13 - Radionuclide Studies of the Lung
Editors: Shields, Thomas W.; LoCicero, Joseph; Ponn, Ronald B.; Rusch, Valerie W.
Title: General Thoracic Surgery, 6th Edition
Copyright 2005 Lippincott Williams & Wilkins
> Table of Contents > Volume I - The Lung, Pleura, Diaphragm, and Chest Wall > Section IV - Diagnostic Procedures > Chapter 17 - Invasive Diagnostic Procedures
Chapter 17
Invasive Diagnostic Procedures
Ronald B. Ponn
Invasive procedures are often indicated for the diagnosis and staging of chest diseases. Bronchoscopy, video-assisted thoracic surgery, and esophagoscopy are discussed in Chapters 16, 18, and 128, respectively. Chapter 69 deals with pericardiocentesis and pericardial biopsy. The utility of transthoracic needle biopsy of the lung for diagnosing solitary pulmonary nodules is covered in Chapter 93. The present section reviews the role of common diagnostic procedures performed by surgeons, including indications and contraindications, technical aspects, complications, and alternatives.
MEDIASTINOSCOPY
Mediastinoscopy is the most common operating room procedure performed by most general thoracic surgeons. Harken and associates (1954) first suggested a way to explore the superior mediastinum from a lateral approach after excision of the scalene fat pad. Carlens (1959) described the classic midline approach that led to the widespread use of this procedure. Its key features are the development of a pretracheal tunnel through a small single cervical incision and passage of a specifically designed lighted speculum, the mediastinoscope, that permits nodal sampling on each side of the trachea as well as access to the subcarinal nodes. Use of the Carlens' procedure spread from Sweden to North America with the strong support of Pearson (1968). Cervical mediastinoscopy has become an integral part of the evaluation and staging of patients with suspected lung cancer as well as diagnosing other causes of mediastinal lymphadenopathy or mass.
Indications
The most common indication for mediastinoscopy is the intial staging and diagnosis of lung cancer (see Chapter 105). Mediastinoscopy for diagnosing other mediastinal diseases is valuable but less frequent. The need for mediastinoscopy in many patients with lung cancer derives from the absence of a reliable noninvasive method for differentiating benign from malignant lymph nodes, the uniformly poor results of primary resection in patients with clinical N2 disease (see Chapter 106), and the frequency of N2 involvement. The nodes accessible to standard cervical mediastinoscopy are levels 2 and 4 (paratracheal), level 7 (subcarinal), and sometimes level 10 (tracheobronchial). Computed tomography (CT) is currently the pivotal imaging study in the early evaluation of patients with known or suspected lung cancer and other thoracic diseases involving the mediastinal structures. CT assessment relies mainly on nodal size. The threshold for positivity has been lowered over time to the currently accepted short-axis diameter of 1 cm. The result is a greater sensitivity, but a lower specificity. The reported usefulness of CT varies widely (Table 17-1). Importantly, the negative predictive value (NPV) of CT is about 90%, whereas the positive predictive value (PPV) is only 74%, as noted by Daly and associates (1987) and by Backer and colleagues (1987). Others, however, have noted less reliability of CT for excluding malignant lymphadenopathy in lung cancer patients. Webb and colleagues (1991) found a sensitivity of only 52% and a specificity of 69%. In a report by Izbicki and colleagues (1992), CT scan correctly predicted the nodal status in only 58% of patients. Similarly, Gdeedo and colleagues (1997), in a study of 100 consecutive patients with non small cell lung cancer (NSCLC) who underwent CT and mediastinoscopy, report a sensitivity and specificity of CT of 63% and 57%, respectively, and of mediastinoscopy, 89% and 100%, respectively. PPV and NPV for CT were 41% and 77%, respectively; and of mediastinoscopy, 100% and 96%, respectively. Accuracy of CT was 59% and of mediastinoscopy, 97%.
The differing findings in the reports noted above, as well as many others, lead some to recommend routine mediastinoscopy, whereas others use a selective approach. In the latter group, common indications for mediastinoscopy in patients without radiographic mediastinal adenopathy include central tumor, known adenocarcinoma, the need for pneumonectomy, enlagement on CT scan of N1 nodes, and
P.300
superior sulcus cancers. A randomized study by the Canadian Lung Oncology Group (1995) comparing the rate of noncurative thoracotomy in routine versus selective mediastinoscopy found a trend favoring the selective strategy by both clinical and cost criteria. This study may be criticized because the routine mediastinoscpy patients did not have CT scans, whereas in current practice, all patients are studied by CT early in their evaluation.
Table 17-1. Approximate Correlations of Radiographic Studies and Mediastinoscopy with Pathologic Nodal Staging in Lung Cancer | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
There has been much recent interest in the use of positron emission tomography (PET) scanning to assess the mediastinum in patients with lung cancer (see Chapter 12). Application of PET may decrease the gap between the results of invasive and noninvasive staging. Although the reported experience with PET scan assessment of the mediastinum varies, the negative predictive value is more than 90% in most series. S. A. Roberts (2000), Gupta (2000), Poncelet (2001), and Kernstine (2002) and their colleagues reported an NPV of 96%, 98%, 94%, and 95%, respectively. In contrast, the PPV of PET in these studies ranged from 43% to 63%. Graeter and associates (2003) reported on 102 patients who had both PET and mediastinoscopy. The NPV for PET in this study was 98.4%, and the PPV, 49%. Of interest is that of 469 nodal levels analyzed, there were only 5 false negative PET studies. In contrast, 81 levels were false positive. Gupta and associates (2000) also noted that PET was equally reliable in detecting malignancy in nodes smaller than 1 cm and nodes larger than 3 cm. If these findings are supported as experience increases, it appears reasonable to bypass invasive mediastinal staging in some patients with a negative PET scan and without enlarged nodes on CT scan. This approach is based on the finding that false-negative PET results will occur in only about 5% of cases, whereas the false-negative rate of mediastinoscopy is approximately 10%. PET positivity, however, is often false positive. Therefore, a positive scan cannot be relied on as definitive, and mediastinoscopy is warranted in the presence of uptake in the mediastinal nodes.
Contraindications and Cautions
Associated conditions that preclude safe mediastinoscopy include huge cervical goiter, extensive calcification or aneurysm of the innominate artery, and permanent tracheostomy after laryngectomy and radiation. Although not contraindications, several other factors may increase the difficulty of mediastinoscopy. A carotid bruit, especially on the right, should prompt extra care to minimize neck extension and innominate artery compression. Prior sternotomy or neck incision may make the midline harder to find, but does not affect the deeper dissection because the pretracheal fascia is generally intact. Prior mediastinoscopy, in contrast, is associated with peritracheal fibrosis extending into the mediastinum. Repeat mediastinoscopy, however, is feasible in most cases. Meersschaut and associates (1992) reported no mortality in 170 patients undergoing repeat mediastinoscopy. Mateu-Navarro and colleagues (2000) experienced no mortality in 24 patients having repeat mediastinoscopy following induction chemotherapy and found mediastinoscopy helpful in deciding which patients might benefit from resection. Care must be taken in separating the innominate artery from the trachea. Sharp dissection of adhesions under direct vision is safer than the blunt dissection approach used in first-time mediastinoscopy. If adhesions are very dense, the midline trachea artery interface can sometimes be avoided by entering the pretracheal space from the left anterolateral aspect of the trachea. Mediastinoscopy can also be performed in the presence of superior vena caval obstruction, as reported by Jahangiri and Goldstraw (1995). The most troublesome bleeding is from the superficial veins divided by the incision rather than the deeper veins, which are retracted laterally. Although generally feasible, redo mediastinoscopy or mediastinoscopy in cases of caval obstruction may be difficult. It may be impossible to perform a complete multilevel nodal sampling. Fortunately, in most patients with caval obstruction, all that is needed is a tissue diagnosis. Repeat mediastinoscopy is usually done to determine the presence or absence of residual cancer after induction therapy and may not require extensive node assessment.
Procedure
Cervical mediastinoscopy is generally performed as an outpatient procedure and can be combined with bronchoscopy, scalene biopsy, or anterior mediastinoscopy as needed. Because of the possibility of significant bleeding, mediastinoscopy should probably be limited to hospital-based rather than free-standing facilities.
