20 - Preoperative Cardiac Evaluation of the Thoracic Surgical Patient
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section VII - Pulmonary Resections > Chapter 26 - General Features of Pulmonary Resections
Chapter 26
General Features of Pulmonary Resections
Thomas W. Shields
Resections of the lungs may vary from a minimal incision of the visceral pleura and enucleation of a hamartoma to a pneumonectomy. Most resections are unilateral, but synchronous bilateral excisions may be carried out. The standard procedures (Table 26-1) may be extended to include excision of a part of the chest wall; one of the thoracic parietes (pleura, pericardium, or diaphragm; an adjacent vascular structure) portion of the atrium or vena cava; and rarely part of the esophagus. Some pulmonary resections may be accomplished using local anesthesia (open-lung biopsy), but most are performed with general, endotracheal anesthetic management (see Chapter 22).
OPERATIVE POSITIONS AND THORACIC INCISIONS
Although each operative procedure has its own unique features, the standard operations are performed with the patient in the lateral decubitus, supine, or prone position. The selection of patient position is determined by the operation planned and, in part, by the patient's physiologic condition. The supine position is associated with fewer physiologic changes in the patient's cardiopulmonary function than are noted with the other positions.
Lateral Decubitus Position
The lateral position permits the best access to the hilus of the lung. The structures contained within the hilus may be approached from either the anterior or posterior aspect; thus, the operator has greater control of the various structures than is afforded by the other approaches. The major disadvantage of the lateral position is that ventilation of the dependent lung is more difficult than in the posterior or supine position; however, perfusion of the dependent lung is increased as a result of the gravitational changes.
Prone Patient
The posterior approach while the patient is prone has a major advantage in that the bronchial secretions will not flood the trachea because of the superior position of the main-stem bronchus. Also, the main-stem bronchus is the most accessible structure and may be isolated and divided as the initial stage in the dissection of the hilar structures. The major disadvantages are that the access to the entire hilus is limited initially and that the vascular structures are the most distant from the operator.
Supine Patient
The anterior approach is used commonly. Bilateral anterior thoracotomy with or without a transverse sternotomy is the procedure of choice for bilateral sequential lung transplantation. In addition, especially in European clinics, a unilateral anterior thoracotomy is used often for the performance of a lobectomy or pneumonectomy. One disadvantage is a somewhat less adequate access to the hilus as compared with the lateral approach.
A median sternotomy incision for various types of pulmonary resection has become popular throughout the world. With controlled deflation of the lung and appropriate packing to elevate the lung anteriorly, the hilar structures are readily accessible on the right side. A left pneumonectomy, a left lower lobectomy, or other procedures on this lobe are difficult because of the position of the hilar structures, particularly that of the inferior pulmonary vein, behind the heart. As a consequence, a median sternotomy approach is not recommended for these procedures.
The various incisions and modifications for conduct of open thoracic pulmonary resections are discussed in Chapter 25. The advent of minimally invasive techniques [video-assisted thoracic surgery (VATS)] has resulted in employment of multiple small (1 to 4 cm in length) ports located at selected sites in the chest wall for introduction of the necessary instrumentation
P.421
for the selected VATS procedures (see Chapters 18, 32, and 33).
Table 26-1. Pulmonary Resections | |
|---|---|
|
With open procedures, the trend is to use shorter posterolateral incisions and to spare the division of the major thoracic muscles as much as is compatible with appropriate operative exposure. The subperiosteal resection of a rib is done infrequently, although I believe better and tighter closure of the thoracic wall and pleural space can be accomplished and is indicated when a pneumonectomy is to be performed. Division or excision of a small posterior portion of a rib or ribs adjacent to the intercostal incision to improve exposure and to prevent a possible fracture of the rib as the intercostal space is retracted is practiced on an individual basis. If a fracture of one or more ribs occurs, control of any bleeding is mandatory, and fixation of the fracture site by sutures is indicated to prevent overriding of the fractured ends to prevent further vascular injury or the occurrence of severe postoperative pain on chest wall movement. As a general rule, the rib cage is closed with pericostal sutures; however, Cerfolio and associates (2003) have suggested the use of intracostal sutures to decrease postoperative incisional pain. In this closure, the sutures are placed through the tissues and periosteum on top of the fifth rib and through small holes drilled in the sixth rib. These investigators found that patients experienced less pain 2 weeks and 1, 2, and 3 months after thoracotomy when compared with that observed in patients with a standard closure. These results, of course, need to be confirmed.
GENERAL TECHNIQUES
The specific techniques of the various standard pulmonary resections are discussed in their respective chapters. The technique of VATS resection is discussed in detail in Chapters 32 and 33. As an introduction, however, a discussion of the general features of the dissection and management of the bronchi, large vessels, and lung surfaces is appropriate.
Dissection and Control of the Major Arteries
Dissection of a major pulmonary artery is carried out with care. The vessel is thin-walled and is easily injured. Simultaneous traction and countertraction on the vessel wall before the proper plane has been established is to be avoided. The fascial envelope can be retracted as the vessel wall is dissected away from it (Fig. 26-1). Pulling on a branch of the artery is also best avoided because the branch may easily be partially or completely avulsed from the main vessel wall. Both sharp and blunt dissection should be used, and finger mobilization of the posterior aspect of the larger vessels is helpful. In dissecting the main pulmonary artery on the right side, the truncus anterior of the artery may be isolated and divided to obtain greater length of the main-stem vessel. On the left side, the pulmonary artery may be isolated up to, or even proximal to, the ligamentum arteriosum, although one must guard against injury to the recurrent laryngeal nerve as it passes underneath the aortic arch from the front to the back of the aorta at this point.
The ligation of a major artery is accomplished in several ways. If it is long enough, the vessel may be doubly ligated with No. 00-0 or No. 0-0 nonabsorbable suture. The proximal end is then suture ligated between the two ligatures before division of the vessel. A simple transfixation suture is not
P.422
sufficient, but a figure-eight suture through the center of the vessel and tied around it is satisfactory. Peterffy and Henze (1983) recommend the use of a purse-string suture. The vessel is then divided. If the vessel is too short to ligate safely in this manner, the artery may be held with two vascular clamps and divided, and the proximal cut end closed with a continuous No. 4-0 or No. 5-0 nonabsorbable monofilament suture. Some surgeons prefer to treat the pulmonary artery in this manner as a routine procedure. A third method of controlling the vessel is the use of a mechanical stapling device such as a TA-30 instrument using 3.5-mm or V staples. Deslauriers (2002a) suggests the use of the Roticulator vascular stapler for the control of short stumps.
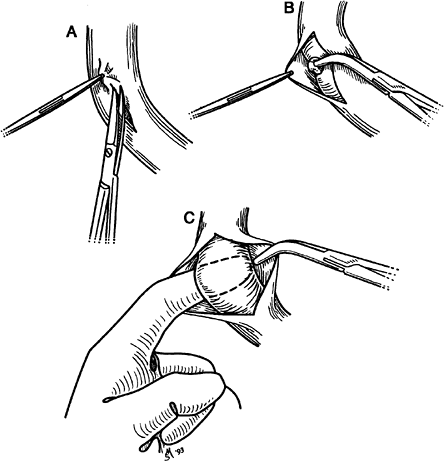 |
Fig. 26-1. Technique of dissection of a major pulmonary artery. A. Fascial envelope is elevated and incised longitudinally. B. Fascial layer is grasped, and the vessel is bluntly dissected from it in the opposite direction. C. After freeing the vessel, the index finger is passed beneath the vessel between the fascial layer and the arterial wall, and a clamp is passed beneath the vessel using the finger as a guide. |
The smaller branches of the artery may be satisfactorily controlled by triple ligation and division between the two most distal ligatures. With moderate-sized vessels, a transfixation suture is appropriate. Tension on any of these ligatures should be avoided because even back-bleeding from the lung side, if a ligature is pulled off, is troublesome.
