56 - Pneumothorax
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XI - The Pleura > Chapter 68 - Malignant Pleural Effusions
Chapter 68
Malignant Pleural Effusions
Steven A. Sahn
DEFINITION AND INCIDENCE
Malignant pleural effusions are diagnosed by finding malignant cells in pleural fluid or pleural tissue by closed needle biopsy, by biopsy through thoracoscopy or thoracotomy, or at autopsy. In some cases of established malignancy with an associated pleural effusion, malignant cells cannot be demonstrated in either pleural fluid or pleural tissue and probably are not present at the time of the diagnostic procedure. I (1985) have termed these effusions paramalignant because they are associated with and caused by the malignancy but do not result from pleural invasion by tumor. Paramalignant effusions can be caused by a direct local effect of the tumor, by systemic manifestations of the malignancy, or as a consequence of therapy (Table 68-1). Impaired pleural space lymphatic drainage is an important mechanism responsible for the formation of both paramalignant and malignant pleural effusions.
Virtually all cancers metastasize to the pleura. Lung cancer is the most common to involve the pleura because of its proximity to the pleural surface and, as Meyer (1966) suggested, its propensity to invade the pulmonary arteries and embolize to the visceral pleura. Breast cancer also frequently metastasizes to the pleura, causing approximately 25% of malignant pleural effusions in large series. Ovarian carcinoma and gastric cancer are next in frequency, and each represents less than 5% of malignant pleural effusions. I (1998) found that approximately 7% of patients with malignant pleural effusions, however, have an unknown primary site at the time of the initial diagnosis of the malignant effusion.
I (1998) have noted that lymphomas account for approximately 10% of all malignant pleural effusions and, according to Valentine and Raffin (1992), are the most common cause of chylothorax. Both Hodgkin's disease and non-Hodgkin's lymphoma have been associated with pleural effusions with variable incidences and usually through different mechanisms.
Diffuse malignant mesothelioma arises from mesothelial cells or possibly from a precursor cell that is situated in the submesothelial connective tissue. The association of asbestos exposure and malignant mesothelioma was established in 1960 by the report of Wagner and colleagues (1960). McDonald and co-workers (1970) recorded that the incidence of malignant mesothelioma is approximately one per million per year in the general population that is not exposed to asbestos. McDonald and associates (1970) reported that the incidence can increase 20-fold in certain populations and is even higher in shipyard communities.
PATHOGENESIS
Impaired lymphatic drainage of the pleural space is an important mechanism responsible for accumulation of large volumes of pleural fluid in malignancy. The lymphatic system can be blocked at any point from the stoma of the parietal pleura to the mediastinal and parasternal (internal mammary) lymph nodes. The autopsy studies by Meyer (1966) and Chernow and myself (1977) have demonstrated the association of mediastinal lymph node involvement and the presence of substantial pleural fluid. Conversely, these studies showed evidence of pleural involvement with tumor in the absence of pleural effusions, lending support to this mechanism. Furthermore, as Meyer (1966) noted, pleural effusions usually do not occur when the pleura is involved by sarcoma because of the absence of lymphatic metastasis. Weick and associates (1973) noted that Hodgkin's disease tends to cause pleural effusions by lymphatic obstruction, and Jenkins and colleagues (1981) and Xaubet and associates (1985) noted that non-Hodgkin's lymphoma tends to produce effusions by both lymphatic obstruction and direct pleural invasion.
The inflammatory response to pleural tumor invasion results in increased microvascular permeability and produces variable volumes of pleural effusion. Chretien and Jaubert (1985)
P.936
suggested that oxygen radicals, arachidonic acid metabolites, proteases, lymphocytes, and immune complexes are probably causative.
Table 68-1. Causes of Paramalignant Pleural Effusions | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
Vascular endothelial growth factor (VEGF) has been shown by Ferrara (1999) to play an important role in the formation of pleural effusions. The cytokine VEGF has been documented to induce vascular leakage in the formation of both pleural effusion and ascites. Tumor cells implanted in the pleura of experimental animals secrete VEGF, leading to microvascular permeability. Blocking the VEGF receptor has inhibited pleural fluid formation in an in vivo model of adenocarcinoma of the lung. Lee and associates (2002) noted that mesothelial cells appear to be an important source of VEGF, and transforming growth factor- has been shown to increase VEGF production both in vivo and in vitro.
Pleural effusion is an early manifestation of a malignant mesothelioma and probably results from a combination of increased capillary permeability from direct pleural invasion and impaired lymphatic drainage of the pleural space. As the tumor progresses and the visceral and parietal pleura fuse, the fluid diminishes or disappears.
Autopsy series have shown that in patients with carcinoma of the lung, pleural metastasis is virtually always found on both the visceral and parietal pleural surfaces. Meyer (1966) noted that rarely is only the visceral pleural surface involved, and isolated parietal pleural metastases were never identified. Visceral pleural metastasis in lung cancer appears to result from contiguous spread or through pulmonary arterial invasion and embolization. Once seeded with tumor, these malignant cells migrate from the visceral to parietal pleural surface along either preformed or tumor-induced pleural adhesions. Alternatively, free tumor cells exfoliated from the visceral pleural surface can adhere to the parietal pleura and multiply. Chernow and I (1977) reported that adenocarcinoma of the lung is the most common cell type to involve the pleura, presumably owing to its peripheral location and propensity for vascular invasion. When bilateral pleural metastases develop in lung cancer, hepatic spread and parenchymal invasion in the contralateral lung usually are causative. Once contralateral lung metastasis occurs, pulmonary artery invasion and embolization follow, as in the initial ipsilateral lesion. The data concerning the laterality of the pleural effusion in relation to the primary lesion support this mechanism.
