89 - Thoracic Mycotic and Actinomycotic Infections of the Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume II > Section XVI - Carcinoma of the Lung > Chapter 104 - Radiologic Evaluation of Lung Cancer
Chapter 104
Radiologic Evaluation of Lung Cancer
Patrick J. Fultz
Richard H. Feins
The radiologic evaluation of lung cancer is directed at determining the best means for diagnosing and staging lung cancer and the best method of treating patients with that diagnosis. Although both the standard chest radiograph and computed tomography (CT) of the chest are the mainstays of radiologic evaluation, neither, alone or in combination, can make a definitive diagnosis of lung cancer. Such a diagnosis requires cytologic or histologic study. Similarly, except for the size criteria (T), neither study can very often stage a given lung cancer definitively. Such staging usually requires either direct operative examination or cytologic or histologic proof. Yet, all too often patients are told they have lung cancer or are assigned to a specific treatment based on radiologic evidence alone. This has been true even of some large-scale cooperative trials. This leap of faith is not only unfounded but also unnecessary, given today's variety of minimally invasive diagnostic procedures.
ROUTINE SCREENING FOR LUNG CANCER
With lung cancer being the most common cause of death by cancer in both men and women and with the belief that the earlier the stage of the tumor, the more curable it is, screening for lung cancer by routine chest radiographs or, more recently, CT, has appeared very attractive. Indeed, several studies have been done to test the hypothesis that routine chest radiographs, especially in a high-risk population, lead to earlier discovery of the disease and more curative treatment. Unfortunately, this has not proved to be the case. Studies were done of large numbers of cigarette-smoking men at the Mayo Clinic by Fontana and associates (1986), at Memorial Sloan Kettering Cancer Center by Melamed and colleagues (1984), at Johns Hopkins University by Tockman and co-workers (1986), and in Czechoslovakia by Kubik and coinvestigators (1990). All studies found that although screening did indeed lead to earlier detection of tumors, increased resectability, and improved length of survival, overall mortality was not decreased from that of controls. Salomaa and colleagues (1998) found only 93 men to have lung cancer out of 33,743 male cigarette smokers screened as part of the Alpha Tocopherol, Beta-Carotene Cancer Prevention Study. Although Strauss (1998) has argued that the significant stage distribution, resectability, and long-term survival advantages in this study prove that chest radiographic screening can save lives, the current American Cancer Society guidelines for cancer screening as reported by Smith and associates (2003) do not recommend routine screening chest radiographs for lung cancer.
Advances in low-dose spiral CT scanning have led to several small studies of the ability of these techniques to detect early tumors. Lung cancers were discovered more frequently with spiral CT than with chest radiographs; the radiographs missed 73% of the lesions. However, only a small number of tumors were discovered in a large number of examinations in the report of Kaneko and associates (1996). Similarly, in the baseline screening study of chest radiographs and CTs of Henschke and co-workers (1999), the radiographs missed 21 (74%) of the 27 malignancies seen by CT.
Management issues regarding the large number of nodules found by CT in at-risk patients are considerable. In the study by Swensen and associates (2002), after a baseline CT and 1 year follow-up CT scan, 2,244 uncalcified nodules were found in 1,000 (66%) of 1,520 study participants. Only 1.7% of patients were proved to have lung cancer.
Studies of the overall effect on mortality are beginning, but given the higher cost of spiral CT versus chest radiography, it remains to be seen if this modality will be shown to be an effective screening tool under current circumstances.
DIAGNOSING LUNG CANCER
Although definitive diagnosis of lung cancer requires histologic or cytologic confirmation, several aspects of a given radiographic finding may lead one to be more suspicious of lung cancer. The most important of these is probably the radiographic history of the lesion. Lesions that can
P.1520
be shown on older studies (either chest radiographs or CT) to be completely stable in size and shape are unlikely to be cancerous. A search for old films should be one of the first steps toward diagnosis. Most films are destroyed after 7 years, however, which makes it difficult to confirm stability. It is hoped that digital archiving of radiographic studies will eliminate the need to purge studies and will make obtaining them for comparison easier. It should be remembered, however, that a change in a lesion, even after a long period of documented stability, could be an indicator of a scar carcinoma or a particularly slow-growing tumor.
Radiographic Features
The radiographic findings caused by carcinoma of the lung may result from the tumor itself, from changes in the pulmonary parenchyma distal to a bronchus obstructed by the tumor (atelectasis, infection, or both), and from spread of the tumor to extrapulmonary intrathoracic sites (e.g., hilar and mediastinal lymph nodes, pleura, chest wall, and other mediastinal structures). The findings vary with location, cell type, and length of time the tumor has been present.
Garland (1966) estimated that when a lung tumor is first detectable on a chest radiograph, it has completed three-fourths of its natural history. Rigler (1957) observed that the radiographic abnormality frequently antedates the first symptoms or signs of the disease by 7 months or more. These early features are subtle and often are appreciated only in retrospect.
Early Radiographic Features
The early signs visible in radiographs of the chest, as Rigler (1966) noted, are produced directly by the tumor itself. The smallest single, solitary lesion that can be seen is approximately 7 mm in diameter, although multiple smaller lesions (diffuse lung lesions) may be recognized. The single lesion frequently is not identified until it is at least 10 mm in diameter; a smaller lesion may be obscured by overlying or adjacent structures. Muhm and associates (1983) studied lung cancer in 92 patients in a chest radiographic lung cancer screening study, in which at least two observers initially reviewed all radiographs, and found that 90% of the peripheral lung cancers were visible in retrospect on prior radiographs.
The early signs of a lung tumor are as follows:
A density within the lung parenchyma
A cavitary mass
A segmental, indistinct, poorly defined dense area
A nodular streaked, local infiltration along the course of a blood vessel
Segmental consolidation
A roughly triangular lesion arising in the apex and extending toward the hilus
A mediastinal mass (an uncommon early sign)
An enlargement of one hilus
Segmental or lobar obstructive emphysema (a rare finding in carcinoma of the lung)
Segmental atelectasis.
The relative incidences of these various early changes are difficult, if not impossible, to discern, because most patients have more advanced disease when first examined. Weiss and associates (1982), however, recorded the incidences of these early features in a screening program to detect lung cancer. A peripheral nodule or mass occurred in 33%, a peripheral infiltrate in 25%, and hilar enlargement in 28% of patients who developed a lung cancer. Atelectasis and a pleural effusion each occurred in 3%, and obstructive emphysema was seen in only 1%.
Usual Radiographic Manifestations
Byrd and associates (1969) classified the radiographic features as hilar, pulmonary parenchymal, and intrathoracic extrapulmonary. In their review of the chest radiographs of 600 patients with carcinoma of the lung, a hilar abnormality either alone or associated with other abnormalities was present in 41% of the patients. Obstructive pneumonitis, collapse, or consolidation was also present in 41%. A large parenchymal mass was present in 21.7%, and a small mass was evident in 20.3%; multiple masses were present in only 1.1%. An apical mass was found in 2.6% of the patients, and in no patient was hypertranslucency seen. The various extrapulmonary intrathoracic manifestations were present in 11%, with mediastinal widening and pleural effusion being more common. Table 104-1 lists the usual radiographic findings. Amemiya and Oho (1982) (Table 104-2) and Swett and associates (1982) have noted that a peripheral nodular mass is now the most common radiographic presentation of bronchial carcinoma. Many, if not most of these, are 3 cm or smaller and fall into the category of solitary pulmonary nodule.
