39 - General Principles of Postoperative Care
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section X - The Diaphragm > Chapter 48 - Embryology and Anatomy of the Diaphragm
Chapter 48
Embryology and Anatomy of the Diaphragm
Thomas W. Shields
The diaphragm serves as the anatomic division between the thoracic and the abdominal cavities and, as such, is a muscular structure that is dealt with by both abdominal and thoracic surgeons. The surgical correction of acquired and congenital abnormalities of the diaphragm may be made from either abdominal or thoracic approaches, depending on the nature of the lesion, the location of the lesion, other abnormalities of the chest or abdomen, and the particular training and experience of the involved surgeon. The diaphragm exists as an anatomic barrier but is not a surgical barrier; a competent surgeon should be able to handle any surgical problem involving the diaphragm and therefore should be versatile enough to approach diaphragmatic lesions from either above or below.
EMBRYOLOGY
The diaphragm originates from an unpaired ventral portion (septum transversum), from paired dorsal lateral portions (pleuroperitoneal folds), and from an irregular medial dorsal portion (dorsal mesentery) (Fig. 48-1). The septum transversum, formed during the third week of gestation, separates the pericardial region from the rest of the body cavity. This part of the diaphragm grows dorsad from the ventral body wall and moves caudad with the other contributors to the diaphragm to reach the normal position of the diaphragm at about 8 weeks. The pleuroperitoneal folds arise on the lateral body walls, at the level where the cardinal veins swing around to enter the sinus venosus of the heart. These folds extend medially and somewhat caudad to join with the septum transversum and the dorsal mesentery to complete the development of the diaphragm at about the seventh week; the right pleuroperitoneal canal closes somewhat earlier than the left. Muscle fibers migrate from the third, fourth, and fifth cervical myotomes, carrying along their innervation, and grow between the two membranes to complete the structures of the diaphragm. During the tenth week, the intestines return from the yolk sac to the abdominal cavity and, at about 12 weeks, rotation and fixation of the intestines occur.
A delay or variation in the described timetable may result in a variety of congenital hernias with or without a hernial sac, or may even result in a congenital eventration of a hemidiaphragm. Early return of the intestines to the abdomen before closure of the pleuroperitoneal membrane results in a hernia through this opening (a so-called foramen of Bochdalek hernia). A sac usually is not present, but if it is, the return of the intestines may have occurred after the closure of the pleuroperitoneal membrane but before the migration of the cervical myotomes between the membranes. Foramen of Morgagni hernias occur anteriorly, almost always have a sac, and therefore probably result from lack of ingrowth of the cervical myotomes. A congenital short esophagus is related to late closure of the diaphragm and early return of the intestine to the abdomen. Congenital eventration may be a total error of ingrowth of cervical myotomes in one or both hemidiaphragms and therefore is actually a large congenital diaphragmatic hernia and not an eventration. An absent diaphragm probably represents an error of growth of the septum transversum and other embryologic elements. Duplication of a hemidiaphragm can occur. The fusion and formation timetable variations also may involve defects in the diaphragm in association with certain vascular anomalies of the lungs and heart.
ANATOMY
Gross Features
The diaphragm is a dome-shaped structure of muscular fibers radiating out from either side of an irregularly shaped central tendon; it consists of the right and the left hemidiaphragms. In structure and function, the diaphragm differs from any other muscle in the body. It is a muscular septum between the abdominal and thoracic cavities, serving as the major muscle of respiration. Its domelike shape allows important abdominal structures, such as the liver and
P.736
the spleen, to have the protection of the lower ribs and the chest wall. The dome of the right hemidiaphragmatic leaf is normally at a higher level than is that of the left. It has been generally thought that this is the result of the liver mass beneath the right leaf. However, this view has been brought into question by the studies of Reddy and colleagues (1994). Their observations support the concept that the cardiac mass is responsible for the lower portion of the left hemidiaphragm, rather than the former hypothesis.
