11. Opioidergic compounds - preclinical data
Editors: Spanagel, Rainer; Mann, Karl F.
Title: Drugs for Relapse Prevention of Alcoholism, 1st Edition
Copyright 2005 Springer
> Table of Contents > Opioidergic compounds: preclinical data
Opioidergic compounds: preclinical data
Petri Hyyti
Department of Mental Health and Alcohol Research, National Public Health Institute, POB 33, FI-00251 Helsinki, Finland
Introduction
Ethanol-induced activation of the endogenous opioid system has been hypothesized to be one of the mechanisms mediating ethanol reinforcement and enhanced ethanol drinking. This hypothesis is supported by many lines of evidence, including ethanol-induced elevation of extracellular levels of -endorphin in the nucleus accumbens [1], and genetically determined differences in the basal levels of -endorphin, enkephalins, and dynorphins in distinct brain areas of rodent lines that differ in ethanol self-administration behavior [2, 3 and 4]. In accordance with this hypothesis, the non-selective opioid receptor antagonists naltrexone, naloxone and nalmefene suppress ethanol-reinforced behavior in widely different experimental conditions.
Opioid receptors are divided into three major classes, the -, - and -opioid receptors, which have all been cloned and sequenced [5, 6, 7 and 8]. Furthermore, on the basis of pharmacological evidence, subtypes of these receptors have been proposed. -endorphin recognizes both - and -receptors with almost equal potency. Also, enkephalins and dynorphins interact with -receptors but with a lower affinity as compared to - and - receptors, respectively [9]. Increasing evidence for the importance of ethanol-induced activation of both -endorphin and enkephalin systems in ethanol reward has further prompted research on the contribution of the different opioid receptor types, especially the - and - receptors, in the reinforcing effect of ethanol. Because the commonly used non-selective antagonists (naltrexone, naloxone and nalmefene) bind to all opioid receptor types as a function of the dose administered [10, 11 and 12], the roles of the opioid receptor types in ethanol reinforcement, in recent studies, have been studied with antagonists selective for these receptors.
In the majority of these studies, the selective antagonists have been tested using behavioral models that measure the direct reinforcing effects of ethanol, including various free-choice drinking and operant self-administration models. These models do not permit assessment of the conditioned ethanol effects, which underlie craving and relapse. However, there is evidence showing that the modulation of the reinforcing effects of ethanol by opioid antagonists may
P.118
also predict their effects on the appetitive conditioned aspects of ethanol consumption [13, 14].
Effects of selective -receptor antagonists on ethanol consumption
Selective -opioid receptor antagonists -funaltrexamine, D-Pen-Cys-Tyr-DTrp-Orn-Thr-Pen-Thr-NH2 (CTOP), and naloxonazine have been shown to reliably suppress ethanol intake in many experimental paradigms. Systemically administered -funaltrexamine (5-20 mg/kg) produced a dose-dependent decrease in ethanol consumption both in the high alcohol drinking (HAD) rat line on a fluid-deprivation schedule with a 2-h daily access to ethanol and water, as well as in Wistar rats, given limited access 1-h to ethanol with ad libitum water and food [15, 16]. Injections of -funaltrexamine were administered 16-20 h before the opportunity to drink, because this antagonist has an initial -agonist effect lasting 2-3 h, followed by a long (2-4 days) -antagonist effect. In both experiments, the highest dose, 20 mg/kg -funaltrexamine, decreased ethanol intake even during the second post-injection session, probably reflecting the long-lasting -antagonist action. Although -funaltrexamine did not attenuate the 2-h scheduled saccharin intake, decrease in 24-h water consumption suggests that this antagonist displays a general suppressive effect on ingestive behavior [15].
Another -opioid receptor antagonist, CTOP, decreased both limited access ethanol drinking and operant responding for ethanol in alcohol-preferring AA (Alko, Alcohol) and Wistar rats after intracerebroventricular (0.3-3 g) administration [17] (Fig. 1). Moreover, systemic administration of this compound
P.119
has been reported to suppress ethanol drinking in mice as well [18]. With repeated administration of CTOP across three sessions, a progressive decline in limited access ethanol drinking was observed, with a transient decrease in 24-h water and food intake [19]. It is not clear, however, whether this finding could be interpreted as an extinction-like decrease caused by an antagonist-induced attenuation of ethanol's reinforcing effects or whether it is due to cumulative drug effects. Moreover, the highest dose, 3- g, had a tendency of slowing the initiation of operant responding for ethanol, suggestive of aversive effects produced by CTOP [17].
