111 - Chemotherapy of Non-Small-Cell Lung Cancer
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XX - Diagnostic Studies > Chapter 128 - Endoscopy of the Esophagus
Chapter 128
Endoscopy of the Esophagus
Arvydas D. Vanagunas
Amit Srivastava
Endoscopic evaluation of the esophagus has become the accepted standard for assessing and diagnosing both mucosal and structural diseases of the esophagus. Current-generation small-diameter video endoscopes provide high- resolution images, improved flexibility, and more comfortable insertion. Some endoscopists will order a barium esophagogram before endoscopy, especially in patients with dysphagia, but endoscopy is increasingly being used as the first step in the evaluation of esophageal disease. However, in patients with symptoms suggestive of a Zenker's diverticulum, a barium esophagogram is a more sensitive diagnostic technique.
In addition to improved diagnostic capabilities, endoscopes can provide such therapeutic options as variceal band ligation, suturing, and mucosal resection. Endoscopic ultrasound has added a second dimension to evaluating the esophageal wall and the paraesophageal structures. Endoscopic ultrasound also permits directed fine-needle aspiration (FNA) of esophageal and paraesophageal lesions. Ultrasonic miniprobes are available for insertion through endoscope biopsy channels for strictured lesions where passage of the diagnostic endoscopic ultrasound is impossible.
For diagnostic esophagoscopy, one must identify both normal and abnormal structures. Therapeutic esophagoscopists require an understanding of stricture and achalasia dilation, botulinum toxin injection, foreign body removal, esophageal stents, variceal sclerotherapy, laser therapy, control of nonvariceal bleeding, and mucosal resection of superficial tumors. Magnification endoscopy, chromoendoscopy, optical coherence tomography, and various spectroscopic techniques have advanced the diagnostic capabilities of endoscopy in the diagnosis of esophageal disease.
DIAGNOSTIC ENDOSCOPY
Flexible endoscopes are available in a variety of sizes. Pediatric endoscopes have an external diameter of 7.9 mm and a working channel of 2.0 mm. Routine endoscopes are 9 to 11 mm in external diameter and have working channels of 2.8 mm. Large double-channel endoscopes are 13 mm in external diameter, with working channels of 2.8 and 3.4 mm (Fig. 128-1). All flexible endoscopes are long enough to examine the esophagus, stomach, and proximal duodenum. Those with a smaller diameter are used for pediatric patients and for adult patients with a narrowed esophageal inlet. Smaller-diameter endoscopes are also helpful in the diagnosis and therapy of strictures. Ultrathin endoscopes with 5.3- to 6-mm insertion tube diameters and 2-mm biopsy channels have been developed, permitting unsedated transnasal insertion in office settings. Double-channel endoscopes are useful in the bleeding patient in that one channel can be used for irrigation to visualize the bleeding site better, leaving the other available for applying the appropriate therapeutic method.
The most common indications for esophagoscopy are evaluation of noncardiac chest pain, persistent symptoms of gastroesophageal reflux, recurrent aspiration pneumonia, dysphagia, odynophagia, and abnormal esophageal radiograph. Both diagnostic and therapeutic endoscopy are used in the evaluation of patients with foreign bodies, bleeding, achalasia, and strictures. Relative contraindications to endoscopy include medically unstable patients, unstable cervical spine, severe coagulopathy, and known or suspected perforation of the gastrointestinal tract.
Routine elective endoscopy of the esophagus is performed on an outpatient basis. The patient usually fasts 6 to 8 hours before the procedure. Conscious sedation with intravenous midazolam or diazepam and fentanyl is used. Some endoscopists will use propofol for conscious sedation because of its rapid elimination and reduced postprocedure recovery time. Sorbi and colleagues (1999) have demonstrated the endoscopic accuracy and feasibility of unsedated endoscopy, using small-caliber (5.3 to 6 mm) endoscopes. Local oropharyngeal anesthesia with lidocaine (Xylocaine) spray is sometimes used.
Direct intubation is achieved by placing the endoscope on the patient's tongue and guiding it into the posterior pharynx.
P.1957
The epiglottis is located, and once the piriform sinuses are identified posterior to the epiglottis, the endoscope is maneuvered into the esophageal inlet, which is between the piriform sinuses. The posterior pharynx and larynx are examined for abnormalities. The patient is asked to swallow, opening the esophageal inlet and enabling the endoscope to be advanced into the esophagus. The esophageal inlet is usually 20 cm from the incisors. As soon as the endoscope is in the esophageal inlet, the endoscopist begins to visualize the esophagus before pushing the endoscope any farther.
 |
Fig. 128-1. Various endoscopes. A. Pediatric endoscope. B. Routine adult endoscope. |
Once intubation is achieved, the endoscope is maneuvered into the stomach and duodenum. Endoscopy is not complete without a careful view of the gastric cardia achieved by retroflexing the instrument 180 degrees in the antrum and pulling back to paradoxically bring the tip of the endoscope up to the proximal stomach. Lesions in the cardia, such as tumors, Mallory-Weiss tears, or lesions within a hiatal hernia, are often very difficult to see without this retroflexion maneuver. At all times during passage of the endoscope, the lumen should be in view. The lumen is examined while the endoscope is withdrawn.
