 | Note: Large images and tables on this page may necessitate printing in landscape mode.
Copyright 2007 The McGraw-Hill Companies. All rights reserved.
Clinician's Pocket Reference > Chapter 8. Blood Gases and Acid Base Disorders >
Blood Gas Basics Blood gases provide information concerning the oxygenation, ventilatory, and acid-base status of the patient. Blood gas results are usually given as pH, PO2, PCO2, [HCO3 ], base excess or deficit (base difference), and O2 saturation. This test gives information on acid base homeostasis (pH, PCO2, [HCO3 ], and base difference) and on blood oxygenation (PO2, O2 saturation). Arterial blood gases (ABG) are most commonly measured; venous, mixed venous, and capillary blood gases are measured less frequently. Indications for blood gas determinations are as follows (Respir Care 2001;46:498 505): - To determine a patient's ventilatory (PaCO2), acid base (pH and PaCO2), and oxygenation and O2-carrying capacity (PaO2 and O2Hb)
- To quantitate the response to therapeutic intervention (eg, supplemental O2 administration, mechanical ventilation) or diagnostic evaluation (eg, exercise desaturation)
- Monitoring the severity and progression of documented disease processes (eg, COPD)
|
Normal Blood Gas Values Normal values for blood gas analysis are given in Table 8 1, and capillary blood gases are discussed in a following section. Mixed venous blood gases are reviewed in Chapter 20. The bicarbonate concentration ([HCO3 ]) from the blood gas is a calculated value and should not be used in interpretation of blood gases; the [HCO3 ] from a concurrent chemistry panel should be used. Note: The HCO3 values on the chemistry panel and those calculated from the blood gases should be about the same. A major discrepancy (> 10% difference) means one or more of the three values is in error (pH, PCO2, or [HCO3 ]). The most common cause of discrepancies is drawing the blood gas and chemistry panel samples at different times. ABGs and chemistry panels [HCO3 ] should be obtained at the same time for the most accurate interpretation. Table 8 1 Normal Blood Gas Values
|
| | Measurement | Arterial Blood | Mixed Venous Blooda
| Venous Blood |
|---|
| pH (range) | 7.40 (7.37 7.44) | 7.36 (7.31 7.41) | 7.36 (7.31 7.41) | PO2 (mm Hg) (decreases with age)
| 80 100 | 35 40 | 30 50 | PCO2 (mm Hg)
| 36 44 | 41 51 | 40 52 | O2 saturation (%) [decreases with age]
| >95 | 60 80 | 60 85 | HCO3 (mEq/L)
| 22 26 | 22 26 | 22 28 | | Base difference (deficit/excess) | 2 to +2 | 2 to +2 | 2 to +2 |
|
aObtained from the right atrium, usually through a pulmonary artery catheter. |
|
Venous Blood Gases There is little difference between arterial and venous pH and [HCO3 ] (except in severe CHF and shock). Venous blood gas levels may occasionally be used to assess acid base status, but venous O2 levels are significantly less than arterial values (see Table 8 1). |
Capillary Blood Gases A CBG is obtained from a highly vascularized capillary bed. CBG is often used for pediatric patients because obtaining the sample is easier (through the heel) and less traumatic (no risk of arterial thrombosis, hemorrhage) than obtaining an ABG sample. (See Chapter 13, Heelstick Technique.) When interpreting a CBG, apply the following rules: - pH: Same as arterial or slightly lower (Normal = 7.35 7.40)
- PCO2: Same as arterial or slightly higher (Normal = 40 45 mm Hg)
- PO2: Lower than arterial (Normal = 45 60 mm Hg)
- O2 saturation: > 70% is acceptable. Saturation is probably more useful than PO2 itself in interpretation of a CBG.
|
General Principles of Blood Gas Determinations Interpretation of O2 values is discussed in Hypoxia - 1. The blood gas analyzers in most labs measure pH and PCO2 as well as PO2. [HCO3 ] and base difference are calculated with the Henderson Hasselbalch equation:
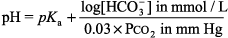
or the Henderson equation: 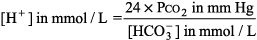
- 2. For a rough estimate of [H+], [H+] = (7.80 pH) x 100, or add 1 mEq/L to 40 mEq for every 0.01 below 7.40 and subtract 1 mEq/L from 40 mEq for every 0.01 above 7.40 (accurate for pH 7.25 7.48); 40 mEq/L = [H+] at the normal pH of 7.40. pH is a log scale; for every change of 0.3 in pH from 7.40 the [H+] doubles or halves. For pH 7.10, [H+] = 2 x 40, or 80 nmol/L, and for pH 7.70, [H+] = 1/2 40, or 20 nmol/L.