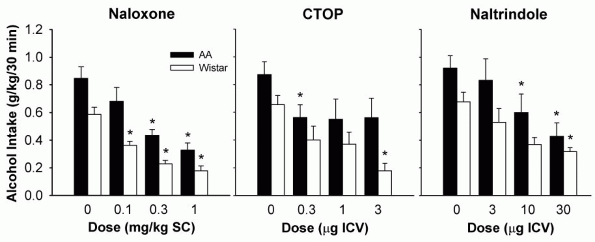 |
Figure 1. Effects of subcutaneous naloxone, and intracerebroventricular CTOP and naltrindole injections on ethanol consumption in alcohol-preferring AA and heterogeneous Wistar rats. The animals were allowed to respond for a 0.1 ml drop of 10% ethanol solution on a FR1 schedule during daily 30-min sessions. Data are expressed as mean ( SEM) ethanol intake (g/kg) during the 30-min session. (* p < 0.05, significantly different from the vehicle conditions). Adapted with permission of Lippincott Williams & Wilkins, Baltimore, from Hyyti P & Kiianmaa K (2001) Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res 25: 25-33. |
Systemic naloxonazine blocks central 1-opioid receptors irreversibly, well over 24 h, but can also block 2-receptors in a reversible manner during the first hours after administration. Thus, when administered 20 h prior to ethanol and concomitant food access, the dose-dependent (1-15 mg/kg) decrease in both ethanol and food intake by naloxonazine could be attributed to a 1-blockade [20]. However, naloxonazine suppressed ethanol and food consumption as well, when given 15 min before the session, suggesting that both 1- and 2-receptor blockade could modulate ethanol reinforcement [21]. When the 15 mg naloxonazine was administered before three successive ethanol sessions, a decrease was seen only during the first session, after which tolerance to the drug's effect developed. This was probably due to a -receptor up-regulation, produced by the prolonged receptor blockade [20].
Effects of selective -receptor antagonists on ethanol consumption
Although -opioid receptor antagonists have been shown to attenuate ethanol reinforcement in many studies, the results are generally more inconsistent when compared to those from experiments with -antagonists. The first -antagonist tested for its effects on ethanol drinking was ICI 174864. When both ethanol and water availability was limited to one 30-min daily access period, ICI 174864 (0.5-3 mg/kg) dose-dependently decreased ethanol but not water intake in the alcohol-preferring HAD rats [22]. Similarly, single injections of this compound (3-8 mg/kg) suppressed ethanol drinking during the first of the three 1-h access periods every 4 h in alcohol-preferring P rats without effects on 24-h water drinking [23]. The effect of naltrindole, another -receptor antagonist (5-20 mg/kg) tested in the same model, lasted for the first two access periods, and no effects were seen on water consumption [23]. However, separate control experiments indicated that naltrindole also affected ethanol-saccharin and saccharin solution intake, suggesting that the suppressive effects were not specific for ethanol.
Attenuation of ethanol consumption by naltrindole in rats and mice has been reported in several other studies [17, 18, 24, 25], but these positive findings have not been uniformly replicated [16, 20, 26, 27]. The reasons for the discrepant findings are not clear. The failure to see decreases in ethanol drinking by naltrindole was probably not always caused by insufficient dosing, as negative
P.120
findings were also reported from experiments where the naltrindole doses matched those employed in positive reports. The genetic background of the animals cannot easily explain the differences either, as indicated by similar naltrindole-induced decreases in operant responding for ethanol both in the alcohol-preferring AA as well as the heterogeneous Wistar rats [17] (Fig. 1). Finally, a meaningful comparison of the various behavioral models used for measuring ethanol consumption is complicated by different periods of ethanol and/or fluid deprivation affecting the motivational state of the animals and by the various combinations of conditioned factors present at the time of ethanol access.