In lung cancer, pleural effusions occur either ipsilaterally or bilaterally and virtually never occur solely in the contralateral pleural space. With other cancers, pleural involvement is usually from tertiary spread from established liver metastases with no predilection for side. Fentiman and associates (1981) summarized the conflicting data in breast carcinoma, with some studies showing a high incidence of ipsilateral pleural effusion and others no predilection for side. Probably two mechanisms are operative: chest wall lymphatic invasion resulting in ipsilateral effusion, and hepatic spread with bilateral, unilateral, or contralateral hematogenous metastasis.
CLINICAL PRESENTATION
The most common presenting symptom of patients with carcinoma or lymphoma of the pleura and a large pleural effusion is dyspnea with exertion. In diffuse pleural mesothelioma, patients generally present with the insidious onset of either chest pain or dyspnea. Taryle and colleagues (1976) noted that almost all patients with a malignant mesothelioma present with some symptoms, whereas Chernow and I (1977) and Weick and associates (1973) reported that up to 25% of patients with carcinoma or lymphoma of the pleura, respectively, may be relatively asymptomatic when the pleural effusion is initially discovered on a routine chest radiograph.
Because malignant involvement of the pleura signals advanced disease, these patients often have weight loss and
P.937
appear chronically ill. A positive pleural cytology provided the initial diagnosis of cancer in almost 50% of these patients.
Patients with carcinoma of the pleura may have chest pain caused by involvement of the parietal pleura, ribs, or chest wall. Elmes and Simpson (1976) emphasized, however, that the chest pain associated with malignant mesothelioma is more common and impressive but is nonpleuritic and frequently referred to the upper abdomen or shoulder.
Chernow and I (1977) noted that signs of a pleural effusion are typically found on physical examination, and cachexia and lymphadenopathy may be seen in cancer, but the examination may be unremarkable in malignant mesothelioma, except for the findings of a moderate to large pleural effusion.
RADIOGRAPHS OF THE CHEST
The pleural effusion associated with lung cancer is ipsilateral to the primary lesion. This may be because of direct pleural involvement, mediastinal lymph node infiltration, or an endobronchial lesion with pneumonia or atelectasis. With other primary sites, with the possible exception of breast cancer, there appears to be no ipsilateral predilection and bilateral effusions are common, and as Chernow and I (1977) pointed out, are usually the result of bilateral mediastinal lymph node metastasis; bilateral parenchymal metastasis, thoracic duct involvement, and malignant ascites can also cause bilateral malignant effusions.
Patients with carcinoma of the pleura usually present with a moderate to large effusion (500 to 2,000 mL; 10% have effusions of < 500 mL, and a similar proportion of patients have massive pleural effusion with complete opacification of the hemithorax). Malignancy is the most common cause of a massive pleural effusion; in a series by Maher and Berger (1972), 67% of 46 massive pleural effusions were caused by malignancy (Fig. 68-1). The radiographic finding of bilateral effusions with a normal heart size suggests malignancy, most commonly carcinoma, which Rabin and Blackman (1957) noted (Fig. 68-2). Nonmalignant effusions associated with these radiographic findings include lupus pleuritis, esophageal rupture, hepatic hydrothorax, nephrotic syndrome, and constrictive pericarditis.
When a patient presents with an apparent large pleural effusion with absence of contralateral mediastinal shift, malignancy is usually the cause. The following diagnoses should be considered in this context: (a) carcinoma of the ipsilateral main-stem bronchus causing atelectasis, (b) a fixed mediastinum caused by malignant lymph nodes, (c) malignant mesothelioma (the density represents mostly tumor with a small effusion), and (d) extensive tumor infiltration of the ipsilateral lung radiographically mimicking a large effusion.
As Whitcomb and associates (1972) and MacDonald (1977) described in Hodgkin's disease, patients with pleural
P.938
effusions usually have associated lymphadenopathy and parenchymal infiltrates. In contrast, Jenkins and colleagues (1981) reported that in non-Hodgkin's lymphoma, intrathoracic lymphadenopathy occurs in few of the cases associated with either pulmonary disease or pleural effusions.
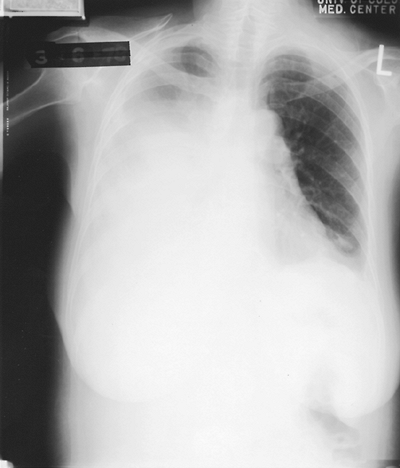 |
Fig. 68-1. A 60-year-old woman with adenocarcinoma of the lung with a massive right pleural effusion. Note the contralateral mediastinal shift. |
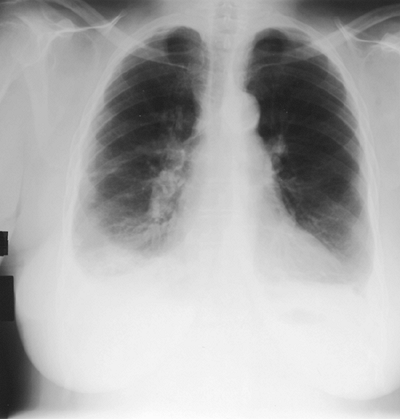 |
Fig. 68-2. A 64-year-old woman who presented with progressive dyspnea on exertion. She had a salivary amylase-rich pleural effusion. Note the bilateral pleural effusions, a cardiac silhouette at the upper limits of normal, and no evidence of congestive heart failure. |
Heller and colleagues (1970) noted that in malignant mesothelioma the initial chest radiograph usually shows a moderate to large unilateral pleural effusion. After therapeutic thoracentesis, the pleura may show thickening and nodularity. Evidence of asbestos exposure, such as interstitial lung disease or pleural plaques, may be identified in the contralateral lung and pleura. Radiographic clues suggesting that the large effusion may be caused by mesothelioma rather than carcinoma are pleural nodularity, absence of contralateral mediastinal shift with an apparent large effusion, and a tendency for loculation.
