9. How do proteins enter and leave membranes?
8.9 How do proteins enter and leave membranes? |
| Key terms defined in this section |
| Integral membrane protein is a protein (noncovalently) inserted into a membrane; it retains its membranous association by means of a stretch of ~25 amino acids that are uncharged and/or hydrophobic. Transmembrane protein is a component of a membrane; a hydrophobic region or regions of the protein resides in the membrane, and hydrophilic regions are exposed on one or both sides of the membrane. |
The association of a protein with a membrane takes several forms. (For an introduction, see Membranes and membrane proteins). Proteins that are secreted from the cell must pass through a membrane, so we have to account for their ability to initiate entry into the lipid bilayer and then to pass across it. Proteins that reside in membranes may start the process in the same way, but then transfer from soluble phase to the hydrophobic environment.
The operational definition of an integral membrane protein is that it requires disruption of the lipid bilayer in order to be released from the membrane. A common feature in such proteins is the presence of at least one transmembrane region, consisting of an α-helical stretch of 21-26 hydrophobic amino acids. A sequence that fits the criteria for membrane insertion can be identified by a hydropathy plot, which measures the cumulative hydrophobicity of a stretch of amino acids. A protein that has domains exposed on both sides of the membrane is called a transmembrane protein. The topography of a membrane protein depends on the number and arrangement of transmembrane regions.
When a protein has a single transmembrane region, its position determines how much of the protein is exposed on either side of the membrane. A protein may have extensive domains exposed on both sides of the membrane or may have a site of insertion close to one end, so that little or no material is exposed on one side. The length of the N-terminal or C-terminal tail that protrudes from the membrane near the site of insertion varies from insignificant to quite bulky.
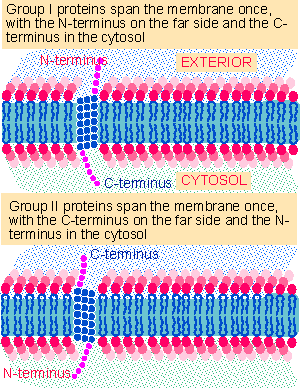 |
Figure 8.27 Group I and group II transmembrane proteins have opposite orientations with regard to the membrane. |
Figure 8.27 shows that proteins with a single transmembrane domain fall into two classes. Group I proteins in which the N-terminus faces the extracellular space are more common than group II proteins in which the orientation has been reversed so that the N-terminus faces the cytoplasm. Orientation is determined during the insertion of the protein into the endoplasmic reticulum.
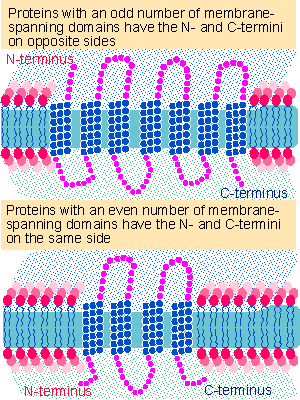 |
Figure 8.28 The orientations of the termini of multiple membrane-spanning proteins depends on whether there is an odd or even number of transmembrane segments. Multiple figure |
Figure 8.28 shows orientations for proteins that have multiple membrane-spanning domains. An odd number means that both termini of the protein are on opposite sides of the membrane, whereas an even number implies that the termini are on the same face. The extent of the domains exposed on one or both sides is determined by the locations of the transmembrane domains. Domains at either terminus may be exposed, and internal sequences between the domains "loop out" into the extracellular space or cytoplasm. One common type of structure is the 7-membrane passage or "serpentine" receptor; another is the 12-membrane passage component of an ion channel.
Does a transmembrane domain itself play any role in protein function besides allowing the protein to insert into the lipid bilayer? In the simple group I or II proteins, it has little or no additional function; often it can be replaced by any other transmembrane domain. However, transmembrane domains play an important role in the function of proteins that make multiple passes through the membrane or that have subunits that oligomerize within the membrane. The transmembrane domains in such cases often contain polar residues, which are not found in the single membrane-spanning domains of group I and group II proteins. Polar regions in the membrane-spanning domains do not interact with the lipid bilayer, but instead interact with one another. This enables them to form a polar pore or channel within the lipid bilayer. Interaction between such transmembrane domains can create a hydrophilic passage through the hydrophobic interior of the membrane. This can allow highly charged ions or molecules to pass through the membrane, and is important for the function of ion channels and transport of ligands. Another case in which conformation of the transmembrane domains is important is provided by certain receptors that bind lipophilic ligands. In such cases, the transmembrane domains (rather than the extracellular domains) bind the ligand within the plane of the membrane.
We have a reasonable understanding of the processes by which secreted proteins pass through membranes and of how this relates to the insertion of the single-membrane spanning group I and group II proteins. We cannot yet explain the details of insertion of proteins with multiple membrane-spanning domains.
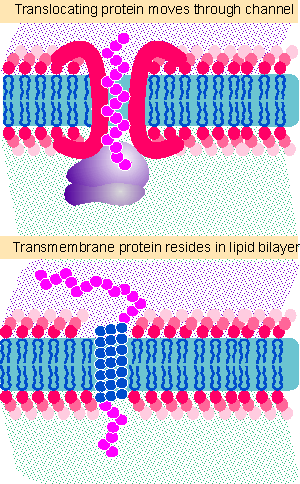 |
Figure 8.29 How does a transmembrane protein make the transition from moving through a proteinaceous channel to interacting directly with the lipid bilayer? |
We understand how a secreted protein passes through a membrane without any conflict, but it is difficult to apply the same model to a protein that resides in the membrane. Figure 8.29 illustrates the difference between the organization of a translocating protein, which is protected from the lipid bilayer by the aqueous channel, and a transmembrane protein, which has a hydrophobic segment directly in contact with the membrane. We do not know how a protein is transferred from its passage through the proteinaceous channel into the lipid bilayer itself. One possibility is that there is some mechanism for transferring hydrophobic transmembrane domains directly from the channel into the membrane, by means of some rearrangement that exposes the hydrophobic region to the surrounding lipids. An alternative is that these domains cause the channel to disaggregate, exposing the hydrophobic amino acids to the lipid bilayer.
It has always been a common assumption that, whatever the exact mechanism for transferring the transmembrane segment into the membrane, it is triggered by the presence of the transmembrane sequence in the pore. However, changes in the pore occur in response to the presence of a transmembrane sequence in the ribosome. When a secreted protein passes through the pore, the channel remains sealed on the cytosolic side but opens on the lumenal side after synthesis of the first 70 residues. However, as soon as a transmembrane sequence has been fully synthesized, that is, while it is still entirely within the ribosome, the pore closes on the lumenal side. How this change relates to the transfer of the transmembrane sequence into the membrane is not clear. And we do not know whether transmembrane region(s) are transferred into the membrane during translation or only after completion of the entire protein sequence. Transfer may take place in stages, as seen by changes in the contacts made by a transmembrane region with the pore.