36 - Anterior Approach to Superior Sulcus Lesions
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section IX - The Chest Wall > Chapter 44 - Thoracoscopic Sympathectomy
Chapter 44
Thoracoscopic Sympathectomy
Scott W. Arnold
Thomas M. Daniel
Surgical sympathectomy for the treatment of autonomic disorders was introduced in the late 19th century by Jaboulay and Jonnesco. Adson and colleagues (1935) were among the first in the United States to describe sympathectomy for vasomotor disorders and hyperhidrosis. Various open approaches to the thoracic sympathetic chain were subsequently described (Table 44-1) but were never widely utilized because of associated pain and morbidity.
Modern thoracoscopic techniques have allowed a rebirth of thoracoscopic sympathectomy. Kux (1954, 1978) was the first to apply thoracoscopic techniques to the treatment of hyperhidrosis. Recent advances in equipment and techniques have resulted in a minimally invasive, safe, quick, anatomically exact, outpatient treatment for specific autonomic disorders of the upper extremity.
Thoracoscopic sympathectomy is now clearly the treatment of choice for the disorder of primary palmar hyperhidrosis, with success rates of greater than 95%. It is also a low-risk option for the treatment of other autonomic disorders such as reflex sympathetic dystrophy (RSD). Current controversies regarding thoracoscopic sympathectomy are primarily technical and involve the number, size, and location of the ports used and the extent and location of the sympathectomy or sympathotomy.
ANATOMY
To understand the physiologic basis of sympathectomy, the controversies surrounding the extent of sympathectomy, and complications related to sympathectomy, one must understand the anatomy of the thoracic autonomic nervous system.
The central autonomic fibers descend in the spinal cord and terminate at cell bodies in the intermediomedial and intermediolateral gray columns of the cord. The peripheral autonomic fibers then exit the cord from T1 and L3 via the ventral roots and enter the sympathetic chain via the white rami. In the sympathetic chain, fibers synapse (or may pass through to synapse higher, lower, or peripherally) and the postganglionic fibers exit the chain via gray rami at all spinal cord segments (Figs. 44-1 and 44-2).
As reviewed by Harati (1993), the preganglionic sympathetic fibers to the upper extremity originate from the intermediolateral cell columns of T2 through T6. They then ascend and either synapse in or pass through the stellate ganglion to enter the lower trunk of the brachial plexus, mainly the medial cord. Sympathetic outflow then travels to the upper extremity primarily via the median and ulnar nerves. The T2 and T3 roots contain most of the vasoconstrictor fibers to the upper extremity. As noted by Lemmens (1932), the T2 outflow is the most important sympathetic innervation for both vasomotor and pseudomotor (sweat) nerves of the hand. Axillary sympathetic innervation originates primarily from T4 and T5. Plantar innervation is less discreet but probably is derived from T2 T7. Splanchnic innervation via the greater splanchnic nerve originates from T5 T8.
As described by Cross (1993), the sympathetic outflow to the ciliary muscle of accommodation and the pupillary constrictor of the eye arises from T1, ascends through the stellate ganglion without synapsing, and travels to the superior cervical ganglion. There the fibers synapse and pass along with branches of the carotid to the eye (see Fig. 44-1).
Finally, there are alternate pathways of sympathetic innervation to the upper extremity. The best described is the nerve of Kuntz described by Kuntz and colleagues in 1937, a variable intrathoracic nerve that arises from approximately T2 and bypasses the sympathetic chain by parallel vertical fibers to flow directly to the lower brachial plexus (Fig. 44-2). A basic knowledge of this anatomy allows one to understand the importance of preservation of the T1 rami and stellate ganglion in preventing Horner's syndrome of ipsilateral ptosis, miosis, and anhidrosis. It also becomes clear why an en bloc T2 T3 ganglionectomy with ablation of Kuntz's fibers can provide a nearly complete autonomic denervation of the upper extremity: it removes T2 and T3 outflow directly and removes ascending fibers from the
P.699
lower thoracic segments of T4 and T5. A selective sympathectomy, or ramisectomy, as reviewed by Wittmoser (1992) and Gossot and associates (1997), involves division of the rami only and theoretically could allow selective T2 T3 denervation and possibly less compensatory hyperhidrosis.
