7.2 Biosensors
|
7.2 Biosensors
In the previous section, we saw that the molecules in living systems transport electrons to keep the metabolism working. It is therefore possible to observe the electrochemical behavior of those molecules using electrochemistry. Biosensors are the most well-studied devices that use biomolecules. Biosensors realized today do not use single molecules. They use molecules as a whole. Various kinds of biomolecules are now used for biosensors, including enzymes, antibodies, receptor proteins, and even microorganisms themselves (Karube and Yokoyama 1993). Each of these can recognize a particular target molecule with the result that some change can occur. For example, a glucose sensor is used to evaluate the glucose concentration in human blood. An enzyme called glucose oxidase is used for the glucose recognition. Glucose is "recognized" by glucose oxidase and is oxidized to gluconolactone. This recognition occurs in the same manner as in most enzymes. Glucose molecules are bound to enzymes by multiple weak attractions, while the active sites of the enzymes are in the shape of clefts or crevices. The specificity of binding depends on the very particular arrangement of atoms at an active site (see chapter 2 of this book). In this case, oxygen is consumed and hydrogen peroxide is produced simultaneously.
![]()
If we measure the change in dissolved oxygen or hydrogen peroxide concentration using an electrochemical device, it is possible to measure the glucose concentration— if we know the relationship between the change and the glucose concentration before measurement. As we can see in the above formula, dissolved oxygen concentration decreases and hydrogen peroxide increases with the increase of glucose. These changes are proportional to the glucose concentration.
Figure 7.4 describes the main structure of this glucose sensor. For typical oxygen electrodes, both the anode (Pb) and the cathode (Pt) are structured so they are dipped in an electrolyte (30% NaOH or KOH). At the cathode end, the tip is constructed with a gas-permeable membrane such that oxygen molecules can get to the cathode layer from the outside and the electrolyte does not leak out. Pb is used as the anode material at a suitable electrochemical potential for the regeneration of oxygen. The top of the gas-permeable membrane is shielded with a membrane containing affixiated oxygen. The enzyme is a protein easily dissolved in water, so the enzyme glucose oxidase must be immobilized in a membrane to prevent its quick outflow in repeated use.
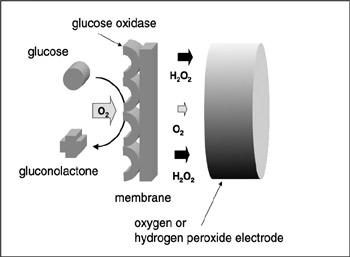
Figure 7.4: Scheme of a glucose sensor. Glucose is oxidized into gluconolactone at the membrane and O2 is consumed. H2O2 is produced at the same time. Both O2 and H2O2 can be measured by the electrode.
After washing, the substrate is removed from the membrane and the sensor is regenerated. If the outflow or the denaturation of the enzyme is controlled to be at a minimum, the lifetime of the sensor increases. In measurement testing, glucose is present and the above-given reaction occurs due to the activity of the enzyme immobilized in the membrane—thus the concentration of oxygen near the membrane drops. This results in a change of the electric current signal received from the oxygen electrodes. From this change in magnitude, the glucose concentration can be swiftly calculated. This type of instrument, where the result of a reaction is linked to the change in an electrical signal, is called a transducer. Using a suitable recognition molecule and a transducer bound together in one unit, a biosensor for a target molecule or reaction can be created. The reaction response of a curved-electrode glucose sensor is given in figure 7.5.
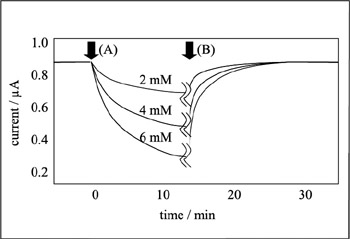
Figure 7.5: Typical response curve of a glucose sensor. Sensor is placed in a buffer solution and stirred using a magnetic stirrer. (A) Standard glucose solution is dropped into the solution. (B) Sensor is washed using a buffer solution. Enough of the buffer solution is added so that the glucose is totally washed away.
