Radiology 118:101, 1976.
Ege GN: Lymphoscintigraphy techniques and applications in the management of breast carcinoma. Semin Nucl Med 13:26, 1983.
Ege GN, Clark RM: Internal mammary lymphoscintigraphy in the conservative surgical management of breast carcinoma. Clin Radiol 31:559, 1980.
Fineman DS, et al: Detection of abnormalities of AIDS patients with fever of unknown origin by In-111 leukocyte and Ga-67 citrate scintigraphy [Abstract]. Radiology 165:73, 1987.
Fischman AJ, Babich JW, Rubin RH: Infection imaging with technetium-99m labeled chemotactic peptide analogs. Semin Nucl Med 24:154, 1994.
Flanagan FL, Dehdashti F, Siegel BA: PET in breast cancer. Semin Nucl Med 28:290, 1998.
Francis IR, et al: Complementary roles of CT and 131I-MIBG scintigraphy in diagnosing pheochromocytoma. AJR 141:719, 1983.
Front D, Bar-Shalom R, Israel O: The continuing clinical role of gallium 67 scintigraphy in the age of receptor imaging. Semin Nucl Med 27:68, 1997.
Front D, et al: Hodgkin disease: prediction of outcome with 67Ga scintigraphy after one cycle of chemotherapy. Radiology 210:487, 1999.
Gimlette TMD, Taylor WH: Localization of enlarged parathyroid glands by thallium-201 and technetium-99m subtraction imaging: gland mass and parathormone levels in primary hyperparathyroidism. Clin Nucl Med 4:237, 1985.
Glass EC, Essner R, Giuliano AE: Sentinel node localization in breast cancer. Semin Nucl Med 29:57, 1999.
Goldsmith SJ, Macapinlac HA, O'Brien JP: Somatostatin-receptor imaging in lymphoma. Semin Nucl Med 25:262, 1995.
Grunwald F, et al: Fluorine-18 fluorodeoxyglucose positron emission tomography in thyroid cancer: results of a multicentre study. Eur J Nucl Med 26:1547, 1999.
Halpern SE, Dillman RO, Hagan PL: The problems and promise of monoclonal antitumor antibodies. Diagn Imag 5:40, 1983.
Hawkins RA, Hoh CK, Phelps ME: PET imaging of the skeletal system. In Sandler MP, et al (eds): Diagnostic Nuclear Medicine. 4th Ed. Philadelphia: Lippincott Williams&Wilkins, 2003, pp. 477 484.
Higashi K, et al: Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med 39:1016, 1998.
Hoffer P: Gallium: mechanisms. J Nucl Med 21:282, 1980a.
Hoffer P: Status of gallium-67 in tumor detection. J Nucl Med 21:394, 1980b.
Hoffmann M, et al: Positron emission tomography with fluorine-18 2-deoxyglucose (F18-FDG) does not visualize extranodal B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT)-type. Ann Oncol 10:1185, 1999.
Hoh CK, et al: Whole body skeletal imaging with [18F] fluoride ion and PET. J Comput Assist Tomogr 17:34, 1993.
Hoh CK, et al: PET in oncology: will it replace the other modalities? Semin Nucl Med 27:94, 1997a.
Hoh CK, et al: Whole-body FDG-PET imaging for staging of Hodgkin disease and lymphoma. J Nucl Med 38:343, 1997b.
Janicek M, et al: Early restaging gallium scans predict outcome in poor-prognosis patients with aggressive non-Hodgkin lymphoma treated with high-dose CHOP chemotherapy. J Clin Oncol 15:1631, 1997.
Jerusalem G, et al: Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin disease and non-Hodgkin lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood 94:429, 1999.
Jerusalem G, et al: Persistent tumor 18F-FDG uptake after a few cycles of polychemotherapy is predictive of treatment failure in non-Hodgkin lymphoma. Haematologica 85:613, 2000.
Keenan AM, Harbert JC, Larson SM: Monoclonal antibodies in nuclear medicine. J Nucl Med 26:531, 1985.
Kelly S, et al: A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 49:534, 2001.