If injury to the vessel occurs, the bleeding should be controlled initially by pressure with a gauze sponge, guarding against any maneuver that might further tear the vessel. Next, adequate exposure is ensured and proximal, and distal control beyond the injury is obtained; or, when possible, a fine vascular clamp is applied directly to control the injured site. Repair is accomplished with an over-and-over fine vascular suture material.
Dissection and Control of the Major Veins
The major pulmonary veins and their branches are managed in a manner not dissimilar to that described for the pulmonary artery. The walls of the veins are stronger than those of the arteries, and injuries to them are less likely to occur. Occasionally, it is necessary to enter the pericardium to obtain sufficient length of the superior or inferior pulmonary vein. After the vessel is free of the pericardial reflections (see Chapter 5), the vein is usually divided between vascular clamps and closed with a fine continuous vascular suture. Even a portion of the left atrial wall may be included in the excision, and the atrial incision is closed in the standard manner. The pericardial defect, if small, is closed. If the opening is too large to close without compromise of the pericardial space, one of two maneuvers must be done to prevent postoperative herniation of the heart through the pericardial defect, with potential strangulation of the vessels and subsequent cardiac arrest. On the left side, the pericardium is opened down to the diaphragm; on the right side, because this maneuver does not prevent herniation, the cut edges of the pericardium are tacked to the surface of the heart, or the defect is closed with a prosthetic soft tissue patch. Piccione and Faber (1991) suggest closing all large defects, either right or left sided, routinely with a soft tissue patch. Harvey (1995) and Sugarbaker (1997) and their colleagues suggest that fenestration of the soft tissue patch be made to permit egress of fluid out of the pericardial sac to avoid the possibility of a pericardial tamponade.
Ligation of the veins as the initial step in a pneumonectomy for carcinoma has been advocated to lessen the possibility of spilling tumor cells into the circulation. The routine use of this maneuver, however, has not been shown to be beneficial, and the maneuver probably is not important. It has been suggested that initial ligation of the veins leads to overfilling of the vascular bed, resulting in an overdistended lung, which would be difficult to manipulate during the operative procedure, as well as in a loss of an excessive amount of blood when the lung is removed. Miller and associates (1968) showed experimentally, however, that with initial ligation of the veins, reflex shunting of the blood from the lung occurs promptly, and thus distention of the vascular bed does not occur.
Dissection and Closure of the Bronchus
Main-Stem Bronchus
The main-stem bronchus is usually the last hilar structure isolated in a pulmonary resection. However, Grismer and Read (1995) have recommended that the older technique of isolation and division of the bronchus be the first step in controlling the hilar structures in the performance of a pneumonectomy, a right upper lobectomy, or a right apical posterior segmentectomy. Regardless, whether the bronchus is the first or last structure mobilized, the technique of handling the bronchus remains essentially the same. On the right side, the dissection can be carried up to the tracheal carina without difficulty, but care is taken, even during lymph node dissection, not to completely denude the bronchus of its investing adventitial tissue and the contained blood supply. On the left side, the main-stem bronchus should likewise be freed to the tracheal bifurcation, but this effort is more tedious because of its position within the aortic window. The proximal site of division of a main-stem bronchus should be close to the bifurcation, and the line of division should be placed across the bronchus to avoid a blind pocket on its lateral side. Moreover, the residual stump should be as short as possible. As a general rule, a clamp need not be placed proximal to the proposed line of division when a manual suture closure of the stump is to be done. Similarly, it is unnecessary if a stapling device is to be used to secure closure.
Takaro (1987) summarized the use and advantages of the mechanical stapler in bronchial closure. Hood (1985) recommended the TA-35 device with a staple size of 4.8 mm if a mechanical stapler is used. Both authors, among others, believe that the use of the stapler has reduced the incidence of breakdown of the bronchial closure. Peterffy and Calabrese (1979) reported the decreased incidence of a bronchopleural fistula with the use of a stapler versus the use of a chromic catgut suture closure. Such a comparison is not
P.423
germane because few would recommend catgut as the suture material of choice (nonabsorbable suture, such as fine monofilament stainless steel wire suture, and synthetic monofilament suture, such as Vicryl or Proline, are the presently acceptable suture materials for closure of a bronchus). Furthermore, the recently noted decrease in incidence of a bronchial stump breakdown is more likely the result of a different selection of patients undergoing operations. With the use of the standard stapler, however, Vester and associates (1991) reported only a 1.6% incidence of bronchial leak.
In the manual closure of the bronchus, an occluding clamp is placed distal to the line of division to prevent soilage of the operative field from any contained material within the distal bronchial tree. A suture is placed in each lateral side of the bronchus just proximal to the line of excision, and the bronchus is divided, either completely before closure or in sequence to avoid a completely open stump. With either method, the posterior membranous wall is approximated to the anterior cartilaginous wall with interrupted single or mattress No. 00-0 or No. 000-0 sutures of the operator's choice. Before complete closure, the proximal stump and trachea should be aspirated by means of a sterile catheter. After the closure is complete, the stump is tested for any persistent air leaks by covering the stump with a sterile solution and having the anesthesiologist apply or increase inspiratory pressure to that side of the tracheobronchial tree. Areas of leakage are controlled by additional sutures as necessary. Fibrin sealant has been used successfully by many surgeons to seal small leaks of the suture or staple line, as reported by Jessen and Sharma (1985) and Matthew and associates (1990). The technique of application of the glue is discussed in detail by the latter authors. Mouritzen and associates (1993) suggested the use of the sealant routinely on all bronchial closures.
Occasionally, a small tear in the membranous wall of the closed stump is identified. A buttress of adjacent tissue or a pledget of synthetic material should then be incorporated into a mattress suture closure of the area.
Frequently, one or two bronchial arteries need to be ligated after the bronchus has been divided. If bleeding is not controlled, these vessels may serve as a significant source of postoperative blood loss; the bronchial arterial system carries about 1% of the cardiac output.
After closure of the proximal end, the bronchial stump is covered with adjacent tissue, such as a pleural flap, the azygos vein, a pedicle graft of pericardial fat, or adjacent pericardium to provide the stump with a viable tissue cover to help prevent the possible development of a leak from the stump, which normally heals by secondary intention. This cover is particularly important on the right side because no natural coverage for the stump is available. On the left side, the short proximal stump recedes into the depth of the aortic window and is surrounded by the adjacent tissues.
Further precautions to protect the stump to ensure healing are indicated in the patient who has received preoperative neoadjuvant chemotherapy and irradiation; who has a positive sputum containing multidrug-resistant Mycobacterium tuberculosis or, even more so, with the presence of environmental mycobacteria such as Mycobacterium avian complex organisms; who is undergoing a completion pneumonectomy for a recurrent or continuing severe inflammatory process after a previous lesser resection; or in whom a bronchopleural fistula is being closed. In all these situations, the risk for bronchial dehiscence is increased. McGovern and associates (1988) have stressed the increased morbidity and problems associated with the bronchial stump in patients undergoing a completion pneumonectomy for an inflammatory disease. As prophylaxis against bronchial dehiscence, coverage of the stump is recommended using a transplanted muscle flap, as described by Pairolero and Payne (1983) and Fujimoto and colleagues (2001). The muscle flap may be obtained from the latissimus dorsi, the pectoralis major, or the serratus anterior muscles. An intercostal muscle flap also may be used, but one must make sure that all periosteum has been removed because ossification may occur from this tissue at a later date. Recently, Sayeed-Shah and associates (2001) have suggested the use of a muscle flap from the hemidiaphragm to cover this stump (Fig. 26-2). Brown and Pomerantz (1995) have extensively used muscle flaps in patients with multidrug-resistant M. tuberculosis infection who undergo major pulmonary resections. The use of an omental flap is also satisfactory in these situations. On the other hand, a pericardial fat pad, as described by Brewer and colleagues (1953) and Brewer and Bai (1955), also has been used by Anderson and Miller (1995), as well as by Saitoh and associates (1996), to achieve protection of a potentially compromised bronchial stump, but as a rule, these are not as satisfactory as the aforementioned muscle flaps.