P.301
General anesthesia is induced. The patient is positioned supine with the occiput at the very top of the operating table. The neck is moderately extended by an interscapular roll in order to draw the trachea from the mediastinum into the neck. The back of the operating table is elevated slightly to decrease venous pressure. The prep includes the anterior chest for possible sternotomy, but this area is not shaved. The endotracheal tube exits the patient's mouth on the anesthesiologist's side. Circulation to the right arm is monitored using a pulse oximeter or radial artery cannula. A dampened waveform may indicate innominate artery compression causing decreased right carotid perfusion. The degree and duration of compression should be minimized. Optical magnification is not required but can be achieved with an attachable swing-away lens. Video-mediastinoscopy is a recent option that allows both direct and monitor viewing. It aids in teaching by obviating the inevitable shifts that occur when the scope is handed back and forth and by assuring that all observers are viewing the same structures.
A 3-cm transverse skin incision is centered between the anterior borders of the sternocleidomastoid muscles 1 cm above the sternoclavicular junctions and carried through the platysma (Fig. 17-1). The avascular space between the strap muscles is opened vertically. Palpation of the trachea aids in locating the narrow midline space between the sternohyoid muscles. The deeper sternothyroid muscles are separated by a wide band of areolar tissue. The anterior wall of the trachea is now in view. If there is insufficient length of trachea caudal to the thyroid isthmus, this structure is retracted cranially. Rarely, the isthmus must be divided and oversewn or ligated. The thyroidea ima artery or a branch of the inferior thyroid vein must be ligated when these vessels cannot be retracted from the midline below the isthmus. It is essential to assess the area just above the sternal notch early in the dissection because cervical extension may draw the innominate artery up into the base of the neck where it can be injured. The pretracheal fascia is incised and elevated. A tunnel is created in the pretracheal space using an index finger (Fig. 17-2). For purposes of palpation and later visualization, mediastinoscopy is a tracheocentric procedure; that is, anatomic orientation is always defined by the relationship to the trachea of the finger, the mediastinoscope, and the instruments. Tough fibrous bands between the trachea and fascia may be encountered proximally. Thereafter, gentle advancement and lateral sweeping clear the pretracheal and paratracheal spaces easily unless there is fibrosis or malignant invasion. The dorsal aspect of the finger senses the tracheal rings in a stepladder fashion as it passes distally. Dissection is carried to the full length of the finger and usually reaches a point 1 or 2 cm proximal to the carina. In short patients, the carina may be reached
P.302
and is identified as a midline posterior depression. Simultaneously, the anteriorly directed volar surface palpates the innominate artery and the aortic arch more distally. Pressures in the superior vena cava and pulmonary artery are too low for these landmarks to be appreciated. During digital dissection, the surgeon formulates a mental map of the area by noting the presence and location of normal structures, as well as lymphadenopathy, mass, or invasion. Because the nodes are external to the pretracheal fascia, access requires penetration of the fascia. This is usually accomplished digitally at a point just beyond the innominate artery (Fig. 17-3). Enlarged nodes can then often be freed bluntly to a significant extent, a maneuver that facilitates subsequent endoscopic retrieval. Firm nodes in the upper right paratracheal area are particularly suitable because the finger can be hooked anteriorly around the innominate artery and blunt dissection performed against the chest wall. Alternatively, the pretracheal fascia can be penetrated later, using an instrument passed through the scope.
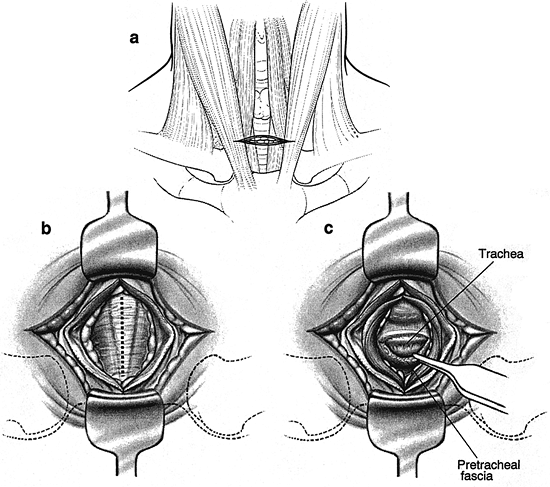 |
Fig. 17-1. Cervical dissection for mediastinoscopy. A. Skin incision. B. Vertical midline incision between the sternothyroid muscles after separation of the sternohyoids. C. The pretracheal fascia is incised and elevated. |
 |
Fig. 17-2. Development of the pretracheal tunnel and identification of normal and abnormal landmarks. A, B. Oblique and anterior views of a finger entering the pretracheal space and palpating the innominate artery. C. Beyond and posterior to the innominate, palpating the trachea, artery, and nodes, and enlarging the tunnel by lateral sweeping. D. Fully inserted, palpating the aortic arch, nodes, and occasionally the carina. |
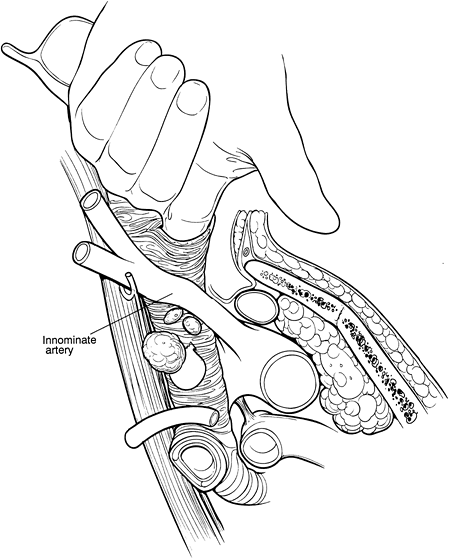 |
Fig. 17-3. Oblique view of a finger piercing the pretracheal fascia to enter the node-containing space. Considerable blunt dissection of enlarged nodes can often be accomplished at this point. |
The mediastinoscope is now introduced. As in all endoscopic procedures, the instrument is advanced only if it passes easily and there is a visible tunnel. Passage is aided by blunt dissection with a metal or plastic suction-cautery device. It is important not to be confused by the position of the scope once it has been fully inserted. The pretracheal fascia and the slope of the trachea from anterior to posterior cause the tip of the scope to be focused directly on the airway or the subcarinal space. The mediastinoscope must be withdrawn slightly and angled anteriorly in order to enter the extrafascial space previously created digitally or to indent the fascia so that it can now be bluntly opened with the sucker (Fig. 17-4). The mediastinum is surveyed to confirm the findings of palpation by visualizing the pretracheal, paratracheal, and subcarinal nodes, azygos vein, pulmonary artery, carina and proximal main-stem bronchi, and sometimes the right upper lobe bronchial takeoff (Fig. 17-5). Lymph nodes are identified by color and consistency and by reference to the map generated during palpation. The blue-gray hue of venous structures may simulate the appearance of an anthracotic node. Pulsations may mislead both by their presence and absence because they may be transmitted to nonvascular structures and may be difficult to appreciate in the vena cava and azygos vein. If there is any doubt about the solid or vascular nature of the structure in question, aspiration is carried out using a 20-gauge spinal needle and small syringe. Saline in the syringe, as opposed to a dry system, facilitates identification of small amounts of blood. In the rare case of a completely solidified mediastinum, when anatomic definition is impossible and biopsy hazardous, aspiration cytology or core tissue histology may be sufficient. In most cases, however, it is possible to obtain substantial biopsy specimens. In order to prevent minor bleeding from obscuring the field, all target nodes are partially dissected before any biopsy specimens are taken. Node
P.303
dissection through the scope is done bluntly, with the suction-cautery, endo dissectors, or biopsy forceps. Once sufficiently freed, the node is grasped with a cupped laryngeal biopsy forceps, and traction is applied under direct vision. If the node cannot be extracted by gentle pulling and twisting, further dissection is carried out. It is occasionally possible and helpful to grasp the node and divide tethering attachments with a simultaneously introduced dissector or second biopsy forceps. If further digital dissection seems helpful, the scope is withdrawn and the finger reintroduced. Although removal of whole lymph nodes theoretically lessens the chance of tumor implantation and is esthetically pleasing, it is not always feasible due to large size, friability, or adherence. In such cases, incisional biopsy specimens are taken with the laryngeal forceps. Particular care is taken when obtaining specimens near the tracheobronchial angles because of the proximity on the right of the azygos vein and apical branch of the anterior pulmonary artery and on the left the recurrent laryngeal nerve. Whenever possible, these structures should be visualized.
