30 - Pancreas
Editors: Mills, Stacey E.
Title: Histology for Pathologists, 3rd Edition
Copyright 2007 Lippincott Williams & Wilkins
> Table of Contents > IX - Genitourinary Tract > 37 - Testis and Excretory Duct System
function show_scrollbar() {}
37
Testis and Excretory Duct System
Thomas D. Trainer
Introduction
The adult testes are paired organs that lie within the scrotum suspended by the spermatic cord (Figure 37.1). The average weight of each is 15 to 19 g, the right usually being 10% heavier than the left (1). The scrotal covering layers are skin, dartos muscle and Colles' fascia, an external spermatic fascia, and the parietal layer of the tunica vaginalis (Figure 37.2). The dartos muscle, of the nonstriated type, is closely attached to the overlying skin but glides freely over the underlying loose fascial layer.
Supporting Structures
The supporting structures of the testis consist of a tough capsule (the tunica) and a number of fibrous septa that extend from the inner surface of the tunica into the parenchyma and divide the testis into approximately 250 lobules, or compartments. The posterior portion of the testis not covered by the capsule is called the mediastinum, which contains blood vessels, nerves, lymphatics, and the extratesticular portion of the rete testis. The capsule has three distinctive layers: the outer serosa, or tunica vaginalis; the thick, collagenous tunica albuginea; and the inner tunica vasculosa. The tunica vaginalis consists of a flattened layer of mesothelial cells overlying a well-developed basement membrane. It forms a sac with two components; a visceral portion covering the testis and head of the epididymis and a parietal portion, formed as the lining reflects posteriorly and superiorly at the mediastinum and epididymis and then covers the internal spermatic fascia. Infrequently, transitional or squamous metaplasia of the surface epithelium may be present (2). The tunica albuginea is composed of a layer of collagen fibers, within which are embedded fibroblasts, myocytes, mast cells, nerve
P.944
fibers, and nerve endings resembling Meissner's corpuscles. The myocytes, found primarily in the posterior aspect of the testis, undergo regular contractions and cause a transient increase in intratesticular pressure. The tunica vasculosa, a loose connective tissue layer containing blood vessels and lymphatics, sends septa into the testicular parenchyma to form the individual lobules. Blood vessels passing through the tunica do so in an oblique plane, which may or may not have some significance with respect to blood flow within the testis. It is well known that the pulse pressure in the testicular parenchyma is extraordinarily low. The tunica varies greatly in thickness with age, averaging 300 g at birth, 400 to 450 g in young adults, and 900 to 950 g in men over 65 years of age (3).
 |
Figure 37.1 Diagrammatic view of testis, epididymis, and portion of ductus (vas) deferens. |
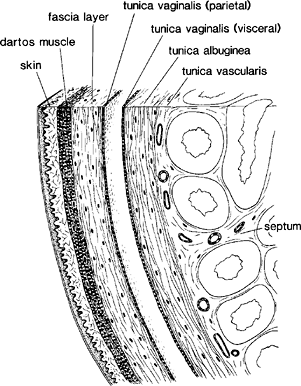 |
Figure 37.2 Scrotal covering layers and capsule of testis. |
Seminiferous Tubules
Each lobule of the testis contains one to four seminiferous tubules (Figure 37.1). The individual tubule is a highly convoluted, closed loop structure, with numerous communications between the arms of the loop but without any blind endings or branches. Each arm of the loop empties into the septal portion of the rete testis, although infrequently seminiferous tubules may empty directly into the mediastinal rete. The total length of seminiferous tubules in each testis has been estimated between 299 and 981 m, with an average around 540 m (4). The average tubule diameter in young adults is 180 m ( 30). The usual open testicular biopsy may encompass tubules from up to five lobules along with portions of the intervening septa. It is important not to interpret the latter as foci of interstitial fibrosis. The seminiferous tubules are composed of germ cells in varying stages of differentiation and Sertoli cells. Each tubule has a distinctive basement membrane and a thin lamina propria (Figure 37.3).
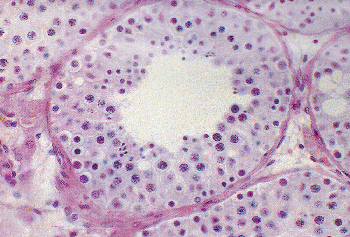 |
Figure 37.3 A cross-sectional view of seminiferous tubule and interstitium. Germ cell maturation is variable around the tubule, a normal finding. |
P.945
Sertoli Cells
Sertoli cells play important and very different roles in the fetal and adult testes, and these divergent roles are reflected in their proliferative activity, cell protein markers, and the nature of cellular intermediate filaments at these periods of the cell's life span. Adult Sertoli cells are nondividing cells. Their number is important since they have the capacity to nurture only a fixed number of germ cells. Genetic, hormonal, and environmental factors appear to play significant roles in determining the final number of Sertoli cells in the adult testis (5). These tall, irregular, columnar cells, with their bases attached to the underlying basal lamina, have an abundant but relatively inconspicuous cytoplasm and an ill-defined cytoplasmic membrane on light microscopic examination. The cells send intricate cytoplasmic extensions around the germ cell elements and continuously alter their contours to accommodate the changing size and shape of the germ cells that they cradle. Adult Sertoli cell nuclei have round to slightly irregularly shapes, with a highly folded nuclear membrane, a homogeneous chromatin distribution, and a prominent, round nucleolus (Figure 37.4). These features are in sharp contast to those of the prepubertal and fetal Sertoli cells, which have an oval or elongated nucleus, a smoothly contoured nuclear membrane, and an inconspicuous nucleolus (Figure 37.15). The Sertoli cell nuclei represent about 10% of the nuclei in a normal adult tubule cross section. They are located toward the basal side of the tubule and lie just central to the nuclei of the spermatogonia and preleptotene spermatocytes. The cytoplasm may contain lipid vacuoles and/or granular eosinophilic material. Much of the phagocytosed material represents remnants of the residual bodies of the spermatid or degenerated earlier germ cell forms. Adult Sertoli cells contain vimentin as intermediate filaments, whereas embryonic and prepubertal Sertoli cells also contain cytokeratins 8 and 18 (6). The transient appearance of cytokeratins is of interest in view of the report of a malignant Sertoli cell tumor containing both cytokeratins and vimentin (7). Low-molecular weight cytokeratins also may be identified in some of the Sertoli cells of atrophic tubules of the adult testis (8), in Sertoli cells of tubules containing in situ germ cell neoplasia (9), and in Sertoli cells of the contralateral testis of patients with germ cell neoplasms. These Sertoli cells have an immature morphological appearance and appear to be part of the testicular dysgenesis syndrome described recently (10). Adult Sertoli cells, unlike fetal or prepubertal Sertoli cells, express androgen receptors within their nuclei but, at the same time, have lost their expression of cytoplasmic anti-m llerian hormone (5,11). A wide variety of other molecules are produced by the Sertoli cells, including inhibin/activin, insulin-like growth factor, platelet-derived growth factor, transforming growth factor, interleukins 1 and 6, and neurofilament proteins (12,13). Still unclear is whether or not the fetal Sertoli cell produces a substance or employs some other mechanism that serves to eventually bring the fetal germ cell into mitotic arrest. Those germ cells that fail to reach the fetal testis or the female germ cells in the ovary do not appear to be exposed to this undefined Sertoli cell product and consequently become arrested in the early phase of the first meiotic division (14,15). Present in the cytoplasm are the crystalloids of Charcot-B ttcher (16). These bundles of filamentous structures, located primarily in the basal portion of mature Sertoli cells, are best seen by ultrastructural examination but occasionally are large enough to be seen by light microscopic studies (Figure 37.5). Ultrastructurally, they appear to merge with vimentin-labeled intermediate filaments, both of which are increased in the cryptorchid testes and the Sertoli cell only syndrome (9).
At puberty, a tight junction complex forms between adjacent Sertoli cells, dividing the seminiferous tubule into
P.946
basal and adluminal compartments; the former contains spermatogonia and preleptotene spermatocytes, and the latter holds the remaining primary spermatocytes, secondary spermatocytes, and spermatids. A transient, intermediate compartment is formed by adjacent Sertoli cells as germ cells move from the basal compartment to the adluminal compartment. A tight junction complex forms behind the germ cells to seal off the intercellular space, thereby ensuring the integrity of the Sertoli cell barrier (17). This junction forms the major blood-testis barrier, preventing access of bloodborne constituents to the germ cells in the adluminal compartment except through the Sertoli cell cytoplasm. Still unclear is the mechanism that serves to promote the movement of germ cells from the basal compartment to the adluminal compartment.
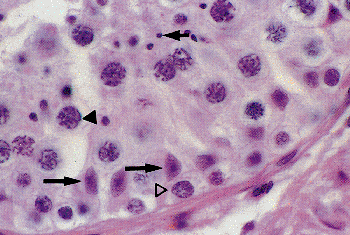 |
Figure 37.4 Seminiferous tubule with Sertoli cells (long arrows), spermatogonia ( ), primary spermatocytes ( ), and spermatids (short arrow). |
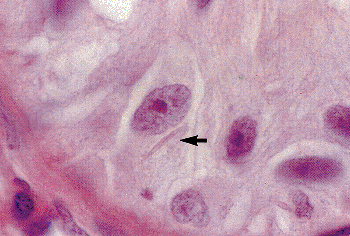 |
Figure 37.5 Sertoli cell with intracytoplasmic Charcot-B ttcher crystalloids (arrow) from a patient with germ cell aplasia. Note the prominent nucleolus and slightly wrinkled nuclear membrane. |
Other cell connections between adjacent Sertoli cells include gap junctions, desmosomes (rarely), and ectoplasm specialization sites (18). Intercellular junctionlike structures are present between Sertoli cells and germ cells, presumably serving as the conduit by which the Sertoli cell plays a role in the regulation of spermatogenesis.
Germ Cells
Germ cells are derived from tissues in the posterior wall of the yolk sac and migrate in early embryonic life to the gonadal ridge (19). Deviations in this migration route account for the appearance in abnormal locations of tumors derived from these cells (Figure 37.6). The germ cell elements comprise the bulk of the cells seen in the adult seminiferous tubule (Figures 37.3,37.4). Maturation in humans covers a period of 70 4 days (20), with no evidence that this time requirement is altered by age or pathologic states. The undifferentiated spermatogonia lie in the basal compartment and undergo proliferation and renewal. Some of them become committed to the process of spermatogensis and give rise to primary spermatocytes, heralding the first meiotic division of spermatogenesis. The earliest of the primary spermatocytes, the preleptotene forms, are also located in the basal compartment. The preleptotene spermatocytes are then moved by an unknown signal into the adluminal compartment, where they sequentially become leptotene, zygotene, pachytene, and diplotene primary spermatocytes, this phase of the cycle involving a period of 24 days (21). After this relatively long period of gametogenesis, the first meiotic division occurs with the formation of secondary spermatocytes. These cells have an extremely short half-life and soon undergo the second meiotic division to form haploid spermatids, which are then converted into spermatozoa through a series of complex steps of metamorphosis (Figure 37.7).
