110 - Radiation Therapy for Carcinoma of the Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > The Esophagus > Section XX - Diagnostic Studies > Chapter 127 - Radiologic Evaluation of the Esophagus
Chapter 127
Radiologic Evaluation of the Esophagus
Richard M. Gore
Yahid Yaghmai
Gary G. Ghahremani
The principal method for the diagnosis of functional or structural abnormalities of the esophagus is fluoroscopic evaluation combined with digital radiography while the patient is drinking a suspension of barium sulfate. This is a simple, cost-effective technique for demonstrating esophageal lesions that typically manifest with dysphagia or odynophagia. Not infrequently, however, the existence of an esophageal disorder is first appreciated during radiologic studies for unrelated or nonspecific clinical symptoms. Maglinte and associates (1983) detected unsuspected esophageal disease in 76 of 500 (15%) consecutive patients who were referred for upper gastrointestinal series because of abdominal pain or digestive complaints. Therefore, most radiologists consider it a prudent practice to routinely survey the entire esophagus as an integral part of any barium examination of the upper gastrointestinal tract.
In addition to the conventional barium esophagography, several other imaging techniques are currently available for evaluation of the esophagus. The selection of an appropriate examination requires a tailored approach based on clinical information about the character and duration of symptoms, their relationships to coexistent systemic diseases, or any previous surgical, diagnostic, or therapeutic procedures involving the esophagus. This chapter provides an overview of the radiologic modalities used for demonstration and differential diagnosis of various functional or organic disorders affecting the esophagus. A discussion of the noninvasive staging of squamous cell and adenocarcinoma of the esophagus is then presented.
RADIOGRAPHIC METHODS OF EXAMINATION
Plain Chest Radiography
The esophageal body contained within the posterior mediastinum has a 3- to 5-mm thick muscular wall, but it is normally invisible on plain films of the chest. This is partly because the esophagus collapses during its resting phase, whereas the upper and lower esophageal sphincters maintain their tonic contraction to prevent aspiration of air and retrograde flow of gastric contents into the esophagus. Nevertheless, the standard posteroanterior and lateral chest radiographs may provide significant clues to the diagnosis of an underlying pathology when the esophageal lumen contains an abnormal collection of air, fluid, or food particles, or harbors an ingested radiopaque object. Furthermore, chest films may show a retrocardiac soft tissue density with an air fluid level, and thereby suggest an otherwise unsuspected hiatal hernia, as noted by Stark and associates (1990).
The term air esophagogram denotes the plain film visualization of an atonic, noncollapsing esophagus by virtue of retained air in its lumen. This finding was initially observed in patients with esophageal involvement by scleroderma. However, the same phenomenon may occur with mediastinal fibrosis secondary to inflammation or irradiation, after thoracic surgery, or when esophageal motility and intraluminal pressure are disturbed by pulmonary infiltrates, which cause diminished compliance of the lungs and poor respiratory excursion.
On chest radiography, diffuse mediastinal widening in the absence of a normal gas collection in the gastric fundus might be a manifestation of a dilated esophagus, usually containing a foamy mixture of air, food, or secretions. These features are highly suggestive of achalasia, but also may be seen with a peptic stricture or infiltrating tumor of the gastroesophageal junction. Carcinoma of the esophagus also may be visible on chest films as a soft tissue mass thickening the esophagopleural stripe, indenting the trachea, or causing mediastinal adenopathy and pulmonary metastasis. Furthermore, plain radiographs of the chest and neck play an important role in the diagnosis of ingested foreign bodies and suspected esophageal perforation as emphasized by one of us (GGG) (1989, 1994).
Most radiologists currently use a biphasic technique of esophagography as outlined by Levine and associates (1990) and Laufer (2000). It includes upright double-contrast
P.1940
views, as well as fluoroscopy and spot imaging of the barium-filled esophagus in prone-oblique positions.
Single-Contrast Esophagram with Barium
The standard contrast material for opacification of the esophageal lumen is a colloidal suspension of a micropulverized barium sulfate in water. Commercial products of various densities and viscosities are available. They usually contain flavoring agents, as well as chemical additives such as aluminum hydroxide, sorbitol, and methylcellulose.
Radiologic examination of the esophagus is performed under fluoroscopic observation. In conventional single-contrast esophagography, the patient is first examined in the upright and then in the recumbent position while taking sequential swallows of a relatively dilute mixture of barium sulfate in water (40% to 80% weight per volume). Images of the well-distended esophagus are obtained, including views of the gastroesophageal segment during suspended respiration, which accentuates any existing hiatal hernia or Schatzki's ring. In infants, a nursing bottle is used for oral administration of nonionic, isotonic iodinated contrast material, but controlled instillation through a soft feeding tube is recommended if esophageal atresia or tracheoesophageal fistula is suspected.
The uniform tubular shape of opacified esophageal lumen and the repetitive nature of its peristaltic activity permit an accurate fluoroscopic analysis of both motility and structural changes. In addition, a permanent digital record of the observed normal or pathologic findings is routinely obtained. Continuous dynamic recording is the preferred technique for the evaluation of the pharyngeal and esophageal motility. Fluoroscopic study of esophageal peristalsis and motility disorders is conducted with the patient in the prone-oblique position. The horizontal placement eliminates the interference of gravity with the passage of contrast material and permits better visibility of the esophagus by projecting it away from the thoracic spine. The patient is instructed to swallow one mouthful of barium at a time. The initiated primary peristaltic wave appears as a lumen-obliterating contraction that propagates distally at 2 to 4 cm per second. In normal adults the bolus transit through the 20- to 24-cm long esophagus is completed in 6 to 8 seconds. Patients with esophageal dysmotility, however, usually show considerable retention and delayed clearing of barium. The associated fluoroscopic findings are decreased incidence and amplitude of peristaltic waves after deglutition, failure of the initiated contraction to progress distally, and repetitive nonpropulsive waves, commonly referred to as tertiary contractions. Ott (1994) and Aksglaede and co-workers (1992) have reported that in contrast to the dilated atonic esophageal body in achalasia, both the entity of nutcracker esophagus and diffuse esophageal spasm are characterized by recurrent high-amplitude contractions that cause dysphagia and cramping retrosternal pain. In this context, it should also be noted, as pointed out by Ott (1990) and Richter (1989) and their associates, that functional and organic abnormalities of the esophagus are common sources of noncardiac chest pain, and barium esophagram can provide the correct diagnosis.
Esophagram with Water-Soluble Iodinated Contrast Media
Iodinated water-soluble preparations, such as Gastrograffin (Bracco Diagnostics, Princeton, NJ) or Hypaque (Amersham Health, Princeton, NJ), have been used in instances of suspected esophageal perforation and anastomotic leakage. These aqueous contrast media are readily absorbed after extravasation into the mediastinal soft tissues and pleural or peritoneal spaces, but rarely define the anatomy of the defect clearly. As documented by Foley (1982) and Buecker (1997) and their colleagues, the mucosal tears and transmural perforations of the esophagus are better diagnosed with barium than with iodinated contrast media. These researchers have pointed out that 25% to 50% of esophageal perforations are unrecognizable or inadequately shown during initial evaluation with water-soluble agents because their low density and rapid diffusion into the surrounding tissues impairs an optimal mucosal coating and visualization of extraluminal leakage. Furthermore, the aspiration of such iodinated hypertonic solutions into the lungs can lead to pulmonary edema and chemical pneumonitis, particularly among patients with esophageal dysmotility or obstruction. Brick and associates (1988), as well as one of us (GGG) (1993), have pointed out that nonionic low-osmolality contrast media, such as Omnipaque (Amersham Health, Princeton, NJ) or Isovue (Bracco Diagnostics, Princeton, NJ), may be used safely. Much more recently, the use of CT oral contrast esophagram followed by an esophagram chest CT has made their argument moot.