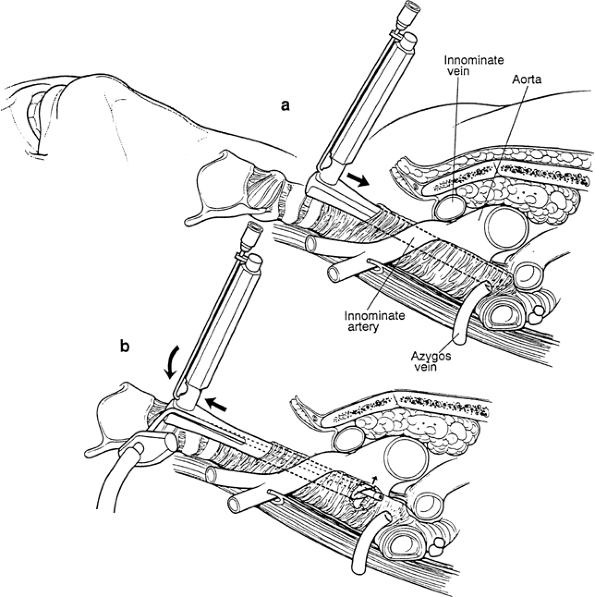 |
Fig. 17-4. A. The passive angle of the mediastinoscope within the pretracheal fascia not previously opened aims the tip of the scope directly at the trachea or subcarinal space. B. The instrument is angled anteriorly to tense the fascia so that it can be penetrated with a sucker. The innominate artery is subject to compression during this maneuver. |
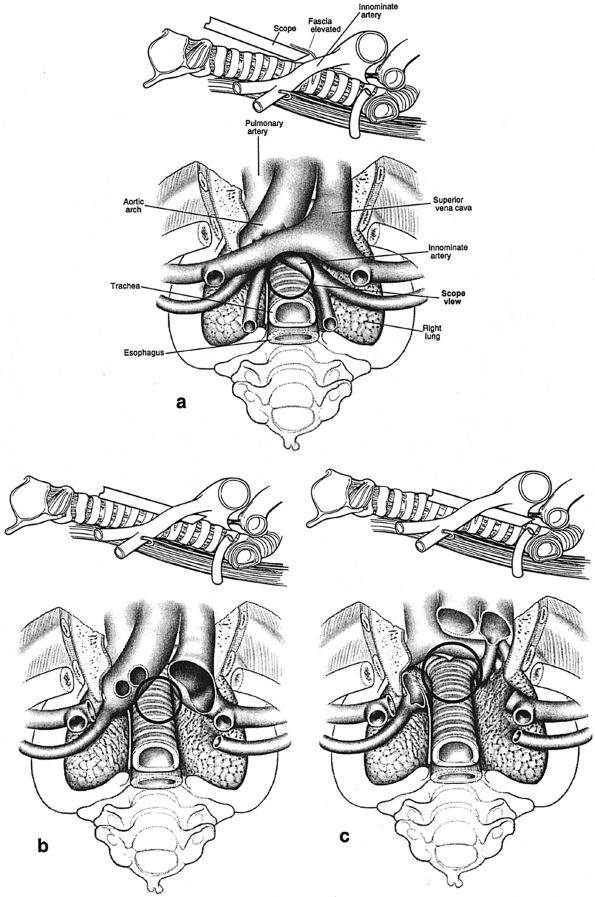 |
Fig. 17-5. Craniocaudal (surgeon's orientation) and corresponding lateral views of superior mediastinal structures at three levels. Circles outline potential views through the mediastinoscope at each level. A. Proximal tunnel. B. Midtrachea. C. Distal trachea, carina, and subcarinal space. |
The extent of biopsies depends on the clinical impression and operative findings. In lymphoma, sarcoidosis, or mediastinal mass, all that is needed is diagnostic tissue. For staging lung cancer, on the other hand, it is necessary to obtain specimens from several locations. Biopsies are taken from the high and low right and left paratracheal regions and from the subcarinal space (levels 2, 4, 7). If there is unequivocal extranodal tumor at a high station, lower levels need not be sampled because staging will not be altered. In general, however, the time taken for frozen-section confirmation is greater than the time and risk of taking more specimens. Routine biopsy of nodes contralateral to the primary tumor is essential in order not to understage N3 cases. Labeling all specimens by numerical station is less subject to error than is using their anatomic designations.
Bleeding is usually minor and requires no treatment. To assess hemostasis, the scope is withdrawn slowly. Most bleeding can be controlled by packing with gauze for a few minutes. Oxidized cellulose can be packed into the subcarinal and paratracheal spaces and left in place. If there is a visible bleeding vessel, it can be clipped. Clipping is most useful for bronchial vessels in the subcarinal space. If cautery is used, the power should be kept low, and it should be applied directly to the bleeding structure in order to avoid arcing and thermal damage.
If there is concern about pleural or pulmonary puncture, the tunnel is filled with saline. Rapid disappearance of the liquid may indicate a pleural rent. Bubbling suggests a parenchymal communication. If the leak is significant, a chest tube is placed. For closure, the strap muscles are reapproximated in the midline with one or two absorbable sutures. Ridge formation is lessened if the platysma is not closed separately. A subcuticular closure is used for the skin. Drainage is not necessary.
Variations
In some cases, standard cervical mediastinoscopy offers inadequate access for staging by one procedure. In such instances, cervical mediastinoscopy can be combined with anterior mediastinoscopy or thoracoscopy. Ginsberg and associates (1987) described an extended cervical mediastinoscopy in which aortopulmonary (level 5) and anterior mediastinal (level 6) nodes are accessible through a cervical incision (Fig. 17-6). Following clearing of the infraisthmic trachea or completion of standard mediastinoscopy, the space anterior to the aortic arch is opened by digitally piercing the fascia between the innominate and left common carotid arteries. The innominate vein, separated from the operative area in standard mediastinoscopy by the arch arteries, now lies directly anterior to the tunnel. Following palpation, the mediastinoscope is passed anterior to the aorta, and appropriate biopsy specimens are taken. In mediastinopleuroscopy, described by Deslauriers and colleagues (1976), the pleural space is intentionally entered
P.304
(Fig. 17-7). On the right, the pleura is opened posterior to the innominate artery, whereas on the left, entry is gained between the left common carotid and left subclavian arteries. In addition to pleural biopsy and fluid sampling, small upper lobe lung biopsies can be obtained. However, the risk for seeding a clean mediastinum by transpleural biopsy of an upper lobe cancer or infection must be considered.
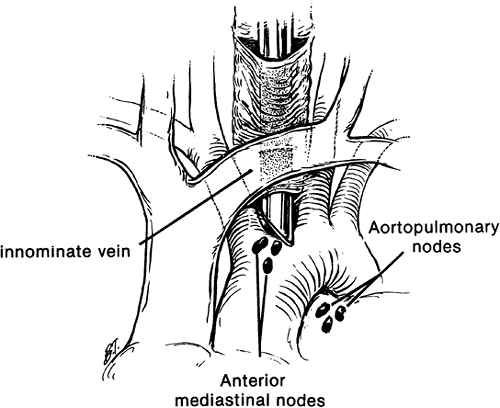 |
Fig. 17-6. Extended mediastinoscopy. |
Postoperative Care
Patients are discharged after 2 to 3 hours of observation that includes standard postanesthetic vital signs, electrocardiogram (ECG) monitoring, and pulse oximetry. Chest radiographs are performed only if there is known or suspected pleural entry, excessive bleeding, abnormal auscultation, or hypoxemia. Similarly, blood tests are done only when specifically indicated.
Complications
Mediastinoscopy is a safe procedure in experienced hands (Table 17-2). The mortality rate in large series ranges from 0% to 0.08%. Complications occur in up to 3% of cases, but are major in 0.5% or less. In a review of 11,000 cases, Specht (1971) found a mortality rate of 0.15%. Puhaka (1989) noted no mortality in a series of 2,021 mediastinoscopies, and a complication rate of 3%. Major complications were noted in only 0.5%. In the series of Basca and colleagues (1974), bleeding requiring operation occurred in 0.1%, pneumothorax in 0.5%, and recurrent nerve paralysis in 0.4%. Luke and associates (1986) had no deaths in 1,000 cases. In a review of 2,137 cases, Hammoud and co-workers (1999) experienced one death related to the procedure and complications in 12 cases. Likewise, in a report on 400 consecutive cases, Porte and associates (1998) experienced no mortality and a 2.5% morbidity rate. Bocage and colleagues (2000) reported only one death in 3,600 cases.
The most feared complication is massive hemorrhage from damage to the aortic arch or its branches, superior vena cava, azygos vein, or pulmonary artery. Innominate and jugular vein lacerations occur less commonly and can be controlled more easily. Injury to a bronchial artery may produce significant but not massive bleeding unless the vessel has been avulsed from the aorta. Although in some series operation for hemorrhage was not required, it is inevitable that significant bleeding will occur on occasion. The incidence should not exceed 0.1%. If control cannot be achieved through the cervical incision, the mediastinum is packed while preparations are made for transfusion and operation. If there is a rent in the superior vena cava, lower extremity venous lines are placed. The decision to proceed with sternotomy or thoracotomy depends on the location of the vascular injury, the resectability of the tumor, and the patient's immediate status. Sternotomy is generally preferable because it does not require repositioning, takes less time to gain entry, and provides better exposure of most injuries.