Lobar and Segmental Bronchi
The surgical closure of the divided lobar or segmental bronchi entails the same principles and techniques of management as for the main-stem bronchi. At present, bronchial closure with a 3.5- or 4.8-mm stapling device is generally preferred, depending on the compliance of the bronchus.
Suture material includes silk, monofilament, polyglactin, or polypropylene. Fell and Kirby (2000) suggest that the sutures be placed about 3 mm apart and 3 mm from the cut edge. As a general rule, it is unnecessary to cover these bronchial stumps with additional tissue when sufficient pulmonary parenchymal tissue is present, which on inflation surrounds the bronchial stump. If one is unsure that this will occur, simple coverage with a freed pleural flap is sufficient. When multidrug-resistant mycobacterial organisms are present in the sputum or when preoperative neoadjuvant therapy including irradiation has been used, a more secure coverage of a lobar bronchial stump is indicated. A transposed muscular flap, a vascularized pericardial
P.424
flap, or even an omental flap should be added to support the closure.
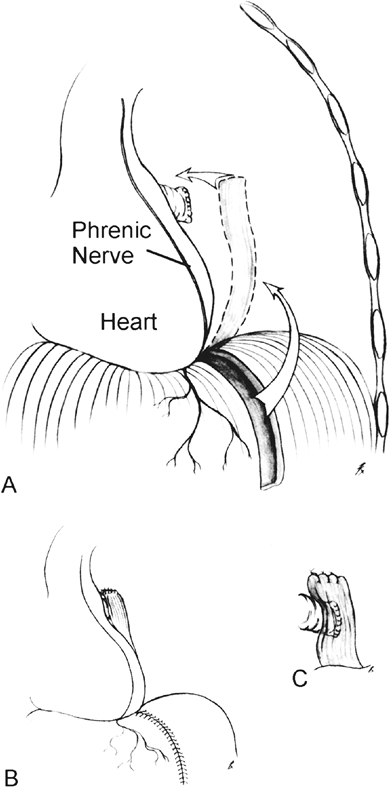 |
Fig. 26-2. Technique of prophylactic diaphragm flap reinforcement for prevention of bronchopleural fistula after pneumonectomy. A. Mobilization of medially based transmural diaphragm flap. B. Closure diaphragm. C. Close-up view of flap over bronchus. From Sayeed-Shah U, Strachan J, Elefteriades JA: Diaphragm flap for routine prophylactic reinforcement of bronchial stump after pneumonectomy [Letter to the editor]. Ann Thorac Surg 71: 2081, 2001. With permission. |
When a residual pleural space or a major air leak is anticipated, one of several maneuvers may be carried out. If an apical space is anticipated (after an upper lobectomy), an apical pleural tent to reduce the pleural space may be constructed as first suggested by Brewer (1956) and Miscall (1956) and their colleagues. Deslauriers (personal communication, 1992) is a strong advocate for its use. Conlan (1990) discussed the technique of constructing the tent and its fate. More recently, Brunelli and associates (2000) have used this procedure to lessen and shorten the period of air leaks. The procedure also has been reported by Robinson and Preksto (1998) and Okur and colleagues (2001). In 2002, Brunelli and associates reported a randomized study of the efficacy and duration of effect in 200 upper lobectomy patients; 100 patients underwent construction of a pleural tent, and 100 did not. The patients with the tent had significant reduction of the mean duration of air leaks (2.5 vs. 7.2 days), number of days a chest tube was required (7.0 vs. 11.2 days), and length of hospital stay (8.2 vs. 11.6 days). Costs were also reduced.
In the absence of sufficient or useful apical parietal pleura, a muscle transplant (latissimus dorsi, pectoralis major flap) or other chest wall muscle flap may be used to reduce the size of the pleural space. The method is described in Chapters 59 and 88.
A third method to reduce the size of the hemithorax, especially when a lower lobe or a middle and lower lobe bilobectomy has been done, is the institution of a pneumoperitoneum to elevate the hemidiaphragmatic leaf. Martini and Ginsberg (2002) suggest this either be done at the time of the operation or later postoperatively. Deslauriers (2002b) suggests that the pneumoperitoneum is best established at the completion of the procedure by a small catheter passed through the hemidiaphragm. The phrenic nerve can be paralyzed by injection of a local anesthetic in the tissue covering the nerve to permit further temporary elevation of the diaphragm. However, the nerve should not be clamped or cut to achieve elevation of the diaphragmatic leaf.
Adjunctive or concomitant tailoring thoracoplasty has also been suggested to reduce the size of the pleural spaces, but its use has been abandoned because of the deranged chest wall physiology postoperatively. Conlan (1990) suggested the use of an osteoplastic thoracoplasty to prevent the chest wall instability. Talamonti and colleagues (1989) favored a modified plombage thoracoplasty using an inflatable prosthesis to fill the space. Most surgeons, as noted, however, recommend a muscle transplant to fill a potentially hazardous residual pleural space.
Lesser Bronchi
The small subsegmental branches of the bronchial tree need only be ligated to obtain adequate and satisfactory closure of the stump. It is important to stress that when an incomplete fissure has been dissected or a segmentectomy has been done, small bronchial openings should be sought carefully and ligated to prevent postoperative air leak.
Raw Surface of the Lung
Parenchymal raw surfaces that are present after a resection should be thoroughly inspected, and significant bleeding should be controlled. A moist sponge is applied to the
P.425
surface, and the lung is expanded. After 5 to 10 minutes, the sponge is removed, and the lung surface is reinspected. If the dissection of the intersegmental plane has been done carefully, only small alveolar air leaks will be present. These tend to seal over promptly with reexpansion of the lung during the postoperative period. Any leakage from small bronchi, however, must be recognized and controlled; otherwise, the leak will persist and predispose to serious postoperative difficulties. Jensik (1986) advocated covering the raw surfaces with pleural flaps or reconstituting the lung by bringing the adjacent segments together. Such a step is generally unnecessary and may even lead to increased postoperative problems.
Matthew and associates (1990) suggested the use of fibrin glue to control air leaks and bleeding from parenchymal raw surfaces, but no data were given as to its efficacy in this particular situation. In a study by Mouritzen and colleagues (1993), the use of fibrin glue applied to the raw lung surfaces reduced the incidence and the persistence of postoperative air leakage, as compared with a control group in which the glue was not applied. Wong and Goldstraw (1997), however, reported that the use of fibrin glue was not helpful in controlling moderate to severe alveolar air leaks after pulmonary resections.