Based on pharmacological and behavioral evidence, the existence of two -opioid receptor subtypes in the rodent brain, 1- and 2-receptors, has been proposed [28, 29]. Naltrindole blocks both subtypes but naltriben is a putative 2-receptor antagonist. Systemic naltriben has been shown to decrease ethanol drinking in a daily 8-h limited access situation and operant responding for ethanol [30, 31]. The effect of naltriben on ethanol reinforcement was relatively specific, as it did not affect intake of saccharin/ethanol or quinine/ethanol solutions in the limited access paradigm nor did it affect responses for the concomitantly available saccharin solution in the operant model.
Effects of selective opioid receptor antagonists on ethanol seeking
Drug-paired environmental stimuli may acquire powerful incentive-motivational properties through classical conditioning and elicit drug craving and relapse in drug abusers as well as in laboratory animals trained to self-administer drugs [32, 33]. There is both clinical and preclinical evidence showing that the endogenous opioid system is not only involved in the direct reinforcing effects of ethanol, but may also partly mediate the effects of conditioned contextual cues on ethanol seeking. For example, naltrexone was demonstrated to attenuate the efficacy of ethanol-associated environmental stimuli to reinstate extinguished responding for ethanol in laboratory rats [13]. Using the same behavioral model, naltrindole and naloxonazine were used for assessing the contribution of - and -opioid receptors in ethanol seeking [14] (Fig. 2). Naltrindole decreased ethanol-seeking behavior at the highest dose (5 mg/kg) under stimuli predictive of ethanol reward, but did not affect responding under the stimulus condition associated with non-reward. In contrast, the effect of naloxonazine was not specific, because the effective dose (15 mg/kg) that suppressed ethanol-seeking behavior also decreased responding under the non-reward stimulus condition.
The conditioned place preference methods have long been used to measure the motivating effects of drug-paired environmental stimuli. The non-selective opioid antagonist naloxone has been shown to attenuate ethanol-induced conditioned place preference in mice [34, 35]. In rats, however, it has been difficult to observe ethanol-induced place preference without special conditioning procedures, including exposure to stress. For example, ethanol produced conditioned
P.121
place preference in rats that were given electric foot shocks prior to ethanol injections (0.3 g/kg) and conditioning. In these animals, pre-treatment with the -antagonist -funaltrexamine or the -antagonist naltrindole dose-dependently reduced preference for the ethanol-paired compartment at doses that have been shown to decrease ethanol drinking [36, 37].
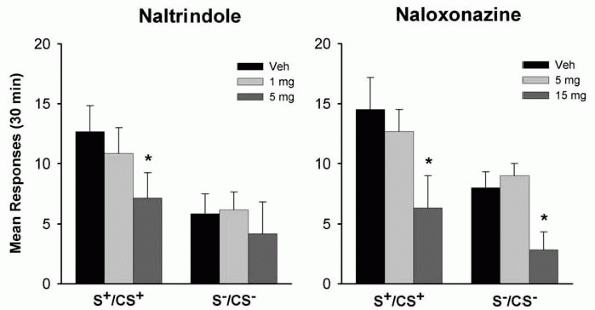 |
Figure 2. Effects of naltrindole and naloxonazine on cue-induced reinstatement of ethanol-seeking behavior. Both drugs were tested under the stimulus conditions previously associated with ethanol (S+/CS+) or non-reward/water (S-/CS-). Data are expressed as mean ( SEM) responses at the active lever during 30-min reinstatement sessions. (* p < 0.05, significantly different from the vehicle conditions). Adapted with permission of Nature Publishing Group, London, from Ciccocioppo R, Martin-Fardon R, Weiss F (2002) Effect of selective blockade of 1 or opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27: 391-399. |
Opioidergic mechanisms of ethanol reinforcement and conditioned ethanol effects
Reductions produced in ethanol consumption and ethanol-seeking behavior by the selective opioid receptor antagonists, generally suggest a role for both the - and -receptors in these phenomena. However, there are also discrepant findings, especially with respect to the involvement of the -receptors in ethanol drinking behavior. Moreover, the experimental data suggest that in most experimental situations, the suppressive effects of opioid receptor antagonists on ethanol consumption are not specific, but reflect the well-known involvement of opioid receptors with regard to ingestive behavior [38].
