XVIII - Anatomy
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XXIII - Benign Esophageal Disease > Chapter 141 - Esophageal Motility Disorders
Chapter 141
Esophageal Motility Disorders
Cameron D. Wright
Esophageal motility disorders are uncommon and usually present with dysphagia or its sequelae (regurgitation and aspiration) or atypical noncardiac chest pain. These disorders are diagnosed by esophageal manometric studies that assess lower esophageal sphincter (LES) pressure and relaxation and the nature of esophageal contraction waves, including amplitude, duration, repetitive nature, the presence of nontransmitted or partially transmitted waves, and the presence of peristalsis in the body of the esophagus. Esophageal motility disorders are classified as primary when not related to a systemic disease and secondary if they are associated with a systemic disease. Table 141-1 lists the primary motility disorders with their manometric profiles. Recent reviews emphasizing the manometric diagnosis and medical management of the primary esophageal motility disorders have been published by Spechler and Castell (2001) and Richter (2001).
ACHALASIA
Achalasia, of Greek origin, means failure to relax. Achalasia was first described and effectively treated (by dilation with whalebone) by Thomas Willis in 1679. The classic findings on manometry are failure of the LES to relax in response to a swallow and absent peristalsis in the smooth muscle of the distal esophagus. The etiology of achalasia is unknown. Possible causes include hereditary, degenerative, autoimmune, and infectious. Achalasia is rare, with an incidence of only about 0.5 per 100,000 people. Although described in the very young and elderly people, it occurs most commonly between the ages of 20 and 50 years. The gender distribution is equal.
History
Payne (1989) reviewed the history of the surgical treatment of achalasia with an emphasis on Heller's contribution. Surgical treatment before the acceptance of Heller's technique was by a variety of anastomotic cardioplasties that relieved obstruction but led to severe esophagitis with its attendant complications. In 1914, Ernst Heller reported a successful result in one patient treated with a transabdominal double (anterior and posterior) esophagomyotomy. His operation did not become standard until the devastating late effects of severe reflux disease were reported by Barrett and Franklin in 1949. Heller's operation was modified to a single anterior esophagomyotomy by Groeneveldt in 1918 and further popularized by Zaaijer in 1923.
Pathophysiology
Pathologic studies have shown abnormalities in the esophageal myenteric (Auerbach's) plexus, which include inflammation, loss of ganglion cells, and fibrosis. Degenerative changes of the vagus nerve and changes of the dorsal motor nucleus of the vagus have also been described. Central vagal dysfunction and peripheral myenteric plexus destruction are the two hypotheses that could explain achalasia; it is unclear which hypothesis is correct. The end result of the destruction of the myenteric plexus is a selective loss of postganglionic inhibitory neurons containing nitric oxide and vasoactive intestinal polypeptide. Postganglionic cholinergic neurons are spared, and cholinergic stimulation continues unopposed, leading to high LES pressures. Impaired LES relaxation is caused by the loss of normal inhibitory input. Loss of normal inhibitory nitric oxide mediated input leads to a loss of peristalsis.
Diagnosis
Clinical Features
Patients with achalasia usually have dysphagia to solids and liquids (76%), as reported by Blam and associates (2002).
P.2147
Regurgitation is frequent (79%) and may contain food or saliva. Regurgitation is especially frequent at night during recumbency and may cause coughing or choking spells. Most patients (79%) learn to eat slowly and may have adaptive mechanisms such as repetitive swallows with the neck extended and using liquids to lubricate solid food. Chest pain occurs in some patients, especially those with early disease. Weight loss is common but not always present. Obesity does not eliminate the possibility of achalasia. A history of food bolus impaction may be present. Heartburn is often present as a symptom (24%), although because the LES does not relax normally, the cause is not classic gastroesophageal reflux. The cause is thought to be from the lactic acid from the fermented retained food. Pulmonary symptoms may predominate, with occasional patients diagnosed after hospitalization for aspiration pneumonia. Most patients live with their symptoms for years, and misdiagnosis of early cases is frequent.
Table 141-1. Classification of Primary Esophageal Motility Disorders and Their Manometric Features | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Chest Radiographs
Chest radiographs are usually normal, especially in the early stages of achalasia. The gastric air bubble may be absent owing to the overly competent LES. As the esophagus dilates, the lateral border of the esophagus may be seen as an abnormal mediastinal contour (Figs. 141-1 and 141-2). An air fluid level may be seen in the esophagus. Megaesophagus formation can lead to suspicion of a mass lesion. Aspiration pneumonia in dependent lung segments may be seen.
Contrast Esophagram
The characteristic findings of a barium swallow in achalasia are a dilated esophagus and a smooth, tapered, distal narrowing at the gastroesophageal junction (Fig. 141-3). Fluoroscopy demonstrates reduced or absent peristalsis in the body of the esophagus and impaired relaxation of the LES. A skilled radiologist can usually suggest the correct diagnosis. Blam and colleagues (2002) noted no correlation between the symptom score and the radiographic severity in achalasia patients. Early achalasia may be difficult to differentiate from other motility disorders (especially pseudoachalasia due to a small gastroesophageal junction cancer). The esophagus progressively dilates and elongates in the disease and can lead to the classic megaesophagus, which eventually can be sigmoid shaped and reach the lateral chest wall (Fig. 141-4). Epiphrenic diverticula may be present in the distal esophagus (Fig. 141-5).
Endoscopy
An upper gastrointestinal endoscopy is necessary in the diagnostic evaluation to exclude mucosal diseases that could mimic achalasia, especially cancer of the esophagogastric junction (pseudoachalasia). In achalasia, the body of the esophagus is usually dilated and often has retained food and liquid. Retention esophagitis may be present with a cobblestone appearance to the mucosa. The LES is usually tonically closed, but it is possible to pass the endoscope with gentle pressure through the esophagogastric junction. Cameron and colleagues (1999) reported on the accuracy of video endoscopy to diagnose achalasia by noting the response of the visualized esophagus to a swallow. In almost all patients, there was an absence of lumen-occluding contractions with failure of the LES to open. A correct diagnosis was made in 96% of the patients. The LES should feel soft as it is traversed. If passage is difficult and there is the suggestion of unusual firmness, a cancer should be suspected and an endoscopic ultrasound performed. If pseudoachalasia is suspected, a computed tomographic (CT) exam should also be performed to look for a mass at the esophagogastric junction.
Manometry
Manometry is the key diagnostic test because endoscopic and radiographic exams are never diagnostic of achalasia. The manometric abnormalities in achalasia are always confined to the distal esophagus because only the smooth muscle is affected. The two manometric features required to make a diagnosis of achalasia are (a) incomplete relaxation of the LES, and (b) aperistalsis in the body of the esophagus characterized by either simultaneous low-amplitude esophageal
P.2148
P.2149
contractions (<40 mm Hg) or no esophageal contractions (Fig. 141-6). The LES pressure is usually high (never low) but can be normal (10 to 45 mm Hg). Absent or incomplete LES relaxation is seen in about 80% of patients. In the remaining 20%, the LES relaxes to the gastric baseline but is of abnormally short duration (<6 seconds), leading to functional obstruction. The term vigorous achalasia is used if high amplitude (>50 mm Hg) simultaneous contraction waves are present in the body of the esophagus. The resting pressure in the body of the esophagus is often noted to be elevated ( pressurized ) above that of the gastric baseline.