P.305
Thoracotomy can be done, however, if the surgeon believes that rapid vascular control can be obtained and that definitive resection is feasible at the time. Operative intervention may also be required for rare nonvascular injuries. Small tracheobronchial tears are treated by packing, but larger lacerations require direct or pedicled flap closure, as reported by Schubach and Landreneau (1992). Esophageal perforation, usually in the subcarinal region, may be detected and repaired early or may elude diagnosis until mediastinal sepsis occurs. Recurrent nerve paralysis is infrequent, usually involves the left side, and is transient in half the cases. As noted earlier, caution must be used when dissecting in the low left paratracheal and tracheobronchial areas because the recurrent nerve may be injured. Pneumothoraces are generally minor and not under tension because most result from a pleural tear not associated with a lung injury. Treatment by observation, aspiration, or chest tube is dictated by the clinical situation. It has been suggested that stroke is a procedure-specific complication, especially of extended mediastinoscopy, and is due to atherosclerotic embolization or cerebral hypoperfusion from aortic arch or branch arterial compression. The incidence, however, is extremely low and probably not higher than expected with any operation and general anesthetic. Tumor seeding of the incision is a very rare complication. Al-Sofyani and associates (2000) reported one case and found five previous instances in the literature.
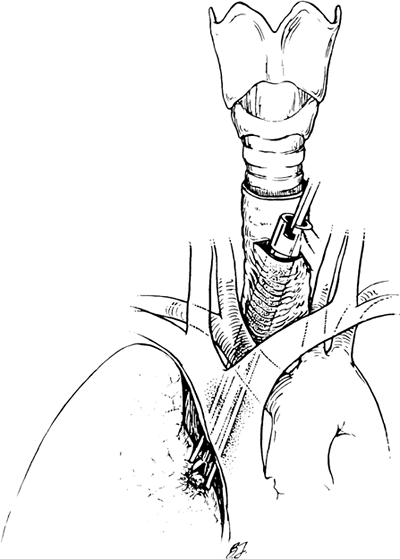 |
Fig. 17-7. Mediastinospleuroscopy. |
Table 17-2. Complications of Mediastinoscopy | |
|---|---|
|
ANTERIOR MEDIASTINOSCOPY AND ANTERIOR MEDIASTINOTOMY
Anterior or parasternal mediastinotomy was devised by McNeill and Chamberlain (1966) to provide access to nodes in the subaortic region on the left, an area not reached by standard cervical mediastinoscopy. This approach can also be used on the right and offers access to the upper hilum, lung, and pleura on both sides. In addition to lung cancer diagnosis and staging, anterior mediastinotomy can be used in biopsy of many anterior mediastinal masses. Anterior mediastinoscopy refers to the common modification of the original operation, in which there is no removal of a costal cartilage and a mediastinoscope is passed through a small incision in the second intercostal space.
Indications
Cancer of the left upper lobe is the most common indication for anterior mediastinoscopy, because of the pattern of nodal metastases from this lobe and possibly better results with resection of localized metastasis in this area as compared with other N2 disease. As emphasized by Ginsberg (1994), the left upper lobe drains not only to the adjacent subaortic and anterior mediastinal stations, but also to the ipsilateral paratracheal nodes. A significant number of patients with left upper lobe cancers have metastases to the latter groups, involvement that is associated with the same very poor surgical prognosis as in other clinical N2 cases (see Chapter 106). In contrast, primary resection of left upper lobe cancers with malignant but not fixed or bulky adenopathy confined to the subaortic level may yield 5-year survival rates of 20% to 30%, as reported by Patterson and associates (1987) and Nakanishi and colleagues (1997). These findings suggest that level 5 node biopsy need not be routine, but can be applied selectively when more than minimal adenopathy is documented by CT scan. Mediastinoscopy, on the other hand, is frequently required for staging left upper lobe cancers. When anterior mediastinoscopy is indicated by CT and PET findings, it is generally preceded by mediastinoscopy. If right or left superior mediastinal nodes obtained through the mediastinoscope are positive, the tumor is unresectable. If mediastinoscopy is negative, the results of anterior mediastinotomy become determinative. The apparent reliable negative predictive value of combined CT and PET scans, as discussed earlier, make it reasonable to forego invasive staging in many cases. Again, there should be a low threshold to proceed to biopsy. In addition to nodal staging, anterior mediastinoscopy allows assessment of invasion of the mediastinum, pulmonary vessels, and phrenic nerve.
Contraindications and Cautions
Prior sternotomy is not a contraindication, but warrants caution. The procedure should generally not be performed, however, in the presence of a patent left internal mammary artery bypass graft. In order to protect the graft in the event of repeat sternotomy, many cardiac surgeons drape it laterally, often opening the pleura to do so. This places the artery anterior to the lung hilum, essentially along the
P.306
course of the phrenic nerve, and subject to injury. Other structures that can be injured include the vagus nerve, the intercostal artery and vein, the superior pulmonary vein, the main pulmonary artery, and the aorta.
Procedure
The patient is supine and under general anesthesia. Selective lung ventilation through a double-lumen endotracheal tube is not essential, but may be helpful, especially if examination of the hilum, lung, or pleura is needed. Anterior mediastinoscopy can also be performed using local anesthesia, as reported by Rendina and associates (2002). This approach is especially useful in cases of large mediastinal masses that might cause airway compression in the setting of general anesthesia and supine position. In the classic procedure, a 6-cm transverse incision is made just lateral to the sternum at the second or third costal levels (Fig. 17-8). The pectoralis muscle is split to expose the cartilage, which is resected from sternochondral to costochondral junction and the mediastinum entered through the posterior perichondrium. In most cases, adequate exposure is achieved through the intercostal space without removing the cartilage. The internal mammary artery and vein are retracted or rarely divided and ligated. The mediastinal pleural reflection is separated bluntly from the posterior table of the sternum and retracted laterally. Finger dissection opens the loose areolar tissue and extends inward until the aorta, pulmonary artery, and intervening space are noted. This area is assessed for fixation, invasion, and adenopathy. During palpation, as in mediastinoscopy, the surgeon formulates a tactile map of normal and abnormal structures and can begin freeing enlarged nodes. Direct inspection and usually placement of a mediastinoscope confirm the anatomy. The vagus and phrenic nerves can sometimes be identified along the aortic arch, and the location of the recurrent laryngeal branch thereby inferred.
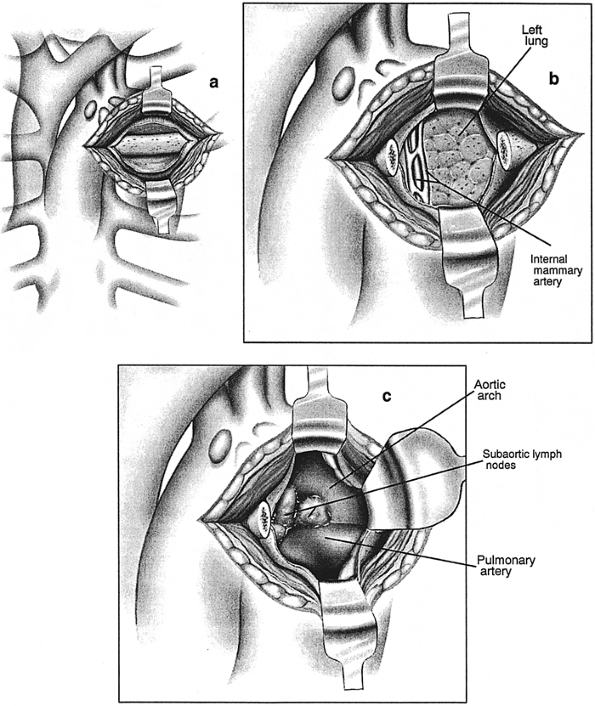 |
Fig. 17-8. Anterior mediastinotomy. A. Incision over the second costal cartilage. B. After resection of cartilage. C. View of enlarged subaortic lymph nodes. |
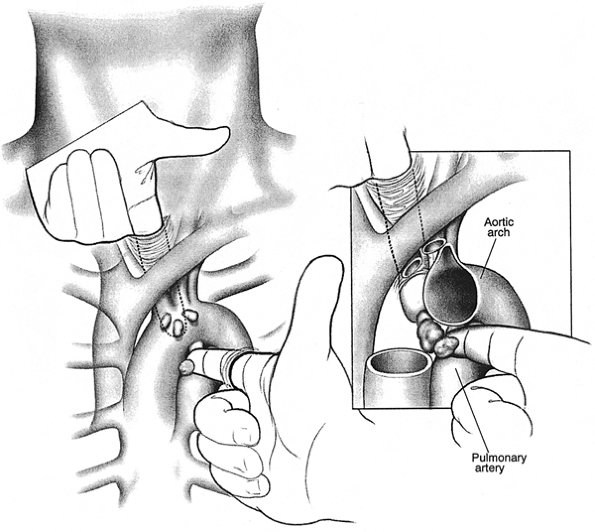 |
Fig. 17-9. Bidigital palpation of the aortopulmonary region at time of combined cervical and left anterior mediastinoscopy. |
Enlarged nodes are sampled directly or through the mediastinoscope using techniques for dissection, identification, and hemostasis described for cervical mediastinoscopy. Anatomic definition may sometimes be clarified by opening the pleura and assessing the area from both the mediastinal and pleural aspects. Some favor opening the pleura and performing biopsy from the pleural side in all cases. When mediastinotomy is done in conjunction with cervical mediastinoscopy, the neck incision is left open to allow bidigital palpation of the aortopulmonary region. (Fig. 17-9). Drainage is usually not needed. If the pleura was entered, a small tube is brought out through one edge of the wound. After closure, pleural air is evacuated by inflating the lungs to a pressure of 30 to 40 cm as the tube is withdrawn. If biopsy of the lung was done at operation, the tube is left in place and connected to underwater suction, underwater seal, or a one-way flutter valve.
