44 - Thoracoscopic Sympathectomy
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section XI - The Pleura > Chapter 54 - Anatomy of the Pleura
Chapter 54
Anatomy of the Pleura
Thomas W. Shields
EMBRYOLOGY
The paired pleural cavities are derivatives of the intraembryonic portion of the primitive coelom. The primitive coelom arises by splitting of the lateral mesoderm on either side of the embryo into splanchnic and somatic layers. These paired cavities are later separated by three partitions into three subdivisions: the pericardial cavity, the pleural cavities, and the peritoneal cavity. The partitions are the unpaired septum transversum, the paired pleuropericardial folds, and the paired pleuroperitoneal folds. The right and left coelomic chambers dorsal to the septum transversum remain relatively unexpanded for a period as the so-called pleural canals; these lie on either side of the mediastinal region. The development of the pleuroperitoneal folds complete the separation of the pleural canals from the other two body cavities: the pericardial cavity and the peritoneal cavity. At the fourth week of development, the laryngotracheal outgrowth from the floor of the pharynx is noted, and in the fifth week, the two lung buds begin to enlarge into the respective pleural canals. According to Patten (1968), the pleural spaces open up in advance of lung growth and the lungs move (bulge) into the space prepared to receive them. With growth, the lungs and the pleuropericardial membranes come to lie on either side of the heart, and the pleuroperitoneal folds become part of the diaphragm. In this process of expansion of the lungs into the pleural canals, the splanchnic mesoderm is pushed out as a covering over the mesenchyma-packed bronchial trees (Fig. 54-1). The splanchnic mesoderm becomes thinned to form the mesothelial layer of the pleura, and the mesenchymal tissue immediately beneath this layer becomes the connective tissue of the pleura. The splanchnic mesoderm is thus the origin of the visceral pleura, and the somatic mesoderm is the origin of most of the parietal pleura.
HISTOLOGY
The two pleural layers have similar histologic features, with the exception of the presence of stomata in the parietal pleura that are absent in the visceral pleura. According to Antony and colleagues (1992), each layer consists of (a) an innermost mesothelial cell layer, (b) a submesothelial interstitial connective tissue layer (macula cribriformis), (c) an inner thin elastic fiber layer, (d) an outer interstitial connective tissue layer, and (e) a thick elastic fiber layer. In addition, according to Hammer (1995), a fatty layer separates the parietal pleura and the muscles of the chest wall. The mesothelial cells in both visceral or parietal pleura, according to Wang (1982, 1985), vary in thickness from less than 1 to more than 4 m and from 16.4 6.8 to 41.9 9.5 m in diameter. Their shape may vary according to their location in the pleural membrane.
Ultrastructurally, the mesothelial cells demonstrate microvilli. Tight apical junctions are present, but gap junctions, desmosomes, or half desmosomes occur infrequently on the basal part of the cell membrane.
Immunohistochemically, the mesothelial cells, as reported by Dervan (1986) and Bolen (1986) and their associates, express both low- and high-molecular-weight cytokeratin. The normal mesothelial cells are negative for reaction to vimentin, epithelial membrane antigen, carcinoembryonic antigen, and factor VIII-related antigen.
Beneath the basal membrane of the mesothelial layer is a collection of loose connective tissue (macula cribriformis). This submesothelial layer contains collagen tissue, elastic fibers, small blood vessels, lymphatic networks, and nerve fibers. The mesenchymal cells in this layer, according to Keating (1978), Said (1984), and England (1989) and their co-workers, have the characteristics of fibroblasts. The cells are negative for cytokeratin, carcinoembryonic antigen, and factor VIII-related antigen.
The visceral and parietal pleural layers are approximately the same thickness, averaging 30 to 40 m according to Staub and colleagues (1985). Large dehiscences, or stomata, have been documented in the parietal pleura. Chretien and Huchon (1990) state that these stomata, ranging from 2 m to more than 6 m in diameter, connect the pleural cavity with the subpleural lymphatic network and permit egress of material into the lymphatics from the pleural space. Wang (1985)
P.786
has described focal accumulations of macrophages, along with pluripotential mesenchymal, lymphoid, and plasma cells (called Kampmeier's foci) in the caudal portions of the mediastinal pleura that may be functionally related to the stomata.