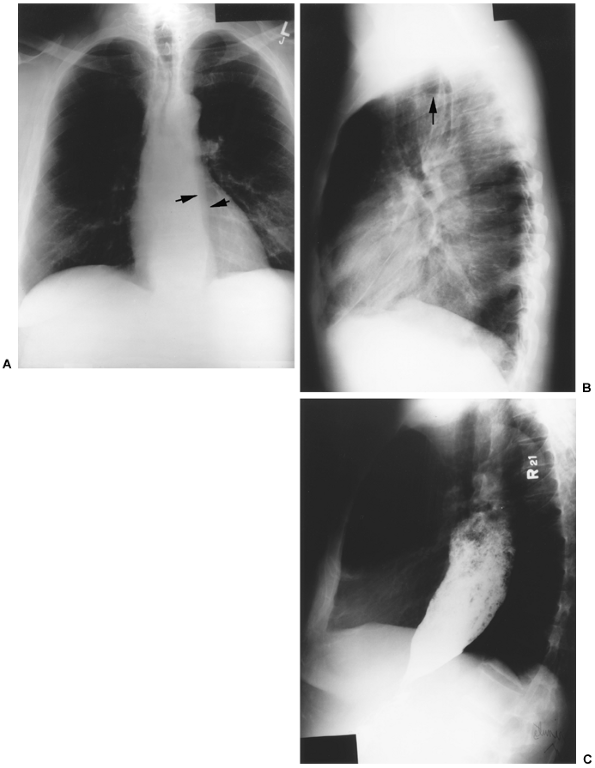 |
Fig. 141-1. A. Chest radiograph in a patient with achalasia illustrating an abnormal left mediastinal contour. The lateral arrow denotes the normal aortic stripe; the medial arrow denotes the abnormal dilated esophagus. B. Lateral chest radiograph from the same patient with an air fluid level (arrow) in a dilated esophagus. C. Barium esophagogram from the same patient demonstrating a massively dilated, food-filled esophagus characteristic of achalasia. |
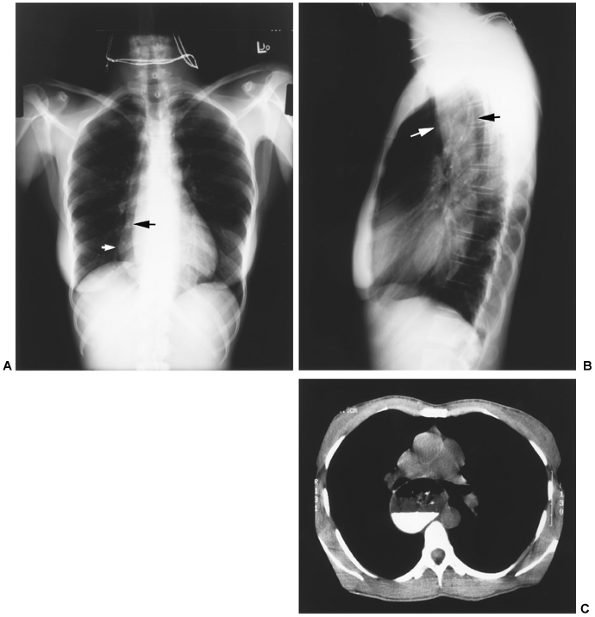 |
Fig. 141-2. A. Chest radiograph in a patient with achalasia illustrating an abnormal right heart border. The white arrow denotes the dilated esophagus, and the black arrow denotes the normal right heart border. B. Lateral chest radiograph from the same patient demonstrating a dilated esophagus (arrows). C. Computed tomographic scan from the same patient demonstrating a very dilated thin-walled esophagus with residual barium and food. |
Treatment
There is no curative treatment for achalasia because it is impossible to restore peristaltic function to the denervated esophagus. Current treatment is directed at reducing the
P.2150
pressure gradient across the LES, thus reducing outflow obstruction and allowing gravity to empty the esophagus. The two most effective treatments are mechanical: balloon dilation and surgical myotomy. Pharmacologic treatment is less effective and includes injection of botulinum toxin into the LES and orally administered drugs that reduce LES pressure. Esophageal resection is rarely needed for failure of primary therapy.
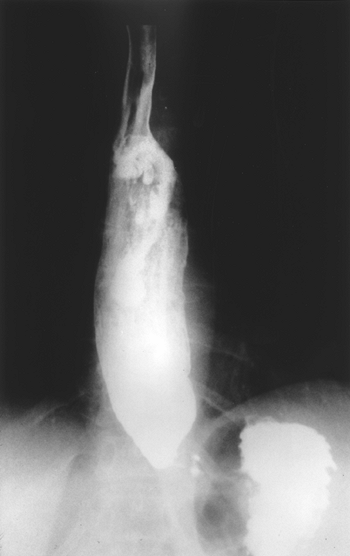 |
Fig. 141-3. Barium esophagogram of a patient with achalasia demonstrating a dilated esophagus with a tapered distal end of esophagus ( bird's beak deformity ). |
Medical Therapy
Several types of drugs are effective at reducing LES pressure; these include agonists, anticholinergics, nitrates, and calcium channel blockers. All have been used in achalasia, but all are of limited use because of either poor efficacy or side effects. Anticholinergics reduce LES pressure but are associated with severe side effects. agonists such as terbutaline sulfate also reduce LES pressure but are of limited clinical use because of poor efficacy, side effects, and tachyphylaxis. Nitrates reduce LES pressure and have been used clinically. Gelfond and associates (1982) reported that isosorbide dinitrate improved symptoms in most patients with achalasia, but headache and postural hypotension limited its use. Calcium channel blockers such as nifedipine and diltiazem reduce LES pressure but clinical improvement has been modest and decreased over time, as noted by Vaezi and Richter (1998). Eherer and co-workers (2002) reported on the effects of sildenafil (Viagra) on the esophageal body and LES. Sildenafil blocks phosphodiesterase, which degrades nitric oxide stimulated 3 5 -cyclic monophosphate, thereby relaxing smooth muscle cells in various organs, including the esophagus. Sildenafil lowered LES pressure and propulsive forces in the esophagus in normal controls and patients with achalasia and nutcracker esophagus. Less than one half of the patients exhibited symptomatic relief, and one half of those experienced intolerable side effects.
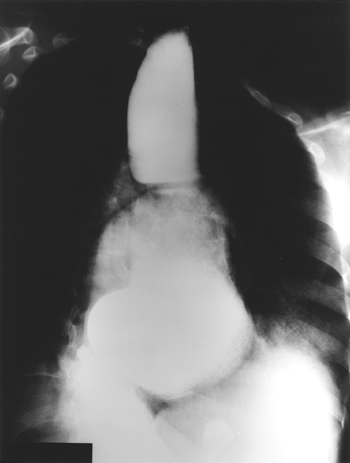 |
Fig. 141-4. Barium esophagogram in a patient with end-stage achalasia. A sigmoid-shaped esophagus is present with marked dilation of the lumen. Note the air bronchograms at the left diaphragm border consistent with aspiration pneumonia. |
Botulinum Toxin Injection
Pasricha and associates in 1994 reported the favorable results of the first trial of botulinum toxin injection in patients with achalasia. Botulinum toxin inhibits acetylcholine
P.2151
release from nerve endings, thereby reducing the cholinergic drive to the LES with resultant sphincter relaxation. The recovery of neurotransmission and subsequent muscle activity requires sprouting of new nerve endings and formation of new synaptic contacts to the adjacent muscle fibers. Botulinum toxin is administered on an outpatient patient by an endoscope through a sclerotherapy needle. Usually, 25 units are delivered into each of the four quadrants of the cardia in 1-mL aliquots. The initial success rate is about 70%, with symptom relief for up to several years. The only common side effects are transient chest pain after the procedure and reflux symptoms. Neubrand and co-workers (2002) reported on medium-term results and prognostic factors with botulinum toxin injection. The LES pressure was significantly reduced from 62 to 43 mm Hg after treatment. Thirty-six patients had symptomatic relief a mean of 2.5 years after treatment, whereas 64% had a good initial result. Retreatment was of no benefit if results were assessed at 6 months. Patients with high LES pressures (mean, 73 mm Hg) and younger patients (mean, 46 years) had poor results. Storr and associates (2001) reported the late results of 23 patients with achalasia treated with botulinum toxin injection. All had an initial favorable symptomatic result, but by 48 months, all had symptomatic relapse. Botulinum toxin injection is best suited for risk-adverse elderly patients with low LES pressures. Retreatment is of some value if there was an initial good response to injection but is of little benefit if the initial response was poor. Increasing the dose of botulinum toxin has been of no benefit in improving the response.