- 3. The calculated [HCO3 ] should be within 2 mEq/L of the [HCO3 ] of a venous measurement drawn at the same time. If not, an error has been made in collection or in determination of the values, and both samples should be recollected.
- 4. Two additional relationships derived from the Henderson Hasselbalch equation should be committed to memory. These two rules are helpful in interpreting blood gas results, particularly in defining a simple versus a mixed blood gas disorder:
Rule I: A change in PCO2 up or down 10 mm Hg is associated with an increase or decrease in pH of 0.08 units. As PCO2 decreases, pH increases; as the PCO2 increases, pH decreases. Rule II: A pH change of 0.15 is equivalent to a base change of 10 mEq/L. A decrease in base (ie, [HCO3 ]) is termed a base deficit, and an increase in base is termed a base excess. |
Acid Base Disorders: Definition - 1. Acid base disorders are common clinical problems. Acidemia is a pH < 7.37, and alkalemia is a pH > 7.44. Acidosis and alkalosis are used to describe the process by which pH changes. The primary causes of acid base disturbances are abnormalities in the respiratory, metabolic, and renal systems. As the Henderson Hasselbalch equation shows, a respiratory disturbance leading to an abnormal PCO2 alters the pH, and similarly a metabolic disturbance altering [HCO3 ] changes the pH.
- 2. Any primary disturbance in acid base homeostasis invokes a normal compensatory response. A primary metabolic disorder leads to respiratory compensation, and a primary respiratory disorder leads to an acute metabolic response due to the buffering capacity of body fluids and chronic compensation (1 2 d) due to alterations in renal function.
- 3. The degree of compensation can be expressed in terms of the degree of primary acid base disturbance. Table 8 2 lists the major categories of primary acid base disorders, the primary abnormality, the secondary compensatory response, and the expected compensation based on the primary abnormality. These changes are defined graphically in Figure 8 1.
|
Mixed Acid Base Disorders - 1. Most acid base disorders result from a single primary disturbance of the normal physiologic compensatory response (called simple acid base disorders). In some cases (eg, serious illness), two or more primary disorders may occur simultaneously, resulting in a mixed acid base disorder. The net effect of mixed disorders can be additive (eg, metabolic acidosis and respiratory acidosis) and result in extreme alteration of pH. Or, the effects can be opposite (eg, metabolic acidosis and respiratory alkalosis) and nullify somewhat the effect of the other on pH.
- 2. To determine the presence of a mixed acid base disorder with a blood gas value, follow the six steps in the Interpretation of Blood Gases. Alterations in either [HCO3 ] or PCO2 that differ from expected compensation levels indicate a second process. Two of the examples given in the following section illustrate the strategies used in identifying a mixed acid base disorder.
|
Interpretation of Blood Gases Use a consistent, stepwise approach to interpretation of blood gases. (See Figure 8 1.) | 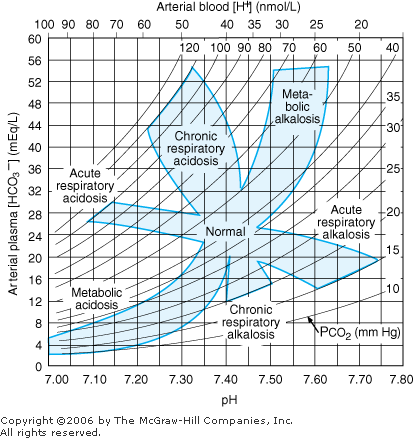 | Nomogram for acid-base disorders. (Reprinted, with permission, from: Cogan MG: Fluid and Electrolytes, Originally published by Appleton & Lange, Copyright 1991 by the McGraw-Hill Companies, Inc.) |
|
Step 1: Determine whether the numbers fit. 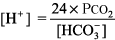
The right side of the equation should be within about 10% of the left side. If the numbers do not fit, obtain another ABG and chemistry panel for [HCO3 ]. Example. pH 7.25, PCO2 48 mm Hg, [HCO3 ] 29 mEq/L. 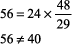
This blood gas cannot be interpreted, and samples for ABG and [HCO3 ] must be recollected. The most common reason for the numbers not fitting is that the ABG and the chemistry panel [HCO3 ] were obtained at different times. Step 2: Next, determine whether acidemia (pH < 7.37) or alkalemia (pH > 7.44) is present. Step 3: Identify the primary disturbance as metabolic or respiratory. For example, if acidemia is present, is the PCO2 > 44 mm Hg (respiratory acidosis), or is the [HCO3 ] < 22 mEq/L (metabolic acidosis)? In other words, identify which component, respiratory or metabolic, is altered in the same direction as the pH abnormality. If both components act in the same direction eg, both respiratory (PCO2 > 44 mm Hg) and