The precise roles of the opioid receptors in ethanol reinforcement and conditioned reinforcement processes are not yet very well known. There is evidence that systemically administered ethanol increases the level of extracellular -endorphin in the nucleus accumbens [1]. Moreover, intra-accumbal infusions
P.122
of methylnaloxonium and naltrindole suppressed ethanol self-administration, suggesting that reductions in ethanol reinforcement by opioid antagonists could be related to their inhibition of the endogenous opioid peptide action in the nucleus accumbens [17, 39]. Infusion of these antagonists into the amygdala also attenuated ethanol reinforcement, which could be related to the role of the amygdala in stimulus-reward associations [17]. Another line of evidence suggests that the suppressive effect of opioid antagonists on ethanol reinforcement could involve interaction with mesolimbic dopamine transmission. For example, ethanol-induced increase in extracellular dopamine level in the nucleus accumbens was attenuated by systemic naltrexone and focal naltrindole administration [40, 41 and 42].
In addition to the direct pharmacological actions of ethanol, ethanol-associated contextual stimuli can increase dopamine levels in the nucleus accumbens as well. This is consistent with the view of the role of the midbrain dopamine neurons in the processing of motivational signals [43, 44]. So far, there are no data on the effects of selective opioid antagonists on cue-induced enhancement in dopamine transmission. Both the - and -receptors are involved in tonic modulation of mesolimbic dopamine transmission [45], and blockade of these receptors could therefore blunt the efficacy of the contextual cues in enhancing dopamine transmission and reinstating ethanol seeking.
References
1 Olive MF, Koenig HN, Nannini MA, Hodge CW (2001) Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 21: 184RC
2 de Waele J-P, Papachristou DN, Gianoulakis C (1992) The alcohol-preferring C57BL/6 mice present an enhanced sensitivity of the hypothalamic -endorphin system to ethanol than the alcohol-avoiding DBA/2 mice. J Pharmacol Exp Ther 261: 788-794
3 Gianoulakis C, de Waele J-P, Kiianmaa K (1992) Differences in the brain and pituitary -endorphin system between the alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res 16: 453-459
4 Nylander I, Hyyti P, Forsander O, Terenius L (1994) Differences between alcohol-preferring (AA) and alcohol-avoiding (ANA) rats in the prodynorphin and proenkephalin systems. Alcohol Clin Exp Res 18: 1272-1279
5 Chen Y, Mestek A, Liu J, Hurley JA, Yu L (1993) Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol 44: 8-12
6 Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH (1992) Cloning of a delta opioid receptor by functional expression. Science 258: 1952-1955
7 Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG (1992) The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA 89: 12048-12052
8 Minami M, Toya T, Katao Y, Maekawa K, Nakamura S, Onogi T, Kaneko S, Satoh M (1993) Cloning and expression of a cDNA for the rat kappa-opioid receptor. FEBS Lett 329: 291-295
9 Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S (1998) Endogenous opioids: overview and current issues. Drug Alcohol Depend 51: 127-140
10 Chang KJ, Cooper BR, Hazum E, Cuatrecasas P (1979) Multiple opiate receptors: different regional distribution in the brain and differential binding of opiates and opioid peptides. Mol Pharmacol 16: 91-104
11 Chang KJ, Cuatrecasas P (1981) Heterogeneity and properties of opiate receptors. Fed Proc 40: 2729-2734
P.123
12 Childers SR, Creese I, Snowman AM, Synder SH (1979) Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol 55: 11-18
13 Katner SN, Magalong JG, Weiss F (1999) Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 20: 471-479
14 Ciccocioppo R, Martin-Fardon R, Weiss F (2002) Effect of selective blockade of 1 or opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology 27: 391-399
15 Krishnan-Sarin S, Wand GS, Li XW, Portoghese PS, Froehlich JC (1998) Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol Biochem Behav 59: 627-635
16 Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP (1998) A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol 15: 281-289
17 Hyyti P, Kiianmaa K (2001) Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res 25: 25-33