PLEURAL FLUID CHARACTERISTICS
Malignant pleural effusions may be serous, serosanguineous, or grossly bloody. A grossly bloody effusion suggests direct pleural involvement, whereas a serous effusion results from either lymphatic obstruction or an endobronchial lesion with atelectasis. Light and co-workers (1973) suggested that when the red blood cell count in the pleural fluid is greater than 100,000 per microliter in the absence of trauma, malignancy is the most likely diagnosis. Most of the nucleated cells (2,500 to 4,000 per microliter) in pleural fluid, as Yam (1967) noted, are lymphocytes, macrophages, and mesothelial cells; more than 50% of the cellular population are lymphocytes in approximately half of the cases. Lymphomatous pleural effusions typically have lymphocyte counts of greater than 80%. The percentage of polymorphonuclear cells (PMNs) usually is less than 25% of the cell population but on rare occasions, when there is intense pleural inflammation, PMNs may predominate. In a prospective study, Rubins and Rubins (1996) reported that pleural fluid eosinophilia occurred in 7.8% (10 of 128) of patients with malignant effusions and that malignancy is as prevalent among eosinophilic as among noneosinophilic effusions.
Carcinomatous pleural effusions typically are exudative; however, the protein concentration is variable. Chernow and I (1977) and Light and associates (1972) reported protein concentrations ranging from 1.5 to 8.0 g/dL. Up to 5% of malignant pleural effusions are transudates. These transudative malignant effusions are caused by early stages of lymphatic obstruction, atelectasis from bronchial obstruction, or concomitant disease, such as congestive heart failure. When an effusion meets exudative criteria by lactate dehydrogenase (LDH) but not protein, Light and associates (1972) emphasized that malignancy should be suspected. The author along with Good (1988) found that approximately one third of patients with malignant pleural effusions have a low pleural fluid pH (<7.30, range of 6.95 to 7.29) and a low glucose concentration (<60 mg/dL or pleural fluid to serum ratio of <0.5) at presentation. These effusions usually have been present for several months and are associated with a large tumor burden and possibly fibrosis of the pleural surface. Good and colleagues (1985) suggested that the abnormal pleural membrane reduces glucose entry into the pleural space and impairs glucose end-product (CO2 and lactic acid) efflux, resulting in a local acidosis. Furthermore, I and my colleague Good (1988) noted that low-pH/low-glucose malignant effusions are associated with shorter survival, higher diagnostic yield on cytologic examination, and a poorer response to chemical pleurodesis compared with normal pH and glucose malignant effusions.
Pleural effusions caused by lymphoma have characteristics similar to those of carcinoma of the pleura. These effusions, however, tend to be less hemorrhagic and less likely to result in pleural fluid acidosis and low glucose concentrations. Both myself (1985) and Gottehrer and colleagues (1990) have pointed out that a pleural effusion in malignant mesothelioma is more likely to have a low pH and low glucose content and greater protein and LDH concentrations than effusions from carcinoma of the pleura. Because of an overlap of values, however, these data are not helpful in separating carcinoma from mesothelioma in an individual patient.
DIAGNOSIS
From my (1998) compilation of several large series totaling more than 500 cases of malignancy, pleural fluid cytology had a diagnostic yield of 66%, and percutaneous pleural biopsy had a diagnostic yield of 46%. When both procedures were performed, a positive diagnosis was obtained in 73% of cases.
With a standardized approach and an experienced cytopathologist, diagnostic yields in cases of proven malignancy may be as high as 90% on the initial pleural fluid examination, with an additional 2% to 4% yield with a second sample, as recorded by Johnston (1985), Hsu (1987), and Starr and Sherman (1991). Several observations can be made from these data: (a) the diagnostic yield is dependent on the extent of disease and primary malignancy; (b) pleural fluid cytology is more sensitive than pleural biopsy; (c) although the tests are complementary, percutaneous pleural biopsy adds little to cytologic examination; (d) the lower yield from pleural biopsy results from the pattern of pleural metastasis, sampling error, and operator technique; and (e) the wide range of incidence of positive results with both tests probably relates to imprecise handling of specimens, expertise of the cytopathologist, and the possibility that the pleural effusion was paramalignant at the time of the procedure. Sallach and co-workers (2002) reported that small volumes (<10 mL) of pleural fluid can be submitted to accurately diagnose malignancy with sensitivity equivalent to that of larger volumes (>1,000 mL) of pleural fluid.
P.939
From the thoracoscopy data of Canto and associates (1983), the yield of percutaneous pleural biopsy probably could be increased by performing the procedure as close to the diaphragm and midline as possible because pleural metastases tend to originate near the diaphragm and spread cephalad toward the costal pleura.