Until spermatozoa are formed, all progeny of a spermatogonium committed to going through the meiotic process are connected together by a narrow cytoplasmic bridge that permits sharing of cytoplasmic organelles and allow for simultaneous maturation of interconnected cells. Of interest is the lack of these intercellular connections in seminomas and intratubular germ cell neoplasm but their occasional presence in spermatocytic seminomas (22). A failure of cell separation, a form of dysmaturation, is reflected in the tubular lumen and seminal fluid by the presence of multinucleated spermatids or multiheaded spermatozoa. In the late spermatid phase, excess cytoplasm is discarded by the spermatid and is phagocytosed by the enveloping Sertoli cells. The sloughing of germ cells into the seminiferous tubule lumen and the presence of immature and abnormal forms observed in patients with varicocele and other pathologic states suggest a failure of Sertoli cell regulation of this maturation process. One must take care to separate this pathologic sloughing process from the artifactual
P.947
sloughing that frequently occurs in open biopsy specimens (Figure 37.8).
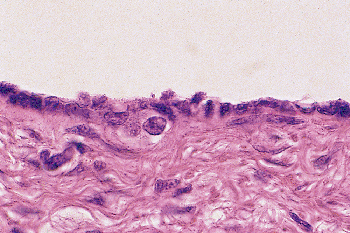 |
Figure 37.6 Germ cells located immediately beneath the mesothelial lining cells of the process vaginalis of a 16-week fetus. |
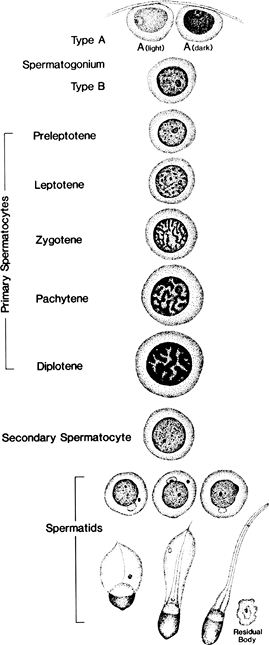 |
Figure 37.7 Steps in spermatogenesis. |
Maturation of germ cells proceeds in an ordered, nonrandom fashion along the length of the seminiferous tubule. Groups of evolving germ cells of one level of development tend to be found in association with developing germ cells of another level of development at any point along the tubule. Clermont (23) described 14 cell association patterns in the rat testis and 6 such cell associations in humans. In the rat, a given cross section of seminiferous tubule shows only one cell association around the circumference of the tubule, whereas in humans up to three cell associations may be seen in a tubule cross section. Initially this arrangement of cell clusters was considered to form an overlapping helical pattern along the length of the tubule (24). That view has been recently challenged (25). Of practical importance is the need to recognize that not all stages of differentiation of germ cells may be seen in any one cross-sectional view of a seminiferous tubule. Mature spermatozoa and late spermatids may be seen in one portion of a tubule cross section, and the opposite wall may show maturation only to the early spermatid level (Figure 37.3). It has been recognized for many years that not all spermatogonia or spermatocytes progress to become spermatozoa and that apoptotic or degenerative changes in these precursor cells can be seen regularly in the seminiferous tubules (26). This normal physiologic process should not be mistaken for maturation arrest. The germ cell elements can be recognized with relative ease, and one should be able to distinguish spermatogonia, primary spermatocytes, secondary spermatocytes, and spermatids.
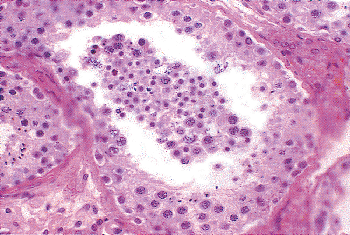 |
Figure 37.8 Seminiferous tubule with artifactual sloughing of germ cell elements into tubule lumen. |
Spermatogonia are located in the basal compartment of the seminiferous tubule, the basal portion of most of the cells being in contact with the tubule basement membrane. They have been subdivided into three types, mainly on the basis of the nuclear chromatin pattern, as type A dark, type A light, and type B. Type A dark cells have a central nuclear cleared area, referred to as the nuclear vacuole, and contain glycogen in their cytoplasm. The type B cells tend to have a more coarsely clumped chromatin pattern than either of the type A cells. It should be pointed out that the nuclear chromatin pattern differences among these spermatogonia is usually evident only with fixatives such as Zencker-formal and that fixatives such as Bouin's produce coarse clumping in the nuclei of all of the spermatogonial subtypes, making subtyping difficult (23). The spermatogonia nuclei are oval to round and contain one or two easily identifiable nucleoli (Figure 37.9). Within the cytoplasm in a perinuclear location are the crystalloids of Lubarsch. These structures, measuring up to 3.0 m in length, may be found in adults and infants as early as the fifth postnatal week.
P.948
They are composed of a mixture of parallel arrays of fibrils 80 to 150 thick and ribosome-like granules (27).
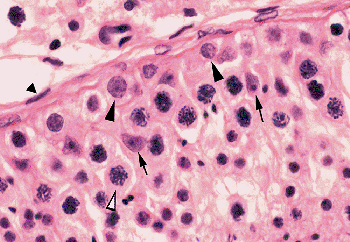 |
Figure 37.9 Portion of seminiferous tubule showing spermatogonia (solid arrowhead), primary spermatocytes (open arrowhead), Sertoli cells (arrow), and fibromyocyte of tunica propria (solid triangle). The smaller cells in the lower right are mainly secondary spermatocytes and early spermatids. |
The classification of primary spermatocytes is based on the alteration of the nuclear chromatin pattern (23). These cells are distinctive because of the doubling of the amount of DNA in their nuclei as a result of the duplication of each chromosome into chromatid pairs in preparation for the first meiotic division. The leptotene primary spermatocytes are characterized by a change in the chromatin pattern into a filamentous structure with a fine-beaded arrangement. Zygotene spermatocytes have an even coarser granularity of the chromosome filaments, and there is a tendency for the chromatin material to gather eccentrically within the nucleus. Pachytene and diplotene spermatocytes are the most easily recognized of the primary spermatocytes because of their large size and their prominent nucleus, containing thick, short chromatin filaments (Figure 37.9).
Secondary spermatocytes, having an extremely short half-life, make up only a small minority of the cells seen in a cross section of the tubule. Their nuclei, substantially smaller than in the primary spermatocytes, have a finely granular chromatin pattern and a haploid number of chromosomes but a diploid amount of DNA because of the presence of the chromatid pairs. These cells, located near the tubule lumen, differ only slightly in appearance from the very early spermatids with which they are closely associated. (Figures 37.4, 37.9).
The spermatids have a heavily stained granular pattern to the nucleus and, if the plane of section is appropriate, a slight depression on the surface of the cell, representing the beginning of the acrosome. The late spermatid form is characterized by a change in the nucleus, first to an oval shape with highly condensed chromatin material, then to an elongated form, and eventually assuming the configuration of the nucleus of a mature spermatozoon. One of the last steps in the maturation process is the separation of the interconnected progeny.
Elaborate methods have been developed for quantitatively assessing the germ cell elements and the relationship of spermatogenesis to seminal fluid sperm density (28,29,30,31,32). The method of Johnson (29) applies a score of 1 to 10 for each tubule cross section examined. The criteria are as follows: 10, complete spermatogenesis and perfect tubules; 9, many spermatozoa present but disorganized spermatogenesis; 8, only a few spermatozoa present; 7, no spermatozoa but many spermatids present; 6, only a few spermatids present; 5, no spermatozoa or spermatids present but many spermatocytes present; 4, only a few spermatocytes present; 3, only spermatogonia present; 2, no germ cells present; 1, neither germ cells nor Sertoli cells present. The mean score count should be at least 8.90 with an average of 9.38, and 60% or more of the tubules should score at 10.
Two relatively simple methods are helpful to the surgical pathologist. The first method (32) involves establishing a germ cell to Sertoli cell ratio by counting at least 30 tubule cross sections. This ratio is relatively constant at approximately 13:1 in young healthy men. An average of 10 to 12 Sertoli cells per tubule cross section is considered normal, and approximately half the germ cell elements within the tubule should be in the spermatid stage. An assumption is made that the Sertoli cell population is stable throughout adult life. A reasonably good assessment of the presence or absence of hypospermatogenesis or maturation arrest can be made with this technique. A second method involves counting spermatids per tubule cross section (33). Only the mature spermatids, that is those with oval nuclei and dark, densely stained chromatin, are counted. Excellent correlations have been made with seminal fluid sperm counts. A spermatid/tubule cross section count of 45 corresponds to a seminal fluid sperm count of 85 106/mL. Spermatid/tubule counts of 40, 20, and 6 to 10 correspond to sperm counts of 45, 10, and 3 106/mL, respectively. A minimum of 20 tubules must be counted.
Interstitium
The interstitium of the testis accounts for 25 to 30% of the testicular mass. It can be divided loosely into intertubular and peritubular regions. Within the former are Leydig cells, blood vessels, lymphatics, nerves, macrophages, and mast cells. The macrophages are often found in close association with Leydig cells (34), where the two cells form complex cell-cell interdigitations. There is increasing evidence for an important paracrine role for macrophages in Leydig cell function (35). Surrounding each seminiferous tubule in a sheathlike fashion is the lamina propria or tunica propria, which consists of an inner basal lamina, surrounded by a
P.949
zone of multilayered spindle-shaped cells, intermingled with collagen and elastic fibers (Figure 37.9). The outermost two layers of cells stain for vimentin and actin but not for desmin. The inner layers of three to five cells stain intensely for desmin as well as actin and vimentin, findings that are characteristic of myofibroblasts (36). These same cells also contain androgen receptors in their nuclei (11). Elastic fibers first appear at puberty in the outermost layer of the lamina propria (37). There is a striking absence of elastic fibers in the lamina propria of sclerotic tubules in patients with Klinefelter's syndrome, in contrast to their abundance in the lamina propria of patients with postpubertal tubular sclerosis of multiple other causes (38).
A common finding in patients with oligozoospermia or azoospermia due to primary testicular failure is the accumulation of eosinophilic, acellular material in the lamina propria. This material is an admixture of increased collagen fibers, elastic fibrils, and basement membrane like material (39). The peritubular tissue of patients with hypogonadotropic hypogonadism is underdeveloped, having only one or two layers of myoid cells mixed with a few collagen fibers. In contrast, there is a large accumulation of collagen in the lamina propria of some of the seminiferous tubules of adult patients with cryptorchidism.
