10. Anchor signals are needed for membrane residence
8.10 Anchor signals are needed for membrane residence |
How are proteins that are secreted through membranes distinguished from those that reside within them? The pathway by which proteins of either type I or type II are inserted into the membrane follows the same initial route as that of secretory proteins, relying on a signal sequence that functions co-translationally. But proteins that are to remain within the membrane possess a second, stop-transfer signal. This takes the form of a cluster of hydrophobic amino acids adjacent to some ionic residues. The cluster serves as an anchor that latches on to the membrane and stops the protein from passing right through.
A surprising property of anchor sequences is that they can function as signal sequences when engineered into a different location. When placed into a protein lacking other signals, such a sequence may sponsor membrane translocation. One possible explanation for these results is that the signal sequence and anchor sequence interact with some common component of the apparatus for translocation. Binding of the signal sequence initiates translocation, but the appearance of the anchor sequence displaces the signal sequence and halts transfer.
Membrane insertion starts by the insertion of a signal sequence in the form of a hairpin loop, in which the N-terminus remains on the cytoplasmic side. Two features determine the position and orientation of a protein in the membrane: whether the signal sequence is cleaved; and the location of the anchor sequence.
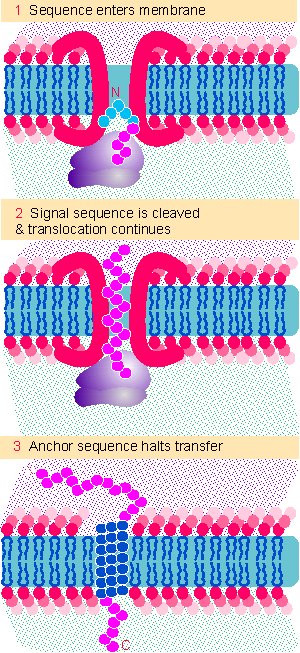 |
Figure 8.30 Proteins that reside in membranes enter by the same route as secreted proteins, but transfer is halted when an anchor sequence passes into the membrane. If the anchor is at the C-terminus, the bulk of the protein passes through the membrane and is exposed on the far surface. |
The insertion of type I proteins is illustrated in Figure 8.30. The signal sequence is N-terminal. The location of the anchor signal determines when transfer of the protein is halted. When the anchor sequence takes root in the membrane, domains on the N-terminal side will be located in the lumen, while domains on the C-terminal side are located facing the cytosol.
A common location for a stop-transfer sequence of this type is at the C-terminus. As shown in the figure, transfer is halted only as the last sequences of the protein enter the membrane. This type of arrangement is responsible for the location in the membrane of many proteins, including cell surface proteins. Most of the protein sequence is exposed on the lumenal side of the membrane, with a small or negligible tail facing the cytosol.
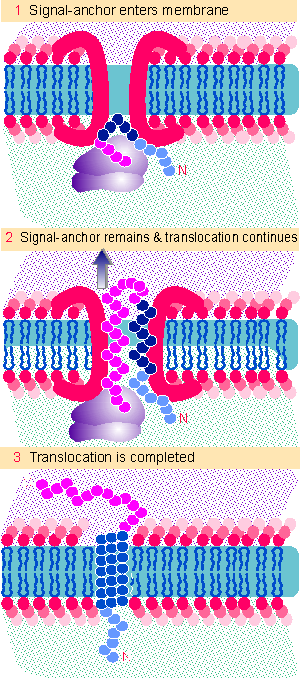 |
Figure 8.31 A combined signal-anchor sequence causes a protein to reverse its orientation, so that the N-terminus remains on the inner face and the C-terminus is exposed on the outer face of the membrane. |
Type II proteins do not have a cleavable leader sequence at the N-terminus. Instead the signal sequence is combined with an anchor sequence. We imagine that the general pathway for the integration of type I proteins into the membrane involves the steps illustrated in Figure 8.31. The signal sequence enters the membrane, but the joint signal-anchor sequence does not pass through. Instead it stays in the membrane (perhaps interacting directly with the lipid bilayer), while the rest of the growing polypeptide continues to loop into the endoplasmic reticulum.
The signal-anchor sequence is usually internal, and its location determines which parts of the protein remain in the cytosol and which are extracellular. Essentially all the N-terminal sequences that precede the signal-anchor are exposed to the cytosol. Usually this cytosolic tail is short, ~6-30 amino acids. In effect the N-terminus remains constrained while the rest of the protein passes through the membrane. This reverses the orientation of the protein with regard to the membrane.
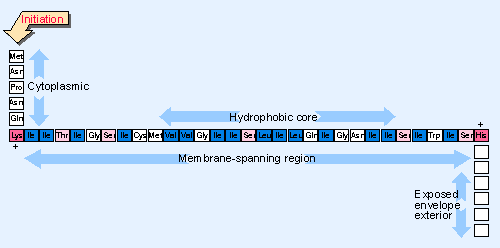 |
Figure 8.32 The signal-anchor of influenza neuraminidase is located close to the N-terminus and has a hydrophobic core. |
The combined signal-anchor sequences of type II proteins resemble cleavable signal sequences. Figure 8.32 gives an example. Like cleavable leader sequences, the amino acid composition is more important than the actual sequence. The regions at the extremities of the signal-anchor carry positive charges; the central region is uncharged and resembles a hydrophobic core of a cleavable leader. Mutations to introduce charged amino acids in the core region prevent membrane insertion; mutations on either side prevent the anchor from working, so the protein is secreted or located in an incorrect compartment.
The distribution of charges around the anchor sequence has an important effect on the orientation of the protein. More positive charges are usually found on the cytoplasmic side (N-terminal side in type II proteins). If the positive charges are removed by mutation, the orientation of the protein can be reversed. The effect of charges on orientation is summarized by the "positive inside" rule, which says that the side of the anchor with the most positive charges will be located in the cytoplasm. The positive charges in effect provide a hook that latches on to the cytoplasmic side of the membrane, controlling the direction in which the hydrophobic region is inserted, and thus determining the orientation of the protein.
The process of insertion into a membrane has been characterized for both type I proteins and type II proteins, in which there is a single transmembrane domain. How is a protein with multiple membrane-spanning regions inserted into a membrane? Much less is known about this process, but we assume that it relies on sequences that provide signal and/or anchor capabilities. One model is to suppose that there is an alternating series of signal and anchor sequences. Translocation is initiated at the first signal sequence and continues until stopped by the first anchor. Then it is reinitiated by a subsequent signal sequence, until stopped by the next anchor. Individual transmembrane regions accumulate in contact with the translocation pore as they are synthesized. We do not know how (and when) they move laterally into contact with the lipid bilayer (for review see Wickner and Lodish, 1985).
| Reviews | |
| Wickner, W. T. and Lodish, H. (1985). Multiple mechanisms of protein insertion into and across membranes. Science 230, 400-407. | |