8. Sigma factors may be organized into cascades
9.8 Sigma factors may be organized into cascades |
| Key terms defined in this section |
| Sporulation is the generation of a spore by a bacterium (by morphological conversion) or by a yeast (as the product of meiosis). |
Sigma factors are used more extensively to control initiation in the bacterium B. subtilis, where ~10 different factors are known. Some are present in vegetative cells; other are produced only in the special circumstances of phage infection or the change from vegetative growth to sporulation.
The major RNA polymerase found in B. subtilis cells engaged in normal vegetative growth has the same structure as that of E. coli, α2ββ′σ. Its sigma factor (described as σ43 or σA) recognizes promoters with the same consensus sequences used by the E. coli enzyme under direction from σ70. Several variants of the RNA polymerase that contain other sigma factors are found in much smaller amounts. The variant enzymes recognize different promoters on the basis of consensus sequences at V35 and V10.
Transitions from expression of one set of genes to expression of another set are a common feature of bacteriophage infection. In all but the very simplest cases, the development of the phage involves shifts in the pattern of transcription during the infective cycle. These shifts may be accomplished by the synthesis of a phage-encoded RNA polymerase or by the efforts of phage-encoded ancillary factors that control the bacterial RNA polymerase. A well characterized example of control via the production of new sigma factors occurs during infection of B. subtilis by phage SPO1.
The infective cycle of SPO1 passes through three stages of gene expression. Immediately on infection, the early genes of the phage are transcribed. After 4-5 minutes, the early genes cease transcription and the middle genes are transcribed. Then at 8-12 minutes, middle gene transcription is replaced by transcription of late genes.
The early genes are transcribed by the holoenzyme of the host bacterium. They are essentially indistinguishable from host genes whose promoters have the intrinsic ability to be recognized by the RNA polymerase α2ββ′σ43.
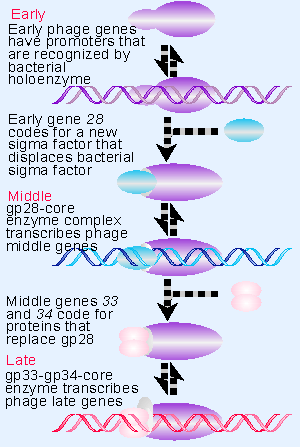 |
Figure 9.21 Transcription of phage SPO1 genes is controlled by two successive substitutions of the sigma factor that change the initiation specificity. |
Expression of phage genes is required for the transitions to middle and late gene transcription. Three regulatory genes, named 28, 33, and 34, control the course of transcription. Their functions are summarized in Figure 9.21. The pattern of regulation creates a cascade, in which the host enzyme transcribes an early gene whose product is needed to transcribe the middle genes; and then two of the middle genes code for products that are needed to transcribe the late genes.
Mutants in the early gene 28 cannot transcribe the middle genes. The product of gene 28 (called gp28) is a protein of 26 kD that replaces the host sigma factor on the core enzyme. This substitution is the sole event required to make the transition from early to middle gene expression. It creates a holoenzyme that can no longer transcribe the host genes, but instead specifically transcribes the middle genes. We do not know how gp28 displaces σ43, or what happens to the host sigma polypeptide.
Two of the middle genes are involved in the next transition. Mutations in either gene 33 or 34 prevent transcription of the late genes. The products of these genes together replace gp28 on the core polymerase. Again, we do not know how gp33 and gp34 exclude gp28 (or any residual host σ43), but once they have bound to the core enzyme, it is able to initiate transcription only at the promoters for late genes.
The successive replacements of sigma factor have dual consequences. Each time the subunit is changed, the RNA polymerase becomes able to recognize a new class of genes, and it no longer recognizes the previous class. These switches therefore constitute global changes in the activity of RNA polymerase. Probably all or virtually all of the core enzyme becomes associated with the sigma factor of the moment; and the change is irreversible.