Leydig Cells
Adult Leydig cells, the source of testicular androgens, only rarely undergo mitotic division (40). They are found singly and in clusters within the interstitium of the testis; some lie immediately adjacent to capillaries, whereas others lie close to the peritubular myofibrocytes (Figure 37.10). They also may be seen in the tunica albuginea, epididymis, spermatic cord, and mediastinum of the testis. They are often located in intimate association with large nerve fibers, sometimes as a large cluster adjacent to the nerve (Figure 37.11) and, at other times, scattered randomly throughout the nerve fiber (41). They may be found at these sites in the fetus as well as the adult. The single nucleus of the cell is round and vesicular, with one to two eccentrically located nucleoli. Occasional binucleated cells are present. The cytoplasm is usually abundant and stains intensely with acid dyes. Lipid droplets and lipofuscin pigment are found in the cytoplasm, first appearing at the time of puberty and increasing in prominence in the aging testis. The characteristic intracytoplasmic Reinke crystalloids are present only in the postpubertal state (Figure 37.10). Their presence is highly variable in the normal testis, and they are frequently absent in Leydig cell tumors. The nature of the material is still unknown, although it is presumed to represent a protein product of the cell (42). The crystalloids stain negatively for actin, vimentin, and desmin.
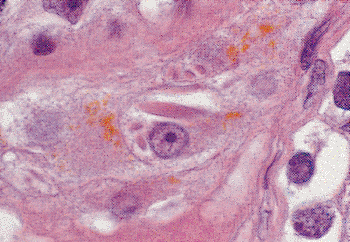 |
Figure 37.10 Leydig cells in interstitium of testis. An eosinophilic crystalloid of Reinke and abundant lipofuscin are prominent features in the cytoplasm of the Leydig cell in the center of this field. |
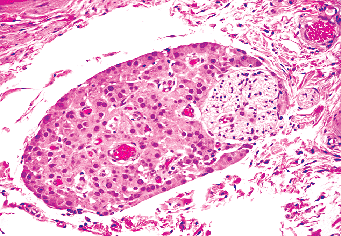 |
Figure 37.11 Leydig cells in intimate association with a nerve in the hilus of the testis. |
Quantitation of Leydig cells has been a difficult parameter to assess. Several indices have been used: mean Leydig cell number or Leydig cell cluster number per seminiferous tubule; mean number of Leydig cells per Leydig cell cluster; Leydig cell to Sertoli cell ratio; and ratio of Leydig cell area to seminiferous tubule area (43). Heller et al. (44), using the Leydig cell to Sertoli cell ratio, found a value of 0.39 and a range of 0.19 to 0.72. In that same study, they also determined the average number of Sertoli cells per tubular cross section to be 10.03 0.6. They made an assumption that the Sertoli cell population was stable in their calculation of the Leydig cell population. As a rule, normal adults should have four to five Leydig cells for each tubular cross section.
Vimentin represents the predominant intermediate filament, but actin filaments and neurofilament triplet proteins have also been identified in both Leydig and Sertoli cells (13). Androgen receptors are found within the nuclei but at a much lower intensity than found in Sertoli cells (11).
P.950
Calretinin, a protein found primarily in the cytoplasm and to a lesser extent in the nucleus, has been shown to be a reliable marker for normal and neoplastic Leydig cells. Theca interna cells of the ovary stain equally well, but Sertoli cells, granulosa cells, and theca externa cells stain little or not at all (45). Relaxin-like factor, also known as the Leydig cell insulin-like factor, stains strongly in the cytoplasm of Leydig cells, as well as theca interna cells and hilar cells in the ovary (46,47). Leydig cells stain positively for S-100, glial fibrillary acidic protein (48), synaptophysin, chromogranin A-B, and neuron-specific enolase but not for vimentin or desmin. A subpopulation of cells will stain intensely for nestin, an intermediate filament seen mainly in nerve and muscle progenitor cells (49). The presence of these substances undoubtedly indicates an important paracrine function that Leydig cells share with Sertoli cells, peritubular cells, macrophages, and nerves.
Vascular Supply
The blood supply to the testis is derived primarily from the internal spermatic (testicular) artery with a smaller contribution from the branches of the vasal portion of the internal (superior) vesicle artery. The testicular artery, arising from the aorta immediately distal to the renal artery, is highly coiled and extremely long relative to its diameter. It has a low pulse pressure as it enters the testis (50). The artery plays an important role in thermal regulation via countercurrent heat exchange with the veins of the pampiniform plexus. The combination of this vascular heat exchange and the heat lost via the thin scrotal covering layers serves to maintain the testicular temperature 2 C below body temperature. Arterial branches of the testicular artery arborize over the surface of the testis, penetrate the capsule, and pass in a centripetal fashion within the septa to the mediastinum, where they form a dense cluster. Only a few branches enter the lobules from these centripetal arteries. From the mediastinum, the small arterial segments then pass in a centrifugal fashion within the parenchyma, where they branch into arterioles and capillaries. The veins run either centrifugally or centripetally to the capsule or mediastinum, respectively, and eventually anastomose posteriorly to form the pampiniform plexus of the testicular vein. Biopsy specimens of the testis of patients with varicoceles often show a striking sclerosis of vascular walls (51). These changes appear to involve both arteries and veins. The significance of these vascular alterations with respect to seminiferous tubule function in patients with varicoceles is uncertain.
At puberty, there is extensive development of the intratesticular microvascular architecture, the most notable features being a marked coiling of the arteries and a great expansion of the peritubular capillary network. Unlike other capillaries of the systemic system, the testicular capillary walls have a prominent basement membrane and an incomplete outer layer of pericytes (52). Arteriovenous anastomoses occur in two locations: (a) beneath the tunica albuginea between branches of the centripetal artery and vein and (b) deep within the parenchyma between branches of the centrifugal artery and vein. The role of these anastomoses is unknown.
The capillary network appears to have a very structured arrangement with respect to the Leydig cells and the seminiferous tubules, ramifying around and within the Leydig cell-macrophage clusters and then penetrating the tunica propria of the seminiferous tubules. Not all areas of the tubule wall appear to be supplied with this elaborate network, and the reason for this is still unclear. The capillaries then reenter the loose interstitium as postcapillary venules, where they receive other capillaries coming from the Leydig cell clusters (53). The intralobular veins then enter the septa, where they move either to the mediastinum or to the tunica vasculosa.
There is a remarkable species-to-species variation in the distribution of lymphatic channels in the testis (54). In humans, ill-defined lymphatic spaces are found in the interstitium adjacent to Leydig cell clusters, but a peritubular lymphatic network is lacking. The lymphatic channels drain into the septa and thence to either the capsule or the mediastinum, where they join on the posterior aspect of the testis. They then anastomose with lymphatic channels from the epididymis, enter the spermatic cord, and drain into the periaortic lymph nodes.
Fetal and Prepubertal Testis
The fetal testis becomes recognizable at seven to eight weeks of gestation, at which time primitive testicular cords become evident. By the ninth week, the primordial germ cells, having migrated from their extraembryonal origin at the base of the allantois, have found their way into the cords, and are now referred to as gonocytes. These mitotically active cells, with a round nucleus and a prominent nucleolus, are scattered throughout the tubuli but usually are found in the center, surrounded by immature Sertoli cells (Figures 37.12,37.13). During the latter half of fetal life and the first six months after birth, the gonocytes undergo a maturation process in which they enter mitotic arrest. They become larger, acquire more cytoplasm, develop a prominent nucleus with a coarse chromatin pattern, and migrate to the periphery of the tubule to reach the basal lamina. At this time, these cells are variously referred to as prespermatogonia, prospermatogonia, M (multiplying) spermatogonia, T (transitional) spermatogonia, or simply spermatogonia (Figure 37.14). At birth, the germ cells average 4 per tubule cross section, two-thirds of them being gonocytes. At 45 days there are about
P.951
equal numbers of gonocytes and spermatogonia, with an average of 4 to 5 per tubule cross section (55). By the sixth postnatal month, virtually all of the germ cells are spermatogonia. This transition from the fetal stem cell pool to the adult stem cell pool is an important first step in the germ cell maturation process and appears to be defective in patients with cryptorchidism (56). From the age of one to four years, the number of germ cells average 1 to 2 per tubule cross section, and this number should double between the ages of 5 and 8 years (55). Although for the most part kept in mitotic arrest until just prior to puberty, occasional germ cells may be found in infancy, childhood, and even in the fetus that have entered the meiotic phase to form spermatocytes or even spermatids. In early puberty, repeated waves of incomplete spermatogenesis take place, with an orderly processs of complete maturation not appearing until the end of puberty (57). Several studies have demonstrated a marked diminution in the number of germ cells in the prepubertal cryptorchid testis (Figure 37.15) and a less predictable decrease of germ cells in the truly ectopic testis (27,58).
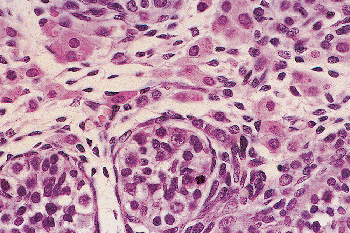 |
Figure 37.12 Fetal testis of 20 weeks' gestation, with numerous Leydig cells throughout the interstitium. |
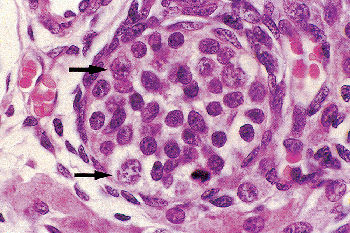 |
Figure 37.13 Same testis as shown in Figure 37.12. Note the mitotic figure, probably of a Sertoli cell. Larger cells (arrows) are undifferentiated germ cells. The remaining cells are immature Sertoli cells. |
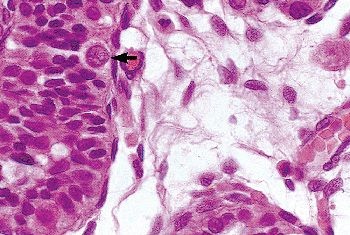 |
Figure 37.14 Testis of an 11-month-old child. A spermatogonium is present adjacent to the basement membrane (arrow). The interstitium contains undifferentiated spindle cells. |
Gonocytes and, less commonly, spermatogonia express a number of markers that are employed in evaluating germ cell neoplasms in adults, including the cytoplasmic placenta-like alkaline phosphatase (PLAP), the cell membrane proto-oncogene receptor c-kit, and the nuclear transcriptional regulator Oct3/4 (also known as Oct3 and Pou5F1). These markers first appear early in the first few weeks of gestation and gradually disappear at birth or soon thereafter. They are not seen in Leydig, Sertoli, or interstitial cells nor are they found in normal adult germ cells. Beginning at about the twentieth week of gestation, PLAP is the first to decrease, followed by Oct3/4, and then by c-kit. These declines correspond to the maturation process of the gonocytes into spermatogonia. Since the rate of disappearance is different for these markers, it is possible to observe germ cells staining positive for all three of these markers
P.952
simultaneously, positive staining only for c-kit and Oct-4 and not PLAP, or positive staining only for c-kit. Corresponding to their mitotic activity and eventual entry into mitotic arrest, gonocytes and spermatogonia both reveal strong nuclear staining with Ki-67 throughout gestation but with only a few cells reacting after birth (59).