 |
Fig. 48-1. Embryologic components of the diaphragm. Redrawn from Shields TW: The diaphragm. In: Nora P (ed): Operative Surgery: Principles and Techniques. Philadelphia: Lea & Febiger, 1972. With permission. |
Voluntary muscular fibers originate from the xiphisternum, from the lateral lower six ribs on each side, and from the external and internal arcuate ligaments that arise from the upper three lumbar vertebrae. Bilaterally, the muscle fibers insert into the central tendon of the diaphragm. The muscle mass of the diaphragm is considered by De Troyer and associates (1982) and Rochester (1985) as comprising two distinct parts: a thin costal muscle mass and a thicker crural portion. Although both muscle masses are innervated by the phrenic nerves, their activity on stimulation is different. The differences that result in diaphragmatic and lower chest wall movement are discussed in Chapter 49. Suffice to mention that the movement of the crural portion has the lesser effect on ventilatory exchange.
The central tendon is a thin aponeurosis of closely interwoven fascial fibers in the form of a three-leaf clover. The two lateral leaves form the dome of the diaphragm, and the third (anterior) leaf is fused with the diaphragmatic surface of the pericardium.
Major interest in the muscular portion of the diaphragm centers on the two crura, which play varying roles in the formation of the esophageal hiatus. The right crus arises from the bodies of the first and second lumbar vertebrae, and the fibers divide as they pass to the left, normally overlapping in front and behind to form the entire esophageal hiatus. Collis and associates (1954), however, found this arrangement in only a little more than half of their subjects. In the others, the left crus contributed to a varying degree to the makeup of the hiatus, and in about 2%, the left crus made up the major portion of the esophageal hiatus.
The hiatal opening is situated at the level of the tenth thoracic vertebra just to the left of the midline and just ventral to where the aorta passes into the abdomen. The inferior vena cava passes through the tendinous portion of the right side of the diaphragm between the anterior leaf and the right lateral leaf at the level of the eighth thoracic vertebra. The other normal openings are the parasternal foramina (i.e., the foramina of Morgagni) through which the internal mammary arteries pass into the abdomen to become the superior epigastric arteries. Evidence suggests that, in some subjects, a variable number of fenestrations or pores are present, or are potentially so, in either hemidiaphragmatic leaf. These are more commonly observed on the right than on the left and are located posteriorly when present. These fenestrations are of no importance normally but may become the route of fluid or air to traverse from the peritoneal cavity into the pleural space, as demonstrated by the studies of Park and Pham (1995) and Urhahn and Gunther (1993) (Fig. 48-2).
The thoracic side of the diaphragm is covered with the parietal pleura, and the abdominal surface with peritoneum, except at the naturally occurring openings, and the bare area is occupied by a portion of the liver.
Blood Supply
The principal blood supply of the diaphragm is derived directly from the aorta or from its most superior abdominal branches (Fig. 48-3), and its venous drainage empties into the inferior vena cava. Both the arterial supply and the venous drainage (the right and left inferior phrenic veins) are found on the undersurface of the diaphragm (Fig. 48-4). The inferior phrenic artery usually bifurcates posteriorly near the dome of the diaphragm, and the branches course along the margins of the central tendon. The smaller posterior division courses laterally above the dorsal and lumbocostal origin of the diaphragm, where it has collateral anastomoses with the lower five intercostal arteries. The
P.737
larger anterior division runs anterosuperiorly to the edge of the central tendon, where it anastomoses freely with the pericardiacophrenic artery. The venous pattern is similar except that the veins generally course along the posterior aspect of the central tendon to join the inferior vena cava. Veins on the inferior surface of the diaphragm communicate with the hepatic veins through the left triangular and coronary ligaments of the liver.