An old dictum of endoscopy is that one only sees what one is looking for; the patient's history directs the visualization at endoscopy. Biopsy samples are taken if any abnormalities or possible lesions are seen. Video endoscopes have facilitated efforts to obtain endoscopic pictures and perform video recording of any segment of the procedure.
Normal Esophagus
The cricopharyngeus muscle forms the beginning of the normal esophagus, which is a tubular structure until it culminates at the diaphragmatic hiatus. At endoscopy, the esophageal mucosa is smooth and whitish pink (Fig. 128-2). The esophageal lumen is 1.5 to 2.0 cm in diameter. Normally, the aorta gives an extrinsic compression at about 25 cm in the esophagus. Sometimes, the left main-stem bronchus can also indent the esophagus below the aorta. The transformation from whitish to reddish mucosa identifies the squamocolumnar junction, or the Z line. Usually, the Z line is closely related to the diaphragmatic hiatus and is 40 cm from the incisors. The anatomic gastroesophageal junction is clinically defined at the proximal margin of the gastric folds. There is mild angulation of the distal esophagus close to the diaphragmatic hiatus.
The final maneuver to evaluate the esophagus is endoscopic retroflexion in the stomach to identify the presence of a hiatal hernia. A sliding hiatal hernia is identified by a displacement greater than 2 cm between the Z line and the diaphragmatic hiatus, as described by Boyce (1987). During the retroflexion in the stomach, if a space between the endoscope and the gastric wall at the cardia is noted, one can also identify a hiatal hernia.
Diseases of the Esophagus
Various disease processes can be identified in patients with dysphagia. Mucosal abnormalities such as gastroesophageal
P.1958
reflux disease (GERD), Barrett's esophagus, Mallory-Weiss tears, infectious esophagitis, pill-induced esophagitis, and malignancy can be diagnosed by esophagoscopy (see Chapter 140, Chapter 143, and Chapter 150). Structural abnormalities such as diverticula, webs, and rings are identified easily by endoscopy and are described in detail by Boyce and Boyce (1991). Endoscopy is an important step in the evaluation of strictures in that biopsies differentiate benign from malignant strictures. Dilations can be performed at the initial endoscopy.
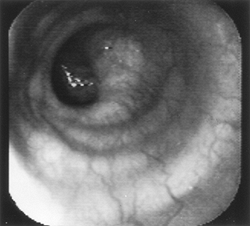 |
Fig. 128-2. Endoscopic view of a normal esophagus. |
A variety of dilators are available for the endoscopist and fall into two categories: mechanical and balloon dilators (Fig. 128-3). Mechanical dilators range in size from No. 15F to No. 60F and are filled with liquid mercury or tungsten and passed without a guidewire. The tapered Maloney dilators are simple to use and are chosen most often for uncomplicated dilations. Savary-Gilliard dilators are made from plastic, have a tapered tip, and are inserted over a guidewire. These type of dilators have generally supplanted the use of the metal olive dilating systems. Through-the-scope (TTS) balloon dilators can be placed directly through the biopsy channel of the endoscope or passed over a guidewire.
Saeed and co-workers (1995) have demonstrated that balloon and mechanical dilators are equally safe for dilating esophageal strictures. Mechanical dilators exert a shearing force and dilate from the proximal to the distal edge of the stricture as the dilator is passed. Balloon dilators exert a uniform, simultaneous radial force throughout the stricture. There is no clear advantage of one system over the other, although strictures with complex features are probably best treated with guidewire-based systems. It is also not clear that fluoroscopy improves the effectiveness of dilation. Complex esophageal strictures that are very long, very narrow, tortuous, or associated with diverticula may benefit from fluoroscopic control.
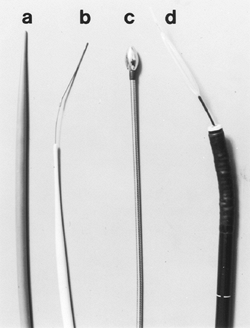 |
Fig. 128-3. Dilators. A. Maloney. B. Savary with a guidewire. C. Puestow. D. Through-the-scope (TTS). |
Excessive force should be avoided while dilating strictures, and some may require more than one session of dilation. Depending on the severity of the stricture, dilations may be done 1 week apart. A generally accepted rule is that no more than three dilators should be passed and that the luminal stenosis should not be increased beyond 2 mm (6F) at one session. Kozarek and colleagues (1995), however, have shown than more aggressive dilation can be safely done in selected circumstances by experienced endoscopists. Some refractory esophageal strictures may respond to endoscopic intralesional injection of corticosteroids, as reported by Zein and co-workers (1995).
Endoscopy is essential in the diagnosis of and therapy for achalasia. The striking endoscopic feature is esophageal dilatation, usually with retained food and debris. The area of
P.1959
the lower esophageal sphincter is typically closed but will open with gentle pressure, permitting inspection of a normal-appearing squamocolumnar junction. Endoscopy is critical in ruling out cancer of the gastric cardia, which can mimic the clinical signs of achalasia. Pneumatic balloon dilation of the lower esophageal sphincter is an effective treatment for achalasia. A number of older balloon dilators, including the Mosher bag and the Brown-McHardy dilators, are no longer being manufactured. The Rigiflex balloon and Witzel dilator (Fig. 128-4) are currently the most popular pneumatic dilators. There is no clear consensus on the optimal method for performing pneumatic dilation, and such parameters as inflation pressure, balloon diameter, and duration of inflation vary widely.
