57 - Physiology of Pleural Fluid Production and Benign Pleural Effusion
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XI - The Pleura > Chapter 69 - Malignant Pericardial Effusion
Chapter 69
Malignant Pericardial Effusion
Robert D. Stewart
Sudhir Sundaresan
Malignant pericardial effusion refers to excessive fluid accumulation in the pericardial sac with associated malignant cells, either in the effusive fluid or in the pericardium or epicardium. This condition must be distinguished from malignancy-associated pericardial effusions, which also occur in patients with cancer, but do not contain malignant cells. The clinical spectrum of malignant pericardial effusion is highly variable, ranging from asymptomatic to hemodynamic compromise from cardiac tamponade. Many of the intermediate clinical presentations are nonspecific and can be easily attributed to other cancer-related problems typically seen in these same patients. For this reason the diagnosis is often made very late when the patients are gravely ill. A raised awareness of the possibility of malignant pericardial effusion is necessary to achieve earlier diagnosis, and subsequent institution of one of several successful therapies. Unfortunately, although acute palliation of the hemodynamic consequences of the effusion is usually successful, the long-term prognosis of patients with malignant pericardial effusion remains poor.
PREVALENCE
The exact prevalence of malignant pericardial effusion is not well established, as many patients with subclinical effusions go undiagnosed. Bisel and colleagues (1953) reported that 64% of the patients with autopsy-proven pericardial metastases were asymptomatic and undiagnosed antemortem. Best estimates of the prevalence come from autopsy series. Klatt and Heitz (1990) reported 28 patients with malignant pericardial effusions among 1,029 autopsies on patients with known malignancy, giving a prevalence of 3%. However, even autopsy series yield wide-ranging estimates of the prevalence (1 to 20%) due to variability in the context of the study and the definition used for malignant pericardial effusion. Among cancer patients presenting with symptomatic pericardial effusions, up to half of the effusions do not contain any evidence of malignancy, as reported by Wilkes and colleagues (1995). Many reports grouped patients with malignancy-related effusions and true malignant effusions together without differentiation.
Although numerous cancers have been associated with malignant pericardial effusion, as listed in Table 69-1, those most commonly implicated are non small cell lung cancer (NSCLC), breast cancer, and lymphoma.
PATHOPHYSIOLOGY
Several factors are believed to act in concert to lead to the development of malignant pericardial effusion. Malignant cells can spread to the pericardial or epicardial tissues by lymphatic channels, by hematogenous spread, or by direct extension. Within the pericardium, the malignant cells can obstruct drainage of the normally produced pericardial fluid by interfering with endothelial cell function or obstructing the lymphatic channels, thus leading to increased pericardial fluid. Additionally, the malignant cells may also elicit an increased effusive response from the mesothelial cells of the pericardium. Tumor invasion of the mediastinal lymph nodes may also promote pericardial effusion though blockage of the normal drainage. It is noteworthy that Klatt and Heitz (1990) reported that only 33% of patients with proven pericardial tumor involvement had associated effusions.
Interestingly, the etiology of nonmalignant pericardial effusions in patients with cancer is usually unknown. Wilkes and colleagues (1995) could determine the etiology of only 15 of 57 such effusions. The most common known causes of nonmalignant effusion were infection (viral and bacterial), irradiation, and uremia.
PRESENTATION
The most common symptom of malignant pericardial effusion is dyspnea, followed by chest discomfort or pressure, cough, fatigue, malaise, and peripheral edema. Although some patients may have large effusions and remain asymptomatic,
P.945
many patients present with tachycardia, hypotension, and respiratory distress due to cardiac tamponade. Fraser and co-workers (1980) reported that 50% of the patients in their series presented with tamponade. Tamponade physiology results from the pericardial effusive fluid-restricting cardiac filling. The decreased cardiac preload leads to depressed cardiac output and eventually shock. The degree of compromise is directly related to the time course of development of the effusion. A slowly developing effusion may be well compensated, while a relatively small but rapidly developing effusion may quickly result in symptoms and hemodynamic consequences.