When a stapler has been used for carrying out a wedge resection in a patient with essentially normal pulmonary parenchyma, a troublesome air leak is infrequent. If it occurs, the use of fibrin glue is a good solution. However, with the advent of lung volume reduction surgery in patients with emphysematous lungs by multiple nonanatomic stapled wedge excisions, serious persistent air leaks have occurred (see Chapter 85). With either unilateral or bilateral procedures, an incidence of prolonged air leaks of 30% to more than 50% has been noted by Naunheim (1996), Cooper (1995), and Miller (1996) and their associates as well as many others. In an effort to reduce this high incidence of air leaks, Cooper (1994) and Cooper and colleagues (1995) suggested the use of bovine pericardial strips placed on the stapling device to buttress the staple lines and thus reduce the incidence of air leaks. Although this technique has been less effective in bilateral than in unilateral procedures, Hazelrigg and colleagues (1997), in a controlled randomized study, found the use of bovine buttressing strips to be more effective in controlling and reducing the duration of air leaks than not using the strips. Disappointingly, Miller and coinvestigators (2001), in a randomized prospective trial in 80 patients (40 buttressed staple lines and 40 nonbuttressed staple lines), showed no statistical differences between the groups, although there was an increased leak duration in the control group. Vaughn and associates (1997) suggested the use of polytetrafluoroethylene (PTFE) sleeves as the buttressing material. Nomori and Horio (1997) have suggested the use of a gelatin-resorcinol-formaldehyde-glutaraldehyde glue spread stapler, and Horsley and Miller (1997) have used cyanoacrylate glue with a pericardial patch to control these air leaks. Each of the aforementioned techniques must be evaluated by additional studies to confirm its usefulness.
MANAGEMENT OF THE PLEURAL SPACE
The management of the pleural space is fundamentally different after a pneumonectomy than after a lobectomy or a lesser resectional procedure. After a pneumonectomy, the major concern is to have the space slowly obliterated by the subsequent anatomic changes in the position of the heart, mediastinal structures, the diaphragm, the contraction of the intercostal spaces, and the accumulation of fluid, without the occurrence of infection. After a lobectomy or a lesser resection, reexpansion of the remaining lung tissue to obliterate the pleural space without any major fluid collections is the desired clinical goal.
Postpneumonectomy Pleural Space
After removal of the specimen, the pleural space is irrigated, and the chest wall is inspected for any sites of continued bleeding. When present, these are controlled with cautery or suture ligation as necessary. Special care is required in the event of persistent bleeding from an intercostal vessel or continued oozing at the posterior angle of the intercostal incision (see Chapter 37).
It is of major importance that the pleural space be dry (absence of continued bleeding) because the development of an acute massive hemothorax that necessitates reexploration is associated with a higher mortality rate and an increased incidence of a bronchopleural fistula, as noted by Peterffy and Henze (1983). After control of any bleeding sites, the pleural space is irrigated once again. Many surgeons instill a broad-spectrum antibiotic in a small amount of sterile solution into the space just before closure. No prospective data support this practice, but its use is reassuring in that the fluid that accumulates within the space is an excellent culture medium.
The postpneumonectomy pleural space usually is closed without drainage. If the development of an infection within the space is likely, a thoracotomy tube is placed into the space along the chest wall just above the hemidiaphragm. This is connected to an underwater-seal drainage system. However, the drainage tube is clamped and opened only periodically to drain the accumulated fluid. Some surgeons (Pellet, personal communication, 1992) routinely drain the space for 24 hours to prevent too rapid accumulation of fluid in the space and to enable the detection of excessive postoperative bleeding if it occurs. Balanced pleural drainage to maintain the mediastinum in a normal midline position, as suggested by Laforet and Boyd (1964), is not recommended. If drainage is used at any time, the negative pressure should not exceed 2 to 4 cm H2O.
After closure of the incision, the pressure within the space is adjusted as necessary to approximate a negative
P.426
pressure of 2 to 4 cm H2O on inspiration and a positive pressure of 2 to 4 cm H2O on exhalation. Adjustment may be made simply by thoracentesis and removal of air until the trachea is in the midline at the sternal notch, or the actual pressures may be measured using a manometer.
Some authors advocate daily adjustment of the intrapleural pressure within the pneumonectomy space for 4 to 5 days after the pneumonectomy. When this is done, antibiotics may be placed within the cavity. Others, including myself, have found this procedure unnecessary and meddlesome, preferring to check the pressure only if clinical signs indicate its need.
Fate of the Pleural Space
After pneumonectomy, elevation of the ipsilateral leaf of the diaphragm, shift of the mediastinum toward the operated side, and narrowing of the intercostal spaces of the ipsilateral side occur. In addition, serosanguineous fluid accumulates in the empty pleural space to fill the residual volume. The rate of accumulation of the fluid and the complete absorption of the air from the space are variable. Generally, the process is completed within 3 to 4 weeks, but it may take as long as 7 months (Fig. 26-3).
In the past, the phrenic nerve on the side of the pneumonectomy was crushed to obtain a more prompt and higher elevation of the diaphragmatic leaf to reduce the residual volume of the postpneumonectomy space. The resultant paralysis of the ipsilateral leaf of the diaphragm, however, permits paradoxical movement of this portion of the thoracic cage. Although this effect is of no real consequence during normal breathing, the paradoxical movement of this paralyzed leaf does interfere with the efficacy of the cough mechanism. Thus, it is not recommended as a routine procedure.
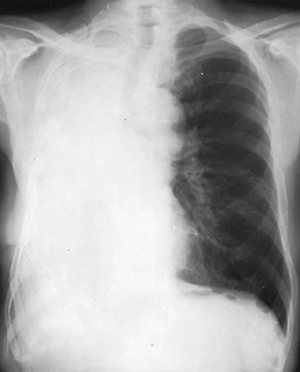 |
Fig. 26-3. Chest radiograph 2 weeks after right pneumonectomy. |
A thoracoplasty often was performed postoperatively to obliterate the residual pleural space, thereby preventing the overdistention of the remaining contralateral lung as well as possibly reducing the incidence of infection of the space. Overdistention of the contralateral lung, however, is in itself not detrimental to lung function, and a standard thoracoplasty does adversely affect the function of the contralateral lung. Gaensler and Strieder (1951) showed a loss of about 25% to 30% of the preoperative vital capacity, and about 20% of the maximum voluntary ventilation in the contralateral lung after a standard thoracoplasty was performed over a nonfunctioning lung. A plombage type of thoracoplasty is followed by less functional loss, but the foreign body frequently becomes associated with infection; consequently, its use is not advised.
The fluid within the pleural space is gradually absorbed so that only a potential space remains. As absorption takes place, the heart and mediastinum shift farther toward the ipsilateral side, and the remaining contralateral lung herniates anteriorly and partially into the postpneumonectomy space to fill this residual thoracic volume (Fig. 26-4). Spirn and colleagues (1988) noted that this anterior herniation occurs after either a left or right pneumonectomy to a variable degree in all cases. Postero-prevertebral lung herniation occurs in about 50% of patients after a left pneumonectomy; its occurrence is not observed after a right pneumonectomy.
Complete absorption of the fluid is uncommon. Suarez and colleagues (1969) found that complete absorption occurred in only 10 of 37 patients who died at varying time intervals after pneumonectomy. In the other 27 individuals, variable amounts of air or fluid remained in simple or loculated spaces. This early observation, that in only one third of postpneumonectomy patients does the space become
P.427
obliterated completely, was confirmed by the computed tomographic (CT) evaluation of the postpneumonectomy space by Biondetti and associates (1982). In the latter study, two thirds of the postpneumonectomy spaces contained a unilocular fluid-filled space of varying size surrounded by thick fibrous margins.