Uncommon Radiographic Manifestations
Woodring (1990a) reviewed the uncommon radiologic presentations of lung cancer. The more important of these
P.1521
were (a) spontaneous regression after initial recognition (extremely rare) or an observed decrease in size of the tumor mass, (b) calcification in psammoma bodies in the tumor or dystrophic calcification in areas of tumor infection or necrosis, and (c) a tumor presenting as a thin-walled cavity (Fig. 104-1). Woodring and Fried (1983) reported a 6% incidence of primary carcinoma in a series of solitary cavities with a wall thickness of 4 mm or less. Other unusual findings were the radiologic presence of satellite nodules or the occurrence of two separate synchronous primary tumors, one being a possible metastasis of the other, in 1% of the initial radiographs. Other rare findings were the presence of a meniscus or crescent sign within the tumor mass and the occurrence of two anatomically separated areas of atelectasis or pulmonary consolidation, which is by far more typical of an inflammatory disease process. Of passing interest is the occurrence of a spontaneous pneumothorax as the initial radiographic feature of a lung cancer. Steinhauslin and Cuttat (1985) reported this occurrence in 0.46% of lung cancer cases. Although dystrophic calcification is considered uncommon, Mahoney and co-workers (1992) identified it on CT scans in 13% of lesions that were subsequently proved to be cancer.
Table 104-1. Chest Radiographic Presentations of Lung Cancer | |
|---|---|
|
Table 104-2. Radiographic Findings in 200 Patientswith Lung Cancer | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Influence of Cell Type
Certain radiographic patterns are characteristic of the various cell types. From the studies of Byrd and associates (1969) and that of Theros (1977), as well as the review by Sider (1990), several generalities can be made.
Squamous cell carcinoma most often presents a picture of obstructive pneumonitis, pulmonary collapse, or parenchymal consolidation because 65% of these tumors occur in a central location in the bronchial tree. A hilar abnormality is often present. Approximately one-third of squamous cell tumors appear as a peripheral mass. Many are small lesions (3 cm or less in diameter), but in previous decades, up to two-thirds of the peripheral squamous cell tumors were larger than 4 cm on initial recognition. Cavitation is more common in peripheral squamous cell carcinomas than in other lung carcinomas, occurring in approximately 10% to 20% of patients with these peripheral tumors. Cavitation in this instance results from necrosis in the tumor mass. Cavitation and abscess formation also may occur distal to a bronchus obstructed by tumor. When both types of cavities
P.1522
are combined, they constitute approximately 50% of all lung abscesses seen in patients older than 50. In 3% to 4% of patients with squamous cell tumors, the radiograph of the chest may show no abnormality, the tumor being located in a main-stem bronchus and producing no changes in the parenchyma distal to it.
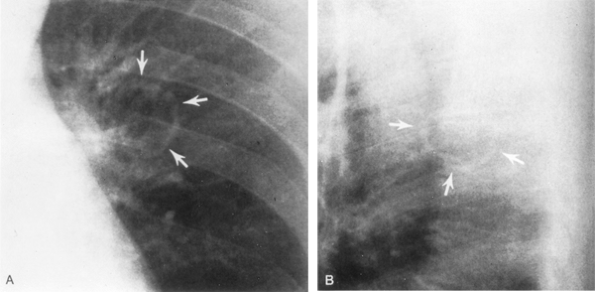 |
Fig. 104-1. Posteroanterior (A) and lateral (B) radiographs reveal a smooth, thin-walled carcinomatous cavity in a 43-year-old man with hypertrophic pulmonary osteoarthropathy with positive sputum cytology. Maximum wall thickness was 2 mm. From Woodring JH: Unusual radiographic manifestations of lung cancer. Radiol Clin North Am 28:599, 1990a. With permission. |
Adenocarcinomas are most often peripheral masses. As with the peripheral squamous cell tumors, a great percentage of these lesions are initially recognized when they are 3 cm or less in diameter. Many still are not discovered, however, until the tumor is larger than 4 cm. Cavitation is rare. These peripheral lesions represent 60% to 70% of all the primary adenocarcinomas of the lung. The lesion most often has a nodular or ill-defined border. A corona radiata, as it was termed by Heitzman and colleagues (1982), or sunburst appearance of the border, is often readily appreciated on the standard radiographs and may represent local lymphatic spread. Eccentric calcification may be present within the mass. A hilar abnormality or an obstructive parenchymal lesion is less common than the peripheral mass, but appears to be increasing in frequency. Woodring and Stelling (1983) reported that 28% of adenocarcinomas presented solely as a central mass, and 51% of all patients had some degree of central involvement: hilar or mediastinal node adenopathy.
A subtype of adenocarcinoma, bronchioloalveolar carcinoma, may present as a solitary peripheral mass. It represents approximately 35% of all small peripheral masses, a localized area of pneumonic infiltrate that may extend to involve an entire lobe or even more of the lung, or less commonly as multiple unilateral or bilateral coalescent multinodular infiltrates. In a study of 136 patients with this tumor, Hill (1984) reported that the presenting radiographic findings were a solitary nodule less than 4 cm in diameter in 23%, a mass in 20%, a localized area of consolidation (less than one lobe) in 7%, diffuse coalescent consolidation in 23%, and diffuse nodules in 27%. Unlike tumors of other cell types, the presence of air bronchograms in the areas of involvement is common. Less frequently, an interstitial pattern of involvement of the lung is noted, which Berkman (1977) reported to be the result of secondary lymphatic spread of the tumor. According to Sider (1990), pleural effusion may be present in 8% to 10% of patients and, rarely, a pneumothorax can occur.
Large cell undifferentiated carcinomas are most likely to be peripheral lesions (approximately 60%); two thirds of these are larger than 4 cm. Cavitation occurs in approximately 6% of these peripheral large cell tumors. A hilar abnormality and parenchymal changes are each present in association with approximately one third of these tumors. Ten percent of patients with this type of tumor have mediastinal widening as one of the presenting features.
Small cell undifferentiated tumors appear primarily as hilar abnormalities (78%), which usually represent hilar or mediastinal lymph node metastases. These tumors are associated with mediastinal widening in more than 13% of patients, and in some of the patients with this finding, a parenchymal abnormality cannot be recognized. Parenchymal obstructive lesions occur as the result of extrinsic compression of a bronchus in slightly less than 40% of patients. A peripheral mass may occur in somewhat less than one third of the patients. Carter and Eggleston (1980) reported that a peripheral mass occurred in only 14%. Three-fourths of the peripheral small cell tumors may be smaller than 4 cm in diameter. This cell type represents the least common cause of a peripheral solitary pulmonary nodule of all cell types of bronchial carcinoma, and the reported incidence, as reviewed by Kreisman and associates (1992), varies between 4% and 12%.
Despite these generalizations, no radiographic feature is diagnostic of the cell type or even of the presence of carcinoma. Nonetheless, these features should alert one's suspicion as to the possibility of a malignancy so that proper cytologic or histologic material can be obtained to make a diagnosis.
Special Radiographic Studies
In addition to the routine posteroanterior and lateral radiographs, other radiographs of the chest can be obtained with the patient in the right or left anterior oblique, lordotic, or other special positions to delineate further any suspected lesion. Contrast-enhanced study of the esophagus may be helpful at times. Other radiographic studies (e.g., laminography, 55-degree oblique tomography, bronchography, angiography, azygography, and pneumomediastinography) can be obtained but are rarely indicated in the evaluation of lung cancer patients at present. These techniques are noted mainly for historic interest.