Background of Biosensors and Their Present Use
The very first biosensor created was a version of the above-outlined glucose sensor, following which various other types of biosensors were developed. The most important aspect of biosensor development has been the development of immobilizing the recognition element in a membrane. More details will be given below, but the first ever reported was that of using electric immobilization of enzymes into a collagen membrane (Karube et al. 1971; Karube and Suzuki 1972a, 1972b; Suzuki et al. Suzuki, Karube, and Watanabe 1972; 1974). Following this, biosensors using various types of recognition elements have been developed. For instance, biosensors can use various materials such as enzymes, antibodies, binding proteins, receptors, organella, organs, microorganisms, or animal/plant cells for the molecular recognition elements. Transducers such as electrodes, thermister, field effect transistor (FIT), a surface acoustic wave (SAW) device, or a quartz crystal microbalance (QCM) can be used to transduce molecular recognition into an electric signal (figure 7.6). Electrodes can receive/give electrons from/to the product molecule. H2O2 is oxidized at the Pt electrode and becomes H+ and O2. Through this oxidization, electrons are taken up by the electrode. When the electrode is connected to a device such as a potentiostat, a current change can be observed. The change in current contains information about the number of electrons transferred during the reaction.
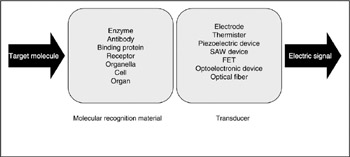
Figure 7.6: Scheme of biosensor. A target molecule is recognized by a molecular recognition element and then the result of recognition is detected by the transducer. Finally, an electric signal is produced from the transducer.
Molecular recognition membranes can be categorized into two kinds. One is a membrane incorporating a catalyst, the other is a membrane incorporating a bioaffinity compound. In the latter case, an enzyme, organella, or microorganism is used for the molecular recognition. In the catalyst-based biosensor, the change due to the catalytic reaction is measured. Dissolved oxygen, hydrogen peroxide, or hydrogen ions are measured by the transducer—in most cases by electrochemical devices. Changes in heat or optical phenomena can also be used. When glucose is oxidized into gluconolactone, energy is released and this results in an increase of the solution temperature. An enzyme-embodied thermistor or optical enzyme sensor is used as a transducer in this case. Careful design of the enzyme membrane is very important in order to have effective transduction of the physical change (optical, heat, chemical) into an electric signal. Molecular permeability, membrane thickness, and the immobilized amount of the enzyme are the main factors in determining this effectiveness.
Glucose sensors using oxygen electrodes or hydrogen peroxide electrodes have now been commercialized and are widely used in the clinical field. Several problems are now being addressed that hold back widespread applications of biosensors, such as the limited lifetime of the enzyme, or the effect of the matrix on the signal.
One solution for these problems is microfabricating the sensor device. Microfabrication of a biosensor requires two techniques—a technique for fabricating the transducer and one to fabricate the enzyme membrane. Immobilizing the enzyme into the membrane can be done in three different ways: binding to resin, crosslinking, and encapsulation (figure 7.7). In the case of binding to resin, either covalent bonding or adsorption of the enzyme onto the resin is performed. Enzymes have amino groups or carboxyl groups on their surfaces, which will form covalent bonds to chemical groups such as amino-, carboxyl-, hydroxyl-, and thiolgroups on the resin surface.
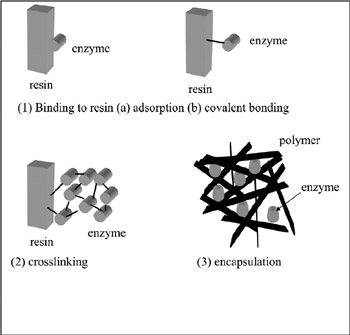
Figure 7.7: Enzyme immobilization methods.