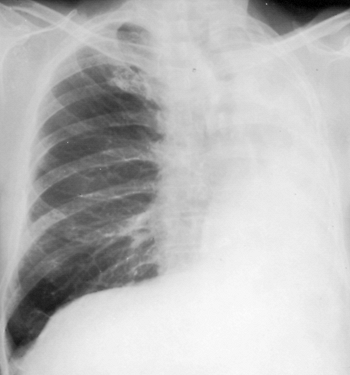 |
Fig. 26-4. Chest radiograph 3 years after left pneumonectomy. |
Postlobectomy Pleural Space
After lobectomy, the pleural space is drained routinely. Most surgeons use two tubes, one inferiorly lying above the diaphragm and passing to the paravertebral area and one placed anteriorly and passing upward into the apex of the chest. However, some, such as Deslauriers (2002b), advocate the use of only one chest drainage tube, whereas others, including Martini and Ginsberg (2002), use three drainage tubes in some patients after a lobectomy that required extensive pleural dissection. The tubes are connected to an underwater-seal drainage system, and as a rule, negative suction of -20cm H2O is applied to the system.
Recently, Alex and colleagues (2003) reported the comparison of the postoperative outcome of using the standard two drains versus the use of a single drain after a lobectomy. In their prospective study, little difference in outcome (i.e., amount of drainage, duration of drainage, and sequela after removal of the tubes) was noted, except for a significantly lower pain score in the patients with a single drainage tube placed in the midportion of he hemithorax and a significantly lower cost in this latter group.
Air leaks are a common problem after lobectomy, especially when incomplete fissures have been divided during the procedure. Most cease after reexpansion of the lung, but some are persistent. Cerfolio and colleagues (1998, 2001) have studied this problem extensively and provided a qualitative and quantitative classification of postoperative air leaks. In a randomized trial, simple water-seal drainage was found to be more effective than the use of suction in stopping an expiratory or forced expiratory air leak, with the exception of the uncommon large air leaks. The randomized study of Marshall and co-workers (2002) at the University of Pennsylvania regarding water seal only versus postoperative suction agrees with the aforementioned conclusion. According to Cerfolio and co-workers (2002), when there is a persistent air leak when the patient is otherwise ready to be discharged, the chest tubes may be attached to a Heimlich valve. Most leaks stop within 2 weeks; even if not, the chest tubes may be removed without adverse effects, as originally reported by Kirschner (1992).
The application of a tissue glue to the raw surface of the lung has also been suggested to reduce the incidence and duration of the air leaks. Fibrin glue has been the most widely used agent to seal alveolar openings and thus prevent the air leaks. However, randomized trials conducted by Fleisher (1990) and Wurtz (1992) and their colleagues found no differences in the occurrence and fate of alveolar air leaks between the treated and control groups. However, Fabian and colleagues (2003) reported the use of pressurized, aerosolized spraying of 5 mL of fibrin glue onto the raw lung surface in a prospective, randomized, blinded trial in 100 patients. The patients who received the aerosolized fibrin glue had significant reductions in incidence (34% vs. 68%), mean duration (1.1 vs. 3.1 days), and mean time of removal of the chest tube (3.5 vs. 5 days) and in the incidence of prolonged alveolar air leaks (2% vs. 16%) than in the control group, respectively. Additional studies are required to confirm these results.
Recently, Porte and associates (2001) reported a randomized, controlled trial using a synthetic sealant (Advanced, Johnson & Johnson, Irvine, CA), in which the control of the air leaks was significantly better in the treated than in the control group. Unfortunately, the use of the synthetic sealant was associated with an increase in postoperative empyema; 4 of 62 patients (6.5%) developed localized empyema and incomplete lung expansion. Thus, this product cannot be recommended for use. Miyamoto and co-workers (2003) reported the use of bioabsorbable polyglycolide felt patches soaked in fibrin glue to cover the leakage site. The leaks were controlled in 86.7% of 60 patients. However, 4 of the 8 patients who continued to have air leaks developed a pyothorax, an incidence of 6.6%, similar to that in the aforementioned study. Again, one must caution against the use of this method.
Postsegmentectomy Pleural Space
The pleural space after a segmentectomy or other lesser resectional procedure should be managed in a fashion similar to that described for the postlobectomy space. Air leaks for a greater or lesser period of time are the major problem in these patients.
Management after Minimal Resections
In most situations, even if minimal or no pulmonary tissue is resected, it is best to drain the pleural space as is done for a lobectomy or a segmentectomy. An exception may be the patient who has had only a lung biopsy or in whom the pleural space was entered by a limited anterior or occasionally a small axillary thoracotomy. In these instances, simple aspiration of the pleural space by use of a small catheter just before complete closure of the incision is all that is necessary. On the other hand, in patients with a standard posterolateral thoracotomy in whom only an exploratory procedure was performed, adequate postoperative thoracostomy tube drainage is indicated; usually, only one lower thoracostomy tube is necessary. Significant amounts of fluid may collect, necessitating subsequent thoracentesis if proper drainage has not been effected.
P.428
ANTIBIOTIC PROPHYLAXIS IN LUNG RESECTION
Intrapleural Antibiotic
Intrapleural antibiotic after resection is generally not recommended. Complete hemostasis and pleural irrigation are essential. At times, a broad-spectrum antibiotic is instilled into the postpneumonectomy space, but proof that this is of value is lacking.
Systemic Antibiotic
Olak and colleagues (1991) conducted a randomized trial of one-dose versus six-dose cefazolin prophylaxis in elective general thoracic surgery and found no difference in the incidence of infectious complications. However, Bernard and associates (1994), in a double-blind trial of two doses of 1.5 g of cefuroxime (one at the time of induction of anesthesia and the second 2 hours later) versus the same regimen, followed by a 1.5-g dose of the drug every 6 hours after the second dose for a total of 48 hours, found that the latter regimen resulted in fewer cases of postoperative empyema and pneumonia than did the former. Elia (1998) and Turna (2003) and their associates recorded their experience with prophylactic use of cephalosporin and cefuroxime, respectively, but the data were insufficient to draw any meaningful conclusion as to the efficacy of either of these drugs.
PHYSIOLOGIC EFFECTS
Ventilatory Changes
Postpneumonectomy
Early after a pneumonectomy, the ventilation of the remaining lung may be improved by a compensatory increase in the depth and rate of breathing. The lung becomes stiffer, however, and the elastic recoil pressure at total lung capacity increases. As a result, the work of breathing is increased. Diffusion capacity (DLCO) is also decreased. As the remaining lung adjusts to the changes in the thoracic volume available to it, the lung becomes hyperinflated. The result is an increase of 10% to 30% in its vital and total capacities.
Van Mieghem and Demedts (1989) found an overall 35% to 40% reduction of the preoperative forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and Dlco after pneumonectomy. These observations are similar to those of Ladurie and Ranson-Bitker (1985), who found that the vital capacity was more than 50% of the predicted value 5 years after pneumonectomy in 98 patients.
In children, the late functional loss after pneumonectomy is less than that observed in adults. Gas exchange is normal when the child is at rest. The total lung capacity and vital capacity are increased well above the values predicted for one lung, and the maximum voluntary ventilation (maximum breathing capacity) is generally normal. Lezama-del Valle (1999) and Eren (2003) and their colleagues, as well as Laros and Westermann (1987), have noted that the function test results, according to the functional volume of the remaining lung, were considered good, although a small degree of respiratory dysfunction was present in many cases.
Diffusion capacity is normal if the pneumonectomy is performed before puberty; this is probably the result of growth of the remaining lung in the young child. Cagle and Thurlbeck (1988) presented a thorough review of the experimental and clinical observations relative to postpneumonectomy compensatory lung growth. Many facets of the mechanisms stimulating this occurrence remain to be elicited, but stretch of the remaining lung is thought to be the initial stimulus for the compensatory growth of the remaining lung tissue. If pneumonectomy is performed after puberty, reduction of the diffusion capacity occurs, as is noted in the adult. After pneumonectomy in children, pulmonary hypertension is not a significant development.