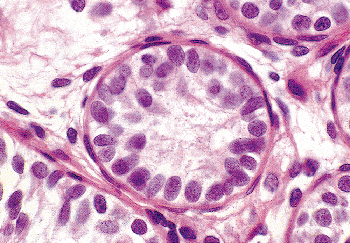 |
Figure 37.15 Thirteen-year-old prepubertal boy with bilateral cryptorchidism. Mature Leydig cells are absent in the interstitium. The tubules lack a lumen. The Sertoli cells are immature, and germ cells are absent. |
At 20 weeks of fetal life, the testis is characterized by abundant, well-developed Leydig cells filling the interstitium (Figure 37.12). The seminiferous tubules are solidly filled with Sertoli cells and germ cells, measure 45 to 50 m in diameter, and lack a well-defined lumen (60). The postnatal tubules slowly increase in size to reach a prepubertal diameter of 64 m (range: 43 70 m), at which time lumens begin to appear (61).
Fetal Sertoli cells outnumber germ cells in a ratio of 7:1 (61) and undergo active mitotic division during this period. Unlike its adult counterpart, the immature Sertoli cell has an oval to round nucleus and an inconspicuous nucleolus (Figure 37.13). Vimentin and cytokeratins 8 and 18 are expressed in the cytoplasm, as noted above. Nuclear androgen receptors are not identified.
Early studies had suggested that the Sertoli cell is mitotically inactive after birth, but stereologic studies indicate their number increases from 260 million late in fetal life, to 1,500 million between 3 months and 10 years, and to 3,700 million in the adult testis (62). They account for 95% of the tubule mass, with the germ cells comprising the other 5%. The testis shows a sixfold increase in size in the first year after birth, primarily as a result of Sertoli cell proliferation and the marked lengthening of the seminiferous tubules rather than a significant increase in tubule diameter (57). To a lesser degree, Sertoli cells continue to proliferate until puberty. Sertoli cells per cross section average 30 in the fetal testis after 20 weeks, increase to 42 at the fourth postnatal month, decrease to 26 at age 13 years, and to 12 to 15 in the adult testis (27,60). At puberty there is a fivefold increase in Sertoli cell volume, at which time Charcot-B ttcher crystalloids first appear. The cryptorchid testis commonly contains microscopic collections, or congeries, of very immature Sertoli cells, having either a round or fusiform-shaped nucleus and an inconspicuous nucleolus (Figure 37.16) (63).
The early prepubertal lamina propria is relatively undeveloped and is separated from the tubules by a thin basement membrane that lacks the knoblike thickening and splitting of the adult testis. The collagen fiber layer is relatively thin, and only one or two spindle-shaped cells are seen in the outer portion of the lamina propria.
Leydig cells are first recognizable by the eighth week of fetal life and become prominent in the fetal testis at 14 to 18 weeks of gestation (Figure 37.12), after which there is a progressive decline in number, with few mature-appearing Leydig cells being evident at birth. A second wave of Leydig cells appears at two to three months of neonatal life, corresponding to the activation of the hypothalamus/pituitary/gonad axis (64). This population is a mix of cells, some with the characteristics of mature Leydig cells, smaller cells with a round nucleus and fairly prominent nuleolus but without the prominent eosinophilic cytoplasm of typical adult Leydig cells, and spindle cells. After this second phase of Leydig cell activity ends at about the fourth neonatal month, only spindle cells (Figure 37.14) are recognized until just before puberty, when the precursor Leydig cells progressively differentiate and eventually take on the appearance of adult Leydig cells. Still unclear is whether adult Leydig cells are derived from dedifferentiated fetal Leydig cells or from the primitive fibroblast cell population (64,65).
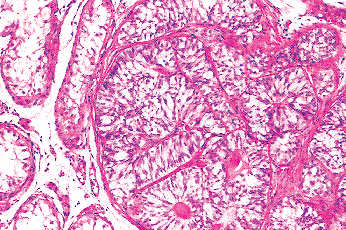 |
Figure 37.16 Sertoli cell collections, or congeries. The tubules are composed of immature Sertoli cells and lack both germ cells and lumens. |
Aging Testis
There is a general consensus that a decline in testicular function occurs with advancing age. These physiologic changes are matched by involutional changes in the testicular parenchyma, including hypospermatogenesis, peritubular fibrosis, and hyalinization of the seminiferous tubules. A few sclerotic tubules are found occasionally in the normal adult testis (66), but larger, focal, or diffuse areas of sclerosis are distinctly pathologic. Although the total number of peritubular cells is maintained in elderly men, there is a sharp decline in the proportion of cells that stain positively for desmin and actin (36).
Thickening of the testicular arterial walls with hyalinization, sometimes seen in otherwise normal testes, is found in over 90% of testes in which there are large zones of tubular fibrosis. The capillary bed in the aged testis becomes sparse and poorly organized (67). These vascular changes probably play a causal role in the peritubular sclerosis and tubular hyalinization.
Substantial controversy exists regarding the Leydig cell population in the aging testis (68,69,70,71). According to
P.953
Neaves et al. (72), Leydig cell numbers and size both progressively decline with advancing age, the total Leydig cell population decreasing by 50% during the first 30 years of adult life. The production of testosterone by Leydig cells is relatively maintained despite a loss of total Leydig cell mass, probably because of the large reserve of these cells in the adult testis. When the Leydig cell mass decreases to a certain threshold point, daily sperm production is depressed. The aged Leydig cell contains large amounts of lipofuscin pigment and numerous vacuoles within the cytoplasm.
Abnormal sperm maturation, sloughing of germ cells into the tubule lumen, degeneration of germ cell elements, and Sertoli cell lipid accumulation and cytoplasmic vacuolization are frequent findings in the aged testis. Earlier studies indicated that the Sertoli cell population is stable throughout the postpubertal years (73,74). However, Johnson et al. (75) found that men 20 to 48 years of age had significantly more Sertoli cells per tubule cross section than did men 50 to 85 years of age, and there was a relatively constant relationship between Sertoli cells and germ cells in both age groups. Furthermore, these investigators suggested that the decline in spermatozoa production in the elderly may be due to a decrease in Sertoli cell function and mass. Sertoli cells of those tubules demonstrating hypospermatogenesis have increased amounts of vimentin microfilaments and the reappearance of cytokeratins 8 and 18, suggesting that the reversion to the intermediate filament pattern of the fetal/prepubertal testis is related to the alteration of the normal spermatogenic process (6).
Rete Testes
The rete testis, a network of channels at the hilus of the testis, receives the luminal contents of the seminiferous tubules (Figure 37.1). It is divided into three components: the septal portion containing the tubuli rete, the mediastinal rete, and the extratesticular portion also known as the bullae retis (76,77). The tubuli rete are short tubules, 0.5 to 1.0 mm in length, that connect the two ends of the seminiferous tubule loop to the mediastinum testis. The terminal end of the seminiferous tubule usually consist only of Sertoli cells, forming an epithelial pluglike structure as it protrudes into the rete lumen (Figure 37.17). There are approximately 1,500 entrances of seminiferous tubules into the rete. A few tubules may enter the mediastinal rete directly without intervening tubuli rete. The mediastinal rete is a cavernous network of interconnecting channels that exits from the testis to form several dilated, vesicular channels or antechamber-like structures called the bullae retis. These structures, measuring up to 3.0 mm in width, anastomose together to form the ductuli efferentia.
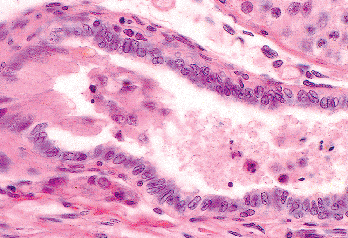 |
Figure 37.17 Junction of septal rete testis and terminal end of seminiferous tubule. Note the Sertoli cells pouting into the lumen of the rete. Rete epithelium is a low columnar type. |
The rete epithelium is a simple squamous or low columnar type (Figure 37.17), the luminal surface of which is studded with microvilli. Each cell contains a single, central flagellum that is inconspicuous on light microscopic examination. The epithelium sits on a relatively thick basal lamina, beneath which are a few fibroblasts and myoid cells intermixed with collagen and elastic fibers. Traversing the mediastinum and the extratesticular rete are epithelium-covered columns or strands called chordae retis. These columns, often appearing as islands on a cross section of the rete testis (Figure 37.18), vary greatly in length (15 100 m) and thickness (5 40 m) and serve to connect opposing walls of the chambers. The cytoplasm of the rete epithelium contains keratin and vimentin intermediate filaments, the former being located primarily in the apical portion of the cell and the latter being found in the basal region. The keratins, primarily of low-molecular weight types, can be first identified at the tenth week of fetal life and precede
P.954
the appearance of vimentin by two to three weeks (78). One would expect coexpression of these two intermediate filaments in the rare carcinoma of the rete or in hyperplasia of the rete. However, a report of nine cases of hyperplasia of the rete testis showed strong cytokeratin and epithelial membrane antigen staining but a negative reaction for vimentin (79). Sometimes found with hyperplastic or otherwise normal rete are hyalin refractile, eosinophilic globules, which are periodic acid-Schiff (PAS) positive, sometimes 1-antitrypsin positive, but -fetoprotein negative (80). These should not be confused with the globules produced by yolk sac tumors of the testis. The rete epithelium, as well that of efferent ductules and the caput (head) of the epididymis, also contains receptors for estrogen, progesterone, and androgen (81), the presence of estrogen receptors perhaps accounting for the hyperplasia of the rete and efferent ductules seen in patients undergoing sex-reversal procedures (82).
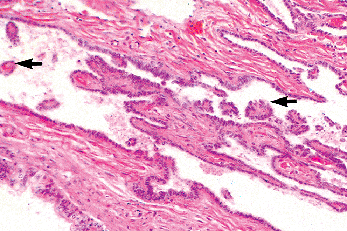 |
Figure 37.18 Rete testis, mediastinal portion, with irregular cavernous channels and cross sections of intratubular chordae (arrows). |
The rete serves multiple functions: (a) as a mixing chamber for the contents of the seminiferous tubules, (b) as a pressure gradient between the seminiferous tubules and the epididymis, (c) as a possible source of as yet unknown components of the seminal fluid, and (d) as a reabsorptive site of proteins from the luminal contents (83).