Table 44-1. Approaches to Thoracic Sympathectom | |
|---|---|
|
Several surgeons have decreased operative time and the size and number of trocars by performing a simple T2-level sympathotomy by cutting or even clipping the chain. This technique is generally successful and may result in less compensatory hyperhidrosis, a known complication of sympathectomy. However, recurrence rates may also be higher due to regeneration of the chain and failure to ablate the accessory fibers of Kuntz, which cross the bodies of ribs 2 and 3.
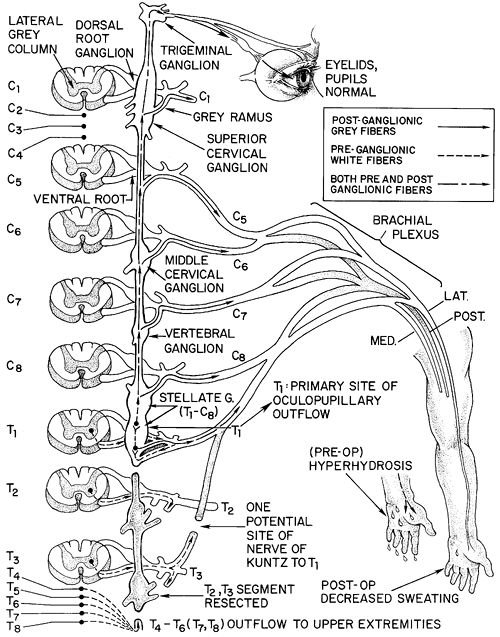 |
Fig. 44-1. Schematic representation of the sympathetic pathway to the upper extremity and face. Note T1 outflow to the eye. |
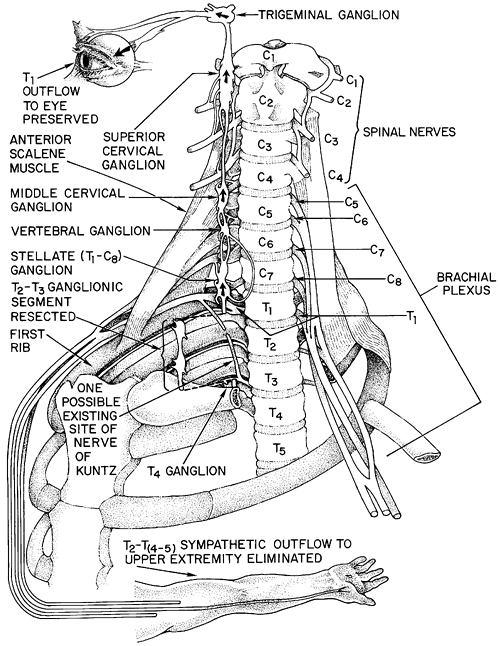 |
Fig. 44-2. Anatomy of the cervicothoracic region, with emphasis on landmarks for sympathectomy. |
Ramicotomy as described by Wittmoser (1992) and Gossot and colleagues (1997) is the division of the T2 and T3 rami only and again theoretically allows selective denervation of T2 and T3 with less compensatory hyperhidrosis but at the expense of added complexity, operative time, and increased recurrences. It is now rarely used.
INDICATIONS
The primary indication for thoracic sympathectomy is primary hyperhidrosis of the upper extremity. Primary palmar hyperhidrosis is a disorder of excessive sweat production of the palms. Hyperhidrosis often also affects the axillae, soles of the feet, and the head and face. As described by Rex and co-workers (1998), an apparently related disorder is social phobia with its associated facial blushing. The triggers for sweating in primary palmar hyperhidrosis are primarily emotional but may also be heat or exercise related. The baseline sweat production is extreme as well. Sweating is profuse and palpable to the patient and visible to others. It is socially debilitating, with patients being extremely self-conscious about shaking hands, and adolescents are often reluctant to date. Symptoms often begin in childhood and become more pronounced
P.700
with adolescence. Young adults often become shy and withdrawn. Employment is often jeopardized. Even the simple process of writing or doing homework is difficult, with sweat ruining the paper or smearing ink. Hyperhidrosis has a slight female predominance, and there is an increased incidence in Asians and Sephardic Jews. There is often a familial predisposition. Medical treatment, as reviewed by White (1986), consists of iontophoresis, systemic or topical anticholinergic therapy, topical therapy with aluminum chloride, or biofeedback. Excision of axillary sweat glands has also been performed.