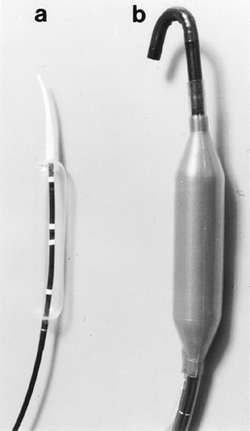 |
Fig. 128-4. Achalasia dilators. A. Rigiflex (Microvasive, Watertown, MA, U.S.A.). B. Witzel (Diagmed, Ltd., Thirsk, United Kingdom). |
Endoscopic injection of botulinum toxin into the lower esophageal sphincter has demonstrated clear short-term symptomatic relief in many patients with achalasia. Controlled trials by Wehrmann (2000) and Annese (1999) and their colleagues comparing botulinum toxin injection with pneumatic dilation have shown similar short-term efficacy for both procedures. However, 50% of patients treated with botulinum toxin will need additional therapy, and objective improvement in esophageal function is more likely after pneumatic dilation.
Endoscopy is very important in detecting and stratifying the extent of suspected GERD (Fig. 128-5). However, the absence of endoscopic features is common and occurs in at least 50% of patients with GERD. Endoscopy therefore
P.1960
should probably be reserved for patients failing to respond to antisecretory therapy, for patients with atypical or alarm symptoms, or for identifying Barrett's esophagus in patients with long-standing symptoms. Endoscopic interobserver variability and the need for consistency in judging treatment response in GERD has led to the development of multiple endoscopic classification schemes. Two of the more popular grading systems (Savary Miller and Los Angeles) are noted in Tables 128-1 and 128-2. Biopsy can be done at the time of endoscopy. Strictures can be dilated, and surveillance for Barrett's epithelium can be performed.
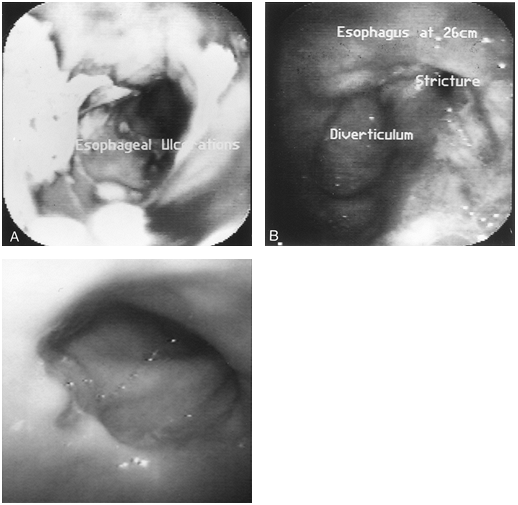 |
Fig. 128-5. A. Grade IV (Savary) or Grade D (Los Angeles) esophagitis with diffuse ulcerations. B. Esophagitis with diverticulum and stricture formation. C. Grade I (Savary) or Grade A (Los Angeles) esophagitis with a single erosion. |
Table 128-1. Savary Miller Grading System for Peptic Esophagitis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
Several minimally invasive endoscopic techniques for the treatment of GERD have been recently introduced. The application of controlled radiofrequency energy (Stretta procedure) to the lower esophageal sphincter induces collagen contraction. Triadafilopoulos and co-workers (2002) have demonstrated symptomatic improvement and a modest decrease in esophageal acid exposure in a nonrandomized group of 118 patients. Several endoscopic techniques involving submucosal suturing or plication have been described and may eventually prove to have some clinical utility. Filipi and associates (2001) have demonstrated that endoscopic esophageal endoluminal plication is a safe and beneficial technique in highly selected patients. The technique requires an esophageal overtube and passage of a preloaded suturing capsule (EndoCinch, C. R. Bard, Inc., Murray Hill, NJ, U.S.A.) mounted on the tip of the instrument. A handle attached to the endoscope's biopsy port serves as a needle driver to create stitches. Stitches to form plications are applied at the three, six, and nine o'clock positions 1 cm above and below the squamocolumnar junction. More information, longer follow-up, and randomized studies will be needed to judge the ultimate benefit of all of these endoscopic techniques for the treatment of GERD.
Table 128-2. Los Angeles Classification of Peptic Esophagitis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Upper endoscopy can be both diagnostic and therapeutic in the evaluation of foreign bodies (see Chapter 137).
Esophageal varices are best recognized by endoscopy (Fig. 128-6). In patients with portal hypertension and esophageal varices, endoscopy is used for diagnosis and therapy of esophageal varices. Bleeding is the primary problem with varices. It is possible to determine the size of the varices endoscopically by the percentage of esophageal lumen occupied by the protruding vessel. In 1981, Beppu described redness on varices as a predictor of a risk of bleeding. Variceal pressure also predicts the risk of bleeding but requires invasive measurements that are not recommended clinically at present. Rigau and co-workers (1989) described new noninvasive measurements of variceal pressure, suggesting that patients with higher pressures are at higher risk of bleeding.