Some patients with exudative pleural effusions remain without a diagnosis after a repeat cytologic examination with or without pleural biopsy. Options at this time include observation, thoracoscopy, or open pleural biopsy. Recommending an invasive procedure is easier psychologically for the physician but creates morbidity and economic burden for the patient. However, with experienced operators, thoracoscopy is a highly effective diagnostic procedure with minimal morbidity and essentially no mortality. Boutin and co-workers (1981) diagnosed 131 of 150 (87%) malignant pleural effusions, both carcinoma and mesothelioma, while pleural fluid cytology and percutaneous needle biopsy performed the day before thoracoscopy provided the diagnosis in only 41% of patients. During the 10 years of study, the diagnostic yield for malignancy increased from 78% to 97% with better instrumentation. Loddenkemper and Boutin (1993) reported a prospective study comparing the diagnostic yield of pleural fluid cytology, percutaneous needle biopsy, and medical thoracoscopy in 208 patients. Cytology had a 62% sensitivity and needle biopsy a 44% sensitivity, with a combined sensitivity of 74%, compared to 95% sensitivity with thoracoscopy. Open pleural biopsy requires a thoracotomy with associated morbidity, a low mortality rate, and economic burden. Treatable causes of exudative effusions, such as tuberculous pleurisy and pulmonary embolism, should be excluded before invasive procedures are undertaken. Approximately 5% to 14% of patients with tuberculous pleural effusions are not diagnosed by pleural fluid and tissue culture and pleural histologic examination; if tuberculous pleurisy is not treated, 65% of patients develop active tuberculosis within 5 years. Therefore, patients with a positive tuberculin skin test and an undiagnosed lymphocyte-predominant exudate should be treated with antituberculous drugs, especially if the pleural fluid adenosine deaminase level exceeds 40 IU/L. Bronchoscopy should be performed before thoracoscopy or open pleural biopsy if there is absence of contralateral shift with a large effusion, evidence of ipsilateral volume loss, a pulmonary lesion in addition to the pleural effusion, or the presence of hemoptysis. According to Feinsilver and associates (1986), the value of bronchoscopy in an undiagnosed pleural effusion without the aforementioned factors is limited.
An alternate approach is observation with repeat cytology and pleural biopsy at a later time if the effusion has not regressed. Malignant pleural effusions almost never resolve spontaneously; an increase in the size of the pleural effusion heightens the suspicion of malignancy. Furthermore, if the clinician does not diagnose a malignant pleural effusion for several weeks, rarely has a disservice been done to the patient who has widespread, incurable disease with a poor prognosis. However, the diagnosis of a malignancy that characteristically is responsive to therapy, such as breast, prostate, thyroid, small cell lung cancer, and germ cell cancer and lymphoma, however, should be pursued more aggressively in the appropriate clinical settings.
Measurements of carcinoembryonic antigen, hyaluronic acid, and LDH isoenzymes have no diagnostic value. Chromosomal analysis of pleural fluid is expensive and not available in all laboratories but may be helpful in the diagnosis of lymphoma and leukemia.
The antemortem diagnosis of malignant mesothelioma requires both clinical and histologic observations. Diagnosis from exfoliative cytology is difficult, and Whitaker and Shilkin (1978) question its value. Even when malignancy is diagnosed, it may be impossible to differentiate metastatic adenocarcinoma from a malignant mesothelioma. Percutaneous needle biopsy, because of the small amount of tissue, does not consistently yield a definitive diagnosis and frequently prompts a misleading diagnosis of adenocarcinoma. High levels of hyaluronic acid have been thought to be helpful in establishing the diagnosis of mesothelioma; however, Rasmussen and Faber (1967) observed that most patients with mesothelioma have intermediate levels, frequently seen in metastatic carcinoma and other inflammatory diseases. Experienced thoracoscopists, such as Boutin and Rey (1993), report a 98% (185 of 188 patients) diagnostic yield in malignant mesothelioma compared with 26% and 21% for cytology and percutaneous needle biopsy, respectively. Mesotheliomas, as Edge and Choudhury (1978) noted, tend to invade surgical sites. Prophylactic irradiation should be given postoperatively. On occasion, even after adequate tissue has been examined from thoracoscopy or thoracotomy, diagnosis remains uncertain. The subsequent course or biopsy from a tumor implant at the surgical site often provides the diagnosis.
Most pathologists can confidently diagnose a sarcomatous or mixed histologic variant but have difficulty in differentiating the epithelial form of mesothelioma from the more common metastatic adenocarcinoma. Special tissue stains, newer immunologic techniques, and electron microscopy aid in the antemortem diagnosis of patients with the epithelial variety of mesothelioma (see Chapter 65).
PROGNOSIS
The author along with Good (1988) noted that the diagnosis of a malignant pleural effusion portends a poor prognosis. Patients with carcinoma of the lung, stomach, and ovary generally survive only a few months from the time of diagnosis of the malignant effusion, whereas patients with breast cancer may survive several months to years, depending on the response to chemotherapy. Patients with lymphomatous pleural effusions tend to have a survival intermediate between that for breast cancer and other carcinomas. Heffner and colleagues (2000),
P.940
in a meta-analysis of 417 patients with malignant effusions, found a median survival of 4 months and a 30.7% 6-month survival rate from time of diagnosis.
Patients with low pH (<7.30) and low glucose (<60 mg/dL) malignant effusions had significantly shorter survival (a few months) compared to those with a normal pH and glucose who survived close to 1 year. Thus, the biochemical findings in the pleural fluid provide the clinician with information that is helpful in deciding on a rational plan of palliative treatment. However, the pleural fluid pH and glucose should only be used in conjunction with other parameters, such as primary tumor, performance status, and comorbid disease in deciding on palliative therapy.