Ductuli Efferentia
The ductuli efferentia consist of five to six tubules that arise from the extratesticular rete testis (Figure 37.1) (84). They are involved primarily in resorption of fluid and do not appear to store spermatozoa for any length of time. These tubules aggregate to form a significant portion of the caput of the epididymis proper (Figure 37.19). Unlike the body of the epididymis, the lumens have an undulating border. The cells of the efferent ductules are composed of ciliated and nonciliated columnar cells, basal cells, and scattered intraepithelial lymphocytes, giving the epithelium a pseudostratified appearance (Figure 37.20). Occasional cells with a Paneth cell like appearance are seen (Figure 37.21). These numerous and brightly eosinophilic globules are PAS positive and diastase resistant and chromogranin A negative; they most likely represent prominent lysosomes. They are less frequently seen in the rest of the epididymis and are most often encountered in patients with epididymal obstruction (85). Coexpression of low-molecular weight cytokeratins and vimentin, as well as epithelial membrane antigen, is evident within the epithelial cytoplasm (78).
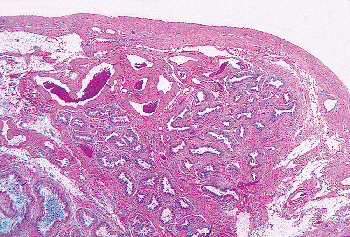 |
Figure 37.19 Caput (head) of epididymis, showing cross sections of distal portions of ductuli efferentia (upper right) and epididymis (lower left). |
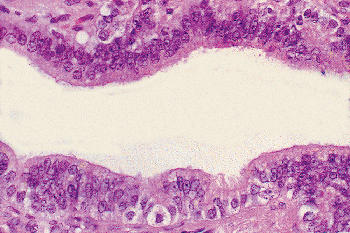 |
Figure 37.20 Epithelium of efferent ductulus. The columnar epithelial cells are mixed with basal cells and occasional intraepithelial lymphocytes, giving the epithelium a pseudostratified appearance. |
The epithelium sits on a thick basement membrane, surrounding which is a coat of smooth muscle cells and fibroblasts, as well as a few scattered macrophages. Intraluminal macrophages, actively phagocytizing spermatozoa, are occasionally present, particularly when duct obstruction exists.
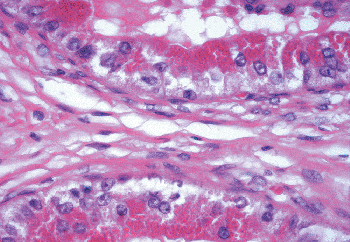 |
Figure 37.21 Epithelium of ductulus with prominent Paneth cell like intracytoplasmic vacuoles. |
P.955
Epididymis
The epididymis, a highly coiled, tubular structure, can be divided anatomically into caput, corpus, and cauda portions (Figure 37.1). The epididymis plays an important role in (a) sperm transport, (b) sperm maturation, including the acquisition of motility, (c) sperm concentration, and (d) sperm storage. The average sperm transit time through the epididymis in humans is 12 days (86). The transport mechanism is by way of muscle contractions of the thick, muscular coat that surrounds the epididymal tubules. There is extensive reabsorption of intraluminal fluid, particularly in the caput portion of the epididymis. Most of the sperm are stored in the caudal segment until ejaculation occurs, and it is in this location in humans that sperm maturation takes place (87). Many spermatozoa apparently undergo senescence and degeneration in the cauda via an unknown mechanism.
The epithelium of the epididymis consists of tall columnar or principal cells, basal cells, clear cells, tall slender or apical cells rich in mitochondria (apical mitochondria-rich cells), and scattered intraepithelial lymphocytes and macrophages (88). The principal cells, comprising over 95% of the columnar cells, have straight stereocilia (Figure 37.22), which are tall and nearly obliterate the lumen in the caput but become progressively shorter as the cauda is reached. The principal cells stain strongly for vimentin, epithelial membrane antigen (EMA), and acid phosphatase. Both basal and principal cells stain positively for low-molecular weight cytokeratins, with more intense staining in the corpus and cauda sections than the caput region (89). In cryptorchid testes the intensity of staining of low-molecular weight keratins, particularly cytokeratin 18, is markedly diminished (90). Intense CD10 paraluminal staining of the columnar cells is evident both in the epididymis and vas deferens, a finding that has been utilized to ascertain the possible origin of glandular structures found adjacent to the epididymis or vas deferens (91). The intensity of the vimentin staining progressively declines in the cauda section (89). The mitochondria-rich apical cells, located primarily in the caput, show intense staining for cytokeratins and acid phosphatase and less intense reactivity for EMA and vimentin. Their configuration varies from slender cells extending from the basement membrane to the lumen to those that appear to be located only in the apex of the duct (Figure 37.23). Small foci of epithelium may rarely have the appearance of prostatic epithelium, including positive immunostaining for prostate-specific antigen. Whether this process represents metaplasia or ectopia is unclear (92). Epididymal cells may contain lipofuscin pigment, which tends to be more prominent in the caput segment and is particularly evident when there is obstruction of the epididymis (93). The lumens are generally round and regular. In up to 50% of individuals, a cribriform pattern is seen, which may represent either a normal variation or a hyperplastic process (85,94). Importantly, this changes should not be mistaken for intraepididymal spread from a testicular germ cell tumor or a primary epididymal carcinoma. The absence of either prominent nucleoli or mitoses should be helpful features supporting a benign process.
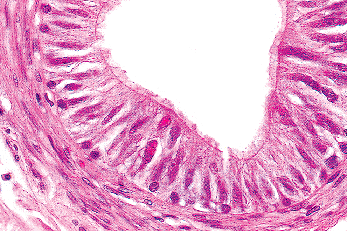 |
Figure 37.22 Epithelium of epididymis. Compare the tall columnar cells of the epididymis with the pseudostratified cells of the efferent ductulus. A few intraepithelial lymphocytes are present. The stereocilia are somewhat short, indicating the caudal segment. A layer of muscle cells forms the wall. |
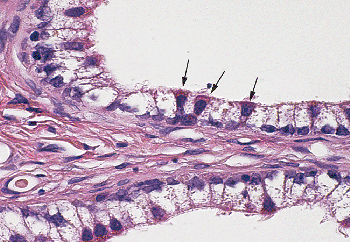 |
Figure 37.23 Epithelium of caput epididymis, demonstrating clear cells and apical mitochondrial-rich cells (arrows). |
Intranuclear, eosinophilic, PAS positive, and diastase-resistant inclusions (Figure 37.24), measuring 1 to 14 m, are found in the columnar cells of the adult epididymis as well as throughout the vas and seminal vesicles. Electron microscopic examination shows the electron-dense globules to be enclosed by a single membrane and to lack any features suggesting viral structures (95). They are most common in the distal epididymis and adjacent vas and least common in the ampulla of the vas and seminal vesicles.
P.956
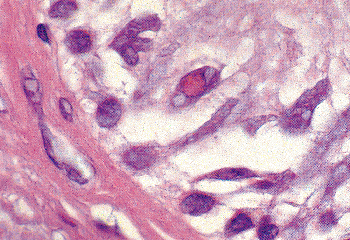 |
Figure 37.24 Intranuclear inclusions present in the epithelium of the epididymis. Similar inclusions are found in the epithelium of the ductus (vas) deferens. |
The epididymis is supported by a thick basement membrane, surrounding which is a well-defined muscular coat. The latter plays an important role in sperm movement through the epididymis. Mast cells are found throughout the connective tissue of the epididymis in a pattern similar to that seen in the tunica and interstitium of the testis (96), being numerous in infancy, decreasing in childhood, and then increasing at the time of puberty. A progressive decline in numbers occurs in adulthood.
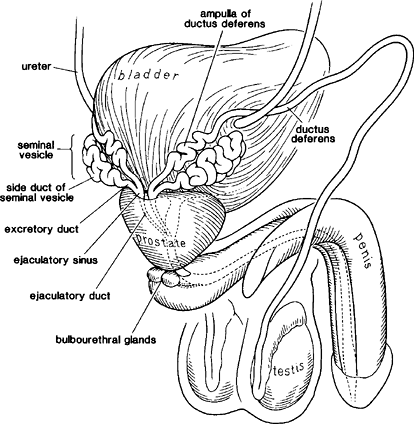 |
Figure 37.25 Diagram of excretory duct system from the vas to the ejaculatory ducts. |
Ductus (VAS) Deferens
The ductus (vas) deferens, a tubular structure arising from the caudal portion of the epididymis, measures 30 to 40 cm in length. The distal 4 to 7 cm portion is enlarged to form the ampulla. The latter joins the excretory duct of the seminal vesicle to form the ejaculatory duct (Figure 37.25). The adult vas is lined by a pseudostratified, columnar epithelium, composed of columnar cells and basal cells by light microscopic examination. Ultrastructural studies show four different cell types: principal cells, pencil or peg cells, mitochondria-enriched cells, and basal cells. The luminal surface of the columnar cell is lined by tall stereocilia throughout most of the vas (97). These stereocilia are substantially shorter and sparser in the ampullary region. Prominent intranuclear inclusions, as described above in the epididymis, may be seen (95). In addition, occasional lipid-positive vacuoles are present within the cytoplasm. The epithelium of the vas is thrown into folds, which are relatively simple in the proximal vas (Figure 37.26) but become much more complicated in the ampullary segment (Figure 37.27). The ampulla has highly complex infoldings and many outpocketings or diverticula that reach into the muscle coat. Beneath the epithelium of the vas is a loose, connective tissue stroma that, after puberty, contains a well-defined, circumferentially oriented layer of elastic fibers (98). These fibers, lacking in infants and children, become frayed and fragmented in the aged vas. The muscle coat is an extraordinary thick structure with inner and outer longitudinal
P.957
coats and a middle oblique or circular zone. The entire muscle mass progressively decreases as one approaches the ampulla, although the inner longitudinal layer becomes somewhat thicker as one progresses distally (97). The epithelium in the ampulla contains significant amounts of lipofuscin pigment and rather closely resembles the epithelium seen in the seminal vesicles. Active phagocytosis of degenerated spermatozoa has been demonstrated in the ampullary region of a number of mammalian species (99).
 |
Figure 37.26 Proximal ductus (vas) deferens. This cross section shows a thick muscle coat, tiny lumen, and slightly folded mucosa. |
Seminal Vesicles
The seminal vesicles are paired, highly coiled, tubular structures, lying posterolateral to the base of the bladder and in a parallel path with the ampulla of the vas deferens (Figure 37.25). Each vesicle measures 3.5 to 7.5 cm in length and 1.2 to 2.4 cm in thickness in the adult. The main duct, which is duplicated in approximately 10% of individuals, measures 10 to 15 cm in length when unraveled. Six to eight first-order side ducts extend off the main duct, and several secondary side ducts are derived from these. The upper part of the main duct is bent backward in a hooklike fashion. A short excretory duct combines with the ampulla of the vas to form the ejaculatory duct (Figure 37.25). The wall of the seminal vesicle has a thin external longitudinal and a thicker internal circular muscle layer. The mucosal folds, relatively simple and shallow in infancy and childhood, become highly complex and alveolus-like in the reproductive years (Figure 37.28) and are blunted in the aged vesicle. The lumen may contain a few sloughed epithelial cells and debris. Commonly seen within the lumens are eosinophilic secretions, often with crystalloid structures. The latter usually have a platelike arrangement but sometimes appear as smaller crystalloids similar to those seen in the lumens of well-differentiated prostate carcinomas. Their significance is unknown, but one should be aware of their presence in biopsies where the seminal vesicle is inadvertantly sampled (100). Spermatozoa, refluxed from the ejaculatory duct, occasionally may be present, although they are not normally stored within the seminal vesicles.
 |
Figure 37.27 Ampullary region of ductus (vas) deferens. Note the complex folding and outpouching of the mucosa into the muscular coat. |
The vesicle epithelium is composed of columnar and basal cells. The former have short microvilli projecting from the surface. The cytoplasm characteristically contains a large amount of lipofuscin pigment, a feature important in recognizing these cells obtained by needle biopsy or aspiration cytology studies of the prostate. Similar lipofuscin pigment is found in the ampulla of the ductus deferens and in the epithelium of the ejaculatory ducts. The pigment has been divided into two different types, based on its appearance. Type 1 consists of coarse, highly refractile, golden brown granules of uniform diameter (1 2 g). Type 2 granules are much more variable in size (0.25 4 g), faintly or nonrefractile and yellow-brown, grey-brown, blue, or pink.