Computed Tomography of Lung Cancer
CT has become a routine examination for patients suspected of having lung cancer. Like any other noninvasive examination, however, it cannot distinguish inflammatory tissue from cancer tissue. In patients with central lesions, CT is useful for demonstrating enlarged hilar lymph nodes, but this finding in itself is not of great import because it does not disqualify the patient for surgical exploration. The demonstration of enlarged nodes may suggest, however, the need for a pneumonectomy as the surgical procedure. The caliber, distortion, and thickening of the wall of the proximal bronchi also can be discerned. Involvement of the pulmonary vessels, even with contrast enhancement, is difficult to determine at times. In patients with peripheral lesions, the margins, presence of calcification, and cavitation can be elicited. Zwirewich and associates (1991) evaluated high-resolution CT in distinguishing malignant from benign peripheral nodules. They found that spiculation of the borders, pleural tags, and bubblelike areas of low attenuation
P.1523
were present in 87%, 58%, and 25% of malignant lesions, respectively. In benign lesions, these percentages were 55%, 27%, and 9%, respectively. Thus, none of these criteria can be used as an unequivocal sign of malignancy.
Im and associates (1990) described the CT angiogram sign in patients with bronchioloalveolar carcinoma. With contrast enhancement in patients with segmented or lobar consolidation, enhanced branching pulmonary vessels are seen in areas of homogeneous low attenuation when the consolidation is related to the presence of this tumor. Air bronchograms may also be seen.
With high-resolution CT and spiral CT, numerous investigators, including Jang and associates (1996), have described a ground-glass appearance as typical of small peripheral nodules that are early bronchioloalveolar carcinomas. This haziness is thought to be due to the lepidic growth pattern of these tumors. In addition, bubblelike hyperlucency or pseudocavitation (representing patent airways) can be present. With growth, areas of consolidation with high attenuation values may be seen. These areas represent so-called scar or sclerotic areas within the tumor. According to Noguchi (1995), Eto (1996), and Yamashiro (1995) and their colleagues, an increasing size of the scar within the tumor portends a worse prognosis.
Calcification occurs as often as 13% of the time in lung cancer, as shown by Mahoney and co-workers (1990) in a CT study of lesions subsequently proved to be cancer. This calcification is most often eccentric. Concentric laminated calcification centered on the middle of a mass, however, is virtually diagnostic of a tuberculous or fungal granuloma, whereas diffuse calcification throughout the mass ( popcorn calcifications) is the hallmark of a benign hamartoma.
However, the most important aspect of the radiologic diagnosis of lung cancer is that radiologic studies alone cannot make the diagnosis. Definitive diagnosis can be made only by cytology or histology. Therefore, the chest radiograph and CT scan should be used primarily to raise the level of suspicion and to direct diagnostic procedures, such as bronchoscopy, for central lesions or removal for peripheral lesions. It has been our practice not to do needle biopsies on new peripheral lesions in otherwise operable candidates without an alternative reason present for having a lung mass, thus saving the patient an operation. This is based on a personal review by one of us (RHF in 1989, unpublished) of 2 years of fine-needle aspirations in this population. In it, only 3% of patients had a definitive benign diagnosis. It has far more often been the case that patients, falsely believing that a negative diagnosis meant benignity, were lost to follow-up until such time as they reappeared, only to find themselves inoperable.
Criticism is also warranted for the practice of following new lesions radiographically for any significant length of time to avoid operation. This is particularly unwarranted in face of the development of thoracoscopic removal of these lesions, which makes definitive diagnosis possible. Indeed, if one believes that the degree of lymphatic invasion can be correlated with curability, allowing lung lesions to grow radiographically in an attempt to show them benign may be ill advised. Ichinose and co-workers (1994) showed that local lymphatic vessel invasion was 25% for tumors less than 1 cm in diameter but rose to 40% in tumors 1.1 to 2.0 cm in diameter. Although the hypothesis that tumors less than 1 cm in diameter are more curable than those 1 to 2 cm in diameter will never be subjected to a prospective randomized trial, it seems far better to remove a lesion and find it benign than to leave in a lesion and find it malignant.
STAGING OF LUNG CANCER BY RADIOGRAPHIC TECHNIQUES
Accurate staging of lung cancer is essential to proper treatment. Just as chest radiographs and CT imaging should be used only as guides to obtaining a definitive diagnosis of lung cancer, so, too, should they be used only as guides to staging. With the possible exception of the size criteria for the T classification of a cancer, neither modality is capable of determining reliable TNM classification and therefore staging. However, nuclear medicine positron emission tomography (PET) scanning is playing an increasingly important role in evaluation of potentially resectable lung cancer.
The TNM staging criteria, as updated by Mountain (1997), are now universally accepted by those treating patients with lung cancer (see Chapter 105). Certain specific radiologic findings should lead the clinician to the appropriate steps to maximize the accuracy of clinical TNM staging.
Primary Tumor (T)
Lesion size can be reliably measured on chest radiograph and CT; therefore, the differentiation between T1 lesions (less than or equal to 3 cm) and T2 lesions (greater than 3 cm) is most often straightforward (Fig. 104-2). Marom and co-workers (2002) found 95% of T1 lung cancers in 185 patients positive by PET scans. These lesions ranged from 0.5 to 3 cm in size (mean, 2 cm).
Invasion of the visceral pleura, another criterion for upstaging to T2, is more difficult to recognize radiographically but is rarely a criterion of operability. The same is true for associated atelectasis or obstructive pneumonia that does not involve the entire lung. This level of clinical staging, although not necessary in the decision to resect a given tumor, may be important for clinical trials of stage IB (T2N0) tumors.
Non small cell cancers that contiguously invade the chest wall (T3) are potentially resectable. In a CT study by H. S. Glazer and co-workers (1985) that used various combinations of criteria for assessing parietal pleural and chest wall invasion, the sensitivity for chest wall invasion was 87% and the specificity was 59%. It is interesting that although CT had limited predictive value, clinical symptoms
P.1524
of focal pain yielded a sensitivity of 67% and a specificity of 94% for parietal pleural or chest wall invasion. Webb and associates (1991) in the Radiology Diagnostic Oncology Group (RDOG) study compared CT with magnetic resonance (MR) imaging for assessing chest wall invasion and found no statistically significant differences between the two modalities. Because surgical resection is the treatment of choice for tumors that locally abut and for those that actually invade the chest wall, making the radiologic distinction between the two probably is not critical for proper treatment.
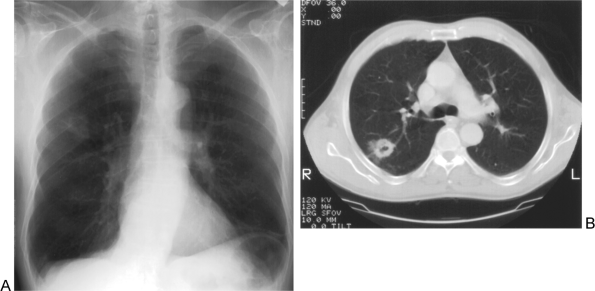 |
Fig. 104-2. T2 disease. A. Chest radiograph demonstrates a 4-cm cavitary spiculated right upper lobe mass. B. Corresponding computed tomographic image in the center of this non small cell lung cancer (NSCLC) also shows the irregular, thick-walled cavitary lesion. |
Perhaps the most important distinction in terms of the T status of a tumor is between T3 (Fig. 104-3) and T4 (Fig. 104-4) because the former (stage IIB) could potentially be resected, whereas the latter (stage IIIB) usually should not be resected. In the absence of gross tumor invasion of the mediastinum or chest wall, these lesions present the greatest challenge because the distinction between tumor abutment and invasion of vital structures is often subtle. Several investigators, including Herman (1994), Glazer (1989), and Webb (1991) and their associates, have indicated that CT is generally insensitive for detecting mediastinal invasion. However, after assessing a number of CT criteria in a limited number of patients, Herman and colleagues (1994) suggested that a mediastinal structure with more than one-half its circumference in contact with the tumor was likely to be invaded. Webb and associates' (1991) study did show, however, that MR imaging was significantly more accurate than CT for assessing mediastinal invasion.