Complications
Because anterior mediastinotomy or mediastinoscopy is performed far less frequently than cervical mediastinoscopy, there are no large series that assess the complication
P.307
rate. It is reasonable to assume that the complication rate is low and similar to that for mediastinoscopy. Mortality should be exceedingly rare. Significant bleeding from injury to the aorta, pulmonary artery, or superior pulmonary vein is possible. Bleeding from the internal mammary vessels is likely more common and more easily controlled. Diaphragmatic paralysis and hoarseness occur occasionally from damage to the phrenic or recurrent laryngeal nerves. Pneumothorax can usually be handled conservatively. Tumor invasion of the incision is rare, but the author has seen one case each with lung cancer, germ cell tumor, and lymphoma.
Scalene Biopsy
Scalene lymph node biopsy is an open procedure for sampling enlarged nodes in the scalene triangle, the low anterior cervical region superficial to the anterior scalene muscle. Scalene fat pad excision denotes resection of all the node-bearing areolar tissue in this region.
Indications
Before the introduction of mediastinoscopy, scalene biopsy, rigid bronchoscopy, plain radiography, and history and physical examination were used to assess the locoregional extent of lung cancer. Daniels (1949) recommended excision of lymph nodes in the scalene fat pad in a variety of intrathoracic diseases. Routine biopsy was performed variously ipsilateral to the tumor, bilateral, on the right for right-sided and left lower lobe tumors, and on the left for left upper lobe cancers. The reported positivity rate in patients without palpable nodes varies widely. Brantigan and colleagues (1973) reported involved scalene nodes in 20% of cases, whereas Bernstein and associates (1985) found positive nodes in only 3.5%. Most thoracic surgeons believe that routine biopsy is not warranted. Certainly, enlarged nodes require confirmation if staging and treatment would be affected. In addition, Lee and Ginsberg (1996) suggested scalene node biopsy at the time of mediastinoscopy in patients with N2 involvement because of an incidence of occult metastasis in as many as 10% of cases. This approach, however, has not become popular, and scalene biopsy is currently limited to sampling of enlarged nodes. In sarcoidosis, in contrast, scalene biopsy in patients without palpable nodes yields a diagnosis in 75% of cases, as reported by Greschuchma and Maasen (1971). Although sarcoidosis can be diagnosed by mediastinoscopy in 98% to 100% of cases, the diagnostic yield of blind scalene biopsy is sufficiently high that the procedure may be substituted for mediastinoscopy when the diagnosis is elusive by bronchoscopy and clinical evidence and general anesthesia must be avoided. Palpable scalene nodes can often be aspirated in the office or clinic (Fig. 17-10). Phillips and Barker (1985) reported success in 86% of 42 such patients. Rohwedder and colleagues (1990) were successful in all 55 patients with palpable nodes, although two required repeat needle biopsy.
 |
Fig. 17-10. Scalene biopsy. A. Skin incision over the sternocleidomastoid muscle. B. Anatomy of the scalene triangle. C. Resection of the fat pad, exposing the anterior scalene muscle and phrenic nerve. D. Alternative percutaneous needle aspiration of enlarged nodes. |
Contraindications and Cautions
Although there are no absolute contraindications to scalene biopsy, poor healing, malignant wound seeding, and chylous fistula can occur in cases of bulky adenopathy and extranodal tumor infiltration with or without prior cervical radiation. Open biopsy should generally be avoided in this setting in favor of needle biopsy.
Procedure
Fat pad excision is described in order to highlight the anatomy (see Fig. 17-10). In the presence of enlarged nodes, however, only abnormal tissue need be sampled by standard methods for incisional or excisional lymph node biopsy. Local anesthesia is adequate. The neck is extended and the head turned to the contralateral side. A 4-cm incision centered between the heads of the sternocleidomastoid muscle and about 2 cm above the clavicle is deepened through the platysma. The muscle heads are retracted in opposite directions, or the entire muscle is retracted medially. The omohyoid is retracted upward. The boundaries of the
P.308
scalene triangle are the jugular vein medially, the subclavian vein inferiorly, and the omohyoid muscle superolaterally. The anterior scalene muscle, with the phrenic nerve coursing along its surface, constitutes the deep margin of dissection. The fat pad is dissected from these structures from below upward. Visible lymphatic channels are clipped or ligated. The transverse cervical artery, arising from the subclavian and entering the fat pad inferiorly, may require ligation. On the left, the thoracic duct can often be identified near its entry into the superior aspect of the subclavian vein. If injured, it must be meticulously ligated. When scalene biopsy is performed along with mediastinoscopy, the mediastinoscopy incision is extended 1 or 2 cm to the appropriate side, and a skin flap is developed. The scalene triangle can then be reached posterior to the sternal head of the sternocleidomastoid muscle by retracting the muscle anteriorly or by transecting it at its insertion.
Complications
Mortality is negligible. Injury to the major veins should be rare. The jugular can be repaired easily, but a subclavian injury may require clavicular resection. Phrenic nerve injury is prevented by avoiding cautery deep to the fat pad. Chylous fistula can be a troublesome problem and usually originates from raw nodal surface following incisional biopsy rather than from injury to a major lymphatic channel. Pneumothorax may result from a rent in the pleural cupola or, less likely, puncture of the lung. Venous air embolism can occur, as in any cervical procedure.
ALTERNATIVES TO SURGICAL BIOPSY
Mediastinoscopy is an ideal staging procedure because of accuracy, safety, and minimal discomfort. In some instances, however, sufficient information can be obtained by other means. At bronchoscopy, enlarged paratracheal, subcarinal, and aortopulmonary nodes can be assessed by transtracheal or transcarinal needle aspiration (TBNA). The technical aspects of TBNA are discussed in Chapter 16. As noted by Mehta and colleagues (1989), the highest yields are obtained by combining aspiration for cytology with biopsy for histology using an 18- or 19-gauge needle. TBNA was assessed in a multicenter study reported by Harrow and associates (2000). The authors found that histology core biopsy samples were positive in 57% of cases and that aspiration cytology was positive in 41%. The negative predictive value was 80%. TBNA avoided further invasive studies in about one third of the cases in this study. False-positive findings have been reported with aspiration cytology, but not with histology specimens. False-positive results are likely due to contamination of the specimen by respiratory secretions containing malignant cells. This problem can be minimized by performing TBNA as the first step in the procedure, before inspecting the distal airways, obtaining other samples, or applying suction. Rapid on-site evaluation of the specimen is required for optimal results.
Early work with virtual bronchoscopy, as reported by McAdams and associates (1998), suggests that this approach may improve the accuracy of TBNA. TBNA has also been performed using CT fluoroscopy guidance to allow real-time imaging. Garpestad and co-workers (2001) were able to obtain positive samples in 28 of 35 patients (87%) who had a previous nondiagnostic standard TBNA. Although there has been some interest in ultrasound-guided TBNA, especially for assessment of small nodes, most centers do not have the necessary equipment or experience. In addition, Shannon and associates (1996) found no significant difference in the yields of ultrasound-guided versus standard TBNA. Transesophageal endoscopic ultrasound (EUS), however, has been used to identify mediastinal lymph nodes to allow for aspiration. As discussed by Roberts (2000), improved technology using ultrasound beams parallel to the long axis of the endoscope allows continuous monitoring and more accurate aspiration of small nodes. In a prospective study, Gress and colleagues (1997) found that operation was avoided in 14 of 24 patients staged by this method. The accuracy was 96%. Midthun and associates (2001) compared EUS and TBNA in patients with lung cancer. The two procedures produced similar results. EUS is limited by its inability to sample anterior lymph nodes, lack of expertise in most centers, and the absence of studies based on large numbers of cases.