Thoracoscopic sympathectomy is highly effective in the treatment of primary hyperhidrosis and should be considered for all patients with the disorder. As reported by Edmondson (1992), Gossot (1997), and Krasna (2000) and their associates, thoracoscopic sympathectomy has a success rate of greater than 90% in the treatment of hyperhidrosis. The complication rates are low, with the most troublesome source of patient dissatisfaction being compensatory hyperhidrosis.
A less common indication for thoracoscopic sympathectomy is RSD. This painful disorder has few successful treatment options. Although sympathectomy is less successful in the treatment of RSD than with hyperhidrosis, it has good success rates and should be considered a valuable treatment option. Schwartzman and colleagues (1997) reported a high success rate with thoracoscopic sympathectomy in the treatment of carefully selected patients with early-phase RSD. In this study, 100% of patients with symptom duration of 12 months or less had long-term relief, whereas only 44% had relief if symptoms had been present longer than 24 months. In a recent study by Bandyk and collaborators (2002), 90% of patients with RSD who responded to preoperative sympathetic nerve blocks had a greater than 50% pain reduction.
As reviewed by van de Wal and associates (1985), sympathectomy is also used in the treatment of Raynaud's syndrome and Buerger's disease. However, these disorders respond more variably to surgery. Recurrence rates after sympathectomy for Raynaud's syndrome are fairly high. Raynaud's syndrome and Buerger's disease are best first treated with smoking cessation, withdrawal of -blockers, avoidance of cold temperatures, and pharmacotherapy with calcium channel blockers or -antagonists. But refractory cases, particularly those with skin ulceration, often do respond to sympathectomy, and given the low risks of the procedure, it should be considered a valid option.
Upper extremity ischemia due to nonrevascularizable arterial embolization to the palmar arch responds well to sympathectomy. Sympathectomy has other rare indications, including refractory angina and long QT syndrome.
TECHNIQUE
Most of the controversies and developments in thoracoscopic sympathectomy over the past several years have involved technical variations to limit port number and size and to limit dissection and extent of sympathectomy with the goals of reducing pain and theoretically reducing the incidence of compensatory hyperhidrosis. The goals of any approach should be a complete autonomic denervation of the hands without complications, no recurrence, minimal or no hospital stay, and the ability to perform single-stage bilateral sympathectomies.
Described variations in techniques include use of progressively smaller needlescopic ports to perform a sympathotomy with simple division of the sympathetic chain just above T2, and selective ramicotomy with division of the T2 T3 sympathectomy with horizontal scoring of the bodies of ribs 2 and 3 to ablate any accessory fibers of Kuntz.