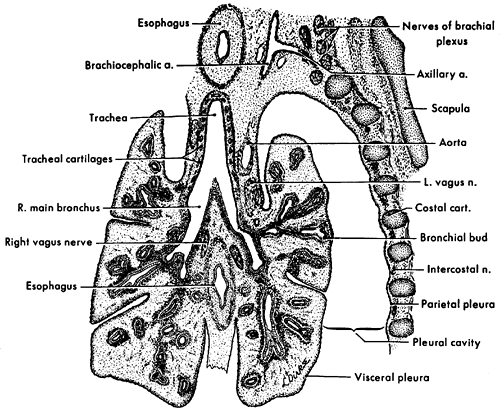 |
Fig. 54-1. Frontal section through the developing lungs in an 8-week-old embryo. Projection drawing (original magnification 25) from University of Michigan Collection, EH 352, CR 25 mm. From Patten BM. Human Embryology. 3rd Ed. New York: McGraw-Hill, 1968, with permission. |
GROSS ANATOMIC FEATURES
The visceral pleura is closely applied to the lung surfaces from the hila outward. It lines the major and minor fissures to the extent that each is developed; the minor fissure on the right may be incomplete to absent in 50% of humans. It also may delineate the various accessory fissures (see Chapter 5) when present. The pulmonary ligament, which extends caudad from each hilus to near the diaphragm, consists of two apposed layers of visceral (splanchnic) pleura and becomes continuous with the parietal pleura.
The visceral pleura adheres to the lung parenchyma. A naturally occurring cleavage plane is absent. When the visceral pleura is stripped from the lung parenchyma, a raw surface with innumerable air leaks, and at times small bleeding vessels, is left behind.
The parietal pleura lines the chest wall (the costal pleura), the mediastinum, and the diaphragm and forms the cupula or plural dome at the thoracic inlet bilaterally. The mediastinal pleura extends from the sternum ventrally to the thoracic spine dorsally and covers the mediastinal structures and the pericardium. The diaphragmatic pleura adheres tightly to the diaphragmatic tissues, and a cleavage plane between the two is basically nonexistent. Similarly, the mediastinal pleura is densely adherent to the pericardium. In contrast, the remainder of the mediastinal pleura, the pleura of the cupula, and the costal pleura can be readily dissected from the underlying tissues. The plane of cleavage from the chest wall is between the loose connective and fatty tissue layer and the endothoracic fascia attached to the underlying osseous, muscular, and vascular structures of the chest wall.
Topographically, the borders of the pleura are formed by continuity of the outer surface of costal, mediastinal, and diaphragmatic pleurae. A sharp anterior border of the pleura is defined along the line at which costal and mediastinal pleurae meet, subjacent to the sternum. The inferior border of the pleura is formed by the line of union between costal and diaphragmatic pleurae. The posterior border is outlined by the meeting of costal pleura with the posterior margin of mediastinal pleura near the thoracic vertebrae. The anterior reflection of mediastinal and costal pleurae forms a thin, sharp costomediastinal recess within the pleural sac. A similar recess, the costodiaphragmatic recess, formed at the base of the pleural sac by reflections of costal and diaphragmatic pleurae, may be related to overlying structures of the thoracic cage. The topographic localization of the anterior, inferior, and posterior borders of the pleura vary somewhat. The anterior pleural borders of the pulmonary cupulae are separated by visceral structures at the base of the neck. As they descend medially behind the sternum, they appose one another at the sternal angle and form the anterior mediastinal line seen on the chest radiograph in Fig. 54-2. The right anterior border continues downward close to the midline. At the lower limits of the body of the sternum, it diverges laterally along the sixth or seventh costal cartilage to become the inferior pleural border. The left anterior pleural border may follow a similar course, but more commonly, it diverges laterally at the fourth costal cartilage, lies at the lateral sternal margin at the fifth, courses still further laterally at the sixth cartilage, and then diverges laterally with increasing severity at the seventh costal cartilage. The lateral displacement of the left anterior pleural border between the fourth and sixth costal cartilages forms the cardiac notch, or cardiac incisura. Radiographically, a lateral view demonstrates this area as a soft tissue shadow bounded by an interface between lung and the heart and adjacent fat. The interface has been termed the retrosternal line by Whalen and associates (1973).