Transthoracic needle biopsy (TNB) of mediastinal and hilar lymph nodes and masses is often an alternative to surgical biopsy. This procedure has a high yield in experienced hands, as discussed by Protopapas and Westcott (1997). Because an anterior parasternal or posterior paravertebral approach is often used, thereby avoiding traversing the lung parenchyma, pneumothorax is less common than following needle biopsy of the lung. TNB has a high yield when the mediastinal or hilar nodes are involved with metastatic carcinoma. Protopapas and Westcott (1996), Morrisey and associates (1993), and Sider and colleagues (1987) reported sensitivities for carcinoma of 98%, 90%, and 95%, respectively. TNB is less likely to provide a specific diagnosis in cases of lymphoma, thymoma, and germ cell tumors of the mediastinum. Using standard cytologic methods, Herman and colleagues (1991) reported sensitivities of only 42% for non Hodgkin's lymphoma and 20% for Hodgkin's disease, as well as difficulty differentiating lymphoma from thymoma in some cases. The addition of immunohistochemical studies, as well as the use of a core biopsy needle, appears to improve the diagnostic yield of TNB. Silverman and associates (1994) were able to provide sufficient information by TNB to allow treatment in 72% of 102 cases of lymphoma. Using a core biopsy needle, Ben-Yehuda and co-workers (1996) obtained diagnostic tissue, leading to treatment in 86 of 100 patients with lymphoma. TNB is less reliable for the diagnosis of thymoma and germ cell tumors.
P.309
Even with core biopsies, the sensitivity for thymoma is only 71%, as reported by Herman and associates (1991). Weisbrod and colleagues (1987) noted a sensitivity of 57% for TNB of germ cell tumors.
It is clear that invasive nonsurgical methods can often provide valuable diagnostic and staging information along with several advantages over open biopsy. Transbronchial biopsy, for example, is performed in association with bronchoscopy and can in some cases diagnose and stage the patient with one procedure. EUS-guided biopsy provides access to posterior mediastinal structures that are not accessible by surgical approaches other than thoracoscopy. TNB can be performed in virtually all mediastinal and hilar locations. The morbidity associated with these procedures is negligible. Nonetheless, nonsurgical biopsy has limitations. In addition to the low yields for many mediastinal masses, a significant problem is that negative findings cannot be depended on to indicate a benign process. This is due to the very limited sample obtained by these methods in cases in which not all of the node is involved with cancer. Although a second needle biopsy often documents cancer, the need for two procedures diminishes the advantage of a nonsurgical approach. In contrast, surgical mediastinal biopsy yields large amounts of tissue taken at several levels and allows assessment of perinodal invasion and fixation of vital structures. Another advantage of open biopsy is that the procedure is performed by the surgeon, who must decide whether operation is indicated initially, after induction therapy, or never. Although TBNA should be part of the thoracic surgeon's staging procedures, the results of EUS and TNB are often used to institute treatment for patients with locoregional cancer without early surgical input. Ideally, surgical and nonsurgical diagnostic and staging procedures should be considered based on the individual case and local expertise. If a needle biopsy is likely to produce sufficient information to institute treatment, based on the entire clinical and imaging picture and the local experience, this option is reasonable. If, on the other hand, the yield is expected to be low or more information than a single aspirate is needed, surgical biopsy is indicated. Surgical staging is safe and versatile. A single outpatient procedure employing appropriate combinations of bronchoscopy, TBNA, cervical mediastinoscopy, anterior mediastinoscopy, and thoracoscopy is almost always able to diagnose and completely stage the local extent of lung cancers and other mediastinal pathology.
THORACENTESIS
Accumulation of fluid in the pleural cavity is a common problem with multiple possible etiologies. Benign and malignant effusions are discussed in Chapters 57 and 68, respectively. A comprehensive discussion of pleural effusion and other pleural diseases is found in the indispensable updated monograph by Light (2001). When systemic disease such as congestive heart failure or renal failure, rather than pleural involvement, is suspected as the etiology of an effusion, treatment is initiated and its effect assessed before proceeding to thoracentesis. Diagnostic thoracentesis is performed when the etiology of an effusion is unknown. Sahn (1988) reported that fluid analysis is diagnostic in 75% of patients. Usually, about 100 mL of fluid is removed for examination. The goal of a therapeutic thoracentesis is the removal of as much fluid as possible in order to relieve symptoms, treat infection, or allow better radiographic assessment of the lung. As noted in a report by the Health and Public Policy Committee of the American College of Physicians (1985), drainage of larger amounts may be associated with a higher rate of complications. Although much attention has been paid to the relative risks of diagnostic versus therapeutic thoracentesis, in most cases that require sampling for diagnosis, it is prudent to withdraw as much fluid as possible during the initial procedure.
Classically, an effusion appears as a convex opacity on an upright chest radiograph. About 250 mL is usually necessary to visualize an effusion. A lateral decubitus film is useful in determining whether the fluid is free flowing. As reported by McLoud and Flower (1991), newer modalities, such as ultrasonography, CT, and magnetic resonance imaging, have increased the sensitivity and accuracy of the diagnosis of pleural effusions. The radiologic and physical findings determine the site of aspiration. Usually, the best site is 2 inches below the superior aspect of the dullness. Small effusions can be aspirated with ultrasound guidance, as reported by Harnsberger (1983) and Grogan (1990) and their associates. CT scan is useful in managing complex loculated effusions.
Indications
As noted, a diagnostic thoracentesis is performed when the etiology of an effusion is unknown. This is usually the case at the time of first presentation. Therapeutic thoracentesis is done to relieve symptoms, whether the diagnosis is known or unknown. Although repeated thoracenteses can be performed for recurrent effusions, these should generally be treated definitively with pleurodesis or other modality, as discussed in Chapter 68. In actual practice, patients with advanced malignancies who present with symptomatic effusions are best served by bypassing thoracentesis and placing a small chest tube for pleurodesis or a Pleurx catheter. The same fluid studies are performed on the drained effusion.
Contraindications and Cautions
The only absolute contraindication to thoracentesis is a severe clotting problem. As shown by McVay and Toy (1991), pleural fluid sampling can be performed safely in the presence of mild coagulation abnormalities. These authors
P.310
found no increased risk for hemorrhage if the coagulation studies were not more than twice normal. Although a low platelet count did not increase the risk, a creatinine above 6 mg/dL was associated with bleeding complications. A small-bore needle is often used in this setting.
Procedure
The patient is generally placed in a sitting position. Debilitated and bedridden patients are brought as upright as possible by elevating the back of the bed or are placed in the lateral decubitus position with the side of the effusion down. Although aspiration of a diagnostic sample can be performed using only a needle and syringe, most thoracenteses are currently done using a kit containing the necessary equipment, including local anesthetic, drapes, needles and syringes, a drainage catheter, and containers for fluid samples. After the instillation of a local anesthetic in the skin and subcutaneous tissue, an 18- or 20-gauge needle is introduced close to the superior border of the rib to avoid the intercostal vessels and nerve (Fig. 17-11). Additional doses of local anesthetic are injected into the periosteum at the superior aspect of the rib. The needle is placed just above the rib, and injection is performed into the parietal pleura. The needle is then introduced into the effusion, and a sample is aspirated. As noted, a catheter also can be placed over the needle and threaded into the pleural space. Use of commercially available catheter kits allows withdrawal of larger amounts of fluid and conversion to a therapeutic thoracentesis if indicated. Alternatively, the needle can be fitted at the outset with a three-way stopcock, thus permitting multiple fillings of the syringe without the needle being open to air. In most cases, the needle is introduced posteriorly several inches lateral to the spine. If air is aspirated, but no fluid, the entry site may be too high, and a lower intercostal space should be tried. If neither air nor fluid is found, the site may be too low, the chest wall too thick for the needle, or the fluid too viscous. These problems can be remedied by moving to a higher site, using a longer needle, or using a larger-bore needle. If the thoracentesis remains unsuccessful, repeat imaging or access under ultrasound guidance is indicated.