Table 69-1. Cancers Associated with Malignant Pericardial Effusion | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Signs associated with pericardial effusion include pulsus paradoxus, decreased heart sounds, jugular venous distention, tachycardia, pericardial rub, peripheral edema, and pulmonary rales. However, patients may be without any abnormal physical findings, and none of these is specific for pericardial effusion.
Complicating the clinical picture is that the majority of patients who present with symptoms from malignant pericardial effusion have other cancer-related problems, most specifically pleural effusions, which can produce these same symptoms. Okamoto and associates (1993) reported that 88% of patients with lung cancer related pericardial effusions also had pleural effusions. Additionally, 76% had other sites of distant metastases. Swanepoel and Apffelstaedt (1997) reported that among breast cancer patients with malignant pericardial effusions, 89% had pleural effusions and overall these patients had a mean of 3.2 sites of distant metastases.
DIAGNOSIS
History and physical examination remain the mainstay of diagnosis; however, with rare exception the nonspecific nature of the symptoms and signs will require further diagnostic testing. The chest radiograph will usually demonstrate some degree of cardiomegaly. Pleural effusions are also commonly seen in these studies. The electrocardiogram may show low voltages associated with large effusion. Both of these tests are adjunctive, but are not specific for effusion.
Echocardiography is the most helpful test in making the definitive diagnosis of pericardial effusion. The test is fast, relatively available, and cost effective. Echocardiography can determine volume of effusion, reveal the presence of loculations, and evaluate cardiac function. Signs of tamponade, which include compression of the right atrium and collapse of the right ventricle during diastole, are discernible by echocardiography.
Other noninvasive imaging modalities include computed tomography (CT) and magnetic resonance (MR) imaging, both of which can demonstrate the presence of effusion but are currently unable to provide any cardiac functional data.
The diagnosis of malignant pericardial effusion requires fluid cytology or pericardial tissue histology demonstrating tumor cells. Diagnostic needle pericardiocentesis can be performed with or without ultrasound guidance. However, in symptomatic individuals, this procedure is not required because fluid and tissue can be obtained as part of a therapeutic procedure. Diagnostic pericardiocentesis is reasonable in patients with small asymptomatic effusions for the purpose of staging and systemic therapeutic planning.
PROGNOSIS
The prognosis of patients with malignant pericardial effusions is bleak. Girardi and colleagues (1997) reported a 97-day median survival of patients treated for malignant pericardial disease. They did note a longer survival in those patients with breast cancer as their primary malignancy. Moores and colleagues (1995) noted a similar poor prognosis for malignant pericardial effusion with a median survival of 56 days. Furthermore, they reported a relatively better but still bleak median survival of 105 days for cancer patients with nonmalignant effusions. There are some long-term survivors. Wilkes and colleagues (1995) reported that 25% of patients surviving more than 1 year had survivals of up to 10 years. These longer survivors all had either hematologic malignancies or breast cancer.
THERAPY
There are several accepted techniques for treating malignant pericardial effusions. The objectives of any therapy are to quickly relieve symptoms related to the effusion (especially tamponade), to obtain sufficient fluid or tissue sample to establish the diagnosis, and to provide lasting resolution of the problem with minimal chance of recurrence. Furthermore, the ideal treatment should have low
P.946
morbidity, create minimal patient discomfort, and be cost effective. The procedures currently used to treat malignant pericardial effusion include pericardiocentesis, balloon pericardiotomy, subxiphoid window, pericardial-pleural window, pericardiectomy, and finally shunts to the peritoneum or to external drainage catheters. Variations and adjuncts to each of these basic procedures have been developed and include sclerotherapy, pericardioscopy, and video-assisted thoracic access.