The inferior borders of both pleural sacs diverge laterally along the seventh costal cartilage and then cross ribs 8, 9, and 10. They reach their lowest level at about the middle of the eleventh rib in the midaxillary line. From this point, they follow an almost horizontal course, cutting across the twelfth rib to meet the posterior pleural border at the twelfth thoracic vertebra. If the twelfth rib is short, the posterior pleural border may lie below it. In some individuals, as noted by Melnikoff (1923), the inferior border may be sufficiently high so that it does not cross the twelfth rib at all; instead, it meets the posterior borders of the pleura at the eleventh thoracic vertebra.
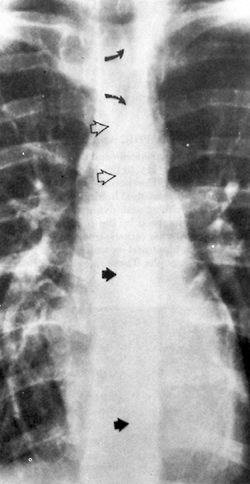 |
Fig. 54-2. Posteroanterior chest radiograph reveals the azygoesophageal recess, superior esophageal recess, and anterior mediastinal line. A slightly curved shadow convex to the right (solid arrows) projected in front of the thoracic spine from below the right main bronchus and extending down to just above the diaphragm is the azygoesophageal recess. Superiorly, a second curved linear shadow convex to the left (curved arrows) projected over the tracheal air column represents the superior esophagopleural recess. Between the two is a third linear shadow projected over the lower half of the tracheal air column (open arrows), representing the anterior mediastinal line. From Fraser RG, Pare JAP. Diagnosis of Diseases of the Chest. 2nd Ed. Philadelphia: WB Saunders, 1983, with permission. |
P.787
The posterior pleural borders ascend alongside or in front of the bodies of thoracic vertebrae until they diverge superiorly near the pulmonary cupulae. They are rounded, in contrast to either anterior or inferior borders. Right and left posterior borders may be in close apposition in front of the vertebral bodies. Where this situation occurs, thin retroesophageal recesses are formed behind the esophagus and in front of the aorta and the hemiazygos and azygos veins.
As a result of these recesses, lines, usually two, are visible within the mediastinal contour on a well-exposed frontal chest radiograph. These lines have been termed the azygoesophageal recess and the superior esophageal recess (see Fig. 54-2). The left paraspinal line, which also is frequently seen, is related to the reflection of the parietal pleura from the vertebral bodies over the descending aorta (Fig. 54-3). Fraser and colleagues (1989) discuss in detail the explanations for these lines as seen on radiographs of the chest.
BLOOD SUPPLY
The visceral pleura was believed to be supplied by both the bronchial and pulmonary arterial systems. The investigations of Albertine and associates (1982) in sheep, however, revealed that the visceral pleural blood supply was entirely from the bronchial arterial system. There is no reason to believe it is otherwise in the human. Venous drainage is by way of the pulmonary veins. The blood supply to the parietal pleura is from the various systemic arterial vessels supplying the chest wall, diaphragm, and mediastinum, as well as from vessels to the cupula from the subclavian arteries in the neck. Venous drainage is to the corresponding veins and, at times, directly into the superior vena cava.
LYMPHATIC DRAINAGE
The subpleural space of the visceral pleura has a large network of lymphatic channels, but only infrequently is a subpleural lymph node identified. Trapnell (1964) reported the incidence of intrapulmonary lymph nodes to be 18%, but in his study no nodes were found in a subpleural location. Greenberg (1961), however, described a lymph node in this location that was removed surgically because it had been identified radiographically as a coin lesion. The identification of subpleural lymph nodes is more common since helical computed tomographic (CT) examination of the chest has become popular. These lymph nodes must be differentiated from small primary and metastatic tumors of the lung. High-resolution CT is of help in this regard. According to Yokomise and associates (1998), these nodes are usually less than 10 mm in size, are ovoid in shape, and are homogeneous in nature as noted in Chapter 6. In the aforementioned study, 72% of the identified subpleural lymph nodes were located in the lower lobes of the lungs.