metabolic [HCO3 ] < 22 mEq/L) acidosis are present then this is a mixed acid base problem (see Step 4). The primary disturbance is the one that varies the most from normal. That is, with a [HCO3 ] of 6 mEq/L and PCO2 of 50 mm Hg, the primary disturbance would be metabolic acidosis; the [HCO3 ] is only 25% of normal, whereas the increase in PCO2 is only 25% above normal. Step 4: After identifying the primary disturbance, use the equations in Table 8 2 to calculate the expected compensatory response. If the difference between the actual value and the calculated value is great, a mixed acid base disturbance is present. Step 5: Calculate the anion gap: 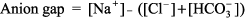
A normal anion gap is 8 12 mEq/L. If the anion gap is increased, proceed to Step 6. Step 6: If the anion gap is elevated, compare the changes from normal between the anion gap and [HCO3 ]. If the change in anion gap is similar to the change in [HCO3 ] from normal, gap acidosis is present and there is no metabolic alkalosis or nongap metabolic acidosis. If the change in anion gap is greater than the change in [HCO3 ] from normal, metabolic alkalosis is present in addition to gap metabolic acidosis. If the change in the anion gap is less than the change in [HCO3 ] from normal, nongap metabolic acidosis is present in addition to gap metabolic acidosis. (See Examples 5, 6, and 7.) Finally, be sure the interpretation of the blood gas is consistent with the clinical setting. |
Metabolic Acidosis: Diagnosis and Treatment Metabolic acidosis represents an increase in acid in body fluids reflected by a decrease in [HCO3 ] and a compensatory decrease in PCO2. Differential Diagnosis The diagnosis of metabolic acidosis (Figure 8 2) can be classified as anion gap or non anion gap acidosis. The anion gap (Normal range, 8 12 mEq/L) is calculated as: 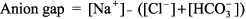
| 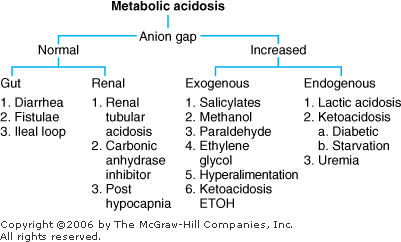 | Differential diagnosis of metabolic acidosis. |
|
Anion Gap Acidosis: Anion gap > 12 mEq/L; caused by a decrease in [HCO3 ] balanced by an increase in an unmeasured acid ion from either endogenous production or exogenous ingestion (normochloremic acidosis). Non Anion Gap Acidosis: Anion gap = 8 12 mEq/L; caused by a decrease in [HCO3 ] balanced by an increase in chloride (hyperchloremic acidosis). Renal tubular acidosis is a type of nongap acidosis that can be associated with a variety of pathologic conditions (Table 8 3). The anion gap is helpful in identifying metabolic gap acidosis, nongap acidosis, and mixed metabolic gap and nongap acidosis. If an elevated anion gap is present, a closer look at the anion gap and [HCO3 ] helps differentiate (a) pure metabolic gap acidosis, (b) metabolic nongap acidosis, (c) mixed metabolic gap and nongap acidosis, and (d) metabolic gap acidosis and metabolic alkalosis. Table 8 3 Renal Tubular Acidosis: Diagnosis and Management
|
| | Clinical Condition | Renal Defect | GFR | Serum [HCO3 ] (mmol/L)
| Serum [K+] (mmol/L) | Minimal Urine pH | Associated Disease States | Treatment |
|---|
| Normal | None | N | 24 28 | 3.5 5 | 4.8 5.2 | None | N/A | | Proximal RTA (type II RTA) | Proximal H+ secretion
| N | 15 18 |  | <5.5 | Drugs, Fanconi syndrome, various genetic disorders, dysproteinemic states, secondary hyperparathyroidism, toxins (heavy metals), tubulointerstitial diseases, nephrotic syndrome, paroxysmal nocturnal hemoglobinuria | NaHCO3 or KHCO3 (10 15 mmol/kg/d), thiazide diuretics
| | Classic distal RTA (type I RTA) | Distal H+ secretion
| N | 20 30 |  | >5.5 | Various genetic disorders, autoimmune diseases, nephrocalcinosis, drugs, toxins, tubulointerstitial diseases, hepatic cirrhosis, empty sella syndrome | NaHCO3 (1 3 mmol/kg/d)
| | Buffer deficiency (type III RTA) | Distal NH3 delivery
|  | 15 18 | N | <5.5 | Chronic renal insufficiency, renal osteodystrophy, severe hypophosphatemia | NaHCO3 (1 3 mmol/kg/d)
| | Generalized distal RTA (type IV RTA) | Distal Na+ reabsorption, K+ secretion, and H+ secretion
|  | 24 28 |  | <5.5 | Primary mineralocorticoid deficiency (eg, Addison disease), hyporeninemic hypoaldosteronism, diabetes mellitus, tubulointerstitial diseases, nephrosclerosis, drugs), salt-wasting mineralocorticoid-resistant hyperkalemia | Fludrocortisone (0.1 0.5 mg/d) dietary K+ restriction, NaHCO3 (1 3 mmol/kg/d) furosemide (40 160 mg/d)
|
|
|
Treatment of Metabolic Acidosis - 1. Correct the underlying disorder (eg, control diarrhea).