Double-Contrast Esophagography
Double-contrast esophagography permits an accurate demonstration of mucosal abnormalities that are usually the hallmark of inflammatory or neoplastic processes. The examination requires specially formulated high-density barium for coating the esophageal mucosa while the lumen is fully distended by gas. For this purpose, carbon dioxide released by ingested effervescent agents, such as citrocarbonate granules, is used together with swallowed air. This serves as a radiolucent intraluminal gas collection to expand the lumen and provide a detailed view of the esophageal inner surface. Any areas of narrowing or rigidity are also better recognized when the otherwise pliable esophageal wall is maximally stretched.
Double-contrast radiography of the normal esophagus (Fig. 127-1A) typically shows a smooth, featureless mucosa and the well-demarcated walls of this tubular structure.
P.1941
P.1942
The longitudinal folds of the esophagus become visible when the esophagus is collapsed or when there is mild esophagitis of the distended esophagus (Fig. 127-1B). Transverse striations (Fig. 127-1C) of the esophagus, so-called feline esophagus are seen in patients with reflux disease and is caused by contraction of longitudinally oriented muscularis mucosae. Some patients develop dilated submucosal glands and when these fill with barium on esophagrams, they appear as multiple small outpouchings (Fig. 127-1D) simulating ulcerations.
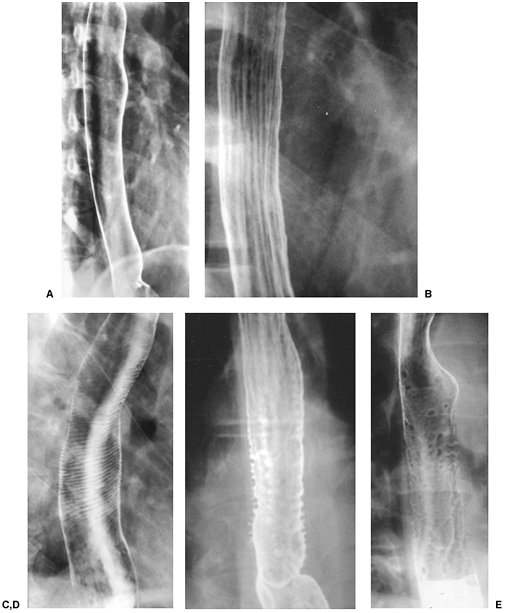 |
Fig. 127-1. The esophagus on double-contrast barium studies. A. The normal thoracic esophagus has a smooth and featureless mucosa pattern. The mucosal folds have been completely effaced. B. Slight thickening of the longitudinal folds of the esophagus in a patient with mild reflux esophagitis. C. Transverse so-called folds, feline esophagus, due to contraction of the longitudinally oriented muscularis mucosae. D. Intramural pseudodiverticulosis. Note the multiple small outpouchings of barium simulating ulcerations. E. Multiple nodular filling defects due to glycogenic acanthosis. |
In approximately 30% of patients over 50 years of age, however, a finely nodular surface pattern may be present (Fig. 127-1E) because of glycogenic acanthosis of the esophagus. This benign asymptomatic condition is characterized by glycogen deposits within multifocal plaques of hyperplastic squamous epithelium.
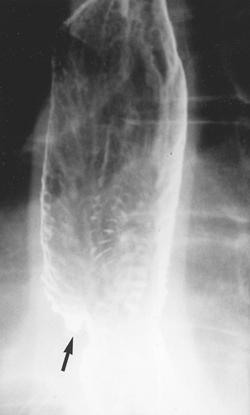 |
Fig. 127-2. Reflux esophagitis with ulcer (arrow) and stepladder appearance with multiple transverse folds in the distal esophagus due to scarring. |
Multiple longitudinal folds are commonly visible in a partially collapsed esophagus, but they become totally effaced and obliterated with progressive luminal distention. Hence, the detection of prominent thickened folds on air contrast views indicates their loss of pliability caused by submucosal edema and diffuse inflammatory changes. Superficial ulcerations and extensive mucosal irregularities are found in severe esophagitis caused by reflux (Fig. 127-2), caustic injury, and infections, such as candidiasis.
The classic radiologic features of Barrett's esophagus are a high esophageal stricture or ulcer (Fig. 127-3A) associated with a sliding hiatal hernia, gastroesophageal reflux or both. A reticular pattern characterized by innumerable, tiny barium-filled grooves or crevices on the esophageal mucosa can also be seen (Fig. 127-3B).
Solid Bolus Test
Subtle areas of narrowing and symptomatic lower esophageal rings can be evaluated by the use of commercially available barium tablets measuring 12.5 mm in diameter, or marshmallows of predetermined size. Ott and colleagues (1991) and one of us (GGG) and colleagues (1996) have reported that this permits more accurate measurement of the narrowed lumen and its functional significance.
EVALUATION OF THE ESOPHAGUS BY OTHER IMAGING TECHNIQUES
Computed Tomography
The esophagus is usually well delineated by computed tomography (CT). The paraesophageal fat is visualized as an interface between the esophagus and adjacent vascular, cardiac, and connective tissue structures. The cervical esophagus lies near the midline, posterior to and occasionally indenting the trachea. It usually does not contain air. As the esophagus enters the thorax, it lies posterior and slightly to the left of the trachea, and the esophagotracheal fat pad is often thin. More inferiorly, the esophagus is situated close to the posterior surface of the left main-stem bronchus, descending thoracic aorta, and thoracic spine. Tumors, adenopathy, and aneurysms can all displace and invade the esophagus.
Small amounts of intraesophageal air are seen in approximately 65% of normal individuals. Megibow (2000) recorded that the presence of an air fluid level, fluid-filled lumen, or lumen caliber greater than 10 mm usually indicates obstruction or severe esophageal dysmotility.
Although there are no normal standards for the cross-sectional diameter of the collapsed esophagus, the wall thickness of a distended esophagus should not exceed 3 mm.
As the esophagus becomes intraabdominal, it courses anteriorly and to the left to enter the stomach at the gastroesophageal
P.1943
junction. This region is often problematic, because in nearly one third of normal individuals, prominent soft tissue in this region projects into the gastric lumen. This soft tissue mass represents the combined thickness of the esophageal walls and medial gastric wall with its nondistended mucosal folds. A hiatal hernia can create a similar appearance. Rescanning the patient in the left lateral decubitus position with more contrast medium or air usually resolves the problem.
Hiatal hernias are manifested by widening of the esophageal hiatus associated with separation of the diaphragmatic crura and an increased distance between the crura and the esophageal wall.
The value of CT in diagnosing various other esophageal disorders has been well described in the radiologic literature. For example, varices in the esophageal wall or periesophageal region are visible on CT in 85% of endoscopically proven cases. Particularly useful, however, as pointed out by one of us (GGG) (1994) and Noh and co-workers (1995), is CT evaluation of accidental or iatrogenic perforations of the esophagus,
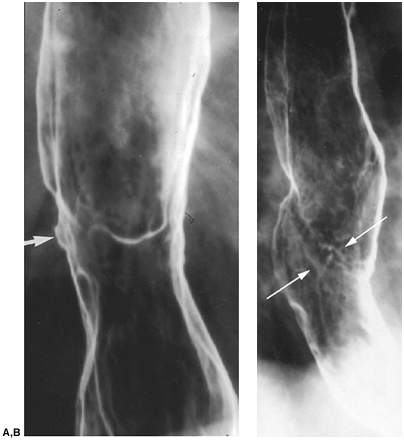 |
Fig. 127-3. Barrett's esophagus. A. There is a high ulcer (arrow) in the midesophagus associated with mild narrowing of the lumen. B. Reticular mucosal pattern (arrows) seen in a minority of patients with Barrett's esophagus. |
Esophageal Endosonography
When performing esophageal endosonography, it is vital to understand the normal sonographic anatomy of the gut and adjacent structures. Sonographically, the normal esophagus is divided into three parts: upper, middle, and lower. The upper portion extends from the oropharynx to the superior region of the aortic arch. The great vessels can be imaged as they emerged from the aortic arch and extend to the neck. The middle portion extends from the aortic arch to the subcarinal region, where the aortic arch and descending aorta project posteriorly. The distal portion extends from the subcarinal region to the level of the cardia. The left atrium projects anteriorly and the descending aorta posteriorly.