Kostakoglu L, Goldsmith SJ: [F-18] fluorodeoxyglucose positron emission tomography in staging and follow-up of lymphoma: is it time to shift gears? Eur J Nucl Med 27:1564, 2000.
Kramer EL, et al: Gallium-67 scans of the chest in patients with acquired immunodeficiency syndrome. J Nucl Med 28:1107, 1978.
Krenning EP, et al: Somatostatin receptor scintigraphy with indium-111 DTPA-D-phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-tyr-3-octreotide. J Nucl Med 33:652, 1992.
Kresnik E, et al: (18)F-FDG positron emission tomography in the early diagnosis of enterocolitis: preliminary results. Eur J Nucl Med Mol Imaging 29:1389, 2002.
Kumar R, et al: Retrospective analysis of sentinel lymph node localization in multifocal, multicentric, palpable, or nonpalpable breast cancer. J Nucl Med 44:7, 2003.
Larson SM: Radiolabeled monoclonal anti-tumor antibodies in diagnosis and therapy. J Nucl Med 26: 538, 1985.
Lindberg S, et al: Methodology and dosimetry in adrenal medullary imaging with iodine-131 MIBG. J Nucl Med 29:1638, 1988.
Lowe VJ, et al: Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol 16:1075, 1998.
Lynn MD, et al: Pheochromocytoma and the normal adrenal medulla: improved visualization with I-123 MIBG scintigraphy. Radiology 156:789, 1985.
Mahajan P, et al: Comparison between F-18 2-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging and Ga-67 citrate scintigraphy in patients with Hodgkin disease and non-Hodgkin lymphoma. Radiology 225(P):658, 2002.
Marcus CS: The status of indium-111 oxine leukocyte imaging studies. Noninvasive Med Imag 3:213, 1984.
Matsuo M, Ushio K, et al: A study on pulmonary hilar and mediastinal lymphoscintigraphy. Radioisotopes 28:562, 1979.
McNeil BJ: Value of bone scanning in neoplastic disease. Semin Nucl Med 14:277, 1984.
Mehta AC, Spies WG, Spies SM: Utility of gallium scintigraphy in AIDS [Abstract]. Radiology 165:72, 1987.
Meyer JE, Munzenrider JE: Computed tomographic demonstration of internal mammary lymph-node metastasis in patients with locally recurrent breast carcinoma. Radiology 139:661, 1981.
Meyer MA, et al: Sequential positron emission tomographic evaluations of brain metabolism in acute herpes encephalitis. J Neuroimaging 4:104, 1994.
P.2424
Muroff LR, Freedman GS: Radionuclide angiography. Semin Nucl Med 6:217, 1976.
Neal CE, et al: Monoclonal antibodies in ovarian and prostate cancer. Semin Nucl Med 23:114, 1993.
Okerlund M: Scintigraphy finds tumors in parathyroid glands. Diagn Imag 6:130, 1988.
Olsen GN, et al: Pulmonary function evaluation of the lung resection candidate: a prospective study. Am Rev Respir Dis 111:379, 1975.
Olsen JO, et al: Somatostatin receptor imaging of neuroendocrine tumors with indium-111 pentetreotide (Octreoscan). Semin Nucl Med 25:251, 1995.
Palestro CJ: The current role of gallium imaging in infection. Semin Nucl Med 24:128, 1994.
Peters AM: The utility of [99mTc] HMPAO-leukocytes for imaging infection. Semin Nucl Med 24:110, 1994.
Pieterman RM, et al: Preoperative staging of non small-cell lung cancer with positron-emission tomography. New Engl J Med 343:254, 2000.
Podoloff DA, et al: Imaging of colorectal carcinoma with technetium-99m radiolabeled Fab fragments. Semin Nucl Med 23:89, 1993.
Rege SD, et al: Imaging of pulmonary mass lesions with whole-body positron emission tomography and fluorodeoxyglucose. Cancer 72:82, 1993.
Reynolds JC, Robbins J: The changing role of radioiodine in the management of differentiated thyroid cancer. Semin Nucl Med 27:152, 1997.
Rosen ST, et al: Monoclonal antibodies in cancer therapy. Lab Med 16:310, 1985.