P.958
Both types 1 and 2 granules are found in the seminal vesicle, vas, and ejaculatory ducts, whereas only the type 2 granules are found in prostatic epithelium (101).
 |
Figure 37.28 Seminal vesicle: alveolus-like arrangement of mucosal folds and cross sections of side ducts. |
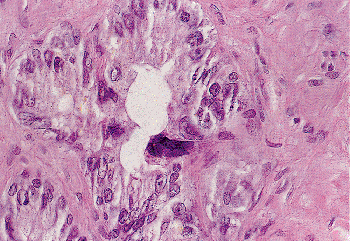 |
Figure 37.29 Seminal vesicle epithelium. Tall columnar cells line the lumen. A single hyperchromatic monster cell is present. These cells should not be mistaken for malignant cells. |
An unusual feature of the seminal vesicular epithelium is the presence of peculiar, monstrous epithelial cells (Figure 37.29). Similar cells may be seen in the ampulla of the vas and, less commonly, more proximally in the vas or epididymis. These cells have enlarged, hyperchromatic, and often irregularly shaped nuclei and are found in approximately three-quarters of adult seminal vesicles. They are not seen in infants or children. Their genesis is unknown but may be related to endocrine influences, similar to the Arias-Stella cells seen in gestational endometrium. Because these cells may be encountered in both needle biopsy and aspiration biopsy specimens, the surgical pathologist must be alert to avoid identifying them as malignant cells (102).
Also encountered in the muscle portion of the seminal vesicle are hyalin, pink globules (Figure 37.30), thought to represent degenerating smooth muscle cells. They also may be seen occasionally in the muscular coat of the vas and within the prostatic parenchyma (103).
Ejaculatory Ducts
The ejaculatory ducts are short (1.5 cm) paired ducts, arising from the confluence of the excretory duct of the seminal vesicle and the ampulla of the vas, that quickly converge and enter the prostate (Figure 37.31). They run through the central zone of the prostatic parenchyma and enter the posterior aspect of the distal prostatic urethra at the verumontanum (104). The outer portions of the ejaculatory ducts have a thin muscle coat that progressively becomes more attenuated as the ducts pass through the prostate. The epithelium of the ejaculatory ducts resembles that of the seminal vesicle and ampulla of the vas (Figure 37.32). On occasion, a needle biopsy of the prostate will sample a portion of one of these ducts, making it imperative that the surgical pathologist be aware of the characteristics of these cells. The presence and character of the lipofuscin pigment in the cytoplasm should give a clue to the cell origin. Immunoperoxidase stains for prostate-specific antigen demonstrate a sharp contrast between the positively stained prostatic parenchyma and the negatively stained cells of the intraprostatic ejaculatory ducts (Figure 37.33).
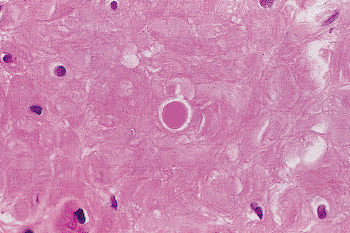 |
Figure 37.30 Muscle coat of seminal vesicle showing a hyalin globule, which probably represents a degenerated smooth muscle cell. |
Testicular Appendages
The testicular and paratesticular appendages are remnants of either the mesonephric duct or the paramesonephric (m llerian) duct (77). The four testicular appendages are
P.959
the appendix testis (hydatid of Morgagni), appendix epididymis, vas aberrans (organ of Haller), and paradidymis (organ of Giraldes) (Figure 37.1).
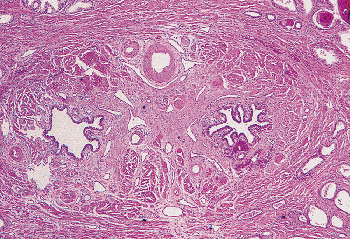 |
Figure 37.31 Paired ejaculatory ducts within the prostatic parenchyma. This section is adjacent to the verumontanum of the prostatic urethra. |
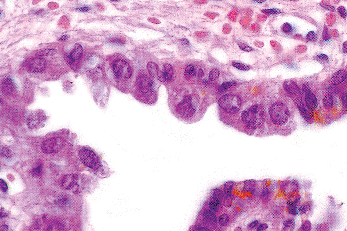 |
Figure 37.32 Epithelium of prostatic portion of ejaculatory duct. This epithelium may be encountered in a needle biopsy or aspiration biopsy of the prostate and should not be misinterpreted as malignant. |
The appendix testis, a remnant of the cranial portion of the m llerian duct, is attached to the tunica vaginalis on the anterosuperior aspect of the testis just below the caput of the epididymis. Occasionally, it is attached to both the testis and the epididymis. It is most often sessile and either oval or fan-shaped (approximately 90%) and, less commonly, pedunculated (approximately 10%) and measures 0.5 to 2.5 cm in greatest dimension (Figure 37.34) (105,106). It is occasionally represented by only a slight roughening or a calcified thickening of the tunica vaginalis. Approximately 80% of individuals have an appendix testis, with bilaterality in one-third of them. The appendix is covered by a cuboidal or columnar epithelium, which may be ciliated (Figure 37.35). The structure has a highly vascular fibrous core, containing variable numbers of smooth muscle cells. Tubular invaginations and small glandlike structures may also be found in the stroma. Rarely, a macroscopic cyst may be present. Because of its pedunculated structure, the appendix testis can become twisted, causing hemorrhagic infarction and producing severe testicular pain (107). This event occurs most often in prepubertal or pubertal boys and may be related to the presence of androgen and estrogen receptors known to be present in the appendix epithelium (and the appendix epididymis) and the possible growth of the appendix as a result of stimulation by androgens and estrogens at this period of time (108).
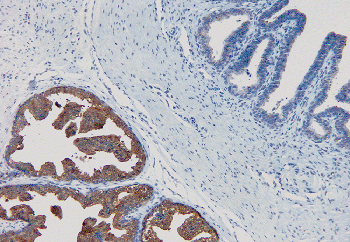 |
Figure 37.33 Intraprostatic ejaculatory duct (right) and adjacent prostatic tissue (left). Prostate-specific antigen immunoperoxidase from the central zone of the prostate is used to demonstrate strongly positive cytoplasmic staining of the prostatic secretory cells and negative staining of ejaculatory duct epithelium. |
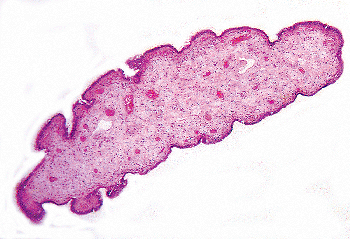 |
Figure 37.34 Appendix testis. This specimen was an incidental finding in a surgically removed testis. It was pedunculated and measures 0.9 cm in greatest length. |
The appendix epididymis (Figure 37.36), a remnant of the most cranial portion of the mesonephric duct, is present in approximately 25% of testes (106). It is almost invariably cystic, the vesicle lumen being filled with amorphous protein secretions. The epithelium lining the cyst is
P.960
columnar and often ciliated (Figure 37.37). The external surface is covered by a flattened layer of serosal cells. Because it may be pedunculated, it is also subject to torsion and infarction.
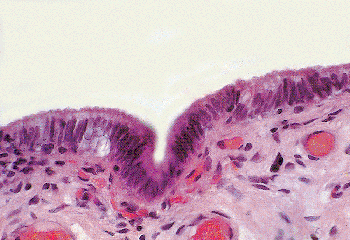 |
Figure 37.35 Appendix testis with covering of low columnar, nonciliated epithelium. |
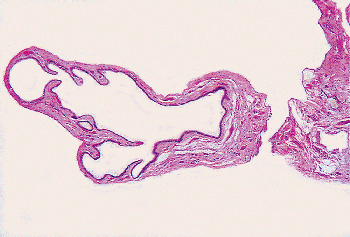 |
Figure 37.36 Appendix epididymis. A pedunculated cystic structure is attached to the caput of the epididymis. |
The other appendicular structures are derived from remnants of either the mesonephric tubules or the paramesonephric ducts and are variably encountered in the fat, usually as microscopic incidental findings. These are the vas aberrans inferior, the vas aberrans superior, and the paradidymis. They all have somewhat similar histologic features, with a low columnar epithelium lining a small cystic space and a thin muscular coat. Some investigators collectively refer to these structures as the paradidymis (109). The vas aberrans inferior is a tubular structure (Figure 37.38) located near the junction of the vas and the caudal portion of the epididymis and which may or may not communicate with either structure.
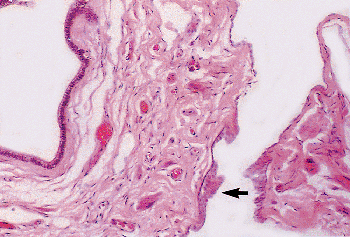 |
Figure 37.37 Appendix epididymis. Low columnar epithelium lines the cystic space. The outer covering of simple squamous epithelium has become stratified at the point of attachment to the caput of the epididymis (arrow). |
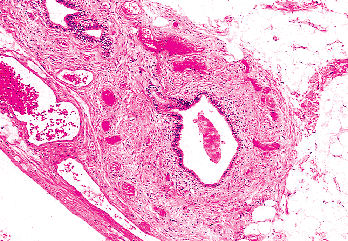 |
Figure 37.38 Vas aberrans inferior. A tubular structure is found near the caudal portion of the epididymis. |
The vas aberrans superior is a small collection of tubules located near the caput or body of the epididymis. It may communicate with the epididymis or the rete testis. Remnants of the vas aberrans may be the origin of cord cysts, seen sporadically in isolated individuals, in patients whose mothers had been treated with diethylstilbestrol (110), or in patients with von Hippel-Lindau syndrome (111).