 |
Fig. 141-5. Barium esophagogram of a patient with achalasia and an epiphrenic diverticulum. Treatment was by a left thoracotomy approach with diverticulectomy and myotomy on the opposite side of the diverticulum closure. |
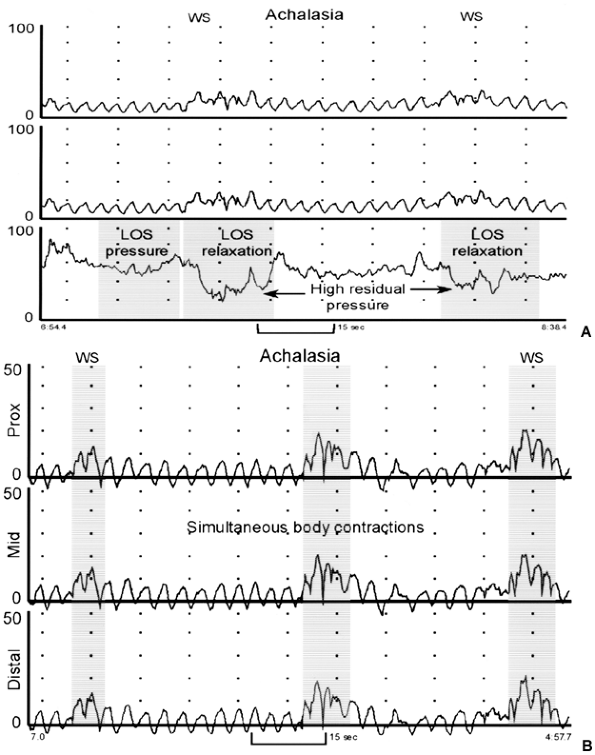 |
Fig. 141-6. A. Esophageal manometry tracing from a patient with achalasia. The distal recording site, positioned in the lower esophageal sphincter (LES), shows high basal LES pressure (about 60 mm Hg). Note that the two wet swallows (WS) are not accompanied by complete relaxation of the LES with residual pressure values of 24 and 36 mm Hg. The two proximal recording sites, located 3 and 8 cm above the LES, confirm an absence of peristaltic waves. B. Esophageal manometry tracing from a patient with achalasia. The three recording sites are positioned 3, 8, and 13 cm above the LES. Note that WS are not followed by peristaltic contraction and that the pressure changes recorded in the esophageal body are simultaneous, low amplitude, and identical (isobaric) in appearance. From permission Spechler SJ, Castell DO: Classification of oesophageal motility disorders. Gut 49:147, 2001. With permission. |
Pneumatic Dilation
Forceful dilation of the LES is a noninvasive, time-tested treatment option in achalasia. Originally a large, relatively compliant bag dilator (Mosher bag) was swallowed and positioned
P.2152
across the cardia and inflated with contrast material under fluoroscopic control until the waist of the LES impression disappeared. The aim of pneumatic dilation is rupture of the muscle fibers of the LES with maintenance of an intact esophageal mucosa. The reason this seemingly contradictory result can occur is the relative different compliances of the esophageal sphincter (less compliant) and the mucosa (more compliant). Good results were obtained initially in about 70% of patients with a perforation rate of 2% to 5%. More recently, balloon dilation has been performed with polyethylene noncompliant-sized balloons that can be passed through the working channel of an endoscope or over a guidewire. The most commonly used balloons are the Rigeflex balloons (Microinvasive, Watertown, MA) in 3-, 3.5-, and 4-cm sizes. The procedure is usually done as an outpatient under sedation. Most patients have rather significant chest pain during the dilation, which rapidly improves. If the pain continues, the patient should be evaluated for perforation. Most gastroenterologists do the dilation under endoscopic but not radiographic control. Results are then judged by relief of dysphagia in follow-up. If there is inadequate relief, the next size of balloon dilator is used. A more precise technique is to use radiographic control with inflation of the balloon with dilute contrast; dilation is continued until the waist of the LES disappears. Kadakia and Wong (2001) reviewed the results of modern balloon dilation. The symptomatic response rate was 74% to the 3-cm balloon, 33% to the 3.5-cm balloon, and 5% to the 4-cm balloon. The perforation rate increased as balloon size increased: 1% with the 3-cm and 15% with the 4-cm balloon. In the short term, relapse occurs at about 6% per year. Troublesome heartburn occurred in only 5% of patients. Sabharwal and colleagues (2002) reported results in 76 patients using radiographic control of balloon dilation. There were no perforations, and 89% of patients reported satisfactory improvement of their swallowing. Fifty-two patients required a single dilation, 22 patients between two and four dilations, and 2 patients needed five dilations. There is very little information on the long-term results of dilation. West and co-workers (2002) recently reported long-term results in patients after dilation. At 5 years, the success rate was only 50%, and at 15 years, the success rate dropped to only 40%. Of the 32 patients who died during the study, 6 (19%) died of esophageal cancer. Sabharwal and associates (2002) have reported safe, effective balloon dilation after failed surgical myotomy. Alternately, Ferguson and colleagues (1996) and Dolan and co-workers (2002) have reported good results with surgical myotomy after failed balloon dilation. Balloon dilation is usually offered as first-line therapy by gastroenterologists because of its minimally invasive nature, good initial results, and low cost compared with myotomy.
Esophagomyotomy
Esophageal myotomy is the definitive treatment for achalasia. The goals of the procedure are twofold: reduce the LES pressure enough to allow gravity drainage of the esophagus and paradoxically maintain (or augment) some control of gastroesophageal reflux. Although the principles of Heller's myotomy are generally accepted, the exact details of the operation remain controversial. The areas of controversy include the approach (laparotomy, thoracotomy, thoracoscopic, or laparoscopic), the extent of the myotomy (limited, just onto the stomach; or extensive, 1 to 2 cm onto the stomach), and the need for an antireflux repair (none or routinely).
Heller's original technique was a double myotomy through a laparotomy. This was abandoned because of late severe reflux complications. A single myotomy is now accepted by all as sufficient to allow enough reduction in LES pressure to relieve outflow obstruction. Many South American and most European surgeons continued with the laparotomy approach because in those areas, historically visceral surgeons usually operated on the esophagus. With a laparotomy approach, because of the more extensive cardial dissection, an antireflux procedure is always added. This is always a partial wrap (either an anterior Dor or a posterior Toupet fundoplication). Excellent early results have been reported by Csendes (1989) and Bonavina (1992) and their associates, with excellent results of myotomy and Dor fundoplication in 95% and 94%, respectively. Most surgeons favor the Dor wrap because less mobilization of the esophagus and gastric fundus is required; the edges of the wrap are sewn to the cut edges of the myotomy, which is thought to prevent healing of the two cut edges together; and any microperforations in the mucosa are covered by the anterior Dor wrap. Ferguson (1991) reviewed multiple series of myotomies, comparing the laparotomy approach to the thoracotomy approach with or without an antireflux repair. The results were essentially equivalent, with good results in 90% (432 patients) with an abdominal approach, 87% (310 patients) with a limited thoracic myotomy, and 91% (280 patients) with an extensive thoracic myotomy with an antireflux procedure. The advantages of an abdominal approach include ease of construction of a fundoplication, ready access to the esophagogastric junction, ability to perform other needed abdominal procedures, and no long-term pain syndromes associated with the incision. The disadvantages are difficulty with exposure in obese patients and inability to do a long myotomy in the rare case of vigorous achalasia.