Pericardiocentesis
Pericardiocentesis is the percutaneous insertion of a needle or catheter into the pericardial space for fluid drainage. The latter is more correctly termed tube pericardiostomy, but is commonly referred to as pericardiocentesis in the literature. The insertion is executed from a subxiphoid approach as demonstrated in Fig. 69-1. The procedure can be performed blindly with a long needle, based on anatomic landmarks, as might be required in the case of sudden hemodynamic collapse due to rapid-onset cardiac tamponade. Under less emergent circumstances, numerous adjuncts to pericardiocentesis can be used to improve effectiveness and safety. The use of an externally draining pigtail catheter inserted over a guidewire has become the first-line therapy for malignant pericardial effusion for many, if not most, surgeons. Ultrasonography has greatly facilitated this procedure, especially when a loculated collection exists, allowing for safe and accurate catheter placement. Fluoroscopy can also be used to guide the insertion of a drainage catheter.
 |
Fig. 69-1. A. Needle pericardiocentesis by the subxiphoid approach. B. Guidewire introduced through needle into the pericardial space. C. Percutaneous catheter drainage of the pericardial space. From Moores DWO, Dziuban SW Jr: Pericardial drainage procedures. Chest Surg Clin North Am 5:539, 1995. With permission. |
Pericardiocentesis requires only local anesthetic, and thus avoids the risks for general anesthesia. However, significant morbidity is associated with pericardiocentesis. Hingorani and Bloomberg (1995) reported on nine patients with ultrasound-guided pigtail catheter pericardiocentesis, and six had minor complications including pleural effusions, atrial fibrillation, and pericardial pain. Celermajer and colleagues (1991) reported on 36 patients managed with pericardiocentesis, of which one patient died as a result of ventricular puncture.
Another potential disadvantage to pericardiocentesis is the high recurrence rate of the effusion, which may be as high as 50%, as noted by Wilkes and associates (1995). This level of recurrence has led to the use of sclerotherapy as an adjunct to pericardiocentesis. Maher and colleagues (1996) reported an 81% freedom from recurrence after tetracycline or doxycycline was instilled into the pericardial sac following pigtail catheter drainage. Celermajer and colleagues (1991) had an initial success rate of 94% using pericardiocentesis, but four of seven patients who did not get sclerotherapy developed recurrent effusions. In the 28 patients who had tetracycline sclerosis following drainage, only 3 cases recurred (11%). Girardi and colleagues (1997) compared percutaneous drainage with sclerotherapy to open drainage in a retrospective review. They found a recurrence rate of only 8.1% in the sclerotherapy group, which was not significantly different from the 12% recurrence rate in the surgical window patients. They also did not find a difference in the complication rate following either procedure; however, the average cost of surgical drainage was $4,200 greater than the average cost of pericardiocentesis.
Subxiphoid Pericardial Window
Subxiphoid pericardial window is an open partial pericardiostomy, approached from the upper abdominal midline with resection or division of the xiphoid process. A large drainage tube is left in the pericardial sac and brought out through a separate stab incision. The technique is depicted in Figs. 69-2 and 69-3. This procedure has several advantages and can be performed either under general anesthesia or with local anesthetic. It uses a small incision, effectively drains the effusion, rapidly resolves related symptoms, and provides both fluid for cytology and tissue for histology. Allen and colleagues (1999) reported excellent results using the subxiphoid window to treat malignant pericardial effusion. In 75 patients with effusions who underwent subxiphoid window, only a single case recurred (1.3%).
The inability to obtain pericardial tissue is cited as a deficiency of percutaneous procedures. However, the need for pericardial tissue is not clear. Campbell and associates (1992) evaluated the subxiphoid window procedure in 25 consecutive patients with malignancy. Local anesthetic
P.947
alone was used in 88% of the cases and there was a 12% recurrence rate of effusions. Malignant cells were found in the fluid cytology in 11 of the 25 patients and tissue histology was positive in only five cases. All of the patients with negative cytology had negative histology.
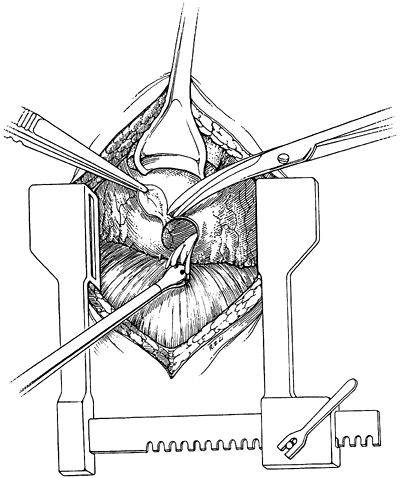 |
Fig. 69-2. Pericardial segment removed via the subxiphoid approach. From Moores DW, et al: Subxiphoid pericardial drainage for pericardial tamponade. J Thorac Cardiovasc Surg 109:546, 1995. With permission. |
The similar rates of recurrent effusions after subxiphoid window and catheter pericardiocentesis with sclerosis lies in the common mechanism of action. While removing the fluid alleviates symptoms, the prevention of recurrent fluid accumulation is achieved by fusion of the pericardium to the epicardium. As described in a pathologic study by Sugimoto and colleagues (1990), the drainage tube used for the pericardial window elicits an inflammatory reaction and thus a pericardial sclerotherapy not unlike that created by the intentional sclerosis using doxycycline.