In the second case, the cross-linking method, a multifunctional compound such as glutaraldehyde is used. Glutaraldehyde has two aldehyde groups in one molecule and will easily form covalent bonds with amino groups of the enzyme. Afterward, an insoluble membrane is formed where the glutaraldehyde is cross-linked with the enzyme. Glutaraldehyde can be mixed with the enzyme solution, or glutaraldehyde vapor can react with enzyme already encapsulated in a polymer matrix. In the third method, the enzyme is encapsulated in a matrix of insoluble polymer. A photocross-linkable polymer such as a derivative of polyvinyl alcohol is used for this purpose. In this method, the enzyme is surrounded by the polymer so that the diffusion of the substrate sometimes can be the determining factor in the rate of reaction. Fabrication of a thinner membrane is therefore desired. It used to be difficult to immobilize enzymes with high spatial resolution because the methods mentioned above are basically manual procedures. At present, electropolymerization of enzymes on the electrode surface can be performed to solve this problem (Shinohara, Chiba, and Aizawa 1988). When a platinum or gold electrode is inserted into a solution of aniline and enzyme, followed by electro-oxidization, a polyaniline membrane containing the enzyme is formed on the electrode surface. Oxygen or hydrogen peroxide can permeate through this membrane, but larger ions or molecules cannot.
Karube and Colleagues have demonstrated the usefulness of a microfabricated glucose sensor (Hiratsuka et al. 1999). This development was triggered by the aging population in Japan and the rapid increase in the number of people potentially susceptible to diabetes. The need for constant monitoring called for a procedure involving less pain. This could be done by requiring a much smaller amount of blood to be drawn from the patient. Moreover, any device used should be disposable to prevent infection of viral disease from one patient to another. A very small, disposable glucose sensor was therefore needed. Of most importance was being able to immobilize the enzyme in a very small area with good reproducibility. Plasma polymerized membrane (PPM) is known as a homogeneous, pinholefree membrane that can load the desired functional chemical groups (Nakanishi, Muguruma, and Karube 1996; Nakanishi et al. 1996a; Nakamura et al. 1997b; Muguruma and Karube 1999). The advantage of this immobilization is that the whole process, from etching to loading of the functional groups, can be performed under vacuum. With this method, several biosensors have been fabricated and have showed good responses to the level of blood glucose. Techniques such as enzyme immobilization using PPM will help create future ultramicrobiosensor devices. Noninvasive techniques using biosensors have also been reported (Ito et al. 1995, 1996).
Enzyme Sensors for Saccharide Detection
The first attempt to fabricate an enzyme sensor was performed for the detection of glucose. Glucose oxidase, an enzyme that catalyzes the oxidation of glucose into gluconolactone, was immobilized in a polyacrylamide gel and was attached on the surface of an oxygen electrode (figure 7.8). The mechanism of this glucose measurement system was described in the preceding section. Oxygen consumed or hydrogen peroxide produced in the membrane is measured using several electrodes. For the measurement of oxygen, usually a Galvanic-type or a Clark-type electrode is used. A platinum working electrode with an applied potential of 700 mV versus Ag/AgCl reference electrode works for this purpose. For the detection of hydrogen peroxide, a platinum electrode with an applied potential of ∼700 mV against Ag/AgCl reference electrode is often used.
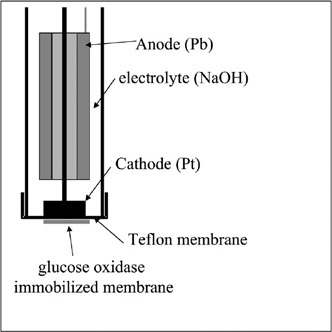
Figure 7.8: Glucose sensor using an enzyme and oxygen electrode.
The mixture of three enzymes—invertase, mutarotase, and glucose oxidase— enables an enzyme sensor for sucrose (Satoh, Karube, and Suzuki 1976). Sucrose is hydrolyzed into α-D-glucose and D-fructose with the activity of invertase. α-Dglucose will then be transformed into β-D-glucose with the function of mutarotase. The final product, β-D-glucose, is then oxidized by glucose oxidase and gluconolactone is produced. Oxygen consumed or hydrogen peroxide produced will be measured electrochemically, and the starting compound, sucrose, is measured.
Other sensors, such as maltose sensor using glucoamirase and glucose oxidase; galactose sensor using galactose oxidase (Yokoyama et al. 1989); and lactose sensor using galactosidase and glucose oxidase are developed. Among these sensors for saccharides, glucose sensor has been the most well studied (Gotoh, Chen, and Karube 1993; Murakami et al. 1994a, 1994b) because of the large number of diabetes patients in the world. Biosensors for sucrose or lactose are used for food analysis (Karube and Takeuchi 1993).