Patients with more than one lesion in a given lobe are now more commonly identified preoperatively with the use of spiral CT scans. Under the new staging criteria, if the lobe contains two or more metastatic sites of the same tumor, the lesion is categorized as a T4 lesion. Confirmation of the histology of the second lesion is important to differentiate a T4 tumor from concurrent benign disease or a second early-stage lung cancer. Lesions in multiple lobes may represent M1 disease (see discussion to follow).
If the chest radiograph or CT demonstrates a pleural effusion in the presence of a suspected or known lung cancer, the possibility exists for the effusion to be malignant and the tumor to be stage T4. It is important to confirm malignant cells cytologically in the fluid before concluding inoperability because benign effusions can accompany malignant tumors. If questions still exist about the cause of a given effusion, a prethoracotomy thoracoscopic evaluation is probably warranted. CT evidence of a pericardial effusion could be indicative of a malignant pericardial effusion (T4) and should be further investigated.
Finally, chest radiographic evidence of an elevated diaphragm can be an indication of phrenic nerve involvement (T4). Many thoracic surgeons, however, believe that isolated invasion of the phrenic nerve in the absence of mediastinal lymph node involvement does not necessarily preclude curative resection.
Lymph Nodes (N)
The American Thoracic Society/European Respiratory Society (ATS/ERS) (1997) recommendations for assessment of patients with suspected lung cancer include the use of chest CT for evaluation of mediastinal lymph node enlargement. In patients with possible or proved lung cancer,
P.1525
the generally accepted threshold for suspecting metastatic nodal involvement is a lymph node 10 mm or greater. Sensitivity and specificity vary depending on whether one chooses to apply that measurement to the long or short axis of a lymph node. In addition, G. M. Glazer and co-workers (1985) showed that variations in size threshold may be applicable based on nodal locations. In the study by G. M. Glazer and co-workers (1985) of CT examinations of 100 patients (excluding those with conditions that may enlarge mediastinal lymph nodes), the short-axis threshold for normal lymph nodes varied from 7 to 11 mm. Many clinical studies have simplified these variable size criteria by using a 10-mm lymph node short-axis threshold. In another study by Glazer and colleagues (1984) using receiver operating characteristic analysis, a short-axis mediastinal lymph node dimension of 10 mm was most efficacious for recognition of nodal metastases in non small cell lung cancers (NSCLCs). It should also be noted that enlarged lymph nodes are by no means necessarily involved by metastatic disease. In the RDOG study findings reported by McCloud and co-workers (1992), 37% of the 19 lymph nodes that measured 2 to 4 cm in their short-axis dimension were benign hyperplastic lymph nodes.
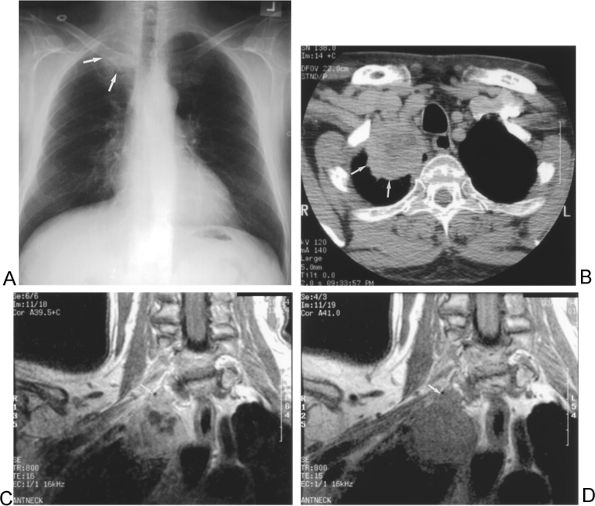 |
Fig. 104-3. T3 disease: Chest wall invasion. A. Chest radiograph demonstrates a right apical mass (arrows). B. Axial computed tomographic image identifies the lesion (arrows), but details regarding possible chest wall involvement are limited. C. Pre- and (D) postgadolinium contrast-enhanced coronal magnetic resonance images show lobular protrusion of the mass into the apical right chest wall (arrows). |
CT surgical correlative studies for staging mediastinal nodal metastases by McCloud (1992) and Seely (1993) and their associates have highlighted some limitations and the relatively low levels of sensitivity and specificity for recognition of metastatic mediastinal lymph nodes by CT. In the study by McCloud and co-workers (1992), intravenous iodinated contrast-enhanced CT was performed with 10-mm
P.1526
slice thickness; a lymph node short-axis size threshold of 10 mm was used. The CT sensitivity for metastases in that study on a per-patient basis was 64%, and the specificity was 62%. Seely and associates (1993) used a similar CT protocol and the same size criterion for short-axis lymph node measurements in a group of 104 patients with T1 lesions; the CT sensitivity was 59%, and specificity was 91%. As we noted previously, when criteria were switched from short- to long-axis lymph node measurement in that same study, CT sensitivity increased (77%) and specificity correspondingly decreased (73%). Previous authors, including G. M. Glazer and colleagues (1984), have reported CT sensitivities for lymph node involvement as high as 93% to 95% using a 1-cm short-axis dimension. Although it is generally accepted that there are limitations in the sensitivity and specificity of CT for detecting mediastinal lymph node metastases, this modality does provide a guide to the appropriate invasive method for definitive diagnostic procedures for the staging of NSCLC.
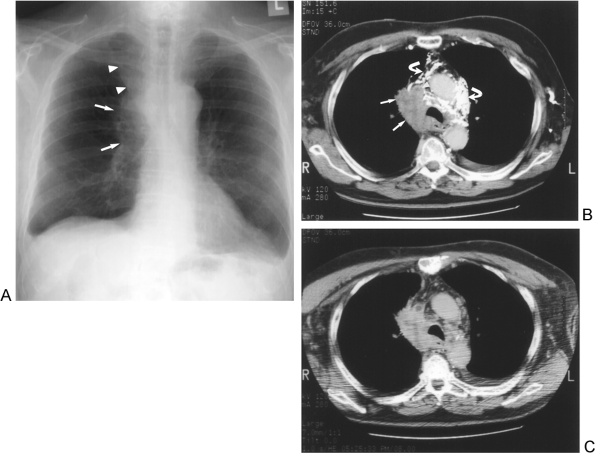 |
Fig. 104-4. T4 disease: Mediastinal invasion. A. Chest radiograph shows a right hilar (arrows) and right upper mediastinal mass (arrowheads). There are also small bilateral pleural effusions in this patient with NSCLC. B. Initial contrast-enhanced CT shows the right mediastinal tumor mass (arrows) with obliteration of the superior vena cava, focal luminal thrombus (arrowhead), and numerous iodinated contrast-filled collateral veins (curved arrows) due to the superior vena caval obstruction. C. A 6-minute-delay post-contrast-enhanced CT scan illustrates clearing of the dense intravenous contrast from the collateral mediastinal vessels in this patient with mediastinal invasion. |
In the RDOG study results reported by McCloud and co-workers (1992) of CT and MR imaging for staging of NSCLC, the sensitivity and specificity of CT for distinguishing N0 or N1 disease (Fig. 104-5) from N2 or N3 mediastinal nodal disease (Figs. 104-6, 104-7, 104-8) were 52% and 69%, respectively. In the same study, there was no statistically significant difference in accuracy between CT and conventional MR imaging for recognizing mediastinal lymph node metastases. As suggested by Jelinek and associates (1990), there may be a role for MR imaging in the global assessment at initial staging of SCLC with the Veterans Administration Lung Study Group simplified dichotomous staging system for both recognition of disease and efficient distinction of localized from extensive disease.