American thoracic surgeons who have historically operated on the esophagus naturally approached a myotomy through the chest. Ellis (1993) championed the limited myotomy just onto the stomach, as recognized by the change in the vessel pattern between the esophagus and stomach. Ellis made the point that this operation is simpler and quicker, reliably reduces LES pressure, does not usually lead to troublesome reflux disease, and avoids the possible obstructive complications sometimes associated with antireflux wraps. Ellis and colleagues (1992) reported very good early results (90% good or excellent) and reasonable
P.2153
late results at 10 to 20 years (67% good or excellent). Troublesome reflux was seen in only 5% of patients. Only 1 patient of 189 required an esophagectomy for late complications of reflux and poor emptying. Pearson of Toronto has been a champion of an extended myotomy with a modified Belsey partial fundoplication. He emphasizes the importance of a complete myotomy (the reason for operation is obstruction), which also cuts the gastric-sling fibers, necessitating a partial wrap to prevent reflux. Malthaner and associates (1994) from Pearson's group reported their early and late results with a minimum follow-up of 10 years. Good or excellent results were recorded in 95% at 1 year but in only 69% at 15 years. Three of 52 patients required an esophagectomy for late complications. Troublesome reflux was seen in 18% at 10 years. The late complications of reflux are likely due to the underlying aperistalsis of the body of the esophagus causing pump failure with poor acid clearance from any refluxate into the esophagus. Paradoxically, a competent LES may make the esophagitis worse because of pump failure in the face of a relatively competent LES. Topart and co-workers (1992) reported severe late obstructive complications requiring reoperation in patients that had a Nissen wrap after myotomy. All now agree that a Nissen total fundoplication results in an excessive degree of obstruction in the face of an aperistaltic esophagus and should not be performed. Ferguson (1991) reported essentially equivalent results with a transthoracic myotomy with or without an antireflux repair, with 90% good results either way. In the end, both approaches are acceptable, and each surgeon should use the technique that leads to the best personal result. The advantages of the thoracic approach include excellent exposure of the esophagus, ability to do a long myotomy or a diverticulectomy, and ease of operation in obese patients. The disadvantages include the temporary need for a chest tube, more postoperative pain, longer hospital stays, and the small incidence of troublesome intercostal neuralgia.
With the advent of the minimally invasive surgery revolution at about 1990, esophageal myotomy was performed with video thoracic surgery (VATS) techniques. Although technically possible, the operation was challenging, occasional mucosal perforations occurred, and there was still a need for a chest tube. Pellegrini and colleagues (1992) were one of the first groups to report on the VATS approach to do a myotomy. They reported on 15 patients. One had a mucosal perforation that was repaired during VATS and subsequently did well. The average length of stay was 3 days. Three patients required reoperation because of an inadequate myotomy. Good results were recorded in only 76% of patients. They found that the lowest part of the myotomy, the most crucial to perform, was the most difficult to see and perform. An indwelling endoscope was found to aid performance of the operation greatly. Ramacciato and associates (2002) compared the VATS and the laparoscopic approaches to myotomy within their own unit. Laparoscopic myotomy was found to be superior in several areas: shorter operation, shorter length of stay, better dysphagia relief, less postoperative heartburn, and less incisional discomfort. Numerous reports from centers around the world have documented the safety and efficacy of laparoscopic myotomy; these include reports by Ramacciato (2002), Zaninotto (2002), Sharp (2002), Champion (2000), and Fernandez (2003) and their associates, as well as by Bowrey and Peters (2000). There is no question that more patients are being referred for minimally invasive myotomy by gastroenterologists who must have been hesitant before about choosing surgical myotomy over pneumatic dilation. There is now a broad consensus that laparoscopic myotomy with a partial wrap is the procedure of choice among most esophageal surgeons. Those who do not perform minimally invasive myotomy have found that their practice has disappeared. Conversion to an open procedure is very rare. Intraoperative mucosal injury occurs in about 5% of patients and can almost always be handled with laparoscopic suturing and buttressing with an anterior Dor wrap. Most surgeons have found intraoperative endoscopy to be helpful, especially early in their experience, to locate the squamocolumnar junction and to gauge the adequacy of the distal extent of the myotomy (the LES is seen, and one can readily see into the stomach from the distal esophagus when the last few gastric fibers are cut). Although there is a loss of feel with the closed approach, the videocamera magnifies the operative field, which actually makes the distinction between the esophageal and stomach submucosa easier to identify. There are various techniques to perform the myotomy, and it is not clear which is best. Myotomy can be performed with scissors, with hook and cautery division, and by tearing muscle fibers between two forceps. It is very important not to injure the mucosa with the cautery, which could present with a delayed perforation. I prefer the scissors. Almost all muscular vessel bleeding will stop with time and pressure during the myotomy, which helps to avoid cautery. It is not clear which partial wrap is best, and at least one randomized trial is in progress to help clarify the issue. Many surgeons obtain a control barium swallow the following morning to document the relief of LES obstruction and the absence of a leak. Liquids are allowed the first day and rapidly advanced to a soft diet. The average length of stay is 2.5 days. Most series report good results in 90% to 95% and heartburn in 5% to 10%, results that duplicate the open approach. Decker and colleagues (2002) reported an interesting study on quality of life before and after laparoscopic myotomy in a series of 73 French patients with achalasia. The quality-of-life score preoperatively was 84 (range, 34 to 129; 126 normal, 144 maximum), whereas postoperatively, it improved to 119 (range, 77 to 143), which was very close to the baseline population. Patients who were the worst preoperatively improved the most postoperatively. Patients had improvement non only of the gastrointestinal symptoms but also of the physical, emotional, and social items. This emphasizes the importance (and joy) of eating normally to one's good overall health.
P.2154
The selection of therapy for an individual patient remains controversial. Many gastroenterologists still favor pneumatic dilation, whereas most surgeons favor laparoscopic myotomy. There are no randomized trials that compare these two treatment options. A previous small randomized trial was reported by Csendes and co-workers (1989) in 81 patients equally split between Mosher bag dilation and laparotomy with myotomy and Dor fundoplication. There were two perforations in the dilation group, and both were successfully repaired. At late follow-up (60 months), 95% of the myotomy patients had good results, whereas only 65% of dilation patients had a good result. This study has been widely quoted as favoring surgical myotomy. It is likely the surgical results could be duplicated with laparoscopic results, and it also is likely that with the modern graded balloon approach, the dilation results might be improved. Two retrospective single-institution studies examined results in their own institution; Okike (1979) and Donahue (1986) and their associates also reported superior results with surgical myotomy. Ferguson (1991), in a review of 11 series between 1980 and 1990 that reported on 899 patients having a pneumatic dilation, found that only 715 had a good result. Redilation was need in 17%, perforation occurred in 1.4%, and the mortality rate was 0.3%. These results were markedly inferior to the 1,032 surgical myotomy patients, who had good results in 90%. It is not surprising that the technical procedural results are better with surgical myotomy because it is done under direct vision and is quite precise. The real question is what price the patient and referring physician are willing to pay for a somewhat better result. A recent interesting report tried to answer that question by means of modern decision and cost analysis techniques. O'Connor and co-workers (2002) compared three treatment options for achalasia: (a) pneumatic dilation, (b) botulinum injection, and (c) laparoscopic myotomy. Based on literature review, they estimated a probability of success the first time of 74% with dilation, 84% with botulinum, and 90% with myotomy. The perforation rate was estimated at 1%. The relapse rate was estimated at 6% per year for dilation, 40% per year for botulinum, and 2% per year for myotomy. The cost of a single procedure was as follows: dilation, $580; botulinum, $723; and myotomy, $18,993. The results of the cost-effectiveness analysis was $7,069 for dilation, $7,011 for botulinum, and $21,047 for myotomy. The quality-of-life adjusted years were the same for all, at 4.6. The incremental costs were $58 for dilation and $14,338 for myotomy. The authors concluded that dilation was the best strategy for the average patient. In the end, the patient needs to be presented with the management options and have a through discussion of the risks and benefits of each technique to help make an individual decision.
Vigorous Achalasia
An atypical variant of classic achalasia is so-called vigorous achalasia, first reported by Sanderson and associates (1967), characterized by esophageal body contractions with amplitudes greater than 40 mm Hg. It is unclear whether this is really a unique clinical entity or just a stage in evolution of achalasia to its classic form. Some investigators have proposed that atypical chest pain is more common in these patients, whereas others, such as Goldenberg and co-workers (1991), suggest that such patients cannot be distinguished from those with classic achalasia. Pasricha and colleagues (1996) reported that botulinum toxin injection is more effective in patients with vigorous as opposed to classic achalasia, whereas Culliere and co-workers (1997) found no difference in response between the patients. If the esophagus is dilated and dysphagia is the clinical problem, the treatment should be the same as for classic achalasia. If the esophagus is not dilated and pain is an important part of the clinical picture, the patient should be considered for a long myotomy to encompass the abnormal manometric area.