Alcohol Sensor
Measuring alcohols is often necessary in areas of medicine, industrial processes, or food preparation processes. Using an enzyme called alcohol oxidase, a biosensor for alcohol can be fabricated. This enzyme oxidizes primary alcohols with low molecular weights, producing aldehyde. Combining this enzyme and a hydrogen peroxide electrode enables one to fabricate an alcohol sensor. The performance of this sensor was tested and ethanol was measured with good reproducibility (relative standard deviation of 3.2%).
A problem with the original sensor was its poor selectivity. The enzyme oxidizes methanol, allylalcohol, n-propanol, and n-butanol, with the sensor reacting with organic acids and aldehydes as well as the target substances. This problem was solved by the use of alcohol dehydrogenase, which is an enzyme that reacts only with alcohols (Kitagawa et al. 1989, 1991). This enzyme requires NAD as coenzyme, with a reduced type of NADH being produced through the reaction. If we measure NADH or a mediator reduced by NADH, an alcohol sensor can be fabricated. This system, however, is a bit complicated and is still at the development stage.
Amino Acid Sensor
The measurement of amino acids is important in the fields of clinical analysis, food processing, and fermentation procedure. In the detection of L-amino acids, L-amino-acid oxidase is used. The combination of this enzyme and an oxygen/ hydrogen peroxide electrode will enable the measurement of L-amino acids. This system, however, lacks selectivity, and the use of other enzymes for amino acid detection is necessary for selective measurement (Matsunaga et al. 1980; Karube and Suzuki 1986; Suzuki 1994).
Nucleic Acid Sensor
Nucleic acid–related compounds are found in living cells or in industrial processes. Measurement of them is therefore important. Among them, hypoxanthine can be measured using immobilized xanthine oxidase and an oxygen electrode. An inosine sensor can be fabricated using nucleoside phosphorylase and a xanthine oxidase immobilized membrane; 5′-nucleotidase, nucleoside phosphorylase, and xanthine oxidase are used for inosine 5′ monophosphate measurement. The combination of adenosine deaminase with an ammonium electrode can measure adenosine. Adenosine deaminase catalyses the decomposition of adenosine into inosine and ammonia, the latter being measured using an ammonium electrode. Other sensors such as guanine sensor using guanidine deaminase and an ammonium electrode, xanthine sensor using xanthine oxidase and a graphite electrode, or ATP (adenosine triphosphate) sensor using glucose oxidase/hexokinase and an oxygen electrode have been reported.
Recently, there has arisen a need to be able to determine the chromosomes of various bacterial strains linked with food poisoning. Because of this, sensors sensitive to special characteristics of particular bacteria have been developed. The material to test will contain exceedingly small amounts of particular DNA, which first must be amplified through PCR (see chapter 5 of this volume for details) to a measurable amount. Combining this with sensors for other DNA in the target chromosome has allowed a biosensor to be developed. By this method, bacteria linked with food poisoning can be monitored in foodstuffs and a measurement can be given in a few hours (Kai et al. 1997). The use of a completely new recognition element—PNA (peptide nucleic acid)—has proven successful in the lab for chromosomal measurement (Sawata et al. 1999).
Urea Sensor
Measurement of urea concentration in the human body is quite useful for the evaluation of kidney function. The conventional method for urea measurement is quite tedious, and therefore a fast and simple method for its measurement was desired. Urea is hydrolyzed by urease and changes into ammonium ion and carbonate ion. Combining this enzyme with an ammonium electrode or a carbon dioxide electrode produces a urea sensor (Hirose et al. 1983; Miyahara et al. 1983).
Fish-Freshness Sensor
The enzyme sensors mentioned above were designed for the measurement of a single compound. If the measurement of several compounds is possible, biosensors could be applied to a wider field. Measurement of, for example, odor or a complicated taste will be possible. Measurement of the freshness of fish can be done using a fishfreshness sensor. ATP (adenosine triphosphate) in fish flesh decomposes after the death of the fish and will change into ADP (adenosine diphosphate), AMP (adenosine monophosphate), inosinic acid, inosine, hypoxanthine, and finally uric acid. Uric acid is ultimately wasted in urine. Fish freshness was already known to be measurable using the ratio of ATP and these related compounds. Freshness value, KI, was defined as
KI = ([inosine] + [hypoxanthine])/([inosine acid] + [inosine] + [hypoxanthine])
where [A] represents the concentration of compound A. As mentioned before, concentration of each compound can be measured using inosine, inosinic acid, and hypoxanthine sensors. Using a system with those three sensors, concentrations of the three compounds can be recorded as the pattern of three values, and KI value is automatically recorded as well. By this indication of freshness as a pattern, a novel identification of freshness was performed (figure 7.9) (Karube et al. 1980; Watanabe et al. 1984a, 1984b; Karube et al. 1984a, 1984b; Suzuki et al. 1989; Chemnitius et al. 1992; Karube and Shimohigoshi 1996).