Postlobectomy
With resection of a lobe of the lung, a part of the total alveolar, bronchial, and vascular masses is removed. Overinflation of the contralateral, as well as of the remaining ipsilateral lung tissue, results.
The remaining lung parenchyma is subjected to increased perfusion, despite an absolute reduction in the diffusion surface. The ratio of the dead space to the total lung volume increases, but a decrease of the dead space with respect to the tidal volume occurs. As a result, ventilatory efficiency is actually improved.
Ali and associates (1980) reported an early disproportionate functional loss after lobectomy that was greater than the predicted loss. They reported a mean fall of about 30% in the FVC and FEV1 when only a 25% reduction was predicted. Markos and colleagues (1989), however, did not observe this disproportionate loss; in fact, the observed mean losses in FVC and FEV1 were less than predicted. The reasons for these discrepancies are unresolved. Van Mieghem and Demedts (1989) reported only a 15% decrease in the FVC after lobectomy. Berend and associates (1980) noted similar findings (decreases of 12% and 10%, respectively, in the total lung capacity and vital capacity) after lobectomy. They observed only a slight reduction of the FEV1 and no change in the Dlco.
The loss may vary in the individual patient, however, and is influenced by the degree of functional loss present preoperatively and the presence or absence of postoperative complications. The occurrence of hemorrhage, effusion, air leak, empyema, fibrothorax, or bronchopleural fistula exerts a serious adverse effect on postoperative pulmonary function.
P.429
Werner and associates (1993) reported that late endurance testing and lung volumes in children and adolescents who had undergone lobectomy in infancy confirmed lung growth as well as lung distention. Good functional recovery was observed, but changes in regional ventilation and perfusion suggested dysplastic parenchyma and vascular bed in the lung that occupied the area of the previous resection.
Postsegmentectomy and Lesser Procedures
The physiologic changes after a segmentectomy are the same as those noted after a lobectomy. The late functional loss is related to the number of segments removed as well as to the occurrence of postoperative complications. The functional gain by the preservation of a segment of lobe is generally less than expected from its volume. The parenchymal tissue saved, however, may play a valuable role in helping to fill the pleural space. Unfortunately, even this is relative because the incidence of postoperative complications occasionally is greater after segmentectomy than after a lobectomy. In the prospective study of the North American Lung Cancer Group reported by Ginsberg and Rubinstein (1995), those patients who underwent a segmentectomy or a lesser resection exhibited an initial ventilating functional advantage over those who underwent a lobectomy. This functional advantage was lost, however, after the first year of observation.
The physiologic changes subsequent to a wedge resection in the absence of postoperative pleural complications are minimal. Those seen are more directly related to the thoracotomy incision than to the removal of the small part of lung. Early in the postoperative period, the lung volume is restricted. Inspiratory capacity and the expiratory reserve volume are decreased. The end-expiratory position is depressed as the result of pain in the chest wall. Alveolar hypoventilation with carbon dioxide retention and some degree of respiratory acidosis occurs. Oxyhemoglobin desaturation occurs and is greatest on the second and third postoperative days; it may persist for as long as 10 days. Compliance is reduced, resulting in an increased work of breathing. This reduction is most remarkable the first few hours after the operation and returns gradually to near normal within the first postoperative week.
Lung Volume Reduction Surgery
The functional changes seen after lung volume reduction surgery (LVRS) depend greatly on the selection of the patients for the procedure. These data are discussed in detail in Chapter 85 and are not discussed in the current presentation.
Lung Growth
Growth of mature lung has been observed following both lung injury and pulmonary resection. According to Adamson and coinvestigators (1988), type II cell proliferation is the major step in the structural and functional restoration after lung epithelial injury. Lung resection initiates compensatory growth that is intended to restore the lung volume, compliance, and mass of the remaining lung tissue. Rannels and Rannels (1988) note that restoration of whole lung levels of protein, RNA, DNA, collagen, and elastin occurs. However, the nature and extent of growth remain elusive. Using a rodent model, Kaza and collaborators (2000) have suggested that epidermal growth factor (EGF) may have a significant role in the postresection lung growth. These aforementioned authors postulate that this process may be mediated by an upregulation of growth factor receptor expression in the remaining lung tissue. In a subsequent experimental study in swine, Kaza and associates (2002) recorded that after a lobectomy, the EGF receptor expression was upregulated relative to that in normal control lobes at 2 weeks; there was also a correlation with upregulation of cell proliferation at this same time. In transplanted lobes, these features were not observed until the third month. The reasons for these differences remain unknown.
Hemodynamic Changes
After a pneumonectomy, the pulmonary artery pressure is usually normal at rest. As noted by Van Mieghem and Demedts (1989), however, maximum effort tolerance decreases after pneumonectomy, and an increase in both the pulmonary artery pressure and pulmonary vascular resistance occurs with effort. Cardiac output and stroke volume decrease during effort. These changes are accompanied by an increase in the peripheral arterial blood pressure as well as in the peripheral vascular resistance. Oxygen saturation decreases on effort, possibly because of an absolute decrease in the Dlco. After a lobectomy, similar hemodynamic changes are seen, but to a lesser degree.
Van Mieghem and Demedts (1989) suggest that the cardiovascular changes can be explained by the hypothesis that the removal of a substantial part of the vascular bed may result in an increase in the afterload of the right ventricle, which may interfere with the emptying of this ventricle. The increased afterload increases both the end-systolic and end-diastolic volumes of the right ventricle, which may in turn cause a shift of the interventricular septum to the left, with resultant changes that result in a decreased cardiac output.
The function of the right ventricle after pulmonary resection has been assessed by thermodilution methods and by right heart pressure studies by both Reed (1992) and Okada (1994) and their associates. From these studies, it was documented that the right ventricular ejection fraction decreases, the right ventricular end-diastolic volume index increases, and the right ventricular stroke volume index decreases as of the first day postoperatively, and this continues for a variable length of time postoperatively.
P.430
At rest, the pulmonary artery pressure, pulmonary vascular resistance index, central venous pressure, and left ventricular function remain unaffected. However, with exercise, the pulmonary artery pressure and the pulmonary vascular resistance index increase with further change in the right ventricular afterload. Okada and associates (1994) believe that at rest, the changes noted in the right ventricular function compensate for the increase in right ventricular volume but cannot with exercise. Furthermore, they suggest that the changes in the right ventricular afterload may be the major cause of right ventricular dysfunction after either a lobectomy or a pneumonectomy. Natriuretic peptides are vasodilator hormones involved in the regulation of blood pressure and volume hemostasis. A-type natriuretic peptide (ANP) is released from the right auricle in response to right atrial stretch or pulmonary artery vasoconstriction and dilates pulmonary artery smooth muscle. B-type natriuretic peptide (BNA) affects the peripheral and central vessels similarly to ANP and is secreted from the cardiac ventricles. Both substances have vasodilatory activity, and in an experimental study in beagle dogs by Tayama and coinvestigators (1998), pneumonectomy resulted in a significant rise in APN levels in the plasma and the remaining contralateral lung and was accompanied by an elevation of the pulmonary artery pressure. Recently, the aforementioned authors [Tayama and colleagues (2002)] recorded the postresection hemodynamic changes and determined the blood (systemic) levels of ANP and BNP by radioimmunoassay in 15 postlobectomy and 10 postpneumonectomy patients. Both the mean pulmonary artery pressure and total pulmonary vascular resistance increased significantly in the pneumonectomy group. The total pulmonary vascular resistance on postoperative day 3 correlated with the plasma BPN concentration in the pneumonectomy group. Overall, the pneumonectomy group exhibited higher concentrations of ANP and BNP than the group undergoing a lobectomy on days 3 and 7. These authors concluded that ANP and BNP effectively compensate for the right ventricular dysfunction noted after pulmonary resection, and this activity is more evident after pneumonectomy than after lobectomy. They also suggest that changes in ventricular activity associated with changes in plasma BNP and total pulmonary vascular resistance are indicative of cardiopulmonary adjustments after pneumonectomy. Whether there is a shift of the interventricular septum to the left with resultant deleterious effect on left ventricular function, as suggested by Van Mieghem and Demedts (1989), remains unresolved.