The appearance and odor of the fluid may provide diagnostic clues. A putrid effusion can be diagnostic of an anaerobic or mixed empyema. A milky appearance is typical of a chylous effusion. Bloody fluid suggests malignancy, tuberculosis, or trauma. Although pleural fluid can be assayed by virtually any laboratory test in the appropriate setting, for example, carcinoembryonic antigen and flow cytometry, the usual initial studies include chemistry, cell count and differential, cytology if malignancy is suspected, and cultures when infection may be etiologic. Chemical analysis includes measurement of the pH, specific gravity, glucose, protein, and lactic dehydrogenase (see Chapter 68). The concentration of the last two substances in the pleural fluid is directly related to their level in the plasma. A blood sample is needed at the time of the thoracentesis to determine the relative serum and pleural fluid concentrations. A primary concern is whether the process is an exudate or a transudate. Infection and neoplasm are the major causes of exudative effusions. Transudative effusions occur as a result of interference with absorption, excessive production, or both. Usually, a specific gravity below 1.015 indicates a transudate. According to Sokolowski and associates (1989), further indication of an exudate may be noted by determining the ratio of pleural fluid protein to serum protein to be greater than 0.5, the pleural fluid lactate dehydrogenase (LDH) to serum LDH ratio to be greater than
P.311
0.6, and the pleural fluid LDH greater than two thirds of the upper limits of normal for serum LDH. Low glucose levels are suggestive of rheumatoid disease, tuberculosis, or bacterial empyema. As noted in Chapter 68, however, there is much overlap of fluid values among various pathologic processes. The cell count and differential can be helpful for determination of empyema, hemorrhagic effusion, and lymphoma, among other diseases. As noted by Ayo and colleagues (1999), this specimen should be placed in a tube containing an anticoagulant because clotting can occur and render the sample useless. A low pH is seen in empyema, complex parapneumonic effusions, and malignancy. Chang and associates (1998) suggest that the pH is optimally determined in the blood gas laboratory, rather than by a pH meter or an indicator strip. The sample must be maintained in an anaerobic environment. Packing in ice is not necessary if the test is performed within an hour of collection.
 |
Fig. 17-11. Diagnostic thoracentesis. A. The skin is injected with a local anesthetic using a 25-guage needle. B. The periosteum is injected. C. The pleural is entered and a fluid sample obtained. D. The attempt is too high; air is aspirated. E. The attempt is too low; neither air nor fluid is aspirated. From Light RW: Pleural Diseases. 4th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001, pp. 362 363. With permission. |
Complications
Pneumothorax is the most frequent complication of thoracentesis. In a report of 506 cases by Barter and associates (1993), postprocedure pneumothorax was identified in 11% and required tube thoracostomy in 2%. Air can enter the pleural space from outside through the drainage system or from a puncture of the lung parenchyma. Bleeding from injury to an intercostal vessel is uncommon but tends to occur more often in older patients whose intercostal arteries may be tortuous, according to Carney and Ravin (1979). Vasovagally mediated bradycardia and hypotension can occur. Rare complications include bleeding from injury to an upper abdominal organ, tumor implantation of the needle tract, and introduction of infection into the pleural space. Reexpansion pulmonary edema is an uncommon but potentially serious problem after therapeutic thoracentesis or rapid expansion of a lung collapsed due to pneumothorax. Although the mechanism of increased capillary permeability in these cases is unproved, reperfusion injury to a substrate of hypoxic tissue most likely plays a role, as discussed by Woodring (1997). Reexpansion pulmonary edema tends to occur with effusions that are present for several days and are large, but there is much overlap. Suggested preventive measures include minimizing negative intrapleural pressure during thoracentesis, limiting the volume of fluid removed per procedure, and terminating the procedure if excessive cough or chest pain develops. There is, however, no consensus with respect to the amount of fluid that be drained safely. Light (2001) suggests that withdrawal be limited to 1 L unless pleural pressures are measured, whereas Moyers and colleagues (1998) found no upper limit in their series. In practice, careful clinical observation of the patient and a low threshold to terminate the procedure are indicated.
NEEDLE BIOPSY OF THE PLEURA
Indications
In the past, blind needle biopsy of the pleura was useful mainly for diagnosing malignancy and tuberculous pleuritis. As discussed by Light (1998), newer approaches have lessened the role of this procedure. In addition, it is not clear that pleural biopsy in the usual case of pleural effusion significantly increases diagnostic accuracy. Prakash and Reiman (1985) found that pleural biopsy was positive in only 17% of 118 patients with malignant effusions but negative pleural fluid cytology. Thoracoscopy has emerged as a far more reliable method for determining malignancy when fluid cytology is negative. Similarly, tuberculous pleural disease is detected in most cases based on elevation of pleural fluid adenosine deaminase (ADA) and interferon- levels, and on the polymerase chain reaction (PCR) for tuberculous DNA in the fluid. Pleural biopsy is still occasionally indicated when these tests are negative or thoracoscopy is not available or feasible.
Contraindications and Cautions
Impaired coagulation is a contraindication to pleural biopsy. Unlike in the case of diagnostic thoracentesis, a small needle cannot be substituted for the large bore devices used for standard biopsy. Also, because of the large needle size, soft tissue abscess can occur in cases of empyema, although there is little reason to perform a pleural biopsy in the presence of frank purulence.
Procedure
Either an Abrams or a Cope needle is used. Both are large-bore metal devices in which a notch near the end of the needle engages the pleura from within as the system is withdrawn. In the former, the notch is located on the large outer cannula, and an inner cutting cannula is then passed through the outer trocar to cut the specimen from the chest wall. In the latter system, the notch is located on an inner biopsy trocar, and the specimen is cut by passing the large outer cannula over the trocar. Both can be connected to a syringe. As for thoracentesis, the entry site is below the superior aspect of percussible dullness posteriorly. The effusion is entered with the needle used for injecting local anesthetic. A small incision is made, and the pleural needle is passed through the parietal pleura, at which time a popping sensation is noted. The device is then withdrawn slightly until the pleural is engaged by the notch. The specimen is then severed as noted. Several samples are obtained. If the effusion is small or loculated, the procedure should be performed under ultrasound guidance.
P.312
Complications
The complications associated with pleural biopsy are similar to those due to thoracentesis and include pneumothorax, bleeding, and injury to upper abdominal organs. The chance of the first two of these is augmented because of the larger size of the needle. In addition, it is important to maintain the notch directed inferiorly, especially during withdrawal, in order to avoid injury to the intercostal bundle.
REFERENCES
Al-Sofyani M, Maziak DE, Shamji FM: Cervical mediastinoscopy incisional metastasis. Ann Thorac Surg 69:1255, 2000.
Ayo DS, et al: Pleural fluid white blood cell count variation using different sample containers and methods at 4 and 24 hours after collection. Chest 116:357S, 1999.
Backer CL, et al: Selective preoperative evaluation for possible N2 disease in carcinoma of the lung. J Thorac Cardiovasc Surg. 93:337, 1987.
Barter T, et al: Lower risk and higher yield for thoracentesis when performed by experienced operators. Chest 103:1873, 1993.
Basca S, Czako Z, Vezendi S: The complications of mediastinoscopy. Panminerva Med 16:402, 1974.
Ben-Yehuda D, et al: Image-guided core-needle biopsy in malignant lymphoma: experience with 100 patients that suggests the technique is reliable. J Clin Oncol 14:2431, 1996.
Bernstein MP, Ferrara JJ, Brown L: Effectiveness of scalene node biopsy for staging of lung cancer in the absence of palpable adenopathy. J Surg Oncol 29:46, 1985.
Bocage JP, McKenzie JW, Nosher JL: Invasive diagnostic procedures. In Shields TW, LoCicero J, Ponn RB (eds): General Thoracic Surgery. 5th Ed. Philadelphia. Lippincott-Williams & Wilkins, 2000, p. 275.
Brantigan JW, Brantigan CO, Brantigan OC: Biopsy of nonpalpable scalene lymph nodes in carcinoma of the lung. Am Rev Respir Dis 107: 962, 1973.
Canadian Lung Oncology Group: Investigation for mediastinal disease in patients with apparently operable lung cancer. Ann Thorac Surg 60: 1382, 1995.
Carlens E: Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 36:343, 1959.
Carney M, Ravin CE: Intercostal artery laceration during thoracentesis. Increased risk in elderly patients. Chest 75:520, 1979.
Chang DS, et al: Comparison of pleural fluid pH values obtained using blood gas machine, pH meter, and pH indicator strip. Chest 114:1368, 1998.
Daly BDT Jr, et al: Mediastinal lymph node evaluation by computed tomography in lung cancer. An analysis of 345 patients grouped by TNM staging, tumor size, and tumor location. J Thorac Cardiovasc Surg 94: 664, 1987.