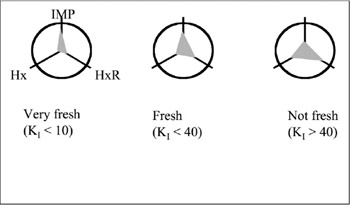
Figure 7.9: Response pattern obtained using a fish-freshness sensor. Fish freshness can be evaluated from the pattern obtained from the concentration pattern of three compounds.
Microfabricated Enzyme Sensor
Work on an enzyme sensor is now on the way to being miniaturized. Small biosensors can be implanted into the human body for clinical analysis, or they can be integrated on one chip. A lot of work has been done using microelectrodes. Anisotropic etching of a silicon wafer enables a microstructure for the device (Karube et al. 1994). A groove formed via this etching is used as a microchannel in which the sample flows. In this groove, electrodes are formed using a sputtering technique. Recently, a three-electrode system—having a platinum working electrode, an Ag/ AgC reference electrode, and a platinum reference electrode—was fabricated on one silicon chip (Suzuki et al. 1999a, 1999b, 1999c). The enzyme was immobilized via γ-aminopropyl triethoxysilane or plasma polymerized film (Hiratsuka et al. 1999; Muguruma, Hiratsuka, and Karube 2000). The latter method in particular enables mass fabrication of the sensor in one vacuum chamber.
Enzyme Sensor Using a Transistor
Another transducer suitable for miniaturization is a transistor. Mass production processes for this device are already very well established. An ion-selective field effect transistor (ISFET) is so far the only silicon device that functions stably under solution. An ISFET has several advantages as a transducer for microbiosensors. First, the smaller impedance compared to that of a glass electrode makes it more suitable for miniaturization (because miniaturization of glass electrodes is accompanied by increasing impedance). Second, ISFETs are produced through a mass production procedure, so quality is consistent; cost is low as well. Third, selective measurement is possible using an ion-selective membrane formed on the device surface. ISFETs were originally developed using an insulator layer, so the addition of a highimpedance membrane does not affect the response speed. Any enzyme that catalyzes a reaction with pH change or ion concentration change can be used in combination with ISFET. For example, lipoprotein lipase hydrolyzes neutral fat in lipoprotein and produces glycerol and long-chain fatty acids. Part of these fatty acids are dissociated, so detecting the accompanying hydrogen ion enables the analysis of the original neutral fat. A urea sensor can be fabricated using the same principle (Gotoh, Tamiya, and Karube 1986; Gotoh et al. 1987, 1988, 1989a, 1989b). In practical measurements, the response of the urea sensor was not stable. When two ISFETs were used, one having urease and the other not, a stable response was obtained regardless of pH, temperature, drift of the baseline, or the disturbance at the injection of the sample. Several other enzyme sensors using ISFET have been reported (Miyahara et al. 1982; Murakami et al. 1986): for example, a glucose sensor using glucose oxidase, a lactate sensor using lactate dehydrogenase, an acetylcholine sensor using acetylcholine esterase, an L-glutamate sensor using L-glutamate decarboxylase, a tributylene sensor using lipase, and an ATP sensor using proton-dependent ATPase.
Enzyme Sensor Using a Thermistor
Most enzymatic reactions are accompanied by an enthalpy change of 5–100 kJ/mol. Measurement of the resulting temperature change of the solution will enable the measurement of substrate concentration. An enzyme thermistor, based on this principle, can be applied to all enzymatic reactions, and its field is very wide. The use of two thermistors, one with an immobilized enzyme and the other with a deactivated enzyme, resulted in the higher performance of the system. This two-thermistor system showed no effects from pH, temperature, and disturbance from the injection of the sample solution (figure 7.10) (Karube and Shimohigoshi 1996).