Chromoscopy and Enhanced Optical Diagnostics
Various dyes can be used to coat the esophageal lining to increase detection of Barrett's esophagus or tumor. Chromoendoscopic techniques have the advantage of low cost
P.1961
but have not been extensively used in clinical practice in the United States. The dye used most commonly is Lugol's 1% to 2% solution, which binds to glycogen in nonkeratinized squamous epithelium. Conditions that deplete glycogen, including ectopic mucosa, ulcers, cancers, and severe dysplasia, will not take up the dye. Yokoyama and colleagues (1995) have demonstrated Lugol dye's usefulness in the diagnosis of early-stage squamous cell cancer of the esophagus. Methylene blue is a vital stain that is taken up by the cytosol of actively absorbing epithelium, including epithelium that has undergone intestinal metaplasia. It does not stain squamous epithelium. Canto (1999) demonstrated that methylene blue spraying at the time of endoscopy will reliably stain Barrett's mucosa and will improve the yield of diagnosing high-grade dysplasia.
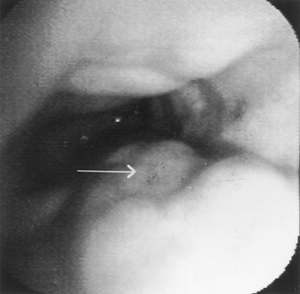 |
Fig. 128-6. Endoscopic view of esophageal varices. |
Interactions of light and tissue may yield visual information about dysplastic mucosa, and revolutionary techniques such as optical coherence tomography, light-induced fluorescence spectroscopy, and light-scattering spectroscopy are being investigated as tools in the endoscopic diagnosis of esophageal disease and may become especially useful in the diagnosis of early esophageal cancer.
THERAPEUTIC ENDOSCOPY
Obstruction or bleeding in the esophagus can be managed endoscopically. Laser therapy, Bicap (biopolar circumactive probe) thermal therapy, or alcohol injections can reduce tumor burden for palliation of esophageal cancer. Stenting and dilation of an obstructing lesion can keep the esophageal lumen patent. Bleeding from the esophagus could be due to varices, ulcer, or a Mallory-Weiss tear. Varices can be managed by endoscopic sclerotherapy or band ligation. Active nonvariceal bleeding can be managed by injection therapy, heater probe, or Bicap therapy.
Malignant Obstruction
Upper endoscopy can be useful not only for the diagnosis of malignant esophageal obstruction (Fig. 128-7) but also as palliative therapy for advanced esophageal carcinoma. Preoperative tumor staging was found to be accurate using the classification of the Japanese Society for Esophageal Diseases, as suggested by Dittler and colleagues (1992). Dilation is possible using a wide array of dilators, as described by Fleischer (1992). Tumor ablation can be performed by applying laser therapy, alcohol injections, intratumoral injection of chemotherapy, or photodynamic therapy (see Chapter 152).
Patients with advanced stages of esophageal cancer can be offered stenting as a palliative option. Self-expanding metal stents of various designs have become the most commonly used means of endoscopic palliation. Dysphagia can be relieved in up to 95% of patients at an acceptable complication rate. The major disadvantages of metallic stenting are its high cost, potential for tumor ingrowth, and stent migration. Covered stents are available and are the treatment of choice in esophageal respiratory fistulae.
Tumor ablation can be achieved by laser therapy, sclerosant injection, or thermal therapy. Laser therapy has been performed using the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser. Ahlquist and co-workers (1987) reported that 90% of patients have improvement of dysphagia. Noncontact and contact methods have been used to achieve ablation. Prograde and retrograde techniques of tumor ablation have been described and have good results in palliation. Prograde therapy may require several sessions of therapy, whereas the retrograde technique can ablate the entire tumor in one session. Retrograde therapy requires dilation of the stricture before ablation; the major complication is perforation. Hemorrhage, fistula formation, sepsis, and pain may also occur. Angelini and colleagues (1991) studied sclerosant therapy with polidocanol injection for palliation in patients with esophageal cancer, and it compared favorably with laser therapy. Injections of 100% alcohol and sodium morrhuate have had variable results in efficacy. Monga and colleagues (2000) demonstrated the efficacy of intratumoral injection of a cisplatin/epinephrine gel in a small number of patients. A sclerotherapy needle is used for multiple injections of the tumor in a prograde fashion. The major risks are perforation, bleeding, and sepsis.
Recent studies suggest that photodynamic therapy (PDT) may offer advantages over all these other techniques. PDT uses a photosensitizing agent such as porfimer sodium in combination with endoscopic low-power laser exposure to destroy malignant esophageal tissue. Photodynamic therapy
P.1962
P.1963
with porfimer sodium induces the production of singlet oxygen, which damages the microcirculation of the cancer and causes ischemic necrosis. Photodynamic therapy is a two-stage process. Porfimer sodium is injected intravenously in a dosage of 2 mg/kg over 5 minutes. After systemic administration, the photosensitizer is absorbed by most tissues, and patients are instructed to avoid direct sunlight exposure over the next 4 to 6 weeks. At approximately 48 hours, the concentration of porfimer sodium is higher in neoplastic tissue than nonneoplastic tissue by a factor of 2:1, and patients undergo endoscopy and application of a 630-nm wavelength of light to the tumor using a tunable dye laser. The light is transmitted by a quartz optical fiber passed through the accessory channel of the endoscope. The tip of the diffusing fiber comes in variable lengths to accommodate the length of the tumor. The procedure generally is well tolerated and can be successful in significantly improving dysphagia. Luketich and associates (2000) described significant improvement in dysphagia scores in greater than 90% of patients undergoing PDT. The most common complications related to PDT include esophageal stricture, opportunistic esophageal infection, symptomatic pleural effusion, and sunburn.