The paradidymis is represented by one or more tubules embedded in the spermatic cord, adjacent to the ductus (vas) deferens, and near the caput of the epididymis. These tubules may be encountered in a section of the wall of an inguinal hernia sac and should not be mistaken for a portion of the ductus deferens (112). The relatively thick muscle coat of the true vas should allow for the correct interpetation. Rarely, macroscopic cysts may form in the spermatic cord (113).
Although not part of the paradidymis, infrequently one may find adrenal cortical rests in the fat of the spermatic
P.961
cord, adjacent to the vas, the epididymis or the rete testis (Figure 37.39). Very rarely, adrenal medullary tissue may also be present. Even less commonly seen is splenogonadal fusion, in which the splenic and gonadal anlages fuse in early embryonal life. This process is almost always on the left side and most commonly is found as an incidental finding in a cryptorchid testis (Figure 37.40). Occasionally it presents as a mass in the scrotum or inguinal canal.
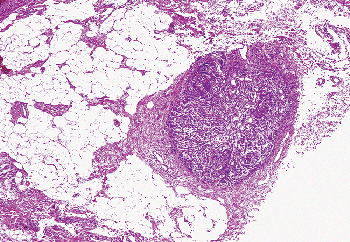 |
Figure 37.39 Adrenal cortical rest in fat adjacent to the epididymis. |
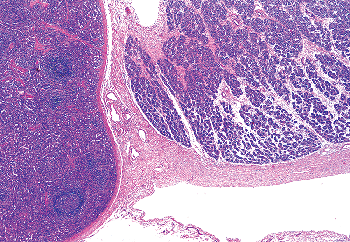 |
Figure 37.40 Splenogonadal fusion of a cryptorchid testis in a 3-year-old child. Spleen is on the left and gonad on the right. |
Gubernaculum
The gubernaculum has been the center of attention with respect to the descent of the testis since it was first described by John Hunter in 1762, and its specific role is still being debated. In the fetus, this cylindrical, gelatinous structure is attached cranially to the testis and epididymis (Figure 37.41) and caudally to the anterior abdominal wall at the site of the inguinal canal. Just before the descent of the testis through the inguinal canal, the gubernaculum increases in net weight disproportionately to the testis, supporting the theory that this structure plays a crucial role in this phase of the passage of the testis into the scrotum.
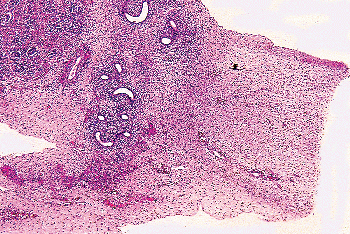 |
Figure 37.41 Gubernaculum and cranial attachment to epididymis and testis in a 26-week-old fetus. |
Histologically, the fetal gubernaculum is composed of a loose undifferentiated mesenchymal tissue similar to Wharton's jelly. Large amounts of glycosaminoglycans fill the extracellular space and separate the individual spindle cells. At the periphery of the gubernaculum, where it attaches to the inguinal wall, a few striated muscle cells can be identified. These fibers are derived from the developing cremaster muscle. The cranial portion of the early fetal gubernaculum is completely devoid of striated muscle. After the testis descends into the scrotum, the gubernaculum undergoes degenerative changes, loses much of the intercellular matrix, and becomes infiltrated by blood vessels, collagen fibers, and striated muscle.
References
1. Handelsman DJ, Staraj S. Testicular size: the effects of aging, malnutrition, and illness. J Androl 1985;6:144 151.
2. Brennan MB, Scrigley JR. Brenner tumors of the testis and paratestis. J Urol Pathol 1999;10:219 228.
3. Sosnik H. Studies of the participation of the tunica albuginea and rete testis (TA and RT) in the quantitative structure of human testis. Gegenbaurs Morphol Jahrb 1985;131:347 356.
4. Lennox B, Ahmad RN, Mack WS. A method for determining the relative total length of the tubules in the testis. J Pathol 1970;102:229 238.
5. Sharpe R, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003;125:769 784.
6. de Miguel M, Bethencourt F, Arenas M, Fraile B, Paniagua R. Intermediate filaments in the Sertoli cells of the ageing human testis. Virchows Arch 1997;431:131 138.
7. Nielsen K, Jacobsen GK. Malignant Sertoli cell tumour of the testis: an immunohistochemical study and a review of the literature. APMIS 1988;96:755 760.
8. Stosiek P, Kasper M, Karsten U. Expression of cytokeratins 8 and 18 in human Sertoli cells of immature and atrophic seminiferous tubules. Differentiation 1990;43:66 70.
9. Rogatsch H, Jezek D, Hittmair A, Mikuz G, Feichtinger H. Expression of vimentin, cytokeratin, and desmin in Sertoli cells of human fetal, cryptorchid, and tumour-adjacent testicular tissue. Virchows Arch 1996;427:497 502.
10. Hoei-Hansen C, Holm M, Rajpert-De Meyts E, Skakkebaek NE. Histological evidence of testicular dygenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol 2003;200:370 374.
11. Suaraz-Quian CA, Martinez-Garcia F, Nistal M, Regadera J. Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab 1999;84:350 358.
12. Emerich D, Hemendinger R, Halberstadt C. The testicular-derived Sertoli cell: cellular immunoscience to enable transplantation. Cell Transplant 2003;12:335 349.
13. Davidoff M, Middendorff R, Pusch W, Muller D, Wichers S, Holstein AF. Sertoli and Leydig cells of the human testis express neurofilament triplet proteins. Histochem Cell Biol 1999;111:173 187.
14. McLaren A. Studies on mouse germ cells inside and outside the gonad. J Exp Zool 1983;228:167 171.
P.962
15. Rey R. Regulation of spermatogenesis. Endocr Dev 2003;5:38 55.
16. Schulze C. Sertoli cells and Leydig cells in man. Adv Anat Embryol Cell Biol 1984;88:1 104.
17. Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood testis barrier. Anat Rec 1973;175:639 656.
18. Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985;94:177 211.
19. Witschi E. Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal fold. Contrib Embryol 1948;32:67 80.
20. Heller CG, Clermont Y. Kinetics of the germinal epithelium in man. Recent Prog Horm Res 1964;20:545 575.
21. Kerr JB, de Kretser DM. The cytology of the human testis. In: Burger MD, de Kretser D, eds. The Testis. New York: Raven Press; 1981:141 169.
22. Gondos B. Ultrastructure of developing and malignant germ cells. Eur Urol 1993;23:68 75.
23. Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972;52:198 236.
24. Schulze W, Riemer M, Rehder U, Hohne K. Computer-aided three-dimensional reconstructions of the arrangement of primary spermatocytes in human seminiferous tubules. Cell Tissue Res 1986;244:1 8.
25. Johnson L, McKenzie KS, Snell JR. Partial wave in human seminiferous tubules appears to be a random occurrence. Tissue Cell 1996;28:127 136.
26. Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology 1995;136:3 4
27. Hadziselimovic F. Ultrastructure of normal and cryptorchid testis development. Adv Anat Embryol Cell Biol 1977;53:3 71.
28. Johnson L, Petty CS, Neaves WB. The relationship of biopsy evaluations and testicular measurements to over-all daily sperm production in human testes. Fertil Steril 1980;34:36 40.
29. Johnsen SG. Testicular biopsy score count a method for registration of spermatogenesis in human testis: normal values and results in 335 hypogonadal males. Hormones 1970;1:2 25.
30. Weissbach L, Ibach B. Quantitative parameters for light microscopic assessment of the tubuli seminiferi. Fertil Steril 1976;27:836 847.
31. Zuckerman Z, Rodriguez-Rigau LJ, Weiss D, Chowdhury AK, Smith KD, Steinberger E. Quantitative analysis of the seminiferous epithelium in human testicular biopsies, and the relation of spermatogenesis to sperm density. Fertil Steril 1978;30:448 455.
32. Skakkebaek NE, Heller CG. Quantification of human seminiferous epithelium. I. Histological studies in 21 fertile men with normal chromosome. J Reprod Fertil 1973;32:379 389.
33. Silber SJ, Rodriguez-Rigau LJ. Quantitative analysis of testicular biopsy: determination of partial obstruction and prediction of sperm count after surgery for obstruction. Fertil Steril 1981;36:480 485.
34. Miller SC, Bowman BM, Rowland HG. Structure, cytochemistry, endocytic activity, and immunoglobulin (Fc) receptors of rat testicular interstitial-tissue macrophages. Am J Anat 1983;168:1 13.
35. Hutson JC. Testicular macrophages. Int Rev Cytol 1994;149:99 143.
36. Arenas MI, Bethencourt FR, De Miguel MP, Fraile B, Romo E, Paniagua R. Immunocytochemical and quantitative study of actin, desmin and vimentin in the peritubular cells of the testes from elderly men. J Reprod and Fertil 1997;110:183 193.
37. De Menezes AP. Elastic tissue in the limiting membrane of the human seminiferous tubule. Am J Anat 1977;150:349 373.
38. Nistal M, Paniagua R. Non-neoplastic diseases of the testis. In: Bostwick DG, Elbe JN, eds. Urologic Surgical Pathology. St Louis: Mosby; 1997:457 465.
39. de Kretser DM, Kerr JB, Paulsen CA. The peritubular tissue in the normal and pathological human testis: an ultrastructural study. Biol Reprod 1975;12:317 324.
40. Amat P, Paniagua R, Nistal M, Martin A. Mitosis in adult human Leydig cells. Cell Tissue Res 1986;243:219 221.
41. Nistal M, Paniagua R. Histogenesis of human extraparenchymal Leydig cells. Acta Anat (Basel) 1979;105:188 197.
42. Nagano T, Otsuki I. Reinvestigation of the fine structure of Reinke's crystal in the human testicular interstitial cell. J Cell Biol 1971;51:148 161.
43. Weiss DB, Rodriguez-Rigau L, Smith KD, Chowdhury A, Steinberger E. Quantitation of Leydig cells in testicular biopsies of oligospermic men with varicocele. Fertil Steril 1978;30:305 312.
44. Heller CG, Lalli MF, Pearson JE, Leach DR. A method for the quantification of Leydig cells in man. J Reprod Fertil 1971;25:177 184.
45. Cao Q, Jones J, Li M. Expression of calretinin in human ovary, testis, and ovarian sex cord-stromal tumors. Int J Gynecol Pathol 2001;20:346 352.
46. Ivell R. Biology of the relaxin-like factor (RLF). Rev Reprod 1997;2:133 138.
47. Bamberger A, Ivell R, Balvers M, et al. Relaxin-like factor (RLF): a new specific marker for Leydig cells in the ovary. Int J Gynecol Pathol 1999;18:163 168.
48. Davidoff MS, Middendorff R, Kofuncu E, Muller D, Jezek D, Holstein AF. Leydig cells of the human testis possess astrocyte and oligodendrocyte marker molecules. Acta Histochem 2002;104:39 49.