Achalasia Associated with Epiphrenic Diverticulum
Epiphrenic diverticulum is almost always associated with an esophageal motility disorder, with about one half of cases characterized as achalasia. Nehra and colleagues (2002) recently reported 21 patients with diverticula and reviewed the recent literature. All of their patients had a motility disorder, and a consistent finding on ambulatory motility studies was a high percentage of simultaneous waveforms with esophageal body contractions of increased amplitude and duration. Pneumatic dilation is not appropriate because the torn esophageal muscle will not extend to the base of the diverticulum, and the diverticulum is left in place. The standard procedure is a left transthoracic myotomy to the base of the diverticulum and a diverticulectomy. There are numerous reports, including that of Rosati and associates (1998), of laparoscopic myotomy and diverticulectomy. For a laparoscopic approach to be feasible, the diverticulum has to be very close to the cardia (as it usually is) for sufficient access through the hiatus. Details of epiphrenic diverticulum management are presented in Chapter 148.
Esophagectomy for Achalasia
Esophagectomy is rarely appropriate as primary therapy for achalasia and occasionally appropriate as a salvage treatment for late complications. Absolute indications for esophagectomy include resectable cancer, multiple failed previous treatments, reflux strictures, intractable esophagitis, obstruction associated with an overly competent fundoplication, and perforation of an end-stage dilated esophagus. Relative indications include a single failed myotomy in an end-stage dilated esophagus and initial treatment of an end-stage sigmoid-shaped esophagus. Most surgeons suggest that the results of dilation or myotomy with a sigmoid esophagus are so poor that the additional risk for esophagectomy is warranted. The massively dilated
P.2155
sigmoid esophagus empties so poorly that a nonperistaltic narrow conduit empties better. Banbury (1999), Miller (1995), Peters (1995), and Devaney (2001) and their co-workers have reported large series of esophagectomies for achalasia. In Devaney's report on 93 patients, the indications for resection were sigmoid esophagus (64%), failed previous procedures (29%), and reflux stricture (7%). Transhiatal resection was possible in 94% of patients. The stomach was used in all but two patients. There were two postoperative deaths. The average hospital stay was 12.5 days. Ninety-five percent of the patients are able to eat a regular diet, 71% had a good or excellent result, and 88% were pleased they had an operation. The authors made several technical points: (a) the esophagus is usually very deviated into the right chest, which complicates resection; (b) the direct esophageal aortic arteries are often quite enlarged and must be carefully controlled; (c) the dilated esophagus makes the cervical esophagus difficult to encircle, and caution must be used to avoid recurrent nerve injuries; and (d) the exposed esophageal submucosa after prior myotomy is often very adherent to the aorta and lung that complicates resection. As noted by Peters and co-workers (1995) at the University of Southern California School of Medicine, DeMeester has advocated use of colon as a better conduit for esophageal replacement in achalasia. He makes the point that the stomach regains its acid-secretion ability despite the vagotomy, which can lead to late esophagitis and strictures of the proximal esophagus. His group reported excellent results with the use of the colon, with no deaths in 19 patients. Most groups, however, report more early complications with the use of colon rather than stomach, especially the occasional devastating ischemic colon graft.
Achalasia and Esophageal Cancer
The end result of years of chronic esophageal mucosal irritation from saliva, undigested food, bacteria, and refluxate in the end-stage achalasia esophagus is cancer. Streitz and associates (1995) reported on 241 collected patients who developed squamous cell cancer of the esophagus in association with achalasia. The patients uniformly presented with advanced disease and had a dismal prognosis accordingly. They made the point that in the dilated obstructed achalasia esophagus, a cancer has to grow to a large size and cause severe dysphagia before the patient can recognize this as a new problem. The risk was estimated to be 14.5 times that of the normal population. Reflux esophagitis is also a late chronic complication of achalasia. It is therefore not surprising that Barrett's mucosa and adenocarcinoma have also been reported by Di Simone and colleagues (1996).
DIFFUSE ESOPHAGEAL SPASM
Diffuse esophageal spasm (DES) is a rare motility disorder characterized by intermittent dysphagia and chest pain with normal peristalsis intermittently interrupted by simultaneous contractions. Diffuse esophageal spasm is rare and is seen in less than 5% of patients with motility disorders. The mean age of affected patients is about 50 years, and more women are affected than men.
History
Osgood (1889) reported the first cases of esophageal spasm with dysphagia. Creamer and co-workers (1958) were the first to report the manometric feature of simultaneous contractions in DES. Richter and Castell (1984) clarified the manometric definition for DES: simultaneous contractions associated with more than 20% of wet swallows (but <100%) and mean simultaneous contraction amplitude greater than 30 mm Hg.
Pathophysiology
The etiology of DES is unknown. Most reports, including those of Friesen (1983) and Eypasch (1992) and their associates, demonstrate no changes in the esophageal muscle and myenteric plexus. Patients with DES are hypersensitive to cholinergic and hormonal (pentagastrin) stimulation. Recent studies implicate decreased available nitric oxide in the etiology of DES, as reported by Beher and Biancani (1993) and Konturek and colleagues (1995).
Diagnosis
Clinical Features
Intermittent chest pain and dysphagia are the common presenting symptoms. The chest pain can be indistinguishable from ischemic cardiac pain and also can respond to nitroglycerine. The pain can vary in intensity, location, and frequency. It is not related to exertion and may relate to meals. The pain is usually substernal but may be epigastric and can radiate to the neck and arm like ischemic pain. The dysphagia is intermittent, is nonprogressive, and can occur with both solids and liquids. Dysphagia may be precipitated by stress, very hot or cold liquids, or rapid eating. Some patients also have other intestinal dysmotility syndromes such as irritable bowel syndrome.
Radiographic Evaluation
Contrast esophagograms can be normal but classically show segmental spasm of the distal esophagus, which has been described as corkscrew esophagus or pseudodiverticulosis of the esophagus (Fig. 141-7). Diffuse esophageal spasm can be suggested by the barium swallow appearance but is not diagnostic, as reported by Fuller and colleagues (1999). CT of the esophagus invariably demonstrates a markedly thickened wall of esophagus.
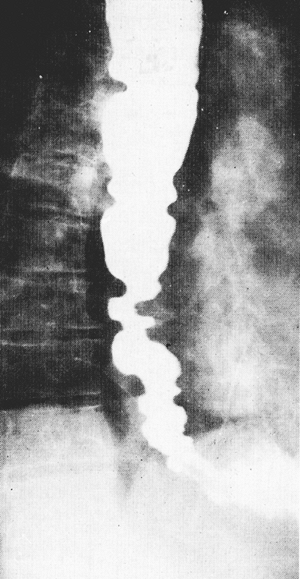 |
Fig. 141-7. Barium esophagram of a patient with diffuse esophageal spasm. Note the nonpropulsive severe segmental contractions that produce functional esophageal obstruction. |
P.2156
Manometry
Diffuse esophageal spasm is defined as more than 20% simultaneous contractions intermixed with normal peristalsis (Fig. 141-8). If all contractions are simultaneous, the diagnosis is achalasia. Those patients with pain tend to have high contraction pressures, whereas those without pain have lower pressures. The simultaneous contractions should exceed 30 mm Hg. Other findings include long-duration contractions, spontaneous non swallow-induced contractions, repetitive waves (three peaks or more), and occasionally incomplete LES relaxation, as reported by Richter (2001). Ambulatory 24-hour manometry is more sensitive for the diagnosis of DES, and meal-induced swallows are especially disordered, as reported by Eypasch and co-workers (1992).