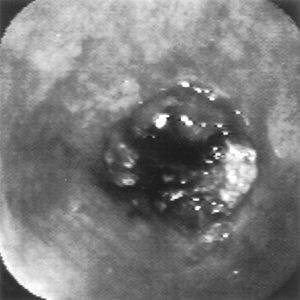 |
Fig. 128-7. Endoscopic view of obstructing esophageal cancer. |
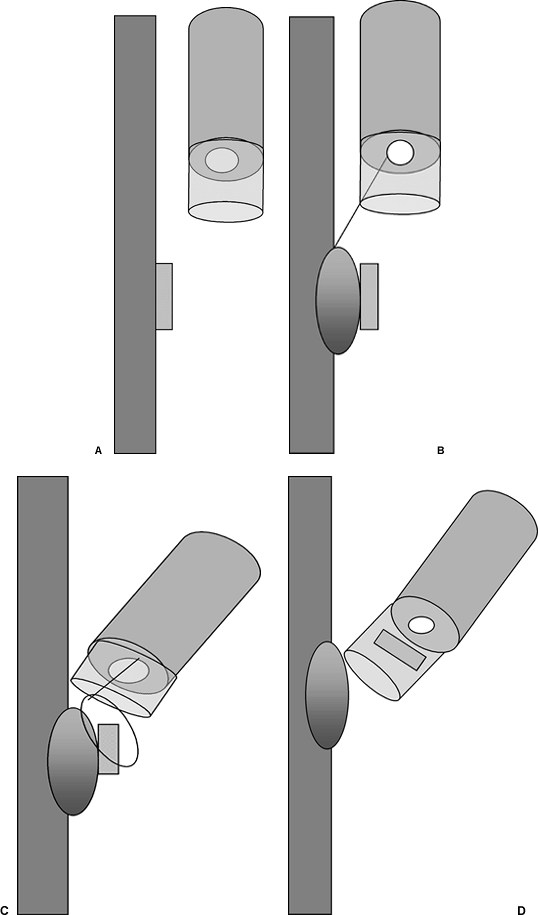 |
Fig. 128-8. Endoscopic mucosal resection. Figure depicting the suck and cut technique. A submucosal saline injection through the endoscope (B) is used to raise the lesion (A), which is aspirated into a hood with a prefitted snare (C). The lesion is excised and suctioned into the hood (D). |
Endoscopic mucosal resection may be a treatment option for patients with early-stage esophageal cancer who are too ill to undergo a standard surgical procedure. Endoscopic mucosal resection should be limited to the early esophageal cancer with a diameter typically less than 2 cm and involvement of less than one-third of the circumference of the esophageal wall. Endoscopic ultrasound should be done to ensure that the lesion is limited to the first endoscopic or mucosal layer of the esophagus. This endoscopic technique can be divided into two major types: the lift and cut technique, in which an esophageal mucosal lesion is injected submucosally with saline, the lesion is grasped with lifting forceps, and electrocautery is used in a freehand manner to cut away the lesion; and the suck and cut (Fig. 128-8) method, in which the lesion is also injected with saline submucosally and aspirated by endoscopic suction into a transparent hood on the tip of the endoscope in which a cautery snare is front-loaded.
Barrett's Esophagus
Endoscopy not only plays a role in surveillance for dysplasia and carcinoma in Barrett's esophagus but has also been proposed for ablative therapy of the disorder. The combination of ablative therapy and high-dose proton pump inhibitor therapy has been used in reversal of Barrett's esophagus as well as eradication of high-grade dysplasia. Heater probes, Nd:YAG laser, argon laser, argon plasma coagulation, cryotherapy, and multipolar electrocoagulation have been shown to effectively ablate columnar epithelium and induce regeneration of squamous epithelium. Although preliminary results as noted by Sampliner and associates (2001) are quite encouraging, foci of residual metaplastic tissue can persist, and caution needs to be exercised. In addition, PDT has some promise as a technique to treat Barrett's dysplasia. These approaches are feasible, but the long-term effectiveness and ultimate reduction in mortality remain unknown. These techniques are not fully proven and are not recommended in routine clinical practice.
Endoscopic mucosal resection offers a mechanical means of removing dysplastic tissue. Using endoscopic mucosal resection, Ell and colleagues (2000) were able to achieve complete remission in 97% of low-risk and 59% of high-risk patients with Barrett's esophagus and early cancer or high-grade dysplasia.
Esophageal Varices
Endoscopy plays an important role in the diagnosis and management of esophageal variceal bleeding. Endoscopic therapy has been applied for acute variceal bleeding. Both endoscopic sclerotherapy and endoscopic band ligation of varices are effective. The large double-channel endoscope is used for endoscopic sclerotherapy. Sclerotherapy needles are long, thin tubes with a needle at the distal end and a connection for a syringe at the proximal end (Fig. 128-9). The endoscopist identifies the varix to be sclerosed and
P.1964
produces an intravariceal or paravariceal injection. The optimal volume of sclerosant injected is not well established, but typically 1 to 2 mL is injected into each varix, for a total of 12 to 20 mL per session. Sclerosant agents used are ethanolamine, sodium morrhuate, tetradecyl sulfate, alcohol, or polidocanol; no one agent has been found to be superior to another. Major complications are fever, dysphagia, chest pain, and ulcerations.