P.1944
Mallery and Van Dam (2000) and Richards and associates (2000) have described the normal appearance of the make-up of the wall of the esophagus as seen on the sonogram. The esophageal wall is stratified into five layers sonographically. The first layer is hyperechoic and corresponds to the superficial mucosa. The second layer is hypoechoic and corresponds to the deep mucosa. The third layer is hyperechoic and corresponds to the submucosa and the interface between the submucosa and the muscularis propria. The fourth, hypoechoic layer corresponds to the muscularis propria, and the fifth layer corresponds to the adventitia.
Magnetic Resonance Imaging
Esophageal magnetic resonance (MR) imaging is compromised by respiratory and cardiac motion. The esophagus is most often imaged in the body coil because the respiratory artifacts produced by a surface coil positioned on the rib cage can be difficult to eliminate. Respiratory artifacts can be minimized by respiratory compensation and single breath-hold pulse sequences. In addition, cardiac gating and gradient moment nulling help reduce artifacts from great vessel and cardiac pulsation. Spatially selective presaturation pulses on spin echo sequences also minimize artifacts from flowing blood, as Semelka and co-workers (2002) noted in Semelka's textbook Abdominal and Pelvic MRI.
The esophagus appears as a low-signal intensity structure contrasted by high-signal intensity fat on T1-weighted images. On T2-weighted images, the muscular wall of the esophagus has low signal intensity, whereas intraluminal contents have high signal intensity. According to Semelka and colleagues (2002), the muscular wall of the esophagus enhances moderately after the injection of intravenous gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA).
Although not a primary means for imaging the esophagus, MR imaging can serve as an alternative technique to CT and represents an important supplemental imaging method.
Positron Emission Tomography
Developed nearly 3 decades ago for research purposes, positron emission tomography (PET) evolved into a powerful clinical imaging tool for evaluating oncology patients. Cancers of the lung, colorectum, breast, brain, head, and neck, as well as lymphoma and melanoma, have proven to be effectively imaged and staged by PET. Recently, the efficacy of PET in cancer of the esophagus has been established by the studies of Antoch and associates (2002).
In vivo PET imaging in malignancies is based on the fact that glucose and hence fluorine 18 fluorodeoxyglucose (FDG) uptake are facilitated by two factors: increased number of glucose transporters on malignant cell surfaces and a block in the intracellular glycolytic pathway due to relative glucose-6-phosphatase deficiency. PET images reflect the relative stage of glycolysis that is increased in tumor cells. Tumors with the highest metabolic rates demonstrate the greatest FDG accumulation.
Positron Emission Tomography/Computed Tomography
Recent technical advances have led to the development of combined PET and CT scanners. This fusion of morphologic (CT) and functional (PET) data dramatically improves the diagnostic potential of either scanner alone. Having both imaging technologies in a single machine allows for more precise localization of metabolic abnormalities. Indeed PET/CT will become an initial staging procedure for tumors if and when reimbursement issues are resolved, as noted by Antoch and co-workers (2002).
Scintigraphy
Gastroesophageal reflux can be detected and quantitatively analyzed by scintigraphy after oral administration of technetium 99m sulfur colloid mixed in 300 to 500 mL of acidified orange juice. This technique is also useful for the evaluation of esophageal motility disorders and postoperative evaluation of esophageal transit time as reported by Datz (1991).
Angiography and Interventional Radiology
The multiplicity of sources for arterial and venous circulation of the esophagus has limited the application of angiographic methods to this organ. As recently reviewed by Nemcek and Vogelzang (2000), selective catheterization of the left gastric or splenic arteries can demonstrate the site of hemorrhage from esophageal varices or Mallory-Weiss tears. Such bleeding also may be treated by transcatheter infusion of vasoconstrictive agents and controlled embolization. Percutaneous transhepatic catheterization of the portal vein and its branches has been widely used for embolotherapy of bleeding gastroesophageal varices.
Transjugular intrahepatic portosystemic shunts (TIPS) is quite useful in treating recurrent variceal hemorrhage that does not respond to endoscopic sclerotherapy, variceal banding, or systemic vasopressin. TIPS is also useful in the setting of intractable ascites and portal gastropathy.
Several interventional techniques that were initially designed for the cardiovascular system have been modified for application to the alimentary tract. For instance, angioplasty balloon catheters are now being used for dilation of esophageal strictures under fluoroscopic guidance. This procedure and its clinical results have been reviewed by Therasse and associates (2003).
P.1945
Abdal and co-workers (2001) used this technique for successful treatment of postsurgical esophageal strictures. Intravascular stents also have been modified by Lee (2001) for the fluoroscopically controlled intraesophageal placement. These endoluminal stents provide an effective means for palliating dysphagia caused by obstructing tumors and for blockage of esophagobronchial fistulae, both having been reported by O'Donnell (2002) and Roy-Choudhury (2001) and their colleagues.
RADIOLOGIC DIAGNOSIS OF ESOPHAGEAL CANCER
Barium Studies
The esophagram has a high sensitivity in the diagnosis of esophageal cancer and accurately predicts lesion length in 59% of patients according to Drudi and collaborators (2002). Polypoid and stenotic tumors are more easily seen than flat, sessile lesions.
Squamous Cell Carcinoma
Drudi and associates (2002) have reported that on double-contrast barium studies, early squamous cell carcinomas of the esophagus appear as small sessile polypoid lesions (Fig. 127-4A) with smoother slightly lobulated contours; plaquelike lesions (Fig. 127-4B) that often have flat, central ulcers that are best visualized in profile; or as superficial spreading lesions with a nodular appearance of the mucosa without a discrete mass. When early esophageal cancer or superficial spreading cancer is suspected on double-contrast barium examinations, endoscopic biopsy should be performed. Unfortunately, these early esophageal cancers are often subtle lesions that can be missed due to interpretive or perceptive error, as noted by Kumbasar (2002).
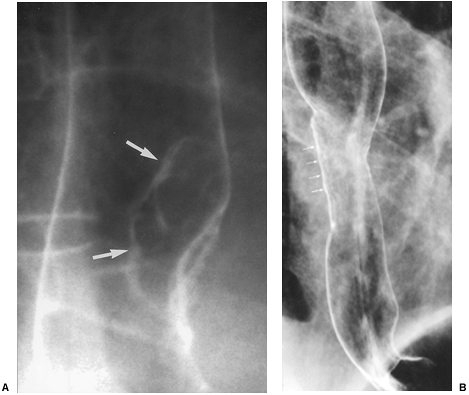 |
Fig. 127-4. Early squamous cell carcinoma: double-contrast barium features. A. Polypoid (arrows). B. Plaque-like (arrows). |
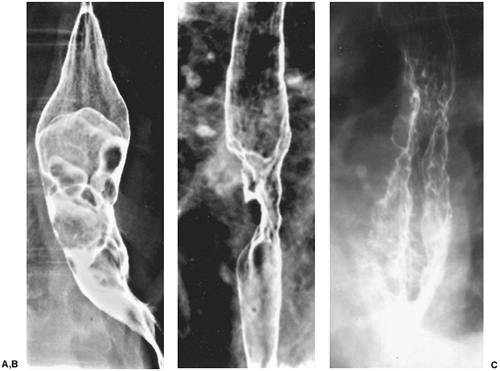 |
Fig. 127-5. Advanced squamous cell carcinoma: double-contrast barium features. A. Infiltrative.B. Polypoid. C. Varicoid. |
Advanced squamous cell carcinoma of the esophagus can manifest on plain chest films by widening of the mediastinum, thickened retrotracheal stripe, anterior tracheal bowing, or by an air fluid level within a dilated, obstructed proximal esophagus.