Daniels AC: A method of biopsy useful in diagnosing certain intrathoracic diseases. Dis Chest 16:360, 1949.
Deslauriers J, et al: Mediastinopleuroscopy: a new approach to the diagnosis of intrathoracic diseases. Ann Thorac Surg 22:265, 1976.
Garpestad E, et al: CT fluoroscopy guidance for transbronchial needle aspiration. An experience in 35 patients. Chest 119:329, 2001.
Gdeedo A, et al: Prospective evaluation of computed tomography and mediastinoscopy in mediastinal lymph node staging. Eur Respir J 10:1547, 1997.
Ginsberg RJ, et al: Extended cervical mediastinoscopy. A single staging procedure for bronchial carcinoma of the left upper lobe. J Thorac Cardiovasc Surg 94:673, 1987.
Ginsberg RJ: The role of preoperative surgical staging in left upper lobe tumors. Ann Thorac Surg 57:526, 1994.
Graeter TP, et al: Mediastinal lymph node staging in suspected lung cancer: comparison of positron emission tomography with F-18 fluorodeoxyglucose and mediastinoscopy. Ann Thorac Surg 75:231, 2003.
Greschuchma D, Maassen W: Results of mediastinoscopy and other biopsies in sarcoidosis and silicosis. In Jepsen O, Sorensen HR (eds): Mediastinoscopy. Denmark: Odense University Press, 1971, pp. 79 82.
Gress FG, et al: Fine needle aspiration biopsy guided by endoscopic ultrasonography and computed tomography in the preoperative staging of non-small cell lung cancer. A comparison study. Ann Intern Med 127: 604, 1997.
Grogan DR, et al: Complications associated with thoracentesis: a prospective, randomized study comparing three different methods. Arch Intern Med 150:873, 1990.
Gupta NC, Graeber GM, Bishop HA: Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm) and large (>3 cm) lymph node lesions. Chest 117:773, 2000.
Hammoud ZT, et al: The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 118:894, 1999.
Harken DE, et al: A simple cervicomediastinal exploration for tissue diagnosis of intrathoracic disease. N Engl J Med 251:1041, 1954.
Harnsberger HR, Lee TG, Mukuno DH: Rapid, inexpensive real-time directed thoracentesis. Radiology 146:545, 1983.
Harrow EM, et al: The utility of transbronchial needle aspiration in the staging of bronchogenic carcinoma. Am J Respir Crit Care Med 161: 601, 2000.
Health and Public Policy Committee, American College of Physicians: Diagnostic thoracentesis and pleural biopsy in pleural effusions. Ann Intern Med 103:799, 1985.
Herman SJ, et al: Anterior mediastinal masses: utility of transthoracic needle biopsy. Radiology 180:167, 1991.
Izbicki JR, et al: Accuracy of computed tomographic scan and surgical assessment for staging of bronchial carcinoma. A prospective study. J Thorac Cardiovasc Surg 104:413, 1992.
Jahangiri M, Goldstraw P: The role of mediastinoscopy in superior vena caval obstruction. Ann Thorac Surg 59:453, 1995.
Kernstine KH, et al: Can FDG-PET reduce the need for mediastinoscopy in potentially resectable nonsmall cell lung cancer? Ann Thorac Surg 73:394, 2002.
Lee JD, Ginsberg RJ: Lung cancer staging: the value of ipsilateral scalene lymph node biopsy performed at mediastinoscopy. Ann Thorac Surg 62: 338, 1996.
Light RW: Closed needle biopsy of the pleura is a valuable diagnostic procedure con. J Bronchol 5:332, 1998.
Light RW. Pleural Diseases. 4th Ed. Philadelphia: Lippincott Williams & Wilkins, 2001, pp. 362 363.
Luke WP, et al: Prospective evaluation of mediastinoscopy for assessment of carcinoma of the lung. J Thorac Cardiovasc Surg 9:53, 1986.
Mateu-Navarro M, et al: Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg 70;391, 2000.
McAdams MP, Goodman PC, Kussin P: Virtual bronchoscopy for directing transbronchial needle aspiration of hilar and mediastinal lymph nodes. A pilot study. AJR Am J Roentgenol 170:1361, 1998.
McLoud TC, Flower DR: Imaging the pleura: sonography, CT, MR imaging. Am J Radiol 156:1145,1991.
McNeill TM, Chamberlain JM: Diagnostic anterior mediastinotomy. Ann Thorac Surg 2:532, 1966.
McVay PA, Toy PT: Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion 31:164, 1991.
Meersschaut D, et al: Repeat mediastinoscopy in the assessment of new and recurrent lung neoplasm. Ann Thorac Surg 53:120, 1992.
Mehta AC, et al: Transbronchial needle aspiration for histology specimens. Chest 96:1228, 1989.
Midthun DE, et al: Transbronchial versus endosonography-guided fine needle aspiration in the evaluation of lung cancer. Am J Respir Crit Care Med 163:957, 2001.
Morrisey B, et al: Percutaneous needle biopsy of the mediastinum: review of 94 procedures. Thorax 48:632, 1993.
Moyers JP, et al: Thoracentesis performed by radiologists using ultrasound guidance is safe regardless of the amount of fluid withdrawn. Chest 114:368S, 1998.
Nakanishi R, et al: Treatment strategy for patients with surgically discovered N2 stage IIIA non-small cell lung cancer. Ann Thorac Surg 64:342, 1997.
Patterson GA, et al: Significance of metastatic disease in subaortic lymph nodes. Ann Thorac Surg 43:155, 1987.
Pearson FG: An evaluation of mediastinoscopy in the management of presumably operable bronchial carcinoma. J Thorac Cardiovasc Surg 55: 617, 1968.
P.313
Phillips MS, Barker V: Extrathoracic lymph node aspiration in bronchial carcinoma. Thorax 40:398, 1985.
Poncelet AJ, et al: PET-FDG scan enhances but does not replace preoperative surgical staging in non-small cell lung cancer. Eur J Cardiothorac Surg 20:468, 2001.
Porte H, et al: The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg 13:196, 1998.
Prakash UBS, Reiman HM: Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 60:158, 1985.
Protopapas Z, Westcott JL: Transthoracic needle biopsy of mediastinal lymph nodes for staging lung and other cancers. Radiology 199:489, 1996.
Protopapas Z, Westcott JL: Transthoracic hilar and mediastinal biopsy. J Thorac Imaging 12:250, 1997.
Puhaka H: Complications of mediastinoscopy. J Laryngol Otol 103:312, 1989.
Rendina EA, et al: Biopsy of anterior mediastinal masses under local anesthesia. Ann Thorac Surg 74:1720, 2002.
Roberts SA: Obtaining tissue from the mediastinum: endoscopic ultrasound-guided transesophageal biopsy. Thorax 55:983, 2000.
Roberts PF, et al: Factors associated with false-positive staging of lung cancer by positron emission tomography. Ann Thorac Surg 70:1154, 2000.
Rohwedder JJ, Handley JA, Kerr D: Rapid diagnosis of lung cancer from palpable metastases by needle thrust. Chest 98:1393, 1990.
Sahn SA: State of the art: the pleura. Am Rev Respir Dis 138:183, 1988.
Schubach SL, Landreneau RJ: Mediastinoscopic injury to the bronchus: use in incontinuity muscle flap repair. Ann Thorac Surg 93:1101, 1992.
Shannon JJ, et al: Endobronchial ultrasound-guided needle aspiration of mediastinal adenopathy. Am Rev Respir Crit Care Med 153:1424, 1996.
Sider L, Davis TM: Hilar masses. Evaluation with CT-guided biopsy after negative bronchoscopic examination. Radiology 164:107, 1987.
Silverman SG, et al: Impact of positive findings at image-guided biopsy of lymphoma on patient care: evaluation of clinical history, needle size, and pathologic findings on biopsy performance. Radiology 190;759, 1994.
Sokolowski JW Jr, et al: Guidelines for thoracentesis and needle biopsy of the pleura. Am Rev Respir Dis 140:257, 1989.
Specht G: Discussion by Carlens. In Jepsen O, Sorenson HR (eds): Mediastinoscopy. Denmark: Odense University Press, 1971, p. 130.
Webb WR, et al: CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 178:705, 1991.
Weisbrod G, et al: Percutaneous fine-needle aspiration biopsy of the mediastinum. Clin Chest Med 8:27, 1987.
Woodring JH: Focal reexpansion pulmonary edema after drainage of large pleural effusions: clinical evidence suggesting hypoxic injury to the lung as the cause of edema. South Med J 90:1176, 1997.
EAN: 2147483647
Pages: 203