Additional sites of potential metastatic lymph node involvement by NSCLC that are not usually addressed in the CT literature are the supraclavicular and upper abdominal lymph nodes. Once they are palpable, supraclavicular lymph nodes are usually involved by metastatic disease in patients with lung cancer. A cytologic diagnosis can be obtained with fine-needle aspiration biopsy. Nonpalpable supraclavicular lymph nodes have a variable prevalence
P.1527
reported in the literature. Brantigan and associates (1973), for example, reported that in their series of 341 consecutive patients with lung cancer, 286 had nonpalpable scalene lymph nodes. Approximately 24% of these 286 patients with pulmonary tumors greater than 3 cm and no palpable scalene lymph nodes had positive scalene lymph node surgical biopsies. The validity of this study, however, must be questioned because this represents a higher incidence of involvement of these nodes than is normally found in lung cancer patients at autopsy. In a study of 47 lung cancer patients with nonpalpable scalene lymph nodes, Shields and Shocket (1958) found an incidence of only 6.3%. In another report by Shields and colleagues (1958), one or more of these clinical findings appeared in all patients with positive nodes: (a) superior mediastinal lymph node enlargement, (b) a large hilar mass, and (c) a diagnosis of small cell carcinoma. More recently, Lee and Ginsberg (1996) identified positive involvement of the
P.1528
P.1529
scalene lymph nodes in 15.4% of patients with centrally located, nonsquamous, non small cell carcinoma with proved N2 disease and in 63% with N3 disease a highly select group of patients. Directing further attention to this area at the time of the initial chest CT evaluation will likely aid in the initial staging of patients with central lesions.
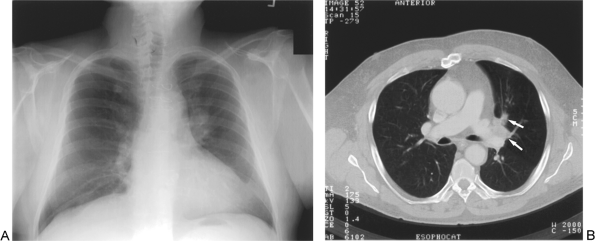 |
Fig. 104-5. N1 disease: Ipsilateral hilar involvement by the left upper lobe tumor. A. Chest radiograph demonstrates a left hilar mass. B. Computed tomographic scan of left hilar involvement by tumor (arrows) also revealed left upper lobe air trapping due to bronchial luminal narrowing. |
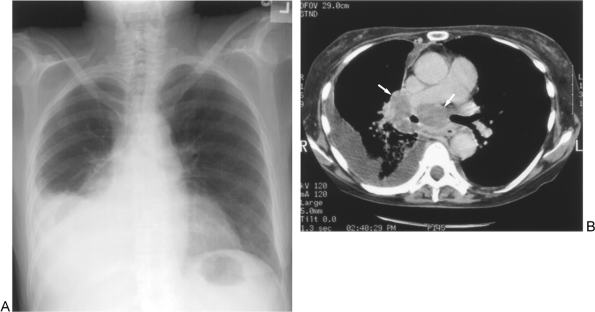 |
Fig. 104-6. N2 disease: Ipsilateral hilar and subcarinal nodal disease. A. Chest radiograph reveals a right hilar mass and large right pleural effusion. B. Large right hilar and subcarinal lymph nodes (arrows) are present in this patient who also has a malignant right pleural effusion (T4). |
 |
Fig. 104-7. N3 disease: Contralateral mediastinal lymph nodes. A. Chest radiograph of a left hilar mass (arrows) and left mediastinal mass (arrowheads) displaying tracheal deviation to the right in this patient with NSCLC. B. Computed tomographic image in the upper chest demonstrates anterior mediastinal mass (arrows) by the tumor (arrowhead). |
 |
Fig. 104-8. N3 disease: Supraclavicular lymph node. A. Chest radiograph of an irregular left hilar mass. B. Computed tomography showed an enlarged left supraclavicular lymph node (arrow) that was not palpable in this patient. C. Ultrasound was used to guide the fine-needle aspiration biopsy of the nonpalpable left supraclavicular lymph node (arrow). The aspiration yielded the diagnosis of NSCLC. |
Ultrasound and ultrasound-guided fine-needle aspiration biopsies may ultimately prove to have a role in assessment of patients with nonpalpable supraclavicular nodes. In a series of 79 patients reported by Monso and co-workers (1992), ultrasound detected an additional 16.7% of patients with enlarged (greater than 1 cm) nonpalpable supraclavicular lymph nodes when compared with physical examination findings. Using a combination of ultrasound and ultrasound-guided fine-needle biopsy techniques, Chang and colleagues (1992) found that 6 of 51 patients (12%) had metastatic nonpalpable supraclavicular lymph nodes from NSCLC. We (PJF, RHF) and our associates (2002) found supraclavicular lymph node metastases in 31% of 55 patients presenting with lung cancer using CT and ultrasound with guided needle biopsies.
 |
Fig. 104-9. N3 disease: Supraclavicular lymph node. A. Computed tomography of the primary left lower lobe lung cancer (arrow). B. Coronal plane positron emission tomographic scan image of this patient with left lung tumor shows abnormal left supraclavicular activity (arrow). C. Computed tomography shows the left supraclavicular lymph node (arrow) that was overlooked at initial interpretation. D. Ultrasound-guided needle sampling (arrow on needle tip) yielded metastatic adenocarcinoma in the supraclavicular lymph node. Arrowhead is on common carotid artery. |
A meta-analysis of the diagnostic performance of PET and CT for mediastinal metastases by Dwamena and associates (1999) yielded mean sensitivities and specificities of 79% and 91% for PET and 60% and 77% for CT, respectively. Sampling of PET-positive sites by radiologic guided procedures may also assist with recognition of patients with occult N3 disease (Fig. 104-9).
Because the status of mediastinal or supraclavicular lymph nodes is so critical to proper staging and treatment, it is sometimes more expedient to perform a biopsy of them even before the main lesion itself: A positive finding not only provides a diagnosis but also stages the patient at the same time.
P.1530
Metastatic Disease (M)
CT is the mainstay for initially directing the workup for M1 metastatic disease (Fig. 104-10). A tumor with multiple foci of lung cancer involving more than one lobe is now staged as metastatic disease (M1) because of Mountain's 1997 revision of the staging system and therefore is believed to be inoperable. There is, however, some question about the validity of this point of view. The histology of a second lung lesion in another lobe must, of course, be definitely determined, as mentioned in the previous discussion of multiple lesions in the same lobe (T4). Again, because of the high frequency of incidental benign nodules on chest CT, the challenges are considerable in this regard. In a study by Kim and co-workers (2002), 62 of 141 patients undergoing lung cancer resection had small (less than or equal to 10 mm) nodules in the nonprimary lobes on preoperative CT scans. Of 138 nodules, only 6 were found to be malignant.