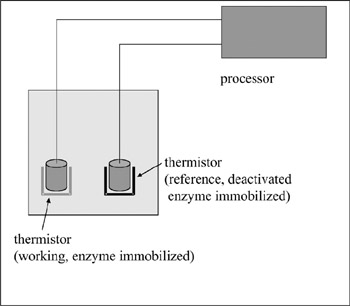
Figure 7.10: Enzyme sensor using thermistors. Two thermistors are used for the measurement.
Enzyme Sensor Using an Optical Device
Chemiluminescence-based measurements are generally more sensitive compared to electrode-based methods because of their high S/N value. The technique is as follows: Several biochemical and chemical reactions are accompanied by optical irradiation. A typical example is the light emission of a firefly. Luciferin is the source of the irradiation, and the reaction is catalyzed by an enzyme called luciferase. Measurement of the light intensity is used to measure the strength of the reaction (Tamiya et al. 1989, 1990; Lee et al. 1992). Chemical luminescence (or chemiluminescence) is often used for measurements rather than biochemical luminescence (Ikebukuro et al. 1996; Nakamura et al. 1997a). For example, luminol (5-amino-2,3-dihydro-1,4-naphthalazinedione) is known to irradiate after the addition of hydrogen peroxide and a metal complex. Hydrogen peroxide is produced from various reactions catalyzed by oxidases, so combining the oxidase and luminol reactions will enable the measurement of substrate concentration or enzyme activity. Glucose, uric acid, cholesterol, L-amino acid, or hypoxanthine were measured using this system (figure 7.11). Luminol (C6H7-N3O2) emits purple light (ν = 460 nm) through the following mechanism:
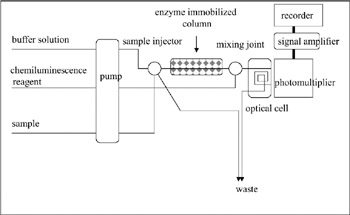
Figure 7.11: Schematic diagram of an enzyme sensor using chemiluminescence.
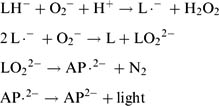
where L = luminol, LH− = monoanionic form of luminol, L.− = radical state of luminol, AP.2− = excited state of aminophthalic acid, and AP.2− = ground state of aminophthalic acid.
Immunosensor
Enzyme sensors are applied to measuring small molecules that can be "recognized" by the enzyme. For molecules that cannot be recognized by them, an immunosensor is useful. An immunoreaction is characterized by the interaction between an antibody and an antigen. Analyses using this interaction, immunoassay, are used in clinical analysis, where a protein, antibody, hormone, or drug is measured in blood. Conventional immunoassay requires labeling using radio isotopes, a fluorescent probe, or enzymes. In radio immunoassay using radio isotope, a picogram-level measurement is possible. This highly sensitive method has a disadvantage of safety. Other immunoassays are accompanied with the disadvantages of complex handling and take a long time. The idea came about of developing an immunosensor. The advantage of using a biosensor is the short measuring time required, because recognition and signal transduction take place at the same time.
Several attempts to fabricate an immunosensor were reported. Electrode-based immunosensors, for example, use the principle of enzyme immunoassay. An electrode with an immobilized antibody is used. A mixture of target antigen and catalase-labeled antigen is then added to the system. Finally, hydrogen peroxide is added. Oxygen is produced from the reaction catalyzed by catalase-labeled antigen, and is measured by the electrode. Competition between target antigen and catalase labeled antigen result in a change of the electric current from the electrode; said change being dependent on the antigen concentration. The same method of competition can be used in a system based on luminescence. As mentioned above, luminol produces chemiluminescence under the existence of hydrogen peroxide and a metal complex such as peroxidase. The use of a peroxidase-labeled antigen competing with the target antigen will produce a luminescence dependent upon antigen concentration. Measurement of the luminescence intensity using a photon counter or photomultiplier is the foundation of this system. The attempts made were, unfortunately, still far from real use—problems have been caused by the nonspecific adsorption of other protein over the electrode, or the steric hindrance caused by the peroxidase resulting in the inhibition of the reaction (figure 7.12) (Karube and Gotoh 1988; Karube, Seki, and Sode 1990).