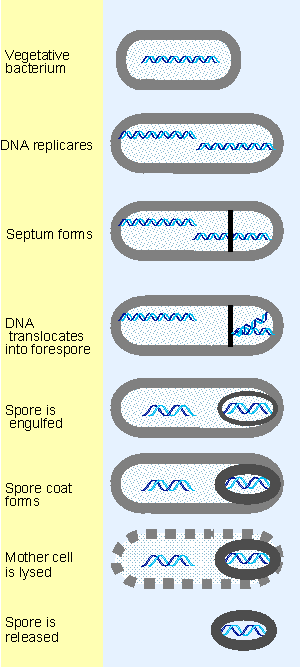 |
Figure 9.22 Sporulation involves the differentiation of a vegetative bacterium into a mother cell that is lysed and a spore that is released. |
Perhaps the most extensive example of switches in sigma factors is provided by sporulation, an alternative lifestyle available to some bacteria. At the end of the vegetative phase in a bacterial culture, logarithmic growth ceases because nutrients in the medium become depleted. This triggers sporulation, as illustrated in Figure 9.22. DNA is replicated, a genome is segregated at one end of the cell, and eventually it is surrounded by the tough spore coat. When the septum forms, it generates two independent compartments, the mother cell and the forespore. At the start of the process, one chromosome is attached to each pole of the cell. The growing septum traps part of one chromosome in the forespore, and then a translocase (SpoIIIE) pumps the rest of the chromosome into the forespore.
Sporulation takes ~8 hours. It can be viewed as a primitive sort of differentiation, in which a parent cell (the vegetative bacterium) gives rise to two different daughter cells with distinct fates: the mother cell is eventually lysed, while the spore that is released has an entirely different structure from the original bacterium.
Sporulation involves a drastic change in the biosynthetic activities of the bacterium, in which many genes are involved. The basic level of control lies at transcription. Some of the genes that functioned in the vegetative phase are turned off during sporulation, but most continue to be expressed. In addition, the genes specific for sporulation are expressed only during this period. At the end of sporulation, ~40% of the bacterial mRNA is sporulation-specific.
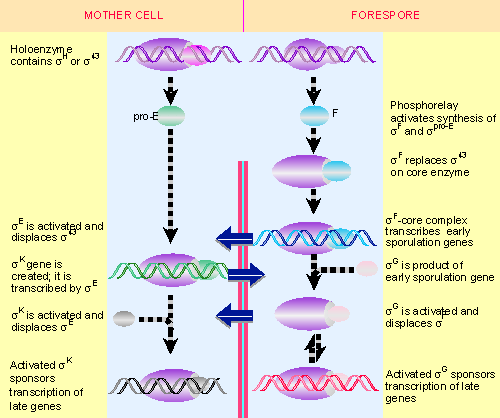 |
Figure 9.23 Sporulation involves successive changes in the sigma factors that control the initiation specificity of RNA polymerase. The cascades in the forespore (left) and the mother cell (right) are related by signals passed across the septum (indicated by horizontal arrows). |
New forms of the RNA polymerase become active in sporulating cells; they contain the same core enzyme as vegetative cells, but have different proteins in place of the vegetative σ43. The changes in transcriptional specificity are summarized in Figure 9.23. The principle is that in each compartment the existing sigma factor is successively displaced by a new factor that causes transcription of a different set of genes. Communication between the compartments occurs in order to coordinate the timing of the changes in the forespore and mother cell (497, 498; for review see 72, 80).
The sporulation cascade is initiated when environmental conditions trigger a "phosphorylation relay," in which a phosphate group is passed along a series of proteins until it reaches SpoOA. (Several gene products are involved in this process, whose complexity may reflect the need to avoid mistakes in triggering sporulation unnecessarily.) SpoOA is a transcriptional regulator whose activity is affected by phosphorylation. In the phosphorylated form, it activates transcription of two operons, each of which is transcribed by a different form of the host RNA polymerase. Under the direction of phosphorylated SpoOA, host enzyme utilizing the general σ 43 transcribes the gene coding for the factor σF; and host enzyme under the direction of a minor factor, σH, transcribes the gene coding for the factor pro-σE. Both of these new sigma factors are produced before septum formation, but become active later.
σF is the first factor to become active in the forespore compartment. It is inhibited by an anti-sigma factor that binds to it; in the forespore, an anti-anti-sigma factor removes the inhibitor. This reaction is controlled by a series of phosphorylation/dephosphorylation events. The initial determinant is a phosphatase (SpoIIE) that is an integral membrane protein, and which accumulates at the pole, with the result that its phosphatase domain becomes more concentrated in the forespore. It dephosphorylates, and thereby activates, SpoIIAA, which in turn displaces the anti-sigma factor SpoIIAB from the complex of SpoIIAB-σF. Release of σF activates it.