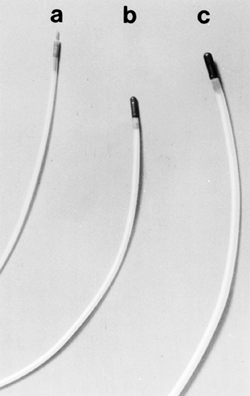 |
Fig. 128-9. A. Sclerotherapy needle. B. 7F heater probe. C. 10F heater probe. |
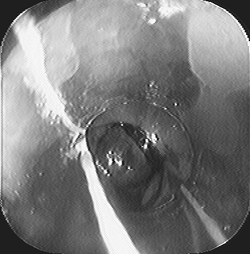 |
Fig. 128-10. Esophageal banding of varices. A dark rubber band has been attached to the neck of a varix seen just beyond the clear hood of the endoscope. |
Endoscopic variceal band ligation has largely supplanted injection sclerotherapy in the treatment of bleeding esophageal varices. A ligating device is loaded on the tip of a standard endoscope, and the application of suction permits placement of a rubber band over the varix (Fig. 128-10). The development of a multiple-band ligator has simplified and improved the safety of this technique. Endoscopic variceal band ligation controls bleeding in 80% to 100% of patients. In a meta-analysis, Laine and Cook (1995) found that compared with sclerotherapy, band ligation reduced the rebleeding rate, mortality rate, and incidence of complications.
Nonvariceal Bleeding
Nonsurgical therapy with injection or thermal coagulation can be used to control bleeding in the esophagus. Usually, the bleeding vessels are associated with ulcers or Mallory-Weiss tears. Injection with epinephrine or thermal coagulation with a heater probe or Bicap probe can control bleeding. For injection therapy, a sclerotherapy needle is used to inject a solution of 1:10,000 epinephrine around the bleeding site. If the thermal device is used, the bleeding vessel is coagulated directly using various probes (see Fig. 128-9). Different methods have similar efficacy for control of bleeding, and at present, injection therapy has the advantage of simplicity and low cost.
ENDOSCOPIC ULTRASONOGRAPHY
Endoscopic ultrasonography (EUS) has added a new dimension to the evaluation of the esophageal wall and paraesophageal structures. The ultrasound probe is attached to the tip of an endoscope (Fig. 128-11). The ultrasound frequency ranges from 5 to 12 MHz. Patient preparation is similar to that for upper endoscopy. Two separate endoscopes are now available. The Olympus system gives a 360-degree view; it is used most often and is the focus of this discussion. An endoscope by Pentax gives the traditional wedge-shaped view and also has the capability of ultrasound-directed biopsies and measurement of blood flow by Doppler technique.
The Olympus system has a 13-mm diameter. The tip is somewhat rigid, so care must be taken during intubation. The lens is on the side of the endoscope tip, so only an oblique endoscopic view is visible. The probe is prepared by placing a balloon over the tip. The balloon is inflated with water during the procedure to achieve a good tissue- water interface, which is required for good sonographic images. For endosonography of the esophagus, the tip of the endoscope is placed in the proximal stomach, the balloon is inflated, the endoscope is slowly withdrawn, and ultrasonographic images are obtained at various levels. A normally layered structure of the esophagus is easily visible (Fig. 128-12). The aorta is used for proper orientation of the images. Routine upper endoscopy is needed to focus on the lesion of interest. In addition, high-frequency miniprobes
P.1965
(20 MHz) are available that can be placed through the biopsy channel of routine diagnostic endoscopes. These probes are particularly useful in stenotic esophageal lesions where the standard EUS cannot pass.
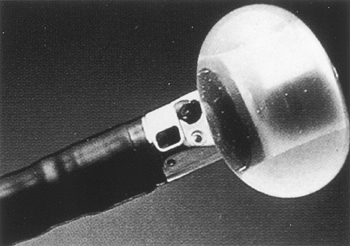 |
Fig. 128-11. Tip of an endoscopic ultrasound probe with the balloon inflated. |
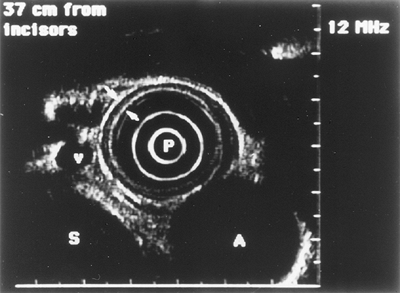 |
Fig. 128-12. Endoscopic ultrasound image of a normal esophageal wall (arrow). A, aorta; P, probe; S, spine; v, varices. |
Submucosal mass evaluation, cancer staging, and detection of recurrent esophageal cancer are well-defined indications for endoscopic ultrasonography. Leiomyomas, lipomas, esophageal duplication cysts, metastatic cancer, lymphoma, bronchial carcinoma, or extrinsic masses compressing the esophagus can be identified by endosonography. Tumor staging using the TNM classification can also be accomplished. Local staging and assessment of resectability for esophageal carcinoma has been studied extensively and found to be reliable. EUS is superior to computed tomographic (CT) scan, magnetic resonance (MR) imaging, or positron emission tomographic (PET) scanning for the locoregional staging of esophageal cancer. In reviewing an extensive international experience, Rosch (1995) noted overall accuracy rates of 84% in T staging. Mistaging on EUS can be due to inadequate scanning (strictures, oblique application of the instrument, microscopic infiltration), understaging, or peritumorous inflammatory and fibrous changes (overstaging).