 |
Fig. 48-2. Thoracoscopy revealed a tiny hole about 3 mm in diameter in the right hemidiaphragm. Adjacent spots were made up of endometrial tissue. Adapted from Sato M, Kase K: Catamenial pneumothorax with diaphragmatic endometriosis: a case report. J Jpn Assoc Chest Surg 11:583, 1997. With permission. |
 |
Fig. 48-3. The arterial supply of the diaphragm from the abdominal aorta with variations in the origin of the inferior phrenic arteries. Redrawn from Anson BJ, McVay C: Surgical Anatomy. 5th Ed. Philadelphia: WB Saunders, 1971. With permission. |
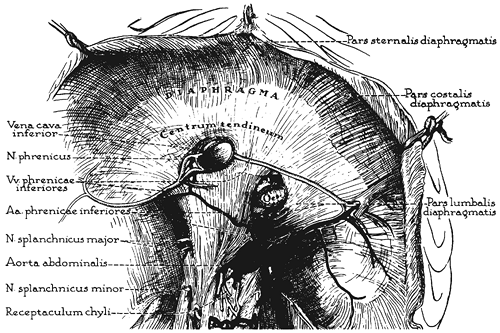 |
Fig. 48-4. The arterial and venous distribution on the undersurface of the diaphragm. Redrawn from Anson BJ, McVay C: Surgical Anatomy. 5th Ed. Philadelphia: WB Saunders, 1971. With permission. |
Nerve Distribution
The right and left phrenic nerves arise from their respective third, fourth, and fifth cervical nerve roots and constitute the total nerve supply for the ipsilateral hemidiaphragm. The distribution of each nerve is important in reference to incisions into the diaphragm. The course of each has been described by Merendino and colleagues (1956). The right phrenic nerve reaches the diaphragm just lateral to the inferior vena cava, and the left just lateral to the left border of the heart. Generally, the nerves divide, either just above or at the level of the diaphragm, into several terminal branches. Some are distributed to the pleural and peritoneal surfaces, but the great bulk of each nerve passes into, or through, the diaphragm and most often divides into four major rami to supply the various muscular portions. Usually, two of the rami share a common trunk for a varying distance so that three muscular branches arise from each phrenic nerve: one anteromedially, one laterally, and the remaining one posteriorly (Fig. 48-5). Injury to any of these branches causes paralysis of the supplied portion of the hemidiaphragm.
Diaphragmatic Lymph Nodes
Lymph nodes of the diaphragm are divided into three groups. The first is the anterior (prepericardiac) group located behind the xiphoid and to the right and left of the pericardium and belongs to the anterior parietal group of nodes of the mediastinum. The second group of lymph nodes is located in proximity to the phrenic nerves bilaterally as the nerves meet the respective hemidiaphragm and is known as the middle or juxtaphrenic group. The third group is the
P.738
posterior or retrocural lymph nodes, which lie behind the left and right crura. These lymph nodes are in continuity with the posterior parietal group of the paravertebral sulcus.
 |
Fig. 48-5. Distribution of the phrenic nerves as seen from above. Redrawn from Merendino KA, et al: The intradiaphragmatic distribution of the phrenic nerve with particular reference to the placement of diaphragmatic incisions and controlled segmental paralysis. Surgery 39:189, 1956. With permission. |
SURGICAL INCISIONS
Incisions into the diaphragm must be made to avoid injury to the major branches of the phrenic nerves. Incision through the central tendon rarely causes diaphragmatic paralysis (Fig. 48-6A, B), but this approach provides only minimal exposure of the adjacent compartment. A more satisfactory access is provided by a circumferential incision at the periphery of the diaphragm, which permits excellent exposure of the upper abdominal contents from the thorax and vice versa with little or no possibility of injury to any major branch of the ipsilateral phrenic nerve (Fig. 48-6C). On the left, the incision may be started at the esophageal hiatus and carried from behind forward circumferentially 2.5 to 3.0 cm away from the attachment of the diaphragm to the chest wall. The crural or posterior branch of the phrenic nerve is divided, but this division is of little consequence. The main branch of the left inferior phrenic artery is usually encountered with this incision and requires division and ligation. Alternatively, the incision may be started anteriorly, just lateral to the pericardium, and extended circumferentially as far posteriorly as necessary. The ipsilateral hemidiaphragm may then be raised as a trapdoor and retracted medially for exposure. Closure of the incision is accomplished readily by approximating the cut edges of the hemidiaphragm with multiple interrupted simple or mattress sutures of 0 0 or 2 0 nonabsorbable material of the surgeon's choice. A similar incision may also be carried out on the right.