- 2. Bicarbonate therapy is reserved for severe metabolic gap acidosis. If the pH < 7.20, correct to above 7.20 with sodium bicarbonate. The total replacement dose of HCO3 can be calculated as follows:

- 3. Replace with one-half the total amount of bicarbonate over 8 12 h and reevaluate. Be aware of sodium and volume overload during replacement. A normal or isotonic bicarbonate drip is made with 3 amp NaHCO3 (50 mEq NaHCO3/amp) in 1 L D5W.
|
Metabolic Alkalosis: Diagnosis and Treatment Metabolic alkalosis represents an increase in [HCO3 ] with a compensatory rise in PCO2. Differential Diagnosis In two basic categories of diseases the kidneys retain HCO3 (Figure 8 3). They can be differentiated in terms of response to treatment with sodium chloride and also by the urinary [Cl ] as determined by ordering a "spot," or "random" urine for chloride (UCl). | 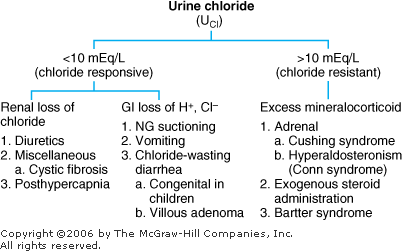 | Differential diagnosis of metabolic alkalosis. |
|
Chloride-Sensitive (Responsive) Metabolic Alkalosis: The initial problem is a sustained loss of chloride out of proportion to the loss of sodium (either by renal or GI losses). This chloride depletion results in renal sodium conservation leading to a corresponding reabsorption of HCO3 by the kidney. In this category of metabolic alkalosis, the urinary [Cl ] is < 10 mEq/L, and the disorders respond to treatment with intravenous NaCl. Chloride-Insensitive (Resistant) Metabolic Alkalosis: The pathogenesis in this category is direct stimulation of the kidneys to retain HCO3 irrespective of electrolyte intake and losses. The urinary [Cl ] > 10 mEq/L, and these disorders do not respond to NaCl administration. Treatment of Metabolic Alkalosis Correct the underlying disorder. | | 1. Chloride-responsive | | a. Replace volume with NaCl if depleted. b. Correct hypokalemia if present. c. NH4Cl and HCl should be reserved for extreme cases. |
2.Chloride-resistant | | a. Correct the underlying problem, such as stopping exogenous steroids. |
|
|
Respiratory Acidosis: Diagnosis and Treatment Respiratory acidosis is a primary rise in PCO2 with a compensatory rise in plasma [HCO3 ]. Increased PCO2 occurs in clinical situations in which decreased alveolar ventilation occurs. Differential Diagnosis Neuromuscular Abnormalities with Ventilatory Failure: Muscular dystrophy, myasthenia gravis, Guillain Barr syndrome, hypophosphatemia Central Nervous System: Drugs (sedatives, analgesics, tranquilizers, ethanol), CVA, central sleep apnea, spinal cord injury (cervical) Airway Obstruction: Chronic (COPD), acute (asthma), upper airway obstruction, obstructive sleep apnea Thoracic Pulmonary Disorders: Bony thoracic cage (flail chest, kyphoscoliosis), parenchymal lesions (pneumothorax, severe pulmonary edema, severe pneumonia), large pleural effusions, scleroderma, marked obesity (pickwickian syndrome) Treatment of Respiratory Acidosis Improve Ventilation: Intubate patient and initiate mechanical ventilation, increase ventilator rate, reverse narcotic sedation with naloxone (Narcan), etc. |