On double-contrast barium studies, advanced squamous cell carcinomas may appear infiltrative (Fig. 127-5A), ulcerative, polypoid (Fig. 127-5B), or, less commonly, varicoid (Fig. 127-5C). Many lesions have mixed morphologic features, resulting in considerable overlap in the radiologic classification of these lesions. Advanced carcinomas most commonly have an infiltrative appearance because they tend to grow circumferentially in the esophageal wall. There is luminal narrowing associated with mucosal nodularity, ulceration, abrupt shelflike proximal and distal borders,
P.1946
and varying degrees of obstruction. By the time the neoplasms compromise the lumen sufficiently to cause dysphagia, they are almost always advanced, unresectable lesions. Some infiltrative lesions have more gradual, tapered borders that may simulate the appearance of a benign stricture. Thus, if an esophageal stricture has any irregular contours or suspicious features, endoscopic biopsy should be performed to exclude a malignant tumor.
These advanced infiltrative squamous cell carcinomas are most commonly found in the upper or middle thoracic esophagus, and less commonly in the cervical or distal thoracic esophagus. Cervical esophageal cancers are more likely to be missed on barium studies because the barium passes so rapidly through the cervical esophagus that it is often difficult to obtain adequate images of this region.
Squamous cell carcinomas can also present as polypoid intraluminal masses often containing areas of ulceration due to necrosis of the tumor. As these lesions enlarge, they tend to have a circumferential pattern of growth that narrows and obstructs the lumen. Accordingly, if a bulky, polypoid esophageal mass expands or dilates the esophagus, then less common lesions such as carcinosarcoma or fibrovascular polyp should be suspected.
Other squamous cell carcinomas can manifest as primarily ulcerative lesions with a giant meniscoid ulcer, surrounded by a thin, radiolucent rind of tumor. They can simulate benign ulcers caused by cytomegalovirus, human immunodeficiency virus, and oral medications (e.g., quinidine potassium chloride or nonsteroidal antiinflammatory agents). The rind of tumor that surrounds a malignant ulcer tends to be thicker and more irregular than the pencil-thin rim of edema surrounding a benign ulcer.
Least commonly, squamous cell carcinomas of the esophagus appear as varicoid defects manifested by multiple submucosal lesions that may simulate varices on a single image. This lesion results from the submucosal spread of tumor. These serpiginous defects have a fixed, unchanging appearance at fluoroscopy, whereas true varices change in size and shape with changes in esophageal distention, peristalsis, and distention. Patients with varicoid carcinoma
P.1947
often present with dysphagia, a rare symptom in patients with varices, since they are soft, compressible structures.
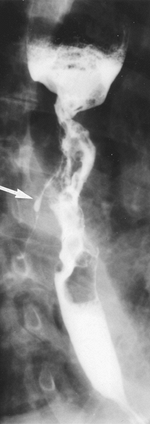 |
Fig. 127-6. Ulcerative advanced squamous cell carcinoma of the esophagus with fistula to tracheobronchial tree (arrow). |
Some 5% to 10% of patients with esophageal cancer develop esophageal airway fistulae involving the trachea or left main bronchus (Fig. 127-6). These complications often occur following radiation therapy.
Adenocarcinoma
On double-contrast barium studies, early adenocarcinoma arising from Barrett's mucosa can manifest as small sessile polyps, plaquelike lesions (Fig. 127-7), or superficial spreading lesions that cause focal nodularity of the mucosa without a discrete mass. Levine and associates (1988) noted that these early cancers can also cause focal irregularity, flattening, or nodularity in a preexisting peptic stricture. Accordingly, early endoscopy and biopsy are necessary to exclude adenocarcinoma whenever any of these suspicious features develop in the region of a peptic stricture.
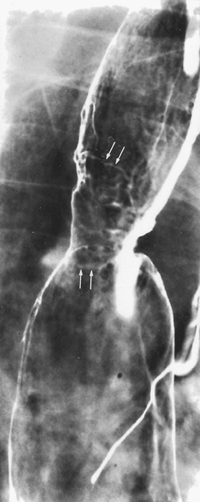 |
Fig. 127-7. Adenocarcinoma of the esophagus arising in the Barrett's mucosa. There is a stricture associated with a plaquelike tumor of the midesophagus (arrows). |
Advanced adenocarcinoma of the esophagus can appear infiltrating, polypoid, ulcerative, or, less commonly, varicoid on barium studies. Many neoplasms have mixed morphologic features so that considerable overlap exists in the radiologic classification of these lesions. Squamous cell carcinomas tend to be located in the upper or middle thoracic esophagus and only rarely invade the adjacent stomach by contiguous spread. Adenocarcinomas develop predominantly in the distal thoracic esophagus and have a marked tendency to invade the gastric cardia and fundus (Fig. 127-8), an important differentiating feature with squamous
P.1948
cell carcinoma. Gastric involvement by esophageal adenocarcinoma may manifest on barium studies as an obvious polypoid or ulcerative mass that is contiguous with the primary tumor in the distal esophagus. In other cases, tumor invasion of the fundus may be recognized only by obliteration or distortion of the normal cardia landmarks associated with nodularity and ulceration in this region.
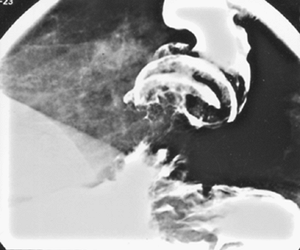 |
Fig. 127-8. Adenocarcinoma of the distal esophagus invades the gastric cardia. |
It may not always be possible to differentiate esophageal adenocarcinoma of the gastric cardia invading the distal esophagus. A primary gastric carcinoma should be suspected when the bulk of the tumor involves the gastric cardia and fundus, and a primary esophageal adenocarcinoma should be suspected when the bulk of tumor involves the distal esophagus. Nevertheless, these tumors have similar pathologic, clinical, and prognostic features.
On barium studies, advanced infiltrative adenocarcinomas present with ulceration, mucosal nodularity, and shelflike or tapered borders that may be indistinguishable from advanced squamous cell carcinomas. The correct diagnosis should be made when the lesion is located in the distal esophagus or is associated with a hiatal hernia, gastroesophageal reflux, or other signs of reflux disease.
RADIOLOGIC STAGING OF ESOPHAGEAL CANCER
Since a multimodality approach is presently used to treat esophageal cancer, a precise histologic diagnosis and highly accurate tumor staging is a prerequisite for the selection of the most suitable treatment option, as stressed by Meyenberger and Fantin (2000) and by Stein and associates (2001).
Esophageal cancer is usually diagnosed by barium studies or upper gastrointestinal endoscopy and confirmed by endoscopically guided biopsy. By virtue of their cross-sectional imaging format and ability to demonstrate the extent of wall invasion, extraluminal tumor spread, and lymph node and distant metastases, CT and endoscopic ultrasonography have become the primary means of pretherapeutic tumor staging, as emphasized by van Overhagen and Becker (1998) and Wu and colleagues (2003).
The Union Internationale Contre le Cancer (UICC) classification, revised in 1987 and mirrored by the American Joint Committee on Cancer, employs the TNM system, which emphasizes the depth of wall invasion by the primary tumor and classifying stages into categories that show similar prognosis and survival statistics.
Computed Tomographic Evaluation
Determining Tumor Stage Status
The normal esophagus has a mural thickness of no more than 3 mm; any wall thickening greater than 5 mm is abnormal. The presence of intraluminal air, contrast, or fluid facilities the measurement of wall thickness. Asymmetric mural thickening is the major but nonspecific CT finding of esophageal cancer. Because CT does not define the esophageal wall, it cannot differentiate between Tis, T1, and T2 tumors. If the paraesophageal fat is obliterated or made ill defined by an abnormal soft tissue mass, T3 disease is suggested. According to Riedel and colleagues (2000), endoscopic ultrasonography is clearly superior to CT in T staging of esophageal neoplasms. CT is somewhat more successful in the depiction of T4 disease.