Treatment
There is no definitive treatment for DES. Most important is to eliminate ischemic cardiac disease in the differential with a cardiology evaluation so that the patient can be reassured that the disease is not fatal. Some patients with mild disease respond to simple reassurance. More symptomatic patients require medical treatment. Patients who have failed a prolonged trial of all medical therapies and who have a persistently poor quality of life are candidates for extended myotomy.
Medical Therapy
Gastroesophageal reflux should be sought by 24-hour pH probe testing and treated, if found, with proton-pump inhibitors. The same drugs that can be used in achalasia also have some degree of efficacy in DES. These include nitrates, anticholinergics, and calcium channel blockers. The frequent presence of anxiety and affective disorders in these patients has lead to the use of anxiolytic agents and antidepressants. Clouse and colleagues (1987) reported a randomized trial of trazodone (an antidepressant) to treat the chest pain. The drug was effective in relieving pain perception but did not alter manometric findings.
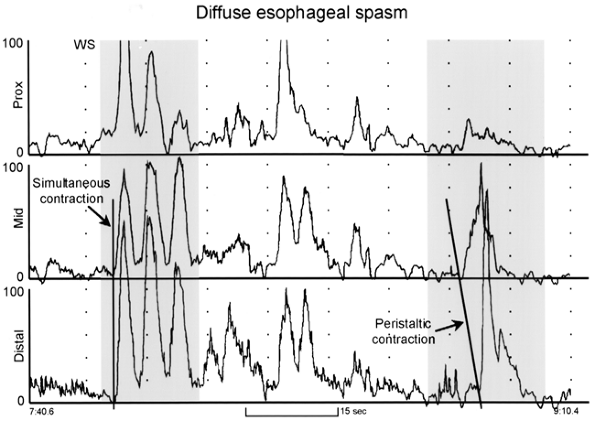 |
Fig. 141-8. Esophageal manometry tracing from a patient with diffuse esophageal spasm. The recording sites are positioned 3, 8, and 13 cm above the lower esophageal sphincter (LES). Note that the first wet swallow (WS) is followed by esophageal contractions that are simultaneous and repetitive. However, some peristaltic activity is preserved, as evidenced by the peristaltic contraction of the esophageal body shown in the sequence on the right. From Spechler SJ, Castell DO: Classification of oesophageal motility disorders. Gut 49:147, 2001. With permission. |
P.2157
Dilation
Simple dilation has been described for patients with dysphagia but has no physiologic basis; any response is likely to be the placebo effect only. Forceful dilation has no role to play in most patients because the entire distal esophagus is affected. The rare patient with impaired LES relaxation and dysphagia may benefit from pneumatic dilation.
Botulinum Toxin Injection
Numerous reports suggest some efficacy for the treatment of both dysphagia and chest pain in patients with DES, including those of Miller (2002) and Storr (2001) and their associates. Storr treated nine patients by multiple injections of botulinum toxin from the LES and along the body of the esophagus. At 1 month, 89% of patients were markedly improved, with reduction in symptom scores from 8 to 2 after treatment. At 6 months, all eight responders were still improved. Four patients needed repeat injections at a mean of 15 months, with similar good results.
Extended Esophagomyotomy
Surgical treatment is reserved for true medical failures because the surgical results are not as good or as predictable as those for achalasia. Patients should be cautioned that the procedure is palliative and is intended to reduce symptoms rather than return them to normal. The extent of myotomy is determined by the preoperative manometry. The myotomy can be extended under the aortic arch through the left chest to the thoracic inlet if needed. The LES does not need to be divided if it is normal on manometry. Most surgeons approach an extended myotomy through the left chest. The extent of myotomy (proximal and distal) and the need for an antireflux repair are areas of controversy. Leonardi and colleagues (1977) reported the results of extended myotomy (sparing the LES and avoiding an antireflux procedure) in 11 patients; 10 patients were improved, and reflux was a problem in only 1 patient. Eypasch and co-workers (1992) reported the results of extended myotomy (dividing the LES and adding a Dor fundoplication) in 15 patients. Chest pain was improved in 12 patients, and dysphagia was improved in 14 patients. Twelve of 14 patients stated they would have the operation again. Patti and associates (1995) reported a thoracoscopic approach to extended myotomy for DES.
HYPERCONTRACTING ESOPHAGUS (NUTCRACKER ESOPHAGUS)
Hypercontracting (or nutcracker) esophagus is a motility disorder with high-amplitude esophageal contractions and chest pain.
Pathophysiology
The etiology of hypercontracting esophagus is unknown. There are no pathologic changes seen in the muscle or myenteric plexus. Some high-pressure contractions are related to either reflux or stress. The correlation between the high-amplitude contractions and pain is unclear because most patients are asymptomatic while undergoing manometry. Relief of chest pain does not reliably correlate with reduction of contraction amplitude following medical or surgical therapy. The abnormal contractions may represent epiphenomenon rather than a true motility disorder. Richter (1986) and Mujica (2001) and their co-workers have reproduced chest pain in these patients with balloon distension of the esophagus. Patients with hypercontracting esophagus had lower pain thresholds than controls. Patients often share psychological profiles similar to patients with irritable bowel syndrome.
Diagnosis
Clinical Features
The average age of the patients is the fifth decade, and most are women. Almost all patients have chest pain and rarely have dysphagia. Cardiac ischemia must be eliminated as a possible cause in these patients, just as in those with DES.
Radiographic Evaluation
Peristalsis is normal in these patients; thus, contrast esophagograms are usually normal.
Manometry
Manometry in a hypercontracting esophagus reveals a mean distal peristaltic wave amplitude greater than 180 mm Hg [measured as the average amplitude of 10 swallows at two recording sites 3 and 8 cm above the LES (Fig. 141-9)]. By definition, the contractions must be peristaltic. Peristaltic contractions of long duration are frequently present. Resting LES pressure is usually normal but can be elevated, in which case a variant diagnosis would be present (hypercontracting esophagus with hypertensive LES).
Treatment
The treatment of the hypercontracting esophagus is similar to the medical treatment of DES with unpredictable results. A small, randomized crossover trial of diltiazem in 14 patients was reported by Cattau and colleagues (1991) and showed reduced chest pain and lower contraction amplitudes. A previous trial of nifedipine by Richter and co-workers (1986) was negative. Trazodone and imipramine
P.2158
have been suggested as beneficial. Winters and associates (1984) reported an interesting trial of placebo dilation with a No. 24F dilator versus a therapeutic No. 54F dilator. Neither would be expected to provide any benefit given the pathophysiology of hypercontracting esophagus. Both groups of patients reported benefit in reducing chest pain, with no difference between the two! This is another example of the power of the placebo effect. Since the contractions are not reliably related to the chest pain, it is difficult to be enthusiastic about extended myotomy in this disease. Traub (1987) and Shimi (1992) and their colleagues reported mixed success of surgical myotomy. Surgery should be an absolute last resort, and the patient should be informed that propulsive contractions will be eliminated by extended myotomy.
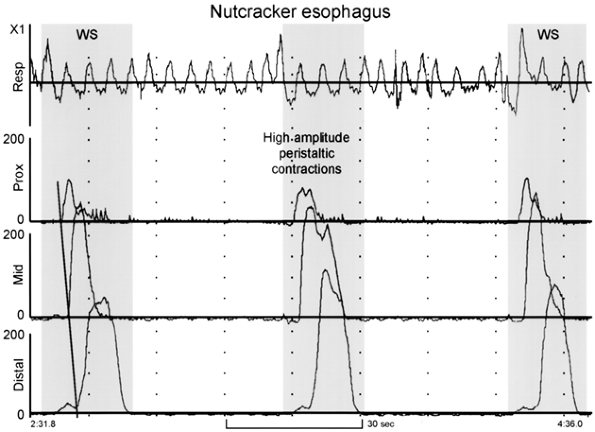 |
Fig. 141-9. Esophageal manometry tracing from a patient with nutcracker esophagus. The recording sites are positioned 3, 8, and 13 cm above the lower esophageal sphincter (LES). Note the high-amplitude peristaltic contractions initiated by wet swallows (WS). From Spechler SJ, Castell DO: Classification of oesophageal motility disorders. Gut 49: 149,2001. With permission. |
HYPERTENSIVE LOWER ESOPHAGEAL SPHINCTER
A hypertensive LES is defined as a mean resting pressure of more than 45 mm Hg measured in mid-respiration using the station pull-through technique. The LES is usually hypertensive in achalasia and often hypertensive in DES, and occasionally with hypercontracting esophagus. Isolated hypertensive LES is rare (<3% of all patients with noncardiac chest pain who undergo manometry) and of uncertain clinical consequences. Bassotti and co-workers (1992) have reported a 9-year experience with hypertensive LES. The rare patient with persistent severe dysphagia should be considered for balloon dilation or limited surgical myotomy.