With high-speed intravenous contrast-enhanced spiral CT, it is now also possible to scan the thorax and upper abdomen, including the liver and adrenal glands. With this capability has come the identification of a large number of lesions in the adrenal glands. The differentiation of benign and malignant adrenal lesions is critical to proper staging. The relative proportion of benign and metastatic adrenal lesion in patients with lung cancer varies with the patient series. In a study by Oliver and associates (1984) of patients with NSCLC with isolated adrenal lesions, fewer than 50% of these lesions were actually metastases.
For purposes of our discussion, the more proper distinction is between adrenal adenoma and nonadenoma (i.e., the nonadenoma is potentially, but not necessarily, a metastatic lesion). Noninvasive assessment of the adrenal glands appears to be able to accurately characterize many of these lesions (Fig. 104-11). Dunnick and colleagues (1996) have outlined noninvasive adrenal imaging options that include non-contrast-enhanced and contrast-enhanced CT, chemical shift MR imaging, and nuclear medicine studies. Currently, the most widely available options are CT and chemical shift MR imaging.
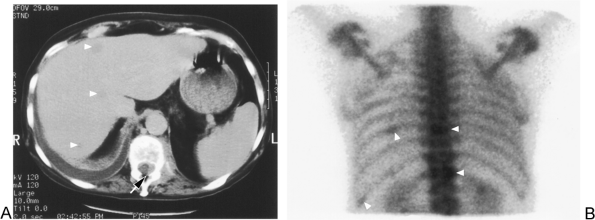 |
Fig. 104-10. M1 disease: Liver and bone metastasis (same patient as in Fig. 104-6). A. Computed tomographic image in the upper liver and lower thoracic spine shows at least three liver metastases (arrowheads) and a left T12 bony metastasis with extraosseous tumor invading the spinal canal (arrow). B. Nuclear medicine bone scan (posterior image of chest) shows abnormal activity in T12, T9, and in two left ribs (arrowheads) consistent with metastasis. |
An extensive review by Boland and colleagues (1998) pooled data from 10 previous studies comprising 495 adrenal lesions (272 benign and 223 malignant) studied by non-contrast-enhanced CT for characterization of adrenal lesions. They correlated the use of various threshold CT Hounsfield unit (HU) values of the adrenal lesions and found that a threshold CT number of 0 HU yielded a sensitivity of 41% and a specificity of 100%. When the threshold was raised to 10 HU, the sensitivity increased to 71%, with a specificity of 98%; at 20 HU, the sensitivity was 88% but the specificity dropped to 84%.
For adrenal lesions between 0 and 20 HU, McNicholas (1995) and Mayo-Smith (1995) and their colleagues have reported the use of chemical shift MR imaging. This technique uses a form of MR imaging that enables recognition of most adenomas because of their higher lipid content. Patients with lesions greater than 20 HU on non-contrast-enhanced CT should undergo an invasive diagnostic procedure because chemical shift MR imaging has not differentiated lesions in this setting. Some concerns regarding chemical shift MR imaging have been expressed by Reinig (1992) because some unknown percentage of nonadenomatous lesions could contain lipid, but this is likely to be the case in only a small minority of instances. Caoili and colleagues (2002) recently
P.1531
summarized their experience with combined unenhanced and delayed enhanced CT to differentiate adenomas from nonadenomatous adrenal masses. In their study, this technique achieved a sensitivity of 98% and specificity of 92% for 166 lesions (127 adenomas and 39 nonadenomatous lesions).
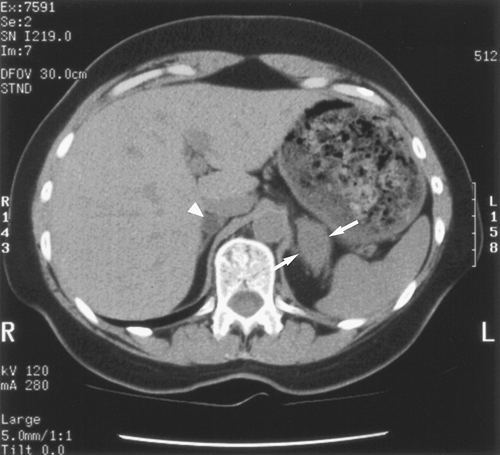 |
Fig. 104-11. M1 disease: Synchronous adrenal metastasis and adrenal adenoma. Non-contrast-enhanced CT image shows a biopsy-proved large left adrenal metastasis from NSCLC (arrows) with a CT density of 23 Hounsfield units (HU). There is also a typical right adrenal adenoma (arrowhead) with a CT density measurement of 0 HU. |
Identification of distant metastasis is a critical part of preoperative evaluation. Sider and Horejs (1988) found that metastasis can be present in 25% of patients, even with preoperative CT scans showing only a solitary mass and no recognizable hilar or mediastinal lymph nodes greater than 1 cm. Routine scanning of bone, brain, and liver in the absence of symptoms and abnormal blood laboratory values, however, has not been found worthwhile. Silvestri and associates (1995), in a multivariant analysis, determined criteria for scanning for metastatic disease. They identified specific parts of the patient history and specific laboratory tests that, when normal, made the yield from a radiologic search for metastasis very low. That has also been our experience, but one must be careful to cover all the points detailed in the study.
The role of PET scanning in preoperative staging of metastatic disease in NSCLC continues to evolve. Weder and associates (1998) used PET as a guide to exclude borderline surgical candidates and those with suspected advanced disease (e.g., weight loss, locally advanced disease, solitary brain metastases). In a review of the literature on the use of PET in lung cancer patients, Lowe and Naunheim (1998) found that more than 10% of patients had unsuspected metastases. Erasmus and colleagues (1997) also suggested a role for PET in the characterization of adrenal lesions in these patients.
FUTURE DIRECTIONS IN IMAGING PATIENTS WITH LUNG CANCER
Endeavors to improve imaging of patients with lung cancer should include efforts to optimize both observer performance and performance of the primary imaging modalities currently in use (i.e., chest radiography and chest CT examinations). Woodring (1990b) indicated that there can be significant limitations and variability in radiologic interpretations. This can occur even among expert readers. A variety of methods to improve observer performance have been proposed by Metz and Shen (1992) and Hessel (1978), Hillman (1976, 1977), Curtis (1988), and Seltzer (1997) and their co-workers with variable degrees of success. Uniform adherence to the American College of Radiology (1995) and ATS/ERS (1997) guidelines for performance of chest CT in this setting should be a goal. In a survey by Chen and colleagues (1997), 13% of U.S. hospitals with at least 300 beds did not routinely carry out CT examinations through the adrenal glands. Improved CT protocols, including thinner slices and uniform delivery of intravenous iodinated contrast via a power injector, may also improve preoperative staging accuracy. With the arrival of helical (spiral) CT technology, inclusion of the supraclavicular lymph node region and liver in standard staging CT examinations may ultimately prove to be efficacious.