Aortic Invasion
The middle and lower intrathoracic esophagus lies in close proximity to the aorta, particularly at the level of the carina and main-stem bronchi. Invasion of the aorta by esophageal carcinoma is found in only 3% of patients at autopsy. Because fat planes are often absent between the esophagus and aorta, the criterion of fat plane obliteration cannot be used to predict tumor invasion of the aorta. Tumor invasion of the aorta is unlikely if the tumor involves the aortic circumference by less than or equal to 45 degrees, contact of greater than or equal to 90 degrees is highly suggestive of invasion, and contact in the range of 45 to 90 degrees is indeterminate. Aortic invasion is also predicted when the small triangle of fat between the esophagus, aorta, and spine is obliterated. Wayman and co-workers (2001) suggest that scanning the patient in the prone as well as supine position will improve diagnostic accuracy by helping to downstage patients with suspected aortic invasion.
Tracheobronchial Invasion
The preoperative identification of tracheobronchial invasion is important because it may make tumor resection, particularly by the transhiatal route, more difficult or even
P.1949
impossible. The demonstration of a tracheobronchial fistula or extension of tumor into the airway lumen are specific signs of invasion, as noted by Schirmer and associates (1999).
The posterior wall of the normal cervical trachea often has a concave (inward bowing) appearance, but the intrathoracic trachea should have a convex (outward bowing) or linear appearance. Tracheobronchial invasion should be suspected according to Griffith and colleagues (1999) if an esophageal tumor either causes inward bowing (Fig. 127-9) of the posterior tracheal or bronchial wall or displaces the trachea or bronchi away from the spine. Invasion may also be present if the fat planes between the esophagus and airway are lost at the level of the tumor but are preserved above and below the tumor. This criterion should be used with caution because there are often no fat planes between the esophagus and trachea. Riedel and colleagues (2000) state that the likelihood of airway invasion increases with the presence of respiratory symptoms, tumor length, and T stage on endoscopic ultrasonography (EUS). Schirmer and associates (1999) reported that CT has an accuracy of 85.1% when compared with bronchoscopy in regard to impingement, displacement, and invasion of the trachea and bronchi.
Pericardial Invasion
Pericardial invasion by esophageal cancer is rare, found in only 1% of patients at autopsy. Invasion should be suspected on CT if a tumor extends directly into the pericardium, obliterating the fat planes at this level, provided that normal fat planes exist immediately above and below the tumor mass. If periesophageal fat planes are absent at all levels, often seen in cachectic patients, CT cannot accurately assess tumor invasion. Other signs suggestive of invasion include pericardial thickening adjacent to tumor, pericardial effusion, and inward deformity of the heart.
Diaphragmatic Invasion
Distal esophageal tumors may invade the diaphragmatic crura and manifest as a soft tissue mass obliterating the fat planes that normally surround the crura. Crural invasion is more commonly seen with adenocarcinoma than squamous cell carcinoma. Because of the oblique course of the gastroesophageal junction, it can be difficult to accurately assess tumor invasion of the diaphragm by means of axial images. This is of minor clinical importance since crural invasions does not necessarily preclude tumor resection.
Determining Metastatic Stage Status
In patients with newly diagnosed esophageal cancer, Quint (1995), Kinkel (2002), and Margolis (1998) and their associates recorded that metastases are seen in the liver in 35%, lung 20%, bone 9%, renal 2%, and brain 2%, and in 1% each in pericardium, pleura, soft tissues, stomach, pancreas, and spleen.
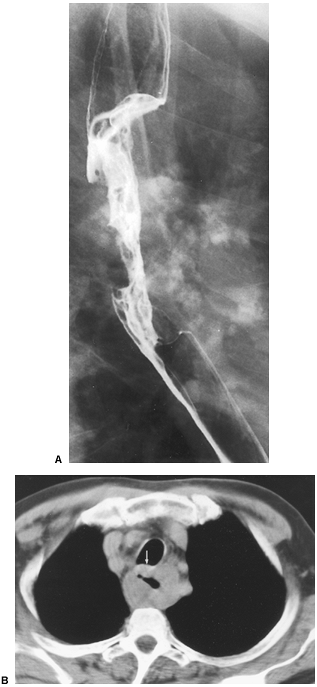 |
Fig. 127-9. Squamous cell carcinoma of the esophagus: CT staging. A. Esophagram shows a malignant stricture.B. CT scan shows the mass bowing the posterior aspect of the trachea (arrow). |
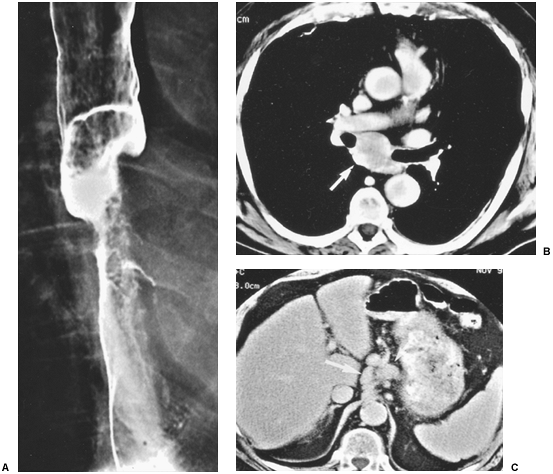 |
Fig. 127-10. Squamous cell carcinoma of the esophagus: CT staging. A. Barium study shows a tumor causing malignant stricture of the midesophagus.B. CT scan shows subcarinal adenopathy (arrow). C. Scan obtained at a lower level shows adenopathy in the gastrohepatic ligament (arrows). |
P.1950
CT scan has a reported sensitivity of 76% and a positive predictive value of 90% in the detection of hepatic metastases. In a cohort of patients with squamous cell carcinoma, the detection of solitary lung metastases was rare at the time of the diagnosis of the primary cancer. Most nodules were either benign or a synchronous primary lung cancer according to Margolis and colleagues (1998).
The presences of ascites, pleural effusion, or nodules in the omentum or pleura are suspicious of metastases to these mesothelial-lined surfaces. Minimally invasive laparoscopy and thoracoscopy can confirm these findings.
Determining Nodal Stage Status
The rich, interconnecting network of lymphatics along the entire esophagus and lack of serosa contribute to the high prevalence (60% to 80%) of mediastinal and extrathoracic lymph node metastases at the time of diagnosis. Lymph node metastases should be suspected if they are enlarged: greater than or equal to 5 mm supraclavicular; greater than or equal to 6 mm retrocrural; greater than or equal to 8 mm gastrohepatic ligament (Fig. 127-9B); and 1 cm in most lymph node groups in the periesophageal chain, other mediastinal regions, and the upper abdomen (Fig. 127-10). Groups of multiple small nodes are suspicious as well. CT accuracy in assessing tumor involvement is limited by a number of factors. False positives may occur in patients with inflammatory or infectious lymphadenopathy. False negatives occur because normal sized nodes may harbor tumor and because small periesophageal lymph nodes are frequently indistinguishable from the primary tumor mass as recorded by Wu and colleagues (2003).
P.1951
Endoscopic Ultrasonography
By virtue of its ability to visualize the various layers of the esophageal wall and periesophageal tissues, EUS has proven very useful in the T and N staging of esophageal carcinoma.
Determining Tumor Stage Status
On EUS, esophageal carcinomas manifest as hypoechoic masses that disrupt the normal five-layer mural stratification of the esophagus. EUS nicely depicts the depth of wall penetration and invasion of tumor into the periesophageal tissues. EUS also shows tumor invasion into most surrounding structures which is important for planning therapy.