HYPOCONTRACTING ESOPHAGUS (INEFFECTIVE ESOPHAGEAL MOTILITY)
Most patients who were previously diagnosed with a nonspecific motility disorder have motility tracings with low-amplitude (<30 mm Hg) peristaltic or simultaneous contractions in the distal esophagus or failed peristalsis (Fig. 141-10). These abnormalities have been renamed ineffective esophageal motility by Leite and co-workers (1997). Patients often have low LES pressure, but it may be normal. Low-amplitude (<30 mm Hg) waves have been demonstrated to be ineffective at esophageal clearance by using simultaneous manometry and barium video radiography by Kahrilas and associates (1988). Most patients with ineffective esophageal motility have gastroesophageal reflux disease and have heartburn and regurgitation. The abnormal acid exposure in these patients correlates better with the poor esophageal clearance than the resting LES pressure. Dysphagia is usually absent or mild in these patients. Severe dysphagia implies that there is an anatomic lesion such as severe esophagitis, stricture, or cancer. It is unclear whether gastroesophageal reflux leads to the development of ineffective esophageal motility by repeated esophageal acid exposure or whether the presence of preexisting poor esophageal peristalsis leads to ineffective esophageal clearance mechanisms, producing abnormal gastroesophageal reflux. Scleroderma is the classic example of severe esophageal hypocontraction. Patients with scleroderma have vascular obliteration and secondary fibrosis of the nerves and
P.2159
the muscle that weakens contractions and results in the loss of normal peristalsis. Similar manometric findings can be seen in other connective tissue diseases as well as a variety of systemic diseases such as alcoholism, diabetes, amyloidosis, and myxedema. Treatment is directed at controlling gastroesophageal reflux with proton-pump inhibitors. There is no effective drug to increase esophageal motility. Cisapride was used to increase motility but was withdrawn because of its rare association with cardiac arrhythmias.
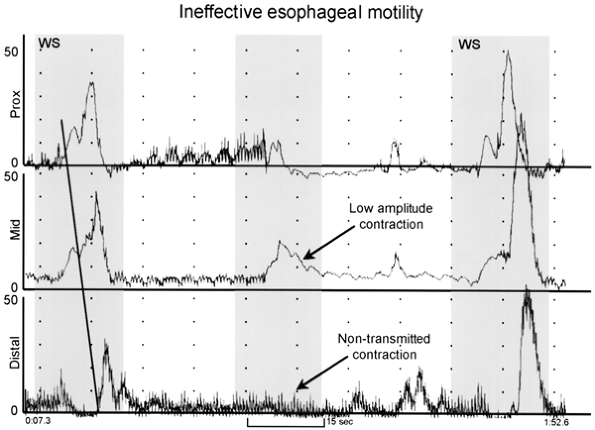 |
Fig. 141-10. Esophageal manometry tracing from a patient with ineffective esophageal motility. The recording sites are positioned 3, 8, and 13 cm above the lower esophageal sphincter (LES). Note that the first and third wet swallows (WS) result in a normal peristaltic sequence. However, the second wet swallow stimulates only low-amplitude contractions in the proximal two leads, and no contraction on the distal lead (nontransmitted or failed peristalsis). From Spechler SJ, Castell DO: Classification of oesophageal motility disorders. Gut 49:149, 2001. With permission. |
SECONDARY ESOPHAGEAL MOTILITY DISORDERS
Patients with secondary disorders of esophageal motility have motility disorders from another systemic disease. Scleroderma is again the classic example. Table 141-2 lists many of these other diseases that affect esophageal motility. Treatment is usually directed at the underlying disease, and reflux symptoms are treated with proton-pump inhibitors. Chagas' disease is a special case and is discussed in detail in Chapter 142.
Pseudoachalasia (Secondary Achalasia)
The radiologic and manometric diagnosis of primary achalasia is not secure because about 4% of patients that present with classic achalasia have instead pseudoachalasia (an achalasialike syndrome that is usually due to an esophagogastric junction cancer) infiltrating the myenteric plexus, as noted by DiBaise and Quigley (1998). It is impossible to differentiate some cases of pseudoachalasia from true achalasia based on barium swallow and manometry alone. This is why all patients with newly suspected achalasia must have endoscopic evaluation of the upper gastrointestinal tract. If there is significant resistance to passage of the scope through the esophagogastric junction, a cancer should be suspected, even in the absence of a mucosal abnormality. An endoscopic ultrasound exam and a computed tomographic exam should be done to evaluate this area thoroughly. Kahrilas and co-workers (1987) compared the clinical features of the two types of achalasia. Clinical features that suggest carcinoma-induced achalasia include older age, rapid symptom development, significant weight loss, progressive dysphagia, and difficulty passing the manometry catheter through the LES. Liu and colleagues (2002) reported 13 cases of pseudoachalasia and found neoplastic infiltration of the myenteric plexus in 11 patients. A paraneoplastic syndrome was thought to be the cause in the other 2 patients. The neoplasms included adenocarcinoma of the esophagogastric junction (7), esophageal cancer (1), metastatic renal cell cancer to the esophagogastric junction (1), breast cancer (1), small cell lung cancer (1), mesothelioma (1), and mediastinal fibrosis (1). The treatment of pseudoachalasia is the treatment of the underlying cancer.
Table 141-2. Secondary Esophageal Motility Disorders | |
|---|---|
|
REFERENCES
Banbury MK, et al: Esophagectomy with gastric reconstruction for achalasia. J Thorac Cardiovasc Surg 117:1077, 1999.
Bassotti G, et al: Isolated hypertensive lower esophageal sphincter. Clinical and manometric aspects of an uncommon esophageal motor disorder. J Clin Gastroenterol 14:285, 1992.
Beher J, Biancani P: Pathogenesis of simultaneous esophageal contractions in patients with motility disorders. Gastroenterology 105:111, 1993.
Blam ME, et al: Achalasia: a disease of varied and subtle symptoms that do not correlate with radiographic findings. Am J Gastroenterol 97: 1916, 2002.
Bonavina L, Nosadini A, Bardini R: Primary treatment of esophageal achalasia. Long-term results of myotomy and fundoplication. Arch Surg 127:222, 1992.
Bowrey DJ, Peters JH: Laparoscopic esophageal surgery. Surg Clin North Am 80:1213, 2000.
Cameron AJ, et al: Videoendoscopic diagnosis of esophageal motility disorders. Gastrointest Endosc 49:62, 1999.
Cattau EL Jr, et al: Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol 86:272, 1991.
Champion JK, Delisle N, Hunt T: Laparoscopic esophagomyotomy with partial posterior fundoplication for primary esophageal motility disorders. Surg Endosc 14:746, 2000.
Clouse RE, et al: Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology 92:1027, 1987.
Creamer B, Donoghue FE, Code CF: Pattern of esophageal motility in diffuse spasm. Gastroenterology 34:782, 1958.
Csendes A, et al: Late results of a prospective randomized study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut 30:299, 1989.
Culliere C, et al: Achalasia: outcome of patients treated with intrasphincteric injection of botulinum toxin. Gut 41:87. 1997.
Decker G, et al: Gastrointestinal quality of life before and after laparoscopic Heller myotomy with partial posterior fundoplication. Ann Surg 236:750, 2002.