Of clinical interest is that, according to Ladurie and Ranson-Bitker (1985), sinoauricular tachycardia is present in more than 75% of the late survivors of a pneumonectomy; in one fourth of the patients, more than 100 beats per minute were recorded. A functional, persistent systolic murmur at the right heart base was observed in 12% of the patients. Right heart overload, determined electrocardiographically, was present in 6% of the patients in their study.
The older the patient at the time of pneumonectomy or the greater the degree of preexistent chronic obstructive airway disease in the remaining lung, the greater the likelihood of functional incapacity. In evaluating the functional capacity, a direct relationship to the pulmonary artery pressure apparently exists; as the functional reserve decreases, the pulmonary artery pressure increases. The reduction in the functional capacity appears to be related more directly to the pulmonary artery pressure and pulmonary blood flow relationships in the remaining lung than to arterial saturation per se. The functional capacity appears to be governed and limited by the expandability of the remaining vascular bed. When the limit of the bed is reached or exceeded, persistent pulmonary hypertension occurs, and cor pulmonale results.
REFERENCES
Adamson IY, Young L, Bowden DH: Relationship of alveolar epithelial injury and repair to the induction of pulmonary fibrosis. Am J Pathol 130:377, 1988.
Alex J, et al: Comparison of the immediate postoperative outcome of using the conventional two drains versus a single drain after lobectomy. Ann Thorac Surg 76:1046, 2003.
Ali MK, et al: Predicting loss of pulmonary function after pulmonary resection for bronchogenic carcinoma. Chest 77:337, 1980.
Anderson TM, Miller JI Jr: Surgical technique and application of pericardial fat pad and pericardiophrenic grafts. Ann Thorac Surg 59:1590, 1995.
Berend N, Woolcock AJ, Marlin GE: Effects of lobectomy on lung function. Thorax 35:145, 1980.
Bernard A, et al: Antibiotic prophylaxis in pulmonary surgery. A prospective randomized double-blind trial of flash cefuroxime versus forty-eight-hour cefuroxime. J Thorac Cardiovasc Surg 107:896, 1994.
Biondetti PR, et al: Evaluation of post-pneumonectomy space by computed tomography. J Comput Assist Tomogr 6:238, 1982.
Brewer LA III, Bai AF: Surgery of the bronchi and trachea. Experience with the pedicled pericardial fat graft reinforcement. Am J Surg 89:331, 1955.
Brewer LA, Bai AF, Jones WM: The development of the pleural partition to prevent overexpansion of the lung following partial pulmonary resection. J Thorac Surg 31:165, 1956.
Brewer LA III, et al: Bronchial closure in pulmonary resection. A clinical and experimental study using a pedicled pericardial fat graft reinforcement. J Thorac Surg 26:507, 1953.
Brown J, Pomerantz M: Extrapleural pneumonectomy for tuberculosis. Chest Surg Clin N Am 5:289, 1995.
Brunelli A, et al: Pleural tent after upper lobectomy: a prospective randomized trial. Ann Thorac Surg 69:1722, 2000.
Brunelli A, et al: Pleural tent after upper lobectomy: a randomized study of efficacy and duration of effect. Ann Thorac Surg 74:1958, 2002.
Cagle PT, Thurlbeck WM: Postpneumonectomy compensatory lung growth. Am Rev Respir Dis 138:1314, 1988.
Cerfolio RJ, Bass C, Katholi CR: Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 71:1613, 2001.
Cerfolio RJ, et al: A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg 66:1726, 1998.
Cerfolio RJ, et al: Predictors and treatment of persistent air leaks. Ann Thorac Surg 73:1727, 2002.
Cerfolio RJ, et al: Intracostal sutures decrease the pain of thoracotomy. Ann Thorac Surg 76:407, 2003.
P.431
Conlan AA: Prophylaxis and management of postlobectomy infected spaces. In Deslauriers J, Lacquet LK (eds): Thoracic Surgery: Surgical Management of Pleural Diseases. St. Louis: CV Mosby, 1990, p. 279.
Cooper JD: Technique to reduce air leaks after resection of emphysematous lung. Ann Thorac Surg 57:1038, 1994.
Cooper JD, et al: Bilateral pneumonectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 109:106, 1995.
Deslauriers J: Discussion of Waters PF: Pneumonectomy. In Pearson FG, et al (eds): Thoracic Surgery. New York: Churchill Livingstone, 2002a, p. 974.
Deslauriers J: Discussion of Martini N, Ginsberg RJ: Lobectomy. In Pearson FG, et al (eds): Thoracic Surgery. New York: Churchill Livingstone, 2002b, p. 981.
Elia S, et al: Preoperative antimicrobial prophylaxis with a long-acting cephalosporin for thoracic surgery in 192 non small cell lung cancer patients. J Chemother 10:58, 1998.
Eren S, Eren MN, Balci AE: Pneumonectomy in children for destroyed lung and the long-term consequences. J Thorac Cardiovasc Surg 126: 574, 2003.
Fabian T, Federico JA, Ponn RB: Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg 75:1587, 2003.
Fell SC, Kirby TJ: Technical aspects of lobectomy. In Shields TW, LoCicero J III, Ponn RB (eds): General Thoracic Surgery. 5th Ed. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 385.
Fleisher AG, et al: Effect of routine fibrin glue use on the duration of air leaks after lobectomy. Ann Thorac Surg 49:133, 1990.
Fujimoto T, et al: Completion pneumonectomy: current indications, complications, and results. J Thorac Cardiovasc Surg 121:484, 2001.
Gaensler EA, Strieder JW: Progressive changes in pulmonary function after pneumonectomy. J Thorac Surg 22:1, 1951.
Ginsberg RJ, Rubinstein L: Randomized trial of lobectomy versus limited resection for T1N0 non small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60:615, 1995.
Grismer JT, Read RC: Evolution of pulmonary resection techniques and review of the bronchus-first method. Ann Thorac Surg 60:1133, 1995.
Harvey JC, Erdman C, Beattie EJ: Pneumonectomy. Chest Surg Clin N Am 5:253, 1995.
Hazelrigg SR, et al: Effect of bovine pericardial strips on air leak after stapled pulmonary resection. Ann Thorac Surg 63:1573, 1997.
Hood RM: Operations involving the lungs. In Hood RM (ed): Techniques in General Thoracic Surgery. Philadelphia: WB Saunders, 1985.
Horsley WS, Miller JI Jr: Management of the uncontrollable pulmonary air leak with cyanoacrylate glue. Ann Thorac Surg 63:1492, 1997.
Jessen C, Sharma P: Use of fibrin glue in thoracic surgery. Ann Thorac Surg 39:521, 1985.
Jensik RJ: The extent of resection for localized lung cancer: Segmental resection. In Kittle CF (ed): Current Controversies in Thoracic Surgery. Philadelphia, WB Saunders, 1986.
Kaza AK, et al: Epidermal growth factors augments postpneumonectomy lung growth. J Thorac Cardiovasc Surg 120:916, 2000.
Kaza AK, et al: Contrasting natures of lung growth after transplantation and lobectomy. J Thorac Cardiovasc Surg 123:288, 2002.