Although a pleural effusion is an ominous sign in lung cancer, usually excluding operability, Decker and colleagues (1978) reported that approximately 5% of these patients have a paramalignant effusion or effusion from another cause and may be operative candidates. The burden falls to the clinician to diagnose the cause of the pleural effusion before making a decision about possible curative surgery. Circumstances suggesting that the pleural effusion in lung cancer is paramalignant and that the patient may still be cured by resection are squamous cell type, radiographic volume loss, serous effusion, transudate, and parapneumonic effusion. If the cause of the effusion cannot be established clinically, thoracoscopy should be performed to investigate the cause.
TREATMENT
When the pleural effusion has been documented to be malignant or paramalignant and the patient is not a surgical candidate, the clinician must make a decision concerning palliation. Factors that must be considered in this decision are the patient's general condition, symptoms, and expected survival. Management options range from observation in the asymptomatic patient to thoracotomy with pleurectomy. Most asymptomatic patients eventually develop progressive pleural effusions, producing dyspnea that requires therapy, while the minority probably reach a new steady state of pleural fluid formation and absorption and do not progress to a symptomatic stage requiring therapy. In the debilitated patient with a short expected survival, based on the extent of disease, general status, and the biochemical characteristics of the fluid, it is more prudent to perform a therapeutic thoracentesis periodically on an outpatient basis than to recommend hospitalization and tube thoracostomy or thoracoscopy with pleurodesis, with their associated morbidity and cost.
Parietal pleurectomy with pleural abrasion is virtually always effective in obliterating the pleural space and controlling recurrence of the effusion. Pleural abrasion with or without talc poudrage or pleurectomy should be performed in most patients who undergo thoracotomy for an undiagnosed pleural effusion and are found to have malignancy because this prevents the subsequent development of a symptomatic pleural effusion. Pleurectomy, however, even when indicated, as Martini and colleagues (1975) and Fry and Khandekar (1995) discussed, is a major surgical procedure associated with substantial morbidity and mortality. Thus, this procedure should be reserved for patients with an expected survival of at least 6 months, who are in relatively good condition, who have a trapped lung, or who have failed pleurodesis, and should be performed with thoracoscopy if possible.
TECHNIQUE OF PLEURECTOMY*
Beattie (1963) has provided an excellent description of pleurectomy. The thorax is entered by a posterolateral incision, with entrance into the pleural space preferably by an intercostal incision in the fifth or sixth interspace. The extrapleural dissection is begun in the plane between the parietal pleura and the extrathoracic fascia at the margins of the intercostal incision before the rib spreader is inserted. The parietal pleura is then stripped circumferentially to the mediastinum; more tumors tend to be present at the diaphragmatic costopleural junction than at the apical pleura, and it is recommended that the upper half of the pleural dissection be completed first. Care must be taken in continuing the pleural dissection over the mediastinal surface to avoid injury to the phrenic, recurrent laryngeal, or sympathetic nerves or stellate ganglion. Damage to the vascular structures of the mediastinum likewise must be avoided. Dissection is continued down from the apex to the pulmonary hilus, which completes the initial phase of the procedure. The inferior portion of the parietal pleura is then dissected free. Care must be taken at the costophrenic sulcus not to remove the diaphragmatic attachment to the chest wall. It is unnecessary, and often impossible, to remove the diaphragmatic pleura, but the reflection of the pleura posteriorly on the lower mediastinal surface in association with the pulmonary ligament should be freed to the inferior border of the hilus. Dissection of the mediastinal pleura from pericardium is difficult and should not even be attempted in the region of the phrenic nerve. If the lung is free and ventilates well, no visceral pleural dissection is indicated. If, on the other hand, the lung is bound down with fibrin, a standard decortication is necessary. After hemostasis is obtained satisfactorily, pleural drainage and closure are completed in the standard manner.
The procedure is applicable only to a highly selected group of patients in good general condition whose malignant pleural effusion has failed to respond to local therapy. Best results are obtained when the primary lesion is a carcinoma of the breast or, occasionally, a melanoma. Results vary but are more often poor in patients with carcinoma of
P.941
the lung. Presently, pleurectomy for treatment of metastatic malignant pleural effusion is considered to be obsolete and rarely indicated.
CHEMOTHERAPY OR IRRADIATION
In general, systemic chemotherapy is disappointing for the control of malignant pleural effusions. Nonetheless, patients with lymphoma [according to Weick (1973) and Xaubet (1985) and their associates], patients with breast cancer [from the reports of Fentiman (1981) and Jones (1975) and their colleagues], or patients with small cell carcinoma of the lung [as Livingston and co-workers (1982) have noted] may respond well to chemotherapy. Information about steroid receptors obtained from malignant pleural fluid in patients with breast cancer can provide a source for determining potential response to hormonal manipulation.
In general, radiation therapy is of limited value in controlling carcinomatous malignant pleural effusions. Roy and associates (1967) suggested, however, that when there is predominantly mediastinal node involvement, irradiation may be valuable for patients with lymphoma or small cell carcinoma of the lung or when the effusion is a chylothorax.