The standard two- or three-port en-bloc T2 T3 sympathectomy is performed using dual-lumen anesthesia with the patient in a modified lateral decubitus position (i.e., leaning forward approximately 15 degrees beyond perpendicular). This allows the ipsilateral lung to fall away from the posteriorly located sympathetic chain. A 30-degree 5- or 10-mm thoracoscope is inserted in the fifth intercostal space at the midaxillary line, and the lung is allowed to passively deflate with the head of the bed elevated to allow the apex to fall away from the chest wall. We then use one or two 5-mm working trocars in the third intercostal space, one anteriorly and one posteriorly. We use either hook cautery or harmonic scissors to open the pleura overlying the sympathetic chain. One important anatomic issue is the identification of the first rib and the upper border of the second rib. Avoidance of Horner's syndrome depends on preservation of the stellate ganglion at T1. If one limits dissection to below the upper border of the second rib, injury to the stellate ganglion can usually be avoided. The first rib is often difficult to visualize thoracoscopically. It is often covered by an area of bright yellow fat at its costovertebral junction, which serves as a useful landmark. The surgeon should thoracoscopically palpate the soft tissue above the apparent first rib to be sure there is no further cephalad rib. Once the first rib is localized, the pleura is opened and the second rib identified. No further dissection is carried out above the upper border of the second rib. This also decreases the chances for injury to the T1 outflow to the lower cord of the brachial plexus which crosses the first rib to join C8 and the lower cord of the plexus (see Fig. 44-2). The main sympathetic chain is then elevated from T2 to T3. It is then clipped and cut with scissors or cut with the harmonic scissors (Fig. 44-3). The rami from T2 and T3 are hemoclipped and divided or divided with the harmonic scissors. Cautery is avoided near the rami to prevent heat transfer to the nerve roots. The bodies of ribs 2 and 3 are then scored horizontally with the cautery from the costovertebral angle laterally for 3 to 4 cm to ablate any accessory fibers of Kuntz. Many surgeons advocate monitoring of the palmar skin temperature to confirm sympathectomy. We have not routinely used this adjunct.
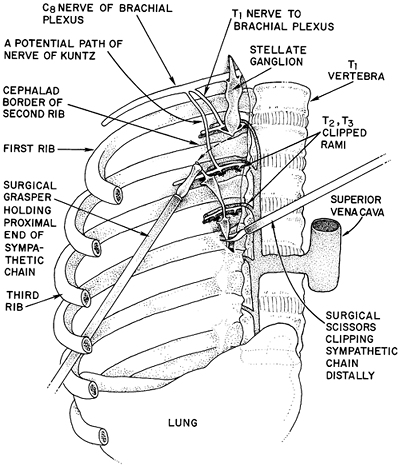 |
Fig. 44-3. Depiction of thoracoscopic T2 T3 ganglionectomy. The second rib is identified, and the rami and main trunk are clipped and cut. |
P.701
Most recently we have been performing bilateral T2 and T3 level sympathotomies using two 5-mm ports and the harmonic scissors to divide the chain at T2 and T3 and to score the ribs with cautery. Upon completion of the first side we place a small Silastic drain through one of the 5-mm ports and then complete the other side. This lung is inflated with a red rubber drain in the pleural space. The drain is removed during a Valsalva maneuver, and the incision closed. The pleural drain is then removed from the first side and the patient is discharged home. Sympathotomy appears to be quick and safe. Rates of compensatory hyperhidrosis may be somewhat less, though persistent symptoms and recurrences may be higher due to regeneration or incomplete division. At present, randomized controlled comparisons of sympathotomy versus resection are lacking.
The operation can be tailored somewhat based on symptoms and anatomy. Craniofacial sweating and facial blushing can be ameliorated with a T2 level lesion. We do not advocate going to T1 because of the increased risk for Horner's syndrome. Predominantly palmar symptoms are treated with a T2 and T3 level operation. Some surgeons include T2 T4 or T5 for axillary denervation. We rarely add these levels, feeling that a T2/T3 level operation will interrupt any ascending fibers to the axillae.
COMPLICATIONS
Specific complications include compensatory hyperhidrosis, which occurs in 60% to 70% of patients. It consists of excessive sweating in nondenervated areas, such as the back and groin. It is often tolerable but can be severe. Its etiology is unclear but may well represent a normal thermoregulatory compensation. Plantar sweating frequently increases as well, but we have interestingly often seen a decrease in plantar sweating with T2/T3 level sympathectomy. Whether this is a direct effect on plantar autonomic innervation or a central feedback phenomenon is unclear. In our experience, if patients are counseled and warned about compensatory hyperhidrosis preoperatively they are generally completely satisfied if their palmar symptoms are alleviated despite some degree of compensatory sweating.