 |
Fig. 48-6. Safe areas for incision into the diaphragm. Redrawn from Merendino KA, et al: The intradiaphragmatic distribution of the phrenic nerve with particular reference to the placement of diaphragmatic incisions and controlled segmental paralysis. Surgery 39:189, 1956. With permission. |
When a combined abdominothoracic approach is used, the incision in the diaphragm may be extended medially between the pericardial attachment to the diaphragm and the entrance of the phrenic nerve into the diaphragm, with severance of only the small sternal division of the nerve (Fig. 48-6D). The incision is then carried to the apex of the esophageal hiatus. To ensure adequate exposure, the phrenic nerve and pericardiacophrenic vessels must be freed from the pericardium proximally and retracted laterally. Care must be exercised to prevent injury to these structures during this retraction. This incision is closed the same as a circumferential incision. Sicular (1992) reported the use of this latter incision with the 90 GIA stapling instrument in more than 50 patients with no compromise of exposure. Moreover, he reported no clinical evidence of phrenic nerve injury postoperatively. Incisions in the diaphragm other than a circumferential or a very medial one must be avoided because the anterolateral and posterolateral branches of the nerve are likely to be divided.
DIAPHRAGMATIC PORES
Significance of Pores in the Diaphragm
Kirschner (1998) has][globally categorized the clinical occurrence of peritoneopleural transphrenic passage of fluids or gases through either congenital or acquired pores in the diaphragm as porous diaphragm syndromes. The numerous clinical entities are listed in Table 48-1. Kirschner (1998) has described these in detail, but only a few of the more important ones are discussed briefly here. The diaphragmatic pores permit the egress of ascitic fluid from the abdomen in some cirrhotic patients into the ipsilateral hemithoracic pleural space, resulting in a cirrhotic hydrothorax. Hydrothorax also may occur during peritoneal dialysis when these pores are present, and Nomoto and associates (1989) reported an incidence of 1.6% of this complication. When identified, closure of the pore is curative, as reported by Lieberman and Peters (1970) and Mouroux and colleagues (1996). Temes (1997) and Tsunezuka (2001) and their associates reported successful video-assisted thoracic surgery (VATS) resection of the diaphragmatic defect and closure thereof in the treatment of hepatic hydrothorax and hydrothorax following ambulatory peritoneal dialysis, respectively. Both groups suggest this approach to be the procedure of choice in the management of such patients.
The role of diaphragmatic pores in the etiology of catamenial pneumothorax is questioned by many, but these pores have been found in more than one third of the cases; in some reports, such as that of Stern and colleagues (1980), all explored patients were found to have these pores, whereas Lillington and associates (1972) identified only 3 of 18 patients as having these diaphragmatic fenestrations. One of the arguments against their role in the development of catamenial pneumothorax has been the failure to identify air in the abdomen. However, Downey and colleagues (1990) reported the
P.739
presence of a pneumoperitoneum associated with a catamenial pneumothorax in a patient on three separate occasions, a finding that tends to refute the aforementioned argument. However, Sato and Kasi (1997) noted that, in the presence of endometriosis of the diaphragm, closure of the diaphragmatic pores is not always successful. In one of their patients with repeated catamenial pneumothoraces, excision and closure of an identified defect associated with endometriosis of the diaphragm was carried out. However, the patient had a recurrent pneumothorax shortly after the surgical repair. They suggested that those patients who suffer this disease be placed on hormonal treatment in addition to undergoing the surgical procedure. Slabbynck (1991) and Espaulella (1991) and their associates suggested the use of a gonadotropin-releasing hormone analogue as the medication of choice.
Table 48-1. Porous Diaphragm Syndromes according to Substance Traversing the Diaphragm | ||
|---|---|---|
|
Finally, the occurrence of a tension pneumothorax during the course of laparoscopy is to be noted. This event may be life-threatening and has been reported by Heddle and Platt (1984), Whiston and associates, (1991), and Childers and Caplinger (1995). This complication must be differentiated from other anesthetic complications presenting with similar clinical features.