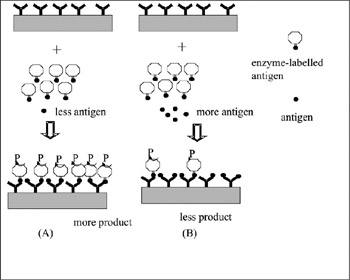
Figure 7.12: Schematic of an immunosensor. Antigen and an enzyme-labeled antigen (fixed concentration) are added to an antibody-immobilized substrate. If the concentration of antigen is low, the enzyme reaction product is high. Conversely, if the concentration of antigen is high, the amount of enzyme reaction product is low. Due to this, measuring the amount of the product gives the amount of antigen. The validity of this measurement depends on whether or not nonspecific protein adsorption occurs, i.e., adsorption of other nondesired proteins to the sensor surface. Some sticky materials in the sample often stick to the metal surface or antibody or enzyme of the sensor. Such interaction occurs due to electrostatic interaction. As a result, the antibody or enzyme on the sensor surface will be blocked.
Immunosensor Using QCM
QCM (quartz crystal microbalance) sensors are based on the measurement of mass change of the surface of a piezoelectric crystal (= QCM). When a certain electric field is applied between two electrodes, sandwiching an AT-cut quartz crystal, a resonant frequency (F) is observed. F changes with the adsorption of compounds on the surface. The relationship is reported as follows:
![]()
Where ΔF (Hz) is the change in the fundamental frequency of the crystal, F (Hz) is the resonant frequency, ΔM (g) is the change of mass, and A is the area of the device. When and antibody is immobilized on the surface of QCM, the interaction of antigen with antibody can be measured using this device. Anti-atrazine (herbicide) antibody was immobilized on a QCM and was used as an atrazine sensor. Because the mass change after the interaction of atrazine and anti-atrazine antibody is small, due to the molecular weight of the atrazine molecule, a competitive method was used. Horseradish-peroxidas-labeled atrazine was added to the system with the target atrazine. Mass change on the surface decreased with the increase of atrazine concentratin, enough to allow measurments to be carried out. QCM systems have been used of several other purposes such as measurement of odorants or cells (Nakanishi et al. 1996b) (figure 7.13). One example is the following: Using an antibody that recognizes the chemical structure of the surrounding membrane, a sensor has been developed to detect the plankton causing red tide. An antibody is immobilized on the surface of the QCM, which links up with the surface chemical structure of the plankton. The resultant change in mass will change the frequency of the crystal and thus can be observed (Nakanishi, Muguruma, and Karube 1996).
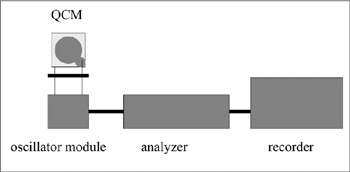
Figure 7.13: Scheme of a QCM sensor system. The antibody is immobilized on the gold electrode surface of a QCM chip.
Immunosensor Using an SPR Device
Highly sensitive immunosensors have been developed using an SPR (surface plasmon resonance) device. The principle of SPR is based on the fact that the intensity of reflected light at glass-gold interface becomes minimum at a certain incident angle (θr). θr changes as the optical index of the media facing gold changes. When an antibody is immobilized on the gold surface, the surface optical index changes after the antigen-antibody interaction, resulting in the change in θr. A proportional relationship between θr and antigen concentration can be observed. Such a system can be used in an immunosensor (figure 7.14) (Nakamura et al. 1997b; Sasaki et al. 1998).
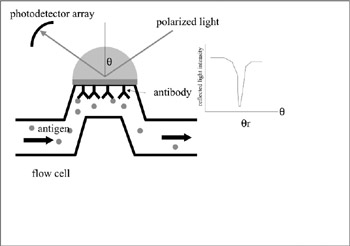
Figure 7.14: Principle of an immunosensor using an SPR device. Interaction between antibody and antigen results in a change in θr.