As with CT, MR technology continues to improve. Use of intravenous contrast-enhanced MR sequences and the development of new MR pulse sequences may provide a growing role for MR imaging in patients with suspected lung cancer. Furthermore, MR lymph node contrast imaging agents are under development and testing and may affect the detection of nodal metastases. One such agent is a form of superparamagnetic iron oxide that is phagocytized by the reticuloendothelial system in the lymph nodes, bone marrow, liver, and spleen, as described by Anzai and colleagues (1994). Normal lymph nodes change on certain imaging sequences performed before and after administration of this contrast agent, but metastatic nodes do not. Van den Brekel and colleagues (1994) and Kernstine and associates (1999) were able to recognize some tumor-bearing (vs. benign) lymph nodes by MR imaging using this technique with lymph node contrast agents.
Advances in imaging are paralleled by advances in image-guided needle biopsy techniques. All major radiologic modalities, including fluoroscopy, CT, ultrasound, and MR imaging as well as nuclear medicine, have the potential to serve as guidance for pathologic confirmation of disease in and outside the chest.
When feasible, real-time ultrasound guidance for biopsy has similar advantages to CT fluoroscopy and is more convenient. In addition, ultrasound guidance for biopsy allows
P.1532
greater flexibility in angled approaches (e.g., at the thoracic inlet). Furthermore, Rubens and associates (1997a, 1997b) emphasized that many lesions that were traditionally sampled using fluoroscopy or CT guidance (e.g., mediastinal and skeletal lesions) are sometimes readily accessible to ultrasound-guided needle biopsy.
CONCLUSIONS
A patient's presenting symptoms and signs and chest radiographic findings, or both, prompt the initial evaluation of patients with suspected lung cancer. The chest radiograph is usually followed by chest CT, which aids in characterization of abnormalities noted on the radiographs (e.g., lung nodule assessment). CT also defines the extent of possible disease and directs invasive procedures to obtain pathologic confirmation of cell type and maximal extent of disease. Further evaluation with MR imaging, nuclear medicine bone scan, or other imaging modalities is currently believed to be most optimally guided by the results of the history, physical, and clinical laboratory evaluations, as per the ATS/ERS guidelines.
In patients for whom a diagnosis has been established, chest CT is an adjunct to directing the final determination of the stage of disease at presentation. CT has had its greatest impact in patient management for those whose history, physical, and laboratory evaluations suggest that they are potential surgical candidates. Likewise, PET scanning appears to be playing an increasingly important role in the staging of possible surgical candidates. When potential sites of disease that would contraindicate surgery are identified on CT or PET scans, a minimally invasive radiology-guided biopsy can frequently be performed to confirm a diagnosis. If that is not the case, initial surgical staging procedures can usually be directed to such sites of concern.
Continuing challenges in the radiologic diagnosis and staging of lung cancer include earlier recognition of disease and minimization of errors in perception and interpretation of radiologic studies. Another challenge is to standardize radiologic techniques and noninvasive characterizations of incidental benign lesions that complicate preoperative staging studies (e.g., benign pulmonary nodules, benign enlarged lymph nodes, and benign adrenal lesions).
Ongoing and future directions of study include radiologic screening of high-risk patients and optimization of observer performance for interpretation of examinations. Future and ongoing research in expanding the use of CT and PET scanning for patients with possible and known lung cancer will further enhance the accuracy of patient staging evaluations.
REFERENCES
Amemiya R, Oho K: X-ray diagnosis of lung cancer. In Hayata Y (ed): Lung Cancer Diagnosis. Tokyo: Igaku-Shoin, 1982, p. 4.
American College of Radiology Task Force on Appropriateness Criteria: Staging of bronchogenic carcinoma, non-small cell lung carcinoma. American College of Radiology Appropriateness Criteria for Imaging and Treatment Decisions, TH2 1, 1995.
American Thoracic Society/European Respiratory Society: Pretreatment evaluation of non-small-cell lung cancer. Am J Respir Crit Care Med 156:320, 1997.
Anzai Y, et al: Initial clinical experience with dextran-coated superparamagnetic iron oxide for detection of lymph node metastases in patients with head and neck cancer. Radiology 192:709, 1994.
Berkman YM: The many faces of bronchiolo-alveolar carcinoma. Semin Roentgenol 12:207, 1977.
Boland GWL, et al: Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 171:201, 1998.
Brantigan JW, Brantigan CO, Brantigan O: Biopsy of nonpalpable scalene lymph nodes in carcinoma of the lung. Am Rev Respir Dis 107:962, 1973.
Byrd RB, et al: Radiographic abnormalities in carcinoma of the lung as related to histological cell type. Thorax 24:573, 1969.
Caoili EM, et al: Adrenal masses: characterization with combined unenhanced and delayed enhanced CT. Radiology 222:629, 2002.
Carter D, Eggleston JC: Tumors of the lower respiratory tract. In Atlas of Tumor Pathology. 2nd Series. Fasc 17. Washington, DC: Armed Forces Institute of Pathology, 1980, pp. 113 160.
Chang D-B, et al: Ultrasonography and ultrasonographically guided fine-needle aspiration biopsy of impalpable cervical lymph nodes in patients with non-small cell lung cancer. Cancer 70:1111, 1992.
Chen MYM, et al: Bronchogenic carcinoma: a survey of CT protocols for staging disease. Acad Radiol 4:687, 1997.
Curtis PB, Ferrell WR, Hillman BJ: Improved imaging diagnosis by sequentially combined confidence judgments. Invest Radiol 23:342, 1988.
Dunnick NR, Korobkin M, Francis I: Adrenal radiology: distinguishing benign from malignant adrenal masses. AJR Am J Roentgenol 167:861, 1996.
Dwamena BA, et al: Metastases from non-small cell lung cancer: mediastinal staging in the 1990s meta-analytic comparison of PET and CT. Radiology 213:530, 1999.
Erasmus JJ, et al: Evaluation of adrenal masses in patients with bronchogenic carcinoma using 18F-fluorodeoxy-glucose positron emission tomography. AJR Am J Roentgenol 168:1357, 1997.
Eto T, et al: The changes of the stromal elastotic framework in the growth of peripheral lung adenocarcinomas. Cancer 77:646, 1996.
Fontana RS, et al: Lung cancer screening: the Mayo program. J Occup Med 28:746, 1986.
Fultz PJ, et al: Detection and diagnosis of nonpalpable supraclavicular lymph nodes in lung cancer at CT and US. Radiology 222:245, 2002.
Garland LH: The rate of growth and natural duration of primary bronchial cancer. Am J Roentgenol Radium Ther Nucl Med 96:604, 1966.
Glazer GM, et al: The mediastinum in non-small cell lung cancer: CT-surgical correlation. AJR Am J Roentgenol 142:1101, 1984.
Glazer GM, et al: Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol 144:261, 1985.
Glazer HS, et al: Pleural and chest wall invasion in bronchogenic carcinoma: CT evaluation. Radiology 157:191, 1985.
Glazer HS, et al: Indeterminate mediastinal invasion in bronchogenic carcinoma: CT evaluation. Radiology 173:37, 1989.
Heitzman ER, et al: Pathways of tumor spread through the lung: radiologic correlation with anatomy and pathology. Radiology 144:3, 1982.
Henschke CI, et al: Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 354:99, 1999.
Herman SJ, et al: Mediastinal invasion by bronchogenic carcinoma: CT signs. Radiology 190:841, 1994.
Hessel SJ, Herman PG, Swensson RG: Improving performance by multiple interpretations of chest radiographs: effectiveness and cost. Radiology 127:589, 1978.
Hill CA: Bronchioloalveolar carcinoma: a review. Radiology 150:15, 1984.
Hillman BJ, et al: The value of consultation among radiologists. AJR Am J Roentgenol 127:807, 1976.