P.788
The lymphatic drainage of the visceral pleura is primarily to the deep pulmonary plexus located in the interlobar and peribronchial spaces. Riquet (1993) and associates (1989), however, described direct subpleural lymphatic connections to the mediastinal nodes in approximately 22% to 25% of lung segments studied. These subpleural connections were present more often in the upper lobes than in the lower lobes.
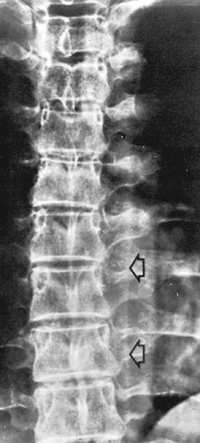 |
Fig. 54-3. Radiograph of the mediastinum (anteroposterior, with patient supine) reveals the longitudinal shadow (arrows) extending from the arch of the aorta to the diaphragm, representing the paraspinal line. From Fraser RG, Pare JAP. Diagnosis of diseases of the chest. 2nd Ed. Philadelphia: WB Saunders, 1983, with permission. |
The lymphatic drainage of the parietal pleura is into the parietal pleural lymphatic chGannels. The aforementioned stomata and Kampmeier's foci play important roles in this process. Miura and associates (2000) have extensively studied by video-assisted thoracoscopy and scanning and transmission electron microscopy the egress and the sites of absorption of carbon particles that had been injected into the pleural space of monkeys. The stomata (Fig. 54-4) in the internal cellular layer of mesothelial cells play an important role in the transfer of the carbon particles into the foramina (Fig. 54-5) in the loose connective tissue layer (the macula cribriformis) and thence into the lymphatic lacunae (Fig. 54-6) of the parietal pleura. These researchers suggest that this probably is the primary pathway of exit of erythrocytes and tumor cells from the pleural space. The lymphatic networks of the chest wall drain into the internal mammary chain anteriorly and the intercostal chain posteriorly. The drainage of the diaphragmatic pleura is to the retrosternal and mediastinal lymph nodes as well as to the celiac lymph nodes in the abdomen.
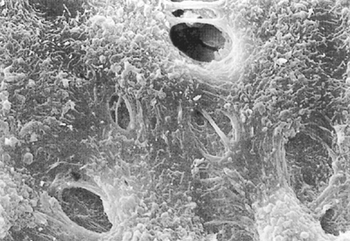 |
Fig. 54-4. Scanning electron microscopic photograph of stomata in the pleura of a monkey that can be seen between cuboidal mesothelial cells. The lymphatic epithelial cells can be seen through the stomata (original magnification 3,300). From Miura T, et al. Lymphatic drainage of carbon particles injected into the pleural cavity of the monkey, as studied by video-assisted thoracoscopy and electron microscopy. J Thorac Cardiovasc Surg 120:437, 2000, with permission. |
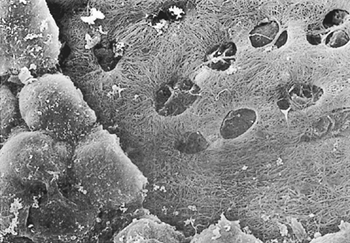 |
Fig. 54-5. Scanning electron microscopic photograph of a monkey's costal pleura treated with 2 N NaOH for 30 minutes at 37 C. Some of the small mesothelial cells are partially removed, and the macula cribriformis with foramina are visible beneath them. (Original magnification x1100.) Used with permission from Miura T, et al. Lymphatic drainage of carbon particles injected into the pleural cavity of the monkey, as studied by video-assisted thoracoscopy and electron microscopy. J Thorac Cardiovasc Surg 120:437, 2000, with permission. |
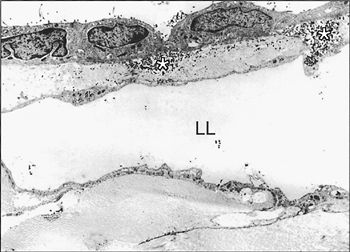 |
Fig. 54-6. Transmission electron microscopic photography of the costal region of a monkey's pleura where intrapleurally injected carbon particles were absorbed. Certain particles can be seen in the macula cribriformis (asterisks) beneath smaller mesothelial cells and in the lumen of the lymphatic lacuna (LL) (original magnification 2,200). From Miura T, et al. Lymphatic drainage of carbon particles injected into the pleural cavity of the monkey, as studied by video-assisted thoracoscopy and electron microscopy. J Thorac Cardiovasc Surg 120:437, 2000, with permission. |
P.789
NERVE SUPPLY
The parietal pleura is innervated by both somatic and sympathetic and parasympathetic fibers via the intercostal nerves. The diaphragmatic pleura is supplied by the phrenic nerves. The visceral pleura is devoid of somatic innervation.