Kirschner PA: Provocative clamping and removal of chest tubes despite persistent air leak [Letter to the editor]. Ann Thorac Surg 53:740, 1992.
Ladurie ML, Ranson-Bitker B: Quality of life following resection for lung cancer. In Delarue NC, Eschapasse H (eds): Lung Cancer. International Trends in General Thoracic Surgery. Vol. 1. Philadelphia: WB Saunders, 1985, p. 296.
Laforet EG, Boyd TF: Balanced drainage of the pneumonectomy space. Surg Gynecol Obstet 118:1051, 1964.
Laros CD, Westermann CJ: Dilatation, compensatory growth, or both after pneumonectomy during childhood and adolescence. A thirty-year follow-up study. J Thorac Cardiovasc Surg 93:570, 1987.
Lezama-del Valle P, Blakely MI, Lobe TE: Physiologic consequences of pneumonectomy. Long-term consequences of pneumonectomy done in children. Chest Surg Clin N Am 9:485, 1999.
Markos J, et al: Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 139:902, 1989.
Marshall MB, et al: Suction vs water seal after pulmonary resection: a randomized prospective study. Chest 121:835, 2002.
Martini N, Ginsberg RJ: Lobectomy. In Pearson FG, et al (eds): Thoracic Surgery. New York: Churchill Livingstone, 2002, p. 981.
Matthew TL, et al: Four years' experience with fibrin sealant in thoracic and cardiovascular surgery. Ann Thorac Surg 50:40, 1990.
McGovern EM, et al: Completion pneumonectomy: indications, complications, and results. Ann Thorac Surg 46:141, 1988.
Miller GE, Aberg THJ, Gerbode F: Effect of pulmonary vein ligation on pulmonary artery flow in dogs. J Thorac Cardiovasc Surg 55:668, 1968.
Miller JI Jr, Lee RB, Mansour KA: Lung volume reduction surgery: lessons learned. Ann Thorac Surg 61:1464, 1996.
Miller JI Jr, et al: A comparative study of buttressed versus nonbuttressed staple line in pulmonary resections. Ann Thorac Surg 71:319, 2001.
Miscall LD, Duffy RW, Nolan RB: The pleural tent as a simultaneous tailoring procedure in combination with pulmonary resection. Am Rev Respir Dis 73:831, 1956.
Miyamoto H, et al: Fibrin glue and bioabsorbable felt patch for intraoperative intractable air leaks. Jpn J Thorac Cardiovasc Surg 51:232, 2003.
Mouritzen C, Dromer M, Keinecke HO: The effect of fibrin glueing to seal bronchial and alveolar leakages after pulmonary resections and decortications. Eur J Cardiothorac Surg 7:75, 1993.
Naunheim KS, et al: Unilateral video assisted thoracoscopic surgical lung reduction. Ann Thorac Surg 61:1092, 1996.
Nomori H, Horio H: Gelatin-resorcinol-formaldehyde-glutaraldehyde glue spread stapler prevents air leakage from the lung. Ann Thorac Surg 63:352, 1997.
Okada M, et al: Right ventricular dysfunction after major pulmonary surgery. J Thorac Cardiovasc Surg 108:503, 1994.
Okur E, et al: Pleural tenting following upper lobectomies or bilobectomies of the lung to prevent residual air space and prolonged air leak. Eur J Cardiothorac Surg 20:1012, 2001.
Olak J, et al: Randomized trial of one dose versus six dose cefazolin prophylaxis in elective general thoracic surgery. Ann Thorac Surg 51:956, 1991.
Pairolero PC, Payne WS: Postoperative care and complications in the thoracic surgical patient. In Glenn WWL, et al (eds): Thoracic and Cardiovascular Surgery. 4th Ed. Norwalk, CT: Appleton-Century-Crofts, 1983, p. 338.
Peterffy A, Calabrese E: Mechanical and conventional manual sutures of the bronchial stump. A comparative study of 298 surgical patients. Scand J Thorac Cardiovasc Surg 13:87, 1979.
Peterffy A, Henze A: Haemorrhagic complications during pulmonary resection. A retrospective review of 1428 resections with 113 haemorrhagic episodes. Scand J Thorac Cardiovasc Surg 17:283, 1983.
Piccione W Jr, Faber LP: Management of complications related to pulmonary resection. In Waldhausen JA, Orringer MB (eds): Complications in Cardiothoracic Surgery. St. Louis: Mosby-Year Book, 1991, p. 336.
Porte HL, et al: Randomized controlled trial of a synthetic sealant for preventing alveolar air leaks after lobectomy. Ann Thorac Surg 71:1618, 2001.
Rannels DE, Rannels SR: Compensatory growth of the lung following partial pneumonectomy. Exp Lung Res 14:157, 1988.
Reed CE, Spinale FG, Crawford FA Jr: Effect of pulmonary resection on right ventricular function. Ann Thorac Surg 53:578, 1992.
Robinson LA, Preksto D: Pleural tenting during upper lobectomy decreases chest tube time and total hospitalization days. J Thorac Cardiovasc Surg 115:319, 1998.
Saitoh Y, et al: Prevention of main bronchial fistula after pneumonectomy: wrapping with a pedicled pericardial flap containing the pericardiophrenic artery. J Jpn Assoc Chest Surg 10:778, 1996.
Sayeed-Shah U, Strachan J, Elefteriades JA: Diaphragm flap for routine prophylactic reinforcement of bronchial stump after pneumonectomy [Letter to the editor]. Ann Thorac Surg 71:2081, 2001.
Spirn PW, et al: Radiology of the chest after thoracic surgery. Semin Roentgenol 23:9, 1988.
Suarez J, Clagett OT, Brown AL Jr: The postpneumonectomy space: factors influencing its obliteration. J Thorac Cardiovasc Surg 57:539, 1969.
Sugarbaker DJ, Norberto JJ, Swanson SJ: Extrapleural pneumonectomy in the setting of multimodality therapy for diffuse malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 9:373, 1997.
Takaro T: Use of staplers in bronchial closure. In Grillo HC, Eschapasse H (eds): International Trends in General Thoracic Surgery. Vol. 2. Philadelphia: WB Saunders, 1987.
P.432
Talamonti MS, et al: A new method of extraperiosteal plombage for atypical pulmonary tuberculosis. Chest 96:237S, 1989.
Tayama K, et al: Natriuretic peptides in the lung modulated by pneumonectomy. Ann Thorac Cardiovasc Surg 4:325, 1998.
Tayama K, et al: Natriuretic peptides after pulmonary resection. Ann Thorac Surg 73:1582, 2002.
Turna A, et al: Antibiotic prophylaxis in elective thoracic surgery: cefuroxime versus cefepime. Thorac Cardiovasc Surg 51:84, 2003.
Van Mieghem W, Demedts M: Cardiopulmonary function after lobectomy or pneumonectomy for pulmonary neoplasm. Respir Med 83: 199, 1989.
Vaughn CC, et al: Prevention of air leaks after pulmonary wedge resection. Ann Thorac Surg 63:864, 1997.
Vester SR, et al: Bronchopleural fistula after stapled closure of the bronchus. Ann Thorac Surg 52:1253, 1991.
Werner HA, et al: Lung volumes, mechanics, and perfusion after pulmonary resection in infancy. J Thorac Cardiovasc Surg 105:737, 1993.
Wong K, Goldstraw P: Effect of fibrin glue in the reduction of postthoracotomy air leaks. Ann Thorac Surg 64:979, 1997.
Wurtz A, et al: Evaluation de l'efficait d'une colle de fibrine en chirurgie de pulmonaire partielle. Lyon Chir 88:368, 1992.
EAN: 2147483647
Pages: 203