Hillman BJ, et al: Improving diagnostic accuracy: a comparison of interactive and Delphi consultations. Invest Radiol 12:112, 1977.
Ichinose Y, et al: The correlation between tumor size and lymphatic vessel invasion in resected peripheral stage I non-small-cell lung cancer. A potential risk of limited resection. J Thorac Cardiovasc Surg 108:684, 1994.
P.1533
Im JG, et al: Lobar bronchioloalveolar carcinoma: angiogram sign on CT scans. Radiology 176:749, 1990.
Jang HJ, et al: Bronchioloalveolar carcinoma: focal areas of ground-glass attenuation at thin-section CT as an early sign. Radiology 199:485, 1996.
Jelinek JS, et al: Small cell lung cancer: staging with MR imaging. Radiology 177:837, 1990.
Kaneko M, et al: Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 201:798, 1996.
Kernstine KH, et al: PET, CT, and MRI with Combidex for mediastinal staging in non-small cell lung carcinoma. Ann Thorac Surg 68:1022, 1999.
Kim YH, et al: Small pulmonary nodules on CT accompanying surgically resectable lung cancer: likelihood of malignancy. J Thorac Imaging 17:40, 2002.
Kreisman H, Wolkove N, Quoix E: Small cell lung cancer presenting as a solitary pulmonary nodule. Chest 101:225, 1992.
Kubik A, et al: Lack of benefit from semi-annual screening for cancer of the lung: follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int J Cancer 45:26, 1990.
Lee JD, Ginsberg RJ: Lung cancer staging: the value of ipsilateral scalene lymph node biopsy performed at mediastinoscopy. Ann Thorac Surg 62:338, 1996.
Lowe VJ, Naunheim KS: Positron emission tomography in lung cancer. Ann Thorac Surg 65:1821, 1998.
Mahoney MC, et al: CT demonstration of calcification in carcinoma of the lung. AJR Am J Roentgenol 154:255, 1990.
Mahoney MC, et al: Computed tomography of the thorax. Chest Surg Clin N Am 2:465, 1992.
Marom EM, et al: T1 lung cancers: sensitivity of diagnosis with fluorodeoxyglucose PET. Radiology 223:2453, 2002.
Mayo-Smith WW, et al: Characterization of adrenal masses (<5 cm) by use of chemical shift MR imaging: observer performance versus quantitative measures. AJR Am J Roentgenol 165:91, 1995.
McCloud TC, et al: Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology 182:319, 1992.
McNicholas MJ, et al: An imaging algorithm for the differential diagnosis of adrenal adenomas and metastases. AJR Am J Roentgenol 165:1453, 1995.
Melamed MR, et al: Screening for early lung cancer: results of the Memorial Sloan Kettering study in New York. Chest 86:44, 1984.
Metz CE, Shen J-H: Gains in accuracy from replicated readings of diagnostic images: prediction and assessment in terms of ROC analysis. Med Decis Making 12:60, 1992.
Monso E, et al: Usefulness of supraclavicular ultrasonography in the staging of lung cancer. Lung 170:243, 1992.
Mountain CF: Revisions in the international system for staging lung cancer. Chest 111:1710, 1997.
Muhm JR, et al: Lung cancer detected during a screening program using four-month chest radiographs. Radiology 148:609, 1983.
Noguchi M, et al: Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 75:2844, 1995.
Oliver TW Jr, et al: Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology 153:217, 1984.
Reinig JW: MR imaging differentiation of adrenal masses: has the time finally come? Radiology 185:339, 1992.
Rigler LG: A roentgen study of the evolution of carcinoma of the lung. J Thorac Cardiovasc Surg 34:283, 1957.
Rigler LG: The earliest roentgenographic signs of carcinoma of the lung. JAMA 195:655, 1966.
Rubens DJ, et al: Effective ultrasonographically guided intervention for diagnosis of musculoskeletal lesions. J Ultrasound Med 16:831, 1997a.
Rubens DJ, et al: Sonographic guidance of mediastinal biopsy: an effective alternative to CT guidance. AJR Am J Roentgenol 169:1605, 1997b.
Salomaa ER, et al: Prognosis of patients with lung cancer found in a single chest radiograph screening. Chest 114:1514, 1998.
Seely JM, et al: T1 lung cancer: prevalence of mediastinal nodal metastases and diagnostic accuracy of CT. Radiology 186:129, 1993.
Seltzer SE, et al: Staging prostate cancer with MR imaging: a combined radiologist-computer system. Radiology 202:219, 1997.
Shields TW, Lees WM, Fox R: The diagnostic value of nonpalpable lymph nodes in chest diseases. Ann Surg 148:184, 1958.
Shields TW, Shocket E: Preoperative evaluation of patients with clinically resectable bronchogenic carcinoma. A role of biopsy of nonpalpable scalene nodes. AMA Arch Surg 76:707, 1958.
Sider L: Radiographic manifestations of primary bronchogenic cancer. Radiol Clin North Am 28:583, 1990.
Sider L, Horejs D: Frequency of extrathoracic metastases from bronchogenic carcinoma in patients with normal-sized hilar and mediastinal lymph nodes on CT. AJR Am J Roentgenol 151:893, 1988.
Silvestri GA, Littenberg B, Colice GL: The clinical evaluation for detecting metastatic lung cancer. A meta-analysis. Am J Respir Crit Care Med 152:225, 1995.
Smith RA, et al: American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin 53:27, 2003.
Steinhauslin CA, Cuttat JF: Spontaneous pneumothorax. A complication of lung cancer? Chest 88:709, 1985.
Strauss GM: The AtBCs of lung cancer screening. Chest 114:1502, 1998.
Swensen SJ, et al: Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 165:508, 2002.
Swett HA, Nagel JS, Sostman HD: Imaging methods in primary lung carcinoma. Clin Chest Med 3:331, 1982.
Theros EG: 1976 Caldwell Lecture: varying manifestation of peripheral pulmonary neoplasms: a radiologic-pathologic correlative study. AJR Am J Roentgenol 128:893, 1977.
Tockman MS: Survival and mortality from lung cancer in a screened population. The Johns Hopkins study. Chest 89:325s, 1986.
Van den Brekel MWM, Castelijns JA, Snow GB: Detection of lymph node metastases in the neck: radiologic criteria. Radiology 192:617, 1994.
Webb WR, et al: CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 178:705, 1991.
Weder W, et al: Detection of extrathoracic metastases by positron emission tomography in lung cancer. Ann Thorac Surg 66:886, 1998.
Weiss W, Bouocot KR, Seidman H: The Philadelphia Pulmonary Neoplasm Research Project. Clin Chest Med 3:243, 1982.
Woodring JH: Unusual radiographic manifestations of lung cancer. Radiol Clin North Am 28:599, 1990a.
Woodring JH: Pitfalls in the radiologic diagnosis of lung cancer. AJR Am J Roentgenol 154:1165, 1990b.
Woodring JH, Fried AM: Significance of wall thickness in solitary cavities of the lung: a follow-up study. AJR Am J Roentgenol 140:473, 1983.
Woodring JH, Stelling CB: Adenocarcinoma of the lung: a tumor with a changing pleomorphic character. AJR Am J Roentgenol 140:657, 1983.
Yamashiro K, et al: Prognostic significance of an interface pattern of central fibrosis and tumor cells in peripheral adenocarcinoma of the lung. Hum Pathol 26:67, 1995.
Zwirewich CV, et al: Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology 179:469, 1991.
EAN: 2147483647
Pages: 203