Pericardioscopy
To improve the diagnostic accuracy of the subxiphoid window, visualization of the pericardial sac using a mediastinoscope or a modified thoracoscope has been reported by several investigators as a useful adjunct. Nugue and colleagues (1996) reported that they were able to increase their diagnostic yield by inspecting the pericardial sac and taking biopsy samples from suspicious sites. They found 5 cases out of 24 where the cytology and histology of the pericardial window specimen were negative, but the pericardioscopy-directed biopsy showed malignancy. They reported minimal morbidity associated with pericardioscopy beyond that of a standard subxiphoid pericardial window. Pericardioscopy, however, does require general anesthesia, which is the major disadvantage beyond that of the additional costs involved.
Balloon Pericardiostomy
Balloon pericardiostomy was proposed as an alternative to pericardiocentesis and to subxiphoid window. The procedure involves percutaneously inserting a catheter into the pericardial sac, aspirating the contents, and then inflating a catheter-based balloon across the pericardium. The balloon creates an 18- to 25-mm hole that allows reaccumulating fluid to drain from the pericardial sac. Ziskind and colleagues (1993) reported the results of the first 50 patients who underwent balloon pericardiostomy. The effusion was successfully treated in 46 patients. However, three patients subsequently required surgery to drain their effusion, and eight required drainage of pleural effusions. Balloon pericardiostomy does not appear to provide a significant benefit over the pericardiocentesis or subxiphoid window in terms of efficacy or freedom from complications.
 |
Fig. 69-3. Subxiphoid pericardial drainage tube brought out through a separate stab incision. From Moores DW, et al: Subxiphoid pericardial drainage for pericardial tamponade. J Thorac Cardiovasc Surg 109:546, 1995. With permission. |
P.948
Video-Assisted Thoracic Surgical Pericardial Window
Video-assisted thoracic surgical (VATS) pericardial window is a minimally invasive approach via the left or right pleural cavity using three port sites in the chest wall to create a pericardial window into the pleural space. The technique is particularly beneficial in situations where ipsilateral pulmonary or pleural pathology is to be simultaneously diagnosed or treated. Liu and associates (1994) used the VATS approach in 28 patients with pericardial effusion, of which 15 were suspected of having a malignancy. They were able to diagnose a lung tumor in 11 and pleural-based tumor in 4. The other advantage for VATS pericardial window is in cases of laterally or posteriorly loculated effusions, which may be drained under direct vision, as noted by Mack and colleagues (1993). The major disadvantages to VATS are the need for general anesthesia and ipsilateral lung deflation, and the equipment costs involved. VATS pericardial window will remain a valuable technique for patients with combined pleural and pericardial disease, and recurrent loculated pericardial effusions.
The same procedure can be accomplished with anterolateral thoracotomy. However, given the generally poor prognosis of the patients and the many satisfactory alternative treatments enumerated in the text, a pericardial window through an anterior or lateral thoracotomy is not a favorable alternative.
Pericardiectomy
Partial or total pericardial resection via sternotomy or an anterior thoracotomy has been used to drain effusions and prevent recurrence, by eliminating the source of the problem and providing extremely wide drainage. However, the invasive nature of such a procedure on patients with poor prognosis makes this approach unappealing. Park and associates (1991) reported two groups of patients who had either a subxiphoid window or pericardiectomy. There was a 10% morbidity rate in the subxiphoid window group compared with a 67% morbidity rate in the pericardiectomy group. The pericardiectomy group had a shorter, though not statistically different, survival compared with the subxiphoid window group. These findings suggest that there are preferable alternatives to pericardiectomy for malignant pericardial effusion.