Respiratory Alkalosis: Diagnosis and Treatment Respiratory alkalosis is a primary fall in PCO2 with a compensatory decrease in plasma [HCO3 ]. Respiratory alkalosis occurs with increased alveolar ventilation. Differential Diagnosis Central Stimulation: Anxiety, hyperventilation syndrome, pain, head trauma or CVA with central neurogenic hyperventilation, tumors, salicylate overdose (often mixed metabolic gap acidosis and respiratory alkalosis), fever, early sepsis Peripheral Stimulation: PE, CHF (mild), interstitial lung disease, pneumonia, altitude, hypoxemia of any cause (see Hypoxia) Miscellaneous: Hepatic insufficiency, PRG, progesterone, hyperthyroidism, iatrogenic mechanical overventilation Treatment of Respiratory Alkalosis Correct the underlying disorder. Hyperventilation Syndrome: Best controlled by having the patient rebreathe into a paper bag to increase PCO2, decrease ventilator rate, increase amount of dead space with ventilator, or manage underlying cause. |
Hypoxia The second type of information gained from a blood gas level, in addition to acid base results, is oxygenation. Results usually are given as PO2 and O2 saturation (see Table 8 1 for normal values). These two parameters are related to each other. Oxygen saturation at any given PO2 is influenced by temperature, pH, and the level of 2,3-diphosphoglycerate (2,3-DPG) as shown in Figure 8 4. | 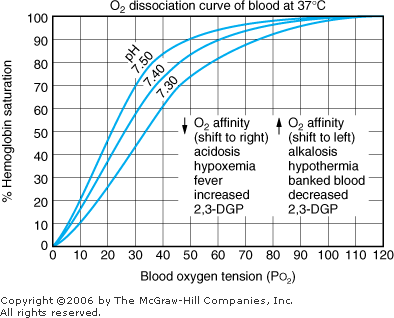 | Oxyhemoglobin dissociation curve. |
|
Oxygenation can also be determined noninvasively with pulse oximetry. Pulse oximetry is used to measure pulse rate and SaO2 and can reduce the need for ABG measurements. The transcutaneous technique (detector placed on the finger, toe, top of the ear, earlobe of adults and the foot, palm, great toe, or thumb of children) is sensitive in the detection of arterial desaturation only. The technology is based on the different red and infrared light absorption characteristics of oxygenated and deoxygenated hemoglobin. The technique may be less accurate in cases of poor perfusion, motion, sensor exposure to ambient light, skin pigmentation (usually at saturations < 80% only), use of IV contrast agents, and the presence of abnormal hemoglobins (carboxy hemoglobin, methemoglobin). Normal pulse oximetry readings should be 95 99% in a healthy person on room air and can vary slightly according to age, state of fitness, and altitude. Anemia, elevated bilirubin, and sickle cell disease do not affect readings. Hypoxia Differential Diagnosis 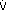 / / Abnormalities: Abnormalities:
COPD (emphysema, chronic bronchitis, asthma), atelectasis, pneumonia, PE, ARDS, pneumothorax, pneumoconiosis, CF, obstructed airway Alveolar Hypoventilation: Skeletal abnormalities, neuromuscular disorders, pickwickian syndrome, sleep apnea Decreased Pulmonary Diffusing Capacity: Pneumoconiosis, pulmonary edema, drug-induced pulmonary fibrosis (bleomycin), collagen vascular diseases Right-to-Left Shunt: Congenital heart disease (eg, tetralogy of Fallot, transposition of the great arteries) |
Sample Acid Base Problems In each of these examples, use the technique in Step 1 of Interpretation of Blood Gases to identify the acid base disorder. Example 1 A patient with COPD has a blood gas of pH 7.34, PCO2 55 mm Hg, and [HCO3 ] 29 mEq/L. Step 1: 
The numbers fit because the difference between the calculated and observed values is < 10%. Step 2: pH < 7.37, acidemia. Step 3: PCO2 > 44 mm Hg, and [HCO3 ] is not < 22 mEq/L, respiratory acidosis. Step 4: Normal compensation for chronic (COPD) respiratory acidosis (from Table 8 2). 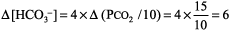
Expected [HCO3 ] is 24 mEq/L + 6 = 30, which is close to the measured [HCO3 ] of 29 mEq/L, simple respiratory acidosis. This patient has chronic respiratory acidosis due to hypoventilation (simple acid base disorder). Example 2 Immediately after cardiac arrest a patient has pH 7.25, PCO2 28 mm Hg, and [HCO3 ] 12 mEq/L. Step 1: 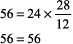