Edmondson and associates (1992) reported a 48% incidence of gustatory sweating (i.e., facial sweating with salivary stimuli). Horner's syndrome is rare with preservation of T1. It may occur in 5% or 10% of patients, however, because of anatomic variability in the formation of the stellate ganglion. Other specific complications include recurrence, intercostal neuralgias, pneumothorax, and injury to the subclavian vessels or the esophagus.
REFERENCES
Adson AW, Craig WM, Brown GE: Essential hyperhidrosis cured by sympathetic ganglionectomy and trunk resection. Arch Surg 31:794, 1935.
Atkins MJB: Sympathectomy by the axillary approach. Lancet 1:538, 1954.
Bandyk DF, et al: Surgical sympathectomy for reflex sympathetic dystrophy syndromes. J Vasc Surg 35:269, 2002
Cloward RB: Hyperhydrosis. J Neurosurg 30:545, 1969.
Cross FA: Autonomic innervation of the eye. In Low PA (ed): Clinical Autonomic Disorders. New York: Little, Brown, 1993.
Edmondson RA, Banerjee AK, Rennie JA: Endoscopic transthoracic sympathectomy in the treatment of hyperhidrosis. Ann Surg 215:289, 1992.
Goetz RH, Marr JAS: The importance of the second thoracic ganglion for the sympathetic supply of the upper extremities: with description of two new approaches for its removal in cases of vascular disease: preliminary report. Clin Proc 3:102, 1944.
Gossot D, et al: Thoracoscopic sympathectomy for upper limb hyperhidrosis: looking for the right operation. Ann Thorac Surg 64:975, 1997.
Harati Y: Anatomy of the spinal and peripheral autonomic nervous system. In Low PA (ed): Clinical Autonomic Disorders. New York: Little, Brown, 1993.
Krasna M, et al: Thoracoscopic sympathectomy. Surg Laparosc Endosc Percutan Tech 10:314, 2000.
Kuntz A, Alexander F, Furcolo L: Role of pre-ganglionic fibers of the first thoracic nerve in sympathetic innervation of the upper extremity. Proc Soc Exp Biol Med 37:282, 1937.
Kux M: Thorakoskopische Engriffe an Nervensystem. Stuttgart: George Thieme Verlag, 1954.
Kux M: Thoracic endoscopic sympathectomy in palmar and axillary hyperhidrosis. Arch Surg 113:264, 1978.
Lemmens HAJ: Importance of the second thoracic segment for the sympathetic denervation of the hand. Vasc Surg 16:23, 1932.
Rex LO, et al: The Boras experience of endoscopic thoracic sympathicotomy for palmar, axillary, facial hyperhidrosis and facial blushing. Eur J Surg Suppl 580:23, 1998.
Roos DB: Experience with first rib resection for thoracic outlet syndrome. Ann Surg 173:429, 1971.
P.702
Schwartzman RJ, et al: Long-term outcome following sympathectomy for complex regional pain syndrome type I (RSD). J Neurol Sci 150:149, 1997.
Smithwick RH: Modified dorsal sympathectomy for vascular spasm of the upper extremities. Am Surg 104:339, 1936.
Telford ED: Technique of sympathectomy. Br J Surg 23:448, 1935.
van de Wal JH, et al: Thoracic sympathectomy as a therapy for upper extremity ischemia. A long-term follow-up study. Thorac Cardiovasc Surg 33:181, 1985.
White JW Jr: Treatment of primary hyperhidrosis. Mayo Clin Proc 61:951, 1986.
Wilkinson HA: Radiofrequency percutaneous upper thoracic sympathectomy: technique and review of indications. N Engl J Med 311:34, 1984.
Wittmoser R. Thoracoscopic sympathectomy and vagotomy. In Cuschieri A, Buess G, Perissat J (eds): Operative Manual of Endoscopic Surgery. New York: Springer, 1992, pp. 110 133.
Reading References
Brown GE: The treatment of peripheral vasomotor disturbances of the extremities. JAMA 87:379, 1926.
Greenwood B: The origins of sympathectomy. Med Hist 11:165, 1967.
EAN: 2147483647
Pages: 203