As stressed by Torres (2000), tumor stenosis that prevents the passage of the endoscope is a major limitation of EUS and occurs in 19% to 63% of examinations. These lesions are usually T3 or T4, and although only the proximal portion of the tumor is seen on EUS, fairly accurate T staging is still possible. Smaller-caliber, endoluminal high-frequency probes may improve tumor visualization in these cases. These 20-MHz probes are passed through the biopsy channel of the endoscope and can accurately determine T stage in 85% to 90% of patients.
The reported accuracy of EUS in assessing depth of tumor penetration (T stage) ranges from 85% to 90%. Tumor overstaging usually results from peritumoral inflammation, and understaging relates to microscopic invasion beneath the spatial resolution of EUS.
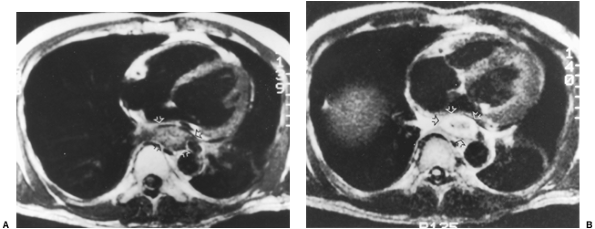 |
Fig. 127-11. Squamous cell carcinoma: MR features. A. Axial T1-weighted scan through the lower esophagus shows a mass (arrows) that abuts but does not invade the pericardium. B. The tumor (arrows) enhances following the administration of gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA). |
Determining Nodal Stage Status
By evaluating nodal morphology and echo architecture, EUS can demonstrate tumor involvement in lymph nodes as small as 4 to 5 mm. Well-marginated, spherical, hypoechoic lymph nodes that are less sharply demarcated are more likely malignant. Lymph nodes that have a bean or elongated shape and have a hyperechoic pattern are more likely benign. False-positive results occur in patients with inflammatory nodes, usually due to granulomatous infection, that mimic the appearance of malignant nodes. Micrometastases in normal-sized malignant nodes beneath the spatial resolution of EUS are responsible for false-negative results. Some investigators use a 5-mm or larger size criterion to indicate malignancy. EUS-guided lymph node fine-needle aspiration (FNA) biopsy should help overcome the limitations of N staging on EUS and improve staging, as pointed out by Richards and coinvestigators (2000).
Determining Metastatic Stage Status
Although EUS can image portions of the liver and celiac lymph nodes, this technique is not useful for the evaluation of distant metastases (M stage) due to limited depth of the transducer field. CT and PET are superior for the diagnosis of distant metastases. Harewood and Wiersema (2002) reported that the use of EUS with FNA of suspicious lymph nodes has become an important aid in the staging of esophageal cancer.
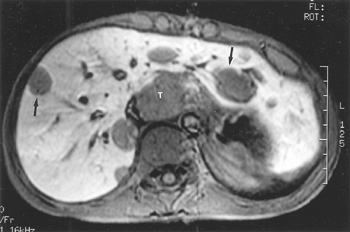 |
Fig. 127-12. Adenocarcinoma of the gastroesophageal junction: MR findings. T1-weighted, fat-suppressed scan shows the tumor mass (T) and multiple hepatic metastases (arrows). |
P.1952
Magnetic Resonance Imaging
MR imaging is useful in staging advanced malignancies, providing information for determining regional resectability and for planning subsequent therapy in patients treated by combined radiation and chemotherapy. Multiplanar MR acquisition, as suggested by Giovagnoni and colleagues (2002), seems better than CT in establishing the longitudinal extent of tumor. CT and MR imaging are relatively equal in their ability to detect invasion of the aorta, tracheobronchial tree, and heart. Standard MR imaging, like CT, cannot reveal the depth of mural invasion because it cannot distinguish the layers of the wall. MR cannot distinguish lesions limited to the submucosa (T1) from those affecting the muscularis (T2) but usually can identify tumors invading the adventitia. The MR endoscope may offer the potential advantage of high-resolution three-dimensional tumor reconstruction, as demonstrated by Inui (1995) and Yamada (2001) and their co-workers.
Esophageal carcinoma appears isointense relative to the normal wall on T1-weighted images, with low to intermediate signal intensity on proton-density images that slightly increases on T2-weighted sequence. Esophageal neoplasms often enhance following intravenous administration of Gd-DTPA (Fig. 127-11).
In patients with adequate mediastinal fat, extraesophageal spread of tumor is suggested when there is hypointense signal extending from the primary mass into the adjacent mediastinal fat coupled with irregularity in the external margins of the lesion and interruption of the periesophageal fat. Tumor masses larger than 4 cm are also always associated with extraesophageal tumor spread, as noted by Balzarini and associates (1998).
The MR criteria used to determine the presence of tracheobronchial, pericardial, and aortic invasion are the same used for CT. Involvement of the prevertebral fascial should be suspected when the angle of contact with the vertebral body is greater than 180 degrees and the interposed fat layer is lost. The size criteria for mediastinal lymphadenopathy is identical for CT and MR imaging, as noted by Giovagnoni and co-workers (2002). MR imaging is more sensitive than CT in the detection of liver metastases (Fig. 127-12).
Positron Emission Tomography
PET scanning in esophageal cancer is based on the fact that malignant tumors have increased glycolytic activity and that [18F] FDG significantly accumulates in cells with active glucose metabolism (Figs. 127-13 and 127-14). FDG accumulates in 92% to 100% of esophageal cancers according
P.1953
to Wallace and associates (2002). In a study by Kato and colleagues (2002a), patients with a high FDG standard uptake value (SUV) of greater than 3 had a lower survival rate than did those with an SUV of less than 3.
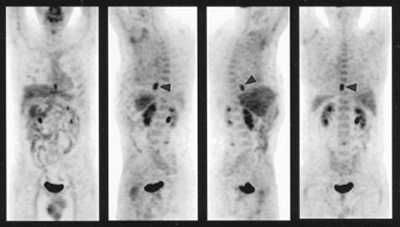 |
Fig. 127-13. Stage N0M0 squamous cell carcinoma of the esophagus. Fluorodeoxyglucose positron emission tomography scan shows an isolated area of intense activity (arrowheads) in the distal esophagus. No adenopathy or distant metastases are identified. (Courtesy of Barry Siegel, St. Louis, MO.) |
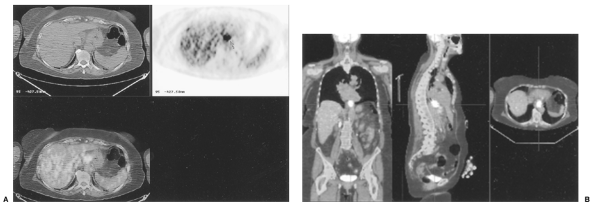 |
Fig. 127-14. PET CT image of adenocarcinoma of the distal esophagus. This technology fuses the axial CT image (A) with the axial PET image(B). The fused image provides anatomic and functional information. Coronal, sagittal, and axial fused images show that the primary tumor has intense uptake and that there are also multiple liver and splenic metastases. (Courtesy of Hedvig Hricak, New York, NY.) |
Determination of Tumor Stage Status
Because FDG PET provides no definition of the esophageal wall or paraesophageal tissue, it has little role in the determination of the T status of esophageal tumors. Himeno and co-workers (2002) reported that PET imaging can detect primary esophageal neoplasms with a depth of invasion of T1b or greater, but Tis and T1a tumors are undetectable.
Determination of Nodal Stage Status
Because the physiologic evaluation of esophageal cancers afforded by FDG PET relies both on the size of the metastatic node as well as the intensity of FDG uptake and decay, it is possible according to Flamen and associates (2000a) to detect tumor in normal-sized lymph nodes. FDG PET cannot differentiate primary tumor from adjacent N1 disease. FDG PET is superior in detecting lymph node metastases in lung cancer when compared with esophageal neoplasm. The reported accuracy in detecting N disease in esophageal cancer is variable: 83% by Choi (2000); 84.4% by Kato (2002b); 83.7% by Kim (2001); and 92.2% by Himeno (2002) and their associates. FDG PET has a greater accuracy in detecting lymph nodes in the neck, upper thorax, and abdomen, with a lower sensitivity in the middle and lower thoracic regions, as reported by Kato and colleagues (2002b).
