4.6 Amazing Belousov-Zhabotinsky Media
|
4.6 Amazing Belousov-Zhabotinsky Media
Two remarkable events in the early 1950s proved the starting points for intense investigations in a new field between physics, chemistry, and biology where systems far from equilibrium demonstrate different and complicated modes of behavior. Chronologically, they were:
-
The discovery of periodic regimes in catalytic reactions of citric acid oxidation by Belousov (Field and Burger 1985; Kapral and Showalter 1995)
-
Publication of the paper "The Chemical Basis of Morphogenesis" by Alan Turing (1952), who first discussed the problem of self-organization in systems far from equilibrium
Later, Zhabotinsky (1964) performed extensive study of the Belousov reaction and developed a very convenient modified version.
The history of the field of oscillating systems is complicated and dramatic (see, for details, Field and Burger 1985; Kapral and Showalter 1995). Different chemical and biological systems have been discovered that demonstrate complicated modes of behavior based on nonlinear dynamic mechanisms. Well-stirred chemical systems have provided examples of behavior based on point-wise kinetics. Using continuousflow stirred-tank reactors (CSTR) has enabled researchers to demonstrate bistability, different types of temporal oscillations, and chaotic behavior (Field and Burger 1985).
Very sophisticated regimes were discovered using unstirred reactors in thin films of a reagent. These included trigger and other types of traveling waves, as well as different spiral structures (Kapral and Showalter 1995).
Finally, the design of a continuous-flow unstirred gel reactor has allowed experiments to be carried out, proving the actual existence of stable dissipative structures that were predicted by Turing some forty years ago (Kapral and Showalter 1995).
Among these different chemical oscillators, Belousov-Zhabotinsky-type media play a principal role. The dynamics of these media are complex enough to demonstrate diverse and complicated behavior (see two important examples in figure 4.1) and thus have became invaluable model systems for excitable media, providing deep insights into the properties of nonlinear dynamic chemical and biological systems.
The Belousov-Zhabotinsky-type reaction (Field and Burger 1985) is a catalytic oxidation of some organic substance (mainly malonic acid) by potassium bromate or some other oxidizing agent. In the original system, a chemical reaction was found that mimicked the predator-prey population periodicity. The system switches periodically from steady state I to steady state II and vice versa. Such a system is usually self-oscillatory, but under appropriate conditions, the inhibitor bromide can be kept above its critical value, and oscillations will not start. Decreasing the bromide concentration at a small spot for a short while (by touching it with a silver wire, or by an equivalent photochemical reaction) will start the autocatalytic process, with chemical waves propagating outward, like ripples in water.
The overall equations of the Belousov-Zhabotinsky reaction catalyzed by metal ions (Ce, Fe, Ru, and some other metals) (Field and Burger 1985) are:
At the beginning of the process:
![]()
After some time:
![]()
But detailed consideration shows that the Belousov-Zhabotinsky reaction includes a set of intermediate stages. The widespread model of the reaction was suggested by Field, Koros, and Noyes (FKN model; Field and Burger 1985). It consists of eleven stages based on several intermediate components.
These media are stable, nonhostile reagents. Furthermore, the temperature range and temporal operation scale of the medium dynamics are convenient for investigation with available physical methods.
B-Z type media based on a light-sensitive catalyst (Kuhnert 1986, 1986b; Kuhnert, Agladze, and Krinsky 1989) are convenient for investigation purposes. The catalyst in the course of reaction changes its electronic state when the medium goes from one stable state to another. As a consequence, the reagent changes its color (from red to blue and vice versa). Therefore it is easy to visualize the process and to observe its spatiotemporal evolution.
The basic important feature of light-sensitive excitable media is that they store input information during a rather long period of time. The periodical process of stored image transformation (figure 4.7) begins after projecting an image onto a thin layer of the medium (Kuhnert, Agladze, and Krinsky 1989; Rambidi and Maximychev 1997).
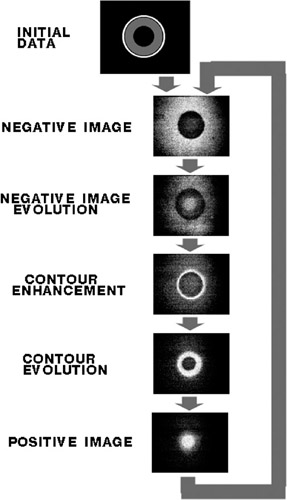
Figure 4.7: Scheme of basic periodic process of image transformation performed by reaction-diffusion medium.
This process represents a combination of three interlaced primitive responses to the stimulus by light (figure 4.8):
-
Contour enhancement of image fragments
-
Alternation of negative and positive images of an input picture
-
Disappearance of small features of the picture
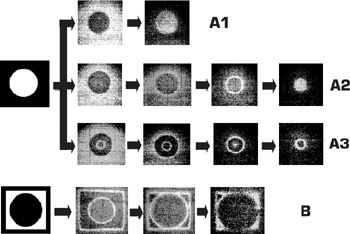
Figure 4.8: Temporal evolution of simple images in thin layers of a light-sensitive Belousov-Zhabotinsky medium depending on the state of the medium (A1–A3 correspond to different acidities of the media) and on the character of the medium illumination (A and B are positive and negative images). Initial images are at the left side of the figure.
The first of these predominates at relatively low medium acidity values and high exposures of light radiation. The second process is revealed at high acidity levels and low light exposures.
The results, which will be discussed in sections 4.7 and 4.8, were obtained using the experimental setup shown in figure 4.9.
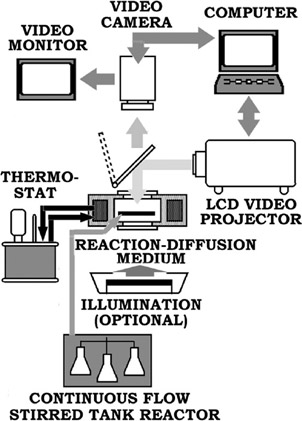
Figure 4.9: Schematic representation of optical and digital video system for investigation of information processing by reaction-diffusion media.
The reaction-diffusion medium was a thin (0.5–1.5 mm), flat nonstirred reagent layer placed in a reaction vessel in which a spatiotemporal oscillating process proceeded. The initial concentrations of the reagent components were: KBrO3: 0.3 M, H2SO4: 0.6–0.3 M, malonic acid: 0.2 M, KBr: 0.05 M. Light-sensitive catalyst Ru(bpy)3Cl2 was used.
The specific feature of this setup was a computer-controlled Sanyo PLC-510M LCD video projector (VGA compatible, 270 ANSI lumens). The high uniformity of the background intensity of this projector improved the reliability of the experiment. Moreover, the computercontrolled projector was indispensable for the elaboration of the technique suitable for finding the shortest paths in complex labyrinths (see below).
An excitation of a thin planar layer of the medium by light radiation was used for the input of initial information. The direction of light was normal to the surface of the medium. The distribution of light intensity on the surface (that is, an image of the labyrinth under processing) determined the initial image stored in the medium.
|