SUMMARY
Malignant pericardial effusion is a relatively common condition in cancer patients most highly associated with NSCLC, breast cancer, and lymphoma. The presentation is highly variable from asymptomatic effusion to hemodynamic collapse from tamponade. Treatment is best achieved either by ultrasound-guided percutaneous pigtail catheter placement followed by sclerotherapy or a subxiphoid pericardial window. In those patients who need a pleural procedure in addition to a pericardial drainage, VATS allows both to be done concurrently. Despite the effective treatments for malignant pericardial effusions, the overall prognosis remains very poor.
REFERENCES
Allen KB, et al: Pericardial effusion: subxyphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg 67:437, 1999.
Bisel HF, Wroblewski F, LaDue JS: Incidence and clinical manifestations of cardiac metastases. JAMA 153:712, 1953.
Campbell PT, et al: Subxiphoid pericardiotomy in the diagnosis and management of large pericardial effusions associated with malignancy. Chest 101:938, 1992.
Celermajer DS, et al: Pericardiocentesis for symptomatic malignant pericardial effusion: a study of 36 patients. Med J Aust 154:19, 1991.
Fraser RS, Viloria JB, Wang NS: Cardiac tamponade as a presentation of extracardiac malignancy. Cancer 45:1697, 1980.
Girardi LN, Ginsberg RJ, Burt ME: Pericardiocentesis and intrapericardial sclerosis: effective therapy for malignant pericardial effusions. Ann Thorac Surg 64:1422, 1997.
Hingorani AD, Bloomberg TJ: Ultrasound-guided pigtail catheter drainage of malignant pericardial effusions. Clin Radiol 50:15, 1995.
Klatt EC, Heitz DR: Cardiac metastases. Cancer 65:1456, 1990.
Liu HP, et al: Thoracoscopic management of effusive pericardial disease: indications and technique. Ann Thorac Surg 58:1695, 1994.
Mack MJ, et al: Video thoracoscopic management of benign and malignant pericardial effusions. Chest 103(suppl):390, 1993.
Maher EA, Shepard FA, Todd TJ: Pericardial sclerosis as the primary management of malignant pericardial effusion and cardiac tamponade. J Thorac Cardiovasc Surg 112:637, 1996.
Moores DWO, Dziuban SW Jr: Pericardial drainage procedures. Chest Surg Clin North Am 5:539, 1995.
Moores DW, et al: Subxiphoid pericardial drainage for pericardial tamponade. J Thorac Cardiovasc Surg 109:546, 1995.
Nugue O, et al: Pericardioscopy in the etiologic diagnosis of pericardial effusion in 141 consecutive patients. Circulation 94:1635, 1996.
Okamoto H, et al: Cardiac tamponade caused by primary lung cancer and the management of pericardial effusion. Cancer 71:93, 1993.
Park JS, Rentschler R, Wilber D: Surgical management of pericardial effusion in patients with malignancies. Comparison of subxiphoid window versus pericardiectomy. Cancer 67:76, 1991.
Sugimoto JT, et al: Pericardial window: mechanisms of efficacy. Ann Thorac Surg 50:442, 1990.
Swanepoel E, Apffelstaedt JP: Malignant pericardial effusion in breast cancer: terminal event or treatable complication? J Surg Oncol 64:308, 1997.
Wilkes JD, et al: Malignancy-related pericardial effusion: 127 cases from Roswell Park Cancer Institute. Cancer 76:1377, 1995.
Ziskind AA, et al: Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. J Am Coll Cardiol 21:1, 1993.
Reading References
DeCamp MM, et al: Malignant effusive pericardial disease of pleural and pericardium. Chest 112(suppl):291, 1997.
Van Trigt P, et al: A prospective trial of subxiphoid pericardiotomy in the diagnosis and treatment of large pericardial effusion. A follow-up report. Ann Surg 218:777, 1993.
Wang N, et al: Pericardial peritoneal shunt: an alternative treatment for malignant pericardial effusion. Ann Thorac Surg 57:289, 1994.
EAN: 2147483647
Pages: 203