REFERENCES
Childers JM, Caplinger P: Spontaneous pneumothorax during operative laparoscopy secondary to congenital diaphragmatic defects: a case report. J Reprod Med 40:151, 1995.
Collis JL, Kelly TD, Wiley AM: Anatomy of the crura of the diaphragm and the surgery of hiatus hernia. Thorax 9:175, 1954.
De Troyer A, et al: Action of costal and crural parts of the diaphragm on the rib cage in dogs. J Appl Physiol 53:30, 1982.
Downey DB, et al: Pneumoperitoneum with catamenial pneumothorax. AJR Am J Roentgenol 155:29, 1990.
Espaulella J, et al: Pulmonary endometriosis: conservative treatment with GnRH agonists. Obstet Gynecol 79:535, 1991.
Heddle RM, Platt AJ: Tension pneumothorax during laparoscopic cholecystectomy. Br J Surg 79:374, 1984.
Kirschner PA: Porous diaphragm syndromes. Chest Surg Clin North Am 8:449, 1998.
Lieberman FL, Peters RL: Cirrhotic hydrothorax. Further evidence that an acquired diaphragmatic defect is at fault. Arch Intern Med 125:114, 1970.
Lillington GA, Mitchell SP, Wood GA: Catamenial pneumothorax. JAMA 219:1328, 1972.
Merendino KA, et al: The intradiaphragmatic distribution of the phrenic nerve with particular reference to the placement of diaphragmatic incisions and controlled segmental paralysis. Surgery 39:189, 1956.
Mouroux J, et al: Management of pleural effusion of cirrhotic origin. Chest 109:1093, 1996.
Nomoto Y, et al: Acute hydrothorax in continuous ambulatory peritoneal dialysis: a collaborative study of 161 centers. Am J Nephrol 9;363, 1989.
Park CH, Pham CD: Hepatic hydrothorax. Scintigraphic confirmation. Clin Nucl Med 20:278, 1995.
Reddy V, Sharma S, Cobanoglu A: What dictates the position of the diaphragm the heart or the liver? A review of sixty-five cases. J Thorac Cardiovasc Surg 108:687, 1994.
Rochester DF: The diaphragm: contractile properties and fatigue. J Clin Invest 75:1397, 1985.
Sato M, Kasi K: Catamenial pneumothorax with diaphragmatic endometriosis: a case report. J Jpn Assoc Chest Surg 11:583, 1997.
Sicular A: Direct septum transversum incision to replace circumferential diaphragmatic incisions in operations on the cardia. Am J Surg 164:167, 1992.
Slabbynck H, et al: Recurring catamenial pneumothorax treated with a gn-RH agonist. Chest 100:851, 1991.
Stern H, Toole AL, Merino M: Catamenial pneumothorax. Chest 78:480, 1980.
Temes RT, et al: Videothoracoscopic treatment of hepatic hydrothorax. Ann Surg 64:1468, 1997.
Tsunezuka Y, et al: Video-assisted thoracoscopic treatment for pleuroperitoneal communication in peritoneal dialysis. Eur J Cardiothorac Surg 20; 205, 2001.
Urhahn R, Gunther RW: Transdiaphragmatic leakage of ascites in cirrhotic patients: evaluation with ultrafast gradient echo MR imaging and intraperitoneal enhancement. Magn Reson Imaging 11:1067, 1993.
Whiston RJ, et al: Tension pneumothorax during laparoscopic cholecystectomy. Br J Surg 78:1325, 1991.
Reading References
Anson BJ. Atlas of Human Anatomy. Philadelphia: WB Saunders, 1950.
Anson BJ, McVay C. Surgical Anatomy. 5th Ed. Philadelphia: WB Saunders, 1971.
Patten B. Human Embryology. 3rd Ed. New York: McGraw-Hill, 1968, p. 406.
EAN: 2147483647
Pages: 203