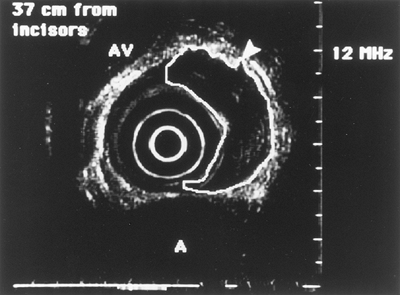 |
Fig. 128-13. Endoscopic ultrasound image of an esophageal tumor (outlined). Pseudopodia (arrow) signifies T3 stage of the tumor. A, aorta; AV, azygos vein. |
The T stage identifies the extent of the tumor through the wall: T1 is limited to the mucosa, T2 indicates extension through the submucosa intact muscularis propria, T3 indicates extension through the muscularis propria (Fig. 128-13), and T4 indicates invasion of adjacent organs (trachea, aorta).
P.1966
N defines the nodal status, and M defines distant metastasis. EUS is not always able to clearly differentiate benign from malignant nodes, and the overall N staging accuracy is approximately 77%. FNA biopsy with linear array instruments has expanded the clinical utility of EUS. Sensitivity, specificity, and diagnostic accuracy of EUS FNA for locoregional lymph nodes are all over 85% in esophageal cancer. In addition, the sensitivity of EUS FNA in celiac nodes is over 85%.
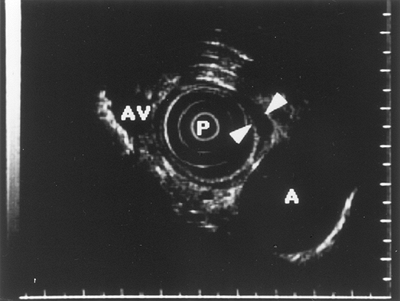 |
Fig. 128-14. Endoscopic ultrasound image of esophageal varices (arrows). A, aorta; AV, azygos vein; P, probe. |
EUS has several limitations in the diagnosis of esophageal malignancy. Detection of invasion into the tracheobronchial tree is not very good, probably because of the acoustic artifacts induced by air. In addition, the instrument may not be capable of being advanced into malignant stenoses. EUS miniprobes (3-mm diameter, 20 MHz), which can be inserted through the biopsy channel of a routine diagnostic endoscope, are useful in this situation, but their limited depth of penetration can lead to incomplete assessment of locoregional spread. Because of its limited depth of penetration, EUS is unsatisfactory for identifying distant metastasis. Endosonography is clearly better than CT scanning for local staging of malignancy, but CT is superior in identifying liver metastasis. Restaging after chemoradiation is inaccurate with EUS. Zuccaro and co-workers (1999) could not easily differentiate between posttreatment fibrosis and inflammation and residual tumor.
Other roles for EUS in various esophageal diseases are still being developed. Its role in portal hypertension (Fig. 128-14) was updated by Calletti and colleagues in 1992, who found it superior to endoscopy in locating gastric varices. Endoscopy, however, is better for finding esophageal varices. EUS also may visualize small, dilated submucosal veins in the stomach in patients with portal hypertensive gastropathy as well as perigastric collateral veins in portal hypertension. EUS may be a helpful adjunct in the diagnosis of achalasia. It helps to differentiate pseudoachalasia from achalasia by noting tumor infiltration in pseudoachalasia and enlargement of the muscularis propria in achalasia.
REFERENCES
Ahlquist DA, et al: Endoscopic laser palliation of malignant dysphagia. A prospective study. Mayo Clin Proc 62:867, 1987.
Angelini G, et al: Nd:YAG laser versus polidocanol injection for palliation of esophageal malignancy: a prospective, randomized study. Gastrointest Endosc 37:607, 1991.
Annese V, et al: Comparison of two different formulations of botulinum toxin A for the treatment of oesphageal achalasia. The Gismad Achalasia Study Group. Aliment Pharmacol Ther 13:1347, 1999.
Beppu K, et al: Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointest Endosc 37:213, 1981.
Boyce GA, Boyce WH: Esophagus: anatomy and structural anomalies. In Yamada T (ed): Textbook of Gastroenterology. 1st Ed. Philadelphia: JB Lippincott, 1991, p. 1075.
Boyce WH: Hiatal hernia and peptic diseases of the esophagus. In Sivak MV (ed): Gastrointestinal Endoscopy. Philadelphia: WB Saunders, 1987, p. 401.
Calletti GC, et al: Value of endoscopic ultrasonography in the management of portal hypertension. Endoscopy 24:342, 1992.
Canto MI: Esophageal cancer screening by chromoendoscopy. Techniques Gastrointest Endosc Esophagea Neoplasm 150, 1999.
Dittler HJ, Pesarini AC, Siewert JR: Endoscopic classification of esophageal cancer: correlation with the T stage. Gastrointest Endosc 38:662, 1992.