Shapiro B, et al: Iodine-131 metaiodobenzylguanidine for the locating of suspected pheochromocytoma: experience in 400 cases. J Nucl Med 26:576, 1975.
Sisson JC, et al: Scintigraphic localization of pheochromocytoma. N Engl J Med 305:12, 1981.
Spies WG, et al: Ventilation-perfusion scintigraphy in suspected pulmonary embolism: correlation with pulmonary angiography and refinement of criteria for interpretation. Radiology 159:383, 1986.
Spies WG: Radionuclide studies of the lung. In Shields TW (ed): General Thoracic Surgery. 3rd Ed. Philadelphia: Lea&Febiger, 1989.
Spies WG, et al: Value of post-therapy whole-body I-131 imaging in the evaluation of patients with thyroid carcinoma having undergone high-dose I-131 therapy. Clin Nucl Med 14:793, 1989.
Stumpe KD, et al: Infection imaging using whole-body FDG-PET. Eur J Nucl Med 27:822, 2000.
Sugawara Y, et al: Rapid detection of human infections with fluorine-18 fluorodeoxyglucose and positron emission tomography: preliminary results. Eur J Nucl Med 25:1238, 1998.
Temmerman OP, et al: Detection of osteomyelitis using FDG and positron emission tomography. J Arthroplasty 16:243, 2001.
Thyrogen Complete Prescribing Information. Genzyme Therapeutics, Genzyme Corporation and Knoll Pharmaceutical Company, 1998.
Toronto Lung Transplant Group: Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 314:1140, 1986.
Tumeh SS, et al: Lymphoma: evaluation with Ga-67 SPECT. Radiology 164:111, 1987.
Wahl RL, et al: Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol 11:2101, 1993.
Wang SC, et al: Perfusion scintigraphy in the evaluation for lung volume reduction surgery: correlation with clinical outcome. Radiology 205:243, 1997.
Webb WR, et al: CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 178:705, 1991.
Wernly JA, et al: Clinical value of quantitative ventilation-perfusion lung scans in the surgical management of bronchogenic carcinoma. J Thorac Cardiovasc Surg 80:535, 1980.
White RI Jr, et al: The significance of unilateral absence of pulmonary artery perfusion by lung scanning. Am J Roentgenol Radium Ther Nucl Med 111:501, 1971.
Williams CD, Brenowitz JB: Prohibitive lung function and major surgical procedures. Am J Surg 132:763, 1976.
Willkomm R, et al: Functional imaging of Hodgkin disease with FDG-PET and gallium-67. Nuklearmedizin 37:251, 1998.
Winzelberg GG, Hydovitz JD: Radionuclide imaging of parathyroid tumors: historical perspectives and newer techniques. Semin Nucl Med 2:161, 1985.
Wiseman et al: Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: a phase II multicenter trial. Blood 99:4336, 2002.
Witzig TE, et al: Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20(+) B-cell non-Hodgkin lymphoma. J Clin Oncol 17:3793, 1999.
Witzig TE, et al: Randomized controlled trial of yttrium-90 labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin lymphoma. J Clin Oncol 20:2453, 2002a.
Witzig TE, et al: Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin lymphoma. J Clin Oncol 20:3262, 2002b.
Yeung HWD, et al: FDG-PET in esophageal cancer. Incremental value over computed tomography. Clin Positron Imaging 2:255, 1999.
Yoon YC, et al: Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. 227:764, 2003.
Young AE, et al: Location of parathyroid adenomas by thallium-201 and technetium-99m subtraction scanning [Abstract]. BMJ 286:1384, 1983.
Zhuang H, Alavi A: 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med 32:47, 2002.
Reading References
Mettler FA Jr, Guiberteau MJ: Essentials of Nuclear Medicine Imaging. 4th Ed. Philadelphia: WB Saunders, 1998.
Murray IPC, Ell PJ (eds): Nuclear Medicine in Clinical Diagnosis and Treatment. 2nd Ed. Edinburgh: Churchill Livingstone, 1998.
Sandler MP, et al (eds): Diagnostic Nuclear Medicine. 4th Ed. Philadelphia: Lippincott Williams&Wilkins, 2003.
EAN: 2147483647
Pages: 203