PLEURODESIS
For most patients, the most cost-effective and least morbid method for controlling a symptomatic, malignant pleural effusion is chest tube drainage with instillation of a sclerosing agent. Tetracycline hydrochloride was most commonly used, but in 1991 Lederle Laboratories, the only manufacturer of intravenous and intramuscular tetracycline hydrochloride, ceased production of this drug as a result of the unavailability of the sterile tetracycline salt, which was now required by the U.S. Food and Drug Administration. Walker-Renard and associates (1994) reported that other tetracyclines (minocycline, 300 mg, and doxycycline, 500 mg) have produced complete response rates of 86% (six of seven patients) and 72% (43 of 60 patients), respectively. Many patients treated with intrapleural doxycycline have required more than one instillation compared with only a single dose of minocycline. The most common adverse effects of the tetracycline drugs have been chest pain and fever. The effectiveness of the tetracyclines depends primarily on their fibrogenicity rather than antineoplastic activity. Dryzer and colleagues (1993b) have shown in a rabbit model that both tetracycline and minocycline produce a marked inflammatory response and extensive pleural fibrosis and symphysis in a dose-dependent manner, as I and my colleague Good (1981) have previously pointed out.
Walker-Renard and colleagues (1994) reported that talc, mainly poudrage but also slurry, resulted in a complete success rate of 93% (153 of 165 patients) in the treatment of malignant pleural effusions. The complete success rate of talc in control of malignant effusions was found superior to the complete success rate of all other chemical agents, including bleomycin at 54% (108 of 199 patients) and the tetracycline drugs at 68% (290 of 427 patients). The complete success rate with all nonantineoplastic agents was 75% (577 of 770 patients) compared with a complete success rate of only 44% (175 of 398 patients) for all antineoplastic agents. The most commonly reported adverse effects were pain (265 of 1,440 patients, 23%) and fever (220 of 1,144 patients, 19%) with variability depending on the chemical agent used.
Kennedy and I (1994) reviewed all published series of talc pleurodesis for the treatment of pleural effusions, the majority being malignant. In this review, success was based on clinical criteria and radiographic findings and both complete and partial success were considered; partial success was defined as some recurrence of pleural fluid but not requiring further pleural space drainage. When analyzed by method of administration, both talc poudrage (418 of 461 patients) and slurry (168 of 185 patients) resulted in similar success rates (complete and partial) of 91% for treatment of pleural effusions. Talc received from chemical suppliers is asbestos-free, with a variable particle size. Although talc is not packaged sterilely by the manufacturer, limitation on the number of microorganisms is a part of specification and total bacterial count cannot exceed 500 organisms per gram of talc. Bacillus species can be routinely cultured from unsterilized talc. Currently, there is no standard method of sterilization; however, dry heat, gamma irradiation, and ethylene oxide gas have all been shown to be effective methods of sterilization by Kennedy and colleagues (1995). Once the packets of talc are sterilized, they remain culture negative for at least 1 year.
The degree of pain associated with talc has been reported from nonexistent to severe; however, in most patients, pain is not a major adverse effect with talc. Fever after talc poudrage or slurry has been reported to occur in 16% to 69% of patients. Fever generally occurs 4 to 12 hours after talc instillation and may last for 72 hours. Despite the efficacy of talc pleurodesis, there are concerns about its short-term safety. There have been a number of reports of respiratory failure after both talc poudrage and talc slurry by Factor (1975) as well as by Rinaldo (1983) and Campos (1997) and their associates. Some of the patients did not survive the episode of respiratory failure. Doses used have been 2 to 14 g. Talc crystals have been found in the bronchoalveolar lavage fluid of some of these patients, and at autopsy, talc crystals have been detected in the lungs and other organs. However, it is uncertain, as noted by Campos and colleagues (1997) and by Kennedy and me (1994), whether talc dissemination from the pleural space is related to the acute respiratory failure. It is doubtful that the method of administration plays a major role in the development of respiratory failure, although the dose and particle size may be important. In my review (2002) of the literature since 1958, over 5,000 talc pleurodeses with both poudrage and slurry have been reported with associated acute respiratory failure in less than
P.942
1% of patients. Virtually all of the cases of acute respiratory failure have been with malignant pleural effusions and virtually none with nonmalignant effusions or pneumothorax. Furthermore, it is unclear whether the acute respiratory failure was caused by talc or due to other causes, such as excess sedation, reexpansion pulmonary edema, severe comorbid disease, or terminal malignancy.
If the clinician has documented that therapeutic thoracentesis results in relief of dyspnea and the rate of recurrence and the return of symptoms is rapid, instillation of talc or doxycycline through a chest tube or talc poudrage via thoracoscopy should be considered. If the expected survival is several months, the patient has a reasonable performance status, there is no severe comorbid disease, and the pleural fluid pH is not low, the patient is a reasonable candidate for pleurodesis. Attempting pleurodesis is useless if the lungs cannot be expanded fully; this would occur in main-stem bronchial obstruction with atelectasis or a trapped lung. I and Good (1988), as well as Sanchez-Armengol and Rodriguez-Panadero (1993), have reported that the documentation of a low pleural fluid pH (<7.30 or <7.20, respectively) not only suggests a limited survival but a poor response to tetracycline pleurodesis and talc poudrage, respectively. The large tumor bulk and fibrosis involving the pleural surfaces in the low-pH effusions diminishes the effectiveness in producing pleural symphysis, which may be caused by trapped lung, the inability of the pleurodesis agent to injure the mesothelial cell, or the blockage of fibroblast migration into the pleural space.