Ell C, et al: Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 118:670, 2000.
Filipi CJ, et al: Transoral, flexible endoscopic suturing for treatment of GERD: a multicenter trial. Gastrointest Endosc 51:416, 2001.
Fleischer D: Esophageal dilatation. In Geenen JE, Fleischer DE, Waye JD (eds): Techniques in Therapeutic Endoscopy. 2nd Ed. New York: Gower Medical Publishing, 1992.
Kozarek RA, et al: Esophageal dilation can be done safely using selective fluoroscopy and single dilating sessions. J Clin Gastroenterol 20:184, 1995.
Laine L, Cook D: Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding. A meta-analysis. Ann Intern Med 123:280, 1995.
Luketich JD, et al: Endoscopic photodynamic therapy for obstructing esophageal cancer: 77 cases over a 2-year period. Surg Endosc 14:653, 2000.
Lundell LR, et al: Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 45:172, 1999.
Monga SP, et al: Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer. Am J Clin Oncol 23:386, 2000.
Ollyo JB, et al: Savary's new endoscopic grading of reflux oesophagitis: a simple, reproducible, logical, complete and useful classification. Gastroenterology 89:A100, 1990.
Rigau J, et al: Endoscopic measurement of variceal pressure in cirrhosis: correlation with portal pressure and variceal hemorrhage. Gastroenterology 96:873, 1989.
Rosch T: Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am 5:537, 1995.
Saeed ZA, et al: Prospective randomized comparison of polyvinyl bougies and through-the-scope balloon for dilation of peptic strictures of the esophagus. Gastrointest Endosc 41:189, 1995.
Sampliner RE, et al: Effective and safe endoscopic reversal of nondysplastic Barrett's esophagus with thermal electrocoagulation combined with high-dose acid inhibition: a multicenter study. Gastrointest Endosc 53: 554, 2001.
Sorbi D, et al: Unsedated small-caliber esophagogastroduodenoscopy (EGD) versus conventional EGD: a comparative study. Gastroenterology 117:1301, 1999.
Triadafilopoulos G, et al: The Stretta procedure for the treatment of GERD: 6 and 12 month follow-up of the U.S. open label trial. Gastrointest Endosc 55:149, 2002.
Wehrmann T, et al: Manometrically-guided endoscopic injection of botulinum toxin for esophageal achalasia: a pilot trial. Z Gastroenterol 38: 899, 2000.
Yokoyama A, et al: Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer 76:928, 1995.
Zein NN, Greseth JM, Perrault J: Endoscopic intralesional steroid injections in the management of refractory esophageal strictures. Gastrointest Endosc 41:596, 1995.
Zuccaro G Jr, et al: Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol 94:906, 1999.
Reading References
Chobanian SJ, et al: In vivo staining with toluidine blue as an adjunct to the endoscopic detection of Barrett's esophagus. Gastrointest Endosc 33:99, 1987.
Cowling MG, et al: Management of malignant oesophageal obstruction with self-expanding metallic stents. Br J Surg 85:264,1998.
Dancygier JT, Classen M: Endoscopic ultrasonography in esophageal diseases. Gastrointest Endosc 35:220, 1989.
P.1967
Eloubeidi MA, et al: The utility of EUS and EUS-guided fine needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer. A single-center experience. Gastrointest Endosc 54:714, 2001.
Kazunori I, Masahiro T: Chromoscopy. In Sivak MV (ed): Gastrointestinal Endoscopy. Philadelphia: WB Saunders, 1987, p. 203.
Luketich JD, et al: Photodynamic therapy for malignant dysphagia. Surg Laparosc Endosc 9:171, 1999.
Miller LS: Endoscopy of the esophagus. In Castell DO (ed): The Esophagus. 1st Ed. Boston: Little, Brown and Company, 1992, p. 89.
Murata Y, et al: Evaluation of endoscopic ultrasonography for the diagnosis of submucosal tumors of the esophagus. Surg Endosc 2:51, 1988.
Pasricha PJ, et al: Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med 322:774, 1995.
Pereira-Lima JC, et al: Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol 94:1497, 1999.
Pfau PR, Sivak MV: Endoscopic diagnostics. Gastroenterology 120:763, 2001.
Rosch T, et al: Local staging and assessment of resectability in carcinoma of the esophagus, stomach, and duodenum by endoscopic ultrasonography. Gastrointest Endosc 38:460, 1992.
Sampliner RE: Ablative therapies for the columnar-lined esophagus. Gastroenterol Clin North Am 26:685,1997.
Silva SA, et al: Endoscopic ultrasonography of esophageal tumors and compressions. J Clin Ultrasound 16:149, 1988.
Snazdy H: Role of endoscopic ultrasonography in diagnosis, staging, and outcomes of gastrointestinal diseases. Gastroenterologist 2:91, 1994.
Stiegmann GV, et al: Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med 326: 1527, 1992.
Takeshita K, et al: Endoscopic treatment of early oesophageal or gastric cancer. Gut 40:123, 1997.
Vazquez-Sequeiros E, et al: Impact of EUS-guided fine needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 53:571, 2001.
Wong RKH, Maydonovitch CL: Achalasia. In Castell DO (ed): The Esophagus. 2nd Ed. Boston: Little, Brown and Company, 1995, p. 219.
EAN: 2147483647
Pages: 203