The numbers fit. Step 2: pH < 7.37, acidemia. Step 3: [HCO3 ] is < 22 mEq/L and PCO2 is not > 44 mm Hg, metabolic acidosis. Step 4: (See Table 8 2) 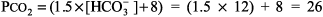
The expected PCO2 of 26 mm Hg is very similar to the measured value of 28 mm Hg, so this condition is simple metabolic acidosis. The patient has lactic acidosis following cardiopulmonary arrest (simple acid base disorder). Example 3 A young man with a fever of 103.2 F and a fruity odor on his breath has a blood gas of pH 7.36, PCO2 9 mm Hg, and [HCO3 ] 5 mEq/L. Step 1: 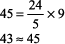
The numbers fit. Step 2: pH < 7.37 indicates acidemia. Step 3: [HCO3 ] < 22 mEq/L and PCO2 is not > 44 mm Hg, thus metabolic acidosis is present. Step 4: The expected compensation in PCO2 can be calculated as follows (see Table 8 2): 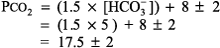
The expected PCO2 is 17.5 mm Hg, but the reading is 9 mm Hg, indicating a second process, respiratory alkalosis. This patient has metabolic acidosis due to DKA and concomitant respiratory alkalosis possibly due to early sepsis and fever (mixed acid base disorder). Example 4 A 30-year-old woman who is 30 wk PRG presents with nausea and vomiting. Blood gas analysis reveals pH 7.55, PCO2 25 mm Hg, and [HCO3 ] 22 mEq/L. Step 1: 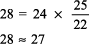
The numbers fit. Step 2: pH < 7.44 indicates alkalemia. Step 3: PCO2 < 36 mm Hg, and [HCO3 ] is not > 26 mEq/L, thus respiratory alkalosis is present. Step 4: The expected compensation for chronic respiratory alkalosis (ie, pregnancy) is calculated from Table 8 2: 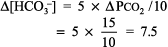
The calculated [HCO3 ] is 24 7.5, or 16 17 mEq, but the actual [HCO3 ] is 22 mEq/L, indicating relative secondary metabolic alkalosis ([HCO3 ] is higher than expected). This patient has respiratory alkalosis due to pregnancy and relative secondary metabolic alkalosis due to vomiting. Example 5 A 19-year-old patient with diabetes has an anion gap of 29 mEq/L and a [HCO3 ] of 6 mEq/L. Step 1: 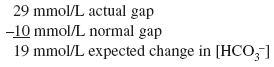
Step 2: 
Actual [HCO3 ] is 6 mEq/L, close to the expected [HCO3 ] of 5 mEq/L. Thus pure metabolic gap acidosis is present, most likely from DKA. Example 6 A 21-year-old patient with diabetes presents with nausea, vomiting, and abdominal pain. The anion gap is 23 mEq/L, and the [HCO3 ] is 18 mEq/L. Step 1: 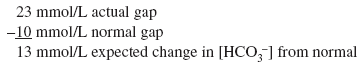
Step 2: 
The [HCO3 ] is 18 mEq/L and not the 11 mEq/L expected from pure metabolic gap acidosis. Because the actual [HCO3 ] is higher than expected, this condition is mixed metabolic gap acidosis and metabolic alkalosis. The patient has metabolic gap acidosis from DKA and metabolic alkalosis from vomiting. Example 7 A 55-year-old patient who drinks a fifth of whiskey per day has a 2-wk history of diarrhea. The anion gap is 17 mEq/L, and the [HCO3 ] is 10 mEq/L. Step 1: 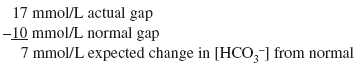
Step 2: 
Actual [HCO3 ] is 10 mEq/L and not the expected 17 mEq/L of pure metabolic gap acidosis. Because the actual [HCO3 ] is lower than expected, mixed metabolic gap acidosis and metabolic nongap acidosis must be present. The patient has metabolic nongap acidosis from diarrhea and metabolic gap acidosis from alcoholic ketoacidosis. |
| |  |