Chapter 7: Bioelectronics and Biocomputers
|
Satoshi Sasaki, Isao Karube
The past ten years has seen remarkable progress in the development of biotechnology in various fields such as genetic engineering, cell engineering, and protein engineering. As the technology advances, the fields to which such technologies can be applied are becoming wider and borderless. Two fields, electronics and biotechnology, have now been mixed to produce a field called bioelectronics. One of the great successes in this field has been the creation of the biosensor, a device that can perform quantitative analysis on certain materials by mimicking processes found in biological organisms. This concept of mimicking is now thought to be applicable to the field of biocomputers. Present semiconductor chips are now running up against the limitation of how much more integration of semiconducter-based computers can actually be carried out. This has stimulated researchers to look for new ways to integrate circuits in a limited area. Much attention has therefore been paid to how electrons (and information) are transferred in living systems. If we could tailor the system to work down at the molecular level, an ultra-super-scale integrated circuit could be realized. In this chapter, a history of bioelectronics, the principles and examples of biosensors, and the idea and feasibility of a future biocomputer is briefly described.
7.1 Electron Transfer in Living Systems
Many living things carry out respiration to live. From the biochemical point of view, this process is called oxidative phosphorylation, because through this process, molecules called adenosine triphosphate (ATP) are synthesized. ATP has a very high chemical energy and acts as the mechanism of energy storage in most living systems. In reverse, a large amount of free energy is liberated when ATP is hydrolyzed to produce adenosine diphosphate (ADP) and orthophosphate (Pi), or when ATP is hydrolyzed to produce adenosine monophosphate (AMP) and pyrophosphate (PPi) (Streyer 1988).
![]()
ATP is used as the currency of energy in living things, using fuel molecules such as fatty acids and glucose for its synthesis. Energy liberated through the oxidation of these fuels can then be used. In such oxidation, the final electron acceptor is oxygen (O2). O2 cannot accept oxygen directly. Instead, electron carrier molecules such as flavins or pyridine nucleotides transfer electrons from the fuel to O2. This process is performed in a chain located in the inner membrane of mitochondria. Mitochondria are organelles with an oval shape, and are 2 μm in length and 0.5 μm in diameter. Molecules such as nichotinamide adenine dinucleotide (NADH), flavin adenine dinucleotide (FADH2) and cytochrome c (Cyt c) transfer electrons finally to O2(figure 7.1). Enzymes such as NADH-Q reductase, cytochrome reductase, and cytochrome oxidase help them carry electrons from one to the other.
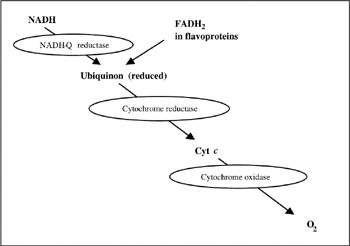
Figure 7.1: Electron transport path in a respiratory chain reaction.
Through this electron transfer, a proton (H+) is also transmitted through the inner mitochondrial membrane (figures 7.2, 7.3). Thus a difference in the pH appears between the matrix (inner side of the membrane) and the intermembrane space (outer side). This proton gradient causes a proton-motive force Δp of ∼0.224 V, as calculated below.
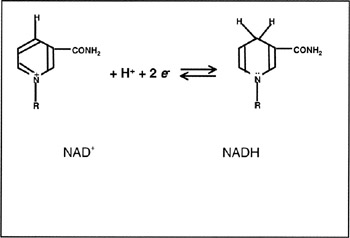
Figure 7.2: Reaction of NAD+ (reactive part) with electrons. R− represents the other nonreactive part of the molecule.
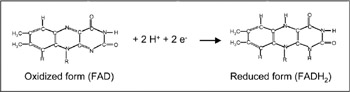
Figure 7.3: Reaction mechanism of the reactive parts of FAD and FADH2.
![]()
In this case, the membrane potential is thought to be 0.14 V, and the pH outside is regarded as being 1.4 units lower than that on the inside. This electric energy potential is used for the synthesis of ATP. The respiratory chain is one example of electron transfer in living systems. What is interesting for us is that the enzyme specifically recognizes the molecule it has to attach to, as well as "knowing" where the electron comes from, and also where it must be donated. This is all done at the molecular level—that is, on the order of several tens of nanometers (ca. the size of the enzyme). Otherwise, oxidation or reduction in a cell would proceed in a small, limited cell space and the reaction would stop in a very short time. This means the death of the cell. Today, many projects are attempting to understand the mechanism of electron transfer among proteins (Zhang et al. 2000; Pletneva et al. 2000; Hu et al. 2000).
|