Soft Nanotechnology
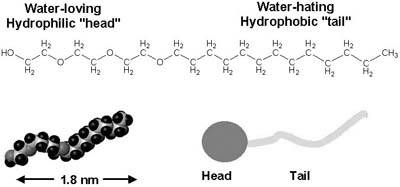
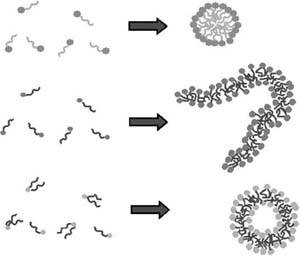
| Fiona Case Many soft or fluid consumer productssuch as foods, paint, detergents, personal care products, and cosmeticscontain nanometer- to micrometer-scale structures. These structures are formed by the spontaneous self-assembly of natural or synthetic surfactants or block copolymers. In many cases, to create the desired structure and performance, complex mixtures of different surfactants and polymers are required. Figure 13-12 shows an example of a simple, nonionic surfactant. Figure 13-13 illustrates some of the soft nanoscale structures that form spontaneously in surfactant or block copolymer solution. The particular structure that is formed depends on the relative sizes of the head and tail and their chemical character. For example, surfactants that have ionic (charged) head groups often form spherical micelles, whereas non-ionic (uncharged) surfactants are more likely to form rodlike structures, and surfactants that have more than one tail are more likely to form vesicles or lamella (almost flat sheets). The soft nanoscale structure can also be influenced by the order of addition of the different ingredients. Figure 13-12. A simple example of a surfactant, in this case a non-ionic ethoxylate surfactant, shown as the chemical structure (top), as a space-filling molecular model (bottom left), and as a cartoon representation (bottom right). Figure 13-13. Cartoon representations of different surfactants forming a spherical micelle (top), a rodlike micelle (middle), and a vesicle (bottom) in water. A slice through each of the nanostructures is shown; in the complete structures, the hydrophilic head groups surround the hydrophobic tails, shielding them from unfavorable interactions with water. These soft nanostructures do work on demand. When you add dish liquid to hot water in a dishpan full of dirty pots and pans, the nanoscale structure of the surfactant micelles change as they migrate to the oily surfaces, encapsulate the grease, and carry it into solution. When you paint a wall with a good-quality emulsion-based paint, the brush moves easily as you spread the paint on the wall, but as soon as you finish brushing an area, the structure changes and the viscosity of the paint increases to reduce the occurrence of drips. Soft nanotechnology is also at work when you mix up a cake or even enjoy the taste and texture of chocolate, ice cream, or yogurt. Designing these complex materials is difficult, and characterizing them is challenging. Until recently, most soft nanostructured products were created by experienced formulators, using empirical rules that were passed down through generations of product developers. Although ideas of self-assembly were invoked to explain behavior, it was almost impossible to predict what structure would be created from a particular mixture of ingredients. And because it was extremely difficult to characterize products that were known to be successful, it was hard to know what structure was desirable for a particular application. Today, new methods of characterization are providing insight into soft nanostructured materialsfor example, dynamic light scattering, NMR diffusion experiments, X-ray and neutron scattering, and electron microscopy. New robotics techniques are allowing developers to explore many more formulas than was possible when each candidate formula had to be created by hand. New theories and computer simulation methods are enabling researchers to construct realistic models of soft nanostructured materials. Taken together, these developments have the potential to revolutionize consumer product development. Other members of the nanotechnology community are also becoming interested in self-assembly and nanostructured fluids, which indeed have the potential to provide robust and inexpensive strategies for creating nanoscale materials. For example, IBM recently highlighted the potential for block-copolymer self-assembly to create nanometer-size structures for electronics applications. Several groups are exploring strategies for creating hard nanoparticles using soft self-assembled structures as templates, and self-assembled block copolymer-stabilized emulsions and vesicles are being proposed as drug- (and nutriceutical-) delivery devices. Moreover, various soft nanotech strategies are being applied to stabilize colloidal systems (nanoparticle solutions and gels). |
EAN: 2147483647
Pages: 204
- Context Management of ERP Processes in Virtual Communities
- Distributed Data Warehouse for Geo-spatial Services
- Healthcare Information: From Administrative to Practice Databases
- A Hybrid Clustering Technique to Improve Patient Data Quality
- Relevance and Micro-Relevance for the Professional as Determinants of IT-Diffusion and IT-Use in Healthcare