The technique for chemical pleurodesis is critical for a successful result in the properly selected patient (Table 68-2). The pleural surfaces must be juxtaposed at the time inflammation is induced and remain in close contact over the ensuing 48 to 72 hours; this is best accomplished with chest tube drainage of the pleural space. If the effusion is large, the fluid probably should be drained slowly over the first several hours with intermittent clamping of the tube to decrease the risk for unilateral pulmonary edema. Pulmonary edema is most likely to occur when there is an endobronchial obstruction or trapped lung that does not allow the lung to expand to the chest wall with removal of fluid, resulting in a precipitous decrease in pleural pressure. Furthermore, pleurodesis should not be attempted in the aforementioned situations because it will not be successful. Talc slurry (2 to 3 g) or doxycycline (500 mg) should be instilled into the pleural space as soon as the lung is expanded fully radiographically, regardless of the volume of pleural space drainage. Lorch and colleagues (1988), working in my laboratory, have radiolabeled tetracycline and demonstrated that after intrapleural instillation, tetracycline is distributed completely throughout the pleural space within seconds and that the distribution is not enhanced by patient rotation. This study suggested that the patient need not be rotated through various positions after tetracycline instillation to ensure adequate distribution of the pleurodesis agent. In a follow-up clinical study from our group, Dryzer and colleagues (1993a) showed no difference in success rate with both tetracycline and minocycline in those patients not rotated compared with those who were rotated through various positions after drug instillation. Thus, my recommendation is that patients receiving doxycycline pleurodesis need not be rotated through various positions after instillation, thus avoiding discomfort for the patient and additional personnel time. No studies have evaluated the optimum dwell time; a 1-hour dwell time for the pleurodesis agent should be adequate because experimental studies have shown immediate mesothelial injury and it is ideal to have the two pleural surfaces in close contact as soon as possible. The chest tube should be removed when pleural space drainage is minimal, approximately less than 100 mL/day. With appropriate patient selection and the use of proper technique, the malignant effusion should be controlled in 80% to 95% of patients. Success need not be defined as the production of complete pleural symphysis but as diminishing the reaccumulation of pleural fluid so that dyspnea is relieved and repeat therapeutic thoracentesis is not required. Wooten and associates (1988) noted that both tetracycline and lidocaine, the latter used by some clinicians in an attempt to ameliorate chest pain, are absorbed systematically and reach therapeutic levels by 30 to 60 minutes; a history of allergic reactions to either drug is a contraindication to its use. The dose of lidocaine should not exceed 150 mg or 3 mg/kg, whichever is less. Strange and associates (1993), in my laboratory, have found similar rapid systemic absorption of minocycline from the rabbit pleural space.
The average wholesale price of talc is less than $1 if sterilized at the institution. Doxycycline, 500 mg, costs approximately $250. In contrast, the usual 1 U/kg dose of bleomycin costs $1,100. Thus, cost consideration must be kept in mind in the management of the malignant effusion.
The management of malignant mesothelioma is discussed in Chapter 65. Judgment in the management of these patients is the keynote of appropriate care.
Table 68-2. Procedure for Pleurodesis | |
|---|---|
|
P.943
REFERENCES
Beattie EJ Jr: The treatment of malignant pleural effusions by partial pleurectomy. Surg Clin North Am 43:99, 1963.
Boutin C, et al: Thoracoscopy in malignant effusion. Am Rev Respir Dis 124:588, 1981.
Boutin C, Rey F: Thoracoscopy in pleural malignant mesothelioma: a prospective study of 188 consecutive patients. Part 1. Diagnosis. Cancer 72:389, 1993.
Campos JRM, et al: Respiratory failure due to insufflated talc. Lancet 349:251, 1997.
Canto A, et al: Points to consider when choosing a biopsy method in cases of pleurisy of unknown origin. Chest 84:176, 1983.
Chernow B, Sahn SA: Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 63:695, 1977.
Chretien J, Jaubert F: Pleural responses in malignant metastatic tumors. In Chretien J, Bignon J, Hirsch A (eds): The Pleura in Health and Disease. New York: Marcel Dekker, 1985, p. 489.
Decker DA, et al: The significance of a cytologically negative pleural effusion in bronchogenic carcinoma. Chest 74:640, 1978.
Dryzer SR, et al: A comparison of rotation and nonrotation in tetracycline pleurodesis. Chest 104:1763, 1993a.
Dryzer SR, et al: Early inflammatory response of minocycline and tetracycline on the rabbit pleura. Chest 104:1585, 1993b.
Edge JR, Choudhury SL: Malignant mesothelioma of the pleura in Barrow-in-Furness. Thorax 33:26, 1978.
Elmes PC, Simpson MJC: The clinical aspects of mesothelioma. Q J Med 45:427, 1976.
Factor SM: Granulomatous pneumonitis. A result of intrapleural instillation of quinacrine and talcum powder. Arch Pathol 99:499, 1975.
Feinsilver SH, Barrows AA, Braman SB: Fiberoptic bronchoscopy and pleural effusion of unknown origin. Chest 90:516, 1986.
Fentiman IS, et al: Pleural effusion in breast cancer: a review of 105 cases. Cancer 47:2087, 1981.
Ferrara N: Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77:527, 1999.
Fry WA, Khandekar JD: Parietal pleurectomy for malignant pleural effusion. Ann Surg Oncol 2:160, 1995.
Gary Lee YCG, et al: Transforming growth factor induces vascular endothelial growth factor elaboration from pleural mesothelial cells in vivo and in vitro. Am J Respir Crit Care Med 165:88, 2002.
Good JT Jr, Taryle DA, Sahn SA: The pathogenesis of low glucose, low pH malignant effusions. Am Rev Respir Dis 131:737, 1985.
Gottehrer A, et al: Hypothyroidism and pleural effusions. Chest 98:1130, 1990.
Heffner JE, Nietert PJ, Barbieri MS: Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest 117:79, 2000.
Heller RM, Janower ML, Weber AL: The radiological manifestations of malignant pleural mesothelioma. AJR 108:53, 1970.
Hsu C: Cytologic detection of malignancy in pleural effusion: a review of 5,255 samples from 3,811 patients. Diagn Cytopathol 3:8, 1987.