Activation of σF is the start of sporulation. Under the direction of σF, RNA polymerase transcribes the first set of sporulation genes instead of the vegetative genes it was previously transcribing. The replacement reaction probably affects only part of the RNA polymerase population, since σF is produced only in small amounts. Some vegetative enzyme remains present during sporulation. The displaced σ43 is not destroyed, but can be recovered from extracts of sporulating cells.
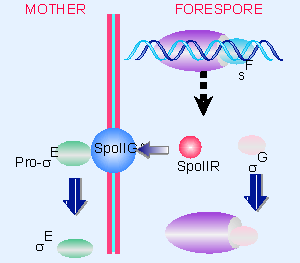 |
Figure 9.24 sF triggers synthesis of the next sigma factor in the forespore (sG) and turns on SpoIIR which causes SpoIIGA to cleave pro-sE. |
Two regulatory events follow from the activity of σF, as detailed in Figure 9.24. In the forespore itself, another factor, σG, is the product of one of the early sporulation genes. σG is the factor that causes RNA polymerase to transcribe the late sporulation genes in the forespore. Another early sporulation gene product is responsible for communicating with the mother cell compartment. σF activates SpoIIR, which is secreted from the forespore. It then activates the membrane-bound protein SpoIIGA to cleave the inactive precursor pro-σE into the active factor σE in the mother cell. (Any σE that is produced in the forespore is degraded by forespore-specific functions.)
The cascade continues when σE in turn is replaced by σK. (Actually the production of σK is quite complex, because first its gene must be created by a recombination event!) This factor also is synthesized as an inactive precursor (pro-σK) that is activated by a protease. Once σK has been activated, it displaces σE and causes transcription of the late genes in the mother cell. The timing of these events in the two compartments are coordinated by further signals. The activity of σE in the mother cell is necessary for activation of σG in the forespore; and in turn the activity of σG is required to generate a signal that is transmitted across the septum to activate σK.
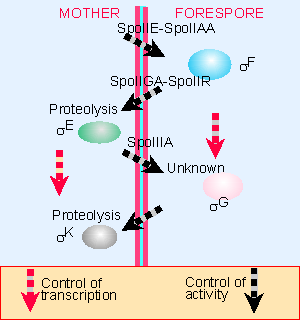 |
Figure 9.25 The criss-cross regulation of sporulation coordinates timing of events in the mother cell and forespore. |
Sporulation is thus controlled by two cascades, in which sigma factors in each compartment are successively activated, each directing the synthesis of a particular set of genes. Figure 9.25 outlines how the two cascades are connected by the transmission of signals from one compartment to the other. As new sigma factors become active, old sigma factors are displaced, so that transitions in sigma factors turn genes off as well as on. The incorporation of each factor into RNA polymerase dictates when its set of target genes is expressed; and the amount of factor available influences the level of gene expression. More than one sigma factor may be active at any time, and the specificities of some of the sigma factors overlap. We do not know what is responsible for the ability of each sigma factor to replace its predecessor (for review see 77, 78, 81).
| Reviews | |
| 72: | Losick, R. et al. (1986). Genetics of endospore formation in B. subtilis. Ann. Rev. Genet. 20, 625-669. |
| 77: | Losick, R. and Stragier, P. (1992). Crisscross regulation of cell-type specific gene expression during development in B. subtilis. Nature 355, 601-604. |
| 78: | Errington, J. (1993). B. subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57, 1-33. |
| 80: | Haldenwang, W. G. (1995). The sigma factors of B. subtilis>. Microbiol. Rev. 59, 1-30. |
| 81: | Stragier, P and Losick, R. (1996). Molecular genetics of sporulation in B. subtilis. Ann. Rev. Genet. 30, 297-341. |
| Research | |
| 497: | Haldenwang, W. G. and Losick, R. (1980). A novel RNA polymerase sigma factor from B. subtilis. Proc. Nat. Acad. Sci. USA 77, 7000-7004. |
| 498: | Haldenwang, W. G., Lang, N., and Losick, R. (1981). A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell 23, 615-624. |