Determination of Metastatic Stage Status
FDG PET is superior to CT in screening for distant metastases, as noted by Flamen and associates (2000a). In a meta-analysis by Kinkel and collaborators (2002) of the various imaging modalities for diagnosing hepatic metastases from colorectal, stomach, and esophageal cancer, FDG PET had a sensitivity of 90%, MR imaging 76%, CT 72%, and ultrasonography 55%.
Monitoring Tumor Response
As noted by Flamen (2000b, 2002) and Brucher (2001) and their associates, PET has been found useful for following the response to induction chemotherapy and radiation therapy for locally advanced esophageal cancer. Low FDG uptake after therapy and reduction in the extent of FDG uptake can provide a reliable assessment of tumor response to therapy, as reported by Kato and colleagues (2002b).
In a study by Wallace and co-workers (2002) of patients undergoing CT, EUS with FNA, FDG PET, thoracoscopy, and laparoscopy, CT and EUS with FNA were the most inexpensive strategy and offered more quality-adjusted life years on average than all other strategies, with the exception of PET and EUS with FNA, which was slightly more effective but also more expensive.
Some studies such as that by Wren and associates (2002) have shown little difference between CT and PET in the initial staging. Others have stated that the relatively low sensitivity for identifying local regional lesions precludes its replacement by conventional CT. The primary advantage of PET as pointed out by Meltzer and colleagues (2000) is its
P.1954
superior sensitivity for tumor detection and improved diagnostic value for distant metastatic sites, features that can substantially affect patient management decisions.
Comparison of Endoscopic Ultrasonography, Computed Tomography, and Magnetic Resonance Imaging
EUS is more accurate than CT in local staging of esophageal carcinoma, and CT is more valuable in the detection of distant metastases. Combining EUS and CT achieves the highest accuracy rate in TNM staging (86%) than either modality alone. Accordingly, CT scan should be used first to find distant metastases, and if none are present, EUS should be used for local staging according to reports of Wakelin (2002) and Hadzijahic (2000) and their coinvestigators.
REFERENCES
Abdal JM, et al: Treatment of malignant esophagorespiratory fistulas with covered stents. Abdom Imaging 26:565, 2001.
Aksglaede K, et al: Diagnosis of esophageal motor disorders: a prospective study comparing barium swallow, food barium mixture, and continuous swallows with manometry. Gastrointest Radiol 17:1, 1992.
Antoch G, et al: Whole-body positron emission tomography-CT: optimized CT using oral and IV contrast materials. AJR 179:1555, 2002.
Balzarini L, Potepan P, Musumeci RN: Diagnosis and staging of esophageal carcinoma by magnetic resonance imaging. In Meyers MA (ed): Neoplasms of the Digestive Tract: Imaging, Staging, and Management. Philadelphia: Lippincott-Raven, 1998, pp. 49 59.
Brick SH, et al: Esophageal disruption: evaluation with iohexol esophagography. Radiology 169:141, 1988.
Brucher BL, et al: Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Oncol 233:300, 2001.
Buecker A, et al: Esophageal perforation: comparison of use of aqueous and barium-containing contrast media. Radiology 202:683, 1997.
Choi JY, et al: Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG-PET. J Nucl Med 41:808, 2000.
Datz F: Gastrointestinal imaging. In Taylor A, Datz F (eds): Clinical Practice of Nuclear Medicine. New York: Churchill Livingstone, 1991, p. 317.
Drudi FM, et al: Esophagogram and CT vs endoscopic and surgical specimens in the diagnosis of esophageal carcinoma. Radiol Med (Torino) 103:344, 2002.
Flamen P, et al: Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 18:3199, 2000a.
Flamen P, et al: The utility of positron emission tomography for the diagnosis and staging of recurrent esophageal cancer. J Thorac Cardiovasc Surg 120:1085, 2000b.
Flamen P, et al: Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 13:361, 2002.
Foley MJ, Ghahremani GG, Rogers LF: Reappraisal of contrast media used to detect upper gastrointestinal perforations: comparison of ionic water-soluble media with barium sulfate. Radiology 144:231, 1982.
Ghahremani GG: Ingested foreign bodies: radiological diagnosis and management. In Thompson WM (ed): Common Problems in Gastrointestinal Radiology. Chicago: Year Book Medical, 1989, p. 152.
Ghahremani GG: Radiologic evaluation of suspected gastrointestinal perforations. Radiol Clin North Am 31:1219, 1993.
Ghahremani GG: Esophageal trauma. Semin Roentgenol 29:387, 1994.
Ghahremani GG, et al: Detection of occult esophageal narrowing with barium tablet during chest radiography. Clin Imaging 20:184, 1996.
Giovagnoni A, Valeri G, Ferrara C: MRI of esophageal cancer. Abdom Imaging 27:361, 2002.
Griffith JF, et al: 3D CT imaging of oesophageal carcinoma. Eur J Radiol 32:216, 1999.
Hadzijahic N, et al: CT or EUS for the initial staging of esophageal cancer? A cost minimization analysis. Gastrointest Endosc 52:715, 2000.
Harewood GC, Wiersema MJ: A cost analysis of endoscopic ultrasound in the evaluation of esophageal cancer. Am J Gastroenterol 97:452, 2002.
Himeno S, et al: Evaluation of esophageal cancer by positron emission tomography. Jpn J Clin Oncol 32:340, 2002.
Inui H, et al: Endoscopic MRI. Preliminary results of a new technique for visualization and staging of gastrointestinal tumors. Endoscopy 27:480, 1995.
Kato H, et al: Usefulness of positron emission tomography for assessing the response of neoadjuvant chemoradiotherapy in patients with esophageal cancer. Am J Surg 184:279, 2002a.
Kato H, et al: Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 94:921, 2002b.
Kim K, et al: Evaluation of lymph node metastases in squamous cell carcinoma of the esophagus with positron emission tomography. Ann Thorac Surg 71:290, 2001.
Kinkel K, et al: Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology 224:748, 2002.
Kumbasar B: Carcinoma of esophagus: radiologic diagnosis and staging. Eur J Radiol 42:170, 2002.
Laufer I: Barium studies of the upper gastrointestinal tract. In Gore RM, Levine MS (eds): Textbook of Gastrointestinal Radiology, 2nd Ed. Philadelphia: WB Saunders, 2000, pp. 272 284.
Lee SH: The role of oesophageal stenting in the non-surgical management of oesophageal strictures. Br J Radiol 74:891, 2001.
Levine MS, et al: Double-contrast upper gastrointestinal examination: technique and interpretation. Radiology 168:593, 1988.
Levine MS, Rubesin SE, Ott DJ: Update on esophageal radiology. AJR 155:933, 1990.
Maglinte DDT, Schultheis TE, Krol KL: Survey of the esophagus during the upper gastrointestinal examination in 500 patients. Radiology 147:65, 1983.
Mallery S, Van Dam J: EUS in the evaluation of esophageal carcinoma. Gastrointest Endosc 52:56, 2000.
Margolis ML, Howlett P, Bubanj P: Pulmonary nodules in patients with esophageal carcinoma. J Clin Gastroenterol 26:245, 1998.
Megibow AJ: CT of the gastrointestinal tract: techniques and principles of interpretation. In Gore RM, Levine MS (eds): Textbook of Gastrointestinal Radiology, 2nd Ed. Philadelphia: WB Saunders, 2000, pp. 77 85.
Meltzer CC, et al: Whole-body FDG positron emission tomographic imaging for staging esophageal cancer: comparison with computed tomography. Clin Nucl Med 25:882, 2000.
Meyenberger C, Fantin AC: Esophageal carcinoma: current staging strategies. Rec Results Cancer Res 155:63, 2002.