REFERENCES
Albertine KH, et al: Structure, blood supply, and lymphatic vessels of the sheep's visceral pleura. Am J Anat 165:277, 1982.
Antony VB, et al: Pleural cell biology in health and disease. Am Rev Respir Dis 145:1236, 1992.
Bolen JW, Hammer SP, McNutt MA: Reactive and neoplastic serosal tissue. A light microscopic, ultrastructural, and immunocytochemical study. Am J Surg Pathol 10:34, 1986.
Chretien J, Huchon GJ: New contributions to the understanding of pleural space structure and junction. In Deslauriers J, Lacquet LK (eds): Thoracic Surgery: Surgical Management of Pleural Diseases. International Trends in General Thoracic Surgery. Vol. 6. St. Louis: CV Mosby, 1990.
Dervan PA, Tobin B, O'Connor M: Solitary (localized) fibrous mesothelioma: evidence against mesothelial cell origin. Histopathology 10:867, 1986.
England DM, Hochholzer L, McCarthy MJ: Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 13:640, 1989.
Fraser RG, et al: Diagnosis of Diseases of the Chest. 3rd Ed. Philadelphia: WB Saunders, 1989.
Greenberg HB: Benign subpleural lymph node appearing as a pulmonary coin lesion. Radiology 77:97, 1961.
Hammer SP: Pleural diseases. In Dail DH, Hammer SP, Colby TV (eds): Pulmonary Pathology Tumors. New York: Springer-Verlag, 1995, p. 405.
Keating S, et al: Solitary fibrous tumor of the pleura: an ultrastructural and immunohistochemical study. Thorax 42:976, 1978.
Melnikoff A: Die chirurgische Anatomie des Sinus costodiaphragmaticus. Arch Klin Chir Berl 123:133, 1923.
Miura T, et al: Lymphatic drainage of carbon particles injected into the pleural cavity of the monkey, as studied by video-assisted thoracoscopy and electron microscopy. J Thorac Cardiovasc Surg 120:437, 2000.
Patten BM. Human Embryology. New York: McGraw-Hill, 1968, pp. 87, 406.
Riquet M: Anatomical basis of lymphatic spread from carcinoma of the lung to the mediastinum: surgical and prognostic implications. Surg Radiol Anat 15:271, 1993.
Riquet M, Hidden G, Debesse B: Direct lymphatic drainage of lung segments to the mediastinal nodes. An anatomic study on 260 adults. J Thorac Cardiovasc Surg 97:623, 1989.
Said JW, et al: Localized fibrous mesothelioma: an immunohistochemical and electron microscopic study. Hum Pathol 15:440, 1984.
Staub NC, Wiener-Kronish JP, Albertine KM: Transport through the pleura: physiology of normal liquid and solute exchange in the pleural space. In Chretien J, Bignon J, Hirsch A (eds): The Pleura in Health and Disease. New York: Marcel Dekker, 1985, pp. 169 193.
Trapnell DH: Recognition and incidence of intrapulmonary lymph nodes. Thorax 19:44, 1964.
Wang NS: Morphological data of the pleura: normal conditions. In Chretien J, Hirsch A (eds): Diseases of the Pleura. Paris: Masson, 1982, pp. 10 24.
Wang NS: Mesothelial cells in situ. In Chretien J, Bignon J, Hirsch J (eds): The Pleura in Health and Disease. New York: Marcel Dekker, 1985, pp. 23 24.
Whalen JP, et al: The retrosternal line: A new sign of an anterior mediastinal mass. AJR 117:861, 1973.
Yokomise H, et al: Importance of intrapulmonary lymph nodes in the differential diagnosis of small pulmonary nodular shadows. Chest 113:703, 1998.
EAN: 2147483647
Pages: 203