Microbial Sensor
Enzyme sensors, as mentioned above, are applicable for many purposes but have disadvantages, such as their relatively higher cost and shorter lifetimes. Enzymes are usually extracted from microorganisms; microorganisms contain various enzymes, allowing them to be used directly as the target recognition device. Microbial sensors using microorganisms for recognition are used in the fields of industrial processing and environmental monitoring. The difference between various types of sensors can be quite large. For instance, a microbial-based BOD sensor is limited by the lifetime of the immobilized microbes—which is about one month even when used intermittently. (Karube and Sode 1988, 1990; Karube and Chang 1991). Conversely, a chemical sensor would have a lifetime of several months.
Electrode-Based Microbial Sensor
Microorganisms carry out functions such as respiration or metabolism. Using these functions enables the selective measurement of chemical compounds. A microbial sensor consists of microorganisms immobilized in a membrane and an electrode. A microrganism that is alive in the immobilization matrix is used and the biochemical reaction inside is generally complicated. A microbial sensor using respiration activity is called a respiration-type microbial sensor. Aerobic microorganisms consume oxygen through respiration; the use of an oxygen electrode equipped with a gaspermeable membrane enables measurement of this activity. When an electrode with a microbial membrane attached is placed into a sample solution, the concentration of the target compound can be measured.
A typical example would be the measurement of an organic compound, which is performed in two steps. First, the microbial electrode is placed in a solution of saturated dissolved oxygen (DO). The response from the electrode is recorded. Second, the electrode is placed in the sample solution saturated with DO. Organic compound diffuses into the membrane and is decomposed by the microorganism. Respiration activity then becomes larger and the amount of oxygen diffusing to the electrode becomes smaller. The concentration of the organic compound and the decrease of oxygen are in a certain relationship, and the measurement of the compound is thus possible via the electrode signal.
Microorganisms also take up organic compounds and produce several metabolites. Among them exist electroactive materials. Metabolites measurable amperometrically are hydrogen, formic acid, or several reduced-type coenzymes. On the other hand, carbon dioxide and organic acids are potentiometrically measurable metabolites. Combining together a microbe-immobilized membrane and electrode will thus enable the fabrication of a microbial sensor (Karube et al. 1976, 1977; Karube, Matsunaga, and Suzuki 1977; Suzuki and Karube 1978).
Alcohol Sensor
Trichosporon blassicae, a type of yeast, is known as an alcohol-assimilating microorganism. This microorganism was immobilized in a porous acetylcellulose membrane and affixed to an oxygen electrode. Finally, the tip of the device was covered with a gas-permeable Teflon membrane. In a neutral pH buffer solution, ethanol evaporated and permeated through the gas-permeable membrane, reaching the microbe-immobilized membrane, and was finally assimilated by the microorganism. Change in the oxygen concentration is observed, resulting in a change in the response. Both the enzymatic saccharide and alcohol sensors are based on oxidation and were developed subsequently to the microbial versions. The stability and capabilities are equivalent to the microbial-based sensors (Hikuma, Kubo, and Yasuda 1979; Kubo and Karube 1988).
Biochemical Oxygen Demand Sensor
BOD (biochemical oxygen demand) is an international index for river water quality. The conventional method of BOD measurement is time consuming (5 days) and is not suitable for real-time monitoring. The authors invented a biosensor for BOD a quarter century ago and this sensor has now been commercialized. This sensor is mainly used for the BOD measurement of wastewater from food factories, which usually contains many organic compounds. Sensor response shows a very good match with the results of conventional method-based BOD monitoring. Trichosporon cutaneum, a yeast used in the wastewater plant, was immobilized in a membrane and was placed on an oxygen electrode. The decrease in current from the electrode was proportionally related to the BOD of the sample. Measurement of BOD can now be performed using this sensor; the time for measurement was reduced to only 20 minutes (Karube et al. 1989). Actual cases of measuring in the middle of a river need to detect a BOD on the scale of parts per million, and the sensor described above was not able to detect down to this level. Because of this, a high-sensitivity microbial-based biosensor was developed (using ozone pretreatment to make the sample more digestable for the microbes; Nomura, Chee, and Karube 1998; Chee et al. 1999). Testing is ongoing to make this available for practical use. Separate from this, there has been work on a BOD sensor using an embedded oxygen-based transducer by incorporating semiconductor production techniques (Yang et al. 1997).
|