18 Kim SG, Stromberg MF, Kim MJ, Volpicelli JR, Park JM (2000) The effect of antagonists selective for mu- and delta-opioid receptor subtypes on alcohol consumption in C57BL/6 mice. Alcohol 22: 85-90
19 Hyyti P (1993) Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav 45: 697-701
20 Honkanen A, Vilamo L, Wegelius K, Sarviharju M, Hyyti P, Korpi ER (1996) Alcohol drinking is reduced by a 1- but not by a -opioid receptor antagonist in alcohol-preferring rats. Eur J Pharmacol 304: 7-13
21 Mhatre M, Holloway F (2003) Microl-opioid antagonist naloxonazine alters ethanol discrimination and consumption. Alcohol 29: 109-116
22 Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK (1991) Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 103: 467-472
23 Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, Froehlich JC (1995) The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology (Berl) 120: 177-185
24 Franck J, Lindholm S, Raaschou P (1998) Modulation of volitional ethanol intake in the rat by central delta-opioid receptors. Alcohol Clin Exp Res 22: 1185-1189
25 Le AD, Poulos CX, Quan B, Chow S (1993) The effects of selective blockade of delta and mu opiate receptors on ethanol consumption by C57BL/6 mice in a restricted access paradigm. Brain Res 630: 330-332
26 Williams KL, Woods JH (1998) Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa-, or delta-receptor. Alcohol Clin Exp Res 22: 1634-1639
27 Middaugh LD, Kelley BM, Groseclose CH, Cuison ER Jr (2000) Delta-opioid and 5-HT3 receptor antagonist effects on ethanol reward and discrimination in C57BL/6 mice. Pharmacol Biochem Behav 65: 145-154
28 Negri L, Potenza RL, Corsi R, Melchiorri P (1991) Evidence for two subtypes of delta opioid receptors in rat brain. Eur J Pharmacol 196: 335-336
29 Sofuoglu M, Portoghese PS, Takemori AE (1991) Differential antagonism of delta opioid agonists by naltrindole and its benzofuran analog (NTB) in mice: evidence for delta opioid receptor subtypes. J Pharmacol Exp Ther 257: 676-680
30 Krishnan-Sarin S, Portoghese PS, Li TK, Froehlich JC (1995) The delta 2-opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol Biochem Behav 52: 153-159
31 June HL, McCane SR, Zink RW, Portoghese PS, Li TK, Froehlich JC (1999) The delta 2-opioid receptor antagonist naltriben reduces motivated responding for ethanol. Psychopharmacology (Berl) 147: 81-89
32 O'Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12: 15-22
33 See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71: 517-529
P.124
34 Cunningham CL, Henderson CM, Bormann NM (1998) Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 139: 62-70
35 Kuzmin A, Sandin J, Terenius L, Ogren SO (2003) Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther 304: 310-318
36 Matsuzawa S, Suzuki T, Misawa M, Nagase H (1998) Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res 803: 169-177
37 Matsuzawa S, Suzuki T, Misawa M, Nagase H (1999) Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol 368: 9-16
38 Cooper SJ (1983) Benzodiazepine-opiate antagonist interactions in relation to feeding and drinking behavior. Life Sci 32: 1043-1051
39 Heyser CJ, Roberts AJ, Schulteis G, Koob GF (1999) Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res 23: 1468-1476
40 Benjamin D, Grant ER, Pohorecky LA (1993) Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 621: 137-140
41 Acquas E, Meloni M, Di Chiara G (1993) Blockade of delta-opioid receptors in the nucleus accumbens prevents ethanol-induced stimulation of dopamine release. Eur J Pharmacol 230: 239-241
42 Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18: 10663-10671
43 Weiss F, Lorang MT, Bloom FE, Koob GF (1993) Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267: 250-258
44 Katner SN, Weiss F (1999) Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res 23: 1751-1760
45 Devine DP, Leone P, Pocock D, Wise RA (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis study. J Pharmacol Exp Ther 266: 1236-1246
EAN: 2147483647
Pages: 26
- ERP Systems Impact on Organizations
- Challenging the Unpredictable: Changeable Order Management Systems
- The Effects of an Enterprise Resource Planning System (ERP) Implementation on Job Characteristics – A Study using the Hackman and Oldham Job Characteristics Model
- Intrinsic and Contextual Data Quality: The Effect of Media and Personal Involvement
- Relevance and Micro-Relevance for the Professional as Determinants of IT-Diffusion and IT-Use in Healthcare