9. Bacterial RNA polymerase has two modes of termination
9.9 Bacterial RNA polymerase has two modes of termination |
| Key terms defined in this section |
| Terminator is a sequence of DNA, represented at the end of the transcript, that causes RNA polymerase to terminate transcription. |
Once RNA polymerase has started transcription, the enzyme moves along the template, synthesizing RNA, until it meets a terminator (t)sequence. At this point, the enzyme stops adding nucleotides to the growing RNA chain, releases the completed product, and dissociates from the DNA template. (We do not know in which order the last two events occur.) Termination requires that all hydrogen bonds holding the RNA-DNA hybrid together must be broken, after which the DNA duplex reforms.
It is difficult to define the termination point of an RNA molecule that has been synthesized in the living cell. It is always possible that the 3′ end of the molecule has been generated by cleavage of the primary transcript, and therefore does not represent the actual site at which RNA polymerase terminated.
The best identification of termination sites is provided by systems in which RNA polymerase terminates in vitro. Because the ability of the enzyme to terminate is strongly influenced by parameters such as the ionic strength, its termination at a particular point in vitro does not prove that this same point is a natural terminator. But we can identify authentic 3′ ends when the same end is generated in vitro and in vivo.
Terminators in bacteria and their phages have been identified as sequences that are needed for the termination reaction (in vitro or in vivo). They vary widely in both their efficiencies of termination and their dependence on ancillary proteins, at least as seen in vitro. Many terminators require a hairpin to form in the secondary structure of the RNA being transcribed. This indicates that termination depends on the RNA product and is not determined simply by scrutiny of the DNA sequence during transcription.
At some terminators, the termination event can be prevented by specific ancillary factors that interact with RNA polymerase. Antitermination causes the enzyme to continue transcription past the terminator sequence, an event called readthrough (the same term used to describe a ribosome’s suppression of termination codons).
In approaching the termination event, we must regard it not simply as a mechanism for generating the 3′ end of the RNA molecule, but as an opportunity to control gene expression. So the stages when RNA polymerase associates with DNA (initiation) or dissociates from it (termination) both are subject to specific control. There are interesting parallels between the systems employed in initiation and termination. Both require breaking of hydrogen bonds (initial melting of DNA at initiation, RNA-DNA dissociation at termination); and both require additional proteins to interact with the core enzyme. In fact, they are accomplished by alternative forms of the polymerase. However, whereas initiation relies solely upon the interaction between RNA polymerase and duplex DNA, the termination event involves recognition of signals in the transcript by RNA polymerase or by ancillary factors.
The sequences at prokaryotic terminators show no similarities beyond the point at which the last base is added to the RNA. The responsibility for termination lies with the sequences already transcribed by RNA polymerase. So termination relies on scrutiny of the template or product that the polymerase is currently transcribing (for review see 69, 73, 74).
Terminators have been distinguished in E. coli according to whether RNA polymerase requires any additional factors to terminate in vitro:
- Core enzyme can terminate in vitro at certain sites in the absence of any other factor. These sites are called intrinsic terminators.
- Rho-dependent terminators are defined by the need for addition of rho (ρ ) factor in vitro; and mutations show that the factor is involved in termination in vivo.
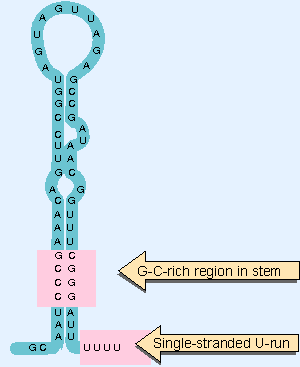 |
Figure 9.26 Intrinsic terminators include palindromic regions that form hairpins varying in length from 7-20 bp. The stem-loop structure includes a G-C-rich region and is followed by a run of U residues. |
Intrinsic terminators have the two structural features evident in Figure 9.26: a hairpin in the secondary structure; and a run of ~6 U residues at the very end of the unit. Both features are needed for termination. The hairpin usually contains a G PC-rich region near the base of the stem. The typical distance between the hairpin and the U-run is 7-9 bases. Sometimes the U-run is interrupted.
Point mutations that prevent termination occur within the stem region of the hairpin. What is the effect of a hairpin on transcription? Probably all hairpins that form in the RNA product cause the polymerase to slow (and perhaps to pause) in RNA synthesis.
Pausing creates an opportunity for termination to occur. Pausing occurs at sites that resemble terminators but have an increased separation (typically 10-11 bases) between the hairpin and the U-run. But if the pause site does not correspond to a terminator, usually the enzyme moves on again to continue transcription. The length of the pause varies, but at a typical terminator lasts ~60 seconds.
A string of U residues in the right location is necessary to allow RNA polymerase to dissociate from the template when it pauses at the hairpin. The rU PdA RNA-DNA hybrid has an unusually weak base-paired structure; it requires the least energy of any RNA-DNA hybrid to break the association between the two strands. When the polymerase pauses, the RNA-DNA hybrid unravels from the weakly bonded rU PdA terminal region. Often the actual termination event takes place at any one of several positions toward or at the end of the U-run, as though the enzyme "stutters" during termination.
The importance of the run of U bases is confirmed by making deletions that shorten this stretch; although the polymerase still pauses at the hairpin, it no longer terminates. The series of U bases corresponds to an A PT-rich region in DNA, so we see that A PT-rich regions are important in intrinsic termination as well as initiation.
Both the sequence of the hairpin and the length of the U-run influence the efficiency of termination. However, termination efficiency in vitro varies from 2-90%, and does not correlate in any simple way with the constitution of the hairpin or the number of U residues in intrinsic terminators. The hairpin and U-run are therefore necessary, but not sufficient, and additional parameters influence the interaction with RNA polymerase.
Less is known about the signals and ancillary factors involved in termination for eukaryotic polymerases. Each class of polymerase uses a different mechanism (see 20 Initiation of transcription). However, the same features used by bacterial (core) RNA polymerase in intrinsic termination recur in eukaryotes: secondary structure and/or runs of U residues in the transcript are important.
| Reviews | |
| 69: | Adhya, S. and Gottesman, M. (1978). Control of transcription termination. Ann. Rev. Biochem 47, 967-996. |
| 73: | Platt, T. (1986). Transcription termination and the regulation of gene expression. Ann. Rev. Biochem 55, 339-372. |
| 74: | Friedman, D. I., Imperiale, M. J., and Adhya, S. L. (1987). RNA 3?/FONT> end formation in the control of gene expression. Ann. Rev. Genet. 21, 453-488. |