P.2160
Devaney EJ, et al: Esophagectomy for achalasia: patient selection and clinical experience. Ann Thorac Surg 72:854, 2001.
DiBaise JK, Quigley EMM: Tumor-related dysmotility: gastrointestinal dysmotility syndromes associated with tumors. Dig Dis Sci 43:1369, 1998.
Di Simone MP, et al: Onset timing of delayed complications and criteria of follow-up after operation for esophageal achalasia. Ann Thorac Surg 61:1106. 1996.
Dolan K, et al: Does pneumatic dilatation affect outcome of laparoscopic cardiomyotomy? Surg Endosc 16:84, 2002.
Donahue PE, et al: Achalasia of the esophagus: treatment controversies and the method of choice. Ann Surg 203:505, 1986.
Eherer AJ, et al: Effect of sildenafil on oesophageal motor function in healthy subjects and patients with oesophageal motor disorders. Gut 50:758, 2002.
Ellis FH Jr: Oesophagomyotomy for achalasia: a 22 year experience. Br J Surg 80:882, 1993.
Ellis FH Jr, et al: Ten to 20-year clinical results after short esophagomyotomy without an antireflux procedure (modified Heller operation) for esophageal achalasia. Eur J Cardiothorac Surg 6:86, 1992.
Eypasch EP, et al: Physiologic assessment and surgical management of diffuse esophageal spasm. J Thorac Surg 104:859, 1992.
Ferguson MK: Achalasia. Current evaluation and therapy. Ann Thorac Surg 52:336, 1991.
Ferguson MK, Reeder LB, Olak J: Results of myotomy and partial fundoplication after pneumatic dilation for achalasia. Ann Thorac Surg 62: 327, 1996.
Fernandez AF, et al: Six years of experience in laparoscopic surgery of esophageal achalasia. Surg Endosc 17:153, 2003.
Friesen DL, Henderson RD, Hanna W: Ultrastructure of the esophageal muscle in achalasia and diffuse esophageal spasm. Am J Clin Pathol 79:319. 1983.
Fuller L, et al: Abnormal esophageal body function: radiographic-manometric correlation. Am Surg 65:911, 1999.
Gelfond M, Rozen P, Gilat T: Isosorbide dinitrate and nifedipine treatment of achalasia: a clinical, manometric and radionuclide evaluation. Gastroenterology 83:963, 1982.
Goldenberg SP, et al: Classic and vigorous achalasia: a comparison of manometric, radiographic, and clinical findings. Gastroenterology 101: 743, 1991.
Kadakia SC, Wong RK: Pneumatic balloon dilation for esophageal achalasia. Gastrointest Endosc Clin N Am 11:325, 2001.
Kahrilas PJ, et al: Comparison of pseudoachalasia and achalasia. Am J Med 82:439, 1987.
Kahrilas PJ, Dodds WJ, Hogan WJ: Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology 94:73, 1988.
Konturek JW, Gillessen A, Domschke W: Diffuse esophageal spasm: a malfunction that involves nitric oxide? Scand J Gastroenterol 30:1041, 1995.
Leite LP, et al: Ineffective esophageal motility (IEM): the primary finding in patients with non-specific motility disorder. Dig Dis Sci 42:1859, 1997.
Leonardi HK, et al: Diffuse spasm of the esophagus. Clinical, manometric, and surgical considerations. J Thorac Cardiovasc Surg 74:736, 1977.
Liu W, et al: The pathogenesis of pseudoachalasia: a clinicopathologic study of 13 cases of a rare disorder. Am J Surg Pathol 26:784, 2002.
Malthaner RA, et al: Long-term results in surgically managed esophageal achalasia. Ann Thorac Surg 58:1343, 1994.
Miller DL, et al: Esophageal resection for recurrent achalasia. Ann Thorac Surg 60:922, 1995.
Miller LS, et al: Treatment chest pain in patients with noncardiac, nonreflux, nonachalasia spastic esophageal motor disorders using botulinum toxin injection the gastroesophageal junction. Am J Gastroenterol 97: 1640, 2002.
Mujica VR, Mudipalli RS, Rao SS: Pathophysiology of chest pain in patients with nutcracker esophagus. Am J Gastroenterol 96:1371, 2001.
Nehra D, et al: Physiologic basis for the treatment of epiphrenic diverticulum. Ann Surg 235:346, 2002.
Neubrand M, et al: Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy 34:519, 2002.
O'Connor JB, et al: The cost-effectiveness of treatment strategies for achalasia. Dig Dis Sci 47:1516, 2002.
Okike N, Payne SW, Neufeld DM: Esophagomyotomy versus forceful dilation for achalasia of the esophagus: results in 899 patients. Ann Thorac Surg 28:119, 1979.
Osgood H: A peculiar form of esophagismus. Boston Med Surg J 120:140, 1889.
Pasricha PJ, et al: Treatment of achalasia with intrasphincteric injection of botulinum toxin: a pilot trial. Ann Intern Med 121:590, 1994.
Pasricha PJ, et al: Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology 110:1410, 1996.
Patti MG, et al: Comparison of medical and minimally invasive surgical therapy for esophageal motility disorders. Arch Surg 130:609, 1995.
Payne WS: Heller's contribution to the surgical treatment of achalasia of the esophagus. Ann Thorac Surg 48:876, 1989.
Pellegrini C, et al: Thoracoscopic esophagomyotomy. Initial experience with a new approach for the treatment of achalasia. Ann Surg 216:291, 1992.
Peters JH, et al: Esophageal resection with colon interposition for end-stage achalasia. Arch Surg 130:632, 1995.
Ramacciato G, et al: The laparoscopic approach with antireflux surgery is superior to the thoracoscopic approach for the treatment of esophageal achalasia. Experience of a single surgical unit. Surg Endosc 16:1431, 2002.
Richter JE: Oesophageal motility disorders. Lancet 358:823, 2001.
Richter JE, Castell DO: Diffuse esophageal spasm: a reappraisal. Ann Intern Med 100:242, 1984.
Richter JE, Barish CF, Castell DO: Abnormal sensory perception in patients with esophageal pain. Gastroenterology 91:845, 1986.
Rosati R, et al: Diverticulectomy, myotomy and fundoplication through laparoscopy: a new option to treat epiphrenic diverticula? Ann Surg 227: 174, 1998.
Sabharwal T, et al: Balloon dilation for achalasia of the cardia: experience in 76 patients. Radiology 224:719, 2002.
Sanderson DR, et al: Syndrome of vigorous achalasia: clinical and physiologic observations. Dis Chest 52:508, 1967.
Sharp KW, et al: 100 Consecutive minimally invasive Heller myotomies: lessons learned. Ann Surg 235:631, 2002.
Shimi SN, Nathanson LK, Cuscheri A: Thoracoscopic long oesophageal myotomy for nutcracker oesophagus: initial experience of a new surgical approach. Br J Surg 79:533, 1992.
Spechler SJ, Castell DO: Classification of oesophageal motility abnormalities. Gut 49:145, 2001.
Storr M, et al: Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc 54:754, 2001.
Streitz JM Jr, et al: Achalasia and squamous cell carcinoma of the esophagus: analysis of 241 patients. Ann Thorac Surg 59:1604, 1995.
Topart P, et al: Long-term effect of total fundoplication on the myotomized esophagus. Ann Thorac Surg 54:1046, 1992.
Traub M, et al: Surgical myotomy in patients with high-amplitude peristaltic contractions: manometric and clinical effects. Dig Dis Sci 32:16, 1987.
Vaezi MF, Richter JE: Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol 88:21, 1998.
West RL, et al: Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol 97:1346, 2002.
Winters C, Artnak EJ, Benjamin SB, Castell DO: Esophageal bougienage in symptomatic patients with the nutcracker esophagus. JAMA 252:363, 1984.
Zaninotto ZG et al: Etiology, diagnosis and treatment of failures after laparoscopic Heller myotomy for achalasia. Ann Surg 235:186, 2002.
EAN: 2147483647
Pages: 203