Jenkins PF, et al: Non-Hodgkin's lymphoma, chronic lymphatic leukemia, and the lung. Br J Dis Chest 75:22, 1981.
Johnston WW: The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 56:905, 1985.
Jones SE, Durie BGM, Salmon SE: Combination chemotherapy with adriamycin and cyclophosphamide for advanced breast cancer. Cancer 36:90, 1975.
Kennedy L, Sahn SA: Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest 106:1215, 1994.
Kennedy L, et al: Sterilization of talc for pleurodesis: available techniques, efficacy and cost analysis. Chest 107:1032, 1995.
Light RW, Erozan YS, Ball WC: Cells in pleural fluid: their value in differential diagnosis. Arch Intern Med 132:854, 1973.
Light RW, et al: Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 77:507, 1972.
Livingston RB, et al: Isolated pleural effusion in small cell lung carcinoma: favorable prognosis. Chest 81:208, 1982.
Loddenkemper R, Boutin C: Thoracoscopy: present diagnostic and therapeutic indications. Eur Respir J 6:1544, 1993.
Lorch DG, et al: The effect of patient positioning on the distribution of tetracycline in the pleural space during pleurodesis. Chest 93:527, 1988.
MacDonald JB: Lung involvement in Hodgkin's disease. Thorax 32:664, 1977.
McDonald JB, et al: Epidemiology of primary malignant mesothelial tumors in Canada. Cancer 26:914, 1970.
Maher GG, Berger HW: Massive pleural effusion: malignant and nonmalignant causes in 46 patients. Am Rev Respir Dis 105:458, 1972.
Martini N, Bains MS, Beattie EJ Jr: Indications for pleurectomy in malignant effusion. Cancer 35:734, 1975.
Meyer PC: Metastatic carcinoma of the pleura. Thorax 21:437, 1966.
Rabin CB, Blackman NS: Bilateral pleural effusion. Its significance in association with a heart of normal size. J Mt Sinai Hosp 24:45, 1957.
Rasmussen KN, Faber V: Hyaluronic acid in 247 pleural fluids. Scand J Respir Dis 48:366, 1967.
Rinaldo JE, Owens GR, Rogers RM: Adult respiratory distress syndrome following intrapleural instillation of talc. J Thorac Cardiovasc Surg 85: 523, 1983.
Roy PH, Carr DT, Payne WS: The problem of chylothorax. Mayo Clin Proc 42:457, 1967.
Rubins JB, Rubins HB: Etiology and prognostic significance of eosinophilic pleural effusions. A prospective study. Chest 110:1271, 1996.
Sahn SA: Malignant pleural effusions. Clin Chest Med 6:113, 1985.
Sahn SA: Malignant pleural effusions. In Fishman AP, et al (eds): Pulmonary Diseases and Disorders. 3rd Ed. New York: McGraw-Hill, 1998.
Sahn SA: Talc should be used for pleurodesis. J Bronchol 9:223, 2002.
Sahn SA, Good JT Jr: The effect of common sclerosing agents on the rabbit pleural space. Am Rev Respir Dis 124:65, 1981.
Sahn SA, Good JT Jr: Pleural fluid pH in malignant effusions: diagnostic, prognostic and therapeutic implications. Ann Intern Med 108:345, 1988.
Sallach SM, et al: Volume of pleural fluid required for diagnosis of pleural malignancy. Chest 122:1913, 2002.
Sanchez-Armengol A, Rodriguez-Panadero F: Survival and talc pleurodesis in metastatic pleural carcinoma, revisited. Report of 125 cases. Chest 104:1482, 1993.
Starr RL, Sherman ME: The value of multiple preparations in the diagnosis of malignant pleural effusions. A cost-benefit analysis. Acta Cytol 35:533, 1991.
Strange C, et al: Minocycline and tetracycline are rapidly absorbed through the rabbit pleural space. Am Rev Respir Dis 147:A795, 1993.
Taryle DA, Lakshminarayan S, Sahn SA: Pleural mesotheliomas. An analysis of 18 cases and review of the literature. Medicine (Baltimore) 55:153, 1976.
Valentine VG, Raffin TA: The management of chylothorax. Chest 102:586, 1992.
Wagner JC, Sleggs CA, Marchand P: Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 17:260, 1960.
Walker-Renard PB, Vaughan LM, Sahn SA: Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 120:56, 1994.
Weick JK, et al: Pleural effusion in lymphoma. Cancer 31:848, 1973.
Whitaker D, Shilkin KB: The cytology of malignant mesothelioma in Western Australia. Acta Cytol 22:67, 1978.
Whitcomb ME, et al: Hodgkin's disease of the lung. Am Rev Respir Dis 106:79, 1972.
Wooten SA, et al: Systemic absorption of tetracycline and lidocaine following intrapleural instillation. Chest 94:960, 1988.
Xaubet A, et al: Characteristics and prognostic value of pleural effusions in non-Hodgkin's lymphomas. Eur J Respir Dis 66:135, 1985.
Yam LT: Diagnostic significance of lymphocytes in pleural effusions. Ann Intern Med 66:972, 1967.
Reading References
Antman KH: Multimodality treatment for malignant mesothelioma based on a study of natural history. Am J Med 68:356, 1980.
Hillerdal G: Malignant mesothelioma 1982: review of 4,710 published cases. Br J Dis Chest 77:321, 1983.
Schienger M, et al: Mesotheliomes pleuraux malins. Bull Cancer (Paris) 56:265, 1969.
*Addendum by the Senior Editor, T.W. Shields.
EAN: 2147483647
Pages: 203