Natsugoe S, et al: Biologic and imaging diagnosis of lymph node metastasis in esophageal carcinoma. J Clin Oncol 81:25, 2002.
Nemcek AA, Vogelzang RL: Angiography and interventional radiology of the hollow viscera. In Gore RM, Levine MS (eds): Textbook of Gastrointestinal Radiology, 2nd Ed. Philadelphia: WB Saunders, 2000, pp. 98 143.
Noh HM, et al: CT of the esophagus: spectrum of disease with emphasis on esophageal carcinoma. Radiographics 15:1113, 1995.
O'Donnell CA, et al: Randomized clinical trial comparing self-expanding metallic stents with plastic endoprostheses in the palliation of oesophageal cancer. Br J Surg 89:985, 2002.
Ott DJ: Esophageal motility disorders. Semin Roentgenol 29:321, 1994.
Ott DJ, et al: Radiologic evaluation of esophageal motility in 170 patients with chest pain. AJR 155:983, 1990.
Ott DJ, et al: Evaluation of the esophagus with a marshmallow bolus: clarifying the cause of dysphagia. Gastrointest Radiol 16:1, 1991.
Quint LE, et al: Incidences and distribution of distant metastases in newly diagnosed esophageal carcinoma. Cancer 76:1120, 1995.
Richards DG, Brown TH, Manson JM: Endoscopic ultrasound in the staging of tumour of the oesophagus and gastro-oesophageal junction. Ann R Coll Surg Engl 82:311, 2000.
P.1955
Richter JE, Bradley LA, Castell DO: Esophageal chest pain: current controversies in pathogenesis, diagnosis, and therapy. Ann Intern Med 110:66, 1989.
Riedel M, Stein HJ, Mounyam L, et al: Predictors of tracheobronchial invasion of suprabifurcal oesophageal cancer. Respiration 67:630, 2000.
Roy-Choudhury SH, et al: Symptomatic malignant gastroesophageal anastomotic leak: management with covered metallic esophageal stents. AJR 176:161, 2001.
Schirmer CC, et al: Efficacy of computed axial tomography in the evaluation of the involvement of respiratory tract in patients with squamous cell carcinoma of esophagus. Dis Esophagus 12:196, 1999.
Semelka RC, et al: Gastrointestinal tract. In Semelka RC (ed): Abdominal-Pelvic MRI. New York: Wiley-Liss, 2002, pp. 319 372.
Stark P, Thordarson S, McKinney M: Manifestations of esophageal disease on plain chest radiographs. AJR 155:729, 1990.
Stein HJ, et al: Esophageal cancer: patient evaluation and pre-treatment staging. Surg Oncol 10I:103, 2001.
Therasse E, et al: Balloon dilation and stent placement for esophageal lesions: indications, methods, and results. Radiographics 23:89, 2003.
Torres GM: Endoscopic ultrasonography. In Gore RM, Levine MS (eds): Textbook of Gastrointestinal Radiology, 2nd Ed. Philadelphia: WB Saunders, 2000, pp. 285 295.
van Overhagen H, Becker CD: Diagnosis and staging of carcinoma of the esophagus and gastroesophageal junction, and detection of postoperative recurrence by computed tomography. In Meyers MA (ed): Neoplasms of the Digestive Tract. Philadelphia: Lippincott-Raven, 1998, p. 31.
Wakelin SJ, et al: A comparison of computerised tomography, laparoscopic ultrasound and endoscopic ultrasound in the preoperative staging of oesophago-gastric carcinoma. Eur J Radiol 41:161, 2002.
Wallace MB, et al: An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg 74:1026, 2002.
Wayman J, et al: Evaluation of local invasion by oesophageal carcinoma a prospective study of prone computed tomography scanning. Postgrad Med J 77:181, 2001.
Wren SM, Stijns P, Srinivas S: Positron emission tomography in the initial staging of esophageal cancer. Arch Surg 137:1001, 2002.
Wu LF, et al: Preoperative TN staging of esophageal cancer. Comparison of miniprobe ultrasonography, spiral CT and MRI. World J Gastroenterol 9:219, 2003.
Yamad I, et al: Superficial esophageal carcinoma: an in vitro study of high-resolution MR imaging at 1.5T. J Magn Reson Imaging 13:225, 2001.
Reading References
Becker CD, Barbier P, Porcellini B: CT evaluation of patients undergoing transhiatal esophagectomy for cancer. J Comput Assist Tomogr 4:114, 1986.
Ghahremani GG, Heck LL, Williams JR: A pharmacologic aid in the radiographic diagnosis of obstructive esophageal lesions. Radiology 103: 289, 1972.
Ghahremani GG, Rushovich AM: Glycogenic acanthosis of the esophagus: radiographic and pathologic features. Gastrointest Radiol 9:93, 1984.
Gore RM: Esophageal cancer: Clinical and pathologic features. Radiol Clin North Am 35:243, 1997.
Gore RM, Yaghmai V: Esophageal cancer: In Bragg DG, Rubin P, Hricak H (eds): Oncologic Imaging. Philadelphia: WB Saunders, 2002, pp. 359 390.
Kaszar-Seibert DJ, et al: Treatment of acute esophageal food impaction with a combination of glucagon, effervescent agent and water. AJR 154:533, 1990.
Ke L: Mortality and incidence trends from esophageal cancer in selected geographic areas of China circa 1970 1990. Int J Cancer 102:271, 2002.
Kim SH, et al: Esophageal resection: indications, techniques and radiologic assessment. Radiographics 21:1119, 2001.
L'Hermine C, et al: Percutaneous transhepatic embolization of gastroesophageal varices: results in 400 patients. AJR 152:755, 1989.
Levine MS: Esophageal cancer: Radiologic diagnosis. Radiol Clin North Am 35:265, 1997.
Levine MS, Halvorsen RA: Carcinoma of the esophagus. In Gore RM, Levine MS (eds): Textbook of Gastrointestinal Radiology, 2nd Ed. Philadelphia: WB Saunders, 2000, pp. 403 434.
Martin IG: Staging of esophageal and gastric carcinoma. In Daly JM, Hennessy TPJ, Reynolds JB (eds): Management of Upper Gastrointestinal Cancer. London: WB Saunders, 1999, pp. 3 35.
Mettler FA Jr: Diagnostic radiology usage and trends in the United States, 1964 1980. Radiology 162:263, 1987.
Ott DJ: Motility disorders of the esophagus. In Gore RM, Levine MS (eds): Textbook of Gastrointestinal Radiology, 2nd Ed. Philadelphia: WB Saunders, 2000, pp. 316 328.
Petrillo R, et al: Esophageal squamous cell carcinoma: MRI evaluation of mediastinum. Gastrointest Radiol 15:275, 1990.
Reed CE, Eloubeidi MA: New techniques for staging esophageal cancer. Surg Clin North Am 82:697, 2002.
Rice TW: Clinical staging of esophageal carcinoma. CT, EUS, and PET. Chest Surg Clin North Am 10:471, 2000.
Saunders HS, Wolfman NT, Ott DJ: Esophageal cancer: radiologic staging. Radiol Clin North Am 35:281, 1997.
Shaffer HA Jr, et al: Basket extraction of esophageal foreign bodies. AJR 147:1010, 1986.
Skehan SJ, et al: Imaging features of primary and recurrent esophageal cancer at FDG-PET. Radiographics 20:713, 2002.
Tio TL: Diagnosis and staging of esophageal carcinoma by endoscopic ultrasonography. In Meyers MA (ed): Neoplasms of the Digestive Tract: Imaging, Staging, and Management. Philadelphia: Lippincott-Raven, 1998, pp. 61 70.
Towbin RB, Dunbar JS, Rice S: Magnetic catheter for removal of magnetic foreign bodies. AJR 154:149, 1990.
Trenkner SW, et al: Esophageal food impaction: treatment with glucagon. Radiology 149:401, 1983.
EAN: 2147483647
Pages: 203