49. Lobo M, Arenas M, Alonso J, et al. Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell Tissue Res 2004;316:369 376.
50. Kormano M, Suoranta H, Reijonen K. Blood supply to testis and excurrent ducts. In: Raspe G, ed. Advances in the Biosciences. Vol 10. Oxford, England: Pergamon Press; 1972:72 83.
51. Andres T, Trainer T, Lapenas D. Small vessel alterations in the testes of infertile men with varicocele. Am J Clin Pathol 1981;76:378 384.
52. Fawcett DW, Leak LV, Heidger PM Jr. Electron microscopic observations on the structural components of the blood testis barrier. J Reprod Fertil Suppl 1979;10:105 122.
53. Ergun S, Stingl S, Holstein A. Microvasculature of the human testis in correlation to Leydig cells and seminiferous tubules. Andrologia 1994;26:255 262.
54. Fawcett DW, Neaves WB, Flores MN. Comparative observations on intertubular lymphatics and the organization of the interstitial tissue of the mammalian testis. Biol Reprod 1973;9:500 532.
55. Hadziselimovic F, Thommen J, Girard J, Herzog B. The significance of postnatal gonadotropin surge for testicular development in normal and cryptorchid testes. J Urol 1986;136(pt 2):274 276.
56. Huff, DS, Fenig DM, Canning DA, Carr MG, Zderic SA, Snyder HM III. Abnormal germ cell development in cryptorchidism. Horm Res 2001;55:11 17.
57. Chemes H. Infancy is not a quiescent period of testicular development. Int J Androl 2001;24:2 7.
58. Nistal M, Paniagua R, Queizan A. Histologic lesions in undescended ectopic obstructed testes. Fertil Steril 1985;43:455 462.
59. Honecker F, Stoop H, de Krijger R, Chris Lau YF, Bokemeyer C, Looijenga LH. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol 2004;203:849 857.
60. Waters B, Trainer T. Development of the human fetal testis. Pediatr Pathol Lab Med 1996;16:9 23.
61. Muller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered sudden death. Int J Androl 1983;6:143 156.
62. Cortes D, Muller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl 1987;10:589 596.
63. Symington T, Cameron K. Endocrine and genetic lesions. In: Pugh CB, ed. Pathology of the Testis. Oxford, England: Blackwell Scientific; 1976:259 303.
64. Nistal M, Paniagua R, Regadera J, Santamaria L, Amat P. A quantitative morphological study of human Leydig cells from birth to adulthood. Cell Tissue Res 1986;246:229 236.
65. Prince F. Commentary. The triphasic nature of Leydig cell development in humans, and comments on nomenclature. J Endocrinol 2001;168:213 216.
66. Paniagua R, Nistal M, Amat P, Rodriguez M, Martin A. Seminiferous tubule involution in elderly men. Biol Reprod 1987;36:939 947.
P.963
67. Suoranta H. Changes in the small blood vessels of the adult human testis in relation to age and to some pathological conditions. Virchows Arch A Pathol Anat 1971;352:165 181.
68. Kothari LK, Gupta AS. Effect of ageing on the volume, structure and total Leydig cell content of the human testis. Int J Fertil 1974;19:140 146.
69. Sokal Z. Morphology of the human testes in various periods of life. Folia Morphol 1964;23:102 111.
70. Sargent JW, McDonald JR. A method for the quantitative estimate of Leydig cells in the human testis. Proc Staff Meet Mayo Clin 1948;23:249 254.
71. Kaler LW, Neaves WB. Attrition of the human Leydig cell population with advancing age. Anat Rec 1978;192:513 518.
72. Neaves WB, Johnson L, Petty C. Seminiferous tubules and daily sperm production in older adult men with varied numbers of Leydig cells. Biol Reprod 1987;36:301 308.
73. Rowley MJ, Heller CG. Quantitation of the cells of the seminiferous epithelium of the human testis employing the Sertoli cell as a constant. Z Zellforsch Mikrosk Anat 1971;115:461 472.
74. Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod 1971;4:84 87.
75. Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 1984;31:785 795.
76. Roosen-Runge EC, Holstein AF. The human rete testis. Cell Tissue Res 1978;189:409 433.
77. Srigley J. The paratesticular region: histoanatomic and general considerations. Semin Diagn Pathol 2000;17:258 269.
78. Dinges H, Zatloukal K, Schmid C, Mair S, Wirnsberger G. Co-expression of cytokeratin and vimentin filaments in rete testis and epididymis. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol 1991;418:119 127.
79. Hartwick RY, Ro JY, Srigley JR, Ondonez NG, Ayala AG. Adenomatous hyperplasia of the rete testis: a clinicopathologic study of nine cases. Am J Surg Pathol 1991;15:350 357.
80. Jones E, Murray S, Young R. Cysts and epithelial proliferations of the testicular collecting system (including rete testis). Semin Diagn Pathol 2000;17:270 293.
81. Hittmair A, Zelger B, Obrist P, Dirnhofer S. Ovarian Sertoli-Leydig cell tumor: a SRY gene-independent pathway of pseudomale gonadal differentiation. Hum Pathol 1997;28:1206 1210.
82. Sapino A, Pagani A, Godano A, Bussolati G. Effects of estrogens on the testis of transsexuals: a pathological and immunocytochemical study. Virchows Arch A 1987;411:409 414.
83. Hinton BT, Keefer DA. Evidence for protein absorption from the lumen of the seminiferous tubule and rete of the rat testis. Cell Tissue Res 1983;230:367 375.
84. Saitoh K, Terada T, Hatakeyama S. A morphological study of the efferent ducts of the human epididymis. Int J Androl 1990;160:369 376.
85. Shah V, Ro J, Amin M, Mullick S, Nazeer T, Ayala AG. Histologic variations in the epididymis: findings in 167 orchiectomy specimens. Amer J Surg Path 1998;22:990 996.
86. Rowley MJ, Teshima F, Heller CG. Duration of transit of spermatozoa through the human male ductular system. Fertil Steril 1970;21:390 396.
87. Hinrichsen MJ, Blaquier JA. Evidence supporting the existence of sperm maturation in the human epididymis. J Reprod Fertil 1980;60:291 294.
88. Regadera J, Cobo P, Paniagua R, Martinez-Garcia F, Palacios J, Nistal M. Immunohistochemical and semiquantitative study of the apical mitochondria-rich cells of the human prepubertal and adult epididymis. J Anat 1993;183(pt 3):507 514
89. Kasper M, Stosiek P. Immunohistochemical investigation of different cytokeratins and vimentin in the human epididymis from the fetal period up to adulthood. Cell Tissue Res 1989;257:661 664.
90. De Miguel M, Marino J, Gonzalez-Peramato P, Nistal M, Regadera J. Epididymal growth and differentiation are altered in human cryptorchidism. J Androl 2001;22:212 225.
91. Cerilli L, Sotelo-Avila C, Mills S. Glandular inclusions in inguinal hernia sacs: morphologic and immunohistochemical distinction from epididymis and vas deferens. Am J Surg Pathol 2003;27:469 476.
92. Lee LY, Tzeng J, Grosman M, Unger PD. Prostate gland-like epithelium in the epididymis: a case report and review of the literature. Arch Pathol Lab Med 2004;128:e60 e62.
93. Rajalakshmi M, Kumar B, Kapur M, Pal P. Ultrastructural changes in the efferent duct and epididymis of men with obstructive infertility. Anat Rec 1993;237:199 207.
94. Oliva E, Young R. Paratesticular tumor-like lesions. Semin Diagn Pathol 2000;17:340 358.
95. Madara JL, Haggitt RC, Federman M. Intranuclear inclusions of the human vas deferens. Arch Pathol Lab Med 1978;102:648 650.
96. Nistal M, Santamaria L, Paniagua R. Mast cells in the human testis and epididymis from birth to adulthood. Acta Anat (Basel) 1984;119:155 160.
97. Paniagua R, Regadera J, Nistal M, Abaurrea MA. Histological, histochemical and ultrastructural variations along the length of the human vas deferens before and after puberty. Acta Anat (Basel) 1981;111:190 203.
98. Paniagua R, Regadera J, Nistal M, Santamaria L. Elastic fibres of the human ductus deferens. J Anat 1983;137(pt 3):467 476.
99. Murakami M, Nishida T, Shiromoto M, Inokuchi T. Scanning and transmission electron microscopic study of the ampullary region of the dog vas deferens, with special reference to epithelial phagocytosis of spermatozoa and latex beads. Anat Anz 1986;162:289 296.
100. Shah R, Lee M, Giraldo A, Amin M. Histologic and histochemical characterization of seminal vesicle intraluminal secretions. Arch Pathol Lab Med 2001;125:141 145.
101. Shidham V, Lindholm P, Kajdacsy-Balla A, Basir Z, George V, Garcia FU. Prostate-specific antigen expression and lipochrome pigment granules in the differential diagnosis of prostatic adenocarcinoma versus seminal vesicle-ejaculatory duct epithelium. Arch Pathol Lab Med 1999;123:1093 1097.
102. Kuo T, Gomez LG. Monstrous epithelial cells in human epididymis and seminal vesicles: a pseudomalignant change. Am J Surg Pathol 1981;5:483 490.
103. Kovi J, Jackson MA, Akberzie ME. Unusual smooth muscle change in the prostate. Arch Pathol Lab Med 1979;103:204 205.
104. McNeal JE. Normal histology of the prostate. Am J Surg Pathol 1988;12:619 633.
105. Rolnick D, Kawanoue S, Szanto P, Bush IM. Anatomical incidence of testicular appendages. J Urol 1968;100:755 756.
106. Sahni D, Jit I, Joshi K, Jeev S. Incidence and structure of the appendices of the testis and epididymis. J Anat 1996;189(pt 2):341 348.
107. Skoglund RW, McRoberts JW, Ragde H. Torsion of testicular appendages: presentation of 43 new cases and a collective review. J Urol 1970;104:598 600.
108. Samnakay N, Cohen R, Orford J, King P, Davies R. Androgen and oestrogen receptor status of the human appendix testis. Pediatr Surg Int 2003;19:520 524.
109. Sadler TW. Langman's Medical Embryology. 7th ed. Baltimore: Williams & Wilkins; 1995:293.
110. Whitehead ED, Leiter E. Genital abnormalities and abnormal semen analysis in male patients exposed to diethylstilbestrol in utero. J Urol 1981;125:47 50.
111. Bernstein J, Gardner KD Jr. Renal cystic disease and renal dysplasia. In: Walsh PC, Gittes RF, Permutter AD, Stamey TA, eds. Campbell's Urology. 5th ed. Vol 2. Philadelphia: WB Saunders; 1986:1760 1803.
112. Popek E. Embryonal remnants in inguinal hernia sacs. Hum Pathol 1990;21:339 349.
113. Wollin M, Marshall F, Fink M, Malhotra R, Diamond D. Aberrant epididymal tissue: a significant clinical entity. J Urol 1987;138:1247 1250.